Key Points
Question
What is the association between the addition of pembrolizumab to ifosfamide, carboplatin, and etoposide chemotherapy and response rates of patients with relapsed or refractory classic Hodgkin lymphoma who are destined for an autologous stem cell transplant?
Findings
In this single-group, phase 2, nonrandomized clinical trial of 42 patients with relapsed or refractory classic Hodgkin lymphoma, pembrolizumab with ifosfamide, carboplatin, and etoposide was associated with a significant improvement in the complete response rate to 86.5% as determined by 18F-fluorodeoxyglucose–positron emission tomography with computed tomography.
Meaning
Findings suggest that concurrent treatment with a checkpoint inhibitor and conventional chemotherapy was associated with improved outcomes in this patient population.
Abstract
Importance
To our knowledge, this is the first clinical trial designed to investigate concurrent treatment with a checkpoint inhibitor and conventional chemotherapy in relapsed or refractory classic Hodgkin lymphoma in patients destined for an autologous stem cell transplant.
Objective
To evaluate the complete response rate as assessed by 18F-fluorodeoxyglucose–positron emission tomography with computed tomography (FDG-PET/CT) after salvage therapy for patients with relapsed or refractory classic Hodgkin lymphoma.
Design, Setting, and Participants
A single-group, phase 2, multi-institutional nonrandomized clinical trial to evaluate the addition of pembrolizumab to ifosfamide, carboplatin, and etoposide (ICE) chemotherapy was conducted from April 20, 2017, to October 29, 2020, at 5 US sites. The 42 patients were aged 18 years or older, with an Eastern Cooperative Oncology Group Performance Status Scale score of 0 or 1 and biopsy-proven relapsed or refractory classic Hodgkin lymphoma after 1 or 2 prior lines of chemotherapy. Patients were required to be appropriate candidates for transplant, with measurable lesions detected by FDG-PET/CT.
Interventions
Two cycles of pembrolizumab (200 mg intravenously on day 1) with ICE chemotherapy every 21 days, followed by stem cell mobilization and collection, and then 1 cycle of pembrolizumab monotherapy followed by FDG-PET/CT response assessment.
Main Outcomes and Measures
The primary end point was complete response rate detected by FDG-PET/CT, defined as a Deauville score of 3 or lower. Patients with a complete response proceeded to an autologous stem cell transplant. Secondary end points included progression-free survival, overall survival, stem cell mobilization, and neutrophil and platelet engraftment. Adverse events were monitored to assess safety.
Results
Forty-two patients were enrolled, with 37 evaluable for the primary end point. The median age was 34 years (range, 19-70 years), 25 patients were female (68%), 6 were African American (16%), and 26 were White (70%). The complete response rate for the 37 patients assessed by FDG-PET/CT imaging was 86.5% (95% CI, 71.2%-95.5%); the overall response rate was 97.3% (36 patients), with 10.8% partial responses (4 patients). New areas of FDG-PET positivity in 2 patients were biopsied, showing noncaseating granuloma in 1 case and a reactive lymph node in a second. Progression-free survival and overall survival 2-year estimates were 87.2% (32 patients; 95% CI, 77.3%-98.3%) and 95.1% (95% CI, 88.8%-100%), respectively. The addition of pembrolizumab to ICE chemotherapy did not negatively affect stem cell mobilization or collection or engraftment, similar to prior experience in this patient population and setting.
Conclusions and Relevance
Results suggest that the addition of pembrolizumab to ICE chemotherapy was well tolerated and highly effective in comparison with prior reports of chemotherapy-only regimens, supporting further investigation in patients with relapsed or refractory classic Hodgkin lymphoma eligible for an autologous stem cell transplant.
Trial Registration
ClinicalTrials.gov Identifier: NCT03077828
This nonrandomized clinical trial investigates the addition of pembrolizumab to ifosfamide, carboplatin, and etoposide chemotherapy and evaluates the response rate of patients with relapsed or refractory Hodgkin lymphoma.
Introduction
Classic Hodgkin lymphoma (cHL) has a favorable prognosis, and most patients reach a durable complete remission with frontline therapy. However, approximately 10% to 15% of patients with early-stage disease and 15% to 30% of those with advanced-stage disease relapse, and an additional 10% to 15% will never achieve remission and have refractory disease.1,2,3,4 Subsequent therapy for both groups typically includes a second-line chemotherapy regimen followed by an autologous hematopoietic stem cell transplant (AHSCT) for patients who are transplant eligible. An AHSCT has remained a standard approach to relapsed or refractory cHL, with curative intent based on prior randomized studies.5,6
Collectively, conventional salvage chemotherapy regimens result in high overall response rates, but complete response (CR) rates are modest. To our knowledge, there are no phase 2 or 3 randomized trials comparing the commonly used salvage chemotherapy regimens. Second-line regimens are typically platinum-based or gemcitabine-based combinations. The combination of ifosfamide, carboplatin, and etoposide (ICE) is the preferred second-line regimen for patients with cHL at many institutions in the US.7
Functional imaging with 18F-fluorodeoxyglucose–positron emission tomography with computed tomography (FDG-PET/CT) has been established as a powerful predictor of post-AHSCT outcomes, including both progression-free survival (PFS) and overall survival (OS).8,9,10,11,12,13 Currently, the goal of salvage chemotherapy in cHL is to reach a CR as assessed by the 5-point Deauville score (FPS) criteria before proceeding to AHSCT.14 The CR rate for standard-dose salvage ICE chemotherapy as assessed by gallium scanning or FDG-PET/CT is no better than approximately 50%.15,16,17 Emerging second-line treatment regimens that include brentuximab vedotin, an antibody-drug conjugate that targets CD30, have resulted in higher CR rates than previously reported, but its increasing use in the frontline setting makes it an inappropriate choice for patients with relapsed or refractory disease.4 Alternative second-line strategies are needed.
