Abstract
Background:
People who have or had the potential to menstruate (PPM) with inherited bleeding disorders (BD) face particular challenges receiving appropriate diagnosis and care and participating in research. As part of an initiative to create a National Research Blueprint for future decades of research, the National Hemophilia Foundation (NHF) and American Thrombosis and Hemostasis Network conducted extensive all-stakeholder consultations to identify the priorities of PPM with inherited BDs and those who care for them.
Research design and methods:
Working group (WG) 4 of the NHF State of the Science Research Summit distilled community-identified priorities for PPM with inherited BDs into concrete research questions and scored their feasibility, impact, and risk.
Results:
WG4 identified important gaps in the foundational knowledge upon which to base optimal diagnosis and care for PPM with inherited BDs. They defined 44 top priority research questions concerning lifespan sex biology, pregnancy and the post-partum context, uterine physiology and bleeding, bone and joint health, health care delivery, and patient-reported outcomes and quality-of-life.
Conclusions:
The needs of PPM will best be advanced with research designed across the spectrum of sex and gender biology, with methodologies and outcome measures tailored to this population, involving them throughout.
Keywords: Gender disparity, Heavy menstrual bleeding, Inherited bleeding disorders, National Hemophilia Foundation, Patient-centered, Sex disparity
Plain language summary
Up to 1% of cisgender women and girls have an inherited bleeding disorder (BD). Common symptoms include heavy menstrual bleeding (HMB), heavy bleeding after giving birth known as post-partum hemorrhage (PPH), nose bleeds, bleeding from the mouth and excessive bleeding after surgery or procedures. They can also experience bleeding into their muscles, joints, and even into the brain. Uterine bleeding such as from HMB and PPH can impact the lives of anyone who has or had a uterus, a group we designate as people who have or had the potential to menstruate (PPM).
Many PPM with an inherited BD do not receive diagnosis, treatment, and care needed due to a lack of expertise among health care professionals and the public, misunderstanding, and bias. Uncertainty about “normal” versus “abnormal” bleeding can contribute to the lack of diagnosis, treatment, and care. Language, such as the label of “carrier,” can be a barrier to access, treatment and care for PPM.
People with inherited BDs, health care professionals with various expertise and focus, and researchers worked together to identify the research that would most improve the lives of PPM, in six focus areas where there are major gaps in knowledge and the lack of standards required for accurate diagnosis.
1. Introduction
1.1. Inherited bleeding disorders in people who have or had the potential to menstruate
Inherited bleeding disorders (BD) may impact every aspect of the lives of the people who live with them; however, individuals who menstruate, ovulate, and experience pregnancy are disproportionately affected due to the bleeding challenges that occur with these events [1, 2]. Biological sex, gender identity, and their interaction influence molecular and cellular processes, clinical characteristics, and health and disease outcomes [3]. The work reported herein focuses on inherited BDs in cisgender women and girls and gender diverse individuals encompassed broadly by the term people who have or had the potential to menstruate (PPM), except where cited. Up to 1% of PPM may have an inherited BD [4], with common symptoms including heavy menstrual bleeding (HMB), post-partum hemorrhage (PPH), epistaxis, gingival bleeding, and excessive bleeding following surgical or dental procedures [5–7].
Up to 40% of menstruating individuals experience HMB [8, 9], most frequently immediately following menarche and during perimenopause [10]. HMB is the most common cause of iron-deficiency anemia in the developed world [11], which may manifest as weakness, fatigue, unexplained weight loss, mood swings, and impaired cognitive function [12], and contributes to increased morbidity and mortality in pregnancy [13]. HMB also negatively impacts health-related quality-of-life (QoL) including educational and vocational attainment, workforce participation, sexual functioning, social and recreational engagement, and mental health [11, 14–17], and can cause substantial pain [18]. The annual direct economic cost of HMB in the United States (US) is conservatively estimated to be $1 billion and the indirect cost, $12 billion [11].
The experience of pregnancy is another area of life disproportionately affected by an inherited BD. The risk of excessive bleeding at pregnancy culmination, whether in miscarriage, abortion, or birth, necessitates a high level of coordination of care. The incidence of PPH is significantly greater among those with inherited BDs than the general population and the maternal mortality rate in the US is ten times higher for them [19–21].
The spectrum of inherited BD identified in PPM includes von Willebrand disease (VWD), platelet dysfunction, coagulation factor deficiencies, and abnormalities of the fibrinolytic pathway [9, 10, 22, 23]. Research in PPM has focused primarily on reproductive tract bleeding, but recognition is growing that PPM historically labeled carriers of hemophilia A or B [24] may be more symptomatic than presumed [25, 26]. Joint bleeds (hemarthroses), traumatic and subclinical, and loss of range of motion are increasingly being reported in a broad portion of PPM with (not only severe forms of) factor deficiencies [27–33].
1.2. It’s complicated
The effects of sex hormones on biological functioning are pervasive [34]. Fluctuations of estradiol and progesterone associated with the menstrual cycle and through the lifespan of PPM influence coagulation factor levels and joint and bone health [35–38]. Extragenic factors including stress, pregnancy, and hormone regimens used for contraception, as hormone replacement therapy, or as part of gender affirmation can also impact hemostasis [38–41]. Hemostatic impairment associated with bleeding in the context of pregnancy is different from trauma-induced bleeding [42]. Menstrual bleeding, a natural process that normally self-terminates with rapid scarless healing, is different from non-uterine bleeding [26, 43]. The research agenda for PPM will best be advanced with research designed across the spectrum of sex and gender biology.
1.3. A knowledge chasm
Inherited BDs in PPM are widely underrecognized and underdiagnosed due to low awareness among health care professionals (HCP) and the public, reluctance to discuss symptoms, uncertainty regarding the definition of normal bleeding, and lack of diagnostic and treatment expertise [44, 45]. Inequitable access to diagnosis and care for people with inherited BDs may affect how they initially present to care [41, 46–48]. Even when they encounter numerous hemostatic challenges, PPM report that they often are not referred to hematologists [7, 49, 50]. Some PPM report feeling that their symptoms are dismissed, and they have to continually self-advocate to be taken seriously, particularly in urgent care situations or for invasive procedures [24, 49, 51, 52]. The PPM who live with inherited BDs, and the people who love and care for them, develop unique expertise, making them truly lived experience experts (LEE) [53]. The trivialization of women’s health complaints is not unique to inherited BDs but a common manifestation of ongoing gender bias in health care [54].
Long-standing sexism in the field has contributed to inequity for affected PPM [26, 47, 55]. Much less research has been conducted into inherited BDs historically thought to affect PPM, such as VWD, as evidenced by the number of PubMed indexed citations, trials listed on clinicaltrials.gov, and products currently approved to treat them in the US [47]. Just as some of the diseases least funded by the US National Institutes of Health (NIH) relative to disease burden are those that affect primarily women [54], bleeding events specifically experienced by PPM, such as menstruation, are under-researched [43]. Furthermore, the context of BD diagnosis and treatment in gender non-conforming folks (e.g., transgender, nonbinary, gender-expansive) is unmeasured but expected to be less than ideal in a health care system that inadequately addresses the needs of this population [41]. Critical to improving the experiences of PPM is the very basis of medicine: phenotypic description. There is an opportunity for prioritized research initiatives to advance diagnosis and care. The people most affected by this knowledge chasm, LEEs, are uniquely qualified to identify areas with the greatest transformative potential [53].
1.4. Community-identified areas of priority research
The National Hemophilia Foundation (NHF) set out to create a National Research Blueprint [56] for the coming decades by gathering input on the issues of greatest concern to people with inherited BDs and those who love and care for them through extensive all-stakeholder listening sessions, surveys, and conversations [10, 57]. In the virtual listening session group composed of people with inherited BDs (86%) and caregivers, 62% of participants identified as female; in the organization and local chapter leaders group (19% people with inherited BDs and 33% caregivers), 66% identified as female [57]. Analyzing the data from all the consultative initiatives, the NHF State of the Science Research Summit (SOSRS) Steering Committee categorized priority community concerns into six key areas and recruited expert Working Groups (WG) to distill each into concrete research questions. WG4 Research Priorities for the Health of People Who Have or Had the Potential to Menstruate were charged with answering the key question: How can we improve care for this group of people with inherited BD through a better understanding of the sex and gender biology of bleeding, as well as through new tools like non-invasive prenatal testing or therapies for reproductive system bleeding [57]?
WG4 was asked to consider issues concerning gender and of particular importance to lesbian, gay, bisexual, transgender, queer, questioning, intersex, asexual and other (LGBTQI+) communities as well as research specific to cisgender women and girls. They were invited to consider the impact of inherited BD symptoms and manifestations throughout the lifespan and across different BDs, hemarthrosis/joint disease and other comorbidities in PPM, the concerns of hemophilia carriers, and the inclusion of PPM in data collection and clinical trials (Figure 1).
Figure 1.
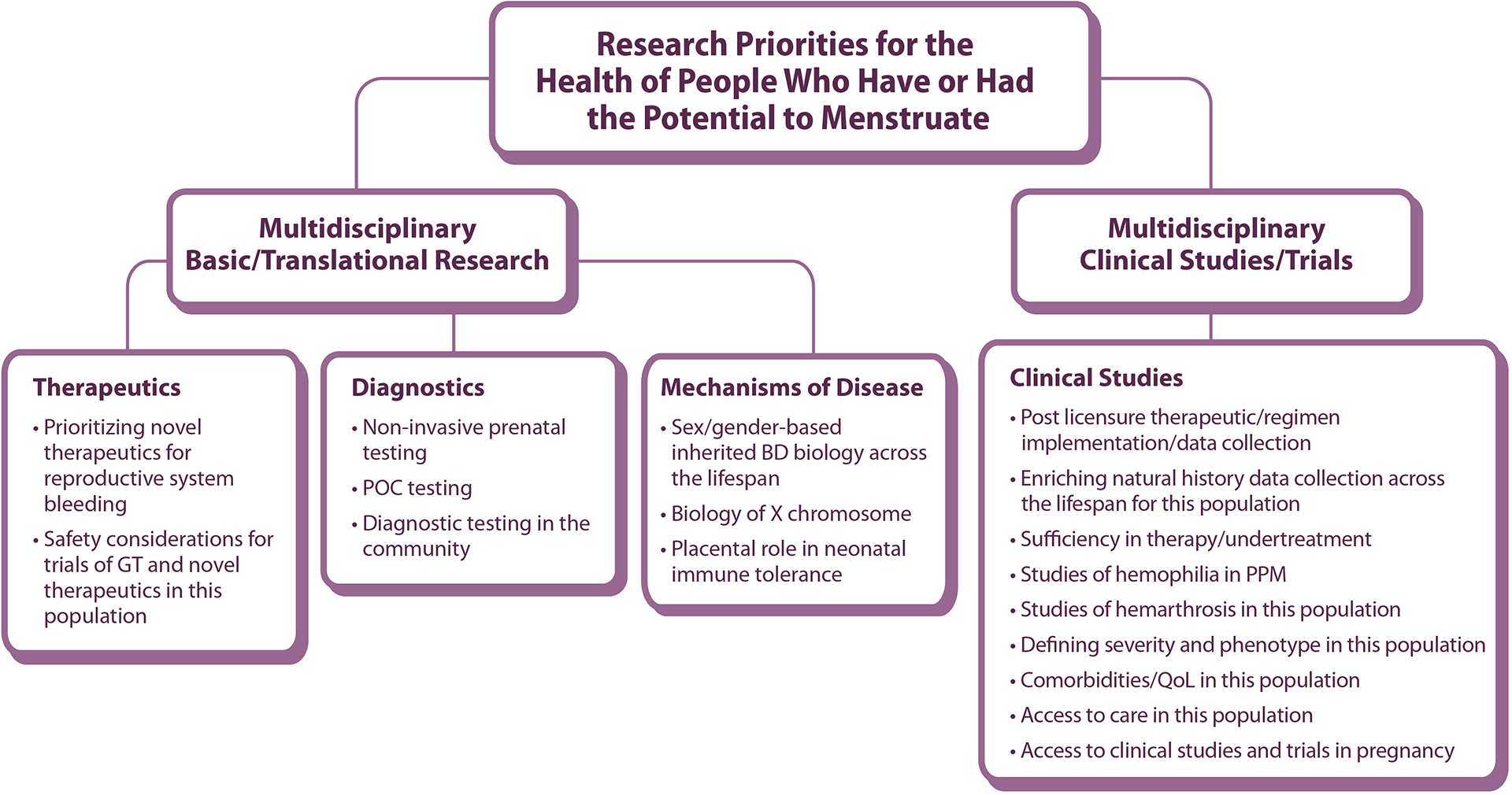
Working Group 4 Research Priorities for the Health of People Who Have or Had the Potential to Menstruate schematic of community-identified areas for priority research framework
GT: gene therapy, BD: bleeding disorder, POC: point-of-care, PPM: people who have or had the potential to menstruate QoL: quality-of-life
In September 2021 WG4 presented the priority research questions derived within this framework at the (virtual) NHF SOSRS [57]. In this paper, WG4 offer their deliberations and conclusions, informed by community feedback and discussions at the Summit, as contributions to the establishment of a National Research Blueprint for Inherited Bleeding Disorders [56].
