Abstract
COVID-19 is an independent risk factor for cardiovascular disease. COVID-19 vaccination may prevent this, but in some cases, COVID-19 vaccination may cause myocarditis or pericarditis. Patients with COVID-19 may present with non-specific symptoms that have a cardiac origin. This review examines the cardiovascular complications of COVID-19 infection and the impact of COVID-19 vaccination. COVID-19 cardiovascular complications include myocardial injury, pericarditis, coagulopathy, myocardial infarction, heart failure, arrhythmias, and persistent post-acute risk of adverse cardiovascular outcomes. Diagnostic and referral pathways for non-specific symptoms, such as dyspnoea and fatigue, remain unclear. COVID-19 vaccination is cardioprotective overall but is associated with myopericarditis in young males, though at a lower rate than following SARS-CoV-2 infection. Increased awareness among primary care physicians of potential cardiovascular causes of non-specific post-COVID-19 symptoms, including in younger adults, such as fatigue, dyspnoea, and chest pain, is essential. We recommend full vaccination with scheduled booster doses, optimal management of cardiovascular risk factors, rapid treatment of COVID-19, and clear diagnostic, referral, and management pathways for patients presenting with non-specific symptoms to rule out cardiac complications.
Keywords: COVID-19, SARS-CoV-2, Heart, Cardiovascular, Vaccines
For the audio file associated with this article, please visit the HTML version of the article online (https://academic.oup.com/eurheartjsupp/issue/25/Supplement_A)
Introduction
COVID-19 is an independent risk factor for cardiovascular disease (CVDs)1 and it may cause significant vascular pathology, cardiac injury, and acute and chronic cardiovascular complications.2 The pathophysiology of SARS-CoV-2 infection involves the binding of viral particles to the angiotensin-converting enzyme-2 (ACE2) receptor, which is expressed in endothelial cells, migratory angiogenic cells, vascular smooth muscle cells, cardiofibroblasts, cardiomyocytes, pericytes, and epicardial adipose cells, resulting in the tropism of SARS-CoV-2 to the heart and vasculature.3 There is emerging evidence that COVID-19 infections can result in both acute and long-term CVD. The risk is highest in patients treated in intensive care, but may also occur in non-hospitalized patients.4 Thus, the role of COVID-19 vaccines in preventing cardiovascular complications, including the potential long-term burden of COVID-19-related chronic CVD, is of clinical and public health importance.
There are many observational studies reporting that individuals with SARS-CoV-2 infection suffer from impairment of myocardial and cardiac function5,6 and cardiovascular complications,1,7,8 including myocarditis, pericarditis, arrhythmias, acute myocardial infarction, stroke, thromboembolism, and sudden cardiac death.9–11 The vascular pathology of SARS-CoV-2 appears to be multi-factorial and includes direct effects of the virus as well as indirect effects of cytokine release and other immune responses. The virus may induce an acute prothrombotic state including excessive coagulation, impaired fibrinolytic ability, and/or an endotheliopathy that can lead to an acute and sometimes chronic coagulopathic state.12,13 More recently, emerging reports have highlighted potential long-term cardiovascular sequelae of COVID-19, which can persist at least 12 months after the acute infection.11,14,15 However, many of these long-term reports have been based on hospitalized patients with a duration of follow-up of <12 months.16–20 Autopsy studies have shed some light on the cardiac pathology due to SARS-CoV-2, revealing a range of abnormalities, including the presence of SARS-CoV-2 virus in the heart, not only within cardiomyocytes but also in interstitial cells, pericytes, and macrophages.21
COVID-19 vaccines became available from late 2020 in some countries.22 In addition to preventing severe SARS-CoV-2 infection, COVID-19 vaccination is associated with reduced risks of cardiovascular outcomes due to a COVID-19 infection.23,24 However, COVID-19 vaccines themselves, especially messenger ribonucleic acid (mRNA) COVID-19 vaccines, have been associated with cardiac complications, such as myocarditis and pericarditis,25–27 although at a much lower rate than after COVID-19 infection.23 However, vaccine-induced immunity wanes rapidly, even after boosters.28,29 Furthermore, the emergence of new SARS-CoV-2 variants of concern, which are antigenically distant from the strain used in the vaccines, has resulted in vaccine escape and immune imprinting, reducing the protective effects of COVID-19 vaccines.30–32 The risk of reinfection has also increased with the Omicron variant of concern.33 The impact of these factors on the incidence of cardiovascular complications is unclear.11,34
This review aims to examine the potential cardiovascular complications of COVID-19 infection and the impact of COVID-19 immunization, particularly in the context of recurrent infections with the continued emergence of new SARS-CoV-2 variants of concern.
