Abstract
Considering the economic and environmental role played by bees and their present threats it is necessary to develop food supplements favoring their health. The aim of this work was to isolate and characterize an immunomodulating probiotic capable to improve the health of honeybee colonies. For this purpose, bacterial strains were isolated from Apis mellifera bees (N = 180) obtained at three apiaries. A total of 44 strains were isolated and 9 of them were identified as Lactobacillus having the capacity to grow under saccharose osmotic stress, at pH 4.0 and possessing a wide susceptibility to antibiotics. Results allowed to select two strains but finally only one of them, strain A14.2 showed a very significant immunomodulating activity. This strain increased the expression of mRNA codifying the antimicrobial peptides 24 h post-administration. We evaluated its growth kinetics under aerobic and microaerobic conditions and its survival in the presence of high concentrations of saccharose. Results demonstrated that Lactobacillus casei A14.2 strain was highly tolerant to oxygen and that it was able to adapt to saccharose enriched environments (50% and 100% w/v). Finally, L. casei A14.2 strain was administered monthly during summer and early fall to 4 honeybee colonies (2 controls and 2 treatments). The results showed a gradual sustained decrease of infestation (p < 0.05) by the pathogenic Nosema spp. but no reduction in the infestation by the mite Varroa destructor. These results suggest that the administration of this potential probiotic, may increase the resistance of honeybee colonies to infectious diseases caused by Nosema spp.
Keywords: Apis mellifera, Probiotic, Immunomodulation, Antimicrobial peptides, Lactobacillus casei
1. Introduction
Apis mellifera is considered an important worldwide pollinator which contributes to the diversity and quality of the human diet and also is a producer of a series of goods, such as honey, propolis, royal jelly and bee venom (Alberoni et al., 2018, Cornara et al., 2017). Regarding its economic importance, the government of the USA estimates the year social gain of the honeybee ranges between US $ 1.6 and 5.7 billion (Chopra et al., 2015). On the other hand, the dependence of the agricultural sector on the pollinizing services is as well considerable, being valued in the range of US $14.2 to 23.8 billion (Southwick and Southwick, 1992).
A number of reasons have considerably reduced the populations of honeybees during the last years, causing the colony collapse disorder (CCD). CCD is characterized by the rapid death of worker bees, high record of deaths in the vicinity of the colonies and finally a kleptoparasitism by nearby colonies (vanEngelsdorp et al., 2017). Factors associated to the development of this disease include malnutrition by monocultures, and the presence of agrochemical, such as the indiscriminate use of pesticides), making it a multifactorial phenomenon (Kane and Faux, 2021, Rucker et al., 2019). Besides, A. mellifera is subjected to a series of pathologies caused by pathogens, such as Nosema spp. Negative effects of nosemosis include reduction of productivity and longevity of adult bees and affect brood rearing and bee behavior. On the other hand, Varroa destructor, reduces the reproductive capacity and the general fitness of the colony (Hristov et al., 2020). Therefore, it becomes necessary to consider different treatments to avoid the pathologies affecting apiaries. Depending on the legislation of different countries, a variety of antibiotics and/or pesticides are usually used to protect bees, but they may have adverse effects on the survival of the colony and, at the same time, in the quality of the products (El-Nahhal, 2020). The presence of traces of pesticides such as insecticides, fungicides, herbicides and acaricides, has been reported in the honey (El-Nahhal, 2020). It has also been reported that oxalic acid, used by the beekeepers in Varroa control treatment, can produce a number of problems to beehives, including a reduced longevity (Rademacher et al., 2017). On the other hand, the constant use of antibiotics can alter the microbiota present in bees, which may have a negative impact in their immune system (Daisley et al., 2020). The immune system of bees includes a physical and a humoral barrier (Tihelka, 2018). The latter includes antimicrobial peptides (AMPs) which provide protection against pathogens (Tihelka, 2018). Two essential mechanisms of action of AMPs are the breakdown of the membrane of prokaryotes and the inhibition of translation of proteins or affecting their proper folding in bacteria (Tihelka, 2018).
Considering the above, the constant use of the presently used treatments, although being effective against pathogens, involve several adverse effects including flight, the reproductive capacity of queens, ability to learn, reduced pollinizing capacity and affected communication capacities (Daisley et al., 2020, Larsen et al., 2019, Mullin et al., 2010). Therefore, it is important to search for new alternatives capable to be a complement or a substitute to the present-day options. Under this scenario, it is possible to consider the use of probiotics, live bacteria which when consumed in adequate quantity provide benefits to the host (Ganguly et al., 2011). The most widely used bacterial species to develop probiotics belong to the genus Lactobacillus (Widyastuti et al., 2021), and they are abundant in the gastrointestinal (GI) tract of bees (Nowak et al., 2021). A specific type of probiotics, the immunobiotics provide a benefic immunomodulation to the host (Clancy, 2003).
The use of probiotics in bees has shown promising results (Han et al., 2022, Motta et al., 2022). For example, the use of probiotics has increased the population of bees and/or the yield of honey per colony (Audisio and Benítez-Ahrendts, 2011, Fanciotti et al., 2018). Other studies have shown that bacterial strains increase the expression of AMPs in A. mellifera; nevertheless, the effect of immunomodulating strains on the sanity of commercial beehives has not yet been described (Evans and Lopez, 2004, Maruščáková et al., 2020).
A. mellifera is a worldwide important insect but honeybee colonies are presently affected by various threats and current treatments have negative consequences on the health of bees. Therefore, the aims of the present work were to isolate and characterize Lactobacillus strains showing immunobiotic potential and to perform a pilot study administering the strain showing the best characteristics as a nutritional supplement to honeybee colonies to analyze its capacity to control infections in commercial beehives.
2. Materials and methods
2.1. Characterization of bacterial strains isolated from the gastrointestinal (GI) tract of bees
2.1.1. Isolation of bacterial strains from the GI tract of bees
All the experiments and methods were designed with the aim of minimizing animal suffering. Bees were collected from three apiaries (located at the localities of Nonguen, Lorenzo Arenas and Pedro del Rio Zañartu) in the province of Concepcion, Chile, between January and April 2018. The three apiaries are under the management of the same beekeeper (Figure S1). The apiaries were selected considering two factors. One of them was their proximity to the laboratory, to maintain the samples integrity during transportation. The other consideration was to sample apiaries surrounded by different vegetation to improve the possibility of collecting microbiotas with variations in their bacterial composition; therefore increasing the probability to find probiotics strains. In each apiary, bees were collected at three hives. Twenty foraging bees were collected at each hive to isolate possible probiotic strains. Foraging bees were selected because they confront the most adverse conditions and they contain bacteria belonging to genus Lactobacillus, known to include potentially probiotic strains. Twenty bees per hive, thus totaling 180 bees, were considered a representative number of individuals to isolate possible probiotic strains. The foraging bees collected at each hive were immediately transported at 4 °C to the Laboratory of Bacterial Pathogenicity, Department of Microbiology, Faculty of Biological Sciences, University of Concepcion, Concepcion, Chile. Once at the laboratory, they were subjected to 4 °C for 20 min.Their digestive tracts were aseptically extracted in a biosafety chamber, macerated in a sterilized mortar containing sterile phosphate buffered saline 1X (PBS), pH 7.3. The macerate was placed in plates containing Man-Rogosa-Sharpe (MRS) agar (Difco, France) and incubated under microaerobic conditions (10% CO2) at 37 °C for two to five days (Olofsson and Vásquez, 2008). Colonies showing a macroscopic and microscopic morphology similar to that of Lactobacillus were isolated by successive subcultures in MRS agar until pure bacterial cultures were obtained. Pure cultures were maintained in MRS broth (Difco, France) plus 20% (v/v) sterile glycerol (Merck KGaA, Darmstadt, Germany) at −20 °C until analysis.
2.2. Identification of Lactobacillus strains isolated from the GI tract of bees
The identification of the pure cultures was done by means of PCR amplification of the DNA obtained from the isolated colonies. For this, the SapphireAmp Fast PCR Master Mix kit was used (TAKARA BIO INC, Japan) following the indications of the manufacturer. The PCR conditions are indicated in Table S1. LbG primers, which allow to identify the different species of the genus Lactobacillus (Table S2) were used. The products of the amplification were analyzed after agarose gel electrophoresis (1.5% p/v) visualized under a UV transilluminator (ENDUROTM GDS). Strains positive for the genus Lactobacillus were characterized in accordance with their tolerance to saccharose, pH and their susceptibility to antibiotics.
