Summary
Background
The combination of chemotherapy and ponatinib in Philadelphia chromosome-positive acute lympho-blastic leukaemia has the potential to be a new standard of care for the disease; however, long-term efficacy and safety data are needed. Our aim was to evaluate the long-term efficacy and safety of this regimen in patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukaemia in this ongoing phase 2 trial.
Methods
In our single-centre, phase 2, single-arm trial in the USA, adult patients with previously untreated Philadelphia chromosome-positive acute lymphoblastic leukaemia were sequentially enrolled. Eligible patients had newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukaemia, were aged 18 years or older, had an Eastern Cooperative Oncology Group performance status of 2 or less, a left ventricular ejection fraction above 50%, and adequate hepatic and renal function (serum bilirubin ≤3·0 mg/dL and serum creatinine ≤3·0 mg/dL, unless higher levels were believed to be due to leukaemia at the discretion of the investigator). Patients received eight cycles of 21 days, alternating between two drug combinations: hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) and high-dose methotrexate and cytarabine. Ponatinib was given orally at 45 mg per day for the first 14 days of cycle 1 then continuously at 45 mg per day for the subsequent cycles. After 37 patients were treated, the protocol was amended to reduce the dose of ponatinib to 30 mg per day at cycle 2, with further reduction to 15 mg once a complete molecular response (defined as absence of quantifiable BCR-ABL1 transcripts) was achieved. Patients in complete remission received maintenance with ponatinib daily (30 mg or 15 mg) indefinitely, and with vincristine (2 mg intravenously on day 1) and prednisone (200 mg orally on days 1–5) monthly for 2 years. The primary endpoint was 3-year event-free survival in the intention-to-treat population. The trial is registered at ClinicalTrials.gov, number NCT01424982, and is ongoing and still enrolling patients.
Findings
76 patients with a median age of 47 years (IQR 39–61) were enrolled and treated between Nov 19, 2011, and April 4, 2018. The 3-year event-free survival was 70% (95% CI 56–80). The most common grade 3 or 4 adverse events were infection (n=65, 86%), increased transaminases (n=24, 32%), increased bilirubin (n=13, 17%), pancreatitis (n=13, 17%), hypertension (n=12, 16%), bleeding (n=10, 13%), and skin rash (n=9, 12%). Six patients died while still on study treatment. Three patients (4%) died from infection and one (1%) from haemorrhage. Two patients died from myocardial infarction related to early ponatinib use; neither death occurred after protocol revision.
Interpretation
The combination of chemotherapy with ponatinib is effective in achieving long-term remission in patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukaemia. This regimen could represent a new standard of care for this population. A randomised, phase 3 study to evaluate the efficacy of this combination compared with chemotherapy plus earlier-generation tyrosine-kinase inhibitors is warranted.
Introduction
Incorporation of BCR-ABL1 tyrosine-kinase inhibitors into chemotherapy regimens has substantially improved survival outcomes of patients with Philadelphia chromo-some-positive acute lymphoblastic leukaemia.1–5 The combination of cytotoxic chemotherapy with tyrosine-kinase inhibitors is now the standard of care for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia.3–10 Despite the high efficacy of this combination, for patients with Philadelphia chromo-some-positive adult acute lymphoblastic leukaemia, when second-generation tyrosine-kinase inhibitors are used, the 3-year event-free survival is 40% and overall survival is 60%, at best.3,5 Further improvement of these outcomes might be driven by an improvement of the complete molecular response, because this is associated with improved survival across different regimens,4,11–15 eliminating the need for allogeneic stem cell trans-plantation.15 Effective regimens for Philadelphia chromo-some-positive acute lymphoblastic leukaemia should also be able to suppress the emergence of clones with Thr315Ile mutations, which are resistant to first-generation and second-generation tyrosine-kinase in-hibitors.2,12,14 The proportion of patients with the Thr315Ile mutation is up to 75% in patients who relapse after being treated with dasatinib-based regimens.2,12,16
Ponatinib is a third-generation BCR-ABL1 inhibitor, which is the most potent of the commercially available BCR-ABL1 inhibitors, with activity observed in Philadelphia chromosome-positive leukaemias with both wild-type and mutated ABL1, including Thr315Ile.17–19 We previously reported the high efficacy and manageable toxicity of the combination of ponatinib with the hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexa-methasone (hyper-CVAD) regimen as a first-line treatment of Philadelphia chromosome-positive acute lymphoblastic leukaemia.14 Important questions are the durability of responses, and whether the regimen can obviate the need for allogeneic stem cell transplantation. We report the long-term safety and efficacy of the hyper-CVAD and ponatinib combination regimen.
Methods
Study design and participants
Adults with previously untreated Philadelphia chromo-some-positive acute lymphoblastic leukaemia, established by the identification of either t(9;22) karyotype or BCR-ABL1 fusion transcript, and patients who had received no more than two courses of previous chemotherapy with or without tyrosine-kinase inhibitors, were eligible and enrolled sequentially at the University of Texas MD Anderson Cancer Center (Houston, TX, USA). Eligible patients were aged 18 years or older, had an Eastern Cooperative Oncology Group performance status of 2 or less, left ventricular ejection fraction above 50%, and adequate hepatic and renal function (serum bilirubin ≤3·0 mg/dL and serum creatinine ≤3·0 mg/dL, unless higher levels were believed to be due to leukaemia at the discretion of the investigator). Patients were excluded if they had an active infection not controlled with antibiotics; clinical evidence of grade 3 or 4 heart failure, as defined by the New York Heart Association criteria;20 uncontrolled hypertension and active cardio vascular comorbidities; active second malignancy; or previous treatment with ponatinib.
All patients were enrolled consecutively and gave written informed consent. The study was done in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Procedures
The details of the hyper-CVAD plus ponatinib regimen have been previously published.14 Patients received eight cycles of treatment (each lasting 21 days), alternating between two drug combinations. In summary, for cycles 1, 3, 5, and 7, treatment consisted of 300 mg/m2 of cyclophosphamide given intravenously over 2–3 h every 12 h for six doses on days 1–3; a continuous daily infusion of sodium mercaptoethanesulfonate at 600 mg/m2 per day for 24 h starting 1 h before cyclophosphamide and completed approximately 12 h after the final cyclophosphamide dose; doxorubicin intra-venously at 50 mg/m2 over 24 h on day 4; 2 mg of vincristine intravenously on days 1 and 11; and 40 mg of dexamethasone intravenously and orally on days 1–4 and days 11–14. For cycles 2, 4, 6, and 8, treatment consisted of methotrexate intravenously at 200 mg/m2 over 2 h, followed by 800 mg/m2 over 22 h on day 1; cytarabine intravenously at 3 g/m2 over 3 h every 12 h for four doses on days 1–2; and 50 mg of citrovorum rescue intravenously or orally, followed by 15 mg every 6 h for eight doses beginning 12 h after methotrexate completion.
