Abstract
Although the past decade has witnessed unprecedented medical advances, achieving rapid and effective hemostasis remains challenging. Uncontrolled bleeding and wound infections continue to plague healthcare providers, increasing the risk of death. Various types of hemostatic materials are nowadays used during clinical practice but have many limitations, including poor biocompatibility, toxicity and biodegradability. Recently, there has been a burgeoning interest in organisms that stick to objects or produce sticky substances. Indeed, applying biological adhesion properties to hemostatic materials remains an interesting approach. This paper reviews the biological behavior, bionics, and mechanisms related to hemostasis. Furthermore, this paper covers the benefits, challenges and prospects of biomimetic hemostatic materials.
Keywords: Biomimetic materials, Hemostasis, Adhesion
Graphical abstract
Highlights
-
•
The biological model related to hemostasis is first elucidated.
-
•
The bionic principle and hemostatic mechanism of bionic hemostatic materials are introduced.
-
•
The latest development and future research direction of biomimetic hemostatic materials are discussed.
1. Introduction
It is well-established that uncontrolled bleeding presents a significant medical challenge associated with trauma, bleeding disorders, or medical conditions [1,2]. Severe trauma causes more than 5.8 million fatalities globally [3]. Furthermore, severe posttraumatic bleeding has a fatality rate of 30–40%, with about half of these patients dying before hospital admission [4,5]. Significant blood loss due to delayed or inefficient bleeding control increases the need for blood products and is conducive to a poor prognosis [6]. In addition, excessive bleeding during and after surgery has been linked to an increased mortality risk [7]. Marietta et al. revealed that severe bleeding accounted for up to 20% of mortalities during elective vascular surgery [8]. However, death caused by severe bleeding can be prevented by achieving prompt and efficient hemostasis [9].
Various hemostats are currently used to control bleeding, encompassing local hemostatic agents, adhesives, sealants, dressings, bandages, hemostatic powder, and glue. Hemostatic agents control bleeding by activating or accelerating the coagulation cascade [2], while adhesive agents and sealants work by physically binding to the tissue or blood vessels [2,10]. Commercially available adhesives used for noninvasive wound closure have benefits and limitations. In this respect, although fibrin glue is biocompatibility (it mimics the crosslinking that occurs during coagulation), it has a low adhesion strength and poor biosafety profile and brings a high risk of infection [11]. Moreover, cyanoacrylate adhesives have high adhesion strength but have been associated with biosafety concerns due to their toxic degradation products, including formaldehyde [11,12]. In recent years, several external hemostats have been developed, including kaolin and zeolite-based materials (QuikClot powder, advanced coagulation sponge, and combat gauze), materials based on chitosan, gelatin, alginate, collagen, and other materials (e. g., HemCon, Celox, GelFoam, Algosteril, Avitene, etc.) [2]. External hemostats promote hemostasis by physical compression or by triggering the coagulation cascade. However, external hemostats have several limitations, including poor biocompatibility, allergic reactions, low adhesion strength, and poor biodegradability [[13], [14], [15], [16]]. Furthermore, gauze has a high capacity to absorb blood, causing additional blood loss and discomfort during removal. Besides, wounds create entry sites for bacteria, predisposing an individual to wound infections [17]. Antibacterial activity should be considered during the functional design of hemostatic materials [18]. Indeed, prompt and effective hemostasis is conducive to better healing of the wound area. Accordingly, further research is warranted on the properties and hemostatic mechanism of materials to design more effective hemostatic materials.
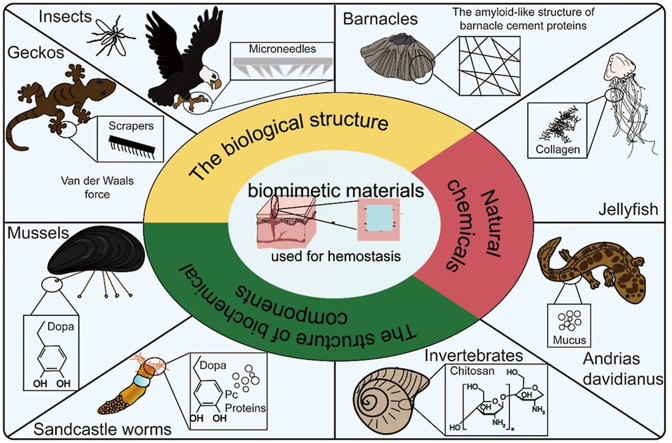
Current evidence suggests that hemostasis can be achieved through different mechanisms, including physical adhesion to tissues and triggering the clotting cascade. Triggering the coagulation cascade is a more traditional and effective hemostatic concept. However, strong bio-adhesives can physically seal bleeding wounds without triggering the clotting cascade, reducing the risk of thrombosis and being more effective against bacteria [19]. Notably, researchers have discovered several organisms with strong adhesive properties for different purposes. For instance, mussels, barnacles, and sandcastle worms produce adhesive substances to promote surface attachment; geckos rely on adhesion to promote crawling, and Andrias davidianus secrete sticky mucus for defense [[20], [21], [22], [23]]. Inspired by these interesting phenomena, this strong adhesive property has been associated with hemostasis in the design of new biological adhesives. This new bio-adhesive (termed biomimetic hemostatic material) can overcome the existing problems of materials used in wounds, including biocompatibility, adhesion (especially wet adhesion), and rapid physical closure hemostatic [20]. Bioinspired materials have unique properties that mimic the structure and chemical composition of biological materials [24]. In addition, hemostasis and wound healing are continuous processes in bleeding wounds. The therapeutic effects of hemostatic materials are divided into immediate short-term effects (rapid hemostasis) and long-term effects (healing of bleeding wounds). Therefore, excellent biomimetic materials should have the following features, i.e. (i) good biocompatibility, (ii) high adhesion strength, (iii) antibacterial properties, (iv) good mechanical properties, and (v) the ability to promote wound healing.
This review presents the characteristics of natural organisms that produce sticky substances, an overview of the recently produced biomimetic materials and their biomimetic mechanisms, and the clinical applications, challenges, and prospects.
2. Bionic model and biomimetic mechanism
This section systematically describes biological models related to hemostasis. The adhesion mechanism related to hemostasis was expounded from the point of view of biological adhesion in nature. Based on different adhesion mechanisms, eight bionic models are roughly classified into three categories: (1) mimic the physical structure of organisms (such as insect mouthparts, gecko foot structure, barnacle cement microstructure), (2) directly extract the chemical composition of organisms (such as jellyfish collagen, Andrias davidianus skin secreted mucus), (3) mimic the structure of the chemical composition of organisms (such as chitin in invertebrates, dopamine in mussel adhesion protein, Pc protein and dopamine in sandcastle worm adhesion components). Furthermore, we comprehensively discussed these 8 biomimetic models in detail, geared towards providing novel biological concepts and ideas when designing biomimetic hemostatic materials in the future.
Comprehensive research on the features of natural organisms has enabled researchers to further improve the substances related to hemostasis. The hemostatic and biomimetic mechanisms involved in these substances are further elaborated, providing a theoretical basis for the design and performance optimization of biomimetic hemostatic materials (Table 1) (see Table 2).
Table 1.
A summary of the inspiration sources of bionic materials.
| Bionic model | The inspiration for the bionics | Characteristic | Mechanism and progress of preparing biomimetic materials | References |
|---|---|---|---|---|
| Insects | The piercing-sucking mouthparts of mosquitoes | Serrated microstructures. The mosquito mouthparts are inserted into the skin through the vibration of the mosquito head. | 1. Transdermal drug delivery or other purposes; 2. Adjusting the insertion angle of the microneedle based on the structure of the eagle claw. |
[26,[36], [37], [38], [39], [40]] |
| Geckos | Gecko bristles | Van der Waals forces, a combination of complex macroscopic, microscopic, and nano-mediated multistage structures in gecko bristles. | 1. The adhesion model based on the cylindrical fiber hypothesis; 2. The flexibility of the fiber; 3. The asymmetric design of bristles; 4. Optimized models of scraper geometry in normal and mixed stripping modes. |
[41,42,[52], [53], [54], [55], [56], [57], [58]] |
| Barnacles | Barnacle cement proteins (BCPs) | A unique combination and arrangement of natural amino acids, self-assemble into nanofibers. | 1. Noncovalent interactions; 2. The microstructure is similar to the nanofiber network of the thrombus microstructure; 3. An enzymatic model of cement polymerization in barnacles; 4. Environment. |
[24,60,69,70,[72], [73], [74], [75], [76], [77], [78], [79], [80], [81]] |
| Jellyfish | Collagen | Substances with good biocompatibility, higher biodegradability, lower antigenicity, and cell-binding properties that can be degraded into physiologically tolerable compounds in vivo. | Mucins extracted from natural sources. | [86,89,91] |
| Andrias davidianus | The skin secretion of Andrias davidianus (SSAD) | Proteins, amino acids, mucopolysaccharides, and antimicrobial peptides. | Extracting the skin secretions of Andrias davidianus. | [[93], [94], [95],97,98] |
| Invertebrates | Chitin | As a deacetylated chitin, CS has good biocompatibility and biodegradability, broad-spectrum antimicrobial resistance, hemostasis, non-toxic, and easy processing. | 1. Unique hemostatic properties; 2. Reduce scar tissue formation, thus achieving good epithelial reformation. |
[99,102,105,108,[114], [115], [116], [117], [118], [119], [120], [121]] |
| Marine mussels | Mussel adhesion protein (MAP) | The amino acid modification 3,4-dihydroxyphenylalanine (DOPA) | PDA | [123,124,134,147,148] |
| Sandcastle worms | Phragmatopoma cement proteins (Pc) and 3,4-dihydroxyphenylalanine (DOPA). | 1. Complex condensation model 2. Separate the opposite charges into different polymers and the high density of phosphate sidechains |
1. Besides a change in amino acid composition or single amino acid change, the final biphasic porous structure and transformation from liquid to solid after secretion, as well as the cellular sorting and packaging processes, control the overall strength and toughness of granulated sandcastle glue. 2. Electrostatic contact between analog polyelectrolytes and divalent cations induces the formation of colloidal nano-complexes in a process analogous to coacervation at the molecular level. |
[22,[135], [136], [137], [138],[143], [144], [145], [146]] |
Table 2.
Acronym table.
| Abbreviations and acronyms | Full name |
|---|---|
| MAP | Mussel adhesion protein |
| DOPA | 3,4-dihydroxyphenylalanine |
| Mfps | Mytilus foot proteins |
| Mefp | Mytilus edulis Linnaeus foot proteins |
| Mcfp | Mytilus californianus foot proteins |
| CLSM | Confocal laser microscope |
| AFM | Atomic force microscope |
| NMR | Nuclear magnetic resonance |
| SEM | Scanning electron microscopy |
| BCPs | Barnacle cement proteins |
| BSE | Bovine spongiform encephalopathy |
| TSE | Transmissible spongiform encephalopathy |
| SSAD | The skin secretion of Andrias davidianus |
| DD | Degree of deacetylation |
| MW | Molecular weight |
| DA | Dopamine |
| l-dopa | Levodopa |
| PDA | Polydopamine |
| DHI | 5,6-dihydroxyindole |
| BCPs | Barnacle cement proteins |
| MMPs | Matrix metalloproteinases |
| ECM | Extracellular matrix |
| CS | Chitosan |
| TMC | Trimethylated chitosan |
| MNs | Microneedles |
| NP-gel | bioadhesive nanoparticle-hydrogel hybrid |
| NPs | Nanoparticles |
| PEG | Polyethylene glycol |
| EGF | Epidermal growth factor |
| OSA-DA | Dopamine-grafted oxidized sodium alginate |
| PAM | Polyacrylamide |
| HA | Hyaluronic acid |
| EPL | ε-polylysine |
| CPAA | Catechol-containing poly(amidoamine) |
| PLGA | Poly(l-glutamic acid) |
| ALG–CHO–Catechol | Catechol- and aldehyde-modified alginate |
| PAAm-TA-KA | Polyacrylamide-tannic acid-kaolin |
| TA | Tannic acid |
| PHEAA | Poly(N-hydroxyethyl acrylamide) |
| PGSA | Poly(glycerol sebacate acrylate) |
| DXTA | Aldehyde-functionalized dextran |
| HxGltn | Hexanoyl group-modified gelatin |
| SiNW | Silicon nanowire |
| FDA | Food and Drug Administration |
| Da-g-Xan | Dopamine-conjugated xanthan gum |
| PAA-NHS eater | Poly (acrylic acid) grafted with N-hydroxysuccinimide ester |
| SF | Silk fibroin |
| XG | Xanthan gum |
| BCI | Blood clotting index |
| FTIR | Fourier Transform Infrared Spectroscopy |
| SEM | Scanning electron microscope |
| GE | Gelatin |
| GS | Gelatin sponge |
| OC | Oxidized cellulose gauze |
| MACS | Microchannel alkylated chitosan sponge |
| SPA | Sodium polyacrylate |
| HBC | Hydroxybutyl chitosan |
| SCF | The supercritical fluid |
| PVM/MA | Poly-(methyl vinyl ether-co-maleic anhydride) |
| DCEC | Dodecyl-modified N-carboxyethyl chitosan |
| OKGM | Oxidized konjac glucomannan |
| TNF | Tumor necrosis factor |
| QCS | Quaternary ammonium chitosan |
| DBAH | Dual bionic adhesive hydrogel |
| PIA | Polysaccharide intercellular adhesin |
| CSMS-Ks | Porous chitosan/kaolin composite microspheres |
| TIPS | Thermally-induced phase separation |
| PCL | Polycaprolactone |
| LM | Liquid metal |
| Pc | Phragmatopoma cement proteins |
| IO | Inverse opall |
| PC | Photonic crystal |
| SH | Superhydrophobic |
| RBC | Red blood cell |
2.1. Mimic the physical structure of organisms
2.1.1. Insects
Insect mouthparts have evolved several functions, including pierce-sucking, sponging, siphoning, grasping, rasping, scratching, and chew-lapping [25]. The proboscis is found in all female mosquitoes and is adapted for penetrating the skin and sucking blood [26]. Besides an extremely sharp tip, the mosquito mouthparts contain serrated microstructures on the sides of the maxilla near the tip. The paired maxillae transform into a pair of micro-saws upon vibration of the mosquito's head, promoting insertion and skin penetration. The saw-like shape of the probe can also reduce the area of contact between the probe surface and the skin tissue, lowering frictional forces [27]. The combination of an exquisite microstructure and a clever insertion mode contributes to the good performance of the mosquito needle mouth and nose [[27], [28], [29]].
Researchers have developed microneedles based on the superior microstructure of mosquito mouthparts and intelligent insertion techniques for application in the medical industry. Mosquito mouthparts, caterpillar spines, Cicadellidae mouthparts, tsetse fly mouthparts, honey bee stingers, and paper wasp stingers are a few examples of insects used in designing MNs' structures [[27], [28], [29], [30], [31], [32]]. Microneedles (MNs) are needle arrays at a microscopic scale used for transdermal medication delivery and other uses [[33], [34], [35]]. Microneedles can nearly painlessly deliver medications while penetrating the skin without leaving scars [[36], [37], [38], [39]]. More importantly, the microneedle structure can improve the strong adhesion of the hemostatic material to the tissue. Therefore, the hemostatic material can act as a physical barrier to control bleeding. With the comprehensive observation of natural organisms, researchers have discovered that the structure of claws can further improve the fixation and closure of wounds with hemostatic materials by mimicking the structure of teeth and claws of several organisms. For instance, the clawed toes of eagles are pointed in the direction of one another, forming a gripping structure that helps to tightly grip their prey. Based on this, the structure of the eagle claw was used to modify the insertion angle of the microneedle. These features may aid in bleeding control and wound closure [40]. The later-mentioned MN patch inspired by shark teeth is similar to that inspired by eagle claws. As a result, researchers are coming up with more interesting ideas for designing materials.
2.1.2. Geckos
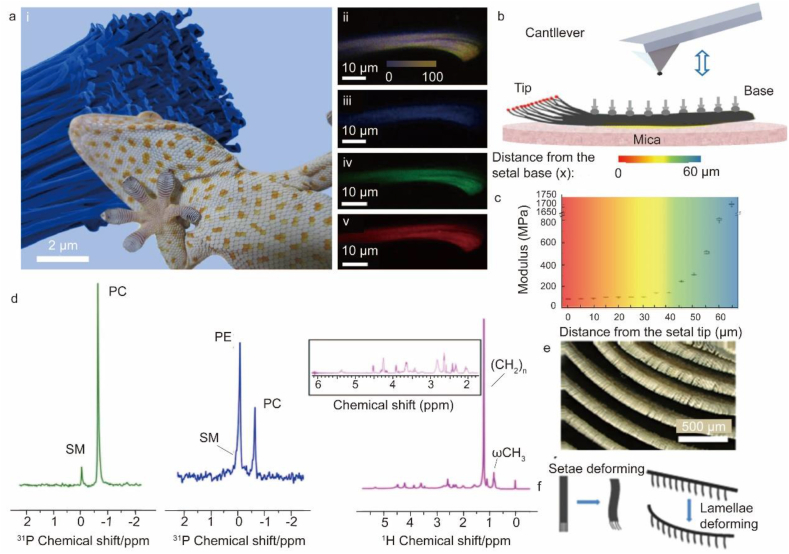
Van der Waals forces account for the dry adhesive properties of geckos [41,42]. The gecko toe pad is covered with hundreds of thousands of tiny hairs called scrapers, which are further divided into millions of bristles (Fig. 1a, e). The gecko bristles are mainly made up of beta-keratin, alpha-keratin, and lipids (Fig. 1d) [43,44]. The individual gecko setal stalk has optimized modulus gradient for achieving robust adhesion with great durability and reusability. This natural design enables the elastic hairs to be both flexible toward the tips (critical to initiate strong frictional adhesion) and rigid at the base (key to achieve high fatigue resistance). The AFM tests showed that Young's modulus of a single setal stalk gradually reduces from the base to the tip by nearly 20 times in magnitude (Fig. 1b and c). Gecko toe pads are widely known to exhibit multilevel structures, with lamellar skin at the macroscale, setal arrays at the microscale, and spatulae brunching out of setal tips at the nanoscale (Fig. 1e). A combination of these complex macroscopic, microscopic, and nanoscale-mediated multistage structures favors effective contact with rough surfaces, leading to strong adhesion. Inspired by gradient bristles, a simple benchmark technique was created to construct functional gradient fiber binder arrays, which improved the adhesive performance and fatigue resistance [41]. Van der Waals forces demonstrate substantial adsorption forces upon contact with a surface. However, van der Waals forces decrease with increasing distance. During high-stress sliding, the gecko foot leaves a lipid-containing imprint that reduces the wear on the bristles. Hard bristles facilitate the self-cleaning and anti-entanglement of the adhesive system [45]. Due to van der Waals force interaction, adhesion is not affected by surface chemistry, which demonstrates that the durability and reusability of this structure. In addition, the super-hydrophobicity and self-cleaning ability of gecko feet are also worthy of researchers' attention [46,47].
Fig. 1.
The principles of gecko adhesion. (a) (i) Picture of a Tokay gecko sticking to an acrylic glass with the background showing the top part of a single seta; (ii) confocal laser microscope (CLSM) image of single gecko seta, showing an overlay image of three different autofluorescence of (iii)–(v). The overall colors become more intense from the base to the tip (i.e., from left to right in (ii)), suggesting an increasing amount of β-keratin from the base to the tips. Therefore, a gradient density distribution exists along the seta, consequently causing a gradient in mechanical properties. (iii)–(v) CLSM images of seta with single excitation wavelengths of 458, 561, and 633 nm, respectively. (b) Schematic of the AFM nanoindentation tests of single gecko seta at different setal stalk positions. (c) Calculated gradient distribution of Young's modulus of the single seta based on the AFM force curve obtained at varied longitudinal positions. (d) NMR spectroscopy reveals the presence and association of lipids and keratin in adhesive gecko setae. (e) SEM image of the gecko lamellar skin. (f) Schematic of the bending/conforming mechanism of gecko setae and lamellae during attachment [[41], [43]].
