Abstract
PURPOSE:
To identify factors influencing visual outcome in patients with neovascular age-related macular degeneration (NVAMD) and subfoveal hemorrhage (SFH) treated with anti–vascular endothelial growth factor (VEGF) agents.
DESIGN:
Retrospective case series.
METHODS:
Anti-VEGF-treated eyes with SFH > 1 disc area (DA) were identified (n = 16) and changes in visual acuity (VA) and central subfield thickness (CST) from baseline to last follow-up, along with SFH area, thickness, minimum distance from fovea to SFH border, and time to resolution, were determined.
RESULTS:
At baseline, mean (± standard error of the mean) size and thickness of SFH were 14.9 ± 2.8 DA and 386.6 ± 46.9 μm, and mean Snellen VA and CST were 20/250 and 591.7 ± 57.0 μm. Median follow-up was 47.6 months. While more than 50% of patients had VA ≤ 20/200 at baseline and all time points through week 48, the percentage of patients with VA ≥ 20/50 increased to 30%–40% at months 6 and 12 and remained stable through month 48. Spearman rank correlation demonstrated 2 independent variables that correlated with good visual outcome, smaller area of SFH at baseline (r = −0.630; P = .009), and high frequency of anti-VEGF injections (r = 0.646; P = .007). In exceptional patients with good visual outcome despite large baseline SFH, shortest distance between the fovea and hemorrhage border significantly correlated with baseline VA (r = −0.503, P = .047) and final VA (r = −0.575, P = .02).
CONCLUSIONS:
Patients with NVAMD and thick SFH, but short distance between fovea and uninvolved retina, can have good visual outcomes when given frequent anti-VEGF injections.
Subretinal hemorrhage is a serious vision-threatening complication of neovascular age-related macular degeneration (NVAMD). It may occur as the initial presentation of NVAMD or at any time during the course. The visual prognosis depends on several factors, including the location, size, and thickness of the hemorrhage, but in general, thick hemorrhages beneath the fovea are associated with a poor prognosis.1,2 Potential reasons for vision loss are photoreceptor shearing by contraction of fibrin clots, iron and hemosiderin toxicity, and interruption of oxygen and nutrient delivery from the choroid.3,4 One approach to attempt to reduce toxicity is to evacuate the hemorrhage. However, a randomized trial comparing observation and surgical removal of subretinal hemorrhage showed no significant visual benefit from removal.5 An alternative approach that is thought to be less invasive is pneumatic displacement of the hemorrhage using an intraocular gas bubble, alone or combined with intravitreous or subretinal injection of tissue plasminogen activator (TPA).6,7 Visual outcomes suggest modest improvement that may be better than reported natural history, but without a controlled clinical trial it is uncertain whether there is any benefit.8,9
The decision of whether to surgically intervene for subretinal hemorrhage in patients with NVAMD has become more complex since the development of vascular endothelial growth factor (VEGF)-neutralizing proteins for the treatment of NVAMD. Subretinal blood constituting ≥50% of lesion size was an exclusion criterion for phase 3 clinical trials investigating the efficacy of anti-VEGF agents, limiting our knowledge of how submacular hemorrhage affects outcomes in patients with NVAMD treated with anti-VEGF agents. The Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) enrolled patients with lesions consisting of ≥50% hemorrhage (B50 group of CATT) as long as best-corrected visual acuity (BCVA) was between 20/25 and 20/320, but the hemorrhage was subfoveal in only 59.5%.10 Baseline BCVA was 56 letters (20/80) in the B50 group compared with 60.9 (20/63) in all others. There was no significant difference in mean improvement from baseline BCVA in the B50 group (9.0 letters) vs others (6.0 letters) at 2 years. These data suggest that anti-VEGF injections provide benefit in patients with NVAMD in whom ≥50% of the neovascular complex consists of hemorrhage when BCVA is ≥20/320; however, there is little to guide treatment decisions in patients with NVAMD who have very poor vision owing to subfoveal hemorrhage (SFH) or those with very thick SFH. This is because although hemorrhage thickness was not provided, it can be estimated from central subfield thickness (CST); and based on those data, it appears that there were few if any patients with SFH > 100–200 μm.10 Thus, it is unknown whether NVAMD patients with poor vision from SFH and/or relatively thick hemorrhages benefit from anti-VEGF injections or if such patients should have hemorrhage displacement as soon as possible. In this study, we reviewed long-term outcomes in NVAMD patients with SFH treated with anti-VEGF injections to assess the impact of SFH characteristics on visual outcome.
METHODS
THIS WAS A RETROSPECTIVE, OBSERVATIONAL CASE SERIES conducted at a single center (The Wilmer Eye Institute, Johns Hopkins Hospital, Baltimore, Maryland, USA). The protocol was approved by Institutional Review Board of the Johns Hopkins Medical Institutions and was conducted in accordance with the Declaration of Helsinki. Initial screening was done to identify patients with NVAMD seen by one of the authors between January and December 2014. All records of these patients are from the first time they were seen at the Wilmer Eye Institute prior to or during the ascertainment period through their last follow-up visit through December 2016.
SUBJECT ELIGIBILITY AND EXCLUSION CRITERIA:
Eligible patients were aged 50 years or older with NVAMD who had SFH > 1 disc area (DA) in size prior to the onset of anti-VEGF treatment or at some time during the course of treatment. To allow adequate assessment of the characteristics of the hemorrhage and anatomic outcomes, spectral-domain optical coherence tomography (SD-OCT) was required at the visit when treatment was begun after the SFH and at last follow-up, at least 6 months after onset of treatment. Patients who had choroidal neovascularization (CNV) secondary to causes other than NVAMD, or had received treatment other than anti-VEGF agents during follow-up for NVAMD. such as pars plana vitrectomy, pneumatic displacement, or intravitreal injection of TPA, were excluded.
