Key Points
Question
Can days alive and at home at day 90 (DAAH90)—a composite of mortality and the number of days at home at day 90 after intensive care unit (ICU) admission—be used to capture the burden of acute hospitalization and long-term functional outcomes in patients who require at least 7 days of mechanical ventilation?
Findings
In this cohort study of 463 patients receiving invasive ventilation across 10 medical or surgical ICUs in Canada, lower DAAH90 was associated with multiple markers of higher ICU intensity and with worse functional outcomes and higher mortality up to 1 year following ICU discharge.
Meaning
The findings of this study suggest that DAAH90 reflects the burden of critical illness and captures the long-term consequences of critical illness for functional status, quality of life, and survival.
This cohort study examines the association of a composite measure of mortality and number of days at home at day 90 after intensive care unit admission with long-term functional outcomes and survival among mechanically ventilated patients.
Abstract
Importance
Many conventional end points in randomized clinical trials of interventions for critically ill patients do not account for patient-centered concerns such as time at home, physical function, and quality of life after critical illness.
Objective
To establish whether days alive and at home at day 90 (DAAH90) is associated with long-term survival and functional outcomes in mechanically ventilated patients.
Design, Setting, and Participants
The RECOVER prospective cohort study was conducted from February 2007 to March 2014, using data from 10 intensive care units (ICUs) in Canada. Patients were included in the baseline cohort if they were aged 16 years or older and underwent invasive mechanical ventilation for 7 or more days. The follow-up cohort analyzed here comprised RECOVER patients who were alive and had functional outcomes ascertained at 3, 6, and 12 months. Secondary data analysis occurred from July 2021 to August 2022.
Exposures
Composite of survival and days alive and at home at day 90 after ICU admission (DAAH90).
Main Outcomes and Measures
Functional outcomes at 3, 6, and 12 months were evaluated with the Functional Independence Measure (FIM), the 6-Minute Walk Test (6MWT), the Medical Research Council (MRC) Scale for Muscle Strength, and the 36-Item Short Form Health Survey physical component summary (SF-36 PCS). Mortality was evaluated at 1 year from ICU admission. Ordinal logistic regression was used to describe the association between DAAH90 tertiles and outcomes. Cox proportional hazards regression models were used to examine the independent association of DAAH90 tertiles with mortality.
Results
The baseline cohort comprised 463 patients. Their median age was 58 years (IQR, 47-68 years), and 278 patients (60.0%) were men. In these patients, Charlson Comorbidity Index score, Acute Physiology and Chronic Health Evaluation II score, ICU intervention (eg, kidney replacement therapy or tracheostomy), and ICU length of stay were independently associated with lower DAAH90. The follow-up cohort comprised 292 patients. Their median age was 57 years (IQR, 46-65 years), and 169 patients (57.9%) were men. Among patients who survived to day 90, lower DAAH90 was associated with higher mortality at 1 year after ICU admission (tertile 1 vs tertile 3: adjusted hazard ratio [HR], 0.18 [95% CI, 0.07-0.43]; P < .001). At 3 months of follow-up, lower DAAH90 was independently associated with lower median scores on the FIM (tertile 1 vs tertile 3, 76 [IQR, 46.2-101] vs 121 [IQR, 112-124.2]; P = .04), 6MWT (tertile 1 vs tertile 3, 98 [IQR, 0-239] vs 402 [IQR, 300-494]; P < .001), MRC (tertile 1 vs tertile 3, 48 [IQR, 32-54] vs 58 [IQR, 51-60]; P < .001), and SF-36 PCS (tertile 1 vs tertile 3, 30 [IQR, 22-38] vs 37 [IQR, 31-47]; P = .001) measures. Among patients who survived to 12 months, being in tertile 3 vs tertile 1 for DAAH90 was associated with higher FIM score at 12 months (estimate, 22.4 [95% CI, 14.8-30.0]; P < .001), but this association was not present for ventilator-free days (estimate, 6.0 [95% CI, −2.2 to 14.1]; P = .15) or ICU-free days (estimate, 5.9 [95% CI, −2.1 to 13.8]; P = .15) at day 28.
Conclusions and Relevance
In this study, lower DAAH90 was associated with greater long-term mortality risk and worse functional outcomes among patients who survived to day 90. These findings suggest that the DAAH90 end point reflects long-term functional status better than standard clinical end points in ICU studies and may serve as a patient-centered end point in future clinical trials.
Introduction
Critical illness is associated with important long-term decrements in functional status and quality of life.1,2 For many patients, avoiding substantial functional impairment is an equal if not greater priority than survival.2,3 Accordingly, measurements used to characterize the outcome of critical illness would ideally reflect both survival and survivorship—the impact of critical illness on long-term functional status and quality of life among survivors.
One measure that may reflect a patient-centered evaluation of the outcome of critical illness is days alive and at home (DAAH); similar constructs include hospital-free days or days alive and out of the hospital.4,5,6,7 Patients highly value time spent at home.8 By contrast, the experience of being hospitalized or admitted to a long-term acute care hospital can impair personal well-being and quality of life, especially if it occurs at the expense of time at home.9 Days alive and at home combines survival with time spent at home into a single measure that can be evaluated over different time points (eg, 3, 6, and 12 months). Patients who die within that period are often (although not necessarily) assigned a value of 0. For survivors, DAAH reflects hospital length of stay (LOS), hospital readmission, and admission to posthospital facilities such as acute rehabilitation units and complex chronic nursing institutions. From a trial design perspective, DAAH offers a comparatively objective outcome measure that can be ascertained using patient self-reports, registries, or health administrative data, making it easier to record than many existing patient-centered measures of functional outcome.10 From a patient perspective, time spent outside the hospital may represent a more meaningful measure of intervention efficacy compared with other end points commonly used in critical care trials, such as ventilator-free days or organ failure–free days.
Days alive and at home and similar end points have been reported across a range of inpatient settings and disease contexts4,9,11,12,13,14 but, to our knowledge, not after critical illness. In this secondary analysis of the RECOVER cohort study,15 we aimed to establish whether DAAH reflects both survival and survivorship in terms of functional status and quality of life after critical illness. We hypothesized that patients with lower DAAH at day 90 (hereafter, DAAH90) would report greater impairments in functional status, lower health-related quality of life, and higher mortality in the year after intensive care unit (ICU) discharge. We further hypothesized that DAAH90 would be more strongly associated with long-term functional outcomes compared with other composite end points routinely used in studies of critical illness, including ventilator-free days and ICU-free days.