Many adhesion models have been developed to describe the gecko's adhesive feature. Further, these models lay the basis for developing biomimetic materials that prevent bleeding and promote wound healing [[48], [49], [50]]. This review covers models that deal with hemostasis and wound healing [51]. According to the adhesion model based on the cylindrical fiber hypothesis, the fiber aspect ratio, tip shape, and contact refinement of the fiber structure can influence the adhesion forces [[52], [53], [54]]. The fiber's flexibility improves its stickiness to uneven surfaces. Furthermore, its multilayered structure improves adaptability and adhesion [[55], [56], [57]]. According to various models, the asymmetric bristle design facilitates quick switching between attachment and detachment [54]. Other researchers have developed optimized models of scraper geometry in normal and mixed stripping modes based on several imperfect previous models to obtain an ideal scraper structure and promote strong adhesion and simple separation [51,58,59]. Based on the physical adhesion properties of the gecko, designed materials can achieve physical blockage of the bleeding wounds and prevent leakage, further promoting wound healing.
2.1.3. Barnacles
Barnacle cement is a polyprotein complex made in cement glands and transported through canals to a small space between the barnacle base shell and the foreign matrix, where it is assembled and crosslinked to create a powerful underwater adhesion [60]. Over the past 20 years, several barnacle cement proteins (BCPs) have been discovered, including cp100 k, cp68 k, cp52 k, cp43 k, cp20 k, and cp19 k [[60], [61], [62], [63], [64], [65]]. Additionally, various BCP orthologs of barnacle cement have been discovered in other barnacle species through gene cloning and RNA sequencing [[66], [67], [68]]. Various amino acids have been identified in different BCPs [69,70]. The attachment process of barnacles relies on a special arrangement and mix of natural amino acids [64,65,69,71]. Additionally, the capacity of adhesives to stick to surfaces depends on their microstructure and chemical composition. Nanofibers constitute the microstructure of barnacle cement [72]. Moreover, one special advantage of BCP is its capacity to self-assemble into nanofibers. Besides these primary BCPs, the remaining trace elements in the barnacle cement, including enzymes and tiny compounds, have not yet been thoroughly characterized [60].
Over the years, several hypotheses have been postulated to explain the adhesion mechanisms of barnacle cements. However, our current understanding of the adhesion process of barnacles is limited. Based on the most widely accepted Kamino's hypothesis, distinct barnacle cement proteins (BCPs) integrate sequence and conformationally tuned noncovalent interactions to collectively achieve underwater adherence [73,74]. However, Dickinson et al. reported that the polymerization of barnacle cement resembles the blood coagulation process, and the microstructure of barnacle cement resembles the nanofiber network of the microstructure of blood clots [75,76]. In addition, So et al. proposed an enzymatic model of barnacles cement polymerization and revealed that BCPs interact non-covalently to self-assemble into amyloid fibers [77]. Subsequently, the fibers are covalently crosslinked, further improving cohesion. The amyloid fibers improve wet adhesion [24,78,79]. However, this model requires further validation. In addition, the adhesion force of barnacle proteins is influenced by pH, ionic concentration, and the order in which the various elements of the barnacle biological adhesions are secreted [80,81]. Under optimal conditions, barnacles can exhibit great adhesion.
2.2. Extract the chemical composition of organisms
2.2.1. Jellyfish
Collagen reportedly has higher biocompatibility biodegradability and adhesion strength and lower antigenicity than synthetic proteins [82]. Many commercially available collagen-based reagents are from pigs and cattle. However, these collagen proteins can cause certain diseases or religious issues [83,84]. Accordingly, it is important to locate a substitute resource to extract collagen, especially from the ocean, which is considerably safer. Jellyfish contains numerous minerals and proteins, including collagen [85]. For instance, the adhesive system in Rhopilema esculentum constitutes a mucilaginous slime with the glycoprotein qniumucin and the protein collagen (mostly collagen I) [86]. The collagen structure is made up of three elongated–chains of helical fibers. The most abundant amino acids in collagen are glycine and (4-hydroxy)proline [87]. The glycoprotein qniumucin, whose function has not been fully elucidated, is the second most conserved macromolecule of jellyfish secretions. Approximately one-third of the molecular weight of qniumucin is composed of glycans [88]. Moreover, the glycoprotein qniumucin also belongs to the mucinous protein family [89].
Jellyfish collagen can be used to prepare collagen-based dressings. On the one hand, jellyfish collagen promotes hemostasis by stimulating platelets and blood cells to adhere to the surface of the hemostatic material. Studies have shown that adequate adhesion strength and collagen concentration can improve the hemostatic capacity of hemostatic materials. On the other hand, highly adhesive collagen enables the hemostatic material to serve as a physical barrier and adhere to the wound surface for hemostasis. Furthermore, collagen-based dressings have been reported to accelerate chronic wound healing. It has been established that matrix metalloproteinases (MMPs) are present in high concentrations during the inflammatory phase of wound healing, especially in chronic or infected wounds, thus making healing difficult. Exogenous collagen competes with endogenous collagen when applied to wounds. Foreign collagen (such as jellyfish collagen) interacts/degrades with matrix metalloproteinases in the wound bed, causing faster production of novel human collagen fibers within the wound. Moreover, qniumucin glycoprotein and human mucin have numerous similar structural characteristics. The amino acid cysteine in the core structure of mucin proteins binds to the collagen-digesting enzymes, including MMPs and neutrophil elastase, promoting the formation of novel collagen in the dermis layer and accelerating wound healing. The collagen-based dressings promote cellular proliferation and migration by mimicking the extracellular matrix (ECM) [90]. Mucins can only be obtained from natural sources because it is difficult to synthesize mucins via biotechnological techniques or chemical synthesis methods [91]. There is a significant heterogeneity in the structure of jellyfish mucins. However, qniumucins are homogeneous with high purity. Thus, unique mucins for biological purposes can be extracted from this marine organism [86].
First, collagen is primarily derived from pigs and cattle, which causes infectious diseases and religious issues. However, their extraction process is often time-consuming and laborious, reducing total protein production and increasing the total cost of the product. However, jellyfish are an accessible, cheap and valuable renewable material. Besides, the use of jellyfish as a valuable raw material is largely confined to relatively small niches in the eastern food industry. It is worth noting that jellyfish have great potential in biomedicine, cosmetics and agriculture. Moreover, the negative impact of the spread of jellyfish on the global economy is internationally recognized and has aroused widespread public concern. The causes of “JF blooms” are varied, including global warming, overfishing, and eutrophication. Thus, the uses of jellyfish should not be overlooked. The utilization of jellyfish in various fields of production will improve global environmental problems. Based on the above findings, jellyfish represent a significant source of biomimetic materials.
2.2.2. Andrias davidianus
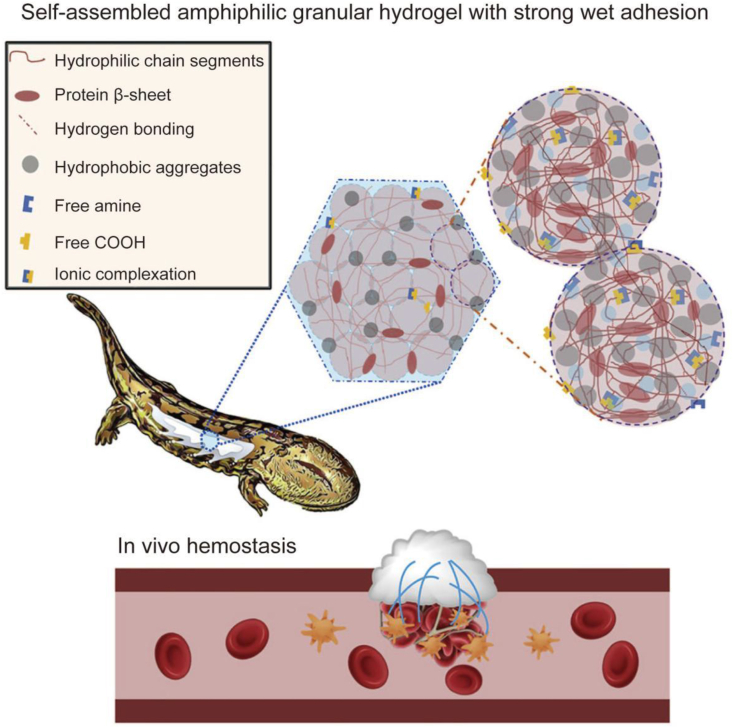
Andrias davidianus (Chinese giant salamander) is the world's largest and oldest amphibian species [92]. Its skin secretes a white mucus for defense (skin secretion of Andrias davidianus (SSAD), which contains proteins, amino acids, mucopolysaccharides, and antimicrobial peptides [93]. A previous proteomics study revealed that the SSAD has 155 proteins with adhesive properties of mucus, including annexin-B11, apolipoprotein A-IV, galectin-2, galectin-7, and talin-2, and ten proteins related to wound healing. The amino acid profile of SSAD revealed that SSAD is made up of 18 amino acid residues, with tyrosine, phenylalanine, and tryptophan accounting for 5.59%, 5.08%, and 0.94%, respectively [94]. Growing evidence suggests that SSAD protein has good hydrophobicity/hydrophilicity, hydrogen bonding and ion complex effect, contributing to the self-assembly and adhesion performance of ADS granule gel.
The hemostatic capacity of SSAD can be attributed to the synergy created by its physical adhesion barrier and procoagulant activity. When SSAD powder is applied to the bleeding site, the dry powder absorbs fluid from the whole blood and self-assembles to form a hydrophobic hydrogel patch within 20 s, which is attached to the wound site to arrest bleeding [93,95]. Several proteins and ECM molecules found at the wound side promote strong adhesion between the SSAD and the wound through hydrogen bonds, van der Waals forces, and/or π–π interactions upon hydration of the adhesive powder by the tissue fluid [[96], [97], [98]]. The interesting self-assembly and wet adhesion behavior of ADS dry particles are distinct from the currently popular wet adhesion bioadhesives that use catecholic bonding or underwater polyelectrolyte condensation. The absorption of liquid components in whole blood by SSAD dry powder also causes plasma protein aggregation. Protein represents the primary component of SSAD, containing several carboxylic acid and amine groups. Charge-based coagulation is also used to achieve rapid hemostasis. The wide charge on the SSAD protein can concentrate the factors around the coagulant viscous hydrogel to achieve clotting and stop bleeding. In addition, SSAD can directly interact with the cellular components in the blood to promote hemostasis due to the presence of cell receptor proteins. Therefore, SSAD is an effective hemostatic material.
2.3. Mimic the structure of the chemical composition of organisms
2.3.1. Invertebrates
Chitin is the second most common natural polymer after cellulose, present in several organisms, including protists, diatoms, sponges, arthropods, mollusks, insects, and arachnoids. It plays a huge role in the structural integrity of the exoskeleton of different marine invertebrates [[99], [100], [101], [102]]. Notably, chitin is a linear polysaccharide that can be extracted through chemical methods, microbial fermentation, and biological enzymatic hydrolysis. It has excellent qualities, including high biocompatibility, bioactivity, biodegradability, and mechanical strength. However, its clinical applicability is limited by poor solubility [[102], [103], [104]]. Therefore, chitosan (CS), a deacetylated derivative of chitin, has been used in various fields because of its excellent biocompatibility and biodegradability, hemostatic capacity, non-toxicity, and ease of processing. The extent of acetylation and the molecular weight of CS significantly affect its functional characteristics, including solubility, biodegradability, and bioactivity [99,105]. CS comprises d-glucosamine, a diacetyl unit, and N-acetyl-d-glucosamine, an acetyl unit. The degree of deacetylation (DD) is calculated as the ratio between the two [103,106]. Due to its reduced molecular weight (MW) and higher deacetylation degree, CS has a high biological activity and water solubility [107]. CS begins to dissolve in an aqueous acidic medium when its deacetylation level reaches approximately 50%. Current evidence suggests that CS is a cationic polymer that dissolves in an acidic environment [108]. CS has potent antibacterial properties against Gram-positive and Gram-negative bacteria and antifungal properties [100,[109], [110], [111], [112]]. It exerts antibacterial activity by interacting with the negatively charged cell membranes of microorganisms. CS can be easily produced through chemical methods. In short, CS provides promising prospects for biomedical uses [113].
The hemostatic process of CS differs from the conventional coagulation cascade in that it induces coagulation without involving the intrinsic pathway. The hemostatic mechanism of CS involves erythrocyte aggregation, platelet activation, the contact activation pathway, and the formation of the space net structure. Notably, CS is basic cationic polysaccharide when the NH3+ positive charge on the CS chain electrostatically interacts with the anion on the surface of erythrocytes, a blood clot is formed, quickly stopping the bleeding. The interaction of chitosan with erythrocytes can be affected by the number of protonated amine groups, molecular weight, and degree of deacetylation of CS which influences its hemostatic effect [114]. Furthermore, biopolymers promote platelet adhesion and aggregation. This complex procedure depends on various variables, including charge density, surface chemical composition, and hydrogen bonding [115]. CS can promote platelet adherence, aggregation, and activation [116]. Sagnella et al. reported that CS requires a high positive charge density to promote platelet adherence and aggregation [117]. CS undergoes a series of metabolic processes in vivo that bind almost all plasma proteins and clotting factors, further strengthening the blood clot [118]. Studies have shown that coagulation factor XI (FXI) and XII (FXII) are necessary for in vitro CS-induced blood coagulation. In addition, the contact activation pathway is affected by the interaction of the biomaterial with the wound surface, altering the functional mechanisms of the adsorption protein functions. These mechanisms have inspired scientists to develop novel hemostatic materials [101].
CS is involved in every stage of wound healing. CS encourages neutrophil and macrophage infiltration, promoting the development and re-epithelialization of granulation and fibrous tissues [119,120]. Excessive collagen production leads to the development of hyperplastic scars during the remodeling period. CS can improve epithelial reformation by reducing the production of scar tissue [113,121]. It is hydrophilic and can be chemically modified due to its free amino groups. Therefore, CS can be modified for clinical applications due to its beneficial properties [100]. For instance, CS derivatives, including trimethylated chitosan (TMC), exhibit improved solubility and potent antibacterial properties [113,122]. CS can speed up wound healing when used as a powder, nanoparticles, microparticles, particles, or sponges [100]. Furthermore, it can be combined with other substances, improving its capacity to promote hemostasis and wound healing.
2.3.2. Marine mussels
Marine mussels are a profound illustration of biological adhesion [123,124]. Since Waite et al. reported their findings in the 1980s, it has been uncovered that marine mussels provide an environment for the organism to secrete mussel adhesion protein (MAP) to stick to wet surfaces in seawater [[125], [126], [127]]. Mefp-1, Mefp-2, Mefp-3, Mefp-4, Mefp-5, and Mefp-6 are MAP components [123]. The three interface proteins, Mefp-3, Mefp-5, and Mefp-6, participate in adhesion. Mefp-2, the primary element of the porosity network [128], interacts with the interface proteins [129,130], while Mefp-4, found in the patch, connects patch Mefp-2 and collagen gland [131]. The coated protein Mefp-1, which forms a tough outer coating to shield the collagen core, is found in the cuticle of the epiphytic line. Mefp-1 can resist wearing [132]. A recent study revealed 15 novel foot proteins (Mcfp), thus widening the scope of mussel retention biochemistry and offering an opportunity to investigate biomimetic wet adhesion [124]. However, these composition gradients and rich structural features have been largely neglected during the design of biomimetic underwater adhesives.
Most mussel foot proteins (Mfps) have 3,4-dihydroxyphenylalanine (DOPA) [123]. The catechol groups in DOPA exhibit exceptional adhesion properties on moist surfaces and biological tissues [124]. Over the years, dopamine has attracted increasing attention in chemical and medicinal research due to its chemical components and capacity to act as an adhesive. The adhesion of DOPA to tissue surface is achieved through metal coordination, covalent crosslinking, hydrogen bonding and π-cationic interaction. Modeling complicated biological wet adhesion requires taking into account various critical elements in addition to molecular catechol bonding, which, while crucial, is not the only source of strong adherence. For example, DOPA and lysine residues in many mfps work synergistically to yield adhesive properties. Recent research has demonstrated that removing hydrated cations from mineral surfaces by vicinal lysine residues is essential for catechol binding to the underlying oxide groups. In addition to the adjacent side chains within the peptide sequences described above, peptide length is very important for mussel adhesion. Besides, it has been reported that the catechol in DOPA is very sensitive to pH. Since DOPA oxidizes to quinones under alkaline conditions, the adhesion of DOPA to the oxide surface via hydrogen bonds and to the surface metal sites via chelation bonds is lost. Coordination bonds and metal ion coordination also increase cohesion as pH increases. Therefore, the utilization of oxidative crosslinking as a mechanism for improved adhesion and cohesion depends on the capacity to manage pH levels [133,134]. Importantly, these factors should be considered during the designing of hemostatic pro-healing materials. Due to the wet adhesion properties of mussels, researchers prefer to use various derivatives of dopamine to design biomimetic hemostatic materials. Currently, various synthetic methods combine catechol groups into the polymer backbone to enhance the material's adhesion properties and thus control bleeding. Indeed, to achieve the functionalization of hemostatic materials, it appears promising to design bionic hemostatic materials by combining the advantages of two or more organisms.
2.3.3. Sandcastle worms
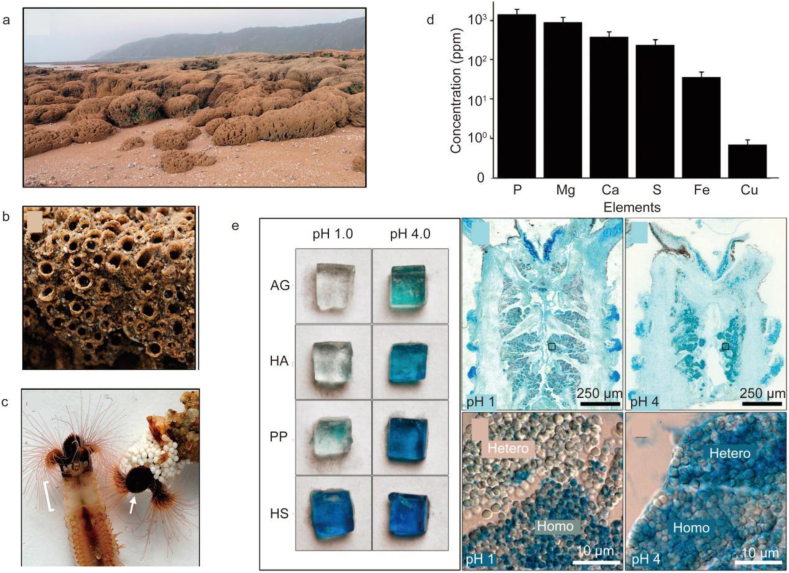
Sandcastle worms and mussels produce sticky secretions. Sandcastle worms use protein glue to bind mineral particles creating a tube-like shelter. The protein glue is secreted by various secretory cells. Interestingly, the protein glue secreted by the Phragmatopoma california species contains various proteins, including five phragmatopoma cement proteins (Pc), Mg2+ and Ca2+ ions, and a sulfated macromolecule (Fig. 2d) [[135], [136], [137]]. To identify potential sulfated macromolecules in the P. californica adhesive gland and other tissues, coronal cryosections through the parathorax were stained with Alcian Blue at pH 1 and 4 (Fig. 2e). At higher magnifications, sulfated components were observed in only homogeneous granules. At pH 4, both granule types stained strongly, as expected (Fig. 2e). Specifically, the generated molecular complexes were condensed at acidic pH, whereas, at pH values around 8, the negatively charged molecules were subjected to repulsive forces, which entailed more extended structures. In this state, binding divalent cations (such as Ca2+) could facilitate the formation of adhesive structures. Therefore, these factors should be considered when designing hemostatic materials. The tyrosine residues of Pc-1 and Pc-2 undergo post-translational modification to form 3,4-dihydroxyphenylalanine (DOPA) [138]. DOPA forms complexes through hydrogen bonding in surface coupling or to metal ions and metal oxides on mineral surfaces and intermolecular crosslinking after oxidation, contributing to the hardening of the cement [123,135]. Pc-3 is slightly acidic because it has an excess of heavily phosphorylated serine residues. Cysteine is present in Pc-1, -2, and -3. Some cysteine residues crosslink with DOPA during the setting process to form 5-S-cysteinyl-Dopa. Dense sub-granules of Mg, the (polyphospho)proteins Pc3A and B, and at least two polybasic proteins, Pc1 and Pc4, can be observed in prominent heterogeneous granules. Pc2 and Pc5 contain two or more polybasic proteins [139]. In addition, the protein glue of sandcastle worms contains enzymes, including tyrosinase and peroxidase, which connect many l-dopa-containing proteins to increase the cohesive and adhesive properties of the glue [140]. Furthermore, the phosphoserine side chains bond to one another through electrostatic interactions [141]. By understanding these rich structural features of sandcastle worms, researchers can design hemostatic materials at the microscopic level.