Each cycle lasted 21 days.14 At the start of the trial, 45 mg of ponatinib was given orally per day for the first 14 days of cycle 1, then continuously at the same dose for cycles 2–8. On Aug 1, 2014, after 37 patients were treated, the protocol was amended because of concern about cardiovascular toxicity with ponatinib due to two ponatinib-related fatal myocardial infarction deaths (decision made on the basis of principal investigator’s judgement). Thereafter, ponatinib was given at 45 mg per day for the first 14 days of cycle 1, then at 30 mg per day continuously from cycle 2 onwards for patients in complete remission or complete remission with incomplete haematological recovery (ie, all patients). The dose was further reduced to 15 mg per day continuously once complete molecular response was achieved.
Rituximab was administered intravenously at 375 mg/m2 during the first four cycles in patients with CD20 expression of at least 20% of leukaemic cells.21 For CNS prophylaxis, intrathecal methotrexate and cytarabine was given alternately on days 2 and 7 of cycles 1–6 for a total of 12 doses. The order of intrathecal chemotherapy was reversed during the evenly numbered cycles 2, 4, and 6 (after the first 50 patients were enrolled); ie, cytarabine on day 2 and methotrexate on day 7, to avoid simultaneous systemic and intrathecal methotrexate, which we suspected to cause rare demyelination and neurotoxicity on the basis of our clinical experience.
Maintenance therapy was given for 2 years with monthly courses of intravenous vincristine on day 1 (2 mg intravenously), oral prednisone (200 mg) on days 1–5, and daily ponatinib at 30 mg in patients with no complete molecular response and at 15 mg in those with complete molecular response. Initiation of maintenance due to treatment-related toxicity before completion of eight cycles was allowed. Months 6 and 13 of maintenance were intensification courses of hyper-CVAD and ponatinib. Patients with complete molecular response or who were poor candidates for such intensification, or both, continued maintenance therapy uninterrupted at the discretion of the treating physician.
Ponatinib dose reductions to 30 mg or 15 mg per day were allowed for substantial drug-related toxicity during both initial therapy and maintenance. At any time during the intensive or maintenance therapy phases, patients with an available matched donor had the option to proceed to allogeneic stem cell transplantation at the discretion of the treating physician. Ponatinib could be given after maintenance, at 30 mg if the patient achieved complete response but no complete molecular response, and at 15 mg if they achieved complete molecular response.
Molecular monitoring with BCR-ABL1 RT-qPCR was done as previously described.14,15 Complete molecular response was defined as the absence of quantifiable BCR-ABL1 transcripts with a sensitivity of 0·01%. Major molecular response was defined as BCR-ABL1 transcripts less than 0·1% by the international scale for patients with p210 transcripts and a 3-log reduction from baseline for patients with p190 transcripts, but not meeting criteria for complete molecular response. BCR-ABL1 kinase domain mutation analysis that covered codons 221–500 was done on cDNA with a nested PCR strategy at the time of relapse.22 Minimal residual disease assessment by six-colour multiparameter flow cytometry was done as previously described, with a sensitivity of at least 0·01%.14,15,23
Outcomes
The primary outcome was 3-year event-free survival, calculated from the beginning of treatment until relapse or death. Secondary outcomes were the proportion of patients who achieved a complete remission, overall survival, and safety. We are reporting these outcomes at a median follow-up of 36 months (IQR 22–63), and this trial is ongoing.
Complete remission was defined as the presence of fewer than 5% blasts in the bone marrow, with more than 1 × 109 neutrophils per L and more than 100 × 109 platelets per L in the peripheral blood, and no extramedullary disease. Relapse was defined by recurrence of more than 5% lymphoblasts in a bone marrow aspirate unrelated to haemopoietic recovery or by the presence of extra medullary disease. Duration of continuous complete remission was calculated from the time of complete remission until relapse, analysed post hoc. Overall survival was calculated from the time of treatment initiation until death from any cause. Allogeneic stem cell transplantation was not censored for any survival estimates in the primary analysis.
Statistical analysis
The trial was continuously monitored, with an early stopping rule in place if the event-free survival was ever likely to be less than that of previous similar trials (the historical 2-year event-free survival was 49%). No stopping rules were met. Survival curves were plotted by the Kaplan-Meier method and compared with the log-rank test. Differences in subgroups by different covariates were evaluated with the χ2 test for nominal values and the Mann-Whitney U and Fisher’s exact tests for continuous variables. Significance was defined as a p value less than 0·05.
The trial is registered at (ClinicalTrials.gov, number NCT01424982, and is ongoing.
