Abstract
Background
In TANGO, switching to dolutegravir/lamivudine (DTG/3TC) demonstrated long-term noninferior efficacy vs continuing tenofovir alafenamide–based regimens in treatment-experienced adults with HIV-1. The phase 3 SALSA study evaluated efficacy and safety of switching to DTG/3TC compared with continuing various 3-/4-drug current antiretroviral regimens (CARs).
Methods
Adults with HIV-1 RNA <50 copies/mL and no previous virologic failure were randomized (1:1, stratified by baseline third agent class) to switch to once-daily fixed-dose combination DTG/3TC or continue CAR (primary endpoint: proportion of participants with HIV-1 RNA ≥50 copies/mL at week 48; Snapshot, intention-to-treat–exposed population, 5% noninferiority margin).
Results
Overall, 493 adults (39% women; 39% aged ≥50 years; 19% African American/African heritage; 14% Asian) were randomized to switch to DTG/3TC (n = 246) or continue CAR (n = 247). At week 48, 1 (0.4%) participant in the DTG/3TC group and 3 (1.2%) in the CAR group had HIV-1 RNA ≥50 copies/mL (Snapshot), demonstrating noninferiority (adjusted difference, −0.8%; 95% CI, −2.4%, .8%). Zero participants met confirmed virologic withdrawal criteria; therefore, no resistance testing was performed. Drug-related adverse events were more frequent with DTG/3TC (20%) than CAR (6%) through week 48 but comparable post–week 24 (5% vs 2%, respectively). Proximal tubular renal function and bone turnover biomarkers improved with DTG/3TC. Both groups had generally minimal changes in lipids and inflammatory biomarkers.
Conclusions
Switching to DTG/3TC was noninferior to continuing CAR for maintaining virologic suppression at week 48 with no observed resistance, supporting the efficacy, good safety, and high barrier to resistance of DTG/3TC.
Clinical Trials Registration
Keywords: 2-drug regimen, dolutegravir/lamivudine, integrase strand transfer inhibitor, treatment-experienced, virologic suppression
In treatment-experienced adults without previous virologic failure, switching to the 2-drug regimen dolutegravir/lamivudine was noninferior to continuing current antiretroviral regimens for maintaining virologic suppression at week 48. No confirmed virologic withdrawals or resistance occurred. Dolutegravir/lamivudine had good safety and tolerability.
People living with human immunodeficiency virus (PLWH) currently require lifelong antiretroviral therapy (ART). While 2-drug regimens (2DRs) minimize the number of drugs in a regimen, they must also demonstrate potent and durable antiviral activity and a high barrier to resistance comparable to that of established 3-/4-drug regimens (3/4DRs) [1]. The 2021 US Department of Health and Human Services, 2021 European AIDS Clinical Society, and 2020 International Antiviral Society–USA guidelines recommend dolutegravir/lamivudine (DTG/3TC) as an initial antiretroviral regimen for most treatment-naive PLWH and as a switch option for virologically suppressed PLWH [2–4]. The phase 3 TANGO study demonstrated that switching to a DTG/3TC fixed-dose combination (FDC) had durable noninferior efficacy compared with continuing tenofovir alafenamide (TAF)–based 3/4DRs in virologically suppressed adults, with good safety and tolerability through 144 weeks [5–7]. No participants who switched to DTG/3TC met confirmed virologic withdrawal (CVW) criteria through 3 years; no resistance was observed. TANGO primarily included participants who were White, male, and aged less than 50 years. Here we present results from the SALSA study, evaluating the efficacy and safety of switching to DTG/3TC FDC compared with continuing various 3-/4-drug (2 nucleoside reverse transcriptase inhibitor [NRTI] backbone) current antiretroviral regimens (CARs) in a diverse population of virologically suppressed adults over 48 weeks.
METHODS
Study Design and Participants
SALSA (ClinicalTrials.gov, NCT04021290) is a phase 3, randomized, open-label, noninferiority study evaluating the efficacy and safety of switching to DTG/3TC compared with continuing CAR in virologically suppressed adults with human immunodeficiency virus 1 (HIV-1). The study was designed in accordance with the International Conference on Harmonization Good Clinical Practice and followed the principles of the Declaration of Helsinki, with protocol approvals and written informed consent obtained before participant screening.
SALSA participants were screened from 119 investigational centers in 17 countries: Argentina, Belgium, Brazil, Canada, China, Denmark, France, Germany, Italy, Mexico, Russia, South Africa, Spain, Sweden, Taiwan, the United Kingdom, and the United States. Virologically suppressed adults with undetectable viral load (≥2 confirmed HIV-1 RNA measurements <50 copies/mL) for 6 months or more and on a stable first or second ART regimen for 3 months or more before screening were eligible. Acceptable stable combination ART regimens before screening included 2 NRTIs plus an integrase strand transfer inhibitor (INSTI), nonnucleoside reverse transcriptase inhibitor (NNRTI), or protease inhibitor (PI). Any prior therapy switch must not have occurred because of suspected or established treatment failure. Prior switches between the following agents were not considered a regimen change: ritonavir and cobicistat, 3TC and emtricitabine (FTC), and tenofovir disoproxil fumarate (TDF) and TAF. Women were eligible if they were not pregnant or lactating and were using protocol-approved contraception.