Methods
An initial search of PubMed was undertaken, followed by analyzing text words contained in the titles and abstracts and index terms used to describe identified articles. We used the following search terms and phrases: ‘SARS-CoV-2’, ‘COVID-19’, ‘cardiovascular’, ‘myocarditis’, ‘pericarditis’, ‘MI*’, ‘myocardial infarct*’, ‘cardiac injur*’, ‘Acute coronary syndrome’, ‘immunization’ ‘vaccination’, ‘mechanism’. The key search terms were used individually and in combination. A comprehensive search strategy, using all identified keywords and index terms, was undertaken and searched for the period between 17 August 2022, and 6 September 2022. A study flow diagram is shown in the Appendix, Figure A1. Our review was restricted to original studies with published results, recent systematic reviews, and those published in English. Additional papers not captured in the search were identified from the reference list of included papers. Any studies not focused predominantly on CVDs were excluded. Three independent reviewers (A.M., M.T., and Z.A.) screened all articles with a title search, from which relavant papers had an abstract review , and those selected as relevant had full text review (Figire A1). Data were extracted from selected papers and reviewed by all co-authors, including two senior cardiologists (O.F. and T.C.T.) and a senior infectious disease expert (C.R.M.).
Results
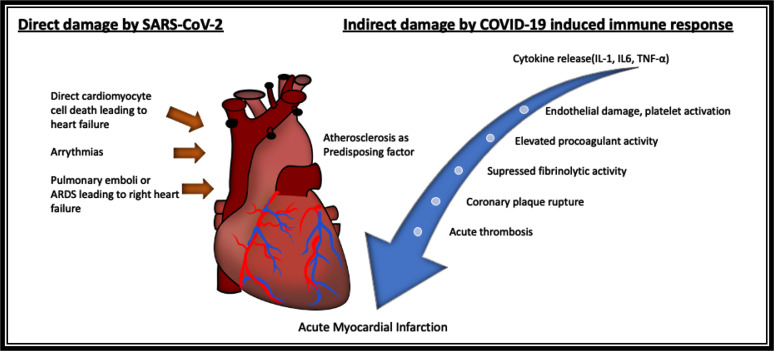
A total of 237 articles were initially identified, from which 49 studies were considered for this review. The pathology of COVID-19 identified in the review includes acute and chronic complications, summarized in Table 1. The mechanisms include acute myocardial infarction, triggered by acute thrombus formation, hypoperfusion, hypoxia, and other mechanisms; left ventricular dysfunction due to direct myocardial injury or myocardial infarction; right ventricular dysfunction due to acute respiratory distress syndrome or pulmonary embolism; diffuse endovascular disease and thromboembolism; arrhythmias; myocarditis, and pericarditis (Figure 1).
Table 1.
Acute and chronic cardiovascular complications of COVID-19
| Cardiovascular complications of COVID-19 | |
|---|---|
| Acute | Chronic |
| Myocardial injury | Left heart failure |
| Myocarditis/pericarditis | Recurrent myocarditis/pericarditis |
| Acute coronary syndrome | Acute coronary syndrome (post-infection) |
| Left heart failure | Congestive cardac failure |
| Right heart failure | Right heart failure |
| Pulmonary hypertension | Hypertension |
| Venous thromboembolism | Thromboembolism eg. pulmonary or deep venous thrombosis |
| Cerebrovascular disorder, stroke | Cerebrovascular disorder, stroke (post-infection) |
| Takotsubo syndrome | Cardiomyopathy |
| Cardiac arrhythmia | Postural orthostatic tachycardia syndrome, arrythmias |
| Sudden cardiac arrest | Sudden cardiac arrest |
Figure 1.
Overview of mechanisms by which SARS-CoV-2 can affect the heart.
Myocardial injuries due to COVID-19
Myocardial injury is common in people with severe COVID-19. It occurs in up to 38% of hospitalized patients6,35,36 and is significantly more prevalent in those with pre-existing CVD.6 Cardiac injury is attributed to the direct invasion of cardiomyocytes35 as a result of viraemia.37 In addition, inflammatory cells have been found in cardiac tissue post-mortem, supporting an immune-mediated mechanism for cardiac injury.38,39 Elevated troponin is a marker of cardiac injury, and some studies showed elevated troponin levels as a predictor of mortality.40 Elevation in troponin predicts a nearly four-fold increased risk of in-hospital mortality compared with those without myocardial injury. In a US-based observational study among 11 159 hospitalized patients with COVID-19, individuals with mildly elevated troponins had greater odds of mortality [adjusted odds ratio, 2.06; confidence interval (CI): 1.68–2.53; P < 0.001] compared with those with normal values.41 A sustained increase in the risk of heart failure has been documented at least 12 months after COVID-19.11 One study of persistent symptoms (including exertional dyspnoea) following recovery from mild COVID-19 infection showed that despite the absence of elevated troponin, diffuse myocardial oedema was seen on cardiac magnetic resonance (CMR) imaging.42
Myocarditis and pericarditis
Myocarditis, defined as inflammation of the myocardium,43 may be caused by the direct invasion of cardiomyocytes by SARS-CoV-235 and an inflammatory response, confirmed by the presence of inflammatory cells in cardiac tissue found in postmortem autopsies.38,39 The fact that myocarditis occurs after vaccination, in the absence of live viruses, also points to an immune-mediated phenomenon and molecular mimicry between the spike protein (present in infection and after vaccination) and auto-antigens in genetically predisposed persons, confirmed by the discovery of autoantibodies in some patients.44 Autopsy-based studies have shown that pericarditis is more common than myocarditis,45 with pericardial effusion and cardiac tamponade being potential additional complications.46 The incidence of myocarditis and pericarditis appears to be highest in teenagers and young adult males, with a higher risk documented following COVID-19 infection (50–180 per 100 000) when compared with COVID-19 vaccination (2–8 per 100 000).23 The male predominance is unexplained but may relate to testosterone.44 Autopsy studies of patients who died of COVID-19 confirm that fulminant myocarditis as a complication of COVID-19 has a reported incidence of 1.4%.47
