Abstract
Despite their potential to prevent or delay the onset and progression of cardiovascular disease (CVD), medicines for CVD remain unavailable and unaffordable to many in low- and middle-income countries (LMICs). We systematically reviewed the literature to identify factors associated with availability and affordability of CVD medicines in LMICs. A protocol for this study was registered on the PROSPERO register of systematic reviews (CRD42019135393). We searched Medline, EMBASE, Global Health, Cumulative Index to Nursing and Allied Health Literature, EconLit, Social Policy and Practice, and Africa Wide Information for studies analyzing factors associated with the presence of medicines (availability) or the price of these medicines as it relates to ability to pay (affordability) in LMICs. We performed a narrative synthesis of the results using an access to medicines framework that examines influences at different levels of the health system. We did not conduct a meta-analysis because of the differences in analytic approaches and outcome measures in different studies. The search was conducted in accordance with PRISMA guidelines. Of 43 studies meeting inclusion criteria, 41 were cross-sectional. Availability and affordability were defined and measured in different ways. A range of factors such as sociodemographic characteristics, facility tier, presence of medicines on national essential medicine lists, and international subsidy programs were examined. The studies had variable quality and findings were often inconsistent. We find gaps in the literature on factors associated with availability and affordability of CVD medicines, particularly at the health program level. We conclude that there is a need for experimental and quasi-experimental studies that could identify causal factors and effective responses. Such studies would help further our understanding of how complex multifactorial influences impact these outcomes, which could inform policy decisions. Along with this, greater standardization of definitions and measurement approaches of availability and affordability are needed to allow for more effective comparisons.
Introduction
Control of blood pressure and lipid levels by pharmacotherapy is a core element of primary and secondary prevention of cardiovascular disease (CVD). Antihypertensive drugs are known to reduce the incidence of cardiac and cerebrovascular diseases [1], as are statins [2] and antiplatelet agents [3]. Despite the known efficacy and cost-effectiveness of these medicines [4], their use remains far from optimal, especially in low- and middle-income countries (LMICs), where 80% percent of CVD-related mortality occurs [5]. The World Health Organization (WHO) has advocated for a target goal of 50% of eligible people receiving medicines for CVD [6]. Yet, in LMICs, fewer than 30% with hypertension receive treatment and less than 8% achieve blood pressure control [7]. In one study of only low-income countries, fewer than 10% of those with a history of coronary heart disease or ischemic stroke received treatment with antiplatelet drugs or lipid-lowering agents, contrary to international guidelines [8].
One reason for this treatment gap is poor access to medicines. Access has multiple dimensions but two that are commonly explored are availability and affordability. A recommended definition of availability compares the quantity of a medicine required in relation to its presence at health facilities. Affordability is captured by a medicine’s price relative to an individual or household’s ability to pay [9]. Research in LMICs shows that the availability and affordability of CVD drugs are associated with greater odds of patients using them and with a lower risk of adverse cardiovascular outcomes [10].
Although the WHO recommends at least 80% medicine availability for CVD [6], this target is not being met in LMICs. Many patients with hypertension require combination therapy but only 13% of communities in low-income countries live in areas where all four main classes of antihypertensives are available and only 30% of households can afford them [11]. While many studies describe the scale of the problem, fewer seek to explain the multitude of factors involved and their interrelationships. Our objective is to systematically review the literature to identify such factors in LMIC to inform appropriate policy responses and future research.
Materials and methods
We conducted a systematic review of the literature on factors associated with availability and affordability of medicines for CVD in LMICs. The protocol for this review was registered and published on the PROSPERO register of systematic reviews (CRD42019135393). Findings are reported according to the PRISMA guidelines.
Inclusion and exclusion criteria
We included published studies in any language that reported original data and analyzed interventions or factors associated with availability and/or affordability of CVD medicines in countries defined as low- or middle-income according to the 2021 World Bank classification. We included quantitative, qualitative, or mixed-method comparative studies, using experimental, quasi-experimental, or observational designs. We included any measure of availability providing information on the physical presence of medicines at health facilities or home. For affordability, we included studies that considered the price of a medicine incurred by an individual or household in relation to their ability to pay [12]. Studies that only provided information on the price of medicines were excluded. Studies that examined an individual or household’s ability to obtain medicines for free (as opposed to having to pay for medicines) were included, because free medicines are, by definition, more affordable than medicines at any price [13]. We included studies that directly measured availability and/or affordability, those that used information from other surveys, and those that asked respondents to report on availability and/or affordability.
Studies with data from more than one country were included if the majority were LMICs. We focused on CVD medicines in the following three categories: antihypertensive agents, platelet aggregation inhibitors, and lipid-lowering agents. Studies that reported data on a basket of medicines were included if CVD medicines were a part of that basket.
Search strategy
We searched MEDLINE, EMBASE, Global Health, Cumulative Index to Nursing and Allied Health Literature, EconLit, Social Policy and Practice, and Africa Wide Information for studies in any language published after the year 2000. We also conducted a grey literature search using the website of the Institute of Development Studies, the WHO Repository, the World Bank Repository, and Google. We restricted our search to publications from the year 2000 onward because of a paucity of health systems literature prior to this date. We performed our initial search in June 2020 and updated the search in July 2021. The references of all included records were manually reviewed. The search strategy was developed in collaboration with a librarian at the London School of Hygiene and Tropical Medicine with expertise in systematic review methodology. The search terms were subsequently peer-reviewed by another information specialist at the London School of Hygiene and Tropical Medicine not involved in developing the search strategy. More details on the search strategy are presented in Table 1 and the complete search terms can be found in Appendix 1 (S1 Table). Non-communicable disease (NCD) medicines were included in our search because an initial scoping review revealed papers that examined availability and/or affordability of a basket of NCD medicines, including CVD drugs.
Table 1. Search strategy combining three themes: Medicines for NCDs and CVD, availability or affordability, and LMICs.
| Main Concept | Components of Main Concept | Sample Terms from Medline Search |
|---|---|---|
| [Terms for CVD Medicines] | [cardiovascular agents/ OR antihypertensive agents/ OR hypolipidemic agents/] | |
| Medicines for NCDs and CVD |
OR [(Terms for CVD) AND (Terms for Medicines)] |
[(cardiovascular diseases/ OR exp hypertension/ OR exp hyperlipidemias/ OR cardiovascular disease*.ti,ab.) AND (exp pharmaceutical preparations/ OR medication*.ti,ab.)] |
| AND |
OR [(Terms for NCD) AND (Terms for Medicines)] |
[(exp chronic disease/ OR chronic disease*.ti,ab. OR chronic condition*.ti,ab. OR NCD.ti,ab.) AND (exp pharmaceutical preparations/ OR medication*.ti,ab.)] |
| Availability/Affordability AND | [Terms for Availability OR Affordability] | [availab*.ti,ab. OR supply.ti,ab. OR drug costs/ OR exp fees, pharmaceutical/ OR affordab*.ti,ab.] |
| LMIC | [Terms for LMIC OR List of LMIC] | [developing countries/ OR exp africa south of the sahara/ OR Armenia/ OR Armenia.ti,ab.] |
The results were screened independently by two reviewers at the title/abstract level and studies not meeting inclusion criteria were excluded. Both reviewers subsequently screened full texts of retained articles independently, excluding those that did not meet inclusion criteria. Discrepancies were discussed with a third reviewer and a consensus was reached.
