Abstract
Obesity dysregulates B cell populations, which contributes toward poor immunological outcomes. We previously reported that differing B cell subsets are lowered in the bone marrow of obese male mice. Here, we focused on how lipid metabolites synthesized from docosahexaenoic acid (DHA) known as specialized pro-resolving lipid mediators (SPMs) influence specific B cell populations in obese male mice. Metabololipidomics revealed that splenic SPM precursors 14-hydroxydocosahexaenoic acid (14-HDHA), 17-hydroxydocosahexaenoic acid (17-HDHA), and downstream protectin DX (PDX) were decreased in obese male C57BL/6J mice. Simultaneous administration of these mediators to obese mice rescued major decrements in bone marrow B cells, modest impairments in the spleen, and circulating IgG2c, which is pro-inflammatory in obesity. In vitro studies with B cells, flow cytometry experiments with ALOX5−/− mice, and lipidomic analyses revealed the lowering of 14-HDHA/17-HDHA/PDX and dysregulation of B cell populations in obesity was driven indirectly via B cell extrinsic mechanisms. Notably, the lowering of lipid mediators was associated with an increase in the abundance of n-6 polyunsaturated fatty acids, which have a high affinity for SPM-generating enzymes. Subsequent experiments revealed female obese mice generally maintained the levels of SPM precursors, B cell subsets, and antibody levels. Finally, obese human females had increased circulating plasma cells accompanied by ex vivo B cell TNFα and IL-10 secretion. Collectively, the data demonstrate that DHA-derived mediators of the SPM pathway control the number of B cell subsets and pro-inflammatory antibody levels in obese male but not female mice through a defect that is extrinsic to B cells.
Keywords: n-3 polyunsaturated fatty acids, 14-HDHA, 17-HDHA, PDX
1 |. INTRODUCTION
Diet-induced obesity negatively impacts innate and adaptive immunity,1–4 which contributes toward a wide range of secondary complications.1,5–8 Several studies show that B cells are one major cellular target of high fat diets.1,9–11 In humans and mice, B cell cytokine secretion and antibody production are dysregulated, which contributes toward increased susceptibility to infections and poor responses to vaccinations.9,12–14 At the cellular level, impairments in B cell responses of obese mice are associated with a loss of differing B cell populations that control humoral immunity.15,16
Strong modifications in lipid metabolism are one potential mechanism by which the number of B cells could become modified upon exposure to high fat diets.17–21 In particular, specialized pro-resolving lipid mediators (SPMs), which provide resolution signals that regulate adaptive immunity, could be impaired in secondary lymphoid organs in obesity.22–27 In support of this hypothesis, specific SPM pathway metabolites are lowered in adipose tissue of obese mice and humans, which prevents the resolution of adipose tissue inflammation.28,29 Furthermore, deficiencies in the levels of the n-3 polyunsaturated fatty acids (PUFA), eicosapentaenoic (EPA) and docosahexaenoic acid (DHA), which are parent molecules of SPMs, could impair B cell development and maturation. Indeed, there are some data demonstrating that EPA and DHA levels are lowered in circulation of obese humans, although their relationship with B cells are unexplored.30–32
There is emerging evidence from our laboratory and others that select SPM precursors and SPMs influence B cell differentiation. To exemplify, the SPM precursors 14-HDHA and 17-HDHA, which are generated through the action of the 12/15 lipoxygenase using DHA as a substrate, promote B cell differentiation toward CD138+ plasma cells.15,33 DHA-derived SPMs also suppress B cell differentiation into IgE producing cells34 and the arachidonic acid-derived SPM, lipoxin A4 (LXA4), inhibits antibody production from memory B cells.35 There is also speculation that resolvins could promote the development of germinal center reactions in specific lymphoid tissues.36 Therefore, SPMs are emerging to have a critical role in regulating B cell phenotypes but their role in obesity is completely unknown.
The first objective of this study was to use metabololipidomics to quantify the levels of various PUFA-derived metabolites in bone marrow and spleen followed by studies in which obese male mice were administered SPM precursors to test their ability to rescue deficits in B cell populations and circulating antibody levels. Mechanistically, we used a combination of cell culture studies, lipidomics, and ALOX5−/− mice to determine if obesity promoted impairments at the level of the B cell or if extrinsic factors such as a loss of dietary DHA were responsible for the observed reduction in SPM precursors. Subsequent studies were conducted with female mice. The rationale was that major differences in immunity between sexes are prevalent but relatively little is known about B cell responses with females, particularly in the context of lipid metabolism and obesity.37 For example, premenopausal obese women display increased class switching from IgM to IgG and increased B cell numbers, which is hypothesized to enhance humoral immunity in women.38 Finally, we assayed for B cell populations and functional outcomes with B cells isolated from obese female mice and compared the results with humans.
2 |. MATERIALS AND METHODS
2.1 |. Mice
All murine experiments fulfilled the guidelines established by East Carolina University and The University of North Carolina at Chapel Hill for euthanasia and humane treatment. Male and female C57BL/6J mice of approximately 5–6 weeks of age were purchased from Jackson Laboratories. Mice were fed control (10% of total kcal from lard) or high fat (60% of total kcal from lard) diets (Research Diets, New Brunswick, NJ) for 15 weeks. ALOX5−/− mice were purchased from Jackson laboratories at 4–6 weeks of age and aged up to ~20 weeks prior to conducting experiments.
2.2 |. Metabolic studies
Echo-MRI for fat and lean mass was conducted as previously described.39 Mice were fasted for 6 h followed by the establishment of baseline glucose values with a glucometer. Dextrose (2.5 g) (Hospira Inc., Lake Forest, IL) per kg lean mass was delivered to each animal via an intraperitoneal injection (i.p.). Glucose measurements were made throughout a 90-min time interval from the tail vein. Fasting insulin levels were assayed with an insulin ELISA kit (Abcam) followed by the calculation of the HOMA-IR index.40
2.3 |. Metabololipidomics
All standards and internal standards used for LC/MS/MS analysis of arachidonic acid, docosahexaenoic acid, and linoleic acid-derived lipid mediators were purchased from Cayman Chemical (Ann Arbor, Michigan, USA). All solvents and extraction solvents were HPLC grade or better.
B cells were pretreated for solid phase extraction (SPE) as follows. Briefly, 400 μl of ice-cold methanol was added along with 10 μl of 10 pg/μl internal standard solution (100 pg total/each of 5(S)-HETE-d8, 8-iso-PGF2a-d4, 9(S)-HODE-d4, LTB4-d4, LTD4-d5, LTE4-d5, PGE2-d4, PGF2a-d9, and RvD2-d5 in ethanol) to isolated B cells that were previously frozen in 1.5 ml microcentrifuge tubes. The samples were allowed to incubate for 15 min on ice, and then centrifuged at 14,000 rpm for 10 min at 4°C. The supernatant was removed and placed into a new microcentrifuge tube. Another 200 μl of icecold methanol was added to the B cell pellet, vortexed and then centrifuged at 14,000 rpm for 10 min at 4°C. The supernatant was removed and then combined with the previously obtained supernatant. The combined supernatants were dried in a vacuum, centrifuged, and then reconstituted with 1.0 ml of 10% methanol prior to solid phase extraction.
Spleen samples were also pretreated for solid phase extraction. Spleen samples were preweighed and transferred into a dry ice chilled, preweighed TissueLyser tube (Qiagen, Hilden, Germany) with a 5 mm stainless steel ball. Chilled methanol (1.0 ml) −20°C and 10 μl of internal standard solution was added and the samples were homogenized at 50 Hz for 2 min. The sample was then centrifuged at 14,000 rpm for 10 min at 4°C. The supernatant was removed and transferred to a new 1.5 ml microcentrifuge tube and dried in a speedvac until completely dry. The dried sample was then reconstituted with 1.0 ml of 10% methanol prior to solid phase extraction.
SPMs from B cells and spleen samples were isolated and purified using SPE. The reconstituted extracts were loaded on a StrataX 33um 30 mg/1 ml SPE column (Phenomenex, Torrance, California, USA) preconditioned with 1.0 ml of methanol followed by 1.0 ml of water. The SPE column was then washed with 10% methanol and then eluted directly into a reduced surface activity/maximum recovery glass autosampler vial with 1.0 ml of methyl formate. The methyl formate was evaporated completely from the vial with a stream of nitrogen and then the SPE cartridge was then eluted with 1.0 ml of methanol directly into the same autosampler vial. The methanol was evaporated to dryness with a stream of nitrogen and then the sample was reconstituted with 20 ul of ethanol. Samples were analyzed immediately or frozen at −70°C until analysis. Liquid chromatography-mass spectrometry of SPMs was performed as previously described.15
2.4 |. Fatty acid extraction and GC/MS lipidomics
Fatty acids were extracted from spleen samples using microwave assisted extraction.41 Fatty acids were extracted using a 4:1 (v/v) ethyl acetate:methanol solution in a Mars 6 microwave system (CEM Corporation, Matthews, NC) with the following heating profile: 1 min ramp to 40°C, 12 min hold with max power of 400 W. Fatty acyl methyl esters were synthesized using the HCl method.42 A 680/600S GC-MS (Perkin Elmer, Waltham, MA) equipped with Agilent Technologies (Santa Clara, CA, USA) DB-23 30-m column was used for data acquisition. The GC parameters were as follows: the initial temperature was set to 100°C for 30 s, ramp was set from 7.0°C/min to 245°C, and a hold for 2 min. Standard curves were created using Supelco 37 Component FAME mix (Sigma). A 22:5 n-3 curve was created using a standard from Cayman Chemical. Seven-point curves were created for all standards. Data analysis was conducted using TargetLynx software. Concentrations were normalized based on the weights of the spleens.
2.5 |. Analysis of B cell SPM precursor production
4 × 106 B cells (purity > 95%) from C57BL/6J mice were resuspended at a concentration of 1 × 106 cells/ml and were placed into 15 ml conical tubes for 0 or 4 h postactivation. Cells were treated with or without 0.5μM DHA complexed to BSA followed by activation with 1 μg/ml CPG 1826 plus 2 μg/ml anti-IgM. Cell pellets were washed twice with 1× PBS and stored in −80°C until samples were analyzed via mass spectrometry as described above.
2.6 |. Flow cytometry
Flow cytometry analyses were conducted using C57BL/6J and ALOX5−/− mice as previously described.15 Briefly, 2.5 × 105 splenocytes and 5 × 105 bone marrow cells were plated on 96-well flat bottom plates followed by one wash in PBS. FcR blocker (Miltenyi Biotech) was added according to manufacturer recommendations. Cells were washed once with cold 1× PBS and stained with fluorescently labeled antibodies for 25 min and subsequently washed three times. A BDLSRII flow cytometer was used for data acquisition.
