Abstract
Type 2 diabetes, obesity-related metabolic syndrome, and insulin resistance are the most common metabolic disorders associated with increased cardiovascular risk. In addition, patients with Type 2 diabetes have an increased risk for a more severe course of influenza virus infection, a common pandemic. There is increasing evidence that influenza vaccination in patients with diabetes can safely and effectively reduce all-cause mortality and cardiovascular death. The effects of vaccination appear to be more effective when using higher-dose and quadrivalent vaccines, although subgroup-specific separate analyses in patients with diabetes are lacking. Clinical recommendations address influenza vaccination in all adults with diabetes. From our point of view, it should be an integral part of treatment strategies in patients with diabetes.
Keywords: Influenza, Diabetes, Cardiovascular death, Vaccination rate
For the audio file associated with this article, please visit the HTML version of the article online (https://academic.oup.com/eurheartjsupp/issue/25/Supplement_A)
Introduction
The most severe influenza A pandemic in recent history was caused by H1N1 in 1918, leading to ∼50 million deaths. The disease and its sequelae can be prevented and modulated, respectively, by a routine vaccination.
In this review, we will focus on patients with diabetes with the following aspects:
Elevated risk for complications of influenza in diabetes.
Clinical effects of influenza vaccination in patients with diabetes.
Efficacy and safety of different vaccination strategies.
Uptake of influenza vaccination over time and in patients with diabetes.
Current recommendations and perspectives in patients with diabetes.
Elevated risk for infection and complications of influenza in diabetes
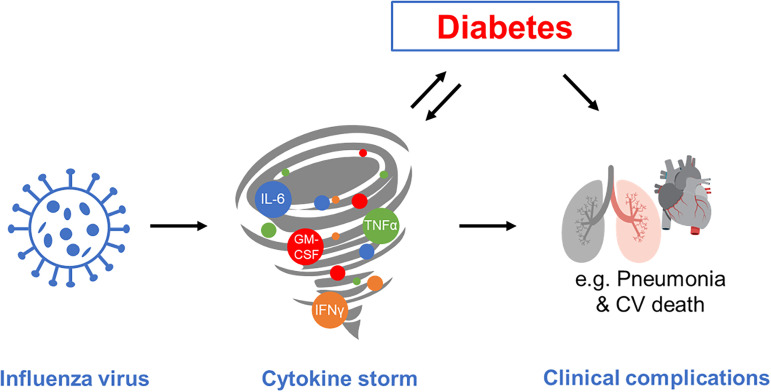
Influenza is a yearly pandemic viral infection, which can have a dramatic clinical course, especially in elderly and susceptible populations, such as patients with diabetes mellitus. Since the viral haemagglutinin binds to sialic receptors on cells lining the superficial epithelium of the respiratory tract, systemic manifestation appears to be mediated by inflammatory reactions. This resembles a ‘cytokine storm’, as has been frequently described for the COVID-19 pandemic. Diabetes and obesity can be modulators and enhancers of inflammatory responses and inflammation can, in turn, affect metabolic control and insulin sensitivity vice versa (Figure 1). Until now, there is no conclusive evidence that patients with diabetes have a higher infection rate. However, there is some observational evidence that diabetes and a possible degree of metabolic control are related to the severity of clinical manifestation and outcome, as has been described for Sars-CoV-2 infections.1–3 There is some evidence that viral infections including influenza viruses might trigger or induce autoimmunity and possibly Type 1 diabetes,4 but this issue is not the scope of this article.
Figure 1.
Modulation of viral influenza infection and systemic complication by inflammatory response (cytokine storm) and diabetes; both are associated with an increased risk for cardiovascular complications and pneumonia and influence each other vice versa like a vicious cycle; for further details, see text.