2. Methods
2.1. Working Group 4 composition, direction, and workings
The NHF SOSRS Steering Committee recruited two co-chairs to lead WG4; together they refined the title of the group to represent cis-women, cis-girls, and gender diverse individuals. They recruited members from across the US and internationally (Table 1) with expertise in obstetrics, gynecology, hematology (adult and pediatric), genetic counseling, maternal fetal medicine, transgender research, family planning, physical therapy, nursing, social work, pathology and lab medicine, sexual and reproductive health research, organizational partners, federal agencies, and contraceptive and clotting factor-based industry. Several LEEs who live with BDs every day [53], were empowered full WG members, accompanied and empowered (virtually) by an NHF staff. The co-chairs proactively sought LEE input as part of a group structure fostering equity and inclusion, and invested in cross-discipline education (e.g., explaining acronyms, providing background, avoiding jargon). All members contributed to a highly respectful atmosphere valuing all input. The group met (virtually) approximately twice per month (May to August, 2021) to discuss the research needs of the target population and define research questions through iterative refinement and prioritization.
Table 1.
Members of Working Group 4
| Member | Stakeholder Group | Affiliation |
|---|---|---|
| Maureen K. Baldwin, MD, MPH (Co-chair) | Obstetrician; Gynecologist; Complex Family Planning specialist | Obstetrics and Gynecology, Oregon Health & Science University, Portland, Oregon |
| Angela C. Weyand, MD (Co-chair) | Pediatric hematologist | Division of Hematology/Oncology, Department of Pediatrics, University of Michigan Medical School, Ann Arbor, Michigan |
| Homa K. Ahmadzia, MD, MPH | Obstetrician; Maternal Fetal Medicine specialist; Researcher | Obstetrics and Gynecology, The George Washington University, Washington, DC |
| Diane L. Bartlett, LCSW | Social worker | St Luke’s Hemophilia Center, Boise, Idaho |
| Debbie Bensen-Kennedy, MD | Hematologist; Industry | CSL Behring, King of Prussia, Pennsylvania |
| Vidhi Desai, MD | Hematologist; Industry | CSL Behring, King of Prussia, Pennsylvania |
| Kristina M. Haley, DO, MCR | Pediatric hematologist | The Hemophilia Center, Oregon Health & Science University, Portland, Oregon |
| Sherry L. Herman-Hilker, PT, MS | Physical therapist | University of Michigan Hemophilia and Coagulation Disorders Program, Ann Arbor, Michigan |
| W. Craig Hooper‡, PhD | Federal agency partner | Division of Blood Disorders, Centers for Disease Control and Prevention |
| Amanda M. Kilgore, PT, DPT | Physical therapist | Bleeding and Clotting Disorders Institute, Peoria, Illinois |
| Roshni Kulkarni, MD | Pediatric hematologist | Center for Bleeding and Clotting Disorders, College of Human Medicine, Michigan State University, East Lansing, Michigan |
| Michelle Lavin, MB, PhD, FRCPath | Hematologist; Researcher | Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, RCSI and National Coagulation Centre, St. James’ Hospital, Dublin, Ireland |
| Shari Luckey, MA | LEE; Chapter staff | Hemophilia Foundation of Michigan, Michigan |
| Kristen A. Matteson, MD, MPH | Obstetrician; Gynecologist | Obstetrics and Gynecology, Warren Alpert Medical School of Brown University, Providence, Rhode Island |
| Kristin Paulyson-Nuñez, MS, CGC | Advanced practice practitioner; Genetic counselor | Obstetrics and Gynecology, Duke University Health Systems, Durham, North Carolina |
| Claire S. Philipp, MD | Hematologist | Division of Hematology, Rutgers Robert Wood Johnson Medical School, New Brunswick, New Jersey |
| Sachiko Ragosta | Sexual and reproductive health researcher | Ibis Reproductive Health, Oakland, California |
| Kimberly Rosen, MD | Industry | Bayer Healthcare, Whippany, New Jersey |
| Dawn Rotellini | LEE; NHF staff | NHF |
NHF SOSRS Steering Committee
NHF SOSRS Advisory Committee
LEE: lived experience expert, NHF: National Hemophilia Foundation, SOSRS: State of the Science Research Summit
2.2. Feasibility-Impact-Risk scoring
In addition to formulating research questions to advance community-defined priorities, WGs were tasked with evaluating the feasibility, impact, and risk of each, using a pre-defined scoring matrix (Supplementary Table S1) [57, 58]. An ideal question would be highly feasible (maximum score: 14, range: 0 to 14), would have great impact on the care and overall health of the target population (maximum score: 14, range: 0 to 14); and would involve very low risk (maximum score: 0, score range: −10 to 0, greater risk is scored with an increasingly negative value) to research participants and to the resources and funding invested. Totaling the scores across the three dimensions yields a single number that can be compared between questions with very different strengths and challenges. Questions formulated as above were scored by a minimum of three members with specific relevant expertise; scores were then reviewed by the co-chairs for discrepancies and finalized through whole WG discussions.
Scoring with the feasibility-risk-impact matrix was challenging. Some criteria were not precisely relevant or difficult to apply consistently across the diverse prioritized questions. The WG elected not to score any of the questions against one particularly problematic criterion (impact criterion 1f: training opportunities, Suppl Table S1). Discussions of overall feasibility and risk/reward balance informed final scoring.
2.3. NHF State of the Science Research Summit
WG4 presented their preliminary findings to the community at the NHF SOSRS. Held (virtually) September 12–15, 2021, it convened over 880 LEEs, physicians, researchers, multidisciplinary care team professionals, and federal and industry partners [10, 57]. NHF, supported by an NHLBI R13 grant (R13HL158209), offered a Remote Participation Group (RPG) option for underrepresented segments of the community to participate [57]. Reports submitted by facilitators of two RPGs of PPM, one from the Hispanic community (conducted in Spanish), and SOSRS live panel discussions of expert presentations and delegate comments and questions, informed the Discussion and Conclusions of this paper.
3. Results
3.1. Foundational research gaps and focused research domains
Group deliberations of the community-identified priorities of PPM derived a long list of specific research questions, including several that did not pertain exclusively to this target population (Suppl Table S2) and identifying major gaps in fundamental research (Suppl Figure S1). To define specific research questions addressing the concerns of PPM with inherited BDs, WG4 targeted six key issues across six research focus domains (Figure 2).
Figure 2.
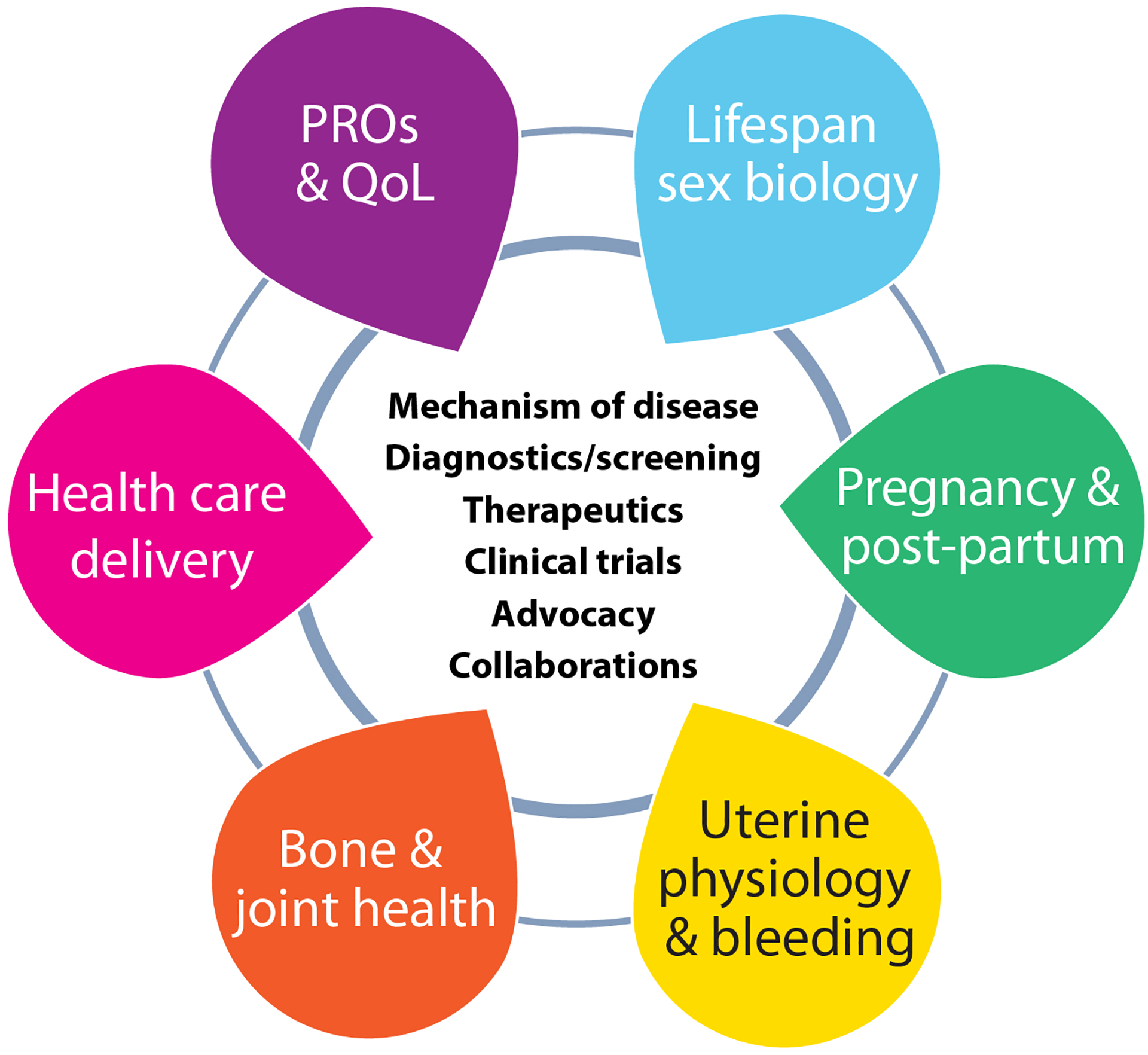
Six focused research domains identified by WG4, and the core issues examined for each
PRO: patient-reported outcome, QoL: quality-of-life
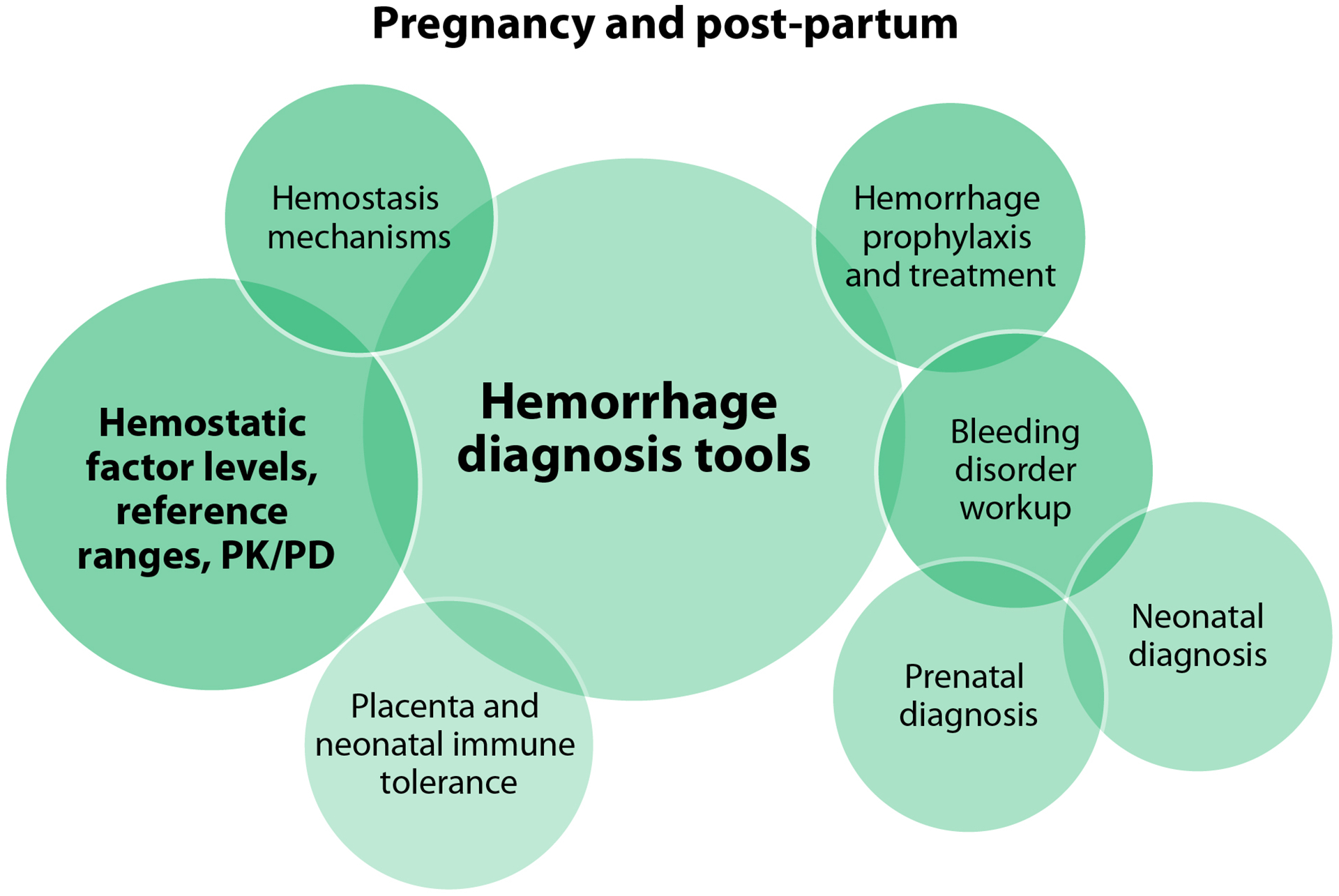
The focused domains are inter-related: uterine bleeding and bone/joint health span the lifespan as do questions of sex biology, including contexts of circulating levels of exogenous and endogenous gonadal steroid hormones (estrogens, progestogens, and androgens) and their effects on hemostatic factors and bleeding symptoms. Pregnancy and post-partum questions were categorized as a separate lifespan experience because of the specific issues of pregnancy-associated bleeding and neonatal diagnosis. The delivery of health care, including locations and organizational structures, emerged as an important domain since it is highly deterministic of access to care. Patient-reported outcomes (PRO) and quality-of-life (QoL) constitute their own focused domain as they span multiple other domains, with PROs and QoL highly influenced by issues of health care delivery and access to care. For each focused research domain, the WG identified mechanisms of disease, diagnosis, screening and therapeutic opportunities, looked at ways to improve clinical trials and inclusion in clinical trials, and discussed opportunities to capitalize upon advocacy and collaboration (Figure 2).