Key exclusion criteria included evidence of Centers for Disease Control and Prevention stage 3 disease (except for cutaneous Kaposi’s sarcoma not requiring systemic therapy and CD4+ cell count <200 cells/mm3), severe hepatic impairment (Child-Pugh class C), hepatitis B virus infection, need for hepatitis C virus therapy, or creatinine clearance of less than 30 mL/min/1.73 m2. Participants with evidence of major NRTI or DTG resistance-associated mutations in any available historical genotypic results as provided by investigators, plasma HIV-1 RNA measurement greater than 200 copies/mL within 12 months of screening after suppression to less than 50 copies/mL, or 2 or more HIV-1 RNA measurements of 50 copies/mL or greater within 12 months of screening after suppression to less than 50 copies/mL were ineligible.
Procedures
After the screening period (≤28 days), eligible participants were randomized 1:1 to switch to once-daily DTG/3TC (50 mg/300 mg) FDC or continue CAR. Randomization was stratified by baseline third agent class (INSTI, NNRTI, PI). No dose reductions, modifications, or changes in frequency of any regimen components were allowed during the study, except for switching between ritonavir and cobicistat or 3TC and FTC in the CAR group. Study visits were planned at baseline (day 1) and weeks 4, 12, 24, 36, 48, and 52. Plasma for HIV-1 RNA quantification was collected at each visit through week 48, with retesting performed within 2 to 4 weeks of each visit if HIV-1 RNA was 50 copies/mL or greater. Additional details regarding study procedures are provided in the Supplementary Methods.
Outcomes
The primary endpoint was the proportion of participants with HIV-1 RNA of 50 copies/mL or greater (US Food and Drug Administration [FDA] Snapshot algorithm in the intention-to-treat–exposed [ITT-E] population) at week 48. Secondary efficacy endpoints included proportion of participants with HIV-1 RNA less than 50 copies/mL (Snapshot, ITT-E), change from baseline in CD4+ cell count and CD4+/CD8+ cell count ratio, and incidence of observed genotypic/phenotypic resistance in participants with CVW. Safety and tolerability were secondary objectives assessed through incidence and severity of adverse events (AEs) and laboratory abnormalities, and discontinuations due to AEs. Change from baseline in renal, bone, and inflammatory biomarkers; fasting lipids; and homeostasis model of assessment–insulin resistance (HOMA-IR) were additional safety endpoints.
Statistical Analysis
All randomized participants receiving 1 or more dose of study treatment were included in the ITT-E population, which was used for efficacy analyses; these participants were also included in the safety population. The per-protocol population included participants with no significant protocol deviations.
The proportion of participants with HIV-1 RNA of 50 copies/mL or greater and less than 50 copies/mL (Snapshot) at week 48 was analyzed using a Cochran-Mantel-Haenszel test adjusting for baseline third agent class. Noninferiority margins for the adjusted difference in proportion of participants with HIV-1 RNA of 50 copies/mL or greater (primary endpoint) and virologic suppression (HIV-1 RNA <50 copies/mL; secondary endpoint) at week 48 were 5% and −12%, respectively. Sensitivity analysis assessing the proportion of participants in the per-protocol population with HIV-1 RNA of 50 copies/mL or greater (Snapshot) was also performed. Incidence and severity of AEs were summarized descriptively. Median change in CD4+ cell count and CD4+/CD8+ cell count ratio at week 48 was assessed. Changes in weight; body mass index (BMI); inflammatory, renal, and bone biomarkers; fasting lipids; and HOMA-IR were estimated as mean change from baseline at week 48 (or geometric mean ratios of week 48 to baseline for log-transformed endpoints) in each group. These estimates were calculated using mixed-model repeated measures, adjusting for relevant covariates (described in Figure 4 and the Supplementary Figures). The proportion of participants with HOMA-IR of 2 or greater at week 48 was compared between groups using a logistic regression model adjusting for baseline variables (treatment, baseline third agent class, CD4+ cell count, age, sex, race, BMI, presence of hypertension, baseline HOMA-IR). No adjustments for multiplicity were used.
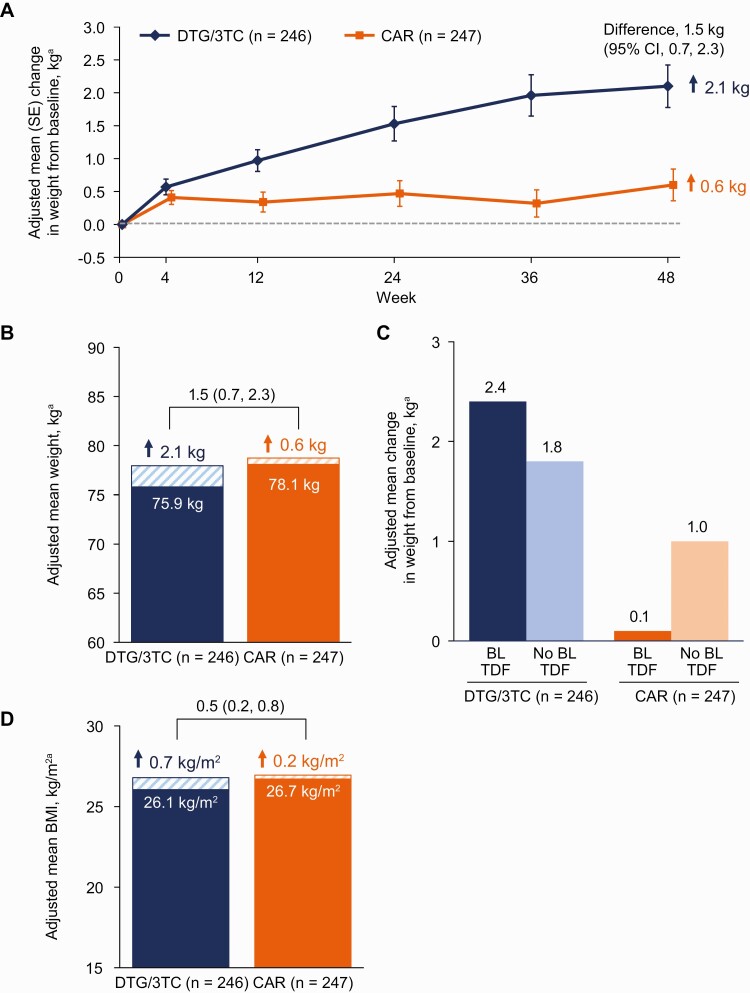
Figure 4.
