Abstract
Arsenic is one of the regulated hazard materials in the environment and a persistent pollutant creating environmental, agricultural and health issues and posing a serious risk to humans. In the present review, sources and mobility of As in various compartments of the environment (air, water, soil and sediment) around the World are comprehensively investigated, along with measures of health hazards. Multiple atomic spectrometric approaches have been applied for total and speciation analysis of As chemical species. The LoD values are basically under 1 μg L−1, which is sufficient for the analysis of As or its chemical species in environmental samples. Both natural and anthropogenic sources contributed to As in air, while fine particulate matter tends to have higher concentrations of arsenic and results in high concentrations of As up to a maximum of 1660 ng m−3 in urban areas. Sources for As in natural waters (as dissolved or in particulate form) can be attributed to natural deposits, agricultural and industrial effluents, for which the maximum concentration of 2000 μg L−1 was found in groundwater. Sources for As in soil can be the initial contents, fossil fuel burning products, industrial effluents, pesticides, and so on, with a maximum reported concentration up to 4600 mg kg−1. Sources for As in sediments can be attributed to their reservoirs, with a maximum reported concentration up to 2500 mg kg−1. It is notable that some reported concentrations of As in the environment are several times higher than permissible limits. However, many aspects of arsenic environmental chemistry including contamination of the environment, quantification, mobility, removal and health hazards are still unclear.
Contamination, mobility, sources, and exposure of arsenic (one of the regulated elements) in the environment are investigated and discussed comprehensively.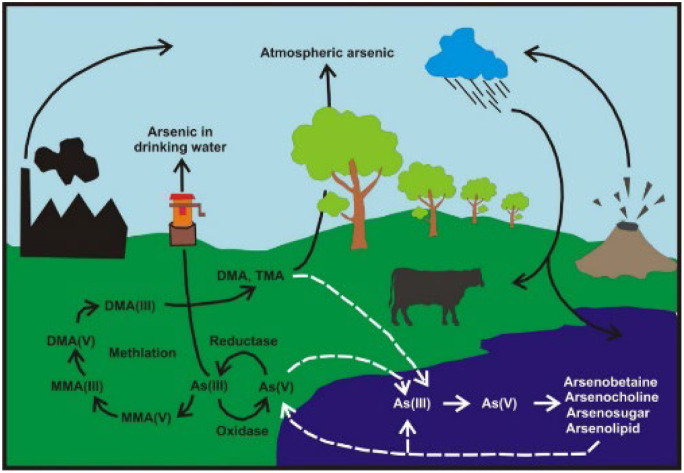
Introduction
Arsenic is a toxic element (metalloid) linked with a broad variety of neurologic, cardiovascular, dermatologic, and carcinogenic effects, including peripheral neuropathy, diabetes, ischemic heart disease, melanosis, keratosis, and impairment of liver function.1 Chronic arsenic pollution is now recognized as a worldwide problem, with 21 countries experiencing arsenic contamination of the environment.2–9 Arsenic susceptibility plays an important role in manifestation of arsenicosis depending on methylation capacity, variation in host genome and individual epigenetic pattern.10
Arsenic is present in the environment in various inorganic and organic chemical forms: arsenite (As(iii)), arsenate (As(v)), monomethylarsonic acid (MMA), dimethylarsinic acid (DMA), trimethyl arsine oxide (TMAO), arsenobetaine (AsB), etc.11 The toxicity, mobility and solubility differ among species, in such a way that inorganic As(iii) is more toxic than As(v), and in turn, organic As is less toxic.12,13
There are several routes of human exposure to arsenic from both natural and anthropogenic sources.14,15 Regarding geogenic sources, the Earth's crust is an abundant natural source of arsenic,16 with an average concentration of As of ≈5 mg kg−1.17 It is present in more than 200 different minerals, the most common of which is arsenopyrite.18 In relation to anthropogenic sources, mining, metal smelting and burning of fossil fuels are the major industrial processes that contribute to arsenic contamination of air, water, and soil.19 Much of the arsenic in the atmosphere comes from high-temperature processes such as coal-fired power plants20,21 and burning vegetation, but also from volcanic activity.22
Prolonged intake of toxic iAs (inorganic arsenic) via air, water and food cause arsenicosis. Analytical techniques for As quantification at low levels in specific environmental samples are required. Many methodologies for detection and quantification of As were reported.23–26
Air is a potential source for As exposure in industrial areas mainly due to emission with airborne particulate matter from smelting of ores and coal combustion. The potential pathways of arsenic exposure and their reduction were reviewed.27,28 Arsenic chemistry, and factors controlling the sorption/desorption, mobility, uptake of As by plants and reduction of translocation in plant tissues and release of As from sediments into groundwater have been reviewed.29–31
The aim of the review is to provide up-to-date information on arsenic contamination of the environment, quantification, sources, mobility and health hazards. The important scientific knowledge gaps and critical areas for future research are discussed.
Results and discussion
Methodology for As quantification
Arsenic contamination of environment is an urgent global issue, and therefore, a reliable and rapid method with good sensitivity and selectivity, portability and robustness to detect As at trace levels both in field and laboratory samples in view of reduced environmental and health risks are required. Several methods, e.g., colorimetric, electrochemical, biological, electrophoretic, surface sensing and spectroscopic methods, are employed for As detection but most of them are suffering with poor sensitivity and selectivity. Many field kits for rapid detection of As at trace levels are used but their reliabilities are not sufficient.32 However, differentiation of the species of As is a quite complex analytical task. Numerous speciation procedures have been studied that include electrochemical, chromatographic, spectrometric and hyphenated techniques (Table 1). It is notable that different concentration units are used in Table 1, i.e., μg L−1, ng g−1, and ng mL−1, such units can be treated approximately equivalent to one another. Regardless the relatively close value to one another, the LoD values depend on the chemical species of As even measured by with an identical method. The LoD values are basically under 1 μg L−1, which are sufficient for the analysis of As or its chemical species as discussed in the following text. The repeatability with a relative standard deviation under 10% and a recovery value over 80% are sufficient for quantitative discussion.
The sample preparation methods, separation and detection for arsenic species in different matrixes (LC-ESI-MS/MS – liquid chromatography-electrospray ionization tandem mass spectrometry; DRC – dynamic reaction cell; CEUV – capillary electrophoresis-ultraviolet detector)a.
| Matrix | Species | Sample pretreatment | Separation/detection technique | Analytical features | Ref. |
|---|---|---|---|---|---|
| Whole blood and urine | As(iii), As(v), MMA, DMA, AB | Dilution with HgCl2 and ultrafiltration | LC-ICP-MS | LoD/μg L−1: <0.3 | 32 |
| Urine | As(iii), As(v), MMA and DMA | Dilution with deionized water and filtration | IC- ICP-MS | LoD/μg L−1: 0.11 As(iii), 0.25 As(v), 0.18 MMA, 0.17 DMA, 0.75 AB; repeatability/%: 1.9 As(iii) 2.7 As(v), 2.1 MMA, 1.9 DMA, 2.8 AB | 34 |
| Urine | As(iii), As(v), MMA, DMA and AB | Dilution with deionized water and filtration | HPLC-ICP-MS | Recovery: 85% to 100% | 33 |
| Urine | As(iii), As(v), MMA, DMA and TMAO | Dilution with mobile phase and filtration | HPLC-HG-AAS | From 1.1 μg L−1 for TMAO to 2.6 μg L−1 for As(v) | 35 |
| Urine | As(iii), As(v), MMA and DMA | Dilution HCl and l-cysteine | HPLC-ET-AAS | LoD/μg L−1: 0.038 | 36 |
| Urine | As(iii), As(v), MMA and DMA | Dilution with deionized water and filtration | HPLC-HG-ICP-MS | LoD/μg L−1: 0.37 As(iii), 0.22 As(v), 0.18 MMA, 0.17 DMA; precision/%: 4.1 As(iii), 5.4 As(v), 6.0 MMA, 6.6 DMA | 37 and 38 |
| Whole blood | MMA, DMA | Centrifuge | CE-ICP-MS | LoD/μg L−1: 1; LoQ/μg L−1: 0.8 As(iii), 1.0 As(v), 1.0 MMA, 0.9 DMA | 39 and 40 |
| Fish and oyster tissues | As(iii), As(v), MMA and DMA | Lyophilization/microwave digestion | CE-ICP-MS | — | 41–44 |
| Fish | As(iii), As(v), MMA and DMA | Ultrasonic extraction and 4 different experimental conditions | HG-AFS | LoD/μg kg−1: 0.62 As(iii), 2.1 As(v), 1.8 MMA, 5.4 DMA; RSD: 6.8% As(iii), 10.3% As(v), 8.5% MMA, 7.4% DMA; recovery: >93% | 45–49 |
| Fish sauce | AB, AC, TMAO | Extraction with water/methanol (1 + 1, v/v)/shaking/centrifugation | HPLC-ICP-MS | LoD/ng g−1:3.07 | 50 |
| Beverages (soft drink, lemon juice, beer) | As(iii), As(v), MMA and DMA | Sample were passed through a C18 sep-pack and filtered | HPLC-ICP-MS | LoD/ng L−1: 0.2, 0.2, 0.3 and 0.5 for As(iii), DMA, MMA and As(v), respectively; RSD of As(iii), DMA, MMA and As(v) were 1.2, 2.1, 2.5 and 3.0%, respectively | 51 |
| Cereals | As(iii), As(v), MMA and DMA | Ultrasonic extraction with H3PO4 and Triton XT-114 | HG-AFS | LoD/ng g−1: 1.3, 0.9, 1.5 and 0.6 for As(iii), As(v), DMA and MMA, respectively; recoveries: >90%; repeatability: 3% for As(iii), 5% for DMA and 6% for As(v) and MMA | 52–55 |
| Wines | As(iii), As(v), MMA and DMA | Treatment with cysteine in HCl for total As; dilution with citrate buffer or acetic acid for As species | HG-AFS | LoD/μg L−1: 0.12, 0.27, 0.15 and 0.13 (as As); RSD: 2–6%, 5–9%, 3–7% and 2–5% for As(iii), As(v), MMA and DMA, respectively | 56 and 57 |
| Vegetables | As(iii), As(v), MMA and DMA | Ultrasonic extraction with H3PO4 and Triton XT-114 | HG-AFS | LoD/ng g−1: 3.1 As(iii), 3.0 As(v), 1.5 DMA and 1.9 MMA; recovery: >91% | 58–61 |
| Milk | As(iii), As(v) | Ultrasonic extraction with/without KI | HG-AFS | LoD/ng g−1: 8.1 and 10.3 for As(iii), As(v); RSD: 5.7 and 5.5% for As(iii) and As(v) | 45, 62 and 63 |
| Water | As(iii), As(v), DMA | Treatment with cysteine, KJ, urea or acids | HG-AAS, CE-UV, LC-ICP-MS | LoD/μg L−1: 0.10 As(iii), As(v), 0.19 (DMA) for HG-AAS, 100 (AsIII, DMA) to 500 (AsV) for CE-UV and 0.1 (DMA, MMA) to 0.2 (AsIII, AsV) for LC-ICP-MS; precision: <5% RSD, recovery: 80–110% except CE-UV where only 50% | 64 |
| Water and urine sample | Inorganic, organic As | Treatment with/without cysteine | HG-ICP-MS | LoD/ng L−1: 6 | 65 and 66 |
| Water and reference materials | As(iii), As(v) | Treatment with HCl and NaBH4 | HG-AAS | LoD/μg L−1: 0.1 for As(iii) and 0.06 for total As. Precision (RSD) level is 2.9% for As(iii) and 3.1% for total As | 67 and 68 |
| Water | As(iii), As(v) | None | HG-AAS | LoD/μg L−1: 0.019 total As, 0.031 As(iii) | 69 |
| Water | As(iii), As(v) | Reaction with cysteine, NaBH4 | HG-AAS | LoD/μg L−1: 0.1 | 70 |
| Water | As(iii), As(v) | Reaction with cysteine | HG-ETAAS | — | 71 |
| Water | As(iii), As(v) | None | HG-AFS | — | 72 |
| Water | As(iii), As(v) | pH adjustment | HG-AAS | LoD/μg L−1: 0.07–0.4 As(v) and 0.1–0.5 As(iii + v); recovery: 90–102% | 69 |
| Water | As(iii), As(v) | Treatment with KMnO4 | ICP-AES | LoD/μg L−1: 0.1–0.6 | 72 and 73 |
| Water | As(iii), As(v), MMA and DMA | None | HPLC-ICP-MS | LoD/μg L−1: 0.22 As(iii), 0.69 As(v); LoQ/μg L−1: 50 | 74 |
| Water | Inorganic As | Pre-reduction of As(v) with cysteine | HG-AAS | LoD/μg L−1: 0.15; LoQ/μg L−1: 0.5; RSD (n = 10) <8% | 75 and 76 |
| Seawater | As(iii) | Complexation with PDC | ETAAS | LoD/μg L−1: 0.008; RSD (n = 11): 4.5% | 76 and 77 |
| Water | As(iii), As(v), MMA and DMA | None | HG-ICP-AES | Recovery: As(v) 97.6%, As(iii) 100%, MMA 99.8%, and DMA 99.9% | 76 and 78 |
| Water | As(iii), As(v) | Treatment with KMnO4 | ETAAS | LoD/μg L−1:0.35; recovery: 93.5–106.4%; RSD: 3–7% | 76 and 79 |
| Water | As(iii), As(v), MMA, DMA and AB | None | HPLC-ICP-MS | LoD/μg L−1: 0.017 As(iii), 0.026 As(v), 0.026 MMA, 0.023 DMA, 0.024 AB; LoQ/μg L−1:0.056 As(iii), 0.085 As(v), 0.088 MMA, 0.076 DMA, 0.080 AB | 76 and 80 |
| Groundwater | As(iii), As(v) MMA, TMAO, PAA and PAO | None | HPLC-ICP-MS | LoQ/μg L−1: 0.2–0.8 | 81 |
| Hot spring water | As(iii), As(v), MMA, DMA, TMAO, TMA, AC and AB | None | HPLC-ICP-MS | LoD (μg L−1): 0.2; RSD (n = 6): <2% | 82 and 83 |
| Human hair | As(iii), As(v) | Reduction of As(v) to As(iii) | HG-AAS | LoD/μg L−1: 0.2 As(iii), 0.5 As(v); RSD: 2.1% As(iii) and 2.5% As(v) | 76 and 84 |
| Sediment and fly ash | Water soluble and phosphate-exchangeable As(iii) and As(v) | Extraction with water and phosphate buffer | HG-AAS | LoD/μg L−1: 0.06–0.10; LoQ/μg L−1: 0.20–0.31. Repeatability expressed as RSD: <1% | 76 and 85 |
| Landfill leachate | As(iii), As(v), MMA, DMA, TMAO and AB | Filtration | HPLC-ICP-MS | LoD vary between 11 ng L−1 for DMA to 27 ng L−1 for As(v). LoQ ranged between 36 and 90 ng L−1 | 81, 86 and 87 |
| Municipal landfill leachates | As(iii), As(v), MMA and DMA | Filtration | LC-ESI-MS/MS and HPLC-DRC-ICP-MS | Recovery: 68–94% | 81 and 88 |
| Soil | Total As | Extraction with HNO3, acetic acid, EDTA and Mehlich II | HG-AAS | — | 89 and 90 |
As(iii): arsenous acid, arsenite, As(v): arsenic acid, arsenate, MMA: monomethylarsenic acid, DMA: dimethylarsenic acid, TMAO: trimethylarsine oxide, TMAP: trimethylarsoniopropionate, TETRA, TMA: tetramethylarsonium ion, AB: arsenobetaine, AC: arsenocholine, PAO: phenylarsine oxide, PAA: phenylarsonic acid, DPAA: diphenylarsonic acid.