Methods
Data Sources
We conducted a secondary analysis of the RECOVER multicenter prospective cohort study of 2-year outcomes after critical illness. The RECOVER study was conducted at 10 university-affiliated, medical or surgical ICUs in Canada from February 2007 to March 2014.15 Patients aged 16 years or older receiving invasive mechanical ventilation for 7 or more days in an ICU and for any medical or surgical admitting diagnosis were eligible for inclusion. Procedures for enrollment and follow-up are detailed in the original report.15 Research ethics approval for the RECOVER study was obtained at each participating center, and written informed consent from the substitute decision-maker was obtained for patient enrollment. This report followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies.
Computation of DAAH
We calculated DAAH90 as the total number of days free of index hospitalization, subsequent hospital readmissions, or admission to a long-term care facility at day 90. The following equation was used:
| DAAH90 = 90 – hospital LOS – hospital LOS90 – long-term care facility LOS90 |
where hospital LOS represents LOS for the index hospitalization, hospital LOS90 reflects total LOS for subsequent hospitalizations, and long-term care facility LOS90 quantifies total LOS in a long-term care facility. Data on hospital readmissions were collected using a combination of retrospective chart review and patient self-reports. Patients who died within 3 months or remained admitted to a hospital or long-term care facility for the full 3-month period were assigned a value of 0 days.
Measurements
Functional outcomes were evaluated at 3, 6, and 12 months. Overall functional status was assessed using the Functional Independence Measure (FIM),15 a patient-centered tool that assesses 6 overall areas of function across motor and cognitive domains. The FIM determines the degree of functional dependence, burden of care in motor and cognitive domains, rehabilitation needs, and outcomes in both critically and noncritically ill patient populations.15,16,17,18 Exercise capacity was quantified with the 6-Minute Walk Test (6MWT),19 and muscle strength was evaluated using the Medical Research Council (MRC) Scale for Muscle Strength (range, 0-60; higher scores indicate normal muscle strength, lower scores indicate weakness and/or absent contractions).20 Quality of life assessed using the 36-Item Short Form Health Survey physical component summary (SF-36 PCS)21 was evaluated as a comparator with the FIM, 6MWT, and MRC measures.22 Posttraumatic stress disorder (PTSD) and depressive symptoms were examined using the Impact Event Scale–Revised (IES-R [range, 0-88; higher scores indicate greater levels of distress cused by traumatic events, lower scores indicate lower levels of distress])23 and the Beck Depression Inventory–II (BDI-II [range, 0-63; higher scores indicate greater depressive symptoms, lower scores indicate fewer depressive symptoms]),24 respectively. Mortality was recorded at 1 year. Phase I of the RECOVER trial identified 4 trajectories of varying functional outcome based on age and ICU LOS15; these disability risk groups were retained for this analysis.
Statistical Analysis
We trichotomized DAAH90 into tertiles to account for skewness of the distribution and the high frequency of 0 values. Patients were compared across tertiles according to their baseline characteristics, comorbidities, illness severity, and ICU characteristics. Descriptive statistics are presented as the mean (SD) or median (IQR). The statistical significance of differences across DAAH90 tertiles was tested using 1-way analysis of variance or the Kruskal-Wallis test, as appropriate. The unadjusted associations of baseline characteristics (eg, age, sex, comorbidity as quantified with the Charlson Comorbidity Index [CCI] score, and illness severity quantified with Acute Physiology and Chronic Health Evaluation II [APACHE II] score) and variables reflecting the burden of critical illness (eg, duration of invasive mechanical ventilation, tracheostomy, receipt of kidney replacement therapy, and ICU LOS) with DAAH90 were evaluated with ordinal logistic regression.
For patients who were alive at day 90, survival at 1 year was compared among DAAH90 tertiles with Kaplan-Meier curves. Between-group differences were assessed for significance using the log-rank test. The independent association of DAAH90 with 1-year mortality in the follow-up cohort was evaluated with Cox proportional hazards regression models adjusted for age, sex, CCI score, and APACHE II score.
For patients who survived to 3 months and who had functional outcomes measured at 3, 6, and 12 months (the follow-up cohort), associations of DAAH90 tertiles with FIM, 6MWT, MRC, and SF-36 scores at 3, 6, and 12 months were quantified with linear regression. To further assess the incremental value of DAAH90 over existing composite metrics, associations of DAAH90, ventilator-free days at day 28 (VFD28), and ICU-free days at day 28 (ICU-FD28) were compared separately with FIM at 1 year. Models were fitted using tertiles of each metric, and correlations are reported using R2 values. These models were further adjusted for age, sex, APACHE II score, and CCI score.
Since long-term care LOS may be less burdensome than acute hospitalization and potentially even preferable to being at home in some contexts,25,26 these associations were also evaluated in a sensitivity analysis that modified DAAH90 computation to exclude long-term care facility LOS. All statistical tests were 2-tailed and were considered significant at P < .05. Statistical analyses were performed using R, version 4.0.5 (R Project for Statistical Computing). Data analysis for this follow-up study was conducted between July 2021 and August 2022.