The fluorescently labeled antibodies used in the experiment were obtained from Biolegend and included: CD19 (PerCP-Cy5.5, Catalog 152406), CD43 (APC, Catalog 143208), CD24 (Pacific Blue, Catalog 101820), IgM (PE, Catalog 406508), IgD (APC, Catalog 405714), CD138 (APC, Catalog 142506), B220+ (FITC, Catalog 103206), CD21/35 (PB, Catalog 123414, CD23 (APC, Catalog 101620), GL7 (AF-647, Catalog 144605), CD80 (SPC, Catalog 104714), CD1d (APC, Catalog 123522), and Zombie NIR (Catalog 423106). All cells were gated off of a Live/Dead cell indicator (Zombie NIR). The following B cell subsets in the bone marrow were analyzed: CD19+CD43+CD24+IgM− (pro), CD19+CD43−CD24+IgM− (pre), CD19+CD24+IgM+IgD− (immature), CD19+IgM+IgD+ (mature), CD19+CD138+ (long lived plasma cells). The following B cell subsets in the spleen were analyzed: B220+IgM+CD21lowCD23− (T1), B220+IgM+CD21−CD23+ (T2), B220+IgM+CD21highCD23+ (T2-MZ), B220+IgMlowCD21intCD23+ (Follicular), B220+IgMhighCD21+CD1dhigh (Marginal), B220+CD19+IgM+GL7+ (Germinal), B220+IgM+CD35+CD80+ (IgM+ Memory), B220lowIgM+CD138+ (Plasmablasts). Data were analyzed by FlowJo V.10.4.
2.7 |. B cell activation
Mouse splenic B cells were isolated using a murine B cell isolation kit (Miltenyi Biotech) and resuspended at a concentration of 1 × 106 cells/ml. 2 × 105 cells were plated in a 96-well U-bottom plate and activated with: (1) 1 μg/ml CPG 1826 (Novus Biologicals, Littleton, CO) plus 2 μg/ml anti-IgM (Jackson Immunoresearch, West Balt Pike, PA); or (2) 1 μg/ml LPS (Sigma Aldrich, St. Louis, MO).
2.8 |. ELISAs
Serum collected from control and experimental groups was used to assay IgG2a, IgG2b, IgG2c, and IgG3 levels via an ELISA (eBioscience). Supernatants from splenic B cells were collected 2 days postactivation and cytokine levels were measured using an ELISA (Biolegend). Leptin levels were measured using an ELISA from Abcam (Cambridge, MA). Supernatants from activated splenic B cells were collected 3 days post activation to measure IgM and IgG levels using ELISAs from eBioscience (San Diego, CA).
2.9 |. qRT-PCR
Total RNA was isolated from murine splenocytes and total splenic B cells using the RNeasy Plus Universal Mini Kit (Qiagen, Valencia, CA). RNA (250 ng) was reverse transcribed and amplified by using a OneStep SYBR green PCR Mix (Biorad). The fold change was calculated by the 2−ΔΔCt method. GAPDH and β-actin were the housekeeping genes used in all PCR experiments, as previously described.15 The primer sets used in the study are presented in Supplemental Table 1.
2.10 |. Rescue studies
SPM precursors and SPMs were injected as previously described at a concentration that leads to elevated levels of 14-HDHA, 17-HDHA, and PDX.43 Briefly, 14(S)-HDHA (0.1 mg/ml), 17R-HDHA (0.1 mg/ml), and Protectin DX (0.1 mg/ml) were purchased from Cayman Chemicals (Ann Arbor, MI). SPMs were prepared in ethanol in the dark and kept on ice at all times. The final cocktail concentration was 900 ng per mouse (300 ng of each SPM precursor and SPM) in PBS or vehicle control (ethanol in PBS) and was administered i.p. to lean and obese mice for 4 consecutive days followed by euthanasia on day 5. Control mice were administered a vehicle control.
2.11 |. PBMC and B cell functional analyses in humans
Human blood samples for B cell studies were procured after obtaining informed consent and were in accordance by the East Carolina University Institutional Review Board. Female subjects were classified as nonobese (BMI < 25 kg/m2) or obese (BMI > 30 kg/m2) (Supplemental Table 2). Subjects were free of chronic/autoimmune disease, not taking n-3 PUFA supplements, free of infection for at least 1 month, and were nonsmokers.
Peripheral blood was collected in vacutainer tubes and diluted in PBS (1:1) followed by separation of the PBMCs by the use of Ficoll Paque (GE Healthcare, Washington, NC) gradient centrifugation. Flow cytometry was used to analyze cell populations from isolated PBMCs. All fluorescently labeled antibodies were purchased from Biolegend (San Diego, CA) or Miltenyi Biotech (San Diego, CA) and were the following: Zombie NIR (Catalog 423106), CD19 (APC, Catalog 982406), CD14 (FITC, Catalog 367116), CD8 (PECy5, Catalog 300910), CD4 (FITC, Catalog 357406), CD2 (Pacific Blue, Catalog 309216), CD45 (PE, Catalog 368510), CD27 (Pacific Blue, Catalog 302822), CD38 (FITC, Catalog 980304), and IgD (PECy7, Catalog 348210). The following populations were analyzed using a BDLSRII flow cytometer: CD45+CD3+CD4+ (helper T cells), CD45+CD3+CD8+ (cytotoxic T cells), CD45+CD3−CD14+ (monocytes), CD45+CD14−CD19+ (B cells), CD19+IgD+CD27+ (memory B cells), and CD19+CD38+CD27+IgD− (plasma B cells).
B cells were isolated from PBMCs using a Human B cell isolation kit (Miltenyi Biotech San Diego, CA) with a resulting purity of > 99% as previously described.15 Briefly, B cells were cultured in RPMI 1640 with 5% FBS, 2 mM L-Glutamine, 5 × 10−5 M 2mercaptoethanol, 10 mM HEPES, and 50 μg/μl gentamicin at a concentration of 3 × 106 cells/ml. B cells were stimulated with: (1) CPG oligodeoxynucleotides (ODN) 2395 (TLR9 agonist) at 1 μg/ml plus BCR stimulation (rabbit anti-human IgM Ab fragment) at 2 μg/ml; (2) PAM3CSK4 (TLR1/2 agonist) at a concentration of 10 μg/ml. 2 × 105 B cells were plated in round bottom inert grade 96-well plates and cultured at different time points upon activation. Supernatant was collected2dayspostactivationtomeasureIL-6,IL-10,andTNFα levels via Luminex Assay Kits (Thermo Fisher Scientific, Waltham, MA). Supernatant was also collected 3 days postactivation to assay IgM and IgG levels via an ELISA (Abcam Cambridge, MA).
2.12 |. Statistics
All data from murine experiments are from multiple cohorts of mice except SPM analyses in female mice, which are from a single cohort. Due to logistics, studies of B cell populations with male and female were not conducted simultaneously. Data were analyzed using Graph Pad Prism Version 7.0. Statistical significance for female human studies, in vitro experiments, and experiments comparing control versus obese mice were analyzed with a two-tailed Student’s unpaired t-test. Statistical significance for lipidomic analysis in the bone marrow comparing control versus obese mice was determined using a paired t-test. This test was used to account for differences in the number of B cells between cohorts of mice. Murine experiments involving SPM injections were analyzed using a one-way ANOVA followed by a post hoc Bonferroni multiple comparisons test. Most data sets displayed normalized distribution as determined by a Kolmogorov-Smirnov test. Those sets that did not display normal distributions were analyzed with a Kruskal–Wallis test followed by a Dunn’s multiple comparison test. Metabolic studies as a function of time were analyzed with a two-way ANOVA. For all analyses, P < 0.05 was considered statistically significant.