A, Adjusted mean change from baseline in weight by visit. B, Baseline and adjusted mean change from baseline in weight at week 48. C, Adjusted mean change from baseline in weight at week 48 by baseline TDF use. D, Baseline and adjusted mean change from baseline in BMI at week 48. aAdjusted mean is the estimated mean change from baseline at each visit in each group calculated using mixed-model repeated measures adjusting for treatment, visit, baseline third agent class, CD4+ cell count (continuous), age (continuous), sex, race, baseline weight or BMI (continuous), treatment-by-visit interaction, and baseline value-by-visit interaction, with visit as the repeated factor. Abbreviations: BL, baseline; BMI, body mass index; CAR, current antiretroviral regimen; CI, confidence interval; DTG/3TC, dolutegravir/lamivudine; SE, standard error; TDF, tenofovir disoproxil fumarate.
RESULTS
Participants
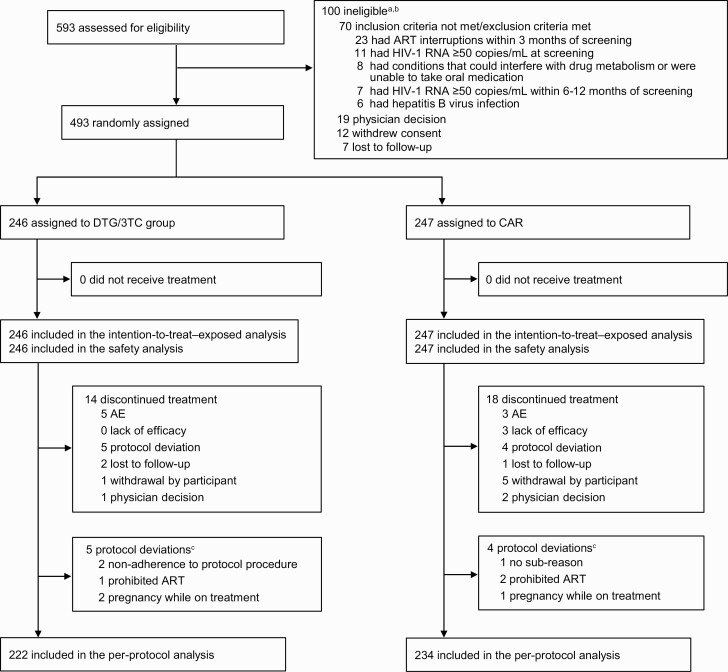
Participant screening began on 11 November 2019. The last participant’s week 52 visit occurred on 23 April 2021. Of the 593 screened participants, 493 were enrolled and randomized to switch to DTG/3TC (n = 246) or continue CAR (n = 247) (Figure 1). In the ITT-E population, 39% of participants were female, with a higher proportion in the DTG/3TC group (44%) than in the CAR group (34%); otherwise, baseline and disease characteristics were well balanced (Table 1). Overall, 39% of participants were aged 50 years or older, 19% were African American or of African heritage, and 14% were Asian. At screening, baseline third agent class was NNRTI for 50% of participants, INSTI for 40%, and PI for 10%; the most commonly used third agents were efavirenz (EFV; 31%) and DTG (17%). The most commonly used NRTIs at screening were FTC (62%), TDF (43%), 3TC (38%), and TAF (35%).
Figure 1.
Trial profile. aParticipants could have multiple reasons for ineligibility. bThe most common reasons for not meeting inclusion criteria or meeting exclusion criteria are listed. All other reasons occurred in <1% of participants. cProtocol deviations leading to exclusion from the per-protocol population; participants could have had ≥1 reason. Abbreviations: AE, adverse event(s); ART, antiretroviral therapy; CAR, current antiretroviral regimen; DTG/3TC, dolutegravir/lamivudine.
Table 1.