Role of the funding source
The funder provided free study drugs and funding for a research nurse for this study. The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author (EJ) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
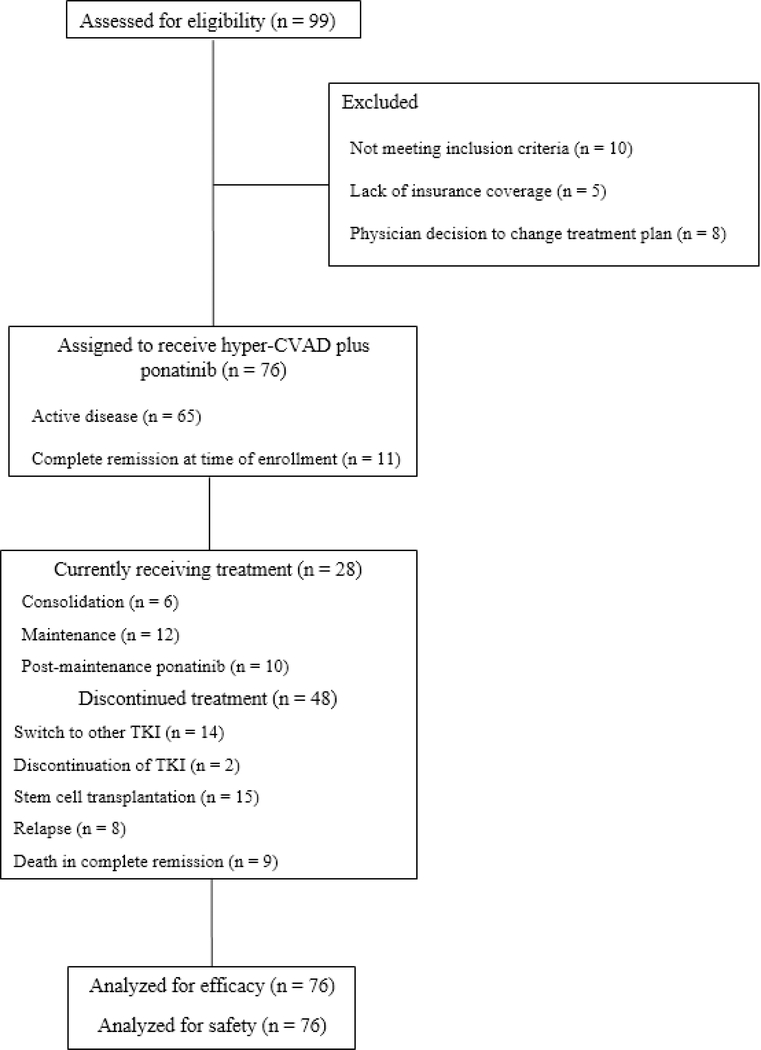
From Nov 19, 2011, to April 4, 2018, 76 patients were consecutively enrolled and allocated to receive hyper-CVAD plus ponatinib (figure 1). The database was locked on May 5, 2018. 63 patients (83%) had untreated Philadelphia chromosome-positive acute lymphoblastic leukaemia and 13 (17%) had been previously treated with one or two cycles of chemotherapy. Of the 13 patients who had previously received treatment, two had active disease status after one previous cycle of chemotherapy without tyrosine-kinase inhibitor (before Philadelphia chromosome-positive or BCR-ABL1 status was identified), and 11 were in complete remission at enrolment (those patients received intensive treatment). Patients were a median age of 47 years (IQR 39–61); 20 patients (26%) were older than 60 years. 26 patients (34%) had CD20-positive acute lymphoblastic leukaemia and received rituximab. 35 patients (46%) had at least one baseline cardiovascular risk factor (hypertension, diabetes, dyslipidaemia, coronary artery disease, or peripheral arterial disease). Patient characteristics are summarised in table 1, and were not different between patients enrolled before and after the study amendment (p>0·1 for all comparisons; data not shown).
Figure 1: Trial profile.
CVAD=cyclophosphamide, vincristine, doxorubicin, and dexamethasone.
*Health insurance coverage was not an exclusion criteria per se, but was necessary for patients to be enrolled on any clinical trial with coverage to receive the therapy.
Table 1:
Patient characteristics
| All patients (N=76) | |
|---|---|
|
| |
| Age, years | 47 (39–61) |
| ≥50 | 35 (46%) |
| ≥60 | 20 (26%) |
| Eastern Cooperative Oncology Group Performance status | |
| 0–1 | 68 (89%) |
| 2 | 8 (11%) |
| White blood cells (x 109 per L) | 13.6 (26–76.6 |
| CNS disease | 5 (7%) |
| CD20 positivity | 26 (34%) |
| BCR-ABL1 transcript | |
| p190 | 56 (74%) |
| p210 | 19 (25%) |
| Cytogenetics | |
| Diploid | 21 (28%) |
| Philadelphia chromosome-positive | 55 (72%) |
| Baseline cardiovascular risk factors | |
| Hypertension | 29 (38%) |
| Diabetes | 13 (17%) |
| Dyslipidaemia | 8 (11%) |
| Coronary artery disease | 6 (8%) |
| Peripheral arterial disease | 1 (1%) |
| Number of cardiovascular risk factors | |
| 1 | 22 (29%) |
| 2 | 7 (9%) |
| 3 | 5 (7%) |
| ≥4 | 1 (1%) |
| Data are median (IQR) or n (%). | |
All 65 patients with active disease at enrolment achieved complete remission; thus, 100% of patients achieved an objective response (table 2). Early mortality (ie, death within 4 weeks of starting treatment) did not occur. Minimal residual disease negativity, assessed by six-colour multiparameter flow cytometry, was achieved in 64 (99%) of 65 patients. Major molecular response (or better) was achieved in 74 (97%) of all 76 patients. Complete molecular response was achieved in 63 patients (83%). Of the 56 patients who had PCR-based minimal residual disease assessment after approximately 3 months of therapy, 41 (73%) achieved a complete molecular response. The median time to major molecular response was 3 weeks (IQR 3–7) and the median time to complete molecular response was 10 weeks (3–14).
Table 2:
Best overall response
| All patients | |
|---|---|
|
| |
| Complete response* | 65/65 (100%) |
| Major molecular response | 74/76 (97%) |
| Complete molecular response | 63/76 (83%) |
| Minimal residual disease flow negativity† | 74/75 (99%) |
| Early death | 0 |
Data are n/N (%).
11 patients were already in complete remission at start.
One patient with no sample.