COVID-19-associated coagulopathy
COVID-induced coagulopathy and endothelial dysfunction are thought to play a greater role than direct viral myocarditis in causing myocardial injury. In addition to thrombosis in larger vessels such as the coronary arteries, microthrombi play an important role in cardiovascular pathology. Post-mortem studies show that fibrin microthrombi are more common than acute ischaemic injury or myocarditis, suggesting that micro-thrombosis has a role in the cardiovascular pathology of COVID-19.21 Cytokine release, especially interleukins: IL-1, IL-16, IL-17, and IL-22, interferon-γ, and tumour necrosis factor-α, may also have a role in endothelial disruption, platelet activation, and coagulopathy.21 The mechanism behind COVID-19-associated coagulopathy is not well understood, but it is hypothesized to be caused by dysregulation of several pathways, including hyperactive coagulation pathways and inflammatory pathways, leading to widespread endothelial damage and potentially multi-organ failure.48,49 The pathogenesis of COVID-19 vasculopathy has been hypothesized to be mediated by SARS-CoV-2-induced dysfunction of the vascular endothelium.50–52 This mechanism is accompanied by hyperinflammation due to viral infection resulting in a hypercoagulable state.48 Multiple studies have documented that elevated procoagulant activity and suppressed fibrinolytic activity in patients are the driving factors for COVID-19-associated coagulopathy.53–55
Myocardial infarction
COVID-19 is an independent risk factor for ischaemic stroke and acute myocardial infarction, both during and after acute infection.1 A matched cohort study showed a three- to eight-fold increase in the risk of acute myocardial infarction and three- to seven-fold increased risk of ischaemic stroke following COVID-19 among hospitalized adult patients.1 The cardiovascular mortality associated with COVID-19 ranged from 6.756 to 73%57 among hospitalized patients with CVD admitted with COVID-19 infection. One study showed that recent SARS-CoV-2 infection might trigger ST-elevation myocardial infarction (STEMI) in patients with fewer traditional cardiovascular risk factors than uninfected patients with STEMI.58 The mechanism is likely similar to that seen in influenza and multi-factorial,59 involving hypoxia, tachycardia, pre-existing coronary artery disease, and prothrombotic cytokine release. One key difference between COVID-19 and influenza is that COVID-19 is associated with more prolonged endothelial dysfunction and coagulopathy,48 which may account for an elevated risk of ischaemic vascular events up to 12 months after the acute infection.11 SARS-CoV-2 is also more directly damaging to the heart than influenza (which acts largely through cytokine effects) and, therefore, may result in greater direct cardiac effects. A large registry-based study of patients with ST-elevation myocardial infarcts (STEMI) found that male patients with COVID-19 had impaired reperfusion and a higher risk of in-hospital mortality than patients without COVID-19.60 Case studies also describe young adults in their 20s with myocardial infarction associated with mild COVID-19.61 Spontaneous coronary artery dissection after COVID-19 has also been described,62 as has myocardial infarction as a complication of the multi-system inflammatory syndrome in children.63
Long-term cardiovascular effects of COVID-19
SARS-CoV-2 infection may cause persistent cardiovascular symptoms following recovery. A German prospective, single-centre cohort study of 346 individuals with prior COVID-19 infection reported that 53% had persistent cardiac symptoms after a mean follow-up period of 329 days post-COVID-19 infection, with 5% reporting new symptoms.42 This study also found diffuse myocardial oedema on CMR imaging, in the absence of elevated troponin, in a cohort with mild COVID-19 infections but persistent symptoms including exertional dyspnoea following recovery from the acute infection.64 A US study involving 153 760 patients found an increased risk of any cardiovascular outcome [hazard ratio (HR) = 1.63, CI: 1.59–1.68] and an incidence rate of 45.29 (CI: 42.22, 48.45) per 1000 persons 12 months after the initial infection.11 Furthermore, after 12 months, there remained an increased risk (HR = 1.55, CI: 1.50–1.60), with 23.48 (CI: 21.54, 25.48) per 1000 persons experiencing a major adverse cardiovascular event.65 Another study from the UK found an increase in cardiovascular diagnoses, including a six-fold increase in atrial arrhythmias and a five-fold increase in venous thromboses, after COVID-19.14 However, in that study, cardiovascular risk returned to baseline levels within 1 year after the infection.14 The persistent risk of cardiovascular events has been postulated to be due to several factors, including persistent virus in the body,64 myocardial injury, or on-going immunological and inflammatory effects that continue to affect the vasculature.
