Abstract
Background
In phase III TIVO-3 trial, tivozanib improved progression-free survival (PFS) compared to sorafenib for patients with metastatic renal cell carcinoma (mRCC). However, the effectiveness of this drug after exposure to other selective VEGFR agents has not yet been defined. Herein, we characterize the clinical efficacy of tivozanib in patients with mRCC previously treated with axitinib.
Methods
We identified patients from the intention to treat (ITT) population, in the TIVO-3 trial, who received treatment with axitinib before enrolment in the study and evaluated PFS, response rate (RR), and safety.
Results
Out of 350 patients, 172 (83:89, tivozanib:sorafenib) had received prior treatment with axitinib in TIVO-3. In this subgroup, PFS was 5.5 months with tivozanib and 3.7 months with sorafenib (HR 0.68). RR was 13% and 8% favoring tivozanib.
Conclusions
Tivozanib is active in the treatment of patients with mRCC who have progressed on prior therapies, including axitinib.
Keywords: metastatic renal cell carcinoma, tivozanib, sorafenib, axitinib, vascular endothelial growth factor inhibitor
Results of the TIVO-3 trial suggested that tivozanib has a progression-free survival advantage compared with sorafenib; however, it has been speculated that prior use of axitinib (which bears mechanistic resemblance to tivozanib) renders tivozanib less effective. This article examines the TIVO-3 dataset for outcomes of patients with prior axitinib therapy.
Introduction
The management of patients with metastatic renal cell carcinoma (mRCC) has evolved in recent years to include combinations of immune checkpoint inhibitors (ICIs) and vascular endothelial growth factor receptor-tyrosine kinase inhibitors (VEGFR-TKIs). Relative to the cytokine era, these agents have significantly improved the outcome among those diagnosed with mRCC. Recently, tivozanib, a potent and highly specific VEGFR-TKI, was approved by the US FDA for patients with relapsed or refractory mRCC following 2 or more systemic therapies1. This was based on data from the phase III TIVO-3 trial which demonstrated an improved progression-free survival (PFS) and response rate (RR) with tivozanib over sorafenib (5.6 months vs. 3.9 months; hazard ratio [HR] 0.73, P = .016) and (18% vs. 8%, P = .017) for PFS and RR, respectively (NCT02627963)2.
The TIVO-3 trial enrolled patients with mRCC with at least 1 prior VEGFR-TKI and 1 or 2 additional lines of therapy in the metastatic setting. The trial included a relatively large proportion of patients with prior ICI, accounting for 26% of the study population. In this setting, tivozanib appeared to have a PFS advantage relative to sorafenib2. Notably, this was also observed in patients with prior TKIs alone (5.5 months vs. 3.3 months; HR 0.57, 95% CI 0.39-0.83)2. However, it has been speculated that prior use of axitinib, which bears mechanistic resemblance to tivozanib, renders tivozanib less effective. To this end, the current analyses specifically examine the TIVO-3 dataset for outcomes of patients with prior axitinib therapy.
Methods
Patients in the TIVO-3 trial were adults with mRCC who had progressed on 2 or 3 lines of systemic therapy, including at least 1 VEGFR-TKI (except for sorafenib or tivozanib). They were randomized in a 1:1 fashion to either tivozanib, administered at 1.5 mg orally once a day for 21 days followed by a 7-day off period (28-day cycles), or sorafenib dosed at 400 mg orally twice daily. Treatment was continued until disease progression, as determined by an independent radiology review committee (IRC), or until unacceptable toxicity. In this post hoc analysis of TIVO-3 data, we identified patients from the intent to treat (ITT) population who received treatment with the VEGFR-TKI axitinib, before enrollment in the study.
Characterization of PFS, RR, dose reductions, treatment interruptions, and treatment discontinuations was performed using descriptive statistics. Patient outcomes based on previous exposure to axitinib were exploratory, and not prespecified in the trial protocol.
Results
Between May 24, 2016, and August 14, 2017, 457 patients were assessed for eligibility, and 350 were randomized to receive tivozanib (n = 175) or sorafenib (n = 175). Prior treatment with axitinib was noted in 172 (49%) patients from the ITT population. Among these, 83 (48%) received tivozanib and 89 (52%) received sorafenib (Table 1). No significant differences were observed between these groups.
Table 1.
