Abstract
Background
Burn injuries result in disruption of the skin barrier against opportunistic infections. Pseudomonas aeruginosa is one of the main infectious agents colonizing burn wounds and making severe infections. Biofilm production and other virulence factors along with antibiotic resistance limit appropriate treatment options and time.
Materials and Methods
Wound samples were collected from hospitalized burn patients. P. aeruginosa isolates and related virulence factors identified by the standard biochemical and molecular methods. Antibiotic resistance patterns were determined by the disc diffusion method and β‐lactamase genes were detected by polymerase chain reaction (PCR) assay. To determine the genetic relatedness amongst the isolates, enterobacterial repetitive intergenic consensus (ERIC)‐PCR was also performed.
Results
Forty P. aeruginosa isolates were identified. All of these isolates were biofilm producers. Carbapenem resistance was detected in 40% of the isolates, and bla TEM (37/5%), bla VIM (30%), and bla CTX‐M (20%) were the most common β‐lactamase genes. The highest resistance was detected to cefotaxime, ceftazidime, meropenem, imipenem and piperacillin, and 16 (40%) isolates were resistant to these antibiotics. The minimum inhibitory concentrations (MIC) of colistin was lower than 2 μg/mL and no resistance was observed. Isolates were categorized to 17 MDR, 13 mono‐drug resistance, and 10 susceptible isolates. High genetic diversity was also observed among the isolates (28 ERIC types) and most carbapenem‐resistant isolates were classified into four main types.
Conclusion
Antibiotic resistance, particularly carbapenem resistance was considerable among the P. aeruginosa isolates colonizing burn wounds. Combining carbapenem resistance with biofilm production and virulence factors would result in severe and difficult‐to‐treat infections.
Keywords: biofilm, burn injuries, carbapenem‐resistant, ERIC‐PCR, Pseudomonas aeruginosa
All of the isolates were biofilm producers. Carbapenem resistance was detected in 40% of the isolates, and bla TEM (37/5%), bla VIM (30%), and bla CTX‐M (20%) were the most common β‐lactamase genes. The minimum inhibitory concentrations of colistin was lower than 2 μg/mL and no resistance was observed. Isolates were categorized to 17 MDR, 13 mono‐drug resistance, and 10 susceptible isolates. High genetic diversity was also observed among the isolates and most carbapenem‐resistant isolates were classified into four main ERIC types.
1. INTRODUCTION
Normal and intact skin is a barrier against infective agents, such as Pseudomonas aeruginosa. 1 Burn injuries destroy the skin protection against infection and disrupt the physiologic function of the immune system, and burn patients are at high risk of acquiring hospital‐associated infections. 2 P. aeruginosa is an aerobic Gram‐negative bacilli and accounts for opportunistic or nosocomial infections in burn patients, cystic fibrosis, and immunocompromised individuals. 3
Pseudomonas aeruginosa possesses a wide range of virulence factors such as elastase, exoenzymes, and exotoxin A which are regulated by cell‐to‐cell signaling systems. The main virulence factor produced by isolates of P. aeruginosa is exotoxin A which has an important role in the pathogenesis of this microorganism. 4 Also, flagella and pili have a key role as virulence factors independently. 5 P. aeruginosa is able to enhance the excretion of virulence determinants in the cytoplasm of target cells through a type III secretion system. These factors are associated with higher mortality, especially in burn patients. 6 Moreover, biofilm formation is a basic and critical virulence factor that improves bacterial survival in harsh circumstances such as dryness or the presence of antiseptics. 7 Biofilm also is one of the main strategies for antibiotic resistance that increases horizontal gene transfer between susceptible and resistant strains. 8 It is a complex aggregate of bacteria encased in alginate polysaccharides and encoded by the algD gene. 7 Biofilm also makes a barrier between bacterial cells and antibiotics or immune responses. 8 P. aeruginosa destroys natural structures of skin or mucous membranes using protease (such as elastase or Las), phospholipase (Plc), neuraminidase (Nan), and exotoxins. They are among those virulence factors that destroy connective tissue proteins, cytokines, cell membranes, and antibodies and modulate P. aeruginosa infections in proper sites such as burned skin or cystic fibrosis lungs. 9 In burn injuries, the natural defense of skin is destructed, and exposed matrix proteins and inflammatory factors accelerate the colonization of P. aeruginosa and infection. 10
Besides these virulence factors that make microorganism a destructive pathogen, antibiotic resistance also complicates the treatment of P. aeruginosa infections. Antibiotic resistance is mediated by various strategies such as β‐lactamases, efflux pumps and mutations, and multi‐drug‐resistant (MDR) isolates harbor several mechanisms for antibiotic resistance. 11 In P. aeruginosa different β‐lactamases like extended spectrum β‐lactamases (ESBLs) and metallo‐β‐lactamases (MBLs) cause resistance to β‐lactam antibiotics. 11 The combination of β‐lactamase‐producing phenotype and virulence factors creates a highly human pathogen, especially in burn patients. 10
Characterization of local epidemiology and determination of genetic relatedness of the drug‐resistant isolates is necessary to control their dissemination in healthcare setting. 12 To determine the genotypic relationship amongst P. aeruginosa isolates, various genotyping methods including, multilocus sequence typing (MLST) and pulsed‐field gel electrophoresis (PFGE) have been used. 13 Furthermore, polymerase chain reaction (PCR)‐based techniques such as enterobacterial repetitive intergenic consensus (ERIC)‐PCR are rapid, cost‐effective, reproducible, and reliable typing methods with acceptable discriminatory power for non‐fermenting Gram‐negative bacilli. 13 , 14
In the current study, we aimed to assess virulence factors, biofilm formation ability, β‐lactamase associated genes, and the genetic relationship amongst P. aeruginosa isolates, obtained from in burn wound infections.