2.3. Tolerance of Lactobacillus to high concentrations of saccharose
Strains belonging to genus Lactobacillus were seeded in MRS broth supplemented with 25% or 50% w/v saccharose. Throughout the text, 25%, 50% and 100% w/v saccharose correspond to 250, 500 or 1000 g of saccharose in 1 L of water, respectively, MRS broth alone was used as control. Lactobacillus strains were suspended at a concentration equivalent to 0.5 McFarland and incubated for 24 h at 37 °C under aerobic conditions and then the turbidity of the culture was evaluated after 24 h incubation. The following criteria were used to evaluate the bacterial growth: (-): no growth, (+): low growth, (++): medium growth, (+++): high growth, (++++): very high growth.
2.4. Resistance of Lactobacillus strains isolated from the GI tract of bees to acidic pH
Lactobacillus strains were seeded, at a concentration equivalent to 0.5 McFarland in MRS broth adjusted to pH 4.0 (using 1 M HCl) or pH 6.9 (using 1 M NaOH), corresponding to the pH of the GI tract of bees, and incubated at 37 °C under aerobic conditions for 24 h. Then, the turbidity of the medium was analyzed as evidence of growth. The turbidity was evaluated following the same procedure described previously (Refer to section 2.3).
2.5. Profile of the antibiotic susceptibility of Lactobacillus strains isolated from the GI tract of bees
The antibiotic susceptibility of Lactobacillus strains isolated from the GI tract of bees was evaluated using the following antibiotics: Cefotaxime, Ampicillin, Gentamicin, Chloramphenicol, Kanamycin, Amikacin,-sSulfamethoxazole-Trimethoprim, Ciprofloxacin, Colistin, Rifampicin, Vancomycin, Neomycin, Streptomycin, Efrotomycin, Clarithromycin and Penicillin (Oxoid, Thermo Fisher Scientific, Waltham, MA, USA). Bacterial suspensions of the strains to be assayed were prepared at a concentration equivalent to 0.5 McFarland in sterile saline solution. One hundred μL of the bacterial suspension were seeded on MRS agar containing dishes and a maximum of 5 antibiotic disks were placed per dish. Then, dishes were incubated under microaerobic conditions at 37 °C for 48 h. After 48 h incubation, the zones of inhibition were measured and recorded in mm. The criterium to evaluate susceptibility was the one described by (Georgieva et al., 2008) expressing sensitivity as R (resistant); MS (intermediate sensitive)- zone of inhibition between 7 and 16 mm; S (sensitive) zone of inhibition between 16 and 25 mm; SS (highly sensitive) zone of inhibition over 25 mm; ND not determined.
2.6. Modulation of AMPs expression in the GI tract of bees by Lactobacillus strains
To determine which probiotic strains showed the capacity to modulate the immune system of the A. mellifera bees, two strains (A14.2 y A8.2) were selected based on of the characteristics shown by them in the previous assays.
2.7. Bioassay cages
The methodology of Williams et al., (2013) with modifications was used to build the cages. Six cages, capable to sustain the worker bees for 5 days, were manufactured in the laboratory using transparent 300 mL plastic containers which were placed inverted, with their caps facing down. Two orifices were made, one at the top of the cage for feeding purposes to allow the administration of 50% w/v saccharose, and the second orifice, on the side of the cage, allowed to introduce the water supply. The feeders were manufactured using 1.5 mL Eppendorf tubes (Fleming et al., 2015). Also, two 15 mL Falcon tube caps were added, one containing 1 mL tap water and one containing 1 mL 50% w/v saccharose. Beeswax was added at the interior top of the cages. Ventilation orifices were made and covered with a plastic mesh. Cages were placed on Petri dishes and fixed in place using adhesive tape (Figure S2) (Williams et al., 2013).
Ten foraging worker bees, obtained from “Apiario Nonguén” (Nonguén valley, Concepcion), were placed inside each cage. Cages were divided into three groups, named A, B or C, of 2 cages each. On day 1, group A bees were administered 1x105 CFU/mL of the strain A14.2 in 50% w/v saccharose, while group B was administered 1x105 CFU/mL of the strain A8.2 in 50% w/v saccharose and group C (control) received only 50% w/v saccharose. Bee cages were maintained at 37 °C in a humidified incubator adjusted to a humidity of 60–70% (Williams et al., 2013). From day 2 onwards, all groups received 50% w/v saccharose (Iansa, Santiago, Chile). The bees of one cage of each group were sacrificed after 24 h and the other cage on day 5 post administration of the bacterial strains. For this, bees were kept at 4 °C for 20 min and then the GI tracts were extracted from seven bees from each cage. The GI tracts were maintained in Ambion RNAlater buffer (Invitrogen, Carlsbad, CA, USA) at 4 °C for 24 h and then at −80 °C until further processing.
2.8. RNA extraction and cDNA synthesis
Whole GI tracts from each bee was removed, resuspended in 1 mL Trizol reagent (TRI reagent) (Sigma-Aldrich) and homogenized using a Precellys Evolution homogenizer (Bertin Technologies) (Kit Precellys CK-14) for 3 cycles of 15 s each at 6500 rpm, with a 10 s pause between cycles. RNA was extracted using Trizol.
Samples were resuspended in 50 μL of nucleases free water and quantified measuring their absorbance at 260 and 280 nm (Synergy 2, BioTek, USA), and stored at −80 °C until use. The cDNA was synthetized according to the protocol of M−MLV Reverse Transcriptase (Thermo FisherScientific Inc) provided by the manufacturer using 0.5 μg RNA per sample as template. The samples of cDNA were stored at −20 °C until use.
2.9. Gene expression analysis (qPCR)
The expression of the abaecin, defensin I, defensin II, apisimin and hymenoptaecin genes was evaluated with specific primers (Table S3). To normalize the data according to the total amount of RNA in each sample an analysis of the consistently expressed Alpha tubulin was performed. Amplification was carried out in a 25 µL reaction volume containing 12.5 µL SYBR Green Master Mix 2X (Applied Biosystems), 2 µL cDNA (diluted 10X), 8.5 µL water and 1 µL (200–600 nmol) of each gene-specific primer. The experimental protocol consisted of an initial denaturation at 95 °C for 10 min, followed by amplification including 40 cycles of 3 steps: denaturation at 95 °C for 30 s, annealing at 60 °C for 1 min and extension at 72 °C for 30 s. The specificity of the PCR products was verified by melting curve analysis for all samples. Relative normalized expression was calculated by the ‘’2 − ΔΔCT’’ method. Results of the gene expression experiment conducted in triplicates were expressed as mean ± standard deviation (SD).
2.10. Growth characteristics of the selected strain with immunomodulating activity
2.10.1. Growth kinetics of the selected probiotic strain A14.2 under aerobic and microaerobic conditions
The growth kinetics strain A14.2, was determined under aerobic and microaerobic conditions using the microdrop technique (Herbert, 1990). For this, the strain was seeded, by streaking, in dishes containing MRS agar and then incubated for 48 h under microaerobic condition. A 108 CFU/mL bacterial suspension was prepared in 5 mL sterile saline solution and 500 µL of this suspension were added to sterile flasks containing 50 mL MRS broth each to obtain an initial concentration of 106 CFU/mL. Flasks were incubated under microaerobic and aerobic conditions at 37 °C for 48 h and dilutions up 10-5were prepared at times 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24 y 48 h and 10 µL were seeded, by triplicate, of each dilution in dishes containing MRS agar. Dishes were incubated at 37 °C under microaerobic conditions and the data obtained allowed to calculate the growth velocity (µ) (h−1) of the strain and its duplication time (dt).
2.11. Survival curve of strain A14.2 in MRS broth supplemented with 50 or 100% w/v saccharose
To evaluate if the selected probiotic strain A14.2 was able to survive at different concentrations of saccharose, the following assay was performed. Dishes containing MRS agar were seeded, by streaking, and incubated under microaerobic condition for 48 h. A 108 CFU/mL bacterial suspension was prepared in 5 mL sterile saline solution and 500 µL of this suspension were added to sterile flasks containing 50 mL MRS broth supplemented with 50% or 100% w/v saccharose each to obtain an initial concentration of 106 CFU/mL. Dilutions up to 10-5 were prepared at times 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24 and 48 h and 10 µL of each dilution were seeded, by triplicate, in dishes containing MRS agar. Dishes containing the microdrops were incubated at 37 °C under aerobic conditions for 48 h and finally the number of colonies was counted.