Commonly used instrumental methods based on atomic absorption, atomic emission and mass spectroscopy methodologies i.e., hydride generation atomic absorption spectrometry (HG-AAS), inductively coupled plasma-mass spectrometry (ICP-MS) and graphite furnace atomic absorption spectrometry (GF-AAS) were reported for monitoring of arsenic at the 0.05 mg L−1 (50 μg L−1) MCL (maximum contaminant level). Among them, ICP-MS is a most sensitive multielement technique with selectivity depending on the version of the instrument whereas HG-AAS and GF-AAS are single element selective technique with relatively lower sensitivity.32,91 The ICP-MS with tandem quadrupole mass spectrometers (ICP-QQQ) is useful to overcome isobaric interferences with reduced background and improved selectivity. The tandem inorganic-organic mass spectrometry, i.e., HPLC-ICP-MS + ESI-MS, is applicable to detect unknown arsenic compounds.92 However, they need extraction/separation of the analyte prior to analysis, depending on nature of the arsenic species (water- or lipid-soluble) and the matrix (biotic or abiotic), except water sample analysis.
For speciation of As in environmental samples, various approaches i.e. liquid–liquid, liquid–solid and solid phase extraction, sonication, pressurized liquid extraction, microwave-assisted extraction, supercritical fluid extraction, enzymatic hydrolysis, conventional sorbent, functional nanomaterials extractant, multi-sorbent based procedure, and derivatization of total arsenic were used.93 Methanol or acetonitrile–water and methanol–chloroform or hexane are generally employed for extraction of polar arsenic and nonpolar species. A 1 mol L−1 phosphoric acid is adequate for extraction of arsenic species from soil and sediment samples. A preconcentration step using freeze-drying or evaporation may be necessary to detect low abundance of arsenic species. Enzymes, e.g. pepsin, trypsin, pronase E, and lipase can be used to digest arsenic associated to lipids, proteins, peptide bonds, or cell walls, to increase extraction efficiencies.
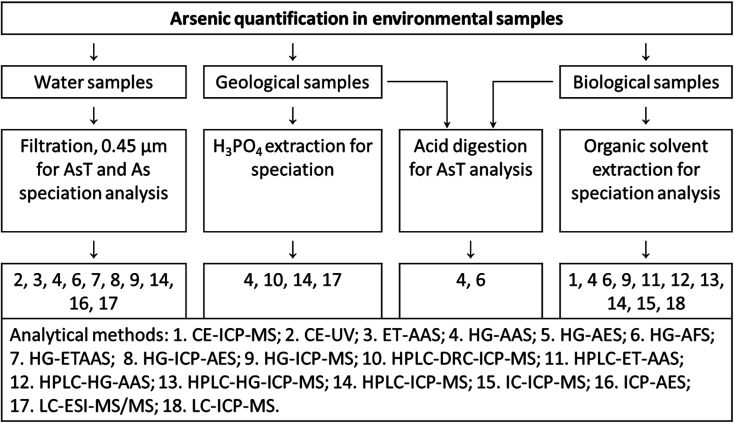
Generally, chromatography and capillary electrophoresis are two major techniques used for separation of As species from complex matrices. Chromatography i.e. anion exchange, cation exchange, reversed-phase, ion pair, and hydrophilic interaction liquid chromatography (HILIC) are proposed for separation of various arsenic species such as As(iii), As(v), MMA, DMA, AsB, arsenocholine (AsC), oxo-arsenosugars (oxoAsS), thio-arsenosugars (thioAsS), phenylarsenicals; AsB, AsC, TMAO, and tetramethylarsonium ion.94 The schematic diagram for quantification of As in environmental samples is shown in Fig. 1.
Fig. 1. Schematic diagram for quantification of As species in environmental samples.
Sample collection, preservation, and pretreatment
The cleaned and sterilized FEP (fluorinated ethylene propylene) container should be used for collection of environmental samples and refrigerated at −20 °C in a dark place to control microbial activities. Water samples collected for As speciation analysis are filtered in the field using a 0.45 μm filter into opaque polyethylene bottles.95 The sample storage duration should be minimized to avoid change in species stability. To prevent loss of arsenic species during sampling, samples (soil, plant, or water) should be collected in a sealed polyethylene bottle/bag. All samples should be stored in the freezer immediately after collection until the sample is prepared for analysis.
The preparation steps before analysis of each type of sample differ. Soil samples are air-dried, crushed lightly and sieved through a 2 mm sieve and used for analysis. The plant sample is placed in an oven dryer at 40 °C to constant weight, the sample is ground, sieved and stored in a desiccator in brown glass bottles to avoid exposure to light and moisture until needed for analysis. Sample preparation for solid samples usually includes procedures such as mincing, freeze-drying, grinding, homogenization, sieving followed by extraction. After sampling of the fresh plant sample, it should be kept in the freezer (−80 °C) to prevent species change. In addition, a dry and ground plant and soil sample can be stored at −20 °C for up to one year.93
Statistical significance of the data is determined using analysis of variance (ANOVA) at p < 0.05 confidence level. Mobile phase concentrations and pH were optimized to get maximum peak separation. The limit of detection (LoD) is determined using the spike and blank based procedures. The standard reference materials (SRMs) and certified reference materials (CRMs) are used to test and validate the accuracy of a method. SRM 1640 (NIST) and various CRM samples are widely employed to check a calibration curve for trace elements in water, while CRMs used depend on the sample matrix and the arsenic species to be analyzed.94
Distribution, mobility and sources of As in the environment
Arsenic exists in the form of various chemical species differing by their physicochemical behavior, in toxicity, bioavailability and biotransformation. The determination of arsenic species is an important issue for environmental, clinical and food chemistry.94,96
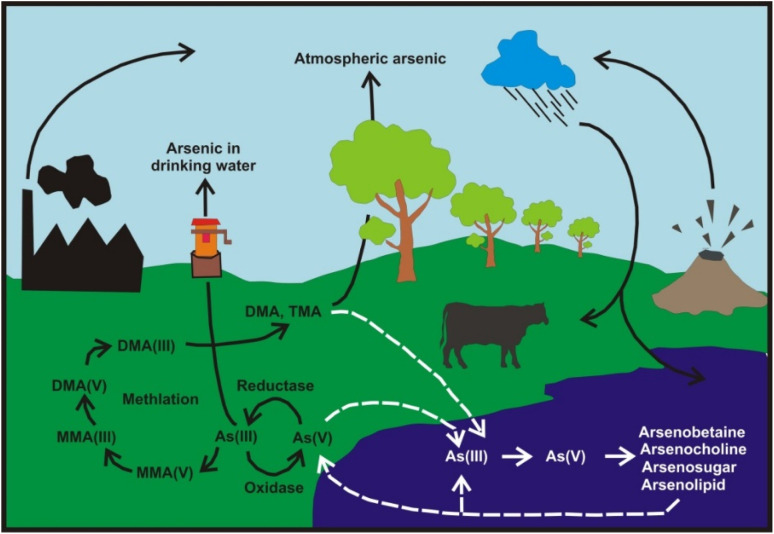
In this review, the distribution, mobility, and sources of As in air, water, soil, sediment, plants, foods and marine organism; remediation of As from water, soil and sediment; disposal of contaminated samples, and health hazard assessment are discussed. The overall cycles of As in different environmental compartments are illustrated in Fig. 2. These cycles involve various chemical species such as As(iii, v), MMA(iii, v), and DMA(iii, v), as well as chemical and/or biological reactions resulting reduction, oxidation, and methylation of As. Transportations of As species among soil, water, plant, animal, air, and sediment should be considered to understand the impact of As in the environment.
Fig. 2. The different mechanism controlling the mobility of arsenic in environmental component.
Air
Arsenic is stable in the air and is emitted by both natural and anthropogenic sources. The natural sources include volcanic eruption, emission from soils or sediments by microbial reduction, dispersion of As containing particles by wind, evaporation from arsenic compounds and marine organism, etc.97 Anthropogenic sources are high-temperature processes such as smelting of non-ferrous metals, burning of fossil fuels, vegetation and wastes, etc. They are emitted into the atmosphere primarily as As2O3 and arsines, and which are further transformed into arsenite, arsenate and organoarsenic compounds. Ultimately, they are transported by winds and settled into earth crusts by dry and wet depositions.98
Arsenic in air is present mainly in particulate forms as inorganic As and released into the atmosphere primarily as oxides that adsorb on particulate matter (PM), which are dispersed by the wind to remote areas.99 The particulates then settle down both by dry and wet deposition. Approximately two thirds of the atmospheric flux of As is of anthropogenic origin.100,101
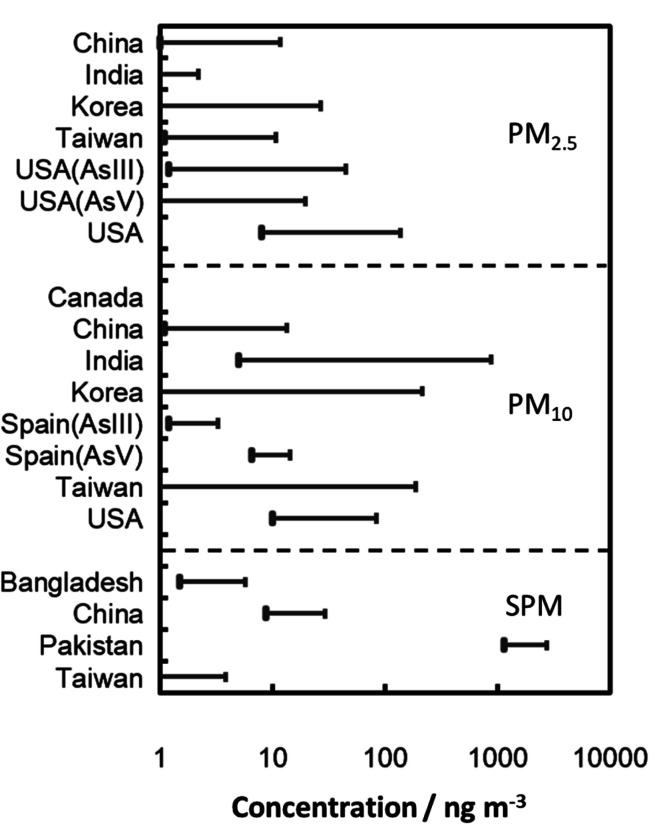
US OSHA regulations regarding iAs are found in 29 Code of Federal Regulations (CFR) 1910.1018. The permissible exposure level (PEL) for iAs is 10 μg m−3 of air, averaged over an 8 hour period without regarding to the use of a respirator. The action level is 5 μg m−3 of air. A medical surveillance program must be established for all employees exposed at or above the action level, for at least 30 days per year without regarding to the use of respirators.102,103 Typical background levels for arsenic in the rural, urban, and industrial areas are in the ranges of 0.2–1.5, 0.5–3, and <50 ng m−3, respectively.101 Arsenic content in ambient air/associated to PM104–121 in different locations worldwide is summarized in Table 2. Concentration range of As in particulate matter around the world is further illustrated in Fig. 3 based on the data given in Table 2. Inorganic As(iii) and As(v) both occurred predominantly in ambient PM and preferably associated to the fine PM in accumulation mode with particle size of 0.2–2.0 μm.118,119,121 Concentrations (associated to ambient SPM, PM10 and PM2.5) in various Chinese, Korean, Mongolian, Taiwanese, American and Canadian cities have been reported over the 2.4–185.2 ng m−3 range, with higher values in fine PM.105,106,113,115,119–121 Similar studies have been carried out for different South Asian cities. In Raipur, total As in the PM10 and PM2.5 ranged from 37.0–501 and 27.0–293 μg m−3, respectively.108 In Dhaka city, As concentration in the SPM varied from 0.0015 to 0.0042 μg m−3.109 In Hyderabad, total As in the PM10 and PM2.5 were in the ranges of 0.685–1.132 and 0.0010–0.0030 μg m−3 with an annual average of 0.866 and 0.0022 μg m−3, respectively.107 The As in the SPM was detected in the range of 1140–1600 ng m−3 in different parts of the city of Lahore.110 The main reason for these high concentrations of As in Lahore city is attributed to anthropogenic pollution. Moreover, in the street, road and indoor dust, several folds higher As content was reported.104,111,112,114,116
Arsenic contents reported in ambient air/particulate matter (PM) over the worlda.