STUDY PROTOCOL:
Each subject had the following information entered into a database: demographics, and medical history including systemic diseases, past ocular history, anticoagulant use, and other medications. Snellen visual acuity (VA) with current correction was recorded for baseline and all follow-up visits. All fundus photographs, fluorescein angiograms, and SD-OCTs were analyzed. The size and thickness of SFH and CST was measured on SD-OCT scans at baseline and all follow-up visits. Fundus photographs and fluorescein angiograms, when available, were used to confirm CNV and assist in determination of the borders of SFH. The type and date of all anti-VEGF injections were recorded. The change from baseline VA and CST was determined at months 6, 12, 24, 48, and 60 and at last follow-up visit.
OPTICAL COHERENCE TOMOGRAPHY:
Heidelberg Spectralis (Heidelberg Engineering, Inc, Heidelberg, Germany) SD-OCT was used to measure the size of hemorrhage, thickness of hemorrhage, and CST. Size of the SFH was measured, in square millimeters (mm2), on infrared images of SD-OCT scans using a free-hand Draw region tool. Size of SFH in DA was calculated by dividing size of SFH by 1.834 mm2 (average area of optic disc, SPECTRALIS Glaucoma Module Premium Edition User Manual, Software Version 6.6: Heidelberg Engineering GmbH, 2016). The shortest distance from the fovea to the hemorrhage border in all directions was also measured on SD-OCT scans using distance measuring calipers provided by the SD-OCT software. The shortest distance to the border of the hemorrhage from the fovea was used for analysis. Thickness of SFH was also manually measured in the fovea on the horizontal SD-OCT section passing through the fovea by using distance measuring calipers. Thickness of SFH was defined as the distance between neurosensory retina and retinal pigment epithelium (RPE) at the fovea. SD-OCT was also used to determine resolution of the subretinal hemorrhage. Subretinal hemorrhage resolution in the fovea was defined as resolution of subretinal hyperreflectivity from presence of hemorrhage in the central 1-mm Early Treatment Diabetic Retinopathy Study (ETDRS) ring of the SD-OCT. Complete resolution of subretinal hemorrhage in the macula was defined as resolution of hyperreflectivity in the subretinal space from presence of hemorrhage in all 3 (1-, 3-, and 6-mm) ETDRS rings of the SD-OCT. CST was measured after manual correction of algorithms and grid alignment, if required, using the Heidelberg Eye Explorer 1.9.10.0, HRA/Spectralis Viewing Module 6.0.9.0. Sequential SD-OCT images were graded by a single examiner (S.K.) for pathology in the fovea and macula.
STATISTICAL ANALYSIS:
All statistical tests were performed using SPSS software version 23.0 (IBM Corp, Armonk, New York, USA) and Stata version 14.1 (Stata Corp, College Station, Texas, USA). Descriptive statistics were calculated for continuous and categorical variables. Comparisons between groups were made using the independent samples t test for parametric variables and the Mann-Whitney U test for nonparametric variables. Categorical variables were compared using Fisher exact test. Spearman’s rho correlation coefficient (r) was used to calculate correlation between variables. Mixed-effects regression models were used to account for correlations among repeated measurements from the same eye. A P value of <.05 was considered to be statistically significant.
RESULTS
PATIENT DISPOSITION:
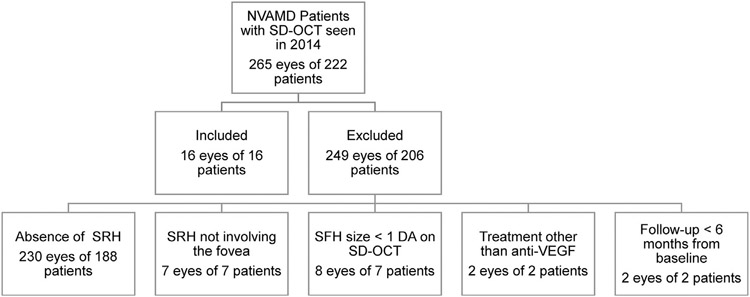
A total of 265 eyes of 222 patients with a diagnosis of NVAMD were seen by 1 of the co-authors at the Wilmer Eye Institute between January and December 2014 and all images and records of those patients prior to and after 2014 were evaluated. Thirty-five eyes of 34 patients had subretinal hemorrhage (13.2%), out of which 19 eyes of 18 patients were excluded for the following reasons: 7 eyes of 7 patients had subretinal hemorrhage not involving the fovea, 8 eyes of 7 patients had SFH < 1 DA, 2 eyes of 2 patients had follow-up duration < 6 months, and 2 eyes of 2 patients received treatments other than anti-VEGF injections (Figure 1). As a result, 16 eyes of 16 patients were included in the analysis. Of these 16 patients, all completed at least 6 months of follow-up, 15 (94%) completed 24 months of follow-up, 11 (69%) completed 36 months of follow-up, 10 (63%) completed 48 months of follow-up, and 5 (31%) completed 60 months of follow-up.
FIGURE 1.
Identification of patients with subfoveal hemorrhage owing to neovascular age-related macular degeneration (NVAMD) treated with intravitreous injections of a vascular endothelial growth factor neutralizing protein. DA = disc area; SD-OCT = spectral-domain optical coherence tomography; SFH = subfoveal hemorrhage; SRH = subretinal hemorrhage; VEGF = vascular endothelial growth factor.