2.12. Pilot study of the putative probiotic effect of L. casei A14.2 on the health status of A. mellifera evaluated in a commercial apiary
2.12.1. Bacterial dose and administration
The methodology of Audisio & Benítez-Ahrendts (2011) with some modifications was used. A 105 CFU/mL dose of the probiotic strain was administered in 100% w/v saccharose. The administration was done once every 30 days during 3 months including summer and early fall. The A14.2 strain was always administered on the same day and at the same time to the four hives considered in the study which were subjected to the same feeding conditions, location, and supervision except for the sun exposure. Group 1 included beehives with more exposition to the sun while Group 2 were less exposed to the sun being more time under the shadow (Audisio and Benítez-Ahrendts, 2011). Two groups of two hives each were analyzed, one group was administered with probiotic and the other group (control) was administered only saccharose.
2.12.2. Quantification of the infestation by Varroa destructor
The percentage of infestation by V. destructor was determined once a month during a 3-month period including summer and early fall (except January due to technical problems). Using containers with 70% v/v ethanol, bees were collected from treated and non-treated hives. Approximately 200 bees were transferred to another container and soapy water was added until all bees were immersed, and the container was agitated for 1 min to detach the adhered mites. Then, the content of each container was poured on a 2.8 mm mesh placed on top of a white fabric to collect the bees on the mesh and the mites on the fabric. Finally, the bees and mites were counted (Dietemann et al., 2013). The infestation percentage was calculated as the quotient between the number of mites and the number of bees analyzed multiplied by 100. In January 2019, all hives were treated against V. destructor using oxalic acids sublimation.
2.12.3. Quantification of the infestation by Nosema spp.
Infestation by Nosema spp. was determined once a month, during a 4 months period including summer and early fall. Twenty bees were collected from each colony, their abdomens cut and placed on a sterile mortar containing 5 mL sterile distilled water to be macerated until a homogeneous product was obtained. From this macerate a 15 µL aliquot was obtained and placed on a Neubauer chamber to count the spores using an optical microscope set at 400X. Then, the following formula was used:
where: Z = number of spores per bee, α = total spores counted, β = number of squares of the Neubauer chamber counted and δ = dilution factor (OIE - World Organisation for Animal Health, 2008).
2.13. Statistical analysis
The number of replicates for each experiment is mentioned in the respective Sections. For standard deviation and statistical significance, calculations and preparation of figures, Infostat and GraphPad Prism 7 software were used. Two-way ANOVA plus Bonferroni post-test was used to analyze the RT-qPCR assay. Paired Two-tailed t-test was used to analyze the growth kinetics of A14.2 strain. Two-way ANOVA multiple comparisons were used to analyze the results of Nosema spp. infestation in hives, comparing each mean of control and treatment, after data normalization. All the analyses were performed with 95% confidence and results were considered significant when p < 0.05.
3. Results
3.1. Isolation and identification of Lactobacillus strains isolated from the GI tract of bees
In accordance with the selection criteria, 44 strains were isolated from the GI tract of A. mellifera bees. All isolates were Gram positive microorganisms having a bacillar morphology, forming white circular colonies and shiny in MRS agar (Figure S3). After the molecular identification, 9 strains (20.5%) were ascribed to the genus Lactobacillus, because the 750 bp amplicon of interest was present in them (Figure S4).
3.2. Tolerance of the Lactobacillus strains isolated from the GI tract of bees to high saccharose concentrations
The nine Lactobacillus strains were subjected to the presence of high saccharose concentrations in the culture medium. All of them (100%) showed to be able to grow under saccharose caused osmotic stress (Table 1). In most cases, the turbidity of cultures supplemented with saccharose exceeded the turbidity of controls cultured in MRS broth alone (Table 1).
Table 1.
Growth of Lactobacillus strains isolated from the gastrointestinal tract of bees subjected to high concentrations of saccharose in MRS broth.
| N° | Strains | MRS Broth (control) | Saccharose 25% | Saccharose 50% |
|---|---|---|---|---|
| 1 | LU2.3 | – | ++ | ++ |
| 2 | A10.3 | + | ++ | ++ |
| 3 | A8.2 | + | ++ | ++ |
| 4 | A7.2 | + | ++ | ++ |
| 5 | A21.2 | + | ++ | ++ |
| 6 | A11.1 | +++ | ++ | ++ |
| 7 | A16.3 | + | +++ | ++ |
| 8 | A10.2 | + | +++ | ++ |
| 9 | A14.2 | ++++ | +++ | ++ |
MRS broth: Man, Rogosa and Sharpe broth, (-): no growth, (+): low growth, (++): moderate growth, (+++): high growth, (++++): very high growth.
3.3. Resistance of Lactobacillus strains isolated from the GI tract of bees to acidic pH
All strains were able to grow at pH 4.0 (Table 2), and 66.7% of them showed a high or very high growth under this pH condition (Table 2). The A14.2 strain was the one reaching the highest turbidity, implying the best growth, followed by LU2.3, A16.3, A11.1 and A10.3 strains showing a high growth (Table 2).
Table 2.
Growth, at pH 4 or 6,9, of te Lactobacillus strains isolated from the gastrointestinal tract of foraging bees.
| N° | Strains | pH 4.0 | pH 6.9 |
|---|---|---|---|
| 1 | A7.2 | ++ | +++ |
| 2 | LU2.3* | +++ | +++ |
| 3 | A16.3* | +++ | +++ |
| 4 | A11.1* | +++ | +++ |
| 5 | A14.2* | ++++ | +++++ |
| 6 | A8.2 | ++ | +++ |
| 7 | A21.2 | ++ | +++ |
| 8 | A10.2* | +++ | +++ |
| 9 | A10.3* | +++ | +++ |
(-): no growth, (+): low growth, (++): moderate growth, (+++): high growth, (++++): very high growth.
3.4. Profile of antibiotic susceptibility of Lactobacillus strains isolated from the GI tract of bees
Table S4 shows the susceptibility to antibiotics profile of Lactobacillus strains assayed. All of them showed a range between intermediate to highly sensitive for most of the antibiotics tested.
3.5. Modulation of the expression of AMPs in the GI tract of bees by Lactobacillus strains
The results obtained in the previous assays allowed to select two Lactobacillus strains, A14.2 and A8.2. The modulation of AMPs expression was analyzed by RT-qPCR. Results indicated that the treatment of bees with the A14.2 strain significantly increased the expression of mRNA for the AMPs Abaecin (p < 0.0001) and Defensin I (p < 0.001) in the GI tract of bees 24 h post administration (Fig. 1). No changes in the expression levels of mRNA were observed when the peptides were analyzed on day 5 (120 h) post administration of the strain A14.2. No changes were observed on the expression levels of mRNA on day 2 and day 5 for the analyzed peptides after administration of the A8.2 strain.
Fig. 1.
Effect of Lactobacillus A14.2 and A8.2 strains on the expression of antimicrobial peptides after or 120 h post probiotic administration. One mRNA Expression (Fold-Change) corresponds to the basal level of AMPs genes expression. Statistical significance is indicated as: * p < 0.05, ** p < 0.01, *** p < 0.001>, **** p < 0.0001.
Therefore, considering its immunomodulatory activity, the Lactobacillus A14.2 strain was selected to continue with the following assays. This strain was sequenced by an external institution and identified as Lactobacillus casei A14.2 (now known as Lacticaseibacillus casei) [35].