| Site | Particulate (n) | Concentration range (mean) | Source type | Ref |
|---|---|---|---|---|
| Berlin Museum, Germany | Ambient air/dust (30) | Max, 48 ng m−3/3507 mg kg−1 | Anthropogenic | 104 |
| Cornwall, UK | Household dust (99) | 3–1079 (84) mg kg−1 | Anthropogenic, natural | 114 |
| Los Angeles, USA | PM2.5 (16) | As(iii)/As(v) = <1.2–44.0 (7.4 ± 10.4)/<0.99–18.7 (5.2 ± 4.6) ng m−3 | Anthropogenic | 117 |
| Huelva, Spain | PM10 | As(v)/As(iii) = (1.2–2.1)/(6.5–7.8) ng m−3 | Anthropogenic | 118 |
| Canada | PM10 | 0.3 μg m−3 | Anthropogenic | 120 |
| Foshan, Hangzhou, Harbin, Yinchuan and Zhengzhou, China | PM10 | 96.9–185.2 ng m−3 | Anthropogenic | 105 |
| Tainan, Taiwan | PM2.5/PM10–2.5/SPM (180) | 1.09–9.51/.18–4.14/0.99–2.85 (1.94) ng m−3 22.7–155.8 (96.7) ng g−1 | Anthropogenic, natural | 119 and 106 |
| Beijing, China | PM2.5/PM10 | 8.1–128 (43.5 ± 28.0)/10–73.4 (61.8 ± 49.7) ng m−3 | Anthropogenic | 115 |
| AlaShan, China | PM2.5/PM10 | 1.0–9.8 (3.7 ± 2.2)/1.1–12.4(5.4 ± 3.0) ng m−3 | Anthropogenic | 115 |
| Seoul, South Korea | PM2.5/PM10 | 1.9–27.0 (9.0 ± 6.4)/2.8–216.4(34.4 ± 58.9) ng m−3 | Anthropogenic, natural | 115 |
| Gosan, South Korea | PM2.5/PM10 | 0–8.9(1.7 ± 1.9)/0.2–6.8 (2.2 ± 2.0) ng m−3 | Anthropogenic, natural | 115 |
| Wuhan, China | PM2.5 (579) | 6.81–10.7 ng m−3 | Anthropogenic | 113 |
| Hyderabad, India | PM10 and PM2.5 (72) | 0.866 and 0.0022 μg m−3 | Anthropogenic | 107 |
| Raipur, India | PM10 (24) | 5–47 (7 ± 1) ng m−3 | Anthropogenic | 108 |
| Ambagarh, Rajnandgaon, India | Road dust (13) | 165–329 (238 ± 29) mg kg−1 | Anthropogenic | 116 |
| Baoji, China | Road dust | 9.0–42.8 (19.8) mg kg−1 | Anthropogenic | 112 |
| China | SPM (918) | 8.67–20.5 (11.7) ng m−3 | Anthropogenic | 121 |
| Dhaka, Bangladesh | SPM (8) | 1.50–4.20 (3.06) ng m−3 | Anthropogenic | 109 |
| Lahore, Pakistan | SPM (20) | 1140–1600 ng m−3 | Anthropogenic | 110 |
| Kolkata, India | Street dust (9) | <2–18.71 (7.96 ± 0.95) mg kg−1 | Anthropogenic | 111 |
SPM = suspended particulate matter, PM10 = coarse particulate matter, PM2.5 = fine particulate matter.
Fig. 3. Concentration range of As reported in particulate matter around the world (concentrations are given for total As, except for those specified).
Water
Water receives As from natural deposits and from both agricultural and industrial effluents.121 It is found in the atmosphere, soils and rocks, natural waters, and other organisms. As is mobilized in the environment through a combination of natural processes and anthropogenic activities, e.g., atmospheric dry and wet depositions, weathering reactions, biological activity and volcanic emissions, mining activity, combustion of fossil fuels, use of arsenical pesticides, herbicides, livestock feed additive, wood preservatives, etc.15
Arsenic in the air gradually precipitates on the soil as arsenic oxides and arsines. Arsenic accumulated on the soil surface is then slowly mixed into the groundwater with the effect of surface waters and rain.123 In groundwater, As is released by rock weathering, long flow paths, use of phosphate fertilizers, irrigation of As enriched soils, etc. and influenced by physical geochemical characteristics of aquifers, water pumping rates, etc.28,124–127
Arsenic in natural waters is mostly found in either dissolved or in particulate form. The most common forms of arsenic in natural waters are arsenite and arsenate.128–130 In well-oxygenated water and sediments, nearly all arsenic is present in the arsenate form.131–134 The MMA and DMA (dimethyl arsenic acid) are also present in some water.15,28,135–137
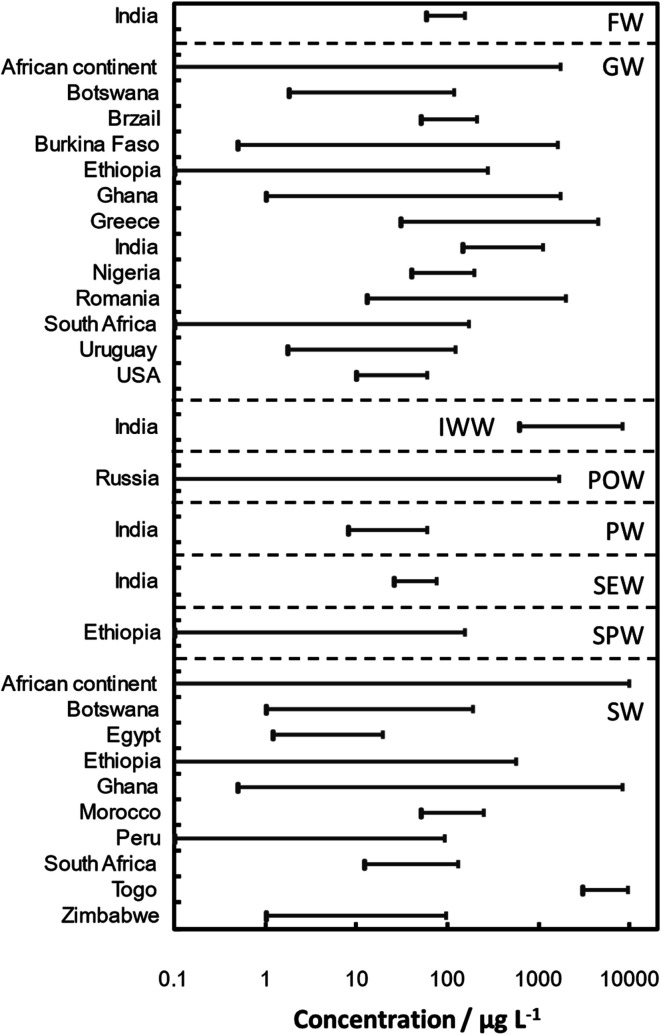
The solubility and mobility of As in the environment increase with increasing alkalinity and salinity. Its movement is controlled by adsorption/desorption and precipitation/dissolution reactions.138 Three major modes of arsenic biotransformation occurred in the environment: redox transformation between As(iii) and As(v), the reduction and methylation of As, and the bioproduction of organ arsenic compounds.8,139–141 Arsenic contamination with sources in water142–169 of some locations over the world are summarized in Table 3. Concentration range of As in water samples around the world is illustrated in Fig. 4 based on the data given in Table 3.
Distribution of As in watera, μg L−1.
| Location | Sample type (n) | Concentration | Sources | Source | Ref. |
|---|---|---|---|---|---|
| Eastern plains of Zimbabwe | SW(20) | 1–96 | Abandoned mine dumps | Natural | 150 |
| Tarkwa, Ghana | GW(40) | <5.2–69.4 | Acid mine drainage | Natural | 144 |
| Kemerovo region, southwestern Siberia, Russia | POW | 1700 | Arsenopyrite leaching | Natural | 153 |
| Okavango Delta, Botswana | SW(9) | 1.1–3.1 | Arsenopyrite weathering | Natural | 149 |
| GW (25) | 1.8–116.6 | ||||
| Ogun state, Nigeria | GW (20) | 40–160 | Arsenopyrite weathering | Natural | 147 |
| Prestea, Ghana | SW(13) | 150–8250 (1568 ± 278) | Arsenopyrites | Natural | 166 |
| Paraiba do Sul delta, Brazil | GW | >50–163 | Authigenic sulfides | Anthropogenic | 156 |
| Korba, India | PW (26) | 8.0–30 (17.2 ± 2.1) | Coal burning | Anthropogenic, natural | 167 |
| Nile Delta, Egypt | SW | 1.2–18.2 | Fertilizers, detergents, herbicides | Anthropogenic | 142 |
| USA | GW(7000) | 10–50 | Igneous rocks | Natural | 168 |
| Hungary, Croatia, Serbia, Romania | GW | 225/610/2000/13–200 | Igneous rocks | Natural | 151 |
| Raipur, India | SEW (7) | 26–51 (40 ± 7) | Industrial activities | Anthropogenic | 158 |
| Raipur, India | IWW (34) | 600–7800 (2900 ± 1400) | Industrial activities | Anthropogenic | 169 |
| Lomé coastal region, Togo | SW (23) | 3000–6460 | Industrial and sea waste | Anthropogenic | 164 |
| Greece, Europe | GW | 30–4500 | Mineral leaching | Natural | 151 |
| Koekemoerspruit, South Africa | SW (40) | 12.3–119 | Minerals | Natural | 145 |
| Upper Moulouya, Morocco | SW (14) | 50.08–199.6 | Minerals | Natural | 146 |
| Tarkwa, Ghana | SW (12) | 0.5–73 | Minerals | Natural | 143 |
| South Africa | GW (10) | 0.1–172.53 (32.21) | Minerals | Natural | 157 |
| Tarkwa, Ghana | GW (16) | <1–1760 | Mining activities | Natural | 152 |
| African continent | SW/GW | 10 000/1760 | Mining activities | Natural | 178 and 179 |
| River water, Peru | SW (151) | 0.1–93.1 (54.5) | Mining and agriculture activities | Anthropogenic, natural | 148 |
| Rift Valley, Ethiopia | SW(53) | <0.1–405 | Rhyolitic rock | Natural | 161 |
| Rift Valley, Ethiopia | SW(11) | 2.39–566 (165 ± 215) | Rhyolitic rock | Natural | 162 |
| GW (54) | 0.60–190 (22.4 ± 33.5) | ||||
| Rift Valley, Ethiopia | SW (05)/GW(25)/SPW(14) | 0.02–96/<0.1–278/<0.1–156 | Rock weathering | Natural | 163 |
| Uruguay | GW (46) | 1.72–120.5 | Rock weathering | Natural | 154 |
| Ambagrh Tehsil, India | FW (20) | 29–98 (58 ± 7) | Sulfide minerals | Natural | 159 |
| Ambagrh Tehsil, India | PW (24) | 10–53 (22 ± 5) | Sulfide minerals | Natural | 165 |
| Ambagrh Tehsil, India | GW (20) | 148–985 (506 ± 118) | Sulfide minerals | Natural | 160 |
| Okavango Delta Botswana | SW | <1 to 188 | Sulfide minerals | Natural | 155 |
| Yatenga and Zondoma provinces, Burkina Faso | GW(45) | <0.5–1630 | Sulfide minerals | Natural | 124 |
FW = field water, GW = groundwater, IWW = industrial wastewater, POW = pore water, PW = pond water, SEW = sewage water, SPW = spring water, SW = surface water.
Fig. 4. Concentration range of As reported in water samples around the world.
The allowable limit values of As for drinking and irrigation purposes recommended are 10 and 100 μg L−1, respectively.170,171 Millions of people, especially from developing countries, use groundwater containing As for drinking purposes. Arsenic in the tube-well, well and surface water of several Asian countries (Bangladesh, Cambodia, China, India, Iran, Nepal, Pakistan, and Vietnam; Bangladesh, China, Cambodia, Iran, Pakistan, and Vietnam; China, Iran, and Pakistan) have been reported due to agricultural and industrial activities.4 In the particular case of China, Bangladesh and India, values of As content in field, surface, pond, ground, sewage and industrial waste water have been reported, and ascribed to mineralization of arsenopyrite.158–160,165,167,169,172–177
In African countries, both surface and groundwater are also seriously polluted with arsenic due to industrial activities.178 Examples for Bostwana, Ethiopia, Ghana, Moroco, Nigeria, South Africa and Tanzania42,145,146,150,155 are presented in Table 3. In some cases, this pollution has a natural origin (rock weathering), such as the As contents of up to 566 μg L−1 in surface water reported in Rift Valley, Ethiopia,162,163 or groundwater pollution, with contents of up to 6150 μg L−1, reported in Johannesburg, South Africa.177,178 Nonetheless, in other cases this contamination is a consequence of mining operations, which, for instance, have led to As contents of up to 8250 μg L−1 in surface waters of Ghana and Togo.164,166
Likewise, in Latin America, the population of various countries, including Argentina, Bolivia, Brazil, Chile, Colombia, Cuba, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Peru, and Uruguay15,143,144,147–149,152,154,156,157,163,180 are facing problems with As contaminated water too (see Table 3). Arsenic pollution is mainly due to natural resources,181 and is particularly serious in Argentina, Chile, and Mexico.182 In North America, contents of up to 2600 μg L−1 have been reported.148,154,156 Western part of USA168 and many countries of Europe,183 such as Greece, Hungary, Romania, Croatia, Siberia, Turkey, and Spain are affected by elevated As levels, ranging from 30 to 4500 μg L−1.151,153,184–186
Soil
The background levels of As depends on soil types, ranging from 5–10 mg kg−1.187 The soil environment is contaminated with As by natural and anthropogenic sources. Arsenic in soils results from anthropogenic activities, and the main sources in the soil are naturally occurring minerals, such as arsenopyrite, gelignite, realgar, orpiment, etc.,17 and the mineralization of FeAsS and As2S3-like species in soil results in its pollution with As.188
The major inputs of As in soil are associated with burning of fossil fuels and biomass, mining activities, industrial effluents, use of pesticides (e.g. monosodium methyl arsenate, disodium methyl arsenate and cacodylic acid) in agriculture, lumber preservatives (e.g. ammoniacal copper zinc arsenate and chromated copper arsenate), manufacturing of glass and ceramics (arsenic trioxide), alloys (brass and bronze), optics and electronic materials (light emitting or laser diodes, gallium arsenide microchips and circuit fiber optic crystals), leatherwork-leather preservative, pigments and paints.8,102
Arsenic fate is a complex due to existence in many chemical forms naturally. In minerals, arsenicals and surface soils occur in arsenate forms, changing valence under different redox conditions, and adsorb to many soil clays and Fe, Mn and Al oxides.189 Arsenic soil mobility and bioavailability is of great interest for human health assessment, and it depends on several physical and chemical factors: e.g. pH, redox potential, organic matter content, clay mineralogy, texture, Ca, Al, Mn, Fe and PO43− content, organic ligands – fulvic and humic acids, and microbial activity of the soil and/or irrigation water.190 The solubility and speciation of As in a contaminated soil is controlled by pH values of the soil extract, in such a way that the mobilization of As is increased in alkaline medium, resulting in an enhanced phytoavailability191 Various elements (e.g., Al, Ca, Fe, and Mn) enact a remarkable effect in As phyto availability in soil, and its translocation in different parts of the plants; root microbe-induced transformation system can also impact the fate of As in the rhizosphere; and phosphate and organic matter in the root system tend to reduce As phytotoxicity.122,192,193
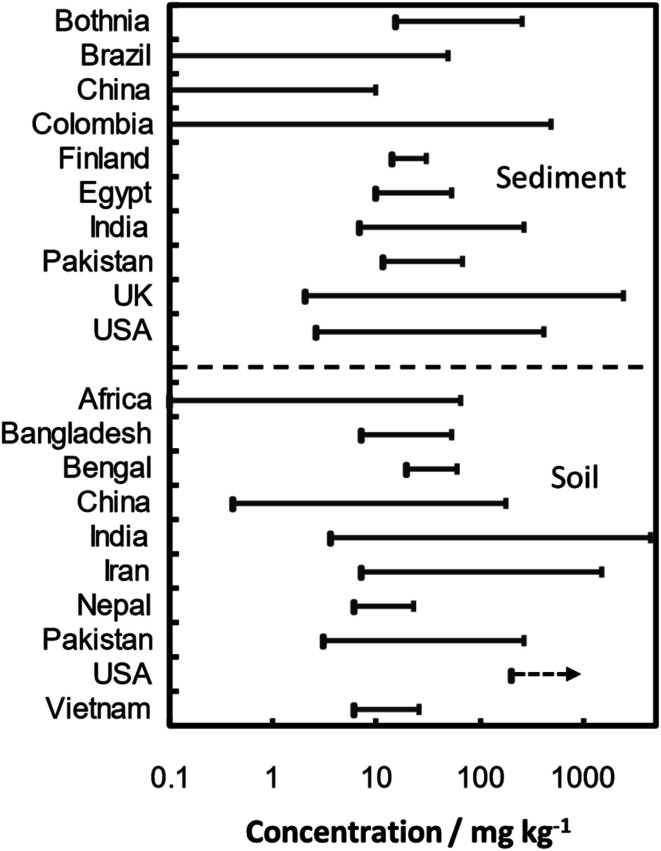
The As soil contamination and sources at various sites of the world are summarized in Table 4. The concentration of total As in 17 locations of surface, agricultural, garden and rhizospheric varied over 126–1600 mg kg−1.159,194,195 The maximum value was observed in rhizospheric soil, probably due to plant and microorganism interactions. Although high concentrations have been reported all over India (in Assam, Bihar, Utter Pradesh and West Bengal states), with values of up to 41.2 mg kg−1,196–198 remarkably high values have been observed in Chhattisgarh state: with As concentrations reaching 164 mg kg−1 in the surface soil of Korba basin199 and of up to 4600 mg kg−1 in Ambagarh Tehsil.159,194 Concerning other countries, contents over the 7–46, 3–266, 6.1–16.7, 9.7–110, 6–20, 7–1500 and 18.3 mg kg−1 intervals have been reported in Bangladesh, Pakistan, Nepal, China, Vietnam, Iran, and South Africa, respectively.195,200–213
Distribution of As in soil and sediment, mg kg−1.
