Abstract
The small-fiber polyneuropathies (SFN) are a class of diseases in which the small thin myelinated (Aδ) and/or unmyelinated (C) fibers within peripheral nerves malfunction and can degenerate. SFN usually begins in the farthest, most-vulnerable axons, so distal neuropathic pain and symptoms from micro-vascular dysregulation are common. It is well known in adults, e.g. from diabetes, human immunodeficiency virus, or neurotoxins, but considered extremely rare in children, linked mostly with pathogenic genetic variants in voltage-gated sodium channels. However, increasing evidence suggests that pediatric SFN is not rare, and that dysimmunity is the most common cause. Because most pediatric neurologists are unfamiliar with SFN, we report the diagnosis and management of 5 Swiss children, aged 6–11y, who presented with severe paroxysmal burning pain in the hands and feet temporarily relieved by cooling—the erythromelalgia presentation. Medical evaluations revealed autoimmune diseases in 3 families and 3/5 had preceding or concomitant infections. The standard diagnostic test (PGP9.5-immunolabeled lower-leg skin biopsy) confirmed SFN diagnoses in 3/4, and autonomic function testing (AFT) was abnormal in 2/3. Blood testing for etiology was unrevealing, including genetic testing in 3. Paracetamol and ibuprofen were ineffective. Two children responded to gabapentin plus mexiletine, one to carbamazepine, two to mexiletine plus immunotherapy (methylprednisolone/IVIg). All recovered within 6 months, remaining well for years. These monophasic tempos and therapeutic responses are most consistent with acute post-infectious immune-mediated causality akin to Guillain-Barré large-fiber polyneuropathy. Skin biopsy and AFT for SFN, neuropathic-pain medications and immunotherapy should be considered for acute sporadic pediatric erythromelalgia.
Keywords: Erythromelalgia, Small-fiber neuropathy, Child, Monophasic, Dysimmune, Acute
1. Introduction
In healthy children, sudden onset of episodic burning pain in hands and feet without prior trauma or skin lesions is puzzling. It may be considered psychogenic or labeled erythromelalgia (red painful extremities in Greek), a syndrome characterized by bilateral, often paroxysmal and burning distal extremity pain, hyperemia and hyperthermia [1], first described in 1878 by S. Weir Mitchell [2]. Not all patients present fully and symptoms are worsened by external heat, or internal heat from exercise or fever. Most use cooling for temporary relief [1,3–5]. Anti-inflammatory and opioid analgesics are generally ineffective [1].
Most erythromelalgia presentations are caused by small-fiber polyneuropathy (SFN) [3,6–12]. SFN’s classic sensory symptoms are pain and itch beginning distally in 3/4 patients. Internal symptoms include tachycardia, abnormal blood pressure, and abdominal distress from gastrointestinal dysmotility and microcirculatory insufficiency. SFN symptoms reflect spontaneous and/or excess firing of the small C-fiber neurons that sense pain and innervate small blood vessels. If prolonged, the excess potentials raise energy demands, permitting entry of excess ions and fluids that lead to distal degeneration of peripheral small-fibers and sensory loss [13,14]. In turn, distal axonal degeneration initiates trans-synaptic downregulation of inhibitory circuits in the dorsal horn and rostrally to compensate for reduced presynaptic input. In SFN, this can amplify or initiate spontaneous pain. In addition, peripheral axonal degeneration may cause sodium channels to accumulate more proximally rendering C-fibers hyperexcitable and spontaneously active [15].
In mature adults, common causes are chronic medical conditions (e.g. diabetes, monoclonal gammopathies) toxic (e.g. some cancer chemotherapies, arsenic, vitamin B6, anti-infectives) and immune-mediated, (e.g. Sjögren’s [16], paraneoplastic). Blood-test screening identifies potentially treatable causes or contributors in 1/3–1/2 of patients [17,18]. Actual adult population prevalence is unknown. The only estimate – 52,95/100 000 – yields a global prevalence of 4 077 150, which is probably too low because ascertainment required specialist documentation of >2 symptoms, confirmatory skin biopsies or thermal thresholds and normal electrodiagnosis [19].
Pre-pubertal SFN is only recently recognized, hence there is no case definition and no epidemiologic prevalence is available [12]. Sensory and internal symptoms are similar to those in adults and erythromelalgia is a well-recognized presentation [1,9,12,20], It has been associated with pathogenic variants in genes coding for alpha subunits of the 3 voltage-gated sodium channels preferentially expressed in small-fiber sensory and sympathetic neurons, that are identifiable by sequencing [21,22]. Among 1139 Dutch adults with objectively confirmed pure SFN 5.1% had SCN9A variants, 3.7% had SCN10A, and 2.9% had SCN11A variants, although a U.S. study did not find elevated prevalence of SCN variants in neuropathy patients suggesting regional variability [23,24].
A few single case observations and 2 larger series link early acute SFN to dysimmunity analogous to Guillain-Barré syndrome (GBS). Additional evidence of dysimmune causality comes from reports, mainly in adolescents and young adults, showing beneficial effects of corticosteroids and intravenous immunoglobulins (IVIg), the primary treatments for immune large-fiber neuropathies [8–10,12,20,25–28] even in some patients with SCN9A variants [29]. Furthermore reports of associated autoantibodies [30,31] and a patient serum transfer study that replicated painful SFN in mice [32] strengthen this hypothesis.
We report five children with sudden onset of episodic burning or stinging pain in their distal extremities and no other illness or interictal abnormalities. All had objective evidence of SFN from lower-leg skin biopsy or physiological testing. Their non-familial, post-infectious, monophasic courses and rapid responses to IVIg and steroids in 2/5 are consistent with dysimmune rather than genetic causality. This case series characterizes among the youngest patients with an erythromelagia presentation of SFN and supports the hypothesis that most cases reflect dysimmune causality, and are thus treatable. Table 1 summarizes other published cases with acute monophasic non-genetic erythromelalgia/SFN under 21 years.
Table 1.