2. MATERIALS AND METHODS
2.1. Bacterial isolates
In this study, clinical isolates of P. aeruginosa were isolated between March 2020 and September 2020 from burn wound samples in the selected hospitals in Tehran, and Ahvaz, Iran. All patients or their legal guardians provided informed written consent, and this study was approved by the Ethics Committee of Ilam University of Medical Sciences (IR.MEDILAM.REC.1399.237). Samples were inoculated on blood agar and MacConkey agar mediums immediately and P. aeruginosa isolates were identified by conventional biochemical tests including, Gram stain, oxidase, catalase, oxidation‐fermentation (OF) test, and the Triple Sugar Iron Agar (TSI) tests.
2.2. Drug susceptibility tests
Antibiotic susceptibility test (DST) was performed for isolates by disc diffusion method according to Clinical Laboratory Standard Institute (CLSI) 2020 guideline. 15 Imipenem (10 μg), meropenem (10 μg), cefotaxime (30 μg), ceftazidime (30 μg), piperacillin (100 μg), ciprofloxacin (5 μg) and gentamycin (10 μg) discs were used to determine the resistance pattern. In addition, the micro‐broth dilution method was applied to determine the susceptibility situation to colistin.
2.3. Phenotypic tests for ESBL, carbapenemase, and metallo β‐lactamase
All isolates were screened for the production of ESBL and MBLs enzymes using the combination disc method. In brief, an overnight incubated suspension of each isolate was inoculated on Muller‐Hinton agar media. Then, ceftazidime and ceftazidime/clavulanic acid discs were used to determine ESBL enzymes. Imipenem and EDTA discs were also used to detect MBLs enzymes. Carbapenemase activity was assessed using the carba‐NP test method, as described previously. 16
2.4. Biofilm assay
Biofilm formation assay was performed as described previously. 17 In brief, P. aeruginosa isolates were inoculated in 5 mL trypticase soy broth (TSB) and overnight incubated at 37°C. Then a concentration equal to 0.5 McFarland standard was prepared in TSB and each well of a flat‐bottomed polystyrene 96‐well microtiter plate was inoculated with 100 μL of these dilutions. After 24 h incubation at 37°C, the supernatant was removed and wells were rinsed with normal saline solution (0.9% NaCl). Adherent biofilms were fixed with 99% ethanol. The solutions were removed, and the plate was air‐dried, and stained with crystal violet (1.5%) for 20 min after that the unbound stain was rinsed with water. The dye was solubilized in 150 μL of 30% (v/v) acetic acid. The optical densities (OD) of the wells were measured by a microplate reader at 550 nm. The whole process was performed in triplicate for each isolate, and P. aeruginosa ATCC 27853 and sterile broth were used as a positive and negative control. A cut‐off value (ODc) was determined and it is defined as three standard deviations (SD) above the mean OD of the negative control: ODc = average OD of negative control + (3 × SD of negative control). The isolates were categorized into the four following groups based on the OD: non‐biofilm producer (OD < ODc); weak‐biofilm producer (ODc < OD <2 × ODc); moderate‐biofilm producer (2 × ODc < OD <4 × ODc); strong‐biofilm producer (4 × ODc < OD). 17 , 18
2.5. Molecular detection of virulence and resistance
The whole genomic DNA was extracted from pure colonies of isolated P. aeruginosa isolates using the boiling method. Briefly, a few colonies were dissolved in sterile distilled water and placed in a dry bath at 95°C for 15 min. Then the isolates were placed at −20°C for 10 min and then centrifuged at 13,000 rpm for 10 min. The supernatant was used as a DNA template. The extracted DNA was kept at −20°C until processed. The quality of the extracted DNA was determined using an absorbance ratio of 260/280 nm by a NanoDrop spectrophotometer. The genes encoding virulence factors (algD, lasB, plcH, nan1, exoS, and exoA) and β‐lactamase resistance genes (ESBL genes [bla CTXM , bla SHV , bla TEM] and carbapenemase genes [bla VIM , bla IMP , bla NDM , bla OXA‐48 , bla OXA‐23, and bla OXA‐11]) were detected by PCR method using the specific primers (Table 1). 19 , 20 , 21 , 22 Then, 1% agarose gel electrophoresis and gel staining (stain load dye (CinnaGen Co, Iran)) were conducted for the analysis of PCR products.
TABLE 1.