Pembrolizumab and nivolumab are anti–programmed cell death protein 1 (PD-1) monoclonal antibodies that have demonstrated effectiveness in patients with heavily treated relapsed or refractory classic Hodgkin lymphoma. In relapsed or refractory cases, nivolumab monotherapy resulted in an overall response rate of 69%, including 16% of patients with CR, whereas pembrolizumab monotherapy showed a similar overall response rate of 72% with a CR rate of 27.6%.18,19 Combinations of checkpoint inhibitors (CPIs) and cytotoxic chemotherapy have proven to be safe.20,21 We therefore hypothesized that the addition of pembrolizumab to conventional second-line ICE chemotherapy for patients with relapsed or refractory cHL would improve complete remission rates and overall outcomes for patients destined for AHSCT.
To our knowledge, this phase 2 study was the first of its kind designed to investigate concurrent pembrolizumab and chemotherapy for patients who are transplant eligible with relapsed or refractory cHL. We report the safety, tolerability, and effectiveness of combined pembrolizumab and ICE chemotherapy with a median follow-up of 24 months (range, 0.5-35.4 months).
Methods
Study Design and Patients
This single-group, phase 2, multi-institutional nonrandomized clinical trial evaluated the addition of pembrolizumab to ICE chemotherapy for patients who were AHSCT eligible with relapsed or refractory cHL (NCT03077828) (trial protocol in Supplement 1). The study completed accrual from April 20, 2017, through October 29, 2020, at 5 US sites. Eligibility criteria included patients aged 18 years or older, an Eastern Cooperative Oncology Group Performance Status Scale score of 0 or 1, and biopsy-proven relapsed or refractory cHL after 1 or 2 lines of chemotherapy. Patients were candidates for AHSCT with measurable lesions detected by FDG-PET/CT. Eligible patients had adequate organ function (absolute neutrophil count >1000/μL, platelet count >75 × 103/μL, hemoglobin level >7 g/dL [to convert to grams per liter, multiply by 10.0], total bilirubin level ≤2 × upper limit of normal, aspartate aminotransferase/alanine aminotransferase level ≤2.5 × upper limit of normal, and creatinine clearance >30 mL/min/1.73 m2 [to convert to milliliters per second per meters squared, multiply by 0.0167]). Exclusion criteria included patients with prior exposure to anti–PD-1 or anti–PD ligand 1 agents, known central nervous system involvement, active HIV, hepatitis B or C, or history of autoimmune disease. The protocol and amendments were approved by the institutional review boards at all participating institutions (Northwestern University Institutional Review Board, Loyola University Chicago Institutional Review Board, Augusta University Institutional Review Board, Emory University Institutional Review Board, and University of Rochester Research Subject Review Board). All patients provided written informed consent before enrollment. This study followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline.
Treatment
Patients received combination pembrolizumab and ICE chemotherapy for 2 cycles. Pembrolizumab at 200 mg intravenously was administered on day 1 of each cycle. Standard ICE salvage chemotherapy included ifosfamide at 5 g/m2 with an equivalent dose of mesna given during 24 hours by continuous intravenous infusion on day 2, carboplatin with area under the curve of 5 intravenously with a maximum of 800 mg on day 2, and etoposide at 100 mg/m2/d intravenously on days 1 to 3. Granulocyte colony-stimulating factor support was administered 24 hours after completion of chemotherapy. Cycles were administered every 21 days. Stem cell mobilization and collection were completed after cycle 2 in accordance with institutional protocols, followed by 1 cycle of pembrolizumab monotherapy. Fourteen to 22 days after pembrolizumab monotherapy, FDG-PET/CT (FDG-PET/CT after 2 cycles of combination pembrolizumab and ICE chemotherapy and 1 cycle of pembrolizumab monotherapy will be referred to as PET2 hereafter) was performed to determine metabolic response by the FPS according to Lugano 2014 criteria.22 Baseline and posttreatment FDG-PET/CT scans were reviewed centrally (H.S.), and in the event of disagreement between the local and central reviews, a third review was obtained (G.L.D.). Patients with an FPS of 3 or lower proceeded to AHSCT. An optional additional cycle of combination pembrolizumab and ICE chemotherapy was allowed according to investigator discretion to provide flexibility in scheduling the AHSCT. Patients with FPS higher than 3 were treated off study at investigator discretion. Initiation of conditioning chemotherapy for the AHSCT was required 21 to 42 days from the last cycle of treatment. The AHSCT conditioning regimen, use of radiation, and post-AHSCT maintenance therapy were not dictated by the study protocol.
Patients had follow-up visits at 30, 60, 90, and 180 days after transplant. Thereafter, they were followed up every 3 months for up to 2 years and then annually for 2 years. Toxic effects were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.23
Statistical Analysis
The primary end point of this phase 2 trial was rate of CR detected by PET2 imaging, defined as an FPS of 3 or lower. The standard response rate was assumed to be 0.50 (null hypothesis) according to prior reports; the new therapy was considered to be worthy of further investigation if the null hypothesis could be rejected in favor of the 1-sided alternative hypothesis that the response rate was at least 0.70. Assuming a 1-sided α (type I error) of .05 and a power of 0.80, the study required a sample size of 37 evaluable patients. For this design and these assumptions, the minimum number of responders was required to be at least 24 to reject the null hypothesis if the true response rate was 0.70.