| Location | Sample type (n) | Concentration | Source | Source type | Ref |
|---|---|---|---|---|---|
| Kinghorn Loch, UK | Sediment | 86–185 | Aluminum industrial waste | Anthropogenic | 226 |
| Sindh, Pakistan | Soil | 8.7–46.2 | Arsenopyrite | Natural | 201 |
| Sindh, Pakistan | Lake sediment | 11.3–55.8 | Arsenopyrite | Natural | 201 |
| China | Agricultural soil (1648) | 0.4–175.8 (10.40) | Atmospheric dusts | Anthropogenic | 204 |
| China | Soil | 110 | Chemical weapons | Anthropogenic | 212 |
| Pakistan | Soil | 110–266 | Coal burning | Anthropogenic | 202 |
| Korba, India | Surface soil (30) | 49–164 (107 ± 32) | Coal mining and burning | Anthropogenic | 199 |
| Korba, India | Pond sediment (26) | 36–154 (95 ± 12) | Coal mining and burning | Anthropogenic | 167 |
| China | Agricultural soil | 9.7 | Fertilizers | Anthropogenic | 213 |
| Bangladesh | Soil (12) | 7–27.5 (14.5) | Groundwater | Natural | 200 |
| Florida, USA | Lake sediment (6) | 39.0–53.5 (47.3) | Herbicides | Anthropogenic | 225 |
| USA | Buffalo river sediment(111) | 2.6–417.0 (14.03 ± 39.21) | Industrial activities | Anthropogenic | 224 |
| Assam, India | Brahmaputra sediment (8) | Up to 37 | Industrial activities | Anthropogenic | 223 |
| China | Coastal sediment | 9.75 | Industrial activities | Anthropogenic | 229 |
| Bothnian Bay/Bothnian Sea/Gulf of Finland/Gulf of Bothnia | Sediment | 109–239/35–63/14–16/15–23 | Industrial activities | Anthropogenic | 228 |
| North Egyptian lake | Lagoon sediment (21) | 10–44 (25) | Industrial and agricultural activities | Anthropogenic | 227 |
| Bihar, middle Gangetic plain, India | Agricultural soil (19) | 3.528–14.690 | Irrigation water | Natural | 196 |
| Ballia, UP, India | Soil (30) | 5.40–15.43 (11.12) | Irrigation water | Natural | 198 |
| Nepal | Soil | 6.1–16.7 | Irrigation water | Anthropogenic | 203 |
| Bangladesh | Paddy soils | 46 | Irrigation water | Anthropogenic | 209 |
| Vietnam | Agricultural soil | 6–20 | Irrigation water | Anthropogenic | 206 |
| Pakistan | Soil | 3.0–3.81 | Minerals | Natural | 210 |
| Iran | Soil (17) | 7–795 | Minerals | Natural | 207 |
| Iran | Soil (18) | 105.4–1500 (1017) | Minerals | Natural | 211 |
| Bihar, India | Ganga sediment (19) | 9.119–20.056 | Minerals | Natural | 196 |
| Bihar middle Gangetic plain, India | Sediment (24) | 6.9–14.2 (11.8) | Minerals | Natural | 222 |
| Colombia | Suratá River sediment | Up to 484 | Mining | Natural | 220 |
| Brazil | Patos Lagoon sediment (15) | Up to 50 (2.5) | Mining | Natural | 230 |
| U.K. estuarine | Sediments | 2–2500 | Mining activities | Natural | 221 |
| USA | Fruit tree soil | >200 | Pesticides | Anthropogenic | 205 |
| Ambagrh Tehsil, India | Surface soil(20) | 58–302 (192 ± 28) | Sulfide minerals | Natural | 195 |
| Ambagrh Tehsil, India | Paddy soil (20) | 44–270 (126 ± 28) | Sulfide minerals | Natural | 159 |
| Ambagrh Tehsil, India | Rhizospheric soil (16) | 220–4600 (1600 ± 700) | Sulfide minerals | Natural | 194 |
| Ambagrh Tehsil, India | Pond sediment (24) | 10–256 (53 ± 26) | Sulfide minerals | Natural | 165 |
| South Africa | Garden soil (4) | 0.1–65.3 (18.3 ± 11.7) | Urban setting | Anthropogenic | 208 |
| West Bengal, India | Agricultural soil | 19.40 ± 0.38–41.24 ± 0.48 | Wet precipitation | Anthropogenic, natural | 197 |
Sediment
As is also present in the sediment bound to clay minerals and Fe, and Mn oxides, As is a redox sensitive element, present in the sediments of surface water reservoirs, formed by continuous weathering and erosion,214 and its bioavailability for biota is dependent on both physical parameters, e.g., pH, redox (200–500 mV), TOC (total organic carbon) and temperature.215–217 In general, when water conditions become oxidizing, at higher pH and organic values and low temperature, mobilized metals (As, Fe, etc.) is typically removed from solution as a solid precipitate, reducing the bioavailability of As for biota. The dissolution of oxides of Fe and Mn is found to be an effective mechanism for As mobility from sediment to groundwater.218,219
Examples of pollution with As of the sediments of various surface water bodies (ponds, lagoons, river estuaries and sea) are summarized in Table 4. Concentration range of As in sediment and soil is illustrated in Fig. 5 based on the data given in Table 4. Over all total As concentration in 17 different types of sediments originated from pond, river, lake, lagoon, sea and estuarine was observed in interval between 2.5-2500 mg kg−1 with maximum value in estuarine sediments due to industrial activities. The highest reported values are associated with metalliferous mining activities: a concentration of 484 mg kg−1 was registered in the stream sediments from Suratá river (southwestern area of Santurbán paramo, Colombia) near a gold mining area,220 and 2500 mg kg−1 was recorded in the sediments of estuaries in southwest England.221 Nonetheless, natural pollution can also lead to dangerous values: for instance, Ganga river – originated from Himalayas – carries sediments contaminated with As at concentrations in the 4.8–19.7 mg kg−1 range,196,222 and contents of up 37 mg kg−1 have been reported for Brahmaputra river – which flows from Southern Tibet.223
Fig. 5. Concentration range of As reported in sediment and soil around the world.
Remarkably high values of As in sediment were reported for two Indian locations, i.e. Ambagrh tehsil and Korba city, with values up to 256 and 154 mg kg−1, respectively. Pollution of As in these locations were attributed to arsenopyrite leaching165 and coal burning and alumina roasting,167 respectively.
As content in the Buffalo bottom sediments (0–90 cm depth) were identified over range of 14.03 to 26.78 mg kg−1 due to industrial activities.224 In the Florida lake sediment (30-45 cm depth), high concentration of As at 147.5 mg kg−1 was accumulated due to use of arsenical herbicide (Na(CH3)2AsO4) to the golf course and lawns throughout the state.225 Similarly, significantly high concentration of As at 160 mg kg−1 was observed in the red mud sediment (by-product of alumina production sludge) of Kinghorn Loch.26 The metalliferous mining activities concentrated remarkable high concentration of As at 2500 mg kg−1 in the estuary's sediments of estuaries of southwest England.221 Industrial activities also released As in the Brazilian lagoon, China coastal area, Egyptian lake, and Northern marine sediments over range of 2.5 to 239 mg kg−1, respectively.227–230
Arsenic health hazards
Arsenic is known to be a human carcinogen involving in the generation of reactive oxygen species (ROS), genetic change and signal transduction pathways. Humans are more sensitive than other animals due to their lower efficiency of arsenic methylation.231 It has been estimated that approximately 94 to 220 million people in the world are potentially exposed to groundwater containing excessive levels of As, most of whom are in Asia (94%) with some in Africa and South America.183
Human exposure to As in contaminated areas occurred through oral, respiratory, or dermal routes.232 Non-occupational human exposure to As is primarily associated with the ingestion of food and water. Food is generally the principal contributor to the daily intake of total arsenic, while drinking water can also be significant source of exposure in some areas.233 The daily intake of total As from food and beverages is generally between 20 and 300 μg per day.234 Pulmonary exposure may contribute up to approximately 10 μg per day in a smoker and about 1 μg per day in a non-smoker, which values can be higher in polluted areas.
The concentration of metabolites of inorganic As in urine (inorganic arsenic, MMA and DMA) was used as a biomarker for the exposure and is generally in the range from 5 to 20 μg As per L.235,241 In workplaces with up-to-date occupational hygiene practices, exposure concentrations generally do not exceed 100 μg m−3.236 Arsenic in hair and nails can be used as indicators of past As exposure, which requires to prevent external arsenic contamination of the samples.
The short-term effects of As exposure may include vomiting, diarrhea having blood, abdominal pain, dizziness, loss of sensation in limbs, skin problems, irritation, hair fall, muscle cramps, etc. Meanwhile, the long term As exposure can cause skin disorders and increased risk of diabetes, high blood pressure, and several types of cancer.237
Arsenic is a protoplasmic poisonous element mainly deactivate the sulfhydryl group of respiration, enzymes and mitosis cells. The toxic iAs (inorganic arsenic compounds) in human is methylated into non-toxic MMA and DMA, and excreted out through urine. However, the MMA is an intermediate species to form other arsenicals. Intake of iAs by air, water and/or food cause toxicity known as arsenicosis. Low exposure can lead adverse physiological effects (i.e., vomiting and nausea, damaging of blood vessels, reduction in production of erythrocytes and leukocytes, change in heartbeat and body sensation, etc.). However, the prolonged exposure can develop lesions in skin, cancers, diabetes mellitus, hypertension, and neurological, pulmonary, vascular, cardiovascular diseases.238 The health effect risk (i.e. composition and size, type, magnitude, frequency, route, and duration of exposure) associated with intake of iAs is assessed by multiple parameters, e.g. average total dose (ATD), estimated daily intake (EDI), hazard quotient (HQ), hazard index (HI), cancer risk (CR) and CRlim methods. Dose–response assessment quantitatively evaluates a relationship between the amount of exposure to a contaminant and the possibility of adverse health effects. The following equations (i)–(vi) are used for evaluation the health hazard parameters.239–241
| ATD = Asw × IR | i |
| EDI = Cm × DI/BW | ii |
| HQ = EDI/RfD | iii |
| HI = ∑HQi | iv |
| CRlim = RfD × BW/Cm | v |
| Cancer risk = CDI × (PF) | vi |
where, scripts Asw, IR, Cm, DI, BW, RfD, CDI, HI, HQi, CRlim and PF represent arsenic contamination of water (mg L−1), water ingestion rate (L per day), mean concentration of As in food, amount of food consumed per day (g per day), mean body weight of a person (kg), reference dose, chronic daily intake, hazard index, summation of HQ of noncarcinogens, the maximum allowable food consumption rate (kg d−1); and potency factor (mg per kg per day), respectively.
The permissible exposure limit for As in air, water and food recommended are 10 μg m−3 (over for 8 hour period), 10 μg L−1, and 0.5 mg kg−1,91,103 respectively. The recommended food dose (g per day) PF (oral route) for arsenic is 1.5 mg per kg per day, supposing that the cancer risk is acceptable when the value of cancer risk (CR) is lie in interval: 10−4 and 10−6. Dietary exposures to total arsenic were highly variable, with a mean of 50.6 μg per day (range of 1.01–1081 μg per day) for females and 58.5 μg per day (range of 0.21–1276 μg per day) for males. U.S. dietary intake of inorganic arsenic has been estimated to range from 1 to 20 μg per day, with grains and produce expected to be significant contributors to dietary inorganic arsenic intake.100,242–245 The permissible arsenic concentrations in water samples are 10 μg L−1 in the World Health Organization246 and United State Environmental Protection Agency (USEPA)240 guidelines for drinking water. In addition, guide levels for the protection of the aquatic biota and drinking water proposed by local authorities are 15 μg L−1 and 10 μg L−1 respectively.247 Accordingly, arsenic can cause adverse effects not only for humans but also for plant and animal species living in aquatic environments. Especially in aquatic ecosystem, arsenic concentration above the permissible limit affects various physiological systems such as growth, reproduction, ion regulation, mortification, gene expression, immune function, enzyme activities and histopathology of fish. Therefore, harmful effects may be seen for people who form the last link of the food chain and for human health.