Primers were used for amplification of virulence and β‐lactamase genes.
| Gene | Primer sequence | Amplicon size (bp) | Reference |
|---|---|---|---|
| exoA |
F: GACAACGCCCTCAGCATCACCAGC R: CGCTGGCCCATTCGCTCCAGCGCT |
396 | 19 |
| nan1 |
F: ATG AAT ACT TAT TTT GAT AT R: CTA AAT CCA TGC TCT GAC CC |
1316 | 20 |
| lasB |
F: AGCCATCACCGAAGTCAAGG R: CGGGAATCAGGTAGGAGACG |
250 | 21 |
| ExoS |
F: CTT GAA GGG ACT CGA CAA GG R: TTC AGG TCC GCG TAG TGA AT |
504 | 21 |
| algD |
F: ATG CGA ATC AGC ATC TTT GGT R: CTA CCA GCA GAT GCC CTC GGC |
1310 | 21 |
| plcH |
F: GAA GCC ATG GGC TACTTCAA R: AGA GTG ACG AGG AGC GGTAG |
307 | 21 |
| bla SHV |
F: GCCCGGGTTATTCTTATTTGTCGC R: TCTTTCCGATGCCGCCGCCAGTCA |
1013 | 22 |
| bla TEM |
F: TCCGCTCATGAGACAATAACC R: ATAATACCGCACCACATAGCAG |
300 | 22 |
| bla CTX‐M |
F: TTTGCGATGTGCAGTACCAGTAA R: CGATATCGTTGGTGGTGCCATA |
455 | 22 |
| bla VIM |
F: GATGGTGTTTGGTCGCATA R: CGAATGCGCAGCACCAG |
390 | 22 |
| bla IMP |
F: AGCCCATAGTTAACCCCGCC R: CTGGCTTAATTCTCAATCCATCCC |
114 | 22 |
| bla NDM |
F: GGTTTGGCGATCTGGTTTTC R: CGGAATGGCTCATCACGATC |
621 | 22 |
| bla oxa‐23 |
F: TGGAAGGGCGAGAAAAGGTC R: TTGCCCAACCAGTCTTTCCA |
400 | 22 |
| bla oxa‐48 |
F: GCGTGGTTAAGGATGAACAC R: CATCAAGTTCAACCCAACCG |
438 | 22 |
| bla oxa‐11 |
F: CGAGTACGGCATTAGCTGGT R: CTCTTGGCTTTCCGTCCCAT |
250 | 22 |
2.6. Enterobacterial repetitive intergenic consensus (ERIC‐PCR)
To characterize the genetic relatedness among the isolates, ERIC‐PCR was performed using followed primers, ERIC1 5′‐ATGTAAGCTCCTGGGGATTCAC‐3′ and ERIC2 5′‐AAGTAAGTGACTGGGGTGAGCG‐3′, as described previously. 13 The PCR protocol consisted of a pre‐denaturation step at 95°C for 5 min, followed by 30 cycles of 60 s at 95°C, 50 s at 59°C, and 60 s at 72°C. A final extension step was done at 72°C for 10 min. PCR products were separated by electrophoresis in 1.5% agarose gels with 0.5× TBE (Tris/Boric acid/EDTA) buffer. DNA bands were visualized using UV light after staining with safe stain load dye. The GelJ software version 2.0 was used to analyze ERIC patterns 23 and the isolates with a similarity coefficient ≥90% were clustered in the same genotypes. In other words, the isolates with equal or more than 90% similarity in their banding patterns were considered the same ERIC type.
2.7. Statistical analysis
The SPSS version 22.0 (SPSS, Inc.) was used to analyze the data. Pearson Chi‐Square test was used to determine the statistically significant correlation between the existence of genes and antibiotic resistance or biofilm production. In addition, p‐value <0.05 was considered as a significance level. The results are presented as descriptive statistics in terms of relative frequency.
3. RESULTS
3.1. Isolates and drug susceptibility
Pseudomonas aeruginosa isolates were identified by various tests that included: Gram‐negative bacilli, motile, oxidase and catalase positive, bluish green pigmentation, and glucose oxidizer. In this study, 40 P. aeruginosa isolates were collected from burn wound samples of 23 male and 17 female hospitalized patients. The mean age of the patients were 26 ± 5 years and 21 patients had Neck and face skin wound, 12 patients had hands and arms wound and 7 patients had full body wounds. The highest resistance was detected for cefotaxime, ceftazidime, meropenem, imipenem and piperacillin, and 16 (40%) isolates were resistant to these antibiotics. The resistance rate to ciprofloxacin and gentamicin was slightly lower and 12 (30%) isolates were resistant to them (Table 2). Minimum inhibitory concentrations (MIC) of colistin against the isolates were lower than 2 μg/mL, and no resistance was seen. According to the DST results, isolates were categorized to 17 MDR, 13 mono‐drug resistance, and 10 susceptible isolates.
TABLE 2.
Antibiotic susceptibility patterns of P. aeruginosa isolates.
| Antibiotics | Sensitive N (%) | Intermediate N (%) | Resistant N (%) |
|---|---|---|---|
| Imipenem | 22 (55) | 0 | 18 (45) |
| Meropenem | 24 (60) | 0 | 16 (40) |
| Ciprofloxacin | 27 (67.5) | 0 | 13 (32.5) |
| Ceftazidime | 24 (60) | 0 | 16 (40) |
| Cefotaxime | 24 (60) | 0 | 16 (40) |
| Gentamicin | 24 (60) | 0 | 14 (35) |
| Piperacillin | 24 (60) | 0 | 16 (40) |
| Colistin | 40 (100) | 0 | 0 (0) |
3.2. Phenotypic assessment of ESBL, metallo‐β‐lactamase, and carbapenemase
While the ESBL activity was not detected in any of the isolates, 12 (30%) isolates were positive for MBL, and 16 (40%) isolates had carbapenemase activity.
