Abstract
Purpose:
To determine whether scatter and grid laser photocoagulation (laser) adds benefit to ranibizumab injections in patients with macular edema from retinal vein occlusion (RVO) and to compare 0.5-mg with 2.0-mg ranibizumab.
Design:
Randomized, double-masked, controlled clinical trial.
Participants:
Thirty-nine patients with central RVO (CRVO) and 42 with branch RVO (BRVO).
Methods:
Subjects were randomized to 0.5 mg or 2.0 mg ranibizumab every 4 weeks for 24 weeks and re-randomized to pro re nata ranibizumab plus laser or ranibizumab alone.
Main Outcome Measures:
Mean change from baseline best-corrected visual acuity (BCVA) at week 24 for BCVA at weeks 48, 96, and 144 for second randomization.
Results:
Mean improvement from baseline BCVA at week 24 was 15.5 and 15.8 letters in the 0.5-mg and 2.0-mg CRVO groups, and 12.1 and 14.6 letters in the 0.5-mg and 2.0-mg BRVO groups. For CRVO, but not BRVO, there was significantly greater reduction from baseline mean central subfield thickness (CST) in the 2.0-mg versus 0.5-mg group (396.1 vs. 253.5 μm; P = 0.03). For the second randomization in CRVO patients, there was no significant difference from week 24 BCVA in the ranibizumab plus laser versus the ranibizumab only groups at week 48 (−3.3 vs. 0.0 letters), week 96 (+0.69 vs. −1.6 letters), or week 144 (+0.4 vs. −6.7 letters), and a significant increase from week 24 mean CST at week 48 (+94.7 vs. +15.2 μm; P = 0.05) but not weeks 96 or 144. For BRVO, there was a significant reduction from week 24 mean BCVA in ranibizumab plus laser versus ranibizumab at week 48 (−7.5 vs. +2.8; P < 0.01) and week 96 (−2.0 vs. +4.8; P < 0.03), but not week 144, and there were no differences in mean CST change from week 24 at weeks 48, 96, or 144. Laser failed to increase edema resolution or to reduce the ranibizumab injections between weeks 24 and 144.
Conclusions:
In patients with macular edema resulting from RVO, there was no short-term clinically significant benefit from monthly injections of 2.0-mg versus 0.5-mg ranibizumab injections and no long-term benefit in BCVA, resolution of edema, or number of ranibizumab injections obtained by addition of laser treatment to ranibizumab.
Central retinal vein occlusions (CRVOs) occur as a result of thrombosis of the main outflow vessel of the eye and result in retinal hemorrhages, cotton wool patches, and variable amounts of retinal nonperfusion throughout the retina. Branch retinal vein occlusions (BRVOs) occur as a result of thrombosis of a branch of the central retinal vein resulting in similar findings throughout the portion of the retina drained by the occluded vessel. The predominant cause of vision loss acutely in patients with CRVO or BRVO is macular edema. Although there is much that we do not understand regarding the pathogenesis of CRVOs and BRVOs, it is well established that vascular endothelial growth factor (VEGF) is an important contributor to macular edema.1–3 In fact, although suppression of VEGF is highly effective in the treatment of neovascular age-related macular degeneration (AMD)4,5 and diabetic macular edema,6–8 effectiveness is probably greatest in patients with macular edema resulting from retinal vein occlusion (RVO) early in the course after occlusion.1–3 In patients with CRVO, the mean improvement from baseline best-corrected visual acuity (BCVA) was 12.7 and 14.9 letters, respectively, after monthly injections of 0.3 mg or 0.5 mg ranibizumab for 6 months,3 and in 2 independent studies in which 2.0 mg aflibercept was injected monthly for 6 months, it was 17.3 and 18.0 letters, respectively.9,10 In patients with BRVO, the mean improvement from baseline BCVA was 16.6 and 18.3 letters, respectively, after monthly injections of 0.3 mg or 0.5 mg ranibizumab for 6 months2 and 17.0 letters after monthly injections of 2.0 mg aflibercept for 6 months.11 An important unanswered question is whether injections of 2.0 mg ranibizumab provide greater benefit than injection of 0.5 mg ranibizumab.
Initially, it was believed that intraocular injections of VEGF antagonists would be needed in patients with RVO for only a relatively short period until recanalization or collateral formation eliminated the need for treatment; however, long-term follow-up demonstrated that this was not the case.12–14 In the RETAIN study (Extended follow-up of patients with macular edema due to bRanch rETinal vein occlusion or centrAl retinal veIn occlusioN previously treated with intravitreal ranibizumab), with a mean follow up of 49 months, 14 of 32 CRVO patients (44%) and 17 of 34 BRVO patients (50%) had edema resolution and no longer required ranibizumab injections.14 The vein occlusion is merely the initiating event that causes retinal ischemia and high levels of VEGF, and the high levels of VEGF cause additional capillary closure and worsening ischemia, resulting in a positive feedback loop and disease worsening over time in some patients.15,16 Scatter photocoagulation reduces retinal ischemia, suggesting that it may provide a way to interrupt the positive feedback loop in patients with RVO and reduce the need for injections of a VEGF antagonist. In this study, we addressed 2 experimental questions: (1) whether injections of 2.0 mg ranibizumab provide greater short-term benefit than injections of 0.5 mg ranibizumab in patients with macular edema resulting from RVO; and (2) whether scatter photocoagulation promotes resolution of macular edema, reduces the need for VEGF antagonists, and improves outcomes in patients with RVO.
Methods
The Ranibizumab Dose Comparison (0.5 mg and 2.0 mg) and the Role of Laser in the Management of Retinal Vein Occlusion (RELATE; ClinicalTrials.gov identifier, NCT01003106) was an investigator-initiated, double-masked, randomized trial sponsored by Genentech, Inc. (South San Francisco, CA), was designed to compare the effects of monthly injections of 0.5 mg ranibizumab with monthly injections of 2.0 mg ranibizumab for 24 weeks in patients with macular edema resulting from RVO and also to determine whether scatter and grid laser photocoagulation (laser treatment) reduces the need for injections and improves long-term outcomes. To address these 2 independent study questions, there were 2 randomizations: 1 at baseline and 1 at week 24 (Fig 1, available at www.aaojournal.org). Eighty-one patients with RVO (39 with CRVO and 42 with BRVO) were enrolled at a single center (The Wilmer Eye Institute, Johns Hopkins Hospital, Baltimore, MD) and were randomized to receive injections of 0.5 mg or 2.0 mg ranibizumab at baseline, with primary end point at 24 weeks when patients were re-randomized to pro re nata (PRN) arms: ranibizumab plus laser or ranibizumab only for recurrent macular edema resulting from RVO. The study was conducted in accordance with the Declaration of Helsinki, applicable United States Food and Drug Administration regulations, and the Health Insurance Portability and Accountability Act. The study protocol was approved by the Johns Hopkins University Institutional Review Board before study initiation, and all participating patients provided informed consent.
Patient Eligibility and Exclusion Criteria
Eligible patients were aged 18 years or older, had BCVA of 20/40 to 20/200, and had central subfield thickness (CST) of 250 μm or more measured by time-domain optical coherence tomography (OCT) using a StratusOCT3 device (Carl Zeiss Meditec, Dublin, CA) in the study eye because of macular edema resulting from RVO and no other cause. Patients were excluded if they had an anti-VEGF injection within 1 month, an intraocular steroid injection within 4 months, or ocular surgery within 3 months.
Randomizations
The study was powered to detect a difference in BCVA of 5 letters or more between RVO patients treated with laser plus ranibizumab versus ranibizumab alone with a probability of 95% or more, and it was determined that the same number of patients would allow detection of a difference of 5 letters or more in BCVA between RVO patients treated with 2.0 mg versus 0.5 mg ranibizumab at 6 months with a probability of 95% or more. Patients were randomized to receive injections of 0.5 mg ranibizumab (22 with BRVO, 19 with CRVO) or 2.0 mg ranibizumab (20 with BRVO and 20 with CRVO) at baseline and weeks 4, 8, 12, 16, and 20. The random allocation sequence was generated using Windows (Microsoft, Inc., Redmond, WA) version 5.0 of block randomization software; the program generated block-stratified assignments with user-selected block size. The pseudorandom number generator is a linear congruent algorithm of Park and Miller with Bays-Durham shuffling. It has a period of more than 2 billion. For the first randomization, the block size was 2, 4, and 8. At week 24, the patients were re-randomized into the laser plus ranibizumab versus ranibizumab only group with a block size of 2, 2, and 2. Treatment groups were double masked until week 24, with patients, care providers, and those assessing outcomes all masked to ranibizumab dose. The only unmasked member of the study team was responsible for enrolling and assigning participants to interventions. After week 24, the patients and investigators were not masked with regard to the second randomization group, but visual acuity examiners remained masked.