Baseline Demographics and Clinical Characteristics in the ITT-E Population
| Characteristics | DTG/3TC (n = 246) | CAR (n = 247) | Overall (N = 493) |
|---|---|---|---|
| Age | |||
| Median (range), y | 45 (22–74) | 45 (23–83) | 45 (22–83) |
| Age ≥50 years, n (%) | 98 (40) | 95 (38) | 193 (39) |
| Female, n (%) | 108 (44) | 84 (34) | 192 (39) |
| Race, n (%) | |||
| African American/African heritage | 45 (18) | 48 (19) | 93 (19) |
| Asian | 31 (13) | 39 (16) | 70 (14) |
| White | 149 (61) | 144 (58) | 293 (59) |
| Other racesa | 21 (9) | 16 (6) | 37 (8) |
| CD4+ cell count, median (range), cells/mm3 | 675 (154–2089) | 668 (94–1954) | 669 (94–2089) |
| CD4+ cell count, n (%) | |||
| <500 cells/mm3 | 60 (24) | 63 (26) | 123 (25) |
| ≥500 cells/mm3 | 185 (75) | 184 (74) | 369 (75) |
| Duration of ART before day 1, median (range), mo | 63 (4–240) | 71 (12–253) | … |
| Baseline third agent class received at screening, n (%) | |||
| NNRTI | 123 (50) | 124 (50) | 247 (50) |
| INSTI | 98 (40) | 98 (40) | 196 (40) |
| PI | 25 (10) | 25 (10) | 50 (10) |
| NNRTIs received at screening in ≥30% of participants | |||
| EFV | 79 (32) | 73 (30) | 152 (31) |
| INSTIs received at screening, n (%) | |||
| DTG | 45 (18) | 41 (17) | 86 (17) |
| EVG + COBI | 24 (10) | 27 (11) | 51 (10) |
| BIC | 24 (10) | 26 (11) | 50 (10) |
| RAL | 6 (2) | 4 (2) | 10 (2) |
| NRTIs received at screening in ≥30% of participants | |||
| FTC | 149 (61) | 156 (63) | 305 (62) |
| TDFb | 109 (44) | 109 (44) | 218 (44) |
| 3TC | 96 (39) | 89 (36) | 185 (38) |
| TAF | 83 (34) | 91 (37) | 174 (35) |
| Weight, median (range), kg | 73 (43–154) | 75 (36–160) | 74 (36–160) |
| BMI, median (range), kg/m2 | 25 (18–51) | 26 (14–69) | 25 (14–69) |
Abbreviations: ART, antiretroviral therapy; BIC, bictegravir; BMI, body mass index; CAR, current antiretroviral regimen; COBI, cobicistat; DTG, dolutegravir; EFV, efavirenz; EVG, elvitegravir; FTC, emtricitabine; INSTI, integrase strand transfer inhibitor; ITT-E, intention-to-treat–exposed; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; RAL, raltegravir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; 3TC, lamivudine.
aIncludes American Indian or Alaska Native or individuals of multiple races.
bIncludes tenofovir disoproxil succinate (DTG/3TC, n = 1; CAR, n = 3).
Efficacy
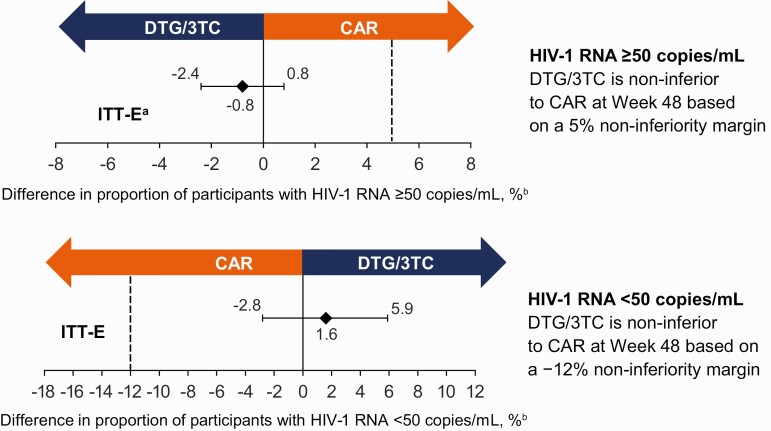
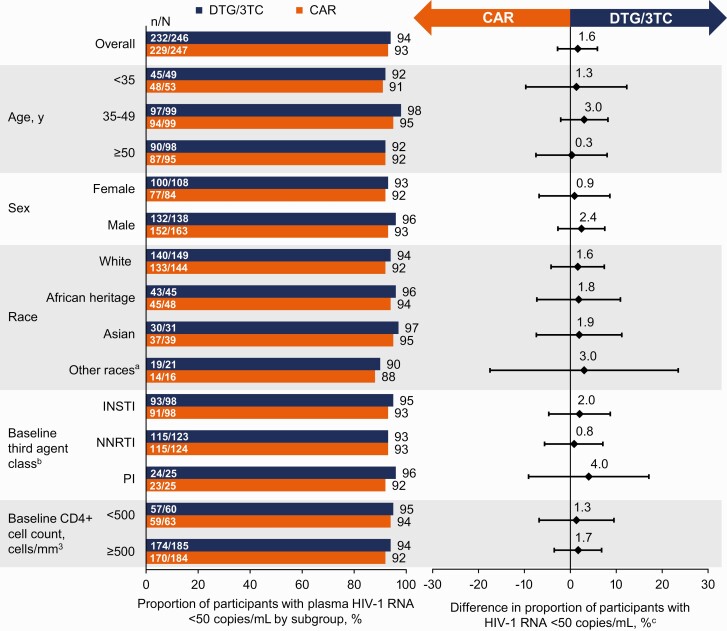
At week 48, 1 (0.4%) participant in the DTG/3TC group and 3 (1.2%) in the CAR group had HIV-1 RNA of 50 copies/mL or greater (Snapshot, ITT-E population), demonstrating noninferiority of DTG/3TC to CAR (adjusted difference, −0.8%; 95% confidence interval [CI], −2.4%, 0.8%) (Table 2, Figure 2). In the per-protocol population, 1 of 222 (0.5%) participants in the DTG/3TC group and 3 of 234 (1.3%) in the CAR group had HIV-1 RNA of 50 copies/mL or greater at week 48 (adjusted difference, −0.8%; 95% CI, −2.5%, 0.9%). The proportion of participants with HIV-1 RNA of less than 50 copies/mL (Snapshot, ITT-E population) was 94% (232/246) in the DTG/3TC group and 93% (229/247) in the CAR group (adjusted difference, 1.6%; 95% CI, −2.8%, 5.9%). Proportions of participants with HIV-1 RNA of less than 50 copies/mL (Snapshot) across demographics, baseline characteristics, and baseline third agent class subgroups were consistent with overall results (Figure 3).