Patients were eligible for inclusion in the analysis to address the primary end point if they had received at least 1 dose of pembrolizumab and had an FDG-PET/CT scan after cycle 3 (N = 37). The proportion of patients with CR was calculated by dividing the number of responders by the number of evaluable patients and was reported as a percentage with the associated 95% Clopper-Pearson CI for the percentage. Similar analyses were used to calculate the observed overall response rate and associated 95% CI.
Adverse event (AE) frequency, severity (grade), timing, and attribution to pembrolizumab were reported at scheduled times during the trial. Patients were eligible for inclusion in the analysis to describe the AE end points if they had received at least 1 dose of pembrolizumab (n = 42). Frequencies and proportions per patient of the worst grade AEs of any attribution were calculated.
Progression-free survival was defined as the length of time from treatment initiation until first occurrence of disease relapse, progression, or death due to disease or until last contact (censored) if the patient did not experience any of these. Overall survival was defined as time from study registration until death or until last contact (censored) if the patient did not die. Kaplan-Meier curves for PFS and OS were calculated, and point estimates and associated SEs for 2-year PFS and OS estimates were derived with these curves. Data were analyzed from April 20, 2017, to October 29, 2022.
Results
Patient Characteristics
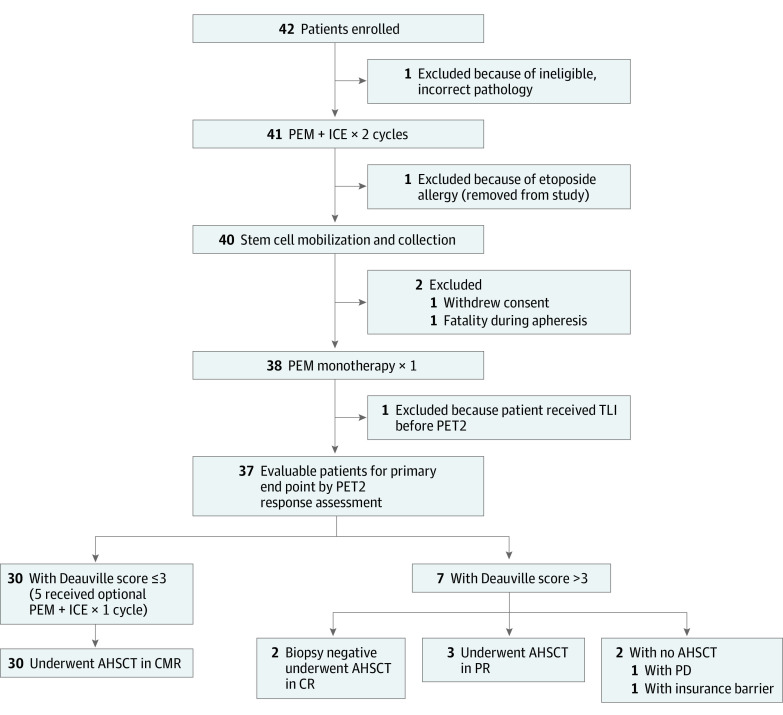
Of the 42 patients enrolled at the 5 participating institutions, 5 ceased protocol or did not have PET2 assessments following the outlined therapy (Figure 1). Of the 37 patients evaluable for our primary end point, the median age was 34 years (range, 19-70 years), 12 were male (32%), 25 were female (68%), 6 were African American (16%), 1 was Asian (3%), 26 were White (70%), and the race and ethnicity of 4 (11%) were unknown or unreported (Table 1). Participants self-reported race and ethnicity to demonstrate diversity of enrolled patients. A total of 16 patients (43%) had primary refractory disease, with an additional 12 patients (32%) having relapsed within 1 year from completing frontline therapy. There were 6 patients (16%) with bulky disease at enrollment in the study. A combination of doxorubicin, bleomycin, vinblastine, and dacarbazine was the frontline therapy for 34 patients (92%).
Figure 1. Study Flow Diagram.
AHSCT indicates autologous hematopoietic stem cell transplant; CMR, complete metabolic response; CR, complete response; ICE, ifosfamide, carboplatin, and etoposide; PD, progressive disease; PEM, pembrolizumab; PET2, 18F-fluorodeoxyglucose–positron emission tomography with computed tomography assessment after 2 cycles of combined PEM and ICE chemotherapy and 1 cycle of PEM monotherapy; PR, partial response; and TLI, total lymphoid irradiation.
Table 1. Baseline Patient Demographic and Clinical Characteristics for Evaluable Patients.
| Characteristic | Patients, No. (%) |
|---|---|
| No. | 37 |
| Age, median (range), y | 34 (19-70) |
| Male | 12 (32) |
| Female | 25 (68) |
| Race and ethnicity | |
| African American | 6 (16) |
| Asian | 1 (3) |
| White | 26 (70) |
| Unknown or not reported | 4 (11) |
| Stage at initial diagnosis | |
| I or II | 17 (46) |
| III or IV | 14 (38) |
| Unknown | 6 (16) |
| Frontline therapy | |
| ABVD and AVD | 34 (92) |
| ABVD and GVDa | 1 (3) |
| ABVD and escBEACOPP | 1 (3) |
| ABVE PC and DECA × 2 | 1 (3) |
| Best response after frontline treatment | |
| Refractory (never with CR) | 16 (43) |
| Early relapse (CR <1 y) | 12 (32) |
| Relapse (CR >1 y) | 9 (24) |
| Ann Arbor stage at enrollment | |
| I or II | 16 (43) |
| III or IV | 21 (57) |
| Bulky disease (>10 cm) | 6 (16) |
| Received third cycle of PEM + ICE (optional) | 5 (14) |
| AHSCT conditioning regimen | |
| No. | 35 |
| BEAM | 25 (71) |
| CCE + TLI | 6 (17) |
| BCV | 3 (9) |
| CyVP + TBI | 1 (3) |
| Radiation therapy | |
| No. | 35 |
| Treatment with AHSCT conditioning | 7 (20) |
| Post-AHSCT consolidation | 4 (11) |
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; ABVE PC, doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide; AHSCT, autologous hematopoietic stem cell transplant; AVD, doxorubicin, vinblastine, and dacarbazine; BCV, busulfan, cyclophosphamide, and etoposide; BEAM, carmustine, etoposide, cytarabine, and melphalan; CCE, carboplatin, cyclophosphamide, and etoposide; CR, complete response; CyVP, cyclophosphamide and etoposide; DECA, dexamethasone, etoposide, cytarabine, and cisplatin; escBEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; GVD, gemcitabine, vinorelbine, and doxorubicin liposomal; ICE, ifosfamide, carboplatin, and etoposide; PEM, pembrolizumab; TBI, total body irradiation; TLI, total lymphoid irradiation.