Cases of acute monophasic pediatric erythromelalgia/SFN with onset before age 21 years (published since 1997 in chronological order).
| First author, publication year | No | Age (y) | Sex M/F | Major somatic symptoms | Dysautonomia signs and symptoms | Illness, vaccination in preceding weeks | Trauma shortly preceding | Pathological confirmation | Autonomic function testing | SFN objectively confirmed | Nerve conduction study | Cerebrospinal fluid | Outcome of immunotherapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Confino, 1997 | 1 | 4.5 | F | acute burning pain, erythema, edema, palms and soles | elevated blood pressure | Influenza vaccination | No data | No biopsies | No testing | No | No data | No data | None administered |
| Wakamoto, 1999 | 1 | 12 | F | acute burning pain, loss of pinprick and temperature sensation, vomiting | hypertension, gastroparesis | Acute febrile illness | No data | Yes (SB) No (NB) |
Not standard | Yes, | normal | normal | None administered |
| Zenz, 1999 | 1 | 5 | M | acute burning pain, erythema hands and feet | hypertension | Gastroenteritis | No | No biopsies | No testing | No | normal | Normal | CR (IVIg) |
| Dabby, 2006 | 1 | 17 | M | acute burning pain, edema hands and feet | No data | No data | No | Yes (SB) | diagnostic AFT | Yes | normal | Normal | NR (IVIg) CR (corticosteroids) |
| Paticoff, 2007 | 1 | 20 | M | acute severe pain hands and feet | hypertension, tachycardia | No | Yes | Yes (SB) | diagnostic AFT | Yes | normal | normal | CR (corticosteroids) |
| Iqbal, 2009 | 2 | 12/8 | M | acute burning pain, erythema hands and feet | No data | No | No | No biopsies | No testing | No data | No testing | No data | None administered |
| Pfund, 2009 | 1 | 12 | F | acute burning pain palms and soles, motor weakness | No data | No | No | No biopsies | No testing | No | abnormal | No data | CR (corticosteroids) |
| Cook-Norris, 2011 | 32 | 5–18 | 10 M/22F | consecutive chronic or acute erythromelalgia, | No data | No data | No data | No neurolabelling of skin biopsies | 4/6 abnormal QSART, 6/14 abnormal TST | Yes (10/32) | 12/12 normal | No data | None administered |
| Morales, 2012 | 1 | 9 | M | acute burning pain, limbs | hypertension | No data | No data | No biopsies | No testing | No data | No testing | No data | CR (corticosteroids) |
| Jakob, 2012 | 1 | 12 | M | acute pain, edema, feet and hands | hypertension | Yes | No | No (SB) | No testing | No | No testing | No data | None administered |
| Elgueta, 2013 | 1 | 9 | M | acute burning pain, edema, erythema, warmth in hands and feet | No data | No data | No data | No biopsies | No testing | No data | No testing | No data- | NR (corticosteroids) |
| Oaklander, 2013 | 41 | 12.3 ± 5.7 | 11 M 30F | consecutive acute and chronic unexplained distal-limb pain, 23% had erythromelalgia | 40/41 with dysautonomic symptoms | 10/41 infections, 14/41 autoimmune diseases | 11/41 | 11/37 SB, 2/2 NB | 18/34 diagnostic AFT | 24/41 definite 7/41 probable 9/41 possible | 2/24 abnormal | Normal 11/41 | 10/15 PR (corticosteroids) 5/8 PR (IVIg) |
| Huh, 2015 | 1 | 12 | F | acute burning pain, erythema with linear pattern | hypertension | Seronegative vasculitis | No data | No biopsies | No testing | No data | No testing | No data | PR (corticosteroids) |
| Hoeijmakers, 2016 | 2 | 14/16 | F | 1 chronic/1 acute burning pain, tingling legs and feet, minor large-fiber involvement | Palpitations, dry eyes, hyperhidrosis, gastrointestinal dysmotility | No/diabetes ketoacidosis 6wks prior | No data | Yes (SB) 2/2 | 1/2 abnormal QST | Yes(2/2) | 1/2 abnormal | No data | None administered |
| Gorlach, 2019 | 26 | 14,2 ± 3,9 | 11 M 15F | 5 acute, 21 chronic distal pain | 18/26 | 2/26 acute 11/26 chronic illness | No data | Yes (SB) 13/26 diagnostic, 9/26 borderline | No testing | Yes (13/26) | 2/11 abnormal | No data | None administered |
Table 1 abbreviations:: y = years, M = male, F = female, SFN = small-fiber polyneuropathy, AFT = autonomic function testing, QST = quantitative sensory testing, SB = skin biopsy, NB = nerve biopsy, IVIg = intravenous immunoglobulins, NR = not responding, CR = complete remission, PR = partial remission.
2. Methods
Patients:
These were 5 consecutive children, admitted to the Centre Universitaire Hospitalier Vaudois, or for whom an opinion was requested, between January 2011 to January 2018 for new-onset, unexplained distal pain. The 2 girls and 3 boys were aged 6–11 years. All were unrelated Caucasians of European origin, three from the nearby Swiss Neuchâtel region. We obtained written parental consent for medical record review and extraction for publication.
Neurodiagnostic Evaluations:
As summarized in Table 2, all had undergone 2–3 mm punch skin biopsies immunolabeled against pan-neuronal marker PGP9.5 to permit morphometric quantitation of epidermal nerve fiber density (END) [33,34]. However, only 4 were removed from the site 5–10 cm above the lateral malleolus for which data are available to permit pathological analysis. Biopsies had been fixed in Zamboni’s fixative solution and mailed to the Massachusetts General Hospital (MGH) Nerve Unit clinical diagnostic laboratory, which has pediatric norms for comparison. Measured ENDs less than the 5th centile of the predicted normal distribution confirmed SFN in suspected patients. Age-matched norms and statistical modeling are essential to reduce false-negative interpretations if children’s biopsies are compared to adult norms. Normal children have 3–4 times higher END that progressively declines until the mid-20’s [35].
Table 2.