3.3. Biofilm formation
All the isolates (100%) were positive for biofilm production. Seventeen (42.5%) isolates were strong biofilm producers and 14 (35%) isolates were moderate producers. Moreover, biofilm production was weak in 9 (22.5%) isolates. The biofilm‐producer isolates had higher levels of antibiotic resistance (Table 3).
TABLE 3.
Distribution of biofilm formation among P. aeruginosa isolates and correlation between biofilm production and antibiotic resistance patterns or co‐presence of virulence factors.
| Biofilm production | Isolates N (%) | Antibiotic resistance phenotype | Number of virulence factors | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MDR | Mono‐drug resistance | S | 1 | 2 | 3 | 4 | 5 | 6 | ||
| Strong N (%) | 17 (42.5%) | 11 | 4 | 2 | 0 | 2 | 5 | 3 | 3 | 4 |
| Moderate N (%) | 14 (35%) | 5 | 6 | 3 | 1 | 3 | 1 | 1 | 5 | 3 |
| Weak N (%) | 9 (22.5%) | 1 | 3 | 5 | 2 | 1 | 2 | 0 | 3 | 1 |
| Total N (%) | 40 (100%) | 17 (42.5%) | 13 (32.5%) | 10 (25%) | 3 | 6 | 8 | 4 | 11 | 8 |
| Pearson Chi‐square p‐value | 0.045 | 0.445 | ||||||||
Note: p‐value <0.05 considered as a significant correlation.
Abbreviations: MDR, Multi‐drug resistant; S, Susceptible phenotype.
3.4. ESBL and carbapenemase‐related genes
Among the ESBL genes, bla TEM, bla CTX, and bla SHV genes were positive in 15 (37.5%), 8 (20%), and 6 (15%) isolates, respectively. MBL and Carbapenemase genes were less frequent and only bla VIM gene was present in the isolates (30%), and bla IMP and bla NDM genes were not detected. Moreover, bla OXA‐48 and bla OXA‐23 genes were found in 7 (17.5%) and 1 (2.5%) isolates and no isolate possessed bla OXA‐11 gene (Table 4). The co‐occurrence of different types of β‐lactamase was seen in 15 isolates and the details are shown in Table 5.
TABLE 4.
Distribution of ESBL, MBL, and carbapenemase genes among 40 P. aeruginosa isolates.
| ESBL | bla TEM | bla SHV | bla CTX‐M | |
| Genotypic | Phenotypic | |||
| 16 (40%) | 0 (0%) | 8 (20%) | 6 (15%) | 15 (37/5%) |
| MBL | bla VIM | bla IMP | bla NDM | |
| Genotypic | Phenotypic | |||
| 12 (30%) | 12 (30%) | 0 | 0 | 12 (30%) |
| Carbapenemase | bla OXA‐48 | bla OXA‐23 | bla OXA‐11 | |
| Genotypic | Carba NP | |||
| 16 (40%) | 16 (40%) | 0 | 1 (2/5%) | 7 (17/5%) |
TABLE 5.
The details of co‐presence of bla genes among P. aeruginosa isolates.
| β‐lactamase class | Detection of genes by PCR assays | Isolates (N) | Phenotype |
|---|---|---|---|
| ESBL + MBL | (bla TEM , bla SHV , bla CTX‐M) + (bla VIM) | 2 | MDR |
| ESBL + MBL | (bla TEM) + (bla VIM) | 3 | MDR |
| ESBL + MBL | (bla TEM , bla SHV) + (bla VIM) | 1 | MDR |
| ESBL + MBL | (bla TEM , bla CTX‐M) + (bla VIM) | 1 | MDR |
| ESBL + Carbapenemase | (bla TEM) + (bla oxa‐48) | 1 | MDR |
| ESBL + Carbapenemase | (bla TEM , bla SHV , bla CTX‐M) + (bla oxa‐23) | 1 | MDR |
| ESBL + Carbapenemase | (bla TEM , bla SHV) + (bla oxa‐48) | 1 | MDR |
| MBL + Carbapenemase | (bla VIM) + (bla oxa‐48) | 1 | MDR |
| ESBL + MBL + Carbapenemase | (bla TEM) + (bla VIM) + (bla oxa‐48) | 2 | MDR |
| ESBL + MBL + Carbapenemase | (bla TEM , bla CTX‐M) + (bla VIM) + (bla oxa‐48) | 1 | MDR |
| ESBL + MBL + Carbapenemase | (bla TEM , bla SHV , bla CTX‐M) + (bla VIM) + (bla oxa‐48) | 1 | MDR |
| Total | 15 | ||
Abbreviation: MDR, Multi‐Drug Resistant.
3.5. Virulence factors
Among the virulence genes, lasB and exoA genes were detected in 38 (95%) isolates. The other genes including plcH, exoS, and nan1 were present in 37 (92%), 36 (90%), and 16 (40%) isolates, respectively. Although we did not find any correlation between the virulence and β‐lactamase genes, the co‐existence of virulence genes (lasB, exoA, plcH, exoS, and nan1) was observed among the isolates. The algD gene was present in 17 (43%) isolates and all of them were strong biofilm producers.