3.2. Prioritized research question Feasibility-Impact-Risk scores and areas of impact
WG4 applied the prescribed feasibility-impact-risk scoring matrix (Suppl Table S1) [57, 58] to the prioritization of questions in each domain (summarized in Table 2, detailed scores in Suppl Table S3). Their very pragmatic approach manifested as questions that scored predominantly in the range of moderate to high feasibility, high impact, and low risk (Figure 3). Feasibility concerns were predominantly around cost rather than challenges to engaging researchers or participants. The key areas of impact of the research questions prioritized in each domain (Figure 4) are detailed below.
Table 2.
Priority research questions in each focused research domain of the health of people who have or had the potential to menstruate, and their total feasibility-impact-risk score
| Lifespan sex biology | ||
|---|---|---|
| # | Research Question | F-I-R Score |
| 1 | What is the impact of aging in inherited BDs for individuals assigned male at birth versus female, particularly peri- and postmenopausal? | 19 |
| 2 | What are the phenotypic differences of inherited BDs across the lifespan based on sex and gender? | 19 |
| 3 | What effects do hormones, pregnancy, and age have on coagulation factor levels and reference ranges? | 17 |
| 4 | How do the pharmacokinetics and pharmacodynamics of coagulation factor products differ in PPM compared to cisgender men? | 17 |
| 5 | What is the impact of coagulation factor levels on bleeding phenotype with aging in PPM? | 15 |
| 6 | What is the impact of comorbidities on bleeding phenotype, beyond clotting factor levels? (e.g., disability, connective tissue disorder, immunotherapy, COVID-19 infection, rheumatologic disorders) | 11 |
| 7 | How do we assess and define phenotype in PPM with BDs? | 10 |
| 8 | What genes and modifying genes are important in impacting phenotype for PPM with BDs? | 8 |
| Pregnancy and the post-partum context | ||
| # | Research Question | F-I-R Score |
| 1 | What is the optimal timing and dosing of antifibrinolytic agents or coagulation factor for prophylaxis and treatment of primary and secondary PPH? | 22 |
| 2 | What is the best tool to identify clinically relevant PPH, indicating need for inherited BD workup? | 21 |
| 3 | What role does local hemostasis play in pregnancy and PPH compared to systemic hemostasis? | 20 |
| 4 | What are the current barriers to prenatal diagnosis of inherited BDs? | 19 |
| 5 | What is the onset and progression of coagulation factor changes throughout pregnancy and post-partum? | 17 |
| 6 | How can we link inherited BD screening to family planning and obstetric care? | 17 |
| 7 | What is the optimal timing of prophylaxis for first trimester procedures? (e.g., D&C) | 16 |
| 8 | What is the risk of VTE for PPM using hormonal treatments or antifibrinolytic agents in the post-partum or post-surgery setting? | 14 |
| 9 | What role does the placenta play in the development of immune tolerance? | 12 |
| 10 | How can we ensure that pregnancy does not exclude participation in clinical trials? | 10 |
| Uterine physiology and bleeding | ||
| # | Research Question | F-I-R Score |
| 1 | What is the relative safety of intrauterine procedures in the setting of inherited BDs? (e.g., ablation, D&C, IUD placement, biopsy) | 22 |
| 2 | What is the optimal dosing and impact of coagulation factors, antifibrinolytic agents, and desmopressin on HMB? | 18 |
| 3 | What is the impact of androgenic hormone therapy on bleeding symptoms? | 16 |
| 4 | What is the safety and effectiveness of combination therapies for HMB management (antifibrinolytic agents, desmopressin, gonadal steroid hormones)? | 16 |
| 5 | What is the relative effectiveness and optimal dosing of gonadal steroid hormone therapies in inherited BDs in the acute and chronic setting? | 15 |
| 6 | Are there histopathological findings on an endometrial biopsy that could be utilized for diagnosis of inherited BDs? | 15 |
| 7 | What are the impacts of various hormone therapies on bleeding workup? | 15 |
| 8 | How does endometrial physiology contribute to HMB and response to treatment both within and outside of inherited BDs? | 14 |
| 9 | Do defects in primary hemostasis have more of an effect on uterine bleeding compared to defects in secondary hemostasis? | 14 |
| 10 | What is the relative accuracy and feasibility for menstrual blood loss measurements? | 13 |
| 11 | What are the expected bleeding patterns in inherited BDs with gonadal steroid hormone therapies? | 13 |
| 12 | How do hemostasis and the levels of coagulation factors in the blood differ from that in the endometrium? | 12 |
| 13 | What is the relative effectiveness of endometrial drug delivery of NSAIDs, antifibrinolytic agents, or other nonhormonal therapies via IUDs in the treatment of HMB? | 8 |
| Bone and joint health | ||
| # | Research Question | F-I-R Score |
| 1 | What is the prevalence of subclinical joint bleeds among PPM with inherited BDs, and what is the most accurate method of assessment? | 18 |
| 2 | What is the impact on bleeding phenotype of concomitant collagen and inherited BDs? | 15 |
| 3 | What effect do inherited BDs have on bone health and what is the best way to study those effects? What role(s) do hormones play? | 15 |
| 4 | How do the biomechanics of PPM change throughout the lifespan, what is the impact on joint bleeding phenotype and which joints are affected? | 14 |
| Health care delivery | ||
| # | Research Question | F-I-R Score |
| 1 | What do young people with inherited BDs need to be prepared for puberty and menarche? | 22 |
| 2 | What are the most effective interventions to increase primary care awareness (screening, knowledge, referral) about HMB and associated BDs? | 20 |
| 3 | What costs are associated with bleeding symptoms in PPM? | 18 |
| 4 | What are the characteristics of people who are diagnosed with BDs early on versus later? | 18 |
| 5 | What care model is most effective and feasible in caring for PPM with inherited BDs? | 10 |
| Patient-reported outcomes and quality-of-life | ||
| # | Research Question | F-I-R Score |
| 1 | What tools are currently available to assess QoL throughout the lifespan and what is missing? | 21 |
| 2 | How does the presence of an inherited BD or bleeding symptoms affect access to education and educational attainment? | 19 |
| 3 | What is the correlation between QoL scores and other outcomes (e.g., PBAC, ferritin)? | 19 |
| 4 | How does an inherited BD, as well as specific symptoms such as HMB, affect QoL and mental health? | 19 |
| 5 | What costs are associated with bleeding symptoms in PPM? | 18 |
| 6 | What are the QoL outcomes from uterine bleeding in trans-masculine people? | 18 |
| 7 | What are the symptom correlations of HMB in inherited BDs with dysmenorrhea/pelvic pain? | 18 |
| 8 | How does an inherited BD affect intimacy, sexual function, and sexual relationships? | 16 |
BD: bleeding disorder, COVID: coronavirus disease 2019, D&C: dilation and curettage, F-I-R: feasibility-impact-risk, HMB: heavy menstrual bleeding, IUD: intrauterine device, NSAID: nonsteroidal anti-inflammatory drug, PBAC: pictorial blood loss assessment chart, PPH: post-partum hemorrhage, PPM: people who have or had the potential to menstruate, QoL: quality-of-life, VTE: venous thromboembolism
Figure 3.
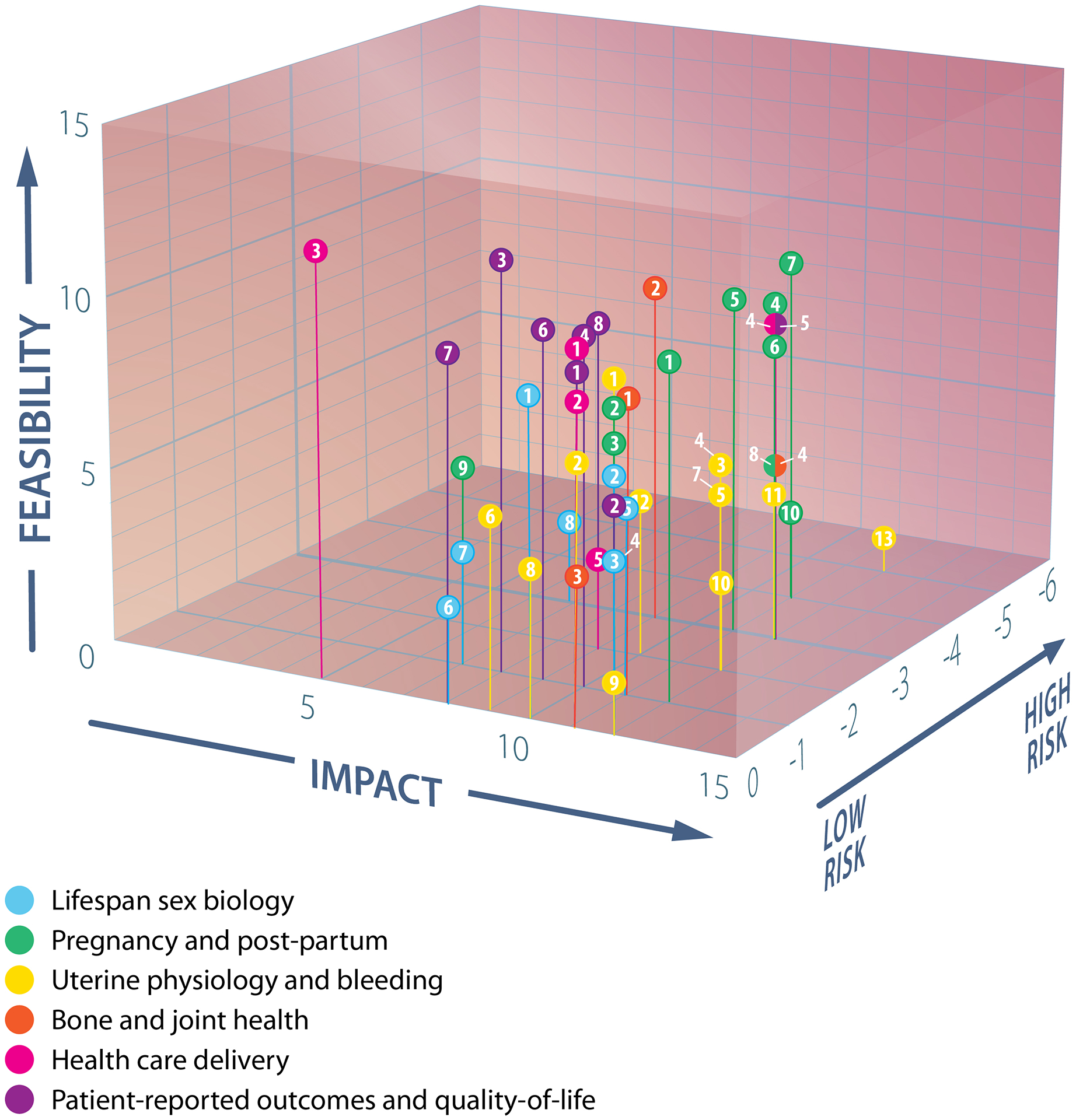
Plot of feasibility, impact, and risk scores of prioritized questions in each focused research domain
Label numbers correspond to those in Table 2, segmented circles indicate data points from different domains with identical coordinates.