3.6. Growth kinetics of Lactobacillus casei A14.2 strain under aerobic and microaerobic conditions
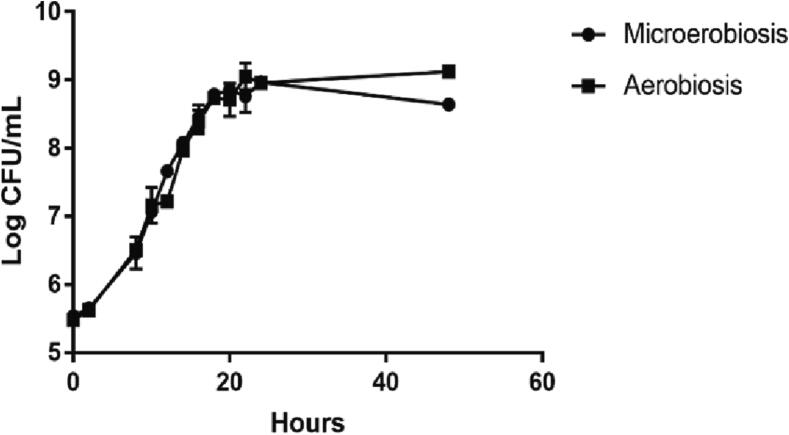
Fig. 2 and Table S5 show the growth kinetics and parameters for the A14.2 strain cultured under microaerobic or aerobic conditions. Results demonstrated that the growth of this strain under one or the other condition is similar (p > 0.05). Thus, the strain L. casei A14.2 is tolerant to oxygen. Its exponential phase growth occurs between 12 and 14 h of culture (Fig. 2), time in which it must be harvested for administration.
Fig. 2.
Growth kinetics of strain A14.2, expressed in Logarithm of Colony Forming Units (CFU) per milliliter per hour of bacterial growth. Curves corresponding to microaerobic, and aerobic conditions are presented.
3.7. Survival curve of L. casei A14.2 strain in MRS broth supplemented with 50% or 100% w/v saccharose
As shown by Fig. 3 the strain L. casei A14.2 remains viable at least for 48 h when subjected to concentrations of 50% w/v or 100 % w/v saccharose. Moreover, at a concentration of 50% w/v saccharose, after 24 h of incubation the exponential phase of the strain started in this medium. This indicates the high tolerance and adaptability of L. casei strain A14.2 to environments enriched with saccharose.
Fig. 3.
Growth kinetics of strain A14.2, expressed in Logarithm of Colony Forming Units (CFU) per milliliter per hour of bacterial growth in MRS broth supplemented with 50% or 100% w/v saccharose.
3.8. Quantification of the infestation by V. destructor
The percentage of the infestation by V. destructor varied during the months of the intervention in the groups 1 and 2 of honeybee colonies. At the end of the study, the infestation percentage was 0% and 2% for the controls and for the beehives treated 0% and 1% for group 1 and 2, respectively (Fig. 4).
Fig. 4.
Quantification of infestation by V. destructor at a commercial apiary during 3 months of administration of the probiotic strain L. casei A14.2. A) Group 1 of hives (more sun exposure). B) Group 2 of hives (less sun exposure).
3.9. Quantification of the infestation by Nosema spp.
Regarding the levels of infestation by Nosema spp., the treated beehives showed a steady decrease of the infestation (Table 3). In general, noticeably decrease in the count of spores was observed after 30 days after the first administration of the strain A14.2 in the beehives of both groups (Table 1). Beehives fed only saccharose showed no clear decrease or increase of infestation by the pathogen (Table 3). At the end of the three months of administration of the strain A14.2, the controls showed an average level of infestation of 1 million spores per bee, as compared to the treated group whose average counts were only thirty thousand spores per bee (Table 3).
Table 3.
Number of Nosema spp. spores per bee.
| Treatment | Mean Spore Number (Spores/Bee) ± SD |
||||
|---|---|---|---|---|---|
| Time 0 | 1 Month | 2 Month | 3 Month | ||
| Group 1 | Negative control | 0.0x100 ± 0.0x100 | 2.4x106 ± 5.3x105 | 1.9x105 ± 2.7x105 | 1.9x106 ± 1.8x105 |
| Probiotic | 2.0x107 ± 9.7x105 | 1.7x106 ± 5.3x104 | 2.6x106 ± 5.3x105 | 6.3x104 ± 8.8x104 | |
| Group 2 | Negative control | 7.5x105 ± 0.0x100 | 1.5x106 ± 1.8x105 | 3.8x106 ± 8.8x104 | 5.0x105 ± 1.8x105 |
| Probiotic | 9.4x105 ± 2.7x105 | 1.9x105 ± 8.8x104 | 6.3x104 ± 8.8x104 | 0.0x100 ± 0.0x100 | |
Bees were fed with saccharose (negative control) or saccharose containing probiotics.
Fig. 5 shows the data with respect to time zero, demonstrating a significant decrease (p < 0.05) in the number of spores per bee in the treated hives. In general, these results suggest that the strain A14.2 has the potentiality to control the infestation of Nosema spp. in bees.
Fig. 5.
Quantification of infestation by Nosema spp. at a commercial apiary during 3 months of administration of the probiotic strain L. casei A14.2. A) Group 1 of hives (more sun exposure). B) Group 2 of hives (less sun exposure). Statistical significance is indicated as: * p < 0.05, ** p < 0.01, *** p < 0.001>, **** p < 0.0001.
4. Discussion
The relationship between microbiota and health related mechanisms in bees caused the intestinal microbiota of bees to become a relevant issue in recent years (Nowak et al., 2021). Among the significant functions of the microbiota of bees is possibly to mention the degradation and use of pollen, the breakdown of toxic compounds and the ability to avoid colonization by pathogens (Royan, 2019). Therefore, the isolation of bacteria from the microbiota of bees to search for those strains having probiotic characteristics to improve the health of bees is an attractive proposal for the beekeeping industry (Di Gioia and Biavati, 2018). Specifically, the immunomodulating strain isolated and characterized in the present study(A14.2) belongs to Lactobacillus casei, a species associated to probiotic characteristics ranging from the treatment for atopic dermatitis to cancer (Hill et al., 2018).
The characteristics of L. casei strains include surviving in a great variety of hostile environments, such as acidic stress (Nezhad et al., 2015). This is a relevant factor for our study because its aim was to isolate a strain to be subjected to conditions of the the GI tract of bees whose pH varies between 5 and 6.8 (Zakaria, 2010). Particularly, the strain A14.2 remains viable under an acidic pH and its growth at a pH of 4.0 or 6.9 was better than that of other strains evaluated. This characteristic of L. casei strains has been associated to the expression of metabolic pathways and surface proteins involved in the adaptation to the acidic stress (Nezhad et al., 2015). Among these pathways, stand out those related to the metabolism of carbohydrates, whose glycolytic enzymes allow these bacteria to generate enough energy to grow when subjected to a low pH condition (Nezhad et al., 2015).
When characterizing a potential probiotic strain, it is necessary to evaluate its susceptibility to antibiotics because they might have and transmit resistance determinants (Campedelli et al., 2018). The strain A14.2 was susceptible to most antibiotics assayed, being resistant only to four antibiotics: Kanamycin, Sulfamethoxazole-Trimethoprim, Colistin and Vancomycin. Resistance to Vancomycin has been described in Lactobacillus species and it is associated to changes in the terminal D-alanine/ D-alanine residue present in peptidoglycan in which D-alanine is replaced by the residues D-lactate or D-serine, preventing the action of the antibiotic (Delcour et al., 1999). The resistance to Vancomycin is considered as intrinsic and, since it is codified in the chromosome, it is not transmissible (Zhou et al., 2005).
The resistance to Sulfamethoxazole-Trimethoprim has also been described as intrinsic within the genus Lactobacillus (Katla et al., 2001). Sulfamethoxazole-Trimethoprim inhibits the metabolic pathway which synthetizes folic acid (Katla et al., 2001). Lactobacillus strains have complex nutritional requirements, including the presence of purines to grow, not possessing a metabolic pathway affected by the mechanism of action of Trimethoprim (Katla et al., 2001), making many Lactobacillus strains naturally resistant to this antibiotic. Finally, the resistance against aminoglycosides (such as Kanamycin) and Polymyxins (such as Colistin) is widely distributed in the Lactobacillus genus and, as both previously mentioned antibiotics, is considered as intrinsic (Anisimova and Yarullina, 2019, Das et al., 2020).
