Abstract
Background:
Residual shunt is observed in up to 25% of patients after patent foramen ovale (PFO) closure, but its long-term influence on stroke recurrence currently is unknown.
Objective:
To investigate the association of residual shunt after PFO closure with the incidence of recurrent stroke and transient ischemic attack (TIA).
Design:
Prospective cohort study comparing stroke or TIA recurrence in patients with and without residual shunt after PFO closure.
Setting:
Single hospital center.
Participants:
1078 consecutive patients (mean age, 49.3 years) with PFO-attributable cryptogenic stroke who were undergoing percutaneous PFO closure were followed for up to 11 years.
Measurements:
Residual shunt was evaluated by transthoracic echocardiography with saline contrast. Primary outcome was a composite of the first recurrent ischemic stroke or TIA after PFO closure.
Results:
Compared with complete closure, the presence of residual shunt after PFO closure was associated with an increased incidence of recurrent stroke or TIA: 2.32 versus 0.75 events per 100 patient-years (hazard ratio [HR], 3.05 [95% CI, 1.65 to 5.62]; P < 0.001). This result remained robust after adjustment for important covariates, namely age; study period; device; presence of atrial septal aneurysm, hypertension, hyperlipidemia, diabetes, hypercoagulability, or hypermobile septum; and medication use (HR, 3.01 [CI, 1.59 to 5.69]; P < 0.001). Further stratification based on shunt size revealed that moderate or large residual shunts were associated with a higher risk for stroke or TIA recurrence (HR, 4.50 [CI, 2.20 to 9.20]; P < 0.001); the result for small residual shunts was indeterminate (HR, 2.02 [CI, 0.87 to 4.69]; P = 0.102).
Limitation:
Nonrandomized study with potential unmeasured confounding.
Conclusion:
Among patients undergoing PFO closure to prevent future stroke, the presence of residual shunt, particularly a moderate or large residual shunt, was associated with an increased risk for stroke or TIA recurrence.
Primary Funding Source:
National Institutes of Health.
Patent foramen ovale (PFO), a congenital right-to-left interatrial shunt, is increasingly recognized as a major etiology of “cryptogenic stroke”—a historical term referring to stroke of unknown cause after exhaustive evaluation (1). The mechanism of PFO-related cryptogenic stroke is attributed to the passage of paradoxical embolism through the PFO shunt into the arterial circulation (1–7). Recent clinical trials and systematic reviews showed efficacy for PFO closure—to eliminate the shunt—in preventing recurrent stroke, particularly in patients with a large shunt (8–12), highlighting the importance of PFO shunt physiology in causing strokes (3, 13, 14).
However, in clinical practice, residual shunt may be observed in up to 25% of patients after PFO closure, and nearly 10% show moderate to large residual shunting, with unclear clinical significance (11–13). We prospectively investigated the long-term association of residual PFO shunt with stroke or transient ischemic attack (TIA) recurrence after percutaneous PFO closure.
Methods
Study Design and Patient Recruitment
This prospective study of patients undergoing PFO closure to prevent stroke recurrence had prespecified outcome measures during the follow-up. The study is reported in accordance with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines (15). Consecutive patients with PFO-attributable cryptogenic stroke and eligible for percutaneous PFO closure were prospectively recruited between January 1995 and November 2017 (per a National Institutes of Health data capture timeline) at Massachusetts General Hospital in accordance with institutional review board approval. A cryptogenic stroke was deemed to be related to PFO after extensive stroke work-up and multidisciplinary discussion to rule out other identifiable mechanisms, such as a definite cardioembolism (such as atrial fibrillation); large artery atherosclerosis; small vessel disease; or another, less common mechanism (such as vasculitis or trauma) (1). All patients had comprehensive neurologic, cardiac, hematologic, and imaging evaluations, including magnetic resonance imaging or computed tomography of the brain, intracranial and extracranial vascular imaging by magnetic resonance angiography or ultrasonography, Holter or prolonged outpatient cardiac monitoring, hypercoagulability work-up (to look for prothrombin gene or factor V Leiden mutation; protein C, protein S, or anti-thrombin III deficiency; antiphospholipid antibodies; and elevated lipoprotein(a) and homocysteine levels) (Supplement Table 1, available at Annals.org), and May–Thurner anatomy screening (16, 17). Each case was presented to the institutional PFO committee, which included external neurologists, cardiologists, hematologists, primary care physicians, and experts in peripheral vascular disease (12 members, rotating to avoid bias), to review patients’ medical records and studies independently, as mandated by the institutional review board. For each patient, the benefits and risks of PFO closure were thoroughly discussed, including the probability that the index event was PFO attributable, the risk for a recurrent thromboembolic event, the patient’s tolerance to anticoagulants, and the patient’s lifestyle and family history. Patent foramen ovale closure was offered only to patients with PFO-related cryptogenic stroke, as determined by 2 independent vascular neurologists after exhaustive work-up. Candidates for a second PFO closure device were not enrolled in the study.
PFO Closure and Antiplatelet or Anticoagulant Treatment
Percutaneous PFO closure was performed under transesophageal echocardiographic guidance by using various closure devices (Supplement Table 1). At discharge, antiplatelet treatment with aspirin, clopidogrel, or both was given to all patients. For patients with a hypercoagulable condition, a single thromboembolic event, and a modifiable clotting risk factor, short-term anticoagulation with warfarin was prescribed for 3 months, followed by aspirin thereafter. Lifelong anticoagulant therapy was prescribed for patients with hypercoagulable status and more than 1 unprovoked thromboembolic event.
Patients were followed at 1, 6, and 12 months and then annually for at least 5 years after closure. After that, patients were invited for further annual clinical visits or followed by telephone interview. Their electronic medical records also were reviewed to identify clinical events. Patients were considered lost to follow-up if they withdrew from the study or were no longer reachable by any means. Patients contributed person-time from the time of PFO closure until the first recurrence of a stroke or TIA, death, loss to follow-up, or the end of the study.
Residual Shunt Evaluation
Transthoracic echocardiogram (TTE) with agitated saline microbubbles was performed 24 hours after device placement and at each follow-up visit under an institutional standardized protocol to evaluate residual shunt at rest and during Valsalva maneuver in accordance with American Society of Echocardiography guidelines (18–21). Shunt size was determined by the maximum number of bubbles appearing in the left atrium within 3 cardiac cycles after agitated saline injection, with 0 bubbles indicating no shunt, 1 to 10 bubbles indicating small shunt, 10 to 30 bubbles indicating moderate shunt, and more than 30 bubbles indicating large shunt. For patients followed for longer than 1 year, shunt severity was classified according to TTE at 1-year follow-up. The 1-year time point was chosen to allow shunt status to stabilize, because residual shunting evident immediately after the procedure often diminishes or resolves over 1 year clinically. For patients with less than 1 year of follow-up due to a recurrent event, death, loss to follow-up, or study termination, echocardiographic results from their last follow-up visit were used. Results were read by cardiologists specializing in echosonography, who were blinded to the study.