The only patient to receive 2 lines of therapy before enrollment.
Five patients received the optional additional cycle of pembrolizumab and ICE chemotherapy. Thirty-five of the 37 evaluable patients proceeded to AHSCT. Seven patients had radiation as part of the conditioning regimen, with an additional 4 patients undergoing radiation after AHSCT.
Effectiveness
The CR rate assessed in the 37 patients by PET/CT imaging after 2 cycles of pembrolizumab and ICE chemotherapy and 1 additional cycle of pembrolizumab monotherapy was 86.5% (95% CI, 71.2%-95.5% [32 patients]), meeting our primary end point of improvement over estimated historical outcomes with second-line chemotherapy regimens from 50% to at least 70% (Table 2). In accordance with protocol, only patients who received at least 1 dose of pembrolizumab and had a PET/CT scan to evaluate response were eligible for the primary end point. The PET2 overall response rate was 97.3% (36 patients), with 4 patients (10.8%) having a partial response and 1 patient (2.7%) having progressive disease. The PET2 FPSs were 1 for 45.9% of patients (n = 17), 2 for 27.0% of patients (n = 10), 3 for 8.1% of patients (n = 3), 4 for 10.8% of patients (n = 4), and 5 for 5.4% of patients (n = 2), and X (a new area of uptake unlikely to be related to lymphoma) for 2.7% of patients (n = 1). New areas of PET positivity in 2 cases were biopsied and were included as CRs. For 1 patient, the biopsy showed noncaseating granuloma, whereas the second patient with an Epstein-Barr virus–negative cHL at diagnosis had an Epstein-Barr virus–positive reactive process on biopsy but no evidence of malignancy or cHL. The CR rates of patients with relapsed and refractory cHL did not differ. Of the 42 registered study patients, 40 were evaluable for the PFS analysis, excluding 1 patient with incorrect histology and 1 patient who experienced anaphylaxis after the first dose of etoposide. After a median follow-up of 24 months (range, 0.5-35.4 months for PFS and 0.5-35.5 months for OS), the 2-year PFS and OS Kaplan-Meier estimates were 87.2% (95% CI, 77.3%-98.3%) and 95.1% (95% CI, 88.8%-100%), respectively (Figure 2). The median PFS and OS estimates were not reached owing to too few events.
Table 2. Primary Effectiveness End Point of Response to Combined PEM and ICE Regimen on PET2 Among 37 Evaluable Patients.
| Characteristics | No. (%) [95% CI] |
|---|---|
| Response rate | |
| Complete responsea | 32 (86.5) [71.2-95.5] |
| Partial response | 4 (10.8) [3.0-25.4] |
| Overall response rate | 36 (97.3) [85.8-99.9] |
| Progressive disease | 1 (2.7) [0.1-14.2] |
| PET2 response score, Deauville 5-point scale | |
| 1 | 17 |
| 2 | 10 |
| 3 | 3 |
| 4 | 4 |
| 5 | 2 |
| Xb | 1 |
| CR by biopsyc | 2 |
Abbreviations: ICE, ifosfamide, carboplatin, and etoposide; PEM, pembrolizumab; PET2, 18F-fluorodeoxyglucose–positron emission tomography with computed tomography after 2 cycles of combined PEM and ICE chemotherapy and 1 cycle of PEM monotherapy.
According to Lugano 2014 criteria, defined as Deauville score 1 to 3 on a 5-point scale.
Deauville score X is a new area of uptake unlikely to be related to lymphoma.
Two patients with Deauville scores X and 5 on PET2 had biopsies showing noncaseating granuloma in 1 patient (Deauville score X) and an Epstein-Barr virus–positive reactive process in a patient with Epstein-Barr virus–negative classic Hodgkin lymphoma at diagnosis (Deauville score 5).
Figure 2. Outcomes Data With Median Follow-up of 24 Months (Range, 0.5-35.4 Months).