When selecting a probiotic for bees, its ability to resist high saccharose concentrations is an important consideration because during periods with reduced availability of flowers the beekeepers usually supplement the diet using carbohydrates, such as saccharose (Taylor et al., 2019). Diets based mainly in saccharoseaffect the presence of bacterial populations in the intestinal microbiota of bees, favoring the presence of bacteria belonging to the family Lactobacillaceae and to the genus Lactobacillus (Taylor et al., 2019, Wang et al., 2020). In this study we observed that strains A14.2, A10.2 and A16.3 increased their growth when subjected to 25% w/v saccharose when compared to the control. It has been previously described that the presence of oligosaccharides favors the growth of some strains of Lactobacillus with potential probiotic activity and producers of lactic acid (Davoodi et al., 2016). All strains evaluated showed a moderate growth when exposed to 50% w/v saccharose(i.e. tolerated high saccharose concentrations). Strain A14.2,for which survival curves in the presence of 50% and 100% w/v saccharose were determined. Demonstrated that it is able to survive, and even to grow, in the presence of 50% w/v saccharose, suggesting that it is highly tolerant to the osmotic stress by saccharose.
It has been reported that bacteria such as Lactobacillus, Bifidobacterium and Gilliamella, symbionts present in the gut of bees, participate in the fermentation of complex carbohydrates and saccharoses which bees cannot digest (Kwong and Moran, 2016). These bacteria possess genes codifying, glycoside hydrolases and polysaccharide lyases, among other carbohydrate degrading enzymes (Engel et al., 2012, Kešnerová et al., 2017, Kwong et al., 2014, Lee et al., 2015). This may facilitate the manufacture of a probiotic food supplement for bees because strain A14.2 could be stored in a matrix containing high concentrations of saccharose and be added directly to the saccharose usually feed to the beehives without modifying the formula regularly used by beekeepers.
Regarding the immunity of A. mellifera, i unfavorable environmental conditions and pesticides make bees susceptible to pathogenic microorganisms (Nowak et al., 2021). Innate immunity of insects is an evolutionary conserved strategy providing prompt responses against invading pathogens (Evans et al., 2006). In particular, AMPs are key components of the humoral immunity of bees (Tihelka, 2018). These components are mainly regulated by the intracellular signaling pathways Toll and Imd/JNK (Evans et al., 2006).
It has also been demonstrated that lactic acid bacteria present in the intestinal community play an important role in the regulation and health equilibrium of bees (Kwong and Moran, 2016). In particular, the connection between the microbiota and the modulation of cells associated to immunity protects against pathogens modulating the expression of AMPs. AMPs damage the membrane of invasive cells (Cederlund et al., 2011) and inhibit the translation or folding of bacterial proteins (Tihelka, 2018). This study evidenced that the L. casei A14.2 probiotic strain is capable to modulate the immune system of A. mellifera. After 24 h of administering the L. casei A14.2 strain theexpression of genes codifying the AMPs Abaecin and Defensin I, participants of the immune response in bees, increased significantly. This modulation of the expression of immunity mediators was observed by Maruščáková and coworkers after administering the probiotic strain Lactobacillus brevis B50 in pollen, with a concomitant increase of the proportion of lactic acid bacteria and enterobacteria in the gut of bees (Maruščáková et al., 2020). With respect of the other two genes evaluated, apisimin and hymenoptaecin, on the contrary to the report of Kwong and coworkers, who reported that the strains Snodgrassella alvi wkB7 and Gilliamella apicola wkB7 strongly modulated the expression of these genes (Kwong et al., n.d.), we did not observe their modulation by the strain L. casei A14.2. Thus, it is possible to suggest that the regulation of immune responses of bees by different members of the microbiota can be mediated by different mechanisms and be strain dependent (Evans et al., 2006, Kwong et al., n.d..).
With respect to the peptides modulated, I is related to the defense against parasites (Hristov et al., 2020). Therefore, the administration of the probiotic L. casei A14.2 strain may protect bees from the parasite V. destructor, the main pathogen presents in beehives (Hristov et al., 2020). Defensin I is also related to the “social” immune system of bees; therefore, it may provide an adaptive advantage for the beehive because, since it acts as a superorganism, also Defensin I is directly related to the behavior and salubriousness of the beehive (Bonoan et al., 2020, Simone-Finstrom, 2017). Moreover, in bees this peptide is associated to the Toll type receptors by means of the DORSAL genes which are, in turn, related to the defense against Gram positive bacteria which are recognized by their specific peptidoglycan molecules (Lourenço et al., 2018). The activation of these pathways by the strain A14.2 could confer resistance to the beehives against the infection of Gram-positive pathogens, such as Paenibacillus larvae (Huang et al., 2021).
With respect to Abaecin, it is regulated by the intracellular signaling pathway Imd/JNK and it is connected with the individual immunity against parasites and Gram-positive bacteria, contributing to maintain the colonies more resistant and healthier (Tihelka, 2018). Hence, an appropriate modulation of the expression of both peptides (Defensin I and Abaecin), should play an important role in the survival of the beehive, selectively protecting it against different pathogens (Evans et al., 2006, Hinshaw et al., 2021).
The intestinal microbial community of bees is horizontally transmitted by social contact, as it is in mammals (Kwong and Moran, 2016). Therefore, the administration of probiotics maydetermine the composition of the microbiota (Kwong et al., n.d.), reinforcing the individual immune system of bees and the social one of the beehives to maintain the homeostasis of the microbiome and improve the health of bees (Arredondo et al., 2018, Maruščáková et al., 2020).
The desired characteristics of a probiotic strain include its capacity to resist different stressing conditions, including environmental conditions of the host and those involved in the production of the probiotic product itself (de Melo Pereira et al., 2018). A factor increasing the production cost of a probiotic is the expensive fermentation of anaerobes which negatively affects an increase in the production of biomass (Ren et al., 2019).The growth of L. casei A14.2 was studied under microaerobic and aerobic conditions and no significant differences in growth were found when comparing both conditions. Oxygen tolerant phenotypes have been described in L. casei and they could be exploited in the food industry (Zotta et al., 2017, Zotta et al., 2014). Moreover, an increased biomass production was reported when the L. casei N87 strain was cultured under aerobic conditions (Siciliano et al., 2019). Thus, it is possible to consider feasible to escalate the production of L. casei strains to be used as widespread probiotics in the future.
V. destructor is the most infecting mite for bees (Hristov et al., 2020). It causes direct damage to bees, consuming and digesting their fat body (Ramsey et al., 2019). Hence, a moderate infestation by this mite reduces the fitness of the beehive (Hristov et al., 2020). On the other hand, Nosema spp. are microsporidia which invade epithelial cells of the midgut of worker bees, queen bees and drones (Hristov et al., 2020, Papini et al., 2017). The presence of both V. destructor and N. ceranae affect the composition of the microbiome of bees (Huang and Evans, 2020, Hubert et al., 2017) and it can lead to the loss of specific functions associated to members of the microbiota. Thus, feeding bees with strains belonging to their normal microbiota may contribute to increase their resistance against the attack by these pathogens. In the present work, the probiotic showed a strong tendency to control the infestation by Nosema spp. but not that by V. destructor.
We were unable to establish if L. casei A14.2 had an effect on the infestation by this mite. Nevertheless, at the end of the three months assay it was possible to observe a lower level of infestation, under 2%, in the treated beehives when compared to the control. It will probably be necessary to administer L. casei A14.2 for a more extended time span and consider a larger number of beehives to ascertain if the probiotic has, in fact, an effect on this pathogen. It has already bee reported that bacterial strains isolated from bees have an acaricidal effect on V. destructor (Saccà and Lodesani, 2020) and assays for at least eight months in apiaries corroborated it (Sabaté et al., 2012, Tejerina et al., 2020). Based on the above it is possible to suppose that L. casei A14.2 may be beneficial against V. destructor, but a long-term assay will be required to confirm it.
It will be also necessary to evaluate other physiological parameters of the bees, such as the percentage of fatty tissue, because V. destructor targets the fat body of bees (Ramsey et al., 2019), using it as an indicator of improvement of the health condition of the bees. L. casei A14.2 may also be favoring the health of bees by other mechanisms, such as production of hydrogen peroxide, lactic acid or short-chain fatty acids or site-specific competence, all of them observed in strains having probiotic potential (de Melo Pereira et al., 2018). These are all interesting possibilities to be investigated in future L. casei A14.2 studies.