Table 2.
Snapshot Outcomes at Week 48 in the ITT-E Population
| DTG/3TC (n = 246) | CAR (n = 247) | |
|---|---|---|
| HIV-1 RNA <50 copies/mL | 232 (94) | 229 (93) |
| HIV-1 RNA ≥50 copies/mL | 1 (<1) | 3 (1) |
| Data in window and HIV-1 RNA ≥50 copies/mL | 1 (<1)a | 1 (<1)b |
| Discontinued for lack of efficacy | 0 | 2 (<1)c |
| Discontinued for other reason and HIV-1 RNA ≥50 copies/mL | 0 | 0 |
| Change in ART | 0 | 0 |
| No virologic data | 13 (5) | 15 (6) |
| Discontinued because of AE or deathd | 5 (2) | 2 (<1) |
| Discontinued for other reasonse | 7 (3) | 10 (4) |
| On study but missing data in windowf | 1 (<1) | 3 (1) |
Data are presented as n (%).
Abbreviations: AE, adverse event; ART, antiretroviral therapy; CAR, current antiretroviral regimen; DTG/3TC, dolutegravir/lamivudine; HIV-1, human immunodeficiency virus 1; ITT-E, intention-to-treat–exposed.
aParticipant had HIV-1 RNA 53 copies/mL at week 48, followed by 2 retests with HIV-1 RNA ≥50 copies/mL, and was withdrawn with HIV-1 RNA <40 copies/mL after week 52.
bParticipant had HIV-1 RNA 90 copies/mL at week 36 and was withdrawn during the week 48 window with HIV-1 RNA 68 copies/mL.
cParticipants were withdrawn after consecutive HIV-1 RNA ≥50 to <200 copies/mL.
dReasons for discontinuation due to AEs in DTG/3TC group: insomnia (n = 2), alcohol abuse/anxiety (n = 1), weight increased (n = 1), and unknown cause of death (n = 1); in CAR group: ulcerative colitis and postoperative complications (n = 1 each); last on-treatment HIV-1 RNA were all <50 copies/mL.
eOther reasons for discontinuation included protocol deviation (n = 6), participant withdrawal (n = 6), pregnancy (n = 2), physician decision (n = 2), and lost to follow-up (n = 1).
fMissing data in window due to coronavirus disease 2019 (COVID-19) pandemic (n = 2 in the CAR group only).
Figure 2.
Adjusted treatment difference in virologic outcomes at week 48 in the ITT-E population. aPrimary endpoint (Snapshot virologic failure, ITT-E). bBased on Cochran-Mantel-Haenszel stratified analysis (DTG/3TC − CAR) adjusting for baseline third agent class. Abbreviations: CAR, current antiretroviral regimen; DTG/3TC, dolutegravir/lamivudine; HIV-1, human immunodeficiency virus 1; ITT-E, intention-to-treat–exposed.
Figure 3.
Proportion of participants with HIV-1 RNA <50 copies/mL by subgroup in the ITT-E population by FDA Snapshot algorithm at week 48. aIncludes American Indian or Alaska Native or individuals of multiple races. bThe study population was stratified by baseline third agent class (PI, INSTI, or NNRTI). cAdjusted difference for overall population (DTG/3TC − CAR) and 95% CIs are based on a stratified analysis (adjusting for baseline third agent class) using Cochran-Mantel-Haenszel weights (meeting noninferiority based on −12% margin); unadjusted difference for subgroups calculated by proportion on DTG/3TC − proportion on CAR. Abbreviations: CAR, current antiretroviral regimen; CI, confidence interval; DTG/3TC, dolutegravir/lamivudine; FDA, Food and Drug Administration; HIV-1, human immunodeficiency virus 1; INSTI, integrase strand transfer inhibitor; ITT-E, intention-to-treat–exposed; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
The median (interquartile range [IQR]) change from baseline to week 48 in CD4+ cell count was 30.0 cells/mm3 (−83.0, 115.5) in the DTG/3TC group and 2.0 cells/mm3 (−105.0, 94.0) in the CAR group. The median (IQR) change from baseline to week 48 in CD4+/CD8+ cell count ratio was 0.04 (−0.06, 0.13) in the DTG/3TC group and 0.05 (−0.06, 0.13) in the CAR group.
Zero participants in either treatment group met CVW criteria; as such, no resistance testing was performed.
Safety
Through week 48, the proportion of participants reporting at least 1 AE in the DTG/3TC group (73%) was similar to that in the CAR group (70%) (Table 3). The most common AEs (≥5%) in the DTG/3TC group were weight increase (8%), headache (7%), coronavirus disease 2019 (COVID-19) (6%), back pain (6%), insomnia (6%), and dizziness (5%), while headache (7%) and upper respiratory tract infection (6%) were the most common AEs in the CAR group. Incidence of drug-related AEs was higher in the DTG/3TC group than in the CAR group through week 48 (20% vs 6%, respectively) but was low and comparable between groups post–week 24 (5% vs 2%, respectively; ad hoc analysis). Proportions of AEs leading to withdrawal (DTG/3TC, 2%; CAR, 1%) and drug-related AEs leading to withdrawal (DTG/3TC, 2%; CAR, <1%) were similar between groups. Proportions of serious AEs were also similar between groups (DTG/3TC, 3%; CAR, 6%), with no drug-related serious AEs reported. One fatal AE of unknown cause occurred in the DTG/3TC group and was not considered treatment related by the investigator. Overall, 4 pregnancies were reported: DTG/3TC, 3 (1 resulted in spontaneous abortion, 1 in elective abortion, 1 participant was lost to follow-up); CAR, 1 (healthy infant delivered at 40 weeks’ gestation).