PFS and RR of tivozanib and sorafenib.
| Patient group | N (subjects) | PFS (months) | HR | RR | |||
|---|---|---|---|---|---|---|---|
| Tivo | Sor | Tivo | Sor | Tivo (%) | Sor (%) | ||
| ITT | 175 | 175 | 5.6 | 3.9 | 0.73 | 18 | 8 |
| Prior axitinib in 3rd-line subgroup | 47 | 46 | 5.5 | 3.9 | 0.71 | 16 | 6 |
| Prior axitinib in 4th-line subgroup | 36 | 43 | 5.5 | 3.6 | 0.64 | 11 | 10 |
| Prior axitinib in 3rd- or 4th-line subgroup | 83 | 89 | 5.5 | 3.7 | 0.68 | 13 | 8 |
Abbreviations: PFS, progression-free survival; HR, hazard ratio; RR, response rate; Tivo, tivozanib; sor, sorafenib; ITT, intention to treat.
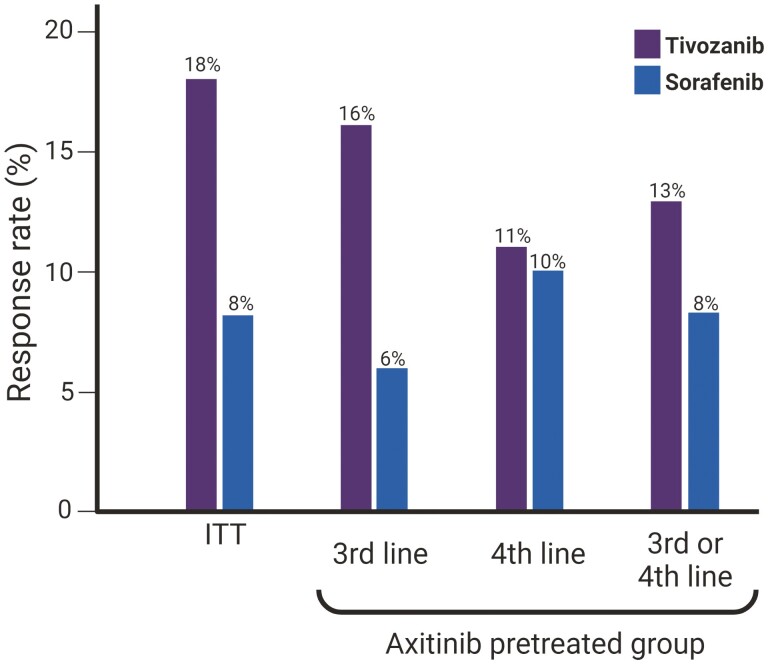
Among the patients who had received treatment with axitinib before the study, tivozanib was also associated with a PFS benefit over sorafenib (5.5 months vs. 3.7 months; HR 0.68). These findings were consistent irrespective of the line in which the study treatment was rendered (Table 1). In line with this PFS advantage, a trend toward increased RR was also seen in the subgroup of patients previously treated with axitinib (13% vs. 8%) (Fig. 1).
Figure 1.
Response rate (RR) of tivozanib and sorafenib in the intention to treat (ITT) population, and patients previously treated with axitinib receiving the study treatment as 3rd-line, 4th-line, and both.
A detailed description of the treatment-related adverse events in the ITT population has been previously reported3. In the axitinib-pretreated population, treatment-related adverse events were reported among 145 patients (84.5%). Within this population, 48 patients (28%) required a dose reduction due to AE, 92 patients (53.3%) required a treatment interruption, and 44 patients (25.6%) required treatment discontinuation. These rates were similar to those in the ITT population with no axitinib exposure (Table 2).
Table 2.
Safety and tolerability in patients with and without prior axitinib treatment.
| AE | Prior axitinib | No prior axitinib |
|---|---|---|
| Treatment-related AE | 84.5% | 92.3% |
| Reduction due to AE | 28.0% | 29.7% |
| Interruption due to AE | 53.5% | 53.8% |
| Discontinuation due to AE | 25.6% | 19.8% |
Abbreviation: AE, adverse event.
Discussion
Our data support the activity of tivozanib in patients previously treated with other VEGFR-specific agents, such as axitinib. Specifically, in TIVO-3, the benefit of tivozanib over sorafenib concerning PFS and RR was maintained in the subset of patients with prior axitinib treatment. Furthermore, there appeared to be no substantial signals of increased toxicity with tivozanib following axitinib (relative to patients without prior axitinib). A potential explanation for the activity of tivozanib following axitinib could lay in subtle but distinct mechanisms of VEGFR blockade at a molecular level. The 2 agents have unique conformational binding characteristics which could theoretically prompt differences in clinical activity4.