PATIENT DEMOGRAPHICS AND BASELINE CHARACTERISTICS:
Mean age was 81.4 ± 2.2 years (range, 55–93 years). Most patients (94%) were white and 56% were female (Table 1). Twelve patients (75%) had hypertension and 2 (12.5%) had diabetes mellitus. None of the patients were using anticoagulants at the time of SFH. Mean size of SFH was 14.9 ± 2.8 DA (median 14.2 DA; range 1.4–33.9 DA), with mean SFH thickness of 386.6 ± 46.9 μm (median 324.0 μm; range 116.0–842.0 μm). Mean Snellen VA was 20/250 (median 20/200; range 20/50 to counting fingers [CF]) and mean CST was 591.7 ± 57.0 μm (median 540.0 μm; range 315.0–1231.0 μm). The range to last follow-up examination was 6.7–64.5 months (median 47.6 months; mean 42.6 months).
TABLE 1.
Baseline Characteristics at Time of Subfoveal Hemorrhage
| Patients With Subfoveal Hemorrhage (N = 16) |
|
|---|---|
| Age (y), mean ± SEM | 81.4 ± 2.2 |
| Female, n (%) | 9 (56.3) |
| Race, n (%) | |
| White | 15 (93.8) |
| African American | 1 (6.3) |
| Systemic disease, n (%) | |
| Diabetes mellitus | 2 (12.5) |
| Hypertension | 12 (75.0) |
| Use of anticoagulation | 0 |
| Thickness of SFH on SD-OCT (μm), n (%) | |
| <250 | 2 (12.5) |
| 250–500 | 11 (68.8) |
| >500 | 3 (18.8) |
| Size of SFH (DA), n (%) | |
| <10 | 7 (43.8) |
| 10–20 | 5 (31.3) |
| >20 | 4 (25.0) |
| VA at time of SFH (Snellen), n (%) | |
| 20/50 or better | 1 (6.3) |
| 20/63 to 20/160 | 5 (31.3) |
| 20/200 or worse | 10 (62.5) |
| CST at time of SFH (μm), mean ± SEM | 591.7 ± 57.0 |
CST = central subfield thickness; DA = disc area; SD-OCT = spectral-domain optical coherence tomography; SFH = subfoveal hemorrhage; VA = visual acuity.
VISUAL OUTCOMES IN ENTIRE POPULATION:
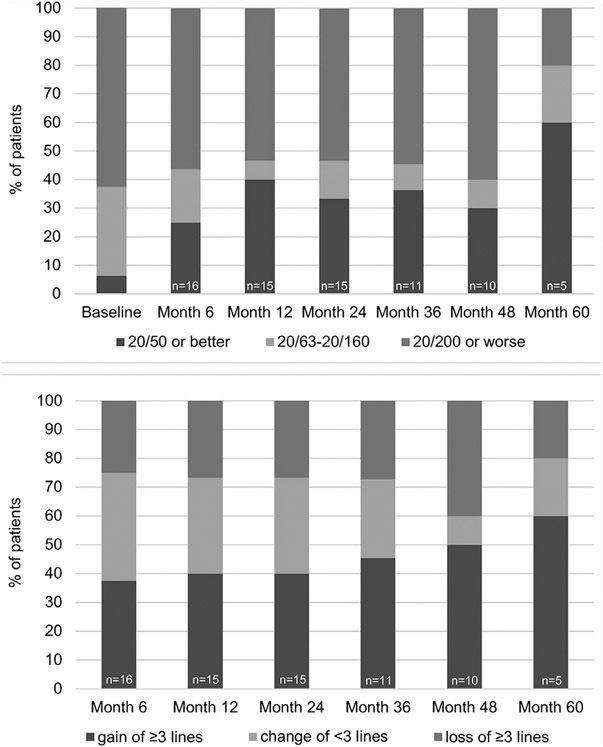
Snellen VA measurements were converted to ETDRS letter score and mean VA in letter score for the entire group of 16 patients was 30.9 ± 4.6 (20/250) at baseline, 35.3 ± 7.1 (20/200) at month 6 (n = 16), 39.0 ± 7.3 (20/160) at month 12 (n = 15), 37.3 ± 7.7 (20/200) at month 24 (n = 15), 35.9 ± 8.9 letters (20/200) at month 36 (n = 11), 33.0 ± 10.5 (20/250) at month 48 (n = 10), and 48.0 ± 13.1 (20/125) at month 60 (n = 5). The percentage of patients with good (VA ≥ 20/50), moderate (20/50 > VA > 20/200), and poor (VA ≤ 20/200) VA at baseline and during follow-up is shown in Figure 2A. More than 50% of patients had poor VA at baseline and at subsequent time points through month 48, the last time point at which data were available for the majority of patients. The percentage of patients with good VA increased at months 6 and 12, and then remained relatively stable at 30%–40% through month 48. The percentage of patients who improved by ≥ 3 lines was roughly 40%–50% through month 48, but the percentage of patients who lost ≥ 3 lines was also substantial (25%–40%) through week 48 (Figure 2B). Three patients gained ≥ 6 lines of VA from baseline and 4 patients lost ≥ 6 lines at last follow-up (Table 2). Thus, there was substantial variability in visual outcome among patients treated with anti-VEGF agents. Spearman rank correlation demonstrated 2 independent variables that correlated with good visual outcome, smaller area of SFH at baseline (r = −0.630; P = .009), and high frequency of anti-VEGF injections (r = 0.646; P = .007).
FIGURE 2.
Visual outcomes in patients with subfoveal hemorrhage owing to neovascular age-related macular degeneration treated with intravitreous injections of a vascular endothelial growth factor neutralizing protein. (Top) Bar graphs show percentage of patients with visual acuity ≥20/50, 20/63 to 20/160, or ≤20/200 at baseline and 6, 12, 24, 36, 48, and 60 months after baseline. (Bottom) Bar graphs show percentage of patients that gained ≥3 lines from baseline, had change ≤3 lines, or lost ≥3 lines at 6, 12, 24, 36, 48, and 60 months after baseline.