Study Protocol
At each study visit, BCVA was measured by Early Treatment Diabetic Retinopathy Study protocol,17 and whereas time-domain OCT was used for eligibility to ensure comparability with prior studies, spectral-domain OCT was carried out with the Spectralis device (Heidelberg Engineering, Inc., Heidelberg, Germany) for outcome analysis. The primary outcome was the mean change from baseline BCVA letter score at week 24. Secondary outcomes were the percentage of patients with letter score improvement of 15 or more and mean improvement in CST.
At week 24, patients were re-randomized to ranibizumab plus laser or ranibizumab alone. In each group, patients received an injection of their originally assigned dose of ranibizumab at each study visit at which there was foveal thickening, intraretinal or subretinal fluid in the macula, or both thickening and fluid. Two years after study initiation, well after all patients had completed the primary end point, Genentech stopped production of the 2.0-mg dose of ranibizumab. At that point, patients in the laser plus ranibizumab or ranibizumab only groups who were receiving 2.0 mg ranibizumab PRN began receiving 1.0 mg ranibizumab.
If patients in the ranibizumab plus laser group required a ranibizumab injection on 2 consecutive visits, they also underwent laser treatment. For the first laser treatment, all areas of nonperfused retina identified by wide-angle fluorescein angiography were treated with 300-μm burns (using a wide-angle lens) 1 burn width apart, and 5 rows of burns were placed in the far periphery as close to the ora serrata as possible for 360° in patients with CRVO and 120° to 180° in patients with BRVO, depending on the area of vascular changes from the BRVO. At each subsequent visit after laser treatment, patients received a ranibizumab injection if there was foveal thickening, intraretinal or subretinal fluid, or both thickening and fluid. Starting 3 months after the first laser treatment, a second laser treatment was administered if a ranibizumab injection was required on 2 consecutive visits. All untreated retina outside a circle centered on the fovea, with the radius extending vertically to 5 disc diameters above or below the temporal arcade vessels. The affected hemisphere or a portion of it was treated in patients with BRVO, and patients with CRVO received treatment for 360° outside the posterior circle. In patients with BRVO, the goal was to treat the superotemporal quadrant for superior BRVOs and the inferotemporal quadrant for inferior BRVO sparing 5 disc diameters adjacent to the temporal arcade vessel, plus a wide margin beyond any visible vascular changes identified by wide-angle fluorescein angiography in the corresponding nasal quadrant. At each subsequent visit, patients in the ranibizumab plus laser group received an injection of ranibizumab if there was foveal thickening, intraretinal or subretinal fluid in the macula, or both thickening and fluid. Starting 3 months after the second laser treatment, if patients required ranibizumab injections on 2 consecutive visits, they underwent a third laser treatment to the untreated retina outside the arcade vessels that had been spared in the second laser treatment. Thus, in patients with CRVO, all peripheral retina outside the macula was treated, and in patients with BRVO, 120° to 180° of peripheral retina was treated with the goal of ensuring that all of the peripheral retina affected by the BRVO, including a healthy margin at the borders, had scatter photocoagulation. In addition, grid laser photocoagulation was administered to all areas of leakage in the macula outside the foveal avascular zone. Patients were seen every 4 weeks through week 96, after which patients were seen at least every 12 weeks, but as frequently as every 4 weeks if judged necessary.
Spectral-Domain Optical Coherence Tomography
Spectral-domain OCT scans were acquired at each visit using the following scan acquisition parameters: volume scan (20° × 20°; roughly 6 × 6 mm) with 25 B-scans in horizontal orientation spaced 240 μm apart, minimum automatic real-time mean of 9, and high speed (512 A-scans/B-scan). All scans after the day 1 visit were acquired with the TruTrack eye tracker (Heidelberg Engineering, Inc., Heidelberg, Germany) using the progression scan function. The spectral-domain OCT images were graded, with manual correction of algorithms and grid alignment if required, using the Heidelberg Eye Explorer version 1.6.4.0 with HRA/Spectralis Viewing Module version 5.3.2.0 at the Johns Hopkins University Retinal Imaging Research and Reading Center (Baltimore, MD).
Statistical Analysis
All statistical analyses were performed using IBM SPSS software version 19.0 (IBM Corp., Armonk, NY). Comparisons between groups were made using the independent samples t test for parametric variables and the Mann–Whitney U test for nonparametric variables. Categorical variables were compared using the chi-square test. Independent analysis was run for each end point at weeks 24, 48, 96, and 144. Analysis for the week 24 end point included data for all patients enrolled in the trial, with the last observation carried forward for patients who missed the week 24 visit. For the week 48, week 96, and week 144 end points, data were included only for patients who were still participating in the trial at least 3 months before the end point. If a patient exited the trial 3 months before the end point or missed the end point visit, the last observation within 3 months of the end point was carried forward. After the initial randomization, mean change from baseline BCVA and CST was compared between the 0.5-mg and 2.0-mg groups at week 24. After the second randomization, mean change from week 24 BCVA, CST, and number of ranibizumab injections were compared between the ranibizumab plus laser versus ranibizumab only groups at weeks 48, 96, and 144.
Results
Patient Demographics and Baseline Characteristics
A total of 81 patients were enrolled: 42 with BRVO, among whom 22 were randomized to receive 0.5 mg ranibizumab and 20 were randomized to receive 2.0 mg ranibizumab, and 39 with CRVO, among whom 19 were randomized to receive 0.5 mg ranibizumab and 20 were randomized to receive 2.0 mg ranibizumab. The patient populations differed from those of BRAVO (The Ranibizumab for the Treatment of Macular Edema following Branch Retinal Vein Occlusion Study) and CRUISE (The Ranibizumab for the Treatment of Macular Edema after Central Retinal Vein Occlusion Study) in that patients were not treatment naïve and were not excluded for duration of disease of 1 year or more. The mean duration of disease at baseline ranged from 12.7 to 18.4 months among the 4 treatment groups (Table 1). By chance, the mean age was 5.4 years more in the 2.0-mg CRVO group compared with the 0.5-mg CRVO group. The mean BCVA at baseline was balanced between the 0.5-mg and 2.0-mg groups and was approximately 20/80 to 20/100 in patients with BRV0 and 20/100 to 20/125 in patients with CRVO. The mean CST obtained by spectral-domain OCT was not significantly different between the 0.5-mg and 2.0-mg groups. The number of patients who had received prior intraocular anti-VEGF treatment or steroid injections was similar among the groups.
Table 1.
Patient Demographics and Baseline Characteristics for Initial 4 Randomization Groups
| Characteristic | Branch Retinal Vein Occlusion |
Central Retinal Vein Occlusion |
||||
|---|---|---|---|---|---|---|
| 0.5 mg RBZ (n = 22) | 2.0 mg RBZ (n = 20) | P Value | 0.5 mg RBZ (n = 19) | 2.0 mg RBZ (n = 20) | P Value | |
| Mean age, yrs | 66.6 ± 2.2 | 69.3 ± 1.8 | 0.35 | 59.0 ± 2.9 | 64.4 ± 2.9 | 0.05 |
| Female sex, no. (%) | 13 (59.1) | 7 (35.0) | 0.21 | 9 (47.4) | 9 (45) | 1.0 |
| Race, no. (%) | ||||||
| White | 14 (63.6) | 8 (40.0) | 0.31 | 12 (63.2) | 13 (65) | 0.55 |
| Black | 6 (27.3) | 9 (45.0) | (15.8) | 5 (25) | ||
| Other | 2 (9.1) | 3 (15.0) | 4 (21.1) | 2 (10) | ||
| Disease duration, mos | 12.7 ± 3.4 | 18.4 ± 5.4 | 0.32 | 15.7 ± 3.8 | 15.0 ± 2.5 | 0.68 |
| Prior anti-VEGF treatment, no. of patients | 8 | 8 | 1.00 | 9 | 9 | 1.00 |
| Prior steroid treatment, no. of patients | 3 | 4 | 0.89 | 4 | 3 | 0.70 |
| BCVA letter score (Snellen equivalent) | 54.3 ± 3.2 (20/80) | 48.9 ± 3.6 (20/100) | 0.27 | 47.1 ± 3.7 (20/100) | 46.7 ± 3.0 (20/125) | 0.93 |
| CST, μm | 539.2 ± 36.5 | 624.0 ± 46.1 | 0.16 | 608.6 ± 40.1 | 672.8 ± 46.5 | 0.33 |
BCVA = best-corrected visual acuity; CST = central subfield thickness; RBZ = ranibizumab; VEGF = vascular endothelial growth factor.