On the other hand, it was possible to observe the protecting effect of the strain L. casei A14.2 against Nosema spp. It may be due to its capacity to stimulate the expression of AMPs in the GI tract of bees, promoting the eradication of pathogens stimulatingthe local immune system. It has been observed that the infection by different Nosema species promotes the expression of AMPs (Antúnez et al., 2009). Specifically, N. ceranae can suppress the expression of the genes abaecin and hymenoptaecin (Antúnez et al., 2009). This study is reporting the ability of L. casei A14.2 to stimulate the expression of Abaecin in the GI tract of bees which may be playing a crucial role to provide protection against Nosema spp. The protecting effect of probiotics against Nosema spp. has already been reported in other studies. An in vitro assay using bees inoculated with N. ceranae showed that probiotics reduced the mortality of bees and studies in beehives showed a decrease in the number of spores (Borges et al., 2021, Tejerina et al., 2020, Tlak Gajger et al., 2020).
5. Conclusions
The present work, aimed to obtain a probiotic for honeybees, evaluated the innocuity and stress tolerance of Lactobacillus strains and their capacity to modulate the expression of antimicrobial peptides in bees, being Lactobacillus casei A14.2 the one showing the best characteristics to modulate health associated aspects in the bees. Moreover, in a small scale pilot study, using a reduced number of beehives, this strain showed to reduce the level of infestation by Nossema spp. Overall, these promising results encourage us to continue our work in this area in order to better characterize the L. casei A14.2 strain and its probiotic effect or to isolate and characterize future probiotic candidates. Nevertheless, larger scale assays are necessary to confirm the health promoting capacity of L. casei A14.2 strain on Apis mellifera.
6. Patents
Invention patent application code 202003192: “Nutritional formulation for pollinating insects including the probiotic strain Lactobacillus casei A14.2”.
CRediT authorship contribution statement
Romina I. Carvajal: Project administration, Writing – original draft, Writing – review & editing, Formal analysis, Methodology, Conceptualization, Investigation, Funding acquisition. Fabiola Silva-Mieres: Funding acquisition, Project administration, Writing – original draft, Writing – review & editing, Formal analysis, Methodology, Investigation, Conceptualization. Alejandra Ilabaca: Funding acquisition, Project administration, Methodology, Conceptualization, Investigation. Jorge Rocha: Investigation, Methodology, Conceptualization, Project administration, Funding acquisition. Luciano Arellano-Arriagada: Investigation, Methodology, Writing – original draft, Writing – review & editing. Felipe A. Zuniga Arbalti: Supervision, Formal analysis, Methodology. Apolinaria García-Cancino: Supervision, Writing – original draft, Writing – review & editing, Investigation, Formal analysis, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors are grateful to Mr. Freddy Toledo for providing the beehives and for his technical assistance to handle the apiary located at Nonguén valley, Concepcion, Chile. The authors are also indebted with the “Asociación Gremial de Apicultores de Concepción - Apiconce A.g.” (Trade Union Association of Beekeepers of Concepcion) for the training provided to our research group on the handling and salubriousness of beehives.
Funding
This research was funded by the “Foundation for Agricultural Innovation (FIA) code PYT-2017-0472, Ministry of Agriculture, Chile.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2023.103612.
Contributor Information
Romina I. Carvajal, Email: rominacarvajal@udec.cl, rcarvajalc@docente.uss.cl.
Fabiola Silva-Mieres, Email: fabiolalsilva@udec.cl, f.silva16@ufromail.cl.
Alejandra Ilabaca, Email: aleilabaca@udec.cl.
Jorge Rocha, Email: jrocha@udec.cl.
Luciano Arellano-Arriagada, Email: lucarellano@udec.cl.
Felipe A. Zuniga Arbalti, Email: fzuniga@udec.cl.
Apolinaria García-Cancino, Email: apgarcia@udec.cl.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Alberoni, D., Baffoni, L., Gaggìa, F., Ryan, P. m., Murphy, K., Ross, P. r., Stanton, C., Di Gioia, D., 2018. Impact of beneficial bacteria supplementation on the gut microbiota, colony development and productivity of Apis mellifera L. Beneficial Microbes 9, 269–278. https://doi.org/10.3920/BM2017.0061 [DOI] [PubMed]
- Anisimova E.A., Yarullina D.R. Antibiotic resistance of LACTOBACILLUS strains. Curr. Microbiol. 2019;76:1407–1416. doi: 10.1007/s00284-019-01769-7. [DOI] [PubMed] [Google Scholar]
- Antúnez K., Martín-Hernández R., Prieto L., Meana A., Zunino P., Higes M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia) Environ. Microbiol. 2009;11:2284–2290. doi: 10.1111/j.1462-2920.2009.01953.x. [DOI] [PubMed] [Google Scholar]
- Arredondo D., Castelli L., Porrini M.P., Garrido P.M., Eguaras M.J., Zunino P., Antúnez K. Lactobacillus kunkeei strains decreased the infection by honey bee pathogens Paenibacillus larvae and Nosema ceranae. Benefic. Microbes. 2018;9:279–290. doi: 10.3920/BM2017.0075. [DOI] [PubMed] [Google Scholar]
- Audisio M., Benítez-Ahrendts M. Lactobacillus johnsonii CRL1647, isolated from Apis mellifera L. bee-gut, exhibited a beneficial effect on honeybee colonies. Benefic. Microbes. 2011;2:29–34. doi: 10.3920/BM2010.0024. [DOI] [PubMed] [Google Scholar]
- Bonoan R.E., Iglesias Feliciano P.M., Chang J., Starks P.T. Social benefits require a community: the influence of colony size on behavioral immunity in honey bees. Apidologie. 2020;51:701–709. doi: 10.1007/s13592-020-00754-5. [DOI] [Google Scholar]
- Borges D., Guzman-Novoa E., Goodwin P.H. Effects of prebiotics and probiotics on honey bees (Apis mellifera) infected with the microsporidian parasite Nosema ceranae. Microorganisms. 2021;9:481. doi: 10.3390/microorganisms9030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campedelli, I., Mathur, H., Salvetti, E., Clarke, S., Rea, M.C., Torriani, S., Ross, R.P., Hill, C., O’Toole, P.W., 2018. Genus-Wide Assessment of Antibiotic Resistance in Lactobacillus spp. Appl Environ Microbiol 85, e01738-18, /aem/85/1/AEM.01738-18.atom. https://doi.org/10.1128/AEM.01738-18. [DOI] [PMC free article] [PubMed]
- Cederlund A., Gudmundsson G.H., Agerberth B. Antimicrobial peptides important in innate immunity. FEBS J. 2011;278:3942–3951. doi: 10.1111/j.1742-4658.2011.08302.x. [DOI] [PubMed] [Google Scholar]
- Chopra S.S., Bakshi B.R., Khanna V. Economic dependence of U.S. industrial sectors on animal-mediated pollination service. Environ. Sci. Technol. 2015;49:14441–14451. doi: 10.1021/acs.est.5b03788. [DOI] [PubMed] [Google Scholar]
- Clancy R. Immunobiotics and the probiotic evolution. FEMS Immunol. Med. Microbiol. 2003;38:9–12. doi: 10.1016/S0928-8244(03)00147-0. [DOI] [PubMed] [Google Scholar]
- Cornara L., Biagi M., Xiao J., Burlando B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017;8:412. doi: 10.3389/fphar.2017.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daisley B.A., Chmiel J.A., Pitek A.P., Thompson G.J., Reid G. Missing microbes in bees: how systematic depletion of key symbionts erodes immunity. Trends Microbiol. 2020;28:1010–1021. doi: 10.1016/j.tim.2020.06.006. [DOI] [PubMed] [Google Scholar]
- Das D.J., Shankar A., Johnson J.B., Thomas S. Critical insights into antibiotic resistance transferability in probiotic Lactobacillus. Nutrition. 2020;69 doi: 10.1016/j.nut.2019.110567. [DOI] [PubMed] [Google Scholar]
- Davoodi S., Behbahani M., Shirani E., Mohabatkar H. Influence of sucrose, glucose, stevia leaf and stevioside on the growth and lactic acid production by Lactobacillus plantarum, Lactobacillus brevis and Lactobacillus casei. Iran. J. Sci. Technol., Trans. A: Sci. 2016;40:275–279. doi: 10.1007/s40995-016-0088-6. [DOI] [Google Scholar]
- de Melo Pereira G.V., de Oliveira Coelho B., Magalhães Júnior A.I., Thomaz-Soccol V., Soccol C.R. How to select a probiotic? a review and update of methods and criteria. Biotechnol. Adv. 2018;36:2060–2076. doi: 10.1016/j.biotechadv.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Delcour, J., Ferain, T., Deghorain, M., Palumbo, E., Hols, P., 1999. The biosynthesis and functionality of the cell-wall of lactic acid bacteria, in: Konings, W.N., Kuipers, O.P., In ’t Veld, J.H.J.H. (Eds.), Lactic Acid Bacteria: Genetics, Metabolism and Applications: Proceedings of the Sixth Symposium on Lactic Acid Bacteria: Genetics, Metabolism and Applications, 19–23 September 1999, Veldhoven, The Netherlands. Springer Netherlands, Dordrecht, pp. 159–184. https://doi.org/10.1007/978-94-017-2027-4_7.