Cardiovascular effects of COVID-19 vaccination
Myocarditis and pericarditis are known complications of mRNA vaccines, especially in young adult and teen males aged 12–17 years,23 with the highest observed incidence within 2–7 days after the second dose at a rate of 3.5–140 per million doses.27,65 New evidence suggests a role of endogenous autoantibodies against interleukin-1 receptor antagonist, IL-1RA and hyperphosphorylated IL-1RA in triggering myocarditis in young male adults.66 The incidence of myocarditis is rare, and the rate of myocarditis is much higher following COVID-19 infection than following vaccination.23 Even in the group at highest risk, males aged 12–17 years, the risk of myocarditis was 1.8–5.6 times as high after SARS-CoV-2 infection than after vaccination.23 In a small study of 63 patients (92% male) with myocarditis following mRNA-based COVID-19 vaccines, 4 developed significant dysrhythmia; 14% had mild left ventricular dysfunction which resolved at discharge; 88% met the diagnostic criteria for myocarditis, and 86% had resolution of symptoms, arryhthmias, and cardiac dysfunction by 35 days post-vaccination.67 Another study of 139 adolescents with suspected myocarditis in the US showed a similar pattern of complications, with a resolution of symptoms and imaging abnormalities during follow-up.68 Cardiac magnetic resonance imaging showed overall mild myocarditis among 15 participants between 3 and 130 days of COVID-19 vaccination.69 In patients who develop pericarditis, pericardial effusion and cardiac tamponade may be further complications.70 A population-based study analyzing electronic health record data from 40 US health care systems for 1 year between 2021 and 2022 found that the risk of cardiac complications was significantly higher after SARS-CoV-2 infection (among patients not vaccinated in the 30 days prior to infection) after mRNA COVID-19 vaccination for both males and females of all age groups. In those aged 12–17, the incidence of myocarditis or pericarditis after SARS-CoV-2 infection (among those not vaccinated in the ≤30 days before a positive SARS-CoV-2 test result) was 1.8–5.6 times higher than post-vaccination.23 Myocarditis was estimated at 1–10 per million persons in the month following vaccination, which was substantially lower than observed post-SARS-CoV-2 infection.10 Cardiovascular events following vaccination are rare and should be considered alongside the overall benefits of COVID-19 vaccination.23,71
Cardioprotective effects of COVID-19 vaccines
Large-scale studies have evaluated the overall cardiovascular risk-benefit ratio between SARS-CoV-2 infection and COVID-19 vaccination. A medical record review of safety surveillance data from the US, including 10 162 227 vaccine-eligible individuals, showed a lower risk of cardiac outcomes after mRNA COVID-19 vaccines than following SARS-CoV-2 infection. The incidence of myocarditis or pericarditis per 1 000 000 person-years was 132 or. 83, with an adjusted rate ratio of 1.39 (95% CI: 1.05–1.82) among unvaccinated compared with the vaccinated cohorts for all ages across similar individuals.72 A similarly elevated risk of post-infection myocarditis was found in a population-based study in Israel, with a post-infection risk ratio of 18.28 (95% CI: 3.95–25.12) among unvaccinated persons; compared with a significantly lower risk ratio of 3.24 (95% CI: 1.55–12.44) for vaccine-induced myocarditis.25
In the UK, a whole-population cohort study analyzed electronic health records from December 2020 to March 2021 and found a lower risk of myocardial infarction >28 days post-vaccination with BNT162b2 (Pfizer BioNTech) among persons below 70 years of age [adjusted HR (aHR) 0.88; 95% CI: 0.80–0.97] and above 70 years of age (aHR 0.75; 95% CI: 0.70–0.80) compared with unvaccinated individuals.73 A lower risk of myocardial infarction was also found following ChAdOx1-S (Aztrazeneca) vaccination among persons below 70 years of age (aHR 0.83; 95% CI: 0.73–0.93) and above 70 years of age (aHR 0.74; 95% CI: 0.68–0.81) compared with unvaccinated individuals.73 Another population-based study from the Korean nationwide COVID-19 registry on infection and vaccination and the Korean National Health Insurance Service database showed that complete vaccination (two doses, 28 days apart) against COVID-19 was associated with a 52% reduced risk of myocardial infarction (aHR 0.48; 95% CI: 0.25–0.94) and ischaemic stroke (aHR 0.40; 95% CI: 0.26–0.63) after COVID-19.24
Discussion
SARS-CoV-2 can result in several cardiovascular events, both during acute infection and as a late complication. Excess cardiovascular deaths during the pandemic are already being seen in many countries.74 In 2020, this observation was thought to be due to decreased access to care for patients with CVD,75 but it is now apparent that deaths attributed to cardiovascular causes during the pandemic likely have COVID-19 as a contributing factor. The cumulative evidence identified in this review suggests that the COVID-19 pandemic has resulted in a substantial excess acute burden of CVD. There may also be an excess chronic burden of disease that impacts populations and health systems, even if long-term cardiovascular effects only occur in a small proportion of patients. With more than 610 million people affected globally76 and no end in sight to the pandemic, this burden of CVD will likely be felt in every nation’s health system and economy for many years.
There are diagnostic and treatment implications for cardiovascular complications during and following COVID-19. Many patients suffering non-specific, on-going symptoms during or after COVID-19, such as fatigue or exertional dyspnoea, are left in diagnostic limbo, as primary care doctors are not provided with clear triage or management pathways. The evidence suggests that non-specific symptoms such as fatigue, dyspnoea, or palpitations should be investigated.
