Abstract
Healthcare facilities are important sites for the transmission of pathogens spread via bioaerosols, such as Mycobacterium tuberculosis. Natural ventilation can play an important role in reducing this transmission. We aimed to measure rates of natural ventilation in clinics in KwaZulu-Natal and Western Cape provinces, South Africa, then use these measurements to estimate Mycobacterium tuberculosis transmission risk. We measured ventilation in clinic spaces using a tracer-gas release method. In spaces where this was not possible, we estimated ventilation using data on indoor and outdoor carbon dioxide levels. Ventilation was measured i) under usual conditions and ii) with all windows and doors fully open. Under various assumptions about infectiousness and duration of exposure, measured absolute ventilation rates were related to risk of Mycobacterium tuberculosis transmission using the Wells-Riley Equation. In 2019, we obtained ventilation measurements in 33 clinical spaces in 10 clinics: 13 consultation rooms, 16 waiting areas and 4 other clinical spaces. Under usual conditions, the absolute ventilation rate was much higher in waiting rooms (median 1769 m3/hr, range 338–4815 m3/hr) than in consultation rooms (median 197 m3/hr, range 0–1451 m3/hr). When compared with usual conditions, fully opening existing doors and windows resulted in a median two-fold increase in ventilation. Using standard assumptions about infectiousness, we estimated that a health worker would have a 24.8% annual risk of becoming infected with Mycobacterium tuberculosis, and that a patient would have an 0.1% risk of becoming infected per visit. Opening existing doors and windows and rearranging patient pathways to preferentially use better ventilated clinic spaces result in important reductions in Mycobacterium tuberculosis transmission risk. However, unless combined with other tuberculosis infection prevention and control interventions, these changes are insufficient to reduce risk to health workers, and other highly exposed individuals, to acceptable levels.
Introduction
Healthcare facilities bring together infectious and susceptible individuals and are important sites for the transmission of airborne pathogens. This is particularly true for Mycobacterium tuberculosis (Mtb) in settings with a high TB burden [1]. Clinic attendees, including vulnerable populations such as people living with HIV, may inhale bioaerosols containing Mtb produced by individuals with pulmonary tuberculosis (TB) attending the same facility [1–5]. Mtb is an important occupational health concern, with health workers at greater risk than the general population of infection and reinfection and, as a result, developing active TB disease [6,7].
By increasing ventilation rates in indoor congregate spaces, transmission of airborne pathogens, such as Mtb, Rubeola virus (measles), and SARS-CoV-2, can be reduced [8–12]. However, ventilation rates are difficult to measure and there are limited data available on ventilation rates in public spaces, such as clinics, in sub-Saharan Africa [13–18].
Natural ventilation plays a key role in low and middle-income settings where the resources and infrastructure needed for mechanical ventilation systems are usually unavailable [19]. Natural ventilation can have comparable or superior performance to mechanical ventilation systems [19]. However, rates will vary with changes in wind speed or direction, and inadequate rates of natural ventilation may occur in poorly designed buildings [20].
We aimed to describe the ventilation of waiting areas, consultation rooms, and other clinical spaces across ten clinics that provide primary healthcare in South Africa. Our ventilation experiments were undertaken as part of an interdisciplinary project called Umoya omuhle (meaning “good air” in isiZulu), which used a whole systems approach to understand Mtb transmission and TB Infection prevention and control in primary healthcare clinics in South Africa [21,22].
Methods
Ethics statement
The Umoya omuhle study received ethical approval from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (ref. BE082/18), the Human Research Ethics Committee of the Faculty of Health Sciences of the University of Cape Town (ref. 165/2018), the Research Ethics Committee of Queen Margaret University (ref. REP 0233), and the Observational/Interventions Research Ethics Committee of the London School of Hygiene & Tropical Medicine (ref. 14872). No data on individuals were captured for this aspect of the project. Consent to undertake these experiments was obtained from facility managers.