TABLE 2.
Outcomes in Patients With Subfoveal Hemorrhage at Last Follow-up Visit
| Patients With Subfoveal Hemorrhage (N = 16) |
|
|---|---|
| Follow-up duration (months), median (range) | 47.6 (6.7–64.5) |
| Baseline VA, n (%) | |
| 20/50 or better | 1 (6.3) |
| 20/63 to 20/160 | 5 (31.3) |
| 20/200 or worse | 10 (62.5) |
| Final VA, n (%) | |
| Better than 20/50 | 6 (37.5) |
| 20/63 to 20/160 | 1 (6.3) |
| Worse than 20/200 | 9 (56.2) |
| Change in VA, n (%) | |
| Gain of ≥6 lines | 3 (18.8) |
| Gain of ≥3 lines | 8 (50.0) |
| Change of >3 lines | 2 (12.5) |
| Loss of ≥3 lines | 6 (37.5) |
| Loss of ≥6 lines | 4 (25.0) |
| Baseline CST (μm), mean ± SEM (range) | 591.7 ± 57.0 (315.0–1231.0) |
| Final CST (μm), mean ± SEM (range) | 285.3 ± 31.9 (158.0–593.0) |
| Time to resolution of SFH in the fovea (months), median (range) | 5.7 (2.8–7.4) |
| Time to complete resolution of SFH (months), median (range) | 7.6 (4.7–13.0) |
| Number of anti-VEGF injections, mean ± SEM (range) | 17.4 ± 3.4 (4.0–48.0) |
| Frequency of anti-VEGF injections, n (%) | |
| Every 4–6 weeks | 5 (31.3) |
| Relatively frequent PRN | 4 (25.0) |
| PRN with long intervals without treatment | 7 (43.8) |
| Final macular status, n (%) | |
| SF fibrosisa | 9 (56.3) |
| Atrophy in area of SF fibrosis, n/n (%) | 5/9 (55.6) |
| SF atrophy | 1 (6.3) |
| Sub-RPE hyperreflective material with atrophy | 1 (6.3) |
| PF fibrosis | 1 (6.3) |
| PF atrophy | 4 (25.0) |
| Location of atrophy, n (%) | |
| No atrophy | 4 (25.0) |
| Within the area of SFH | 9 (56.3) |
| Within and outside the area of SFH | 3 (18.8) |
CST = central subfield thickness; PF = perifoveal; PRN = pro re nata; SF = subfoveal; SFH = subfoveal hemorrhage; VA = visual acuity.
One patient had small area of atrophy that was not contiguous with area of SF fibrosis.
VISUAL OUTCOMES IN INDIVIDUAL PATIENTS:
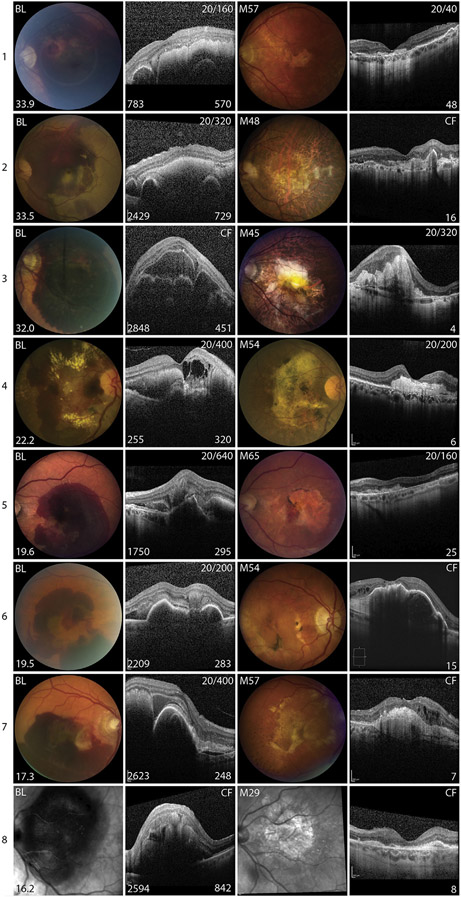
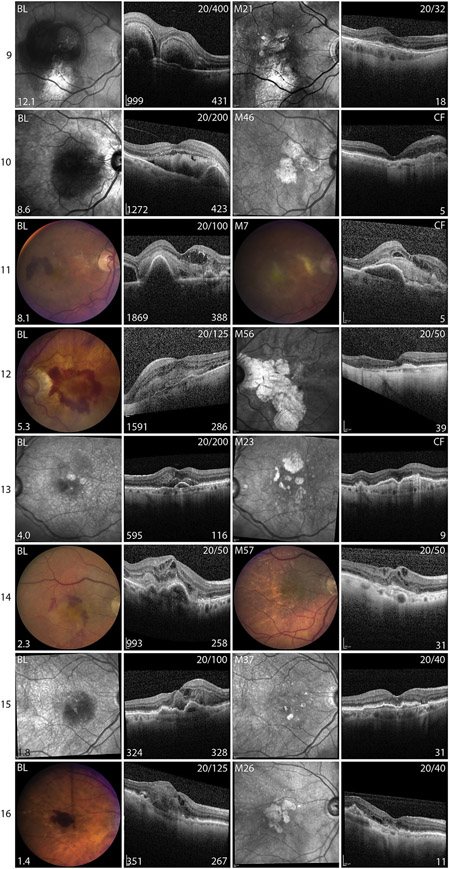
Because the characteristics of SFH vary among patients, it is necessary to examine the course of each patient in great detail to determine how different characteristics of SFH or its treatment might affect outcomes. Since large SFH size correlated with poor visual outcome, the images and outcomes of patients were organized in order from those with largest to smallest SFH at baseline (Figure 3). As indicated by Spearman rank correlation, patients with SFH that occupied a large area of retina tended to have a poor visual outcome, but there were a few exceptions. The patient in whom the hemorrhage occupied the largest overall area, 33.9 DA, had a good visual outcome (Figure 3, Patient 1). This patient was unusual in that the baseline VA was better than all other patients with a large SFH and although the hemorrhage was large, it was asymmetric. It was much thicker on the temporal side of the fovea compared with the nasal side, and the fovea was on the downslope of the hemorrhage. The hemorrhage was still quite thick beneath the center of the fovea, 570 μm, but at the nasal border of the fovea the thickness dropped precipitously and ended at the border of a pigment epithelial detachment (PED), 783 μm nasal to the fovea. Fifty-seven months later and after 48 anti-VEGF injections, VA was 20/40. There was atrophy on the temporal side of the fovea, but the nasal retina appeared relatively normal.