‘Long COVID’ was a name given to persisting symptoms early in the pandemic, but these symptoms may reflect heterogeneous pathology, ranging from pulmonary, cardiac, and neurocognitive to immunological effects. Better diagnostic decision support is required for doctors dealing with ‘Long COVID’ to know which tests are appropriate for which symptoms, which would then guide referral pathways. Routine tests, such as chest X-rays, may not be helpful; lung and cardiac function tests, as well as CMR imaging, may be required to detect more subtle abnormalities. We recommend the development of clinical and diagnostic decision support pathways for patients suffering post-acute symptoms, so that appropriate tests and referrals can be done.
An electrocardiogram and cardiac biomarkers may be appropriate initial tests, and depending on symptoms and other findings, clinicians can consider an echocardiogram or CMR imaging. If these are normal, it may indicate respiratory or other causes of persistent symptoms, which should be appropriately investigated.
For adult patients with COVID-19, especially adults with known cardiac risk factors, guideline-directed optimal medical management of primary and secondary prevention of coronary artery disease or cardiovascular risk factors are key to management. Regarding COVID-19-related cardiac injury, there are no novel treatments as yet. New research has shown a marked reduction of antithrombin protein (ADAMTS13) may have a role in myocardial injury during COVID-19 infections77 and replenishing this protein may be a future innovative therapeutic approach.
Influenza is also associated with adverse cardiovascular outcomes, which can be prevented by vaccination.78 Co-infection with influenza is documented,79–82 and there is evidence that co-infection with influenza A results in greater COVID-19 disease severity83 Influenza should be prevented by ensuring patients who meet age- or risk-based indications are vaccinated annually against influenza. There is also evidence that pneumococcal infection and herpes zoster can also trigger cardiovascular events,84,85 so vaccination against these infections is also indicated.
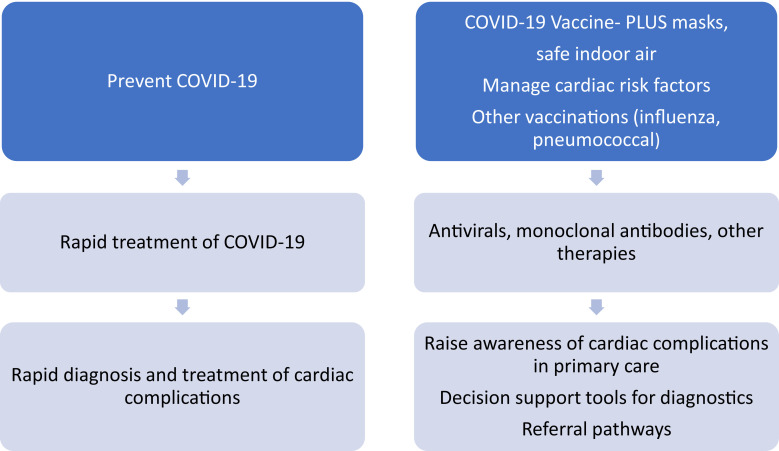
With new variants of concern, such as Omicron B.A5 and XBB, reinfection is more common.33 Studies suggest that reinfection may result in worse outcomes, including cardiovascular outcomes.86 Preventing COVID-19 is, therefore, an important strategy for patients with cardiovascular risk factors. Primary COVID-19 prevention requires vaccination, including periodic boosters but this alone may not be enough given the waning29 and substantial vaccine escape of new variants.31,32 A ‘vaccine-plus strategy’87 of vaccines and layered mitigating measures, such as masks, safe indoor air, and tailoring COVID-19 control measures based on the level of community transmission, will provide better prevention. Rapid treatment with COVID-19 antivirals or other therapies may also result in faster viral clearance88 and a lower risk of cardiovascular events, but studies are needed to confirm this. In addition, clear diagnostic and management pathways for cardiac complications are warranted to guide primary care physicians. We recommend a hierarchy of controls to reduce the impact of COVID-19-related cardiac pathology in people with cardiac risk factors (Figure 2). This includes prevention of COVID-19 and other preventable infections, management of cardiac risk factors, rapid diagnosis and treatment of infection, raising awareness of common cardiac complications, and clear decision support tools, diagnostic, and referral pathways for patients who present with potential cardiac complications.
Figure 2.
Approaches to the prevention and management of cardiovascular disease complications.
Supplementary Material
Appendix
Figure A1 A study flow diagram for article selection in the review.
Contributor Information
Zubair Akhtar, Biosecurity Program, The Kirby Institute, University of New South Wales (UNSW), 2052, Sydney Australia; Programme on Emerging Infections, Infectious Diseases Division, icddr,b, Dhaka, Bangladesh 1212.
Mallory Trent, Biosecurity Program, The Kirby Institute, University of New South Wales (UNSW), 2052, Sydney Australia.
Aye Moa, Biosecurity Program, The Kirby Institute, University of New South Wales (UNSW), 2052, Sydney Australia.
Timothy C Tan, Department of Cardiology, Blacktown Hospital, University of Western Sydney, 2148, Blacktown, NSW, Australia; School of Medical Sciences, Faculty of Medicine, University of New South Wales, 2052, Sydney, NSW, Australia; Department of Cardiology, Westmead Hospital, Sydney University, 2145, Westmead, NSW, Australia.