3 |. RESULTS
3.1 |. Metabolic phenotype of obese male mice
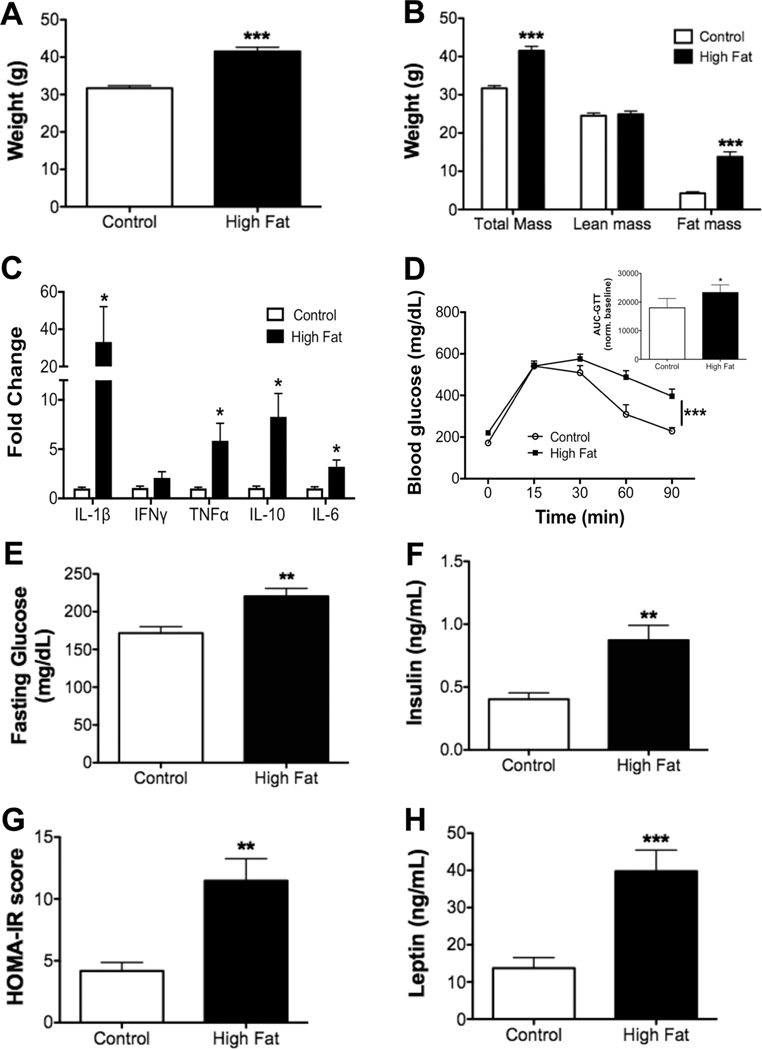
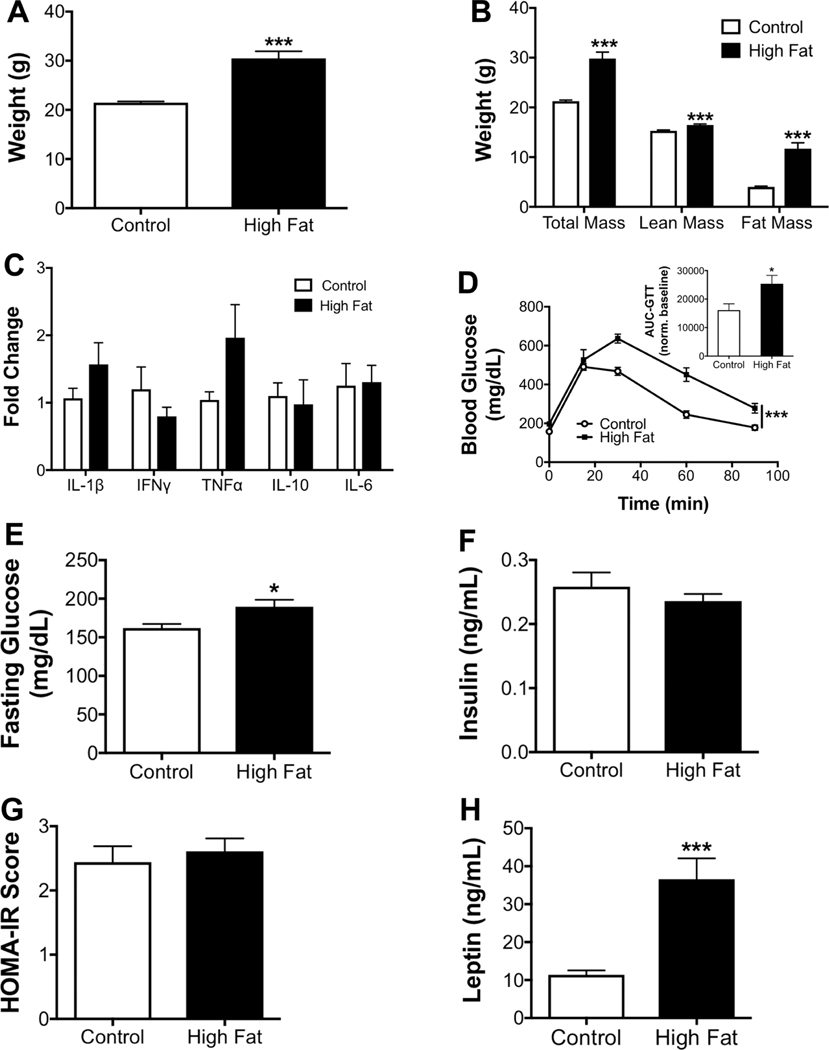
The metabolic profile for obese male mice was first established. Obese mice showed a significant increase in body weight after a 15-week feeding period compared to controls (Fig. 1A). The increase in total body mass was driven by elevated fat mass (Fig. 1B). qRT-PCR analysis revealed that obese mice had strong upregulation of IL-1β, TNFα, IL-6, and IL-10 in white adipose tissue (Fig. 1C). The increase in IL-10 secretion was consistent with a counter regulatory effect of obesity.44 Glucose clearance (Fig. 1D) was also impaired, as quantified by the area under the curve (Fig. 1D inset). Fasting glucose levels (Fig. 1E) were modestly increased and fasting insulin was increased 2-fold with the high fat diet relative to the lean controls (Fig. 1F). Obese male mice displayed a 2-fold increase in the HOMA-IR score, a measure of insulin sensitivity, compared to controls (Fig. 1G). Finally, leptin levels were increased by ~4-fold with obese mice relative to lean animals (Fig. 1H).
FIGURE 1. Metabolic profile of male C57BL/6J mice consuming a high fat diet.
(A) Body weights of male mice at the completion of the study. (B) Body composition assayed by Echo-MRI after 15 weeks of consuming experimental diets. (C) qRT-PCR analysis of inflammatory cytokines in the adipose tissue of control and obese mice. (D) Glucose tolerance test (GTT), performed by intraperitoneal injection of glucose after a 6 h fast. Inset shows the area under the curve (AUC), calculated by integration of the glucose curves shown in subpart (D), normalized to baseline values. (E) Fasting blood glucose and (F) insulin levels determined after a 6 h fast. (G) HOMA-IR scores. (H) Fasting leptin levels. N = 9–10 mice per diet (A, B, and D), N = 8 mice per diet (C), N = 6–8 mice per diet (E–H). Data are average ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001 by a Student’s unpaired t-test (A–C, E–H) or a two-way ANOVA analysis followed by a post hoc t-test (D)
3.2 |. SPM precursor levels are lowered in obese male C57BL/6J mice
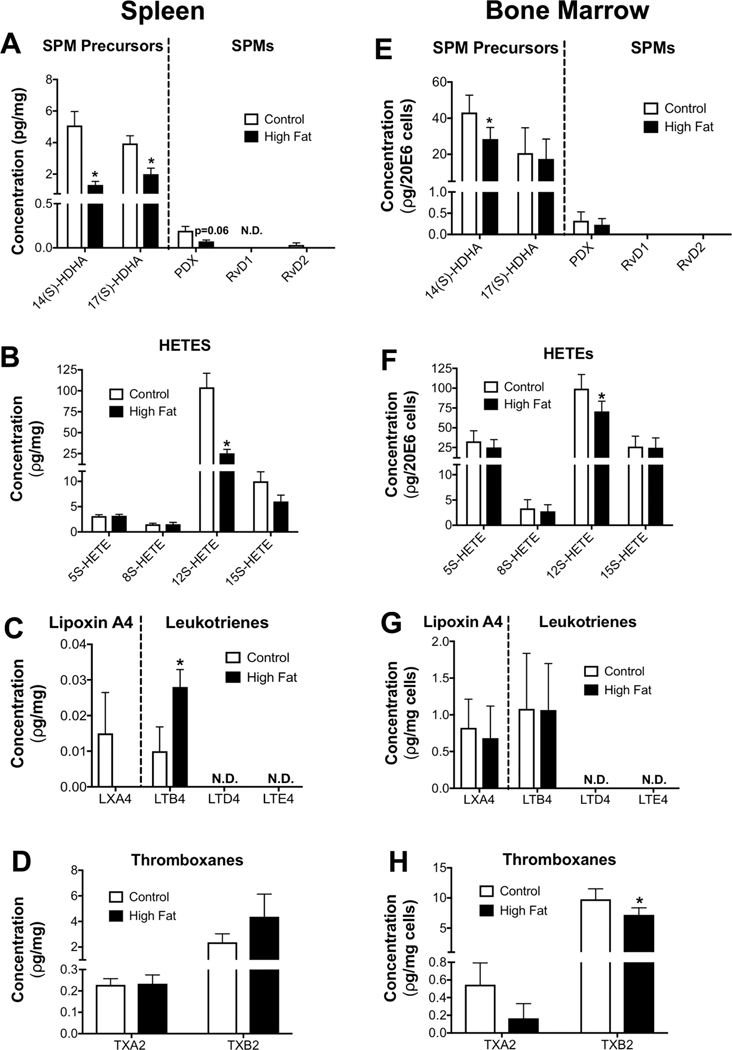
We investigated if diet-induced obesity lowered splenic SPM precursors and SPMs that may regulate B cell populations, in addition to analyses of other key PUFA-derived metabolites.29,45–47 14-HDHA and 17-HDHA were lowered by 2–2.5-fold relative to controls in the spleen (Fig. 2A). Protectin DX (PDX) trended (P = 0.06) to be lowered in obese mice compared to controls whereas resolvin D2 (RvD2) did not show a significant effect. In addition, RvD1 was at the detection limit (Fig. 2A). Other SPMs (except LXA4) were not detectable in the spleen. We also measured mediators synthesized from arachidonic and linoleic acids (LAs). For simplicity, we present key molecules. Obesity lowered 12-HETE by 4-fold (Fig. 2B) and increased LTB4 by 2.8-fold (Fig. 2C) but had no influence on thromboxanes (Fig. 2D). Comparative analysis of total bone marrow cells showed that 14-HDHA was modestly decreased in male obese mice by 1.5-fold with no change in 17-HDHA or PDX between the two groups (Fig. 2E). 12-HETE was modestly lowered with obese mice by 1.4-fold (Fig. 2F). There was no effect of obesity on LTB4 (Fig. 2G) and TXB2 was slightly decreased by 1.3-fold relative to the lean control (Fig. 2H).
FIGURE 2. DHA-derived SPM precursors and SPMs are lowered in obese male mice.