Data are mean ± SD unless otherwise indicated.
Patient Disposition Regarding First Randomization Groups
Three patients with BRVO (all in the 0.5-mg ranibizumab group) and 1 patient with CRVO (2.0-mg ranibizumab group) withdrew consent and exited the study before the month 6 primary end point (Table 2). The change from baseline in Early Treatment Diabetic Retinopathy Study letter score at last visit was carried forward to week 24 and was 3, 11, and 9 letters for the 3 BRVO patients and 20 letters for the CRVO patient. These patients withdrew consent for personal reasons that made it difficult to continue follow-up, not because they were dissatisfied with treatment response.
Table 2.
Summary of Patients Who Discontinued the Trial
| Patient Identification | Dose (mg) |
Laser Plus RBZ vs RBZ Alone | Last Visit |
Months Since Last Injection | Edema Status | Reason for Discontinuation | |||
|---|---|---|---|---|---|---|---|---|---|
| Week | Best-Corrected Visual Acuity * |
Change in Best-Corrected Visual Acuity
(No. of Letters) |
Central Subfield Thickness
(μm) |
||||||
| BV-004 | 0.5 | Laser + RBZ | 60 | 43 | 7 | 156 | 9 | Resolved | Stable |
| BV-011 | 2.0 | Laser + RBZ | 48 | 38 | 4 | 262 | 1 | Unresolved | Withdrew consent |
| BV-012 | 0.5 | RBZ | 112 | 69 | 40 | 241 | 9 | Resolved | Withdrew consent |
| BV-016 | 0.5 | — | 20 | 53 | 3 | 417 | 0 | Unresolved | Withdrew consent |
| BV-021 | 2.0 | Laser + RBZ | 48 | 65 | 10 | 321 | 0 | Unresolved | Withdrew consent |
| BV-032 | 0.5 | — | 16 | 41 | 11 | 530 | 0 | Unresolved | Withdrew consent |
| BV-033 | 2.0 | RBZ | 32 | 49 | 7 | 488 | 0 | Unresolved | Diagnosed with brain tumor |
| BV-035 | 0.5 | — | 20 | 82 | 9 | 247 | 2 | Unresolved | Withdrew consent |
| BV-037 | 2.0 | RBZ | 108 | 68 | 19 | 251 | 2 | Unresolved | Transportation difficulties |
| CV-004 | 0.5 | RBZ | 48 | 81 | 13 | 268 | 6 | Resolved | Withdrew consent |
| CV-007 | 2.0 | Laser + RBZ | 132 | 55 | 0 | 231 | 1 | Unresolved | Transportation difficulties |
| CV-009 | 2.0 | RBZ | 132 | 69 | −4 | 280 | 21 | Resolved | Patient was sick |
| CV-011 | 2.0 | Laser + RBZ | 96 | 74 | 29 | 263 | 0 | Unresolved | Withdrew consent |
| CV-016 | 0.5 | Laser + RBZ | 40 | 71 | 17 | 440 | 0 | Unresolved | Withdrew consent |
| CV-017 | 2.0 | RBZ | 84 | 26 | −12 | 1200 | 0 | Unresolved | Nonresponsive to anti-VEGF therapy |
| CV-021 | 2.0 | RBZ | 92 | 65 | 12 | 167 | 1 | Unresolved | Withdrew consent |
| CV-024 | 2.0 | — | 12 | 69 | 20 | 271 | 0 | Unresolved | Withdrew consent |
| CV-029 | 2.0 | RBZ | 32 | 37 | −10 | 258 | 0 | Unresolved | Withdrew consent |
| CV-030 | 0.5 | Laser + RBZ | 76 | 50 | 5 | 239 | 2 | Unresolved | Died |
| CV-040 | 2.0 | RBZ | 72 | 77 | 42 | 279 | 1 | Unresolved | Withdrew consent |
RBZ = ranibizumab; VEGF = vascular endothelial growth factor.
Dash (–) indicates that the patient exited the trial prior to the second randomization.
Early Treatment Diabetic Retinopathy Study score.
Comparison of Visual Outcomes in Patients Treated with 0.5 mg versus 2.0 mg Ranibizumab
Mean BCVA improved rapidly between baseline and week 4 and then improved gradually thereafter in a manner that was very similar in 0.5-mg versus 2.0-mg ranibizumab patients with BRVO (Fig 2A) or CRVO (Fig 2B). The primary outcome, the mean change in BCVA between baseline and week 24, was 12.1 ± 2.9 letters in the 0.5-mg BRVO group versus 14.6 ± 2.3 letters in the 2.0-mg BRVO group (P = 0.31) and 15.5 ± 2.4 letters in the 0.5-mg CRVO group versus 15.8 ± 2.4 letters in the 2.0-mg CRVO group (P = 0.94; Table 3). The percentage of patients who gained 15 letters or more in BCVA was roughly 50% to 60% in all of the groups except the 0.5-mg BRVO group, in which it was 27.3%; this could be in part because the mean baseline BCVA letter score was slightly better in the 0.5-mg BRVO group compared with the 2.0-mg BRVO group (54.3 vs. 48.9 letters) and partly because of chance.
Figure 2.
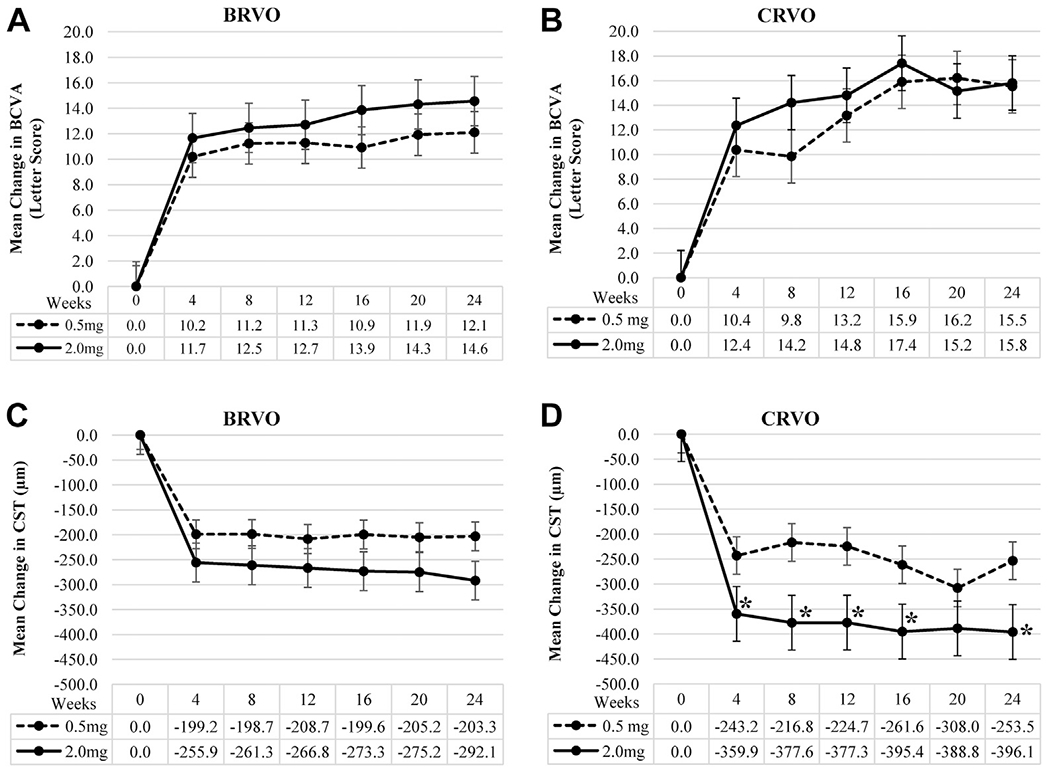
Graphs comparing visual and anatomic outcomes after injections of 2.0 mg versus 0.5 mg ranibizumab in patients with macular edema resulting from retinal vein occlusion. Patients with macular edema resulting from (A and C) branch retinal vein occlusion (BRVO) or (B and D) central retinal vein occlusion (CRVO) were randomized to receive an injection of 2.0 or 0.5 mg ranibizumab every 4 weeks. There was no significant difference between the 2.0-mg and 0.5-mg groups in mean change from baseline best-corrected visual acuity (BCVA) measured in Early Treatment Diabetic Retinopathy Study letter score at any time point through the week 24 end point in patients with (A) BRVO or (B) CRVO. C, In patients with BRVO, there was no significant difference between the 2.0-mg and 0.5-mg groups in mean change from baseline central subfield thickness (CST) measured by spectral-domain optical coherence tomography at any time point through the week 24. D, However, patients with CRVO who received injection of 2.0 mg ranibizumab had a significantly greater reduction in mean CST at several time points (*P = 0.03, independent samples t test), including week 24. Error bars show the standard error of the mean.