Data extraction
The following information was extracted from all studies: language, study implementation year, country, study design, study setting, sample size, survey method used, factors analyzed, methodology, medicines studied, outcome measure, definition of availability and/or affordability, how availability and/or affordability was quantified, and study findings including the statistical parameters used (e.g. odds ratios, proportions, chi-squared values, p-values, and confidence intervals). Studies that were not in English were translated using Google Translate. Where translations were not clear, we used a dictionary and consulted colleagues with knowledge of the language.
Risk of bias assessment
We assessed quality for observational studies using a method previously published by Maimaris [14], which examines three domains: selection bias, information bias (differential and non-differential misclassification), and confounding, thereby providing more precise information on studies than is done by some other instruments whose primary purpose is to determine whether to include a study or not. To evaluate non-differential misclassification, we assessed the reliability of the measures used for availability and affordability. The risk of bias tool for observational studies is presented in Appendix 2 (S2 Table). For randomized controlled trials, the revised Cochrane tool was used [15]. This tool measures bias in the following domains: randomization, timing of identification/recruitment in relation to timing of randomization, deviations in intended interventions, missing outcome data, outcome measurements, and selection of the reported results. Two reviewers independently performed risk of bias assessment and resolved discrepancies with discussion.
Conceptual framework and narrative synthesis
We organized our findings according to a framework of health systems constraints adapted to access to medicines in LMICs [16]. This framework proposes different levels of the health system as follows: 1) individuals, households, and communities, 2) health service delivery, 3) health sector (or program), 4) national context (public policies cutting across sectors), 5) international context. We considered sociodemographic factors and geographic location at level 1. At level 2, we included characteristics of a health facility or service delivery arrangements at that facility. At the health sector level, we examined country or region-wide health programs or policies. At the national context, policies extending beyond the health sector were examined. At level 5, international programs or arrangements that were related to the availability or affordability of medicines were examined. We coded factors associated with availability and affordability into the different levels and performed a narrative synthesis of the data.
Results
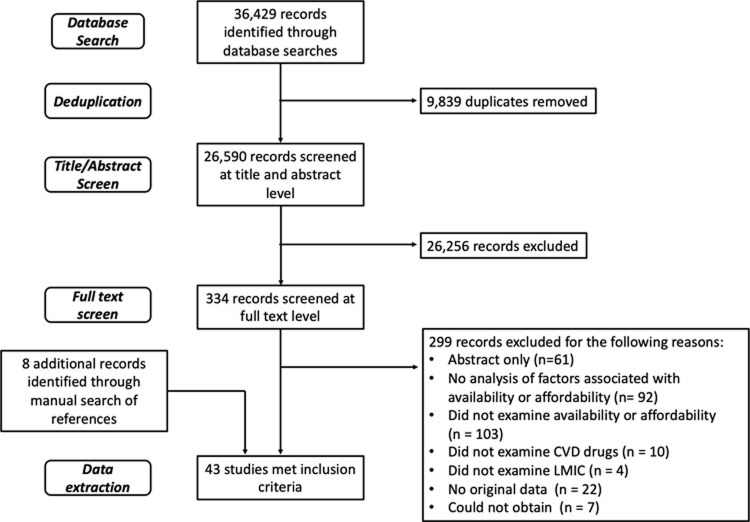
The results of the screening process are reported in the PRISMA flowchart (Fig 1). The initial search yielded 32,352 results and an additional 4,077 were retrieved from the updated search, resulting in 36,429 titles. Of these, 9,839 were duplicates, leaving 26,590 citations. Title and abstract screening resulted in 334 studies, of which 327 were retrieved for full-text screening. The remaining seven studies could not be accessed because they were in journals to which our library and no affiliated libraries subscribe. Thirty-five studies met inclusion criteria and an additional eight were included through a manual search of the references.
Fig 1. PRISMA flowchart.
Flowchart of the selection and screening process of studies on availability and affordability of CVD medicines.
Study characteristics
Of the included studies, 40 were in English [17–56], two were in Spanish [57, 58], and one was in Portuguese [59]. Thirty-four studies focused on availability [17, 18, 20–24, 26–36, 38, 40–42, 44, 45, 47, 48, 50–54, 56, 58, 59], seven on affordability [19, 25, 37, 39, 43, 46, 55], and two on both [49, 57]. Risk of bias assessment revealed heterogeneity in the quality of the included studies. Fifteen of the cross-sectional studies had one or more domains with high risk of bias [23, 24, 27, 32, 35, 37, 43, 49–55, 57] while another seven had unclear risk of bias in at least one domain [20, 22, 30, 44, 53, 54, 59]. Detailed characteristics of included studies and the results of risk of bias assessments are described in Table 2. Seven of the included studies used the WHO Health Action International survey [20, 22, 23, 26, 28, 30, 31, 53, 56] and six used the Service Availability and Readiness Assessment tool to measure medicine availability [17, 18, 24, 27, 29, 38, 52]. Definitions and quantification approaches used to measure availability and affordability are presented in Tables 3 and 4, respectively.
Table 2. Characteristics of identified studies.
| Author Year Country | Study Design (Sample Size and Setting) | Medicines Studied | Key Findings | Risk of Bias |
|---|---|---|---|---|
| Availability | ||||
| Adinan [17] 2019 Tanzania | Cross-sectional (34 Facilities) | Antihypertensive agents | No significant difference in availability at different tier facilities | Low risk of bias in all domains |
| Albelbeisi [50] 2020 West Bank and Gaza | Cross-sectional (52 Facilities) | CVD Medicines | No significant difference in availability in different regions | High risk for confounding |
| Armstrong-Hough [18] 2018 Uganda | Cross-sectional (196 Facilities) | NCD medicines | Being a higher tier facility, being a private facility (versus public), having greater infrastructure, and integrating NCD care with HIV testing and counseling associated with significantly greater availability; providing HIV care associated with significantly lower availability | Low risk of bias in all domains |
| Bazargani [20] 2014 23 LMICs | Cross-sectional (2290 Facilities) | Basket of medicines for chronic and acute conditions | Significantly higher median availability of medicines on the national essential medicine list than those not on the list | Unclear risk for selection bias |
| Bintabara [51] 2020 Tanzania | Cross-sectional (770 Facilities) | CVD Medicines | Significantly greater availability at private facilities than at public facilities | High risk for confounding |
| Bintabara [21] 2018 Tanzania | Cross-sectional (725 Facilities) | Antihypertensive agents | Significantly greater odds of preparedness for hypertension services at private facilities, at higher tier facilities, and at urban facilities | Low risk of bias in all domains |
| Cameron [22] 2011 40 Countries | Cross-sectional (2779 Facilities) | Medicines for chronic conditions | Significantly higher mean availability at private facilities than public facilities | Unclear risk for selection bias |
| Cepuch [23] 2012 Kenya | Cross-sectional (56 Facilities) | NCD medicines CVD medicines | No significant difference in mean availability of NCD medicines at private mission and public facilities; significantly higher availability of hydrochlorothiazide at urban facilities but no significant differences for other CVD medicines | High risk for confounding |
| Duong [24] 2019 Vietnam | Cross-sectional (89 Facilities) | CVD medicines | Significantly lower availability in the mountainous region as compared to other regions of the country | High risk for confounding and selection bias |
| Ekenna [52] 2020 Nigeria | Cross-sectional (60 Facilities) | Basic essential medicines | Significantly greater availability at urban facilities than at rural ones | High risk for confounding |
| Fang [26] 2013 China | Cross-sectional (80 Facilities in First Survey, 144 Facilities in Second Survey) | Basic essential medicines | Significant decrease in availability after implementation of a policy of zero-mark-ups on drugs and introduction of a provincial essential medicine list | Low risk of bias in all domains |
| Ibrahim [53] 2020 Yemen | Cross-sectional (30 Facilities) | CVD Medicines | No significant different in availability in different cities, significantly greater availability at private facilities | Unclear risk for selection bias and high risk of confounding |
| Jigjidsuren [27] 2019 Mongolia | Cross-sectional (146 Facilities) | Basic essential medicines | No significant difference in availability between higher and lower tier facilities | High risk for confounding |
| Kasonde [28] 2019 Bangladesh | Cross-sectional (135 Facilities) | Basket of medicines for chronic and acute conditions | Significantly lower availability of medicines at public facilities compare to private facilities, no significant difference in availability of medicines on the national essential medicine list and those not on the list | Low risk of bias in all domains |
| Katende [29] 2015 Uganda | Cross-sectional (28 Facilities) | Antihypertensive agents | Significantly higher medicine availability at higher tier facilities | Low risk of bias in all domains |