Figure 4.
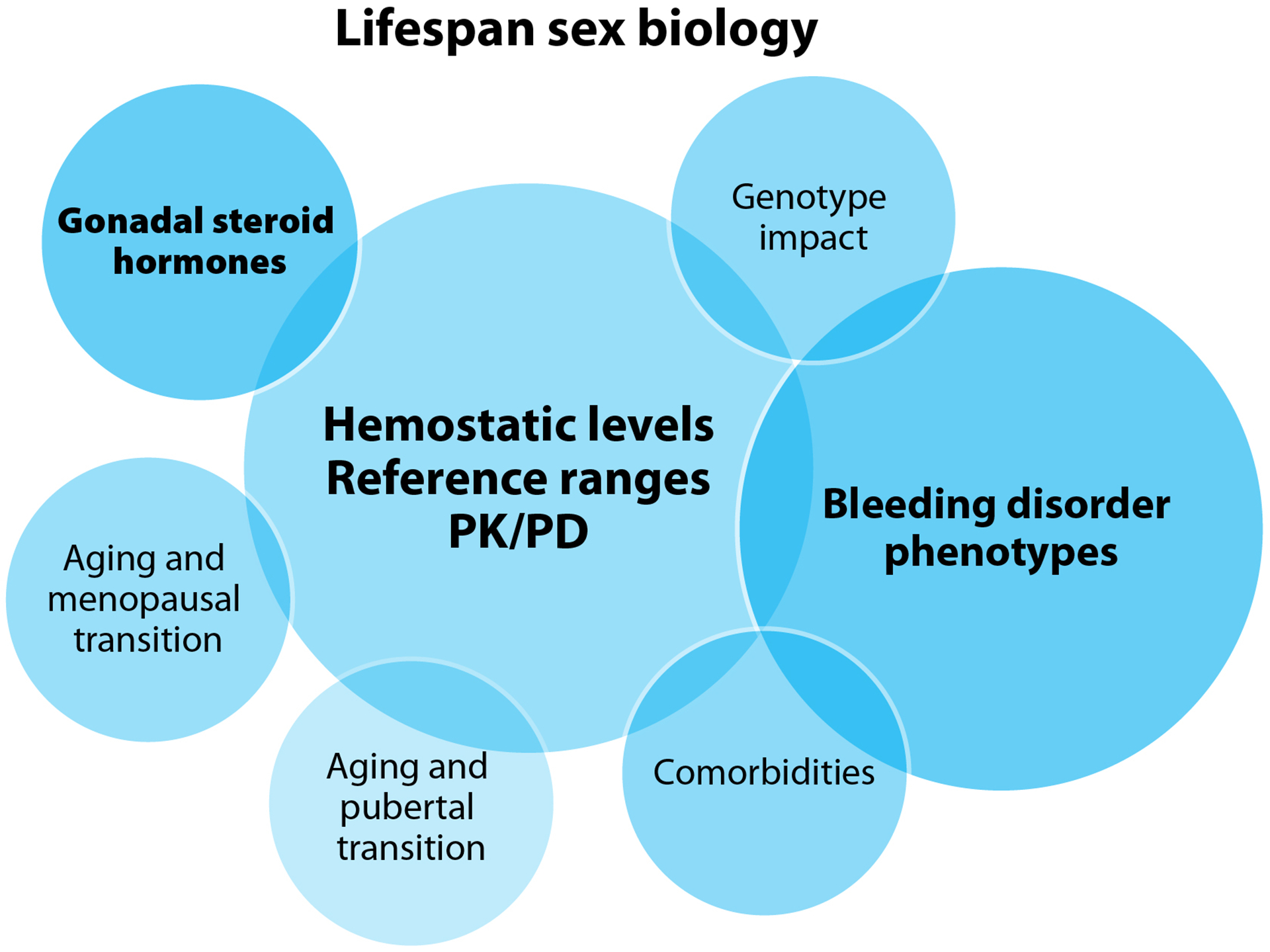
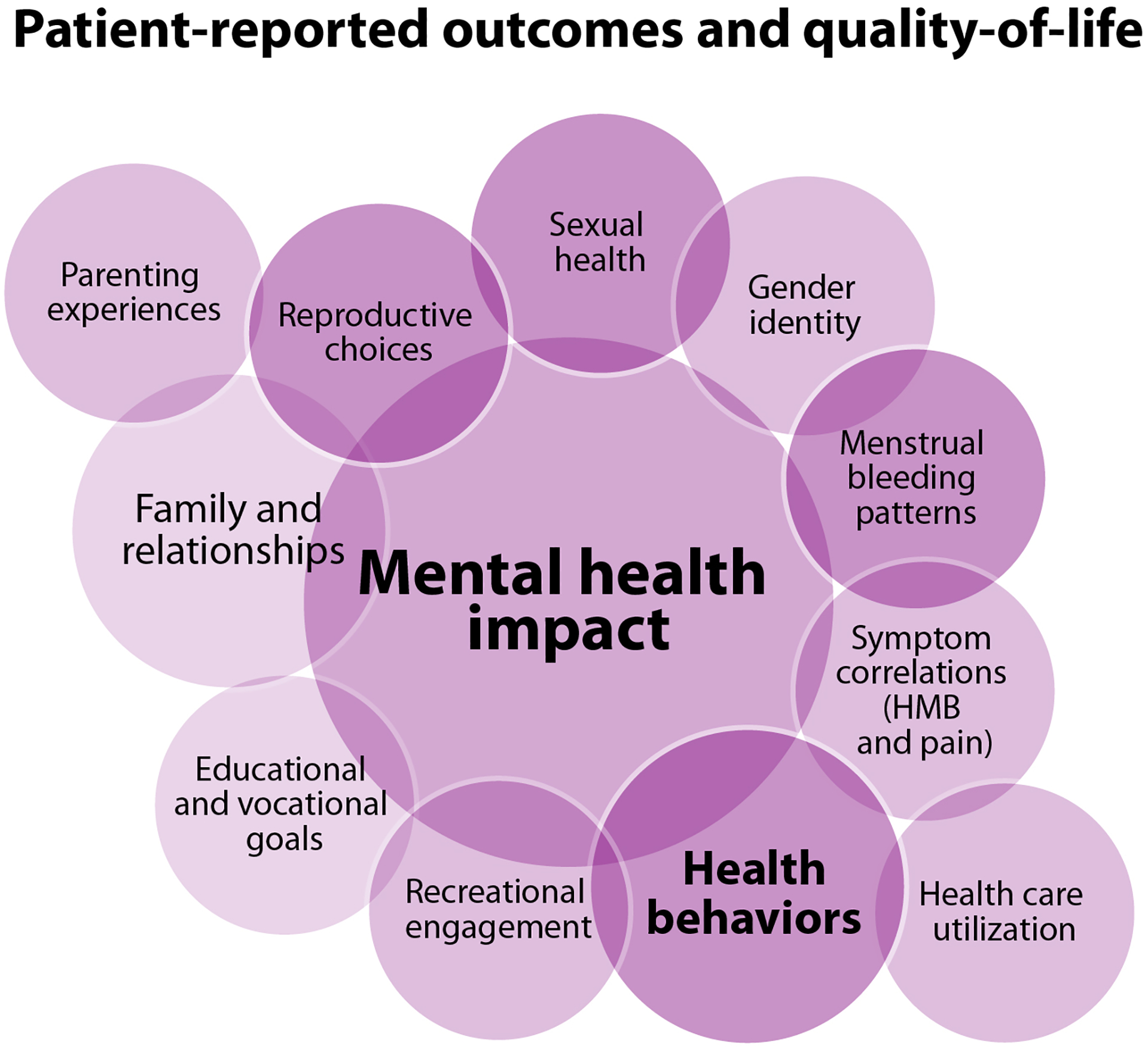
Venn diagrams of areas of impact of the prioritized research questions in each focused research domain
A. Lifespan sex biology B. Pregnancy and the post-partum context C. Uterine physiology and bleeding research questions D. Bone and joint health E. Health care delivery F. Patient-reported outcomes and quality-of-life
D&C: dilation and curettage, HMB: heavy menstrual bleeding, PD: pharmacodynamics, PK: pharmacokinetics, IUD: intrauterine device
3.3. Lifespan sex biology
Three core issues centered the research priorities in lifespan sex biology (Figure 4A). The need to better understand the levels of coagulation factors throughout the lives of PPM, how different therapeutics impact circulating coagulation factor levels, reference ranges and parameters for diagnosis, and individual pharmacokinetic and pharmacodynamic profiles (Table 2). These issues are key to understanding the experiences and phenotype severity in this population. Important opportunities to advance knowledge exist at the pubertal transition, during pregnancy, at the menopausal transition, and throughout the lives of PPM. The impact of comorbidities on BDs and their pathophysiology also remains largely unexplored in this population.
The high cost of laboratory analyses lowered the feasibility scores of many of these potential studies. Answering many of these questions is anticipated to pave the way for clinical trials investigating effective therapeutics, contributing to high impact scores.
3.4. Pregnancy and post-partum
Pregnancy and post-partum were accorded their own focused research domain in addition to being a focus in lifespan sex biology (Table 2). The dissemination of feasible tools to diagnose hemorrhage in PPM during pregnancy and in the post-partum context are prerequisite to conducting research into prevention of post-partum hemorrhage (PPH) and relevant hemostatic mechanisms (Figure 4B). This research may then investigate the optimization of coagulation factor or antifibrinolytic agent prophylaxis in the context of PPH or for first trimester procedures, how and when coagulation factor levels change throughout pregnancy and the post-partum period, and the roles of primary versus secondary hemostasis in pregnancy and PPH. The risk of venous thromboembolism (VTE) in post-partum PPM with BDs in the context of hormonal and/or antifibrinolytic treatments requires specific investigation. Widely feasible blood loss quantitation methodology is also key to determining the screening criteria for performing a BDs workup, and to optimizing the identification of bleeding problems related to pregnancy.
Pregnancy was determined to be an important opportunity for fetal and neonatal BD screening and diagnosis, and to engage the pregnant person in an investigation of their own bleeding tendency. Prioritized research questions in this domain also target identifying and overcoming barriers to the integration and coordination of prenatal diagnosis and genetic testing with family planning (including pre-conception evaluations) and obstetric care. Elucidating any role the placenta (and the coagulation factor level of the pregnant person) might play in determining the lifetime risk of the fetus to develop neutralizing alloantibodies, or inhibitors, to factor concentrates has the potential to be particularly impactful as this remains the treatment-related complication of greatest concern [59, 60]. The group felt it was important to address the fact that pregnancy often means exclusion from clinical trials, and the need for strategies to change that.
These research questions share elements of risk with other pregnancy studies, and the feasibility of many is somewhat lowered by high costs, but the downstream impact was assessed to be consistently high.
3.5. Uterine physiology and bleeding
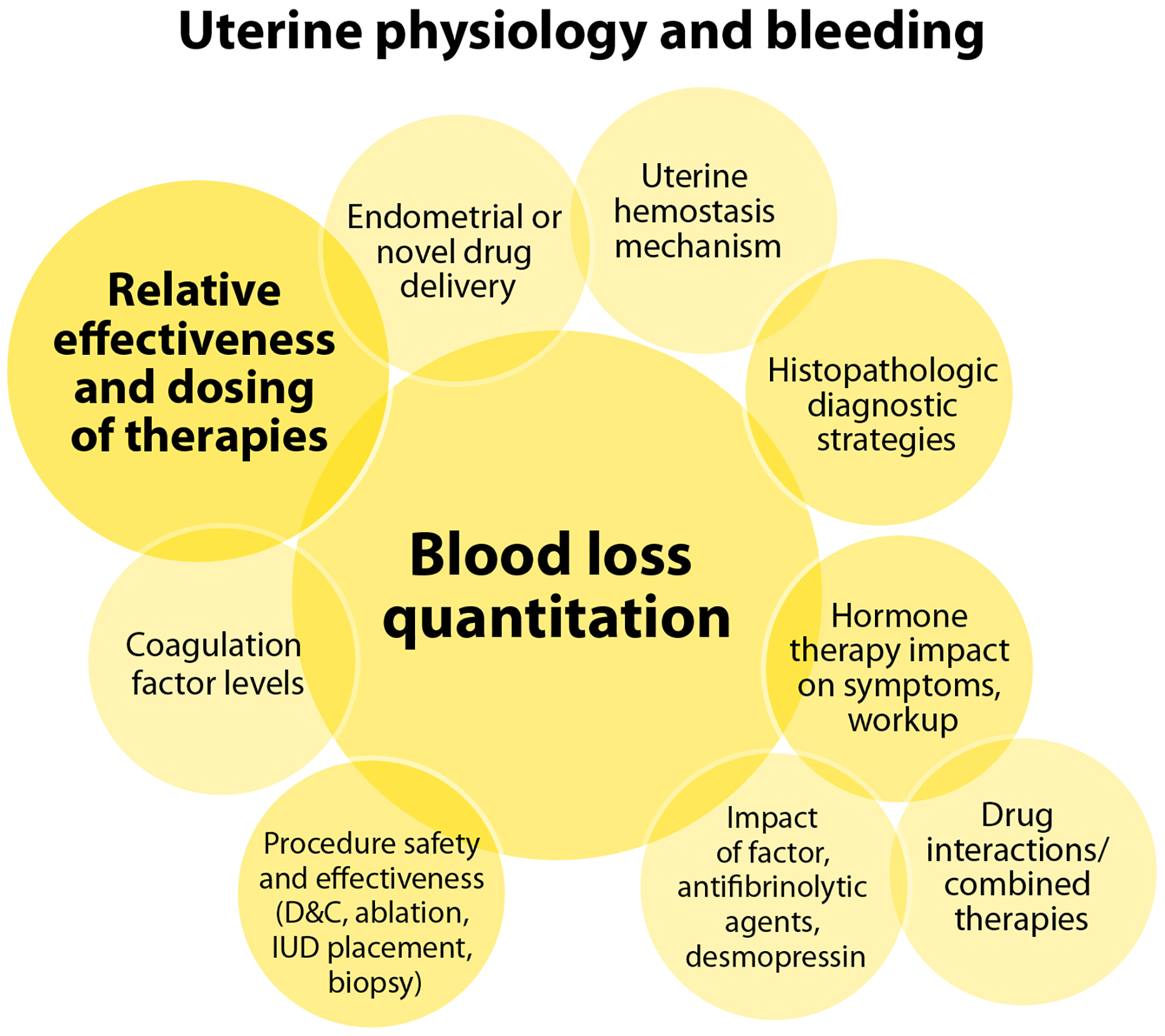
Discussions of uterine physiology and bleeding resulted in the greatest number of prioritized research questions of any single focus domain (Table 2). Tools to efficiently quantitate blood loss, are key to investigating the relative effectiveness and dosing of treatments. The impacts of variables on uterine bleeding, such as coagulation factor levels or gonadal steroid hormones are central to this discussion (Figure 4C).