Table 3.
Comparison of Visual and Anatomic Outcomes in Patients Treated with 0.5 mg versus 2.0 mg Ranibizumab
| Outcome | Branch Retinal Vein Occlusion |
Central Retinal Vein Occlusion |
||||
|---|---|---|---|---|---|---|
|
0.5 mg RBZ (n = 22) |
2.0 mg RBZ (n = 20) |
P Value |
0.5 mg RBZ (n = 19) |
2.0 mg RBZ (n = 20) |
P Value | |
| BCVA at baseline (letter score) | 54.3±3.2 | 48.9±3.6 | 0.27 | 47.1±3.7 | 46.7±3.0 | 0.78 |
| BCVA at 24 wks (letter score) | 66.4±3.4 | 63.4±2.7 | 0.49 | 62.6±4.2 | 62.5±3.5 | 0.97 |
| Change from baseline BCVA | 12.1±2.9 | 14.6±2.3 | 0.31 | 15.5±2.4 | 15.8±2.4 | 0.94 |
| Percentage of patients gaining in letter score (no. of letters) | ||||||
| ≥15 | 27.3 | 50.0 | 0.23 | 52.6 | 60.0 | 0.89 |
| ≥10 | 54.5 | 70.0 | 0.48 | 84.2 | 75.0 | 0.70 |
| ≥5 | 63.6 | 90.0 | 0.07 | 84.2 | 85.0 | 1.0 |
| CST at baseline (μm) | 539.2±36.5 | 624.0±46.1 | 0.16 | 608.6±40.1 | 672.8±46.5 | 0.33 |
| CST at 24 wks (μm) | 336.0±29.1 | 331.9±20.0 | 0.67 | 355.1±34.4 | 276.7±12.4 | 0.14 |
| Change from baseline CST | −203.3±41.0 | −292.1±51.7 | 0.19 | −253.5±43.0 | −396.1±48.1 | 0.03 |
| Percentage (no.) of patients with CST ≤320 μm at 24 wks | 68.2 (15) | 55.0 (11) | 0.58 | 52.6 (10) | 90.0 (18) | 0.03 |
BCVA = best-corrected visual acuity; CST = central subfield thickness; RBZ = Ranibizumab.
Comparison of Anatomic Outcomes in Patients Treated with 0.5 mg versus 2.0 mg Ranibizumab
In patients with BRVO, the 0.5-mg ranibizumab group showed a rapid reduction in mean CST between baseline and week 4, with little change thereafter, and although the 2.0-mg ranibizumab group showed a slightly greater initial reduction and further reduction over time, these differences were not statistically significant (Fig 2C). The mean reduction in CST between baseline and week 24 was 203.3 ± 41.0 μm in the 0.5-mg group versus 292.1 ± 51.7 μm in the 2.0-mg group (P = 0.19; Table 3). In patients with CRVO, the initial reduction in mean CST was greater in patients treated with 2.0 mg ranibizumab compared with those treated with 0.5 mg ranibizumab, and a statistically significant difference remained at all time points except week 20 (Fig 2D). At week 24, the mean improvement in CST was 253.5 ± 43.0 μm in the 0.5 mg ranibizumab group versus 396.1 ± 48.1 μm in the 2.0-mg ranibizumab group (P = 0.03; Table 3). The percentage of patients with week 24 CST of 320 μm or less was 68.2% and 55.0% in the 0.5-mg and 2.0-mg BRVO groups, respectively, and 52.6% and 90% in the 0.5-mg and 2.0-mg CRVO groups, respectively (P = 0.03).
Patient Demographics and Disposition Regarding Second Randomization Groups
At week 24, patients were re-randomized to 2 PRN treatment groups, ranibizumab plus laser or ranibizumab only for recurrent edema. The new randomization groups were well balanced with regard to 0.5-mg versus 2.0-mg ranibizumab treatment and duration of disease (Table 4). The week 24 BCVA letter score was well balanced between ranibizumab plus laser and ranibizumab only groups in patients with CRVO but was slightly better in the ranibizumab plus laser versus ranibizumab only group in patients with BRVO (69.9 vs. 60.8 letters; P = 0.04; Table 4). Among patients with CRVO, the ranibizumab group had a slightly higher mean age than the ranibizumab plus laser group (66.2 vs. 56.3 years; P = 0.02).
Table 4.
Patient Demographics and Characteristics at 24 Weeks for Second 4 Randomization Groups
| Characteristic | Branch Retinal Vein Occlusion |
Central Retinal Vein Occlusion |
||||
|---|---|---|---|---|---|---|
|
Ranibizumab plus Laser (n = 20) |
Ranibizumab (n = 19) |
P Value |
Ranibizumab plus Laser (n = 18) |
Ranibizumab (n = 20) |
P Value | |
| Mean age (yrs) | 69.8 ± 2.2 | 66.3 ± 2.1 | 0.33 | 56.3 ± 3.4 | 66.2 ± 2.3 | 0.02 |
| No. of women (%) | 8 (40.0) | 9 (47.4) | 0.89 | 6.0 (33.3) | 11.0 (55.0) | 0.31 |
| Disease duration (mos) | 16.8 ± 5.5 | 12.2 ± 2.9 | 0.99 | 15.3 ± 3.1 | 16.1 ± 3.3 | 0.98 |
| 2.0-mg Ranibizumab group (no. of patients) | 10 | 9 | 9 | 10 | ||
| 0.5-mg Ranibizumab group (no. of patients) | 10 | 10 | 9 | 10 | ||
| BCVA letter score (Snellen equivalent) | 69.9 ± 3.1 (20/40) | 60.8 ± 2.9 (20/63) | 0.04 | 63.1 ± 3.3 (20/50) | 61.7 ± 4.4 (20/50) | 0.92 |
| CST (μm) | 311.4 ± 18.7 | 347.7 ± 31.6 | 0.82 | 323.0 ± 19.6 | 309.8 ± 32.5 | 0.16 |
BCVA = best-corrected visual acuity; CST = central subfield thickness.
Only 1 BRVO patient who underwent the second randomization (ranibizumab group) failed to remain in the study through week 48. This patient (BV33; Table 2) had improvement in BCVA of 7 letters from week 24 with persistent or recurrent edema and was diagnosed with a brain tumor, requiring withdrawal after the week 32 visit. Two CRVO patients who underwent the second randomization exited the trial before week 48: 1 in the ranibizumab only group at week 32, with a 10-letter loss in BCVA between weeks 24 and 32, and 1 in the ranibizumab plus laser group at week 40, at which the patient had a gain in BCVA of 17 letters (Table 2).
Three patients with BRVO in the ranibizumab plus laser group exited the trial between weeks 48 and 92. Two patients exited at week 48 with unresolved edema, but improvements from week 24 BCVA of 10 and 4 letters. One patient exited at week 60 with resolved edema and a 7-letter gain in BCVA from week 24. Five patients with CRVO exited the trial between weeks 48 and 92. Four of the patients were in the ranibizumab only group and showed improvements in BCVA from week 24 of 42, 13, 12, and −12 letters, and thus on average were doing well (Table 2). A patient in the ranibizumab plus laser group died after the week 76 visit, when there was no edema and an improvement from week 24 BCVA of 5 letters.
