Abstract
While effective at preventing Zaire ebolavirus (ZEBOV) disease, cellular immunity to ZEBOV and vector-directed immunity elicited by the recombinant vesicular stomatitis virus expressing ZEBOV glycoprotein (rVSVΔG-ZEBOV-GP) vaccine remain poorly understood. Sera and peripheral blood mononuclear cells were collected from 32 participants enrolled in a prospective multicenter study [ClinicalTrials.gov NCT02788227] before vaccination and up to six months post-vaccination. IgM and IgG antibodies, IgG-producing memory B cells (MBCs), and T cell reactivity to ZEBOV glycoprotein (ZEBOV-GP), vesicular stomatitis virus-Indiana strain (VSV-I) matrix (M) protein, and VSV-I nucleoprotein (NP) were measured using ELISA, ELISpot, and flow cytometry, respectively. 11/32 (34.4%) participants previously received a different investigational ZEBOV vaccine prior to enrollment and 21/32 (65.6%) participants were ZEBOV vaccine naïve. Both ZEBOV vaccine naïve and experienced participants had increased ZEBOV-GP IgG optical densities (ODs) post-rVSVΔG-ZEBOV-GP vaccination while only ZEBOV vaccine naïve participants had increased ZEBOV-GP IgM ODs. Transient IgM and IgG antibody responses to VSV-I M protein and NP were observed in a minority of participants. All participants had detectable ZEBOV-GP specific IgG-producing MBCs by 6 months post-vaccination while no changes were observed in the median IgG-producing MBCs to VSV-I proteins. T cell responses to ZEBOV-GP differed between ZEBOV vaccine experienced and ZEBOV vaccine naïve participants. T cell responses to both VSV-I M protein and VSV-I NP were observed, but were of a low magnitude. The rVSVΔG-ZEBOV-GP vaccine elicits robust humoral and memory B cell responses to ZEBOV glycoprotein in both ZEBOV vaccine naïve and experienced individuals and can generate vector-directed T cell immunity. Further research is needed to understand the significance of pre-existing vector and target antigen immunity on responses to booster doses of rVSVΔG-ZEBOV-GP and other rVSV-vectored vaccines.
Keywords: Ebola Vaccine, Vesicular stomatitis-Indiana virus, Recombinant vaccines, Antibodies, T Cell, B cell
Introduction
Ebolaviruses continue to pose a threat to human health since they were first identified in 1976, notably causing large-scale outbreaks in West Africa from 2014–2016 involving over 28,000 cases and 11,325 deaths and in the Democratic Republic of Congo from 2018–2020 with over 3400 cases and 2200 deaths [1, 2]. These large-scale epidemics accelerated clinical data collection on the efficacy of Zaire ebolavirus (ZEBOV) vaccines, leading to European Medicines Agency and the U.S. Food and Drug Administration approval of a recombinant vesicular stomatitis virus expressing ZEBOV glycoprotein (rVSVΔG-ZEBOV-GP) vaccine in 2019 as a single dose regimen for the prevention of Ebola virus disease (EVD) due to infection with Zaire ebolavirus [3, 4]. This vaccine utilizes a replication-competent vesicular stomatitis virus – Indiana strain (VSV-I) from which the VSV-I glycoprotein (GP) gene has been removed and replaced by the ZEBOV-GP gene (Kikwit strain). The efficacy of the rVSVΔG-ZEBOV-GP vaccine for prevention of EVD is estimated to be 97.5% beginning ten days or more post-vaccination and 88.1% at any time post-vaccination when measured over approximately 11 months [5]. ZEBOV-GP antibodies primarily mediate the protection conferred by the rVSVΔG-ZEBOV-GP vaccine although the vaccine can elicit both CD4+ and CD8+ T cell responses to ZEBOV-GP [6, 7]. CD4+ T cells play an important role in vaccine-induced antibody development, but not in protection at the time of ZEBOV challenge, while CD8+ T cells may play a minor role in protection [7, 8]. A combination of ZEBOV-GP antibody titers ≥ 200 EU/mL plus a minimum 2-fold rise in ZEBOV-GP antibody titers post-vaccination has been proposed as a vaccine-induced seroresponse in humans, although a minimum antibody titer necessary to confer protection has not been established [9].
Knowledge gaps remain regarding vector-induced immunity elicited by rVSVΔG-ZEBOV-GP vaccination. Understanding vaccine vector-induced immunity is important to assess for any unintended, potentially adverse consequences associated with use of vectored vaccines, such as modification of vaccine-induced target antigen immunity in the setting of pre-existing vector immunity or the potential to enhance susceptibility to infection, as was observed among adenovirus type 5 vectored HIV vaccine recipients [10–13]. Human infection with VSV-I is rare and the prevalence of naturally acquired VSV-I antibodies is generally low with a few, geographically restricted exceptions [14]. The rVSVΔG-ZEBOV-GP vaccine can elicit anti-VSV-I antibodies in non-human primates, although these did not interfere with protection induced by a subsequently administered, similarly designed recombinant vesicular stomatitis virus (rVSV) vaccine expressing Lassa virus GP [15]. In a retrospective analysis of a phase I clinical trial using three different doses of rVSVΔG-ZEBOV-GP vaccine, transient, non-neutralizing antibodies directed against the VSV-I matrix (M) protein and T cell responses against the VSV-I nucleoprotein (NP) were elicited in a third of vaccine recipients [16].
Expanding our understanding of immunity elicited by the rVSVΔG-ZEBOV-GP vaccine against the target antigen and the VSV-I vector is critical for understanding vaccine-induced immunity, the potential for rVSVΔG-ZEBOV-GP vaccine to be used as a booster among people who previously received ZEBOV vaccines, and the potential of future doses of other rVSV-based vaccines to successfully initiate or boost immunity to the same or different target antigens. Although the rVSVΔG-ZEBOV-GP vaccine is currently approved as a single dose vaccine, the duration of protection following vaccination is unclear; clinical trials assessing the immunological benefit of booster doses of rVSVΔG-ZEBOV-GP vaccination are underway [ClinicalTrials.gov NCT02788227, NCT02876328]. Here we describe humoral, memory B cell, and T cell responses directed against ZEBOV-GP, VSV-I M protein, and VSV-NP elicited in the first 6 months following rVSVΔG-ZEBOV-GP vaccine administration from a subset of volunteers participating in an open-label, phase 2 clinical trial to assess the durability of the immune response from rVSVΔG-ZEBOV-GP, including individuals who previous received heterologous ZEBOV vaccines.
Materials and Methods
Specimen Collection, Processing, and Storage:
Blood specimens for this sub-study were obtained from a subset of participants enrolled in the PREPARE study [ClinicalTrials.gov NCT02788227]. Written informed consent for the PREPARE study was obtained from all participants, which permitted the study site to collect additional research specimens at scheduled study visits. The PREPARE study was approved by the US Food and Drug Administration and the Emory University Institutional Review Board.
Study participants received the rVSVΔG-ZEBOV-GP vaccine at a dose of 2 × 107 plaque forming units (PFU) at their first study visit. Additional sera, plasma, and peripheral blood mononuclear cells (PBMCs) were collected at baseline prior to rVSVΔG-ZEBOV-GP vaccination, and one month, three months, and six months after vaccination (Figure S1). Serum samples were obtained using serum-separating tubes containing silica clot activator and polymer gel (BD, 367983) and were processed by sitting at room temperature until clot formation was demonstrated followed by centrifugation. Plasma and PBMCs were obtained using CPT™ tubes with sodium heparin (BD 362753) following centrifugation. PBMCs were washed with phosphate-buffered saline (PBS), and cryopreserved in 90% fetal bovine serum (FBS) with 10% DMSO using a StrataCooler (Agilent) at −80°C, after which PBMCs were stored in liquid nitrogen prior to use. All samples were processed within two hours of collection. Aliquots of sera and plasma were stored at −80°C until ready for initial use and additional aliquots that underwent one freeze-thaw cycle were subsequently stored at −20°C.