Metabololipidomic analyses of (A) DHA-derived SPM precursors and SPMs, (B) AA-derived HETES, (C) AA-derived LXA4 and leukotrienes, and (D) AA-derived thromboxanes in the spleens of mice consuming a control or high fat diet. Metabololipidomic analyses of (E) DHA-derived SPM precursors and SPMs, (F) AA-derived HETEs, (G) AA-derived LXA4 and leukotrienes, and (H) AA-derived thromboxanes in the bone marrow of control and obese mice. N.D.: not detectable. Mice consumed experimental diets for 15 weeks. N = 6 mice per diet (A–H). Data are average ± S.E.M. *P < 0.05 by an unpaired Student’s t-test
3.3 |. Administration of 14-HDHA/17-HDHA/PDX modestly increases germinal center B cells in obese mice accompanied by a rescue of pro-inflammatory IgG2c
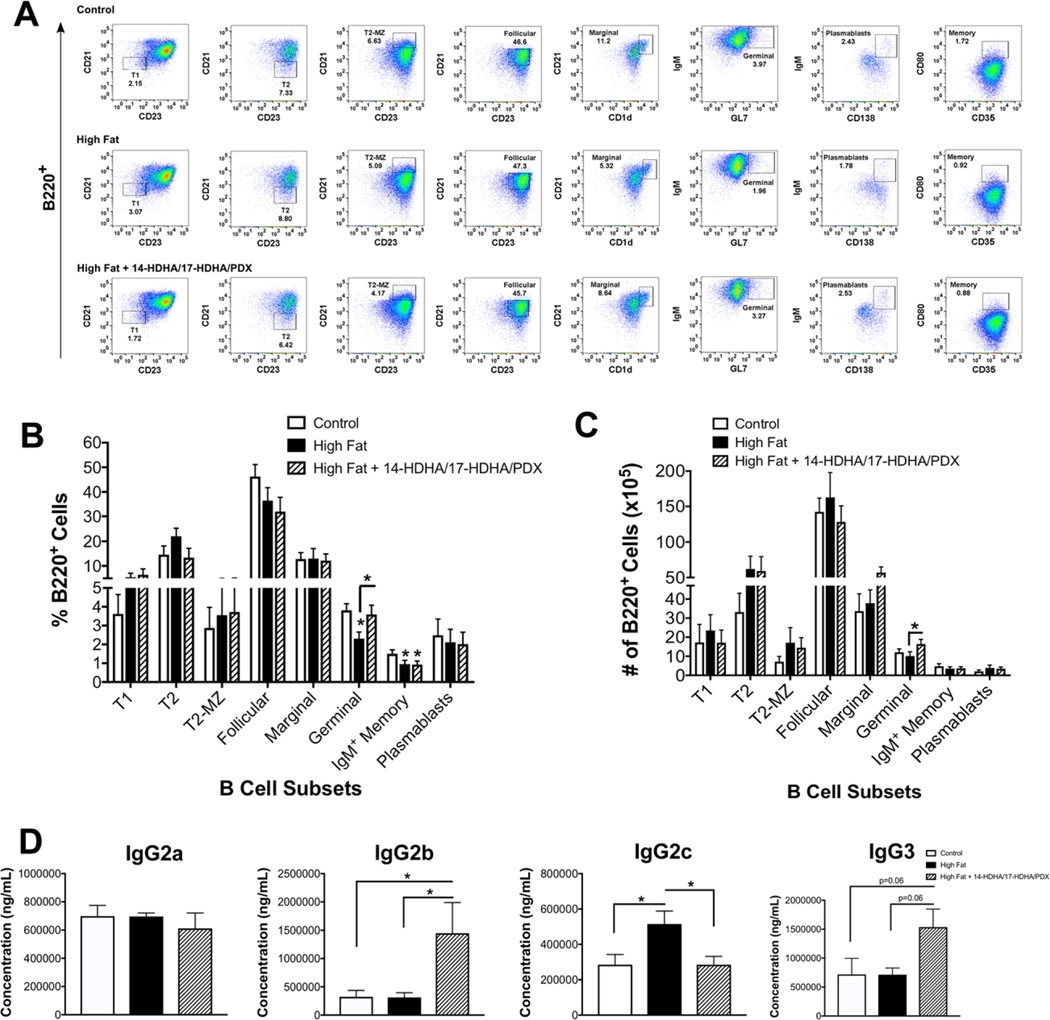
Next, we tested if obesity lowered select B cell subsets and if 4 days of simultaneous administration of 14-HDHA, 17-HDHA, and PDX could influence the number of key B cell populations. The rationale for 4 days of administration was based on a previous report to show this time range is adequate to increase SPM levels in obese mice.48 We confirmed that our protocol of injecting 14-HDHA, 17-HDHA, and PDX increased splenic levels of these mediators (Supplemental Table 3).
Obese mice receiving SPMs had no change in the number of total B220+ cells in the spleen (data not shown). Splenic B cell populations were analyzed by flow cytometry for mice consuming the control, high fat, and high fat diet + 14-HDHA/17-HDHA/PDX (Supplemental Fig. 1, Fig. 3A). A modest 1.65-fold decrease was observed in the percentage of B220+IgM+CD19+GL7+ germinal center B cells in male obese mice compared to the lean controls, which was increased with 14-HDHA/17-HDHA/PDX (Fig. 3B). The number of germinal center B cells were also modestly elevated with 14-HDHA/17HDHA/PDX administration relative to the mice consuming the high fat diet (Fig. 3C).
FIGURE 3. DHA-derived SPM precursors modestly increase germinal center B cells of male obese mice accompanied by increased IgG2b and a rescue of IgG2c.
(A) Sample flow cytometry plots of splenic B cell subsets for mice consuming a control, high fat, or high fat + 14-HDHA/17-HDHA/PDX including: B220+IgM+CD21lowCD23− (T1), B220+IgM+CD21−CD23+ (T2), B220+IgM+CD21highCD23+ (T2-MZ), B220+IgMlowCD21intCD23+ (Follicular), B220+IgMhighCD21+CD1dhigh (Marginal), B220+CD19+IgM+GL7+ (Germinal), B220+IgM+CD35+CD80+ (IgM+ Memory), B220lowIgM+CD138+ (Plasmablasts). (B) Percentage and (C) number of splenic B cell subsets. (D) Serum concentrations of IgG2a, IgG2b, IgG2c, and IgG3. Mice consumed experimental diets for 15 weeks. Mice received either an injection of vehicle control or a cocktail (900 ng/mouse) consisting of the SPM precursors 14-HDHA, 17-HDHA, and the SPM PDX once every day for 4 consecutive days. N = 6–10 mice per condition. Data are average ± S.E.M. *P < 0.05 by a one-way ANOVA followed by a Bonferroni post test (B and C)
We determined if mice receiving the 14-HDHA/17-HDHA/PDX had modifications to their serum IgG antibody subclasses (Fig. 3D). Mice consuming the high fat diet that received 14-HDHA/17HDHA/PDX had elevated IgG2b and IgG3 (P = 0.06) compared to their control and high fat counterparts (Fig. 3D). Notably, administration of 14-HDHA/17-HDHA/PDX to obese mice rescued the high fat diet-induced elevation in IgG2c, which is pathogenic in obesity (Fig. 3D).
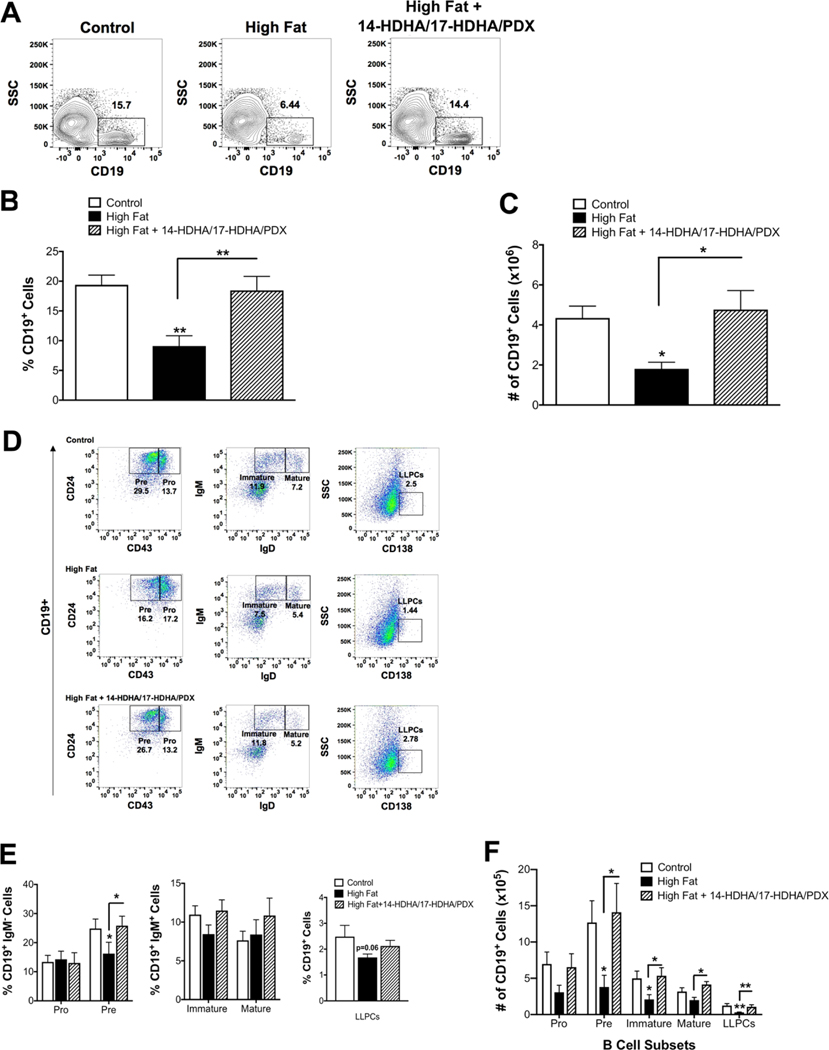
3.4 |. Administration of 14-HDHA/17-HDHA/PDX rescues the number of bone marrow B cells in obese mice
We also evaluated the effects of the SPM precursors and SPM on bone marrow B cells. The total CD19+ B cell population (Fig. 4A) in the bone marrow was decreased by ~2.3-fold when calculated as the percentage (Fig. 4B) and total number (Fig. 4C) with obese mice compared to controls. The male mice receiving 14-HDHA/17-HDHA/PDX had a 2–2.5-fold rescue in the percentage (Fig. 4B) and number (Fig. 4C) of CD19+ cells in the bone marrow compared to obese mice. Flow cytometry analysis of the B cell subsets in the bone marrow (Fig. 4D) revealed that the DHA-derived mediators rescued the percentage of pre B cells (Fig. 4E). Administration of 14-HDHA/17-HDHA/PDX to obese mice strongly rescued the reductions in the number of pre, immature, mature, and CD138+ long-lived plasma cells in the bone marrow (Fig. 4F). Collectively, these results establish that DHA-derived mediators are not just targeting plasma cells, as previously reported, and can influence several B cells subsets, notably in the bone marrow.15,35,46
FIGURE 4. DHA-derived SPM precursors and SPM rescue the decrease in the number of bone marrow B cell subsets of obese male mice.