3.6. ERIC‐PCR typing
ERIC‐PCR typing indicated high genetic diversity among the isolates. The results of genotyping showed that 36 isolates were classified into 28 ERIC types according to a 90% cut‐off (Figure 1). No band was detected following ERIC‐PCR in four isolates, and thereby they were non‐typeable. According to our analysis, 12 isolates were clustered in four main genotypes (A–D). The predominant type was type A, and it contained five isolates, followed by B (three), C (two), and D (two). Other 24 isolates possessed different banding patterns and they were distributed in 24 single types (Figure 1).
FIGURE 1.
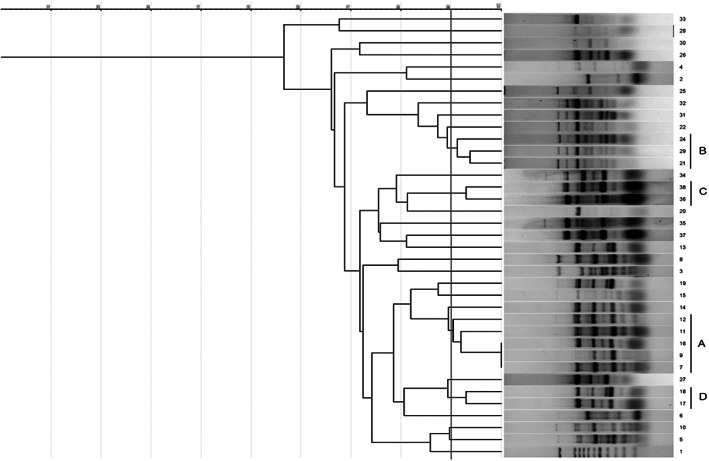
Dendrogram showing relatedness between ERIC‐PCR patterns of 36 P. aeruginosa isolates; Lane 1, 100 bp size marker; A–D, Four ERIC types.
4. DISCUSSION
The growing rates of antibiotic resistance in P. aeruginosa neutralize antibiotic efficacy against infections caused by this opportunistic agent. In this study, we isolated P. aeruginosa from burn wounds and determined antibiotic resistance rate, biofilm production, and their virulence factors. Resistance to carbapenem antibiotics such as imipenem and meropenem was 45% and 40%, respectively. While these rates in a study conducted in Poland were 41% and 61.6%, 24 and the International Nosocomial Infection Control Consortium (INICC) has reported a resistance rate of 47.2% for imipenem among clinical P. aeruginosa isolates, collected from different geographical regions, including Europe, Africa, Asia, and South America. 25 , 26 , 27 In a previous study in the USA, meropenem resistance of 23.7% was reported among P. aeruginosa isolates. 28
Interestingly, meropenem resistance has been demonstrated to be higher in P. aeruginosa isolates from cystic fibrosis patients. 29 It is speculated that a complicated environment and a chronic infection in cystic fibrosis lungs are responsible for the higher resistance rates. 29 Local studies from Iran have reported higher carbapenem resistance in P. aeruginosa isolates from burn wounds. Moreover, the imipenem resistance rate was found to be 58% and 94% in Shahrekord and Isfahan, respectively. 30 , 31
Also in a report from India, 61% of P. aeruginosa isolates from burn wounds were imipenem‐resistant. 11 Altogether, it seems that the prevalence of carbapenem resistance depends on the geographical area of studies. MBL and carbapenemase enzymes are considered to be the main underlying carbapenem resistance. In the current study, 40% of isolates were positive for encoding at least one of the MBL or carbapenemase enzymes, and co‐existence of ESBL, MBL, and carbapenemase genes was observed in 37.5% of isolates. The co‐existence of these enzymes resulted in high levels of β‐lactam resistance, and as shown in Table 5, the co‐presence of these genes was related to the formation of the MDR phenotype. Also, bla TEM (37.5%), bla VIM (30%), and bla CTX‐M (20%) were the most common β‐lactamase genes among the isolates. In other study by Peymani et al., the bla TEM‐1 (26.7%) and bla CTX‐M‐15 (17.3%), were the most common genes. 32 The prevalence rate of ESBL in the study performed by Senthamaria et al., 33 Begum et al., 34 and Mirsalehian et al., 35 was 42.3%, 37.8%, and 39.4%, respectively.