Furthermore, when 1 considers purported mechanisms of resistance to VEGFR-inhibitors, there is good rationale to suspect that re-challenge with these agents (after treatment interruption) may inhibit tumor growth. Conditions of hypoxia (induced by VEGFR-blockade) could lead to upregulation of hypoxia-inducible Factor α (HIFα), with downstream production of VEGF and other proangiogenic agents leading to angiogenic escape5. Once VEGFR TKIs are stopped, the increased production of VEGF can override other mechanisms of angiogenesis leading to restoration of the tumor vasculature dependence on VEGF. This could render the tumor once again sensitive to VEGFR inhibitors after a period of temporary cessation. Beyond our study, this could explain the persistent clinical benefit observed in other studies. For example, the “resume” study included 52 patients who had received sunitinib in the 1st-line setting; of these patients, 54% of patients had achieved an objective response6. With rechallenge in the 3rd-line setting and beyond, a RR of 15% was observed.
The rising prominence of the use of anti-angiogenic therapy/immunotherapy combinations in the upfront setting raises the challenge of selecting agents that will still be active in the salvage setting7. The analysis presented here supports that tivozanib preserves its efficacy even after exposure to axitinib, a mechanistically similar agent. This data is especially relevant in the context of upfront contemporary regimens, such as axitinib with pembrolizumab or axitinib with avelumab, after which tivozanib could still provide benefit. Furthermore, ongoing investigation examining tivozanib in combination with immune checkpoints inhibitors, such as the phase III TiNivo-2 trial, will provide insights on the utility of such regimens in 2nd-line following other IO/TKI combinations8.
Limitations of the current study include the post-hoc and unplanned nature of the analysis, our limited sample size, and lack of stratification based on axitinib exposure. Consequently, results should be considered hypothesis-generating. Notably, despite these limitations, efficacy assessments favored tivozanib, even among patients previously treated with axitinib. In conclusion, tivozanib is active in the treatment of mRCC, conferring a substantial clinical benefit as compared to sorafenib in previously treated disease even in the context of prior axitinib therapy another highly selective VEGFR-TKI.
Contributor Information
Luis Meza, Department of Medical Oncology & Therapeutics, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
David F McDermott, Department of Medicine, Beth Israel Deaconess Medical Center, Dana-Farber/Harvard Cancer Center, Boston, MA, USA.
Bernard Escudier, Department of Medical Oncology, Gustave Roussy, Villejuif, France.
Thomas E Hutson, Department of Hematology and Medical Oncology, Texas A&M University College of Medicine, Bryan, TX, USA.
Camillo Porta, Department of Biomedical Sciences and Human Oncology, University of Bari Aldo Moro and Policlinico Consorziale di Bari, Bari, Italy.
Elena Verzoni, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy.
Michael B Atkins, Department of Medical Oncology, Georgetown Lombardi Comprehensive Cancer Center, Washington, DC, USA.
Vijay Kasturi, Clinical Development and Medical Affairs, Aveo Oncology, Boston, MA, USA.
Sumanta K Pal, Department of Medical Oncology & Therapeutics, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Brian Rini, Division of Hematology Oncology, Vanderbilt-Ingram Cancer Center, Nashville, TN, USA.
Funding
This research did not receive any specific grant from funding agencies in the public or not-for-profit sectors. The study was funded by Aveo Oncology.
