Abstract
This cohort study examines whether preinfection anti–SARS-CoV-2 spike antibody titer is associated with protection against Omicron BA.5 infection among staff members of a medical and research center in Tokyo, Japan.
Introduction
The number of patients with COVID-19 has surged during the Omicron pandemic worldwide. It remains unclear whether and how previous SARS-CoV-2 infection and vaccine-induced humoral immunity can protect against Omicron BA.5 infection. We examined the association between preinfection anti–SARS-CoV-2 spike antibody titers and protection against Omicron BA.5 infection among the staff of a medical and research center in Tokyo, Japan.
Methods
A total of 2610 staff members participated in a serosurvey in June 2022 (baseline), were evaluated for anti–SARS-CoV-2 antibodies (spike and nucleocapsid proteins; Abbott Japan and Roche Diagnostics), and answered a questionnaire.1 Diagnosed infection was defined as a history of COVID-19 that was self-reported (confirmed against in-house COVID-19 registry), whereas undiagnosed infection was defined as antinucleocapsid seropositive at any of the first (July 2020) through sixth (June 2022: baseline) surveys among those without a history of COVID-19. In July 2022, a large epidemic caused by the Omicron BA.5 variant hit Japan. Using an in-house COVID-19 registry, we followed up the study participants for SARS-CoV-2 infection from baseline until the date of COVID-19 diagnosis, additional vaccination, or September 21, 2022, whichever occurred first. Additional methods can be found in the eMethods in Supplement 1. Written informed consent was obtained from all participants. This study was approved by the ethics committee of the National Center for Global Health and Medicine and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Results
Of the 2610 participants at baseline (1845 [70.7%] female; median [IQR] age, 37 [28-48] years), 2401 (92.0%) had received 3-dose vaccinations, and 418 (16.0%) had previous SARS-CoV-2 infection. Of the 230 previously diagnosed infections, 202 (88.0%) occurred during the Omicron BA.1/BA.2 predominant epidemic. Those with previously diagnosed and undiagnosed infections had higher antispike antibody titers than infection-naive individuals (median titer, 29 528 [previously diagnosed], 24 556 [previously undiagnosed], and 4849 [infection naive]).
During the follow-up period (ie, Omicron BA.5 predominant wave), 288 participants were diagnosed with COVID-19; the incidence rate was 13.8 per 10 000 person-days. Higher antispike antibody titers were associated with higher protection against Omicron BA.5 infection (3.7% per 1000 titers; 95% CI, 3.3%-4.2%) (Table). Fifty percent protection was achieved at 17 000 AU/mL among previously infected participants and 27 000 AU/mL among infection-naive participants, respectively, and 80% protection was achieved at 50 000 AU/mL among previously infected participants but not among infection-naive participants (Figure).
Table. Protection Against Omicron BA.5 Infection Across Baseline SARS-CoV-2 Immunity Status.
| Baseline SARS-CoV-2 immunity status | Total, No. infected of participants | Infection during Omicron BA.5 dominant wave | Protection against infection, % (95% CIs) | |||
|---|---|---|---|---|---|---|
