Abstract
The purpose of this review is to update the recent information regarding the role of influenza vaccination (IV) as a strategy to reduce cardiovascular (CV) events. During the last 2 years, new meta-analysis, guidelines, and two randomized controlled trials (RCTs) were published. The IAMI trial added information regarding the safety and efficacy of IV right after an acute myocardial infarction hospitalization. A significant reduction in the primary endpoint—including mortality—was observed. More recently, the influenza vaccine to prevent vascular events trial (IVVE) trial did not meet the primary CV endpoint in patients with heart failure (HF). However, a significant reduction was observed during the seasonal peaks of Influenza circulation. COVID-19 pandemic provoked recruitment difficulties in these trials, as well as an altered influenza seasonality and incidence. Further analysis of IVVE trial is needed to clarify the precise role of IV in patients with HF. A recent meta-analysis of RCTs and observational studies indicated that IV was safe and effective to reduce CV events, and it was included in the most updated guideline. Despite these benefits, and the recommendations for its prescription by scientific societies and health regulatory agencies, the vaccination rate remains below than expected globally. The correct understanding of implementation barriers, which involve doctors, patients, and their context, is essential when continuous improvement strategies are planned, in order to improve the IV rate in at-risk subjects.
Keywords: Influenza vaccination, Cardiovascular events, Implementation
For the audio file associated with this article, please visit the HTML version of the article online (https://academic.oup.com/eurheartjsupp/issue/25/Supplement_A)
Introduction
Cardiovascular (CV) mortality continues to be the main cause of death in developed countries as well as in emerging economies. Cardiovascular disease (CVD) takes ∼18 million lives each year, which accounts for one-third of all deaths worldwide. In order to achieve the global target of ‘25 by 25’ and ‘1/3 by 30’, reducing a quarter of premature deaths from non-communicable diseases by 2025 and one-third of them by 2030, effective interventions need to be identified and implemented in the most vulnerable populations.1 The economic burden of CVD is projected to be more than $1 trillion in 2030, half of which relates to direct medical costs. Cost-effective interventions are needed to flatten the rising curve of healthcare costs for CVD.2
Respiratory infections, particularly those caused by the Influenza virus, contribute significantly to morbidity and mortality throughout the world. Numerous deaths and CV complications take place during flu epidemics, especially in vulnerable populations. Patients with chronic CVD are particularly at risk during this period and represent a population that could benefit the most from vaccination.3
In the last 15 years, influenza vaccination (IV) in high-risk populations has become an effective strategy to reduce the incidence of respiratory infections and therefore associated CV complications. However, the prescription of IV is not a usual cardiologists’ practice, and vaccination rates vary widely among high-risk vulnerable populations in different regions of the world. Influenza vaccine coverage rates (VCRs) are variable and often low in populations with pre-existing CVD. For example, recent influenza VCR in patients with heart failure (HF) ranged from nearly 0% in Asia to ∼80% in Europe. In particular, most low- and middle-income countries have not reached the target of 70% VCR set by WHO for high-risk groups. This reluctance of cardiologists to incorporate immunization as a routine CV prevention strategy for their patients has also been observed in Latin American countries.4–6
The purpose of this review is to update the evidence regarding the efficacy of IV in preventing CVD morbidity and mortality especially in high-risk populations, so healthcare providers may be able to make recommendations to individual patients with more certainty. The barriers that prevent putting into practice the recommendations of the guidelines will also be analysed.