- Di Gioia, D., Biavati, B., 2018. Probiotics and Prebiotics in Animal Health and Food Safety: Conclusive Remarks and Future Perspectives, in: Di Gioia, D., Biavati, B. (Eds.), Probiotics and Prebiotics in Animal Health and Food Safety. Springer International Publishing, Cham, pp. 269–273. https://doi.org/10.1007/978-3-319-71950-4_11.
- Dietemann V., Ellis J.D., Neumann P. The COLOSS BEEBOOK volume I, standard methods for Apis mellifera research: introduction. J. Apic. Res. 2013;52:1–4. doi: 10.3896/IBRA.1.52.4.23. [DOI] [Google Scholar]
- El-Nahhal Y. Pesticide residues in honey and their potential reproductive toxicity. Sci. Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.139953. [DOI] [PubMed] [Google Scholar]
- Engel P., Martinson V.G., Moran N.A. Functional diversity within the simple gut microbiota of the honey bee. PNAS. 2012;109:11002–11007. doi: 10.1073/pnas.1202970109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.D., Aronstein K., Chen Y.P., Hetru C., Imler J.-L., Jiang H., Kanost M., Thompson G.J., Zou Z., Hultmark D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006;15:645–656. doi: 10.1111/j.1365-2583.2006.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.D., Lopez D.L. Bacterial probiotics induce an immune response in the honey bee (Hymenoptera: Apidae) J. Econ. Entomol. 2004;97:752–756. doi: 10.1093/jee/97.3.752. [DOI] [PubMed] [Google Scholar]
- Fanciotti, M.N., Tejerina, M., Benítez-Ahrendts, M. r., Audisio, M. c., 2018. Honey yield of different commercial apiaries treated with Lactobacillus salivarius A3iob, a new bee-probiotic strain. Beneficial Microbes 9, 291–298. https://doi.org/10.3920/BM2017.0089 [DOI] [PubMed]
- Fleming J.C., Schmehl D.R., Ellis J.D. Characterizing the impact of commercial pollen substitute diets on the level of Nosema spp. in honey bees (Apis mellifera L.) PLoS One. 2015;10:e0132014. doi: 10.1371/journal.pone.0132014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly N.K., Bhattacharya S.K., Sesikeran B., Nair G.B., Ramakrishna B.S., Sachdev H.P.S., Batish V.K., Kanagasabapathy A.S., Muthuswamy V., Kathuria S.C., Katoch V.M., Satyanarayana K., Toteja G.S., Rahi M., Rao S., Bhan M.K., Kapur R., Hemalatha R. ICMR-DBT guidelines for evaluation of probiotics in food. Indian J. Med. Res. 2011;134:22–25. [PMC free article] [PubMed] [Google Scholar]
- Georgieva R.N., Iliev I.N., Chipeva V.A., Dimitonova S.P., Samelis J., Danova S.T. Identification and in vitro characterisation of Lactobacillus plantarum strains from artisanal Bulgarian white brined cheeses. J. Basic Microbiol. 2008;48:234–244. doi: 10.1002/jobm.200700355. [DOI] [PubMed] [Google Scholar]
- Han K., Wang H., Liu Z., Chi X., Wang Y., Cui X., Xu B. A study about the application of probiotics on Apis mellifera. J. Apic. Res. 2022:1–12. doi: 10.1080/00218839.2022.2088933. [DOI] [Google Scholar]
- Herbert R.A. In: Methods in Microbiology, Techniques in Microbial Ecology. Grigorova R., Norris J.R., editors. Academic Press; 1990. 1 Methods for Enumerating Microorganisms and Determining Biomass in Natural Environments; pp. 1–39. [DOI] [Google Scholar]
- Hill D., Sugrue I., Tobin C., Hill C., Stanton C., Ross R.P. The Lactobacillus casei group: history and health related applications. Front. Microbiol. 2018 doi: 10.3389/fmicb.2018.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw C., Evans K.C., Rosa C., López-Uribe M.M. The role of pathogen dynamics and immune gene expression in the survival of feral honey bees. Front. Ecol. Evol. 2021;8:505. doi: 10.3389/fevo.2020.594263. [DOI] [Google Scholar]
- Hristov P., Shumkova R., Palova N., Neov B. Factors associated with honey bee colony losses: a mini-review. Veter. Sci. 2020;7:166. doi: 10.3390/vetsci7040166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-H., Chen Y.-H., Chen J.-H., Hsu P.-S., Wu T.-H., Lin C.-F., Peng C.-C., Wu M.-C. A potential probiotic Leuconostoc mesenteroides TBE-8 for honey bee. Sci. Rep. 2021;11:18466. doi: 10.1038/s41598-021-97950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q., Evans J.D. Targeting the honey bee gut parasite Nosema ceranae with siRNA positively affects gut bacteria. BMC Microbiol. 2020;20:258. doi: 10.1186/s12866-020-01939-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert J., Bicianova M., Ledvinka O., Kamler M., Lester P.J., Nesvorna M., Kopecky J., Erban T. Changes in the bacteriome of honey bees associated with the parasite Varroa destructor, and pathogens Nosema and Lotmaria passim. Microb. Ecol. 2017;73:685–698. doi: 10.1007/s00248-016-0869-7. [DOI] [PubMed] [Google Scholar]
- Kane T.R., Faux C.M. John Wiley & Sons; 2021. Honey Bee Medicine for the Veterinary Practitioner. [Google Scholar]
- Katla A.-K., Kruse H., Johnsen G., Herikstad H. Antimicrobial susceptibility of starter culture bacteria used in Norwegian dairy products. Int. J. Food Microbiol. 2001;67:147–152. doi: 10.1016/S0168-1605(00)00522-5. [DOI] [PubMed] [Google Scholar]
- Kešnerová L., Mars R.A.T., Ellegaard K.M., Troilo M., Sauer U., Engel P. Disentangling metabolic functions of bacteria in the honey bee gut. PLoS Biol. 2017;15:e2003467. doi: 10.1371/journal.pbio.2003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong W.K., Engel P., Koch H., Moran N.A. Genomics and host specialization of honey bee and bumble bee gut symbionts. PNAS. 2014;111:11509–11514. doi: 10.1073/pnas.1405838111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong, W.K., Mancenido, A.L., Moran, N.A., n.d. Immune system stimulation by the native gut microbiota of honey bees. Royal Society Open Science 4, 170003. https://doi.org/10.1098/rsos.170003. [DOI] [PMC free article] [PubMed]
- Kwong W.K., Moran N.A. Gut microbial communities of social bees. Nat. Rev. Microbiol. 2016;14:374–384. doi: 10.1038/nrmicro.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen A., Reynaldi F.J., Guzmán-Novoa E. Bases del sistema inmune de la abeja melífera (Apis mellifera) Rev. RMCP. 2019;10:705–728. doi: 10.22319/rmcp.v10i3.4785. [DOI] [Google Scholar]
- Lee F.J., Rusch D.B., Stewart F.J., Mattila H.R., Newton I.L.G. Saccharide breakdown and fermentation by the honey bee gut microbiome: Fermentation by honey bee gut microbes. Environ. Microbiol. 2015;17:796–815. doi: 10.1111/1462-2920.12526. [DOI] [PubMed] [Google Scholar]
- Lourenço A.P., Florecki M.M., Simões Z.L.P., Evans J.D. Silencing of Apis mellifera dorsal genes reveals their role in expression of the antimicrobial peptide defensin-1. Insect Mol. Biol. 2018;27:577–589. doi: 10.1111/imb.12498. [DOI] [PubMed] [Google Scholar]
- Maruščáková I.C., Schusterová P., Bielik B., Toporčák J., Bíliková K., Mudroňová D. Effect of application of probiotic pollen suspension on immune response and gut microbiota of honey bees (Apis mellifera) Probiotics & Antimicro. Prot. 2020 doi: 10.1007/s12602-019-09626-6. [DOI] [PubMed] [Google Scholar]