Table 3.
Summary of Adverse Events in the Safety Populations
| Day 1 to Week 48 | Week 24 to Week 48 | |||
|---|---|---|---|---|
| DTG/3TC (n = 246) | CAR (n = 247) | DTG/3TC (n = 236) | CAR (n = 242) | |
| Any AE | 180 (73) | 172 (70) | 110 (47) | 100 (41) |
| AEs occurring in ≥5% of participants in either group | ||||
| Weight increased | 20 (8) | 5 (2) | 8 (3) | 2 (<1) |
| Headache | 16 (7) | 17 (7) | 7 (3) | 6 (2) |
| COVID-19 | 15 (6) | 11 (4) | 7 (3) | 7 (3) |
| Back pain | 14 (6) | 7 (3) | 4 (2) | 4 (2) |
| Insomnia | 14 (6) | 4 (2) | 4 (2) | 0 |
| Dizziness | 13 (5) | 6 (2) | 2 (<1) | 1 (<1) |
| Upper respiratory tract infection | 11 (4) | 15 (6) | 6 (3) | 5 (2) |
| Drug-related AEs | 48 (20) | 16 (6) | 11 (5) | 4 (2) |
| Drug-related grade 2–5 AEs | 12 (5) | 6 (2) | 2 (<1)a | 2 (<1)b |
| Drug-related grade 2–5 AEs occurring in ≥2 participants in either group | ||||
| Weight increased | 3 (1) | 0 | 0 | 0 |
| Insomnia | 3 (1) | 1 (<1) | 0 | 0 |
| Glomerular filtration rate decreased | 2 (<1) | 0 | 0 | 0 |
| AEs leading to withdrawalc | 5 (2) | 3 (1) | 1 (<1) | 2 (<1) |
| Drug-related AEs leading to withdrawal | 4 (2) | 1 (<1) | 0 | 1 (<1) |
| Any SAEs | 7 (3) | 16 (6) | 3 (1) | 7 (3) |
| Drug-related SAEs | 0 | 0 | 0 | 0 |
Data are presented as n (%).
Abbreviations: AE, adverse event; CAR, current antiretroviral regimen; COVID-19, coronavirus disease 2019; DTG/3TC, dolutegravir/lamivudine; SAE, serious adverse event.
aTwo drug-related grade 2–5 AEs occurred in the DTG/3TC group: grade 2 hypertriglyceridemia (n = 1) and grade 2 renal impairment (n = 1).
bTwo drug-related grade 2–5 AEs occurred in the CAR group: grade 2 creatinine renal clearance increased (n = 1) and grade 3 hypercholesterolemia (n = 1).
cThe following AEs leading to withdrawal from the study were observed in ≥1 participant in either treatment group (DTG/3TC, n [%]; CAR, n [%]): insomnia (2 [<1%]; 0), alcohol abuse (1 [<1%]; 0), anxiety (1 [<1%]; 0), suicidal ideation (0; 1 [<1%]), ulcerative colitis (0; 1 [<1%]), death (1 [<1%]; 0), postprocedural complication (0; 1 [<1%]), and weight increased (1 [<1%]; 0); ulcerative colitis, death, and postprocedural complication were considered unrelated to study treatment.
Adverse events of increased weight or obesity were reported in 9% and 2% of participants in the DTG/3TC and CAR groups, respectively, all of which were grade 1 or 2 in severity and led to discontinuation in 1 DTG/3TC participant (0 in the CAR group). Absolute mean (SD) weight at baseline vs week 48 was 75.9 (16.9) vs 77.2 (15.7) kg in the DTG/3TC group and 78.1 (17.6) vs 78.4 (17.3) kg in the CAR group, respectively. Adjusted mean weight change from baseline to week 48 was 2.1 kg in the DTG/3TC group and 0.6 kg in the CAR group (adjusted difference, 1.5 kg; 95% CI, .7–2.3; P < .001) (Figure 4A and 4B). Among participants switching to DTG/3TC, adjusted mean weight change was higher in those switching from TDF-containing regimens at baseline compared with those with no baseline TDF use (Figure 4C). Adjusted mean change in BMI from baseline to week 48 was 0.7 kg/m2 in the DTG/3TC group and 0.2 kg/m2 in the CAR group (adjusted difference, .5 kg/m2; 95% CI, .2–.8; P < .001) (Figure 4D). Three participants in the DTG/3TC group and 1 in the CAR group reported an AE of new-onset diabetes mellitus. Changes in insulin resistance were minimal in both groups (Supplementary Figure 1). At week 48, 49% of participants in the DTG/3TC group and 55% in the CAR group had insulin resistance defined as HOMA-IR of 2 or greater (odds ratio, 1.02; 95% CI, .63–1.66; P = .930).
At week 48, changes in inflammatory biomarkers were generally small and similar between groups, with the exception of reductions in serum soluble CD14 (sCD14) favoring DTG/3TC (Supplementary Figure 2). Change from baseline to week 48 in lipid parameters was minimal and similar between groups (Supplementary Figure 3). For renal biomarkers, small and similar changes in estimated glomerular filtration rate from cystatin C were observed in both groups (Supplementary Figure 4A). Improvements were observed from baseline in all proximal tubular renal function (Supplementary Figure 4B) and bone turnover biomarkers (Supplementary Figure 5) after switching to DTG/3TC.