Patient clinical presentation and results.
| Case 1, girl, 6 years | Case 2, boy, 9 years | Case 3, boy, 10 years | Case 4, girl, 7 years | Case 5, boy, 11 years | |
|---|---|---|---|---|---|
| Chief complaint | Burning pain in one foot, spread to hands and feet | Needles and pins in both fingertips and toe tips | Burning pain in palms and soles | Needles and pins, burning pain in one foot, rapidly spreading to hands and feet | Needles and pins, burning pain, initially palms and soles, later palms only |
| Circumstances at onset | After a day of skiing No trauma | None | Concomitant streptococcal infection | Diarrhea 2 weeks before onset (sister with rotavirus) | Concomitant streptococcal infection |
| - frequency | ↑, all 10–15min, also night | ↑, several times/d also night | ↑, up to continuous pain | ↑, up to continuous pain | ↑, >30 episodes/d |
| - duration | 10–30min | 30min | 20 min, later constant | Minutes, later constant | 10–15min |
| Patients self-treatment | Cold water immersion | Activity in the cold (ice hockey) | Rubbing limbs, contact with cold (tiles) | Cold water immersion | Cold water immersion |
| Autonomic signs | ↑, blood pressure (persistent) | None | ↑ blood pressure, tachycardia (during symptomatic episodes only) | Persistent ↑ blood pressure, tachycardia, unexplained hyperthermia | ↑ blood pressure and ↑ body temperature (during symptomatic episodes only) |
| Sleep disturbance | Yes | Yes | Yes | Yes | Yes |
| Local skin lesions | Maceration due to cold water | None | None | Maceration due to cold water | None |
| Attempted treatment (chronological order, helpful drugs in bold italics) | paracetamol/ibuprofen tramadol/morphine ASA/carbamazepine chloral hydrate chlorpromazine gabapentin mexiletine | gabapentin carbamazepine | paracetamol/ibuprofen ASA/cetirizine gabapentin/pregabalin lidocaine patch morphine/fentanyl iv ketamine/clonidine lidocaine iv/mexiletine + methylprednisolone/prednisone | carbamazepine ASA gabapentin mexiletine | paracetamol/ibuprofen tramadol/morphine gabapentin carbamazepine chlorpromazine lorazepam mexiletine IVIg |
| Time to symptom resolution | 3 months | 6 months | 3 months | 24 days | 44 days |
| Relapse | No | No | No | No | No |
| Duration of follow up | 8 years | 6 years | 4 years | 3,5 years | 1 year |
| Family history | Multiple sclerosis (aunt) | Negative | Negative | Diabetes mellitus type 1 (father) | lupus (grandfather) rheumatoid arthritis (grandmother) |
| Other investigations (only pathologic results) | ANA 1/80 Low IgG * | ANA 1/320 | Thrombocytosis (450 G/l) * | – | * |
| Skin biopsy END (neurites/mm2 skin surface area) and centile on predicted normal distribution | Day 79 Two from distal leg 68 ENF/mm2 each <1st centile 2/2 diagnostic | 6 months after onset Distal leg skin biopsy with 269 ENF/mm2 3.8th centile diagnostic |
Day 35 Palm of hand and sole of foot Sites not valid for interpretation |
Day 30 Two from distal leg 444 ENF/mm2 and 352 ENF/mm [2], 4.68th and 0.9th centile 2/2 diagnostic |
Day 40 460 ENF/mm2 41st centile normal |
| Electrochemical skin conductance ** | Not performed | Not performed | Not performed | Not performed | No response, suggestive of SFN |
| Sympathetic skin response | Not tolerated | Normal | No response, suggestive of SFN | Not performed | Not performed |
Patients 1, 3 and 5 had negative genetic testing for SCN9A (cases 1, 3, 5), SCN10A, SCN11A and TRPA1 (cases 3 and 5),
Sudoscan®,
SLE = systemic Lupus Erythematosus, ANA = antinuclear antibody (elevated if 1/80, significant if > 1/320). ASA = acetylsalicylic acid; IVIg = intravenous immunoglobulin.
Other testing included nerve-conduction study (1/5) and measuring sympathetic skin responses (SSR) on the soles and palms (3/5), although not tolerated in one 6-year-old. One child had electrochemical skin conductance measured by Sudoscan® (Impeto Medical, Paris, France) [36].
3. Representative case
This healthy 6-year-old girl (Table 2, case 1, Fig. 1) complained of sudden severe burning pain in one foot developing a few minutes after skiing. There had been no recent trauma, fever or illness. During the next few days, she had several daily recurrences in the soles and toes of both feet and in the palms and fingertips; each lasting 2–10 min and preventing walking and playing. No redness or swelling were visible during or between attacks. On day 9, pain severity prompted community hospital admission. Between episodes she was symptom free with normal vital signs and general and neurological examination. Attempted analgesia (Fig. 1) was ineffective except for hand and foot immersion in cold water. On day 22 continued worsening prompted University Hospital transfer; pain episodes were occurring every 60–90min and lasting ≥30 min. Continual immersion of hands and feet in cold water interfered with activities and sleep (Fig. 1). Examination revealed only persistent tachycardia (100–110bpm) and hypertension (99th percentile for age and height) [37]. Normal results were obtained from lumbar puncture, electroencephalography, nerve conduction study, capillary microscopy of the nail-beds, blood tests for auto-immunity and infection (HIV, hepatitis and Lyme borreliosis), Fabry disease, thyroid dysfunction, and SCN9A sequencing. The only abnormal result was anti-nuclear antibody (ANA titer 1:80). Initiating gabapentin (36mg/kgBW/d) plus mexiletine (27mg/kgBW/d) provided pain relief, and chloral hydrate and chlorpromazine improved sleep. At hospital discharge 2 months post-onset she had only 1–2 painful episodes/day relieved by cold pack-applications. A lower leg skin biopsy on Day 79 was interpreted as diagnostic for SFN (Fig. 2). At 3 months post-onset, pain attacks ceased but were followed by total-body itching, on hands and feet for 2 weeks, perhaps to indicate small-fiber regeneration. All medications were slowly weaned and discontinued during recovery and she remains symptom-free for 9 years.
Fig. 1. Case 1: Clinical presentation and time course.