Influenza vaccination in patients with cardiovascular disease: recent emerging evidence
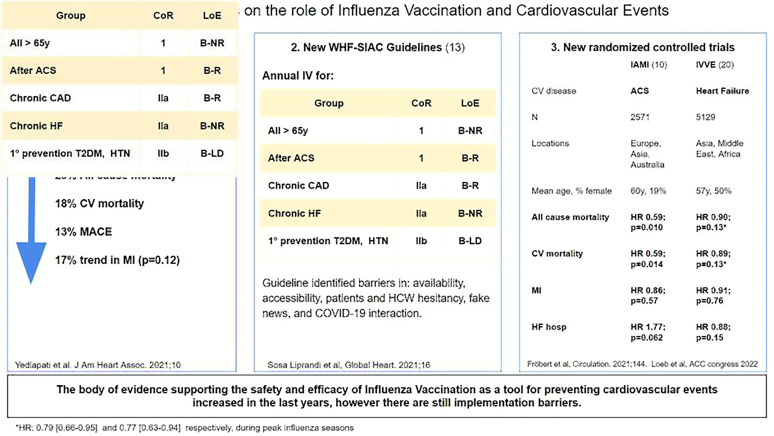
A recently published systematic review and meta-analysis included a total of 16 studies (n = 237 058) comprising four randomized clinical trials (RCTs n = 1667) and 12 observational studies (n = 235 391). The studies had at least 50% of patients with established CVD: atherosclerotic CVD or HF. Four RCTs (n = 1667) and eight observational studies (n = 164 047) reported all-cause mortality.7–18 Overall, IV was associated with a 25% reduction in all-cause mortality compared with control [Risk Reduction (RR): 0.75; 95% Confidence Interval (CI): 0.60–0.93 (P = 0.01)] with high heterogeneity (I2 = 97%). Four RCTs (n = 1667) and three observational studies (n = 136 082) reported 18% reduction in CV mortality compared with control [RR: 0.82; 95% CI: 0.80–0.84 (P < 0.001)] (I2 = 31%).19
Moreover, IV was associated with a 13% reduction in major adverse cardiovascular events compared with control [RR: 0.87; 95% CI: 0.80–0.94 (P < 0.001)] (I2 = 51%) in four RCTs (n = 1667) and three observational studies (n = 27 207). These benefits were consistent among study designs but more pronounced in RCTs than observational studies on mortality and CV end points. While a numerical reduction in myocardial infarction (MI) was associated with the IV, statistical significance was not achieved [RR: 0.73; 95% CI: 0.49–1.09 (P = 0.12)] (I2 = 64%). The influence of IV on HF was not reported in the main results due to lack of data from RCTs (only one RCT). However, the summary estimate was consistent with a 29% RR in HF, predominantly driven by the 27% reduction noted from observational data. One of the distinctive features of this meta-analysis is that the follow-up duration was 12 months compared with other meta-analyses where the follow-up time was shorter.7 This becomes relevant when the objective is to demonstrate the impact on end points such as mortality, requiring a longer observation time.
The recently published IAMI trial was a randomized, double-blind, placebo-controlled, investigator-initiated trial designed to evaluate the efficacy of IV after MI or percutaneous coronary intervention (PCI) in high-risk patients with coronary artery disease.20 The trial was conducted at 30 centres in eight countries. The influenza vaccine/placebo was administered within 72 h of coronary angiography/PCI or, in Bangladeshi centres, on hospital admission. The trial was early terminated because of the COVID-19 pandemic before attaining the target sample size (4372 patients).
A total of 1348 (54.5%) patients were admitted with ST segment elevation MI, 1119 (45.2%) with non-ST segment elevation MI, and 8 (0.3%) with stable coronary artery disease. A total of 1868 participants (74.3%) were treated with PCI, and 587 (23.4%) received medical treatment only. Over the 12-month follow-up, the primary outcome (the composite of all-cause death, MI, or stent thrombosis) occurred in 67 participants (5.3%) assigned to IV and 91 participants (7.2%) in the placebo group [hazard ratio (HR): 0.72 (95% CI: 0.52–0.99); P = 0.040]. Influenza vaccination resulted in a 41% reduction in CV mortality [HR: 0.59 (95% CI: 0.39–0.90); P = 0.014], and a 41% reduction in all-cause mortality [HR: 0.59 (95% CI: 0.39–0.89); P = 0.010]. There was a trend to a reduction in MI [HR: 0.86 (95% CI: 0.50–1.46); P = 0.57]. Although it was not statistically significant, it may have been attributable to low numbers of events and limited statistical power.
One of the limitations of the study is that it was interrupted early, being able to incorporate only 2571 individuals of the 4372 originally planned. This may have positively influenced the results of the study. On the other hand, once again, women were under-represented constituting only 19% of the sample, limiting the generalizability of the results to this subgroup.