Fig. 2.
Reef-building sabellariid tubeworms. (a) The lateral growth of isolated dome-shaped colonies of S. alveolota (foreground) causes colony fusion into a continuous tabular surface covering the beach. (b) Closer view of a colony of P. californica. Each tube contains an individual worm. (c) Left: P. californica removed from the tubular shell. The white bracket indicates the parathorax region that contains the adhesive gland. Right: Zirconium oxide beads have been glued onto the anterior end of the natural tube. The arrow indicates the operculum. (d) Elemental composition of sandcastle glue. (e) Alcian Blue staining. Left: agarose gels containing equal concentrations of known polyelectrolytes stained with AB at pH 1 and 4: agarose only (AG), hyaluronic acid (HA), polyphosphate (PP), heparan sulfate (HS). Right: coronal cryosection and closer view of secretory granules stained with AB at pH 1 and 4. Homo = homogeneous granules; Hetero = heterogeneous granules [137].
The glue comprises different sets of condensed, oppositely charged polyelectrolytes, granulated individually and maintained at a high concentration in different types of cells. With little mixing, the pre-organized adhesive modules separately were packaged in granules and fused after release to form a fluid that could penetrate cracks. The fluid glue transforms into a porous sticky structure within less than 30 min of its release into the salty water [135,142,143]. The nanoporous and microporous matrix of the foamy solid glue increases the tensile strength and stability of the glue through different mechanisms. Catechol oxidase, which covalently crosslinks the adhesive and turns into a structural component of the finished bonded junction, is co-packaged with bonding grains. Besides the change in amino acid composition or single amino acid change, the final biphasic porous structure and transformation from liquid to solid after secretion, as well as the cellular sorting and packaging processes, control the overall strength and toughness of granulated sandcastle glue [22]. Complex coacervates can be formed in test tubes by integrating comparably diluted solutions of oppositely charged colloids or polyelectrolytes. However, complex coacervation can only occur up to a certain copolyelectrolyte concentration. Although complex coacervation might not be a factor in the development of sandcastle glue, it acts as a beneficial model for replicating the glue using artificial glue protein analogs [141,144].
The glue structurally comprises repetitive protein sequences analogous to poly(meth)acrylates. The most important features of sandcastle glue are the separation of the opposite charges into different polymers and the high density of phosphate sidechains. These features can be easily and artificially duplicated using synthetic poly(meth)acrylates that are affordable, scalable, and water-soluble [143]. Additionally, it is widely thought that electrostatic contact between analog polyelectrolytes and divalent cations stimulates the formation of colloidal nano-complexes in a process analogous to coacervation at the molecular level [145,146]. Further studies are ongoing to elucidate the dynamics and nano- and micro-phase arrangement of the biomimetic glue coacervates. According to the characteristics of sandcastle worm adhesion protein and the complex coagulation model of sandcastle gum, researchers can design the preparation method of biomimetic hemostatic material skillfully to improve the adhesion of hemostatic material to control bleeding. Similarly, combining the advantages of two or more organisms to design biomimetic hemostatic materials has huge significance.
3. Application of bionic materials on hemostasis and promoting wound healing
The latest findings on biomimetic hemostatic materials designed by researchers are systematically described here. The application scenarios of biomimetic hemostatic materials depend on their composition and form. The performance of hemostatic material has been improved by the addition of bionic material. In addition, several substances with excellent properties can be combined to improve material properties. For example, combining several biological materials can improve the functional limitations of a single material.
3.1. Bionic materials inspired by insects
Researchers have devoted significant effort to developing microneedles inspired by insect life. With progress in research, they added numerous novel features to the structure of microneedles to expand their application in various industries. Interestingly, researchers have designed an eagle claw-inspired liquid metal (LM)-encapsulated microneedle patch to accelerate linear wound healing. A microneedle patch with a claw-like clamping structure was made from two gauze-connected, tip-tilted hydrogel microneedle sections. This endowed it with the capacity to fix and tighten the injured area to prevent secondary wound dehiscence. The claw-inspired MN patch included a liquid metal with negligible toxicity that could deliver electric stimulation directing cell function and promoting wound closure. Electrical stimulation is currently used by researchers in numerous clinical domains, including the treatment of mental disease, neuron regeneration, and wound healing [40].
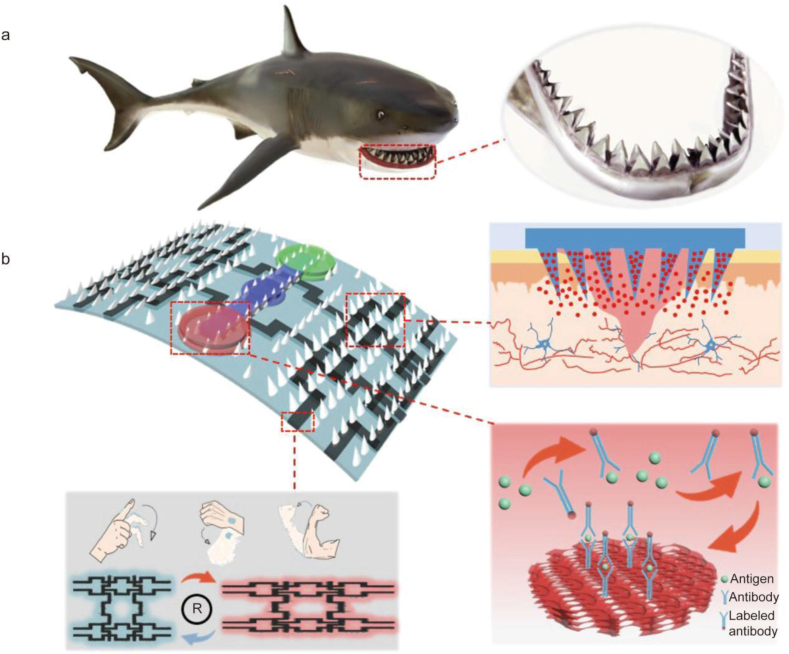
Cha et al. presented a hydrogel-based adhesive MN patch with a double-layered structure that could firmly anchor onto various tissues. This material was inspired by the mechanisms by which endoparasites connect to the host intestines by swelling their proboscis. The two-layered structure consisted of a non-swellable core made of silk fibroin and a swellable sticky shell embedded with mussel protein. The fracture strength analysis of this MN patch revealed its outstanding mechanical strength. The novel adhesive MN patch successfully demonstrated its superior adhesion onto wet and dynamic biological surfaces through swelling-mediated mechanical interlocking, excellent biocompatibility, biodegradability, and optional transdermal drug delivery ability through ex vivo and in vivo evaluations [149]. This caused prompt sealing and functional healing of external and internal wounds. To treat difficult-to-heal chronic wounds, researchers have designed a MN patch inspired by shark teeth (Fig. 3) [150]. Similar to the eagle-inspired microneedles described earlier, this MN patch has also a tilted special structure that allows easy penetration of the skin, especially by preserving a stable adhesion during long-term recovery from chronic wounds. The patch has microfluidic channels and inverse opall (IO) photonic crystal (PC) structures, which allow researchers to analyze specific biomarkers contained in wound secretions and increase the loading capacity of the patch (Fig. 3b). Most importantly, the movement of the wound area can be monitored by developing flexible circuits on the MN patch (Fig. 3b), increasing the prospect of managing wounds. However, further improvements are still needed to make its clinical use a reality. The design of microneedles mimicking biological teeth, claws or mouthparts can be added to the hemostatic material, which can be used in the future to rapidly and firmly close the wound in the case of acute bleeding to control bleeding. Of course, the challenges of the physical environment also need to be taken into account. For example, how do researchers adjust the Angle of the microneedles based on the smoothness of the bleeding wound and the wet environment? How can hemostatic materials be inserted in minimally invasive surgery?
Fig. 3.
Schematic diagram of shark tooth-inspired microneedle dressing for intelligent wound management. (a) The designed wound dressing was inspired by the shark teeth. (b) Application of wound dressing, including motion sensing, biochemical analysis, and healing [150].
3.2. Bionic materials inspired by geckos
Inspired by adhesion properties of the gecko, from the microstructure of the gecko foot and van der Waals forces, researchers prepared several tourniquet bandages. These materials can control wound bleeding caused by internal surgery or accidental body injuries by improving the tissue adhesion ability of the material [151]. To design a biocompatible, biodegradable, and extremely sticky tissue adhesive, researchers used poly(glycerol sebacate acrylate) (PGSA) to mimic the nanomicrostructure of the gecko [152]. Due to its microscopic pattern, the material demonstrated adhesive strength twice as high as a previously unpatterned material. However, animal experiments have shown that introducing this pattern did not increase the tissue response to the implant. Aldehyde-functionalized dextran (DXTA) promotes the adhesion of hydrogels to tissues. Therefore, to increase the capacity of PGSA to adhere to tissues, the researchers coated it with DXTA. Animal experiments showed that materials coated with DXTA exhibited two times stronger adhesion strength than that without coating. DXTA-coated PGSA has the potential to seal wounds and replace or improve surgical sutures. Notably, based on the properties of PGSA, growth factors, antibiotics, and anti-inflammatory drugs can be added to bandages, which may exert multiple benefits, especially in tissue regeneration. In addition, Xi et al. prepared a material comprising hexanoyl group-modified gelatin (HxGltn) and silicon nanowire (SiNW), mimicking the nanostructure surface of gecko feet [153]. They showed that the bond strength of samples containing HxGltn/silicon nanowires was five times stronger than samples with non-nanostructured surfaces. This biomimetic adhesive surface works incredibly well for connecting soft tissue. This adhesive is reversible in gecko feet since the van der Waals force produced by the nanostructures provides the adhesion force. Therefore, nanostructured surfaces of gecko feet are worth studying to improve these materials. In addition, although the gecko feet exhibit strong adhesion on a dry surface, the adhesion force of the materials based on the gecko microstructure is greatly reduced in a humid environment. Therefore, the wet adhesion of the material could be improved by loading some substances with wet adhesion properties, including a chitosan membrane monolayer and mussel-mimetic polymer [154,155]. Additionally, the adhesive capabilities of geckos have been applied in various fields including robotics, intelligent bonding systems, and health detection systems [[156], [157], [158], [159]]. Nanoarray structural materials are useful alternatives but have some disadvantages, including brittleness and loss of adhesion mass under large loads. As a result, more adjustments are required to address the real flaws in the adhesion process. It is worth noting that current hemostatic materials based on the adhesion properties of geckos are expensive and not durable, and most related materials are still in the exploratory stage. Developing cheaper and more durable hemostatic materials is key to research progress in this field.
3.3. Bionic materials inspired by barnacles
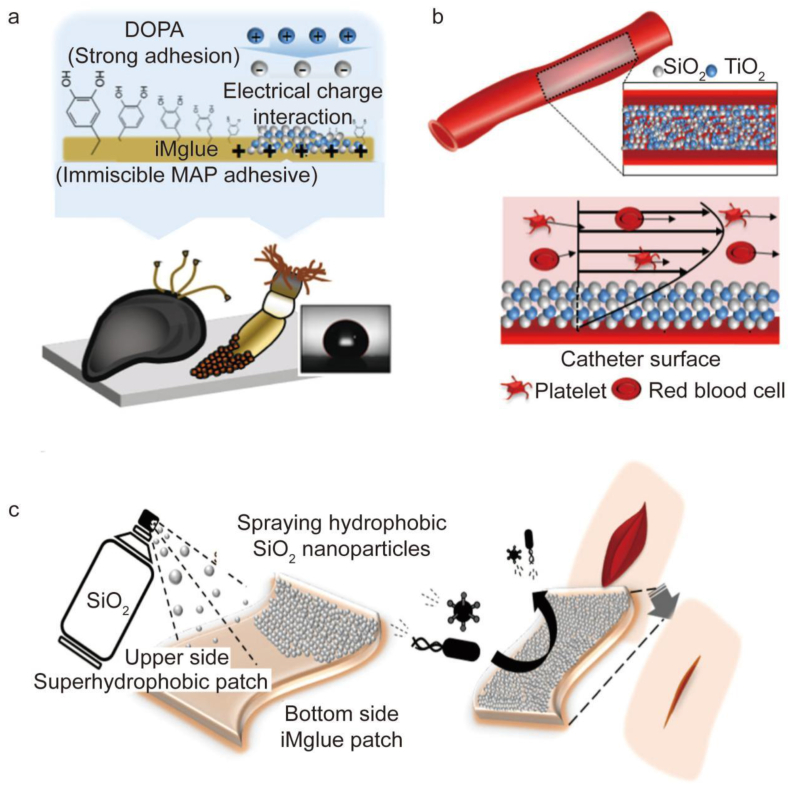
It is well-recognized that barnacles have strong adhesion capabilities. To create a favorable microenvironment by eliminating organic impurities and water layers from the substrate, barnacles and their larvae first release hydrophobic lipids. Protein binders can be added to create powerful surface adhesion forces and intermolecular crosslinking. However, current commercially available tissue adhesives generally do not efficiently attach to tissues exposed to blood or other bodily fluids. Barnacle-inspired hemostatic materials do not depend on coagulation mechanisms but control bleeding primarily via strong adhesion to the tissue. Based on this characteristic of barnacles, Yuk et al. developed an injectable paste made of crosslinked networks of poly (acrylic acid) grafted with N-hydroxysuccinimide ester (PAA-NHS eater) and chitosan [160]. In vivo animal experiments showed that this material can control bleeding within 5 s in the mouse liver and heart and within 15 s in pig liver. This material is associated with a shorter hemostasis time than commercial hemostatic materials and does not depend on the hemostatic capacity of blood coagulation. Experiments indicate that the material has better adhesion properties than commercial hemostatic adhesive materials, including an interface toughness of over 240 J m−2 and tensile strength of over 50 kPa. Therefore, this material has clinical application prospects due to its excellent adhesion capability and superior hemostatic properties.
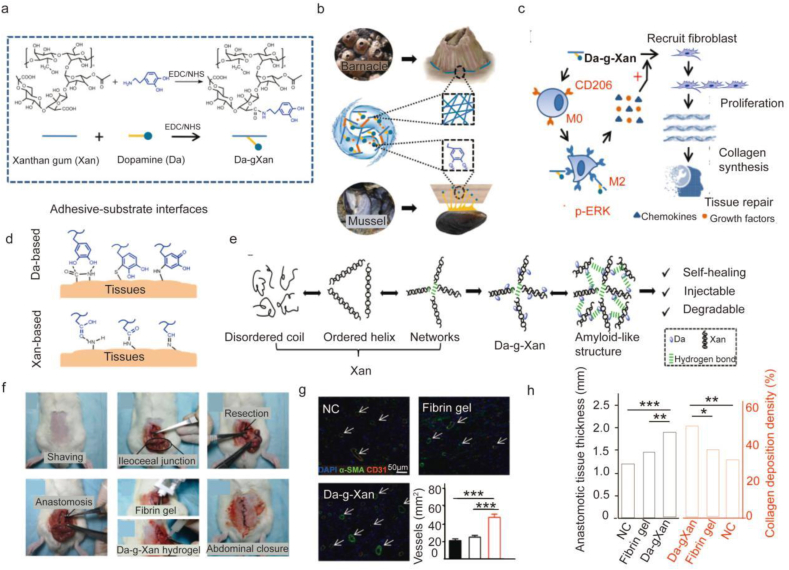
Designing biomimetic composites of amyloid structures and other adhesion techniques has emerged as a promising research area. After surgery, some patients experience inadequate anastomosis healing, a challenging complication that hinders recovery. To address this issue, Huang et al. developed a biomimetic adhesive using dopa and xanthan gum [161]. This design uniquely exploits the different adhesion principles of mussel and barnacle cement proteins to develop new materials. As an analog of DOPA, dopamine has functional catechol groups responsible for mussel-mediated underwater adhesion. A bacterial extracellular polysaccharide with intrinsic helical structures resembling the amyloid fibers in barnacle cement, xanthan gum is an FDA-approved product (Fig. 4b and d). Through many intermolecular hydrogen interactions, the dopamine-conjugated xanthan gum (Da-g-Xan) creates injectable, biodegradable, highly sticky, and self-healing hydrogels (Fig. 4e). These hydrogels induce M2 macrophage polarization in vivo, consistently release Da-g-Xan monomers in situ, and improve the repair of injured internal organs by promoting vascularization and collagen deposition (Fig. 4c, g, 4h). Unlike other groups, the regenerated intestinal granulation tissues of the Da-g-Xan hydrogel were thicker with visible collagen fibers deposition. Additionally, additional neovessels developed in this group (Fig. 4g and h). This finding revealed that the Da-g-Xan hydrogel adhesive enhances fibroblast and vascular endothelial cell activities. As a result, it has excellent potential for accelerating healing following surgical anastomosis. Animal experiments showed a better healing effect of the hydrogel group than the control group.
Fig. 4.
A biomimetic adhesive material inspired by barnacles and mussels. (a) Dopamine (Da) and xanthan gum (Xan) are conjugated through the activation of carboxyl groups by EDC/NHS catalysts. (b) To function as an adhesive, Da-g-Xan simultaneously mimics the mfps of mussels and the amyloid-like structure of barnacle cement proteins. (c) Da-g-Xan has a cascade of amplification effects for fibroblasts on tissue repair mediated by the paracrine action of macrophages. (d) Several interfacial interactions mediate the strong tissue adhesion between the tissue surface and the Da-g-Xan adhesive. (e) The Da-g-Xan hydrogel has a modified microstructure and advantageous qualities like self-healing, injectability, and degradability thanks to the improved intermolecular hydrogen bonding. (f) The precise surgical techniques used to create colonic anastomosis models in rats with various interventions, including basic suture, suture with the protection of a fibrin gel, and suture with the protection of a 10%wt Da-g-Xan hydrogel. (g) Quantitative analysis of neovessel formation in regenerated granulation tissues by staining the vascular biomarkers (CD31, α-SMA) for the different interventions; n = 5 for each replicate, ∗∗∗p < 0.001. (h) Quantitative analysis of the granulation tissues and collagen fiber deposition for the different interventions; n = 5 for each replicate, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 [161].
Other scientists have designed similar biomimetic adhesives based on two different adhesion principles of mussels and barnacles [162]. However, due to the amino acid similarity between silk fibroin (SF) and barnacle cement, the researchers used SF to simulate the adhesion principle of the barnacle. This mixture adhesive does not require complex synthesis steps and exhibits effective adhesive properties on both dry surfaces and underwater. Nonetheless, for these materials to be applied in clinical practice, additional experiments are required to validate their biocompatibility and degradability.
Although mussel system-based proteins have high adhesion strength (300–500 kPa), several disadvantages, including low yield, lack of self-assembly, and self-regeneration have limited their application in clinical practice. Researchers have designed a live-cell adhesive using three self-assembling substances, including, barnacle cp19 k, E. coli CsgA, and Xanthan gum (XG) [163]. Fusion proteins expressed after the fusion of the genes encoding two native adhesion proteins (CsgA and cp19 k) derived from E. coli and barnacle cement can self-assemble into amyloid nanofiber networks at the cell surface. However, E. coli enters this fiber network by covalently crosslinking with XG to generate living-cell biological tissue binders. Through cell experiments, this biological tissue adhesive has been proved to have the self-regenerative capability and adhesion strength of up to 278 kPa. Therefore, based on its low bio-toxicity, the capacity to resist the in vivo liquid environment, and high adhesion, this adhesive has huge application prospects as an adhesive for intestinal repair. However, research on barnacle biomimetic materials is still in its infancy. Therefore, additional work is required to understand the adhesive mechanisms and to investigate various manufacturing techniques for biomimetic hemostatic materials and anterior wound healing materials.