Ole Fröbert, Department of Cardiology, Faculty of Medicine and Health, Örebro University, Örebro 8200, Sweden; Department of Clinical Medicine, Faculty of Health, Aarhus University, Aarhus, Denmark; Department of Clinical Pharmacology, Aarhus University Hospital, Arhus, Denmark; Steno Diabetes Center Aarhus, Aarhus University Hospital, Arhus, Denmark.
C Raina MacIntyre, Biosecurity Program, The Kirby Institute, University of New South Wales (UNSW), 2052, Sydney Australia.
Supplementary material
Supplementary material is available at European Heart Journal Supplements online.
Funding
This paper was published as part of a supplement financially supported by Sanofi. Manuscripts were accepted after rigorous peer review process that was managed by an expert Guest Editor independently appointed by the Editor-in-Chief. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of Sanofi.
Data availability
All papers and data used in this review are available, with some being open access papers but others requiring paid access. Abstracts are available for all studies reviewed.
References
- 1.Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet 2021;398:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Task Force for the management of COVID-19 of the European Society of Cardiology . European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 1—epidemiology, pathophysiology, and diagnosis. Eur Heart J 2021;43:1033–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner ATet al. . Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 2020;126:1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022;28:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang Fet al. . Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo T, Fan Y, Chen M, Wu X, Zhang L, He Tet al. . Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y, Bowe B, Maddukuri G, Al-Aly Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: cohort study. BMJ 2020;371:m4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawatet TSet al. . Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017–1032. [DOI] [PubMed] [Google Scholar]
- 9.Boehmer TK, Kompaniyets L, Lavery AM, Hsu J, Ko JY, Yusuf Het al. . Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep 2021;70:1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hariet Met al. . Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med 2022;28:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022;28:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini Eet al. . Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost 2021;19:2546–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pretorius E, Vlok M, Venter C, Bezuidenhout JA, Laubscher GJ, Steenkamp Jet al. . Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol 2021;20:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezel-Potts E, Douiri A, Sun X, Chowienczyk PJ, Shah AM, Gulliford MC. Cardiometabolic outcomes up to 12 months after COVID-19 infection. A matched cohort study in the UK. PLoS Med 2022;19:e1004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021;594:259–264. [DOI] [PubMed] [Google Scholar]
- 16.Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond Iet al. . Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ 2021;372:n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C, Huang L, Wang Y, Li X, Ren L, Gu Xet al. . 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021;397:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA 2020;324:603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daugherty SE, Guo Y, Heath K, Dasmariñas MC, Jubilo KG, Samranvedhya Jet al. . Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 2021;373:n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akhtar Z, Chowdhury F, Aleem MA, Ghosh PK, Rahman M, Rahman Met al. . Undiagnosed SARS-CoV-2 infection and outcome in patients with acute MI and no COVID-19 symptoms. Open Heart 2021;8:e001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raman B, Bluemke DA, Lüscher TF, Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J 2022;43:1157–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.BIO S . Status of COVID-19 vaccines within WHO EUL/PQ evaluation process. Guidance Document 2021.
- 23.Block JP, Boehmer TK, Forrest CB, Carton TW, Lee GM, Ajani UAet al. . Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YE, Huh K, Park YJ, Peck KR, Jung J. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA 2022; 328:887-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana Ret al. . Safety of the BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl J Med 2021;385:1078–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Yet al. . Myocarditis after covid-19 vaccination in a large health care organization. N Engl J Med 2021;385:2132–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KRet al. . Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA 2022;327:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magen O, Waxman JG, Makov-Assif M, Vered R, Dicker D, Hernán MAet al. . Fourth dose of BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med 2022;386:1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit Set al. . Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N Engl J Med 2021;385:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds CJ, Pade C, Gibbons JM, Otter AD, Lin K-M, Muñoz Sandoval Det al. . Immune boosting by B. 1.1. 529 (Omicron) depends on previous SARS-CoV-2 exposure. Science 2022;377:eabq1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wibmer CK, Ayres F, Hermanus T, Madzivhandila M, Kgagudi P, Oosthuysen Bet al. . SARS-CoV-2 501Y. V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med 2021;27:622–625. [DOI] [PubMed] [Google Scholar]