(A) Sample flow cytometry plots for total CD19+ B cells in the bone marrow of mice consuming a control, high fat, and high fat diet +14-HDHA/17-HDHA/PDX. (B) Percentage and (C) number of CD19+ cells in the bone marrow. (D) Sample flow cytometry plots for B cell subsets in the bone marrow including CD19+CD43+CD24+IgM− (pro), CD19+CD43−CD24+IgM− (pre), CD19+CD24+IgM+IgD− (immature), CD19+IgM+IgD+ (mature), and CD19+CD138+ (long-lived plasma cells, LLPCs). (E) Percentage and (F) number of B cell subsets in the bone marrow. Mice received either an injection of vehicle control or an SPM cocktail (900 ng/mouse) consisting of 14-HDHA, 17-HDHA, and PDX once every day for 4 consecutive days. N = 7–10 mice per condition. Data are average ± S.E.M. *P < 0.05, **P < 0.01 by a one-way ANOVA followed by a Bonferroni post test
3.5 |. Splenic B-cell LOX levels are lowered in obese mice but do not account for the loss of SPM precursors or impairments in B cell populations
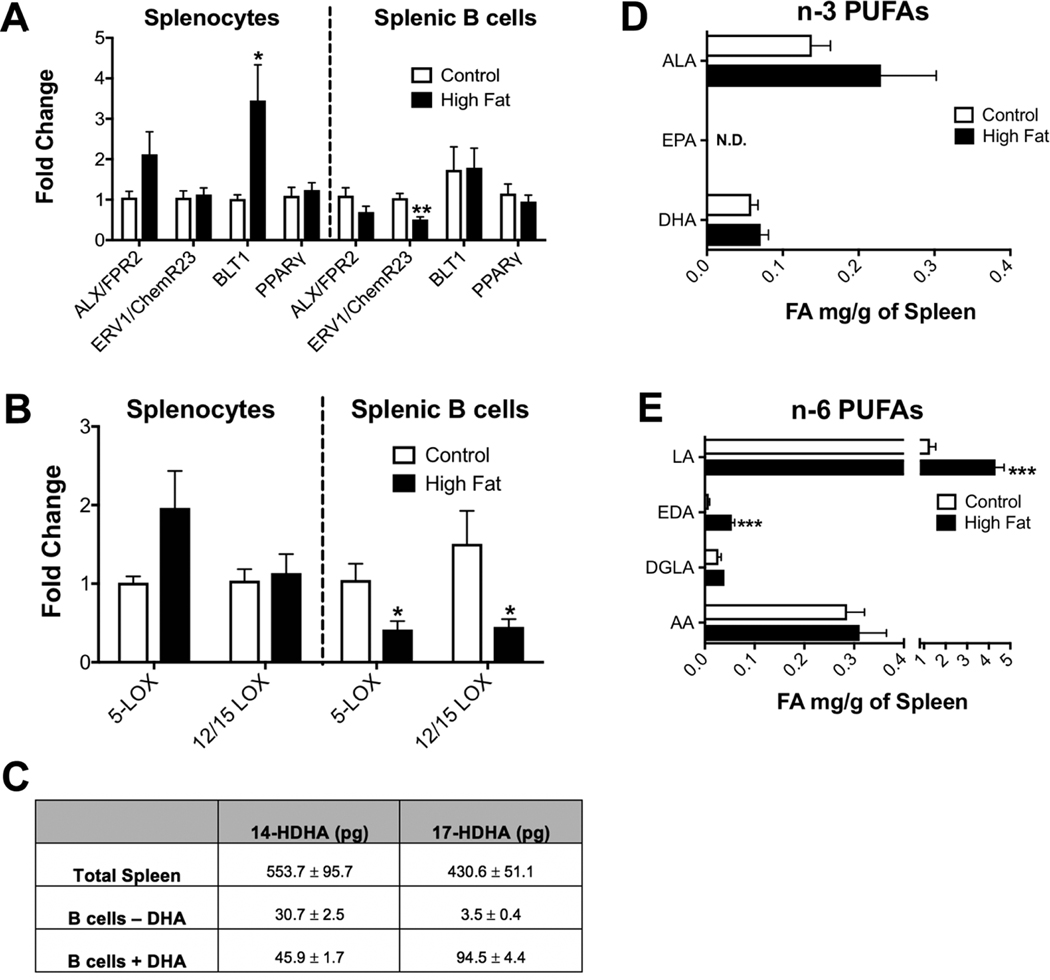
The next set of experiments addressed if the reduction in splenic SPM precursors with obesity was due to a deficiency in the transcript levels of SPM receptors of splenic B cells compared to splenocytes (Fig. 5A). The specific receptors for the SPM precursors 14-HDHA and 17-HDHA are unknown; thus, we conducted a general analysis of major known lipid mediator receptors. qRT-PCR analysis revealed a 3-fold increase in the transcript of BLT1 (a receptor for the leukotriene LTB4) in splenocytes, which may reflect changes in immune cell populations (Fig. 5A, left panel). ERV1/ChemR23, a receptor for RvE1, was decreased in splenic B cells of obese mice by 2-fold compared to their lean counterparts (Fig. 5A, right panel). Transcripts of ALX/FPR2, a receptor for RvD1 and LXA4, were identical between control and obese mice in splenocytes and splenic B cells (Fig. 5A). There were no differences in transcript expression of the RvD2 receptor, DRV2/GPR18, in splenic B cells between control and obese mice (data not shown). SPMs can potentially mediate their effects via PPAR𝛾, which serves as a natural ligand for parent n-3 PUFAs.49 However, there was no effect of obesity on splenocyte or B cell PPARγ transcripts between control and obese mice (Fig. 5A).
FIGURE 5. The reduction in SPM precursor production of male mice is not driven by a defect in dietary DHA or B cell production of 14-HDHA and 17-HDHA.
(A) qRT-PCR analysis of SPM receptors in splenocytes and splenic B cells. (B) qRT-PCR analysis of 5-LOX and 12/15-LOX in splenocytes and splenic B cells. (C) Concentration of B cell SPM precursor production with or without substrate (DHA) relative to the whole spleen. (D) GC/MS analyses of splenic ALA (18:3 n-3), EPA (20:5 n-3), and DHA (22:6 n-3). (E) Fatty acid analysis of the major splenic n-6 polyunsaturated fatty acids including linoleic acid (LA) (18:2 n-6), eicosadienoic acid (EDA) (20:2 n-6), dihomo-gamma-linolenic acid (DGLA) (20:3 n-6), and arachidonic acid (AA) (20:4 n-6). Fatty acid analyses show milligrams of fatty acid (FA) per gram of spleen. Mice consumed experimental diets for 15 weeks. N = 7 mice per diet (A and B), N = 3 mice per condition (C) and N = 10 mice per diet (D and E). Data are average ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001 by an unpaired Student’s t-test
We investigated if a defect in lipoxygenase (LOX) enzymes, which are responsible for the production of DHA-derived mediators could be driving SPM precursor deficiencies in obesity.50–53 Analysis of total splenocytes revealed no difference in the transcript levels of 5-LOX and 12/15-LOX in lean and obese male mice (Fig. 5B, left panel). Obese mice showed a 2–2.5-fold decrease in the transcript levels of 5- and 12/15-LOX in total splenic B cells (Fig. 5B, right panel).
To determine if a reduction in B cell SPM production could account for the defect in splenic 14-HDHA, 17-HDHA, and PDX levels, we calculated SPM precursor production from B cells relative to the whole spleen. Compared to the concentration in the whole spleen, 30.7 pg of the total concentration of 14-HDHA was attributable to B cells, which was increased to 45.9 pg upon addition of the LOX substrate DHA (Fig. 5C). B cells treated without or with DHA did not produce any detectable amount of PDX (data not shown). B cells in the absence of DHA accounted for 3.5 pg of the total splenic 17-HDHA. Upon addition of DHA, B cells accounted for 94.5 pg of the total splenic 17-HDHA (Fig. 5C). These data demonstrate for the first time that B cells can generate SPM precursors although the concentration of 14-HDHA and 17HDHA produced by splenic B cells was a minor proportion of the total concentration in the whole spleen (Fig. 5C).
Experiments were also conducted with ALOX5−/− mice to test if this enzyme is key for generation of splenic and bone marrow B cells. Studies with ALOX12/15−/− mice were not pursued since B cell subsets are not decreased in this model.54 Analyses of key B cell subsets from ALOX5−/− mice did not reveal a decrease in the percentage (Supplemental Fig. 2A) or number (Supplemental Fig. 2B) of B cell subsets in a manner similar to obese mice. Thus, these results suggest that the obesity-driven defect in SPM precursor levels and B cell populations was not at the level of the B cell or driven by 5-LOX, despite the ability of B cells to produce SPM precursors and a reduction in B cell 5-LOX levels.
3.6 |. Male obese mice have elevated levels of splenic n-6 PUFAs that have a high affinity for LOX
We hypothesized that the levels of dietary DHA, the parent molecule for 14-HDHA and 17-HDHA, were reduced in obese mice to lower 14-HDHA and 17-HDHA levels. Strikingly, GC/MS analyses revealed DHA and its parent molecule alpha-linolenic acid (ALA), were maintained in obese male mice (Fig. 5D). Alternatively, n-6 PUFAs could be elevated and thus simply outcompete n-3 PUFAs for key enzymes that synthesize SPM precursors. Indeed, obese mice had a 4.5-fold increase in the total amount of the parent n-6 PUFA, linoleic acid (Fig. 5E), and a 4.5-fold increase in its elongation product eicosadienoic acid (EDA) (Fig. 5E). There was no difference in the total amount of dihomo-γ-linoleic acid (DGLA) (Fig. 5E) or arachidonic acid (AA) (Fig. 5E) in the spleens of lean versus obese mice. These results suggest that male obese mice have elevated levels of linoleic acid that are abundant in the western diet and known to bind with a higher affinity to LOX enzymes than n-3 PUFA when present in excessive amounts.55–57
3.7 |. SPM precursors levels, antibody concentration, and B cell subsets are generally not lowered in female obese mice
The next set of studies focused on female mice. The metabolic phenotype of the female mice was first established. Female mice showed a significant increase in body weight after consuming experimental diets (Fig. 6A). The increase in total body mass was driven by elevated lean and fat mass (Fig. 6B). There were no changes in adipose tissue inflammatory transcripts between the two groups (Fig. 6C). These mice had impaired glucose clearance (Fig. 6D), as quantified by the area under the curve (Fig. 6D inset). Fasting glucose levels (Fig. 6E) were modestly increased and fasting insulin was not influenced by the obese female mice (Fig. 6F). Obesity did not influence the HOMA-IR score compared to controls (Fig. 6G). Finally, leptin levels were increased by 3-fold with obese female mice relative to controls (Fig. 6H).
FIGURE 6. Metabolic profile of female mice consuming a high fat diet.