Results
Cohort Characteristics and Outcomes
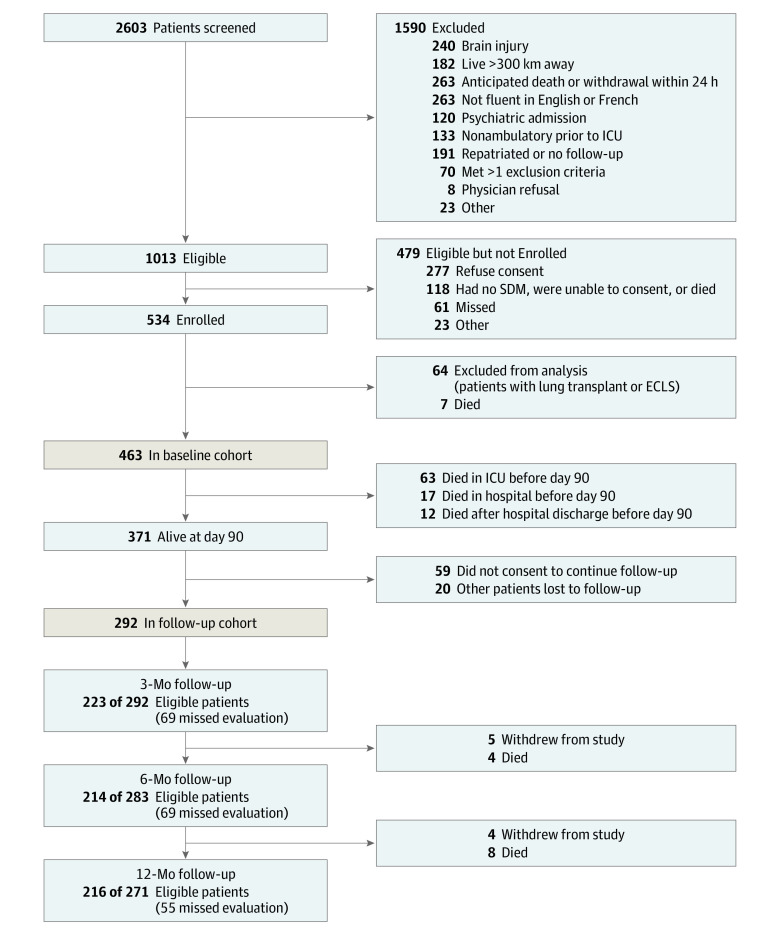
A total of 463 patients were included in the baseline cohort (Figure 1). Their median age was 58 years (IQR, 47-68 years); 278 patients (60.0%) were men and 185 (40.0%) were women. Of these individuals, 292 were included in the follow-up cohort with functional outcomes measured at 3, 6, and 12 months (Figure 1). The median age in the follow-up cohort was 57 years (IQR, 46-65 years); 169 patients (57.9%) were men and 123 (42.1%) were women.
Figure 1. Participant Recruitment Flowchart.
ECLS indicates extracorporeal life support; ICU, intensive care unit; SDM, surrogate decision-maker.
The distribution for DAAH90 is presented in eFigure 1 in Supplement 1. The median value was 11 days (IQR, 0-53 days). A total of 214 patients (46.2% of the overall cohort) had a DAAH90 of 0 days; 92 (19.9%) died and 109 (23.5%) remained in the hospital or other inpatient facility at 3 months. Thirteen patients (2.8%) were admitted to a long-term care facility. Removing long-term care LOS from the calculation of DAAH90 did not appreciably alter the distribution of days alive and at home (eFigure 1 in Supplement 1).
Patient Characteristics and DAAH90 in the Baseline Cohort
Older age, higher CCI score, and higher APACHE II score were associated with lower DAAH90 (Table 1 and eFigure 2 in Supplement 1). Additionally, DAAH90 was not substantially different between men and women but varied considerably according to admission diagnosis (Table 1 and eFigure 2 in Supplement 1).
Table 1. Baseline and Critical Illness Characteristics According to Days Alive and at Home at Day 90 (DAAH90) in the Baseline Cohorta.
| Characteristic | DAAH90 (n = 463) | P value | ||
|---|---|---|---|---|
| Tertile 1 (n = 214) | Tertile 2 (n = 102) | Tertile 3 (n = 147) | ||
| DAAH90, median (range), d | 0 (0-0) | 27 (1-46) | 63 (47-78) | NA |
| Age, median (IQR), y | 61 (50-70) | 59 (47-66) | 54 (41-62) | <.001 |
| Sex | ||||
| Men | 134 (62.6) | 64 (62.7) | 80 (54.4) | .24 |
| Women | 80 (37.4) | 38 (37.3) | 67 (45.6) | |
| BMI, median (IQR) | 26.7 (23.5-30.7) | 27.7 (24.0-33.3) | 28.0 (24.6-33.4) | .09 |
| Education level | ||||
| Less than secondary | 18 (8.4) | 18 (17.6) | 24 (16.3) | .001 |
| Secondary or some postsecondary | 33 (15.4) | 27 (26.5) | 39 (26.5) | |
| Postsecondary | 163 (76.2) | 57 (55.9) | 84 (57.1) | |
| Prior employment | ||||
| Part-time or full-time | 44 (48.4) | 40 (50.6) | 56 (50.5) | .11 |
| Retired | 27 (29.7) | 23 (29.1) | 20 (18.0) | |
| Disability | 9 (9.9) | 9 (11.4) | 10 (9.0) | |
| Other | 11 (12.1) | 7 (8.9) | 25 (22.5) | |
| Income, $ | ||||
| <50 000 | 22 (24.2) | 22 (27.8) | 45 (40.5) | .11 |
| 50 000-70 000 | 10 (11.0) | 11 (13.9) | 11 (9.9) | |
| >70 000 | 26 (28.6) | 20 (25.3) | 32 (28.8) | |
| Unknown | 33 (36.3) | 26 (32.9) | 23 (20.7) | |
| Charlson Comorbidity Index score, median (IQR) | 2 (1-3) | 1 (0-3) | 1 (0-2) | <.001 |
| Admission diagnosis category | ||||
| Cardiac | 22 (10.3) | 5 (4.9) | 13 (8.8) | .08 |
| Gastrointestinal | 29 (13.6) | 15 (14.7) | 20 (13.6) | |
| Neurological | 32 (15.0) | 19 (18.6) | 21 (14.3) | |
| Respiratory | 60 (28.0) | 28 (27.5) | 51 (34.7) | |
| Sepsis | 38 (17.8) | 11 (10.8) | 9 (6.1) | |
| Trauma | 16 (7.5) | 14 (13.7) | 14 (9.5) | |
| Other | 17 (7.9) | 10 (9.8) | 19 (12.9) | |
| Acute Physiology and Chronic Health Evaluation II score, median (IQR) | 24 (18-28) | 21 (17-26) | 22 (15-28) | .03 |
| Lowest Pao2/FiO2, median (IQR) | 100 (66-142) | 105 (74-173) | 100 (74-144) | .23 |
| Kidney replacement therapy | 81 (37.9) | 18 (17.6) | 16 (10.9) | <.001 |
| Tracheostomy | 133 (62.1) | 55 (53.9) | 36 (24.5) | <.001 |
| Duration of mechanical ventilation among ICU survivors, median (IQR), d | 24 (14-36) | 19 (13-27) | 12 (9-17) | <.001 |
| Ventilator-free days to day 28, median (IQR), d | 4 (0-15) | 9 (0-15) | 17 (13-19) | <.001 |
| ICU LOS among survivors, median (IQR), d | 26 (18-45) | 24 (16-33) | 15 (11-19) | <.001 |
| ICU-free days to day 28, median (IQR), d | 0 (0-10) | 4 (0-12) | 13 (9-17) | <.001 |