One BRVO patient in the ranibizumab group exited the trial at week 112 with resolved edema and improvement from week 24 BCVA of 40 letters. Three patients with CRVO exited between weeks 96 and 144: 2 in the ranibizumab plus laser group who had improvements from week 24 BCVA of 29 and 0 letters and 1 patient in the ranibizumab group who had a change from week 24 BCVA of −4 letters when exiting at week 132 (Table 2). Throughout the entire 2.5-year follow-up period after the second randomization, there were 7 dropouts from the ranibizumab plus laser group and 8 from the ranibizumab only group. These patients had a mean improvement in BCVA from baseline to last follow-up of 10.3 and 11.0 letters, respectively. This suggests that dropouts had little effect on the results of the study.
Effect of Scatter and Grid Laser Photocoagulation on Visual and Anatomic Outcomes
Between weeks 24 and 28 in patients with BRVO, there was a small decrease in mean BCVA in both the ranibizumab only and ranibizumab plus laser groups, but although the ranibizumab only group recovered quickly and showed small improvements compared with week 24 at most time points through week 144, the ranibizumab plus laser group showed a small decline in mean BCVA compared with week 24 at each time point through week 144 (Fig 3A). The mean change from week 24 BCVA in the ranibizumab plus laser group versus the ranibizumab only group for patients who remained in the study for the following time points was −7.5 versus +2.8 letters for week 48 (P < 0.001), −2.0 versus +4.8 letters for week 96 (P = 0.03), and −2.6 versus +3.1 letters for week 144 (P = 0.19; Table 5). Among patients with CRVO, the ranibizumab plus laser group showed an initial decline in mean BCVA compared with week 24 and remained depressed compared with the ranibizumab group through week 44, but thereafter, there was little difference between the 2 groups (Fig 3B). The mean change from week 24 BCVA in the ranibizumab plus laser group versus the ranibizumab only group for patients who remained in the study for the following time points was −3.3 versus 0.0 letters for week 48 (P = 0.34), +0.69 versus −1.6 letters for week 96 (P = 0.60), and +0.4 versus −6.7 letters for week 144 (P = 0.22; Table 5). Using a last observation carried forward analysis, the mean change from week 24 BCVA letter score for the 2 groups of BRVO and CRVO patients at each of the 3 time points was very similar (Table 6, available at www.aaojournal.org), suggesting that there was not a major impact on the results as a result of patient dropout.
Figure 3.
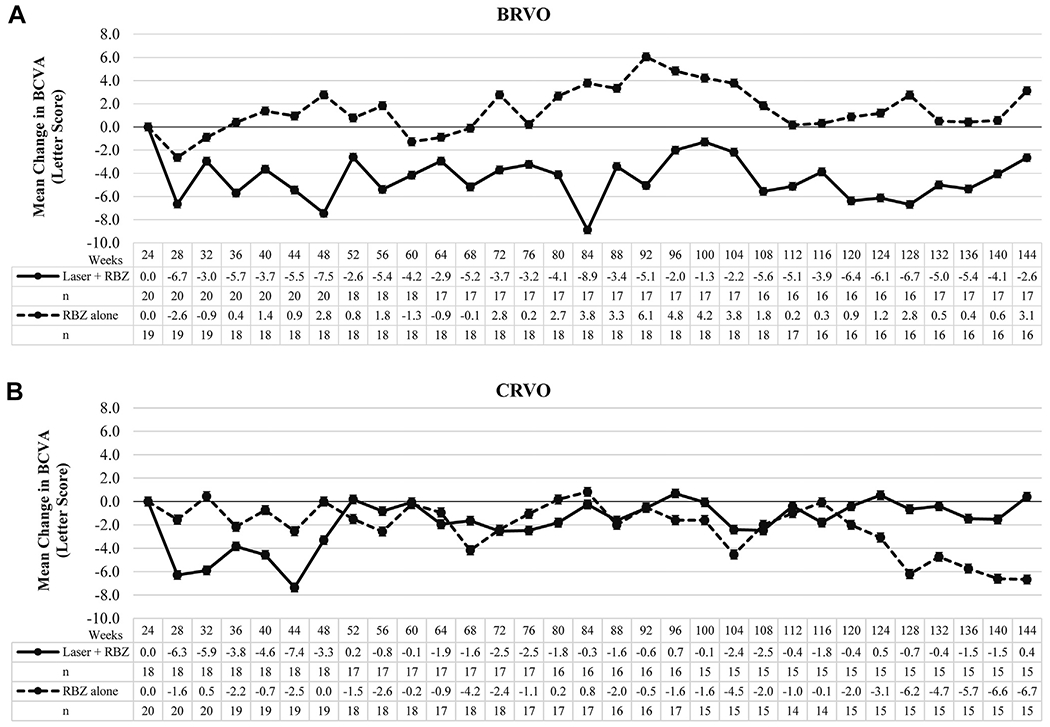
Graphs showing long-term visual outcomes in patients with macular edema resulting from retinal vein occlusion treated with a combination of ranibizumab and scatter and grid laser photocoagulation versus ranibizumab alone. Patients were re-randomized at week 24 to pro re nata (PRN) ranibizumab plus scatter and grid laser photocoagulation (laser + RBZ) or PRN ranibizumab alone RBZ alone. The graphs show the mean (±standard error of the mean) change from week 24 in best-corrected visual acuity (BCVA) in Early Treatment Diabetic Retinopathy Study letter score at all time points through week 144 in patients with (A) macular edema resulting from branch retinal vein occlusion (BRVO) or (B) central retinal vein occlusion (CRVO). There was no significant difference between laser plus ranibizumab and ranibizumab only groups at week 144 in patients with BRVO or CRVO.
Table 5.
Comparison of Visual Outcomes in Patients Treated with Laser plus Ranibizumab versus Ranibizumab Alone
| Outcome | Branch Retinal Vein Occlusion |
Central Retinal Vein Occlusion |
||||
|---|---|---|---|---|---|---|
| Ranibizumab + Laser | Ranibizumab Alone | P Value | Ranibizumab + Laser | Ranibizumab Alone | P Value | |
| Week 48 completers | ||||||
| Mean BCVA (letter score) | (n = 20) | (n = 18) | (n = 18) | (n = 19) | ||
| Week 24 | 69.9 ± 3.1 | 61.3 ± 3.0 | 0.06 | 63.1 ± 3.3 | 62.8 ± 4.5 | 0.87 |
| Week 48 | 62.4 ± 2.7 | 64.1 ± 3.4 | 0.70 | 59.8 ± 4.1 | 62.8 ± 4.7 | 0.31 |
| Change from baseline to week 48 | −7.5 ± 1.9 | 2.8 ± 2.1 | <0.001 | −3.3 ± 2.7 | 0.0 ± 2.0 | 0.34 |
| Week 96 completers | ||||||
| Mean BCVA (letter score) | (n = 17) | (n = 18) | (n = 16) | (n = 17) | ||
| Week 24 | 73.0 ± 2.9 | 61.3 ± 3.0 | 0.01 | 63.1 ± 3.4 | 60.8 ± 4.8 | 0.71 |
| Week 96 | 71.0 ± 2.9 | 66.1 ± 2.8 | 0.24 | 63.8 ± 3.7 | 59.2 ± 5.0 | 0.89 |
| Change from baseline to week 96 | −2.0 ± 1.9 | 4.8 ± 2.8 | 0.03 | 0.69 ± 3.1 | −1.6 ± 3.1 | 0.60 |
| Week 144 completers | ||||||
| Mean BCVA (letter score) | (n = 17) | (n = 16) | (n = 15) | (n = 15) | ||
| Week 24 | 73.0 ± 2.9 | 61.0 ± 3.3 | 0.01 | 62.5 ± 3.6 | 61.7 ± 5.3 | 0.90 |
| Week 144 | 70.4 ± 2.9 | 64.1 ± 3.9 | 0.21 | 62.9 ± 4.3 | 55.1 ± 6.5 | 0.69 |
| Change from baseline to week 144 | −2.6 ± 2.2 | 3.1 ± 3.3 | 0.19 | 0.4 ± 4.3 | −6.7 ± 3.7 | 0.22 |
BCVA = best-corrected visual acuity.