Transesophageal echocardiography was not used to gauge shunt, because patients under anesthesia often cannot perform the Valsalva maneuver satisfactorily to open the PFO for shunt size quantification. It also is more invasive over longitudinal follow-up (Supplement Methods, available at Annals.org).
Outcome
The prespecified primary outcome was the composite of first recurrent ischemic stroke or TIA after PFO closure. A neurologist was consulted if any recurrent neurologic event occurred or was reported by a patient. In these cases, a full stroke work-up was initiated if the patient was deemed to have had a stroke or TIA; evaluation included brain imaging, cardiac monitoring, coagulation studies, and TTE bubble study to assess residual shunt. The etiology or cause of recurrent stroke was determined by 2 independent vascular neurologists on the basis of TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria (22). For recurrent events treated at outside hospitals, a copy of patient records, including all stroke work-up, imaging, laboratory, and echocardiography data, was reviewed by 2 independent vascular neurologists for adjudication. The neurologists evaluating outcome events were blinded to patients’ shunt status and were not involved in data analysis.
Statistical Analysis
Statistical analyses included all patients with successful device placement—that is, those in whom the closure device was successfully deployed and well seated, regardless of subsequent shunt status. Between-group comparisons were performed by using the Fisher exact test for categorical variables and the t test for continuous measures. Time-to-event analyses were performed according to the Kaplan–Meier method. A Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% CIs. Proportional hazard assumptions were tested by using Schoenfeld residuals, and no violations were observed. Covariate adjustment was performed by using a propensity score calculated from study period, device, traditional stroke risk factors (age, hypertension, hyperlipidemia, diabetes), high-risk PFO features (atrial septal aneurysm, hypermobile septum, hypercoagulability), and medication use (aspirin, clopidogrel, warfarin) (23–25). The association of these covariates with residual shunt and clinical outcome was assessed by using logistic regression and the Cox model (Supplement Table 2, available at Annals.org). Sensitivity analysis was performed to assess the effect of loss to follow-up on the analysis of recurrent events (Supplement Table 3, available at Annals.org). The association of different shunt sizes with outcome was evaluated by the Cox model, followed by post hoc pairwise comparison with the no-shunt group as the reference. Statistical analyses were performed with SPSS Statistics, version 25.0 (IBM). P values less than 0.050 were considered statistically significant.
Role of the Funding Source
This study was funded by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NIH/NINDS grants R01NS067139 and R01NS093415). The funder had no role in the design, conduct, or analysis of the study or in the decision to publish the manuscript.
Results
Study Population
The study consecutively recruited 1088 patients who had PFO-related cryptogenic stroke and were eligible for PFO closure. Ten patients (0.9%) were referred to surgical repair because of unfavorable anatomy, such as an extremely large PFO, for which appropriate endovascular closure devices were not available at the time. The remaining 1078 patients (99.1%; mean age, 49.3 years [SD, 13.7]) had PFO closure with successful device placement and were followed for up to 11 years, with an average duration of 3.7 years (SD, 2.9) and a total observation period of 3988 patient-years (Figure 1).
Figure 1.
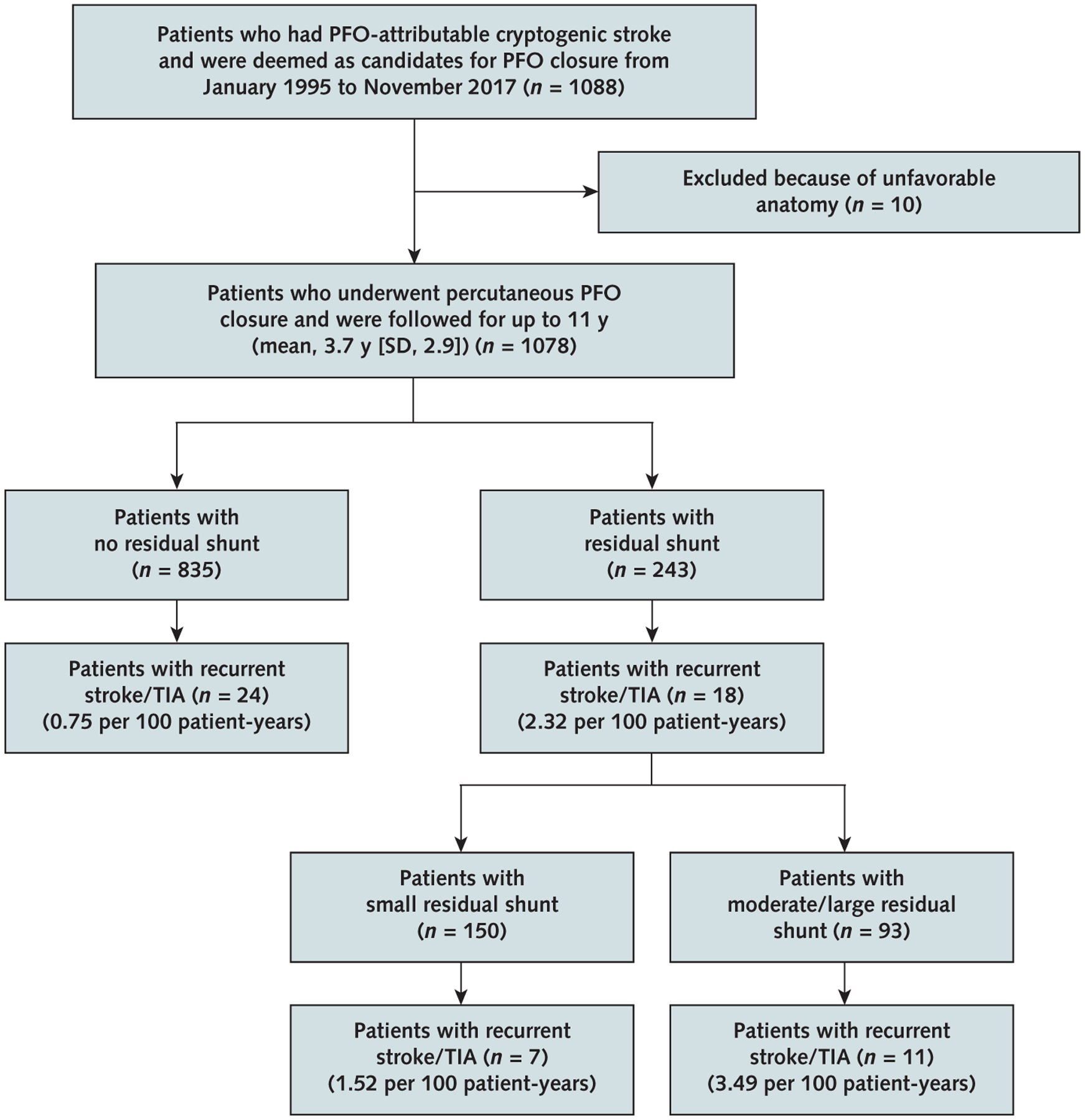
Study flow diagram.