| No. of participants | No. of Person-days | Incident rate per 10 000 person-daysa | Unadjusted | Adjusted | ||
| Antispike IgG antibody titer, AU/mL | ||||||
| Total participants | ||||||
| Quarter 1 (antibody titer range, 0-2879 AU/mL) | 653 | 98 | 50 962 | 19.2 | 1 [Reference] | 1 [Reference] |
| Quarter 2 (antibody titer range, 2880-5911 AU/mL) | 652 | 79 | 51 356 | 15.4 | 21.0 (−6.2 to 41.3) | 21.9 (−5.5 to 42.1) |
| Quarter 3 (antibody titer range, 5922-13 733 AU/mL) | 653 | 78 | 51 868 | 15.0 | 22.4 (−4.5 to 42.4) | 21.7 (−5.8 to 42.0) |
| Quarter 4 (antibody titer range, 13 745-80 000 AU/mL) | 652 | 33 | 53 802 | 6.1 | 68.2 (52.8 to 78.6) | 70.0 (55.3 to 79.9) |
| Per 1000-AU/mL increase | 3.6 (3.3 to 3.8) | 3.7 (3.3 to 4.2) | ||||
| Infection-naive participants | ||||||
| Quarter 1 (antibody titer range, 0-2564 AU/mL) | 542 | 84 | 42 319 | 19.8 | 1 [Reference] | 1 [Reference] |
| Quarter 2 (antibody titer range, 2567-4849 AU/mL) | 541 | 68 | 42 177 | 16.1 | 19.9 (−10.2 to 41.9) | 21.3 (−8.8 to 43.1) |
| Quarter 3 (antibody titer range, 4857-9522 AU/mL) | 541 | 69 | 42 345 | 16.3 | 18.9 (−11.5 to 41.0) | 19.9 (−10.6 to 41.9) |
| Quarter 4 (antibody titer range, 9550-80 000 AU/mL) | 541 | 48 | 44 393 | 10.8 | 45.4 (22.2 to 61.7) | 41.7 (16.2 to 59.5) |
| Per 1000-AU/mL increase | 2.7 (1.6 to 3.7) | 2.3 (0.6 to 4.1) | ||||
| Previously infected participants | ||||||
| Quarter 1 (antibody titer range, 0-12 182 AU/mL) | 112 | 11 | 9283 | 11.8 | 1 [Reference] | 1 [Reference] |
| Quarter 2 (antibody titer range, 12 264-27 771 AU/mL) | 111 | 4 | 9083 | 4.4 | 64.0 (−13.0 to 88.5) | 56.2 (−156.7 to 92.5) |
| Quarter 3 (antibody titer range, 27 918-45 362 AU/mL) | 111 | 2 | 9242 | 2.2 | 82.1 (19.4 to 96.0) | 78.7 (59.1 to 88.9) |
| Quarter 4 (antibody titer range, 46 691-80 000 AU/mL) | 111 | 2 | 9146 | 2.2 | 82.1 (19.3 to 96.0) | 84.3 (60.4 to 93.8) |
| Per 1000-AU/mL increase | 3.3 (1.5 to 5.0) | 3.0 (0.4 to 5.5) | ||||
| Previous infection status | ||||||
| Infection naive | 2165 | 269 | 171 234 | 15.7 | 1 [Reference] | 1 [Reference] |
| Undiagnosed infection | 215 | 14 | 17 569 | 7.9 | 48.8 (12.4 to 70.1) | 58.0 (27.9 to 75.6) |
| Diagnosed infection | 230 | 5 | 19 185 | 2.6 | 83.4 (59.9 to 93.2) | 86.9 (68.3 to 94.6) |
| At pre–Omicron-dominant waves | 27 | 2 | 2233 | 9.0 | 42.0 (−133.1 to 85.6) | 41.2 (−137.5 to 85.4) |
| At Omicron BA.1/BA.2–dominant waves | 203 | 3 | 16 952 | 1.8 | 88.8 (65.0 to 96.4) | 91.4 (73.2 to 97.3) |
Diagnosed infection was defined as a self-report of a positive polymerase chain reaction or antigen test result (confirmed against in-house registry), whereas undiagnosed infection was ascertained using anti-nucleocapsid assays. Protection was calculated as (1 − hazard ratio) × 100. The hazard ratio was estimated using a Cox proportional hazards regression model, adjusted by age, sex, job (physicians, nurses, allied health professionals, administrative staff, or others), occupational SARS-CoV-2 exposure risk (low, moderate, or high), body mass index, coexisting diseases (no or having any of cancer, cardiovascular diseases, diabetes, hypertension, immunosuppressant diseases, chronic kidney disease, or lung disease), smoking status (nonsmoker or current smoker), alcohol consumption status (none, occasional, or weekly drinker), number of households, child-related household status (living without school-age children, living with younger school-aged children, or living with older school-aged children), spending 30 minutes or more in the crowded places, close-contact settings, and confined and enclosed spaces without a mask (none, 1-5 times, or ≥6 times), and having dinner in a group of 5 or more people for more than 1 hour (none, 1-5 times, or ≥6 times).