| Index hospitalization LOS, median (IQR), d | 67 (33-111) | 53 (43-68) | 28 (22-37) | <.001 |
| Days in hospital for subsequent hospital admissions, median (IQR) | 21 (0-43) | 19 (6-33) | 0 (0-6) | <.001 |
| Patients admitted in long-term care | 12 (5.6) | 1 (1.0) | 0 (0.0) | .003 |
| Functional Independence Measure score at day 7 after ICU discharge, median (IQR) | 43 (30-57) | 49 (31-66) | 85 (55-107) | <.001 |
| Total Medical Research Council Scale for Muscle Strength score, median (IQR) | 38 (25-48) | 46 (38-50) | 52 (46-58) | <.001 |
| Disability risk groupb | ||||
| Young (<42 y), short LOS (<2 wk) | 3 (1.4) | 2 (2.0) | 19 (12.9) | <.001 |
| Mixed age (≥42 or ≤45 y), variable LOS (<2 or ≥2 wk) | 53 (24.8) | 36 (35.3) | 70 (47.6) | |
| Older (46-66 y), long LOS (≥2 wk) | 84 (39.3) | 40 (39.2) | 44 (29.9) | |
| Oldest (>66 y), long LOS (≥2 wk) | 74 (34.6) | 24 (23.5) | 14 (9.5) | |
| Discharge disposition | ||||
| Acute or chronic care | 32 (27.6) | 8 (7.8) | 18 (12.3) | <.001 |
| Home | 12 (10.3) | 28 (27.5) | 88 (60.3) | |
| Referring hospital | 19 (16.4) | 17 (16.7) | 20 (13.7) | |
| Rehabilitation | 53 (45.7) | 49 (48.0) | 20 (13.7) | |
| Died in ICU | 61 (45.5) | 0 (0.0) | 0 (0.0) | <.001 |
| Died in hospital | 5 (2.3) | 0 (0.0) | 0 (0.0) | .05 |
| Mortality at 1 year | 126 (58.9) | 8 (7.8) | 6 (4.1) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ICU, intensive care unit; LOS, length of stay; NA, not applicable; Pao2/FiO2, ratio of the partial pressure of arterial oxygen to fraction of inspired oxygen concentration.
Unless noted otherwise, data are expressed as No. (%) of patients.
Defined in the original RECOVER study.15
Burden of Critical Illness and DAAH90 in the Baseline Cohort
In the baseline cohort, patients with a more prolonged duration of mechanical ventilation or ICU admission and those with lower VFD28 or ICU-FD28 had lower DAAH90 (Table 1 and eTable 1 in Supplement 1). Patients who required tracheostomy were more likely to be in a lower DAAH90 tertile (adjusted proportional odds ratio [OR], 0.31 [95% CI, 0.21-0.45]). Patients who required kidney replacement therapy were also more likely to be in a lower DAAH90 tertile (adjusted proportional OR, 0.23 [95% CI, 0.14-0.38]). The distribution of RECOVER disability risk groups and ICU LOS varied notably between DAAH90 tertiles in the original cohort (Table 1). Patients in higher risk groups had substantially lower DAAH90 (eFigure 3 in Supplement 1).
DAAH90 and Long-term Functional Outcomes in the Follow-up Cohort
Baseline and critical illness characteristics of the follow-up cohort are reported in eTable 2 in Supplement 1. In both unadjusted and adjusted analyses, lower DAAH90 was associated with lower FIM scores at 3, 6, and 12 months (Figure 2 and Table 2). Among patients who survived to 12 months, being in tertile 3 vs tertile 1 for DAAH90 was associated with higher FIM score (estimate, 22.4 [95% CI, 14.8-30.0]; P < .001), but this association was not present for VFD28 (estimate, 6.0 [95% CI, −2.2 to 14.1]; P = .15) or ICU-FD28 (estimate, 5.9 [95% CI, −2.1 to 13.8]; P = .15) (eTable 3 in Supplement 1). Lower tertiles of DAAH90 were also associated with lower 6MWT, MRC, and SF-36 scores at 3, 6, and 12 months (eg, at 3 months: 6MWT tertile 1 vs tertile 3, 98 [IQR, 0-239] vs 402 [IQR, 300-494], P < .001; MRC tertile 1 vs tertile 3, 48 [IQR, 32-54] vs 58 [IQR, 51-60], P < .001; SF-36 tertile 1 vs tertile 3, 30 [IQR, 22-38] vs 37 [IQR, 31-47], P = .001) (Figure 2 and Table 2). However, DAAH90 was not associated with IES-R or BDI-II scores at any of the time points measured (Table 2). In a sensitivity analysis in the follow-up cohort excluding long-term care LOS from DAAH90 calculation, the association of DAAH90 with functional outcomes persisted (eTable 4 in Supplement 1). In a sensitivity analysis partitioning DAAH90 into deciles, FIM, SF-36, and MRC scores progressively increased with increasing DAAH90, although the magnitude of increase varied across the range of deciles (eFigure 5 in Supplement 1).
Figure 2. Association Between Tertiles of Days Alive and at Home at Day 90 (DAAH90) and Functional Outcomes at 3, 6, and 12 Months.