PFO = patent foramen ovale; TIA = transient ischemic attack.
Complete PFO closure (no shunt) was observed in 835 patients (77.5%). Residual shunt was observed in 243 patients (22.5%), with a small shunt in 150 patients (13.9%) and moderate or large shunt in 93 patients (8.6%) (Figure 1). Effective closure (no or small residual shunt) was achieved in 985 patients (91.4%), similar to previous trials (11, 12, 26, 27). As shown in Table 1 and Supplement Tables 1 and 2, patient characteristics and medication use generally did not differ between the shunt and no-shunt groups. Residual shunts diminished over time, especially during the first year (Supplement Table 4, available at Annals.org), and shunt rates improved over the course of the study (Supplement Table 5, available at Annals.org). The shunt group had a shorter mean follow-up than the no-shunt group (3.2 years [SD, 3.0] vs. 3.8 years [SD, 2.9]; P = 0.002). Paroxysmal atrial fibrillation developed in 25 patients (2.3%) within 30 days after closure and resolved spontaneously. Device embolization occurred in 3 patients (0.3%) the day after the procedure; they received repeated intervention with successful device replacement. During follow-up, 37 deaths occurred, none of which was related to recurrent events (Table 1).
Table 1.
Patient Characteristics
| Characteristic | Total (n = 1078) | No-Shunt Group (n = 835) | Shunt Group (n = 243) | P Value |
|---|---|---|---|---|
| Mean age (SD), y | 49.3 (13.7) | 49.4 (13.4) | 49.1 (14.5) | 0.71 |
| Male sex, n (%) | 589 (54.6) | 466 (55.8) | 123 (50.6) | 0.164 |
| Study period, n (%) | 0.002 | |||
| 1995–2002 | 134 (12.4) | 87 (10.4) | 47 (19.3) | |
| 2003–2010 | 696 (64.6) | 551 (66.0) | 145 (59.7) | |
| 2011–2017 | 248 (23.0) | 197 (23.6) | 51 (21.0) | |
| Smoking status, n (%) | 0.60 | |||
| Never | 705 (65.4) | 548 (65.6) | 157 (64.6) | |
| Former | 254 (23.6) | 199 (23.8) | 55 (22.6) | |
| Current | 119 (11.0) | 88 (10.5) | 31 (12.8) | |
| Stroke/TIA before closure, n (%) | 0.37 | |||
| Stroke | 807 (74.9) | 627 (75.1) | 180 (74.1) | |
| TIA | 218 (20.2) | 171 (20.5) | 47 (19.3) | |
| Stroke + TIA | 53 (4.9) | 37 (4.4) | 16 (6.6) | |
| Comorbid conditions, n (%) | ||||
| Hypercoagulability | 470 (43.6) | 369 (44.2) | 101 (41.6) | 0.51 |
| Atrial septal aneurysm | 303 (28.1) | 225 (26.9) | 78 (32.1) | 0.124 |
| Hypermobile septum | 254 (23.6) | 187 (22.4) | 67 (27.6) | 0.103 |
| Deep venous thrombosis | 56 (5.2) | 44 (5.3) | 12 (4.9) | 1.00 |
| May-Thurner anatomy | 187 (17.3) | 146 (17.5) | 41 (16.9) | 0.92 |
| Hypertension | 285 (26.4) | 227 (27.2) | 58 (23.9) | 0.32 |
| Hyperlipidemia | 317 (29.4) | 255 (30.5) | 62 (25.5) | 0.150 |
| Diabetes | 65 (6.0) | 48 (5.7) | 17 (7.0) | 0.45 |
| Coronary artery disease | 63 (5.8) | 48 (5.7) | 15 (6.2) | 0.76 |
| Myocardial infarction | 9 (0.8) | 7 (0.8) | 2 (0.8) | 1.00 |
| Migraine | 215 (19.9) | 174 (20.8) | 41 (16.9) | 0.20 |
| Congestive heart failure | 5 (0.5) | 3 (0.4) | 2 (0.8) | 0.32 |
| Chronic obstructive pulmonary disease | 10 (0.9) | 6 (0.7) | 4 (1.6) | 0.25 |
| Medication on discharge, n (%) | ||||
| Aspirin | 1007 (93.4) | 775 (92.8) | 232 (95.5) | 0.185 |
| Clopidogrel | 96 (8.9) | 79 (9.5) | 17 (7.0) | 0.25 |
| Warfarin | 0.80 | |||
| Short-term | 188 (17.4) | 143 (17.1) | 45 (18.5) | 0.63 |
| Long-term | 99 (9.2) | 79 (9.5) | 20 (8.2) | 0.62 |
| Apixaban | 4 (0.4) | 3 (0.4) | 1 (0.4) | 1.00 |
| Dabigatran | 2 (0.2) | 1 (0.1) | 1 (0.4) | 0.40 |
| Rivaroxaban | 6 (0.6) | 5 (0.6) | 1 (0.4) | 1.00 |
| Atrial fibrillation, n (%) | 46 (4.3) | 40 (4.8) | 6 (2.5) | 0.32 |
| 0–30 d | 25 (2.3) | 22 (2.6) | 3 (1.2) | 0.33 |
| After 30 d | 21 (1.9) | 18 (2.2) | 3 (1.2) | 0.44 |
| Death, n (%) | 37 (3.4) | 28 (3.4) | 9 (3.7) | 0.84 |
TIA = transient ischemic attack.
Primary Outcome: Composite Stroke and TIA
The total observation period was 775 patient-years for the shunt group and 3213 patient-years for the no-shunt group. The primary outcome (composite of recurrent stroke or TIA) occurred in 18 patients in the shunt group (2.32 events per 100 patient-years) and 24 in the no-shunt group (0.75 events per 100 patient-years). Residual shunt thus was associated with an increased risk for recurrent stroke or TIA (HR, 3.05 [95% CI, 1.65 to 5.62]; P < 0.001) (Table 2 and Figure 2). The cumulative probability of recurrent stroke or TIA 5 years after closure was 9.3% for patients with residual shunt and 2.5% for those without it (Figure 2).