However, in health index evaluation method factors i.e., the life style, age and diet quality are not considered. For example, young children, elderly and people with long-term illnesses are at greater risk. In addition, interactions of other toxicants, and bioavailability, bioaccumulation, background concentration level of the toxicant are not considered. Toxicity data are often unavailable, and evaluated data is not sufficient to verify dose–response curve and action mode.248
Conclusions
In this review, data on natural and anthropogenic sources and mobility of As in the various compartments of the environment (air, water, soil and sediment, and biota) in different parts of the World has been presented, together with their main biogeochemical relations. It's apparent that arsenic contamination is an alarming problem on a global scale for animal and human health. The smelters, coal based thermal power plants and biomass burnings are potential sources for arsenic emissions, and their uses should be minimized. The contaminated water, food and soil dusts poses the greatest threat to public health from arsenic. New insights on As contamination, exposure sources, mobility and toxicity mechanisms at molecular and gene levels for understanding of its adverse effect spectrum are required.
The scientific understanding of As is still evolving even with the foundation of knowledge already established by years of research on its origin, toxicities, mobility, distribution patterns, quantification, and exposure. Further study on its interactions should help in the development of methods of safe clean-up and exposure prevention all the way down to the trophic level of ecosystem.
Ethics approval
No ethical issue is declared in this review.
Data availability
All data are available in this review paper, and further information could be obtained from the corresponding author (https://docs.google.com/document/d/1kz2tTt-zfVyxRTJbbxKtPA-S7LSxPosA/edit?usp=sharing&ouid=102850718200296775047&rtpof=true&sd=true).
Author contributions
Khageshwar Singh Patel: conceptualization, data curation, investigation, methodology, validation, visualization, writing – original draft. Piyush Kant Pandey and Warren T. Corns: investigation, visualization, validation, editing. Pablo Martín-Ramos: data curation, graphic drafting, editing original draft. Simge Varol: software, graphics drafting, editing – original draft. Prosun Bhattacharya: review & editing. Yanbei Zhu: preparing figures, reviewing, and editing.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could influenced the work reported in this paper.
Supplementary Material
Acknowledgments
The University Grants Commission (UGC), New Delhi is gratefully acknowledged for providing financial support through BSR grant no. F.18-1/2011(BSR)2016.
Biographies
Biography
Khageshwar Singh Patel.

Dr Khageshwar Singh Patel is currently, research Professor in Department of Applied Sciences, Amity University, Baloda Bazar Road, Raipur-493225, CG, India. He is an Alexander von Humboldt (Germany), Asia Foundation (Sans Francisco) and BSF, UGC, New Delhi awardee fellow, and run several national and international projects. He published >150 research papers in journals of international repute and supervised 34 PhD students in his credit. His recent research interest is phytochemistry (identification of bioactive compounds), biofuel production and characterization as well as source appointment of toxic and strategic elements in environment.
Biography
Simge Varol.

Dr Simge Varol She was born in 1978 in Istanbul/Turkey and graduated from Geological Engineering Department of Süleyman Demirel University (SDU, Isparta, Turkey) in 2000. She completed her master's and doctorate degrees in 2006 and 2011, respectively, in Geological Engineering department of SDU. Her areas of expertise include topics such as hydrogeology, hydrology, hydrogeochemistry, medical geology, ground and surface water quality, and water chemistry. She has 80 national and international scientific articles, 35 scientific papers, and 4 scientific book chapters related to her field of expertise. She has also been the Deputy Director of Water Institute in SDU since 2018. She is married and has 2 sons.
Biography
Yanbei Zhu.

Yanbei Zhu, Senior researcher, National Metrology Institute of Japan (NMIJ), National Institute of Advanced Industrial Science and Technology (AIST), Japan. Yanbei received his PhD in March of 2005 from Nagoya University. After two years of postdoc research in Nagoya University, he joined NMIJ/AIST in April of 2007 and started the research on development of certified reference materials (CRMs) and related techniques for elemental analysis in food and environmental samples. Yanbei is focusing on quantitative elemental analysis based on ICP-MS related techniques, as well as development of devices and instruments fascinating the sample pretreatment process and on-site analysis.
References
- Ratnaike R. N. Postgrad. Med. J. 2003;79:391–396. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]; , https://pmj.bmj.com/content/postgradmedj/79/933/391.full.pdf
- Bhattacharyya P. and Alam M. M., Arsenic-contaminated soil toxicity and its mitigation through monocot crops, in Contaminants in Agriculture, ed. M. Naeem, A. Ansari and S. Gill, Springer, 2020 [Google Scholar]
- Mandal B. K. Suzuki K. T. Talanta. 2002;58:201–235. doi: 10.1016/S0039-9140(02)00268-0. [DOI] [PubMed] [Google Scholar]
- Shahid M., Imran M., Khalid S., Murtaza B., Niazi N. K., Zhang Y., Hussain I., Arsenic environmental contamination status in South Asia, Arsenic in Drinking Water and Food, 2020, pp. 13–39 [Google Scholar]
- Nordstrom D. K. Science. 2002;296:2143–2145. doi: 10.1126/science.1072375. [DOI] [PubMed] [Google Scholar]
- Pandey P. K. Yadav S. Nair S. Bhui A. Environ. Int. 2002;28:235–245. doi: 10.1016/s0160-4120(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Rahman M. M. Naidu R. Bhattacharya P. Environ. Geochem. Health. 2009;S1:9–21. doi: 10.1007/s10653-008-9233-2. [DOI] [PubMed] [Google Scholar]
- Shankar S. Shanker U. Sci. World J. 2014:304524. doi: 10.1155/2014/304524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A. Barla A. Singh S. Mandraha S. Bose S. J. Hazard. Mater. 2017;324:526–534. doi: 10.1016/j.jhazmat.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Sanyal T. Bhattacharjee P. Paul S. Bhattacharjee P. Front. Public Health. 2020;8:464. doi: 10.3389/fpubh.2020.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter K. F. Owens G. Davey D. E. Naidu R. Rev. Environ. Contam. Toxicol. 2005;184:97–149. doi: 10.1007/0-387-27565-7_3. [DOI] [PubMed] [Google Scholar]
- Alka S. Shahir S. Ibrahim N. Ndejiko M. J. Vo D. V. Manan F. A. J. Cleaner Prod. 2021;278:123805. doi: 10.1016/j.jclepro.2020.123805. [DOI] [Google Scholar]
- Sharma V. K. Sohn M. Environ. Int. 2009;35:743–759. doi: 10.1016/j.envint.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Baker B. A. Cassano V. A. Murray C. J. Occup. Environ. Med. 2018;60:e634–e639. doi: 10.1097/JOM.0000000000001485. [DOI] [PubMed] [Google Scholar]
- Smedley P. L. Kinniburgh D. G. Appl. Geochem. 2002;17:517–568. doi: 10.1016/S0883-2927(02)00018-5. [DOI] [Google Scholar]
- Bhadauria R. Horticult. Int. J. 2019;3:20–22. doi: 10.15406/hij.2019.03.00106. [DOI] [Google Scholar]
- Masuda H. Prog. Earth Planet. Sci. 2018;5:68. doi: 10.1186/s40645-018-0224-3. [DOI] [Google Scholar]
- IARC (International Agency for Research on Cancer), 2012, https://www.ncbi.nlm.nih.gov/books/NBK304375/
- Upadhyay M. K. Shukla A. Yadav P. Srivastava S. Food Chem. 2019;276:608–618. doi: 10.1016/j.foodchem.2018.10.069. [DOI] [PubMed] [Google Scholar]
- Wang C. Liu H. Zhang Y. Zou C. Anthony E. J. Prog. Energy Combust. Sci. 2018;68:1–28. doi: 10.1016/j.pecs.2018.04. [DOI] [Google Scholar]
- Yudovich Y. E. Ketris M. P. Arsenic in coal: a review. Int. J. Coal Geol. 2005;61:141–196. doi: 10.1016/j.coal.2004.09.003. [DOI] [Google Scholar]
- ENVIS (Environmental Information System), publisher (Ministry of Environment & Forest, Govt. of India), 2020, Arsenic -https://nbrienvis.nic.in [Google Scholar]
- D'Amato M. Forte G. Caroli S. Identification and quantification of major species of arsenic in rice. J. AOAC Int. 2004;87:238–243. doi: 10.1093/jaoac/87.1.238. [DOI] [PubMed] [Google Scholar]
- Davis W. Zeisler R. Sieber J. Yu L. Anal. Bioanal. Chem. 2010;396:3041–3050. doi: 10.1007/s00216-010-3541-y. [DOI] [PubMed] [Google Scholar]
- Mehouel F. Fowler S. W. Environ. Sci. Pollut. Res. 2022;29:3288–3301. doi: 10.1007/s11356-021-17130-0. [DOI] [PubMed] [Google Scholar]
- Rahman M. S. Kumar A. Kumar R. Ali M. Ghosh A. K. K Singh S. Indian J. Occup. Environ. Med. 2019;23:126–132. doi: 10.4103/ijoem.IJOEM_240_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J.-Y. Yu S.-D. Hong Y.-S. J. Prev. Med. Public Health. 2014;47:253–257. doi: 10.3961/jpmph.14.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju N. J. Environ. Res. 2022;203:111782. doi: 10.1016/j.envres.2021.111782. [DOI] [PubMed] [Google Scholar]
- Aftabtalab A. Rinklebe J. Shaheen S. M. Niazi N. K. Moreno-Jiménez E. Schaller J. Knorr K. H. Chemosphere. 2022;286:131790. doi: 10.1016/j.chemosphere.2021.131790. [DOI] [PubMed] [Google Scholar]
- Dadwal A. Mishra V. Clean: Soil, Air, Water. 2017;45:1600364. doi: 10.1002/clen.201600364. [DOI] [Google Scholar]
- Pigna M. Caporale A. Cavalca L. Sommella A. Violante A. Environ. Eng. Sci. 2015;32:150505074828006. doi: 10.1089/ees.2015.0018. [DOI] [Google Scholar]
- Yogarajah N. Tsai S. S. H. Environ. Sci.: Water Res. Technol. 2015;1:426–447. doi: 10.1039/C5EW00099H. [DOI] [Google Scholar]
- Harrington C. F. Clough R. Drennan-Harris L. R. Hilld S. J. Tysonc J. F. J. Anal. At. Spectrom. 2011;26:1561–1595. [Google Scholar]
- Xie R. Johnson W. Spayd S. Hall G. S. Buckley B. Anal. Chim. Acta. 2006;578:186–194. doi: 10.1016/j.aca.2006.06.076. [DOI] [PubMed] [Google Scholar]
- Sur R. Dunemann L. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2004;807:169–176. doi: 10.1016/j.jchromb.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Shi Y., Speciation of arsenic by chemical separations and neutron activation analysis, 2001, http://hdl.handle.net/10222/55802 [Google Scholar]
- Francesconi K. A. Kuehnelt D. Analyst. 2004;129:373–395. doi: 10.1039/b401321m. [DOI] [PubMed] [Google Scholar]
- Radke B. Jewell L. Namieśnik J. Crit. Rev. Anal. Chem. 2012;42:162–183. doi: 10.1080/10408347.2011.634637. [DOI] [Google Scholar]
- Dressler V. L. Antes F. G. Moreira C. M. Pozebon D. Duarte F. A. Int. J. Mass Spectrom. 2011;307:149–162. doi: 10.1016/j.ijms.2011.01.026. [DOI] [Google Scholar]
- Mesko M. F. Hartwig C. A. Bizzi C. A. Pereira J. S. Mello P. A. Flores E. M. Int. J. Mass Spectrom. 2011;307:123–136. doi: 10.1016/j.ijms.2011.03.002. [DOI] [Google Scholar]
- Yuan C. G. Jiang G. B. J. Anal. At. Spectrom. 2005;20:103–110. [Google Scholar]
- Simon S. Tran H. Pannier F. Potin-Gautier M. J. Chromatogr. A. 2004;1024:105–113. doi: 10.1016/j.chroma.2003.09.068. [DOI] [PubMed] [Google Scholar]
- Ackley K. L. B'Hymer C. Sutton K. L. Caruso J. A. J. Anal. At. Spectrom. 1999;14:845–850. doi: 10.1039/A807466F. [DOI] [Google Scholar]
- Gomez-Ariza J. L. Sanchez-Rodas D. Giraldez I. Morales E. Analyst. 2000;125:401–407. doi: 10.1039/A908884. [DOI] [Google Scholar]
- Cava-Montesinos P. Nilles K. Cervera M. L. de la Guardia M. Talanta. 2005;66:895–901. doi: 10.1016/j.talanta.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Gómez-Ariza J. L. Sánchez-Rodas D. Beltrán R. Giráldez I. Int. J. Environ. Anal. Chem. 1999;74:203–213. doi: 10.1080/03067319908031426. [DOI] [Google Scholar]
- Yuan C. G. Jiang G. B. J. Anal. At. Spectrom. 2005;20:103–110. doi: 10.1039/B416102E. [DOI] [Google Scholar]
- Bohari Y. Astruc A. J. Anal. At. Spectrom. 2001;16:774–778. doi: 10.1039/b101591p. [DOI] [Google Scholar]
- Johannes T. Elterena V. Water Res. 2000;36:2967. doi: 10.1016/S0043-1354(01)00527-9. [DOI] [Google Scholar]
- Muñoz O. Vélez D. Montoro R. Analyst. 1999;124:601–607. doi: 10.1039/A809426H. [DOI] [PubMed] [Google Scholar]
- Coelho N. M. M. Coelho L. M. De Lima E. S. Pastor A. De La Guardia M. Talanta. 2005;66:818–822. doi: 10.1016/j.talanta.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Reyes M. M. Cervera M. L. Campos R. C. De la Guardia M. Spectrochim. Acta, Part B. 2007;62:1078–1082. doi: 10.1016/j.sab.2007.07.011. [DOI] [Google Scholar]
- Heitkemper D. T. Vela N. P. Stewart K. R. Westphal C. S. J. Anal. At. Spectrom. 2001;16:299–306. doi: 10.1039/B0072411. [DOI] [Google Scholar]
- Vela N. P. Heitkemper D. T. J. AOAC Int. 2004;87:244–252. [PubMed] [Google Scholar]; , PMID: 15084107.