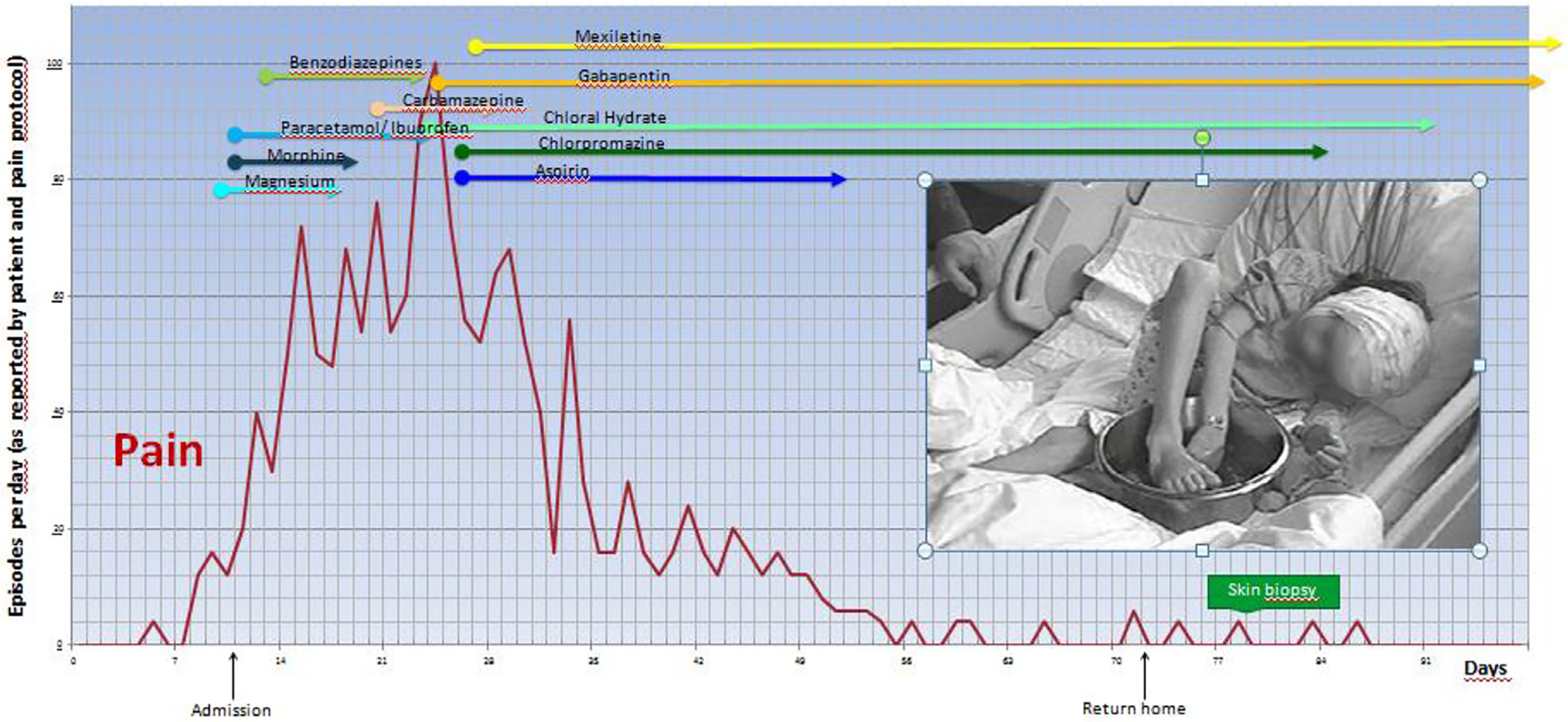
The graph illustrates the painful episodes (up to 100) reported per day and the numerous medications tried within 90 days. The cold-water immersion depicted was replaced by wrapped dry icepacks to prevent skin maceration.
Fig. 2. Case 1: lower leg PGP9.5-immunolabeled skin biopsy.
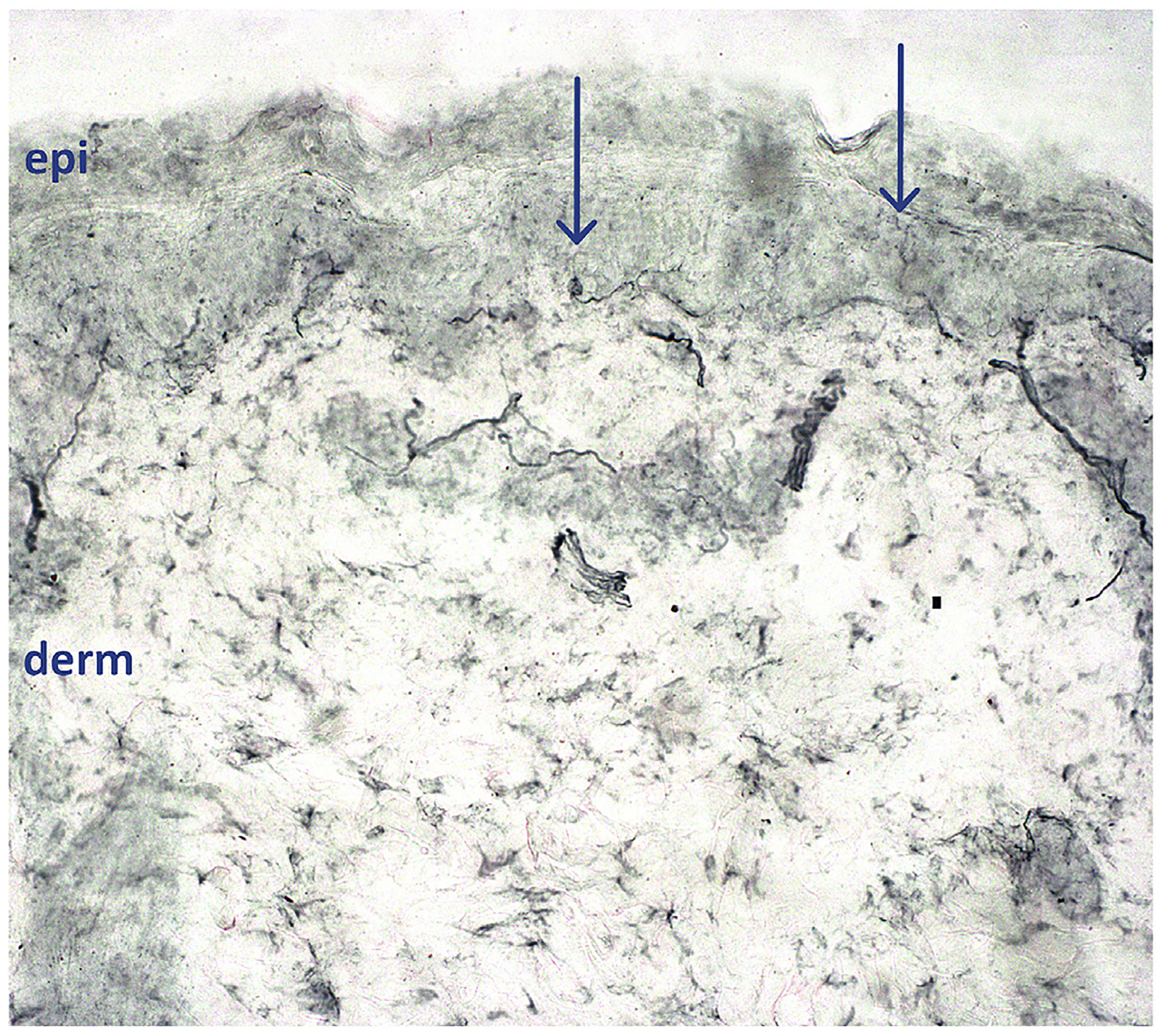
epi = epidermal layer, derm = dermal layer. All neurites crossing the dermalepidermal junction (arrows) are counted by a blinded morphometrist, and measurements compared to those from age-matched healthy volunteers. Virtually all epidermal innervation is sensory small-fiber. The arrow at left demonstrates an epidermal neurite with intraaxonal swelling, a potential pre-degenerative sign. Small-fiber epidermal innervation was nearly depleted, with END of 68 epidermal neurites/mm2 skin surface area, below the 1st centile of the predicted normal distribution, calculated from END of 76 healthy children aged 8–20y. END ≤5th centile are interpreted as confirming suspected clinical cases of SFN. 40x magnification.