The impact of gonadal steroid hormones on bleeding symptoms and workup, and the safety, effectiveness, and optimal dosing in acute and chronic setting of hormonal therapies alone or in combination with other therapies, underpin several priority questions. Similarly, the optimal dosing and duration of coagulation factor replacement, desmopressin, and/or fibrinolytic agents in the treatment of HMB requires further investigation. A number of fascinating basic biology questions were prioritized, such as whether defects in primary or secondary hemostasis have a greater impact on uterine bleeding, how uterine factor levels compare to systemic levels, and how the physiology of the endometrium may contribute to HMB and response to treatments. The determination of the safety and effectiveness of common intrauterine procedures, in the context of an inherited BD, could be tackled through a systematic review and/or registry study. Some of the prioritized questions require more challenging methodology. These include investigations of uterine hemostatic mechanisms and factor levels, capitalizing upon the potential for endometrial histopathologic diagnostic strategies to assist with BD diagnosis and treatment outcome determinations, as well as innovative approaches such as endometrial drug delivery, especially of novel therapies, to treat HMB.
In addition to challenging methodology, anticipated high costs negatively impacted the feasibility scores of several prioritized questions, though this was highly variable between questions. They were all, however, assessed as having high impact and low risk.
3.6. Bone and joint health
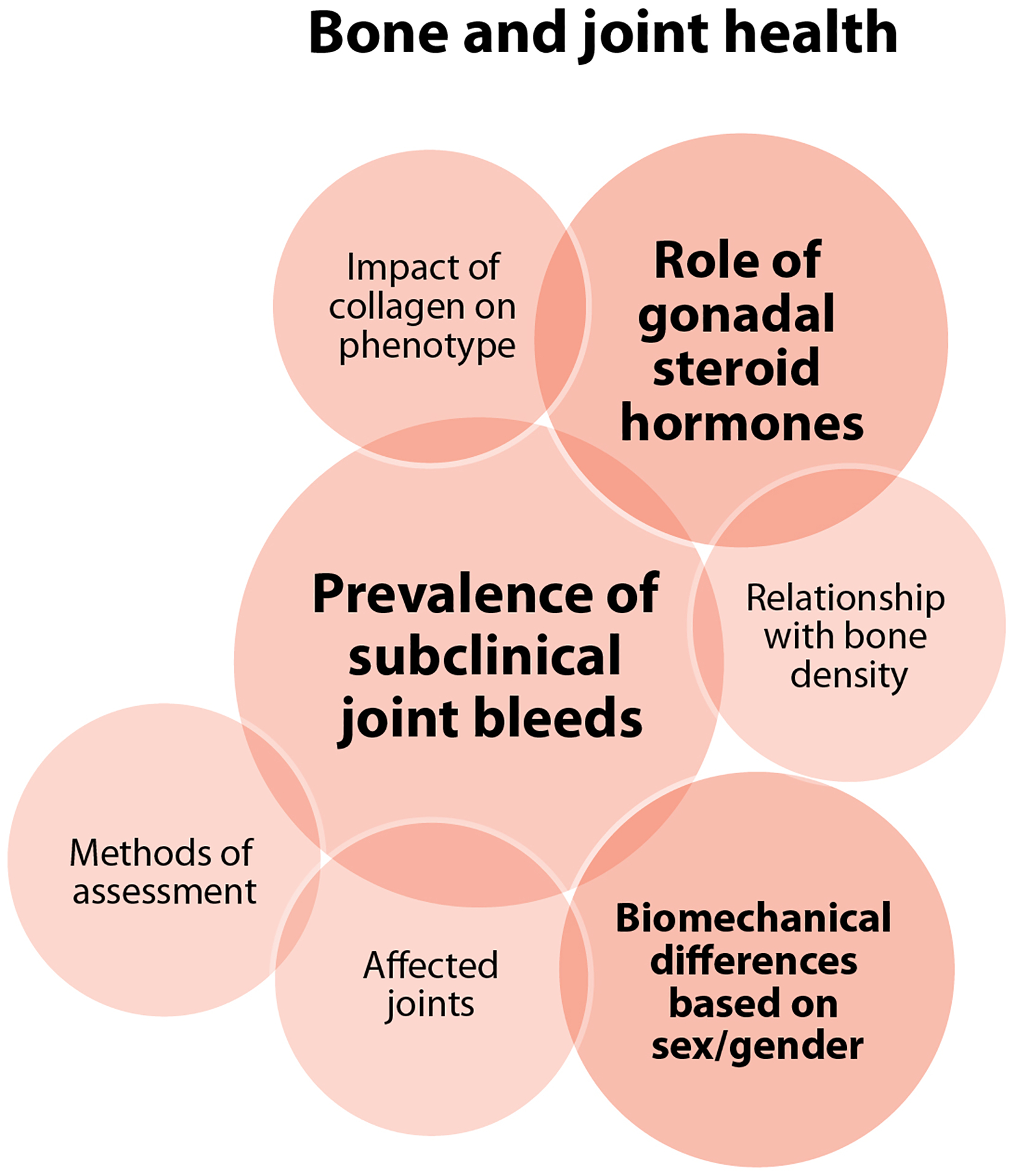
WG4 invites the reader to consult the arthropathy, pain, and bone health questions identified by WG1 in their exploration of research priorities to transform the care of people with hemophilia [61]. WG4 research priorities to meet the bone and joint health needs of PPM with inherited BDs coalesced around characterizing the prevalence of subclinical joint bleeds and how and this is unique to PPM (Figure 4D and Table 2). Establishing the best methods to assess these bleeds and the joints most often affected are important initial steps, with potential implications for similar or collaborative studies in mild hemophilia. The effects of endogenous and exogenous gonadal steroid hormones, and their varying levels throughout the lives of PPM on joints and bone density in the context of BDs merit particular investigation. The identification of other relevant variables, such as coagulation factor levels, and how they might influence outcomes would be an important extension of this type of study. Similarly, potential interplay with other disorders, such as the hypermobility associated with collagen disorders (addressed in greater depth by WG2) [62], and bleeding phenotypes should be explored. Potential individual biomechanical differences, how these might contribute to specific bone and joint outcomes, and how they evolve throughout the lifespan and based on sex biology are also important lines of inquiry. These four research questions were assessed to be highly feasible with low risk, while their impact was more uncertain and felt to depend very much on the outcomes.
3.7. Health care delivery
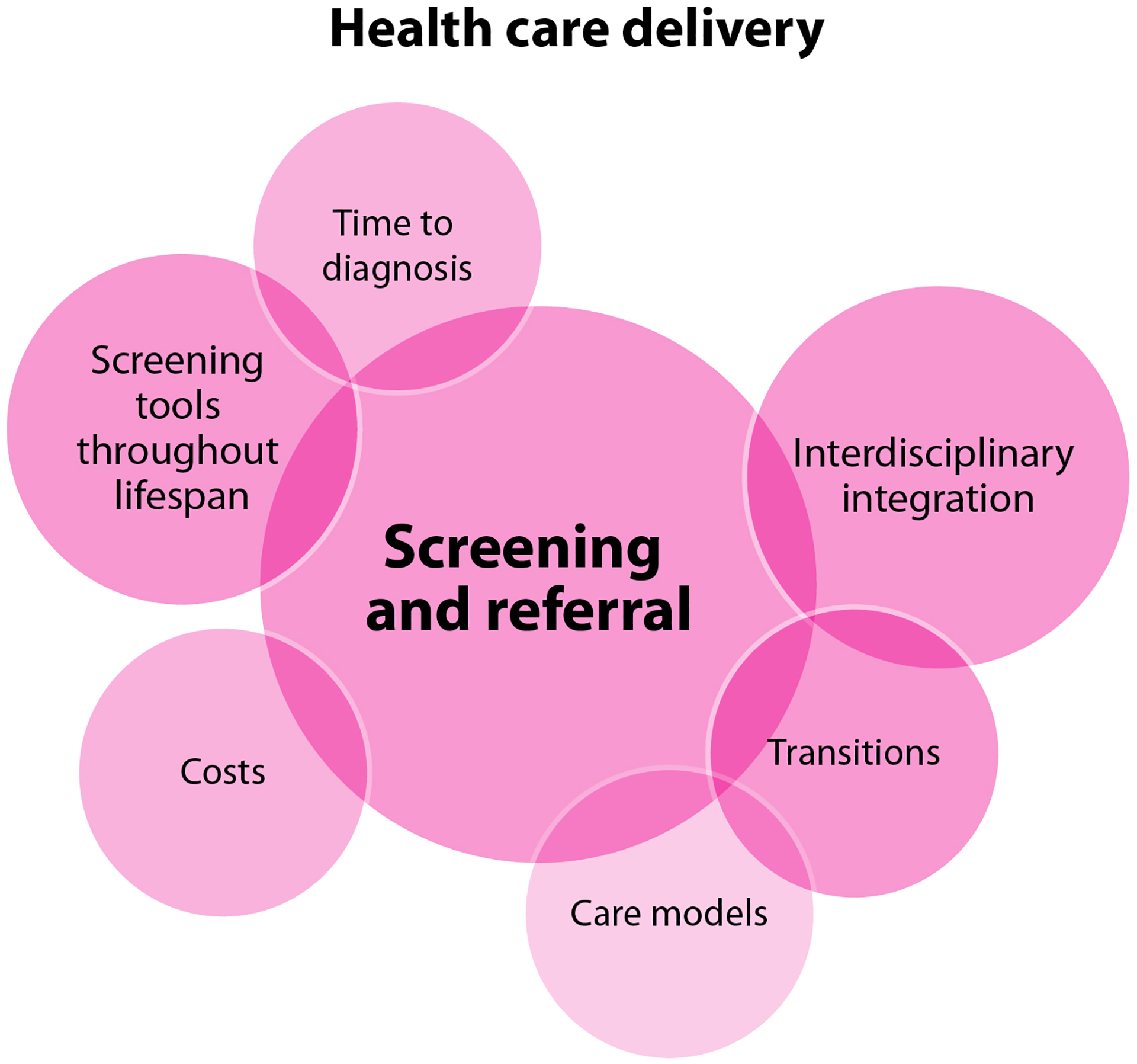
WG4 identified a substantial knowledge gap regarding the impact of screening and referral tools and practices on PPM with inherited BDs at the center of research opportunities in health care delivery (Figure 4F and Table 2). Existing screening tools have not been optimized for different phases of the lifespan in this target population and will benefit from refinement for specific age groups. There is an important opportunity to study the characteristics of PPM diagnosed early in their experience of bleeding symptoms versus those for whom the diagnostic journey takes many years. As addressed in greater detail by WG5 Diversity, Equity, and Inclusion; Health Services Research; and Implementation Science, social determinants of health, such as availability of transportation, socioeconomic status, and access to culturally appropriate care, likely result in diagnostic delays and further health inequity [46, 48, 63]. Transitions between life phases also present focus points for research to improve care delivery, especially menarche. Identifying the most effective interventions to increase primary care awareness, through initial and ongoing education of HCPs, as well as improvements in referral processes, constitute another promising research avenue. Similarly, deepening interdisciplinary integration and determining the optimal care model for the effective and feasible diagnosis and management of inherited BDs in this target population has the potential to significantly impact the experiences of PPM. The question of costs, particularly the relative merits of investing extensively in screening versus treatments, must also be examined.