ELISA:
Antibodies to ZEBOV-GP, VSV-I GP, VSV-I NP, and VSV-I M proteins were assessed by indirect enzyme-linked immunosorbent assay (ELISA). VSV-I GP is not present in the rVSVΔG-ZEBOV-GP vaccine and served as a control for natural VSV-I infection. In brief, Nunc Maxisorb plates (Fisher 439454) were coated with 1 μg/mL of recombinant ZEBOV-GP minus the trans-membrane region (IBT Bioservices 0501-015), VSV-I glycoprotein (Mudd-Summers strain, MyBioSource MBS1060254), VSV-I M protein (98COE North America strain, MyBioSource MBS1317470), or VSV-I NP (Glasgow strain, MyBioSource MBS1026155) diluted with PBS and incubated at 4°C. Plates were blocked with PBS containing 0.05% Tween-20 (PBS-T), 5% dry milk, and 4% whey for 1 hour, after which diluted serum was added in three-fold dilutions starting at 1:100 and incubated at room temperature for 1 hours. Pooled serum from healthy volunteers from influenza vaccine study served as a negative control and serum from an EVD convalescent patient (provided courtesy of the Centers for Disease Control and Prevention), murine anti-VSV-I GP antibodies (Sigma-Aldrich V5507), anti-VSV-I M protein antibodies (Kerafast EB0011), anti-VSV-I NP antibodies (Kerafast EB0009) diluted 1:2000 were used as positive controls. Plates were washed with PBS-T and incubated with horseradish peroxidase (HRP) goat anti-human IgG antibody (Jackson ImmunoResearch 109-036-098) diluted 1:10000, HRP goat anti-mouse IgG Fc (ThermoFisher A16084) diluted 1:20000, or HRP goat anti-human IgM antibody (Jackson ImmunoResearch 109-035-129) diluted 1:20000 for 1 hour. KPL SureBlue TM TMB Microwell Peroxidase Substrate (KPL 52-00-00) was added for 5 minutes, the reaction was stopped with 1N hydrochloric acid (VWR BDH3202-2), and optical densities at 450 nm were read using a Biotek EL808 ELISA plate reader at room temperature. All samples were run in duplicate. We defined a positive antibody response to vaccination by an optical density (OD) at 450 nm at 1:100 dilution at least three standard deviations above the mean OD at baseline from all participants for VSV-I antibodies and from ZEBOV vaccine naïve participants only for ZEBOV-GP antibodies. Mean values between ZEBOV experienced and ZEBOV naïve vaccines groups were compared using T-tests with Welch’s correction. All statistical analysis was performed using Prism 9.2 (GraphPad, San Diego, CA).
PBMC Thawing:
Cryopreserved PBMCs were thawed in RPMI 1640 with L-glutamine and HEPES (Fisher MT10041CV) with 10% fetal bovine serum (Hyclone SH30088.03) and penicillin and streptomycin solution (Cellgro 30-002-CI), hereafter referred to as R10, containing DNase I (Roche 04716728001), washed, and resuspended in R10 with DNase I. Cell counting and viability assessment was performed using a Guava easyCyte counter (Luminex) per the manufacturer instructions. For flow cytometry, freshly thawed cells were rested overnight in an incubator at 37°C with 5% carbon dioxide and repeat counting and viability assessment were performed prior to use.
IgG-Producing Memory B Cell ELISpot:
IgG-producing memory B cell (MBC) ELISpots were performed using recombinant ZEBOV-GP minus the trans-membrane region, VSV-I M protein, and VSV-I NP as described above on PBMCs at baseline and 6 months post-rVSVΔG-ZEBOV-GP vaccination. In brief, cryopreserved PBMCs were thawed, stimulated with B-poly-S containing IL-2 and R848 (Cellular Technology Limited CTL-hBPOLYS-200) and 2-mercaptoethanol (Sigma-Aldrich M3148) in a 24 well flat-bottom cell culture plate (Fisher Scientific 07-200-740), and incubated for 5 days at 37°C. 96-well multiscreen-HA plates (Millipore MSHAN4B50) were coated with 2.5 μg/mL ZEBOV-GP, 2 μg/mL of VSV-I M protein, VSV-I NP, or donkey anti-human IgG (Jackson Immuno Research Laboratory 709-005-149) and stored at 4°C for 1–7 days prior to use. On day 6, plates were washed with PBS-T and blocked with R10. Three-fold dilutions of PBMCs were added to the 96-well plates starting at 1:3 dilution for viral antigen wells and 1:30 dilution for anti-human IgG wells and incubated for 6 hours at 37°C. Plates were washed with PBS and PBS-T, and incubated overnight at 4°C with biotin-conjugated donkey anti-human IgG Fc antibody (Jackson Immuno Research Laboratory 709065098). After washing with PBS-T, samples were incubated with Avidin D conjugated with HRP (Vector Laboratories A-2004) for 1 hour at room temperature, plates were washed with PBS-T and PBS. 3-amino-9-ethylcarbazole substrate (Sigma A-5754) was added for 5 minutes and plates were washed with water and allowed to dry in the dark prior to being analyzed with an Immunospot S6 Universal Analyzer (Cellular Technology Limited). All samples were run in duplicate. To account for differences in the quantity of IgG-producing memory B cells present per million PBMCs, the number of antigen-specific spots per million PBMCs was divided by the total number of IgG-producing spots per million PBMCs and multiplied by 100 to produce an antigen-specific/total IgG-producing memory B cell percentage. Median MBC responses were compared using Mann-Whitney-U tests and Pearson’s correlation co-efficient was used to calculate the correlation between ZEBOV-GP IgG secretion MBC responses and ZEBOV-GP antibody ODs.
Flow Cytometry:
PBMCs collected at baseline and one month post-vaccination were used to assess for production of interferon γ (IFN-γ), interleukin 2 (IL-2), and tumor necrosis factor α (TNF-α) from T cells stimulated with peptide pools representing full-length Zaire ebolavirus GP (Mayinga strain, JPT PM-ZEBOV-GPMay), VSV-I M protein, or VSV-I NP by flow cytometry. Peptides sequences matching the recombinant VSV-I M protein and NP antigens used for ELISA and ELISpot assays were custom prepared by Genscript as 15-mer peptides with 10-mer overlaps sequence and pooled to create a concentration of 200 μg/mL. Stimulated cells without antigen and staphylococcal enterotoxin B (Sigma-Aldrich S4881) served as negative and positive controls. In brief, cryopreserved PBMCs were thawed and rested overnight as above. Cells were incubated with antigen-specific peptide pools, CD28 (BD 555725), and CD49d (BD 555501) for 6 hours at 37°C; brefeldin A and monensin (eBioscience 5537) were added after 2 hours of incubation. Cells were washed, stained with viability dye (Zombie Aqua, Biolegend L423101), and fixated/permeabilized using Cytofix/Cytoperm (BD, 554722). Staining was performed using fluorescent-conjugated antibodies to CD3 (SP34-2, 562877), CD4 (L200, 560836), CD8 (RPA-T8, 555367), IL-2 (MQ1-17H12, 554567), and TNF-α (Mab11, 560679) from BD Bioscience and IFN-γ (4S.B3, 47731942) from eBioscience. Flow cytometry was performed on a LSRII (BD Bioscience) and data analysis was performed using FlowJo. The gating strategy is outlined in Figure S2 and Boolean gating was used to identify monofunctional and polyfunctional T cells. Median T cell responses were compared using Mann-Whitney-U tests.
Results
Blood samples were obtained at baseline immediately pre-vaccination, one month post-vaccination, and three months post-vaccination with 2 × 107 PFU of rVSVΔG-ZEBOV-GP from 32 PREPARE participants and at six months post-vaccination for 31 participants (Figure S1). 21/32 (65.6%) participants had never received an ZEBOV vaccine prior to enrollment [ZEBOV vaccine naïve cohort] while 11/32 (34.4%) participants had previously received at least one heterologous investigational ZEBOV vaccine, either a single dose (2 × 1011 PFU) of replication-defective chimpanzee adenovirus 3 vector vaccine expressing ZEBOV-GP (ChAd3-EBO-Z) alone or ChAd3-EBO-Z followed 8 weeks later by a modified vaccinia Ankara virus expressing ZEBOV-GP and other filovirus antigens (MVA-BN-Filo, 1 × 108 PFU) within 2–3 years prior to enrollment [ZEBOV vaccine experienced cohort]. The median age of participants was 40 years (age range: 24 – 67 years; Table 1).
Table 1:
Demographics of Study Participants
| All Participants (%) N = 32 |
ZEBOV Vaccine Experienced Participants (%) N = 11 |
ZEBOV Vaccine Naive Participants (%) N = 21 |
|
|---|---|---|---|
| Median Age in Years (Interquartile range) | 40 (34.5, 46.8) | 37 (34, 46) | 42 (37, 47.5) |
| Gender | |||
| Female | 17 (53.1%) | 5 (45.5%) | 12 (57.1%) |
| Male | 15 (46.9%) | 6 (54.5%) | 9 (42.9%) |
| Prior Ebola Vaccine | |||
| None | 21 (65.6%) | 0 (0%) | 21 (100%) |
| ChAd3-EBO-Za only | 2 (6.3%) | 2 (18.2%) | 0 (0%) |
| ChAd3-EBO-Z with MVA-BN-Filob booster | 9 (28.1%) | 9 (81.8%) | 0 (0%) |
ChAd3-EBO-Z: replication-defective chimpanzee adenovirus 3 vector vaccine expressing ZEBOV-GP. MVA-BN-Filo: modified vaccinia Ankara virus expressing ZEBOV and other filovirus antigens (GPs from Marburg virus, Sudan virus, and Tai Forest virus).