- 32.Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol 2021;21:626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruff J, Zhang Y, Kappel M, Rathi S, Watkins K, Zhang Let al. . Rapid increase in suspected SARS-CoV-2 reinfections, Clark County, Nevada, USA, December 2021. Emerg Infect Dis 2022;28:1977-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai M, Bowe B, Xie Y, Al-Aly Z. Temporal trends of COVID-19 mortality and hospitalisation rates: an observational cohort study from the US Department of Veterans Affairs. BMJ Open 2021;11:e047369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanson PJ, Liu-Fei F, Ng C, Minato TA, Lai C, Hossain ARet al. . Characterization of COVID-19-associated cardiac injury: evidence for a multifactorial disease in an autopsy cohort. Lab Invest 2022;102:814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capone F, Cipriani A, Molinari L, Poretto A, Sella N, Boscolo Aet al. . Cardiac injury and COVID-19 associated coagulopathy in patients with acute SARS-CoV-2 pneumonia: a rotational thromboelastometry study. Adv Med Sci 2022;67:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myhre PL, Prebensen C, Jonassen CM, Berdal JE, Omland T. SARS-CoV-2 viremia is associated with inflammatory, but not cardiovascular biomarkers, in patients hospitalized for COVID-19. J Am Heart Assoc 2021;10:e019756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox SE, Falgout L, Vander Heide RS. COVID-19 myocarditis: quantitative analysis of the inflammatory infiltrate and a proposed mechanism. Cardiovasc Pathol 2021;54:107361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellegrini D, Kawakami R, Guagliumi G, Sakamoto A, Kawai K, Gianatti Aet al. . Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation 2021;143:1031–1042. [DOI] [PubMed] [Google Scholar]
- 40.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter Fet al. . Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020; 76: 533-546. [DOI] [PMC free article] [PubMed]
- 41.Majure DT, Gruberg L, Saba SG, Kvasnovsky C, Hirsch JS, Jauhar Ret al. . Usefulness of elevated troponin to predict death in patients with COVID-19 and myocardial injury. Am J Cardiol 2021;138:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puntmann VO, Martin S, Shchendrygina A, Hoffmann J, Ka MM, Giokoglu Eet al. . Long-term cardiac pathology in individuals with mild initial COVID-19 illness. Nat Med 2022;28:2117-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol 1987;18:619–624. [DOI] [PubMed] [Google Scholar]
- 44.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basso C, Leone O, Rizzo S, De Gaspari M, Van Der Wal AC, Aubry MCet al. . Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J 2020;41:3827–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdelmottaleb W, Salmon JT, Rodriguez BSQ, Portillo I, Mushiyev S. COVID-19 myopericarditis with pericardial effusion complicated with cardiac tamponade and rhabdomyolysis. Cureus 2022;14:e27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol 2021;50:107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conway EM, Mackman N, Warren RQ, Wolberg AS, Mosnier LO, Campbell RAet al. . Understanding COVID-19-associated coagulopathy. Nat Rev Immunol 2022; 10:639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicosia RF, Ligresti G, Caporarello N, Akilesh S, Ribatti D. COVID-19 vasculopathy: mounting evidence for an indirect mechanism of endothelial injury. Am J Pathol 2021;191:1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teuwen L-A, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol 2020;20:389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans PC, Rainger GE, Mason JC, Guzik TJ, Osto E, Stamataki Zet al. . Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res 2020;116:2177–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagashima S, Mendes MC, Camargo Martins AP, Borges NH, Godoy TM, Miggiolaro AFRd.Set al. . Endothelial dysfunction and thrombosis in patients with COVID-19—brief report. Arterioscler Thromb Vasc Biol 2020;40:2404–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bouck EG, Denorme F, Holle LA, Middelton EA, Blair AM, De Laat Bet al. . COVID-19 and sepsis are associated with different abnormalities in plasma procoagulant and fibrinolytic activity. Arterioscler Thromb Vasc Biol 2021;41:401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nougier C, Benoit R, Simon M, Desmurs-Clavel H, Marcotte G, Argaud Let al. . Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J Thromb Haemost 2020;18:2215–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blasi A, von Meijenfeldt FA, Adelmeijer J, Calvo A, Ibañez C, Perdomo Jet al. . In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation. J Thromb Haemost 2020;18:2646–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Behrendt CA, Seiffert M, Gerloff C, L'Hoest H, Acar L, Thomalla G. How does SARS-CoV-2 infection affect survival of emergency cardiovascular patients? A cohort study from a German insurance claims database. Eur J Vasc Endovasc Surg 2021;62:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chotalia M, Ali M, Alderman JE, Patel JM, Parekh D, Bangash MN.. Cardiovascular subphenotypes in patients with COVID-19 pneumonitis whose lungs are mechanically ventilated: a single-centre retrospective observational study. Anaesthesia 2022;77:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Díaz-Domínguez KA, Cruz-Melendez A, Amezcua-Castillo LM, Guerra-López JA, Tavera-Alonso C, González-Pacheco Het al. . Should a recent SARS-CoV-2 infection be considered a risk or prognostic factor for ST-segment elevation myocardial infarction? Arch Cardiol Mex 2022. Sep 8. doi: 10.24875/ACM.22000153. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 59.MacIntyre CR, Mahimbo A, Moa AM, Barnes M. Influenza vaccine as a coronary intervention for prevention of myocardial infarction. Heart 2016;102:1953–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Luca G, Silverio A, Verdoia M, Siudak Z, Tokarek T, Kite TAet al. . Angiographic and clinical outcome of SARS-CoV-2 positive patients with ST-segment elevation myocardial infarction undergoing primary angioplasty: a collaborative, individual patient data meta-analysis of six registry-based studies. Eur J Intern Med 2022;105:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolsky RM, Bateman CT. A myocardial infarction following a mild case of COVID-19 in a 26-year-old male. Cureus 2022;14:e27026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pettinato AM, Ladha FA, Zeman J, Ingrassia JJ. Spontaneous coronary artery dissection following SARS-CoV-2-associated multisystem inflammatory syndrome. Cureus 2022;14:e26479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merlocco A, Philip R, Beasley G, Ryan K, Shah S, Sathanandam Set al. . Myocardial infarction at mid-term follow-up in a teenager with multisystem inflammatory syndrome in children after COVID-19. Circ Cardiovasc Imaging 2022;15:e014010. [DOI] [PubMed] [Google Scholar]