Another point of discussion is that this group of patients had a precise indication to receive IV according to guidelines; therefore it would be unethical to consider the placebo group. However, the investigators included patients with an acute coronary event, a setting where the opportunity for vaccination was not yet a standard of care, and also, per protocol it was allowed for patients to be vaccinated throughout the study if they wished. Interestingly, only 13% of the patients in the placebo group crossed over to receive the vaccine. In any case, this could have diminished the magnitude of the results.
Consistent with the presentation of the results, the authors performed an exploratory meta-analysis for the key secondary endpoint of CV death at 1 year, combining the results with prior RCTs in patients with CV disease. The pooled estimate of CV death of the HR from the fixed-effect meta-analysis of all four trials was 0.51 (95% CI: 0.36–0.71); P = 0.0001. The results were consistent across subgroups and in agreement with the meta-analysis previously mentioned.19
No new alerts of side effects were described with this strategy of prescribing IV during an acute CV disease and in the context of strong antithrombotic therapy. This is a very important observation since it allows the vaccine to be safely prescribed prior to discharge in patients hospitalized due to MI. Another interesting point mentioned by the authors refers to the early separation of the event rate curves since the administration of the vaccine, stabilizing at 3 months, perhaps suggesting an anti-inflammatory mechanism during the vulnerable phase after MI.
The impact of the IV in the prevention of CV events has a similar or even greater scope than other strategies that we usually apply in the context of secondary prevention of CVD, such as the prescription of beta-blockers, statins, aspirin, angiotensin converting enzyme inhibitors, or smoking cessation.8,21–24 These results, particularly the 41% relative reduction in the risk of all-cause mortality and CV mortality, reinforce the concept of adopting an active approach and recommending vaccination against Influenza in patients with MI before hospital discharge, ensuring patient adherence to guidelines recommendations.
In this sense, our group carried out a pilot study, and it was proposed that consulting a specialist, or being hospitalized in a coronary care unit (CCU) was an important opportunity to improve the rate of adherence to the indication of the vaccines. Eighty patients were included, of which 68% were men, with a median age of 65 years. In 25 patients (31%), the indication of IV was upon hospital discharge and in the remaining at the outpatient clinic. The most frequent indications were acute coronary syndromes (59%), age >65 years (50%), active smoking (16%), diabetes (12%) and HF (10%), overlapping several of them in some cases. At 30 days, 73% of the patients received the vaccine. The main reason referred by patients for non-vaccination was ‘personal decision’. The indication of the vaccine from the CCU was associated with a trend towards a higher vaccination rate (52 vs. 42%), although the 10% rate difference was not significant probably due to low statistical power.9
In March 2022, in the context of the annual meeting of the American College of Cardiology, Mark Loeb presented the study ‘A Randomized Controlled Trial of Influenza Vaccine to Prevent Adverse Vascular Events’ (IVVE).10 It was a pragmatic, double-blind trial that evaluated if the IV was effective to reduce CV events in patients with symptomatic HF in low- and middle-income countries. Patients were recruited from 10 countries in Asia, the Middle East, and Africa over three influenza seasons. The trial included 5129 patients with symptomatic HF, 50% were women, 95% were in New York Heart Association (NYHA) Class II or III, including preserved or reduced left ventricular ejection fraction. The vaccine was administered annually for three influenza seasons.
The authors reported that there were no differences in the primary outcomes (composite of CV death, non-fatal MI and non-fatal stroke) or secondary outcomes (first co-primary and HF hospitalizations) at 36 months. The influenza group showed a 15% reduction rate in all-cause hospitalizations (HR: 0.85; 95% CI: 0.74–0.97; P < 0.01), and 42% reduction rate in pneumonia (HR: 0.58; 95% CI: 0.42–0.80; P < 0.0006). However, during periods of peak influenza circulation, there was a significant reduction in first primary outcome (HR: 0.82; 95% CI: 0.68–0.99), all-cause hospitalizations, and pneumonia in the IV group compared with placebo.