- Pizarro I. Gómez M. Palacios M. A. Cámara C. Anal. Bioanal. Chem. 2003;376:102–109. doi: 10.1007/s00216-003-1870-9. [DOI] [PubMed] [Google Scholar]
- Rezende H. C. Almeida I. L. Coelho L. M. Coelho N. M. Marques T. L. Sample Prep. 2014;2:31–48. doi: 10.2478/sampre-2014-0004. [DOI] [Google Scholar]
- Vieira M. A. Grinberg P. Bobeda C. R. Reyes M. N. Campos R. C. Spectrochim. Acta, Part B. 2009;64:459–476. doi: 10.1016/j.sab.2009.04.010. [DOI] [Google Scholar]
- Reyes M. N. M. Cervera M. L. Campos R. C. de la Guardia M. Talanta. 2008;75:811–816. doi: 10.1016/j.sab.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Vela N. P. Heitkemper D. T. Stewart K. R. Analyst. 2001;126:1011–1017. doi: 10.1039/B102420P. [DOI] [PubMed] [Google Scholar]
- Bohari Y. Astruc A. J. Anal. At. Spectrom. 2001;16:774–778. doi: 10.1039/B101591P. [DOI] [Google Scholar]
- Caruso J. A. Heitkemper D. T. B'Hymer C. Analyst. 2001;126:136–140. doi: 10.1039/b009825f. [DOI] [PubMed] [Google Scholar]
- Ai X. Wu L. Zhang M. Hou X. Yang L. Zheng C. J. Agric. Food Chem. 2014;62:8586–8593. doi: 10.1021/jf501638k. [DOI] [PubMed] [Google Scholar]
- Yu H. Li C. Tian Y. Jiang X. Microchem. J. 2020;152:104312. doi: 10.1016/j.microc.2019.104312. [DOI] [Google Scholar]
- Clough R. Harrington C. F. Hill S. J. Madrid Y. Tyson J. F. J. Anal. At. Spectrom. 2013;28:1153–1195. doi: 10.1039/C3JA90039H. [DOI] [Google Scholar]
- Mihaltan A. I. Frentiu T. Ponta M. Petreus D. Frentiu M. Darvasi E. Marutoiu C. Talanta. 2013;109:84–90. doi: 10.1016/j.talanta.2013.01.056. [DOI] [PubMed] [Google Scholar]
- Leybourne M. I. Johannesson K. H. Asfaw A. Rev. Mineral. Geochem. 2014;79:371–390. doi: 10.2138/rmg.2014.79.6. [DOI] [Google Scholar]
- Samanta G. Chowdhury T. R. Mandal B. K. Biswas B. K. Chowdhury U. K. Basu G. K. Chanda C. R. Lodh D. Chakraborti D. Microchem. J. 1999;62:174–191. doi: 10.1006/mchj.1999.1713. [DOI] [Google Scholar]
- Abdel-Lateef A. M. Mohamed R. A. Mahmoud H. H. Adv. Chem. Sci. 2013;2:110–113. [Google Scholar]
- Akter K. F. Owens G. Davey D. E. Naidu R. Rev. Environ. Contam. Toxicol. 2005;184:97–149. doi: 10.1007/0-387-27565-7_3. [DOI] [PubMed] [Google Scholar]
- Bühl V. Álvarez C. Kordas K. Pistón M. Mañay N. J. Environ. Pollut. Hum. Health. 2015;3:46–51. doi: 10.12691/jephh-3-2-4. [DOI] [Google Scholar]
- Pitzalis E. Ajala D. Onor M. Zamboni R. D'Ulivo A. Anal. Chem. 2007;79:6324–6333. doi: 10.1021/ac070513p. [DOI] [PubMed] [Google Scholar]
- Sutherland D. Swash P. M. Macqueen A. C. McWilliam L. E. Ross D. J. Wood S. C. Environ. Technol. 2002;23:385–403. doi: 10.1080/09593332508618444. [DOI] [PubMed] [Google Scholar]
- Rupasinghe T. Cardwell T. J. Cattrall R. W. Potter I. D. Kolev S. D. Anal. Chim. Acta. 2004;510:225–230. doi: 10.1016/j.aca.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Komorowicz I. Barałkiewicz D. Rapid Commun. Mass Spectrom. 2014;28:159–168. doi: 10.1002/rcm.6774. [DOI] [PubMed] [Google Scholar]
- Bortoleto G. G. Cadore S. Talanta. 2005;67:169–174. doi: 10.1016/j.talanta.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Gonzalvez A. Cervera M. L. Armenta S. De la Guardia M. Anal. Chim. Acta. 2009;636:129–157. doi: 10.1016/j.aca.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Herbello-Hermelo P. Barciela-Alonso M. C. Bermejo-Barrera A. Bermejo-Barrera P. J. Anal. At. Spectrom. 2005;20:662–664. doi: 10.1039/B416772D. [DOI] [Google Scholar]
- Narvaez J. Richter P. Toral M. I. Anal. Bioanal. Chem. 2005;381:1483–1487. doi: 10.1007/s00216-005-3122-7. [DOI] [PubMed] [Google Scholar]
- Coelho L. M. Coelho N. M. Arruda M. A. de la Guardia M. Talanta. 2007;71:353–358. doi: 10.1016/j.talanta.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Ronkart S. N. Laurent V. Carbonnelle P. Mabon N. Copin A. Barthélemy J. P. Chemosphere. 2007;66:738–745. doi: 10.1016/j.chemosphere.2006.07.056. [DOI] [PubMed] [Google Scholar]
- Komorowicz I. Barałkiewicz D. Talanta. 2011;84:247–261. doi: 10.1016/j.talanta.2010.10.065. [DOI] [PubMed] [Google Scholar]
- Morita Y. Kobayashi T. Kuroiwa T. Narukawa T. Talanta. 2007;73:81–86. doi: 10.1016/j.talanta.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Popp M. Hann S. Koellensperger G. Anal. Chim. Acta. 2010;668:114–129. doi: 10.1016/j.aca.2010.04.036. [DOI] [PubMed] [Google Scholar]
- Li X. Jia J. Wang Z. Anal. Chim. Acta. 2006;560:153–158. doi: 10.1016/j.aca.2005.12.054. [DOI] [Google Scholar]
- González J. C. Lavilla I. Bendicho C. Talanta. 2003;59:525–534. doi: 10.1016/S0039-9140(02)00541-6. [DOI] [PubMed] [Google Scholar]
- Hoek E. V. Elteren J. T. Comansi R. N. J. Int. J. Environ. Anal. Chem. 1996;63:67–79. doi: 10.1080/03067319608039811. [DOI] [Google Scholar]
- Ponthieu M. Pinel-Raffaitin P. Hecho I. Mazeas L. Amouroux D. Donard O. F. X. Potin-Gautier M. Water Res. 2007;41:3177–3185. doi: 10.1016/j.watres.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Li Y. Low G. K. Scott J. A. Amal R. Chemosphere. 2010;79:794–801. doi: 10.1016/j.chemosphere.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Leschber R., Davis R. D. and L'Hermite P., Chemical methods for assessing bio-available metals in sludges and soils, 1985 [Google Scholar]
- Száková J. Tlustoš P. Goessler W. Frková Z. Najmanová J. J. Hazard. Mater. 2009;172:1244–1251. doi: 10.1016/j.jhazmat.2009.07.143. [DOI] [PubMed] [Google Scholar]
- USEPA (US Environmental Protection Agency), The Arsenic Rule, 2015, https://www.epa.gov/sites/production/files/2015-09/documents/train1-background.pdf [Google Scholar]
- Ardini F. Dan G. Grotti M. J. Anal. At. Spectrom. 2020;35:215–237. doi: 10.1039/C9JA00333A. [DOI] [Google Scholar]
- Kamal M. Z. U., Miah M. Y., Arsenic Speciation Techniques in Soil Water and Plant: An Overview, Arsenic Monitoring, Removal and Remediation, Intech Open, 2021, 10.5772/intechopen.99273 [DOI] [Google Scholar]
- Reid M. S. Hoy K. S. Schofield J. R. M. Uppal J. S. Lin Y. Lu X. Peng H. Le X. C. TrAC, Trends Anal. Chem. 2020;123:115770. doi: 10.1016/j.trac.2019.115770. [DOI] [Google Scholar]
- Stetson S. J. Lawrence C. Whitcomb S. Kanagy C. MethodsX. 2021;8:101183. doi: 10.1016/j.mex.2020.101183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi K. A. Kuehnelt D. Analyst. 2004;129:373–395. doi: 10.1039/b401321m. [DOI] [PubMed] [Google Scholar]
- IPCS (International Programme on Chemical Safety), Arsenic and arsenic compounds, WHO, 2001 [Google Scholar]
- Frankenberger W. T., Environmental Chemistry of Arsenic, Marcel Dekker, Inc, New York, 2002 [Google Scholar]
- Matschullat J. Sci. Total Environ. 2000;249:297–312. doi: 10.1016/S0048-9697(99)00524-0. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry), Toxicological profile for arsenic, Update, U.S. Department of Health & Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, 2007, http://www.atsdr.cdc.gov/toxprofiles/tp2.html [Google Scholar]
- WHO (World Health Organization), Arsenic and arsenic compounds, World Health Organization, International Programme on Chemical Safety, Geneva, 2nd edn, 2001, Environmental Health Criteria 224 [Google Scholar]
- Baker B. A. Cassano V. A. Murray C. J. Occup. Environ. Med. 2018;60:e634–e639. doi: 10.1097/JOM.0000000000001485. [DOI] [PubMed] [Google Scholar]
- USOSHA (US Occupational Safety and Health Administration), 29 CFR 1910.1018. OSHA Standard for inorganic arsenic, 2001, https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=10023, accessed August 8, 2022 [Google Scholar]
- Deering K. Spiegel E. Quaisser C. Nowak D. Schierl R. Reilly S. B. Garí M. Environ. Monit. Assess. 2019;191:375. doi: 10.1007/s10661-019-7495-z. [DOI] [PubMed] [Google Scholar]
- Duan J. Tan J. Atmos. Environ. 2013;74:93–101. doi: 10.1016/j.atmosenv.2013.03.031. [DOI] [Google Scholar]
- Fang G.-C. Lin C.-C. Huang J.-H. Huang Y.-L. Aerosol Air Qual. Res. 2011;11:218–229. doi: 10.4209/aaqr.2010.09.0075. [DOI] [Google Scholar]
- Gummeneni S. Yusup Y. B. Chavali M. Samadi S. Z. Atmos. Res. 2011;101:752–764. doi: 10.1016/j.atmosres.2011.05.002. [DOI] [Google Scholar]
- Gupta S., Studies on trace element composition and sources of aerosols, PhD thesis submitted to Pt. Ravishankar Shukla University, Raipur, India, 2010 [Google Scholar]
- Islam M. F. Majumder S. S. Al Mamu A. Khan Md. B. Rahman M. A. Salam A. Open J. Air Pollut. 2015;4:86–98. doi: 10.4236/ojap.2015.42009. [DOI] [Google Scholar]
- Jalees M. I. Asim Z. Earth Sci. 2016;75:842. doi: 10.1007/s12665-016-5604-7. [DOI] [Google Scholar]
- Kar S. Nath B. Samal A. C. Santra S. C. Curr. Sci. 2006;90:158–160. [Google Scholar]
- Lu X. Li L. Y. Wang L. Lei K. Huang J. Zhai Y. Atmos. Environ. 2009;43:2489–2496. doi: 10.1016/j.atmosenv.2009.01.048. [DOI] [Google Scholar]
- Mao X. Hu X. Wang Y. Xia W. Zhao S. Wan Y. Environ. Sci. Pollut. Res. 2020;17:21654–21665. doi: 10.1007/s11356-020-08626-2. [DOI] [PubMed] [Google Scholar]
- Middleton D. R. S. Watts M. J. Beriro D. J. Hamilto E. M. Leonardi G. S. Fletcher T. Close R. M. Polya D. A. Environ. Sci.: Processes Impacts. 2017;19:517–527. doi: 10.1039/C6EM00690F. [DOI] [PubMed] [Google Scholar]
- Park E. Kim D. Park C. Song S. Lee B. Hon Y. Pan X. Wang J. Zhang Y. Park K. Korean J. Environ. Health Sci. 2010;36:60–372. doi: 10.5668/JEHS.2010.36.5.360. [DOI] [Google Scholar]
- Patel K. S. Yadav A. Sahu Y. K. Lata L. Milosh H. Corns W. T. Martín-Ramos P. J. Hazard., Toxic Radioact. Waste. 2020;24:05019006. doi: 10.1061/(ASCE)HZ.2153-5515.0000475. [DOI] [Google Scholar]
- Rabano E. S. Castillo N. T. Torre K. J. Solomon P. A. J. Air Waste Mange. 1989;39:76–80. doi: 10.1080/08940630.1989.10466511. [DOI] [PubMed] [Google Scholar]
- Sánchez-Rodas D. Ana M. Campa S. Rosa J. D. Oliveira V. Gómez-Ariza J. L. Querol X. Alastuey A. Chemosphere. 2007;66:1485–1493. doi: 10.1016/j.chemosphere.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Tsai Y. Kuo S. Lin Y. Atmos. Environ. 2003;37:3401–3411. doi: 10.1016/S1352-2310(03)00358-3. [DOI] [Google Scholar]
- Wang S. Mulligan C. N. Sci. Total Environ. 2006;366:701–721. doi: 10.1016/j.scitotenv.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Wang J. Wan Y. Cheng L. Xia W. Li Y. Xu S. Atmos. Pollut. Res. 2020;11:785–792. doi: 10.1016/j.apr.2020.01.006. [DOI] [Google Scholar]
- Roble A. D. Polizzi P. Romero M. B. Boudet L. N. C. Medici S. Costas A. Gerpe M. Environ. Earth Sci. 2016;75:1479. doi: 10.1007/s12665-016-6273-2. [DOI] [Google Scholar]
- Nriagu J. O. Bhattacharya P. Mukherjee A. B. Bundschuh J. Zevenhoven R. Loeppert R. H. Trace Met. Other Contam. Environ. 2007;9:3–60. doi: 10.1016/S1875-1121(06)09001-8. [DOI] [Google Scholar]