Figure. Association Between Previous SARS-CoV-2 Infection– and Vaccine-Induced Immunity and Protection Against Omicron BA.5 Infection .
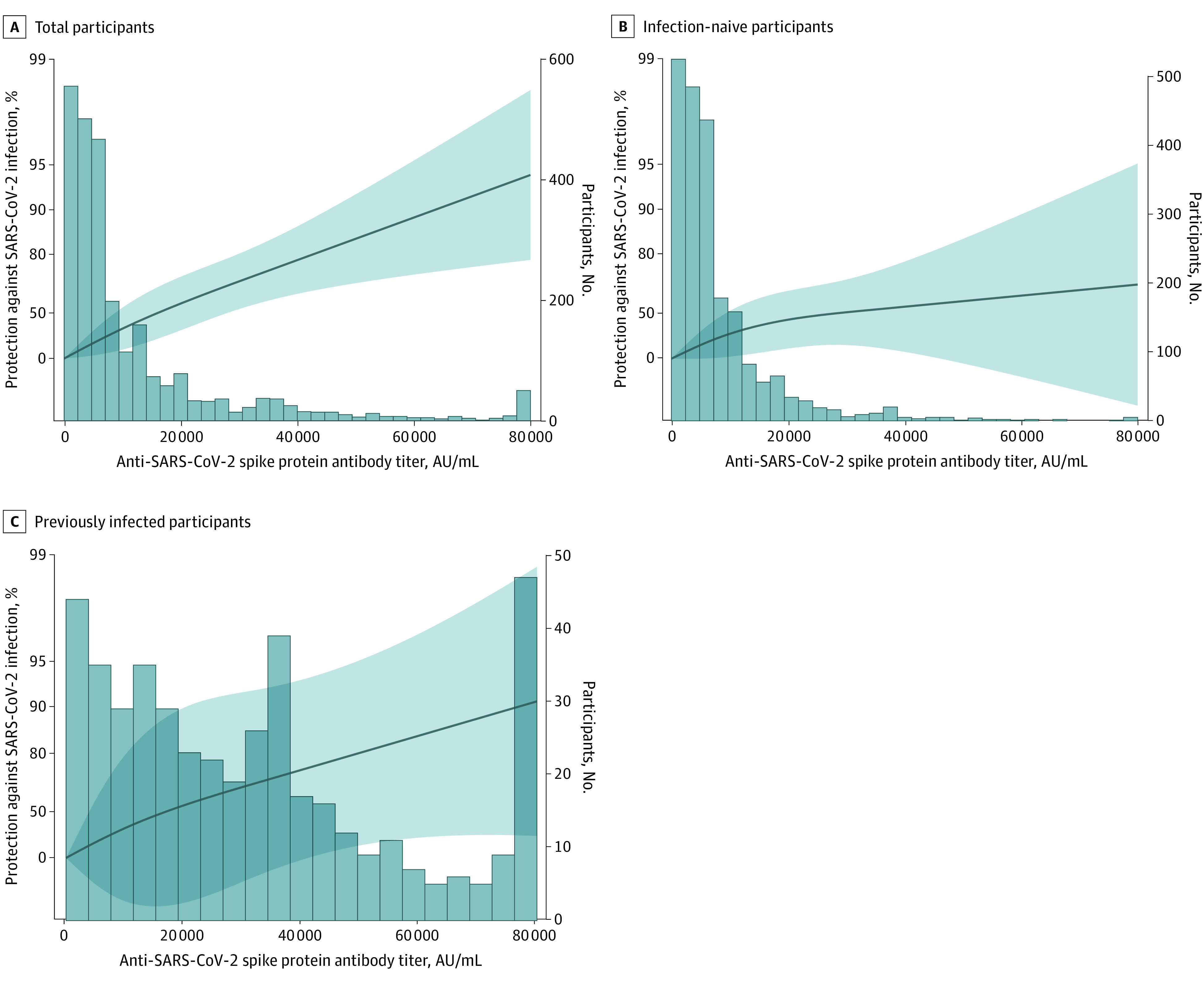
Antibody titers were 16 000 AU/mL for 50% protection, 31 000 AU/mL for 70% protection, 43 000 AU/mL for 80% protection, and 63 000 AU/mL for 90% protection for total participants; 27 000 AU/mL for 50% protection and not applicable at the other protection levels for infection-naive participants; and 17 000 AU/mL for 50% protection, 35 000 AU/mL for 70% protection, 50 000 AU/mL for 80% protection, and 76 000 AU/mL for 90% protection for previously infected participants.
Protection against Omicron BA.5 reinfection was 87.0% (95% CI, 68.0%-95.0%) in participants with previously diagnosed infections and 58.0% (95% CI, 28.0%-76.0%) among those with undiagnosed infections. The protection of the previous infection during the Omicron BA.1/BA.2 epidemic was 91.0% (95% CI, 73.0%-97.0%).
Discussion
In vaccine recipients (mainly 3-dose) who had experienced the Omicron BA.1/BA.2 wave, we found that higher antispike antibody titers were associated with a lower risk of Omicron BA.5 infection, and the association was enhanced by previous infection (mainly occurred during the Omicron BA.1/BA.2 epidemic). In the current study, the antispike antibody titer required for a 50% reduction in the risk of infection during the Omicron BA.5 epidemic was lower among those with previous infection than among infection-naive individuals. These results are compatible with laboratory data showing that Omicron BA.1/BA.2 convalescent patients had higher neutralizing capacity against Omicron BA.5 than infection-naive 3-dose recipients.2,3 Limitations of this study include the lack of active surveillance during the study period and generalizability of the findings. These data suggest that preinfection spike antibody titers indicate the risk of Omicron BA.5 infection and that high protection can only be achieved with hybrid immunity from infection and 3-dose vaccination.
eMethods. Supplemental Methods
Data Sharing Statement
References
- 1.Yamamoto S, Maeda K, Matsuda K, et al. Coronavirus disease 2019 (COVID-19) breakthrough infection and post-vaccination neutralizing antibodies among healthcare workers in a referral hospital in Tokyo: a case-control matching study. Clin Infect Dis. 2022;75(1):e683-e691. doi: 10.1093/cid/ciab1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hachmann NP, Miller J, Collier AY, et al. Neutralization escape by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022;387(1):86-88. doi: 10.1056/NEJMc2206576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muik A, Lui BG, Bacher M, et al. Omicron BA.2 breakthrough infection enhances cross-neutralization of BA.2.12.1 and BA.4/BA.5. Sci Immunol. 2022;7(77):eade2283. doi: 10.1126/sciimmunol.ade2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
Data Sharing Statement