As described above, the impact of influenza on medical outcomes is mostly estimated by using different observational data and recorded clinical care or reported data of infections. Only a handful of estimates are evaluated in patients with Type 2 diabetes (T2DM). Recently, a retrospective cohort analysis used conventional claims data and linked them with patient generated health data including information from wearable sensors on sleep and physical activity from a large US health company.5 One focus of this study was to compare data in 54 656 patients with known T2DM with age- and gender-matched controls (n = 113 016). Patients with diabetes had a significantly higher number of glycaemic events, 7.4-fold higher rate of pneumonia, and 5.7-fold higher rate of sepsis during the peri-influenza period. The latter period was defined as 2 weeks before and 4 weeks after an incident influenza diagnosis, which was compared with a 6-week baseline control period. A comparison of the difference in rates between peri-influenza and baseline periods showed that there was a 2.1% gain for ischaemic heart disease in patients with T2DM, which was eight times higher than seen in controls. Impact on physical activity and sleep behaviours showed that people with diabetes had, in the 14-days pre- and post-influenza period, a shift to 30 min earlier sleep start time and an increase in restless nights and naps taken. Fewer steps or physical activity could be observed in the 4-day pre-influenza and 7-day post-influenza period compared with baseline, the lowest step count being ∼2 days after influenza diagnosis.
Clinical effects of influenza vaccination in patients with diabetes
Observational and clinical studies suggest that influenza vaccination might reduce cardiovascular events.6 The first prospective randomized trial in patients with cardiovascular disease was performed recently, 20% of them had diabetes.7 Two thousand five hundred and seventy-one patients received influenza vaccine vs. saline placebo during hospital stay shortly after myocardial infarction (MI). The relative risk for the composite primary endpoint, which consisted of all-cause death, MI, or stent thrombosis at 12 months, was reduced by 28%. In patients with diabetes, the relative risk reduction was 27% with no evidence for interaction. However, the incidence rates of events were higher in patients with diabetes compared with patients without diabetes, i.e. 11.0 vs. 3.6% in the vaccine group and 14.6 vs. 5.5% in the placebo group. These results correspond to an absolute risk reduction of even 3.6% or a number to treat of below 30 within a year in patients with diabetes. In addition, they performed a meta-analysis7 combining data from three other influenza vaccination studies8–10 in patients with cardiovascular disease and found a robust lower relative risk for cardiovascular complications by 49% associated with vaccination, although patients with or without diabetes were not analysed separately.
A nationwide register data from Denmark was used to evaluate clinical risks in patients with diabetes during nine consecutive influenza seasons from 2007 to 2016.11 A total of 241 551 patients were monitored for a median of four seasons with a vaccine coverage ranging from 24 to 36%. Vaccination was significantly associated with reduced all-cause mortality and cardiovascular death. However, MI was not reported separately. In this Danish National Registry, vaccination in patients with diabetes was also associated with reduced hospital admissions due to acute diabetes complications, like ketoacidosis, hypoglycaemia, or coma.11 Using data from health insurance, data from 61 002 patients with diabetes were analysed in Taiwan over the years 2008–13. Influenza vaccination was associated significantly with reduced 30-day in-hospital mortality, especially in patients with prior hospitalization because of diabetes, inadequate glucose control, and other diabetes-related comorbidities.12 An analysis from surveillance network (FluSurv-Net) from 2012–13 to 2016–17 in 13 US states in patients aged ≥65 years based on laboratory-confirmed influenza-associated hospitalizations reported about a pooled database of 31 934 hospitalized patients, 34% of which had diabetes.13 Patients with diabetes had significantly higher rates of influenza-associated hospitalization (1.57-fold), admission to intensive care unit (ICU; 1.84-fold) or mechanical ventilation (relative risk 1.95), 57% increased risk for pneumonia and 48% risk for in-hospital death. In an older study with 239 hospitalized patients with polymerase chain reaction–confirmed influenza A (H1N1), the odds ratio for ICU admission was 4.29 among hospitalized patients with diabetes compared with those without.14 In other words, diabetes tripled the risk of hospitalization after influenza A (H1N1) and quadrupled the risk of ICU admission once hospitalized.14 A systematic review and meta-analysis of 1444 articles, 11 observational studies with a total of 170 924 participants showed, that in patients with diabetes in