4. Discussion
These five children were ultimately diagnosed with early-onset SFN. The characteristic tachycardia and elevated blood pressure in three patients persisted between episodes in two. Although initially attributed to pain, these specific abnormalities are common in pediatric SFN, providing clues to diagnosis (Table 1) [9,12] whereas adults more often have orthostatic hypotension or blood pressure swings [3,13,38]. None of our patients had distal redness or heat, typically part of adult erythromelalgia, but cold relieved their pain as expected. We hypothesize that their absence of distal flushing and edema reflects children’s shorter stature, which reduces the orthostatic pressure that potentiates neuropathic capillary and venous congestion [13,39,40].
Because of pain’s subjective nature, objective confirmation of SFN using lower leg skin biopsies and/or autonomic nerve function testing (AFT) is preferred in clinical care and required for research [1,3,12,13,41–43].Skin biopsies have the advantage of not requiring patient cooperation, special equipment or expertise. However, in two large skin biopsy studies of adults with erythromelalgia, only 10% of lower-leg biopsies were diagnostic, whereas 81% had reduces finger and toe END, showing that a biopsy taken above the ankle can miss far distal abnormalities [41,43]. In addition, as axonal degeneration is typically later and longer-lasting than excess C-fiber firing, skin biopsies performed early in the disease course can be less sensitive than AFT, such as quantitative sudomotor axon reflex testing (QSART). Multiple large studies indeed report impaired far-distal small-fiber control of sweating in 60–94% of erythromelalgia patients and a smaller proportion (34%) have additional cardiovascular abnormalities [14,40,41,43].
Objective confirmation of SFN is however difficult in children because pediatric norms for skin biopsies and sudomotor function testing such as QSART are scant [35,44,45]. SSR tends to provide an “all-none” rather than graded response, like in our cases 2 and 3. Both QSART and SSR are obtained via electrical stimuli and thus require the child’s cooperation [45]. Although diagnostic in one child here, with published pediatric normative values from a little cohort [36], preliminary data suggest that Sudoscan® measurements of sweating have low specificity and sensitivity for detecting SFN in children (Klein et al. unpublished data).
The disease course in all patients was acute and monophasic, peaking within days or a few weeks, and then plateauing for weeks and recovery within 6 months without relapse. Patients’ prior and subsequent normal health, essentially discards genetic, metabolic, paraneoplastic or rheumatological causes. Although GBS is predominantly a large-fiber demyelinating, dysimmune neuropathy, the time course is similar to our young SFN patients and overlap cases are increasingly recognized [3,26,32,38,46]. This continuum between erythromelalgia/SFN and GBS is also supported by albuminocytologic dissociations found in the cerebrospinal fluid in one series of 6 adults [27], even if most patients have bland lumbar punctures.
Inflammatory or dysimmune causality is the most commonly reported cause in children and adolescents with acute early-onset SFN with and without erythromelalgia (Table 1) [8,12,20,26]. The simultaneous or recent mild infections in 3 of our patients are indeed consistent with autoreactive immunity, reported in other acute-onset cases (Table 1) [7,8,10,20,26,32,38,47–52]. In 4/8 pediatric SFN patients, Epstein-Barr virus was implicated [30] and immunization against human papilloma virus is strongly linked to onset of SFN symptoms worldwide [53–55]. In Oaklander et al.’s large cohort, of 41 patients below 21 years with unexplained pain syndromes, almost all with evidence of SFN, 89% had blood-test markers of disordered immunity including 45% with ANA titer ≥ 1:80 and 25% with shortly preceding infections [12]. In our series, 2 children had ANA titers ≥ 1:80, 3 had preceding or concomitant infections and 3/5 families had autoimmune histories, consistent with the 52% family history prevalence of Oaklander’s cohort. Anti-fibroblast growth factor receptor 3 (FGFR3) is linked to SFN in adults, but was unrevealing in one of our patients (Case 5) [56,57].
SFN-treatment is two-pronged: symptom relief plus disease modification. Cooling, gabapentin and sodium-channel blockers best relieved symptoms in our patients. Regarding immunotherapies, a few children with erythromelalgia presentations of SFN received steroids and improved (Table 1). Typical for hospitalized patients is the intravenous methylprednisolone dose of 500mg/1,73m2/day during 3 days used here, with prednisone 1–2 mg/kg/day given in outpatients [8,10,26]. One study of 15 corticosteroid-treated children and adolescents with biopsy-confirmed SFN reported 67% sustained improvement [12]. IVIg is the primary treatment overall for autoimmune neuropathy, and the evidence, also uncontrolled, is even stronger in adults [12,25,58], A randomized clinical trial of IVIg for idiopathic SFN is underway in the Netherlands [59]. In our 2 patients, IVIg was administered once at standard adult and pediatric doses of 2g/kgBW over 1–5 consecutive days. In the largest uncontrolled study, of 55 adults and children treated with ≥1g/kgBW/4 weeks for ≥3 months, 74% of patients rated themselves “improved”, their neurologist labeled 77% as IVIg responders, and 16% remained in remission after IVIg withdrawal. Their proportion of abnormal autonomic testing dropped from 89% at baseline to 55% (p ≤ 0,001) [25]. Although both of our immunotherapy-treated patients improved quickly, benefits were difficult to measure with mexiletine given concomitantly.