| Khanal [30] 2019 Nepal | Cross-sectional (60 Facilities) | NCD Medicines | No significant difference in medicine availability in different geographic regions of the country | Unclear risk for selection bias |
| Kibridge [31] 2017 Uganda | Cross-sectional (155 Facilities) | CVD medicines | Significantly higher medicine availability at private facilities compared to public ones only for certain classes of CVD medicines | Low risk of bias in all domains |
| Mendes [59] 2014 Brazil | Cross-sectional (29,228 Facilities) | Basic essential medicines CVD medicines | Significantly higher odds of essential medicine availability at facilities with greater levels of infrastructure and at facilities dispensing psychotropic medicines; significantly higher availability of antihypertensives and cardiology drugs at higher tier health facilities | Unclear risk for selection bias |
| Minaei [32] 2019 Iran | Cross-sectional (60 Facilities) | Basic essential medicines | No significant difference in availability at public and private facilities or across difference cities | High risk for confounding |
| Musinguzi [33] 2015 Uganda | Cross-sectional (126 Facilities) | Antihypertensive agents | Significantly higher availability at higher tier facilities | Low risk of bias in all domains |
| Mutale [34] 2018 Zambia | Cross-sectional (46 Facilities) | CVD medicines | Significantly higher availability of calcium channel blockers at urban facilities but no significant difference for other CVD medicine classes | Low risk of bias in all domains |
| Oliveira [35] 2016 Brazil | Cross-sectional (12,725 Households) | Medicines for chronic conditions | Significantly higher availability at private facilities and at facilities adopting the Farmacia Popular Programa | High risk for information bias |
| Oyekale [36] 2017 Nigeria | Cross-sectional (2480 Facilities) | Basic essential medicines | Significantly higher availability at higher tier facilities and facilities with greater infrastructure; no significant difference in availability between urban and rural facilities or between private and public facilities | Low risk of bias in all domains |
| Peck [38] 2014 Tanzania | Cross-sectional (24 Facilities) | Antihypertensive agents | No significant difference in availability at different tier facilities | Low risk of bias in all domains |
| Resendez [58] 2000 Mexico | Cross-sectional (67 Facilities) | Antihypertensive agents | No significant difference in availability between urban and rural facilities and different tier facilities | Low risk of bias in all domains |
| Restinia [54] 2021 Indonesia | Cross-sectional (Unknown number of facilities) | Antihypertensive agents | Significant increase in the availability of amlodipine and lower dose captopril, a decrease in the availability of hydrochlorothiazide, and no change in availability of higher dose captopril or nifedipine after implementation of the National Health Insurance scheme which changed payments from fee for service to diagnosis-based payments and required drugs to be ordered from the national formulary | Unclear risk for selection bias, unclear risk for differential and non-differential misclassification, and high risk for confounding |
| Rockers [40] 2019 Kenya | Cluster Randomized Trial (571 Individuals, 127 Facilities) | Antihypertensive agents | Novartis access program providing medicines at $1 per treatment per month was associated with significantly greater odds of availability of amlodipine but not other medicines at health facilities; the intervention was not associated with significantly greater odds of availability of any medicines at home | Low risk of bias in all domains |
| Rockers [41] 2018 Kenya | Cross-sectional (639 Individuals) | Antihypertensive agents | Significantly higher likelihood of availability in higher socioeconomic households | Low risk of bias in all domains |
| Saeed [56] 2021 Pakistan | Cross-sectional (81 Facilities) | CVD Medicines | Significantly lower mean medicine availability at public facilities than at private facilities, significantly lower availability of generics than originator brands, significantly greater availability of medicines on the national essential medicine list | Low risk of bias in all domains |
| Su [42] 2017 China | Cross-sectional (3362 Facilities) | Antihypertensive agents | Significantly higher availability at higher tier health facilities and of medicines recommended by national guidelines | Low risk of bias in all domains |
| Uzuchukwo [44] 2012 Nigeria | Cross-sectional (33 Facilities) | Basic essential medicines | Significantly higher availability at facilities adopting a revolving drug fund | Unclear risk for confounding and information bias |
| Vialle-Valentin [45] 2015 5 LMICs | Cross-sectional (1867 Individuals) | Medicines for chronic conditions | No significant difference in odds of medicine availability at home based on household education level in any country; significantly greater odds of medicine availability in poorer households in Jordan and lower odds of medicine availability in poorer households in Kenya, no differences observed in other countries | Low risk of bias in all domains |
| Wirtz [47] 2018 Kenya | Cross-sectional (445 Individuals) | Antihypertensive agents | Significantly greater odds of medicine availability in households with higher education level and higher socioeconomic status; no significant difference in odds of availability between urban and rural facilities | Low risk of bias in all domains |
| Yang [48] 2015 China | Cross-sectional (90 Facilities) | Basic essential medicines | Significant positive association between being in a central province and medicine availability | Low risk of bias in all domains |
| Affordability | ||||
| Ashigbie [19] 2020 Kenya | Cross-sectional (137 Facilities) | Basket of medicines for chronic and acute conditions | Significantly greater likelihood of receiving medicines for free at public facilities than private facilities | Low risk of bias in all domains |
| Emmerick [25] 2020 Brazil | Retrospective interrupted time series (25,150 Facilities) | Antihypertensive agents | Significant reduction of out of pocket payment for medicines to zero after elimination of co-payments | Low risk of bias in all domains |
| Leao Tavares [43] 2016 Brazil | Cross-sectional (12,725 Individuals) | Medicines for chronic conditions | Significantly higher likelihood of obtaining medicines for free among individuals from lower socioeconomic backgrounds and those without health insurance | High risk for information bias |
| Paniz [37] 2010 Brazil | Cross-sectional (2460 Individuals) | Medicines for hypertension and diabetes | Significantly greater likelihood of obtaining medicines for free at health units providing more comprehensive NCD care with greater follow-up | High risk for information bias |
| Perlman [39] 2011 Russian Federation | Cross-sectional (4215 Households) | Basic essential medicines | Significantly greater likelihood of household-reported affordability of medicines among those from higher socioeconomic backgrounds; significantly greater likelihood of household reported affordability among men with compulsory health insurance in 1994 but not in 1998 or 2004 | Low risk of bias in all domains |
| Restrepo 2020 Brazil | Cross-sectional (289 individuals) | Basic essential medicines | No difference in affordability based on sex, age, or socioeconomic status, significantly greater affordability for those with lower education, chronic disease, no supplementary health insurance plan, and those who primarily obtained medicines from the Sistema Unico de Saudeb | High risk for confounding |
| Viana [46] 2015 Brazil | Cross-sectional (27,333 Individuals) | Medicines for chronic conditions | Significantly greater odds of obtaining medicines for free among those from lower socioeconomic backgrounds and those without health insurance | Low risk of bias in all domains |
| Both | ||||
| Contreras-Loya [57] 2013 Mexico | Cross-sectional (30 Facilities) | Basic essential medicines | No significant difference in availability of medicines at facilities with outsourced pharmacies and those operated by the state health service; significantly greater likelihood of obtaining medicines for free at outsourced pharmacies | High risk for confounding |
| Fernandopulle [49] 2019 Sri Lanka | Cross-sectional (1008 Individuals) | NCD medicines | No significant differences in availability or affordability of medicines reported by men or women | High risk for confounding and information bias |
a Programs adopting the Farmacia Popular Program provide a wider range of medicines and charge a fee for some of them, their counterparts provide fewer medicines all at no cost
b The Sistema Unico de Saude is a program that aims to increase access to medicines by providing medicines for free
Table 3. Approaches to measuring availability by different studies.