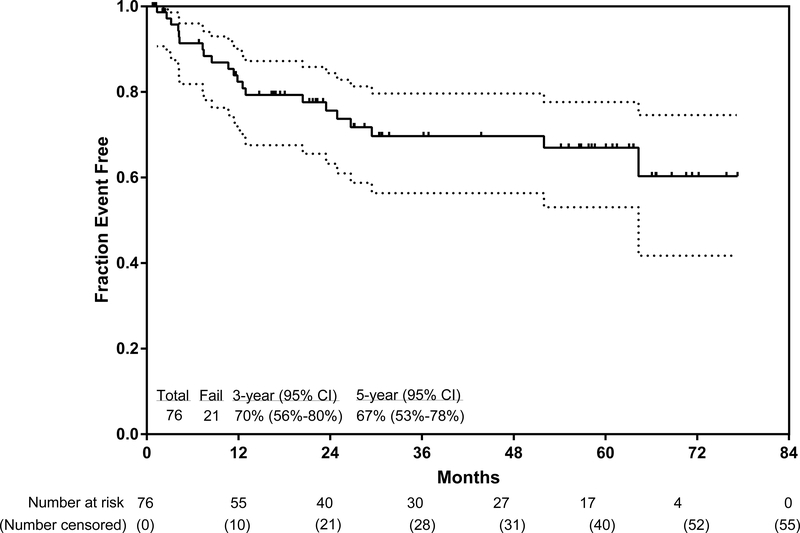
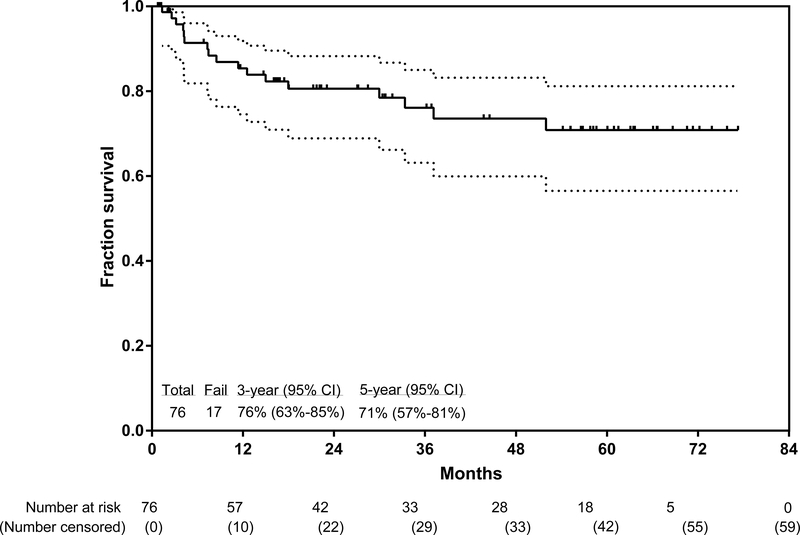
The median follow-up since the start of the trial was 36 months (IQR 22–63). Among the 59 patients who were alive at the time of this analysis (May 5, 2018), 37 (63%) were followed up for more than 2 years. The longest follow-up was 77·3 months. 55 (72%) of 76 patients are in continuous complete remission at the time of this analysis, for a 3-year continuous complete remission of 83% (95% CI 69–91), 3-year event-free survival of 70% (56–80; figure 2A), and 3-year overall survival of 76% (63–85; figure 2B). The 5-year continuous complete remission was 83% (95% CI 69–91), 5-year continuous event-free survival was 67% (53–78), and 5-year overall survival was 71% (57–81). In a post-hoc analysis, the 3-year overall survival for patients with a complete molecular response at 3 months follow-up was 81% (95% CI 64–91), and for patients without at the same timepoint was 72% (41–88; p=0·27). In a post-hoc analysis, 3-year overall survival did not differ between patients aged 59 years or younger and those aged 60 years or older (p=0·90, appendix p4).
Figure 2: Kaplan-Meier plot of event-free survival (A) and overall survival (B).
The lighter shade indicates 95% CI.
Eight patients (11%) relapsed after a median of 21 months (IQR 13–26) in remission. Three patients relapsed while on ponatinib after 13, 24, and 26 months from the start of therapy; two patients had an Glu255Lys kinase domain mutation at the time of relapse. The Thr315Ile mutation was not detected in any of these three patients. Four patients relapsed after switching to a different tyrosine-kinase inhibitor, which was allowed at the time of the August, 2014, amendment because of concerns about the vascular events encountered in the ponatinib trials. At the time of relapse, these four patients were on dasatinib (n=2), imatinib (n=1), and nilotinib (n=1); three of the four had been in complete molecular response at the time of switch. They relapsed after a median of 22 months from diagnosis (range 11–65, IQR 20–24) and 19 months (range 6–41, IQR 19–19) from tyrosine-kinase inhibitor switch. At the time of failure, a Thr315Ile kinase domain mutation was found in two of the four patients on alternative tyrosine-kinase inhibitors (one on dasatinib and one on imatinib). One patient relapsed while not on a tyrosine-kinase inhibitor, and stopped ponatinib after 7·5 months and relapsed 4 months later. In addition to the eight patients with morphological relapse, one patient had a molecular relapse with BCR-ABL1 transcripts of 0·08%. This patient was taken off study, treated with a combination of cytotoxic chemotherapy plus ponatinib, regained complete molecular response, and was alive 36 months later without allogeneic stem cell transplantation.
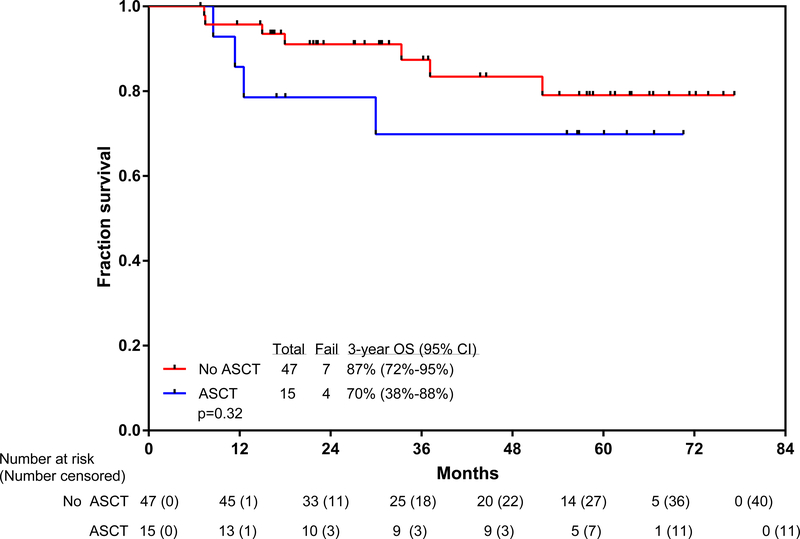
Overall, 15 patients (20%) underwent allogeneic stem cell transplantation while in first complete remission (nine with major molecular response and six with complete molecular response before allogeneic stem cell transplantation) after a median of 6 months (IQR 4–8) from the start of therapy. The donor was matched and related for eight patients, matched and unrelated for five patients, and haploidentical for two patients. After transplant, continuation of tyrosine-kinase inhibitor was at the discretion of the physician. Five patients continued ponatinib, three received dasatinib, two received imatinib, one received nilotinib, and four discontinued tyrosine-kinase inhibitors. 11 patients who received transplants were alive and disease-free after transplantation. Four patients died after stem cell transplantation: one from relapsed and progressive disease and three from stem cell transplantation-related complications. A post-hoc 6-month landmark analysis, done to assess the effect of stem cell transplantation, did not show a difference in overall survival by whether or not patients received a subsequent allogeneic stem cell transplantation (figure 3). The 3-year overall survival was 70% (95% CI 38–88) for patients who received allogenic stem cell transplantation and 87% (72–95) for patients who did not receive stem cell transplantation (hazard ratio [HR]=0·54, 95% CI 0·13–2·17; p=0·32).