FIGURE 3.
Images from baseline and last follow-up visit for patients with subfoveal hemorrhages owing to neovascular age-related macular degeneration. Color fundus photographs (CFP) or infrared (IR) images and horizontal spectral-domain optical coherence tomography (SD-OCT) scans through fovea in patients with subfoveal hemorrhage (SFH) organized in descending order of area of hemorrhage from 1 to 16. First column shows CFP or IR image at baseline (BL) visit and the area of SFH in disc areas is in the lower left corner. The second column shows the horizontal SD-OCT scan through the fovea at BL. Snellen visual acuity (VA) at BL is shown in the upper right corner, thickness of the SFH (μm) is shown in the lower right corner, and the distance from the fovea to the edge of the hemorrhage (μm) is shown in the lower left corner. The third column shows CFP or IR image at last follow-up visit, with time in months (M) after baseline in the upper left corner. The fourth column shows horizontal SD-OCT scan through the fovea at last follow-up visit, with VA shown in the upper right corner and number of anti-VEGF injections shown in the lower right corner.
A second patient with a large area of SFH who had a good visual outcome also had a very asymmetric hemorrhage with 2 separate mounds of blood in the horizontal meridian through the fovea (Figure 3, Patient 9). The fovea was located on the crest of the nasal mound 431 μm thick at the fovea, but the mound itself was quite narrow so that the retina relatively close to the fovea on each side contacted the RPE, with the minimum distance from the foveal center to the edge of hemorrhage equal to 999 μm. After 21 months and 18 anti-VEGF injections, VA was 20/32.
A third patient with a good visual outcome had a large asymmetric hemorrhage extending from the border of peripapillary atrophy to the temporal side of the fovea (Figure 3, Patient 12). The hemorrhage was thickest nasal to the fovea, and the fovea was elevated 286 μm on the downslope of the hemorrhage, 1591 μm from the temporal edge of the hemorrhage. Fifty-six months later after 39 anti-VEGF injections, VA was 20/50 and while there was severe atrophy nasal to the fovea the retina on the temporal side of the fovea appeared relatively normal. Three other patients with good visual outcome had relatively small hemorrhages, which were asymmetric with respect to the foveal center (Figure 3, Patients 14–16). These observations suggest the hypothesis that patients with relatively thick SFH may have a relatively favorable prognosis provided the fovea is relatively close to the border of the hemorrhage and they receive frequent anti-VEGF injections. It is useful to ask, “Were there any patients who fit these criteria, but had a poor visual outcome?” Patients with relatively thick hemorrhages with minimum distance from hemorrhage border to the fovea ≥ 2000 μm had poor visual outcomes (Figure 3, Patients 2, 3, 6, 7, and 8). Some patients had retinal/RPE contact < 2000 μm to the fovea, but did not fit the criteria because they did not receive aggressive anti-VEGF treatment (Figure 3, Patients 4, 10, and 11). One patient had an RPE tear (Figure 3, Patient 5) and another patient had a small subretinal hemorrhage adjacent to a small hemorrhagic PED, but after 23 months and only 9 anti-VEGF injections, there was a large PED containing hyperreflective material with overlying thinning of the retina and CF vision (Figure 3, Patient 13). To test the hypothesis that shortest distance from the fovea to the hemorrhage border correlates with visual outcome, the distance was measured in all directions on SD-OCT scans. Shortest distance between the fovea and the border of hemorrhage was significantly correlated with baseline VA (r = −0.503, P = .047) and final VA (r = −0.575, P = .02).
ANATOMIC OUTCOME:
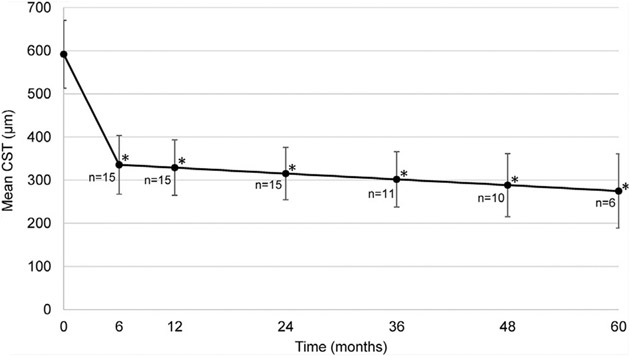
Mean baseline CST for the entire population was 591.7 ± 57.0 μm and correlated with SFH thickness (r = 0.809, P < .001) and SFT size (r = 0.629, P = .009). There was a significant reduction from mean baseline CST at all follow-up time points (P < .001, Figure 4), which decreased to 285.3 ± 31.9 μm at last follow-up visit. Grading of SD-OCT scans at every visit showed that the median time from baseline to elimination of hemorrhage beneath the fovea was 5.7 months (range, 2.8–7.4 months) and median time from baseline to complete resolution of hemorrhage was 7.6 months (range, 4.7–13.0 months, Table 2). Time to resolution of SRH beneath the fovea correlated with time to complete hemorrhage resolution (r = 0.758; P = .001). Compared with patients with SFH < 500 μm thick, those with SFH thickness ≥ 500 μm required a significantly longer time to elimination of hemorrhage beneath the fovea (P = .049) and complete hemorrhage resolution (P = .001).