In our study, all the isolates were biofilm producers, however, the intensity of biofilm was different among the isolates. In previous studies, 77.5%, 86.5%, and 100% of P. aeruginosa isolates were reported to be the biofilm producers, which supports our finding. 36 , 37 Similar to Ratajczak, et al.'s study, we found that the formation of biofilm is significantly stronger in MDR isolates, and 64.7% (11 out of 17 isolates) of strong biofilm producer isolates were MDR. 24
The synergistic effect of antibiotic resistance and biofilm formation has been reported in P. aeruginosa and other bacterial pathogens, and several studies have displayed that biofilm formation is stronger in MDR strains of P. aeruginosa. 3 While biofilm protects the bacterial cell from exposure to antibiotics and increases the probability of horizontal transfer of antibiotic‐resistance genes, antibiotic‐resistant bacteria form stronger biofilms. In addition, biofilm‐forming isolates have different MIC amounts than planktonic cells, and a combination of antibiotics probably contributes to the elimination of biofilm‐forming strains. 3
In the present study, the most frequent virulence genes were lasB and toxA, which were present in 95% of isolates. In Ratajczak and colleagues' survey, 24 lasB gene was present in 93.1% of P. aeruginosa clinical isolates, but this rate was estimated to be 86% and 75% in the other studies from China and India, respectively. 38 , 39 Although the rates reported by the above‐mentioned studies are lower than our results, elastase seems to be an important and frequent virulence factor of clinical P. aeruginosa isolates. We found that 95% and 90% of our isolates were positive for toxA and toxS genes. Both genes are common virulence factors among the P. aeruginosa isolates and other studies have also reported that most of the clinical and environmental isolates harbor virulence traits. 7 , 40 In other study conducted by Bogiel et al., PCR results indicated 58.9% and 96.3% of the isolates harbored toxS and toxA genes, respectively. 41
Although Khosravi and colleagues 40 have demonstrated that the existence of toxA and toxS genes is related to high antibiotic resistance in P. aeruginosa isolates, we did not find any significant correlation between the presence of these virulence factors and high antibiotic resistance rate.
The plcH gene is a source of hemolytic phospholipase C in P. aeruginosa. 42 This virulence factor has a link to the high growth rate and pathogenicity, and mutant isolates have attenuated pathogenicity and slow growth rate. 42 We found the plcH gene in 92.5% of the isolates, and this factor as well as toxA, lasB, and toxS could be related to the high pathogenicity of the studied isolates.
We investigated the genetic relatedness of the P. aeruginosa isolates using ERIC‐PCR fingerprinting, and the results showed high genetic diversity. Most carbapenem‐resistant isolates (12/16) were classified into four ERIC types (A‐D). ERIC patterns of other carbapenem‐resistant isolates were also comparable with type A (lanes no. 14 and 19) and type B (lanes no. 31 and 32) (Figure 1). It seems that these genotypes are circulating strains among hospitalized patients in various wards of the hospitals. Notable antimicrobial resistance and biofilm formation ability were identified in these types (Table 6), and these factors are associated with long‐term persistence in a medical setting. 43 , 44
TABLE 6.
Characteristics of the predominant genotypes.
| ERIC type (Included isolates) | Virulence genes | Resistance profile | Biofilm formation |
|---|---|---|---|
| A (5) | toxA, toxS, lasB, plcH, algD | MDR | Moderate |
| B (3) | toxA, toxS, lasB, nan1, plcH, algD | MDR | Strong |
Abbreviation: MDR, Multi‐Drug Resistant.
According to the cut‐off, most of the isolates (n = 24) showed high‐level heterogeneity. These isolates, classified into 24 single types, were susceptible or did not show high‐level antimicrobial resistance. This diversity could be due to environmental or exogenous sources of the isolates. Based on the ERIC‐PCR method, four isolates were nontypeable; therefore, 90% (36/40) efficiency was calculated for this method in this study.
5. CONCLUSION
Antibiotic resistance of P. aeruginosa is considerable among the burn wound samples. Biofilm production is a synergistic factor that amplifies antibiotic resistance in these isolates, and alternative treatment for the elimination of biofilm could help decrease the antibiotic resistance rate in the life‐threatening burn infections by P. aeruginosa. Also, the high prevalence of virulence factors such as toxA, plcH, toxS, and lasB in our isolates shows that these factors are important in the pathogenesis of these bacteria in burn wounds. Innovation of new strategies for the inhibition of these virulence factors could be also beneficial for the treatment of burn infections by P. aeruginosa.
AUTHOR CONTRIBUTIONS
SGH, HH, SKH, SGH, and MKZ substantially contributed to the conceptualization, methodology, validation, and investigation of the work. EK and HK have been involved in data curation, supervision, writing, and acquisition of data or revised the review article for intellectual content. All authors agreed and confirmed the manuscript for publication.
CONFLICT OF INTEREST
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. The authors report no conflict of interest in this study.
ACKNOWLEDGMENTS
We would like to thank the Clinical Microbiology Research Center, Ilam University of Medical Sciences, for their cooperation. This study was supported by Ilam University of Medical Sciences (Project no. 1188).
Ghasemian S, Karami‐Zarandi M, Heidari H, et al. Molecular characterizations of antibiotic resistance, biofilm formation, and virulence determinants of Pseudomonas aeruginosa isolated from burn wound infection. J Clin Lab Anal. 2023;37:e24850. doi: 10.1002/jcla.24850
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article.