Figure 3: Kaplan-Meier plot of overall survival with or without allogeneic stem cell transplantation.
ASCT=allogeneic stem cell transplantation.
All 76 patients were evaluable for safety analyses. Overall, patients received a median of seven cycles of intensive chemotherapy (IQR 4–8). At the time of the amendment (August, 2014), 11 of the 37 patients enrolled switched to other tyrosine-kinase inhibitors (eight to dasatinib, two to imatinib, and one to nilotinib) in response to safety amendment at investigator and patient discretion. The remaining patients continued ponatinib therapy, but with dose modification as described in the amendment (eg, 15 mg for patients in complete molecular response and 30 mg for patients in complete remission but not complete molecular response).
Most adverse events were grades 1–2 (table 3). The most common grade 3 or 4 adverse events were infection (n=65, 86%), increased transaminases (n=24, 32%), increased bilirubin (n=13, 17%), pancreatitis (n=13, 17%), hypertension (n=12, 16%), bleeding (n=10, 13%), and skin rash (n=9, 12%). Nine patients (12%) died while in complete remission (six patients died while still on study treatment; appendix); seven of the deaths were unrelated to ponatinib. Three patients died from sepsis (cycle 2, day 13; cycle 5, day 21; and cycle 6, day 77); the patient who died at cycle 6, day 77 also had haemophagocytic lymphohistiocytosis. In a post-hoc analysis, four of the 20 patients aged at least 60 years (20%) died in complete remission, compared with five of 56 patients younger than age 60 years (9%; p=0·16).
Table 3:
Treatment-related toxicities during induction and consolidation chemotherapy cycles regardless of causality
| Any grade | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|---|
|
| |||||
| Increased aspartate aminotransferase or alanine aminotransferase | 71 (93%) | 47 (62%) | 21 (27%) | 3 (4%) | 0 |
| Infections overall | 68 (89%) | 0 | 58 (76%) | 7 (9%) | 3 (4%) |
| Infections during induction | 36 (47%) | 0 | 35 (46%) | 1 (1%) | 0 |
| Increased bilirubin | 58 (76%) | 45 (59%) | 10 (13%) | 3 (4%) | 0 |
| Skin rash | 56 (74%) | 47 (62%) | 9 (12%) | 0 | 0 |
| Nausea | 51 (67%) | 43 (57%) | 8 (10%) | 0 | 0 |
| Peripheral neuropathy | 50 (66%) | 48 (63%) | 2 (3%) | 0 | 0 |
| Constipation | 49 (64%) | 68 (63%) | 1 (1%) | 0 | 0 |
| Headache | 49 (64%) | 44 (58%) | 5 (6%) | 0 | 0 |
| Diarrhoea | 48 (63%) | 40 (53%) | 8 (10%) | 0 | 0 |
| Hypertension | 38 (50%) | 26 (34%) | 12 (16%) | 0 | 0 |
| Abdominal pain | 35 (46%) | 30(39%) | 5 (7%) | 0 | 0 |
| Anorexia | 33 (43%) | 29 (38%) | 4 (5%) | 0 | 0 |
| Vomiting | 33 (43%) | 29 (38%) | 4 (5%) | 0 | 0 |
| Bleeding | 31 (41%) | 20 (26%) | 10 (13%) | 0 | 1 (1%) |
| Mucositis | 27 (36%) | 21 (28%) | 6 (8%) | 0 | 0 |
| Dyspnoea | 25 (33%) | 22 (29%) | 3 (4%) | 0 | 0 |
| Sinus tachycardia | 22 (29%) | 22 (29%) | 0 | 0 | 0 |
| Increased amylase or lipase | 19 (25%) | 6 (8%) | 7 (9%) | 6 (8%) | 0 |
| Visual changes | 18 (24%) | 18 (24%) | 0 | 0 | 0 |
| Sinus bradycardia | 17 (22%) | 17 (22%) | 0 | 0 | 0 |
| Dry skin | 17 (22%) | 17 (22%) | 0 | 0 | 0 |
| Pancreatitis | 16 (21%) | 3 (4%) | 12 (16%) | 1 (1%) | 0 |
| Pleural effusion | 13 (17%) | 13 (17%) | 0 | 0 | 0 |
| Pericardial effusion | 10 (13%) | 9 (12%) | 1 (1%) | 0 | 0 |
| Venous thrombotic events | 10 (13%) | 7 (9%) | 1 (1%) | 2 (3%) | 0 |
| Atrial fibrillation | 9 (12%) | 5 (7%) | 4 (5%) | 0 | 0 |
| Renal injury | 5 (7%) | 3 (4%) | 2 (3%) | 0 | 0 |
| Arterial cardiovascular events* | 5 (7%) | 1 (1%) | 2 (3%) | 0 | 2 (3%) |
| Acute respiratory distress syndrome | 2 (3%) | 0 | 1 (1%) | 1 (1%) | 0 |
Data are n (%).
Includes two fatal ponatinib-related myocardial infarctions, two episodes of unstable angina (one in the context of severe sepsis), and one episode of stable angina.
Data are n (%).
The cumulative incidence of arterial cardiovascular events is shown in the appendix (p 5). Two patients, both of whom had no known cardiovascular risk factors, died from arterial vascular events possibly related to ponatinib. These patients were two of the nine who died in complete remission. The first patient was aged 37 years, was receiving 45 mg of ponatinib per day, and had a non-ST elevation myocardial infarction on cycle 2, day 41. The second patient was aged 54 years, was receiving 30 mg of ponatinib per day, and had unexplained chest pain at cycle 4, day 42 that preceded death. This was the second death related to myocardial infarction. Both patients died before the protocol amendment in which risk-adapted ponatinib dosing was implemented. A third patient, aged 79 years, died in complete remission, from myocardial infarction 40 months after being off study because of poor adherence to treatment visits.
After the protocol was amended (August, 2014), two patients with several cardiovascular risk factors had a cardiovascular event. One patient had grade 2 stable angina; this patient had been on ponatinib for 42 months at the time of the event (at a dose of 15 mg for the preceding 35 months) and was subsequently switched to 300 mg of imatinib per day. The other patient had grade 3 unstable angina requiring stent placement after 5 years of ponatinib; this patient had been on 30 mg of ponatinib per day and was switched to 50 mg of dasatinib per day after the event.