3.4. Bionic materials inspired by jellyfish
Many organisms containing collagen have been used as raw materials for biomimetic hemostatic materials [164], including jellyfish [87], tilapia, duck, pig, cow. Jellyfish, which are rich in collagen, have attracted significant attention. Jellyfish collagen has been utilized in medical procedures, either by itself (e.g., as a material for scaffolds) or in combination with other molecules (such as agarose or alginate), to promote the regeneration of bone or cartilage tissue [165,166]. Additionally, jellyfish collagen is utilized to create microparticles that carry medicinal medicines and as a sensor to detect thrombin [167,168]. Jellyfish adhesive compounds (qniumucin and collagen) have been analyzed as potential components of hemostatic and wound healing materials. Cheng et al. developed collagen sponges and assessed their structure using a scanning electron microscope (SEM) and Fourier Transform Infrared Spectroscopy (FTIR). Studies on hemostatic mechanisms have shown that platelets and hemocytes may stick to and congregate on the surface of the collagen sponge. It has been established that the collagen sponge has a higher rate of water absorption, significantly lower blood clotting index (BCI) (p < 0.05), and a greater capacity for hemostasis than medical gauze [87]. Collagen sponge from jellyfish is excellent for hemostasis and wound healing. Silver nanoparticles are also incorporated with this substance to generate mats with strong antibacterial characteristics. In a pig wound healing model, preclinical tests using the acquired pads revealed quick and thorough wound healing [90]. Moreover, other studies revealed that jellyfish mucin-based electrospun (sugar) protein scaffolds could promote cardiac cell proliferation without causing harmful effects. Notably, a composite of jellyfish collagen and collagen from pigs or cattle significantly accelerate wound healing by promoting epidermal regeneration and granulation tissue formation [169,170]. Therefore, jellyfish collagen is an excellent biomaterial for hemostasis and wound healing. Nonetheless, the use of jellyfish collagen as a hemostatic material is in its infancy. This cheap and easily accessible material should be taken seriously and could be an important source of hemostatic materials in the future.
3.5. Bionic materials inspired by Andrias davidianus
Rapid hemostasis is crucial to saving the lives of trauma patients in need of emergency medical care. Zhang et al. developed SSAD, a hemostatic powder with good biodegradability, antimicrobial properties, and wound-healing capabilities, from the epidermal secretion of Andrias davidianus [93]. Instantaneous and efficient hemostasis can be achieved by the local application of SSAD hemostatic powder with the right particle size. Researchers optimized the particle size of the hemostatic powder since too tiny or too large particles did not satisfy the requirements of controlled water absorption and gelation. Initial dehydration of the blood is rapid, speeding up the aggregation of platelets and blood solids by quickly wetting powders with optimal particle sizes to form uniform hydrogels. Subsequently, these SSAD hemostatic powders create gelled adhesive matrices that mechanically stop additional bleeding. As an alternative, researchers have discovered several growth factors and bioactive substances in SSAD, including thrombin, which is crucial for hemostasis. In addition, in vivo exothermic effects, systemic toxicity, and degradability of SSAD have been investigated, indicating that the hydration procedure of SSAD dressing does not generate any additional heat. The SSAD hydrogel was entirely decomposed 14 days after implantation with no evidence of long-term material toxicity. Notably, SSAD is a natural, affordable, and sustainable option since the salamander is not killed during the extraction of the mucus needed to make the hemostatic powder. The application of the unaltered SSAD-based tissue adhesive for wound healing has been documented in the literature. SSAD was successfully used to treat a full-skin deficit in diabetic SD rats and quickly seal a bleeding skin cut in rats. Moreover, SSAD could be comprehensively degraded in vivo with mild inflammation. As demonstrated by current research, we predict that due to its low cost, environmentally friendly manufacture, healing-promotion potential, and superior biocompatibility, SSAD is a potential material for sutureless wound closure. Moreover, SSAD can treat wounds in weak internal organs and tissues [95]. In addition, researchers prepared a hemostatic adhesive inspired by Andrias davidianus [171]. The wet adhesion of the material is attributed to the good hydrophobicity/hydrophilicity, hydrogen bonding and ion complexation of SSAD proteins (Fig. 5). When SSAD powder is applied to the bleeding site, the dry powder absorbs fluid from the whole blood and self-assembles to form a hydrophobic hydrogel patch within 20 s, attached to the wound site to arrest bleeding. The absorption of liquid components in whole blood by SSAD dry powder causes plasma protein aggregation. The hemostatic capacity of SSAD is attributed to the synergistic effect between its physical adhesion barrier and procoagulant activity. Upon contact with water, hemostatic materials form a hydrophobic hydrogel that can firmly adhere to the tissue surface. This material exhibited excellent hemostatic properties and wet adhesion during in vivo animal experiments. Its 72 h underwater adhesion strength to porcine skin tissue was about 47 kPa.
Fig. 5.
Dry granule adhesive of Andrias davidianus skin secretion builds strong wet-adhesive properties [171].
Other researchers have loaded drugs into SSAD wound dressings to accelerate wound healing [172]. SSAD-based materials exhibit significant value during hemostasis and accelerate wound healing, potentially overcoming drawbacks of commercially available surgical hemostasis glues and materials.
3.6. Bionic materials inspired by chitosan
Currently available chitosan-based hemostasis products include Celox®, TraumaStat®, and HemCon® bandages [2]. Due to its excellent properties, CS is an excellent candidate for hemostatic materials. The hemostatic capacity and mechanical qualities of CS-based materials can be further improved by combining them with additional substances to develop composite materials. At present, several CS-based hemostatic materials have been designed, including sponges, hydrogels, thin films, particles, and fibers.
3.6.1. CS-based composite hemostatic sponges
CS sponge is an efficient hemostatic dressing, due to its interconnected porous architecture, high swelling capacity, and strong antibacterial activity. However, during its removal as a wound-healing dressing, the poor hydrophilic nature of the sponge fails to keep the wound wet, causing additional injury. Researchers designed a CS sponge based on fly-larva shells [173]. Cytotoxicity experiments revealed that the hemostatic material based on CS is cytocompatible. In the liver bleeding model, CS hemostatic agents exhibited better performance than the absorbable gelatin sponge (GS) and oxidized cellulose gauze (OC) due to the ability of CS to improve platelet activation, red blood cell aggregation, altered morphology, and thrombin generation. For these reasons, this material can be used as an absorbable surgical hemostatic agent for internal use. Besides, researchers have used 3D printing to design and surface-actively modify a microchannel alkylated chitosan sponge (MACS) to treat incompressible bleeding and promote wound healing [174]. In vitro procoagulant experiments showed higher numbers of red blood cells and platelets attached to MACS than controls. Since the hydrophobic alkyl chains and microchannel structure can promote the aggregation of erythrocytes and platelets, they work in tandem with CS, potentiating the ability of MACS to promote blood coagulation. In the in vivo liver bleeding model, the total blood loss and hemostasis time were significantly lower in the MACS group than in the control group. In vitro and in vivo experiments have demonstrated the excellent hemostasis performance of MACS. In addition, researchers have demonstrated the antibacterial capability of MACS via contact-killing experiments. It was found that S. aureus and E. coli were significantly lower in the MACS group than in the control group. The pro-regenerative capacity of the MACS-2 was assessed using an in situ liver regeneration model. This material achieves the versatile objectives of rapid hemostasis, antibacterial properties, and tissue regeneration capacity. In addition, Hu et al. developed a composite sponge by physically integrating hydroxybutyl chitosan (HBC) and CS using vacuum freeze-drying to address the issue at hand [175]. HBC/CS sponges are characterized by high porosity (about 85%), high water absorption (approximately 25 times higher), exceptional softness, negligible cytotoxicity, and strong antibacterial capabilities. Additionally, the 3D porous structure of the composite sponge improves nutrient preservation, keeps the wound wet, promotes the crawling and proliferation of epithelial cells in composite sponges, and speeds up wound healing in rat wound healing trials, suggesting its potential use in wound dressings. Besides these composites, materials based on cellulose and polymers (such as sodium polyacrylate (SPA), and PEG) can be integrated with CS to form an excellent hemostatic sponge material [176,177]. SPA- and PEG-based sponges also exert rapid hemostasis capacity to promote wound healing and good biocompatibility. Supercritical fluid (SCF) technology has been developed and widely applied in tissue engineering and biomedical applications over the last few decades. A composite sponge made of CS and poly-(methyl vinyl ether-co-maleic anhydride) (PVM/MA) was effectively developed using the supercritical CO2 technique with ammonium bicarbonate particles as the pore-generating agent [178]. The CS-PVM/MA sponge exhibited a porous structure (around 80% porosity), which promoted the formation of a red blood cell clot or embolization of the red blood cells. Further, in vitro and in vivo tests indicate that CS-PVM/MA sponge exhibits a strong coagulability as Avitene, a commercially available collagen hemostatic agent. Polysaccharide hydrogels have become research hotspots due to their exceptional biodegradability and biocompatibility, high swelling performance, quick hemostasis, and capacity to develop a moist environment.
3.6.2. CS-based composite hemostatic hydrogels
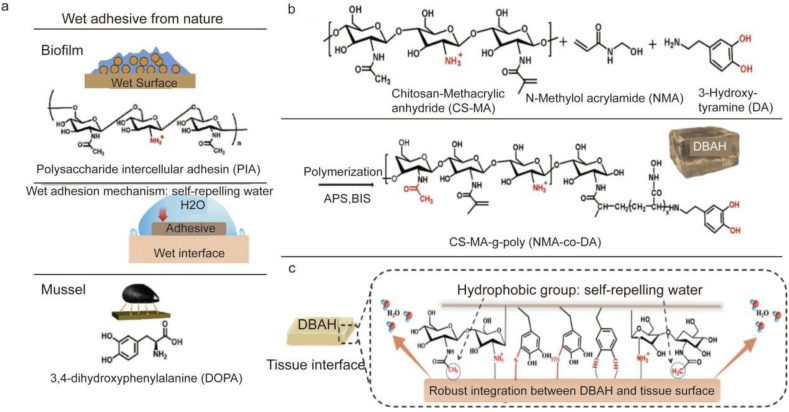
Due to their excellent properties, various CS-based hydrogels have been designed to improve the hemostasis and wound-healing properties of materials. CS-based materials are loaded with cellulose [[179], [180], [181]], polysaccharides [182,183], polymers [184,185], metal ions [186], and nanoparticles [187,188] to improve their hemostatic and pro-healing capacities. In addition, the hemostatic properties of the material can be improved by adding some groups, including sulfa, quaternary ammonium, and dodecyl, to chitosan [[189], [190], [191]]. The introduction of these groups can increase red blood cell (RBC) adhesion and aggregation, thereby promoting blood coagulation. For example, Xiang et al. produced a hydrogel using oxidized konjac glucomannan (OKGM) and dodecyl-modified N-carboxyethyl chitosan (DCEC) using the Schiff base reaction [192]. The hydrogels made from DCEC and OKGM exhibited good hemostasis and adhesion properties during experiments. Due to the electrostatic and hydrophobic interactions between the cell membrane and the amine/dodecyl group, DCEC equips the hydrogel with outstanding antibacterial and hemostatic properties. However, DCEC/OKGM hydrogels exhibit anti-adhesive properties, which may be attributed to their role as a physical barrier between the damaged tissues and the adjacent organs. This barrier action prevents adhesion directly caused by cell proliferation and migration during wound healing and inhibits tumor necrosis factor (TNF) expression. Zhang et al. developed a Gel/TA hydrogel with high adhesion, low distensibility, biocompatibility, antibacterial, antioxidant, and hemostatic properties [193]. The addition of silk fibroin (SF) was shown to promote blood coagulation and the hydrogel's hemostatic capacity. Gel/TA hydrogel adhesives with good antibacterial properties are formed through the joint action of tannic acid (TA) and quaternary ammonium chitosan (QCS). As an antioxidant, TA helps accelerate wound healing by reducing the production of inflammatory factors in the wound. Therefore, this hydrogel is a good candidate for hemostatic and wound dressing. Researchers have also revealed a brand-new dual bionic adhesive hydrogel (DBAH) powered by mussel adhesion protein (Dopa) and polysaccharide intercellular adhesin (PIA) [194]. Among them, DA is a derivative of L-3,4-dihydroxyphenylalanine, whereas CS-MA is a biomimetic polymer that mimics the PIA structure (DOPA). It has been documented that several hydrophobic residues (-CH3) in DBAH immediately form coacervates when it comes in contact with water, self-repelling the water molecules on the substrate surface (Fig. 6c). At this stage, more catechol groups of DA and cationic-free amine groups NH3+ of CS-MA are externally exposed, promoting adequate interaction with the adhering substrate to ensure quick and powerful wet tissue integration (Fig. 6a). CS-MA and DA promoted wet adhesion, providing DBAH with excellent hemostasis to stop bleeding even in the moist and active milieu of the rabbit heart. During burst pressure testing, DBAH could sustain pressures of up to 680 mmHg, which is higher than the systolic pressure in most clinical settings (60–160 mmHg) and beyond that of currently available surgical sealants. According to in vivo bleeding experiments, DBAH can stop liver bleeding in mice within 30 s and heart bleeding in rabbits within 1 min. The excellent hemostatic properties of DBAH are due to its excellent wet adhesion and the inherent hemostatic properties of CS. Additionally, the strong adhesion of DBAH maintains the wound moist and prevents bacterial infection at the wound site. These characteristics highlight its significant potential in the field of wound healing.
Fig. 6.
Schematic diagram of the synthesis of a novel dual bionic adhesive hydrogel (DBAH). (a) The structure of polysaccharide intercellular adhesin (PIA) derived from biofilm and DOPA derived from mussel protein plays a key role in wet adhesion. (b) A biometic biopolymer, chitosan grafted with methacrylate (CS-MA) from PIA, and Dopamine, a catecholamine containing a catechol group of DOPA, was conjugated with NMA for hydrogel formation. (c) Schematic illustration of strong underwater bioinspired adhesion based on the self-repelling water function of CS-MA [194].
3.6.3. CS-based composite hemostatic films
A hemostatic film is an important form of surgical hemostatic material. For various substances (such as drugs, growth factors, etc.), composite films are used as their carriers for intraluminal hemostasis and wound healing. Li et al. prepared composite films from chitosan (CS) and gelatin (GE) containing ibuprofen [195]. Antibacterial tests revealed that material coated with ibuprofen exhibited stronger antimicrobial properties against S. aureus than E. coli. Therefore, this composite membrane can act as a drug delivery system for infected wounds. Cellular experiments revealed that raw materials, CS/GE complex membranes, and vehicle-loaded compound membranes with ibuprofen concentrations lower than 2.5 mg/ml showed no apparent cytotoxicity. Crosslinking of glutaraldehyde influenced the rate of drug release and wound healing, as assessed by in vitro wound release. The CS/GE composite membrane coated with ibuprofen effectively prevented bleeding during surgical procedures based on hemostasis impact evaluation.
3.6.4. Other CS-based composite hemostatic materials
As a powdery hemostat, hemostatic particles can be applied to wounds with any shape and depth, which cannot be achieved by traditional hemostatic materials such as sponges and films. For example, the minerals kaolinite and silicate make up Kaolin. The surface charge characteristics of Kaolin have a significant impact on its ability to clot. When exposed to plasma, Kaolin activates the endogenous coagulation cascade's coagulation factor XII (FXII). Sun et al. developed porous chitosan/kaolin composite microspheres (CSMS-Ks) using a combination of reverse-phase emulsion and thermally induced phase separation (TIPS) procedures to improve the hemostatic action of CS [196]. The physical and physiological mechanisms of chitosan electrostatic adsorption and kaolin-activated coagulation cascade function in concert to provide CSMS-Ks a stronger hemostatic potential than the widely used chitosan hemostatic agent Celox. Notably, CSMS-Ks have huge potential for application as quick hemostatic agents for stopping traumatic bleeding.
Nanofibers have a similar morphology to the natural extracellular matrix of the skin and have the advantages of high porosity, variable pore size distribution, and high surface area ratio. As biomaterials for skin substitutes, nanofiber matrix exhibits tremendous potential. Park et al. loaded β-chitosan on the nanofiber coatings prepared from Polycaprolactone (PCL) and CaCO3 to prepare hemostatic material [197]. It was found that CS could promote both the improvements in blood coagulability and the transformation of surface wetting from hydrophobic to hydrophilic. The calcium ions contained in CaCO3 are an important factor in hemostasis, and CS can improve the wet adhesion of the material to promote coagulation. Thus, the PCL/CaCO3 nanofibers coated with CS are effective hemostatic agents.
CS-based hemostatic materials have become research hotspots in recent years. Different forms of hemostatic materials are used in different scenarios, and different materials groups are synthesized into multifunctional composite materials. It is worth noting that combining various bionic models represents a promising approach for designing efficient and novel bionic hemostatic materials. Indeed, an in-depth understanding of the physical and chemical properties of various materials can help to design hemostatic materials and solve clinical hemostatic problems.
3.7. Bionic materials inspired by mussels
Current commercial hemostatic materials are not effective in blocking the rapid bleeding in a wet environment with high bleeding, and the wet adhesion properties of mussels turn the dilemma. Several studies have designed different types of hemostatic materials that can be used in different scenarios, including hydrogels, sponges [198], nanomaterials, and particles. Hydrogels offer adaptable and optimal physical and chemical properties for promoting wound healing due to their high surface area, customizable substrate, intrinsic flexibility, regulated mechanical strength, biocompatibility, capacity for self-healing, and ability to repair injured tissues.
Hemostatic materials have been designed to rapidly promote wound hemostasis and contain different active ingredients that promote healing. Multiple polymers contain polymer catechol moiety, which helps improve the material's hemostatic properties [199]. Using the Schiff base reaction, Yan et al. developed injectable PLGA/ALG–CHO–catechol hydrogels with exceptional mechanical qualities, adhesive behavior, self-healing potential, and strong cytocompatibility. Trials have demonstrated potential anti-hemorrhagic effects of the hydrogels because of their strong bioadhesion. The injectable PLGA/ALG-CHO/catechol hydrogel system has demonstrated potential efficacy as a hemostatic agent [200]. Significant research efforts focus on finding a method of controlling severe bleeding. Fan et al. developed a polyacrylamide-tannic acid-kaolin (PAAm-TA-KA) hydrogel mimicking the traits of mussels and Arion subfuscus. Among them, tannins serve as a chemical crosslinker and provide the hydrogel with strong and long-lasting adhesion strength due to their abundant catechol groups. As an alternative, kaolin nanoparticles can promote hemostasis by activating the coagulation factor XII (FXII) and acting as physical crosslinkers. This hydrogel shows great application potential as a hemostatic material in traumatic bleeding due to its superior mechanical properties, high hemostatic efficiency, and biocompatibility [201]. Tannic acid (TA) and the polymer (N-hydroxyethyl acrylamide) (PHEAA) were combined by Zhang et al. to develop a viscous hydrogel known as TAHE. PHEAA exhibited good antibacterial activities due to the formation of stronger hydrogen bonds at the interface between water and PHEAA. The TAHE hydrogel firmly adhered to various substrates, including ceramics, PMMA, tin sheets, and pig skin. The researchers successfully proved that TAHE accelerated the hemostatic process and reduced the bleeding time more efficiently than TA by testing the hemostatic efficacy of TAHE powder in a rat liver bleeding model. However, the starch-based hemostatic powder cannot effectively exert its hemostatic effects in an aqueous physiological environment. Inspired by mussel adhesion proteins, researchers developed a novel injectable tissue-adhesion hydrogel (St-Dopa hydrogel) from starch, succinate anhydride, and dopamine by enzymatic crosslinking. The St-Dopa hydrogel demonstrated better in vivo hemostasis capacity in a rabbit liver bleeding model than the chitin hydrogel, promoting quick and efficient hemostasis due to its qualities of rapid sol-gel transition, strong adhesion strength, porous structure, and high swelling degree [202]. Choi et al. proposed a tissue-adhesive gelatin hydrogel made from human adipose tissue's ECM by first generating l-dopa using enzymes before crosslinking it with Fe3+ ions. Adding Fe3+ ions stimulated the DOPA-modified gelatin to produce a viscous hydrogel. Using a hemorrhagic liver rat model, it was demonstrated that DOPA-Fe3+ gelatin hydrogel has outstanding elastic and hemostatic properties and is a potential tissue adhesive in various surgical procedures [203]. It is well-established that CS promotes thrombus formation by producing electrostatic interactions when in contact with blood, which increases the adhesion and aggregation of platelets and red blood cells at the injury site. The catechol groups speed up hemostasis by increasing the adherence of chitosan to wet tissue and constricting blood vessels. Researchers have reported an adhesive hydrogel made of poly (acrylic acid-co-catechol) and chitosan. Due to the covalent/electrostatic attraction/π–π/cationic–π/hydrogen bonding, the hydrogel exhibited high cohesion strength and strong adhesion. The 180-degree peeling adhesion energy of hydrogel to blood-soaked pig skin was significant (1010 J m−2). A bursting pressure of up to 520 mmHg was documented, and the hydrogel could stick to the pig's small intestine tightly and smoothly [204].