Table 2.
Outcomes: Stroke and/or TIA Recurrence in the Shunt Versus the No-Shunt Group
| Outcome* | No-Shunt Group (n = 835) | Shunt Group (n = 243) | Unadjusted | Adjusted† | ||||
|---|---|---|---|---|---|---|---|---|
| Events, n | Event Rate per 100 Patient-Years | Events, n | Event Rate per 100 Patient-Years | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Recurrent ischemic stroke/TIA | 24 | 0.75 | 18 | 2.32 | 3.05 (1.65–5.62) | <0.001 | 3.01 (1.59–5.69) | <0.001 |
| Ischemic stroke | 13 | 0.40 | 10 | 1.29 | 3.16 (1.38–7.21) | 0.006 | 3.33 (1.41–7.84) | 0.006 |
| Cryptogenic‡ | 3 | 0.09 | 7 | 0.90 | 9.62 (2.48–37.27) | 0.001 | 10.17 (2.54–40.64) | 0.001 |
| Noncryptogenic‡§ | 10 | 0.31 | 3 | 0.39 | 1.23 (0.34–4.47) | 0.75 | 1.26 (0.33–4.83) | 0.73 |
| TIA | 11 | 0.34 | 8 | 1.03 | 2.92 (1.17–7.25) | 0.021 | 2.66 (1.02–6.91) | 0.045 |
HR = hazard ratio; TIA = transient ischemic attack.
Outcome is the first event to occur in patients after patent foramen ovale closure.
All values were adjusted for age; study period; device; presence or absence of atrial septal aneurysm, hypertension, hyperlipidemia, diabetes, hypercoagulable state, and hypermobile septum; and medication use (aspirin, clopidogrel, warfarin) by using the propensity score method.
Recurrent ischemic strokes were adjudicated as cryptogenic or noncryptogenic according to TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria.
Includes 7 patients with cardioembolism, 1 patient with large artery atherosclerosis, 4 patients with small vessel occlusion, and 1 patient with trauma.
Figure 2.
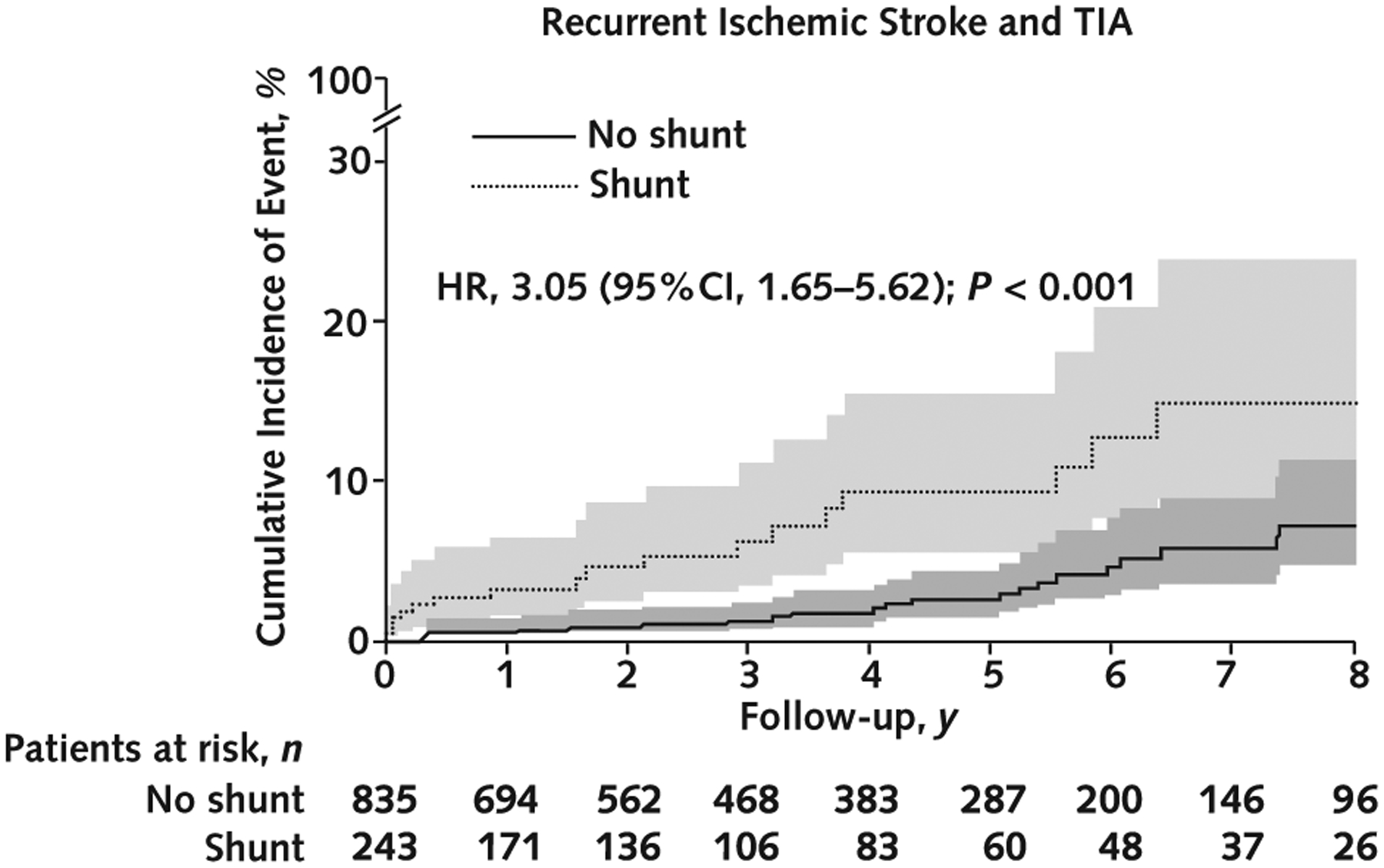
Cumulative incidence of recurrent stroke or TIA in patients with and without residual shunt after percutaneous PFO closure.
HR = hazard ratio; PFO = patent foramen ovale; TIA = transient ischemic attack.
After covariate adjustment using a propensity score calculated from confounders associated with PFO closure (study period and device), traditional stroke risk factors (age, hypertension, hyperlipidemia, and diabetes), high-risk PFO features (atrial septal aneurysm, hypermobile septum, and hypercoagulability), and medication use (aspirin, clopidogrel, and warfarin), the result remained robust—residual shunt was associated with increased stroke or TIA recurrence (HR, 3.01 [CI, 1.59 to 5.69]; P < 0.001) (Table 2).