REFERENCES
- 1. Lachiewicz AM, Hauck CG, Weber DJ, Cairns BA, Van Duin D. Bacterial infections after burn injuries: impact of multidrug resistance. Clin Infect Dis. 2017;65(12):2130‐2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19(2):403‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Memar MY, Adibkia K, Farajnia S, et al. In‐vitro effect of imipenem, fosfomycin, colistin, and gentamicin combination against carbapenem‐resistant and biofilm‐forming Pseudomonas aeruginosa isolated from burn patients. Iran J Pharm Res. 2021;20(2):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corehtash ZG, Khorshidi A, Firoozeh F, Akbari H, Aznaveh AM. Biofilm formation and virulence factors among Pseudomonas aeruginosa isolated from burn patients. Jundishapur J Microbiol. 2015;8(10):e22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sato H, Okinaga K, Saito H. Role of pili in the pathogenesis of Pseudomonas aeruginosa burn infection. Microbiol Immunol. 1988;32(2):131‐139. [DOI] [PubMed] [Google Scholar]
- 6. Elnagar RM, Elshaer M, Osama Shouman O, Sabry El‐Kazzaz S. Type III secretion system (exoenzymes) as a virulence determinant in Pseudomonas aeruginosa isolated from burn patients in Mansoura University hospitals, Egypt. Iran J Med Microbiol. 2022;16(6):520‐527. [Google Scholar]
- 7. Gholami M, Zeighami H, Bikas R, Heidari A, Rafiee F, Haghi F. Inhibitory activity of metal‐curcumin complexes on quorum sensing related virulence factors of Pseudomonas aeruginosa PAO1. AMB Express. 2020;10(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Molin S, Tolker‐Nielsen T. Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure. Curr Opin Biotechnol. 2003;14(3):255‐261. [DOI] [PubMed] [Google Scholar]
- 9. Mauch RM, Jensen PØ, Moser C, Levy CE, Høiby N. Mechanisms of humoral immune response against Pseudomonas aeruginosa biofilm infection in cystic fibrosis. J Cyst Fibros. 2018;17(2):143‐152. [DOI] [PubMed] [Google Scholar]
- 10. D'Abbondanza JA, Shahrokhi S. Burn infection and burn sepsis. Surg Infect (Larchmt). 2021;22(1):58‐64. [DOI] [PubMed] [Google Scholar]
- 11. Bhatt P, Rathi KR, Hazra S, Sharma A, Shete V. Prevalence of multidrug resistant Pseudomonas aeruginosa infection in burn patients at a tertiary care Centre. Indian J Burns. 2015;23(1):56. [Google Scholar]
- 12. Mokhtari A, Amini K. Genotyping of Pseudomonas aeruginosa strains as a multidrug resistant (MDR) bacterium and evaluating the prevalence of ESBLs and some virulence factors encoding genes by PFGE and ERIC‐PCR methods. Iran J Pharm Res. 2019;18(3):1580‐1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silbert S, Pfaller MA, Hollis RJ, Barth AL, Sader HS. Evaluation of three molecular typing techniques for nonfermentative gram‐negative bacilli. Infect Control Hosp Epidemiol. 2004;25(10):847‐851. [DOI] [PubMed] [Google Scholar]
- 14. Motamedifar M, Heidari H, Yasemi M, Sedigh Ebrahim‐Saraie H. Molecular epidemiology and characteristics of 16 cases with Stenotrophomonas maltophilia bacteraemia in pediatric intensive care units. Ann Ig. 2017;29(4):264‐272. [DOI] [PubMed] [Google Scholar]
- 15. Clinical & Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing M100. 2020.
- 16. Dortet L, Poirel L, Nordmann P. Rapid detection of carbapenemase‐producing pseudomonas spp. J Clin Microbiol. 2012;50(11):3773‐3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Banar M, Emaneini M, Satarzadeh M, et al. Evaluation of mannosidase and trypsin enzymes effects on biofilm production of Pseudomonas aeruginosa isolated from burn wound infections. PLoS One. 2016;11(10):e0164622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamali E, Jamali A, Ardebili A, Ezadi F, Mohebbi A. Evaluation of antimicrobial resistance, biofilm forming potential, and the presence of biofilm‐related genes among clinical isolates of Pseudomonas aeruginosa . BMC Res Notes. 2020;13(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tarashi S, Heidary M, Dabiri H, Nasiri MJ. Prevalence of drug‐resistant Pseudomonas aeruginosa in Iranian burned patients: a meta‐analysis. Arch Trauma Res. 2017;6(3):1‐7. [Google Scholar]
- 20. Mahdavi Z, Hemati S, Sadeghifard N, et al. The association between lasB and nanI genes with biofilm formation in Pseudomonas aeruginosa clinical isolates. J Clin Diagn Res. 2020;14(5):DC01‐DC03. [Google Scholar]
- 21. Benie C, Dadie A, Guessennd N, Kouame N, N'gbesso‐Kouadio N. Molecular identification and virulence factors of Pseudomonas aeruginosa strains isolated from animal products. J Bacteriol Mycol. 2017;4(3):91‐96. [Google Scholar]
- 22. Kazemian H, Heidari H, Ghanavati R, et al. Phenotypic and genotypic characterization of ESBL‐, AmpC‐, and carbapenemase‐producing Klebsiella pneumoniae and Escherichia coli isolates. Med Princ Pract. 2019;28(6):547‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heras J, Domínguez C, Mata E, et al. GelJ–a tool for analyzing DNA fingerprint gel images. BMC Bioinformatics. 2015;16(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ratajczak M, Kamińska D, Nowak‐Malczewska DM, Schneider A, Dlugaszewska J. Relationship between antibiotic resistance, biofilm formation, genes coding virulence factors and source of origin of Pseudomonas aeruginosa clinical strains. Ann Agric Environ Med. 2021;28(2):306‐313. [DOI] [PubMed] [Google Scholar]