DISCUSSION
In SALSA, switching to DTG/3TC FDC was noninferior to continuing 3- or 4-drug antiretroviral regimens in virologically suppressed adults through 48 weeks, meeting the primary endpoint (HIV-1 RNA ≥50 copies/mL; Snapshot, ITT-E) (Supplementary Graphical Abstract). TANGO demonstrated 3-year durable efficacy and safety of switching to DTG/3TC FDC from TAF-based 3/4DRs in virologically suppressed adults, with most participants in the comparison group continuing rilpivirine/TAF/FTC or elvitegravir/cobicistat/TAF/FTC [5–7]. SALSA results are consistent with TANGO and broaden the existing evidence by demonstrating noninferiority of switching to DTG/3TC FDC compared with continuing a variety of 3/4DR CAR, including non–TAF-based regimens, in a diverse population at week 48.
In the SALSA subgroup analysis, female participants, participants aged 50 years or older, and participants who were African American or of African heritage or Asian were well represented (39%, 39%, 19%, and 14%, respectively, in the overall population) and demonstrated maintenance of virologic suppression consistent with the overall analysis. This is important because clinical trials can lack diversity, with enrolled participants not reflecting the demographics of the intended patient population, thereby limiting interpretation of published outcomes [8]. The proportion of participants maintaining virologic suppression was also similar across baseline third agent class subgroups (NNRTI, INSTI, PI); in the DTG/3TC group, the proportion maintaining virologic suppression using INSTIs before switch (10% bictegravir, 10% elvitegravir/cobicistat, and 18% DTG-containing regimens) was similar to that observed in the overall analysis. Immune reconstitution (change from baseline in CD4+ cell count and CD4+/CD8+ ratio) was also similar between the 2DR and 3/4DR groups in SALSA. Consistent with TANGO, no SALSA participants met CVW criteria; therefore, there was no need for resistance testing. Together, the data from SALSA and TANGO at 1 and 3 years, respectively, support the high efficacy and barrier to resistance of DTG/3TC in a switch setting.
Virologic outcomes in SALSA and TANGO are consistent with a meta-analysis of 6 real-world studies, which found that DTG + 3TC as maintenance therapy had a 1% virologic failure rate (defined as 2 consecutive HIV-1 RNA measurements ≥50 copies/mL and/or 1 measurement >1000 copies/mL) in treatment-experienced PLWH (N = 2033) at either week 48 or week 96 [7, 9]. These real-world studies included populations with characteristics that would typically be excluded from randomized clinical trials (eg, presence of resistance mutations).
Although overall AEs were generally similar between groups, drug-related AEs were more frequent in participants switching to a new regimen of DTG/3TC than those continuing CAR, which is expected in a switch study setting where participants in the CAR group were stable on their baseline regimens and 56% of participants in the DTG/3TC group switched from a baseline regimen not containing DTG or 3TC. This is supported by post–week 24 analyses of AEs in which the incidence of AEs was low and more comparable across groups (DTG/3TC, 5%; CAR, 2%; ad hoc analysis). A similar pattern was observed in the TANGO study.
In SALSA, the adjusted mean weight increase from baseline to week 48 was 1.5 kg higher in the DTG/3TC group than in the CAR group; however, absolute weight was comparable between groups at week 48 (unadjusted values of 77.2 vs 78.4 kg, respectively). This difference in weight gain between groups may be partly explained by participants in the DTG/3TC group switching from regimens known to be associated with weight-gain suppression (eg, TDF and EFV) [10, 11]. This is supported by the subgroup analysis, demonstrating higher weight gain in participants switching from TDF-containing regimens to DTG/3TC compared with no baseline TDF use. In TANGO, the adjusted mean weight gain from baseline to week 48 was 0.8 kg in both groups. However, differences in the TANGO and SALSA study designs (including switching from a TAF-based regimen in TANGO) and diversity of participant demographics influence the ability to directly compare results between trials. Despite the 1.5-kg difference in weight increase, metabolic health (as assessed by insulin resistance and lipids) was generally unchanged from baseline across both treatment groups in SALSA.
Changes from baseline to week 48 in inflammatory biomarkers were generally small and similar between the DTG/3TC and CAR groups, with the exception of sCD14, which favored DTG/3TC. However, the clinical meaning of this change is unknown. Overall, there was no increase in inflammatory or immune activation biomarkers after switching to DTG/3TC, as expected given the similar and high proportions of participants maintaining virologic suppression in both groups.
Improvements in proximal tubular renal function and bone turnover biomarkers were observed in the DTG/3TC group. This could also be partly explained by the high proportion of participants (44%) in the DTG/3TC group switching from TDF-containing regimens, which have been associated with renal toxicity and decreased bone mineral density [12]. Similarly, in the GEMINI studies, treatment initiation with DTG + 3TC had favorable effects on renal and bone health compared with DTG + TDF/FTC in treatment-naive individuals through 3 years [13]. These data indicate that switching from TDF to DTG/3TC can potentially improve tubular renal function and bone biomarkers in PLWH.
A potential limitation of SALSA is that the CAR group was composed of a heterogenous population taking a variety of ART regimens, expanding the generalizability of the results but possibly increasing variability such that treatment differences for individual regimens are more difficult to ascertain.