Recurrent Ischemic Stroke and Recurrent TIA
We stratified the primary outcome further to assess ischemic stroke and TIA separately (Table 2; Supplement Figure 1, available at Annals.org). The incidence of recurrent ischemic stroke was 1.29 events per 100 patient-years for the shunt group and 0.40 events per 100 patient-years for the no-shunt group. Residual shunt thus was associated with an increased risk for recurrent ischemic stroke (HR, 3.16 [CI, 1.38 to 7.21]; P = 0.006). After neurologic adjudication, the incidence of recurrent cryptogenic stroke was 0.90 events per 100 patient-years for the shunt group, higher than the 0.09 events per 100 patient-years for the no-shunt group (HR, 9.62 [CI, 2.48 to 37.27]; P = 0.001). In contrast, the result for noncryptogenic stroke (with well-defined causes) was indeterminate between study groups: 0.39 versus 0.31 events per 100 patient-years (HR, 1.23 [CI, 0.34 to 4.47]; P = 0.75). Residual shunt likewise was associated with higher TIA recurrence, with an incidence of 1.03 events per 100 patient-years for the shunt group and 0.34 events per 100 patient-years for the no-shunt group (HR, 2.92 [CI, 1.17 to 7.25]; P = 0.021).
After covariate adjustment, residual shunt remained associated with increased recurrence of ischemic stroke (HR, 3.33 [CI, 1.41 to 7.84]; P = 0.006), particularly cryptogenic stroke (HR, 10.17 [CI, 2.54 to 40.64]; P = 0.001), and TIA (HR, 2.66 [CI, 1.02 to 6.91]; P = 0.045), whereas the result was indeterminate for noncryptogenic stroke (HR, 1.26 [CI, 0.33 to 4.83]; P = 0.73) (Table 2).
Shunt Size and Outcome
With regard to residual shunt size, the total observation period was 315 patient-years for the group with small shunts and 460 patient-years for the group with moderate or large shunts. The primary outcome occurred in 7 patients with a small shunt (1.52 events per 100 patient-years) and 11 with a moderate or large shunt (3.49 events per 100 patient-years). Increased shunt size was associated with increased stroke or TIA recurrence (HR, 2.11 [CI, 1.48 to 3.01]; P < 0.001) (Supplement Table 6 and Supplement Figure 2, available at Annals.org). Compared with patients with no shunt, those with a moderate or large shunt showed a higher incidence of recurrent stroke or TIA (HR, 4.50 [CI, 2.20 to 9.20]; P < 0.001), whereas the result was indeterminate for those with a small shunt (HR, 2.02 [CI, 0.87 to 4.69]; P = 0.102) (Supplement Table 6).
In our cohort, patients with a moderate or large shunt were older than those with a small shunt (52.2 years [SD, 15.4] vs. 47.1 years [SD, 13.6]; P = 0.009) and had higher rates of atrial septal aneurysm (43.0% vs. 25.3%; P = 0.004), hypertension (38.7% vs. 14.7%; P < 0.001), hyperlipidemia (33.3% vs. 20.7%; P = 0.029), and diabetes (12.9% vs. 3.3%; P = 0.008) (Supplement Table 7, available at Annals.org). However, after these confounders were included for covariate adjustment, larger shunt size was still associated with a higher rate of stroke or TIA recurrence (HR, 2.06 [CI, 1.43 to 2.96]; P < 0.001), with an HR of 1.90 (CI, 0.80 to 4.49) (P = 0.146) for small and 4.28 (CI, 2.07 to 8.88) (P < 0.001) for moderate and large shunts (Supplement Table 6).
When ischemic stroke and TIA were analyzed separately, greater shunt size was associated with an increased recurrence of both stroke (HR, 2.28 [CI, 1.43 to 3.66]; P < 0.001) and TIA (HR, 1.92 [CI, 1.12 to 3.29]; P = 0.018). The association again was significant for cryptogenic stroke (HR, 4.15 [CI, 2.05 to 8.40]; P < 0.001) but was indeterminate for noncryptogenic stroke (HR, 1.29 [CI, 0.59 to 2.80]; P = 0.53). These results remained robust after covariate adjustment—ischemic stroke: HR, 2.23 (CI, 1.37 to 3.62) (P = 0.001); TIA: HR, 1.87 (CI, 1.08 to 3.25) (P = 0.027); cryptogenic stroke: HR, 4.26 (CI, 2.09 to 8.72) (P < 0.001); and noncryptogenic stroke: HR, 1.16 (CI, 0.51 to 2.63) (P = 0.72) (Supplement Table 6 and Supplement Figure 3, available at Annals.org).
Exploratory Subgroup Analysis
To explore the interaction of stroke risk factors with PFO shunt, exploratory subgroup analyses were performed by stratifying patients on the basis of age (≤50 years and >50 years) and the presence or absence of traditional stroke risk factors (hypertension, hyperlipidemia, and diabetes) and other high-risk PFO features (atrial septal aneurysm, hypermobile septum, and hypercoagulability). As shown in Figure 3, the association of residual shunt with stroke or TIA recurrence was particularly evident in younger patients (≤50 years) (HR, 4.78 [CI, 1.98 to 11.54]; P < 0.001) and those without known stroke risk factors (atrial septal aneurysm: HR, 3.55 [CI, 1.76 to 7.15]; P < 0.001; hypertension: HR, 4.65 [CI, 2.18 to 9.94]; P < 0.001; hyperlipidemia: HR, 4.45 [CI, 1.92 to 10.30]; P < 0.001; diabetes: HR, 3.25 [CI, 1.68 to 6.28]; P < 0.001; hypercoagulability: HR, 3.95 [CI, 1.74 to 8.96]; P = 0.001; hypermobile septum: HR, 3.29 [CI, 1.51 to 7.17]; P = 0.003). This may suggest that residual shunt is associated with risk for stroke or TIA recurrence, regardless of any other traditional or PFO-related high-risk features. However, the association was indeterminate in older patients with comorbid conditions (P > 0.050).
Figure 3.