Setting
South Africa has one of the highest incidence of both TB disease and HIV-associated TB in the World [23]. The prevalence of bacteriologically confirmed pulmonary TB in South Africa was 852 (95% CI 679–1026) per 100 000 population in the 2017–19 national prevalence survey [24]. As part of the Umoya omuhle project, a TB prevalence study was performed at two clinics in 2018–19. The prevalence of TB in clinic attendees in rural KwaZulu-Natal was 1.0%, which was similar to TB prevalence in the surrounding community [25].
In this study, ventilation experiments were performed in five clinics in KwaZulu-Natal and five in Western Cape province, South Africa, between December 2018 and December 2019. These clinics were built between the 1980s and early 2010s and serve both urban and rural populations. The rural clinics serve an estimated 3000 to 8000 attendees per month and the peri-urban/urban clinics serve 1000 to 30,000 attendees per month (Table 1). They were selected to be broadly representative of clinics in the two provinces with respect to location, size, age of building, and type of clinic. Each clinic had a unique design, though some clinics had several rooms that were identical in their layout. Many clinics had a mixture of permanent and temporary buildings. Temporary buildings included pre-fabricated structures that were used to rapidly expand clinic capacity as the antiretroviral therapy programme was being rolled out in the 2000’s [26].
Table 1. Characteristics of facilities (n = 10) and rooms (n = 33) in which ventilation experiments were conducted.
| Facility code | Decade built | Location | Estimated monthly headcount, n (1000s) | Type of clinic | Rooms studied (n = 33), n | Rooms where CO2 release experiments performed (n = 26), n | Rooms where rebreathed fraction experiments performed (n = 8), n |
|---|---|---|---|---|---|---|---|
| KZN1 | 1990s | Peri-urban | 11–14 | PHC | 5 | 4 | 1 |
| KZN2 | 1980s | Rural | 5–8 | PHC | 7 | 7 | 0 |
| KZN3 | 2000s | Peri-urban | 27–30 | CHC | 3 | 2 | 1 |
| KZN4 | 1980s | Urban | 27–30 | CHC | 2* | 2 | 1 |
| KZN6 | 2000s | Rural | 3–6 | PHC | 2 | 2 | 0 |
| WC1 | 2010s | Peri-urban | 25–28 |
PHC |
3 | 1 | 2 |
| WC2 | 2000s | Urban | 1–3 | PHC | 2 | 1 | 1 |
| WC3 | 1980s | Peri-urban | 2–5 | PHC | 5 | 4 | 1 |
| WC5 | 2010s | Urban | 27–30 | CHC | 2 | 1 | 1 |
| WC6 | 2000s | Peri-urban | 25–28 | PHC | 2 | 2 | 0 |
CHC: Community health clinic; PHC: Primary healthcare clinic.
*Both tracer gas release and rebreathed fraction experiments were performed in the same room.
Measurements were undertaken in spaces where patients either waited or interacted with clinic staff. These included: rooms where patient vital signs were measured; rooms where phlebotomy was performed; formal and informal waiting areas; and consultation rooms. The timing of experiments was agreed in advance with clinic managers.
Weather
KwaZulu Natal has a subtropical climate, while the Western Cape has a more temperate climate. Data on wind speed and temperature were provided by the South African Weather Services for the period 01 January 2018 to 31 December 2020. These consisted of hourly measurements taken at the nearest weather station to each clinic (median Euclidean distance: 20.5km [range: 5-35km]). Using these data, the wind speed and temperature at the time experiments were undertaken were compared to the distribution observed during typical clinic opening hours (0600–1800) across this three-year period.
Describing spaces
The dimensions of each room were measured using a Bosch PLR 40R digital laser measure (Bosch, Gerlingen, Germany; accuracy +/-2 mm) and room volumes calculated. The configuration of the windows and doors when the room was in routine use was recorded–these were then used as the ‘usual’ conditions in our experiments.
To summarise these data, window configuration was classified into one of four categories: 1) open (all windows fully open); 2) closed (all windows closed); 3) half or less open; or 4) more than half open. For example, if there were three windows, one closed and two half-open, the windows would be 33% open [(0 + 0.5 + 0.5) / 3], so the windows would be classified as ‘half or less open’. The same classification was used for doors.