Since bacterial infections around wounds are common wound complications, researchers have sought develop antimicrobial nanomaterials that can be locally delivered. By administering effective antimicrobial treatment at the site of wound infection, wound healing can be accelerated. Zhang et al. developed a bioadhesive nanoparticle-hydrogel hybrid (NP-gel) inspired by a delivery system that resembles marine mussel adhesion that could efficiently deliver antimicrobial drugs locally. Under high shear pressures, this material exhibits good adhesion and antibiotic retention on biological surfaces. In vitro experiments have demonstrated that this material can inhibit the growth of E. coli. Bioadhesive NP-gel delivery methods using adhesion forces to counter strong shear forces provide fresh perspectives for treating infected wounds [205]. Lin and colleagues, developed an injectable and self-repairing hydrogel based on catechin-functional polyethylene glycol (PEG). This hydrogel exhibits antibacterial, antifouling, and self-healing properties. The self-repair capacity of this material is attributed to catechol-mediated hydrogen bonding and aromatic interactions rather than catechol-metal coordination. Importantly, this material has low cytotoxicity since it does not contain any metal. Overall, these findings suggest that PEG has significant potential in bioengineering and implant-related applications [206]. Besides, Chen et al. developed an OSA-DA-PAM hydrogel using physical and chemical crosslinking. This hydrogel had a distinctive cell affinity and tissue adhesion because of several catechin groups on the OSA-DA chain. On the other hand, the catechol groups in the material immobilize EGF through chemical reactions and noncovalent interactions and promote the stable release of EGF, thus accelerating tissue regeneration (Fig. 7b). The remaining wound ratios in the groups treated with hydrogel reduced from 100% to 81.2–83.8% from day 0 to day 5 but only slightly declined in the blank group (Fig. 7c) [207]. This finding reveals that the substance could promote tissue regeneration and wound healing. Fig. 7 shows the features of the hydrogel.
Fig. 7.
A mussel-inspired bionic material is used to promote wound healing. (a) Synthesis Route of OSA-DA. (b) Synthetic Process and Schematic Structure of OSA-DA-PAM Hydrogel. (c) Residual wound rates in each group at 0, 5, and 15 days after surgery [207].
Due to its high-water absorption rate, hyaluronic acid can increase the concentration of platelets and coagulation factors. The catechol moiety of dopamine interacts and bonds with amino or sulfhydryl groups in tissue, providing hyaluronic acid greater adhesion to wet tissue and prompt hemostasis. By integrating modified hyaluronic acid (HA) with ε-polylysine (EPL), Liu et al. developed an antibacterial hydrogel wound dressing. EPL-Dopa inhibited and even killed bacteria by altering the permeability of bacterial membranes and the electrostatic interaction between NH3+ and functional groups on bacterial membrane surfaces. In an antibacterial trial, EPL-Dopa effectively killed most germs, indicating its significant level of antibacterial activity [208]. ROS overproduction at the wound site can cause oxidative stress, which slows down wound healing. Dopamine (DA) derived from mussels and polyphenols exhibits antioxidant properties by suppressing free radicals through electron transfer [204,209]. Bo et al. developed a catechol-containing poly(amidoamine) (CPAA) tissue adhesive that can be used in place of a suture to lessen extra tissue damage and scarring. In vivo experiments revealed that the CPAA tissue glue may successfully speed up the healing of the back incision wound and decrease scar formation in rats unlike sutures [210]. Deeper wounds, however, only heal at the edge of the site, and the area inside the wound is coated in scar tissue. Therefore, it is a promising research direction to produce dopa-modified materials for deep and extensive wounds. Mussel-inspired materials including PDA are designed to be multifunctional by integrating them with natural polymers, synthetic polymers, metal ions, and silicon-based materials to quickly stop bleeding and promote wound healing. However, these materials have drawbacks, including poor degradability, toxicity, and immunogenicity. These problems will need to be addressed in the future to improve the efficiency of the materials.
3.8. Bionic materials inspired by sandcastle worms
Sandcastle worms secrete oppositely charged polyelectrolytes, which undergo complex condensation to form viscous adhesives. Interestingly, researchers have prepared biomimetic adhesives with strong adhesive properties [211,212]. A major challenge is how to close bleeding wounds during minimally invasive surgery. The key to solving this problem is that tissue glue must be delivered using a small-bore needle or trocar to close the bleeding wound. Inspired by sandcastle worms, a viscous hydrophobic light-activated glue based on poly(glycerol sebacate)-acrylate was designed as a nanoparticulate composition [212]. Alginate is a negatively charged agent that stabilizes the nanoparticulate surface and can considerably reduce viscosity and increase small-bore needle injectability. When activated by a positively charged polymer, nanoparticulate adhesives readily aggregate to form a viscous glue with rheological, mechanical, and adhesive properties. This method facilitates accurate control of the glue application dose and duration [212]. The use of sandcastle worm-inspired materials in minimally invasive tissue repair, tissue closure, fetal membrane damage repair, bone regeneration, and other areas have been documented [[213], [214], [215]]. Researchers have developed various medicinal tissue adhesives with wet adhesion and anti-fouling, particularly based on the traits of sandcastle worms. Currently, any functional hydrogel requires both the polymers pre-modified with specific reactive/binding sites and the functional molecules bearing the corresponding reactive/binding sites, which restricts the flexibility to develop functional hydrogels. Pc-1, a crucial adhesion protein found in the secretions of sandcastle worms, contains lysine and DOPA in a 1:1 M ratio. Inspired by sandcastle worms, scientists developed a straightforward one-step method for functionalizing wet hydrogels utilizing molecules bearing an adhesive dibutylamine-DOPA-lysine-DOPA tripeptide [216]. This tripeptide can be easily replaced with other functional groups to start different kinds of polymerizations and generate functional polymers with sticky tripeptides at the terminal position. Wet hydrogels can be directly modified with these functional molecules to provide biological properties including antibacterial, cell adhesion, and wound healing. Notably, adhesion is a common feature of hydrogels that promotes wound closure and hemostasis to achieve hemorrhage control. Therefore, by modifying the hydrogel, the adhesion of the material can be improved to refine its hemostatic and pro-healing capacity [216].
Similarly, researchers combined sandcastle worms' adhesion properties with other organisms' excellent properties to design multifunctional bionic adhesives [213,217]. A superhydrophobic (SH) patch, inspired by lotus leaves, sandcastle worms, and mussels, was designed by researchers (Fig. 8) [213]. In addition, the hydrophobic SiO2 was sprayed on the patch surface, which strengthens the material, and provides the material with antibacterial and antithrombotic properties (Fig. 8b and c). Due to the water-immiscibility properties of the sandcastle worm secretion gum and the wet adhesion and positively charged properties of the mussel adhesion proteins, the materials can strongly adhere to the surface of different materials (Fig. 8a). Based on in vitro adhesion testing on porcine skin tissue, the wound site of pig skin treated with this substance had 26 times more adhesion strength than the control group. Antimicrobial experiments showed that the SH patch was completely free of bacterial biofilm formation after 30 min in an E. coli solution. Thus, this highly adhesive material with antibacterial properties has huge potential for seamless wound closure.
Fig. 8.
Comprehensive summary and biomedical multifunctionality of the robust superhydrophobic surface with water-immiscible underwater adhesive. (a) The material was prepared based on the adhesion properties of mussels and sandcastle worms. (b) The material has anti-thrombosis properties. (c) The material was conferred antibacterial properties by spraying SiO2 nanoparticles on the material surface [213].
The formation process of sandcastle gum has been used by researchers to design the synthesis process of hemostatic materials, which is a novel idea for designing hemostatic materials. Based on the in-depth study of the microscopic composition of the chemical composition of natural organisms, researchers can design hemostatic materials from a microscopic perspective. For example, peptides are synthesized as functional polymers at the amino acid level to stop bleeding. In addition, it is worth noting that, as described above, combining multiple biological properties can broaden the application range of hemostatic materials.
4. Discussion
The practice of “biomimicry” involves adapting natural design ideas and concepts for technological creations [218,219]. This strategy is based on the idea that a deeper comprehension of the mechanical, structural, and chemical principles at work in natural materials will promote better translation of desirable properties to controlled synthetic systems, which can then be optimized to meet technological demands that current synthetic materials cannot satisfy. For surgeons, achieving quick hemostasis and effective wound healing is presently a highly challenging task. The adhesive properties of natural organisms are cleverly linked to the design of hemostatic materials to address various problems existing in hemostatic materials (such as biocompatibility, wet adhesion, etc.).
A deep understanding of the molecular background and adhesion principles of organisms is critical to mimicking natural ingredients and designing more efficient hemostatic materials for different bleeding scenarios. From designing microneedle materials by imitating insect mouthparts to adjusting microneedle structures by imitating eagle claws and shark teeth, it is a valuable imitation of nature. In a comprehensive study of geckos and barnacles adhesion models, the researchers designed hemostatic materials with nano-microstructure to enhance adhesion. In addition, directly extracting the biological components of jellyfish and giant salamanders is a simple, environmentally friendly, renewable and low-cost strategy for designing hemostatic materials. Moreover, current research focuses on the wet adhesion of mussels and sandcastle worms. Researchers explored their biological composition and adhesion mode to reasonably improve and design bionic hemostatic materials. With the further study of biological properties and hemostatic materials, mimicking a single biological property cannot satisfy the functional design of hemostatic materials. Researchers have integrated the benefits of two or three organisms during the design of a biomimetic hemostatic material to improve its performance and clinical needs. Adhesion models of these organisms in nature are unique and novel. Three factors are vital in designing materials: chemical composition, nano/microstructure, and structure. The development of new materials, diversification of existing materials, and elimination of current restrictions on weight, toughness, strength, and environmental tolerance are all possibilities since it is possible to combine structural control found in nature with a wide range of synthetic compounds. Understanding natural structures and determining how to mimic them will lead to innovations that could advance materials science in the future.
Even though our understanding of the key components in natural glues that convey adhesive behavior is constantly expanding, there exist still several unanswered questions, particularly for the less-studied variants of these bio-adhesives. For instance, how do the various materials interact with various (natural or artificial) surfaces? How are the chemical characteristics of the glue/material contact and outside factors like variations in moisture content, temperature, or ionic milieu used to determine the lifespan of the adhesive function? A deeper comprehension of the principles underlying the function of biological adhesives has recently enabled researchers to develop specialized, multifunctional glues with various properties, in conjunction with current developments in materials science. Similar advancements in the field of bio-derived glues may be useful for biomedical applications. It is equally crucial to satisfy diverse biological requirements and attain the necessary mechanical properties while at the same time developing biomedical adhesives. Hazardous chemicals and particularly sturdy, non-degradable formulations are therefore rarely appropriate for medical applications due to biological constraints of sensitive tissues. Instead, bioinspired adhesives with (semi-)synthetic polymer backbones and biological features may offer the best of both worlds in terms of customizable material characteristics and biological compatibility/degradability.
The drawbacks of current tissue adhesives, including non-degradability, cytotoxicity, incompatibility with wet surfaces, inability to accommodate dynamic movements of tissues, etc., can be addressed by bioinspired adhesive formulations with strong adhesion performance and outstanding biocompatibility. These bioinspired adhesives can be used as a wound dressing and as medical glue for promoting wound closure. Nonetheless, there remains much work to be performed before clinical use. Furthermore, additional factors must be considered, including the stability of materials during storage, the dependability of adhesion performance, large-scale production techniques, patient compliance, and others. The material must also withstand the stresses of the in vivo environment, including shear pressures, liquid flux, pH and temperature variations, as well as enzyme attack, even if all the other requirements are met. Some bioinspired adhesives are difficult to remove, which might result in secondary harm. Besides, it is challenging to change the adhesive property on demand. By altering their chemical composition to make their stickiness customizable, bioinspired adhesive formulations may be combined with the property of environmentally sensitive materials in the near future to address this issue.
5. Future perspectives
The design of cheap, environmentally friendly, high-yield, and easily manufactured bionic hemostatic materials is essential for improving the outcomes of patients. The combination of multiple biomimetic models represents a promising idea for designing efficient and novel biomimetic hemostatic materials, which can help to overcome the limitations of current hemostatic materials. In biomaterials, a range of crosslinked compounds with excellent adhesion or mechanical properties can be utilized. For instance, hydrogels made from crosslinked polymers are widely used because they have good adhesion and contain factors that promote wound healing or rapid hemostasis. Moreover, materials with strong mechanical properties can be transformed into microneedles for wound suturing. In addition, various factors and cells loaded into formulations can also be inspired by nature. For instance, hydrogels loaded with mesenchymal stem cells are widely used for hemostasis and wound healing. The study of bionic hemostatic materials is a very interesting topic, which deserves more attention from researchers. In short, the application of bionic models in hemostasis has broad prospects.
Author contributions
Simin Jiao: Writing - original draft; Writing - review & editing; Conceptualization; Software; Revision of the manuscript; Xi Zhang: Revision of the manuscript; Hang Cai: Revision of the manuscript; Siyu Wu: Revision of the manuscript; Xiaolan Ou: Revision of the manuscript; Guangda Han: Revision of the manuscript; Jie Zhao: Revision of the manuscript; Yan Li: Revision of the manuscript; Wenlai Guo: Revision of the manuscript; Tianzhou Liu: Revision of the manuscript; Wenrui Qu: Revision of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financed by Jilin Provincial Science and Technology Department [grant numbers: YDZJ202201ZYTS572, YDZJ202301ZYTS499], Jilin Provincial Development and Reform Commission [grant numbers: 2023C040-2], Jilin Province People’s Government Department of Education [grant numbers: JJKH20211123KJ], Jilin Province Department of Finance [grant numbers: 2020SCZT051], and Norman Bethune Program of Jilin University (grant numbers: 2022B44).
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Contributor Information
Wenlai Guo, Email: guowl19@jlu.edu.cn.
Tianzhou Liu, Email: liutianzhou@jlu.edu.cn.
Wenrui Qu, Email: quwenrui@jlu.edu.cn.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1.Montazerian H., Davoodi E., Baidya A., Baghdasarian S., Sarikhani E., Meyer C.E., Haghniaz R., Badv M., Annabi N., Khademhosseini A., Weiss P.S. Engineered Hemostatic Biomaterials for Sealing Wounds. Chem. Rev. 2022 doi: 10.1021/acs.chemrev.1c01015. [DOI] [PubMed] [Google Scholar]
- 2.Hickman D.A., Pawlowski C.L., Sekhon U.D.S., Marks J., Gupta A. Biomaterials and advanced technologies for hemostatic management of bleeding (vol 30, 1700859, 2018) Adv. Mater. 2018;30(36) doi: 10.1002/adma.201804635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spahn D.R., Bouillon B., Cerny V., Duranteau J., Filipescu D., Hunt B.J., Komadina R., Maegele M., Nardi G., Riddez L., Samama C.M., Vincent J.L., Rossaint R. The European guideline on management of major bleeding and coagulopathy following trauma. fifth edition, Critical Care. 2019;23:74. doi: 10.1186/s13054-019-2347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stokes M.E., Ye X., Shah M., Mercaldi K., Reynolds M.W., Rupnow M.F.T., Hammond J. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv. Res. 2011;11:13. doi: 10.1186/1472-6963-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kauvar D.S., Lefering R., Wade C.E. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J. Trauma Inj. Infect. Crit. Care. 2006;60(6) doi: 10.1097/01.ta.0000199961.02677.19. S3-S9. [DOI] [PubMed] [Google Scholar]