(A) Body weights of female mice at the completion of the 15-week study. (B) Body composition assayed by Echo-MRI. (C) qRT-PCR analysis of inflammatory markers from white adipose tissue of control and obese mice. (D) Glucose tolerance test (GTT), performed by intraperitoneal injection of glucose after a 6 h fast. (Inset) Area under the curve (AUC), calculated by integration of the curve normalized to baseline values. (E) Fasting blood glucose levels determined after a 6 h fast. (F) Fasting insulin levels determined after a 6 h fast. (G) HOMA-IR scores. (H) Fasting leptin levels. N = 8–10 mice per diet (A–D), N = 6–8 mice per diet (E–H). Data are average ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001 by a Student’s unpaired t-test (A–C, E–H) or a two-way ANOVA analysis followed by a post hoc t-test (D)
Metabololipidomics revealed no difference in the levels of 14-HDHA or 17-HDHA between control and obese female mice (Supplemental Fig. 3A). The levels of PDX, RvD1, and RvD5 were maintained in obese female mice (Supplemental Fig. 3B). Experiments were also conducted to determine if antibody production and cytokine levels were impaired in response to obesity as previously reported in male mice.15 There was no change in murine ex vivo IgM or IgG production with the high fat diet upon stimulation with either CpG-ODN + anti-IgM or LPS (Supplemental Fig. 3C). Furthermore, the splenic B cells of control and obese female mice showed no differences in the expression of inflammatory transcripts in the absence of activation (Supplemental Fig. 3D).
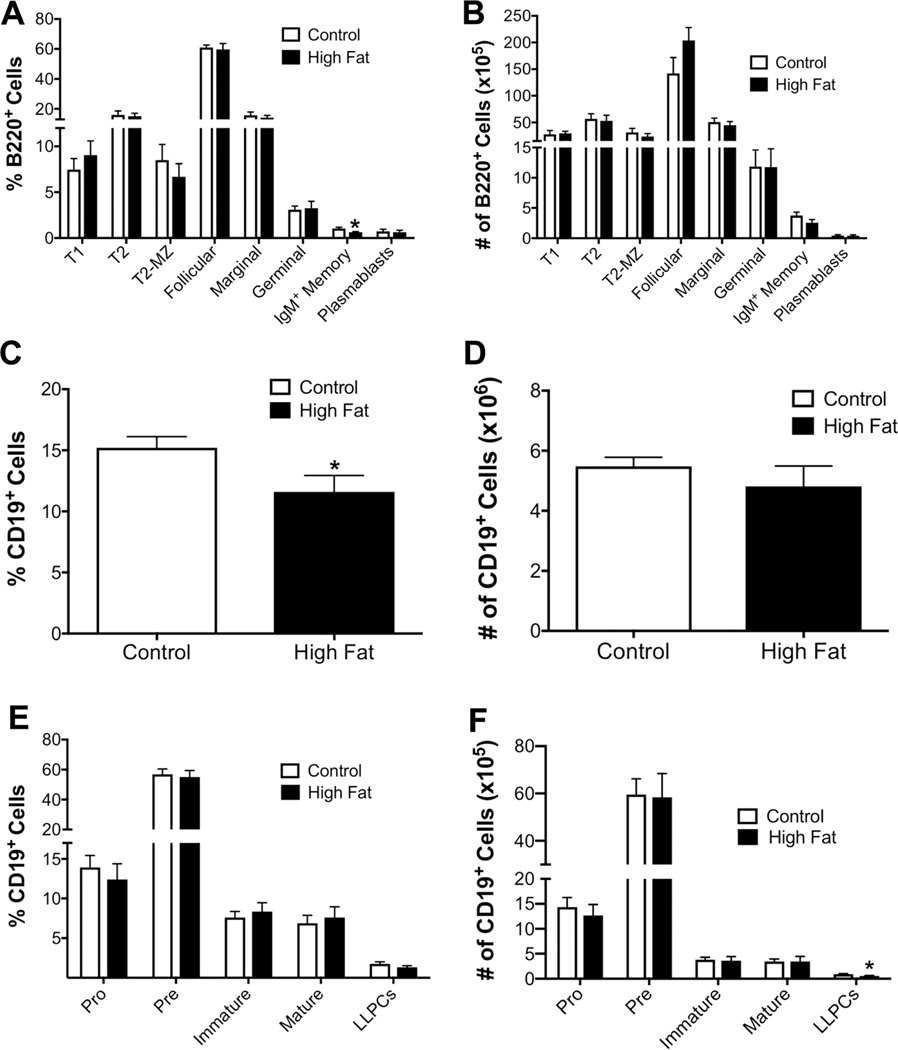
Subsequent experiments tested if obesity impaired the percentage and number of select B cell subsets in the spleen and bone marrow of female C57BL/6J mice. Flow cytometry analysis revealed a modest decrease in the percentage of splenic IgM+ memory B cells (Fig. 7A) by 1.65-fold in obesity with no change in the number of splenic B cell subsets (Fig. 7B). In the bone marrow, the percentage of CD19+ cells was statistically lowered by 3.6% (Fig. 7C); however, the number of CD19+ cells (Fig. 7D) was not influenced with obesity. Flow cytometry analysis of bone marrow B cell populations revealed that female obese mice showed no differences in the percentages of B cell subsets in the bone marrow (Fig. 7E). Female obese mice displayed a 1.62-fold decrease in the number of long-lived plasma cells in the bone marrow (Fig. 7F).
FIGURE 7. Obese female mice display small changes in splenic and bone marrow B cell subsets.
(A) Percentages and (B) numbers of B cell subsets in the spleen of control and obese female C57BL/6J mice. (C) Percentage and (D) number of total CD19+ B cells in the bone marrow. (E) Percentages and (F) number of B cell subsets in the bone marrow. Mice were fed lean control or high fat diets for 15 weeks. N = 7–8 mice per diet (A and B) and N = 9–10 for (C–F). Data are average ± S.E.M. *P < 0.05 by a Student’s unpaired t-test
3.8 |. Obese female humans have increased percentage of CD38+IgD−CD27+ B cells and elevated B cell cytokine secretion
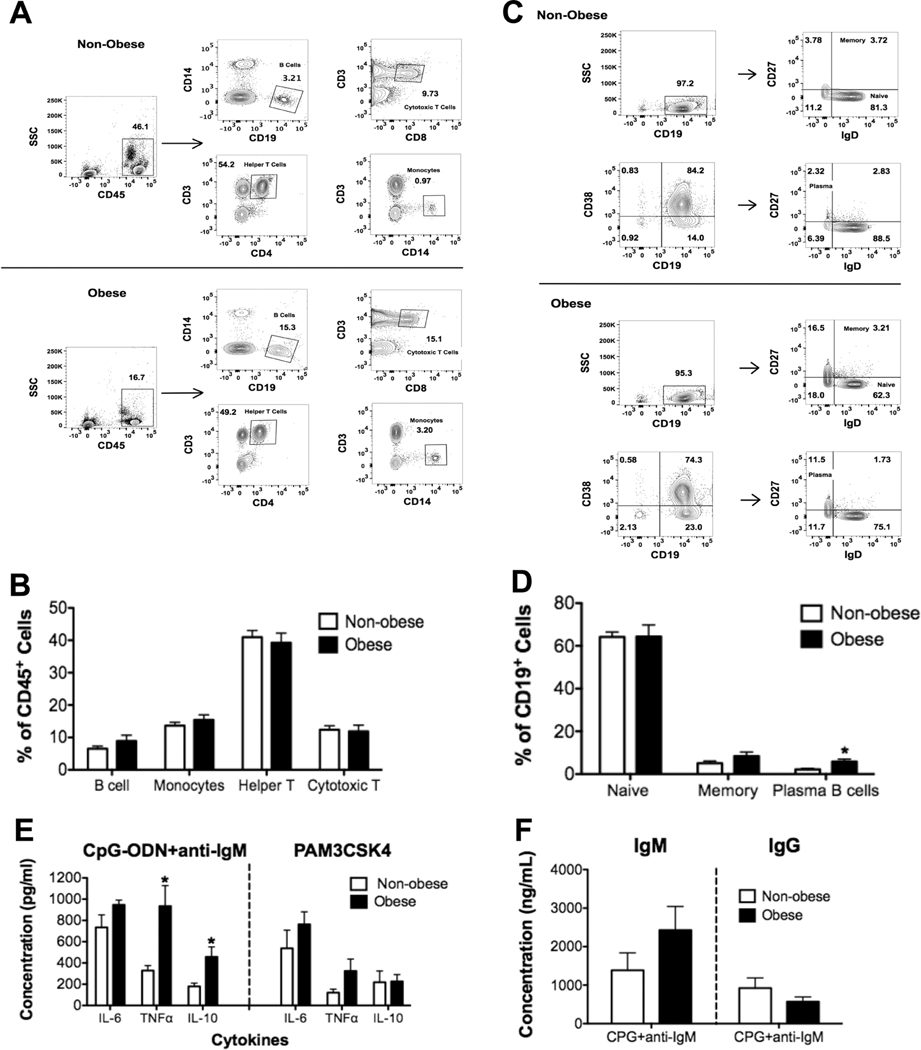
We finally determined if female obese subjects, relative to nonobese controls, displayed impairments in circulating B cell populations as we have previously measured in males.15 Flow cytometry analysis of PBMC populations (Fig. 8A) revealed no significant changes in the percentages of monocytes, helper T, or cytotoxic T cells (Fig. 8B). In addition, percentages of total B cells were not influenced by obesity. Flow cytometry analysis (Fig. 8C) of B cell subsets in obese females revealed a 5-fold increase in the percentage of plasma CD19+CD38+IgD−CD27+ B cells, but no changes in naïve or memory B cells, compared to nonobese subjects (Fig. 8D).
FIGURE 8. Obese females have increased levels of plasma B cells relative to nonobese females and elevated levels of select cytokines.
(A) Sample flow cytometry data showing the percentage of CD45+CD14−CD19+ (B cells), CD45+CD3+CD8+ (cytotoxic T cells), CD45+CD3+CD4+ (helper T cells), and CD45+CD3−CD14+ (monocytes) in peripheral blood mononuclear cells. (B) Quantification of the percentages of B cells, monocytes, helper T cells, and cytotoxic T cells. (C) Sample flow cytometry plots of memory (CD19+IgD+CD27+) and plasma B cells (CD19+CD38+IgD−CD27+). (D) Quantification of the percentages of naïve, memory, and plasma B cells in peripheral blood mononuclear cells. (E) Ex vivo B-cell cytokine secretion upon CpG-ODN + anti-IgM or PAM3CSK4 stimulation. (F) Ex vivo IgM and IgG concentrations upon stimulation of B cells with CpG-ODN + anti-IgM for nonobese and obese females. N = 10 female human subjects per group for (A–D). N = 9 female human subjects per group for (E and F). Data are average ± S.E.M. *P < 0.05 by unpaired Student’s t test
B cells from female humans were also challenged ex vivo with CPG-ODN+anti-IgM and Pam3CSK4 (TLR1/2 agonist). Obese females had increased ex vivo B-cell TNFα and IL-10 secretion by 2.8- and 2.5-fold, respectively, when challenged with CPG-ODN+anti-IgM but not when challenged with PAM3CSK4 (Fig. 8E). Activation of B cells with CPG-ODN+anti-IgM did not reveal any difference with IgM or IgG levels between obese and nonobese subjects (Fig. 8F).