working-age (18–64 years), influenza vaccination prevented all-cause hospitalization by 58% and hospitalization due to influenza or pneumonia by 43%, whereas no effects on all-cause mortality was observed in this younger population.15 In the elderly patients of 65 years and above, influenza vaccination prevented also all-cause mortality by 38%.16 A retrospective cohort study using primary and secondary care data from the Clinical Practice Research Datalink in England, over a 7-year period between 2003–04 and 2009–10 enrolled 124 503 adults with T2DM.17 Outcome measures included admission to hospital for acute MI, stroke, heart failure or pneumonia/influenza, and death. After adjustment for covariates and residual confounding, vaccination was associated with significantly lower admission rates for stroke by 30%, heart failure by 22%, and pneumonia or influenza by 15%. Clinical effectiveness of influenza vaccination in adults with diabetes was reported from the Prevention of Influenza, Surveillance, and Management study, a nested case–control study conducted during the 1999–2000 influenza A epidemic, among 75 235 patients from primary care of any age recommended for vaccination.18 Among 9238 adult patients with diabetes, vaccination was associated with a 56% reduction in any complication, a 54% reduction in hospitalizations, and 58% reduction in deaths compared with individuals without diabetes. In a population-based cohort study, individual-level data from several national registers for all Norwegian residents aged ≥30 years as of January 2009 were linked and people with or without T2DM at baseline (n = 2 992 228) were followed until December 2013.15 Pandemic influenza hospitalization was 2.46-fold higher in individuals with T2DM. The rate of hospitalization for pandemic influenza was 78% lower in those vaccinated compared with nonvaccinated among people with T2DM. Mortality was 25% lower in those vaccinated compared with nonvaccinated patients with diabetes. In a recent systematic review and meta-analysis, hospital-acquired influenza infections had a worse prognosis that nonhospital acquired and was especially susceptible in patients with metabolic disorders.19 A meta-analysis of observational studies until April 2019 for patients with diabetes showed that influenza vaccination was associated with significantly lower all-cause mortality and hospitalization for pneumonia.20
Efficacy and safety of different vaccination strategies
Responses to influenza vaccination vary between 10 and 60% in large part due to antigen drifts. ESC/EMA high-dose vaccines and quadrivalent vaccines are usually more effective and are recommended in individuals aged 65 years and older, in Germany for people 60 years and older. Although there are many possible diabetes-related factors, like glucose levels, adipokines and cytokine responses, as well as possible impairments in humoral or cell-mediated immune responses, there is no clinical relevant and robust evidence that patients with diabetes have a lower vaccination response than without diabetes.21 However, larger trials investigating stratified head-to-head comparisons between patient populations are lacking.
The antibody response to vaccination in healthy and elderly participants with diabetes were studied in two groups of elderly participants with and without diabetes (healthy N = 119 and diabetes N = 102). Immunogenicity of influenza vaccine was measured by haemagglutination inhibition assays. No difference was found between antibody responses in elderly with diabetes compared with elderly without diabetes.22 This was also seen for B-cell response. Immune response was evaluated to the seasonal influenza vaccine in young and elderly patients with T2DM. The study showed that in vivo responses, as well as B-cell-specific markers decreased by age in healthy individuals but not in patients with T2DM.23 B-cell-specific biomarkers of optimal vaccine response were measured ex vivo by switched memory B cells, plasmablasts, and in vitro by activation-induced cytidine deaminase in stimulated cells as well as markers of systemic and B-cell-intrinsic inflammation. The results suggest that valid protection against influenza can be achieved in patients with T2DM. To study persistence of immune responses in individuals with diabetes, a randomized controlled study was conducted at two university hospitals during the 2012–13 influenza season.24 The study vaccine was a standard-dose trivalent subunit inactivated intramuscular vaccine. Serum haemagglutination-inhibiting antibodies were measured at the time of vaccination and 1 and 6 months after vaccination. A total of 105 patients with diabetes and 108 controls were included. One month after vaccination, both individuals with and without diabetes showed similar immunogenicity profiles. Furthermore, there was no statistical difference between the two groups in the long-term immunogenicity profiles. In multivariate analysis, long-term immunogenicity was associated with age and pre-vaccination titre, but not of diabetes status.