- Smedley P. L. Knudsen J. Maiga D. Appl. Geochem. 2007;22:1074–1092. doi: 10.1016/j.apgeochem.2007.01.001. [DOI] [Google Scholar]
- Chakraborty M. Mukherjee A. Ahmed K. M. Curr. Pollut. Rep. 2015;1:220–247. doi: 10.1007/s40726-015-0022-0. [DOI] [Google Scholar]
- Akhtar N. Syakir Ishak M. I. Bhawani S. A. Umar K. Water. 2021;13:2660. doi: 10.3390/w13192660. [DOI] [Google Scholar]
- Pradhan B. Chand S. Chand S. Rou P. R. Naik S. K. Groundwater Sustain. Develop. 2023;20:100868. doi: 10.1016/j.gsd.2022.100868. [DOI] [Google Scholar]
- Wang Y. Y., Chai L. Y. and Yang W. C., in Arsenic Pollution Control in Nonferrous Metallurgy, ed. L.Y. Chai, Springer, Singapore, 2019, pp. 1–15, 10.1007/978-981-13-6721-2_1 [DOI] [Google Scholar]
- Penezić A. Tercier-Waeber M. L. Abdou M. Bossy C. Dutruch L. Bakker E. Schäfer J. Mar. Chem. 2020;223:103804. doi: 10.1016/j.marchem.2020.103804. [DOI] [Google Scholar]
- Qaiser F. U. R. Zhang F. Pant R. R. Zeng C. Khan N. G. Wang G. Sci. Total Environ. 2023;857:159408. doi: 10.1016/j.scitotenv.2022.159408. [DOI] [PubMed] [Google Scholar]
- Chen Y. W. Yu X. Belzile N. J. Geochem. Explor. 2019;205:106349. doi: 10.1016/j.gexplo.2019.106349. [DOI] [Google Scholar]
- Zheng Q. Tu S. Hou J. Ni C. Wang M. Ren L. Wang M. Cao M. Xiong S. Tan W. Water Res. 2021;203:117558. doi: 10.1016/j.watres.2021.117558. [DOI] [PubMed] [Google Scholar]
- Astles B. C. Chételat J. Palmer M. J. Vermaire J. C. PLoS One. 2022;17:e0279412. doi: 10.1371/journal.pone.0279412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung S. R. Hull E. A. Burkart K. Gawel J. E. Horner-Devine A. R. Neumann R. B. Water Resour. Res. 2022;58:e2022WR032564. doi: 10.1029/2022WR032564. [DOI] [Google Scholar]
- Raber G. Francesconi K. A. Irgolic K. J. Goessler W. Fresenius' J. Anal. Chem. 2000;367:181–188. doi: 10.1007/s002160051621. [DOI] [PubMed] [Google Scholar]
- Flora S. J., Arsenic: chemistry, occurrence, and exposure, in Handbook of arsenic toxicology, Academic Press, 2015, pp. 1–49 [Google Scholar]
- Jang Y. C. Somanna Y. Kim H. J. I. J. Int. J. Appl. Environ. Sci. 2016;11:559–581. [Google Scholar]
- Brusseau M. L. Chorover J. Chapter 8-Chemical Processes Affecting Contaminant Transport and Fate. Environ. Pollut. Sci. 2020:113–130. doi: 10.1016/B978-0-12-814719-1.00008-2. [DOI] [Google Scholar]
- Alonso D. L. Pérez R. Okio C. K. Y. A. Castillo E. J. Environ. Manage. 2020;264:110478. doi: 10.1016/j.jenvman.2020.110478. [DOI] [PubMed] [Google Scholar]
- Hussain M. M. Wang J. Bibi I. Shahid M. Niazi N. K. Iqbal J. Mian I. A. Shaheen S. M. Bashir S. Shah N. S. Rinklebe J. J. Hazard. Mater. 2021;403:124027. doi: 10.1016/j.jhazmat.2020.124027. [DOI] [PubMed] [Google Scholar]
- Meng X. Dupont R. R. Sorensen D. L. Jacobson A. R. McLean J. E. Appl. Geochem. 2016;66:250–263. doi: 10.1016/j.apgeochem.2016.01.004. [DOI] [Google Scholar]
- Abdel-Moati A. R. Water, Air, Soil Pollut. 1990;51:117–132. doi: 10.1007/BF00211509. [DOI] [Google Scholar]
- Asante K. A. Agusa T. Subramanian A. Ansa-Asare O. D. Biney C. A. Tanabe S. Chemosphere. 2007;66:1513–1522. doi: 10.1016/j.chemosphere.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P. Sracek O. Eldvall B. Asklund R. Barmen G. Jacks G. Koku J. Gustafsson J.-E. Singh N. Balfors B. B. J. Afr. Earth Sci. 2012;66–67:72–84. doi: 10.1016/j.jafrearsci.2012.03.005. [DOI] [Google Scholar]
- Dzoma B. M. Moralo R. A. Motsei L. E. Ndou R. V. Bakunzi F. R. J. Anim. Vet. Adv. 2010;9:3026–3033. doi: 10.3923/javaa.2010.3026.3033. [DOI] [Google Scholar]
- El Hachimi M. L. El Founti L. Bouabdli A. Saidi N. Fekhoui M. Tassé N. Rev. Sci. Eau. 2007;20:1–13. doi: 10.7202/014903ar. [DOI] [Google Scholar]
- Gbadebo A. M., 32nd International Geological Congress, Florence, Italy, CRC Press, 2005, pp. 85–92, 10.1201/9780203970829 [DOI] [Google Scholar]
- George G. Gqaza B. M. J. Adv. Agric. Technol. 2015;2:29–35. doi: 10.12720/joaat.2.1.29-33. [DOI] [Google Scholar]
- Huntsman-Mapila P. Mapila T. Letshwenyo M. Wolski P. Hemond C. Appl. Geochem. 2006;21:1376–1391. doi: 10.1016/j.apgeochem.2006.05.003. [DOI] [Google Scholar]
- Jonnalagadda S. B. Nenzou G. J. Environ. Sci. Health, Part A: Environ. Sci. Eng. Toxic Hazard. Subst. Control. 1996;11:2547–2555. doi: 10.1080/10934529609376509. [DOI] [Google Scholar]
- Katsoyiannis I. A. Mitrakas M. Zouboulis A. I. Desalin. Water Treat. 2015;54:2100–2107. doi: 10.1080/19443994.2014.933630. [DOI] [Google Scholar]
- Kusimi J. M. Kusimi B. A. J. Geochem. Explor. 2012;112:252–261. doi: 10.1016/j.gexplo.2011.09.003. [DOI] [Google Scholar]
- Lazareva E. V. Shuvaeva O. V. Tsimbalist V. G. Geochem.: Explor., Environ., Anal. 2002;2:263–268. doi: 10.1144/1467-787302-030. [DOI] [Google Scholar]
- Machado I. Falchi L. Bühl V. Mañay N. Sci. Total Environ. 2020;721:137787. doi: 10.1016/j.scitotenv.2020.137787. [DOI] [PubMed] [Google Scholar]
- Mladenov N. Wolski P. Hettiarachchi G. M. Murray-Hudson M. Enriquez H. Damaraju S. Masamba W. J. Hydrol. 2014;518:326–341. doi: 10.1016/j.jhydrol.2013.09.026. [DOI] [Google Scholar]
- Mirlean N. Andrus V. E. Baisch P. Griep G. Casartelli M. R. Mar. Pollut. Bull. 2003;46:1480–1484. doi: 10.1016/S0025-326X(03)00257-1. [DOI] [PubMed] [Google Scholar]
- Mudzielwana R. Gitari M. W. Akinyemi S. A. Talabi A. O. Ndungu P. Groundwater Sustain. Develop. 2020;10:100336. doi: 10.1016/j.gsd.2020.100336. [DOI] [Google Scholar]
- Patel K. S. Dahariya N. S. Chakradhari S. Sahu P. K. Rajhans K. P. Ramteke S. Lata L. Milos H. Am. J. Anal. Chem. 2015;6:787–796. doi: 10.4236/ajac.2015.610075. [DOI] [Google Scholar]
- Patel K. S. Sahu B. L. Ramteke S. Bontempi E. J. Environ. Prot. 2016;7:689–698. doi: 10.4236/jep.2016.75061. [DOI] [Google Scholar]
- Patel K. S. Sahu B. L. Dahariya N. S. Bhatia A. Patel R. K. Matini L. Sracek O. Bhattacharya P. Appl. Water Sci. 2017;7:1817–1826. doi: 10.1007/s13201-015-0355-2. [DOI] [Google Scholar]
- Rango T. Bianchini G. Beccaluva L. Tassinari R. J. Afr. Earth Sci. 2010;57:479–491. doi: 10.1016/j.jafrearsci.2009.12.005. [DOI] [Google Scholar]
- Rango T. Vengosh A. Dwyer G. Bianchini G. Water Res. 2013;47:5801–5818. doi: 10.1016/j.watres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Reimann C. Bjorvatn K. Frengstad B. Melaku Z. Tekle-Haimanot R. Siewers U. Sci. Total Environ. 2003;311:65–80. doi: 10.1016/S0048-9697(03)00137-2. [DOI] [PubMed] [Google Scholar]
- Rezaie-Boroon M. H. Gnandi K. Folly K.-M. Fresenius Environ. Bull. 2011;20:1853–1865. [Google Scholar]; . http://www.psp-parlar.de
- Sahu B. L. Ramteke S. Rajhans K. P. Patel K. S. Wysocka I. Jaron I. Water Resour. 2018;45:992–1001. doi: 10.1134/S0097807818060131. [DOI] [Google Scholar]
- Serfor-Armah Y. Nyarko B. J. B. Dampare S. B. Adomako D. Water, Air, Soil Pollut. 2006;175:181–192. doi: 10.1007/s11270-006-9127-9. [DOI] [Google Scholar]
- Sharma R. Patel K. S. Lata L. Milosh H. J. Environ. Prot. 2017;8:358–379. doi: 10.4236/jep.2017.83027. [DOI] [Google Scholar]
- Welch A. H. Lico M. S. Hughes J. L. Groundwater. 1988;26:333–347. doi: 10.1111/j.1745-6584.1988.tb00397.x. [DOI] [Google Scholar]
- Yadav A. Sahu P. Chakradhari S. Rajhans K. P. Ramteke S. Dahariya N. Patel K. S. Agnihotri G. J. Environ. Prot. 2016;7:52–59. doi: 10.4236/jep.2016.71005. [DOI] [Google Scholar]
- FAO (Food and Agricultural Organization), Water quality guidelines for Maximum Crop Production, Food and Agricultural Organization/UN, Rome, Italy, 1985 [Google Scholar]
- WHO (World Health Organization), Arsenic in Drinking-water, World Health Organization, Geneva, Switzerland, 2011 [Google Scholar]
- Ahmad S. A. Khan M. H. Haque M. Risk Manag. Healthc. Pol. 2018;11:251–261. doi: 10.2147/RMHP.S153188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. Lu Y. Wang C. Zhang M. Yuan J. Zhang A. Song S. Baninla Y. Khan K. Wang Y. Ecotoxicol. Environ. Saf. 2019;186:109628. doi: 10.1016/j.ecoenv.2019.109628. [DOI] [PubMed] [Google Scholar]
- Saha R. Dey N. C. Rahman M. Bhattacharya P. Rabbani G. H. Front. Environ. Sci. 2019;7:57. doi: 10.3389/fenvs.2019.00057. [DOI] [Google Scholar]
- Shahid M. Niazi N. K. Dumat C. Naidu R. Khalid S. Rahman M. M. Bibi I. Environ. Pollut. 2018;242(A):307–319. doi: 10.1016/j.envpol.2018.06.083. [DOI] [PubMed] [Google Scholar]
- Singh A. K., Proceedings of 11th National Symposium on Hydrology with focal theme on Water Quality, Roorkee, 2004 [Google Scholar]
- Thakur A. K. Gupta V. Bhattacharya P. Jakariya M. Islam M. T. Groundwater Sustain. Develop. 2021;12:100504. doi: 10.1016/j.gsd.2020.100504. [DOI] [Google Scholar]
- Irunde R. Ijumulana J. Ligate F. Maity J. Ahmad A. Mtamba J. Mtalo F. Bhattacharya P. Groundwater Sustain. Develop. 2022;18:100746. doi: 10.1016/j.gsd.2022.100746. [DOI] [Google Scholar]
- Abiye T. A. Bhattacharya P. Arsenic concentration in groundwater: Archetypal study from South Africa. Groundwater Sustain. Develop. 2019;9:100246. doi: 10.1016/j.gsd.2019.100246. [DOI] [Google Scholar]
- Quino Lima I. Ormachea Muñoz M. Ramos Ramos O. E. Bhattacharya P. Quispe Choqu R. Quintanilla Aguirre J. Sracek O. Groundwater Sustain. Develop. 2019;8:281–293. doi: 10.1016/j.gsd.2018.11.013. [DOI] [Google Scholar]
- Litter M. I. Armienta M. A. Villanueva-Estrada R. E. Lepori E. C. V. Olmos V. Sci. Rev. 2019;1:54–73. [Google Scholar]; . https://www.researchgate.net/publication/337714294
- Litter M. I. Ingallinella A. M. Olmos V. Savio M. Difeo G. Botto L. Farfan M. Taylor S. Frangie S. Herkovits J. Schalamuk I. González M. J. Berardozzi E. Einschlag F. S. G. Bhattacharya P. Ahmad A. Sci. Total Environ. 2019;676:756–766. doi: 10.1016/j.scitotenv.2019.04.262. [DOI] [PubMed] [Google Scholar]
- Podgorski J. Berg M. Science. 2020;368:845–850. doi: 10.1126/science.aba1510. [DOI] [PubMed] [Google Scholar]
- Rowland H. Omoregie E. Millot R. Jimenez C. Mertens J. Baciu C. Hug S. Berg M. Appl. Geochem. 2011;26:1–17. doi: 10.1016/j.apgeochem.2010.10.006. [DOI] [Google Scholar]
- Molinari A. Guadagnini L. Marcaccio M. Guadagnini A. Water Res. 2019;149:522–532. doi: 10.1016/j.watres.2018.09.049. [DOI] [PubMed] [Google Scholar]
- Reyes F. A. P. Crosta G. B. Frattini P. Basiricò S. Pergola D. R. J. Hydrol. 2015;529:1530–1549. doi: 10.1016/j.jhydrol.2015.08.029. [DOI] [Google Scholar]
- Abbas G. Murtaza B. Bibi I. Shahid M. Niazi N. K. Khan M. I. Amjad M. Hussain M. Natasha T. Int. J. Environ. Res. Public Health. 2018;15:59. doi: 10.3390/ijerph15010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itabashi T. Li J. Hashimoto Y. Ueshima M. Sakanakura H. Yasutaka T. Imoto Y. Hosomi M. Environ. Sci. Technol. 2019;53:14186–14193. doi: 10.1021/acs.est.9b03864. [DOI] [PubMed] [Google Scholar]