Significant venous thromboembolic events were observed in ten patients (13%; appendix p 3). The types of venous thromboembolic events included: two pulmonary embolisms, one renal vein thrombosis, and seven deep venous thromboembolisms. Four of the seven deep venous thromboembolisms were line-associated upper extremity venous thromboembolic events. 38 patients (50%) had hypertension; 12 of the episodes were grade 3. Among the patients who had hypertension, 26 (68%) had no previous history of hypertension.
14 patients developed pancreatitis during the study, and pancreatitis events totalled 16. One patient developed grade 4 pancreatitis. 13 of 14 patients continued ponatinib therapy after developing pancreatitis; one patient was switched to dasatinib after the first episode of pancreatitis. Two patients developed recurrent pancreatitis after dose reduction (the recurrence episode occurred after 11 additional cycles in one patient and 22 additional cycles in the other patient). In both patients with recurrent pancreatitis, ponatinib was switched to dasatinib without further recurrence.
After the August, 2014, protocol amendment, the number of several grade 3 or 4 adverse events decreased, including elevation of aspartate transaminase or alanine aminotransferase (14 [38%] vs 10 [26%]), elevated bilirubin (9 [24%] vs 4 [10%]), rash (7 [19%] vs 2 [5%]), and venous thromboembolic events [3 (8%) vs 0]. The numbers of grade 3 and 4 pancreatitis and hypertension event were similar before and after study amendment (six patients with each adverse event before and after amendment). Overall, 28 patients (37%) required dose reductions because of adverse events; the most common causes included rash (n=7), liver function test abnormalities (n=5), pancreatitis (n=3), deepvenous thromboembolisms (n=2), and thrombocytopenia (n=2).
Discussion
In this phase 2 study, durable remissions and long-term survival were achieved with the combination of hyper-CVAD with ponatinib. The overall proportion of patients who achieved a major molecular response was 97%, and 83% achieved a complete molecular response. The combination of hyper-CVAD and ponatinib was found to be safe with no fatal cardiovascular toxicities when ponatinib was used at low risk-adapted doses of 30 mg and 15 mg per day. The 3-year overall survival was 76%, with no difference in overall survival, irrespective of whether patients were censored at the time of stem cell transplantation.
After a 3-year follow-up, only three patients on ponatinib had relapsed, with the Glu255Lys mutation detected in two patients and the Thr315Ile mutation not detected at the time of relapse. This is a substantial improvement compared with previous studies with first-generation and second-generation tyrosine-kinase inhibitors, and indicates the long-term ability of ponatinib to suppress resistant Thr315Ile clones.1–3,5,11–13,16 Previous studies17,24 have suggested the Glu255Lys mutation might confer an intermediate level of resistance to ponatinib, which might explain the relapses in the present study. One patient was found to not have a BCRABL1 kinase domain mutation after relapsing on ponatinib, suggesting a non-BCR-ABL1-dependent mechanism of resistance. Future studies are needed to evaluate mechanisms of relapse for patients with Philadelphia chromosome-positive leukaemias treated with ponatinib-based regimens.
With a median follow-up of 36 months, the 3-year complete remission was 83%, event-free survival was 70%, and overall survival was 76%. These results compare favourably with previous studies in similar patients treated with hyper-CVAD and imatinib or dasatinib.2–3,5 Although no randomised studies have compared different tyrosine-kinase inhibitor regimens in Philadelphia chromosome-positive acute lymphoblastic leukaemia, a propensity score-matched analysis comparing a prospective phase 2 trial25 of hyper-CVAD plus dasatinib with an earlier dataset of this hyper-CVAD plus ponatinib study showed the ponatinib cohort to be superior (2-year event-free survival of 76% vs 49%, p=0·04; 2-year overall survival of 83% vs 61%, p=0·03). The superior outcomes with ponatinib were probably driven by the higher complete molecular response with this regimen—a response endpoint strongly associated with improved survival in Philadelphia chromosome-positive acute lymphoblastic leukaemia.4,11–13,15
A phase 2 trial (GIMEMA LAL1811)26 of 45 mg of ponatinib per day plus steroids in 42 patients (median age 67 years) with newly diagnosed Philadelphia chromo-some-positive acute lymphoblastic leukaemia, reported a complete molecular response of 40% and an estimated 2-year survival of 62%. These results, albeit in an older population, are inferior to those obtained in our study, suggesting a potential benefit for a more intensive approach in patients able to tolerate it. Furthermore, 13 serious adverse events (31%) including two deaths related to ponatinib occurred in GIMEMA LAL1811. This number of events is more than expected, and is possibly due to the higher dose of ponatinib administered (45 mg per day) than that used in our study. Low-intensity regimens in which corticosteroids or low-intensity chemotherapy plus risk-adapted dosing of ponatinib (as used in our study), or both, are used might result in improved outcomes by decreasing adverse events related to ponatinib and allowing more patients to remain on continuous tyrosine-kinase inhibitor therapy.
Overall, an increased incidence of hypertension and vascular side-effects were noted. The major safety concern of ponatinib is the development of vascular occlusive events, which were reported at a cumulative rate of 27% in the PACE study18,19 of patients with relapsed or refractory Philadelphia chromosome-positive leukaemia. These events have been observed with all tyrosine-kinase inhibitors, although at different frequencies.27 Although two myocardial infarctions were encountered at the initial dose of ponatinib, the use of a lower dose of ponatinib (median dose of 15 mg per day) reduced the incidence of vascular events.
Allogeneic stem cell transplantation has improved the outcome of patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia in the pre-tyrosine-kinase inhibitor era and in some studies using first-generation1,11 or second-generation3 tyrosine-kinase inhibitors. However, suitable candidates for this procedure in first complete remission are debated. No randomised studies of transplant versus no transplant in patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia exist; therefore, the decision to pursue transplant is often dependent on the physician and institution. In an analysis15 of patients treated with chemotherapy and different tyrosine-kinase inhibitors, who did not undergo allogeneic stem cell transplantation, the achievement of 3-month complete molecular response was the only favourable prognostic factor for overall survival (hazard ratio 0·42, p=0·01) by multivariate analysis. Patients who achieved complete molecular response by 3 months had a favourable 4-year overall survival of 66%, despite not undergoing allogeneic stem cell transplantation in first remission. Therefore, the use of a ponatinib-based regimen, through the improvement of the 3-month complete molecular response (73% in our study), might reduce the need for consolidation with allogeneic stem cell transplantation. More sensitive techniques to detect low levels of minimal residual disease (eg, next-generation sequencing) might further improve the identification of patients in whom allogenic stem cell transplantation in first remission can be safely deferred.