Clinical trial comparing high vs. standard dose of trivalent vaccine25 showed higher relative effectiveness, immunogenicity, and seroprotection, but there was no separate analysis for the about 20% of patients with known diabetes, who participated in this study. A recent meta-analysis reviewed the relative vaccine efficacy of high-dose inactivated trivalent influenza vaccine compared with standard dose influenza vaccines in adults aged ≥65 years.26 Fifteen publications were meta-analysed after screening 1293 studies, providing data on 10 consecutive influenza seasons and over 22 million individuals received high-dose trivalent vaccine in randomized and observational settings. The relative vaccination efficacy was 15.9% higher to protect from influenza-like disease, 11.7% to prevent hospitalization because of influenza, pneumonia (27.3%), or cardiorespiratory problems (17.9%). These data show robust evidence that high dose is consistently more effective than standard dose at reducing influenza in individuals aged ≥65 years. Again, also in this meta-analysis, there were no data provided for patients with or without diabetes.
One recent study compared immunogenicity and safety of quadrivalent high-dose vaccine with a standard-dose quadrivalent influenza vaccine (IIV4-SD) in adults aged ≥60 years.27 This trial enrolled 1528 participants aged ≥60 years and was randomized 1:1 to a single injection of high or standard dose. High-dose vaccine induced a superior immune response vs. standard dose in terms of increase in geometric mean titres and seroconversion rates in participants aged 60–64 years and those aged ≥65 years for all four influenza strains. Both vaccines were well tolerated in participants ≥60 years of age, with no safety concerns identified. Accordingly, high-dose quadrivalent influenza vaccine is assumed to offer improved protection against influenza compared with standard dose in adults aged ≥60 years, as was previously assessed for adults aged ≥65 years. No data were provided for patients with or without diabetes.
Uptake of influenza vaccination over time and in diabetes
The rates of flu vaccination in general population in the USA, England, and Germany have been consistently low and estimates show that only 40–48% of adults received their influenza vaccines in the last few seasons (Table 1). In the season 2018–19, Centers for Disease Control and Prevention (CDC) reported that 62% of adults with diabetes received their flu vaccines. In UK, patients aged 6 months to <65 years in a clinical risk group remain stable (44–49%) in the last few seasons (i.e. diabetes, pregnancy, chronic kidney disease, heart failure, and chronic obstructive pulmonary disease). Similar to the USA, about two-thirds of patients with diabetes in the UK received their influenza vaccine in the last few seasons. In Germany, estimates for influenza vaccination rates show to be among the lowest. Around one-third of adults reported to receive the flu vaccine in the last few seasons. Among healthcare workers, the vaccination rates in nursing staff remain consistently low.
Table 1.
Reported rates in selected population groups in the USA, England, and Germany
| Population groups | 2016–17 (%) | 2017–18 (%) | 2018–19 (%) | 2019–20 (%) | 2020–21 (%) | 2021–22 (%) |
|---|---|---|---|---|---|---|
| CDC | ||||||
| Adults | 42 | 43 | 46 | 48.4 | 44.6 | 44.3 |
| Adults with diabetes | 61.6 | 65 | 62 | N/A | N/A | N/A |
| Public Health England | ||||||
| Patients (6 months to <65 years) with clinical riska | 45.1 | 48.6 | 48.0 | 44.9 | N/A | N/A |
| Patients with diabetes | 64 | 65 | 63 | 60 | N/A | N/A |
| Germany | ||||||
| Adults with clinical riska | 30 | 29 | 31 | 32 | N/A | N/A |
| Doctors | 60.8 | 59.4 | 76.0 | 79.3 | N/A | N/A |
| Nursing staff | 32.7 | 31.1 | 46.0 | 46.7 | N/A | N/A |
N/A, not available.