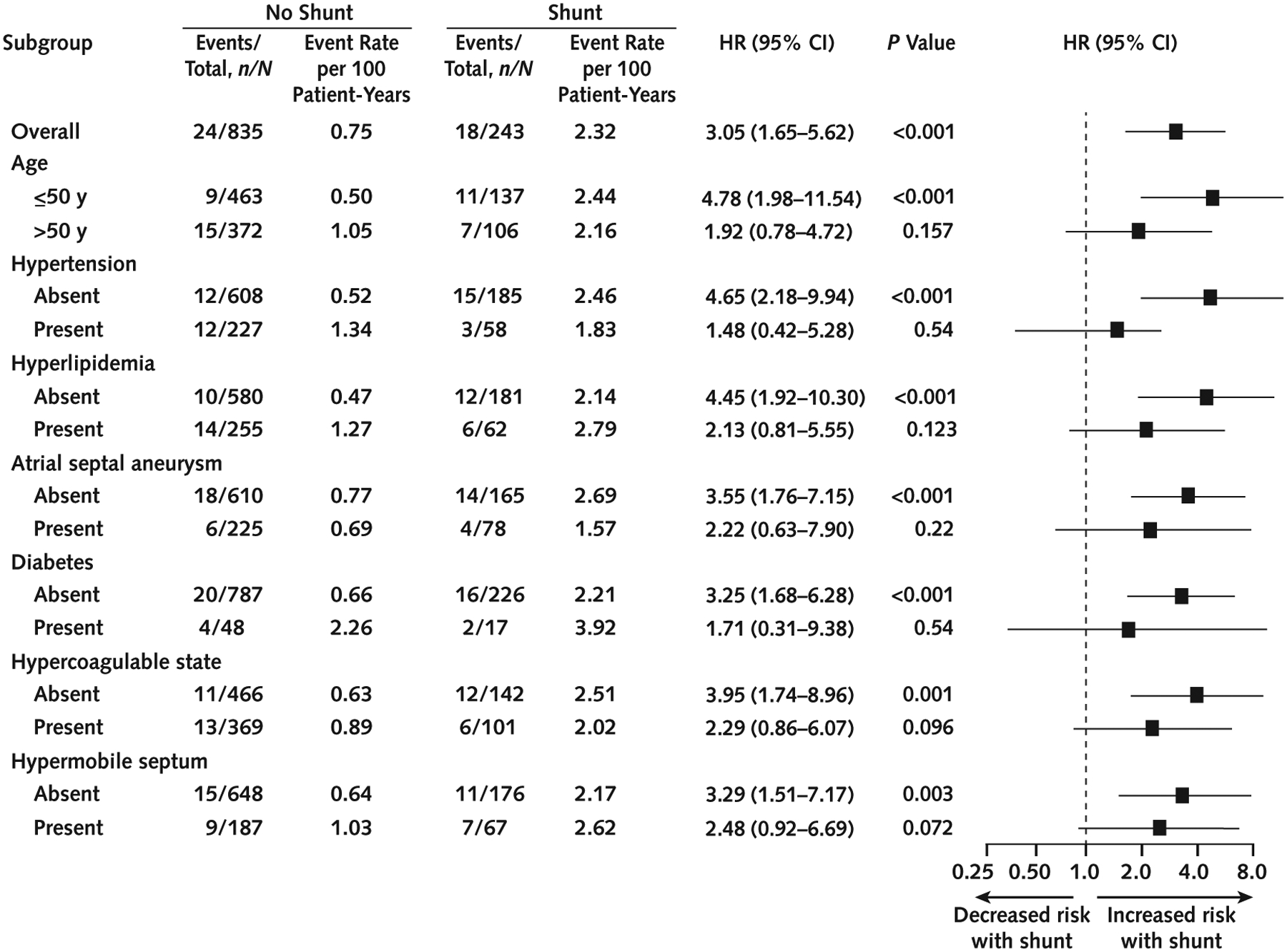
Subgroup analysis of recurrent stroke or TIA.
HR = hazard ratio; TIA = transient ischemic attack.
Discussion
In this prospective cohort study of patients undergoing percutaneous PFO closure for secondary stroke prevention, we found residual shunt, particularly of moderate or large size, to be a novel risk factor independently associated with long-term stroke or TIA recurrence. Recent randomized trials (RESPECT [Patent Foramen Ovale Closure or Medical Therapy After Stroke], REDUCE [GORE Septal Occluder Device for PFO Closure in Stroke Patients], and CLOSE [Patent Foramen Ovale Closure or Anticoagulants Versus Antiplatelet Therapy to Prevent Stroke Recurrence]) showed that PFO closure may prevent an average of 0.8 strokes per 100 patient-years (range, 0.49 to 1.32 strokes per 100 patient-years) (10–12). Our results are similar—residual shunt increased recurrence by 0.89 strokes per 100 patient-years and by 1.57 strokes or TIAs (combined primary outcome) per 100 patient-years—suggesting that residual PFO shunt, especially if moderate or large, continues to pose a risk for paradoxical embolism.
It is important to emphasize that PFO shunt matters in the context of other risk factors. Both clinical and biochemical evidence suggests a critical role for PFO shunt in the pathophysiology of stroke. Here, we report clinical evidence of increased stroke or TIA recurrence in patients with residual shunt over long-term clinical follow-up. However, in addition to serving as a conduit for venous clots, the presence of PFO shunt, as we reported previously, also may allow inappropriate procoagulable and oxidative factors to avoid pulmonary filtration and persist in the arterial circulation over time, further creating and propagating a hypercoagulable state (2, 28–34).
In our cohort, most recurrence occurred within the first few years (26.2% in <1 year and 78% in <6 years; Supplement Table 8, available at Annals.org), making clinical follow-up critical. The association of recurrent cryptogenic stroke with residual shunt suggests that PFO is an important mechanism for cryptogenic stroke. However, the field is rapidly evolving, and the historical designation of “cryptogenic stroke,” in our opinion, should continue to be reclassified and diminish in importance as emerging causes, such as PFO, atrial fibrillation, and genetic causes, are validated.
That our data are indeterminate regarding the interaction of PFO shunt with noncryptogenic risk factors and older age suggests that shunt stands out more prominently in the absence of other risk factors—but it does not cease to be a risk factor when other risks are present. Although traditional risks, such as hypertension, hyperlipidemia, and diabetes, may become more salient as patients age, making strokes less likely to be cryptogenic, the simultaneous presence of such comorbid conditions does not disqualify PFO shunt as a contributing factor. To the contrary, it stands to reason that the risk for PFO-mediated stroke would increase with age and disease, when more circulating clots are available to potentially cross via a PFO shunt.
Our analysis also suggests that the preexistence of traditional risk factors may sustain a larger residual shunt over the long term, possibly by delaying epithelialization after PFO closure, and thus prolong patients’ risk for paradoxical embolism. However, our and others’ experience suggests that large residual shunts can be managed successfully by placing a second device (35, 36) or, in rare instances, by surgical intervention. A multidisciplinary approach to PFO management with regular long-term follow-up remains of paramount importance for the prevention of recurrent events (2, 16, 34, 35).