- 6.Gruen R.L., Brohi K., Schreiber M., Balogh Z.J., Pitt V., Narayan M., Maier R.V. Haemorrhage control in severely injured patients. Lancet. 2012;380(9847):1099–1108. doi: 10.1016/s0140-6736(12)61224-0. [DOI] [PubMed] [Google Scholar]
- 7.Mannucci P.M., Levi M. Drug therapy: prevention and treatment of major blood loss. N. Engl. J. Med. 2007;356(22):2301–2311. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 8.Marietta M., Facchini L., Pedrazzi P., Busani S., Torelli G. Pathophysiology of bleeding in surgery. Transplant. Proc. 2006;38(3):812–814. doi: 10.1016/j.transproceed.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 9.Fisher A.D., Bulger E.M., Gestring M.L. Stop the bleeding educating the public. JAMA, J. Am. Med. Assoc. 2018;320(6):589–590. doi: 10.1001/jama.2018.7301. [DOI] [PubMed] [Google Scholar]
- 10.Pourshahrestani S., Zeimaran E., Kadri N.A., Mutlu N., Boccaccini A.R. Polymeric hydrogel systems as emerging biomaterial platforms to enable hemostasis and wound healing. Advanced Healthcare Materials. 2020;9(20):52. doi: 10.1002/adhm.202000905. [DOI] [PubMed] [Google Scholar]
- 11.Spotnitz W.D., Burks S. Hemostats, sealants, and adhesives: components of the surgical toolbox. Transfusion. 2008;48(7):1502–1516. doi: 10.1111/j.1537-2995.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 12.Cerda D.G., Ballester A.M., Aliena-Valero A., Caraben-Redano A., Lloris J.M. Use of cyanoacrylate adhesives in general surgery. Surg. Today. 2015;45(8):939–956. doi: 10.1007/s00595-014-1056-4. [DOI] [PubMed] [Google Scholar]
- 13.Älgå A., Haweizy R., Bashaireh K., Wong S., Lundgren K.C., von Schreeb J., Malmstedt J. Negative pressure wound therapy versus standard treatment in patients with acute conflict-related extremity wounds: a pragmatic, multisite, randomised controlled trial. Lancet Global Health. 2020;8(3):e423–e429. doi: 10.1016/S2214-109X(19)30547-9. [DOI] [PubMed] [Google Scholar]
- 14.Clark R.A.F. To scar or not to scar. N. Engl. J. Med. 2021;385(5):469–471. doi: 10.1056/NEJMcibr2107204. [DOI] [PubMed] [Google Scholar]
- 15.Ma Z.W., Yang Z., Gao Q.M., Bao G.Y., Valiei A., Yang F., Huo R., Wang C., Song G.L., Ma D.L., Gao Z.H., Li J.Y. Bioinspired tough gel sheath for robust and versatile surface functionalization. Sci. Adv. 2021;7(15):14. doi: 10.1126/sciadv.abc3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu B.C., Wang Y., Miao Y., Zhang X.Y., Fan Z.X., Singh G., Zhang X.Y., Xu K.G., Li B.Y., Hu Z.Q., Xing M. Hydrogen bonds autonomously powered gelatin methacrylate hydrogels with super-elasticity, self-heal and underwater self-adhesion for sutureless skin and stomach surgery and E-skin. Biomaterials. 2018;171:83–96. doi: 10.1016/j.biomaterials.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Polk H.C., Jr. Prevention of surgical wound infection. Ann. Intern. Med. 1978;89(5 Pt 2 Suppl):770–773. doi: 10.7326/0003-4819-89-5-770. [DOI] [PubMed] [Google Scholar]
- 18.Yang R., Wang X.D., Yan S.J., Dong A., Luan S.F., Yin J.H. Advances in design and biomedical application of hierarchical polymer brushes. Prog. Polym. Sci. 2021;118 doi: 10.1016/j.progpolymsci.2021.101409. [DOI] [Google Scholar]
- 19.Tomizawa Y. Clinical benefits and risk analysis of topical hemostats: a review. J. Artif. Organs : the official journal of the Japanese Society for Artificial Organs. 2005;8(3):137–142. doi: 10.1007/s10047-005-0296-x. [DOI] [PubMed] [Google Scholar]
- 20.Ma X.L., Bian Q., Hu J.Y., Gao J.Q. Stem from nature: bioinspired adhesive formulations for wound healing. J. Contr. Release. 2022;345:292–305. doi: 10.1016/j.jconrel.2022.03.027. [DOI] [PubMed] [Google Scholar]
- 21.Ahn B.K. Perspectives on mussel-inspired wet adhesion. J. Am. Chem. Soc. 2017;139(30):10166–10171. doi: 10.1021/jacs.6b13149. [DOI] [PubMed] [Google Scholar]
- 22.Stewart R.J., Wang C.S., Song I.T., Jones J.P. The role of coacervation and phase transitions in the sandcastle worm adhesive system. Adv. Colloid Interface Sci. 2017;239:88–96. doi: 10.1016/j.cis.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gohad N.V., Aldred N., Hartshorn C.M., Lee Y.J., Cicerone M.T., Orihuela B., Clare A.S., Rittschof D., Mount A.S. Synergistic roles for lipids and proteins in the permanent adhesive of barnacle larvae. Nat. Commun. 2014;5:9. doi: 10.1038/ncomms5414. [DOI] [PubMed] [Google Scholar]
- 24.DeBenedictis E.P., Liu J., Keten S. Adhesion mechanisms of curli subunit CsgA to abiotic surfaces. Sci. Adv. 2016;2(11) doi: 10.1126/sciadv.1600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma G.J., Wu C.W. Microneedle, bio-microneedle and bio-inspired microneedle: a review. J. Contr. Release. 2017;251:11–23. doi: 10.1016/j.jconrel.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Kong X.Q., Wu C.W. Mosquito proboscis: an elegant biomicroelectromechanical system. Phys. Rev. 2010;82(1) doi: 10.1103/PhysRevE.82.011910. [DOI] [PubMed] [Google Scholar]
- 27.Oka K., Aoyagi S., Arai Y., Isono Y., Hashiguchi G., Fujita H. Fabrication of a micro needle for a trace blood test. Sensors and Actuators a-Physical. 2002;97–8:478–485. doi: 10.1016/s0924-4247(01)00872-x. [DOI] [Google Scholar]
- 28.Ramasubramanian M.K., Barham O.M., Swaminathan V. Mechanics of a mosquito bite with applications to microneedle design. Bioinspiration Biomimetics. 2008;3(4) doi: 10.1088/1748-3182/3/4/046001. [DOI] [PubMed] [Google Scholar]
- 29.Aoyagi S., Izumi H., Fukuda M. Biodegradable polymer needle with various tip angles and consideration on insertion mechanism of mosquito's proboscis. Sensors and Actuators a-Physical. 2008;143(1):20–28. doi: 10.1016/j.sna.2007.06.007. [DOI] [Google Scholar]
- 30.Ma G.J., Shi L.T., Wu C.W. Biomechanical property of a natural microneedle: the caterpillar spine. Journal of Medical Devices-Transactions of the Asme. 2011;5(3) doi: 10.1115/1.4004651. [DOI] [Google Scholar]
- 31.Leopold R.A., Freeman T.P., Buckner J.S., Nelson D.R. Mouthpart morphology and stylet penetration of host plants by the glassy-winged sharpshooter, Homalodisca coagulata, (Homoptera : Cicadellidae) Arthropod Struct. Dev. 2003;32(2–3):189–199. doi: 10.1016/s1467-8039(03)00047-1. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Z.L., Zhao H.P., Ma G.J., Wu C.W., Yang K., Feng X.Q. Structures, properties, and functions of the stings of honey bees and paper wasps: a comparative study. Biology Open. 2015;4(7):921–928. doi: 10.1242/bio.012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machekposhti S.A., Soltani M., Najafizadeh P., Ebrahimi S.A., Chen P. Biocompatible polymer microneedle for topical/dermal delivery of tranexamic acid. J. Contr. Release. 2017;261:87–92. doi: 10.1016/j.jconrel.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Raphael A.P., Crichton M.L., Falconer R.J., Meliga S., Chen X.F., Fernando G.J.P., Huang H., Kendall M.A.F. Formulations for microprojection/microneedle vaccine delivery: structure, strength and release profiles. J. Contr. Release. 2016;225:40–52. doi: 10.1016/j.jconrel.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 35.Lee K., Lee H.C., Lee D.S., Jung H. Drawing lithography: three-dimensional fabrication of an ultrahigh-aspect-ratio microneedle. Adv. Mater. 2010;22(4):483. doi: 10.1002/adma.200902418. [DOI] [PubMed] [Google Scholar]
- 36.Ye Y.Q., Wang C., Zhang X.D., Hu Q.Y., Zhang Y.Q., Liu Q., Wen D., Milligan J., Bellotti A., Huang L., Dotti G., Gu Z. A melanin-mediated cancer immunotherapy patch. Science Immunology. 2017;2(17) doi: 10.1126/sciimmunol.aan5692. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X.X., Wang F.Y., Yu Y.R., Chen G.P., Shang L.R., Sun L.Y., Zhao Y.J. Bio-inspired clamping microneedle arrays from flexible ferrofluid-configured moldings. Sci. Bull. 2019;64(15):1110–1117. doi: 10.1016/j.scib.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Dai B., Li S.H., Xu T.L., Wang Y.F., Zhang F.L., Gu Z., Wang S.T. Artificial asymmetric cilia array of dielectric elastomer for cargo transportation. ACS Appl. Mater. Interfaces. 2018;10(49):42979–42984. doi: 10.1021/acsami.8b13419. [DOI] [PubMed] [Google Scholar]
- 39.Wang J.Q., Yu J., Zhang Y.Q., Zhang X.D., Kahkoska A.R., Chen G.J., Wang Z.J., Sun W.J., Cai L.L., Chen Z.W., Qian C.G., Shen Q.D., Khademhosseini A., Buse J.B., Gu Z. Charge-switchable polymeric complex for glucose-responsive insulin delivery in mice and pigs. Sci. Adv. 2019;5(7) doi: 10.1126/sciadv.aaw4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X.X., Chen G.P., Sun L.Y., Ye F.F., Shen X., Zhao Y.J. Claw-inspired microneedle patches with liquid metal encapsulation for accelerating incisional wound healing. Chem. Eng. J. 2021;406 doi: 10.1016/j.cej.2020.126741. [DOI] [Google Scholar]
- 41.Dong X.X., Zhang R., Tian Y., Ramos M.A., Hu T.S., Wang Z.H., Zhao H., Zhang L.P., Wan Y.Y., Xia Z.H., Xu Q. Functionally graded gecko setae and the biomimics with robust adhesion and durability. ACS Appl. Polym. Mater. 2020;2(7):2658–2666. doi: 10.1021/acsapm.0c00282. [DOI] [Google Scholar]
- 42.Autumn K., Sitti M., Liang Y.C.A., Peattie A.M., Hansen W.R., Sponberg S., Kenny T.W., Fearing R., Israelachvili J.N., Full R.J. Evidence for van der Waals adhesion in gecko setae. Proc. Natl. Acad. Sci. U.S.A. 2002;99(19):12252–12256. doi: 10.1073/pnas.192252799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain D., Stark A.Y., Niewiarowski P.H., Miyoshi T., Dhinojwala A. NMR spectroscopy reveals the presence and association of lipids and keratin in adhesive gecko setae. Sci. Rep. 2015;5:8. doi: 10.1038/srep09594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alibardi L. Immunolocalization of specific keratin associated beta-proteins (beta-keratins) in the adhesive setae of Gekko gecko. Tissue Cell. 2013;45(4):231–240. doi: 10.1016/j.tice.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L.W., Liu M., Zhang Y.J., Pei R.J. Recent progress of highly adhesive hydrogels as wound dressings. Biomacromolecules. 2020;21(10):3966–3983. doi: 10.1021/acs.biomac.0c01069. [DOI] [PubMed] [Google Scholar]
- 46.Hansen W.R., Autumn K. Evidence for self-cleaning in gecko setae. Proc. Natl. Acad. Sci. U.S.A. 2005;102(2):385–389. doi: 10.1073/pnas.0408304102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwak M.K., Jeong H.E., Suh K.Y. Rational design and enhanced biocompatibility of a dry adhesive medical skin patch. Adv. Mater. 2011;23(34):3949. doi: 10.1002/adma.201101694. [DOI] [PubMed] [Google Scholar]
- 48.Kwaki J.S., Kim T.W. A review of adhesion and friction models for gecko feet. Int. J. Precis. Eng. Manuf. 2010;11(1):171–186. doi: 10.1007/s12541-010-0020-5. [DOI] [Google Scholar]
- 49.Zhou M., Pesika N., Zeng H.B., Tian Y., Israelachvili J. Recent advances in gecko adhesion and friction mechanisms and development of gecko-inspired dry adhesive surfaces. Friction. 2013;1(2):114–129. doi: 10.1007/s40544-013-0011-5. [DOI] [Google Scholar]
- 50.Li Y.S., Krahn J., Menon C. Bioinspired dry adhesive materials and their application in robotics: a review. JBE. 2016;13(2):181–199. doi: 10.1016/s1672-6529(16)60293-7. [DOI] [Google Scholar]
- 51.Wang W., Liu Y., Xie Z.W. Gecko-like dry adhesive surfaces and their applications: a review. JBE. 2021;18(5):1011–1044. doi: 10.1007/s42235-021-00088-7. [DOI] [Google Scholar]
- 52.Jagota A., Bennison S.J. Mechanics of adhesion through a fibrillar microstructure. Integr. Comp. Biol. 2002;42(6):1140–1145. doi: 10.1093/icb/42.6.1140. [DOI] [PubMed] [Google Scholar]
- 53.Arzt E., Gorb S., Spolenak R. From micro to nano contacts in biological attachment devices. Proc. Natl. Acad. Sci. U.S.A. 2003;100(19):10603–10606. doi: 10.1073/pnas.1534701100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao H.J., Wang X., Yao H.M., Gorb S., Arzt E. Mechanics of hierarchical adhesion structures of geckos. Mech. Mater. 2005;37(2–3):275–285. doi: 10.1016/j.mechmat.2004.03.008. [DOI] [Google Scholar]
- 55.Bhushan B., Peressadko A.G., Kim T.W. Adhesion analysis of two-level hierarchical morphology in natural attachment systems for 'smart adhesion. J. Adhes. Sci. Technol. 2006;20(13):1475–1491. doi: 10.1163/156856106778666408. [DOI] [Google Scholar]
- 56.Takahashi K., Berengueres J.O.L., Obata K.J., Saito S. Geckos' foot hair structure and their ability to hang from rough surfaces and move quickly. Int. J. Adhesion Adhes. 2006;26(8):639–643. doi: 10.1016/j.ijadhadh.2005.12.002. [DOI] [Google Scholar]
- 57.Kim T.W., Bhushan B. Effect of stiffness of multi-level hierarchical attachment system on adhesion enhancement. Ultramicroscopy. 2007;107(10–11):902–912. doi: 10.1016/j.ultramic.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Mergel J.C., Sauer R.A., Saxena A. Computational optimization of adhesive microstructures based on a nonlinear beam formulation. Struct. Multidiscip. Optim. 2014;50(6):1001–1017. doi: 10.1007/s00158-014-1091-1. [DOI] [Google Scholar]
- 59.Mergel J.C., Sauer R.A. On the optimum shape of thin adhesive strips for various peeling directions. J. Adhes. 2014;90(5–6):526–544. doi: 10.1080/00218464.2013.840538. [DOI] [Google Scholar]
- 60.Gan K.S., Liang C., Bi X.Y., Wu J.Z., Ye Z.H., Wu W.J., Hu B.R. Adhesive materials inspired by barnacle underwater adhesion: biological principles and biomimetic designs. Front. Bioeng. Biotechnol. 2022;10:18. doi: 10.3389/fbioe.2022.870445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamino K., Inoue K., Maruyama T., Takamatsu N., Harayama S., Shizuri Y. Barnacle cement proteins - importance of disulfide bonds in their insolubility. J. Biol. Chem. 2000;275(35):27360–27365. doi: 10.1074/jbc.M910363199. [DOI] [PubMed] [Google Scholar]
- 62.Kamino K. Novel barnacle underwater adhesive protein is a charged amino acid-rich protein constituted by a Cys-rich repetitive sequence. Biochem. J. 2001;356:503–507. doi: 10.1042/0264-6021:3560503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urushida Y., Nakano M., Matsuda S., Inoue N., Kanai S., Kitamura N., Nishino T., Kamino K. Identification and functional characterization of a novel barnacle cement protein. FEBS J. 2007;274(16):4336–4346. doi: 10.1111/j.1742-4658.2007.05965.x. [DOI] [PubMed] [Google Scholar]
- 64.Kamino K., Nakano M., Kanai S. Significance of the conformation of building blocks in curing of barnacle underwater adhesive. FEBS J. 2012;279(10):1750–1760. doi: 10.1111/j.1742-4658.2012.08552.x. [DOI] [PubMed] [Google Scholar]
- 65.So C.R., Fears K.P., Leary D.H., Scancella J.M., Wang Z., Liu J.L., Orihuela B., Rittschof D., Spillmann C.M., Wahl K.J. Sequence basis of barnacle cement nanostructure is defined by proteins with silk homology. Sci. Rep. 2016;6:14. doi: 10.1038/srep36219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dominguez-Perez D., Almeida D., Wissing J., Machado A.M., Jansch L., Antunes A., Castro L.F., Vasconcelos V., Campos A., Cunha I. Proteogenomic characterization of the cement and adhesive gland of the pelagic gooseneck barnacle lepas anatifera. Int. J. Mol. Sci. 2021;22(7):19. doi: 10.3390/ijms22073370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin H.C., Wong Y.H., Sung C.H., Chan B.K.K. Histology and transcriptomic analyses of barnacles with different base materials and habitats shed lights on the duplication and chemical diversification of barnacle cement proteins. BMC Genom. 2021;22(1):18. doi: 10.1186/s12864-021-08049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schultzhaus J.N., Hervey W.J., Taitt C.R., So C.R., Leary D.H., Wahl K.J., Spillmann C.M. Comparative analysis of stalked and acorn barnacle adhesive proteomes. Open Biol. 2021;11(8):17. doi: 10.1098/rsob.210142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kamino K. Underwater adhesive of marine organisms as the vital link between biological science and material science. Mar. Biotechnol. 2008;10(2):111–121. doi: 10.1007/s10126-007-9076-3. [DOI] [PubMed] [Google Scholar]
- 70.Liang C., Strickland J., Ye Z.H., Wu W.J., Hu B.R., Rittschof D. Biochemistry of barnacle adhesion: an updated review. Front. Mar. Sci. 2019;6:20. doi: 10.3389/fmars.2019.00565. [DOI] [Google Scholar]
- 71.Mohanram H., Kumar A., Verma C.S., Pervushin K., Miserez A. Three-dimensional structure of Megabalanus rosa Cement Protein 20 revealed by multi-dimensional NMR and molecular dynamics simulations. Philos. Trans. R. Soc. B-Biol. Sci. 2019;374(1784):9. doi: 10.1098/rstb.2019.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barlow D.E., Dickinson G.H., Orihuela B., Kulp J.L., Rittschof D., Wahl K.J. Characterization of the adhesive plaque of the barnacle Balanus amphitrite: amyloid-like nanofibrils are a major component. Langmuir. 2010;26(9):6549–6556. doi: 10.1021/la9041309. [DOI] [PubMed] [Google Scholar]
- 73.Kamino K. 2006. Barnacle Underwater Attachment, Biological Adhesives; pp. 145–166. [DOI] [Google Scholar]
- 74.Kamino K. Barnacle Underwater Attachment. Biological Adhesives. 2016:153–176. doi: 10.1007/978-3-319-46082-6_7. [DOI] [Google Scholar]
- 75.Dickinson G.H., Vega I.E., Wahl K.J., Orihuela B., Beyley V., Rodriguez E.N., Everett R.K., Bonaventura J., Rittschof D. Barnacle cement: a polymerization model based on evolutionary concepts. J. Exp. Biol. 2009;212(21):3499–3510. doi: 10.1242/jeb.029884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamino K. Absence of cross-linking via trans-glutaminase in barnacle cement and redefinition of the cement. Biofouling. 2010;26(7):755–760. doi: 10.1080/08927014.2010.514335. [DOI] [PubMed] [Google Scholar]
- 77.So C.R., Scancella J.M., Fears K.P., Essock-Burns T., Haynes S.E., Leary D.H., Diana Z., Wang C.Y., North S., Oh C.S., Wang Z., Orihuela B., Rittschof D., Spillmann C.M., Wahl K.J. Oxidase activity of the barnacle adhesive interface involves peroxide-dependent catechol oxidase and lysyl oxidase enzymes. ACS Appl. Mater. Interfaces. 2017;9(13):11493–11505. doi: 10.1021/acsami.7b01185. [DOI] [PubMed] [Google Scholar]
- 78.Fukuma T., Mostaert A.S., Jarvis S.P. Explanation for the mechanical strength of amyloid fibrils. Tribol. Lett. 2006;22(3):233–237. doi: 10.1007/s11249-006-9086-8. [DOI] [Google Scholar]
- 79.Zhang Y., Wang A., DeBenedictis E.P., Keten S. Bending energy penalty enhances the adhesive strength of functional amyloid curli to surfaces. Nanotechnology. 2017;28(46) doi: 10.1088/1361-6528/aa8f72. [DOI] [PubMed] [Google Scholar]
- 80.Liang C., Ye Z.H., Xue B., Zeng L., Wu W.J., Zhong C., Cao Y., Hu B.R., Messersmith P.B. Self-assembled nanofibers for strong underwater adhesion: the trick of barnacles. ACS Appl. Mater. Interfaces. 2018;10(30):25017–25025. doi: 10.1021/acsami.8b04752. [DOI] [PubMed] [Google Scholar]
- 81.Anne Marie Power W.K., Zheden Vanessa, Jonker Jaimie, Byern P.M.a.J.v. 2010. Mechanisms of Adhesion in Adult Barnacles, Biological Adhesive Systems; pp. 153–168. [DOI] [Google Scholar]