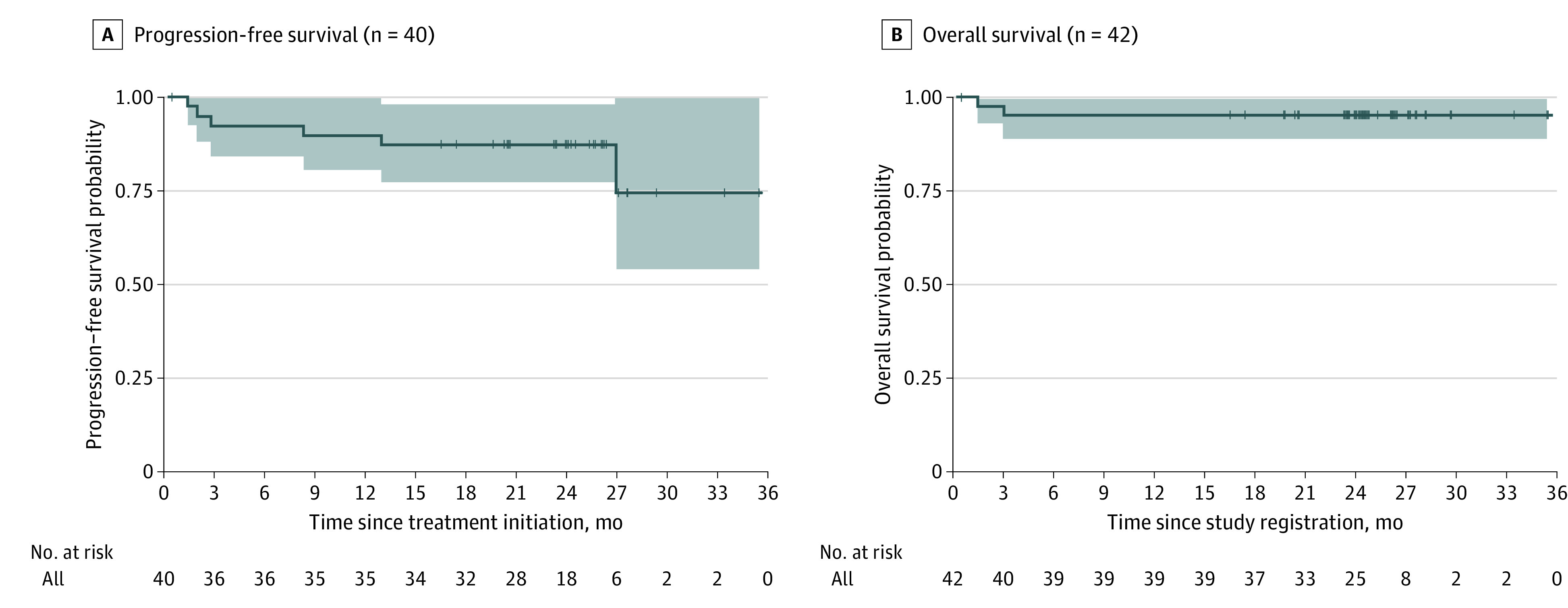
A, Progression-free survival 2-year estimate was 87.2% (95% CI, 77.3%-98.3%). B, Overall survival 2-year estimate was 95.1% (95% CI, 88.8%-100%). Shaded areas indicate 95% CIs.
Treatment with pembrolizumab and ICE chemotherapy did not impair stem cell mobilization. All 40 patients who underwent stem cell harvest had an adequate amount for autograft, with 35 (87%) completing collection within 2 apheresis sessions (range, 1-7 sessions). Patients had a median of 9.6 million cells per kilogram (range, 1.6-46.1 cells per kilogram), adequate for a single AHSCT. No patients had engraftment delays or failure. The median time to absolute neutrophil count recovery was 11 days (range, 9-24 days), and the median time to platelet count recovery was 12 days (range, 8-23 days).
Safety
There were 42 patients who received at least 1 dose of pembrolizumab and were therefore eligible for toxic effects analyses. The most common AEs for which grade 3 or 4 occurrences were reported are summarized in Table 3. Overall, the toxic effects profile was similar to what one would expect in patients receiving ICE chemotherapy, AHSCT, or both.
Table 3. Most Common Adverse Event Categories for Which Grade 3 and Grade 4 Toxic Effects Were Reported Among 42 Patientsa.
| Adverse effect | Patients, No. (%) | |||||
|---|---|---|---|---|---|---|
| Before conditioning | Any time during treatment | |||||
| Grades 1 and 2 | Grades 3 and 4 | Total | Grades 1 and 2 | Grades 3 and 4 | Total | |
| Anemia | 20 (48) | 20 (48) | 40 (95) | 9 (21) | 32 (76) | 41 (98) |
| ALC decreased | 15 (36) | 24 (57) | 39 (93) | 2 (5) | 39 (93) | 41 (98) |
| Platelet count decreased | 8 (19) | 32 (76) | 40 (95) | 1 (2) | 40 (95) | 41 (98) |
| WBC count decreased | 12 (29) | 17 (40) | 29 (69) | 1 (2) | 39 (93) | 40 (95) |
| Neutropenia | 5 (12) | 15 (36) | 20 (48) | NA | 38 (91) | 38 (91) |
| Nausea | 29 (69) | NA | 29 (69) | 34 (81) | 1 (2) | 35 (83) |
| Elevated AST or ALT | 26 (62) | 2 (5) | 28 (67) | 29 (69) | 4 (10) | 33 (79) |
| Hypoalbuminemia | 26 (62) | NA | 26 (62) | 32 (76) | 1 (2) | 33 (79) |
| Hypokalemia | 13 (31) | 4 (10) | 17 (45) | 17 (40) | 16 (38) | 33 (79) |
| Hyperglycemia | 28 (67) | 1 (2) | 29 (69) | 29 (69) | 3 (7) | 32 (76) |
| Hypocalcemia | 22 (52) | NA | 22 (52) | 29 (69) | 2 (5) | 31 (74) |
| Diarrhea | 5 (12) | 1 (2) | 6 (14) | 28 (67) | 1 (2) | 29 (69) |
| Hypophosphatemia | 7 (17) | 3 (7) | 10 (24) | 13 (31) | 11 (26) | 24 (57) |
| Hypertension | 14 (33) | 4 (10) | 18 (43) | 17 (40) | 6 (14) | 23 (55) |
| Hyponatremia | 14 (33) | NA | 14 (33) | 17 (40) | 3 (7) | 20 (48) |
| Mucositis | NA | NA | NA | 10 (24) | 10 (24) | 20 (48) |
| Fatigue | 12 (29) | 1 (2) | 13 (31) | 16 (38) | 1 (2) | 17 (40) |
| Hypotension | 3 (7) | NA | 3 (7) | 13 (31) | 2 (5) | 15 (36) |
| Abdominal pain | 5 (12) | NA | 5 (12) | 13 (31) | 1 (2) | 14 (33) |
| Febrile neutropenia | NA | NA | NA | NA | 12 (29) | 12 (29) |
Abbreviations: ALC, absolute lymphocyte count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NA, not available; WBC, white blood cell.