| Study | Availability Definition | Availability Quantification Approach | Verified through direct observation | Checked for medicine expiry date? |
|---|---|---|---|---|
| Adinan 2019 [17] | Physical presence of drug | Proportion of facilities where at least one of three classes of medicines was available | Yes | Yes |
| Albelbeisi 2020 [50] | Physical presence of drug | Proportion of facilities where medicine was available | Not stated | Not stated |
| Armstrong-Hough 2018 [18] | Physical presence of drug | Proportion of medicines available | Not stated | Not stated |
| Bazargani 2014 [20] | Physical presence of drug | Proportion of facilities where medicine was available | Not stated | Not stated |
| Bintabara 2020 [51] | Physical presence of drug | Proportion of facilities where at least one type of medicine was available | Yes | Not stated |
| Bintabara 2018 [21] | Physical presence of drug | Incorporated into preparedness score, where drug availability was counted as one of three equally weighted components of facility preparedness | Not stated | Not stated |
| Cameron 2011 [22] | Physical presence of drug | Proportion of facilities where medicine was available | Not stated | Not stated |
| Cepuch 2012 [23] | Physical presence of drug | Proportion of facilities where medicine was available | Not stated | Not stated |
| Contreras-Loya 2013 [57] | Physical presence of drug | Proportion of medicines available; proportion of facilities where all medicines from a predetermined list were available | Not stated | Not stated |
| Duong 2019 [24] | Not stated | Not stated | Not stated | Not stated |
| Ekenna 2020 [52] | Physical presence of drug | Proportion of medicines available | Not stated | Not stated |
| Fang 2013 [26] | Physical presence of drug | Proportion of facilities where medicine was available | Not stated | Not stated |
| Fernandopulle 2019 [49] | Patient-reported availability of medicines at health facility | Proportion of individuals reporting medicine was available | Not stated | Not stated |
| Ibrahim 2020 [32] | Physical presence of drug | Proportion of facilities where each medicine was available | Yes | Not stated |
| Jigjidsuren 2019 [27] | Not stated | Proportion of medicines available | Not stated | Not stated |
| Kasonde 2019 [28] | Not stated | Proportion of medicines available | Not stated | Not stated |
| Katende 2015 [29] | Physical presence of drug | Proportion of facilities where at least one type of medicine was available | Not stated | Yes |
| Khanal 2019 [30] | Not stated | Proportion of facilities where medicine was available | Not stated | Not stated |
| Kibridge 2017 [31] | Not stated | Proportion of facilities where medicine was available | Not stated | Not stated |
| Mendes 2014 [59] | Physical presence of drug | The proportion of health units where greater than 80% of all key drugs from 12 categories were available | Yes | Not stated |
| Minaei 2019 [32] | Not stated | Proportion of medicines available | Not stated | Not stated |
| Musinguzi 2015 [33] | Two definitions used, first definition not stated, second definition is presence of reported stock-outs in the prior three months | Proportion of facilities where medicine was available; proportion of facilities reporting stock-outs in prior three months | Not stated | Not stated |
| Mutale 2018 [34] | Not stated | Proportion of facilities where medicine was available | Not stated | Not stated |
| Oliveira 2016 [35] | Patient-reported availability of medicines at health facility | Proportion of individuals reporting medicine was available | Not stated | Not stated |
| Oyekale 2017 [36] | Not stated | Proportion of facilities where medicine was available | Not stated | Yes |
| Peck 2014 [38] | Physical presence of drug | Proportion of facilities where medicine was available | Not stated | Not stated |
| Resendez 2000 [58] | Physical presence of drug | Proportion of medicines available; proportion of facilities where zero, 1, 2, or 3 types of medicines were available | Not stated | Not stated |
| Restinia 2021 [54] | Not stated | Not stated | Not stated | Not stated |
| Rockers 2019 [40] | Physical presence of drug | Proportion of facilities or households where medicine was available | Yes | Not stated |
| Rockers 2018 [41] | Physical presence of drug | Proportion of facilities or households where medicine was available | Yes | Not stated |
| Saeed 2021 [56] | Physical presence of drug | Proportion of facilities where medicine was available | Yes | Not stated |
| Su 2017 [42] | Physical presence of drug | Proportion of facilities where medicine was available | Not stated | Not stated |
| Uzuchukwo 2012 [44] | Estimated expected shelf life of a drug | Proportion of medicines available; mean estimated shelf life of a drug across facilities | Not stated | Not stated |
| Vialle-Valentin 2015 [45] | Physical presence of drug | Proportion of households where medicine was available | Not stated | Not stated |
| Wirtz 2018 [47] | Physical presence of drug | Proportion of households where at least one type of medicine was available | Yes | Not stated |
| Yang 2015 [48] | Physical presence of drug | Proportion of medicines available | Not stated | Not stated |
Table 4. Approaches to measuring affordability used by different studies.
| Study | Affordability Definition | Affordability Quantification Approach |
|---|---|---|
| Ashighbie 2020 [19] | Ability to obtain medicines for free | Proportion of facilities providing medicines for free |
| Contreras-Loya 2013 [57] | Ability to obtain medicines for free | Proportion of individuals who obtained medicines for free, proportion of facilities where medicines were available for free |
| Emmerick 2020 [25] | Paying no co-payment for medicines | Mean monthly co-payments (paying any amount versus zero) |
| Fernandopulle 2019 [49] | Patient-reported affordability of medicines | Proportion of individuals reporting medicine was affordable |
| Leao Tavares 2016 [43] | Ability to obtain medicines for free | Proportion of individuals who obtained all of their medicines for free |
| Paniz 2010 [37] | Ability to obtain medicines for free | Proportion of individuals who obtained medicines for free |
| Perlman 2011 [39] | Patient-reported affordability of medicines | Proportion of individuals reporting medicine was affordable |
| Restrepo 2020 [55] | Income impairment as defined by the average amount spent on medicines divided by the family income per capita multiplied by 100 | Mean income impairment |
| Viana 2015 [46] | Ability to obtain medicines for free | Proportion of individuals who obtained all their medicines for free |
Factors associated with availability
Individual, household, and community level
Sociodemographic characteristics associated with medicine availability were assessed in four studies, with inconsistent findings [41, 45, 47, 49]. Age was examined in two and was only associated with greater availability in three of five countries in one of the studies [45, 47]. Associations between socioeconomic status and availability varied in the same five-country study, but two other studies found positive associations [41, 45, 47]. Education was also positively associated with availability in one of two studies but not in the other [45, 47]. There was no association between sex and availability of medicines in the three studies looking at this variable [45, 47, 49]. At the community level, seven studies compared medicine availability in urban and rural settings [21, 23, 34, 36, 47, 52, 58], with three reporting no differences [36, 47, 58]. In two studies from Zambia and Kenya that looked at different CVD medicine classes separately, only calcium channel blockers and hydrochlorothiazide had significantly lower availability in rural facilities, respectively [23, 34].