- 82.Lee C.H., Singla A., Lee Y. Biomedical applications of collagen. Int. J. Pharm. 2001;221(1–2):1–22. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 83.Ogawa M., Portier R.J., Moody M.W., Bell J., Schexnayder M.A., Losso J.N. Biochemical properties of bone and scale collagens isolated from the subtropical fish black drum (Pogonia cromis) and sheepshead seabream (Archosargus probatocephalus) Food Chem. 2004;88(4):495–501. doi: 10.1016/j.foodchem.2004.02.006. [DOI] [Google Scholar]
- 84.Trevitt C.R., Singh P.N. Variant Creutzfeldt-Jakob disease: pathology, epidemiology, and public health implications. Am. J. Clin. Nutr. 2003;78(3):651S–656S. doi: 10.1093/ajcn/78.3.651S. [DOI] [PubMed] [Google Scholar]
- 85.Hsieh Y.H.P., Leong F.M., Rudloe J. Jellyfish as food. Hydrobiologia. 2001;451(1–3):11–17. [Google Scholar]
- 86.Lutz T.M., Kimna C., Casini A., Lieleg O. Bio-based and bio-inspired adhesives from animals and plants for biomedical applications. Materials Today Bio. 2022;13 doi: 10.1016/j.mtbio.2022.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng X.C., Shao Z.Y., Li C.B., Yu L.J., Raja M.A., Liu C.G. Isolation, characterization and evaluation of collagen from jellyfish Rhopilema esculentum kishinouye for use in hemostatic applications. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Masuda A., Baba T., Dohmae N., Yamamura M., Wada H., Ushida K. Mucin (Qniumucin), a glycoprotein from jellyfish, and determination of its main chain structure. J. Nat. Prod. 2007;70(7):1089–1092. doi: 10.1021/np060341b. [DOI] [PubMed] [Google Scholar]
- 89.Uzawa J., Urai M., Baba T., Seki H., Taniguchi K., Ushida K. NMR study on a novel mucin from jellyfish in natural abundance, qniumucin from aurelia aurita. J. Nat. Prod. 2009;72(5):818–823. doi: 10.1021/np800601j. [DOI] [PubMed] [Google Scholar]
- 90.Nudelman R., Alhmoud H., Delalat B., Fleicher S., Fine E., Guliakhmedova T., Elnathan R., Nyska A., Voelcker N.H., Gozin M., Richter S. Jellyfish-based smart wound dressing devices containing in situ synthesized antibacterial nanoparticles. Adv. Funct. Mater. 2019;29(38) doi: 10.1002/adfm.201902783. [DOI] [Google Scholar]
- 91.Kiminori Ushida T.M. Materials science and engineering of mucin: a new aspect of mucin chemistry. Stud. Nat. Prod. Chem. 2013:115–159. doi: 10.1016/B978-0-444-62615-8.00004-7. [DOI] [Google Scholar]
- 92.Rose C.S., James B. Plasticity of lung development in the amphibian, Xenopus laevis. Biology Open. 2013;2(12):1324–1335. doi: 10.1242/bio.20133772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang X.M., Jiang L., Li X., Zheng L.W., Dang R.Y., Liu X., Wang X.P., Chen L.L., Zhang Y.S., Zhang J.X., Yang D.Q. A bioinspired hemostatic powder derived from the skin secretion of Andrias davidianus for rapid hemostasis and intraoral wound healing. Small. 2022;18(3) doi: 10.1002/smll.202101699. [DOI] [PubMed] [Google Scholar]
- 94.Geng X.F., Wei H., Shang H.T., Zhou M.H., Chen B., Zhang F.C., Zang X.Y., Li P.F., Sun J.Y., Che J., Zhang Y.P., Xu C.S. Proteomic analysis of the skin of Chinese giant salamander (Andrias dauidianus) J. Proteonomics. 2015;119:196–208. doi: 10.1016/j.jprot.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 95.Deng J., Tang Y.Y., Zhang Q., Wang C., Liao M., Ji P., Song J.L., Luo G.X., Chen L., Ran X.H., Wei Z.M., Zheng L.W., Dang R.Y., Liu X., Zhang H.M., Zhang Y.S., Zhang X.M., Tan H. A bioinspired medical adhesive derived from skin secretion of Andrias davidianus for wound healing. Adv. Funct. Mater. 2019;29(31) doi: 10.1002/adfm.201809110. [DOI] [Google Scholar]
- 96.Forooshani P.K., Lee B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Polym. Chem. 2017;55(1):9–33. doi: 10.1002/pola.28368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brennan M.J., Kilbride B.F., Wilker J.J., Liu J.C. A bioinspired elastin-based protein for a cytocompatible underwater adhesive. Biomaterials. 2017;124:116–125. doi: 10.1016/j.biomaterials.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 98.Ooka A.A., Garrell R.L. Surface-enhanced Raman spectroscopy of DOPA-containing peptides related to adhesive protein of marine mussel. Mytilus edulis, Biopolymers. 2000;57(2):92–102. doi: 10.1002/(SICI)1097-0282. 2000. [DOI] [PubMed] [Google Scholar]
- 99.Ahsan S.M., Thomas M., Reddy K.K., Sooraparaju S.G., Asthana A., Bhatnagar I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018;110:97–109. doi: 10.1016/j.ijbiomac.2017.08.140. [DOI] [PubMed] [Google Scholar]
- 100.Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Zabermawi N.M., Arif M., Batiha G.E., Khafaga A.F., Abd El-Hakim Y.M., Al-Sagheer A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Int. J. Biol. Macromol. 2020;164:2726–2744. doi: 10.1016/j.ijbiomac.2020.08.153. [DOI] [PubMed] [Google Scholar]
- 101.Hu Z., Zhang D.Y., Lu S.T., Li P.W., Li S.D. Chitosan-based composite materials for prospective hemostatic applications. Mar. Drugs. 2018;16(8) doi: 10.3390/md16080273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khan F.I., Rahman S., Queen A., Ahamad S., Ali S., Kim J., Hassan M.I. Implications of molecular diversity of chitin and its derivatives. Appl. Microbiol. Biotechnol. 2017;101(9):3513–3536. doi: 10.1007/s00253-017-8229-1. [DOI] [PubMed] [Google Scholar]
- 103.Bedian L., Villalba-Rodriguez A.M., Hernandez-Vargas G., Parra-Saldivar R., Iqbal H.M.N. Bio-based materials with novel characteristics for tissue engineering applications - a review. Int. J. Biol. Macromol. 2017;98:837–846. doi: 10.1016/j.ijbiomac.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 104.Sayari N., Sila A., Abdelmalek B.E., Ben Abdallah R., Ellouz-Chaabouni S., Bougatef A., Balti R. Chitin and chitosan from the Norway lobster by-products: antimicrobial and anti-proliferative activities. Int. J. Biol. Macromol. 2016;87:163–171. doi: 10.1016/j.ijbiomac.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 105.Negm N.A., Hefni H.H.H., Abd-Elaal A.A.A., Badr E.A., Abou Kana M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020;152:681–702. doi: 10.1016/j.ijbiomac.2020.02.196. [DOI] [PubMed] [Google Scholar]
- 106.Verlee A., Mincke S., Stevens C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017;164:268–283. doi: 10.1016/j.carbpol.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 107.Kou S., Peters L.M., Mucalo M.R. Chitosan: a review of sources and preparation methods. Int. J. Biol. Macromol. 2021;169:85–94. doi: 10.1016/j.ijbiomac.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 108.Cazon P., Velazquez G., Ramirez J.A., Vazquez M. Polysaccharide-based films and coatings for food packaging: a review. Food Hydrocolloids. 2017;68:136–148. doi: 10.1016/j.foodhyd.2016.09.009. [DOI] [Google Scholar]
- 109.Qin Y.K., Li P.C. Antimicrobial chitosan conjugates: current synthetic strategies and potential applications. Int. J. Mol. Sci. 2020;21(2) doi: 10.3390/ijms21020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Amato A., Migneco L.M., Martinelli A., Pietrelli L., Piozzi A., Francolini I. Antimicrobial activity of catechol functionalized-chitosan versus Staphylococcus epidermidis. Carbohydr. Polym. 2018;179:273–281. doi: 10.1016/j.carbpol.2017.09.073. [DOI] [PubMed] [Google Scholar]
- 111.Cheah W.Y., Show P.L., Ng I.S., Lin G.Y., Chiu C.Y., Chang Y.K. Antibacterial activity of quaternized chitosan modified nanofiber membrane. Int. J. Biol. Macromol. 2019;126:569–577. doi: 10.1016/j.ijbiomac.2018.12.193. [DOI] [PubMed] [Google Scholar]
- 112.Wei L.J., Tan W.Q., Wang G., Li Q., Dong F., Guo Z.Y. The antioxidant and antifungal activity of chitosan derivatives bearing Schiff bases and quaternary ammonium salts. Carbohydr. Polym. 2019;226 doi: 10.1016/j.carbpol.2019.115256. [DOI] [PubMed] [Google Scholar]
- 113.Patrulea V., Ostafe V., Borchard G., Jordan O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015;97:417–426. doi: 10.1016/j.ejpb.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 114.Hattori H., Ishihara M. Changes in blood aggregation with differences in molecular weight and degree of deacetylation of chitosan. Biomed. Mater. 2015;10(1) doi: 10.1088/1748-6041/10/1/015014. [DOI] [PubMed] [Google Scholar]
- 115.Sagnella S., Kwok J., Marchant R.E., Kottke-Marchant K. Shear-induced platelet activation and adhesion on human pulmonary artery endothelial cells seeded onto hydrophilic polymers. J. Biomed. Mater. Res. 2001;57(3):419–431. doi: 10.1002/1097-4636. [DOI] [PubMed] [Google Scholar]
- 116.Chou T.C., Fu E., Wu C.J., Yeh J.H. Chitosan enhances platelet adhesion and aggregation. Biochem. Biophys. Res. Commun. 2003;302(3):480–483. doi: 10.1016/s0006-291x(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 117.Sagnella S., Mai-Ngam K. Chitosan based surfactant polymers designed to improve blood compatibility on biomaterials. Colloids Surf. B Biointerfaces. 2005;42(2):147–155. doi: 10.1016/j.colsurfb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 118.Fischer T.H., Connolly R., Thatte H.S., Schwaitzberg S.S. Comparison of structural and hemostatic properties of the poly-N-acetyl glucosamine Syvek Patch with products containing chitosan. Microsc. Res. Tech. 2004;63(3):168–174. doi: 10.1002/jemt.20017. [DOI] [PubMed] [Google Scholar]
- 119.Simard P., Galarneau H., Marois S., Rusu D., Hoemann C.D., Poubelle P.E., El-Gabalawy H., Fernandes M.J.G. Neutrophils exhibit distinct phenotypes toward chitosans with different degrees of deacetylation: implications for cartilage repair. Arthritis Res. Ther. 2009;11(3) doi: 10.1186/ar2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park C.J., Clark S.G., Lichtensteiger C.A., Jamison R.D., Johnson A.J.W. Accelerated wound closure of pressure ulcers in aged mice by chitosan scaffolds with and without bFGF. Acta Biomater. 2009;5(6):1926–1936. doi: 10.1016/j.actbio.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 121.Howling G.I., Dettmar P.W., Goddard P.A., Hampson F.C., Dornish M., Wood E.J. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials. 2001;22(22):2959–2966. doi: 10.1016/s0142-9612(01)00042-4. [DOI] [PubMed] [Google Scholar]
- 122.Benediktsdottir B.E., Baldursson O., Masson M. Challenges in evaluation of chitosan and trimethylated chitosan (TMC) as mucosal permeation enhancers: from synthesis to in vitro application. J. Contr. Release. 2014;173:18–31. doi: 10.1016/j.jconrel.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 123.Lee B.P., Messersmith P.B., Israelachvili J.N., Waite J.H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 2011;41:99–132. doi: 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.DeMartini D.G., Errico J.M., Sjoestroem S., Fenster A., Waite J.H. A cohort of new adhesive proteins identified from transcriptomic analysis of mussel foot glands. J. R. Soc. Interface. 2017;14(131) doi: 10.1098/rsif.2017.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tamarin A., Keller P.J. An ultrastructural study of the byssal thread forming system in Mytilus. J Ultrastruct Res. 1972;40(3):401–416. doi: 10.1016/s0022-5320(72)90110-4. [DOI] [PubMed] [Google Scholar]
- 126.Waite J.H. Mussel adhesion - essential footwork. J. Exp. Biol. 2017;220(4):517–530. doi: 10.1242/jeb.134056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Priemel T., Degtyar E., Dean M.N., Harrington M.J. Rapid self-assembly of complex biomolecular architectures during mussel byssus biofabrication. Nat. Commun. 2017;8:12. doi: 10.1038/ncomms14539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rzepecki L.M., Hansen K.M., Waite J.H. Characterization of a cystine-rich polyphenolic protein family from the Blue mussel Mytilus edulis L. Biol. Bull. 1992;183(1):123–137. doi: 10.2307/1542413. [DOI] [PubMed] [Google Scholar]
- 129.Nicklisch S.C.T., Das S., Rodriguez N.R.M., Waite J.H., Israelachvili J.N. Antioxidant efficacy and adhesion rescue by a recombinant mussel foot protein-6. Biotechnol. Prog. 2013;29(6):1587–1593. doi: 10.1002/btpr.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu Q.Y., Danner E., Waite J.H., Israelachvili J.N., Zeng H.B., Hwang D.S. Adhesion of mussel foot proteins to different substrate surfaces. J. R. Soc. Interface. 2013;10(79):11. doi: 10.1098/rsif.2012.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao H., Waite J.H. Proteins in load-bearing junctions: the histidine-rich metal-binding protein of mussel byssus. Biochemistry. 2006;45(47):14223–14231. doi: 10.1021/bi061677n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miller D.R., Das S., Huang K.Y., Han S., Israelachvili J.N., Waite J.H. Mussel coating protein-derived complex coacervates mitigate frictional surface damage. ACS Biomater. Sci. Eng. 2015;1(11):1121–1128. doi: 10.1021/acsbiomaterials.5b00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang C., Xiang L., Zhang J.W., Liu C., Wang Z.K., Zeng H.B., Xu Z.K. Revisiting the adhesion mechanism of mussel-inspired chemistry. Chem. Sci. 2022;13(6):1698–1705. doi: 10.1039/d1sc05512g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dreyer D.R., Miller D.J., Freeman B.D., Paul D.R., Bielawski C.W. Elucidating the structure of poly(dopamine) Langmuir. 2012;28(15):6428–6435. doi: 10.1021/la204831b. [DOI] [PubMed] [Google Scholar]
- 135.Zhao H., Sun C.J., Stewart R.J., Waite J.H. Cement proteins of the tube-building polychaete Phragmatopoma californica. J. Biol. Chem. 2005;280(52):42938–42944. doi: 10.1074/jbc.M508457200. [DOI] [PubMed] [Google Scholar]
- 136.Endrizzi B.J., Stewart R.J., Glueomics An expression survey of the adhesive gland of the sandcastle worm. J. Adhes. 2009;85(8):546–559. doi: 10.1080/00218460902996457. [DOI] [Google Scholar]
- 137.Wang C.S., Stewart R.J. Multipart copolyelectrolyte adhesive of the sandcastle worm, phragmatopoma californica (fewkes): catechol oxidase catalyzed curing through peptidyl-DOPA. Biomacromolecules. 2013;14(5):1607–1617. doi: 10.1021/bm400251k. [DOI] [PubMed] [Google Scholar]
- 138.Waite J.H., Jensen R.A., Morse D.E. Cement precursor proteins of the reef-building polychaete Phragmatopoma californica (Fewkes) Biochemistry. 1992;31(25):5733–5738. doi: 10.1021/bi00140a007. [DOI] [PubMed] [Google Scholar]
- 139.Wang C.S., Stewart R.J. Localization of the bioadhesive precursors of the sandcastle worm, Phragmatopoma californica (Fewkes) J. Exp. Biol. 2012;215(2):351–361. doi: 10.1242/jeb.065011. [DOI] [PubMed] [Google Scholar]
- 140.Buffet J.P., Corre E., Duvernois-Berthet E., Fournier J., Lopez P.J. Adhesive gland transcriptomics uncovers a diversity of genes involved in glue formation in marine tube-building polychaetes. Acta Biomater. 2018;72:316–328. doi: 10.1016/j.actbio.2018.03.037. [DOI] [PubMed] [Google Scholar]
- 141.Shao H., Bachus K.N., Stewart R.J. A water-borne adhesive modeled after the sandcastle glue of P-californica. Macromol. Biosci. 2009;9(5):464–471. doi: 10.1002/mabi.200800252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stewart R.J., Weaver J.C., Morse D.E., Waite J.H. The tube cement of Phragmatopoma californica: a solid foam. J. Exp. Biol. 2004;207(26):4727–4734. doi: 10.1242/jeb.01330. [DOI] [PubMed] [Google Scholar]
- 143.Stewart R.J., Wang C.S., Shao H. Complex coacervates as a foundation for synthetic underwater adhesives. Adv. Colloid Interface Sci. 2011;167(1–2):85–93. doi: 10.1016/j.cis.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shao H., Stewart R.J. Biomimetic underwater adhesives with environmentally triggered setting mechanisms. Adv. Mater. 2010;22(6):729. doi: 10.1002/adma.200902380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weinbreck F., de Vries R., Schrooyen P., de Kruif C.G. Complex coacervation of whey proteins and gum Arabic. Biomacromolecules. 2003;4(2):293–303. doi: 10.1021/bm025667n. [DOI] [PubMed] [Google Scholar]
- 146.Bohidar H., Dubin P.L., Majhi P.R., Tribet C., Jaeger W. Effects of protein-polyelectrolyte affinity and polyelectrolyte molecular weight on dynamic properties of bovine serum albumin-poly(diallyldimethylammonium chloride) coacervates. Biomacromolecules. 2005;6(3):1573–1585. doi: 10.1021/bm049174p. [DOI] [PubMed] [Google Scholar]
- 147.Yu F., Chen S.G., Chen Y., Li H.M., Yang L., Chen Y.Y., Yin Y.S. Experimental and theoretical analysis of polymerization reaction process on the polydopamine membranes and its corrosion protection properties for 304 Stainless Steel. J. Mol. Struct. 2010;982(1–3):152–161. doi: 10.1016/j.molstruc.2010.08.021. [DOI] [Google Scholar]
- 148.Hong S., Na Y.S., Choi S., Song I.T., Kim W.Y., Lee H. Non-covalent self-assembly and covalent polymerization Co-contribute to polydopamine formation. Adv. Funct. Mater. 2012;22(22):4711–4717. doi: 10.1002/adfm.201201156. [DOI] [Google Scholar]
- 149.Jeon E.Y., Lee J., Kim B.J., Joo K.I., Kim K.H., Lim G., Cha H.J. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials. 2019:222. doi: 10.1016/j.biomaterials.2019.119439. [DOI] [PubMed] [Google Scholar]
- 150.Guo M.Z., Wang Y.Q., Gao B.B., He B.F. Shark tooth-inspired microneedle dressing for intelligent wound management. ACS Nano. 2021;15(9):15316–15327. doi: 10.1021/acsnano.1c06279. [DOI] [PubMed] [Google Scholar]
- 151.Yanik M.F. Towards gecko-feet-inspired bandages. Trends Biotechnol. 2009;27(1):1–2. doi: 10.1016/j.tibtech.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 152.Mahdavi A., Ferreira L., Sundback C., Nichol J.W., Chan E.P., Carter D.J.D., Bettinger C.J., Patanavanich S., Chignozha L., Ben-Joseph E., Galakatos A., Pryor H., Pomerantseva I., Masiakos P.T., Faquin W., Zumbuehl A., Hong S., Borenstein J., Vacanti J., Langer R., Karp J.M. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc. Natl. Acad. Sci. U.S.A. 2008;105(7):2307–2312. doi: 10.1073/pnas.0712117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen X., Mizuta R., Fukata N., Taguchi T. Design of bio-inspired adhesive surface composed of hexanoyl group-modified gelatin and silicon nanowire. Colloids Surf. B Biointerfaces. 2019;178:111–119. doi: 10.1016/j.colsurfb.2019.02.053. [DOI] [PubMed] [Google Scholar]
- 154.Frost S.J., Mawad D., Higgins M.J., Ruprai H., Kuchel R., Tilley R.D., Myers S., Hook J.M., Lauto A. Gecko-inspired chitosan adhesive for tissue repair. NPG Asia Mater. 2016;8 doi: 10.1038/am.2016.73. [DOI] [Google Scholar]
- 155.Lee H., Lee B.P., Messersmith P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature. 2007;448(7151) doi: 10.1038/nature05968. 338-U4. [DOI] [PubMed] [Google Scholar]
- 156.Raut H.K., Baji A., Hariri H.H., Parveen H., Soh G.S., Low H.Y., Wood K.L. Gecko-inspired dry adhesive based on micro-nanoscale hierarchical arrays for application in climbing devices. ACS Appl. Mater. Interfaces. 2018;10(1):1288–1296. doi: 10.1021/acsami.7b09526. [DOI] [PubMed] [Google Scholar]