Several investigators reported underlying genomic lesions in Philadelphia chromosome-positive acute lymphoblastic leukaemia that substantially influence outcomes, such as deletions of IKZF1 and CDKN2A/B.28–31 However, these studies were done in patients receiving first-generation or second-generation tyrosine-kinase inhibitors (eg, imatinib or dasatinib). Baseline Thr315Ile mutations have also been detected in up to 23% of patients aged 55 years or older with Philadelphia chromosome-positive acute lymphoblastic leukaemia in one study.12 Patients who have Thr315Ile mutations identified in pretreatment samples would be the most appropriate candidates for therapy with a ponatinib-based regimen, because these patients are highly likely to relapse.2,12 Comprehensive genomic profiling was not done in the present study; therefore, the effect of these alterations on outcomes in patients treated with hyper-CVAD plus ponatinib cannot be assessed. However, given the low proportion of patients who relapsed on ponatinib (three [4%] of 76 patients), it is possible that ponatinib might overcome the negative prognostic effect of these genomic changes. Correlative genomic analyses are currently being done to test this hypothesis.
The study has several limitations. First, this is a single-arm phase 2 study; thus, our findings need to be confirmed in future work. Confirmation of our previous results with intensive chemotherapy and dasatinib was reported by Ravandi and colleagues.3 Second, although the median follow-up was 36 months (IQR 22–63), some of the patients reported were recently enrolled with short follow-up (ie, <1 year). Among the 59 patients who were alive at the time of analyses, 37 (63%) had been followed up for more than 2 years. Third, although we are reporting high long-term efficacy, questions about the long-term safety of this combination are yet to be addressed. This regimen could also potentially be improved. With the use of an intensive chemotherapy backbone, the number of deaths in complete remission is similar to previous studies using chemotherapy alone in patients with Philadelphia-negative acute lymphoblastic leukaemia or the combination of chemotherapy and dasatinib in similar patient populations.2
Methods to deintensify the regimen, but maintain efficacy, would probably improve the long-term survival of patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia. A less intensive chemotherapy regimen, as delivered by Chalandon and colleagues11 and Rousselot and colleagues,12 might reduce the number of deaths in complete remission and, as such, improve long-term survival.
In the phase 2 ALCANTARA study,32 blinatumomab was shown to be active in heavily pretreated, relapsed, or refractory Philadelphia chromosome-positive acute lymphoblastic leukaemia, with a complete remission or complete remission with incomplete haematological recovery of 36% and median overall survival of 7·1 months (95% CI 5·6–not estimable). Blinatumomab was safely combined with ponatinib in retrospective series of patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukaemia or chronic myeloid leukaemia in lymphoid blast phase.33 Among ten patients treated for morphological relapse and ten patients treated for minimal residual disease relapse, the overall complete molecular response rate of blinatumomab plus a tyrosine-kinase inhibitor was 65%; the median survival was 14 months, and the 1-year overall survival was 75%.33 A phase 2 study is currently ongoing to evaluate the combination of blinatumomab and ponatinib as first-line therapy for patients and 65 years or older with Philadelphia chromosome-positive acute lymphoblastic leukaemia or patients of any age with relapsed or refractory disease (NCT03263572). The combination of low-intensity chemo-therapy with ponatinib and blinatumomab as first-line therapy for adults with Philadelphia chromosome-positive acute lymphoblastic leukaemia is also planned, with the hypothesis that the addition of blinatumomab might further improve complete molecular responses by eradicating low levels of minimal residual disease.
In summary, combining chemotherapy with ponatinib in patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia is safe and effective. The regimen resulted in sustained responses, with 83% of patients achieving a complete molecular response rate and 3-year overall survival of 76%. Among responding patients, long-term survival was not affected by allogeneic stem cell transplantation. New strategies, including dose titration of ponatinib, optimal control of vascular risk factors, and the addition of new monoclonal antibodies might further improve outcomes.
Supplementary Material
Research in context.
Evidence before this study
A systemic review was not done before this trial was designed; however, we searched PubMed for studies on the outcomes of adults with Philadelphia chromosome-positive acute lymphoblastic leukaemia and clinical trials in this population without date or language restrictions. Tyrosine-kinase inhibitors, in combination with chemotherapy, are a primary therapy for Philadelphia chromosome-positive acute lymphoblastic leukaemia. Unfortunately, many malignancies acquire resistance to most tyrosine-kinase inhibitors through the Thr315Ile mutation in the BCR-ABL1 fusion protein. We addressed this clinical issue by combining chemotherapy with the tyrosine-kinase inhibitor, ponatinib, which can effectively target both wild-type and Thr315Ile BCR-ABL1. Resistance mechanisms to ponatinib are not well understood.
Added value of this study
We previously reported the high efficacy and manageable toxicity of the combination of ponatinib with the hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) regimen as a first-line treatment of Philadelphia chromosome-positive acute lymphoblastic leukaemia. Important questions are the durability of responses, and whether the regimen can obviate the need for allogeneic stem cell transplantation. We report the long-term safety and efficacy of the hyper-CVAD and ponatinib combination regimen.
Implications of all the available evidence
The combination of chemotherapy with ponatinib is highly effective in achieving long-term remission in patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukaemia. Knowledge of the long-term efficacy and safety results of this combination might help to establish a new standard of care for patients with this disease.
Acknowledgments
Funding Takeda Oncology.
EJ, NJS, FR, JC, and HK received research grants from ARIAD/Takeda Oncology. EJ, NJS, FR, and GG-M received consulting fees from ARIAD/Takeda Oncology. ARIAD Pharmaceuticals/Takeda Oncology provided free drug and financial support.
Footnotes
Declaration of interests
All other authors declare no competing interests.
Data sharing
Patient-level data will not be shared.
Contributor Information
Elias Jabbour, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Nicholas J Short, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Farhad Ravandi, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Xuelin Huang, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Naval Daver, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Courtney D DiNardo, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Marina Konopleva, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Naveen Pemmaraju, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
William Wierda, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Guillermo Garcia-Manero, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Koji Sasaki, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Jorge Cortes, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Rebecca Garris, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Joseph D Khoury, Department of Hematopathology, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Jeffrey Jorgensen, Department of Hematopathology, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Nitin Jain, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Joie Alvarez, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
Susan O’Brien, Chao Family Comprehensive Cancer Center, University of California, Irvine, Orange, CA, USA.