Serology:
The mean IgG OD to ZEBOV-GP was significantly higher in ZEBOV vaccine experienced participants compared to ZEBOV vaccine naïve participants at baseline (mean OD 0.847 to 0.153, p=0.03) and one month post-rVSVΔG-ZEBOV-GP vaccination (mean OD 2.137 to 1.351, p=0.04; Figure 1A), but not three or six months post-vaccination (mean OD 1.809 to 1.106, p=0.07; 1.540 to 0.925, p=0.15, respectively). ZEBOV vaccine experienced participants had significantly smaller mean fold increases in their ZEBOV-GP IgG ODs post-vaccination compared to ZEBOV vaccine naïve participants (mean fold change at 1 month: 3.5 to 8.6, p=0.002; 3 months: 2.4 to 6.8, p=0.002; 6 months: 1.4 to 5.5, p<0.001; Figure 1B). ZEBOV-GP IgG antibodies met our positive antibody response to vaccination criterion (OD ≥ 0.341 at 1:100 dilution) in 0/21 (0%), 20/21 (95.2%), 21/21 (100%), and 20/21 (95.2%) of ZEBOV vaccine naïve participants and in 7/11 (63.6%), 11/11 (100%), 11/11 (100%), and 9/10 (90%) ZEBOV vaccine experienced participants at baseline, one month, three months, and six months post-vaccination, respectively.
Figure 1:
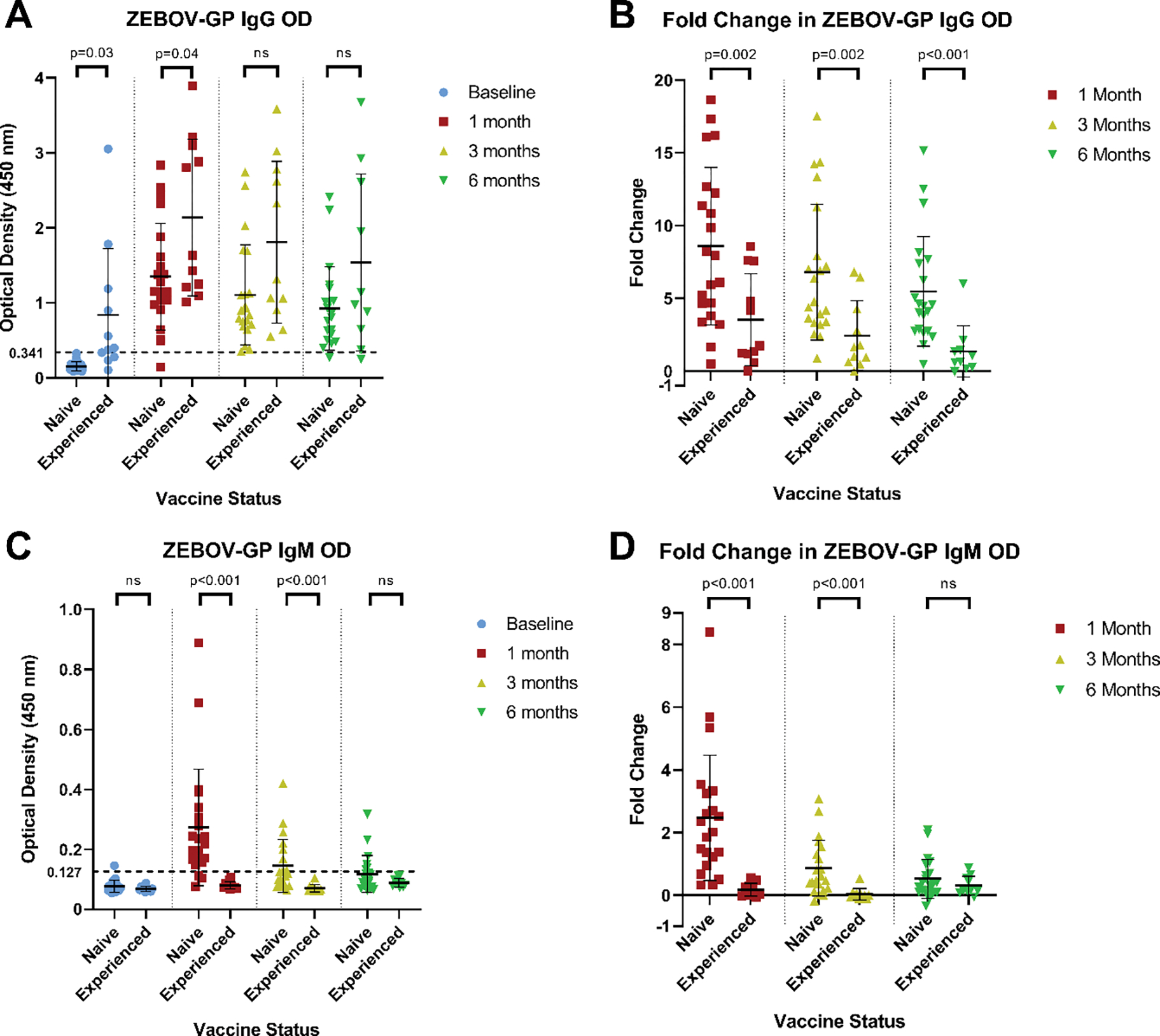
Antibody responses to ZEBOV-GP by ELISA
A: Comparison of ZEBOV-GP IgG ODs between ZEBOV vaccine naïve and ZEBOV vaccine experienced participants. B: Fold changes in ZEBOV-GP IgG ODs post-vaccination compared to baseline according to ZEBOV vaccination status at enrollment. C: Comparison of ZEBOV-GP IgM ODs between ZEBOV vaccine naïve and ZEBOV vaccine experienced participants. D: Fold changes in ZEBOV-GP IgM ODs post-vaccination compared to baseline according to ZEBOV vaccination status at enrollment. All ODs reflect a 1:100 serum dilution. Dotted line indicates the OD criterion used to define a positive antibody response to vaccination (at least three standard deviations above the mean OD at baseline value of ZEBOV vaccine naïve participants)
In contrast, the mean IgM OD to ZEBOV-GP was significantly lower among ZEBOV vaccine experienced participants compared to ZEBOV vaccine naïve participants at one month (0.080 to 0.274, p<0.001) and three months (0.071 to 0.146, p<0.001) post-rVSVΔG-ZEBOV-GP vaccination, but not at baseline (0.069 to 0.078, p=0.10) or six months post vaccination (0.089 to 0.119, p=0.05; Figure 1C). The mean fold change in ZEBOV-GP IgM OD post-vaccination was significantly lower among ZEBOV vaccine experienced participants compared to ZEBOV vaccine naïve participants at one month (0.2 to 2.5, p<0.001) and three months (<0.1 to 0.9, p<0.001) but not at six months post-vaccination (0.3 to 0.5, p=0.20; Figure 1D). No ZEBOV vaccine experienced participants met our criterion for a positive ZEBOV-GP IgM response to vaccination (OD ≥ 0.127 at 1:100 dilution) at any time post-vaccination while 18/21 (85.7%), 8/21 (38.1%), and 6/21 (28.6%) of ZEBOV vaccine naïve participants met this criterion at one month, three months, and six months post-vaccination, respectively.
No participants met our criterion for a positive IgG response to vaccination to VSV-I M protein (OD ≥ 2.773 at 1:100 dilution; Figure 2A). 1/32 (3.1%) participants met the criterion for a positive IgM response to vaccination to VSV-I M protein (OD ≥ 0.173 at 1:100 dilution) at one and three months post-vaccination, but not at baseline, while 1/32 (3.1%) participants met this OD threshold only at the baseline visit (Figure 2B). 1/32 (3.1%) participants met the criterion for a positive IgG response to vaccination to VSV-I NP (OD ≥ 2.359 at 1:100 dilution) at one month post-vaccination, but not at baseline (Figure 2C). 2/32 (6.3%) participants met the criterion for a positive IgM response to vaccination to VSV-I NP (OD ≥ 0.297 at 1:100 dilution) at the one month visit only while a separate participant (1/32 participants, 3.1%) met this OD threshold for IgM antibodies to VSV-I NP only at baseline (Figure 2D). No participants met the criterion for an IgG antibody response to vaccination to VSV-I GP (OD ≥ 1.584) during the study period (Figure 2E).