5. Conclusion
We describe 5 children with acute onset, episodic erythromelalgia pain in the extremities without other illness, shown to be SFN. Their monophasic courses are consistent with immune, perhaps post-infectious etiology akin to GBS. Lower-leg skin biopsy and sweating quantitation provide best diagnostic confirmation, although better norms from healthy children would improve accuracy. Identification of specific autoantibodies remains scarce, especially in children.
Acknowledgements
We warmly thank our colleagues D. Mercati, M. Hofer, I. El Faleh, T. Kuntzer and graphic designer E. Alibrandi for their contributions to our manuscript.
Funding
NIH 1-NS093653, 2-NS093653 (Oaklander), U.S. DoD GW140169 (Oaklander).
Abbreviations:
- SFN
Small-fiber polyneuropathy
- GBS
Guillain-Barré syndrome
- NSAIDs
non-steroidal anti-inflammatory drugs
- IVIg
intravenous immunoglobulins
- AFT
autonomic nerve function testing
- END
epidermal nerve fiber density
- FGFR3
fibroblast growth-factor 3
- kgBW
kilograms body-weight
- PGP9.5
protein gene product 9.5
- ESC
electrochemical skin conductance
- SSR
sympathetic skin response
- QSART
quantitative sudomotor axon reflex testing
Footnotes
Financial disclosure
The authors have indicated they have no financial relationships relevant to this article to disclose.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article to disclose.
References
- [1].Cook-Norris RH, Tollefson MM, Cruz-Inigo AE, et al. , Pediatric erythromelalgia: a retrospective review of 32 cases evaluated at Mayo Clinic over a 37-year period, J. Am. Acad. Dermatol 66 (3) (2012) 416–423, 10.1016/j.jaad.2011.01.010, published Online First: 2011/07/30. [DOI] [PubMed] [Google Scholar]
- [2].Mitchell SW, On a rare vaso-motor neurosis of the extremeties, and on maladies with which it may be confounded, Am. J. Med. Sci 151 (1878) 17–36. [Google Scholar]
- [3].Davis MD, Sandroni P, Rooke TW, et al. , Erythromelalgia: vasculopathy, neuropathy, or both? A prospective study of vascular and neurophysiologic studies in erythromelalgia, Arch. Dermatol 139 (10) (2003) 1337–1343, 10.1001/archderm.139.10.1337, published Online First: 2003/10/22. [DOI] [PubMed] [Google Scholar]
- [4].Elgueta F, de la Cuadra-Fontaine JC, Clede L, et al. , Erythromelagia: a rare and hard-to-treat condition: a 9-year-old boy responsive to intravenous lidocaine and oral mexilitene, Pain Med 14 (2) (2013) 311–312, 10.1111/pme.12030, published Online First: 2013/02/02. [DOI] [PubMed] [Google Scholar]
- [5].Iqbal J, Bhat MI, Charoo BA, et al. , Experience with oral mexiletine in primary erythromelalgia in children, Ann. Saudi Med 29 (4) (2009) 316–318, 10.4103/0256-4947.55316, published Online First: 2009/07/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gorlach J, Amsel D, Kolbel H, et al. , Diagnostic utility of small fiber analysis in skin biopsies from children with chronic pain, Muscle Nerve 61 (2) (2020) 173–181, 10.1002/mus.26766, published Online First: 2019/11/22. [DOI] [PubMed] [Google Scholar]
- [7].Hoeijmakers JG, Faber CG, Miedema CJ, et al. , Small fiber neuropathy in children: two case reports illustrating the importance of recognition, Pediatrics 138 (4) (2016), 10.1542/peds.2016-1215 published Online First: 2016/09/24. [DOI] [PubMed] [Google Scholar]
- [8].Paticoff J, Valovska A, Nedeljkovic SS, et al. , Defining a treatable cause of erythromelalgia: acute adolescent autoimmune small-fiber axonopathy, Anesth. Analg 104 (2) (2007) 438–441, 10.1213/01.ane.0000252965.83347.25, published Online First: 2007/01/24. [DOI] [PubMed] [Google Scholar]
- [9].Wakamoto H, Hirai A, Manabe K, et al. , Idiopathic small-fiber sensory neuropathy in childhood: a diagnosis based on objective findings on punch skin biopsy specimens, J. Pediatr 135 (2 Pt 1) (1999) 257–260, 10.1016/s0022-3476(99)70032-6, published Online First: 1999/08/04. [DOI] [PubMed] [Google Scholar]
- [10].Dabby R, Gilad R, Sadeh M, et al. , Acute steroid responsive small-fiber sensory neuropathy: a new entity? J. Peripher. Nerv. Syst 11 (1) (2006) 47–52, 10.1111/j.1085-9489.2006.00062.x, published Online First: 2006/03/08. [DOI] [PubMed] [Google Scholar]
- [11].Kumar N, Davis MD, Erythromelalgia: an underrecognized manifestation of small-fiber neuropathy, Mayo Clin. Proc 81 (8) (2006) 1001, published Online First: 2006/08/12. [DOI] [PubMed] [Google Scholar]
- [12].Oaklander AL, Klein MM, Evidence of small-fiber polyneuropathy in unexplained, juvenile-onset, widespread pain syndromes, Pediatrics 131 (4) (2013) e1091–e1100, 10.1542/peds.2012-2597, published Online First: 2013/03/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Oaklander AL, Nolano M, Scientific advances in and clinical approaches to small-fiber polyneuropathy: a review, JAMA Neurol (2019), 10.1001/jamaneurol.2019.2917 published Online First: 2019/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Orstavik K, Weidner C, Schmidt R, et al. , Pathological C-fibres in patients with a chronic painful condition, Brain 126 (Pt 3) (2003) 567–578, 10.1093/brain/awg060, published Online First: 2003/02/05. [DOI] [PubMed] [Google Scholar]
- [15].Akin EJ, Higerd GP, Mis MA, et al. , Building sensory axons: delivery and distribution of NaV1.7 channels and effects of inflammatory mediators, Sci Adv 5 (10) (2019), 10.1126/sciadv.aax4755 eaax4755, published Online First: 2019/11/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lefaucheur JP, Sene D, Oaklander AL, Primary sjogren’s syndrome, N. Engl. J. Med 379 (1) (2018) 96, 10.1056/NEJMc1804598, published Online First: 2018/07/06. [DOI] [PubMed] [Google Scholar]