Hagop Kantarjian, Department of Leukemia, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
References
- 1.Fielding AK, Rowe JM, Buck G, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood 2014; 123: 843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravandi F, O’Brien S, Thomas D, et al. First report of phase II study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood 2010; 116: 2070–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravandi F, Othus M, O’Brien S, et al. US intergroup study of chemotherapy plus dasatinib and allogeneic stem cell transplant in Philadelphia chromosome positive ALL. Blood Adv 2016; 1: 250–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz KR, Bowman WP, Heerema NA, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031. Leukemia 2014; 28: 1467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daver N, Thomas D, Ravandi F, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica 2015; 100: 653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanguy-Schmidt A, Rousselot P, Chalandon Y, et al. Long-term follow up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: a GRAALL study. Biol Blood Marrow Transplant 2013; 19: 150–55. [DOI] [PubMed] [Google Scholar]
- 7.Bassan R, Rossi G, Pogliani EM, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: northern Italy Leukemia Group protocol 09/00. J Clin Oncol 2010; 28: 3644–52. [DOI] [PubMed] [Google Scholar]
- 8.Pfeifer H, Goekbuget N, Volp C, et al. Long-term outcome of 335 patients receiving different schedules of imatinib and chemotherapy as front-line treatment for Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood 2010; 116: abstract 173. [Google Scholar]
- 9.Wassmann B, Pfeifer H, Goekbuget N, et al. Alternating versus concurrent schedules of imatinib and chemotherapy as front-line therapy for Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood 2006; 108: 1469–77. [DOI] [PubMed] [Google Scholar]
- 10.Dombret H, Gabert J, Boiron JM, et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia—results of the prospective multicenter LALA-94 trial. Blood 2002; 100: 2357–66. [DOI] [PubMed] [Google Scholar]
- 11.Chalandon Y, Thomas X, Hayette S, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood 2015; 125: 3711–19. [DOI] [PubMed] [Google Scholar]
- 12.Rousselot P, Coudé MM, Gokbuget N, et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood 2016; 128: 774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiaretti S, Vitale A, Elia L. Multicenter total therapy Gimema LAL 1509 for de novo adult Ph+ acute lymphoblastic leukemia (ALL) patients. Updated results and refined genetic-based prognostic stratification. Blood 2015; 126: 81 (abstr). [Google Scholar]
- 14.Jabbour E, Kantarjian H, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-centre, phase 2 study. Lancet Oncol 2015; 16: 1547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Short N, Jabbour E, Sasaki K, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 2016; 128: 504–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foa R, Vitale A, Vignetti M, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 2011; 118: 6521–28. [DOI] [PubMed] [Google Scholar]
- 17.O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell 2009; 16: 401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med 2013; 369: 1783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood 2018; 132: 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Criteria Committee of the New York Heart Association. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th edn. Little, Brown & Co: Boston, MA, 1994: 253–56. [Google Scholar]
- 21.Thomas DA, O’Brien S, Faderl S, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol 2010; 28: 3880–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luthra R, Sanchez-Vega B, Medeiros LJ. TaqMan RT-PCR assay coupled with capillary electrophoresis for quantification and identification of Bcr-Abl transcript type. Mod Pathol 2004; 17: 96–103. [DOI] [PubMed] [Google Scholar]
- 23.Ravandi F, Jorgensen JL, O’Brien SM, et al. Minimal residual disease assessed by multi-parameter flow cytometry is highly prognostic in adult patients with acute lymphoblastic leukaemia. Br J Haematol 2016; 172: 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki Jabbour E, Ravandi F, et al. Hyper-CVAD + ponatinib vs. hyper-CVAD + dasatinib as frontline therapy for Ph-positive ALL: a propensity score analysis. Cancer 2016; 122: 3650–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Hare T, Pollock R, Stoffregen EP, et al. Inhibition of wild-type and mutant Bcr-Abl by AP23464, a potent ATP-based oncogenic protein kinase inhibitor: implications for CML. Blood 2004; 104: 2532–39. [DOI] [PubMed] [Google Scholar]
- 26.Martinelli G, Piciocchi A, Papayannidis C, et al. First report of the Gimema LAL1811 phase II prospective study of the combination of steroids with ponatinib as frontline therapy of elderly or unfit patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 2017; 130: 99. [Google Scholar]
- 27.Valent P, Hadzijusufovic E, Schernthaner G, et al. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood 2015; 125: 901–06. [DOI] [PubMed] [Google Scholar]
- 28.Martinelli G, Iacobucci I, Storlazzi CT, et al. IKZF1 (Ikaros) deletions in BCR-ABL1-positive acute lymphoblastic leukemia are associated with short disease-free survival and high rate of cumulative incidence of relapse: a GIMEMA AL WP report. J Clin Oncol 2009; 27: 5202–07. [DOI] [PubMed] [Google Scholar]
- 29.Iacobucci I, Ferrari A, Lonetti A, et al. CDKN2A/B alterations impair prognosis in adult BCR-ABL1-positive acute lymphoblastic leukemia patients. Clin Cancer Res 2011; 17: 7413–23. [DOI] [PubMed] [Google Scholar]
- 30.Pfeifer H, Raum K, Markovic S, et al. Genomic CDKN2A/2B deletions in adult Ph(+) ALL are adverse despite allogeneic stem cell transplantation. Blood 2018; 131: 1464–75. [DOI] [PubMed] [Google Scholar]
- 31.Fedullo AL, Messina M, Vitale A, et al. Prognostic impact of additional molecular lesions in Ph+ acute lymphoblastic leukemia (ALL) . European Hematology Association; Madrid, Spain; June 22–25, 2017. Abstr S800. [Google Scholar]
- 32.Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase ii, single-arm, multicenter study. J Clin Oncol 2017; 35: 1795–802. [DOI] [PubMed] [Google Scholar]
- 33.Assi R, Kantarjian HM, Short NJ, et al. Safety and efficacy of blinatumomab in combination with a tyrosine kinase inhibitor for the treatment of relapsed Philadelphia chromosome-positive leukemia. Blood 2017; 130 (suppl 1): 2598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.