4 |. DISCUSSION
The data from this study advance the fields of B cell immunology, lipid metabolism, and obesity by demonstrating that: (1) 14-HDHA, 17-HDHA, and PDX are decreased in male obese mice and that administration of these molecules rescues some of the reductions in differing B cell subsets and IgG2c, which is pathogenic in obesity; (2) the underlying reduction in 17-HDHA and 14-HDHA levels and thereby dysregulated B cell subsets are not due to a defect at the level of the B cell or driven by a loss of dietary DHA; (3) B cells can generate SPM precursors; (4) female obese mice have no change in levels of SPMs and their precursors accompanied by small to modest impairments in splenic and bone marrow B cell populations; and (5) obese female mice and humans show no defects in ex vivo antibody production although humans have elevated plasma cells and some elevated B cell cytokines upon BCR/TLR9 stimulation.
The results suggest a complex model by which several cellular and molecular factors regulate SPM precursor levels and thereby B cell populations in the spleen and bone marrow of male mice. Strikingly, DHA and its precursor ALA, which regulate metabolic responses, were not lowered in obese male mice.58 This demonstrated that a reduction in dietary DHA or ALA levels is not driving the reduction in murine SPM precursors. This may not hold true in humans given that essential and conditionally essential fatty acid deficiencies exist with obese subjects, which could lead to impaired SPM pathway metabolites.30–32
DHA in membrane phospholipids is liberated and subsequently hydroxylated by LOX enzymes to generate SPM precursors. B cell 12/15-and 5-LOX transcripts were lowered in obese male mice suggesting a potential defect at the level of the B cell. However, we found no evidence that B cell production of 17-HDHA or 14-HDHA could account for the loss of SPMs. Furthermore, experiments with ALOX5−/− mice showed that a defect in this enzyme was not responsible for the reduction in splenic and bone marrow B cell subsets. We did not pursue experiments with ALOX12/15−/− mice since it is reported that some B cell populations actually increase upon the loss of B cell 12/15-LOX.54
Future studies will need to focus on how other key cells in the spleen such as macrophages, monocytes, neutrophils, and T cells are contributing toward lowering of DHA-derived mediators.24,53,59 In the bone marrow, mesenchymal stromal cells can produce SPMs, which could contribute toward reduced levels of SPM precursors.60 It is also possible that reduced levels of SPMs in obese mice are limiting leukocyte trafficking and signaling to primary and secondary organs such as the bone marrow and spleen, respectively.61–63 The discovery that SPM precursors were decreased in the spleen and bone marrow of obese mice was highly consistent with data demonstrating diminished SPMs in mouse and human adipose tissue depots.29,48
Select splenic n-6 PUFAs were elevated several fold compared to DHA, which modeled how humans consume considerably larger quantities of n-6 to n-3 fatty acids.64 Linoleic acid was dramatically increased relative to DHA in obese male but not female mice. This was driven by the high levels of linoleic acid present in the mouse diet. LOX prefers n-3 PUFAs if n-6 PUFA abundance is relatively low; however, linoleic acid is more likely to bind 12/15-LOX when it is in excess.55,57 As a result, DHA likely did not serve as the primary substrate for the LOX with differing cell types, resulting in less hydroxylation and subsequent generation of 14-HDHA and 17-HDHA. In addition, the consistently high levels of n-6 PUFAs could prevent lipid mediator class-switching to n-3-derived SPMs in obesity, which is needed to promote resolution of the inflammatory response.
The finding that SPM precursors increased B cell population numbers in the bone marrow and B220+IgM+CD19+GL7+ germinal center B cells of obese mice strongly support the emerging notion that SPMs are critical for humoral immunity. For instance, germinal center B cells are an essential source for the development of plasma and memory B cells, which both provide protection upon exposure to pathogens.65,66 In addition, it was recently reported that splenic B cells from obese/type 2 diabetic mice administered S. aureus displayed inadequate class switching.67 Therefore, it is possible that SPMs are aiding the process of somatic hypermutation in the germinal center of obese mice, which produces highly specialized plasma and memory B cells.46 We did not measure a change in the production of plasma and memory B cells, or in the number of IgG+ memory B cells. Subsequent experiments will need to address this using different antigens that will stimulate differing B cell subsets including germinal center, plasma, and IgG+ memory B cells. Furthermore, we will need to further investigate the flow cytometry data with immunohistochemistry analyses of the spleen. We also did not determine if the increase in B cell populations with the cocktail was due to increased trafficking of B cells into differing tissues such as the adipose or driven by enhanced proliferation. Future studies will also need to dissect which lipid mediator is rescuing each B cell subset. It is plausible that each lipid mediator has unique effects on each B cell subset.
A notable finding was that administration of 14-HDHA/17-HDHA/PDX rescued the obesity-induced elevation in IgG2c. The increase in IgG2c was highly consistent with previous work by Winer et al., to show that pro-inflammatory IgG2c is elevated in obesity.14 The ability of the DHA-derived mediators to rescue this was notable as these molecules are highly potent immunoresolvants.24 The DHA-derived mediators also increased other IgG subclasses, providing evidence that SPMs regulate antibody production.33 These results open the door to investigating the role of DHA and its metabolites on B cell class switching.
Females generally exhibit a stronger humoral and cellular immune response after infection as well as improved vaccination compared to males during premenopausal years.38,68 Our findings, compared to our previous work, significantly expand on this notion by establishing that female obese mice did not display large reductions in B cell populations of the bone marrow and spleen.15 Similarly, obese women had increased numbers of plasma B cells relative to their controls, differing from obese men.15 Females are known to have elevated B cell numbers compared to males.69 Therefore, obese females could have increased B cell pools in the bone marrow and spleen resulting in a constant flux of B cells, which maintains adequate B cell numbers.
There are likely several mechanistic factors that could explain why female mice did not show the same results as the males. The females displayed no deficiencies in 14-HDHA, 17-HDHA, PDX, or RvD1 with obesity. It is reported that overall synthesis of DHA is higher in women than men and is independent of dietary differences, offsetting the increased flux of n-6 PUFAs present in a high fat diet.70–73 Furthermore, the capacity to generate DHA from the parent n-3 PUFA, ALA, is higher in women than men suggesting that DHA could be converted to downstream mediators more efficiently in obese females than obese males. The results open a new area study; that is, investigating differences in fatty acid and SPM metabolism between sexes in the context of obesity, which could have strong implications for the use of drugs that target PUFA metabolic pathways. For instance, Pace et al., recently demonstrated that females are more responsive to leukotriene biosynthesis inhibitors than males due to differences in androgens between sexes.74 Similarly, there are data to show that female humans are protected from inflammation induced endothelial impairments due to elevated levels of specific SPMs.75
The males and females had different metabolic profiles, which would influence the levels of SPM precursors. Notably, male mice tended to weigh more than the females after administering the diets for the same period of time although the amount of fat mass was comparable between sexes. Furthermore, the obese males had increased insulin levels and elevated adipose tissue inflammatory gene expression compared to females, which would then influence glucose uptake. Finally, obese females may have protective B cell responses due to the effects of estrogen and the increased production of IL-10. For instance, estrogen increases somatic hypermutation and class switching recombination resulting in high affinity-Ig producing cells in females.37,76,77
Collectively, the data establish that a reduction in SPM pathway metabolites synthesized from DHA contribute toward some of the impairments in the proportion of B cell subsets and antibody production of obese male, but not female mice. Mechanistically, the defect is not at the level of the B cell or due to a decrease in the levels of DHA and may be due extrinsic factors such elevated levels of n-6 fatty acids. The results set the foundation for future studies on underlying metabolic factors that may drive abnormal B cell responses in obesity through changes in DHA metabolism in male obese mice.69
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by: NIH R01AT008375 (S.R.S.), Foundation Award from Caroline Raby (S.R.S.), funds from UNC Chapel Hill Gillings School of Global Public Health (S.R.S.), P30DK056350 (S.R.S.), NIH S10RR026522 (N.R.), and NIH UL1TR001082 (N.R.). The UNC Flow Cytometry Core Facility is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center.
We thank the UNC Flow Cytometry Core for advice in study design, use of flow cytometry and instrument funding.
Abbreviations:
- 12-HETE
12-hydroxyeicosatetraenoic acid
- 14-HDHA
14(S)-hydroxydocosahexaenoic acid
- 17-HDHA
17(S)-hydroxydocosahexaenoic acid
- AA
arachidonic acid
- ALA
alpha-linolenic acid
- BCR
B cell receptor
- ChemR23
chemerin receptor 23
- DGLA,
dihomo-γ-linoleic acid
- DHA
docosahexaenoic acid
- EDA
eicosadienoic acid
- EPA
eicosapentaenoic acid
- LA
linoleic acid
- LOX
lipoxygenase
- LPS
lipopolysaccharide
- LTB4
leukotriene B4
- LXA4
lipoxin A4
- PDX
protectin DX
- PUFA
polyunsaturated fatty acids
- RvD1
resolvin D1
- RvD5
resolvin D5
- SPMs
specialized pro-resolving lipid mediators
- TLR9
toll-like receptor 9
- TxB2
thromboxane B2
Footnotes
DISCLOSURES
The authors declare that they have no conflicts of interest with the contents of this article.