Figure 2:
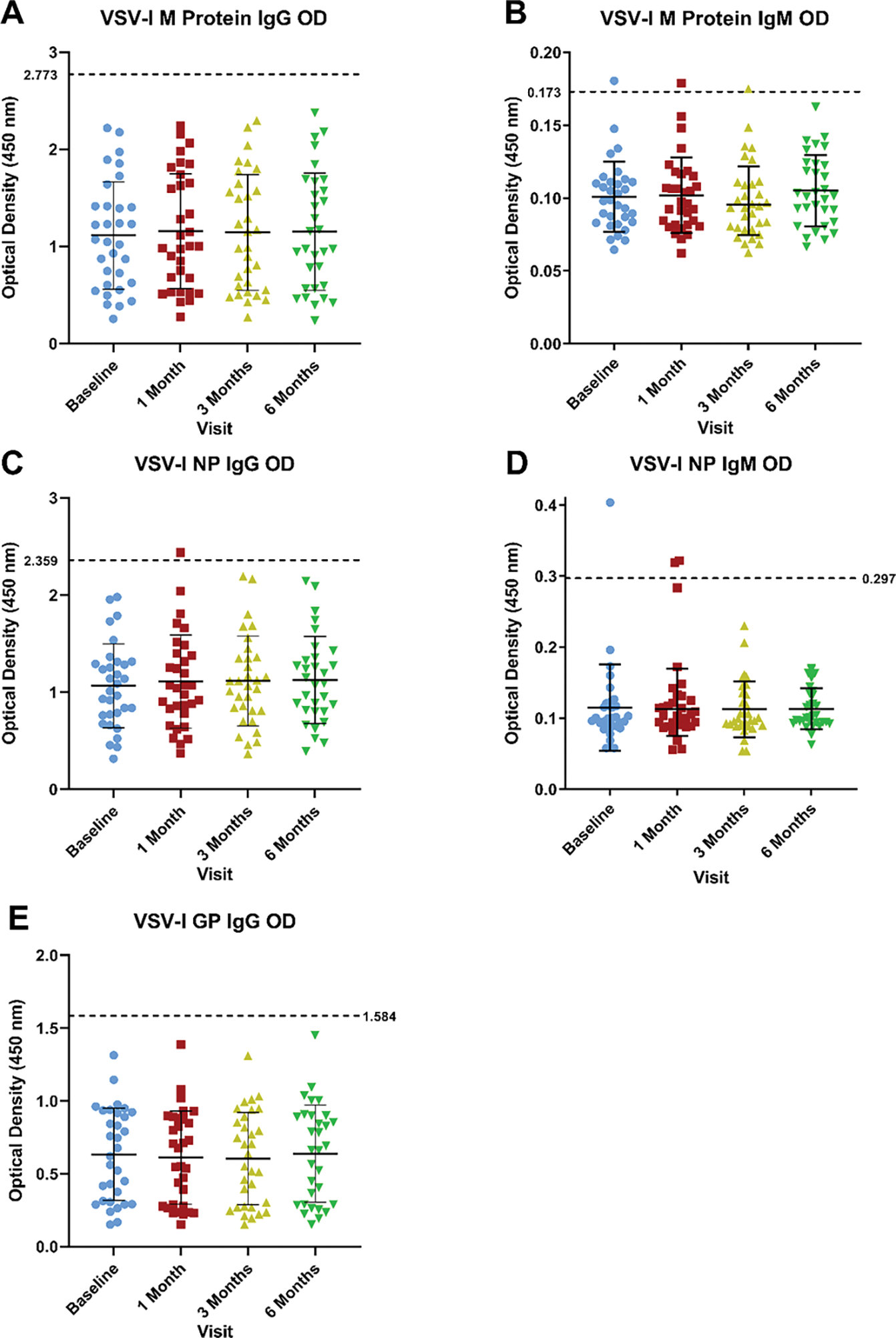
Antibody responses to VSV-I M protein, VSV-I NP, and VSV-I GP by ELISA
Optical densities from baseline and one, three, and six months post-vaccination at 1:100 dilution for A) IgG antibodies to VSV-I M protein, B) IgM antibodies to VSV-I M protein, C) IgG antibodies to VSV-I NP, D) IgM antibodies to VSV-I NP, and E) IgG antibodies to VSV-I GP. All ODs reflect a 1:100 serum dilution. Dotted line indicates the OD criterion used to define a positive antibody response to vaccination (at least three standard deviations above the mean OD at baseline value of all participants). The VSV-I M protein and NP are present in the rVSVΔG-ZEBOV-GP vaccine while the VSV-I GP is absent from the vaccine.
IgG-Producing MBCs:
No ZEBOV vaccine naïve participants (n=21) had detectable ZEBOV-GP specific IgG-producing MBCs at baseline while all ZEBOV vaccine experienced participants (n=10) had detectable ZEBOV-GP specific IgG-producing MBCs at baseline (median percentage of antigen-specific / total IgG-producing MBCs: 0.00% to 0.15%, p<0.001). Significant increases in ZEBOV-GP specific IgG-producing MBCs were observed 6 months post-rVSVΔG-ZEBOV-GP vaccination in both ZEBOV vaccine naïve (median 0.00% to 0.49%, p<0.001) and ZEBOV vaccine experienced (median 0.15% to 0.29%, p=0.03; Figure 3A) participants. No difference in the median percentage of ZEBOV-GP specific IgG-producing MBCs were observed between vaccine naïve and vaccine experienced participants at 6 months post-vaccination (0.49% to 0.29%, p=0.09). The percentage of ZEBOV-GP specific MBCs weakly correlated with ZEBOV-GP IgG ODs at 6 months post-vaccination (r=0.06). No significant differences in median VSV-I M protein (0.02% to 0.03%, p=0.47) or VSV-I NP (0.00% to 0.02%, p=0.44) specific IgG-producing MBCs were observed 6 months post-vaccination (Figure 3B). The range of IgG-producing MBC responses to VSV-I proteins were of a lower magnitude (0–0.20% antigen-specific/total IgG-producing MBCs) compared to the IgG-producing MBC response elicited by ZEBOV-GP (0.11–2.68% antigen-specific/total IgG-producing MBCs; Table S1).
Figure 3:
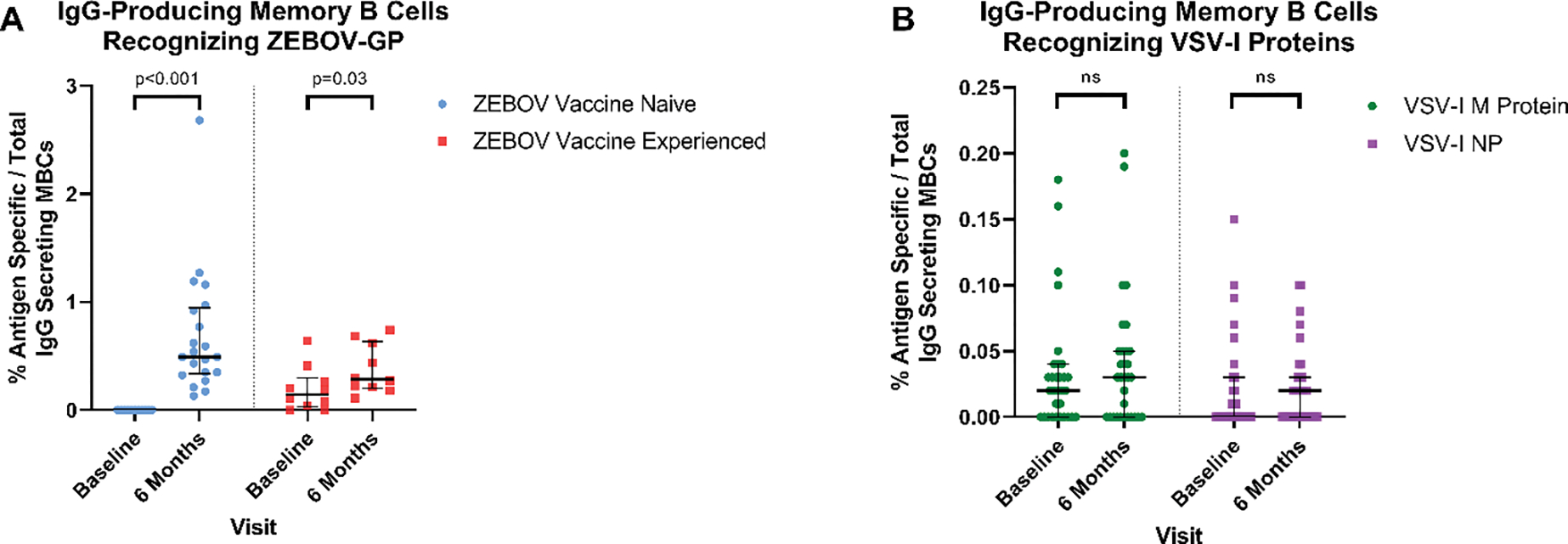
IgG-producing MBCs at baseline and 6 months post-rVSVΔG-ZEBOV-GP vaccination
A: The percentage of ZEBOV-GP specific MBCs out of the total IgG-producing MBCs for ZEBOV vaccine naïve and experienced participants at baseline and 6 months post-rVSVΔG-ZEBOV-GP vaccination. B The percentage of VSV-I M protein and VSV-I NP specific MBCs out of the total IgG-producing MBCs at baseline and 6 months post-rVSVΔG-ZEBOV-GP vaccination. Note that the scale used for VSV-I MBC results differs from that of ZEBOV-GP MBC results.