The questions prioritized in this domain are of varying feasibility, depending heavily on the level of engagement of the health care system with the research process and these objectives. Their potential to improve outcomes for PPM with BDs is high, while the risk to patients was assessed to be low. The group also assessed the risk to institutions as low, but expressed concern that institutions may not necessarily initially recognize this, creating an opportunity for impactful advocacy.
3.8. Patient-reported outcomes and quality-of-life
The domain of PROs and QoL encompasses many concerns highlighted in the community consultation sessions [57]. They constitute top priorities in a research agenda intended to transform wellbeing (Table 2), with impacts on mental health anchoring this discussion (Figure 4F). Much of this work requires further development of tools to assess QoL throughout the lifespan and may inform investigations into correlations with other outcomes such as extent of bleeding or iron deficiency.
There are opportunities to improve our understanding of how an inherited BD may impact the reproductive choices of PPM, as well as their sexual health, intimate relationships, sexual function, and gender dysphoria. We need to learn more about how menstrual bleeding patterns are impacted in these contexts, and how they impact fundamental aspects of daily life and mental health, including recreational engagement and educational and vocational access and goal attainment. The impact of uterine bleeding on QoL is of high importance. The impact of uterine bleeding on trans-masculine gender dysphoria and the correlation between HMB and pain merit particular attention. The impact on PPM’s family relationships, particularly parenting experiences in a family where more than one member has a BD, remains inadequately investigated. All these elements are expected to impact health behaviors and health care utilization, the characterization of which also constitutes an important research avenue. The costs to PPM of their bleeding symptoms and its impact on their lives, likely varied and many, remain poorly understood.
These research questions are largely highly feasible with the opportunity for moderate to high impact. Many of them rely heavily on access to the health care described above, including effective screening and diagnosis. Some risks may lie in the willingness of PPM to engage with some of these questions, highlighting the importance of building research contexts characterized by trust and support that are sensitive to the potential personal impact of examining intimate issues.
4. Discussion
PPM with inherited BDs become true lived experience experts (LEE) of their disorders [53] while remaining largely unrecognized, undiagnosed, uncounted and often without access to optimal care [2, 44, 49]. LEEs and a subset of dedicated HCPs have developed detailed experience in the management of abnormal bleeding in this population, however the formal evidence base upon which to establish and disseminate best practices remains strikingly thin. The ultimate outcome of this knowledge gap is that health equity, a life unimpaired by disease complications [55], is unimaginable for many PPM, and their families, who live with diminished daily QoL and significant mental health implications [14, 18, 44, 64].
WG4 defined, evaluated, and prioritized 48 specific questions across six focused research domains (Table 2) with the potential to transform the experiences of PPM living with BDs in the areas of greatest concern raised in NHF’s community consultations [57]. Some of these studies should be undertaken immediately. Others rely on the resolution of foundational knowledge gaps (Suppl Figure S1):
Understanding the prevalence of inherited BDs and their phenotypes and how they impact PPM throughout their lifespan
Defining the hemostatic norms and standards key to diagnosing and assessing treatment effectiveness in this population
Establishing feasible, affordable methods to quantitate bleeding in PPM, and disseminating these widely
Identifying the patient-centered outcomes of greatest importance to this population and effective tools to measure them
The research with the greatest transformative potential faces a number of obstacles. Studies generating the data to fill the foundational knowledge gaps are impeded by an inability to recruit sufficient participants and prohibitive methodological issues. Contributors to these barriers, opportunities to overcome them, and potential partners (examples presented in Table 3) in these solution-oriented endeavors are presented below.
Table 3.
Examples of potential collaborators in efforts to overcome barriers to important research to advance the health of people who have or had the potential to menstruate with inherited bleeding disorders
| American College of Obstetricians and Gynecologists (ACOG) |
| American Thrombosis and Hemostasis Network (ATHN) |
| Centers for Disease Control and Prevention (CDC) Office of Women’s Health and Division of Blood Disorders |
| Contraceptive Clinical Trials Network (CCTN) |
| Fenway Institute |
| Foundation for Women and Girls with Blood Disorders (FWGBD) |
| Hemophilia Federation of America (HFA) |
| Hemostasis and Thrombosis Research Society (HTRS) |
| International Federation of Gynecology and Obstetrics (FIGO) Committee for Menstrual Disorders |
| International Society on Thrombosis and Haemostasis (ISTH) Scientific and Standardization Committee (SSC) Subcommittee on Women’s Health Issues in Thrombosis and Haemostasis |
| Maternal Fetal Medicine Units Network (MFMU) |
| National Hemophilia Foundation (NHF) |
| National Institutes of Health (NIH): Office of Research on Women’s Health (ORWH) |
| Obstetric Fetal Pharmacology Research Network |
| Partners in Bleeding Disorders Education |
| PRIDEnet |
| World Federation of Hemophilia (WFH) |
| World Professional Association for Transgender Health (WPATH) |
4.1. Shortening the diagnostic odyssey
PPM often live for years with abnormal bleeding negatively affecting their physical and mental health and QoL prior to diagnosis with an inherited BD. The average delay between symptom onset and BD diagnosis for females in the US is reported to be 10–16 years [65, 66]. Despite a similar age of first bleeding event, the diagnostic delay is on average six years longer for women than men [1]. With an accurate diagnosis and in partnership with a multidisciplinary care team, PPM with inherited BDs can live happy, healthy, fulfilling lives [44, 52]. A coordinated educational campaign targeting all HCPs that PPM may encounter after a bleeding event could substantially smoothen and shorten their diagnostic odyssey.
PPM from marginalized and minoritized populations (e.g., gender diverse individuals, racialized groups) face additional challenges and barriers to optimal care [63]. Understanding and addressing these disparities is an unmet research priority; identifying and dismantling these barriers must be an important focus of education and advocacy initiatives.
HMB is one of the most common symptoms of an inherited BD in PPM [1, 64, 65, 67], however societal stigmatization and lack of HCP and PPM knowledge/comfort discussing menstruation contribute to low rates of diagnosis [47, 49, 68]. A 2012 survey of US obstetricians/gynecologists reported >50% underestimated the true prevalence of VWD and <40% would likely consider BDs in women with HMB [69], despite multiple international gynecologic and hematologic guidelines recommending inherited BDs screening of PPM presenting with HMB [4, 70]. Excellent educational initiatives have been spearheaded by advocacy organizations such as the Foundation for Women and Girls with Blood Disorders (FWGBD) and NHF with the (US) Centers for Disease Control and Prevention (CDC), and some smaller research group initiatives (e.g., Letstalkperiod.ca, WeThrive) [71, 72]. Broader public campaigns might seek industry partners that market products for the management of menstrual bleeding, targeting those that consume their products most. Hormone therapy industry partners might also contribute to efforts to diagnose more PPM with BDs.
Centers that treat PPM with BDs may lack screening processes to identify people at risk and potential research candidates. A clinical pathway at one institution to screen all adolescents presenting with acute HMB to the emergency department or inpatient unit for potential BD resulted in a significant rate of new VWD diagnoses [73]. Standardized definitions and parameters for disease would also facilitate diagnosis. There is considerable heterogeneity between the symptoms and presentation of various bleeding disorders. As such, we should consider thoughtful evaluation of grouping characteristics based on phenotype versus pathology. Refining the understanding of biomarker and phenotypic characteristics is an ongoing effort.
Family planning, pre-conception evaluation, pregnancy, birth, and the post-partum period tend to focus on diagnosis of potential offspring, but present numerous other opportunities to investigate bleeding tendencies. For example, PPH inconsistently prompts neonatal testing for a potential BD, and should also trigger BD testing in the recently pregnant person.
Recent advances in therapeutic technologies for hemophilia (e.g., gene therapy) prompted the establishment of an efficient and effective network of HCP educational partners in the US, including ATHN, NHF, and Partners in Bleeding Disorders Education [74]. This network should expand to develop and disseminate resources to raise awareness about BDs in PPM. PPM with BDs often initially take their concerns to primary care physicians, therefore raising awareness among these gatekeepers is incredibly important [1]. Emergency room physicians, pediatricians, dental professionals, obstetricians and gynecologists, midwives, genetic counselors, and other specialists would also benefit from a greater awareness of the hallmarks of a BD in PPM and how to refer individuals for specialized follow-up. Professional associations and societies constitute valuable potential partners in the effective education of their members.
4.2. Recruiting for research
The NIH has required the “appropriate participation of women in clinical studies” since 1993 and implemented a separate policy in 2016 requiring that sex as a biological variable be factored into the designs, analyses, and reporting of human studies or that applicants provide strong justification for single-sex investigations [34]. This policy recognize the pervasive effect of sex on biological functioning and that sex must be considered for research to be reproducible and relevant for all people [75].
In the experience of these authors, many PPM with an inherited BD are keen to be involved in research. Frustration with confusing or incorrect diagnoses, feeling that their symptoms are sometimes dismissed, and difficulty accessing appropriate treatment [24, 49, 51] motivate them to contribute to advancing knowledge. LEEs are uniquely positioned to engage other potential participants with the value of research and to (re)build trust. Their expertise is invaluable in identifying meaningful outcomes, feasible methodologies, and effective recruitment strategies [53].
4.3. Problematic methodologies
4.3.1. Menstrual blood loss quantitation
In clinical practice, HMB is defined as menstrual blood loss (MBL) that interferes with an individual’s physical, emotional, social, and material QoL and its management is guided by the impact on QoL [8, 76, 77]. The pivotal trials for the few hormonal therapies approved for HMB by the U.S. Food and Drug Administration (FDA) [78, 79] measured MBL via the 1960s alkaline hematin method [80]. The definitive “gold standard” objective quantitative method and a valuable research tool, it has been used for decades to define HMB treatment outcomes [78, 79, 81]. However, MBL does not measure the outcomes of greatest importance to the management of HMB, the impact on PROs and QoL [76]. Validated pictorial HMB assessment methods combined with QoL assessments appear well suited to clinical studies [77, 82–85], and have contributed to at least one European Medicines Agency approval [84]. We can perform meaningful research into the relative effectiveness of treatments for abnormal bleeding without cumbersome volumetric measurements of blood loss. Regulatory acceptance of efficacy demonstrations through slightly less quantitative methods may encourage more trials leading to expansions of indications of hormonal therapies to include HMB. However, the primary obstacle to these indications remains the infrastructure, personnel, and resource costs of running traditional pivotal clinical trials. WG4 refers readers to the discussions of potential solutions to cost, time, and complexity burdens facing trials for therapeutics for small populations reported by WG3 [86] the strategies proposed by WG6 [87] and the potential offered by national and international collaborations detailed by WG2 [62].
Evolving the HMB assessment paradigm and expanding therapeutic options with approved regulatory indications will likely require extensive coordinated advocacy. Fortunately, PPM may look to formidable allies such as NHF, Hemophilia Federation of America (HFA), ATHN, FWGBD and many dedicated and influential HCPs to champion this change. Industry partners may see value in the potential to expand access to novel markets for therapeutics with proven safety records. This is also an exciting opportunity for collaboration between the BDs and family planning communities working in the realm of contraceptive-induced menstrual changes (CIMC) [88].
4.3.2. Data collection
The outcomes commonly measured for cisgender boys and men in BD research [89] do not accurately reflect the pathophysiology or the lived experience of PPM [1]. The CDC Community Counts Public Health Surveillance of Bleeding Disorders project recently expanded to include data collection on some outcomes specific to people assigned female at birth such as use of prophylactic treatment for menstrual bleeding and history of hysterectomy [64]. Identifying the patient-centered outcomes of greatest importance to PPM and effective tools to measure them is requisite to the collection and interpretation of meaningful data [15].
5. Conclusions
WG4 offers the priorities reported herein as contributions to a National Research Blueprint for Inherited Bleeding Disorders [56] to advance the health of people who have or had the potential to menstruate. To truly serve the needs of this community, it may be constructive to consider the following principles:
“I am more than my uterus”: PPM with inherited BDs may also experience bleeding associated with procedures or traumatic injury, and musculoskeletal and intracranial bleeds. Caring for PPM as whole people means understanding how inherited BDs impact all aspects of their mental and physical health throughout their lifespan.
“Women are not smaller men”: Understanding, diagnosing, and treating inherited BDs in PPM will best be advanced by studies designed with and for this population. Support for research into health concerns specific to PPM must be equitable and meaningful.
“Baking mental health in everywhere”: The impacts of inherited BDs on the mental health of PPM is clinically significant and consistently prioritized by LEEs. Improving mental health outcomes must be an intentional priority of research and care initiatives.
“Just because bleeding is expected doesn’t mean it should be accepted”: While some bleeding in PPM is normal, abnormal bleeding may be less straightforward to characterize and explain. PPM may not realize that their bleeding is abnormal, and treatment goals must reflect individually appropriate outcomes.