FIGURE 4.
Anatomic outcomes in patients with subfoveal hemorrhage owing to neovascular age-related macular degeneration treated with intravitreous injections of a vascular endothelial growth factor neutralizing protein. Points show the mean (±95% confidence interval) central subfield thickness (CST) at baseline and 6, 12, 24, 36, 48, and 60 months after baseline. *P < .001 for difference from baseline by unadjusted mixedeffect regression model.
MACULAR MORPHOLOGIC OUTCOME:
Macular morphology was assessed to identify central macular changes that could have affected VA. Hemorrhage beneath the fovea persisted in 20% of eyes (3/15) at month 6 and was not seen in any patient thereafter. None of the patients had recurrence of SFH > 1 DA or had development of breakthrough vitreous hemorrhage during the follow-up period. Using infrared images and SD-OCT scans, grading was done for RPE changes, atrophy, and subretinal hyperreflective material (SRHRM), defined as hyperreflective material in the subretinal space above the RPE. When SRHRM was highly reflective with well-demarcated, sharp borders and loss or disruption of overlying ellipsoid zone and external limiting membrane, it was designated as subretinal fibrosis,11,12 and was the most common macular finding after resolution of SFH. It was present in 33.3% of eyes (5/15) at month 6, 40.0% (6/15) at month 12, 46.7% (7/15) at month 24, 54.5% (6/11) at month 36, 60.0% (6/10) at month 48, and 40.0% (2/5) at month 60. Three eyes had RPE changes at month 6 after resolution of SFH and in 2 eyes the changes evolved into subfoveal atrophy, 1 at month 12 and the other at month 24, whereas the third eye evolved into subretinal fibrosis at month 12 (Table 3). An RPE tear in the macula was identified in 2 patients after resolution of the subretinal hemorrhage. A summary of visual, anatomic, and morphologic outcomes in all patients is described in Supplemental Table 1 (Supplemental Material available at AJO.com).
TABLE 3.
Macular Changes After Subfoveal Hemorrhage Due to Neovascular Age-related Macular Degeneration
| Pathology in the Fovea, N (%) | Month 6 (N = 15)b | Month 12 (N = 15) | Month 24 (N = 15) | Month 36 (N = 11) | Month 48 (N = 10) | Month 60 (N = 5) |
|---|---|---|---|---|---|---|
| SR hemorrhage | 3 (20.0) | 0 | 0 | 0 | 0 | 0 |
| Atrophy | 0 | 1 (6.7) | 2 (13.3) | 2 (18.2) | 2 (20.0) | 1 (20.0) |
| RPE changes | 3 (20.0) | 1 (6.7) | 0 | 0 | 0 | 0 |
| SRHRM | 5 (33.3) | 8 (53.3) | 8 (53.3) | 6 (54.5) | 6 (60.0) | 2 (40.0) |
| SR fibrosis,a N/n (%) | 5/5 (100) | 6/8 (75.0) | 7/8 (87.5) | 6/6 (100) | 6/6 (100) | 2/2 (100) |
| Sub-RPE HRM | 0 | 1 (6.7) | 1 (6.7) | 0 | 0 | 0 |
CNV = choroidal neovascularization; HRM = hyperreflective material; RPE = retinal pigment epithelium; SR = subretinal; SRHRM = subretinal hyperreflective material.
Subretinal fibrosis was defined as subretinal hyperreflective material with well-demarcated, sharp borders and loss or disruption of overlying ellipsoid zone and external limiting membrane on spectral-domain optical coherence tomography.
Images for 1 patient were unavailable for month 6 visit.
NUMBER OF INJECTIONS AND COMPLICATIONS:
All patients with SFH were treated with anti-VEGF injections. The median number of anti-VEGF injections was 5.0 (range, 4–6) between baseline and month 6; 2.0 between months 6 and 12 (range, 0–6); 4.0 (range, 0–10) between months 12 and 24; 1.0 (range, 0–11) between months 24 and 36; 0.5 (range, 0–12) between months 36 and 48; and 6.0 (range, 0–8) between months 48 and 60. There was a significant correlation between better VA at last follow-up visit and higher total number of anti-VEGF injections (r = 0.632, P = .009). At each of the yearly endpoints, the number of anti-VEGF injections received by the patients during the preceding 6 months (month 12 endpoint) to the preceding 12 months (all other endpoints) showed significant correlation with improvement in vision; month 12 (r = 0.592, P = .020); month 36 (r = 0.637, P = .035); month 48 (r = 0.677, P = .032); and month 60 (r = 0.892, P = .042). No significant ocular or systemic adverse events were noted with anti-VEGF injections during the course of follow-up.
DISCUSSION
COMPARED TO PRIOR INDUSTRY-SPONSORED CLINICAL trials investigating anti-VEGF agents for NVAMD, the inclusion of B50 patients in CATT has provided greater guidance for treatment of NVAMD patients with subretinal blood. There was no significant difference in visual or anatomic outcomes in the 84 B50 patients compared with 1101 patients in the other category suggesting that a larger area of subretinal blood within the CNV lesion complex does not adversely affect outcomes of anti-VEGF treatment. Since SFH was allowed in each group, and the thickness and size of SFH for each patient is unspecified, it is not possible to fully assess the impact of SFH thickness and size on outcomes.