The initial decline in mean BCVA in the ranibizumab plus laser group compared with the ranibizumab only group was accompanied by an initial increase in mean CST, but there was little difference between the groups after week 48 (Fig 4). The increase from week 24 mean CST was significantly greater for the CRVO ranibizumab plus laser group compared with the ranibizumab only group, but there were no significant differences at other time points and no significant differences at any time points between the BRVO groups (Table 7). Differences in mean CST between groups was similar when data were analyzed with the last observation carried forward method, suggesting that patient dropout did not have a major impact on this parameter (Table 8, available at www.aaojournal.org). Patients were considered to have resolution of macular edema if they had no intraretinal or subretinal fluid in the macula and no thickening for at least 6 months before exit from the trial, so that a ranibizumab injection was not required for at least 6 months. Using these criteria, 13 BRVO patients, 7 (35.0%) in the ranibizumab plus laser group and 6 (31.6%) in the ranibizumab group, had resolution of edema, and 4 patients with CRVO, 2 (11.1%) in the ranibizumab plus laser group and 2 (10.0%) in the ranibizumab group, had resolution of edema. Scatter photocoagulation failed to reduce the number of ranibizumab injections needed, and in fact, the mean number of ranibizumab injections between weeks 24 and 144 was significantly greater in the ranibizumab plus laser group compared with the ranibizumab only group in patients with CRVO (Table 9).
Figure 4.
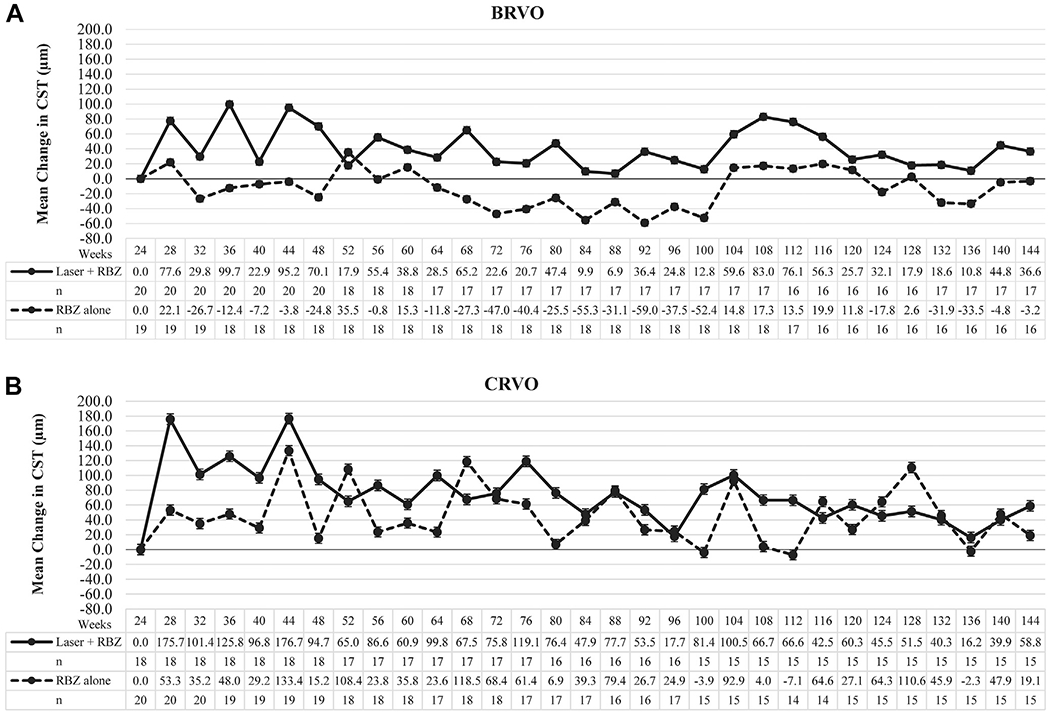
Graphs showing long-term anatomic outcomes in patients with macular edema resulting from retinal vein occlusion treated with a combination of ranibizumab plus scatter and grid laser photocoagulation versus ranibizumab alone. The graphs show the mean (±standard error of the mean) change from week 24 central subfield thickness (CST) at all time points after re-randomized to pro re nata (PRN) ranibizumab plus scatter and grid laser photocoagulation (laser + RBZ) or PRN ranibizumab alone (RBZ alone). There was no significant difference between the laser + ranibizumab and ranibizumab only groups at week 144 in patients with (A) BRVO or (B) CRVO.
Table 7.
Comparison of Anatomic Outcomes in Patients Treated with Laser plus Ranibizumab versus Ranibizumab Alone
| Outcome | Branch Retinal Vein Occlusion |
Central Retinal Vein Occlusion |
||||
|---|---|---|---|---|---|---|
| Ranibizumab + Laser | Ranibizumab Alone | P Value | Ranibizumab + Laser | Ranibizumab Alone | P Value | |
| Week 48 completers | ||||||
| Mean CST (μm) | (n = 20) | (n = 18) | (n = 18) | (n = 19) | ||
| Week 24 | 311.4 ± 18.7 | 335.8 ± 31.0 | 0.95 | 323.0 ± 19.6 | 315.8 ± 33.7 | 0.24 |
| Week 48 | 381.5 ± 34.8 | 311.0 ± 22.2 | 0.10 | 417.7 ± 33.5 | 331.1 ± 35.4 | 0.02 |
| Change from baseline to week 48 | 70.1 ± 27.9 | −24.8 ± 40.9 | 0.19 | 94.7 ± 27.4 | 15.2 ± 38.8 | 0.05 |
| Percentage (no.) of patients with CST ≤320 μm at 48 wks | 45.0 (9.0) | 66.7 (12.0) | 0.31 | 38.9 (7.0) | 68.4 (13.0) | 0.14 |
| Week 96 completers | ||||||
| Mean CST (μm) | (n = 17) | (n = 18) | (n = 16) | (n = 17) | ||
| Week 24 | 322.8 ± 19.7 | 335.8 ± 31.0 | 0.64 | 325.6 ± 21.6 | 325.5 ± 37.0 | 0.46 |
| Week 96 | 347.6 ± 29.4 | 298.3 ± 29.0 | 0.11 | 343.3 ± 23.8 | 350.4 ± 60.0 | 0.37 |
| Change from baseline to week 96 | 24.8 ± 31.0 | −37.5 ± 43.7 | 0.41 | 17.7 ± 17.3 | 24.9 ± 51.8 | 0.26 |
| Percentage (no.) of patients with CST ≤320 μm at 96 wks | 52.9 (9.0) | 83.3 (15.0) | 0.12 | 50.0 (8.0) | 70.6 (12.0) | 0.39 |
| Week 144 completers | ||||||
| Mean CST (μm) | (n = 17) | (n = 16) | (n = 15) | (n = 15) | ||
| Week 24 | 322.8 ± 19.7 | 343.3 ± 34.2 | 0.75 | 330.8 ± 22.4 | 327.5 ± 40.7 | 0.38 |
| Week 144 | 359.5 ± 30.1 | 340.1 ± 41.0 | 0.22 | 389.6 ± 36.3 | 346.6 ± 40.1 | 0.43 |
| Change from baseline to week 144 | 36.6 ± 29.2 | −3.2 ± 54.7 | 0.94 | 58.8 ± 38.5 | 19.1 ± 50.3 | 0.54 |
| Percentage (no.) of patients with CST ≤320 μm at 144 wks | 58.9 (10.0) | 75.0 (12.0) | 0.54 | 40.0 (6.0) | 60.0 (9.0) | 0.47 |
CST = central subfield thickness.
Table 9.