“Equity in everything”: Research, diagnosis, and care of PPM with inherited BDs have been neglected due to sexism for centuries. Achieving equitable access to diagnosis, care, and the opportunity to participate in research requires recognition of the impact on, not just cis-girls and cis-women, but also gender diverse individuals, and intentional investment in initiatives and advocacy that strenuously counter these forces, valuing and uplifting the voices of all PPM.
Inherited BDs in PPM are not well understood, and the evidence base on which to optimize diagnosis and management is thin. Opportunities to conduct research that advances the priorities of this target population abound, and while substantial obstacles exist, potential collaborators in surmounting those obstacles are also plentiful, not least of all the LEEs themselves who are keen to contribute.
Supplementary Material
LEE Perspective.
It was a privilege to be invited to WG4 Research Priorities for the Health of People who Have or Had the Potential to Menstruate (PPM). While initially being in discussions with physicians at the top of their field felt intimidating, the lived experience experts (LEE) quickly felt heard and understood. The group answered questions and clarified information in real-time and via email. The researchers demonstrated their interest and concern for the LEEs’ experiences regarding bleeding issues.
At the first bi-monthly meeting, it became clear how passionate and dedicated the researchers were to “get it right”, when we discussed the name of our group and how to be inclusive of all who could, do, or have menstruated. It was remarkable to be a part of stimulating conversations and active problem solving across disciplines. LEEs were particularly impactful in the discussion that identified gaps in foundational research, such as patient-centered outcomes and feasible quantitative methods; the focused research domain of patient-reported outcomes, quality-of-life issues; and the impact on mental health of being a PPM with a bleeding disorder (BD).
Involving LEEs from the beginning had a significant impact on the outcomes of this project. Learning what PPM’s real-life experiences are helped guide the path to relevant research questions. This is an important first step in advancing the health and wellbeing of PPM as they will have the opportunity to participate in research specific to their inherited BD and benefit from research findings.
Acknowledgments
MK Baldwin and AC Weyand presented highlights of the deliberations of Working Group 4 at the National Hemophilia Foundation State of the Science (virtual) Research Summit (SOSRS), September 12-15, 2021. Summit discussions informed the Discussion and Conclusions of this paper. Fiona Robinson, PhD provided professional medical writing support during manuscript development; medical illustrations were created by Matt Evans; both paid by NHF. The authors thank Maria E. Santaella, MSN, RN-BC, CPHON for her review of the manuscript.
Funding
The entire State of the Science Research Summit and this manuscript were funded by National Hemophilia Foundation. Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number NIH_NOA_1R13HL158209-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of interests
The authors are integrated members of the inherited bleeding disorders community: people with inherited bleeding disorders, their family members, health care providers and researchers (including physicians, nurses, physical therapists, pharmacists, social workers/psychologists, geneticists/genetic counselors, etc.), industry partners, government officials/regulators, local community organization representatives and others. MK Baldwin discloses: grants or contracts from any entity: NIH K12HD085809; consulting fees: Tremeau Pharmaceuticals, Exeltis Pharmaceuticals; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: National Hemophilia Foundation Medical and Scientific Advisory Committee, Foundation for Women and Girls with Bleeding Disorders. AC Weyand discloses: grants or contracts from any entity: Pfizer, Sanofi, NovoNordisk, Takeda; consulting fees: Takeda, Sanofi; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Genentech, Sanofi; Support for attending meetings and/or travel: Sanofi, Genentech; participation on a Data Safety Monitoring Board or Advisory Board: Bayer, Genentech, Sanofi. CS Philipp discloses: consulting Fees: Hemabiologics, Takeda, Coagulant Therapeutics; participation on a Data Safety Monitoring Board or Advisory Board: Spark. D Bensen-Kennedy discloses: other financial or non-financial interests: an employee of CSL Behring, a manufacturer of products used to treat bleeding disorders. HK Ahmadzia discloses: grants or contracts from any entity: NIH K23 HL141640; consulting fees: Coagulant Therapeutics, Haemosonics. K Rosen discloses: grants or contracts from any entity: Bayer Healthcare; stock or stock options: Bayer Healthcare. KA Matteson discloses: grants or contracts from any entity: OHSU; consulting fees: Myovant; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Maine Medical Center, Baystate Medical Center, UTMB; support for attending meetings and/or travel: Maine Medical Center, American College of ObGyn. K Paulyson-Nuñez discloses: consulting fees: Hemophilia of Georgia; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: University of North Carolina Greensboro; participation on a Data Safety Monitoring Board or Advisory Board: Foundation for Women and Girls with Blood Disorders; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: American Board of Genetic Counseling. KM Haley discloses: grants or contracts from any entity: ATHN/HTRS; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Comprehensive Health Education - Ladybugs Program. M Lavin discloses: consulting fees: Band Therapeutics, Sobi, CSL Behring, Takeda; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Pfizer, CSL Behring; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: EAHAD Co-Chair Women and Girls with Bleeding Disorders Committee, ISTH VWF Scientific SubCommittee Co-Chair, WFH Chair VWD and Rare Bleeding Disorders Committee. R Kulkarni discloses: payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: CSL Behring; participation on a Data Safety Monitoring Board or Advisory Board: Sanofi Genzyme, Novo Nordisk, Pfizer. S Ragosta discloses: all support for the present manuscript (e.g., funding, provision of study materials, medical writing, article processing charges, etc.): Ibis Reproductive Health; grants or contracts from any entity: National Institutes of Health, Collaborative for Gender and Reproductive Equity; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: TRANS-ARC steering committee member. S Luckey discloses: leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid: Hemophilia Federation of American Board member. V Desai discloses: other financial or non-financial interests: an employee of CSL Behring, a manufacturer of products used to treat bleeding disorders. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
List of abbreviations
- ACOG
American College of Obstetricians and Gynecologists
- ATHN
American Thrombosis and Hemostasis Network
- BD
Bleeding disorder
- CCTN
Contraceptive Clinical Trials Network
- CDC
Centers for Disease Control and Prevention
- CIMC
Contraceptive-induced menstrual changes
- COVID
Coronavirus disease 2019
- D&C
Dilation and curettage
- FDA
US Food and Drug Administration
- FIGO
International Federation of Gynecology and Obstetrics
- F-I-R
Feasibility-impact-risk
- FWGBD
Foundation for Women and Girls with Blood Disorders
- GT
Gene therapy
- HCP
Health care professional
- HFA
Hemophilia Federation of America
- HMB
Heavy menstrual bleeding
- HTRS
Hemostasis and Thrombosis Research Society
- ISTH
International Society on Thrombosis and Haemostasis
- IUD
Intrauterine device
- LEE
Lived experience expert
- LGBTQI+
Lesbian, gay, bisexual, transgender, queer, questioning, intersex, asexual and other
- MBL
Menstrual blood loss
- MFMU
Maternal Fetal Medicine Units Network
- NHF
National Hemophilia Foundation
- NIH
National Institutes of Health
- NSAID
Nonsteroidal anti-inflammatory drug
- ORWH
Office of Research on Women’s Health (NIH)
- PBAC
Pictorial blood loss assessment chart
- PD
Pharmacodynamics
- PK
Pharmacokinetics
- POC
Point-of-care
- PPH
Post-partum hemorrhage
- PPM
People who have or had the potential to menstruate
- PRO
Patient-reported outcome
- QoL
Quality-of-life
- RPG
Remote Participation Group
- SOSRS
State of the Science Research Summit
- SSC
Scientific and Standardization Committee
- US
United States
- VTE
Venous thromboembolism
- VWD
Von Willebrand disease
- WFH
World Federation of Hemophilia
- WPATH
World Professional Association for Transgender Health
- WG
Working group
References
- 1.Atiq F, Saes JL, Punt MC, et al. Major differences in clinical presentation, diagnosis and management of men and women with autosomal inherited bleeding disorders. EClinicalMedicine. 2021;32:100726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulkarni R Improving care and treatment options for women and girls with bleeding disorders. Eur J Haematol. 2015;95 Suppl 81:2–10. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes for Health. Consideration of Sex as a Biological Variable in NIH-funded Research (additional guidance for NOT-OD-15–102) [cited 2022 May 3]. Available from: https://orwh.od.nih.gov/sites/orwh/files/docs/NOT-OD-15-102%20Guidance.pdf.
- 4.James PD, Connell NT, Ameer B, et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021;5(1):280–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abe K, Dupervil B, O’Brien SH, et al. Higher rates of bleeding and use of treatment products among young boys compared to girls with von Willebrand disease. Am J Hematol. 2020;95(1):10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James AH. Heavy menstrual bleeding: work-up and management. Hematology Am Soc Hematol Educ Program. 2016;2016(1):236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadir RA, Economides DL, Sabin CA, et al. Frequency of inherited bleeding disorders in women with menorrhagia. Lancet. 1998;351(9101):485–9. [DOI] [PubMed] [Google Scholar]
- 8.Munro MG, Critchley HOD, Fraser IS, Figo Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018;143(3):393–408. [DOI] [PubMed] [Google Scholar]
- 9.Haamid F, Sass AE, Dietrich JE. Heavy Menstrual Bleeding in Adolescents. J Pediatr Adolesc Gynecol. 2017;30(3):335–40. [DOI] [PubMed] [Google Scholar]
- 10.Rauch A, Valentino LA, Mills K, et al. Big picture initiatives in bleeding disorders. Haemophilia. 2022;28 Suppl 4:53–60. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health. 2007;10(3):183–94. [DOI] [PubMed] [Google Scholar]
- 12.Cohen BJ, Gibor Y. Anemia and menstrual blood loss. Obstet Gynecol Surv. 1980;35(10):597–618. [PubMed] [Google Scholar]
- 13.Smith C, Teng F, Branch E, et al. Maternal and Perinatal Morbidity and Mortality Associated With Anemia in Pregnancy. Obstet Gynecol. 2019;134(6):1234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadir RA, Sabin CA, Pollard D, et al. Quality of life during menstruation in patients with inherited bleeding disorders. Haemophilia. 1998;4(6):836–41. [DOI] [PubMed] [Google Scholar]
- 15.van Hoorn ES, Houwing ME, Al Arashi W, et al. Patient-reported outcomes in autosomal inherited bleeding disorders: A systematic literature review. Haemophilia. 2022;28(2):197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGrath M, Quint EH, Weyand AC. Depression in adolescents and young adults with heavy menstrual bleeding in a referral clinic setting. Am J Hematol. 2021;96(4):E105–E8. [DOI] [PubMed] [Google Scholar]
- 17.Weyand AC, Fitzgerald KD, McGrath M, et al. Depression in Female Adolescents with Heavy Menstrual Bleeding. J Pediatr. 2022;240:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kouides PA, Phatak PD, Burkart P, et al. Gynaecological and obstetrical morbidity in women with type I von Willebrand disease: results of a patient survey. Haemophilia. 2000;6(6):643–8. [DOI] [PubMed] [Google Scholar]
- 19.Hews-Girard JC, Galica J, Goldie C, et al. Determining the incidence of postpartum haemorrhage among Ontario women with and without inherited bleeding disorders: A population-based cohort study. Haemophilia. 2022;28(5):832–41. [DOI] [PubMed] [Google Scholar]
- 20.James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5(6):1165–9. [DOI] [PubMed] [Google Scholar]
- 21.Malec LM, Moore CG, Yabes J, et al. Postpartum haemorrhage in women with von Willebrand disease: an observational study of the Pennsylvania Health Care Cost Containment Council (PHC4) database. Haemophilia. 2015;21(5):e442–5. [DOI] [PubMed] [Google Scholar]
- 22.Miller CH, Philipp CS, Stein SF, et al. The spectrum of haemostatic characteristics of women with unexplained menorrhagia. Haemophilia. 2011;17(1):e223–9. [DOI] [PubMed] [Google Scholar]
- 23.United Kingdom Haemophilia Centre Doctors’ Organisation and National Haemophilia Database. UKHCDO Annual Report 2020 & Bleeding Disorder Statistics for 2020/2021. Manchester, Unite Kingdom; 2021. Available from: http://www.ukhcdo.org/wp-content/uploads/2021/12/2021-UKHCDO-Annual-Report-2020-21-Data.pdf. [Google Scholar]
- 24.Renault NK, Howell RE, Robinson KS, Greer WL. Qualitative assessment of the emotional and behavioural responses of haemophilia A carriers to negative experiences in their medical care. Haemophilia. 2011;17(2):237–45. [DOI] [PubMed] [Google Scholar]