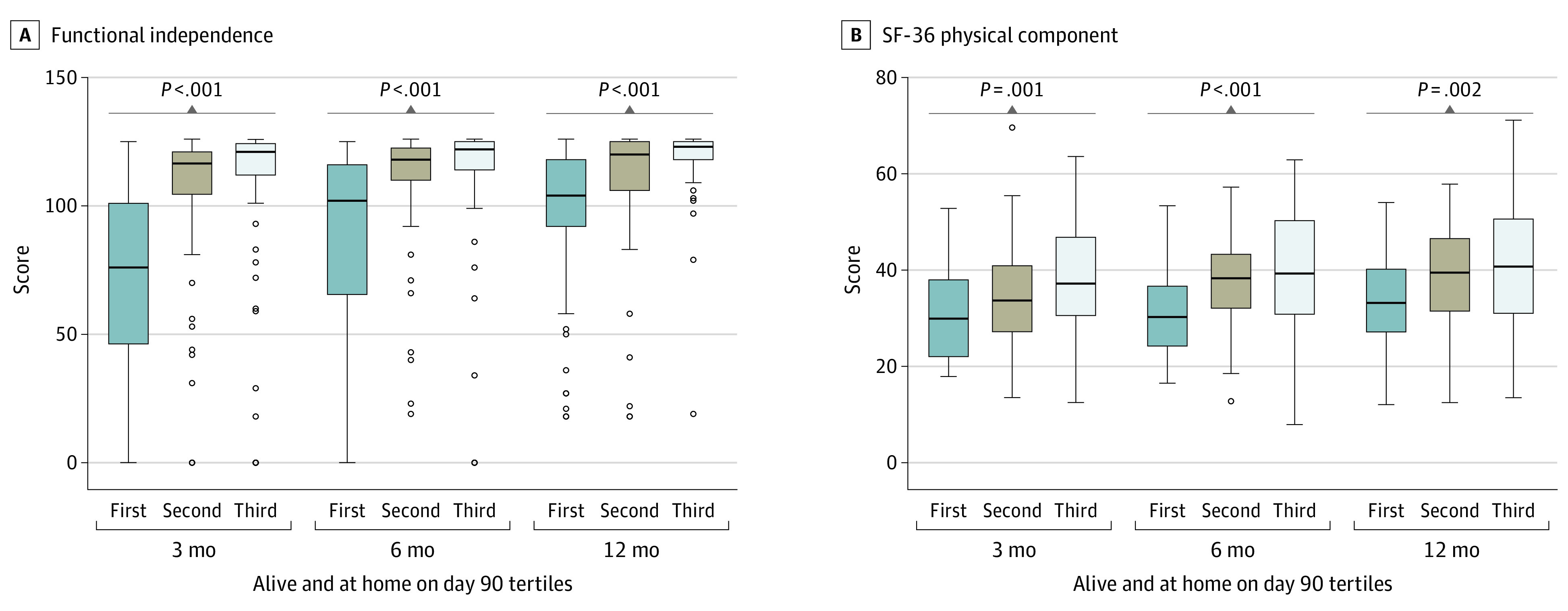
A and B, Data are derived from patients in the RECOVER follow-up cohort who survived to 3 months and who had functional outcomes measured at 3, 6, and 12 months using the Functional Independence Measure (FIM; A) and the 36-Item Short Form Health Survey physical component summary (SF-36 PCS; B). Higher FIM and SF-36 PCS scores indicate a greater level of functional independence. Lower values of DAAH90 are consistently associated with worse functional outcomes. Horizontal lines represent medians; the lower and upper bounds of the boxes represent the first and third quartiles of the data, respectively; whiskers encompass data extending to 1.5 times the IQR (derived as the difference between the first and third tertiles); and circles represent outliers.
Table 2. Functional Outcomes at 3, 6, and 12 Months According to Days Alive and at Home at Day 90 (DAAH90) for Patients Alive at 3 Months in the Follow-up Cohort.
| Outcome measure | DAAH90 (n = 292)a | P value | |||
|---|---|---|---|---|---|
| Tertile 1 (n = 98) | Tertile 2 (n = 97) | Tertile 3 (n = 97) | Unadjusted | Adjustedb | |
| DAAH90, median (range), d | 0 (0-1) | 30 (2-52) | 65 (53-78) | NA | NA |
| Functional Independence Measure score | |||||
| 3 mo | 76 (46.2-101) | 116.5 (104.5-121) | 121 (112-124.2) | <.001 | .04 |
| 6 mo | 102 (65.6-116) | 118.00 (110-122.5) | 122 (114-125) | <.001 | .03 |
| 12 mo | 104 (92-118) | 120 (106-125) | 123 (118-125) | <.001 | <.001 |
| 6-Minute Walk Test score | |||||
| 3 mo | 98 (0-239) | 337 (240-451) | 402 (300-494) | <.001 | <.001 |
| 6 mo | 264 (101-354) | 400 (272.2-491.2) | 443 (296-516) | <.001 | <.001 |
| 12 mo | 325 (144-409) | 408 (252-507) | 449.5 (370-548) | <.001 | <.001 |
| Medical Research Council Scale for Muscle Strength score | |||||
| 3 mo | 48 (32-54) | 53 (48-58) | 58 (51-60) | <.001 | <.001 |
| 6 mo | 48 (44-58) | 58 (48-60) | 60 (56-60) | <.001 | <.001 |
| 12 mo | 55 (48-60) | 60 (50-60) | 60 (58-60) | <.001 | <.001 |
| 36-Item Short Form Health Survey physical component summary score | |||||
| 3 mo | 30 (22-38) | 34 (27-41) | 37 (31-47) | .001 | .001 |
| 6 mo | 30 (24-37) | 38 (32-43) | 39 (31-50) | <.001 | <.001 |
| 12 mo | 33 (27-40) | 40 (32-47) | 41 (31-51) | .002 | <.001 |
| Impact Event Scale–Revised score | |||||
| 3 mo | 15 (3-26) | 13 (4-27) | 18 (5-31) | .58 | .83 |
| 6 mo | 12 (4-28) | 13 (4-28) | 15 (5-28) | .69 | .56 |
| 12 mo | 14 (8-27) | 10 (2-23) | 11 (4-27) | .12 | .12 |
| Beck Depression Inventory–II score | |||||
| 3 mo | 12 (7-18) | 9 (6-16) | 9 (6-17) | .37 | .24 |
| 6 mo | 11 (5-18) | 8 (3-13) | 10 (4-19) | .13 | .05 |
| 12 mo | 10 (6-18) | 8 (4-14) | 8 (2-17) | .28 | .33 |
| Mortality at 1 year | 11 (11.2) | 5 (5.2) | 1 (1.0) | .009 | .006 |
Abbreviation: NA, not applicable.
Unless noted otherwise, data are expressed as the median (IQR).
Multivariable models were adjusted for age, sex, Acute Physiology and Chronic Health Evaluation II score, and Charlson Comorbidity Index score.