Flow Cytometry:
At baseline, ZEBOV vaccine naïve participants had lower median percentages of CD4+ T cells producing IFN-γ (0.001% to 0.019%, p<0.001) and IL-2 (0.003% to 0.040%, p=0.03), but not TNF-α (0.014% to 0.123%, p=0.05), when stimulated with ZEBOV-GP peptide pools than ZEBOV vaccine experienced participants. ZEBOV vaccine naïve participants had significant increases in the median percentage of CD4+ T cells producing IFN-γ (0.001% to 0.012%, p<0.001), IL-2 (0.003% to 0.046%, p<0.001), and TNF-α (0.014% to 0.080%, p=0.03) post-vaccination. ZEBOV vaccine experienced participants had a significant increase in the median percentage of CD4+ T cells producing IL-2 (0.040% to 0.082%, p=0.02), but not IFN-γ (0.019% to 0.026%, p=0.10) or TNF-α (0.123% to 0.120%, p=0.44). (Figure 4A). One month post-vaccination, ZEBOV vaccine experienced participants had higher median percentages of CD4+ T cells producing IFN-γ (0.012% to 0.026%, p=0.005) and IL-2 (0.046% to 0.082%, p=0.03) than ZEBOV vaccine naïve participants, however, the median percentage of TNF-α producing CD4+ T cells were not different between the vaccination cohorts (0.080% [naïve] to 0.120% [experienced], p=0.07).
Figure 4:
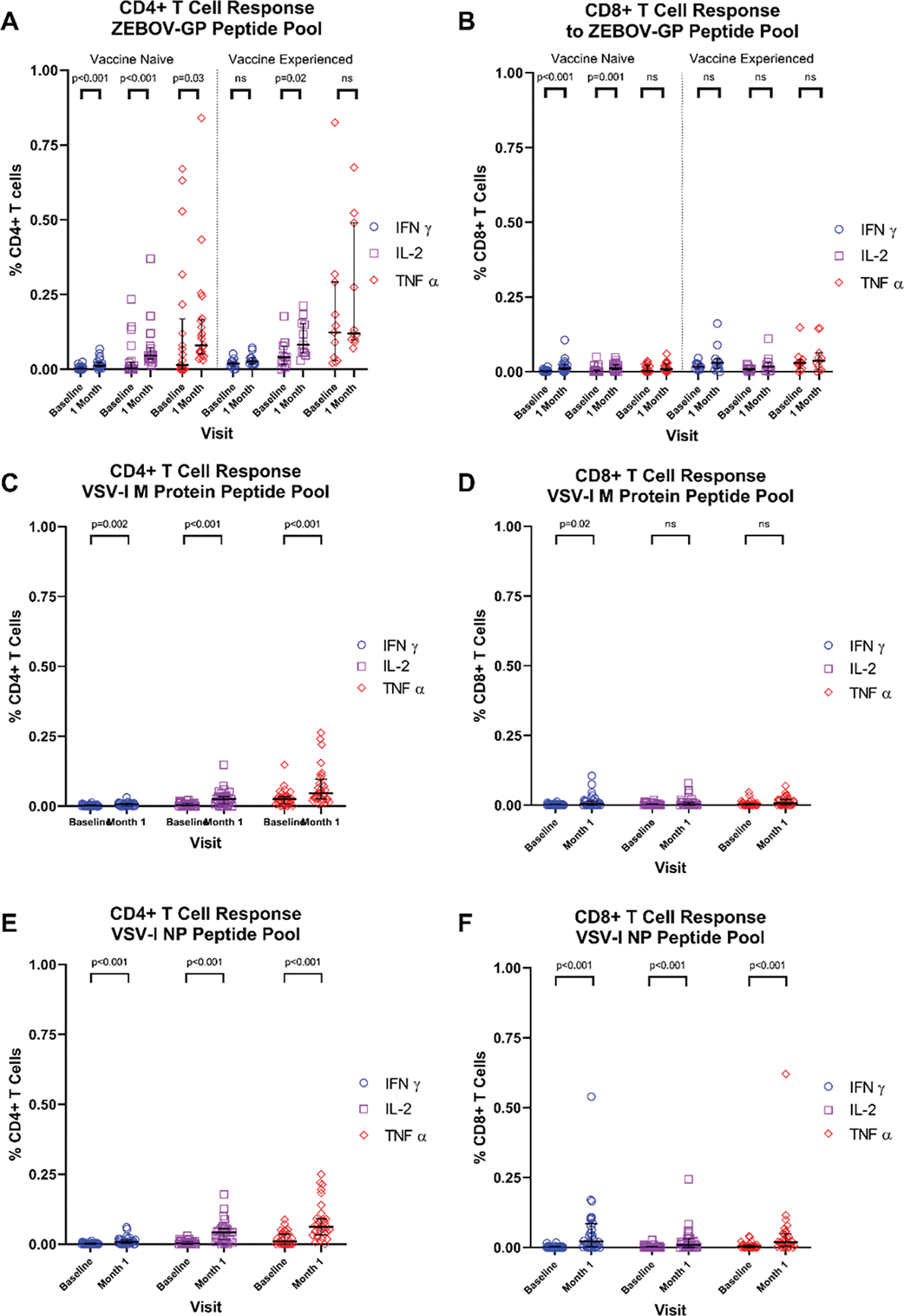
Flow cytometry for IFN-γ, IL-2, and TNF-α from CD4+ and CD8+ T cells
Peripheral blood mononuclear cells from vaccinees at baseline and one month following rVSVΔG-ZEBOV-GP vaccination were assessed for production of IFN-γ, IL-2, and TNF-α from CD4+ (A, C, E) or CD8+ (B, D, F) T cells by flow cytometry when stimulated with pooled peptides representing the full-length ZEBOV-GP (A, B), VSV-I M protein (C, D), or VSV-I NP (E, F).
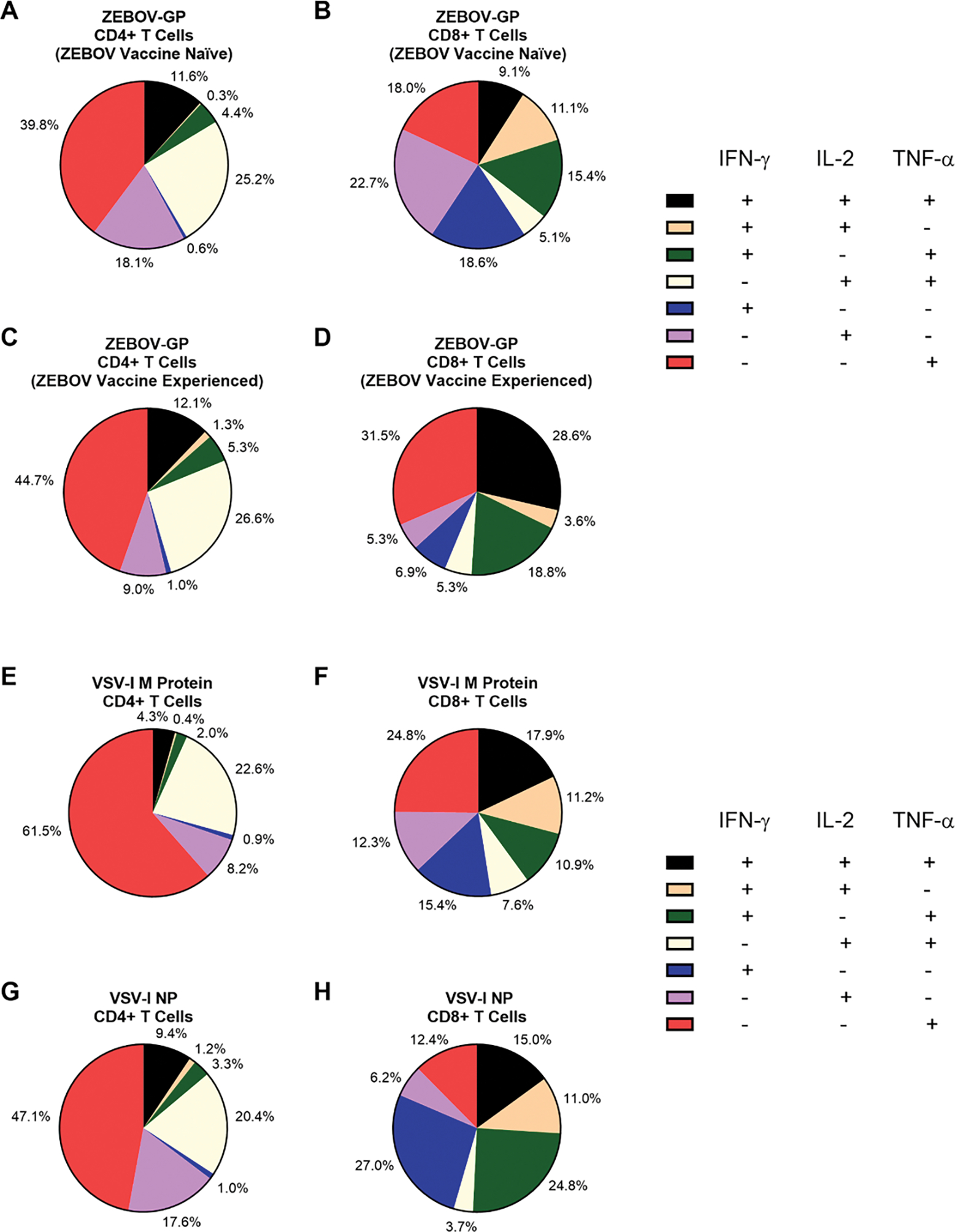
ZEBOV vaccine experienced participants had a higher median percentage of CD8+ T cells producing IFN-γ (0.002% to 0.017%, p<0.001), IL-2 (0.000% to 0.008%, p=0.016), and TNF-α (0.003% to 0.030%, p=0.006) in response to ZEBOV-GP peptide pools at baseline compared to ZEBOV vaccine naïve participants. Among ZEBOV vaccine naïve participants, significant increases in the median percentage of CD8+ T cells producing IFN-γ (0.002% to 0.011% p<0.001) and IL-2 (0.000% to 0.011%, p=0.001), but not TNF-α (0.003% to 0.009%, p=0.10), were observed 1 month post-vaccination. In contrast, ZEBOV vaccine experienced participants had no significant increases in the median percentage of CD8+ T cells producing IFN-γ (0.017% to 0.030%, p=0.30), IL-2 (0.008% to 0.017%, p=0.26), or TNF-α (0.030% to 0.037%, p=0.62; Figure 4B). One month post-vaccination, the median percentage of CD8+ cells producing TNF-α on stimulation with ZEBOV-GP peptide pools was lower among ZEBOV vaccine naïve participants compared to vaccine experienced participants (0.009% to 0.037%, p=0.047) but no significant differences in IFN-γ (0.011% to 0.030%, p=0.07) or IL-2 (0.011% to 0.017%, p=0.51) responses were observed. Post-vaccination CD4+ T cell responses to ZEBOV-GP peptides were primarily mono-cytokine, while mean CD8+ T cell responses were predominantly mono-cytokine for ZEBOV vaccine naïve participants and polyfunctional for heterologous ZEBOV vaccine experienced participants (Figures 5A–D).
Figure 5:
Pie charts representing the mean functionality of CD4+ and CD8+ T cells at one month post-rVSVΔG-ZEBOV-GP vaccination
Pie charts representing the mean functionality of CD4+ T cells (A, C, E, G) and CD8+ T cells (B, D, F, H) at one month following rVSVΔG-ZEBOV-GP vaccination to ZEBOV-GP for ZEBOV vaccine naïve (A, B, n=21) or ZEBOV vaccine experienced (C, D, n=10) participants, and to VSV-I M protein (E, F, n=31) and VSV-I NP (G, H, n=31) among all participants. Due to rounding, the total mean percentage of cells of each functionality may not add up precisely to 100%.
Stimulation of PBMCs with peptide pools representing the VSV-I M protein led to significant increases in the median percentage of CD4+ % cells producing of IFN-γ (0.001% to 0.007%; p=0.002), IL-2 (0.004% to 0.026%, p<0.001), and TNF-α (0.026% to 0.047%, p<0.001; Figure 4C) one month post-vaccination, but only significant increases in IFN-γ (0.000% to 0.004%, p=0.02) from CD8+ T cells (IL-2: 0.000% to 0.002%, p=0.09; TNF-α: 0.001% to 0.007%, p=0.6; Figure 4D). In contrast, stimulation with peptide pools representing the VSV-I NP produced significant increases in the median percentage of IFN-γ (0.001% to 0.008%; p<0.001), IL-2 (0.002% to 0.043%, p<0.001), and TNF-α (0.010% to 0.062%, p<0.001; Figure 4E) producing CD4+ T cells and CD8+ T cells (IFN-γ: 0.000% to 0.021%; p<0.001; IL-2: 0.000% to 0.009%, p<0.001; TNF-α: 0.003% to 0.019%, p<0.001; Figure 4F). T cell responses to VSV-I Matrix protein were predominantly mono-cytokine for both CD4+ and CD8+ T cells (Figures 5E, 5F). CD4+ T cell responses to VSV-I NP were predominantly mono-cytokine while CD8+ T cell responses were predominantly polyfunctional (Figures 5G, 5H).
Discussion
To our knowledge, this is the first study to describe the use of the rVSVΔG-ZEBOV-GP vaccine among heterologous ZEBOV vaccine recipients. All 32 participants in this study, regardless of their previous ZEBOV vaccination status, met our criteria for a positive IgG response to vaccination at three months post-rVSVΔG-ZEBOV-GP vaccination. Increases in ZEBOV-GP IgG ODs were observed among ZEBOV vaccine experienced participants, raising the potential for the rVSVΔG-ZEBOV-GP vaccine to be used as a booster for recipients of heterologous ZEBOV vaccines with waning immunity. Two-fold or larger increase in ZEBOV-GP IgG antibodies were also observed following rVSVΔG-ZEBOV-GP vaccination in a post-hoc analysis of clinical trial data for ZEBOV vaccine naïve individuals with ZEBOV-GP antibodies at baseline [17]. Taken together with our data, this suggests that individuals with pre-existing ZEBOV-GP IgG antibodies, either from prior filovirus infection or vaccination, may still achieve some immunological benefit from rVSVΔG-ZEBOV-GP vaccination.
While the duration of protection against EVD conferred by the rVSVΔG-ZEBOV-GP vaccine remains unclear, we demonstrated that the VSVΔG-ZEBOV-GP vaccine generates B cell memory to ZEBOV-GP. All participants had detectable, circulating IgG-producing MBCs recognizing ZEBOV-GP following vaccination, although the correlation between levels of ZEBOV-GP IgG-producing MBCs with ZEBOV-GP IgG antibody ODs, the established mediator of protection for the rVSVΔG-ZEBOV-GP vaccine, was weak [7]. Whether this immunological memory will be sufficient to provide long-term protection against EVD in the setting of waning serum antibody levels over time has yet to be determined.
IgM antibodies to ZEBOV-GP generated by the rVSVΔG-ZEBOV-GP vaccine have been demonstrated to provide up to 50% of antibody-mediated neutralization of ZEBOV and therefore may contribute to early vaccine-mediated protection from disease [19]. IgM antibodies to ZEBOV-GP were detectable only among participants who were ZEBOV vaccine naïve prior to administration of the rVSVΔG-ZEBOV-GP vaccine. In contrast, IgM was the primary antibody type identified four weeks following two doses of rVSVΔG-ZEBOV-GP vaccine given twenty-eight days apart [19]. The differences in the observed results could be due to longer durations between ZEBOV vaccine doses among ZEBOV vaccine experienced participants in this study or the type of ZEBOV vaccine used as the priming dose. Alternatively, it is possible that heterologous ZEBOV vaccine experienced participants in this study mounted transient IgM antibody responses to rVSVΔG-ZEBOV-GP vaccine that resolved prior to the first post-vaccination visit. Among ZEBOV vaccine naïve participants, the percentage meeting our criterion for an IgM response to vaccination declined after the one month visit, but this threshold was still met in nearly one-fifth of participants at six months post-vaccination. Prolonged detection of ZEBOV-GP IgM following rVSVΔG-ZEBOV-GP vaccination has been noted in other studies, even out to one year post-vaccination, therefore caution should be used when interpreting diagnostic tests for ZEBOV infection that include ZEBOV-GP IgM antibodies among rVSVΔG-ZEBOV-GP vaccinees [19, 20].
Similar to previous studies, we found the rVSVΔG-ZEBOV-GP vaccine can elicit CD4+ and CD8+ T cells to ZEBOV-GP producing IFN-γ and IL-2 [6]. In this study, the cytokine profile produced by T cells differed between ZEBOV vaccine experienced and vaccine naïve individuals. ZEBOV-GP CD8+ T cell responses to ZEBOV-GP were more polyfunctional in nature in this study compared to those documented by Dahlke et al., especially among heterologous ZEBOV vaccine experienced participants [6]. CD8+ T cells play a minor role in protection from the rVSVΔG-ZEBOV-GP vaccination, but a critical role in adenovirus-based and MVA-based ZEBOV vaccines [8, 21, 22]. Given the differences in the role of antibodies and CD8+ T cells in mediating protection between these vaccine platforms, further research on the respective contributions to protection and changes in the quality of antibodies and CD8+ T cells among vaccinees who received both a rVSV-based and an adenovirus or MVA-based platform could help shed light on the nature of the immunological response required for protection against EVD. Notably, the T cell responses to ZEBOV-GP were of a higher magnitude than those to VSV-I targets, suggesting that ZEBOV-GP is the predominant T cell target in the rVSVΔG-ZEBOV-GP vaccine.
Increases in vaccine vector-directed antibodies to the VSV-I M protein and NP were uncommon among vaccine recipients. No participants in this study developed IgG antibodies directed at the VSV-I M protein, in contrast to 28% of rVSVΔG-ZEBOV-GP vaccinees in a study conducted by Poetsch et al, which observed a dose effect with increased VSV-I M protein IgG antibodies at lower vaccine doses compared to high vaccine doses [16]. Only one participant developed transient IgG antibodies to VSV-I NP; to our knowledge, the development of anti-VSV-I NP antibodies has not previously been assessed following rVSVΔG-ZEBOV-GP vaccination. Animal models suggest antibodies to VSV NP are non-neutralizing [23], but the neutralizing capacity of human VSV NP antibodies in humans has not been assessed to our knowledge. Antibodies to VSV-I M protein induced by the rVSVΔG-ZEBOV-GP vaccine have been shown to be non-neutralizing in humans [16]. Animal models have shown that VSV GP is the main target of neutralizing antibodies [24–26] and VSV GP antibodies mediate protection against VSV infection [27–29] while pseudotyped VSV viruses in which the VSV GP gene has been removed do not elicit neutralizing antibodies to VSV, even after multiple doses [14, 30]. In this study we did not observe increases in VSV-I GP IgG antibodies among any participants, as expected given that VSV-I GP is not contained in the rVSVΔG-ZEBOV-GP vaccine. Additionally, detectable IgG-producing memory B cells to VSV-I proteins contained in the vaccine were rare. Taken together, our data indicate that most rVSVΔG-ZEBOV-GP vaccinees do not acquire humoral immunity to viral vector proteins after one dose of rVSVΔG-ZEBOV-GP vaccine at the licensed vaccine dose. In a non-human primate model, antibodies to whole VSV were induced by a rVSV vaccine expressing ZEBOV-GP, but did not impair protection from a subsequently given rVSV vaccine expressing Lassa virus GP, although only three primates were challenged [15]. Whether or not anti-VSV-I antibodies negatively impair responses to booster doses of rVSVΔG-ZEBOV-GP vaccine, or future doses other replication-competent, rVSV-based vaccines, in humans remains to be determined.
The VSV-I nucleoprotein is the most common target for T cell responses in mice infected with VSV-I and infected with VSV-vectored vaccines [32–35], although no pre-existing data exists on cellular immunity in humans naturally infected with VSV-I to our knowledge. We found significant increases in IFN-γ, IL-2, and TNF-α production from CD4+ T cells to both VSV-I M protein and VSV-I NP and CD8+ T cells to VSV-I NP following rVSVΔG-ZEBOV-GP vaccination. A retrospective analysis of a phase I rVSVΔG-ZEBOV-GP clinical trial using three different vaccine doses found that 36% of vaccinees demonstrated CD8+ T cell responses to VSV-I nucleoprotein [16]. Interestingly, we observed more polyfunctional VSV-I NP CD4+ and CD8+ T cells in our study than had previously been reported following vaccination, where predominantly mono-cytokine production of TNF-α was observed [16]. To our knowledge, no previous studies have examined T cell response to the VSV M protein in humans following rVSVΔG-ZEBOV-GP vaccination. Overall, T cell responses to VSV-I observed in the study were of a low magnitude. Further work is needed to assess whether these vector-directed T cell responses negatively affect the immune response to subsequent doses of rVSV-based vaccines.
Limitations of the study include the small number of participants assessed, especially of heterologous ZEBOV vaccine experienced individuals. The ZEBOV vaccine experienced individuals had previously received vaccine constructs other than the rVSVΔG-ZEBOV-GP vaccine, therefore, the immune responses among ZEBOV vaccine experienced individuals reported in this study may not be reflective of vaccine responses provided by homologous prime and boost with the rVSVΔG-ZEBOV-GP vaccine. No time points prior to one month post-vaccination were scheduled in the PREPARE study to assess whether pre-existing ZEBOV-GP IgG antibodies among ZEBOV vaccine experienced participants altered the course of rVSVΔG-ZEBOV-GP vaccine viremia or the development of a circulating follicular helper T cell response post-vaccination, which correlates with ZEBOV-GP antibody titers 4 weeks after vaccination [36]; these are important areas for future research to determine who may be most likely to benefit from rVSVΔG-ZEBOV-GP vaccine booster doses. Given the lack of data on immunity to VSV-I infection in humans and the absence of meaningful cut-offs for antibody titers, we presented data in optical density format to be comparable with previously reported studies and selected our lowest ELISA dilution as the most comprehensive for analysis, although we suspect the wide distribution of IgG ODs to all VSV-I proteins at baseline by ELISA indicates non-specific antigen cross-reactivity with non-VSV-I antibodies, which has previously been reported [37].
In conclusion, the rVSVΔG-ZEBOV-GP vaccine frequently elicits ZEBOV-GP IgG antibody and IgG-producing memory B cell responses regardless of whether an individual has previously received an ZEBOV vaccine, although the CD4+ and CD8+ T cell responses elicited by vaccination vary depending on whether an individual has previously received ZEBOV vaccines or not. The rVSVΔG-ZEBOV-GP vaccine elicits low magnitude T cell responses to VSV-I proteins while vaccine vector-directed humoral and IgG-producing memory B cell responses are rare. Additional research is needed to understand the implications of vaccine-elicited immunity, both to the vector and target antigens, on the immune response to booster doses of the rVSVΔG-ZEBOV-GP vaccine and other rVSV-vectored vaccines.
Supplementary Material
Acknowledgements:
We also wish to acknowledge the main study team at the NIH Clinical Center and at the University of Minnesota Data Center under whose auspices the PREPARE trial has been conducted. We would also like to thank our study participants for their volunteerism and acknowledge the important contributions of the following Emory Hope Clinic staff members: Dawn Battle, Mary Bower, Justin Colwell, Rijalda Deovic, Tamera Franks, Tigisty Girmay, Mari Hart, Christopher Huerta, Hannah Huston, Sara Jo Johnson, Brandi Johnson, Dean Kleinhenz, Pam Lankford-Turner, Hollie Macenczak, Regina Mosley, Eileen Osinski, Michele McCullough, Divyanshu Raheja, Brittany Robinson, Sirajud-Deen Talib and Juton Winston.
Funding:
This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID), including funding from the Division of Intramural Research, National Institutes of Health (NIH), Department of Health and Human Services [grant number T32AI074492 to V.R, N.R., and M.J.M.], NIH/NIAID contract number HHSN272201300018I/HHSN27200021 to Emory University Vaccine and Treatment Evaluation Unit and NIAID grant AI148574 (MJM, VR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Abbreviations:
- ChAd3-EBO-Z
Replication-defective chimpanzee adenovirus 3 vector vaccine expressing Zaire ebolavirus glycoprotein
- ZEBOV
Zaire ebolavirus
- ELISA
Enzyme-linked immunosorbent assay
- EVD
Ebola virus disease
- GP
Glycoprotein
- IFN-γ
Interferon-γ
- IL-2
Interleukin-2
- M
Matrix
- MBC
Memory B cell
- MVA-BN-Filo
Modified vaccinia Ankara virus expression Zaire ebolavirus glycoprotein and other filovirus antigens
- NP
Nucleoprotein
- OD
Optical density
- PBMCs
Peripheral blood mononuclear cells
- PFU
plaque forming units
- PREPARE
The Multicenter Study of the Immunogenicity of Recombinant Vesicular Stomatitis Vaccine for Ebola-Zaire for Pre-Exposure Prophylaxis in Individuals at Potential Occupational Risk for Ebola Virus Exposure
- rVSV
Recombinant vesicular stomatitis virus
- rVSVΔG-ZEBOV-GP
Recombinant vesicular stomatitis virus Zaire ebolavirus vaccine
- TNF-α
Tumor necrosis factor α
- VSV-I
Vesicular stomatitis virus Indiana strain
Footnotes
Competing Interests: MJM received research contracts from Lilly, Pfizer, and Sanofi, and serves on advisory boards for Pfizer and Meissa Vaccines. VR received a research contract from Sanofi and serves an advisory board for Novavax. NR received research contracts from Lilly, Pfizer, Merck, Quidel, and Sanofi.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Vanessa Raabe reports financial support was provided by National Institute of Allergy and Infectious Diseases. Mark J. Mulligan reports financial support was provided by National Institute of Allergy and Infectious Diseases. Nadine Rouphael reports financial support was provided by National Institute of Allergy and Infectious Diseases. Nadine Rouphael reports a relationship with Merck & Co Inc that includes: funding grants. Vanessa Raabe reports a relationship with Sanofi Pasteur Inc that includes: funding grants. Vanessa Raabe reports a relationship with Novavax that includes: board membership. Nadine Rouphael reports a relationship with Sanofi Pasteur Inc that includes: funding grants. Mark J Mulligan reports a relationship with Sanofi Pasteur Inc that includes: funding grants. Mark J Mulligan reports a relationship with Eli Lilly and Company that includes: funding grants. Mark J Mulligan reports a relationship with Pfizer Inc that includes: board membership and funding grants. Nadine Rouphael reports a relationship with Eli Lilly and Company that includes: funding grants. Nadine Rouphael reports a relationship with Pfizer Inc that includes: funding grants. Nadine Rouphael reports a relationship with Quidel Corp that includes: funding grants. Mark J Mulligan reports a relationship with Meissa Vaccines that includes: board membership. Additional funding supporting this work was provided by National Institute of Allergy and Infectious Diseases to the Emory University Vaccine and Treatment Evaluation Unit.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Centers for Disease Control and Prevention. 2014–2016 Ebola Outbreak in West Africa. 2019.
- [2].World Health Organization. 10th Ebola outbreak in the Democratic Republic of the Congo declared over; vigilance against flare-ups and support for survivors must continue. 2020.
- [3].European Medicines Agency. Ebola. 2019. [Google Scholar]
- [4].Sharp Merck & Corp Dohme. ERVEBO®(Ebola Zaire Vaccine, Live) Suspension for intramuscular injection [package insert]. US Food and Drug Administration Website; https://wwwfdagov/media/133748/download Accessed 1 July 2021. [Google Scholar]
- [5].World Health Organization. Preliminary results on the efficacy of rVSV-ZEBOV-GP Ebola vaccine using the ring vaccination strategy in the control of an Ebola outbreak in the Democratic Republic of the Congo: an example of integration of research into epidemic response. 2019.
- [6].Dahlke C, Kasonta R, Lunemann S, Krahling V, Zinser ME, Biedenkopf N, et al. Dose-dependent T-cell Dynamics and Cytokine Cascade Following rVSV-ZEBOV Immunization. EBioMedicine. 2017;19:107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marzi A, Engelmann F, Feldmann F, Haberthur K, Shupert WL, Brining D, et al. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci U S A. 2013;110:1893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Menicucci AR, Sureshchandra S, Marzi A, Feldmann H, Messaoudi I. Transcriptomic analysis reveals a previously unknown role for CD8(+) T-cells in rVSV-EBOV mediated protection. Sci Rep. 2017;7:919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Antonello J, Grant-Klein RJ, Nichols R, Kennedy SB, Dubey S, Simon JK. Serostatus cutoff levels and fold increase to define seroresponse to recombinant vesicular stomatitis virus - Zaire Ebola virus envelope glycoprotein vaccine: An evidence-based analysis. Vaccine. 2020;38:4885–91. [DOI] [PubMed] [Google Scholar]
- [10].Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet (London, England). 2008;372:1881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Auclair S, Liu F, Niu Q, Hou W, Churchyard G, Morgan C, et al. Distinct susceptibility of HIV vaccine vector-induced CD4 T cells to HIV infection. PLoS Pathog. 2018;14:e1006888–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Frahm N, DeCamp AC, Friedrich DP, Carter DK, Defawe OD, Kublin JG, et al. Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J Clin Invest. 2012;122:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Perreau M, Pantaleo G, Kremer EJ. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J Exp Med. 2008;205:2717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roberts A, Buonocore L, Price R, Forman J, Rose JK. Attenuated vesicular stomatitis viruses as vaccine vectors. Journal of virology. 1999;73:3723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Marzi A, Feldmann F, Geisbert TW, Feldmann H, Safronetz D. Vesicular stomatitis virus-based vaccines against Lassa and Ebola viruses. Emerg Infect Dis. 2015;21:305–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Poetsch JH, Dahlke C, Zinser ME, Kasonta R, Lunemann S, Rechtien A, et al. Detectable vesicular stomatitis virus (VSV)-specific humoral and cellular immune responses following VSV-Ebola virus vaccination in humans. J Infect Dis. 2019;219:556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Simon JK, Kennedy SB, Mahon BE, Dubey SA, Grant-Klein RJ, Liu K, et al. Immunogenicity of rVSVΔG-ZEBOV-GP Ebola vaccine (ERVEBO®) in African clinical trial participants by age, sex, and baseline GP-ELISA titer: A post hoc analysis of three Phase 2/3 trials. Vaccine. 2022. [DOI] [PubMed] [Google Scholar]
- [18].Regules JA, Beigel JH, Paolino KM, Voell J, Castellano AR, Hu Z, et al. A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med. 2017;376:330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Khurana S, Fuentes S, Coyle EM, Ravichandran S, Davey RT, Jr., Beigel JH. Human antibody repertoire after VSV-Ebola vaccination identifies novel targets and virus-neutralizing IgM antibodies. Nat Med. 2016;22:1439–47. [DOI] [PubMed] [Google Scholar]
- [20].Koch T, Rottstegge M, Ruibal P, Gomez-Medina S, Nelson EV, Escudero-Pérez B, et al. Ebola virus disease survivors show more efficient antibody immunity than vaccinees despite similar levels of circulating immunoglobulins. Viruses. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sullivan NJ, Hensley L, Asiedu C, Geisbert TW, Stanley D, Johnson J, et al. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med. 2011;17:1128–31. [DOI] [PubMed] [Google Scholar]
- [22].Kupke A, Volz A, Dietzel E, Freudenstein A, Schmidt J, Shams-Eldin H, et al. Protective CD8+ T cell response induced by modified Vaccinia virus Ankara delivering Ebola virus nucleoprotein. Vaccines (Basel). 2022;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bachmann MF, Hengartner H, Zinkernagel RM. Immunization with recombinant protein: conditions for cytotoxic T cell and/or antibody induction. Med Microbiol Immunol. 1994;183:315–24. [DOI] [PubMed] [Google Scholar]
- [24].Kelley JM, Emerson SU, Wagner RR. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. Journal of virology. 1972;10:1231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lefrancios L, Lyles DS. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology. 1982;121:157–67. [PubMed] [Google Scholar]
- [26].Vandepol SB, Lefrancois L, Holland JJ. Sequences of the major antibody binding epitopes of the Indiana serotype of vesicular stomatitis virus. Virology. 1986;148:312–25. [DOI] [PubMed] [Google Scholar]
- [27].Steinhoff U, Müller U, Schertler A, Hengartner H, Aguet M, Zinkernagel RM. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. Journal of virology. 1995;69:2153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gobet R, Cerny A, Rüedi E, Hengartner H, Zinkernagel RM. The role of antibodies in natural and acquired resistance of mice to vesicular stomatitis virus. Exp Cell Biol. 1988;56:175–80. [DOI] [PubMed] [Google Scholar]
- [29].Lefrancois L Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: Distinct mechanisms of action in vivo. Journal of virology. 1984;51:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tober R, Banki Z, Egerer L, Muik A, Behmuller S, Kreppel F, et al. VSV-GP: A potent viral vaccine vector that boosts the immune response upon repeated applications. Journal of virology. 2014;88:4897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Davis C, Tipton T, Sabir S, Aitken C, Bennett S, Becker S, et al. Postexposure prophylaxis with rVSV-ZEBOV following exposure to a patient with Ebola virus disease relapse in the United Kingdom: An operational, safety, and immunogenicity Report. Clin Infect Dis. 2020;71:2872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Puddington L, Bevan MJ, Rose JK, Lefrancois L. N protein is the predominant antigen recognized by vesicular stomatitis virus-specific cytotoxic T cells. J Virol. 1986;60:708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cobleigh MA, Bradfield C, Liu Y, Mehta A, Robek MD. The immune response to a vesicular stomatitis virus vaccine vector is independent of particulate antigen secretion and protein turnover rate. Journal of virology. 2012;86:4253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Van Bleek GM, Nathenson SG. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990;348:213–6. [DOI] [PubMed] [Google Scholar]
- [35].Yewdell JW, Bennink JR, Mackett M, Lefrancois L, Lyles DS, Moss B. Recognition of cloned vesicular stomatitis virus internal and external gene products by cytotoxic T lymphocytes. J Exp Med. 1986;163:1529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Farooq F, Beck K, Paolino KM, Phillips R, Waters NC, Regules JA, et al. Circulating follicular T helper cells and cytokine profile in humans following vaccination with the rVSV-ZEBOV Ebola vaccine. Scientific reports. 2016;6:27944-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hardgrave KL, Neas BR, Scofield RH, Harley JB. Antibodies to vesicular stomatitis virus proteins in patients with systemic lupus erythematosus and in normal subjects. Arthritis Rheum. 1993;36:962–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