Service delivery level
Twenty one studies examined service delivery level arrangements [17, 18, 21–23, 27–29, 31–33, 35, 36, 38, 42, 47, 51, 53, 56, 58, 59]. Thirteen of these compared availability at private and public facilities with mixed results [18, 21–23, 28, 31, 32, 35, 36, 47, 51, 53, 56]. Additionally, 11 studies looked at the relationship between facility tier and medicine availability [17, 18, 21, 27, 29, 33, 36, 38, 42, 58, 59]. In seven studies from Tanzania, Uganda, Nigeria, China, and Brazil, higher tier facilities staffed by medical doctors or providing a wider range of services were significantly more likely to have antihypertensive medicines or a basket of medicines available [18, 21, 29, 33, 36, 42, 59]. Among these, three also looked at correlations between availability and the level of amenities at a facility such as equipment, a pharmacy refrigerator, or solar panels. The presence of such amenities was associated with greater availability of medicines in all three studies [18, 36, 59]. The relationship between service integration and medicine availability was examined in two studies. In Uganda, facilities providing HIV counseling and testing had greater availability of NCD medicines while those offering HIV care had lower availability [18]. In Brazil, primary healthcare units dispensing psychotropic drugs had 3.16 (95% CI: 2.85–3.51) times greater odds of having essential medicines available [59].
Health sector (program) level
Eight studies examined health sector level arrangements [20, 26, 28, 35, 42, 44, 54, 56]. Three studies from Brazil, China, and Nigeria all point to an association between facility revenues and medicine availability [26, 35, 44]. For example, in China, the introduction of a policy that eliminated facility mark-ups on drug prices (and thus decreased revenue) was associated with a decrease in mean availability of lowest-priced generics from 25.5% to 20.5% (p < 0.0001) [26]. In a fourth study, from Indonesia, implementation of a national policy that included a shift from fee-for-service to more limited reimbursements based on the type of diagnosis was associated with variable results for different types of antihypertensive agents. This policy also included a requirement that medicines be ordered from the national formulary, however, the study provided little detail on the methodology employed [54].
The relationship between the presence of drugs on essential medicine lists and availability was examined in four studies [20, 26, 28, 56]. In China, the introduction of a provincial essential medicine list was associated with decreases in medicine availability [26]. In Bangladesh, availability of medicines was 50.3% for those on the national essential medicine list and 57% for those not on the list (p > 0.05) [28]. But in a survey of 23 LMICs, median availability of generic medicines on national lists was significantly greater [20]. In Pakistan as well, health facility availability of CVD medicines on the essential medicine list was significantly greater than those not on the list [56].
National and international levels
We found no studies assessing the relationship between national policies from outside the health sector and availability. In terms of international factors, one cluster-randomized controlled trial that evaluated a Novartis-sponsored program providing medicines for NCDs in Kenya at $1 per treatment per month found increased availability of amlodipine–but not other CVD medicines–at health facilities [40].
Factors associated with affordability
Individual, household, and community level
Five studies examined sociodemographic factors associated with affordability with four examining socioeconomic status [39, 43, 46, 49, 55]. Two from Brazil revealed a negative association between socioeconomic status and affordability [43, 46], while a third study showed no association [55]. Conversely, a study from Russia revealed a positive association [39].
Service delivery level
Three studies examined correlations between affordability and service delivery level indicators [19, 55, 57]. In Kenya, public facilities were more likely to provide free NCD medicines than private facilities (47% v 9%, p<0.0001) [19]. In Mexico, medicines were obtained for free by 62% of those visiting pharmacies that were operated by the state and by 70% of those visiting outsourced pharmacies (p < 0.01) [57]. In Brazil, medicines were more affordable for individuals who reported primarily using public health facilities as part of the Sistemo Unico de Saude to procure medicines than those who used private pharmacies. The Sistemo Unico de Saude is a national program that aims to increase access to medicines by providing certain medications free of charge [55].
Health sector (program) level
Four studies examined the association between affordability and the presence of health insurance [39, 43, 46, 55]. In Brazil, not having a health insurance plan was associated with greater medicine affordability [43, 46, 55]. An interrupted time series analysis from Brazil examining a policy removing copayments for antihypertensives found significant reductions in mean out-of-pocket expenditures after the policy was implemented [25].
National and international levels
We did not identify any studies that examined factors at the national level (non-health sector) or international level associated with medicine affordability.
Discussion
To the best of our knowledge, this is the first systematic review of health system factors associated with availability and affordability of CVD medicines in LMICs. Our findings highlight significant gaps in evidence and heterogeneity in results as well as variable quality of existing studies. While certain factors seemed consistently associated with availability, findings regarding other factors were inconsistent.
There are a number of possible reasons for these inconsistencies. First, most studies were cross-sectional and subject to potential confounding, precluding establishment of causal relationships. Intervention studies are needed but the feasibility of implementing them will depend on context, so they should be accompanied by qualitative policy analyses.
Second, availability and affordability of medicines are unlikely to be affected by a single factor. Instead, a multitude of factors must be aligned in complex relationships at different health systems levels [16]. This was evident in several studies, including the sole cluster-randomized trial included, where subsidies from Novartis had little effect on medicine availability in Kenya [40]. This differs from a similar intervention involving subsidies for artemisinin-based combination therapy in sub-Saharan Africa, which achieved notable increases in medicine availability [60]. As the authors of the first study acknowledge, subsidization is only one element in a complex system which also has to account for factors such as physician, pharmacist, and patient awareness of specific medicines, procurement, delivery mechanisms, and inclusion in clinical practice guidelines. This may also explain why the presence of medicines on national essential medicine lists did not translate to greater availability in all included studies. Different countries may go about deciding what medicines to include on these lists differently [20]. It may be that in some contexts, drugs on essential medicine lists are not included in clinical guidelines or are less accepted by prescribers and patients. Other factors may also influence whether medicines on essential lists are more available. One included study demonstrated an inverse relationship between country-income level and availability of medicines on these lists [20]. The authors posit that in countries with fewer resources, essential medicine lists drive prioritization. Interestingly, in two studies we identified from Pakistan [56] and Bangladesh [28], availability of medicines positively correlated with their presence on essential lists only in Pakistan, which has the lower national income level of the two [61]. Similarly, medicines on essential lists tend to be more available at public facilities [20]. Yet, the studies we included analyzed a heterogeneous sample of public and private facilities as well as different tier facilities together. This may explain some of the inconsistencies observed. In a study from China [26], where implementation of an essential medicine list was associated with lower availability, the policy was accompanied by elimination of mark-ups on drug prices at health facilities, which may have reduced revenues for medicine procurement.
We also noted inconsistencies and counterintuitive findings for affordability. In Brazil, the absence of health insurance was associated with greater affordability of medicines [43, 46, 55]. Individuals from lower socioeconomic backgrounds who lack health insurance rely more heavily on Brazilian public health programs like the Sistema Unico de Saude, which provide medicines for free particularly for NCDs. Those with private insurance, on the other hand, are more likely to procure medicines through the private sector where they will be required to pay [62]. Future research must take a more holistic and dynamic approach, looking at how individual correlates of inadequate access are influenced by the characteristics of the health systems in which they operate.
A third possible reason for inconsistent findings is the heterogeneity in measures of availability and affordability. For example, some studies on availability assess the percentage of facilities where a drug is available while others report the proportion of drugs available from a list of medicines. Employing even slightly different measures can yield widely disparate estimates in the same country [63]. This problem is compounded by the fact that most current measures are binary. Many of the included studies considered a medicine available if it was present on the day of the survey regardless of quantity, expiry date, or reliability of supply.