DAAH90 and 1-Year Mortality
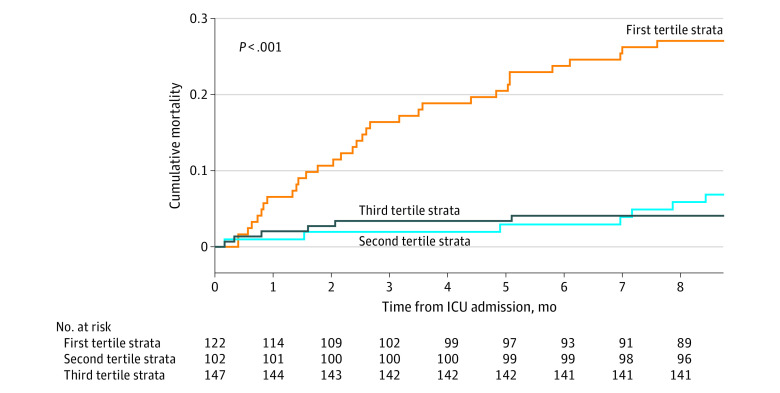
After restricting the unadjusted analysis to the 371 patients alive at day 90, lower DAAH90 was associated with a higher risk of death at 1 year (tertile 2 vs tertile 1: adjusted HR, 0.28 [95% CI, 0.12-0.64]; P = .012, log-rank test of difference; Figure 3). In the adjusted analysis, higher DAAH90 tertiles were associated with a lower risk of death at 1 year compared with the lowest DAAH90 tertile (eTable 5 in Supplement 1). In a sensitivity analysis of patients alive at day 90 that excluded long-term care facility LOS from the computation of DAAH90, lower DAAH90 was persistently associated with higher mortality (eFigure 4 in Supplement 1). Finally, in patients alive at day 90, 1-year mortality was highest in lower deciles of DAAH90 (eFigure 6 in Supplement 1).
Figure 3. Cumulative Mortality Up to 12 Months After Intensive Care Unit (ICU) Discharge According to Tertiles of Days Alive and at Home at Day 90 Among Patients Alive at Day 90.
Patients in the lowest tertile of days alive and at home at day 90 had a significantly (P < .001) higher risk of death at 1 year compared with patients in higher tertiles.
Discussion
In this secondary analysis of the RECOVER prospective cohort study of adults receiving mechanical ventilation for 7 or more days across 10 Canadian ICUs,15 we observed that DAAH90 was associated with multiple measures of functional status and quality of life in ICU survivors over the first year after critical illness and with mortality at 1 year. In addition, we observed that DAAH90 reflected the severity of critical illness as represented by multiple metrics of ICU intensity. In this study, other common metrics of ICU outcomes (VFD28 and ICU-FD28) were not associated with long-term functional status. These findings support the use of DAAH90 as a readily ascertained patient-centered outcome in future clinical studies and clinical trials involving critically ill patients.
The DAAH measure prioritizes time spent at home while offering information about mortality and length of hospitalization.5,13,27 Prior studies have explored DAAH as a measure of intervention efficacy across various hospitalized populations.5,6,9 To our knowledge, this is the first study to evaluate the role of DAAH90 in the critical care setting. The DAAH90 outcome measure is attractive in critical care for several reasons. First, DAAH90 prioritizes an outcome that most individuals would consider important (time spent at home). Many conventional end points in critical care research do not capture priorities of direct importance to patients and may be difficult for patients to comprehend.28 Mortality is convenient to measure and important to patients, but it may be superseded by other priorities that are more relevant to patients in certain contexts, such as avoiding pain or preserving quality of life.2,29,30 Organ failure–free days, ventilator-free days, and ICU-free days have similar shortcomings. Indeed, we observed that these end points were not associated with long-term functional status in ICU survivors. Because health is widely regarded as more than mere survival, outcomes such as DAAH90 may be useful for measuring what matters most to patients.31
Second, from a trial design perspective, DAAH90 can be easily computed from site-submitted data or health administrative registries without time-intensive adjudication.14 Critical care end points often require central event adjudication according to specific criteria (eg, classification of organ failures), potentially creating challenges in comparing outcomes across studies where adjudication criteria were different.14 Our results suggest that the use of DAAH90 overcomes this limitation because it incorporates data on outcomes that are objective, easy to obtain, and comparable across studies.
Third, use of DAAH90 as a common end point may enable comparisons across studies in which interventions serve different goals.32 Studies of therapeutic interventions frequently evaluate outcomes in terms of morbidity or mortality, whereas those evaluating palliative interventions often report outcomes in terms of health-related satisfaction or quality of life. Direct comparisons between these interventions may be challenging because the 2 outcomes evaluate different patient priorities. In this context, the use of DAAH90 across studies is likely to be appreciated by patients because it portrays the risk of undesirable outcomes (eg, death or longer inpatient stays) alongside a desirable outcome (eg, being at home), thus permitting a fairer comparison and selection of the more goal-concordant intervention.32
Clinicians and investigators seeking to use DAAH90 should consider the following additional points to ensure that the metric is appropriate for their intended uses. First, DAAH90 assumes that time spent at home is preferable to any time in the clinical setting, which is not always true. Some patients may find the home environment uncomfortable or improperly equipped to deal with their care needs and may prefer to receive care in a hospital.32 Second, composite outcomes such as DAAH90 assign equal weight to the components included in the calculation, and this weighting may not be precisely aligned with individual patient values.28,33 As with all composite outcomes, the individual components of DAAH90 should be reported. Some have also argued that it is not appropriate to automatically assign a value of 0 for all patients who die within the time period considered, since time at home before death may be of real value to patients.32 Third, the statistical gains that make composite measures such as DAAH90 appealing may disappear entirely if the intervention causes the components of the composite to change in different directions.28 A study evaluating a rapid discharge strategy from acute care settings could potentially decrease hospital LOS at the expense of increasing long-term care LOS; such an intervention might be ill suited for evaluation with DAAH90. Careful a priori consideration of how the proposed intervention might affect each component of the composite outcome is therefore required in order to maximize the statistical utility of this measure.
Limitations
This study has some limitations. The original RECOVER cohort included patients who required mechanical ventilation for at least 7 days. The median duration of mechanical ventilation among survivors discharged from the ICU was 16 days, and the median hospital LOS for the overall cohort was 49 days.15 These durations are substantially longer than those from comparable cohort studies, in which the median duration of mechanical ventilation ranged from 3.0 to 4.1 days and hospital LOS was 18.9 to 22.5 days.34,35 Whether DAAH90 is a relevant outcome among nonventilated patients or those with shorter hospitalizations remains unknown. Future studies could aim to explore DAAH90 in more heterogeneous ICU cohorts. Our calculation of DAAH90 also assumes that any days not spent in a hospital or long-term care facility were spent at home. In reality, 5.2% of patients in the original cohort not discharged home or to long-term care were transferred to rehabilitation, assisted living, or chronic care.15 Obtaining data on discharges to these alternative institutions is not always possible and may limit the applicability of the described measure. Nevertheless, to offer the most realistic portrayal of a proposed intervention, it should be made clear to patients how much home time is truly contributed by time at home vs time spent in a nonacute inpatient facility.36 In addition, DAAH90 does not consider the impact of home time on patient caregivers, their ability to provide care outside the hospital, and the potential for caregiver burnout, which may limit the success of home discharge.37,38 Data on caregiver status were only collected for a portion of patients enrolled in the original RECOVER study.15 Finally, our findings suggest that DAAH90 does not adequately capture the long-term burden of mental illness following critical illness. We did not observe an association between DAAH90 and IES-R or BDI-II scores at any of the follow-up time points that are specific to PTSD and depression.