- ITRC (Technology and Regulatory Council), Arsenic: Fate and transport, Washington, DC, 2017, https://bcs-1.itrcweb.org/7-1-fate-and-transport/ [Google Scholar]
- Violante A., in Advances in agronomy, ed. D. L. Sparks, Elsevier Inc., Newark (DE), 2013, pp. 111–176, 10.1016/B978-0-12-405942-9.00003-7 [DOI] [Google Scholar]
- Masscheleyn P. H. Delaune R. D. Patrick W. H. Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environ. Sci. Technol. 1991;25:1414–1419. doi: 10.1021/es00020a008. [DOI] [Google Scholar]
- Azam S. M. G. G. Sarker T. C. Naz S. Toxicol. Mech. Methods. 2016;26:565–579. doi: 10.1080/15376516.2016.1230165. [DOI] [PubMed] [Google Scholar]
- B Singh S. Srivastava P. K. SN Appl. Sci. 2020;2:153. doi: 10.1007/s42452-019-1932-z. [DOI] [Google Scholar]
- Patel K. S. Sahu B. L. Ramteke S. Jaiswal N. K. Borgese L. Gianoncelli A. Bontempi E. Am. J. Anal. Chem. 2015;6:822–829. doi: 10.4236/ajac.2015.610078. [DOI] [Google Scholar]
- Yadav A. Sahu P. K. Patel K. S. Lata L. Milosh H. Corns W. T. Allen J. Martín-Ramos P. J. Hazard., Toxic Radioact. Waste. 2020;24 doi: 10.1061/(ASCE)HZ.2153-5515.0000478. [DOI] [Google Scholar]
- Kumar M. Ramanathan A. L. Rahman M. M. Naidu R. Sci. Total Environ. 2016;573:1103–1114. doi: 10.1016/j.scitotenv.2016.08.109. [DOI] [PubMed] [Google Scholar]
- Shrivastava A. Barla A. Singh S. Mandraha S. Bose S. J. Hazard. Mater. 2017;324:526–534. doi: 10.1016/j.jhazmat.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Srivastava S. Sharma Y. K. Environ. Monit. Assess. 2013;185:4995–5002. doi: 10.1007/s10661-012-2920-6. [DOI] [PubMed] [Google Scholar]
- Sharma R. Yadav A. Ramteke S. Patel K. S. Lata L. Milosh H. Corns W. T. Martín-Ramos P. J. Hazard., Toxic Radioact. Waste. 2019;23 doi: 10.1061/(ASCE)HZ.2153-5515.0000460. [DOI] [Google Scholar]
- Ali M. A., Badruzzaman A. B. M., Jalil M. A., Hossain M. D., Ahmed M. F., Masud A. A., Kamruzzaman Md. and Rahman M. A., Arsenic in Plant-Soil Environment in Bangladesh, 2003, https://www.researchgate.net/publication/237455644_Arsenic_in_Plant-Soil_Environment_in_Bangladesh [Google Scholar]
- Arain M. B. Kazi T. G. Baig J. A. Jamali M. K. Afridi H. I. Shah A. Q. Jalbani N. Sarfraz R. A. Food Chem. Toxicol. 2009;47:242–248. doi: 10.1016/j.fct.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Brahman K. D. Kazi T. G. Baig J. A. Afridi H. I. Khan A. Arain S. S. Arain M. B. Chemosphere. 2014;110:182–189. doi: 10.1016/j.chemosphere.2013.11.035. [DOI] [PubMed] [Google Scholar]
- Dahal A. M. Fuerhacker M. Mentler A. Karki K. B. Shrestha R. R. Blum W. E. H. Environ. Pollut. 2008;155:157–163. doi: 10.1016/j.envpol.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Gong Y. Qu Y. Yang S. Tao S. Shi T. Liu Q. Chen Y. Wu Y. Ma J. Environ. Res. 2020;186:109525. doi: 10.1016/j.envres.2020.109525. [DOI] [PubMed] [Google Scholar]
- Higgins M. A. Metcalf M. J. Robbins G. A. J. Environ. Qual. 2022;51:66–77. doi: 10.1002/jeq2.20304. [DOI] [PubMed] [Google Scholar]
- Huang Y. Miyauchi K. Endo G. Le Don D. Manh N. C. Inoue C. Earth Sci. 2016;75:757. doi: 10.1007/s12665-016-5535-3. [DOI] [Google Scholar]
- Karimi N. Ghaderian S. M. Schat H. Int. J. Environ. Sci. Technol. 2013;10:743–752. doi: 10.1007/s13762-013-0227-y. [DOI] [Google Scholar]
- Mathee A. Kootbodien T. Kapwata T. Naicker N. Environ. Res. 2018;167:524–527. doi: 10.1016/j.envres.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Meharg A. A. Rahman Md. M. Environ. Sci. Technol. 2003;37:229–234. doi: 10.1021/es0259842. [DOI] [PubMed] [Google Scholar]
- Rehman Z. U. Khan S. Qin K. Brusseau M. L. Shah M. T. Din I. Sci. Total Environ. 2016;550:321–329. doi: 10.1016/j.scitotenv.2016.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandsalimi S. Karimi N. Kohandel A. Int. J. Environ. Sci. Technol. 2011;8:331–338. doi: 10.1007/BF03326220. [DOI] [Google Scholar]
- Zhang Y. Zhu Y. Zhao S. Li D. Xi H. Wang Y. Environ. Sci. Pollut. Res. Int. 2022;29:28957–28972. doi: 10.1007/s11356-021-18482-3. [DOI] [PubMed] [Google Scholar]
- Zhou Y. Niu L. Liu K. Yin S. Liu W. Sci. Total Environ. 2018;616–617:56–163. doi: 10.1016/j.scitotenv.2017.10.232. [DOI] [PubMed] [Google Scholar]
- Dargahi A., Reservoir Sedimentation, in Encyclopedia of Lakes and Reservoirs. Encyclopedia of Earth Sciences Series, ed. L. Bengtsson, R. W. Herschy and R. W. Fairbridge, Springer, Dordrecht, 2012, 10.1007/978-1-4020-4410-6 [DOI] [Google Scholar]
- John A. and Leventhal J. S., Bioavailability of metals. Preliminary Compilation of Descriptive Geoenvironmental Mineral Deposit Models, U.S. Geological Survey Open-File Report, 1995, vol. 95–831, pp. 10–19, https://pubs.usgs.gov/of/1995/0831/report.pdf [Google Scholar]
- Kominkova B., Nabelkova J. and Starmanova D., in Urban Environment. Alliance for Global Sustainability Bookseries (Science and Technology: Tools for Sustainable Development), ed. S. Rauch and G. Morrison, Springer, Dordrecht, 2012, vol. 19, 10.1007/978-94-007-2540-9_28 [DOI] [Google Scholar]
- Lamie P. O., Proceedings of the annual international conference on soils, sediments, water and energy, 2012, vol. 17, article 3, https://scholarworks.umass.edu/soilsproceedings/vol17/iss1/3 [Google Scholar]
- Hossain S. Oguchi C. T. Hachinohe S. Ishiyama T. Hamamoto H. Geophys. Res. 2014;16:4807. [Google Scholar]
- Nikolaidis N. P. Dobbs G. M. Chen J. Lackovic J. A. Environ. Pollut. 2004;129:479–487. doi: 10.1016/j.envpol.2003.11.005. [DOI] [PubMed] [Google Scholar]; , PMID: 15016468.
- Alonso A. L. Pérez R. Okio C. K. Y. A. Castillo E. J. Environ. Manage. 2020;264:110478. doi: 10.1016/j.jenvman.2020.110478. [DOI] [PubMed] [Google Scholar]
- J Langston W. J. Mar. Biol. Assoc. 1980;60:869–881. doi: 10.1017/S0025315400041953. [DOI] [Google Scholar]
- Kumar M. Ramanathan A. L. Int. J. Environ. Res. Public Health. 2018;15:183. doi: 10.3390/ijerph15020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borah R. Taki K. Gogoi A. Das P. Kumar M. Ecotoxicol. Environ. Saf. 2018;161:769–776. doi: 10.1016/j.ecoenv.2018.06.038. [DOI] [PubMed] [Google Scholar]
- Gawedzki A. Forsythe K. W. Int. J. Ecol. 2012:496740. doi: 10.1155/2012/496740. [DOI] [Google Scholar]
- Whitmore T. J. Riedinger-Whitmore M. A. Smoak J. M. Kolasa K. V. Goddard E. A. Bindler R. J. Paleolimnol. 2008;40:869–884. doi: 10.1007/s10933-008-9204-8. [DOI] [Google Scholar]
- Olszewska J. P. Meharg A. A. Heal K. V. Carey M. Gunn I. D. M. Searle K. R. Winfield I. J. Spears B. M. Environ. Sci. Technol. 2016;50:9044–9052. doi: 10.1021/acs.est.6b00942. [DOI] [PubMed] [Google Scholar]
- El-Badry A. E.-M. A. Khalifa M. M. Ecologia. 2017;7:20–28. doi: 10.3923/ecologia.2017.20.28. [DOI] [Google Scholar]
- Leivuori M. and Vallius H., in: Arsenic in marine sediments, ed. K. Loukola-Ruskeeniemi and P. Lahermo, Arsenic in Finland: Distribution, Environmental Impacts and Risks, Geological Survey of Finland, 2004, pp. 89–96. (in Finnish with synopsis in English) [Google Scholar]
- Liu X. Zeng B. Lin G. Mar. Pollut. Bull. 2022;175:113350. doi: 10.1016/j.marpolbul.2022.113350. [DOI] [PubMed] [Google Scholar]
- Mirlean N. Andrus V. E. Baisch P. Griep G. Casartelli M. R. Mar. Pollut. Bull. 2003;46:1480–1484. doi: 10.1016/S0025-326X(03)00257-1. [DOI] [PubMed] [Google Scholar]
- Zhou Q. Xi S. Regul. Toxicol. Pharmacol. 2018;99:78–88. doi: 10.1016/j.yrtph.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Hong Y.-S. Song K.-H. Chung J.-Y. J. Prev. Med. Public Health. 2014;47:245–252. doi: 10.3961/jpmph.14.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Darani K. Rehman Y. Katsoyiannis I. A. Kokkinos E. Zouboulis A. I. Water. 2022;14:1884. doi: 10.3390/w14121884. [DOI] [Google Scholar]
- Ng J., Gomez-Caminero A., Howe P., Hughes M., Kenyon E. M., Lewis D. R., R Moore M.., Aitio A. and Becking G.. Environmental Health Criteria 224: Arsenic and Arsenic Compounds Beds. WHO, ILO & UNEP, Geneva, 2001 [Google Scholar]
- Hughes M. F. Environ. Health Perspect. 2006;114:1790–1796. doi: 10.1289/ehp.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch K. Higgins I. Oh M. S. Burchfiel C. Arch. Environ. Health. 2013;37:325–335. doi: 10.1080/00039896.1982.10667586. [DOI] [PubMed] [Google Scholar]
- Abdul K. S. M. Jayasinghe S. S. Chandana E. P. De Silva C. Jayasumana P. M. C. Environ. Toxicol. Pharmacol. 2015;40:828–846. doi: 10.1016/j.etap.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Jaishankar M. Tseten T. Anbalagan N. Mathew B. B. Beeregowda K. N. Interdiscip. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia S. Balamurugan G. Baranwal A. Front. Environ. Sci. 2014;2:49. doi: 10.3389/fenvs.2014.00049. [DOI] [Google Scholar]
- USEPA (US Environmental Protection Agency), Columbia river basin fish contaminant survey; 1996-1998, USEPA, Seattle, WA, USA, 2002 [Google Scholar]
- Varol S. J. Water Health. 2021;19:97–107. doi: 10.2166/wh.2020.107. [DOI] [Google Scholar]
- Schoof R. A., Eickhoff J., Yost L. J., Crecelius E. A., Cragin D. W., Meacher D. M. and Menzel D. B., Dietary exposure to inorganic arsenic, in Arsenic exposure and health effects III, Elsevier Science Ltd, 1999, pp. 81–88 [Google Scholar]
- Tao S. S. H. Michael Bolger P. Food Addit. Contam. 1999;16:465–472. doi: 10.1080/026520399283759. [DOI] [PubMed] [Google Scholar]
- Del Razo L. M. Garcia-Vargas G. G. Garcia-Salcedo J. Sanmiguel M. F. Rivera M. Hernandez M. C. Cebrian M. E. Food Chem. Toxicol. 2002;40:1423–1431. doi: 10.1016/s0278-6915(02)00074-1. [DOI] [PubMed] [Google Scholar]
- Muñoz O. Vélez D. Montoro R. Analyst. 1999;124:601–607. doi: 10.1039/A809426H. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), Chemical fact sheets: Arsenic, World Health Organization, Geneva, 3rd edition incorporating 1st and 2nd addenda, 2008, vol. 1, Recommendations, pp. 306–308b, http://www.who.int/water_sanitation_health/dwq/GDW12rev1and2.pdf [Google Scholar]
- SRH (Subsecretaria de Recursos Hı ´dricos de Argentina), Niveles Guı ´a de Calidad de Aguas, 2006, http://hidricos.obraspublicas.gov.ar/calidad_del_agua_actividades.htm, accessed 11 July 2021 [Google Scholar]
- Sterner T. R., Robinson P. J., Mattie D. R. and Burton G. A., The Toxicology of chemical mixtures risk assessment for human and ecological receptors, 2005, p. 101, 10.21236/ada458544 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in this review paper, and further information could be obtained from the corresponding author (https://docs.google.com/document/d/1kz2tTt-zfVyxRTJbbxKtPA-S7LSxPosA/edit?usp=sharing&ouid=102850718200296775047&rtpof=true&sd=true).