- Motta E.V.S., Powell J.E., Leonard S.P., Moran N.A. Prospects for probiotics in social bees. Philos. Trans. R. Soc., B. 2022;377:20210156. doi: 10.1098/rstb.2021.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin C.A., Frazier M., Frazier J.L., Ashcraft S., Simonds R., vanEngelsdorp D., Pettis J.S. High levels of miticides and agrochemicals in North American Apiaries: implications for honey bee health. PLoS One. 2010;5:e9754. doi: 10.1371/journal.pone.0009754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezhad M.H., Hussain M.A., Britz M.L. Stress responses in probiotic Lactobacillus casei. Crit. Rev. Food Sci. Nutr. 2015;55:740–749. doi: 10.1080/10408398.2012.675601. [DOI] [PubMed] [Google Scholar]
- Nowak A., Szczuka D., Górczyńska A., Motyl I., Kręgiel D. Characterization of Apis mellifera gastrointestinal microbiota and lactic acid bacteria for honeybee protection—a review. Cells. 2021;10:701. doi: 10.3390/cells10030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE - World Organisation for Animal Health (Ed.), 2008. Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees). 2: Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees), 6. ed. ed. OIE, Paris [PubMed]
- Olofsson T.C., Vásquez A. Detection and identification of a novel lactic acid bacterial flora within the honey stomach of the honeybee Apis mellifera. Curr. Microbiol. 2008;57:356–363. doi: 10.1007/s00284-008-9202-0. [DOI] [PubMed] [Google Scholar]
- Papini R., Mancianti F., Canovai R., Cosci F., Rocchigiani G., Benelli G., Canale A. Prevalence of the microsporidian Nosema ceranae in honeybee (Apis mellifera) apiaries in Central Italy. Saudi J. Biol. Sci. 2017;24:979–982. doi: 10.1016/j.sjbs.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher E., Harz M., Schneider S. Effects of oxalic acid on Apis mellifera (Hymenoptera: Apidae) Insects. 2017;8:84. doi: 10.3390/insects8030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey S.D., Ochoa R., Bauchan G., Gulbronson C., Mowery J.D., Cohen A., Lim D., Joklik J., Cicero J.M., Ellis J.D., Hawthorne D., vanEngelsdorp D. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. PNAS. 2019;116:1792–1801. doi: 10.1073/pnas.1818371116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, Zentek, Vahjen, 2019. Optimization of Production Parameters for Probiotic Lactobacillus Strains as Feed Additive. Molecules 24, 3286. https://doi.org/10.3390/molecules24183286 [DOI] [PMC free article] [PubMed]
- Royan M. Mechanisms of probiotic action in the honeybee. CRE. 2019;29 doi: 10.1615/CritRevEukaryotGeneExpr.2019025358. [DOI] [PubMed] [Google Scholar]
- Rucker R.R., Thurman W.N., Burgett M. Colony collapse and the consequences of bee disease: market adaptation to environmental change. J. Assoc. Environ. Resour. Econ. 2019;6:927–960. doi: 10.1086/704360. [DOI] [Google Scholar]
- Sabaté D.C., Cruz M.S., Benítez-Ahrendts M.R., Audisio M.C. Beneficial effects of Bacillus subtilis subsp. subtilis Mori2, a honey-associated strain, on honeybee colony performance. Probiotics & Antimicro. Prot. 2012;4:39–46. doi: 10.1007/s12602-011-9089-0. [DOI] [PubMed] [Google Scholar]
- Saccà, M. l., Lodesani, M., 2020. Isolation of bacterial microbiota associated to honey bees and evaluation of potential biocontrol agents of Varroa destructor. Beneficial Microbes 11, 641–654. https://doi.org/10.3920/BM2019.0164 [DOI] [PubMed]
- Siciliano R.A., Pannella G., Lippolis R., Ricciardi A., Mazzeo M.F., Zotta T. Impact of aerobic and respirative life-style on Lactobacillus casei N87 proteome. Int. J. Food Microbiol. 2019;298:51–62. doi: 10.1016/j.ijfoodmicro.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Simone-Finstrom M. Social immunity and the superorganism: behavioral defenses protecting honey bee colonies from pathogens and parasites. Bee World. 2017;94:21–29. doi: 10.1080/0005772X.2017.1307800. [DOI] [Google Scholar]
- Southwick E.E., Southwick L., Jr. Estimating the economic value of honey bees (Hymenoptera: Apidae) as agricultural pollinators in the United States. J. Econ. Entomol. 1992;85:621–633. doi: 10.1093/jee/85.3.621. [DOI] [Google Scholar]
- Taylor M.A., Robertson A.W., Biggs P.J., Richards K.K., Jones D.F., Parkar S.G. The effect of carbohydrate sources: Sucrose, invert sugar and components of mānuka honey, on core bacteria in the digestive tract of adult honey bees (Apis mellifera) PLoS One. 2019;14:e0225845. doi: 10.1371/journal.pone.0225845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejerina M.R., Benítez-Ahrendts M.R., Audisio M.C. Lactobacillus salivarius A3iob reduces the incidence of Varroa destructor and Nosema Spp. in commercial Apiaries located in the Northwest of Argentina. Probiotics & Antimicro. Prot. 2020;12:1360–1369. doi: 10.1007/s12602-020-09638-7. [DOI] [PubMed] [Google Scholar]
- Tihelka, E., 2018. EFFECTS OF SYNTHETIC AND ORGANIC ACARICIDES ON HONEY BEE HEALTH: A REVIEW. SLOVENIAN VETERINARY RESEARCH 55. https://doi.org/10.26873/SVR-422-2017.
- Tlak Gajger I., Vlainić J., Šoštarić P., Prešern J., Bubnič J., Smodiš Škerl M.I. Effects on some therapeutical, biochemical, and immunological parameters of honey bee (Apis mellifera) exposed to probiotic treatments, in field and laboratory conditions. Insects. 2020;11:638. doi: 10.3390/insects11090638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanEngelsdorp D., Traynor K.S., Andree M., Lichtenberg E.M., Chen Y., Saegerman C., Cox-Foster D.L. Colony Collapse Disorder (CCD) and bee age impact honey bee pathophysiology. PLoS One. 2017;12:e0179535. doi: 10.1371/journal.pone.0179535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Liu C., Liu Z., Wang Y., Ma L., Xu B. The different dietary sugars modulate the composition of the gut microbiota in honeybee during overwintering. BMC Microbiol. 2020;20:61. doi: 10.1186/s12866-020-01726-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widyastuti Y., Febrisiantosa A., Tidona F. Health-promoting properties of Lactobacilli in fermented dairy products. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.673890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G.R., Alaux C., Costa C., Csáki T., Doublet V., Eisenhardt D., Fries I., Kuhn R., McMahon D.P., Medrzycki P., Murray T.E., Natsopoulou M.E., Neumann P., Oliver R., Paxton R.J., Pernal S.F., Shutler D., Tanner G., van der Steen J.J.M., Brodschneider R. Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J. Apic. Res. 2013;52:1–36. doi: 10.3896/IBRA.1.52.1.04. [DOI] [Google Scholar]
- Zakaria, M.E., 2010. The Physiological Structure Differences of the Honey Stomach Tissue at Different Developmental Stages of Worker Honey Bees (Apis mellifera L.) 6.
- Zhou J.S., Pillidge C.J., Gopal P.K., Gill H.S. Antibiotic susceptibility profiles of new probiotic Lactobacillus and Bifidobacterium strains. Int. J. Food Microbiol. 2005;98:211–217. doi: 10.1016/j.ijfoodmicro.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Zotta T., Ricciardi A., Ianniello R.G., Parente E., Reale A., Rossi F., Iacumin L., Comi G., Coppola R. Assessment of aerobic and respiratory growth in the Lactobacillus casei Group. PLoS One. 2014;9:e99189. doi: 10.1371/journal.pone.0099189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotta T., Parente E., Ricciardi A. Aerobic metabolism in the genus Lactobacillus: impact on stress response and potential applications in the food industry. J. Appl. Microbiol. 2017;122:857–869. doi: 10.1111/jam.13399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.