Conclusions
In this cohort study, DAAH90 was associated with long-term functional status and survival after critical illness. Given its comparative ease of ascertainment, DAAH90 represents a patient-centered end point that may be useful in future clinical studies and clinical trials in critical care.
eFigure 1. Frequency Distributions for Days Alive and at Home at Day 90 (DAAH90) With or Without Including Length of Stay (LOS) in Long-term Care
eFigure 2. Distribution of Days Alive and at Home at Day 90 (DAAH90) According to Baseline Characteristics of the Study Population
eTable 1. Association of Measures of Burden of Critical Illness With Tertiles of Days Alive and at Home at Day 90
eTable 2. Baseline Characteristics of the 292 Patients in the Follow-up Cohort
eFigure 3. Distribution of Days Alive and at Home at Day 90 (DAAH90) According to Disability Risk Groups Defined in the RECOVER Study
eTable 3. Adjusted Association of Composite Metrics With Functional Independence Measure Score at 1 Year
eTable 4. Sensitivity Analysis Removing Long-Term Care Length of Stay at Day 90
eTable 5. Cox Proportional Hazards Regression Model for 1-Year Mortality
eFigure 4. Survival at 12 Months Among Survivors at Day 90 According to Tertiles of Days Alive and at Home at Day 90 Removing Long-Term Care Length of Stay
eFigure 5. Association of Deciles of Days Alive and at Home at Day 90 (DAAH90) With Functional Outcomes at 3 Months
eFigure 6. Mortality at 1 Year in Survivors at Day 90 According to Deciles of Days Alive and at Home at Day 90 (DAAH90)
Data Sharing Statement
References
- 1.Rubin EB, Buehler A, Halpern SD. Seriously ill patients’ willingness to trade survival time to avoid high treatment intensity at the end of life. JAMA Intern Med. 2020;180(6):907-909. doi: 10.1001/jamainternmed.2020.0681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin EB, Buehler AE, Halpern SD. States worse than death among hospitalized patients with serious illnesses. JAMA Intern Med. 2016;176(10):1557-1559. doi: 10.1001/jamainternmed.2016.4362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns KE, Jacob SK, Aguirre V, Gomes J, Mehta S, Rizvi L. Stakeholder engagement in trial design: survey of visitors to critically ill patients regarding preferences for outcomes and treatment options during weaning from mechanical ventilation. Ann Am Thorac Soc. 2016;13(11):1962-1968. doi: 10.1513/AnnalsATS.201606-445OC [DOI] [PubMed] [Google Scholar]
- 4.Ariti CA, Cleland JGF, Pocock SJ, et al. Days alive and out of hospital and the patient journey in patients with heart failure: insights from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Am Heart J. 2011;162(5):900-906. doi: 10.1016/j.ahj.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 5.Wasywich CA, Gamble GD, Whalley GA, Doughty RN. Understanding changing patterns of survival and hospitalization for heart failure over two decades in New Zealand: utility of ‘days alive and out of hospital’ from epidemiological data. Eur J Heart Fail. 2010;12(5):462-468. doi: 10.1093/eurjhf/hfq027 [DOI] [PubMed] [Google Scholar]
- 6.Yu AYX, Rogers E, Wang M, et al. Population-based study of home-time by stroke type and correlation with modified Rankin score. Neurology. 2017;89(19):1970-1976. doi: 10.1212/WNL.0000000000004631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guttman MP, Tillmann BW, Nathens AB, et al. Alive and at home: five-year outcomes in older adults following emergency general surgery. J Trauma Acute Care Surg. 2021;90(2):287-295. doi: 10.1097/TA.0000000000003018 [DOI] [PubMed] [Google Scholar]
- 8.Groff AC, Colla CH, Lee TH. Days spent at home—a patient-centered goal and outcome. N Engl J Med. 2016;375(17):1610-1612. doi: 10.1056/NEJMp1607206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien EC, Xian Y, Xu H, et al. Hospital variation in home-time after acute ischemic stroke: insights from the PROSPER study (Patient-Centered Research Into Outcomes Stroke Patients Prefer and Effectiveness Research). Stroke. 2016;47(10):2627-2633. doi: 10.1161/STROKEAHA.116.013563 [DOI] [PubMed] [Google Scholar]
- 10.Bell M, Eriksson LI, Svensson T, et al. Days at home after surgery: an integrated and efficient outcome measure for clinical trials and quality assurance. EClinicalMedicine. 2019;11:18-26. doi: 10.1016/j.eclinm.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIsaac DI, Fottinger A, Sucha E, McDonald B. Association of frailty with days alive at home after cardiac surgery: a population-based cohort study. Br J Anaesth. 2021;126(6):1103-1110. doi: 10.1016/j.bja.2021.02.011 [DOI] [PubMed] [Google Scholar]
- 12.Jerath A, Austin PC, Wijeysundera DN. Days alive and out of hospital: validation of a patient-centered outcome for perioperative medicine. Anesthesiology. 2019;131(1):84-93. doi: 10.1097/ALN.0000000000002701 [DOI] [PubMed] [Google Scholar]
- 13.Fonarow GC, Liang L, Thomas L, et al. Assessment of home-time after acute ischemic stroke in Medicare beneficiaries. Stroke. 2016;47(3):836-842. doi: 10.1161/STROKEAHA.115.011599 [DOI] [PubMed] [Google Scholar]
- 14.Fanaroff AC, Cyr D, Neely ML, et al. Days alive and out of hospital: exploring a patient-centered, pragmatic outcome in a clinical trial of patients with acute coronary syndromes. Circ Cardiovasc Qual Outcomes. 2018;11(12):e004755. doi: 10.1161/CIRCOUTCOMES.118.004755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herridge MS, Chu LM, Matte A, et al. ; RECOVER Program Investigators (Phase 1: Towards RECOVER); Canadian Critical Care Trials Group . The RECOVER program: disability risk groups and 1-year outcome after 7 or more days of mechanical ventilation. Am J Respir Crit Care Med. 2016;194(7):831-844. doi: 10.1164/rccm.201512-2343OC [DOI] [PubMed] [Google Scholar]
- 16.Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6-18. [PubMed] [Google Scholar]