- 25. Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability‐adjusted life‐years caused by infections with antibiotic‐resistant bacteria in the EU and the European economic area in 2015: a population‐level modelling analysis. Lancet Infect Dis. 2019;19(1):56‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenthal VD, Rodrigues C, Madani N, et al. Effectiveness of a multidimensional approach for prevention of ventilator‐associated pneumonia in adult intensive care units from 14 developing countries of four continents: findings of the international nosocomial infection control consortium. Crit Care Med. 2012;40(12):3121‐3128. [DOI] [PubMed] [Google Scholar]
- 27. Hong DJ, Bae IK, Jang I‐H, Jeong SH, Kang H‐K, Lee K. Epidemiology and characteristics of metallo‐β‐lactamase‐producing Pseudomonas aeruginosa . Infect Chemother. 2015;47(2):81‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sader HS, Carvalhaes CG, Streit JM, Doyle TB, Castanheira M. Antimicrobial activity of ceftazidime‐avibactam, ceftolozane‐tazobactam and comparators tested against Pseudomonas aeruginosa and Klebsiella pneumoniae isolates from United States medical centers in 2016–2018. Microb Drug Resist. 2021;27(3):342‐349. [DOI] [PubMed] [Google Scholar]
- 29. Richardot C, Plésiat P, Fournier D, Monlezun L, Broutin I, Llanes C. Carbapenem resistance in cystic fibrosis strains of Pseudomonas aeruginosa as a result of amino acid substitutions in porin OprD. Int J Antimicrob Agents. 2015;45(5):529‐532. [DOI] [PubMed] [Google Scholar]
- 30. Golshani Z, Ahadi AM, Sharifzadeh A. Antimicrobial susceptibility pattern of Pseudomonas aeruginosa isolated from patients referring to hospitals. 2012.
- 31. Fazeli H, Moslehi Z, Irajian G, Salehi M. Determination of drug resistance patterns and detection of Bla‐VIM gene in Pseudomonas aeruginosa strains isolated from burned patients in the Emam Mosa Kazem hospital, Esfahan, Iran (2008‐9). Iran J Med Microbiol. 2010;3(4):1‐8. [Google Scholar]
- 32. Peymani A, Naserpour‐Farivar T, Zare E, Azarhoosh K. Distribution of blaTEM, blaSHV, and blaCTX‐M genes among ESBL‐producing P. aeruginosa isolated from Qazvin and Tehran hospitals, Iran. J Prev Med Hygiene. 2017;58(2):E155. [PMC free article] [PubMed] [Google Scholar]
- 33. Senthamarai S, Sivasankari S, Anitha C, et al. Resistance pattern of Pseudomonas aeruginosa in a tertiary care hospital of Kanchipuram, Tamilnadu, India. J Clin Diagn Res. 2014;8(5):DC30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Begum S, Salam MA, Alam KF, Begum N, Hassan P, Haq JA. Detection of extended spectrum β‐lactamase in pseudomonas spp. isolated from two tertiary care hospitals in Bangladesh. BMC Res Notes. 2013;6(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mirsalehian A, Feizabadi M, Nakhjavani FA, Jabalameli F, Goli H, Kalantari N. Detection of VEB‐1, OXA‐10 and PER‐1 genotypes in extended‐spectrum β‐lactamase‐producing Pseudomonas aeruginosa strains isolated from burn patients. Burns. 2010;36(1):70‐74. [DOI] [PubMed] [Google Scholar]
- 36. da Silva Carvalho T, Perez LRR. Impact of biofilm production on polymyxin B susceptibility among Pseudomonas aeruginosa clinical isolates. Infect Control Hosp Epidemiol. 2019;40(6):739‐740. [DOI] [PubMed] [Google Scholar]
- 37. Lima JLC, Alves LR, Jacomé PRLA, Bezerra Neto JP, Maciel MAV, Morais MMC. Biofilm production by clinical isolates of Pseudomonas aeruginosa and structural changes in LasR protein of isolates non biofilm‐producing. Braz J Infect Dis. 2018;22:129‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, Gao L, Rao X, et al. Characterization of lasR‐deficient clinical isolates of Pseudomonas aeruginosa . Sci Rep. 2018;8(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sabharwal N, Dhall S, Chhibber S, Harjai K. Molecular detection of virulence genes as markers in Pseudomonas aeruginosa isolated from urinary tract infections. Int J Mol Epidemiol Genet. 2014;5(3):125. [PMC free article] [PubMed] [Google Scholar]
- 40. Khosravi AD, Shafie F, Montazeri EA, Rostami S. The frequency of genes encoding exotoxin a and exoenzyme S in Pseudomonas aeruginosa strains isolated from burn patients. Burns. 2016;42(5):1116‐1120. [DOI] [PubMed] [Google Scholar]
- 41. Bogiel T, Depka D, Rzepka M, Kwiecińska‐Piróg J, Gospodarek‐Komkowska E. Prevalence of the genes associated with biofilm and toxins synthesis amongst the Pseudomonas aeruginosa clinical strains. Antibiotics. 2021;10(3):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kida Y, Shimizu T, Kuwano K. Cooperation between LepA and PlcH contributes to the in vivo virulence and growth of Pseudomonas aeruginosa in mice. Infect Immun. 2011;79(1):211‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Santajit S, Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int. 2016;2016:2475067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thi MTT, Wibowo D, Rehm BHA. Pseudomonas aeruginosa Biofilms. Int J Mol Sci. 2020;21(22):8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