There have been several case series reporting effects of anti-VEGF agents in patients with SFH from NVAMD.13-19 Three of these reports were on the same patient population, with 1 reporting outcomes at 6 months17 and the other 2 reporting outcomes in a subpopulation of the original study group at 1218 and 24 months.19 The baseline characteristics and outcomes for these studies are summarized in Supplemental Table 2 (Supplemental Material available at AJO.com). Follow-up was 12 months or less in all but 1 of the studies, in which it was at least 24 months. In 1 study, hemorrhage extended under the fovea in only 6 of 23 eyes; visual outcome was substantially worse in those eyes compared to those in which the fovea was not involved.16 Median or mean baseline and final VAs for the other 3 independent studies were 20/80 to 20/80, 20/320 to 20/250, and 20/300 to 20/125. The 3 reports on the same population had VA of 20/479 at baseline, 20/182 at 6 months, 20/132 at 12 months, and 20/214 at 24 months.17-19 None of these reports provided information regarding the thickness of hemorrhage in the fovea, but instead reported mean (± standard error of the mean, SEM) central foveal thickness at baseline and at various time points after baseline, which provide an indication of SFH thickness. The study with mean baseline VA of 20/80 had thin SFH in the range of 50–70 μm.13 In the other studies, it appears that average thickness of blood beneath the fovea ranged from roughly 100 μm to 400 μm, with large SEMs indicating large variability in hemorrhage thickness in the fovea. These large differences within and among studies makes interpretation difficult, but the aggregate analyses suggest that visual improvement can occur in some patients, but in most patients improvement occurs from a very poor baseline VA to poor VA at final follow-up ranging from 6 to 24 months. Baseline characteristics reported to correlate with poor visual outcome were longer duration of symptoms, greater extent of hemorrhage, and greater foveal thickness.17,18
In this study, we sought to carefully document all characteristics of SFH in each patient and assess visual and anatomic outcomes with anti-VEGF treatment over time. There was substantial variability in VA and SFH characteristics at baseline and a wide range of visual outcomes. This provided an opportunity to assess for correlations between baseline characteristics, treatment differences, and VA outcomes. Smaller area of subfoveal hemorrhage and greater frequency of anti-VEGF injections were the only variables that correlated with good visual outcome. Thickness of hemorrhage at the fovea did not correlate with visual outcome, but the number of subjects in the study and the range of SFH thickness among patients were small. Images from each patient were carefully studied, and it was noted that patients with large SFH that had good outcomes tended to have asymmetric hemorrhages with a relatively short minimum distance from the foveal center to the border of the hemorrhage and received a high number of anti-VEGF injections. Measurements of minimum distance from the foveal center to the hemorrhage border confirmed that a relatively short distance correlated with good visual outcome. Interestingly, published images from patients with thick SFHs that had good visual outcomes after anti-VEGF injections show asymmetric hemorrhages with a relatively short distance between the fovea and hemorrhage border (reference 15, Figure 115; reference 17, Figure 117).
The weaknesses of our study are that the number of subjects was small, the data were collected retrospectively, there was not a defined protocol for anti-VEGF treatment so that it varied considerably among patients, and there was no control group. Therefore, any conclusions or recommendations do not carry the same weight as those based on data from a prospective, controlled clinical trial. However, it is worthwhile to consider possible conclusions and recommendations that deserve further testing. These data support the hypothesis that SFH that exceeds a critical thickness exerts its damaging effects in part by impairing oxygen and nutrient diffusion from the choroid; and if the border of the hemorrhage is close enough to the fovea, sufficient diffusion from the choroid may occur so that central vision can be salvaged by frequent anti-VEGF injections. Among our patients who obtained a good visual outcome, the longest distance from the fovea to the border of SFH was 1591 μm for an SFH that elevated the fovea 286 μm. For other patients with a good visual outcome, the distance from fovea to SFH border and SFH thickness were 783 μm/570 μm, 999 μm/431 μm, 993 μm/258 μm, 324 μm/328 μm, and 351 μm/267 μm. These data begin to provide some parameters to guide clinicians trying to decide whether to displace SFH and treat with anti-VEGF injections or to treat with anti-VEGF injections alone.
There was correlation between good visual outcome and high frequency of VEGF injections. We cannot determine if high frequency helped to improve outcome or whether the treating physician had some reason to suspect a good visual prognosis in those patients and therefore treated more frequently. The latter appears to be a likely explanation, because all patients who received frequent injections had a good visual outcome. This confounding factor, along with the small data set, limits our ability to determine the upper limit of SFH thickness and distance from fovea to hemorrhage border for which SFH displacement should be combined with VEGF suppression. For now, we recommend that when a decision is made not to displace SFH in a patient with NVAMD, the patient be treated with monthly anti-VEGF injections for at least 12–24 months, and we encourage other investigators to publish SFH thickness and distance from fovea to hemorrhage border, along with visual outcomes for patients, to establish the limits of anti-VEGF treatment alone and establish better guidelines for displacement of SFH.
Supplementary Material
FUNDING/SUPPORT:
SUPPORTED BY BIOSTATISTICS CORE GRANT EY01765 TO THE WILMER EYE INSTITUTE.