- 17.Chumney D, Nollinger K, Shesko K, Skop K, Spencer M, Newton RA. Ability of functional independence measure to accurately predict functional outcome of stroke-specific population: systematic review. J Rehabil Res Dev. 2010;47(1):17-29. doi: 10.1682/JRRD.2009.08.0140 [DOI] [PubMed] [Google Scholar]
- 18.Hall KM, Cohen ME, Wright J, Call M, Werner P. Characteristics of the Functional Independence Measure in traumatic spinal cord injury. Arch Phys Med Rehabil. 1999;80(11):1471-1476. doi: 10.1016/S0003-9993(99)90260-5 [DOI] [PubMed] [Google Scholar]
- 19.Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. BMJ (Clin Res Ed). 1982;284(6329):1607-1608. doi: 10.1136/bmj.284.6329.1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. BMJ. 1959;2(5147):257-266. doi: 10.1136/bmj.2.5147.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teo BJX, Koh JSB, Jiang L, Allen JC, Yeo SJ, Howe TS. Association of the 36-Item Short Form Health Survey physical component summary score with patient satisfaction and improvement 2 years after total knee arthroplasty. JAMA Netw Open. 2019;2(2):e190062. doi: 10.1001/jamanetworkopen.2019.0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware JE. Scoring the SF-36. In: SF-36 Health Survey: Manual and Interpretation Guide. Nimrod Press; 1993:6:3-6:19. [Google Scholar]
- 23.Horowitz M, Wilner N, Alvarez W. Impact of Event scale: a measure of subjective stress. Psychosom Med. 1979;41(3):209-218. doi: 10.1097/00006842-197905000-00004 [DOI] [PubMed] [Google Scholar]
- 24.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. doi: 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 25.Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160(11):774-784. doi: 10.7326/M14-0083 [DOI] [PubMed] [Google Scholar]
- 26.Connor SR, Pyenson B, Fitch K, Spence C, Iwasaki K. Comparing hospice and nonhospice patient survival among patients who die within a three-year window. J Pain Symptom Manage. 2007;33(3):238-246. doi: 10.1016/j.jpainsymman.2006.10.010 [DOI] [PubMed] [Google Scholar]
- 27.Greene SJ, O’Brien EC, Mentz RJ, et al. Home-time after discharge among patients hospitalized with heart failure. J Am Coll Cardiol. 2018;71(23):2643-2652. doi: 10.1016/j.jacc.2018.03.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200(7):828-836. doi: 10.1164/rccm.201810-2050CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halpern SD. Goal-concordant care—searching for the holy grail. N Engl J Med. 2019;381(17):1603-1606. doi: 10.1056/NEJMp1908153 [DOI] [PubMed] [Google Scholar]
- 30.Patrick DL, Starks HE, Cain KC, Uhlmann RF, Pearlman RA. Measuring preferences for health states worse than death. Med Decis Making. 1994;14(1):9-18. doi: 10.1177/0272989X9401400102 [DOI] [PubMed] [Google Scholar]
- 31.Dzau VJ, McClellan MB, McGinnis JM, et al. Vital directions for health and health care: priorities from a National Academy of Medicine initiative. JAMA. 2017;317(14):1461-1470. doi: 10.1001/jama.2017.1964 [DOI] [PubMed] [Google Scholar]
- 32.Auriemma CL, Taylor SP, Harhay MO, Courtright KR, Halpern SD. Hospital-free days: a pragmatic and patient-centered outcome for trials among critically and seriously ill patients. Am J Respir Crit Care Med. 2021;204(8):902-909. doi: 10.1164/rccm.202104-1063PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cordoba G, Schwartz L, Woloshin S, Bae H, Gøtzsche PC. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ. 2010;341:c3920. doi: 10.1136/bmj.c3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esteban A, Anzueto A, Frutos F, et al. ; Mechanical Ventilation International Study Group . Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345-355. doi: 10.1001/jama.287.3.345 [DOI] [PubMed] [Google Scholar]
- 35.Burns KEA, Rizvi L, Cook DJ, et al. ; Canadian Critical Care Trials Group . Ventilator weaning and discontinuation practices for critically ill patients. JAMA. 2021;325(12):1173-1184. doi: 10.1001/jama.2021.2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time—measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. doi: 10.1056/NEJMp1703423 [DOI] [PubMed] [Google Scholar]
- 37.van Beusekom I, Bakhshi-Raiez F, de Keizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care. 2016;20(1):16. doi: 10.1186/s13054-016-1185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turnbull AE, Sepulveda KA, Dinglas VD, Chessare CM, Bingham CO III, Needham DM. Core domains for clinical research in acute respiratory failure survivors: an international modified Delphi consensus study. Crit Care Med. 2017;45(6):1001-1010. doi: 10.1097/CCM.0000000000002435 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Frequency Distributions for Days Alive and at Home at Day 90 (DAAH90) With or Without Including Length of Stay (LOS) in Long-term Care
eFigure 2. Distribution of Days Alive and at Home at Day 90 (DAAH90) According to Baseline Characteristics of the Study Population
eTable 1. Association of Measures of Burden of Critical Illness With Tertiles of Days Alive and at Home at Day 90
eTable 2. Baseline Characteristics of the 292 Patients in the Follow-up Cohort
eFigure 3. Distribution of Days Alive and at Home at Day 90 (DAAH90) According to Disability Risk Groups Defined in the RECOVER Study
eTable 3. Adjusted Association of Composite Metrics With Functional Independence Measure Score at 1 Year
eTable 4. Sensitivity Analysis Removing Long-Term Care Length of Stay at Day 90
eTable 5. Cox Proportional Hazards Regression Model for 1-Year Mortality
eFigure 4. Survival at 12 Months Among Survivors at Day 90 According to Tertiles of Days Alive and at Home at Day 90 Removing Long-Term Care Length of Stay
eFigure 5. Association of Deciles of Days Alive and at Home at Day 90 (DAAH90) With Functional Outcomes at 3 Months
eFigure 6. Mortality at 1 Year in Survivors at Day 90 According to Deciles of Days Alive and at Home at Day 90 (DAAH90)
Data Sharing Statement