- 157.Tao D.S., Gao X., Lu H.Y., Liu Z.Y., Li Y., Tong H., Pesika N., Meng Y.G., Tian Y. Controllable anisotropic dry adhesion in vacuum: gecko inspired wedged surface fabricated with ultraprecision diamond cutting. Adv. Funct. Mater. 2017;27(22) doi: 10.1002/adfm.201606576. [DOI] [Google Scholar]
- 158.Wang Y., Lehmann S., Shao J.Y., Sameoto D. Adhesion circle: a new approach to better characterize directional gecko-inspired dry adhesives. ACS Appl. Mater. Interfaces. 2017;9(3):3060–3067. doi: 10.1021/acsami.6b11708. [DOI] [PubMed] [Google Scholar]
- 159.Zhang Y.L., Ma S.H., Li B., Yu B., Lee H., Cai M.R., Gorb S.N., Zhou F., Liu W.M. Gecko's feet-inspired self-peeling switchable dry/wet adhesive. Chem. Mater. 2021;33(8):2785–2795. doi: 10.1021/acs.chemmater.0c04576. [DOI] [Google Scholar]
- 160.Yuk H., Wu J.J., Sarrafian T.L., Mao X.Y., Varela C.E., Roche E.T., Griffiths L.G., Nabzdyk C.S., Zhao X.H. Rapid and coagulation-independent haemostatic sealing by a paste inspired by barnacle glue. Nature Biomedical Engineering. 2021;5(10):1131. doi: 10.1038/s41551-021-00769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang J.J., Jiang Y.G., Liu Y., Ren Y.H., Xu Z.Y., Li Z.G., Zhao Y., Wu X.W., Ren J.N. Marine-inspired molecular mimicry generates a drug-free, but immunogenic hydrogel adhesive protecting surgical anastomosis. Bioact. Mater. 2021;6(3):770–782. doi: 10.1016/j.bioactmat.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lo Presti M., Rizzo G., Farinola G.M., Omenetto F.G. Bioinspired biomaterial composite for all-water-based high-performance adhesives. Adv. Sci. 2021;8(16) doi: 10.1002/advs.202004786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li F., Ye L.N., Zhang L.Y., Li X.Y., Liu X.X., Zhu J.R., Li H.H., Pang H.M., Yan Y.J., Xu L., Yang M., Yan J.Y. Design of a genetically programmed barnacle-curli inspired living-cell bioadhesive. Materials Today Bio. 2022;14 doi: 10.1016/j.mtbio.2022.100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Montazerian H., Davoodi E., Baidya A., Baghdasarian S., Sarikhani E., Meyer C.E., Haghniaz R., Badv M., Annabi N., Khademhosseini A., Weiss P.S. Engineered hemostatic biomaterials for sealing wounds. Chem. Rev. 2022;40 doi: 10.1021/acs.chemrev.1c01015. [DOI] [PubMed] [Google Scholar]
- 165.Rastian Z., Putz S., Wang Y.J., Kumar S., Fleissner F., Weidner T., Parekh S.H. Type I collagen from jellyfish catostylus mosaicus for biomaterial applications. ACS Biomater. Sci. Eng. 2018;4(6):2115–2125. doi: 10.1021/acsbiomaterials.7b00979. [DOI] [PubMed] [Google Scholar]
- 166.Pustlauk W., Paul B., Gelinsky M., Bernhardt A. Jellyfish collagen and alginate: combined marine materials for superior chondrogenesis of hMSC. Materials Science and Engineering C-Materials for Biological Applications. 2016;64:190–198. doi: 10.1016/j.msec.2016.03.081. [DOI] [PubMed] [Google Scholar]
- 167.Derkus B., Arslan Y.E., Bayrac A.T., Kantarcioglu I., Emregul K.C., Emregul E. Development of a novel aptasensor using jellyfish collagen as matrix and thrombin detection in blood samples obtained from patients with various neurodisease. Sensor. Actuator. B Chem. 2016;228:725–736. doi: 10.1016/j.snb.2016.01.095. [DOI] [Google Scholar]
- 168.Calejo M.T., Almeida A.J., Fernandes A.I. Exploring a new jellyfish collagen in the production of microparticles for protein delivery. J. Microencapsul. 2012;29(6):520–531. doi: 10.3109/02652048.2012.665089. [DOI] [PubMed] [Google Scholar]
- 169.Sunniyoshi H., Nakao S., Endo H., Yanagawa T., Nakano Y., Okamura Y., Kawaguchi A.T., Inagaki Y. A novel composite biomaterial made of jellyfish and porcine collagens accelerates dermal wound healing by enhancing reepithelization and granulation tissue formation in mice. Adv. Wound Care. 2020;9(6):295–311. doi: 10.1089/wound.2019.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sumiyoshi H., Okamura Y., Kawaguchi A.T., Kubota T., Endo H., Yanagawa T., Yasuda J., Matsuki Y., Nakao S., Inagaki Y. External administration of moon jellyfish collagen solution accelerates physiological wound healing and improves delayed wound closure in diabetic model mice. Regenerative Therapy. 2021;18:223–230. doi: 10.1016/j.reth.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu Y.Q., Li Y.H., Shang H.T., Zhong W., Wang Q., Mequanint K., Zhu C.H., Xing M., Wei H. Underwater instant adhesion mechanism of self-assembled amphiphilic hemostatic granular hydrogel from Andrias davidianus skin secretion. iScience. 2022;25(10) doi: 10.1016/j.isci.2022.105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu X., Mao X., Ye G., Wang M.H., Xue K., Zhang Y., Zhang H.M., Ning X.Q., Zhao M., Song J.L., Zhang Y.S., Zhang X.M. Bioinspired Andrias davidianus-Derived wound dressings for localized drug-elution. Bioact. Mater. 2022;15:482–494. doi: 10.1016/j.bioactmat.2021.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gu R.L., Sun W.H., Zhou H., Wu Z.N., Meng Z.Y., Zhu X.X., Tang Q., Dong J., Dou G.F. The performance of a fly-larva shell-derived chitosan sponge as an absorbable surgical hemostatic agent. Biomaterials. 2010;31(6):1270–1277. doi: 10.1016/j.biomaterials.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 174.Du X.C., Wu L., Yan H.Y., Jiang Z.Y., Li S.L., Li W., Bai Y.L., Wang H.J., Cheng Z.J., Kong D.L., Wang L.Y., Zhu M.F. Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nat. Commun. 2021;12(1) doi: 10.1038/s41467-021-24972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hu S.H., Bi S.C., Yan D., Zhou Z.Z., Sun G.H., Cheng X.J., Chen X.G. Preparation of composite hydroxybutyl chitosan sponge and its role in promoting wound healing. Carbohydr. Polym. 2018;184:154–163. doi: 10.1016/j.carbpol.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 176.Sukul M., Ventura R.D., Bae S.H., Choi H.J., Lee S.Y., Lee B.T. Plant-derived oxidized nanofibrillar cellulose-chitosan composite as an absorbable hemostat. Mater. Lett. 2017;197:150–155. doi: 10.1016/j.matlet.2017.03.102. [DOI] [Google Scholar]
- 177.Qian Z.Y., Wang H.P., Tuo X.Y., Guo H.Y., Xu P., Liu D.H., Wei Y., Liu H.F., Fan Y.B., Guo X.M. A porous sodium polyacrylate-grafted chitosan xerogel for severe hemorrhage control synthesized from one-pot reaction. J. Mater. Chem. B. 2017;5(25):4845–4851. doi: 10.1039/c7tb00802c. [DOI] [PubMed] [Google Scholar]
- 178.Song H.F., Chen A.Z., Wang S.B., Kang Y.Q., Ye S.F., Liu Y.G., Wu W.G. Preparation of chitosan-based hemostatic sponges by supercritical fluid technology. Materials. 2014;7(4):2459–2473. doi: 10.3390/ma7042459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Guo Y., Zhao C.Y., Yan C., Cui L. Construction of cellulose/carboxymethyl chitosan hydrogels for potential wound dressing application. Cellulose. 2021;28(15):10013–10023. doi: 10.1007/s10570-021-04149-2. [DOI] [Google Scholar]
- 180.Deng L.L., Wang B.X., Li W.Y., Han Z.L., Chen S.Y., Wang H.P. Bacterial cellulose reinforced chitosan-based hydrogel with highly efficient self-healing and enhanced antibacterial activity for wound healing. Int. J. Biol. Macromol. 2022;217:77–87. doi: 10.1016/j.ijbiomac.2022.07.017. [DOI] [PubMed] [Google Scholar]
- 181.Patel D.K., Ganguly K., Jin H.X., Dutta S.D., Patil T.V., Lim K.T. Functionalized chitosan/spherical nanocellulose-based hydrogel with superior antibacterial efficiency for wound healing. Carbohydr. Polym. 2022;284 doi: 10.1016/j.carbpol.2022.119202. [DOI] [PubMed] [Google Scholar]
- 182.Liu S.X., Jiang N., Chi Y.Q., Peng Q., Dai G.R., Qian L., Xu K.M., Zhong W.Y., Yue W.Q. Injectable and self-healing hydrogel based on chitosan-tannic acid and oxidized hyaluronic acid for wound healing. ACS Biomater. Sci. Eng. 2022;8(9):3754–3764. doi: 10.1021/acsbiomaterials.2c00321. [DOI] [PubMed] [Google Scholar]
- 183.Hu Y., Zhang Z.Y., Li Y., Ding X.K., Li D.W., Shen C.N., Xu F.J. Dual-crosslinked amorphous polysaccharide hydrogels based on chitosan/alginate for wound healing applications. Macromol. Rapid Commun. 2018;39(20) doi: 10.1002/marc.201800069. [DOI] [PubMed] [Google Scholar]
- 184.Zheng K.K., Tong Y., Zhang S.H., He R.Y., Xiao L., Iqbal Z., Zhang Y.H., Gao J., Zhang L., Jiang L.B., Li Y.L. Flexible bicolorimetric polyacrylamide/chitosan hydrogels for smart real-time monitoring and promotion of wound healing. Adv. Funct. Mater. 2021;31(34) doi: 10.1002/adfm.202102599. [DOI] [Google Scholar]
- 185.Lu J.W., Chen Y., Ding M., Fan X.K., Hu J.W., Chen Y.H., Li J., Li Z.H., Liu W.Y. A 4arm-PEG macromolecule crosslinked chitosan hydrogels as antibacterial wound dressing. Carbohydr. Polym. 2022;277 doi: 10.1016/j.carbpol.2021.118871. [DOI] [PubMed] [Google Scholar]
- 186.Yang E.R., Hou W., Liu K., Yang H., Wei W.Y., Kang H.F., Dai H.L. A multifunctional chitosan hydrogel dressing for liver hemostasis and infected wound healing. Carbohydr. Polym. 2022;291 doi: 10.1016/j.carbpol.2022.119631. [DOI] [PubMed] [Google Scholar]
- 187.Wang Y.J., Liu S.J., Yu W. Functionalized graphene oxide-reinforced chitosan hydrogel as biomimetic dressing for wound healing. Macromol. Biosci. 2021;21(4) doi: 10.1002/mabi.202000432. [DOI] [PubMed] [Google Scholar]
- 188.Feng W.J., Wang Z.K. Shear-thinning and self-healing chitosan-graphene oxide hydrogel for hemostasis and wound healing. Carbohydr. Polym. 2022;294 doi: 10.1016/j.carbpol.2022.119824. [DOI] [PubMed] [Google Scholar]
- 189.Lin Z., Li R.W., Liu Y., Zhao Y.W., Ao N.J., Wang J., Li L.H., Wu G. Histatin1-modified thiolated chitosan hydrogels enhance wound healing by accelerating cell adhesion, migration and angiogenesis. Carbohydr. Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115710. [DOI] [PubMed] [Google Scholar]
- 190.Zhong Y., Seidi F., Wang Y., Zheng L., Jin Y., Xiao H. Injectable chitosan hydrogels tailored with antibacterial and antioxidant dual functions for regenerative wound healing. Carbohydr. Polym. 2022;298 doi: 10.1016/j.carbpol.2022.120103. [DOI] [PubMed] [Google Scholar]
- 191.Du X.C., Liu Y.J., Wang X., Yan H.Y., Wang L.N., Qu L.J., Kong D.L., Qiao M.Q., Wang L.Y. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Materials Science and Engineering C-Materials for Biological Applications. 2019;104 doi: 10.1016/j.msec.2019.109930. [DOI] [PubMed] [Google Scholar]
- 192.Wei X., Cui C., Fan C., Wu T., Li Y., Zhang X., Wang K., Pang Y., Yao P., Yang J. Injectable hydrogel based on dodecyl-modified N-carboxyethyl chitosan/oxidized konjac glucomannan effectively prevents bleeding and postoperative adhesions after partial hepatectomy. Int. J. Biol. Macromol. 2022;199:401–412. doi: 10.1016/j.ijbiomac.2021.12.193. [DOI] [PubMed] [Google Scholar]
- 193.Zhang L.W., Zhang Y.J., Ma F.S., Liu X.Z., Liu Y.Z., Cao Y., Pei R.J. A low-swelling and toughened adhesive hydrogel with anti-microbial and hemostatic capacities for wound healing. J. Mater. Chem. B. 2022;10(6):915–926. doi: 10.1039/d1tb01871j. [DOI] [PubMed] [Google Scholar]
- 194.Han W., Zhou B., Yang K., Xiong X., Luan S.F., Wang Y., Xu Z., Lei P., Luo Z.S., Gao J., Zhan Y.J., Chen G.P., Liang L., Wang R., Li S., Xu H. Biofilm-inspired adhesive and antibacterial hydrogel with tough tissue integration performance for sealing hemostasis and wound healing. Bioact. Mater. 2020;5(4):768–778. doi: 10.1016/j.bioactmat.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li H.B., Cheng F., Gao S.Z., Wu Z.H., Dong L.L., Lin S.H., Luo Z., Li X.Y. Preparation, characterization, antibacterial properties, and hemostatic evaluation of ibuprofen-loaded chitosan/gelatin composite films. J. Appl. Polym. Sci. 2017;134(42) doi: 10.1002/app.45441. [DOI] [Google Scholar]
- 196.Sun X., Tang Z.H., Pan M., Wang Z.C., Yang H.Q., Liu H.Q. Chitosan/kaolin composite porous microspheres with high hemostatic efficacy. Carbohydr. Polym. 2017;177:135–143. doi: 10.1016/j.carbpol.2017.08.131. [DOI] [PubMed] [Google Scholar]
- 197.Park J.Y., Kyung K.H., Tsukada K., Kim S.H., Shiratori S. Biodegradable polycaprolactone nanofibres with beta-chitosan and calcium carbonate produce a hemostatic effect. Polymer. 2017;123:194–202. doi: 10.1016/j.polymer.2017.07.013. [DOI] [Google Scholar]
- 198.Sun W., Mu C.J., Zhang X., Shi H.C., Yan Q.Y., Luan S.F. Mussel-inspired polysaccharide-based sponges for hemostasis and bacteria infected wound healing. Carbohydr. Polym. 2022;295 doi: 10.1016/j.carbpol.2022.119868. [DOI] [PubMed] [Google Scholar]
- 199.Barros N.R., Chen Y., Hosseini V., Wang W.Y., Nasiri R., Mahmoodi M., Yalcintas E.P., Haghniaz R., Mecwan M.M., Karamikamkar S., Dai W., Sarabi S.A., Falcone N., Young P., Zhu Y.Z., Sun W.J., Zhang S.M., Lee J.M., Lee K., Ahadian S., Dokmeci M.R., Khademhosseini A., Kim H.J. Recent developments in mussel-inspired materials for biomedical applications. Biomater. Sci. 2021;9(20):6653–6672. doi: 10.1039/d1bm01126j. [DOI] [PubMed] [Google Scholar]
- 200.Yan S.F., Wang W.D., Li X., Ren J., Yun W.T., Zhang K.X., Li G.F., Yin J.B. Preparation of mussel-inspired injectable hydrogels based on dual-functionalized alginate with improved adhesive, self-healing, and mechanical properties. J. Mater. Chem. B. 2018;6(40):6377–6390. doi: 10.1039/c8tb01928b. [DOI] [PubMed] [Google Scholar]
- 201.Fan X.M., Wang S.B., Fang Y., Li P.Y., Zhou W.K., Wang Z.C., Chen M.F., Liu H.Q. Tough polyacrylamide-tannic acid-kaolin adhesive hydrogels for quick hemostatic application. Materials Science and Engineering C-Materials for Biological Applications. 2020;109 doi: 10.1016/j.msec.2020.110649. [DOI] [PubMed] [Google Scholar]
- 202.Cui R.H., Chen F.P., Zhao Y.J., Huang W.J., Liu C.S. A novel injectable starch-based tissue adhesive for hemostasis. J. Mater. Chem. B. 2020;8(36):8282–8293. doi: 10.1039/d0tb01562h. [DOI] [PubMed] [Google Scholar]
- 203.Choi Y.C., Choi J.S., Jung Y.J., Cho Y.W. Human gelatin tissue-adhesive hydrogels prepared by enzyme-mediated biosynthesis of DOPA and Fe3+ ion crosslinking. J. Mater. Chem. B. 2014;2(2):201–209. doi: 10.1039/c3tb20696c. [DOI] [PubMed] [Google Scholar]
- 204.Fan X.M., Fang Y., Zhou W.K., Yan L.Y., Xu Y.H., Zhu H., Liu H.Q. Mussel foot protein inspired tough tissue-selective underwater adhesive hydrogel. Mater. Horiz. 2021;8(3):997–1007. doi: 10.1039/d0mh01231a. [DOI] [PubMed] [Google Scholar]
- 205.Zhang Y., Zhang J.H., Chen M.G., Gong H., Tharnphiwatana S., Eckmann L., Gao W.W., Zhang L.F. A bioadhesive nanoparticle-hydrogel hybrid system for localized antimicrobial drug delivery. ACS Appl. Mater. Interfaces. 2016;8(28):18367–18374. doi: 10.1021/acsami.6b04858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li L., Yan B., Yang J.Q., Huang W.J., Chen L.Y., Zeng H.B. Injectable self-healing hydrogel with antimicrobial and antifouling properties. ACS Appl. Mater. Interfaces. 2017;9(11):9221–9225. doi: 10.1021/acsami.6b16192. [DOI] [PubMed] [Google Scholar]
- 207.Chen T., Chen Y.J., Rehman H.U., Chen Z., Yang Z., Wang M., Li H., Liu H.Z. Ultratough, self-healing, and tissue-adhesive hydrogel for wound dressing. ACS Appl. Mater. Interfaces. 2018;10(39):33523–33531. doi: 10.1021/acsami.8b10064. [DOI] [PubMed] [Google Scholar]
- 208.Liu S., Liu X., Ren Y.H., Wang P.H., Pu Y.J., Yang R., Wang X.X., Tan X.Y., Ye Z.W., Maurizot V., Chi B. Mussel-inspired dual-cross-linking hyaluronic acid/epsilon-polylysine hydrogel with self-healing and antibacterial properties for wound healing. ACS Appl. Mater. Interfaces. 2020;12(25):27876–27888. doi: 10.1021/acsami.0c00782. [DOI] [PubMed] [Google Scholar]
- 209.Wang Y., Chen L., Ren D.Y., Feng Z.X., Zhang L.Y., Zhong Y.F., Jin M.Y., Xu F.W., Feng C.Y., Du Y.Z., Tan W.Q. Mussel-inspired collagen-hyaluronic acid composite scaffold with excellent antioxidant properties and sustained release of a growth factor for enhancing diabetic wound healing. Materials Today Bio. 2022;15 doi: 10.1016/j.mtbio.2022.100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Peng B., Lai X.Y., Chen L., Lin X.M., Sun C.X., Liu L.X., Qi S.H., Chen Y.M., Leong K.W. Scarless wound closure by a mussel-inspired poly(amidoamine) tissue adhesive with tunable degradability. ACS Omega. 2017;2(9):6053–6062. doi: 10.1021/acsomega.7b01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dompe M., Cedano-Serrano F.J., Heckert O., van den Heuvel N., van der Gucht J., Tran Y., Hourdet D., Creton C., Kamperman M. Thermoresponsive complex coacervate-based underwater adhesive. Adv. Mater. 2019;31(21) doi: 10.1002/adma.201808179. [DOI] [PubMed] [Google Scholar]
- 212.Lee Y., Xu C.J., Sebastin M., Lee A., Holwell N., Xu C.V., Nieves D.M., Mu L.Y., Langer R.S., Lin C.P., Karp J.M. Bioinspired nanoparticulate medical glues for minimally invasive tissue repair. Advanced Healthcare Materials. 2015;4(16):2587–2596. doi: 10.1002/adhm.201500419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Han K., Park T.Y., Yong K., Cha H.J. Combinational biomimicking of Lotus leaf, mussel, and sandcastle worm for robust superhydrophobic surfaces with biomedical multifunctionality: antithrombotic, antibiofouling, and tissue closure capabilities. ACS Appl. Mater. Interfaces. 2019;11(10):9777–9785. doi: 10.1021/acsami.8b21122. [DOI] [PubMed] [Google Scholar]
- 214.Mann L.K., Papanna R., Moise K.J., Byrd R.H., Popek E.J., Kaur S., Tseng S.C.G., Stewart R.J. Fetal membrane patch and biomimetic adhesive coacervates as a sealant for fetoscopic defects. Acta Biomater. 2012;8(6):2160–2165. doi: 10.1016/j.actbio.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 215.Kim H.J., Choi B.H., Jun S.H., Cha H.J. Sandcastle worm-inspired blood-resistant bone graft binder using a sticky mussel protein for augmented in vivo bone regeneration. Advanced Healthcare Materials. 2016;5(24):3191–3202. doi: 10.1002/adhm.201601169. [DOI] [PubMed] [Google Scholar]
- 216.Zhang D.H., Liu J.J., Chen Q., Jiang W.N., Wang Y.B., Xie J.Y., Ma K.Q., Shi C., Zhang H.D., Chen M.Z., Wan J.L., Ma P.C., Zou J.C., Zhang W.J., Zhou F., Liu R.H. A sandcastle worm-inspired strategy to functionalize wet hydrogels. Nat. Commun. 2021;12(1) doi: 10.1038/s41467-021-26659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hofman A.H., van Hees I.A., Yang J., Kamperman M. Bioinspired underwater adhesives by using the supramolecular toolbox. Adv. Mater. 2018;30(19) doi: 10.1002/adma.201704640. [DOI] [PubMed] [Google Scholar]
- 218.Martin-Palma R.J., Lakhtakia A. Progress on bioinspired, biomimetic, and bioreplication routes to harvest solar energy. Appl. Phys. Rev. 2017;4(2):9. doi: 10.1063/1.4981792. [DOI] [Google Scholar]
- 219.Seidi K., Ayoubi-Joshaghani M.H., Azizi M., Javaheri T., Jaymand M., Alizadeh E., Webster T.J., Yazdi A.A., Niazi M., Hamblin M.R., Amoozgar Z., Jahanban-Esfahlan R. Bioinspired hydrogels build a bridge from bench to bedside. Nano Today. 2021;39:33. doi: 10.1016/j.nantod.2021.101157. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.