A consistent and comprehensive definition of affordability is also needed. In all but one study, affordability was either ascertained subjectively from respondents or by measuring the ability to obtain medicines for free, which does not fully encapsulate the degree to which medicines are unaffordable for those who have to pay. Many measures of affordability, including those employed by the studies included here, rely on a single measure at one point in time. These measures may not provide a full picture of the long-term financial burden that purchasing medications for chronic conditions places on families [64]. Other metrics of affordability have been described in the literature, however, no study meeting our inclusion criteria used such definitions. For example, the WHO Health Action International Survey measures affordability as the number of days of wages the lowest-paid government worker has to spend on medicines [65]. Many of these approaches involve making arbitrary judgments on thresholds for affordability. Doing so without taking into account households’ disposable income risks overestimating true affordability [13]. The downside of more comprehensive definitions is challenges in measurement. Yet, without them, interventions may be deemed effective if they meet certain predetermined benchmarks, even if they do not truly reflect how available or affordable medicines are for those who need them.
An important finding from our study is the paucity of factors studied that could influence availability and affordability. For example, no study examined the role of corruption, although this has been shown to be negatively associated with access to HIV antiretroviral therapy [66]. Nor were supply chain factors investigated, despite several studies showing how relevant interventions affected availability of medicines for other diseases [67, 68]. Similarly, intellectual property provisions may have implications on availability and affordability of CVD medicines. Different studies have shown that the vast majority of medicines on the WHO Essential Medicine List are not under patent protection [69, 70]. These findings, coupled with extensive evidence that medicines on the essential medicine list remain unavailable and unaffordable in most LMICs, imply that patent provisions are unlikely to be the sole contributor to non-availability and affordability. Nevertheless, patents on newer therapeutics or fixed-dose combination therapies for CVD that are not on essential medicine lists may impact their availability and affordability and warrant further investigation [71].
Our study has some limitations. We restricted our search to factors associated with availability and affordability but did not examine other parameters of access. Though beyond the scope of this review, aspects such as acceptability are also important in ensuring that those who need CVD medicines can receive them [9]. Furthermore, while we did not restrict our search based on language, studies in languages other than English may not be captured in the databases used. Finally, availability and affordability are conceptualized in a multitude of ways. Some studies on this topic may have used terms that were missed by our search terms.
Conclusion
Evidence concerning factors that influence the availability or affordability of CVD medicines is limited. The majority of studies are observational and while they have identified a number of potential associations, they cannot establish causality. Factors at different levels of the health system likely act together in a multifactorial way to influence availability and affordability of CVD medicines as part of complex health systems. Future research involving uniform definitions and measurement approaches is needed with a particular focus on experimental and quasi-experimental methods that provide insight into causal mechanisms.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Acknowledgments
We thank Jane Falconer from the London School of Hygiene & Tropical Medicine Library for her expertise and support in developing the search strategy.
Data Availability
All relevant data is presented within the manuscript. Extracted data is included as supplementary material.
Funding Statement
AM was supported by a Wellcome Trust Research Fellowship (number 104349/Z/14/Z). BP, DB and MM were supported by a grant under the Wellcome Trust/Newton Fund-MRC Humanities & Social Science Collaborative Award scheme (200346/Z/15/Z). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370(9587):591–603. doi: 10.1016/S0140-6736(07)61299-9 [DOI] [PubMed] [Google Scholar]
- 2.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301–7. doi: 10.1056/NEJM199511163332001 [DOI] [PubMed] [Google Scholar]
- 3.Antithrombotic Trialists Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–60. doi: 10.1016/S0140-6736(09)60503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aminde LN, Takah NF, Zapata-Diomedi B, Veerman JL. Primary and secondary prevention interventions for cardiovascular disease in low-income and middle-income countries: a systematic review of economic evaluations. Cost Eff Resour Alloc. 2018;16:22. doi: 10.1186/s12962-018-0108-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global status report on noncommunicable diseases 2010. Geneva: World Health Organization; 2011 [cited 2020 Sept 14]. Available from: https://www.who.int/nmh/publications/ncd_report2010/en/. [Google Scholar]
- 6.Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Geneva: World Health Organization; 2013. [cited 2020 Sept 14]. Available from: https://apps.who.int/iris/bitstream/handle/10665/94384/9789241506236_eng.pdf?sequence=1. [Google Scholar]
- 7.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation. 2016;134(6):441–50. doi: 10.1161/CIRCULATIONAHA.115.018912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, Diaz R, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378(9798):1231–43. doi: 10.1016/S0140-6736(11)61215-4 [DOI] [PubMed] [Google Scholar]
- 9.Wirtz VJ, Kaplan WA, Kwan GF, Laing RO. Access to Medications for Cardiovascular Diseases in Low- and Middle-Income Countries. Circulation. 2016;133(21):2076–85. doi: 10.1161/CIRCULATIONAHA.115.008722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow CK, Nguyen TN, Marschner S, Diaz R, Rahman O, Avezum A, et al. Availability and affordability of medicines and cardiovascular outcomes in 21 high-income, middle-income and low-income countries. BMJ Glob Health. 2020;5(11). doi: 10.1136/bmjgh-2020-002640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attaei MW, Khatib R, McKee M, Lear S, Dagenais G, Igumbor EU, et al. Availability and affordability of blood pressure-lowering medicines and the effect on blood pressure control in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet Public Health. 2017;2(9):e411–e9. doi: 10.1016/S2468-2667(17)30141-X [DOI] [PubMed] [Google Scholar]
- 12.Embrey M. Chapter 1 Managing Access to Medicines and Health Technologies. Embrey M, Ryan M, editors. Arlington, VA: Management Sciences for Health; 2012. [Google Scholar]