Clinical risk includes diabetes, CKD, HF, and pregnancy.28–33
Although diabetes is associated with higher risk of hospitalization, morbidity, and mortality from influenza, and despite specific recommendations for influenza vaccination among people with diabetes, more than one-third of adults with diabetes are unvaccinated.34 Influenza vaccination coverage was assessed among adults aged ≥18 years with diabetes during the 2007–08 through 2017–18 influenza seasons in the USA. Influenza vaccination coverage among adults aged ≥18 years with diabetes ranged from 62.6 to 64.8%. In the 2017–18 influenza season, coverage was significantly higher among adults with diabetes (64.8%) compared with those without diabetes (43.9%). Vaccination uptake for influenza is far beyond recommendation. While WHO recommends a target ≥75, in Germany, for example, vaccination rate over the years or seasons from 2008–09 to 2018–19 decreased from 47.9 to 35% for adults ≥60 years. For the season 2019–20, the rate was 38.8% in the aged ≥60 years and only 32.3% in the individuals ≥18 years with a specific recommendation because of risk condition.28 The rate in the group for healthcare providers in the season 2019–20 in hospitals was 79.3% for doctors and between 46.7 and 48% in non-doctoral healthcare providers (Table 1).
Influenza vaccination uptake among individuals with a wide spectrum of chronic diseases was assessed using a nationwide statutory health insurance physician outpatient claims data from the years 2009 to 2018 covering 87% of the total German population.35 Influenza vaccination coverage varied across patient populations and was below 45% in the influenza season 2017–18, which was also seen in the group of patients with diabetes. The influenza vaccine uptake among the population of patients with diabetes in Spain from 2011 to 2020 showed an overall coverage among patients with diabetes of 52.1% compared with 40.6% for matched participants without diabetes.36 The vaccine uptake among adults with diabetes was 52.6% in 2011, 54.38% in 2014, and 53.4% in 2017. Influenza vaccination coverage rates across the UK in adults aged 16–64 years in a clinical at-risk group have been consistently low over the past 5 years.33 In 2018–19, vaccination rates in all at-risk adults aged 16–64 years were 48.0% in England, 42.4% in Scotland, 44.1% in Wales, and 52.4% in Northern Ireland. Influenza vaccine coverage was lowest in patients with morbid obesity and highest in patients with diabetes in 2018–19. In patients with diabetes, vaccination in England and Wales were 63.7 and 59.4%, respectively. Therefore, coverage rates were below current national ambitions of ≥75% in all clinical risk groups. In these clinical at-risk groups, influenza vaccine coverage decreased between 2015 and 2019, and there was considerable regional variation.
Current recommendations and perspectives
The 2020–21 recommendations of the Advisory Committee on Immunization Practices regarding the use of seasonal influenza vaccines in the USA37 recommend influenza vaccination for all persons aged ≥6 months who do not have contraindications. They prioritized patients with diabetes, because patients with diabetes have a higher risk for medical complications.