All patients who received at least 1 dose of pembrolizumab were eligible for toxic effects analysis. There were 2 grade 5 events: 1 patient with unsuspected severe coronary artery disease had cardiac arrest during apheresis, and 1 patient died of respiratory failure suspected to represent engraftment syndrome.
Thirty-four of the 42 patients (81%) experienced AEs that were attributed to pembrolizumab by the site investigators (L.J.B., C.C., P.B.A., S.E.S., and J.N.W.). For 22 patients (52.4%), grade 3 to grade 4 AEs were reported, composed of cytopenias (thrombocytopenia, n = 15; anemia, n = 11; neutropenia, n = 9; lymphopenia, n = 10; and leukopenia, n = 12), elevated aspartate aminotransferase and alanine aminotransferase levels (n = 2), hyponatremia (n = 1), hypophosphatemia (n = 1), and fatigue (n = 1). Five patients had 6 serious AEs judged as possibly associated with pembrolizumab. One patient with unappreciated significant coronary artery disease experienced ventricular fibrillation during stem cell collection, and a second had acute respiratory failure during white blood cell count recovery after AHSCT, possibly associated with engraftment syndrome. The serious AEs were all grades 1 to 2 and included anemia, decreased left ventricular ejection fraction, fever, and infection. Apart from the episode of respiratory failure possibly associated with engraftment syndrome and pembrolizumab, there were no significant pembrolizumab-related autoimmune events that delayed a patient’s treatment on protocol. There were no cases of hypothyroidism or hyperthyroidism, hepatitis, or colitis. Maculopapular rashes occurred in 13 of the 42 patients (31%) and were all grades 1 to 2.
Discussion
Although a combination of doxorubicin, bleomycin, vinblastine, and dacarbazine or its derivatives may cure most patients with cHL, a significant proportion will prove refractory to upfront therapy or relapse and be candidates for potentially curative therapy with AHSCT.1,2,3,4 Multiple studies have demonstrated that achievement of a CR by FDG-PET/CT to second-line treatment is a powerful predictor of outcome with AHSCT.8,9,10,11,12,13 Hence, improving the CR rate to salvage regimens is a high priority that is expected to translate into better clinical outcomes, including OS. The addition of pembrolizumab to conventional ICE chemotherapy was associated with a CR rate of 86.5% (32 of 37 patients) in this high-risk patient population, which is substantially higher than historical controls.15,16,17,24,25 Our findings are consistent with the recently reported early results of pembrolizumab with gemcitabine, vinorelbine, and liposomal doxorubicin. The addition of CPIs has the potential to substantially increase the number of patients with relapsed or refractory disease who are eligible for AHSCT and the overall success rate of salvage therapy.
Clinical trials of pembrolizumab and nivolumab monotherapy for patients with relapsed or refractory cHL, including those previously treated with AHSCT and brentuximab vedotin, have established that checkpoint inhibition alone is associated with high response rates among heavily pretreated patients, albeit CRs occurred in few of the individuals.18,19 Because ICE chemotherapy is the preferred regimen at many institutions,7 we chose to study the addition of pembrolizumab to ICE chemotherapy. Although the resulting CR rate of 86.5% (32 of 37 patients) is excellent and met our predetermined primary end point by exceeding the estimated historical rate of 50%, it may in fact underrepresent the true CR rate. Use of CPIs may result in inflammatory processes, leading to FDG-PET/CT positivity in the absence of disease. On our protocol, biopsies of PET-avid sites showed no evidence of lymphoma, suggesting that assessment of the true CR rate in cHL will be challenging. Response assessment in the setting of CPI therapy can be difficult given the possibility of tumor flare or what has been called pseudoprogression.26 One additional patient with very limited residual uptake and radiologic characteristics suggestive of a reactive process did not undergo biopsy and may very likely not have had residual disease. Whereas a biopsy is not always possible, it will be challenging to compare clinical outcomes with CPI-containing regimens with clinical outcomes with chemotherapy-only regimens.
Our results confirm those recently reported by Moskowitz et al,27 who combined pembrolizumab with gemcitabine, vinorelbine, and liposomal doxorubicin for similar patients with relapsed or refractory disease and reported a 95% complete remission rate. Their 95% CR rate would have allowed all patients to proceed to transplant, although 2 declined to do so. Five of the 36 patients undergoing transplant in the pembrolizumab with gemcitabine, vinorelbine, and liposomal doxorubicin trial developed new PET-avid lesions 7 to 15 months after transplant that led to biopsies showing nonnecrotizing granuloma in 2 patients, reactive changes in 2 patients, and follicular hyperplasia in 1 patient, underscoring the importance of biopsies in assessing PET-positive lesions in patients treated with CPIs.