Number of Ranibizumab Injections Received by Laser plus Ranibizumab versus Ranibizumab Alone Groups
| No. of Injections By Time Point | Branch Retinal Vein Occlusion |
Central Retinal Vein Occlusion |
||||
|---|---|---|---|---|---|---|
| Ranibizumab ± Laser | Ranibizumab Alone | P Value | Ranibizumab ± Laser | Ranibizumab Alone | P Value | |
| Between 24 and 48 wks | 3.8 ± 0.4 (n = 20) | 3.7 ± 0.5 (n = 18) | 0.96 | 4.0 ± 0.3 (n = 18) | 3.0 ± 0.4 (n = 19) | 0.07 |
| Between 48 and 96 wks | 7.4 ± 0.9 (n = 17) | 6.9 ± 0.7 (n = 18) | 0.73 | 7.8 ± 0.9 (n = 16) | 5.5 ± 0.7 (n = 17) | 0.03 |
| Between 96 and 144 wks | 5.2 ± 1.1 (n = 17) | 4.0 ± 0.8 (n = 16) | 0.41 | 5.7 ± 1.0 (n = 15) | 3.9 ± 0.7 (n = 15) | 0.15 |
| Total between 24 and 144 wks | 15.6 ± 2.0 (n = 17) | 14.9 ± 1.8 (n = 16) | 0.79 | 17.9 ± 2.1 (n = 15) | 12.4 ± 1.6 (n = 15) | 0.05 |
Discussion
The first experimental question addressed in this study was whether injections of 2.0 mg ranibizumab provide greater short-term benefit than injections of 0.5 mg in patients with macular edema resulting from RVO. The answer to this question is that in patients with BRVO or CRVO with mean disease duration of 12 to 18 months who have recurrent edema despite many prior intraocular anti-VEGF or steroid injections, or both, visual outcomes are no better after 24 weeks of injections of 2.0 mg ranibizumab every 4 weeks compared with injections every 4 weeks of 0.5 mg ranibizumab. This is similar to visual outcome results in patients with neovascular AMD, in whom injections of 2.0 mg ranibizumab provided no advantage over injections of 0.5 mg ranibizumab.18,19 Patients with neovascular AMD treated with 2.0 mg ranibizumab also had no anatomic benefits compared with those treated with 0.5 mg ranibizumab; however, among patients with CRVO, injections of 2.0 mg ranibizumab caused a significantly greater reduction in mean CST than did injections of 0.5 mg ranibizumab, and 90% of the 2.0 mg ranibizumab group had CST of 320 μm or less compared with 52.6% of the 0.5-mg group (P = 0.03). Among patients with BRVO, there was a similar trend, but no statistically significant differences. On average, intraocular VEGF levels are higher in patients with CRVO than in patients with BRVO,20 so that 0.5 mg ranibizumab may be sufficient to neutralize VEGF for 1 month in most patients with BRVO, but not in a substantial number with CRVO. It is more difficult to document an average difference in VEGF levels between CRVO and neovascular AMD, but some patients with CRVO have particularly high levels that could account for the relative difference in anatomic benefit between neovascular AMD and CRVO patients treated with 2.0 mg ranibizumab. Despite inferior edema reduction in eyes with CRVO injected with 0.5 mg ranibizumab compared with those injected with 2.0 mg ranibizumab, injections with 0.5 mg ranibizumab were sufficient to achieve similar functional improvement. It is not clear whether similar outcomes would be maintained in the long term because patients with CRVO who have persistent or recurrent edema lose vision over time.14
The second experimental question was whether scatter photocoagulation treatment promotes resolution of macular edema, reduces the need for VEGF antagonists, and improves outcomes in patients with RVO. Among the population of patients studied, the answer was no. In fact, patients treated with scatter photocoagulation experienced a transient increase in mean CST and reduction in mean BCVA. This lessened over time, so that there was no significant difference in mean change from week 24 CST or BCVA in ranibizumab plus laser groups versus ranibizumab only groups 2.5 years after randomization, but there was clearly no benefit to BCVA resulting from scatter photocoagulation, to the number of patients who had resolution of edema and no longer needed injections, or to the mean number of PRN ranibizumab injections that were given to control edema. Among CRVO patients, the mean number of ranibizumab injections was significantly greater in the ranibizumab plus laser group than in the ranibizumab only group. Because there were no major differences in outcomes, and because when differences occurred, they favored ranibizumab only rather than ranibizumab plus laser, it is unlikely that a larger study would demonstrate that scatter photocoagulation provides benefit.
Scatter photocoagulation can be delivered in many different ways, and it is useful to consider whether a modified approach from that used in this study could have given a different result. In this study, patients randomized to ranibizumab plus laser were treated in a stepwise fashion, treating the far periphery and areas of retinal nonperfusion first, followed by the midperiphery, and finally treating more posteriorly so that all retina outside the arcade vessels was treated with dense laser (1 burn width between burns) and grid laser was administered to areas of leakage in the macula outside the foveal avascular zone. This stepwise approach was carried out to try to identify the minimal amount of laser needed to control edema and to reduce or eliminate ranibizumab injections while minimizing the chance of exacerbating the edema. There is no reason to believe that treatment of the entire retina in one session or in multiple sessions over the span of weeks rather than months would have been more effective. Despite the graduated approach that we used, there was transient exacerbation of edema in many patients, and it is possible that more rapid completion of the laser could have caused greater exacerbation of edema. Among patients with proliferative diabetic retinopathy, it is not the rate at which laser is completed, but rather the total area of retina treated with adequate density that causes regression of retinal neovascularization and long-term stability. Scatter photocoagulation to all areas of the retina outside the temporal arcade vessels with burns 1 burn width apart is quite extensive treatment, and because this did not show any evidence of partial benefit, it seems unlikely that denser treatment would provide benefit.
Prior small studies have been carried out to investigate the potential role of scatter photocoagulation in patients with macular edema resulting from RVO. In a small uncontrolled trial, 10 patients with chronic or recurrent edema resulting from CRVO underwent scatter photocoagulation to peripheral areas of retinal nonperfusion. Comparison of the 6 months before laser treatment with the 6 months after laser treatment showed no significant difference in visual acuity or number of PRN ranibizumab injections required.21 Little can be discerned from this study because of the small numbers, lack of a control group, and short follow-up, but if any conclusion can be made, it would be consistent with the week 48 results of our study. In another small study, patients with CRVO with a duration of 8 months or less and an area of retinal nonperfusion between 1 and 10 disc areas were randomized to ranibizumab plus laser (n = 10) or ranibizumab (n = 12).22 At baseline, patients in the ranibizumab group had an injection of 0.5 mg ranibizumab and patients in the ranibizumab plus laser group had an injection of 0.5 mg ranibizumab plus scatter photocoagulation to all areas of retinal nonperfusion outside the temporal arcade vessels identified by wide-angle fluorescein angiography. In both groups, repeat ranibizumab injections were mandated at the week 4 and week 8 visits and were administered at the week 12, 16, and 20 visits only if re-treatment criteria were met. At the week 24 primary end point, the mean improvement in BCVA was 7.3 ± 15.0 letters in the ranibizumab plus laser group versus 2.3 ± 18.59 letters in the ranibizumab only group. These differences were not statistically significant, but the authors concluded that scatter photocoagulation to areas of nonperfusion was beneficial. However, given the small number of subjects, the enormous variability in the visual outcome in both groups, and the lack of statistical significance, it is more likely that the difference is the result of chance. Also, the small numbers and short duration of the study with the lack of a mandated injection at week 20 makes the outcome highly dependent on how many patients in each group happened to receive a ranibizumab injection at week 20. Furthermore, the visual outcomes were so poor that the results are uninterpretable and provide little confidence that the treatment regimen in either arm of the study should be recommended.
A strength of this study is that it addressed 2 important, well-defined study questions and provided unequivocal answers. The long follow-up after laser photocoagulation was particularly valuable because it demonstrated that the initial exacerbation of edema and decline in BCVA in the laser groups eventually recovered, and it also provided confidence in the conclusion that scatter photocoagulation does not hasten edema resolution and does not reduce treatment burden. As with all long-duration studies, early exit of some patients was an inevitable weakness, but this was mitigated by the modest dropout rate that was well-balanced between the ranibizumab plus laser group (n = 7) and the ranibizumab only group (n = 8), with mean improvement in BCVA from baseline to last follow-up of 10.3 and 11.0 letters, respectively. This indicates that patients who exited from each arm on average had similar outcomes, and thus this did not influence the result of the study. This was also demonstrated by the very similar outcomes of the observed data analysis and the last observation carried forward analysis for the ranibizumab plus laser group versus ranibizumab only group comparisons. We are confident that our results are generalizable to patients with a BRVO or CRVO duration approximately 12 months or longer and chronic or recurrent edema despite prior anti-VEGF treatment, intraocular steroid treatment, or both. We cannot rule out the possibility that scatter photocoagulation given earlier in the course of RVO provides a different outcome than that seen in our patient population; however, given the unequivocal results among the patients with a mean disease duration of 12 to 18 months, the excellent outcomes that result from monthly injections of VEGF-neutralizing proteins in patients with RVO of short duration, and the potential for exacerbation of edema by scatter photocoagulation, the rationale for conducting such a study is not compelling.