- 13.Niens LM, Brouwer WB. Measuring the affordability of medicines: importance and challenges. Health Policy. 2013;112(1–2):45–52. doi: 10.1016/j.healthpol.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 14.Maimaris W, Paty J, Perel P, Legido-Quigley H, Balabanova D, Nieuwlaat R, et al. The influence of health systems on hypertension awareness, treatment, and control: a systematic literature review. PLoS Med. 2013;10(7):e1001490. doi: 10.1371/journal.pmed.1001490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 16.Bigdeli M, Jacobs B, Tomson G, Laing R, Ghaffar A, Dujardin B, et al. Access to medicines from a health system perspective. Health Policy Plan. 2013;28(7):692–704. doi: 10.1093/heapol/czs108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adinan J, Manongi R, Temu GA, Kapologwe N, Marandu A, Wajanga B, et al. Preparedness of health facilities in managing hypertension & diabetes mellitus in Kilimanjaro, Tanzania: a cross sectional study. BMC Health Serv Res. 2019;19(1):537. doi: 10.1186/s12913-019-4316-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong-Hough M, Kishore SP, Byakika S, Mutungi G, Nunez-Smith M, Schwartz JI. Disparities in availability of essential medicines to treat non-communicable diseases in Uganda: A Poisson analysis using the Service Availability and Readiness Assessment. PLoS One. 2018;13(2):e0192332. doi: 10.1371/journal.pone.0192332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashigbie PG, Rockers PC, Laing RO, Cabral HJ, Onyango MA, Buleti JPL, et al. Availability and prices of medicines for non-communicable diseases at health facilities and retail drug outlets in Kenya: a cross-sectional survey in eight counties. BMJ Open. 2020;10(5):e035132. doi: 10.1136/bmjopen-2019-035132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazargani YT, Ewen M, de Boer A, Leufkens HG, Mantel-Teeuwisse AK. Essential medicines are more available than other medicines around the globe. PLoS One. 2014;9(2):e87576. doi: 10.1371/journal.pone.0087576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bintabara D, Mpondo BCT. Preparedness of lower-level health facilities and the associated factors for the outpatient primary care of hypertension: Evidence from Tanzanian national survey. PLoS One. 2018;13(2):e0192942. doi: 10.1371/journal.pone.0192942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron A, Roubos I, Ewen M, Mantel-Teeuwisse AK, Leufkens HG, Laing RO. Differences in the availability of medicines for chronic and acute conditions in the public and private sectors of developing countries. Bull World Health Organ. 2011;89(6):412–21. doi: 10.2471/BLT.10.084327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cepuch C. Availability of essential medicines for chronic disease vs. communicable disease in Kenya as an indicator of age-related inequities in access [Master’s Thesis]: University of the Western Cape; 2012. [Google Scholar]
- 24.Duong DB, Minh HV, Ngo LH, Ellner AL. Readiness, Availability and Utilization of Rural Vietnamese Health Facilities for Community Based Primary Care of Non-communicable Diseases: A CrossSectional Survey of 3 Provinces in Northern Vietnam. Int J Health Policy Manag. 2019;8(3):150–7. doi: 10.15171/ijhpm.2018.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emmerick ICM, Campos MR, da Silva RM, Chaves LA, Bertoldi AD, Ross-Degnan D, et al. Hypertension and diabetes treatment affordability and government expenditures following changes in patient cost sharing in the "Farmacia popular" program in Brazil: an interrupted time series study. BMC Public Health. 2020;20(1):24. doi: 10.1186/s12889-019-8095-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang Y, Wagner AK, Yang S, Jiang M, Zhang F, Ross-Degnan D. Access to affordable medicines after health reform: evidence from two cross-sectional surveys in Shaanxi Province, western China. Lancet Glob Health. 2013;1(4):e227–37. doi: 10.1016/S2214-109X(13)70072-X [DOI] [PubMed] [Google Scholar]
- 27.Jigjidsuren A, Byambaa T, Altangerel E, Batbaatar S, Saw YM, Kariya T, et al. Free and universal access to primary healthcare in Mongolia: the service availability and readiness assessment. BMC Health Serv Res. 2019;19(1):129. doi: 10.1186/s12913-019-3932-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasonde L, Tordrup D, Naheed A, Zeng W, Ahmed S, Babar ZU. Evaluating medicine prices, availability and affordability in Bangladesh using World Health Organisation and Health Action International methodology. BMC Health Serv Res. 2019;19(1):383. doi: 10.1186/s12913-019-4221-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katende D, Mutungi G, Baisley K, Biraro S, Ikoona E, Peck R, et al. Readiness of Ugandan health services for the management of outpatients with chronic diseases. Trop Med Int Health. 2015;20(10):1385–95. doi: 10.1111/tmi.12560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanal S, Veerman L, Ewen M, Nissen L, Hollingworth S. Availability, Price, and Affordability of Essential Medicines to Manage Noncommunicable Diseases: A National Survey From Nepal. Inquiry. 2019;56:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kibirige D, Atuhe D, Kampiire L, Kiggundu DS, Donggo P, Nabbaale J, et al. Access to medicines and diagnostic tests integral in the management of diabetes mellitus and cardiovascular diseases in Uganda: insights from the ACCODAD study. Int J Equity Health. 2017;16(1):154. doi: 10.1186/s12939-017-0651-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minaei H, Peikanpour M, Yousefi N, Peymani P, Peiravian F, Shobeiri N, et al. Country Pharmaceutical Situation on Access, Quality, and Rational Use of Medicines: An Evidence from a middle-income country. Iran J Pharm Res. 2019;18(4):2191–203. doi: 10.22037/ijpr.2019.111636.13273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musinguzi G, Bastiaens H, Wanyenze RK, Mukose A, Van Geertruyden JP, Nuwaha F. Capacity of Health Facilities to Manage Hypertension in Mukono and Buikwe Districts in Uganda: Challenges and Recommendations. PLoS One. 2015;10(11):e0142312. doi: 10.1371/journal.pone.0142312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mutale W, Bosomprah S, Shankalala P, Mweemba O, Chilengi R, Kapambwe S, et al. Assessing capacity and readiness to manage NCDs in primary care setting: Gaps and opportunities based on adapted WHO PEN tool in Zambia. PLoS One. 2018;13(8):e0200994. doi: 10.1371/journal.pone.0200994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliveira MA, Luiza VL, Tavares NU, Mengue SS, Arrais PS, Farias MR, et al. Access to medicines for chronic diseases in Brazil: a multidimensional approach. Rev Saude Publica. 2016;50(suppl 2):6s. doi: 10.1590/S1518-8787.2016050006161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oyekale AS. Assessment of primary health care facilities’ service readiness in Nigeria. BMC Health Serv Res. 2017;17(1):172. doi: 10.1186/s12913-017-2112-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paniz VM, Fassa AG, Facchini LA, Piccini RX, Tomasi E, Thume E, et al. Free access to hypertension and diabetes medicines among the elderly: a reality yet to be constructed. Cad Saude Publica. 2010;26(6):1163–74. doi: 10.1590/s0102-311x2010000600010 [DOI] [PubMed] [Google Scholar]
- 38.Peck R, Mghamba J, Vanobberghen F, Kavishe B, Rugarabamu V, Smeeth L, et al. Preparedness of Tanzanian health facilities for outpatient primary care of hypertension and diabetes: a cross-sectional survey. Lancet Glob Health. 2014;2(5):e285–92. doi: 10.1016/S2214-109X(14)70033-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perlman F, Balabanova D. Prescription for change: accessing medication in transitional Russia. Health Policy Plan. 2011;26(6):453–63. doi: 10.1093/heapol/czq082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rockers PC, Laing RO, Ashigbie PG, Onyango MA, Mukiira CK, Wirtz VJ. Effect of Novartis Access on availability and price of non-communicable disease medicines in Kenya: a cluster-randomised controlled trial. Lancet Glob Health. 2019;7(4):e492–e502. doi: 10.1016/S2214-109X(18)30563-1 [DOI] [PubMed] [Google Scholar]
- 41.Rockers PC, Laing RO, Wirtz VJ. Equity in access to non-communicable disease medicines: a cross-sectional study in Kenya. BMJ Glob Health. 2018;3(3):e000828. doi: 10.1136/bmjgh-2018-000828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su M, Zhang Q, Bai X, Wu C, Li Y, Mossialos E, et al. Availability, cost, and prescription patterns of antihypertensive medications in primary health care in China: a nationwide cross-sectional survey. Lancet. 2017;390(10112):2559–68. doi: 10.1016/S0140-6736(17)32476-5 [DOI] [PubMed] [Google Scholar]