This important observation follows the same direction as the studies previously discussed that highlight the importance of vaccination against influenza in these high-risk populations as a strategy to prevent CV events, especially during peak influenza season. The authors considered that the results of the trial, reflecting a significant difference in outcomes only during peak influenza seasons, were interpreted not only as non-negative results, but also as a strong support to the body of evidence regarding the association of influenza and CV events (Figure 1).
Figure 1.
Updates on the role of influenza vaccination and cardiovascular events. ACS, acute coronary syndrome; CAD, coronary artery disease; CV, cardiovascular; HCW, healthcare workers; HF, heart failure; HR, hazard ratio; HTN, hypertension; IAMI, influenza vaccination after myocardial infarction trial; IVVE, influenza vaccine to prevent vascular events trial; MACE, major adverse cardiovascular events; MI, myocardial infarction; RCTs, randomized clinical trials; T2DM, type 2 diabetes mellitus; WHF-SIAC, World Heart Federation, InterAmerican Society of Cardiology; CoR, class of recommendation; LoE, level of evidence; R, randomized; NR, not randomized; LD, limited data.
Barriers to influenza vaccination implementation related to physicians, patients, and their context
The American Heart Association/American College of Cardiology Guidelines for Secondary Prevention for Patients With Coronary Disease recommend IV for all patients with established coronary artery disease since 2006 (Class I, Level of Evidence B), consistent with the CDC guidelines.11,12 A recently published Consensus document of the Inter-American Society of Cardiology and the Word Heart Federation reinforce the use of the IV in adults in order to reduce the rate of CV events and its effect on the burden of the disease in the region.13 The European Society of Cardiology and individual initiatives from other countries are aligned in the same direction.14,15 Despite the benefits related to IV and the recommendations for its prescription by scientific societies and health regulatory agencies, vaccination rates globally, remains far below the expected effective rate of vaccination.4,13
As with other prevention measures, medical knowledge through continuous education, clear regulations, and conviction regarding the risk–benefit ratio seem to be the main determinants of the implementation of an intervention. The personal experience of the physician, as well as that of other health workers with IV also appears to be determining factors in future recommendations for patients. When ‘missed opportunities’ were analysed in unvaccinated patients, lack of recommendation during medical visits was identified as the main cause. Seen in another way, when doctors have a positive attitude and recommend the vaccine, the vaccination rate increases considerably, generating an effective vaccination rate between 50 and 93% of cases in different series.13 Among physicians, specialists may be reluctant to perform primary care interventions.16
Another key limitation in effective vaccination involves complex behavioural attributes related to the psychological aspects of patients.13 There are ∼500 articles that analyse behavioural aspects that determine hesitancy in the vaccination decision. These aspects are grouped into complacency (low perceived risk of becoming ill or presenting serious complications, or not having presented the disease), inconvenience (self-efficacy, cost, behavioural aspects), lack of confidence (aspects such as distrust in the efficacy and adverse effects, psychological aspects regarding the relation with the authorities and the indications, greater acceptance of negative myths) and calculation (individual and social risk–benefit ratio).17
Sociocultural factors were also identified such as economic strata, education level (paradoxically, highest educational level patients reject vaccination to a greater extent), religion, and demographic variables (ethnic differences have been reported in the USA, with a lower vaccination rate among Hispanic-Latino populations). Among countries of the Americas, trust in vaccination by patients is unevenly distributed. Recent data from the CorCOVID-LATAM study, conducted in 13 Latin American countries, has reported differences in the vaccination rate according to economic incomes and educational level.6
Fake news in the media and social networks and people who advocate against vaccination are key aspects in the hesitation process, with potentially harmful effects on population health.13 Years of scientific research can be overshadowed by a simple fake article developed in 1 min and massively disseminated on social media.13 In an analysis of 450 000 health-related fake news articles collected on social media in Poland, the majority were related to vaccines. Cautious dissemination of recent scientific articles, review of data or publications by experts, social media campaigns and alliances with influential subjects in social networks, as well as public commitment by doctors, are some of the suggestions to overcome these obstacles.