- [17].de Greef BT, Hoeijmakers JG, Gorissen-Brouwers CM, et al. , Associated conditions in small fiber neuropathy - a large cohort study and review of the literature, Eur. J. Neurol 25 (2) (2018) 348–355, 10.1111/ene.13508, published Online First: 2017/11/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lang M, Treister R, Oaklander AL, Diagnostic value of blood tests for occult causes of initially idiopathic small-fiber polyneuropathy, J. Neurol 263 (12) (2016) 2515–2527, 10.1007/s00415-016-8270-5, published Online First: 2016/10/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Peters MJ, Bakkers M, Merkies IS, et al. , Incidence and prevalence of small-fiber neuropathy: a survey in The Netherlands, Neurology 81 (15) (2013) 1356–1360, 10.1212/WNL.0b013e3182a8236e, published Online First: 2013/09/03. [DOI] [PubMed] [Google Scholar]
- [20].Morales PS, Escobar RG, Lizama M, et al. , Paediatric hypertension-associated erythromelalgia responds to corticosteroids and is not associated with SCN9A mutations, Rheumatology 51 (12) (2012) 2295–2296, 10.1093/rheumatology/kes098, published Online First: 2012/06/22. [DOI] [PubMed] [Google Scholar]
- [21].Waxman SG, Dib-Hajj S, Erythermalgia: molecular basis for an inherited pain syndrome, Trends Mol. Med 11 (12) (2005) 555–562, 10.1016/j.molmed.2005.10.004, published Online First: 2005/11/10. [DOI] [PubMed] [Google Scholar]
- [22].Yang Y, Wang Y, Li S, et al. , Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia, J. Med. Genet 41 (3) (2004) 171–174, published Online First: 2004/02/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Eijkenboom I, Sopacua M, Hoeijmakers JG, et al. , Yield of peripheral sodium channels gene screening in pure small fibre neuropathy, J. Neurol. Neurosurg. Psychiatry 90 (3) (2019) 342–352, 10.1136/jnnp-2018-319042, published Online First: 2018/12/17. [DOI] [PubMed] [Google Scholar]
- [24].Wadhawan S, Pant S, Golhar R, et al. , NaV channel variants in patients with painful and nonpainful peripheral neuropathy, Neurol Genet 3 (6) (2017), e207, 10.1212/NXG.0000000000000207 published Online First: 2017/12/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu X, Treister R, Lang M, et al. , IVIg for apparently autoimmune small-fiber polyneuropathy: first analysis of efficacy and safety, Ther Adv Neurol Disord 11 (2018), 10.1177/1756285617744484, 1756285617744484, published Online First: 2018/02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pfund Z, Stankovics J, Decsi T, et al. , Childhood steroid-responsive acute erythromelalgia with axonal neuropathy of large myelinated fibers: a dysimmune neuropathy? Neuromuscul. Disord 19 (1) (2009) 49–52, 10.1016/j.nmd.2008.10.005, published Online First: 2008/12/06. [DOI] [PubMed] [Google Scholar]
- [27].Seneviratne U, Gunasekera S, Acute small fibre sensory neuropathy: another variant of Guillain-Barre syndrome? J. Neurol. Neurosurg. Psychiatry 72 (4) (2002) 540–542, 10.1136/jnnp.72.4.540, published Online First: 2002/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shinkarevsky Fleitman I, Nevo Y, Harel L, et al. , Small-fiber neuropathy associated with autoinflammatory syndromes in children and adolescents, Muscle Nerve (2020), 10.1002/mus.26857 published Online First: 2020/03/07. [DOI] [PubMed] [Google Scholar]
- [29].Kelley MA, Oaklander AL, Association of small-fiber polyneuropathy with three previously unassociated rare missense SCNA9 variants, Canadian Journal of Pain 4 (1) (2020) 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kafaie J, Al Balushi A, Kim M, et al. , Clinical and laboratory profiles of idiopathic small fiber neuropathy in children: case series, J. Clin. Neuromuscul. Dis 19 (1) (2017) 31–37, 10.1097/CND.0000000000000178, published Online First: 2017/08/23. [DOI] [PubMed] [Google Scholar]
- [31].Malik A, Lopate G, Hayat G, et al. , Prevalence of axonal sensory neuropathy with IgM binding to trisulfated heparin disaccharide in patients with fibromyalgia, J. Clin. Neuromuscul. Dis 20 (3) (2019) 103–110, 10.1097/CND.0000000000000236, published Online First: 2019/02/26. [DOI] [PubMed] [Google Scholar]
- [32].Yuki N, Chan AC, Wong A Hiu Yi, et al. , Acute painful autoimmune neuropathy: a variant of Guillain-Barre syndrome, Muscle Nerve 57 (2) (2018) 320–324, 10.1002/mus.25738, published Online First: 2017/07/02. [DOI] [PubMed] [Google Scholar]
- [33].England JD, Gronseth GS, Franklin G, et al. , Practice Parameter: evaluation of distal symmetric polyneuropathy: role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation, Neurology 72 (2) (2009) 177–184, 10.1212/01.wnl.0000336345.70511.0f, published Online First: 2008/12/06. [DOI] [PubMed] [Google Scholar]
- [34].Lauria G, Hsieh ST, Johansson O, et al. , European federation of neurological societies/peripheral nerve society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European federation of neurological societies and the peripheral nerve society, Eur. J. Neurol 17 (7) (2010) 903–912, 10.1111/j.1468-1331.2010.03023.x, e44–9, published Online First: 2010/07/21. [DOI] [PubMed] [Google Scholar]
- [35].Klein MM, Downs H, O’Neil K, et al. , Skin biopsy of normal children demonstrates inverse correlation between age and epidermal nerve fiber density, meaning age-specific norms are needed, Ann. Neurol 76 (2014) S69. [Google Scholar]
- [36].Leclair-Visonneau L, Bosquet T, Magot A, et al. , Electrochemical skin conductance for quantitative assessment of sweat function: normative values in children, Clin Neurophysiol Pract 1 (2016) 43–45, 10.1016/j.cnp.2016.07.001, published Online First: 2016/07/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Barba G, Buck C, Bammann K, et al. , Blood pressure reference values for European non-overweight school children: the IDEFICS study, Int. J. Obes 38 (Suppl 2) (2005) S48eS56, 10.1038/ijo.2014.135, 2014, published Online First: 2014/09/16. [DOI] [PubMed] [Google Scholar]