We can only speculate as to why scatter photocoagulation did not improve visual outcomes or reduce treatment burden. One possibility is that areas of posterior retina that were not treated with scatter photocoagulation were hypoxic and secreted sufficient VEGF to cause persistent or recurrent edema after scatter photocoagulation of peripheral retina. Another possibility is that chronic hypoxia, high levels of VEGF, and recurrent leakage resulted in structural changes in retinal vessels that made them more prone to leakage. This could be combined with the first hypothesis listed above, so that despite reduction of VEGF levels by scatter photocoagulation, even mild elevation of VEGF levels was sufficient to induce leakage from compromised paramacular vessels. A third possibility is that any scatter photocoagulation–induced reduction of VEGF secretion by the peripheral retina was countered by photocoagulation-induced inflammation and production of propermeability factors. One would expect that photocoagulation-induced edema would decrease over time, and there was gradual edema reduction in the photocoagulation plus ranibizumab arms, but the reduction was not greater than that occurring in the ranibizumab only arms. Although the 3.5 years of follow-up after the second randomization is quite long, it is conceivable that longer follow-up might be needed to see any benefit from scatter photocoagulation.
In summary, this study failed to find a short-term (24 weeks) visual benefit among patients with chronic or recurrent edema resulting from RVO treated with 2.0 mg ranibizumab versus 0.5 mg ranibizumab, but there was a significantly greater reduction in edema in CRVO patients treated with 2.0 mg ranibizumab. This suggests that VEGF may not be completely neutralized by monthly injections of 0.5 mg ranibizumab in all patients with CRVO, but the amount of edema reduction achieved with 0.5 mg ranibizumab, although less than that achieved with 2.0 mg ranibizumab, was sufficient to improve BCVA a similar amount over a 6-month period. We also failed to identify any evidence of long-term benefit from scatter photocoagulation in patients with chronic or recurrent edema resulting from RVO. The short-term anatomic benefits from high-dose ranibizumab in CRVO, the lack of evidence that scatter photocoagulation can provide an exit strategy to achieve resolution of edema, and the loss of short-term visual gains during long-term follow-up and PRN therapy in many patients with CRVO14 suggest that new technologies designed to achieve sustained release of VEGF antagonists may be particularly appealing in patients with CRVO.
Supplementary Material
Financial Disclosure(s):
The author(s) have made the following disclosure(s):
P.A.C.: Financial support – Genentech, Inc. (South San Francisco, CA); Regeneron (Tarrytown, NY); Aerpio (Cincinnati, OH); Roche (Basel, Switzerland); Abbvie (North Chicago, IL); Allergan (Irvine, CA); Clearside Biomedical, Inc. (Alpharetta, GA); GlaxoSmithKline (Middlesex, UK); Genzyme (Cambridge, MA); Oxford BioMedica (Oxford, UK); Santen Pharmaceutical Co, Ltd. (Emeryville, CA); AsclepiX (Baltimore, MD); Ocata Therapeutics, Inc. (Marlborough, MS); Eleven (Cambridge, MA); Applied Genetic Technologies Corporation (Gainesville, FL); Alimera (Atlanta, GA); Allegro (San Diego, CA); Novaritis (Basel, Switzerland); Kala (Cambridge, MA); Graybu (Baltimore, MD)
H.Y.: Financial support – Regeneron; Lutronics
A.W.: Financial support – Genentech, Inc. (South San Francisco, CA)
D.V.D.: Consultant – Allergan; Genentech, Inc. (South San Francisco, CA); Regeneron; Bayer (Leverkusen, FRG); Financial support – Allergan; Genentech; Regeneron
Q.D.N.: Consultant – Allergan; Genentech, Inc. (South San Francisco, CA); Regeneron; Santen; Bayer; Financial support: Allergan; Genentech, Inc.; Regeneron; Santen
Supported by Genentech, Inc, South San Francisco, CA.
Abbreviations and Acronyms:
- AMD
age-related macular degeneration
- BCVA
best-corrected visual acuity
- BRVO
branch retinal vein occlusion
- CRVO
central retinal vein occlusion
- CST
central subfield thickness
- OCT
optical coherence tomography
- PRN
pro re nata
- RBZ
ranibizumab
- RVO
retinal vein occlusion
- VEGF
vascular endothelial growth factor
References
- 1.Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions; implication of VEGF as a critical stimulator. Molec Ther 2008;16:791–9. [DOI] [PubMed] [Google Scholar]
- 2.Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: 6-month primary endpoint results of a phase III study. Ophthalmology 2010;117:1102–12. [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Campochiaro PA, Singh RP, et al. Efficacy and safety of ranibizumab in the treatment of macular edema secondary to central retinal vein occlusion: 6-month results of the phase III CRUISE study. Ophthalmology 2010;117:1124–33. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31. [DOI] [PubMed] [Google Scholar]
- 5.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432–44. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen QD, Tatlipinar S, Shah SM, et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol 2006;142:961–9. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen QD, Shah SM, Heier JS, et al. Primary end point (6 months) results of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) study. Ophthalmology 2009;116:2175–81. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema. Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119:789–801. [DOI] [PubMed] [Google Scholar]
- 9.Boyer D, Heier J, Brown DM, et al. Vascular endothelial growth factor trap-eye for macular edema secondary to central retinal vein occlusion. Six-month results of the phase 3 COPERNICUS study. Ophthalmology 2012;119:1024–32. [DOI] [PubMed] [Google Scholar]
- 10.Holz FG, Roider J, Ogura Y, et al. VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study. Br J Ophthalmol 2014;97:278–84. [DOI] [PubMed] [Google Scholar]
- 11.Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology 2015;122:538–44. [DOI] [PubMed] [Google Scholar]
- 12.Heier JS, Campochiaro PA, Yau L, et al. Ranibizumab for macular edema due to retinal vein occlusions: long term follow-up in the HORIZON trial. Ophthalmology 2012;119:802–9. [DOI] [PubMed] [Google Scholar]
- 13.Campochiaro PA, Hafiz G, Channa R, et al. Antagonism of vascular endothelial growth factor for macular edema caused by retinal vein occlusion: two-year outcomes. Ophthalmology 2010;117:2387–94. [DOI] [PubMed] [Google Scholar]
- 14.Campochiaro PA, Sophie R, Pearlman J, et al. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN study. Ophthalmology 2014;121:209–19. [DOI] [PubMed] [Google Scholar]
- 15.Campochiaro PA, Bhistikul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology 2013;120:795–802. [DOI] [PubMed] [Google Scholar]
- 16.Sophie R, Hafiz G, Scott A, et al. Long term outcomes in ranibizumab-treated patients with retinal vein occlusion; the role of progression of retinal nonperfusion. Am J Ophthalmol 2013;156:693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Early Treatment Diabetic Retinopathy Study Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 1985;103:1796–806. [PubMed] [Google Scholar]
- 18.Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2013;120:1046–56. [DOI] [PubMed] [Google Scholar]
- 19.Ho AC, Busbee BG, Regillo CD, et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014;121:2181–92. [DOI] [PubMed] [Google Scholar]
- 20.Campochiaro PA, Choy DF, Do DV, et al. Monitoring ocular drug therapy by analysis of aqueous samples. Ophthalmology 2009;116:2158–64. [DOI] [PubMed] [Google Scholar]
- 21.Spaide RF. Prospective study of peripheral panretinal photocoagulation of areas of nonperfusion in central retinal vein occlusion. Retina 2013;33:56–62. [DOI] [PubMed] [Google Scholar]
- 22.Rehak M, Tilgner E, Franke A, et al. Early peripheral laser photocoagulation of nonperfused retina improves vision in patients with central retinal vein occlusion (results of a proof of concept study). Graefes Arch Clin Exp Ophtalmol 2014;252:745–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