Footnotes
Financial disclosures: Adrienne W. Scott is a paid advisor for Allergan. Peter A. Campochiaro is a paid advisor for Aerpio Therapeutics, Alimera, Allergan, Applied Genetic Technologies, AsclipiX, Genentech/Roche, Graybug, Rxi, Merck, Allegro, Intrexon, Novartis, Astellas, and Exonate. JHU receives grants on his behalf from Alimera, Allergan, Aerpio Therapeutics, Genentech/Roche, Genzyme, AsclipiX, Oxford Biomedica, Regeneron, Regenxbio, and Clearside. He has equity in Graybug and Allegro. The following authors have no disclosures: Saleema Kherani, Adam S. Wenick, Ingrid Zimmer-Galler, Christopher J. Brady, Akrit Sodhi, Catherine Meyerle, Sharon D. Solomon, Rimsha Shaukat, Roomasa Channa, Olukemi Adeyemo, James T. Handa, and Jiangxia Wang. The authors attest that they meet the current ICMJE criteria for authorship.
Supplemental Material available at AJO.com.
Contributor Information
SALEEMA KHERANI, The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland.
ADRIENNE W. SCOTT, The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland
ADAM S. WENICK, The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland
INGRID ZIMMER-GALLER, The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland.
CHRISTOPHER J. BRADY, The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland
AKRIT SODHI, The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland.
CATHERINE MEYERLE, The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland.
SHARON D. SOLOMON, The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland
RIMSHA SHAUKAT, The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland.
ROOMASA CHANNA, The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland.
OLUKEMI ADEYEMO, The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland.
JAMES T. HANDA, The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland
JIANGXIA WANG, Johns Hopkins Biostatistics Center, Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
PETER A. CAMPOCHIARO, The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland
REFERENCES
- 1.Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina 1996;16(3):183–189. [DOI] [PubMed] [Google Scholar]
- 2.Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica 1999;213(2):97–102. [DOI] [PubMed] [Google Scholar]
- 3.Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol 1990;109(1):33–37. [DOI] [PubMed] [Google Scholar]
- 4.Toth CA, Morse LS, Hjelmeland LM, Landers MB III. Fibrin directs early retinal damage after experimental subretinal hemorrhage. Arch Ophthalmol 1991;109(5):723–729. [DOI] [PubMed] [Google Scholar]
- 5.Submacular Surgery Trials (SST) Research Group. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: Ophthalmic Findings SST Report No. 13. Ophthlmology 2004;111(5):1993–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan AS, Johnson MW, Schneiderman TE, et al. Management of submacular hemorrhage with intravitreous tissue plaminogen activator injection and pneumatic displacement. Ophthalmology 1999;106(10):1900–1906. [DOI] [PubMed] [Google Scholar]
- 7.Haupert CL, McCuen BW II, Jaffe GJ, et al. Pars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid-gas exchange for displacement of thick submacular hemorrhage in age-related macular degeneration. Am J Ophthalmol 2001;131(2):208–215. [DOI] [PubMed] [Google Scholar]
- 8.Treumer F, Roider J, Hillenkamp J. Long-term outcome of subretinal coapplication of rtPA and bevacizumab followed by repeated intravitreal anti-VEGF injections for neovascular AMD with submacular haemorrhage. Br J Ophthalmol 2012;96(5):708–713. [DOI] [PubMed] [Google Scholar]
- 9.Chang W, Garg SJ, Maturi R, et al. Management of thick submacular hemorrhage with subretinal tissue plasminogen activator and pneumatic displacement for age-related macular degeneration. Am J Ophthalmol 2014;157(6):1250–1257. [DOI] [PubMed] [Google Scholar]
- 10.Altaweel MM, Daniel E, Martin DF, et al. Outcomes of eyes with lesions composed of >50% blood in the comparison of age-related macular degeneration treatments trials (CATT). Ophthalmology 2015;122(2):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oishi A, Hata M, Shimozono M, et al. The significance of external limiting membrane status for visual acuity in age-related macular degeneration. Am J Ophthalmol 2010;150(1):27–32. [DOI] [PubMed] [Google Scholar]
- 12.Keane PA, Patel PJ, Liakopoulos S, et al. Evaluation of age-related macular degeneration with optical coherence tomography. Surv Ophthalmol 2012;57(5):389–414. [DOI] [PubMed] [Google Scholar]
- 13.Stifter E, Michels S, Prager F, et al. Intravitreal bevacizumab therapy for neovascular age-related macular degeneration with large submacular hemorrhage. Am J Ophthalmol 2007;144(6):886–892. [DOI] [PubMed] [Google Scholar]
- 14.Chang M, Do DV, Bressler SB, et al. Prospective one-year study of ranibizumab for predominantly hemorrhagic choroidal neovascular lesions in age-related macular degeneration. Retina 2010;30(8):1171–1176. [DOI] [PubMed] [Google Scholar]
- 15.Shienbaum G, Garcia Filho CA, Flynn HW Jr, et al. Management of submacular hemorrhage secondary to neovascular age-related macular degeneration with anti–vascular endothelial growth factor monotherapy. Am J Ophthalmol 2013;155:1009–1013. [DOI] [PubMed] [Google Scholar]
- 16.Iacono P, Parodi MB, Introini U, et al. Intravitreal ranibizumab for choroidal neovascularization with large submacular hemorrhage in age-related macular degeneration. Retina 2014;34(2):281–287. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Chang YS, Kim JW, et al. Intravitreal anti-vascular endothelial growth factor for submacular hemorrhage from choroidal neovascularization. Ophthalmology 2014;212:926–935. [DOI] [PubMed] [Google Scholar]
- 18.Kim HS, Cho HJ, Yoo SG, et al. Intravitreal anti-vascular ednothelial growth factor monotherapy for large submacular hemorrhage secondary to neovascular age-related macular degeneration. Eye 2015;29(8):1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim KH, Kim JH, Chang YS, et al. Clinical outcomes of eyes with submacular hemorrhage secondary to age-related macular degeneration treated with anti-vascular endothelial growth factor. Korean J Ophthalmol 2015;29(5):315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.