Conflict of Interest
David F. McDermott reported honoraria from BMS, Pfizer, Merck, Alkernes, EMD Serono, Eli Lilly and Company, Iovance, Eisai, Werewolf Therapeutics, Calithera Biosciences, and research funding from BMS, Merck, Genentech, Pfizer, Exelixis, X4 Pharma, and Alkermes. Bernard Escudier reported consulting/advisory relationships with Pfizer, BMS, Ipsen, Aveo, and Eisai. Thomas E. Hutson reported consulting/advisory relationships with Aveo, Pfizer, Exelixis, BMS, EMD Serono, Eisai, Jansen, Gilead, and Astellas. Camillo Porta reported consulting/advisory relationships with Angelini Pharma, AstraZeneca, Bristol Myers Squibb, Eisai, EUSA Pharma, General Electric, Ipsen, Janssen, Merck, Merck Sharp & Dohme, Novartis, and Pfizer; expert testimony for EUSA Pharma and Pfizer; Protocol Steering Committee Member: Merck Sharp & Dohme, Bristol Myers Squibb, Eisai, and EUSA Pharma; and travel support from Roche. Elena Verzoni reported consulting/advisory relationships with Bristol Myers Squibb, Eisai, Janssen, Merck, MSD, and Ipsen. Michael B. Atkins reported consulting/advisory relationships with Eisai, Aveo, Pfizer, Werewolf Therapeutics, Fathom, Pneuma, Leads, Pyxis Oncology, PACT, Elpis, X4Pharma, ValoHealth, ScholarRock, Surface, Takeda, Simcha, Bristol Myers Squibb, Merck, Novartis, Pfizer, Roche, Exelixis, Iovance, Idera, Agenus, Asher Bio, Neoleukin, AstraZeneca, Calithera, SeaGen, SAB Bio Oncorena, and Sanofi; and ownership interests in Werewolf Therapeutics and Pyxis Oncology. Brian Rini reported research funding from Pfizer, Hoffman-LaRoche, Incyte, AstraZeneca, Seattle Genetics, Arrowhead Pharmaceuticals, Immunomedics, BMS, Mirati Therapeutics, Merck, Surface Oncology, Dragonfly Therapeutics, Aravive, Exelixis, Jannsen; Consulting: BMS, Pfizer, GNE/Roche, Aveo, Synthorx, Compugen, Merck, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, GSK, Shionogi, Eisai, Nikang Therapeutics; and ownership interests in PTC Therapeutics. The other authors indicated no financial relationships.
Author Contributions
Conception/design: L.M., V.K., S.K.P., B.R. Provision of study material or patients: All authors. Collection and/or assembly of data: All authors. Data analysis and interpretation: L.M., D.F.M., B.E., C.P., M.B.A., V.K., S.K.P., B.R. Manuscript writing: L.M., D.F.M., B.E., C.P., M.B.A., V.K., S.K.P., B.R. Final approval of manuscript: All authors.
Data Availability
The data underlying this article were provided by Aveo Oncology. Data will be shared on request to the corresponding author with permission of Aveo Oncology.
References
- 1. Research C for DE and FDA approves tivozanib for relapsed or refractory advanced renal cell carcinoma. FDA. Published online June 11, 2021. Accessed January 27, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tivozanib-relapsed-or-refractory-advanced-renal-cell-carcinoma
- 2. Rini BI, Pal SK, Escudier BJ, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21(1):95-104. 10.1016/S1470-2045(19)30735-1. [DOI] [PubMed] [Google Scholar]
- 3. Pal SK, McDermott DF, Escudier B, et al. Temporal characteristics of treatment-emergent adverse events and dose modifications with tivozanib and sorafenib in the phase 3 TIVO-3 study of relapsed or refractory mRCC. J Clin Oncol. 2021;39(15_suppl):4567-4567. 10.1200/JCO.2021.39.15_suppl.4567. [DOI] [Google Scholar]
- 4. McTigue M, Murray BW, Chen JH, et al. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc Natl Acad Sci USA. 2012;109(45):18281-18289. 10.1073/pnas.1207759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rini BI, Atkins MB, Atkins MB.. Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol. 2009;10(10):992-1000. 10.1016/S1470-2045(09)70240-2. [DOI] [PubMed] [Google Scholar]
- 6. Oudard S, Geoffrois L, Guillot A, et al. Clinical activity of sunitinib rechallenge in metastatic renal cell carcinoma-Results of the REchallenge with SUnitinib in MEtastatic RCC (RESUME) Study. Eur J Cancer. 2016;62:28-35. 10.1016/j.ejca.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 7. Meeting Library. Real-world assessment of changing treatment patterns and sequence for patients with metastatic renal cell carcinoma (mRCC) in the first-line (1L) setting. Accessed April 13, 2022. https://meetinglibrary.asco.org/record/205591/abstract
- 8. Choueiri TK, Albiges L, Hammers HJ, et al. TiNivo-2: a phase 3, randomized, controlled, multicenter, open-label study to compare tivozanib in combination with nivolumab to tivozanib monotherapy in subjects with renal cell carcinoma who have progressed following one or two lines of therapy where one line has an immune checkpoint inhibitor. J Clin Oncol. 2022;40(Suppl 6):TPS405-TPS405. 10.1200/JCO.2022.40.6_suppl.TPS405. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article were provided by Aveo Oncology. Data will be shared on request to the corresponding author with permission of Aveo Oncology.