Findings from the SALSA study build upon the evidence supporting 3-year efficacy and safety of DTG/3TC in treatment-experienced individuals in the TANGO study and in treatment-naive individuals in the GEMINI-1 and GEMINI-2 studies as well as real-world evidence supporting the effectiveness of DTG/3TC [6, 9, 13, 14]. These results provide further support for use of this 2DR as a treatment option in a diverse population of virologically suppressed individuals with HIV-1, including high proportions of women, participants aged 50 years and older, and those of African American/African heritage or Asian backgrounds, with no previous virologic failure or known NRTI or DTG resistance-associated mutations, switching from various ART regimens.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants and their families and caregivers; the investigators and site staff who participated in the study; and the ViiV Healthcare, GSK, and Pharmaceutical Product Development study team members. Editorial assistance was provided under the direction of the authors by Aarthi Gobinath, PhD, and Jennifer Rossi, MA, ELS, MedThink SciCom, and was funded by ViiV Healthcare.
Financial support. This work was supported by ViiV Healthcare.
Contributor Information
Josep M Llibre, Hospital Universitari Germans Trias i Pujol, Barcelona, Spain.
Carlos Brites, Universidade Federal da Bahia, Salvador, Brazil.
Chien-Yu Cheng, Department of Infectious Diseases, Taoyuan General Hospital, Ministry of Health and Welfare, Taoyuan, Taiwan; Institute of Public Health, School of Medicine, National Yang-Ming Chiao Tung University, Taipei, Taiwan.
Olayemi Osiyemi, Triple O Research Institute PA, West Palm Beach, Florida, USA.
Carlos Galera, Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain.
Laurent Hocqueloux, Centre Hospitalier Régional d’Orléans, Orléans, France.
Franco Maggiolo, ASST Papa Giovanni XXIII, Bergamo, Italy.
Olaf Degen, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany.
Stephen Taylor, Birmingham Heartlands Hospital, Birmingham, United Kingdom; University of Birmingham, Birmingham, United Kingdom.
Elizabeth Blair, ViiV Healthcare, Durham, North Carolina, USA.
Choy Man, ViiV Healthcare, Durham, North Carolina, USA.
Brian Wynne, ViiV Healthcare, Durham, North Carolina, USA.
James Oyee, GSK, Brentford, United Kingdom.
Mark Underwood, ViiV Healthcare, Durham, North Carolina, USA.
Lloyd Curtis, GSK, Brentford, United Kingdom.
Gilda Bontempo, ViiV Healthcare, Durham, North Carolina, USA.
Jean van Wyk, ViiV Healthcare, Brentford, United Kingdom.
References
- 1.Back D. 2-Drug regimens in HIV treatment: pharmacological considerations. Germs 2017; 7:113–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. 2021. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf. Accessed 24 September 2021.
- 3.European AIDS Clinical Society. EACS guidelines version 11.0. October 2021. Available at: https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf. Accessed 7 November 2021.
- 4.Saag MS, Gandhi RT, Hoy JF, et al. . Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society–USA panel. JAMA 2020; 324:1651–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Wyk J, Ajana F, Bisshop F, et al. . Switching to DTG/3TC fixed-dose combination (FDC) is non-inferior to continuing a TAF-based regimen (TBR) in maintaining virologic suppression through 96 weeks (TANGO study) [abstract O441]. In: Program and abstracts of HIV Drug Therapy Glasgow 2020 (virtual). Geneva, Switzerland: International AIDS Society, 2020:14. [Google Scholar]
- 6.van Wyk J, Ait-Khaled M, Santos J, et al. . Metabolic health outcomes at week 144 in the TANGO study, comparing a switch to DTG/3TC versus maintenance of TAF-based regimens [abstract PEB164]. In: Program and abstracts of 11th IAS Conference on HIV Science (virtual). Geneva, Switzerland: International AIDS Society, 2021. [Google Scholar]
- 7.van Wyk J, Ajana F, Bisshop F, et al. . Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide–based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO study. Clin Infect Dis 2020; 71:1920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin E. Striving for diversity in research studies. N Engl J Med 2021; 385:1429–30. [DOI] [PubMed] [Google Scholar]
- 9.Punekar YS, Parks D, Joshi M, et al. . Effectiveness and safety of dolutegravir two-drug regimens in virologically suppressed people living with HIV: a systematic literature review and meta-analysis of real-world evidence. HIV Med 2021; 22:423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sax PE, Erlandson KM, Lake JE, et al. . Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang S-H, Huang W-C, Lin S-W, et al. . Impact of efavirenz mid-dose plasma concentration on long-term weight change among virologically suppressed people living with HIV. J Acquir Immune Defic Syndr 2021; 87:834–41. [DOI] [PubMed] [Google Scholar]
- 12.Tao X, Lu Y, Zhou Y, Zhang L, Chen Y.. Efficacy and safety of the regimens containing tenofovir alafenamide versus tenofovir disoproxil fumarate in fixed-dose single-tablet regimens for initial treatment of HIV-1 infection: a meta-analysis of randomized controlled trials. Int J Infect Dis 2020; 93:108–17. [DOI] [PubMed] [Google Scholar]
- 13.Cahn P, Sierra Madero J, Arribas JR, et al. . Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy-naive adults with HIV-1 infection. AIDS 2022; 36:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel R, Evitt L, Mariolis I, et al. . HIV treatment with the two-drug regimen dolutegravir plus lamivudine in real-world clinical practice: a systematic literature review. Infect Dis Ther 2021; 10:2051–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.