It remains unclear whether a sequential response-adapted approach to CPI-based therapy is superior to administration of CPIs concomitantly with chemotherapy. Responses to CPI monotherapy in patients with relapsed or refractory disease have been variable. For heavily pretreated patients treated in KEYNOTE-087, the CR rate to pembrolizumab monotherapy was 27.6%, with most remissions (84.5%) occurring after 6 or more months of treatment and 63.8% after 12 months or more.19 In KEYNOTE-204, a randomized clinical trial comparing pembrolizumab with brentuximab vedotin among patients with relapsed or refractory disease who had either failed AHSCT or were not considered candidates for transplant, the CR rate to monotherapy was nearly identical, at 26% of treated patients.28 For patients relapsing after AHSCT, nivolumab monotherapy led to complete remissions in only 16% of cases.18 Others have studied nivolumab monotherapy for patients with relapsed or refractory disease who were eligible for transplant and report complete remissions in 62% of patients after only 3 cycles of immunotherapy and 78% after 6 cycles.29 Patients not in remission proceeded to combination therapy with nivolumab plus ICE chemotherapy. The unexpected high CR rate may be attributable to small numbers, patient selection, or issues associated with the interpretation of PET/CT images. Nevertheless, the high CR rate among patients with relapsed or refractory disease treated with immunotherapy alone before AHSCT suggests that some patients may be spared conventional chemotherapy.
In a randomized phase 2 trial comparing sequential and concomitant therapy for patients with previously untreated early-stage unfavorable cHL, there appeared to be no significant difference in outcomes between the 2 approaches.30 In the sequential group, 4 doses of nivolumab resulted in complete metabolic responses in 51% of cases. In a phase 2 trial of sequential pembrolizumab and doxorubicin, vinblastine, and dacarbazine chemotherapy for previously untreated patients with early unfavorable and advanced-stage disease, two-thirds of all patients achieved complete or near-complete remission after only 3 doses of pembrolizumab monotherapy and 100% were in remission after 2 cycles of subsequent doxorubicin, vinblastine, and dacarbazine.31 Despite variable response rates reported with CPI monotherapy, these studies speak to the potential power of checkpoint inhibition in cHL.
When designing this trial, we were concerned that pembrolizumab would negatively affect stem cell mobilization or that use of growth factor might be associated with autoimmune toxic effects in the presence of a CPI. Neither proved to be the case, and engraftment did not appear to be delayed. The complicated and extensive toxic effects profile associated with conditioning chemotherapy followed by AHSCT makes it difficult to determine any difference in the frequency of AEs attributable to pembrolizumab. The 1 case of what appeared to be engraftment syndrome is consistent with the experience with nivolumab-ICE reported recently, in which 4 of 33 patients experienced engraftment syndrome a median of 8 days after transplant (range, 7-10 days).29 Only 1 of these patients was treated with corticosteroids. Two additional patients experienced febrile syndromes near the time of engraftment and were treated with corticosteroids, although neither met the traditional diagnostic criteria for engraftment syndrome.32 Similarly, extremely high rates of engraftment syndrome treated with corticosteroids were observed in the trial of pembrolizumab with gemcitabine, vinorelbine, and liposomal doxorubicin, with 68% of patients with transplant experiencing symptoms of the syndrome at a median of 10 days (range, 8-17 days), with successful resolution in all.27 Because an increasing number of patients are treated with CPIs as part of second-line therapy, it will be important to track this particular complication and to be vigilant after AHSCT for this uncommon complication. Early recognition and treatment with steroids will be important in limiting morbidity and mortality.
Limitations
Study limitations include the single-group nonrandomized study design, which makes assessment of response rates difficult in comparison to reported historical rates. The study included a small number of participants with a short follow-up period. As discussed, repeated biopsies may be required to confirm false-positive PET/CT imaging when CR rates are assessed.
Conclusions
In this phase 2 nonrandomized clinical trial, we found that pembrolizumab administered concurrently with conventional ICE chemotherapy was a well-tolerated and effective treatment strategy for patients with relapsed or refractory cHL, resulting in high CR rates and nearly all patients proceeding to AHSCT. Pseudoprogression, flare reactions, and granulomatous and reactive processes may obscure the true complete metabolic response rate in the presence of CPIs, making it difficult to directly compare outcomes with those of conventional regimens. Transplant physicians should pay special attention to signs and symptoms suggestive of “engraftment syndrome” in patients previously treated with CPIs. Randomized phase 3 trials comparing conventional regimens with those containing CPIs are needed but will be challenging to design.