- 25.van Galen KPM, d’Oiron R, James P, et al. A new hemophilia carrier nomenclature to define hemophilia in women and girls: Communication from the SSC of the ISTH. J Thromb Haemost. 2021;19(8):1883–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weyand AC, Sidonio RF Jr., Sholzberg M. Health issues in women and girls affected by haemophilia with a focus on nomenclature, heavy menstrual bleeding, and musculoskeletal issues. Haemophilia. 2022;28 Suppl 4:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudhury A, Sidonio R Jr., Jain N, et al. Women and girls with haemophilia and bleeding tendencies: Outcomes related to menstruation, pregnancy, surgery and other bleeding episodes from a retrospective chart review. Haemophilia. 2021;27(2):293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.d’Oiron R, O’Brien S, James AH. Women and girls with haemophilia: Lessons learned. Haemophilia. 2021;27 Suppl 3:75–81. [DOI] [PubMed] [Google Scholar]
- 29.Gilbert L, Rollins L, Hilmes M, et al. Haemophilia A carriers demonstrate pathological and radiological evidence of structural joint changes. Haemophilia. 2014;20(6):e426–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirtava A, Drews C, Lally C, et al. Medical, reproductive and psychosocial experiences of women diagnosed with von Willebrand’s disease receiving care in haemophilia treatment centres: a case-control study. Haemophilia. 2003;9(3):292–7. [DOI] [PubMed] [Google Scholar]
- 31.Olsson A, Hellgren M, Berntorp E, et al. Clotting factor level is not a good predictor of bleeding in carriers of haemophilia A and B. Blood Coagul Fibrinolysis. 2014;25(5):471–5. [DOI] [PubMed] [Google Scholar]
- 32.Puetz J, Cheng D. Descriptive analysis of bleeding symptoms in haemophilia carriers enrolled in the ATHNdataset. Haemophilia. 2021;27(6):1045–50. [DOI] [PubMed] [Google Scholar]
- 33.Sidonio RF, Mili FD, Li T, et al. Females with FVIII and FIX deficiency have reduced joint range of motion. Am J Hematol. 2014;89(8):831–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnegard ME, Whitten LA, Hunter C, Clayton JA. Sex as a Biological Variable: A 5-Year Progress Report and Call to Action. J Womens Health (Larchmt). 2020;29(6):858–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dasarathy J, Labrador H. Bone Health in Women. Prim Care. 2018;45(4):643–57. [DOI] [PubMed] [Google Scholar]
- 36.Knol HM, Kemperman RF, Kluin-Nelemans HC, et al. Haemostatic variables during normal menstrual cycle. A systematic review. Thromb Haemost. 2012;107(1):22–9. [DOI] [PubMed] [Google Scholar]
- 37.Miller CH, Dilley AB, Drews C, et al. Changes in von Willebrand factor and factor VIII levels during the menstrual cycle. Thromb Haemost. 2002;87(6):1082–3. [PubMed] [Google Scholar]
- 38.Dupuis M, Severin S, Noirrit-Esclassan E, et al. Effects of Estrogens on Platelets and Megakaryocytes. Int J Mol Sci. 2019;20(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.James AH, Ragni MV, Picozzi VJ. Bleeding disorders in premenopausal women: (another) public health crisis for hematology? Hematology Am Soc Hematol Educ Program. 2006:474–85. [DOI] [PubMed] [Google Scholar]
- 40.Oral C, Hemostasis Study G. The effects of seven monophasic oral contraceptive regimens on hemostatic variables: conclusions from a large randomized multicenter study. Contraception. 2003;67(3):173–85. [DOI] [PubMed] [Google Scholar]
- 41.Connors JM, Middeldorp S. Transgender patients and the role of the coagulation clinician. J Thromb Haemost. 2019;17(11):1790–7. [DOI] [PubMed] [Google Scholar]
- 42.Collis RE, Collins PW. Haemostatic management of obstetric haemorrhage. Anaesthesia. 2015;70 Suppl 1:78–86, e27–8. [DOI] [PubMed] [Google Scholar]
- 43.Critchley HOD, Babayev E, Bulun SE, et al. Menstruation: science and society. Am J Obstet Gynecol. 2020;223(5):624–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zia A, Lau M, Journeycake J, et al. Developing a multidisciplinary Young Women’s Blood Disorders Program: a single-centre approach with guidance for other centres. Haemophilia. 2016;22(2):199–207. [DOI] [PubMed] [Google Scholar]
- 45.Sidonio RF Jr., Zia A, Fallaize D. Potential Undiagnosed VWD Or Other Mucocutaneous Bleeding Disorder Cases Estimated From Private Medical Insurance Claims. J Blood Med. 2020;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arya S, Wilton P, Page D, et al. Healthcare provider perspectives on inequities in access to care for patients with inherited bleeding disorders. PLoS One. 2020;15(2):e0229099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weyand AC, James PD. Sexism in the management of bleeding disorders. Res Pract Thromb Haemost. 2021;5(1):51–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez K, Norris K, Hardy M, Valentino LA. Defining the Impact of Social Drivers on Health Outcomes for People with Inherited Bleeding Disorders. J Clin Med. 2022;11(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arya S, Wilton P, Page D, et al. “Everything was blood when it comes to me”: Understanding the lived experiences of women with inherited bleeding disorders. J Thromb Haemost. 2020;18(12):3211–21. [DOI] [PubMed] [Google Scholar]
- 50.James AH. Von Willebrand: An underdiagnosed disorder 2017. [cited 2022 May 3]. Available from: https://www.contemporaryobgyn.net/view/von-willebrand-underdiagnosed-disorde.
- 51.Arya S, Wilton P, Page D, et al. “They don’t really take my bleeds seriously”: Barriers to care for women with inherited bleeding disorders. J Thromb Haemost. 2021;19(6):1506–14. [DOI] [PubMed] [Google Scholar]
- 52.Khair K, Holland M, Pollard D. The experience of girls and young women with inherited bleeding disorders. Haemophilia. 2013;19(5):e276–81. [DOI] [PubMed] [Google Scholar]
- 53.Vázquez E, Kim M, Santaella ME. Lived Experience Experts: A name created by us for us. Expert Rev Hematol. 2023; In Press. [DOI] [PubMed] [Google Scholar]
- 54.Mirin AA. Gender Disparity in the Funding of Diseases by the U.S. National Institutes of Health. J Womens Health (Larchmt). 2021;30(7):956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skinner MW, Nugent D, Wilton P, et al. Achieving the unimaginable: Health equity in haemophilia. Haemophilia. 2020;26(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valentino LA, Witkop ML, Santaella ME, et al. The National Hemophilia Foundation State of the Science Research Summit Initiative: Executive Summary. Expert Rev Hematol. 2023; In Press. [DOI] [PubMed] [Google Scholar]
- 57.Valentino LA, Witkop ML, Santaella ME, et al. Building the blueprint: Formulating a community-generated national plan for future research in inherited bleeding disorders. Haemophilia. 2022;28(5):760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valentino LA, Witkop ML, Santaella ME, et al. National Hemophilia Foundation State of the Science Research Summit Initiative: The Foundation of an Inherited Bleeding Disorders National Research Blueprint. Expert Rev Hematol. 2023; In Press. [DOI] [PubMed] [Google Scholar]
- 59.Lillicrap D, Fijnvandraat K, Young G, Mancuso ME. Patients with hemophilia A and inhibitors: prevention and evolving treatment paradigms. Expert Rev Hematol. 2020;13(4):313–21. [DOI] [PubMed] [Google Scholar]
- 60.Sabatino DE, Pipe SW, Nugent DJ, et al. Origins and organization of the NHLBI State of the Science Workshop: Generating a national blueprint for future research on factor VIII inhibitors. Haemophilia. 2019;25(4):575–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tran DQ, Benson CC, Boice JA, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: Research priorities to transform the care of people with hemophilia. Expert Rev Hematol. 2023; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sidonio RF Jr., Bryant PC, Di Paola J, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: Research priorities for mucocutaneous bleeding disorders. Expert Rev Hematol. 2023; In Press. [DOI] [PubMed] [Google Scholar]
- 63.Byams VR, Baker JR, Bailey C, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: Research priorities in health services; diversity, equity, and inclusion; and implementation science. Expert Rev Hematol. 2023; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Byams VR, Miller CH, Bethea FM, et al. Bleeding Disorders in Women and Girls: State of the Science and CDC Collaborative Programs. J Womens Health (Larchmt). 2022;31(3):301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirtava A, Crudder S, Dilley A, et al. Trends in clinical management of women with von Willebrand disease: a survey of 75 women enrolled in haemophilia treatment centres in the United States. Haemophilia. 2004;10(2):158–61. [DOI] [PubMed] [Google Scholar]
- 66.Srivaths LV, Zhang QC, Byams VR, et al. Differences in bleeding phenotype and provider interventions in postmenarchal adolescents when compared to adult women with bleeding disorders and heavy menstrual bleeding. Haemophilia. 2018;24(1):63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ragni MV, Bontempo FA, Hassett AC. von Willebrand disease and bleeding in women. Haemophilia. 1999;5(5):313–7. [DOI] [PubMed] [Google Scholar]
- 68.American Academy of Pediatrics Committee on Adolescence and American College of Obstetricians and Gynecologists Committee on Adolescent Health Care, Diaz A, Laufer MR, Breech LL. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Pediatrics. 2006;118(5):2245–50. [DOI] [PubMed] [Google Scholar]
- 69.Byams VR, Anderson BL, Grant AM, et al. Evaluation of bleeding disorders in women with menorrhagia: a survey of obstetrician-gynecologists. Am J Obstet Gynecol. 2012;207(4):269 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Screening and Management of Bleeding Disorders in Adolescents With Heavy Menstrual Bleeding: ACOG COMMITTEE OPINION, Number 785. Obstet Gynecol. 2019;134(3):e71–e83. [DOI] [PubMed] [Google Scholar]
- 71.James P About Let’s Talk Period [cited Oct 4, 2022]. Available from: https://letstalkperiod.ca/about/.
- 72.IWK Health. WeThrive: Period tracking for quality of life [cited 2022 Oct 4]. Available from: https://youriwk.com/2022/06/02/we-thrive-period-tracking-for-quality-of-life/.
- 73.Brown MC, White MH, Friedberg R, et al. Elevated von Willebrand factor levels during heavy menstrual bleeding episodes limit the diagnostic utility for von Willebrand disease. Res Pract Thromb Haemost. 2021;5(4):e12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Indiana Hemophilia & Thrombosis Center. Partners in Bleeding Disorders Education [cited 2022 Sept 17]. Available from: https://www.partnersprn.org/.
- 75.Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505(7485):612–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.National Institute for Health and Care Excellence. Heavy Menstrual Bleeding: Assessment and Management [cited 2022 May 4]. Available from: https://www.nice.org.uk/guidance/ng88/resources/heavy-menstrual-bleeding-assessment-and-management-pdf-1837701412549. [PubMed]
- 77.Magnay JL, O’Brien S, Gerlinger C, Seitz C. A systematic review of methods to measure menstrual blood loss. BMC Womens Health. 2018;18(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.U.S. Food and Drug Administration. Prescribing Information. Mirena (levonorgestrel-releasing intrauterine system) [cited 2022 May 4]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021225s042lbl.pdf.
- 79.U.S. Food and Drug Administration. Prescribing Information. Natazia (estradiol valerate and estradiol valerate/dienogest) tablets, for oral use [cited 2022 May 4]. Available from: https://labeling.bayerhealthcare.com/html/products/pi/natazia_pi.pdf.
- 80.Hallberg L, Nilsson L. Determination of Menstrual Blood Loss. Scand J Clin Lab Invest. 1964;16:244–8. [PubMed] [Google Scholar]
- 81.Hallberg L, Hogdahl AM, Nilsson L, Rybo G. Menstrual blood loss--a population study. Variation at different ages and attempts to define normality. Acta Obstet Gynecol Scand. 1966;45(3):320–51. [DOI] [PubMed] [Google Scholar]
- 82.Magnay JL, O’Brien S, Gerlinger C, Seitz C. Pictorial methods to assess heavy menstrual bleeding in research and clinical practice: a systematic literature review. BMC Womens Health. 2020;20(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abu Hashim H, Alsherbini W, Bazeed M. Contraceptive vaginal ring treatment of heavy menstrual bleeding: a randomized controlled trial with norethisterone. Contraception. 2012;85(3):246–52. [DOI] [PubMed] [Google Scholar]
- 84.European Medicines Agency. Summary of product characteristics. Esmya 5mg tablets, INN-Ulipristal [cited 2022 May 4]. Available from: https://www.ema.europa.eu/en/documents/product-information/esmya-epar-product-information_en.pdf.
- 85.Hoaglin DC, Filonenko A, Glickman ME, et al. Use of mixed-treatment-comparison methods in estimating efficacy of treatments for heavy menstrual bleeding. Eur J Med Res. 2013;18:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nugent D, Acharya SS, Baumann KJ, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: Research priorities for ultra-rare inherited bleeding disorders. Expert Rev Hematol. 2023; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ragni MV, Young G, Batsuli G, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders. Facilitating research through infrastructure, workforce, and resources and funding. Expert Rev Hematol. 2023; In Press. [DOI] [PubMed] [Google Scholar]
- 88.Johns Hopkins Center for Communication Programs. Technical Consultation on Contraceptive-Induced Menstrual Changes: New Perspectives on Research that Can Improve Women’s Lives Globally [cited 2022 Jun 12]. Available from: https://knowledgesuccess.org/2021/01/20/technical-consultation-on-contraceptive-induced-menstrual-changes/.
- 89.Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