More recently, the COVID-19 pandemic occurred in a completely virtual era with a high global penetration rate of internet and social networks, where scepticism about the disease, as well as the safety and efficacy of new vaccines, could influence short-term IV rates. Recently, three of the largest platforms (YouTube, Facebook, and Twitter) unified criteria to prevent the spread of vaccine related fake news.25
The direct cost of the vaccine is a major barrier. However, PAHO has created a revolving fund to facilitate lower-cost access to both trivalent and quadrivalent vaccines. In this way, 41 countries and territories of Latin America and the Caribbean have a facilitated vaccine acquisition programme. For adult vaccinations against Influenza, most (21) European Union Member States provide vaccinations free of charge at the point of delivery for those groups of the population targeted by the respective national vaccination programme, e.g. people aged 65 years and older. However, in some countries targeted patients need to pay at least part of the costs for adult vaccinations against influenza.13
Effective strategies in increasing influenza vaccination rate
It is understood that physicians’ conviction seems to be more influential than the perceptions of the patient when analysing effective vaccination rates. The strong and positive attitude by the doctors at the moment of vaccine prescription seems decisive.13 The implementation of continuous medical education programmes aimed at general practitioners and specialists addressing the benefits and opportunities of IV should be considered as a primary objective, as well as its incorporation into clinical practice guidelines.
Vaccination should be considered an essential topic of undergraduate medical and nursing schools. The study of immunological aspects, as well as pharmacology, should be expanded and standardized. The onboarding of advanced medical and nursing students in vaccination campaigns could contribute in this regard.26
Patient’s adherence to treatment while undergoing acute-high-mortality diseases such as an acute coronary syndrome, is almost complete upon discharge from the CCU, but drastically falls during follow-up.13 Therefore, and in the light of emergent safety data from IAMI trial, implementation of IV prior to discharge or immediately after it would be a highly effective measure to increase vaccination rates, considering this strategy as standard-of-care procedure.
The correct understanding of implementation barriers, which involve doctors, patients, and their context, is essential when designing continuous improvement strategies in order to optimize the effective rate of IV.
Conclusions
In the last years, the body of evidence increased and added strength to the usefulness of IV as a CV prevention tool. Further in depth analysis of IVVE trial may help us understand why primary endpoint was not reached and if IV for coronary disease patients has more utility than for HF patients, which a priori lacks a logical support. COVID pandemic completely altered influenza’s seasonality and circulation, interfering with the development of further clinical trials. However, positive and negative experiences of COVID vaccine platforms and vaccination campaigns, as well as the results of the commented trials, may help reinforce, and improve IV rates for CVD patients.
Acknowledgements
The authors thank Dr. Adrian Baranchuk for critical review of the manuscript.
Contributor Information
María Inés Sosa-Liprandi, Department of Cardiology, Sanatorio Güemes, Acuña de Figueroa 1228 C1180AAX, Buenos Aires, Argentina.
Ezequiel José Zaidel, Department of Cardiology, Sanatorio Güemes, Acuña de Figueroa 1228 C1180AAX, Buenos Aires, Argentina.
Álvaro Sosa-Liprandi, Department of Cardiology, Sanatorio Güemes, Acuña de Figueroa 1228 C1180AAX, Buenos Aires, Argentina.
Funding
This paper was published as part of a supplement financially supported by Sanofi. Manuscripts were accepted after rigorous peer review process that was managed by an expert Guest Editor independently appointed by the Editor-in-Chief. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of Sanofi.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
- 1.Bennett JE, Kontis V, Mathers CD, Guillot M, Rehm J, Chalkidou Ket al. NCD Countdown 2030: pathways to achieving sustainable development goal target 3.4. Lancet 2020;396:918–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghebreyesus TA. Acting on NCDs: counting the cost. Lancet 2018;391:1973–1974. [DOI] [PubMed] [Google Scholar]
- 3.Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow Tet al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 2018;378:345–353. [DOI] [PubMed] [Google Scholar]
- 4.Aguado TM, Barratt J, Beard JR, Blomberg BB, Chen WH, Hickling Jet al. Report on WHO meeting on immunization in older adults: Geneva, Switzerland, 22–23 March 2017. Vaccine 2018;36:921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaidel EJ, Cacia SL, Pérez GE, Costabel JP, Failo M, Rosende Aet al. Vacuna antineumocócica en adultos: encuesta a residentes de cardiología de Argentina. Rev CONAREC 2014;15:101–104. [Google Scholar]
- 6.Liprandi A S, Zaidel EJ, Lopez Santi R, Araujo JJ, Baños González MA, Busso JMet al. Influenza and pneumococcal vaccination in non-infected cardiometabolic patients from the Americas during the COVID-19 pandemic. A sub-analysis of the CorCOVID-LATAM study. Vaccines (Basel) 2021;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul Aet al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013;310:1711–1720. [DOI] [PubMed] [Google Scholar]
- 8.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 2003;290:86–97. [DOI] [PubMed] [Google Scholar]
- 9.Villarreal R, Zaidel EJ, Cestari HG, Mele EF, Sosa Liprandi MI, Sosa Liprandi A. Vacunación antigripal y antineumocócica en pacientes con enfermedad cardiovascular: proyecto piloto. Rev Argent Cardiol 2016;84:607–609. [Google Scholar]
- 10.Presented by Dr. Mark Loeb at the American College of Cardiology Annual Scientific Session (ACC 2022), Washington, DC, April 3, 2022.