SUPPORTING INFORMATION
Additional information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Sheridan PA, Paich HA, Handy J, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond). 2012;36:1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang H, Youm Y-H, Vandanmagsar B, et al. Obesity accelerates thymic aging. Blood. 2009;114:3803–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Cir-culating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–1571. [DOI] [PubMed] [Google Scholar]
- 4.Weber DJ, Rutala WA, Samsa GP, Santimaw JE, Lemon SM. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA. 1985;254:3187–3189. [PubMed] [Google Scholar]
- 5.Bandaru P, Rajkumar H, Nappanveettil G. The impact of obesity on immune response to infection and vaccine: an insight into plausible mechanisms. Endocrinol Metab Syndr. 2013;2:113. [Google Scholar]
- 6.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15: 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fatis enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bremer AA, Jialal I. Adipose tissue dysfunction in nascent metabolic syndrome. J Obes. 2013:393192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frasca D, Diaz A, Romero M, Vazquez T, Blomberg BB. Obesity induces pro-inflammatory B cells and impairs B cell function in old mice. Mech Ageing Dev. 2017;162:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frasca D, Ferracci F, Diaz A, Romero M, Lechner S, Blomberg BB. Obesity decreases B cell responses in young and elderly individuals. Obesity. 2016;24:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenhill C. Metabolism: contribution of B cells to obesity and insulin resistance. Nat Rev Endocrinol. 2013;9:315. [DOI] [PubMed] [Google Scholar]
- 12.DeFuria J, Belkina AC, Jagannathan-Bogdan M, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of Tcell function and an inflammatory cytokine profile. Proc Natl Acad Sci. 2013;110:5133–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagannathan M, McDonnell M, Liang Y, et al. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia. 2010;53:1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winer DA, Winer S, Shen L, et al. B lymphocytes promote insulin resistance through modulation of T lymphocytes and production of pathogenic IgG antibody. Nat Med. 2011;17:610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosaraju R, Guesdon W, Crouch MJ, et al. B cell activity is impaired in human and mouse obesity and is responsive to an essential fatty acid upon murine influenza infection. J Immunol. 2017;198: 4738–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler BJ, Green DE, Pagnotti GM, Chan ME, Rubin CT. High fat diet rapidly suppresses B lymphopoiesis by disrupting the supportive capacity of the bone marrow niche. PLoS One. 2014;9(3):e90639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rockett BD, Salameh M, Carraway K, et al. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. J Lipid Res. 2010;51:1284–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Sanctis JB, Blanca I, Rivera H, Bianco NE. Expression of low-density lipoprotein receptors in peripheral blood and tonsil B lymphocytes. Clin Exp Immunol. 1998;113:206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98: 800–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teague H, Fhaner CJ, Harris M, Duriancik DM, Reid GE, Shaikh SR. n-3 PUFAs enhance the frequency of murine B-cell subsets and restore the impairment of antibody production to a T-independent antigen in obesity. J Lipid Res. 2013;54:3130–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teague H, Harris M, Fenton J, Lallemand P, Shewchuk BM, Shaikh SR. Eicosapentaenoic and docosahexaenoic acid ethyl esters differentially enhance B-cell activity in murine obesity. J Lipid Res. 2014;55: 1420–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serhan CN, Clish CB, Brannon J, Colgan SP, Gronert K, Chiang N. Anti-microinflammatory lipid signals generated from dietary N-3 fatty acids via cyclooxygenase-2 and transcellular processing: a novel mechanism for NSAID and N-3 pufa therapeutic actions. J Physiol Pharmacol. 2000;51:643–654. [PubMed] [Google Scholar]
- 23.Serhan CN, Dalli J, Karamnov S, et al. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. J Fed Am Soc Exp Biol. 2012;26:1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27:200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. LipoxinA4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993;92:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programs. Nature. 2007;447:869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clària J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol. 2012;189:2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neuhofer A, Zeyda M, Mascher D, et al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes. 2013;62:1945–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Micallef M, Munro I, Phang M, Garg M. Plasma n-3 polyunsaturated fatty acids are negatively associated with obesity. Br J Nutr. 2009;102:1370–1374. [DOI] [PubMed] [Google Scholar]
- 31.Albert BB, Derraik JGB, Brennan CM, et al. Higher omega-3 index is associated with increased insulin sensitivity and more favourable metabolic profile in middle-aged overweight men. Sci Rep. 2014;4:6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Y, Cai Z, Zheng J, et al. Serum levels of polyunsaturated fatty acids are low in Chinese men with metabolic syndrome, whereas serum levels of saturated fatty acids, zinc, and magnesium are high. Nutr Res. 2012;32:71–77. [DOI] [PubMed] [Google Scholar]
- 33.Ramon S, Baker SF, Sahler JM, et al. The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant? J Immunol. 2014;193:6031–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim N, Ramon S, Thatcher TH, Woeller CF, Sime PJ, Phipps RP. Specialized pro-resolving mediators (SPMs) inhibit human B-cell IgE production. Eur J Immunol. 2016;46:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramon S, Bancos S, Serhan CN, Phipps RP. Lipoxin A(4) decreases human memory B cell antibody production via an ALX/FPR2dependent mechanism: a link between resolution signals and adaptive immunity. Eur J Immunol. 2014;44:357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diaz LA, Altman NH, Khan W, Serhan CN, Adkins B. Specialized proresolving mediators (SPM) rescue infant mice from lethal Citrobacter rodentium infection and promote immunity against re-infection. Infect Immun. 2017;85:464–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. [DOI] [PubMed] [Google Scholar]
- 38.Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drew Rockett B, Harris M, Raza Shaikh S. High dose of an n-3 polyunsaturated fatty acid diet lowers activity of C57BL/6 mice. Prostaglandins Leukot Essent Fat Acids. 2012;86:137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kane DA, Lin CT, Anderson EJ, et al. Progesterone increases skeletal muscle mitochondrial H2O2 emission in nonmenopausal women. Am J Physiol Endocrinol Metab. 2011;300:528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medina AL, da Silva M, de Sousa Barbosa H, Arruda M, Marsaioli A, Bragagnolo N. Rapid microwave assisted extraction of meat lipids. Food Res Int. 2015;78:124–130. [DOI] [PubMed] [Google Scholar]
- 42.Ichihara K, Fukubayashi Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J Lipid Res. 2010;51:635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kilburg-Basnyat B, Reece SW, Crouch MJ, et al. Specialized pro-resolving lipid mediators regulate ozone-induced pulmonary and systemic inflammation. Toxicol Sci. 2018;163:466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juge-Aubry CE, Somm E, Pernin A, et al. Adipose tissue is a regulated source of interleukin-10. Cytokine. 2005;29:270–274. [DOI] [PubMed] [Google Scholar]
- 45.Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-α secretion from human T Cells. Immunol. 2003;170:6266–6272. [DOI] [PubMed] [Google Scholar]
- 46.Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody secreting cells. J Immunol. 2012;189:1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Titos E, Rius B, López-Vicario C, et al. Signaling and immunoresolving actions of resolvin D1 in inflamed human visceral adipose tissue. J Immunol. 2016;197:3360–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.González-Périz A, Horrillo R, Ferré N, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by ω−3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H, Ruan XZ, Powis SH, et al. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2017;67:867–874. [DOI] [PubMed] [Google Scholar]
- 50.Fredman G, Hellmann J, Proto JD, et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. 2016;7:12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levy BD, Romano M, Chapman HA, Reilly JJ, Drazen J, Serhan CN. Human alveolar macrophages have 15-lipoxygenase and generate 15(S)-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid and lipoxins. J Clin Invest. 1993;92:1572–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong S, Gronert K, Devchand PR, Moussignac R-L, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. [DOI] [PubMed] [Google Scholar]
- 54.Lauder SN, Tyrrell VJ, Allen-Redpath K, et al. Myeloid 12/15-LOXregulates B cell numbers and innate immune antibody levels in vivo. Wellcome Open Res. 2017;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Das UN. Biological significance of essential fatty acids. J Assoc Physicians India. 2006;54:309–319. [PubMed] [Google Scholar]
- 56.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. [DOI] [PubMed] [Google Scholar]
- 57.Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J Nutr Metab. 2012:539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang C-W, Chien Y-S, Chen Y-J, Ajuwon KM, Mersmann HM, Ding S-T. Role of n-3 polyunsaturated fatty acids in ameliorating the obesityinduced metabolic syndrome in animal models and humans. Int J Mol Sci. 2016;17(1):1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ariel A, Li P-L, Wang W, et al. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280:43079–43086. [DOI] [PubMed] [Google Scholar]
- 60.Tsoyi K, Hall SRR, Dalli J, et al. Carbon monoxide improves efficacy of mesenchymal stromal cells during sepsis by production of specialized proresolving lipid mediators. Crit Care Med. 2016;44: 1236–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serhan CN, Chiang N, Dalli J, Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harb Perspect Biol. 2015;7(2):a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121: 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dalli J, Zhu M, Vlasenko NA, et al. The novel 13S,14S-epoxy-maresinisconverted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013;27:2573–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simopoulos AP. Overview of evolutionary aspects of omega 3 fatty acids in the diet. World Rev Nutr Diet. 1998;83:1–11. [DOI] [PubMed] [Google Scholar]
- 65.Kaji T, Ishige A, Hikida M, et al. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J Exp Med. 2012;209:2079–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dogan I, Bertocci B, Vilmont V, et al. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292–1299. [DOI] [PubMed] [Google Scholar]
- 67.Farnsworth CW, Schott EM, Benvie A, et al. Exacerbated Staphylococcus aureus foot infections in obese/diabetic mice are associated with impaired germinal center reactions, Ig class switching, and humoral immunity. J Immunol. 2018;201(2):560–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26:3551–3555. [DOI] [PubMed] [Google Scholar]
- 69.Abdullah M, Chai P-S, Chong M-Y, et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol. 2012;272:214–219. [DOI] [PubMed] [Google Scholar]
- 70.Giltay EJ, Gooren LJG, Toorians AWFT, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr. 2004;80:1167–1174. [DOI] [PubMed] [Google Scholar]
- 71.Tavendale R, Lee AJ, Smith WCS, Tunstall-Pedoe H. Adipose tissue fatty acids in Scottish men and women: results from the Scottish heart health study. Atherosclerosis. 1992;94:161–169. [DOI] [PubMed] [Google Scholar]
- 72.Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docos apentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br J Nutr. 2002;88:355–363. [DOI] [PubMed] [Google Scholar]
- 73.Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002;88:411–420. [DOI] [PubMed] [Google Scholar]
- 74.Pace S, Pergola C, Dehm F, et al. Androgen-mediated sex bias impairs efficiency of leukotriene biosynthesis inhibitors in males. J Clin Invest. 2017;127:3167–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rathod KS, Kapil V, Velmurugan S, et al. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J Clin Invest. 2017;127:169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mai T, Zan H, Zhang J, Hawkins JS, Xu Z, Casali P. Estrogen receptors bind to and activate the HOXC4/HoxC4 promoter to potentiate HoxC4-mediated activation-induced cytosine deaminase induction, immunoglobulin class switch DNA recombination, and somatic hypermutation. J Biol Chem. 2010;285:37797–37810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pauklin S, Petersen-Mahrt SK. Progesterone inhibits activation-induced deaminase by binding to the promoter. J Immunol. 2009;183:1238–1244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.