- 64.Stein SR, Ramelli SC, Grazioli A, Chung JY, Singh M, Yinda CKet al. . SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022;612:758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fiolet T, Kherabi Y, MacDonald C-J, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect 2021;28:202-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thurner L, Kessel C, Fadle N, Regitz E, Seidel F, Kindermann Iet al. . IL-1RA antibodies in myocarditis after SARS-CoV-2 vaccination. N Engl J Med 2022; 387:1524-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jain SS, Steele JM, Fonseca B, Huang S, Shah S, Maskatia SAet al. . COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics 2021;148:e2021053427. [DOI] [PubMed] [Google Scholar]
- 68.Truong DT, Dionne A, Muniz JC, McHugh KE, Portman MA, Lambert LMet al. . Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults: suspected myocarditis after COVID-19 vaccination. Circulation 2022;145:345–356. [DOI] [PubMed] [Google Scholar]
- 69.Shiyovich A, Witberg G, Aviv Y, Eisen A, Orvin K, Wiessman Met al. . Myocarditis following COVID-19 vaccination: magnetic resonance imaging study. Eur Heart J Cardiovasc Imaging 2022;23:1075–1082. [DOI] [PubMed] [Google Scholar]
- 70.Patel K, Dalal S, Tran D, Patel A, McMackin C. COVID-19 mRNA vaccine induced pericarditis with large pericardial effusion followed by new-onset arrhythmia. Cureus 2022;14:e24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pillay J, Gaudet L, Wingert A, Bialy L, Mackie AS, Paterson DIet al. . Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: living evidence syntheses and review. BMJ 2022;378:e069445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KEet al. . Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 2021;326:1390–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whiteley WN, Ip S, Cooper JA, Bolton T, Keene S, Walker Vet al. . Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England. PLoS Med 2022;19:e1003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Digital Actuaries . Excess mortality continues for March 2022, albeit not as high as earlier in 2022. https://www.actuaries.digital/2022/06/30/excess-mortality-continues-for-march-2022-albeit-not-as-high-as-earlier-in-2022/ (20 September 2022).
- 75.Raisi-Estabragh Z, Mamas MA. Cardiovascular health care implications of the COVID-19 pandemic. Cardiol Clin 2022;40:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.World Health Organization , WHO coronavirus (COVID-19) dashboard [Internet]. https://covid19.who.int (14 September 2022).
- 77.Roh JD, Kitchen RR, Guseh JS, McNeill JN, Aid M, Martinot AJet al. . Plasma proteomics of COVID-19-associated cardiovascular complications: implications for pathophysiology and therapeutics. JACC Basic Transl Sci 2022;7:425–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fröbert O, Götberg M, Erlinge D, Akhtar Z, Christiansen EH, MacIntyre CRet al. . Influenza vaccination after myocardial infarction: a randomized, double-blind, placebo-controlled, multicenter trial. Circulation 2021;144:1476–1484. [DOI] [PubMed] [Google Scholar]
- 79.Wu X, Cai Y, Huang X, Yu X, Zhao L, Wang Fet al. . Co-infection with SARS-CoV-2 and influenza A virus in patient with pneumonia, China. Emerg Infect Dis 2020;26:1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dadashi M, Khaleghnejad S, Abedi Elkhichi P, Goudarzi M, Goudarzi H, Taghavi Aet al. . COVID-19 and influenza co-infection: a systematic review and meta-analysis. Front Med (Lausanne) 2021;8:971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akhtar Z, Islam MA, Aleem MA, Mah-E-Muneer S, Ahmmed MK, Ghosh PKet al. . SARS-CoV-2 and influenza virus coinfection among patients with severe acute respiratory infection during the first wave of COVID-19 pandemic in Bangladesh: a hospital-based descriptive study. BMJ Open 2021;11:e053768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swets MC, Russell CD, Harrison EM, Docherty AB, Lone N, Girvan Met al. . SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet 2022;399:1463–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bai L, Zhao Y, Dong J, Liang S, Guo M, Liu Xet al. . Coinfection with influenza A virus enhances SARS-CoV-2 infectivity. Cell Res 2021;31:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jaiswal V, Ang SP, Lnu K, Ishak A, Pokhrel NB, Chia JEet al. . Effect of pneumococcal vaccine on mortality and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Med 2022;11:3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim J, Jeon J, Lee HS, Lee K-Y. Association between the risk for cardiovascular events and antiviral treatment for herpes zoster. Clin Infect Dis 2021;73:758–764. [DOI] [PubMed] [Google Scholar]
- 86.Al-Aly Z, Bowe B, Xie Y. Long Covid after breakthrough SARS-CoV-2 infection. Nat Med. 2022 Jul;28(7):1461-1467. [DOI] [PMC free article] [PubMed]
- 87.Greenhalgh T, Griffin S, Gurdasani D, Hamdy A, Katzourakis A, McKee Met al. . Covid-19: an urgent call for global “vaccines-plus” action. BMJ 2022;376:o1. [DOI] [PubMed] [Google Scholar]
- 88.Fischer WA II, Eron JJ Jr, Holman W, Cohen MS, Fang L, Szewczyk LJet al. . A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med 2022;14:eabl7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All papers and data used in this review are available, with some being open access papers but others requiring paid access. Abstracts are available for all studies reviewed.