- 11.Smith SC, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GCet al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation 2006;113:2363–2372. [DOI] [PubMed] [Google Scholar]
- 12.Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019;68:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sosa Liprandi A, Sosa Liprandi MI, Zaidel EJ, Aisenberg GM, Baranchuk A, Costa Duarte Barbosa Eet al. Influenza vaccination for the prevention of cardiovascular disease in the Americas: consensus document of the Inter-American Society of Cardiology and the Word Heart Federation. Glob Heart 2021;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano Cet al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. Erratum in: Eur Heart J. 2020;41(44):4242. [DOI] [PubMed] [Google Scholar]
- 15.García-Zamora S, Sosa Liprandi MI, Picco JM, Matta GM, Villarreal R, Col Pulido LY. Consenso de Inmunizaciones en Adultos con Cardiopatías Sociedad Argentina de Cardiología (SAC). Rev Argent Cardiol 2020;88:1–36. [PubMed] [Google Scholar]
- 16.Srivanichakom W, Asavathitanonta K, Washirasaksiri C, Chaisathaphon T, Chouriyagune C, Phisalprapa Pet al. Prescribing rate of influenza vaccine among internal medicine residents for outpatient continuum care. J Med Assoc Thai 2014;97:1281–1289. [PubMed] [Google Scholar]
- 17.Schmid P, Rauber D, Betsch C, Lidolt G, Denker M-L. Barriers of influenza vaccination intention and behavior – a systematic review of influenza vaccine hesitancy, 2005–2016. PLoS One 2017;12:e0170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill JA, Agewall S, Baranchuk A. Medical misinformation: vet the message! Int J Cardiol 2019;277:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yedlapati SH, Khan SU, Talluri S, Lone AN, Khan MZ, Khan MSet al. Effects of influenza vaccine on mortality and cardiovascular outcomes in patients with cardiovascular disease: a systematic review and meta-analysis. J Am Heart Assoc 2021;10:e019636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fröbert O, Götberg M, Erlinge D, Akhtar Z, Christiansen EH, MacIntyre CRet al. Influenza vaccination after myocardial infarction. Circulation 2021;144:1476–1484. [DOI] [PubMed] [Google Scholar]
- 21.Freemantle N, Cleland J, Young P, Mason J, Harrison J. Beta blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 1999;318:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pignone M, Phillips C, Murrow C. Use of lipid lowering drugs for primary prevention of coronary heart disease: a meta-analysis of randomized trials. BMJ 2000;321:983–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flather MD, Yusuf S, Køber L, Pfeffer M, Hall A, Murray Get al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-inhibitor myocardial infarction collaborative group. Lancet 2000:355:1575–1581. [DOI] [PubMed] [Google Scholar]
- 24.Antithrombotic Trialists’ Collaboration . Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.YouTube, Facebook and Twitter align to fight Covid vaccine conspiracies. BBC News. 2020. https://www.bbc.com/news/technology-55005385 (15 July 2022).
- 26.SIAC|GUÍAS|SIAC. http://www.siacardio.com/category/educacion/guias/ (14 July 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.