- 43.Tavares NU, Luiza VL, Oliveira MA, Costa KS, Mengue SS, Arrais PS, et al. Free access to medicines for the treatment of chronic diseases in Brazil. Rev Saude Publica. 2016;50(suppl 2):1–10. doi: 10.1590/S1518-8787.2016050006118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uzochukwu BS, Onwujekwe OE, Akpala CO. Effect of the Bamako-Initiative drug revolving fund on availability and rational use of essential drugs in primary health care facilities in south-east Nigeria. Health Policy Plan. 2002;17(4):378–83. doi: 10.1093/heapol/17.4.378 [DOI] [PubMed] [Google Scholar]
- 45.Vialle-Valentin CE, Serumaga B, Wagner AK, Ross-Degnan D. Evidence on access to medicines for chronic diseases from household surveys in five low- and middle-income countries. Health Policy Plan. 2015;30(8):1044–52. doi: 10.1093/heapol/czu107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viana KP, Brito Ados S, Rodrigues CS, Luiz RR. Access to continued-use medication among older adults, Brazil. Rev Saude Publica. 2015;49:14. doi: 10.1590/s0034-8910.2015049005352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wirtz VJ, Turpin K, Laing RO, Mukiira CK, Rockers PC. Access to medicines for asthma, diabetes and hypertension in eight counties of Kenya. Trop Med Int Health. 2018;23(8):879–85. doi: 10.1111/tmi.13081 [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Liu C, Ferrier JA, Zhang X. Organizational barriers associated with the implementation of national essential medicines policy: A cross-sectional study of township hospitals in China. Soc Sci Med. 2015;145:201–8. doi: 10.1016/j.socscimed.2015.08.044 [DOI] [PubMed] [Google Scholar]
- 49.Fernandopulle BMR, Gunawardena N, de Silva SHP, Abayawardana C, Hirimuthugoda LK. Patient experiences of access to NCD medicines in Sri Lanka: Evidence of the success story towards universal coverage. Med Access @ Point of Care. 2019;3:1–10. [Google Scholar]
- 50.Albelbeisi AH, Albelbeisi A, El-Bilbeisi AH, Takian A, Akbari-Sari A. Capacity of palestinian primary health care system to prevent and control of non-communicable diseases in Gaza Strip, Palestine: a capacity assessment analysis based on adapted WHO-PEN tool. Int J Health Plann Manage. 2020;35(6):1412–25. doi: 10.1002/hpm.3022 [DOI] [PubMed] [Google Scholar]
- 51.Bintabara D, Ngajilo D. Readiness of health facilities for the outpatient management of non-communicable diseases in a low-resource setting: an example from a facility-based cross-sectional survey in Tanzania. BMJ Open. 2020;10(11):e040908. doi: 10.1136/bmjopen-2020-040908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ekenna A, Itanyi IU, Nwokoro U, Hirschhorn LR, Uzochukwu B. How ready is the system to deliver primary healthcare? Results of a primary health facility assessment in Enugu State, Nigeria. Health Policy Plan. 2020;35(Supplement_1):i97–i106. doi: 10.1093/heapol/czaa108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohamed Ibrahim MI, Alshakka M, Al-Abd N, Bahattab A, Badulla W. Availability of essential medicines in a country in conflict: A quantitative insight from Yemen. Int J Environ Res Public Health. 2021;18(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Restinia M, Laksmitawati DR, Anggriani Y, Sekowati Y. The availability of antihypertensive drug in era of nhi: A study in the primary health care centre jakarta-indonesia. International Journal of Applied Pharmaceutics. 2021;13(Special Issue 2):93–8.33451053 [Google Scholar]
- 55.Restrepo SF, Vieira MRdS, Barros CRdS, Bousquat A. Medicines’ private costs among elderly and the impairment of family income in a medium-sized municipality in the state of Sao Paulo. Revista Brasileira de Epidemiologia. 2020;23(39). [DOI] [PubMed] [Google Scholar]
- 56.Saeed A, Saeed F, Saeed H, Saleem Z, Yang C, Chang J, et al. Access to Essential Cardiovascular Medicines in Pakistan: A National Survey on the Availability, Price, and Affordability, Using WHO/HAI Methodology. Front Pharmacol. 2020;11:595008. doi: 10.3389/fphar.2020.595008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Contreras-Loya D, Reding-Bernal A, Gomez-Dantes O, Puentes-Rosas E, Pineda-Perez D, Castro-Tinoco M, et al. Abasto y surtimiento de medicamentos en unidades especializadas en la atención de enfermedades crónicas en México en 2012. Salud Publica Mex. 2013;55(6):618–26. [PubMed] [Google Scholar]
- 58.Resendez C, Garrido F, Gomez-Dantes O. Disponibilidad de medicamentos esenciales en unidades de primer nivel de la Secretaría de Salud de Tamaulipas, México. Salud Publica Mex. 2000;42(4):298–308. [PubMed] [Google Scholar]
- 59.Mendes LV, Campos MR, Chaves GC, Mendes da Silva R, da Silva Freitas P, Costa KS, et al. Disponibilidade de medicamentos nas unidades básicas de saúde e fatores relacionados: uma abordagem transversal. Saúde Debate. 2014;38:109–23. [Google Scholar]
- 60.Sabot OJ, Mwita A, Cohen JM, Ipuge Y, Gordon M, Bishop D, et al. Piloting the global subsidy: the impact of subsidized artemisinin-based combination therapies distributed through private drug shops in rural Tanzania. PLoS One. 2009;4(9):e6857. doi: 10.1371/journal.pone.0006857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Bank Country and Lending Groups [Internet] Washington, D.C.: World Bank; 2020. [cited 2020 Sept 14]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519. [Google Scholar]
- 62.Luiza VL, Chaves LA, Campos MR, Bertoldi AD, Silva RM, Bigdeli M, et al. Applying a health system perspective to the evolving Farmacia Popular medicines access programme in Brazil. BMJ Glob Health. 2017;2(Suppl 3):e000547. doi: 10.1136/bmjgh-2017-000547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robertson J, Mace C, Forte G, de Joncheere K, Beran D. Medicines availability for non-communicable diseases: the case for standardized monitoring. Global Health. 2015;11:18. doi: 10.1186/s12992-015-0105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy A, McGowan C, McKee M, Suhrcke M, Hanson K. Coping with healthcare costs for chronic illness in low-income and middle-income countries: a systematic literature review. BMJ Glob Health. 2019;4(4):e001475. doi: 10.1136/bmjgh-2019-001475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Measuring medicine prices, availability, affordability and price components Geneva: World Health Organization; 2008 [cited 2020 Sept 14]. Available from: https://www.who.int/medicines/areas/access/OMS_Medicine_prices.pdf.
- 66.Levi J, Pozniak A, Heath K, Hill A. The impact of HIV prevalence, conflict, corruption, and GDP/capita on treatment cascades: data from 137 countries. J Virus Erad. 2018;4(2):80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shieshia M, Noel M, Andersson S, Felling B, Alva S, Agarwal S, et al. Strengthening community health supply chain performance through an integrated approach: Using mHealth technology and multilevel teams in Malawi. J Glob Health. 2014;4(2):020406. doi: 10.7189/jogh.04.020406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vledder M, Friedman J, Sjoblom M, Brown T, Yadav P. Improving Supply Chain for Essential Drugs in Low-Income Countries: Results from a Large Scale Randomized Experiment in Zambia. Health Syst Reform. 2019;5(2):158–77. doi: 10.1080/23288604.2019.1596050 [DOI] [PubMed] [Google Scholar]
- 69.Attaran A. How do patents and economic policies affect access to essential medicines in developing countries? Health Aff (Milwood). 2004;23(3):155–66. doi: 10.1377/hlthaff.23.3.155 [DOI] [PubMed] [Google Scholar]
- 70.Mackey TK, Liang BA. Patent and exclusivity status of essential medicines for non-communicable disease. PLoS One. 2012;7(11):e51022. doi: 10.1371/journal.pone.0051022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beall RF, Schwalm JD, Huffman MD, McCready T, Yusuf S, Attaran A. Could patents interfere with the development of a cardiovascular polypill? J Transl Med. 2016;14(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data is presented within the manuscript. Extracted data is included as supplementary material.