Given the important clinical implications of residual shunting and the lack of guidelines for management, here we briefly share our clinical practice and recommendations. On the basis of the authors’ combined experience and the data presented here noting that recurrence rates are highest within the first few years, we suggest the following approach for patients with a moderate to large residual shunt after PFO closure (2).
First, we recommend long-term clinical follow-up (at least 5 years) with a multidisciplinary team involving primary care physicians to ensure adherence. To gauge shunt size, TTE with bubbles should be performed every 3 to 6 months during the first year and every 6 to 12 months thereafter.
Second, because residual shunt diminishes over time as a closure device becomes further epithelialized (Supplement Table 9, available at Annals.org), stepping up medical treatment, such as anticoagulant or dual-antiplatelet therapy, for the first year is reasonable until the shunt stabilizes.
Third, we suggest maximizing the management of PFO-specific risk factors, such as hypercoagulable states; deep venous thrombosis prevention; and, as patients age, treatment of traditional stroke risk factors and acquired hypercoagulability (such as age-appropriate cancer screening and management of hyperhomocysteinemia).
Finally, for high-risk patients with a persistent moderate or large shunt, we recommend multidisciplinary assessment by neurologists, cardiologists, hematologists, vascular specialists, and primary care clinicians to determine the optimal management plan, whether with second device closure or lifelong anticoagulant therapy.
This study had several limitations. As with all longitudinal observational studies, bias for patient selection may have been present. We used a rigorous prospective study protocol to minimize bias, using the following: prespecified clinical outcomes of recurrent stroke or TIA, because when the study was initiated more than 2 decades ago, PFO closure was not standard care but these end points were clearly known to be important long-term outcomes of interest; rigorous criteria to include only patients with PFO-attributed cryptogenic stroke who were deemed likely to benefit from PFO closure after exhaustive stroke work-up and multidisciplinary discussion; the same protocol, with no preferential treatment given to shunt status; and blind adjudication of shunt size and outcome events. However, in this long-term noninterventional study, a clear decline was observed in the rate of residual shunt over the years, probably because of advances in closure devices and technologies that have enhanced the efficacy of PFO closure. In addition, follow-up rates differed between study groups, most commonly because of patients relocating over a decade. However, the shorter follow-up in the shunt group probably underestimates the strength of our findings, because it is much more likely that stroke and TIA recurrences went uncaptured among shunt-group patients lost to follow-up than that they went unnoticed among no-shunt patients who remained in the study longer. We also used statistical methods, such as sensitivity analysis and proportional hazards modeling, to adjust for both known and unknown bias and confounders, with results remaining robust. Although younger age and fewer risk factors highlight the importance of residual shunt for recurrent events, our subgroup analyses of interaction between residual shunt and other stroke risk factors are purely exploratory and were not powered to determine the role of shunt in older adults with such risk factors. Lastly, we are constrained in shunt classification by current echocardiographic technology. Although the gold standard TTE bubble test is relatively reliable and noninvasive for accurately quantifying shunt size (37), counting bubbles with absolute accuracy is difficult, even for experts. Thus, misclassification of shunt size is possible and may result in decreased power to detect the “dose effect” of shunt. The combination of better echocardiographic quantification and other, more finely tuned quantitative circulatory biochemical markers of shunt (such as homocysteine) (28, 29, 31–33) may offer solutions to better quantify PFO shunt in future studies.
In conclusion, our prospective cohort study suggests that patients with residual shunt, particularly those with a moderate or large shunt, face continued risk, and PFO-related shunt seems to be critical in the pathophysiology of recurrent stroke. Thus, we suggest that patients with a moderate or large residual shunt be followed long term with multidisciplinary care.
Supplementary Material
Acknowledgment:
The authors thank Dr. Joseph Locascio for his expert statistics consultation. They remember Dr. Zareh Demirjian for his many contributions to this work and for his hematologic expertise in helping their stroke patients through the years.
Footnotes
Disclosures: Authors have disclosed no conflicts of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M19-3583.
Reproducible Research Statement: Study protocol: Available from Dr. Ning (e-mail, mmning@mgh.harvard.edu). Statistical code: Not available. Data set: Available on reasonable request from Dr. Ning (e-mail, mmning@mgh.harvard.edu).
Current author addresses and author contributions are available at Annals.org.
References
- 1.Saver JL. Clinical practice. Cryptogenic stroke. N Engl J Med. 2016; 374:2065–74. doi: 10.1056/NEJMcp1503946 [DOI] [PubMed] [Google Scholar]
- 2.Ning M, Lo EH, Ning PC, et al. The brain’s heart - therapeutic opportunities for patent foramen ovale (PFO) and neurovascular disease. Pharmacol Ther. 2013;139:111–23. doi: 10.1016/j.pharmthera.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ropper AH. Tipping point for patent foramen ovale closure [Editorial]. N Engl J Med. 2017;377:1093–1095. doi: 10.1056/NEJMe1709637 [DOI] [PubMed] [Google Scholar]
- 4.Meier B Optimal stroke prevention in patients with patent foramen ovale. Lancet Neurol. 2018;17:1027–1028. doi: 10.1016/S1474-4422(18)30369-7 [DOI] [PubMed] [Google Scholar]
- 5.Mas JL, Arquizan C, Lamy C, et al. ; Patent Foramen Ovale and Atrial Septal Aneurysm Study Group. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740–6. [DOI] [PubMed] [Google Scholar]
- 6.Tobis J Patent foramen ovale and the risk of cryptogenic stroke. Cleve Clin J Med. 2014;81:425–6. doi: 10.3949/ccjm.81a.14066 [DOI] [PubMed] [Google Scholar]
- 7.Le Moigne E, Timsit S, Ben Salem D, et al. Patent foramen ovale and ischemic stroke in patients with pulmonary embolism: a prospective cohort study. Ann Intern Med. 2019;170:756–763. doi: 10.7326/M18-3485 [DOI] [PubMed] [Google Scholar]