Measuring ventilation
Tracer gas release experiments
Our primary means of measuring ventilation was a tracer gas release technique using carbon dioxide (CO2; Table A in S1 Text) [19,27]. Ventilation was estimated under both ‘usual’ conditions (the configuration of doors and windows observed when the room was in routine use) and ‘ideal’ conditions (with existing windows and doors maximally open; Table B in S1 Text). Air-conditioners were not switched on during experiments. Standard air-conditioners circulate air and have little effect on room ventilation.
At the start of each experiment, windows and doors were closed and any remaining openings were taped closed with plastic sheeting. CO2 monitors were placed at two different central locations within the room (Datalogging Indoor Air Quality Meter– 800050, Sper Scientific, Scottsdale, Arizona, USA; accuracy +/- 75 parts per million [ppm] CO2). The concentration of CO2 in the room was then increased by releasing CO2 fire extinguishers for 5–10 seconds. CO2 was mixed with room air using a paddle fan for two minutes, with the aim of achieving a stable and homogeneous CO2 concentration of 3,000–10,000 ppm throughout the space. The fan was then turned off and, after five minutes, any plastic sheeting was removed, and the windows and doors were placed in either the usual or ideal configuration. CO2 levels were then recorded every second for a period of approximately five minutes. The aim was to perform three experiments under usual conditions, and three under ideal conditions, yielding six CO2 decay curves under each set of conditions.
Rebreathed fraction approach
Tracer gas release experiments were not possible in spaces that 1) could not be vacated by patients or clinic staff or 2) were large and very open to the outdoors, meaning high levels of CO2 could not be attained at baseline. Where tracer gas release experiments were not possible, an established approach [28,29] was adapted to estimate the ventilation rate using data on indoor CO2 concentrations, outdoor CO2 concentrations, and occupancy [30]. These experiments were undertaken in eight main waiting areas during working hours when spaces were occupied by patients and staff.
In these waiting areas, three CO2 monitors were placed at different central locations and one monitor placed immediately outside the building to capture the CO2 concentration in the replacement air. Measurements were obtained every second. The number of individuals in the room was counted every ten minutes, categorising individuals as aged <1 year, 1–5 years, or >5 years. Data were collected for between one and three hours under each set of conditions (usual and ideal).
Statistical analysis
The tracer gas release experiments produced data similar to those presented in Fig A in S1 Text. Analysis focused on the right-hand side of these curves. Specifically, data were included from 30 seconds after the doors and windows were opened until CO2 levels reached 200 ppm above baseline or, where no baseline value was available, until CO2 levels reached 800 ppm.
Prior to analysis, the CO2 data were smoothed, using a sixty second moving average (mean), consisting of the 30 seconds before and after each measurement. Using these smoothed data, the natural logarithms of the CO2 concentration measurements were plotted against time in hours. A linear regression line was then fitted through each trace, with the gradient of this line providing an estimate of the number of air changes per hour (ACH). With (typically) six such estimates per experiment (three experiments, two CO2 meters per experiment), a pooled estimate of the number of air changes per hour, with an associated 95% confidence interval (CI), was obtained using random effects meta-analysis.
The absolute ventilation rate was then calculated as the product of the number of air changes per hour and the room volume (see Eq 1). In this study, we have presented estimates of the absolute ventilation rate in m3/hour. We convert these estimates to litres/second, where needed, to make comparisons with published guidelines.
| (Eq 1) |
Q = absolute ventilation (volume/time); ACH = air changes per hour; Volume = room volume
Whilst ACH are commonly mentioned in discussions about ventilation and the transmission of airborne pathogens, the absolute ventilation rate is the ventilation parameter that determines risk of airborne infection. Use of ACH as a measure of ventilation can be misleading, as a small space with a high number of ACH may be less well ventilated than a larger space with fewer ACH. However, we report data on room volumes and ACH, allowing readers to understand what is driving differences in the absolute ventilation rate.
To analyse the paired indoor and outdoor CO2 measurements, we developed an adaptation of the Persily and de Jonge method [29] to allow for non-steady state conditions. The outdoor CO2 concentration was estimated as a mean obtained through curve fitting, using linear interpolation. The indoor CO2 concentration in each space was calculated at each time point by taking the mean value from the three indoor monitors. The number of individuals in each age group in the room at each time point was multiplied by their assumed CO2 generation rate to estimate the total CO2 generation rate at each time point. Age-specific CO2 production rates were taken from Persily and de Jonge [29], assuming an activity rate of 1.2 MET (occupants sitting quietly). A simple linear regression model was then fitted describing how, at each time point, the difference between the indoor CO2 concentration and the outdoor CO2 concentration related to the total CO2 generation rate. The slope of this regression line estimated the absolute ventilation rate. More details of this method are given elsewhere [30]. Note, outdoor CO2 concentrations in our experiments were measured and found to be stable. In settings where outdoor CO2 levels are more variable, investigators using this method may prefer to update the outdoor CO2 measurement at each timestep.
Most spaces were only visited on one occasion and, as such, our estimates of ventilation do not fully capture variation in ventilation rates associated with, for example, changes in wind speed or direction. For this reason, when combining data from different spaces, we opted for a descriptive presentation of our results. We avoided calculating pooled estimates with associated confidence intervals, as we did not wish to overestimate precision.
Estimates of ventilation rates per person were calculated by dividing the absolute ventilation rates by the number of people in the room. For consulting rooms, we used two people (as this was the most frequent observation) and for waiting areas, we used the highest number of people observed during our time at the clinics.
Estimating transmission risk
We related our calculated absolute ventilation rate to the risk of Mtb transmission using the Wells Riley Equation [31] (Eq 2). The Wells-Riley equation models transmission risk for airborne infections as a Poisson process. It takes into account the time spent in a space, the number of infectious individuals present, the number of ‘infectious quanta’ they produce per unit time (usually assumed), the volume of air susceptible individuals breathe per unit time, and the absolute ventilation rate [31]. Here, quanta are defined as ‘the number of infectious airborne particles required to infect which may be one or more airborne particles’ [31]. The Wells-Riley equation assumes a well-mixed airspace so cannot be used to model variation in transmission risk within a room [32].
| (Eq 2) |
P = probability for each susceptible individual of being infected over time t; I = Number of infectious individuals; q = number of infectious quanta they produce per hour; p = pulmonary ventilation rate of susceptible individuals (volume/time); t = exposure time; Q = absolute room ventilation (volume/time)
In estimating transmission risk using the Wells-Riley equation, we assumed a single infectious individual was present and that susceptible individuals had a pulmonary ventilation rate of 0.6m3/hr. With TB prevalence in these clinics approximately 1% [25] and 1000 to 30,000 attendees per month, 0–10 individuals with viable Mtb in sputum might be expected to visit these clinics each day.
The infectiousness of people with pulmonary TB is known to be heterogeneous. The standard assumption is that infectious individuals produce 1.25 infectious quanta per hour, based on data from early studies at Johns Hopkins [33]. A similar study of HIV-positive adults with pulmonary TB in Lima, Peru, reported a mean quanta production rate of 8.2 per hour, and the most infectious individual in that study produced 226 quanta per hour [34]. We, therefore, estimated transmission risk at 1.25, 8.2, and 226 quanta/hr.
We presented estimates of transmission risk in two different ways. First, we used heat maps to describe transmission risk across a range of plausible ventilation rates and visit durations. Second, we used ‘illustrative scenarios’, presenting estimated risk of Mtb infection following typical exposures: the risk of a health worker becoming infected during one month at work, and the risk of a patient becoming infected during a typical clinic visit. For the first scenario, we presented the estimated risk of a health worker becoming infected with Mtb if, on each of 25 working days, they spent 15 minutes in a consulting room with an individual with infectious pulmonary TB. For the second scenario, we presented the estimated risk of becoming infected with Mtb during 2.5 hours, a typical visit duration [35], in a waiting room with one individual with infectious pulmonary TB. In both scenarios, we assumed that neither the infectious individual nor the susceptible individual wore a mask or respirator. Risk was estimated using each of the three quanta production rates and using the ventilation rate for each room type under both usual and ideal conditions. The main estimate used data from the consulting room or waiting room with the median estimated absolute ventilation rate, but we also produced estimates of transmission risk using the best and worst ventilation rates observed under each set of conditions. Note, we did not apply the 226 q/hr quanta production rate to the health worker scenario, as it is implausible that a health worker would encounter 25 such patients in a single month.
Tools and code
Stata version 14.2 (StataCorp, College Station, Texas, USA) was used to analyse the tracer gas release experiments and to produce the heat maps. This Stata code is available on GitHub (https://github.com/tayates/uo_ventilation). Analysis of paired indoor-outdoor CO2 measurements was carried out using R version 3.6.0 [36]. This R code is also available on GitHub (https://github.com/ArminderD/ventilation.git). The data are available via https://datacompass.lshtm.ac.uk/.
Results
Setting
Ventilation experiments were performed in 33 clinic spaces between December 2018 and December 2019. Clinic characteristics are detailed in Table 1. None of these spaces were mechanically ventilated. Tracer gas release experiments were performed in 26 different clinic spaces comprising 13 (50%) consulting rooms; nine (35%) waiting areas; and four (15%) other spaces, including corridors, rooms where patients’ vital signs were measured, and phlebotomy rooms. The layout and dimensions of these rooms are described in S2 Text. Eight main waiting areas were assessed using the rebreathed fraction approach. Note, in one waiting area, we performed both tracer gas release and rebreathed fraction experiments.
Under usual conditions in the 26 rooms where the CO2 release technique was used, four rooms (15%) had no windows, two (8%) had all existing windows closed, 12 (46%) had their windows less than half open, and eight (31%) had their windows maximally open. All consulting rooms had their doors closed when attending to patients and no waiting rooms had more than half of their doors open when the room was in use (Table 2). There were two consulting rooms with wall mounted air-conditioners.
Table 2. The configuration of windows and doors under usual conditions in rooms where tracer gas release experiments were performed (n = 26 experiments on 12 days).
| Type of room | Configuration of windows | Configuration of door in rooms with one door (n = 13), n |
Configuration of doors in rooms with multiple doors (n = 13), n |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No windows |
All windows closed | Windows open half or less | All windows maximally open | Door closed | Door open | All doors closed | Half or less doors open | Greater than half doors open | |
| Consulting room (n = 13) |
0 | 2 | 6 | 5 | 11 | 0 | 0 | 1 | 1 |
| Waiting area (n = 9) | 3 | 0 | 4 | 2 | 0 | 0 | 0 | 7 | 2 |
| Other (n = 4)* | 1 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 2 |
| Total (n = 26) | 4 | 2 | 12 | 8 | 12 | 1 | 0 | 8 | 5 |
*Other rooms included: Two rooms where patient vital signs were measured, one phlebotomy room, one corridor where patients waited.
Temperature and wind speed
We compared temperature and wind speed measured at the nearest weather station at the time we undertook our experiments to the 3-year mean temperature and wind speed measured during typical clinic opening hours at the same weather station.
Temperatures at the times tracer gas release experiments were conducted were within one standard deviations (SD) of the 3-year mean temperature on 10/12 (83%) experiment days and within two SDs on 12/12 (100%) experiment days. Wind speeds were within one and two SDs of the 3-year mean wind speed on 10/12 (83%) and 11/12 (92%) experiment days, respectively (Fig 1).
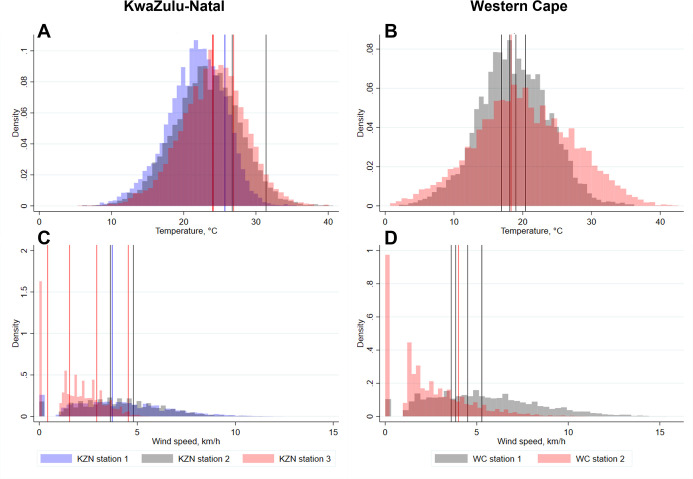
Fig 1. Histograms showing the distribution of temperatures and wind speeds in KwaZulu-Natal and Western Cape during working hours from January 2018–December 2020.