Trial Protocol
Data Sharing Statement
References
- 1.Stephens DM, Li H, Schöder H, et al. Five-year follow-up of SWOG S0816: limitations and values of a PET-adapted approach with stage III/IV Hodgkin lymphoma. Blood. 2019;134(15):1238-1246. doi: 10.1182/blood.2019000719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engert A, Plütschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363(7):640-652. doi: 10.1056/NEJMoa1000067 [DOI] [PubMed] [Google Scholar]
- 3.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31(6):684-691. doi: 10.1200/JCO.2012.43.4803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connors JM, Jurczak W, Straus DJ, et al. ; ECHELON-1 Study Group . Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2018;378(4):331-344. doi: 10.1056/NEJMoa1708984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341(8852):1051-1054. doi: 10.1016/0140-6736(93)92411-L [DOI] [PubMed] [Google Scholar]
- 6.Schmitz N, Pfistner B, Sextro M, et al. ; German Hodgkin’s Lymphoma Study Group; Lymphoma Working Party of the European Group for Blood and Marrow Transplantation . Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065-2071. doi: 10.1016/S0140-6736(02)08938-9 [DOI] [PubMed] [Google Scholar]
- 7.Hoppe RT, Advani RH, Ai WZ, et al. Hodgkin lymphoma, version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18(6):755-781. doi: 10.6004/jnccn.2020.0026 [DOI] [PubMed] [Google Scholar]
- 8.Adams HJ, Kwee TC. Prognostic value of pretransplant FDG-PET in refractory/relapsed Hodgkin lymphoma treated with autologous stem cell transplantation: systematic review and meta-analysis. Ann Hematol. 2016;95(5):695-706. doi: 10.1007/s00277-016-2619-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akhtar S, Al-Sugair AS, Abouzied M, et al. Pre-transplant FDG-PET–based survival model in relapsed and refractory Hodgkin’s lymphoma: outcome after high-dose chemotherapy and auto-SCT. Bone Marrow Transplant. 2013;48(12):1530-1536. doi: 10.1038/bmt.2013.88 [DOI] [PubMed] [Google Scholar]
- 10.Jabbour E, Hosing C, Ayers G, et al. Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer. 2007;109(12):2481-2489. doi: 10.1002/cncr.22714 [DOI] [PubMed] [Google Scholar]
- 11.Mocikova H, Pytlik R, Markova J, et al. Pre-transplant positron emission tomography in patients with relapsed Hodgkin lymphoma. Leuk Lymphoma. 2011;52(9):1668-1674. doi: 10.3109/10428194.2011.573889 [DOI] [PubMed] [Google Scholar]
- 12.Smeltzer JP, Cashen AF, Zhang Q, et al. Prognostic significance of FDG-PET in relapsed or refractory classical Hodgkin lymphoma treated with standard salvage chemotherapy and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(11):1646-1652. doi: 10.1016/j.bbmt.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentzler RD, Evens AM, Rademaker AW, et al. F-18 FDG-PET predicts outcomes for patients receiving total lymphoid irradiation and autologous blood stem-cell transplantation for relapsed and refractory Hodgkin lymphoma. Br J Haematol. 2014;165(6):793-800. doi: 10.1111/bjh.12824 [DOI] [PubMed] [Google Scholar]
- 14.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048-3058. doi: 10.1200/JCO.2013.53.5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97(3):616-623. doi: 10.1182/blood.V97.3.616 [DOI] [PubMed] [Google Scholar]
- 16.Moskowitz CH, Yahalom J, Zelenetz AD, et al. High-dose chemo-radiotherapy for relapsed or refractory Hodgkin lymphoma and the significance of pre-transplant functional imaging. Br J Haematol. 2010;148(6):890-897. doi: 10.1111/j.1365-2141.2009.08037.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehrzad V, Ashrafi F, Farrashi AR, Pourmarjani R, Dehghani M, Shahsanaei A. Comparison of ifosfamide, carboplatin and etoposide versus etoposide, steroid, and cytarabine cisplatin as salvage chemotherapy in patients with refractory or relapsed Hodgkin’s lymphoma. Adv Biomed Res. 2017;6:30. doi: 10.4103/2277-9175.201687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36(14):1428-1439. doi: 10.1200/JCO.2017.76.0793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen R, Zinzani PL, Lee HJ, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134(14):1144-1153. doi: 10.1182/blood.2019000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdel-Rahman O. Toxicity patterns associated with chemotherapy/immune checkpoint inhibitor combinations: a meta-analysis. Immunotherapy. 2019;11(6):543-554. doi: 10.2217/imt-2018-0186 [DOI] [PubMed] [Google Scholar]
- 21.Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30(2):219-235. doi: 10.1093/annonc/mdy551 [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Fisher RI, Barrington SF, et al. ; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin’s Study Group; Japanese Lymphorra Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute . Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. doi: 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CTCAE files. Accessed July 28, 2022. http://evs.nci.nih.gov/ftp1/CTCAE/About.html
- 24.Bartlett NL, Niedzwiecki D, Johnson JL, et al. ; Cancer Leukemia Group B . Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin’s lymphoma: CALGB 59804. Ann Oncol. 2007;18(6):1071-1079. doi: 10.1093/annonc/mdm090 [DOI] [PubMed] [Google Scholar]
- 25.Baetz T, Belch A, Couban S, et al. Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin’s disease: a phase II study by the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 2003;14(12):1762-1767. doi: 10.1093/annonc/mdg496 [DOI] [PubMed] [Google Scholar]
- 26.Cheson BD, Ansell S, Schwartz L, et al. Refinement of the Lugano classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128(21):2489-2496. doi: 10.1182/blood-2016-05-718528 [DOI] [PubMed] [Google Scholar]
- 27.Moskowitz AJ, Shah G, Schöder H, et al. Phase II trial of pembrolizumab plus gemcitabine, vinorelbine, and liposomal doxorubicin as second-line therapy for relapsed or refractory classical Hodgkin lymphoma. J Clin Oncol. 2021;39(28):3109-3117. doi: 10.1200/JCO.21.01056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuruvilla J, Ramchandren R, Santoro A, et al. ; KEYNOTE-204 investigators . Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2021;22(4):512-524. doi: 10.1016/S1470-2045(21)00005-X [DOI] [PubMed] [Google Scholar]
- 29.Mei MG, Lee HJ, Palmer JM, et al. Response-adapted anti-PD-1-based salvage therapy for Hodgkin lymphoma with nivolumab alone or in combination with ICE. Blood. 2022;139(25):3605-3616. doi: 10.1182/blood.2022015423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bröckelmann PJ, Goergen H, Keller U, et al. Efficacy of nivolumab and AVD in early-stage unfavorable classic Hodgkin lymphoma: the randomized phase 2 German Hodgkin Study Group NIVAHL trial. JAMA Oncol. 2020;6(6):872-880. doi: 10.1001/jamaoncol.2020.0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen PB, Savas H, Evens AM, et al. Pembrolizumab followed by AVD in untreated early unfavorable and advanced-stage classical Hodgkin lymphoma. Blood. 2021;137(10):1318-1326. doi: 10.1182/blood.2020007400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27(9):893-898. doi: 10.1038/sj.bmt.1703015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement