Abstract
The field of stem cell therapy is growing rapidly and hopes to offer an alternative solution to diseases that are historically treated medically or surgically. One such focus of research is the treatment of medically refractory epilepsy, which is traditionally approached from a surgical or interventional standpoint. Research shows that stem cell transplantation has potential to offer significant benefits to the epilepsy patient by reducing seizure frequency, intensity, and neurological deficits that often result from the condition. This review explores the basic science progress made on the topic of stem cells and epilepsy by focusing on experiments using animal models and highlighting the most recent developments from the last 4 years.
Keywords: stem cell therapy, epilepsy, neurological disease
Introduction
Epilepsy, defined by high occurrence of seizures secondary to aberrant electoral neural signaling, affects 50 to 60 million people around the world1. There are several described seizure types, including generalized, focal, and epileptic spasms. Generalized seizures can be further broken down into tonic-clonic, absence, myoclonic, and atonic seizures2. Furthermore, epilepsy can be categorized as idiopathic, acquired (through changes in brain structure, infection, or metabolic disorders), and immunological3. Despite these multiple classifications, seizures can often be related to a common underlying pathophysiology: neuronal hyperactivation. Thus, most therapeutic interventions aim to remove the aberrant signaling area, via surgery, or reduce neuronal action potentials, using pharmacological interventions2. Unfortunately, only two of three patients obtain complete remittance from their seizures3. Initial treatment approaches for epilepsy are usually conservative. The availability of anti-epileptic drugs, costs, and insurance coverage for pharmaceutical treatments appear generally addressed by health care systems around the world although there are patients whose insurance does not cover anti-epileptic drugs. However, the clinical outcomes of pharmaceutical treatments remain variable, indicating the need for novel and more effective therapeutic approaches. To this end, while first-line treatments for epilepsy include pharmaceutical management, surgical interventions stand as an alternative treatment option when medical management fails. Unfortunately, cell-based therapy represents an experimental surgical intervention, which probably may pose as a challenge for uninsured patients warranting case management resolution. Even surgical interventions only result in complete absence of seizures in 58% of patients with medically refractory temporal lobe epilepsy (TLE)4,5. Over time, repeated epileptic episodes can result in cell death, activation of astrocytes, reactive oxidative species production, and changes to mitochondria function3. This aberrant functioning at the cellular level, alongside a plethora of patients with refractory epilepsy, implores researchers to consider novel treatment strategies, such as cell-based therapies3. This review will discuss current methods of surgical and pharmacological approaches for treating epilepsy while also delving into the less developed concept of using stem cells to reprogram and remodel neuronal networks to reduce epileptic burden.
Pharmaceutical Treatments for Epilepsy
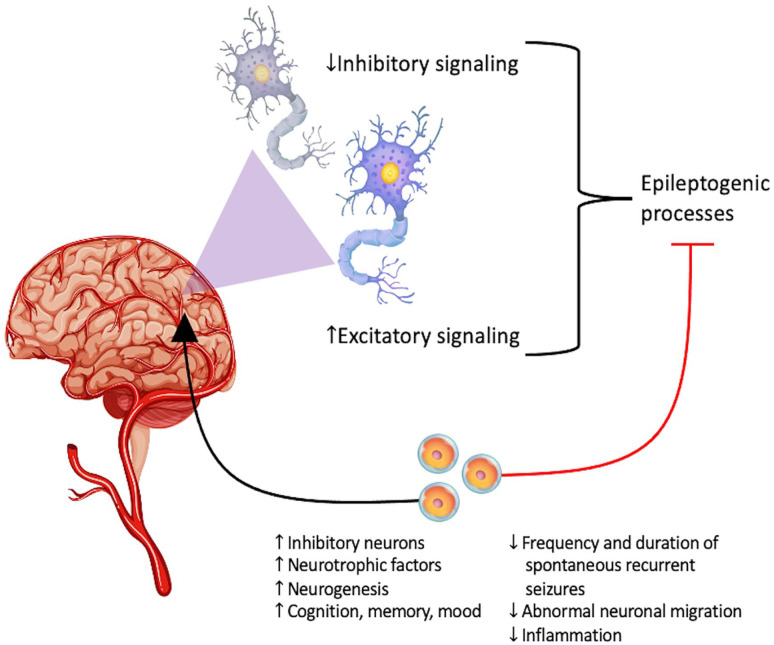
The primary goal of anti-seizure drugs (ASDs) is to eliminate seizures while minimizing adverse side effects6. Medication is considered after two documented cases of unprovoked seizures or one seizure that occurred during sleep or in the presence of the electrocardiogram (ECG) or magnetic resonance imaging (MRI)6. The best practice is to wait for reoccurrence before starting treatment, as treatment after the first seizure will not alter prognosis7. However, those with a higher risk of occurrence, including individuals with an identified structural abnormality, an abnormal electroencephalograph (EEG), or pre-existing neurological deficit, should start sooner. Considerations when choosing a medication include factors such as age, sex, existing comorbidities, seizure type, and tolerability7. The two main types of seizures, focal and generalized, are neurologically different, and specific medications may work better for one type over the other. Furthermore, new-generation drugs are often better tolerated and have similar efficacy as previous generation drugs8. Previous work demonstrated that up to 47% of patients become seizure free on the first appropriately selected ASD9. It is therefore crucial that physicians understand the classes and mechanisms of ASDs to choose the right option for their patient. Monotherapy (single drug) is the best option as polytherapy (multiple drugs) may increase the risk of poor adherence, adverse drug interactions, and the potential for long-term toxicity. If polytherapy is required, the physician must make sure to not prescribe drugs in the same class to avoid cross sensitivity; use combinations that have synergistic effects; combine drugs with different mechanisms of action; and anticipate drug doses appropriately based on potential side effects9. Each drug should be introduced slowly with the dosage gradually raised to the maximum tolerated dose. If the patient does not receive benefit at the maximally tolerated dose, an alternative first-line drug should then be tried7. Drug-resistant epilepsy is defined as the failure of adequate trials of two appropriately chosen ASDs7. Surgical intervention is the next likely choice for these individuals (Figure 1).
Figure 1.
The primary underlying pathophysiology of epilepsy: aberrant excitatory and inhibitory signaling at the neural level. Stem cells may ameliorate this imbalance in signaling pathways, while also promoting brain repair secondary to epileptic episodes.
There are currently 26 US Food and Drug Administration (FDA) approved ASDs which fall into four main categories. Eight (benzodiazepines, lamotrigine, levetiracetam, perampanel, phenobarbital, topiramate, sodium valproate, and zonisamide) are used for focal and most generalized epileptic conditions. An additional 10 (brivaracetam, carbamazepine, eslicarbazepine acetate, gabapentin, lacosamide, oxcarbazepine, phenytoin, pregabalin, tiagabine, and vigabatrin) are used in focal conditions only. Ethosuximide is used in absence seizures only, and the additional five (cannabidiol, everolimus, felbamate, rufinamide, and stiripentol) are used in special encephalopathies7. These drugs are classified into four main types of mechanisms9. Ion channel modulators include those that exert their effects on sodium, calcium, and potassium channels. The sodium channel modulators are phenytoin, carbamazepine, and lacosamide. The calcium channel modulators are lamotrigine and ethosuximide9. A second class of drugs enhances the effect of inhibitory neurotransmission. For example, phenobarbital and benzodiazepines bind to the GABA-A complex at distinct sites, felbamate and topiramate activate the GABA-A receptor, and vigabatrin irreversibly inhibits GABA-transaminase9. Perampanel is the sole ASD that acts on glutamate to attenuate excitatory neurotransmission while others such as felbamate and topiramate have multiple mechanisms that include the attenuation of excitatory neurotransmission. Finally, the new generation of ASDs have novel mechanisms such as the ability of levetiracetam and brivaracetam to bind to synaptic vesicle 2A (SV2A) on presynaptic vesicles. This is believed to regulate vesicle shape and neurotransmitter trafficking9.
As with all medications, potential side effects and drug–drug interactions must be considered when choosing which ASD to prescribe. In all, 20% to 50% of users experience neurologic, psychiatric, cognitive, or mental disorders such as mood, anxiety, attention deficit, and migraines6. Additional side effects such as fatigue, dizziness, unsteadiness, and irritability may also occur7. Furthermore, Cytochrome P450 ASDs such as carbamazepine and phenytoin may worsen comorbid coronary and cerebrovascular disease due to hyperlipidemia6. Also, women with childbearing potential should avoid valproate due to the potential side effect of teratogenicity8. Additional research is needed to develop new ASDs to increase their success rate while decreasing the potential side effects.
Surgical Treatments of Epilepsy
Three different randomized control trials have shown that surgery for epileptic patients is effective, but this treatment option is often underutilized5,10,11. These studies show that surgical treatment is safe, but less than 1% of epilepsy patients are referred for surgery12. Misconceptions or fears about surgery may cause this underutilization, but results have shown that the seizure-free rate of individuals after surgery ranges from 50% to 80%7. With the development of advanced imaging techniques such as functional magnetic resonance imaging (fMRI), surgeons can locate the epileptic region and determine any post-surgical deficits that may result from surgery in that area of the brain. The prototypical surgical patient is one with TLE due to unilateral hippocampal sclerosis; but recent advances have widened the range of patients who may benefit from surgical intervention7. Of the roughly 60% of patients with focal epilepsy, 15% do not receive the relief they need from medications alone13. Individual cases which may benefit from surgery include TLE with focal lesions, lesional extratemporal epilepsies, hemispherical epilepsies, and gelastic epilepsy with hypothalamic hamartoma. Furthermore, corpus callosotomy and vagal nerve stimulation may be beneficial for children with diffuse and multifocal epilepsies13. Those with an electro-clinically concordant structural lesion on MRI have the best outcomes with seizure reduction in 60% to 70% of cases, whereas those with extratemporal lobe foci, focal-to-bilateral tonic-clonic seizures, normal imaging, psychiatric comorbidities, and learning disabilities often have lower success rates4. The future of epilepsy surgery includes techniques such as stereotactic radiosurgery, radiofrequency thermocoagulation, and laser interstitial thermal therapy7. More research is required in these fields, but they have the potential to improve surgical outcomes while lessening the chance of unintended brain damage. Surgical treatments for epilepsy are underused but should seriously be considered for patients with focal epilepsy where two to three medications have failed.
Neurostimulative Interventions for Epilepsy
A variety of interventional treatments for epilepsy exist, including deep brain stimulation (DBS), responsive neurostimulation (RNS), vagus nerve stimulation therapy (VNS), and trigeminal nerve stimulation (TNS). In the 2010 SANTE trial, DBS of the anterior nuclei of the thalamus (ANT) reduced seizures by 29% more compared with the control group14. When a follow-up was performed 2 years later, the researchers found a median 56% reduction in seizure frequency. These results led to FDA approval for DBS in epilepsy patients15. Further work in the field of DBS agreed that stimulation of the ANT reduced seizures between 46% and 90%. Furthermore, stimulation of the hippocampus resulted in a 48% to 95% reduction in seizures16. The ANT connects to the hippocampus via the mammillothalamic tract and fornix before projecting to the cingulate cortex and neocortex. This area is involved in emotional processing and seizure propagation16. However, stimulation of this area is associated with higher rates of self-reported depression and subjective memory impairment; thus, more work is needed to elucidate these causes to help prevent them in the future17. Furthermore, the hippocampus is a target for DBS due to its role in the Papez circuit, as hippocampal stimulation may attenuate epileptic activity originating in the medial temporal region16. Other areas of possible DBS such as the centromedian nucleus of the thalamus, the cerebellum, and the nucleus accumbens have yet to show significant beneficial results17.
RNS was approved in 2013 as a potential therapy for adults with uncontrolled focal seizures that are limited to one or two foci. RNS works through an implantable neurostimulator connected to one or two cortical strip leads each containing four electrodes18. These leads are surgically placed at the seizure foci where they continually monitor electrical activity and provide responsive electrical stimulation when necessary. The measurement thresholds and response parameters are individually tailored to suit the needs of the patient18. Studies have shown a reduction in seizure frequency of 44% after 1 year, 53% after 2 years19, and 48% to 66% in years 3 to 620. The most common side effect after a 5.4-year follow-up was implant site infection which occurred in around 9% of patients20.
VNS entails a pulse generator implanted below the clavicle with an attached lead that is wrapped around the vagus nerve in the carotid sheath21,22. Complete seizure freedom is rare, but previous work revealed that 26% to 40% of patients see a 50% reduction in seizure frequency23. External trigeminal nerve stimulation (ETNS), a new experimental treatment, demonstrated a benefit in small sample size studies24. This therapy entails a non-invasive neurostimulation therapy that is delivered bilaterally with adhesive skin electrodes. Experimental results show a drop of 43.5% in seizure frequency in the treated group versus the control group. The same study shows that ETNS improved quality of life without an increase in anxiety or depression, and no relevant adverse events were observed. Additional research into this field is required to determine the efficacy and safety of this therapy as it relates to the larger population before widespread use.
Stem Cell Therapy in Epilepsy
The application of stem cells for neurological disorders is a rapidly growing field. Research has uncovered potential benefits for degenerative disorders such as Parkinson’s and Alzheimer’s disease, stroke recovery, and epilepsy among other diseases25. Stem cells exist at various levels of differentiation such as pluripotent, multipotent, and oligopotent cells with varying potential to develop into target tissue. They can often proliferate much more often than target tissue, aiding in the recovery of damaged tissue26. Stem cells are an attractive option for use in the treatment of epilepsy because of their potential to replace damaged cells, restore cognitive function, and stabilize focal electrical activity. Indeed, early animal models display potential for restricting the duration and frequency of focal seizures27,28. This can be life-changing for patients with medically refractory epilepsy because it provides an alternative to invasive or stimulatory techniques. In fact, the use of stem cells has a distinct advantage over certain stimulatory control techniques because stem cells do not require real-time detection of seizures to deliver a therapeutic inhibitory signal.
Grafting embryonic striatal precursor cells collected from the lateral ganglionic eminence into adult rats with induced status epilepticus (SE) reduced the frequency of spontaneous recurrent major motor seizures by up to 89%, at 12 months post-SE27. This is likely due to the presence of inhibitory GABA-ergic neurons in the graft which could control the frequency and intensity of subsequent seizures. These findings only looked at the improvements on major motor seizures, but the grafting of GABA progenitors was later found to also decrease the frequency of electrographic seizures, with the added benefit of restoring behavioral deficits such as aggression and hyperactivity29. Evidence shows that the benefits of stem cell therapy also extend to absence seizures seen in stargazer mouse models. The transplantation of medial ganglion eminence (MGE) cells into the primary visual cortex of mice produces a significant post-synaptic inhibitory current which holds promise for the treatment of absence seizures. In fact, stargazer mouse models grafted with MGE cells experienced significant rescue from absence seizures and experienced significantly lower excitability in the primary visual cortex during potassium-induced excitability challenges when compared with control stargazer mice30.
The findings discussed so far have been restricted to mouse models and mouse-derived cell grafts, but use of human cells, although much less studied, yields similar results. For example, human fetal fibroblast cells can be converted into induced neuronal cells in vitro and subsequently matured, with robust survival in the short term after being transplanted into adult rats31. Promisingly, fetal human fibroblast-derived induced neurons transplanted into rat hippocampi progressively matured and displayed robust functionality and integration into their transplant sites32. This highlights the feasibility of using induced neuron cell grafts as a potential human source of stem cells for cell replacement therapy. Interestingly, human adipose stem cell extract administered to mouse models prior to pilocarpine-induced SE seems to reduce abnormal behavior induced by SE33. This is likely due to the cytokines and growth factors contained within these cells which have been shown to protect against neuroinflammation and rescue damaged neurons34.
Human umbilical mesenchymal stem cells (HUMSCs) can also display positive effects on epileptic rat models. These cells can be obtained from Wharton’s jelly of fetal umbilical cords, which can raise ethical concerns although certain ethically secure techniques can be used, such as obtaining them from medical waste after delivery. These cells have displayed neuroprotective properties in other applications such as stroke35, with possible use in epilepsy. Pilocarpine-treated rats transplanted with HUMSCs experienced lower frequencies of recurrent motor seizures and experienced less tissue damage than control rats, showing that this neuroprotective effect extends to damage caused by recurrent seizures36. HUMSC implantation also corrected the dysregulated adenosine receptor expression seen in rat models of chronic epilepsy, showing potential for the treatment of medically refractory epilepsy37. Recently, autologous mesenchymal stem cells (MSCs) were tested in human patients with medically refractory epilepsy and caused most patients in the treatment group to either experience remission or begin responding to ASDs38–40.
Mechanisms of Stem Cell Therapy for Epilepsy
Stem cells may provide therapeutic and neuroprotective benefits to epileptic patients in various ways. Endogenous neural stem cells (NSCs) are activated as a result of seizure activity41,42. Following a seizure, the brain undergoes many pathological changes, including excitotoxicity, neuroinflammation, oxidative damage, and ultimately neurodegeneration43. These changes may contribute to the initial increase in adult hippocampal neurogenesis, causing an activation of endogenous NSC that normally remains quiescent in the dentate gyrus41. These endogenous stem cells offer insufficient neuroprotection, as persistently elevated adult hippocampal neurogenesis eventually depletes the NSC pool, resulting in a diminished neuroregenerative capacity41.
Both exogenous and endogenous stem cells exert much of their effects by redefining the aberrant neuronal circuitry characteristic of epilepsy. Specifically, stem cells function to restore inhibitory GABA-ergic neurons and provide a counter to the hyperexcitability that characterizes seizures44,45. MSCs may attenuate glutamate excitotoxicity following seizures, as they have been shown to migrate to rat hippocampi and protect neurons by downregulating N-methyl D-aspartate (NMDA) receptor expression and glutamate-induced calcium activity44. This is a significant therapeutic effect, as excitotoxicity leads to neuronal death and an inflammatory environment incompatible with neuroregeneration46. Furthermore, NSCs, human embryonic stem cells (hESCs), and MGE-derived interneuron precursors can replenish hippocampal GABA-ergic interneurons in the epileptic rats47–49. Specifically, these cells can integrate into pre-existing neuronal networks47,49 and provide nearby excitatory cells with inhibitory post-synaptic signals50, reducing epileptogenic processes. Given the loss of GABA-ergic interneurons in the epileptic brain48, protecting and replenishing them may serve as a significant mechanism in ameliorating epilepsy-induced damage. Stem cells may also exert their effects via astrocytes44. NSCs can differentiate into astrocytes, which play a key role in regulating neuronal homeostasis, neuroinflammation, and synaptic activity51. Astrocytes may also directly decrease seizure activity, as they secrete anti-convulsant protective proteins, such as glial-derived neurotrophic factor (GDNF)44. In addition, MSCs inhibit astrogliosis, a response to pathological conditions that is associated with neuroinflammation51.
The ability of stem cells to attenuate neuroinflammation and oxidative damage is key to their therapeutic effects. Seizures cause increased reactive oxygen species (ROS), which cause neuronal apoptosis, cell loss, and mitochondrial damage52. Given the susceptibility of hippocampal CA1 pyramidal neurons to oxidative stress52, reducing such stress may preserve hippocampal architecture and function. Transplanted MSCs can reduce oxidative stress and neuroinflammation in SE mice. Specifically, MSC-derived exosomes (MSC-Exo) protect against neuroinflammation and oxidative stress via nuclear factor erythroid 2–related factor 2 (Nrf2) signaling51,52. Nrf-2, an antioxidant transcription factor, regulates redox homeostasis by inducing the expression of antioxidant proteins53. By inducing Nrf-2 signaling, MSC exosomes diminish neuroinflammation and promote antioxidant activity, creating an environment conducive to tissue repair51,52.
Recent Developments in Stem Cell Therapy
Outlined in the following text and Table 1 are pertinent preclinical studies that focus on stem cell treatment in epilepsy models from 2018 to 2022. These papers were identified via a PubMed search using the terms “stem cell and seizure” and “stem cell and epilepsy.” A subsequent review of the resulting 873 studies to include only those that relate to translational models of stem cell therapy results in the studies described below and in Table 1. A variety of different types of stem cells are used in experiments from different sources, most often murine or human. Epilepsy models typically use pilocarpine, pentylenetetrazol, or kainate to induce SE. As stated below, stem cells and related cell products (such as extracellular vesicles and exosomes) provide therapeutic benefits in epilepsy models, often reducing seizure frequency and duration, and improving neurobehavioral outcomes.
Table 1.
In Vivo Stem Cell Studies Targeting Epileptic Disease Models.
| Citation | Sample | Cell type | Route | Dosage | Results | Mechanism |
|---|---|---|---|---|---|---|
| Fukumura et al.54 | Pilocarpine-induced SE rats | MSCs | Intravenous | 1 × 106 cells | Epileptogenesis is inhibited with preservation of cognitive function. Abnormal mossy fiber sprouting is suppressed. | Paracrine neuroprotective effect |
| Backofen-Wehrhahn et al.47 | PTZ-induced seizure rats with or without pilocarpine-induced SE | Human/rat/porcine MGE-derived and VM-derived NPCs | Intracranial | 8 × 104 or 1.5 × 105 (STN); 8 × 104 or 5 × 105 (aSNr) |
MGE-derived and VM-derived NPCs migrate and differentiate into inhibitory interneurons. Anti-convulsant effects are produced with human and porcine VM-derived NPCs in the STN. | Direct GABA-ergic activity |
| Anderson et al.49 | Pilocarpine-induced TLE mice | hESC-derived interneuron progenitors | Intrahippocampal | 2 × 105 cells | Transplanted progenitors differentiate into GABA-ergic interneurons. Cognitive function improves without suppression of seizures. | Direct GABA-ergic activity |
| Upadhya et al.28 | KA-induced SE | hiPSC-derived MGE-like interneuron precursors | Intrahippocampal | 3 × 105 cells | Transplantation ameliorates spontaneous recurrent seizures, and improves cognition, memory, and mood. Interneuron loss in the hippocampus and aberrant neurogenesis is decreased. | Direct integration as GABA-ergic interneurons as well as a paracrine neuroprotective effect |
| Xu et al.55 | Pilocarpine-induced epileptic rats | NSCs | Intrahippocampal | 3 × 106 cells | NSCs decrease the frequency of electroencephalography in addition to the frequency and duration of spontaneous recurrent seizures. | Direct GABA-ergic activity |
| Ali et al.46 | PTZ-induced epileptic rats | EPCs | Intravenous | 2 × 106 cells | EPCs migrate to the hippocampus to improve memory and motor deficits. BDNF is upregulated, neurotransmitter activity is stabilized, and autophagy is upregulated. | Paracrine neuroprotective effect |
| Xian et al.56 | Pilocarpine-induced SE rats | MSC-derived exosomes | Intraventricular | 3.2 × 109 particles | MSC-derived exosomes ameliorate learning and memory deficits. | Paracrine/exosomal neuroprotective effect |
| Kodali et al.57 | KA-induced SE rats | MSC-derived EVs | Intranasal | 1 × 1010 particles | Unilateral intranasal injections of MSC-derived EVs leads to bilateral incorporation in all regions of the forebrain, with favorable migration to the hippocampal CA subfield and entorhinal cortex. | Paracrine/exosomal neuroprotective effect |
| Hattiangady et al.48 | KA-induced SE rats | NSCs | Intrahippocampal | 3.2 × 105 cells | NSCs differentiate into GABA-ergic interneurons, astrocytes, and oligodendrocytes and express FGF-2, BDNF, IGF-1, and GDNF. This reduces abnormal neuronal migration, maintains normal neurogenesis, and diminishes progression of epileptogenic processes. | Direct integration as GABA-ergic interneurons as well as a paracrine neuroprotective effect |
| Liu et al.51 | Pilocarpine-induced SE mice | IL-1β-treated MSC exosomes | Intraventricular | 1.5 μL particles | IL-1β-treated MSC exosomes reduce neuroinflammation and improve cognitive function. | Paracrine/exosomal neuroprotective effect |
| Luo et al.52 | Pilocarpine-induced SE mice | MSC-derived EVs | Intravenous | 50 μg particles | MSC-EVs upregulate the Nrf2 pathway to promote neurorestoration against oxidative damage from seizure-induced hippocampal injury. | Paracrine/exosomal neuroprotective effect |
| Wang et al.58 | KA-induced TLE rats | ADSCs | Intrahippocampal | 5 × 104 cells | ADSCs inhibit seizure activity and restore learning capacity, associated with a release of BDNF, NT3, NT4, and anti-apoptotic factors. | Paracrine neuroprotective effect |
| Waloschková et al.50 | KA-induced SE rats | hESC-derived GABA-ergic interneuron precursors | Intrahippocampal | 1 × 105 cells | GABA-ergic precursors promoted inhibitory synaptic connections between host neurons, reduced the rate of epileptiform discharges, and decreased both the frequency and length of spontaneously recurring seizures. | Direct GABA-ergic activity |
| Venugopal et al.59 | KA-induced SE mice | Dental pulp stem cells or BM-MSCs | Intrahippocampal | 1 × 105 cells | Dental pulp stem cells are more effective than BM-MSCs in preventing hippocampal neurodegeneration and neuroinflammation while promoting neurogenesis and cognitive function through downregulation of apoptosis. | Paracrine neuroprotective effect |
| Upadhya et al.60 | KA-induced TLE rats | hiPSC-derived MGE-like interneuron precursors | Intrahippocampal | 3 × 105 cells | Interneuron grafting reduces spontaneous recurrent seizures and ameliorates cognitive function. | Direct integration as GABA-ergic interneurons as well as a paracrine neuroprotective effect |
| Arshad et al.61 | Pilocarpine-induced TLE mice | MGE-derived GABA-ergic progenitors | Intrahippocampal | 1 × 105 cells/μL | GABA-ergic progenitors reduce aberrant neurogenesis patterns, as evidenced through reductions in inverted type 1, type 2, and hilar ectopic type 3 cells. | Direct integration as GABA-ergic interneurons as well as a paracrine neuroprotective effect |
This table outlines cell-based preclinical trials finding improved functional outcomes in epileptic disease from 2018 to 2022.
SE: status epilepticus; STN: subthalamic nucleus; PTZ: pentylenetetrazol; KA: kainic acid; GABA: γ-aminobutyric acid; NSC: neural stem cell; BDNF: brain-derived neurotrophic factor; FGF-2: fibroblast growth factor 2; IGF-1: insulin-like growth factor 1; GDNF: glial cell–derived neurotrophic factor; NT: neurotrophin; TLE: temporal lobe epilepsy; MSC: mesenchymal stem cell; BM-MSC: bone marrow–derived mesenchymal stem cell; NPC: neural progenitor cell; EPC: endothelial progenitor cell; hESC: human embryonic stem cell; hiPSC: human-induced pluripotent stem cell; ADSC: adipose-derived stem cell; MGE: medial ganglionic eminence; VM: ventral mesencephalon; EV: extracellular vesicle; Nrf2: nuclear factor erythroid 2–related factor 2; CA: cornu ammonis.
Fukumura et al.54 sought to understand whether the systemic infusion of MSCs could attenuate epileptogenesis in a lithium-pilocarpine rat model of SE. Seizure frequency was quantified using a video-monitoring system, and a Morris water maze test was used to assess cognitive function of animals. The findings showed that MSC therapy reduced seizures and improved cognitive function of SE rats. Green flourescent protein staining also confirmed that the MSCs localized to the rat hippocampus, and thus promoted neuronal survival. The exact mechanism for this neuroprotective effect is unclear, but it may involve the direct preservation of GABA-ergic interneurons by the infused MSCs. Manganese-enhanced MRI and Timm staining also confirmed that MSC treatment decreased aberrant mossy fiber sprouting in the dentate gyrus of SE rats. Therefore, MSCs may have attenuated epileptogenesis by controlling neuronal death and preventing aberrant mossy fiber sprouting.
Seeing that various partial seizure types can be remotely controlled via inhibition of the subthalamic nucleus or substantia nigra pars reticulata, Backofen-Wehrhahn and others47 investigated whether the transplantation of neural precursor cells (NPCs) into the above-mentioned brain sites could ameliorate seizures in pentylenetetrazol-injected rats. The group used NPCs harvested from the ventral mesencephalon and MGE of humans, pigs, and rats. The findings of their study showed that transplanted NPCs migrated as expected, transformed into GABA-ergic inhibitory interneuron phenotypes, and survived ≥4 months post-transplantation. Temporary anti-epileptic effects were also observed in rats that received bilateral grafting of NPCs derived from human and pig ventral mesencephalon into their subthalamic nucleus, but not into the substantia nigra. Unfortunately, NPCs derived from the MGE did not produce significant effects in either injection site, despite pretreatment of these cells with retinoic acid and potassium chloride to promote their differentiation into GABA-ergic neurons.
Anderson et al.49 investigated whether hESC-derived interneuron progenitors could appropriately differentiate into GABA-ergic cells, correct hippocampus-dependent spatial memory deficits, and attenuate seizures in pilocarpine-treated mice. In their study, they used hESC-derived interneuron progenitors and transplanted them into the hippocampi of animals. The findings showed that the transplanted cells differentiated into GABA-ergic interneurons. Mice that received these cells showed improvement in Morris water maze performance, but not attenuation of seizures, contrary to the findings of most preclinical studies. The lack of effect of the transplant on seizures was attributed to cell type heterogeneity of the transplants.
In another study, Upadhya et al.28 investigated the effects of human-induced pluripotent stem cell (hiPSC)–derived MGE-like interneurons in a TLE model. The hiPSC-derived MGE-like interneuron precursors were grafted into rat hippocampi following SE. They reported that grafted cells survived well, migrated into the different areas of the hippocampus, and differentiated into various types of inhibitory interneurons that express calcium-binding proteins and neuropeptides. Behaviorally, grafting of the cells also controlled spontaneous recurrent seizures (SRSs), and improved cognitive and memory deficits of SE animals. In addition, it reduced interneuron death and aberrant mossy fiber sprouting in the dentate gyrus, displaying a paracrine neuroprotective effect via the release of neurotrophic factors. Furthermore, efficient graft–host synaptic integration was observed as evidenced by axons from graft-derived interneurons forming synapses with dendrites of host excitatory neurons in the dentate gyrus and CA1 regions of the hippocampus. A recent study by the group further confirmed the grafted cells’ direct regulation of SRS and improvement of cognitive functions of rat models of TLE60. Using hiPSC-derived MGE-like GABA-ergic progenitors that were engineered to express inhibitory designer receptors exclusively activated by designer drugs (DREADDs), they showed that activation of these DREADDs-expressing cells transplanted into the hippocampi reduced SRS and memory impairment. Deactivating them through the use of a designer drug produced the opposite effect, that is, it increased SRS and impaired memory functions of TLE rats.
Waloschkova and others50 examined effects of hESC-derived GABA-ergic interneuron precursors grafted into rat hippocampi following kainate-induced SE. They observed that grafted cells were forming inhibitory GABA-ergic synaptic connections onto host neurons. This treatment reduced SRS frequency and duration compared with non-transplanted controls.
Xu and colleagues55 examined whether GABA-ergic neurons generated in vitro from NSCs reduces SRS frequency and duration and compared their effects with those of intrahippocampal transplantation of NSCs. Using a pilocarpine-injection TLE model, they showed that transplantation of the differentiated cells decreased the seizure frequency and duration of SRS and achieved the highest number of GABA interneurons expression in the hippocampus compared with NSC transplantation and phosphate-buffered saline treatment (control). Moreover, GABA levels were also increased in the hippocampus of GABA interneuron-transplanted rats. Thus, the differentiated cells successfully grafted into the hippocampi of epileptic rats and produced anti-epileptic effects by enhancing GABA-mediated inhibition.
Ali et al.46 explored the effects of intravenously administered endothelial progenitor cells (EPCs) in a pentylenetetrazol-induced epilepsy model in rats. They reported that EPCs migrated into the hippocampus and improved the memory and motor deficits of rats. There was a neuroprotective paracrine effect leading to an increase in brain-derived neurotrophic factor (BDNF) expression as well as autophagy-related proteins: light chain protein-3, beclin-1, and autophagy-related gene-7. Increasing autophagy activity is beneficial, allowing neurons to properly ensure equilibrium between synthesis and degradation of intracellular substances.
MSC-Exo exert substantial anti-inflammatory effects in the treatment of neurological disorders, including epilepsy. Xian et al.56 investigated the mechanism behind MSC-Exo’s robust anti-inflammatory effects in in vitro and in vivo epilepsy models. They showed that MSC-Exo integrated well into hippocampal astrocytes and decreased reactive astrogliosis and inflammatory responses. Importantly, MSC-Exo improved learning and memory impairments of mice subjected to pilocarpine-induced SE. Inhibiting the Nrf2 pathway via ML385 injection in SE rats decreased the putative therapeutic effects of MSC-Exo. They proposed that the Nrf2 signaling pathway mediates the anti-inflammatory effects of MSC-Exo, and the therapeutic value of MSC-Exo in controlling seizures. In a different study, Liu et al.51 explored the mechanism underlying the therapeutic effects of exosomes derived from Interleukin-1β treated MSCs (IL-1-Exo) on SE mice. The IL-1-Exo reduced the inflammatory response and enhanced cognitive functions of SE mice. Moreover, they also showed that the Nrf2 pathway is involved in the anti-inflammatory effects of IL-1 Exo.
MSC-derived extracellular vesicles (MSC-EVs) attenuate oxidative stress-induced neuronal damage and show promise as neurological disorders treatment. Luo et al.52 further showed that MSC-EVs restored oxidative stress-induced dendritic spine alterations, electrophysiological changes, mitochondrial changes, and other functional and structural impairments in hippocampal neurons. Using mouse seizure models, they also showed that MSC-EVs improved cognitive functions of rats. Moreover, their studies showed that the antioxidant activity of MSC-EVs was exerted through the Nrf2 signaling pathway. This study further demonstrates the value of MSC-derived nanotherapeutics as epilepsy treatments.
In view of these outcomes, Kodali et al.57 examined the impact of intranasally administered MSC-EVs in SE rats. Intranasal administration is non-invasive and allows repetitive administration and rapid delivery of EVs into multiple forebrain regions. The group administered 10 billion EVs via the left nares of control rats and those that underwent 2 h of kainate-induced SE. Six hours post administration, EVs were observed bilaterally in nearly all regions of the forebrain in both SE mice and controls. The percentage of neurons that incorporated EVs was comparable between groups for most forebrain regions, except in the hippocampal CA1 region and the entorhinal cortex, in which the percentage was higher in the SE group. Notably, these brain regions are most vulnerable to neurodegeneration after experimental SE. Therefore, unilateral intranasal administration of EVs is an effective method to deliver EVs to forebrain regions. That the integration of EVs was higher in injured neurons indicates that injury-related signals help in the penetration and incorporation of EVs in neurons. Moreover, the findings further demonstrate the therapeutic potential of MSC-EVs in epilepsy.
Hattiangady et al.48 performed bilateral hippocampal grafting of neural progenitor cells 6 days after SE in animals to examine the effect of the transplant on the chronic phase of epilepsy. They showed that 8 months post-SE, the transplantation attenuated frequency and severity of SRS and improved memory. The grafted cells migrated into the hippocampus and differentiated into GABA-ergic interneurons, astrocytes, and oligodendrocytes, and also exhibited a neuroprotective paracrine effect. There was an increase in BDNF, fibroblast growth factor-2, insulin-like growth factor-1, and glial cell line–derived neurotrophic factor. The graft also improved host neurogenesis and reduced aberrant mossy fiber sprouting in the dentate gyrus.
Wang et al.58 investigated the efficacy of adipose-derived stem cells in kainate-induced epilepsy rat models. They showed that the transplanted cells inhibited EEG seizure activity and restored learning capacity and memory of rats as measured by the Morris water maze conducted 2 weeks and 2 months post-transplantation. The proposed mechanism points to a neuroprotective effect due to the release of BDNF, NT3, and NT4 to reduce apoptosis within the hippocampus. Proapoptotic BAX was reduced, whereas anti-apoptotic Bcl-2 and Bcl-xL were increased.
Knowing that transplanted GABA-ergic interneurons form synaptic contacts with host cell, and help control seizures, Arshad et al.61 investigated whether grafted GABA-ergic progenitors into the dentate gyrus ameliorated existing abnormalities in adult neurogenesis due to TLE. Accordingly, TLE alters neurogenesis by increasing neuronal proliferation and abnormal inversion of Type 1 progenitors, leading to the ectopic migration of Type 3 progenitors into the hilus of the dentate gyrus. Upon their maturation into granule cells, they become hyperexcitable and thus contribute to SRS. Arshad et al. showed that GABA-ergic progenitors transplanted into intact mice increased GABA-ergic interneurons in the dentate gyrus and significantly reduced type 2 progenitors. Type 3 progenitors were also increased. However, in epileptic mice, the transplantation resulted in reductions of inverted type 1, type 2, and hilar ectopic type 3 cells. It also resulted in increased radial migration of type 3 cells into the granule cell layer of the hippocampus. These effects are presumed to be due to paracrine signaling changes caused by the implanted progenitors. Therefore, hilar transplantation of GABA-ergic interneurons may correct aberrant adult neurogenesis due to TLE and prevent seizures.
The recent research on stem cell therapy has elucidated new target pathways, methods of administration, and increased our knowledge on the integration of transplanted stem cells in translational epilepsy models. Other areas of neurological disease, such as stroke, have expert consensus and recommendations that direct future research in the field. For example, stem cell therapy as an emerging paradigm for stroke (STEPS) is a report generated to guide translational research on cell-based restorative therapy in stroke62,63. This was later expanded via the RIGOR guidelines in 201364. A similar consensus and set of guidelines are missing in epilepsy stem cell research and could be beneficial for the structured development of the field. One of the important future directions of research will be to assess the safety and efficacy of stem cell therapy when combined with current established pharmaceutical and surgical interventions. Most patients who will qualify for stem cell therapy will have undergone several prior lines of therapy, and it is important to ensure their safety. Combining different treatments with stem cells may also result in a better outcome, so it is important to identify the most efficacious combination therapies for different epilepsy models.
Conclusion
Stem cell therapy is an emerging field with a promising future. Epilepsy has long been treated with medical, surgical, or interventional techniques, but among the emerging therapies lies the potential for stem cells to offer a potent and effective new way to treat the condition. Experiments with animal models display a striking capacity for stem cell grafts to inhibit major motor, electrographic, and absence seizures while also exhibiting neuroprotective and regenerative properties. Cells can be administered both before and after the onset of SE for varying neuroprotective effects. More recently, focus has shifted from animal-derived cell lines to the usage of human-derived cells such as mesenchymal and fibroblast cells. Limited human trials have taken place but as the body of evidence matures, it will become critical to apply the accumulated knowledge to humans in clinical trials to assess the safety of this therapy in humans and how well these results can translate to real-world applications.
Acknowledgments
We acknowledge the work of brgfx who designed the vectors used in Figure 1. The vectors were downloaded from freepik.com.
Footnotes
Author Contributions: Conceptualization, A.A. and C.V.B.; investigation, A.A., G.L., J.G., J.C., M.M., S.S., and I.P.; writing—original draft preparation, A.A., G.L., J.G., J.C., M.M., S.S., and I.P.; writing—review and editing, A.A., G.L., J.G., J.C., M.M., S.S., I.P., and C.V.B.; visualization, A.A., G.L., J.G., J.C., M.M., S.S., and I.P.; supervision, C.V.B. All authors have read and agreed to the published version of the manuscript.
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.V.B. declared leadership position with University of South Florida, patents holder and patent applications on stem cell biology and its therapeutic applications, consultant to a number of stem cell–based companies, and research funding from the NIH. All of the other authors declared no potential conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Adam Alayli  https://orcid.org/0000-0002-6986-788X
https://orcid.org/0000-0002-6986-788X
Jonah Gordon  https://orcid.org/0000-0002-9161-5160
https://orcid.org/0000-0002-9161-5160
Ike Dela Peña  https://orcid.org/0000-0003-2046-522X
https://orcid.org/0000-0003-2046-522X
Cesar V. Borlongan  https://orcid.org/0000-0002-2966-9782
https://orcid.org/0000-0002-2966-9782
References
- 1. WHO. Epilepsy In WHO Fact Sheets; 2022. https://www.who.int/en/news-room/fact-sheets/detail/epilepsy [accessed 2022 Nov 15].
- 2. Stafstrom CE, Carmant L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med. 2015;5(6):a022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang BL, Chang KH. Stem cell therapy in treating epilepsy. Front Neurosci. 2022;16:934507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rugg-Gunn F, Miserocchi A, McEvoy A. Epilepsy surgery. Pract Neurol. 2020;20(1): 4–14. [DOI] [PubMed] [Google Scholar]
- 5. Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness Efficiency of Surgery for Temporal Lobe Epilepsy Study G. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5): 311–18. [DOI] [PubMed] [Google Scholar]
- 6. Kanner AM, Bicchi MM. Antiseizure medications for adults with epilepsy: a review. JAMA. 2022;327(13): 1269–81. [DOI] [PubMed] [Google Scholar]
- 7. Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393(10172): 689–701. [DOI] [PubMed] [Google Scholar]
- 8. Hakami T. Neuropharmacology of antiseizure drugs. Neuropsychopharmacol Rep. 2021;41(3): 336–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sankaraneni R, Lachhwani D. Antiepileptic drugs—a review. Pediatr Ann. 2015;44(2): e36–42. [DOI] [PubMed] [Google Scholar]
- 10. Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G, Fried I, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307(9): 922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dwivedi R, Ramanujam B, Chandra PS, Sapra S, Gulati S, Kalaivani M, Garg A, Bal CS, Tripathi M, Dwivedi SN, et al. Surgery for drug-resistant epilepsy in children. N Engl J Med. 2017;377(17): 1639–47. [DOI] [PubMed] [Google Scholar]
- 12. Engel J., Jr. The current place of epilepsy surgery. Curr Opin Neurol. 2018;31(2): 192–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jayalakshmi S, Vooturi S, Gupta S, Panigrahi M. Epilepsy surgery in children. Neurol India. 2017;65(3): 485–92. [DOI] [PubMed] [Google Scholar]
- 14. Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5): 899–908. [DOI] [PubMed] [Google Scholar]
- 15. Salanova V. Deep brain stimulation for epilepsy. Epilepsy Behav. 2018;88s:21–24. [DOI] [PubMed] [Google Scholar]
- 16. Li MCH, Cook MJ. Deep brain stimulation for drug-resistant epilepsy. Epilepsia. 2018;59(2): 273–90. [DOI] [PubMed] [Google Scholar]
- 17. Sprengers M, Vonck K, Carrette E, Marson AG, Boon P. Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev. 2017;7(7):CD008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skarpaas TL, Jarosiewicz B, Morrell MJ. Brain-responsive neurostimulation for epilepsy (RNS(®) System). Epilepsy Res. 2019;153:68–70. [DOI] [PubMed] [Google Scholar]
- 19. Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, Salanova V, Cole AJ, Smith MC, Gwinn RP, Skidmore C, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55(3): 432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, Srinivasan S, Jobst B, Gross RE, Shields DC, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84(8): 810–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. González HFJ, Yengo-Kahn A, Englot DJ. Vagus nerve stimulation for the treatment of epilepsy. Neurosurg Clin N Am. 2019;30(2): 219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Englot DJ. A modern epilepsy surgery treatment algorithm: Incorporating traditional and emerging technologies. Epilepsy Behav. 2018;80:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pérez-Carbonell L, Faulkner H, Higgins S, Koutroumanidis M, Leschziner G. Vagus nerve stimulation for drug-resistant epilepsy. Pract Neurol. 2020;20(3): 189–98. [DOI] [PubMed] [Google Scholar]
- 24. Gil-López F, Boget T, Manzanares I, Donaire A, Conde-Blanco E, Baillés E, Pintor L, Setoaín X, Bargalló N, Navarro J, Casanova J, et al. External trigeminal nerve stimulation for drug resistant epilepsy: a randomized controlled trial. Brain Stimul. 2020;13(5): 1245–53. [DOI] [PubMed] [Google Scholar]
- 25. De Gioia R, Biella F, Citterio G, Rizzo F, Abati E, Nizzardo M, Bresolin N, Comi GP, Corti S. Neural stem cell transplantation for neurodegenerative diseases. Int J Mol Sci. 2020;21(9):3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85(1): 3–10. [DOI] [PubMed] [Google Scholar]
- 27. Hattiangady B, Rao MS, Shetty AK. Grafting of striatal precursor cells into hippocampus shortly after status epilepticus restrains chronic temporal lobe epilepsy. Exp Neurol. 2008;212(2): 468–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Upadhya D, Hattiangady B, Castro OW, Shuai B, Kodali M, Attaluri S, Bates A, Dong Y, Zhang SC, Prockop DJ, et al. Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration. Proc Natl Acad Sci USA. 2019;116(1): 287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A, Baraban SC. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat Neurosci. 2013;16(6): 692–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hammad M, Schmidt SL, Zhang X, Bray R, Frohlich F, Ghashghaei HT. Transplantation of GABAergic interneurons into the neonatal primary visual cortex reduces absence seizures in stargazer mice. Cereb Cortex. 2015;25(9): 2970–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pereira M, Pfisterer U, Rylander D, Torper O, Lau S, Lundblad M, Grealish S, Parmar M. Highly efficient generation of induced neurons from human fibroblasts that survive transplantation into the adult rat brain. Sci Rep. 2014;4:6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Avaliani N, Pfisterer U, Heuer A, Parmar M, Kokaia M, Andersson M. Directly converted human fibroblasts mature to neurons and show long-term survival in adult rodent hippocampus. Stem Cells Int. 2017;2017:5718608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jeon D, Chu K, Lee ST, Jung KH, Kang KM, Ban JJ, Kim S, Seo JS, Won CH, Kim M, Lee SK, et al. A cell-free extract from human adipose stem cells protects mice against epilepsy. Epilepsia. 2011;52(9): 1617–26. [DOI] [PubMed] [Google Scholar]
- 34. Lee ST, Chu K, Jung KH, Im WS, Park JE, Lim HC, Won CH, Shin SH, Lee SK, Kim M, Roh JK. Slowed progression in models of Huntington disease by adipose stem cell transplantation. Ann Neurol. 2009;66(5): 671–81. [DOI] [PubMed] [Google Scholar]
- 35. Lin YC, Ko TL, Shih YH, Lin MY, Fu TW, Hsiao HS, Hsu JY, Fu YS. Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke. 2011;42(7): 2045–53. [DOI] [PubMed] [Google Scholar]
- 36. Huang PY, Shih YH, Tseng YJ, Ko TL, Fu YS, Lin YY. Xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly as a potential therapy for rat pilocarpine-induced epilepsy. Brain Behav Immun. 2016;54:45–58. [DOI] [PubMed] [Google Scholar]
- 37. Huicong K, Zheng X, Furong W, Zhouping T, Feng X, Qi H, Xiaoyan L, Xiaojiang H, Na Z, Ke X, Zheng Z, et al. The imbalanced expression of adenosine receptors in an epilepsy model corrected using targeted mesenchymal stem cell transplantation. Mol Neurobiol. 2013;48(3): 921–30. [DOI] [PubMed] [Google Scholar]
- 38. Hlebokazov F, Dakukina T, Ihnatsenko S, Kosmacheva S, Potapnev M, Shakhbazau A, Goncharova N, Makhrov M, Korolevich P, Misyuk N, Dakukina V, et al. Treatment of refractory epilepsy patients with autologous mesenchymal stem cells reduces seizure frequency: an open label study. Adv Med Sci. 2017;62(2): 273–79. [DOI] [PubMed] [Google Scholar]
- 39. Hlebokazov F, Dakukina T, Potapnev M, Kosmacheva S, Moroz L, Misiuk N, Golubeva T, Slobina E, Krasko O, Shakhbazau A, Hlavinski I, et al. Clinical benefits of single vs repeated courses of mesenchymal stem cell therapy in epilepsy patients. Clin Neurol Neurosurg. 2021;207:106736. [DOI] [PubMed] [Google Scholar]
- 40. DaCosta JC, Portuguez MW, Marinowic DR, Schilling LP, Torres CM, DaCosta DI, Carrion MJM, Raupp EF, Machado DC, Soder RB, Lardi SL, et al. Safety and seizure control in patients with mesial temporal lobe epilepsy treated with regional superselective intra-arterial injection of autologous bone marrow mononuclear cells. J Tissue Eng Regen Med. 2018;12(2): e648–56. [DOI] [PubMed] [Google Scholar]
- 41. Lourenço DM, Ribeiro-Rodrigues L, Sebastião AM, Diógenes MJ, Xapelli S. Neural stem cells and cannabinoids in the spotlight as potential therapy for epilepsy. Int J Mol Sci. 2020;21(19):7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dietrich J, Kempermann G. Role of endogenous neural stem cells in neurological disease and brain repair. Adv Exp Med Biol. 2006;557:191–220. [DOI] [PubMed] [Google Scholar]
- 43. Farrell JS, Wolff MD, Teskey GC. Neurodegeneration and pathology in epilepsy: clinical and basic perspectives. Adv Neurobiol. 2017;15:317–34. [DOI] [PubMed] [Google Scholar]
- 44. Sadanandan N, Saft M, Gonzales-Portillo B, Borlongan CV. Multipronged attack of stem cell therapy in treating the neurological and neuropsychiatric symptoms of epilepsy. Front Pharmacol. 2021;12:596287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Papazian I, Kyrargyri V, Evangelidou M, Voulgari-Kokota A, Probert L. Mesenchymal stem cell protection of neurons against glutamate excitotoxicity involves reduction of NMDA-triggered calcium responses and surface GluR1, and is partly mediated by TNF. Int J Mol Sci. 2018;19(3):651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ali SO, Shahin NN, Safar MM, Rizk SM. Therapeutic potential of endothelial progenitor cells in a rat model of epilepsy: role of autophagy. J Adv Res. 2019;18:101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Backofen-Wehrhahn B, Gey L, Bröer S, Petersen B, Schiff M, Handreck A, Stanslowsky N, Scharrenbroich J, Weißing M, Staege S, Wegner F, et al. Anticonvulsant effects after grafting of rat, porcine, and human mesencephalic neural progenitor cells into the rat subthalamic nucleus. Exp Neurol. 2018;310:70–83. [DOI] [PubMed] [Google Scholar]
- 48. Hattiangady B, Kuruba R, Shuai B, Grier R, Shetty AK. Hippocampal neural stem cell grafting after status epilepticus alleviates chronic epilepsy and abnormal plasticity, and maintains better memory and mood function. Aging Dis. 2020;11(6): 1374–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anderson NC, Van Zandt MA, Shrestha S, Lawrence DB, Gupta J, Chen CY, Harrsch FA, Boyi T, Dundes CE, Aaron G, et al. Pluripotent stem cell-derived interneuron progenitors mature and restore memory deficits but do not suppress seizures in the epileptic mouse brain. Stem Cell Res. 2018;33:83–94. [DOI] [PubMed] [Google Scholar]
- 50. Waloschková E, Gonzalez-Ramos A, Mikroulis A, Kudláček J, Andersson M, Ledri M, Kokaia M. Human stem cell-derived GABAergic interneurons establish efferent synapses onto host neurons in rat epileptic hippocampus and inhibit spontaneous recurrent seizures. Int J Mol Sci. 2021;22(24):13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu K, Cai GL, Zhuang Z, Pei SY, Xu SN, Wang YN, Wang H, Wang X, Cui C, Sun MC, Guo SH, et al. Interleukin-1β-treated mesenchymal stem cells inhibit inflammation in hippocampal astrocytes through exosome-activated Nrf-2 signaling. Int J Nanomedicine. 2021;16:1423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luo Q, Xian P, Wang T, Wu S, Sun T, Wang W, Wang B, Yang H, Yang Y, Wang H, Liu W, et al. Antioxidant activity of mesenchymal stem cell-derived extracellular vesicles restores hippocampal neurons following seizure damage. Theranostics. 2021;11(12): 5986–6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans. 2015;43(4): 621–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fukumura S, Sasaki M, Kataoka-Sasaki Y, Oka S, Nakazaki M, Nagahama H, Morita T, Sakai T, Tsutsumi H, Kocsis JD, Honmou O. Intravenous infusion of mesenchymal stem cells reduces epileptogenesis in a rat model of status epilepticus. Epilepsy Res. 2018;141:56–63. [DOI] [PubMed] [Google Scholar]
- 55. Xu K, Liu F, Xu W, Liu J, Chen S, Wu G. Transplanting GABAergic neurons differentiated from neural stem cells into hippocampus inhibits seizures and epileptiform discharges in pilocarpine-induced temporal lobe epilepsy model. World Neurosurg. 2019;128:e1–11. [DOI] [PubMed] [Google Scholar]
- 56. Xian P, Hei Y, Wang R, Wang T, Yang J, Li J, Di Z, Liu Z, Baskys A, Liu W, Wu S, et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019;9(20): 5956–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kodali M, Castro OW, Kim DK, Thomas A, Shuai B, Attaluri S, Upadhya R, Gitai D, Madhu LN, Prockop DJ, et al. Intranasally administered human MSC-derived extracellular vesicles pervasively incorporate into neurons and microglia in both intact and status epilepticus injured forebrain. Int J Mol Sci. 2019;21(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang L, Zhao Y, Pan X, Zhang Y, Lin L, Wu Y, Huang Y, He H. Adipose-derived stem cell transplantation improves learning and memory via releasing neurotrophins in rat model of temporal lobe epilepsy. Brain Res. 2021;1750:147121. [DOI] [PubMed] [Google Scholar]
- 59. Venugopal C, Shobha K, Rai KS, Dhanushkodi A. Neurogenic and cognitive enhancing effects of human dental pulp stem cells and its secretome in animal model of hippocampal neurodegeneration. Brain Res Bull 2022;180:46–58. [DOI] [PubMed] [Google Scholar]
- 60. Upadhya D, Attaluri S, Liu Y, Hattiangady B, Castro OW, Shuai B, Dong Y, Zhang SC, Shetty AK. Grafted hPSC-derived GABA-ergic interneurons regulate seizures and specific cognitive function in temporal lobe epilepsy. NPJ Regen Med. 2022;7(1): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Arshad MN, Oppenheimer S, Jeong J, Buyukdemirtas B, Naegele JR. Hippocampal transplants of fetal GABAergic progenitors regulate adult neurogenesis in mice with temporal lobe epilepsy. Neurobiol Dis. 2022;174:105879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stem Cell Therapies as an Emerging Paradigm in Stroke Participants. Bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40(2): 510–15. [DOI] [PubMed] [Google Scholar]
- 63. Savitz SI, Chopp M, Deans R, Carmichael T, Phinney D, Wechsler L, STEPS Participants. Stem cell therapy as an emerging paradigm for stroke (STEPS) II. Stroke. 2011;42(3): 825–29. [DOI] [PubMed] [Google Scholar]
- 64. Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl Stroke Res. 2013;4(3): 279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]