The annually updated medical standards of the American Diabetes Association recommend flu vaccination with the following wording: ‘…Given the benefits of the annual influenza vaccination, it is recommended for all individuals ≥6 months of age who do not have a contraindication … patients with chronic conditions such as diabetes are cautioned against taking the live attenuated influenza vaccine and are instead recommended to receive the inactive or recombinant influenza vaccination. For individuals ≥65 years of age, there may be additional benefit from the high-dose quadrivalent inactivated influenza vaccine…’38
There is growing evidence that vaccination for influenza is associated with reduced clinical and cardiovascular complications in elderly individuals or patients with cardiovascular disease or diabetes. Study evidence from randomized prospective double-blind trials regarding clinical complications of cardiovascular disease is growing, but larger trials are needed. Particularly, these trials should investigate subgroups with diabetes. In addition, influenza season monitoring data by health authorities should depict susceptible subgroups including patients with diabetes. Considering the growing evidence of the health benefits of flu vaccination overall and in patients with diabetes, there should be a call for action in clinical medicine and respective recommendations to increase vaccination uptake in these patient groups. Therefore, one should address not only medical professionals but also patients, for example, by providing them education and the necessary information using digital platforms or mobile devices. This might offer new opportunities to reduce influenza-related burden of our health systems and improve patient outcomes and global health. With regard to the latter aspect, we were recently involved in a study addressing the question whether a digitally administered intervention strategy can increase the influenza vaccination rate in people with diabetes. In this prospective randomized controlled trial spread over 6 months, the intervention group received monthly messages through an online platform with educational content and a call to action for the participants to complete. As many as 31 404 patients with diabetes were enrolled and randomized and 64.2% of the intervention group reported vaccination after 6 months compared with 61.1% in the control group.39 The completion of one or more messages was even associated with an increase of up to 8% in the vaccination rate. These data provide direct evidence in people with diabetes that digital tools providing education and information can improve the uptake of influenza vaccine significantly, especially in digitally responsive patient populations.
Contributor Information
Marlo Verket, Department of Medicine I for Cardiology, Angiology and Intensive Care Medicine, University Hospital Aachen, Pauwelsstr. 30, Aachen 52074, Germany.
Malte Jacobsen, Department of Medicine I for Cardiology, Angiology and Intensive Care Medicine, University Hospital Aachen, Pauwelsstr. 30, Aachen 52074, Germany.
Katharina Schütt, Department of Medicine I for Cardiology, Angiology and Intensive Care Medicine, University Hospital Aachen, Pauwelsstr. 30, Aachen 52074, Germany.
Nikolaus Marx, Department of Medicine I for Cardiology, Angiology and Intensive Care Medicine, University Hospital Aachen, Pauwelsstr. 30, Aachen 52074, Germany.
Dirk Müller-Wieland, Department of Medicine I for Cardiology, Angiology and Intensive Care Medicine, University Hospital Aachen, Pauwelsstr. 30, Aachen 52074, Germany.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This paper was published as part of a supplement financially supported by Sanofi. Manuscripts were accepted after rigorous peer review process that was managed by an expert Guest Editor independently appointed by the Editor-in-Chief. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of Sanofi.
Data availability
No new data were generated or analysed in support of this research.
References
- 1.Goeijenbier M, van Sloten TT, Slaobbe L, Mathieu C, Gendereren van P, Beyer WEPet al. Benefits of flu vaccination for persons with diabetes mellitus: a review. Vaccine 2017;35:5095–5101. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya I, Ghayor C, Dominguez AP, Weber FE. From influenza virus to novel corona virus (SARS-CoV-2)—the contribution of obesity. Front Endocrinol 2020;11:556962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller-Wieland D, Marx N, Dreher M, Fritzen K, Schnell O. COVID-19 and cardiovascular comorbidities. Exp Clin Endocrinol Diabetes 2022;130:178–189. [DOI] [PubMed] [Google Scholar]