- 8.De Rosa S, Sievert H, Sabatino J, et al. Percutaneous closure versus medical treatment in stroke patients with patent foramen ovale: a systematic review and meta-analysis. Ann Intern Med. 2018;168:343–350. doi: 10.7326/M17-3033 [DOI] [PubMed] [Google Scholar]
- 9.Shah R, Nayyar M, Jovin IS, et al. Device closure versus medical therapy alone for patent foramen ovale in patients with cryptogenic stroke: a systematic review and meta-analysis. Ann Intern Med. 2018; 168:335–342. doi: 10.7326/M17-2679 [DOI] [PubMed] [Google Scholar]
- 10.Saver JL, Carroll JD, Thaler DE, et al. ; RESPECT Investigators. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377:1022–1032. doi: 10.1056/NEJMoa1610057 [DOI] [PubMed] [Google Scholar]
- 11.Mas JL, Derumeaux G, Guillon B, et al. ; CLOSE Investigators. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377:1011–1021. doi: 10.1056/NEJMoa1705915 [DOI] [PubMed] [Google Scholar]
- 12.Søndergaard L, Kasner SE, Rhodes JF, et al. ; Gore REDUCE Clinical Study Investigators. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377:1033–1042. doi: 10.1056/NEJMoa1707404 [DOI] [PubMed] [Google Scholar]
- 13.Windecker S, Wahl A, Nedeltchev K, et al. Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke. J Am Coll Cardiol. 2004;44:750–8. [DOI] [PubMed] [Google Scholar]
- 14.Serena J, Segura T, Perez-Ayuso MJ, et al. The need to quantify right-to-left shunt in acute ischemic stroke: a case-control study. Stroke. 1998;29:1322–8. [DOI] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7. [DOI] [PubMed] [Google Scholar]
- 16.Inglessis I, Elmariah S, Rengifo-Moreno PA, et al. Long-term experience and outcomes with transcatheter closure of patent foramen ovale. JACC Cardiovasc Interv. 2013;6:1176–83. doi: 10.1016/j.jcin.2013.06.013 [DOI] [PubMed] [Google Scholar]
- 17.Ning M, Gonzalez RG. Case records of the Massachusetts General Hospital. Case 34–2013. A 69-year-old man with dizziness and vomiting. N Engl J Med. 2013;369:1736–48. doi: 10.1056/NEJMcpc1302431 [DOI] [PubMed] [Google Scholar]
- 18.Johansson MC, Eriksson P, Guron CW, et al. Pitfalls in diagnosing PFO: characteristics of false-negative contrast injections during transesophageal echocardiography in patients with patent foramen ovales. J Am Soc Echocardiogr. 2010;23:1136–42. doi: 10.1016/j.echo.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 19.Waggoner AD, Ehler D, Adams D, et al. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: recommendations of the American Society of Echocardiography Council on Cardiac Sonography. J Am Soc Echocardiogr. 2001;14: 417–20. [DOI] [PubMed] [Google Scholar]
- 20.Porter TR, Abdelmoneim S, Belcik JT, et al. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: a focused update from the American Society of Echocardiography. J Am Soc Echocardiogr. 2014;27:797–810. doi: 10.1016/j.echo.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 21.Silvestry FE, Cohen MS, Armsby LB, et al. ; American Society of Echocardiography. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale: from the American Society of Echocardiography and Society for Cardiac Angiography and Interventions. J Am Soc Echocardiogr. 2015;28:910–58. doi: 10.1016/j.echo.2015.05.015 [DOI] [PubMed] [Google Scholar]
- 22.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 23.Marchese N, Pacilli MA, Inchingolo V, et al. Residual shunt after percutaneous closure of patent foramen ovale with AMPLATZER occluder devices - influence of anatomic features: a transcranial Doppler and intracardiac echocardiography study. EuroIntervention. 2013;9:382–8. doi: 10.4244/EIJV9I3A61 [DOI] [PubMed] [Google Scholar]
- 24.Taggart NW, Reeder GS, Lennon RJ, et al. Long-term follow-up after PFO device closure: outcomes and complications in a single-center experience. Catheter Cardiovasc Interv. 2017;89:124–133. doi: 10.1002/ccd.26518 [DOI] [PubMed] [Google Scholar]
- 25.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores [Editorial]. Ann Intern Med. 2002;137:693–5. [DOI] [PubMed] [Google Scholar]
- 26.Meier B, Kalesan B, Mattle HP, et al. ; PC Trial Investigators. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083–91. doi: 10.1056/NEJMoa1211716 [DOI] [PubMed] [Google Scholar]
- 27.Carroll JD, Saver JL, Thaler DE, et al. ; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–100. doi: 10.1056/NEJMoa1301440 [DOI] [PubMed] [Google Scholar]
- 28.Ning M, Navaratna D, Demirjian Z, et al. Serotonin as neurovascular mediator in patent foramen ovale related stroke. Circulation. 2011;124(Suppl 21):A618. [Google Scholar]
- 29.Ning M, Lopez M, Cao J, et al. Application of proteomics to cerebrovascular disease. Electrophoresis. 2012;33:3582–97. doi: 10.1002/elps.201200481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buonanno FS, Meng R, Feeney K, et al. Antiphospholipid associated hypercoagulability and subclinical white matter lesions in patent foramen ovale related stroke. Stroke. 2012;43:ANS2. [Google Scholar]
- 31.Lopez MF, Sarracino DA, Vogelsang M, et al. Heart-brain signaling in patent foramen ovale-related stroke: differential plasma proteomic expression patterns revealed with a 2-pass liquid chromatography-tandem mass spectrometry discovery workflow. J Investig Med. 2012;60:1122–30. doi: 10.2310/JIM.0b013e318276de0e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez MF, Krastins B, Sarracino DA, et al. Proteomic signatures of serum albumin-bound proteins from stroke patients with and without endovascular closure of PFO are significantly different and suggest a novel mechanism for cholesterol efflux. Clin Proteomics. 2015; 12:2. doi: 10.1186/1559-0275-12-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng W, Beecher C, Burant C, et al. Metabolomic analysis of PFO-related stroke shows immediate and persistent decrease of homocysteine post PFO closure. Neurology. 2015;84:P2.277. [Google Scholar]
- 34.Chen L, Deng W, Palacios I, et al. Patent foramen ovale (PFO), stroke and pregnancy. J Investig Med. 2016;64:992–1000. doi: 10.1136/jim-2016-000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz T, Cubeddu RJ, Rengifo-Moreno PA, et al. Management of residual shunts after initial percutaneous patent foramen ovale closure: a single center experience with immediate and long-term follow-up. Catheter Cardiovasc Interv. 2010;76:145–50. doi: 10.1002/ccd.22475 [DOI] [PubMed] [Google Scholar]
- 36.Susuri N, Obeid S, Ulmi M, et al. Second transcatheter closure for residual shunt following percutaneous closure of patent foramen ovale. EuroIntervention. 2017;13:858–866. doi: 10.4244/EIJ-D-17-00061 [DOI] [PubMed] [Google Scholar]
- 37.Ha JW, Shin MS, Kang S, et al. Enhanced detection of right-to-left shunt through patent foramen ovale by transthoracic contrast echocardiography using harmonic imaging. Am J Cardiol. 2001;87:669–71, A11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