- [38].Makonahalli R, Seneviratne J, Seneviratne U, Acute small fiber neuropathy following Mycoplasma infection: a rare variant of Guillain-Barre syndrome, J. Clin. Neuromuscul. Dis 15 (4) (2014) 147–151, 10.1097/CND.0000000000000031, published Online First: 2014/05/30. [DOI] [PubMed] [Google Scholar]
- [39].Dori A, Lopate G, Keeling R, et al. , Myovascular innervation: axon loss in small-fiber neuropathies, Muscle Nerve 51 (4) (2015) 514–521, 10.1002/mus.24356, published Online First: 2014/08/06. [DOI] [PubMed] [Google Scholar]
- [40].Mork C, Kalgaard OM, Kvernebo K, Impaired neurogenic control of skin perfusion in erythromelalgia, J. Invest. Dermatol 118 (4) (2002) 699–703, 10.1046/j.1523-1747.2002.01726.x, published Online First: 2002/03/29. [DOI] [PubMed] [Google Scholar]
- [41].Davis MD, Weenig RH, Genebriera J, et al. , Histopathologic findings in primary erythromelalgia are nonspecific: special studies show a decrease in small nerve fiber density, J. Am. Acad. Dermatol 55 (3) (2006) 519–522, 10.1016/j.jaad.2006.04.067, published Online First: 2006/08/16. [DOI] [PubMed] [Google Scholar]
- [42].Gewandter J, Freeman R, Dworkin R, et al. , Diagnostic criteria for idiopathic distal sensory polyneuropathy and idiopathic small fiber polyneuropathy (1798), Neurology 94 (15 Supplement) (2020) 1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mantyh WG, Dyck JB, Dyck PJ, et al. , Epidermal nerve fiber quantification in patients with erythromelalgia, JAMA Dermatol (2016), 10.1001/jamadermatol.2016.4404 published Online First: 2016/12/08. [DOI] [PubMed] [Google Scholar]
- [44].Devigili G, Tugnoli V, Penza P, et al. , The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology, Brain 131 (Pt 7) (2008) 1912–1925, 10.1093/brain/awn093, published Online First: 2008/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kuntz NL, Patwari PP, Laboratory evaluation of pediatric autonomic disorders, Semin. Pediatr. Neurol 20 (1) (2013) 35–43, 10.1016/j.spen.2013.01.004, published Online First: 2013/03/08. [DOI] [PubMed] [Google Scholar]
- [46].Oh SJ, LaGanke C, Claussen GC, Sensory guillain-barre syndrome, Neurology 56 (1) (2001) 82–86, published Online First: 2001/01/10. [DOI] [PubMed] [Google Scholar]
- [47].Confino I, Passwell JH, Padeh S, Erythromelalgia following influenza vaccine in a child, Clin. Exp. Rheumatol 15 (1) (1997) 111–113, published Online First: 1997/01/01. [PubMed] [Google Scholar]
- [48].Huh S, Jung MK, Eun LY, et al. , Erythromelalgia with a linear pattern in a 12-year-old girl, Pediatr. Int 57 (4) (2015) 706–708, 10.1111/ped.12661, published Online First: 2015/09/01. [DOI] [PubMed] [Google Scholar]
- [49].Jackson AL, Oates JA, A patient with adult erythermalgia: evidence suggesting an autoimmune etiology, Am. J. Med. Sci 335 (4) (2008) 320–322, 10.1097/MAJ.0b013e31812f65e7, published Online First: 2008/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jakob A, Creutzfeldt R, Staszewski O, et al. , Primary erythromelalgia in a 12-year-old boy: positive response to sodium channel blockers despite negative SCN9A mutations, Klin. Pädiatr 224 (5) (2012) 309–312, 10.1055/s-0031-1287823, published Online First: 2011/12/16. [DOI] [PubMed] [Google Scholar]
- [51].Kafaie J, Kim M, Krause E, Small fiber neuropathy following vaccination, J. Clin. Neuromuscul. Dis 18 (1) (2016) 37–40, 10.1097/CND.0000000000000130, published Online First: 2016/08/24. [DOI] [PubMed] [Google Scholar]
- [52].Zenz W, Zohrer B, Zobel G, et al. , Acute erythromelalgia with hypertension in a 5-year old boy, Klin. Pädiatr 211 (6) (1999) 469–472, 10.1055/s-2008-1043837, published Online First: 1999/12/11. [DOI] [PubMed] [Google Scholar]
- [53].Blitshteyn S, Postural tachycardia syndrome following human papillomavirus vaccination, Eur. J. Neurol 21 (1) (2014) 135–139, 10.1111/ene.12272, published Online First: 2013/10/10. [DOI] [PubMed] [Google Scholar]
- [54].Kinoshita T, Abe RT, Hineno A, et al. , Peripheral sympathetic nerve dysfunction in adolescent Japanese girls following immunization with the human papillomavirus vaccine, Intern. Med 53 (19) (2014) 2185–2200, 10.2169/internalmedicine.53.3133, published Online First: 2014/10/03. [DOI] [PubMed] [Google Scholar]
- [55].Palmieri B, Poddighe D, Vadala M, et al. , Severe somatoform and dysautonomic syndromes after HPV vaccination: case series and review of literature, Immunol. Res 65 (1) (2017) 106–116, 10.1007/s12026-016-8820-z, published Online First: 2016/08/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Antoine JC, Boutahar N, Lassabliere F, et al. , Antifibroblast growth factor receptor 3 antibodies identify a subgroup of patients with sensory neuropathy, J. Neurol. Neurosurg. Psychiatry 86 (12) (2015) 1347–1355, 10.1136/jnnp-2014-309730, published Online First: 2015/01/30. [DOI] [PubMed] [Google Scholar]
- [57].Delmont E, New antibodies in peripheral neuropathies, Pratique Neurologique FMC 9 (2) (2018) 95–99, 10.1016/S1878-7762(18)30062-1. [DOI] [Google Scholar]
- [58].Moody S, Pacheco S, Butler IJ, et al. , Secondary erythromelalgia successfully treated with intravenous immunoglobulin, J. Child Neurol 27 (7) (2012) 922–923, 10.1177/0883073811427784, published Online First: 2011/12/14. [DOI] [PubMed] [Google Scholar]
- [59].de Greef BT, Geerts M, Hoeijmakers JG, et al. , Intravenous immunoglobulin therapy for small fiber neuropathy: study protocol for a randomized controlled trial, Trials 17 (1) (2016) 330, 10.1186/s13063-016-1450-x, published Online First: 2016/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]