- 4.Thomas S, Ouhtit A, Khatib HHA, Eid AH, Mathew S, Nasrallah GKet al. Burden and disease pathogenesis of influenza and other respiratory viruses in diabetic patients. J Infect Public Health 2022;15:412–424. [DOI] [PubMed] [Google Scholar]
- 5.Samsoan SI, Konty K, Lee W-N, Quisel T, Foschini L, Kerr Det al. Quantifying the impact of influenza among persons with type 2 diabetes mellitus: a new approach to determine medical and physical activity impact. J Diabetes Sci Technol 2021;15:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clar C, Oseni Z, Flowers N, Keshtkar-Jahromi M, Rees K. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst Rev 2015; 2015:CD005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fröbert O, Götberg M, Erlinge D, Akhtar Z, Christansen EH, MacIntyre CRet al. Influenza vaccination after myocardial infarction. A randomized, double-blind, placebo-controlled, multicenter trial. Circulation 2021;144:1476–1484. [DOI] [PubMed] [Google Scholar]
- 8.Gurfinkel EP, Leon de la Fuente R, Mendiz O, Mautner B. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) study. Eur Heart J 2004;25:25–31. [DOI] [PubMed] [Google Scholar]
- 9.Ciszewski A, Bilinska ZT, Brydak LB, Kepka C, Kruk M, Romanowska Met al. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur Heart J 2008;29:1350–1358. [DOI] [PubMed] [Google Scholar]
- 10.Phrommintikul A, Kuanprasert S, Wongcharoen W, Kanjanavanit R, Chaiwarith R, Sukonthasarn A. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur Heart J 2011;32:1730–1735. [DOI] [PubMed] [Google Scholar]
- 11.Modin D, Claggett B, Kober L, Schou M, Jensen JUS, Solomon SCet al. Influenza vaccination is associated with reduced cardiovascular mortality in adults with diabetes. A nationwide cohort study. Diabetes Care 2020;43:2226–2233. [DOI] [PubMed] [Google Scholar]
- 12.Lin C-S, Chang C-C, Yeh C-C, Chang Y-C, Chen T-L, Liao C-C. Outcomes following diabetes admission in patients who had influenza vaccination: a population-based cohort study. Diabetes Res Clin Pract 2022; 189:109930. [DOI] [PubMed] [Google Scholar]
- 13.Owusu D, Rolfes MA, Arriola CS, Kirley PD, Alden NB, Meek Jet al. Rates of severe influenza-associated outcomes among older adults living with diabetes—influenza hospitalization surveillance network (FluSurv-NET), 2012–2017. Open Forum Infect Dis 2022; 9:ofac131. doi: 10.1093/ofid/ofac131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allard R, Leclerc P, Tremblay C, Tannenbaum T-N. Diabetes and the severity of pandemic influenza A (H1N1) infection. Diabetes Care 2010;33:1491–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiz PLD, Bakken IJ, Håberg SE, Tapia G, Hauge SH, Birkeland KIet al. Higher frequency of hospitalization but lower relative mortality for pandemic influenza in people with type 2 diabetes. J Intern Med 2020;287:78–86. [DOI] [PubMed] [Google Scholar]
- 16.Remschmidt C, Wichmann O, Harder T. Vaccines for the prevention of seasonal influenza in patients with diabetes: systematic review and meta-analysis. BMC Med 2015;13:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vamos EP, Pape UJ, Curcin V, Harris MJ, Valabhji J, Majeed Aet al. Effectiveness of the influenza vaccine in preventing admission to hospital and death in people with type 2 diabetes. CMAJ 2016;188:E342–E351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Looijmans-Van den Akker I, Verheij TJM, Buskens E, Nichol KL, Rutten GEHM, Hak E. Clinical effectiveness of first and repeat influenza vaccination in adult and elderly diabetic patients. Diabetes Care 2006;29:1771–1776. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Wang L-L, Yie L-L, Hou W-L, Liu X-Y, Yin S.. The epidemiological and clinical characteristics of hospital-acquired influenza infections. Medicine (Baltimore) 2021;100:e25142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bechini A, Ninci A, del Riccio M, Biondi I, Bianchi J, Bonanni Pet al. Impact of influenza vaccination on all-cause mortality and hospitalization for pneumonia in adults and the elderly with diabetes: a meta-analysis of observational studies. Vaccines (Basel) 2020;8:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiggins KB, Smith MA, Schultz-Cherry S. The nature of immune responses to influenza vaccination in high risk populations. Viruses 2021;13:1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McElhaney JE, Garneau H, Camous X, Dupuis G, Pawelec G, Baehl Set al. Predictors of the antibody response to influenza vaccination in older adults with type 2 diabetes. BMJ Open Diabetes Res Care 2015;3:e000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frasca D, Diaz A, Romero M, Mendez NV, Landin AM, Ryan JGet al. Young and elderly patients with type 2 diabetes have optimal B cell responses to the seasonal influenza vaccine. Vaccine 2013;31:3603–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo YB, Baek JH, Lee J, Song JS, Lee JS, Cheong HJet al. Long-term immunogenicity and safety of a conventional influenza vaccine in patients with type 2 diabetes. Clin Vaccine Immunol 2015;22:1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Tranor J, Collins A. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014;371:635–645. [DOI] [PubMed] [Google Scholar]
- 26.Lee JKH, Lam GKL, Shin T, Samsons SI, Greenberg DP, Chit A. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: an updated systematic review and meta-analysis. Vaccine 2021;39:A24–A35. [DOI] [PubMed] [Google Scholar]
- 27.Pepin S, Nicolas J-F, Szymanski H, Leroux-Roels I, Schaum T, Bonten M. Immunogenicity and safety of a quadrivalent high-dose inactivated influenza vaccine compared with a standard-dose quadrivalent influenza vaccine in healthy people aged 60 years or older: a randomized Phase III trial. Hum Vaccin Immunother 2021;17:5475–5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rieck T, Steffen A, Schmid-Küpke N, Feig M, Feig M, Wichmann Oet al. Impfquoten bei Erwachsenen in Deutschland—Aktuelles aus der KV-Impfsurveillance und der Onlinebefragung von Krankenhauspersonal OKaPII. Epid Bull 2020;47:3–26. [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC) . 2018 BRFSS survey data and documentation. https://www.cdc.gov/brfss/annual_data/annual_2018.html (3 August 2022).
- 30.Centers for Disease Control and Prevention (CDC) . https://www.cdc.gov/nchs/nhis/nhis_2018_data_release.htm (3 August 2022).
- 31.Centers for Disease Control and Prevention (CDC) . Vaccination coverage among adults with diagnosed diabetes: United States, 2015. NCHS Data Brief No. 265. 2016. https://www.cdc.gov/nchs/products/databriefs/db265.htm (3 August 2022).
- 32.Centers for Disease Control and Prevention (CDC) . Flu & people with diabetes. https://www.cdc.gov/flu/highrisk/diabetes.htm (3 August 2022).
- 33.Oakley S, Bouchet J, Costello P, Parker Jet al. Influenza vaccine uptake among at-risk adults (aged 16–64 years) in the UK: a retrospective database analysis. BMC Public Health 2021;21:1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hung M-C, Lu P-J, Srivastav A, Cheng YJ, Williams WW. Influenza vaccination coverage among adults with diabetes, United States, 2007–08 through 2017–18 seasons. Vaccine 2020;38:6545–6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akmatov MK, Holstiege J, Steffen A, Bätzing J. Utilization of influenza vaccination among chronically ill individuals in Germany: a nationwide claims-based analysis. Vaccine 2021;39:952–960. [DOI] [PubMed] [Google Scholar]
- 36.Zamorano-Leon JJ, Jimenez-Garcia R, Lopez-de-Andres A, de-Miguel-Diez J, Carabantes-Alarcon D, Albaladejo-Vicente R. Low levels of influenza vaccine uptake among the diabetic population in Spain: a time trend study from 2011 to 2020. J Clin Med 2022;11:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grohskopf LA, Alyanak E, Ferdinands JM, Broder KR, Blanton LH, Talbot HKet al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 influenza season. MMWR Recomm Rep 2021;70:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Diabetes Association, Professional Practice Committee . Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care 2022;45:S46–S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JL, Foschini L, Kumar S, Juusola J, Liska J, Mercer M. Digital intervention increases influenza vaccination rates for people with diabetes in a decentralized randomized trial. NPJ Digit Med 2021;4:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.