Vertical lines show the mean temperatures and wind speeds on the 12 days when tracer gas release experiments were conducted. A: Temperatures in KwaZulu-Natal; B: Temperatures in Western Cape; C: Wind speeds in KwaZulu-Natal; D: Wind speeds in Western Cape. Vertical lines indicate mean temperature or wind speed at the weather station closest to each clinic during working hours on the day the data were collected. Colour of histograms and vertical lines corresponds to specific weather stations in each province. C: Centigrade; km/h: Kilometres per hour; KZN: KwaZulu-Natal; WC: Western Cape.
Temperatures at the time we performed rebreathed fraction experiments were within one and two SDs of the 3-year mean temperature for 7/8 (88%) and 8/8 (100%) experiment days and wind speeds within one and two SDs of the 3-year mean wind speed on 7/8 (88%) and 8/8 (100%) of experiment days (Fig B in S1 Text).
Whilst not far from the seasonal average, the days on which we undertook tracer gas release experiments in KwaZulu-Natal were all towards the warmer end of the distribution.
Tracer gas release experiments
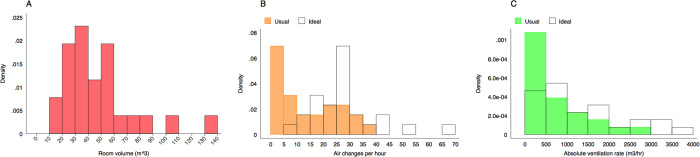
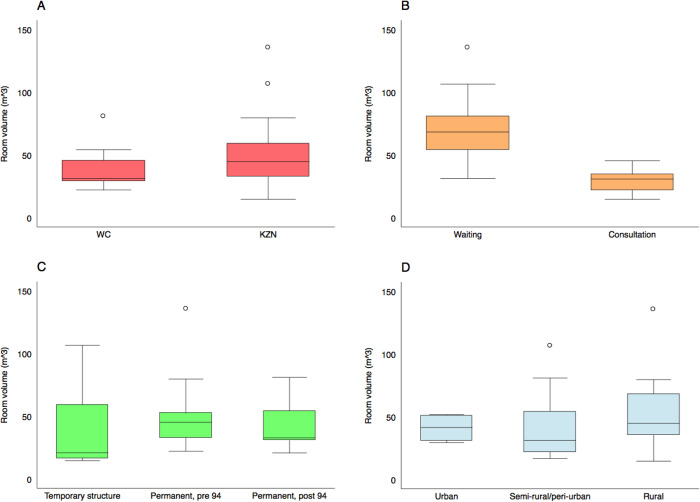
The distribution of room volumes across the 26 spaces in which we performed tracer gas release experiments is presented in Fig 2A. Box and whisker plots in Fig 3 present the same results disaggregated by province, type of room, the age of the building, and whether the community is urban or rural. The distribution of room sizes was skewed with a small number of much larger rooms. As might be expected, consultation rooms (n = 13; median volume 31 m3, range 15–45 m3) were smaller than waiting rooms (n = 9; median volume 68m3, range 31–135 m3). Rooms in temporary structures (n = 5; median volume 21 m3, range 15–106 m3) were smaller than those in permanent structures (n = 21; median volume 43 m3, range 21–135 m3).
Fig 2.
Histograms describing the distribution of room volumes (Fig 2A), the number of air changes per hour (Fig 2B) and the absolute ventilation rate (Fig 2C) in the 26 clinical spaces where we conducted tracer gas release experiments. Ventilation rates are described under usual conditions and ideal conditions (all doors and windows fully open).
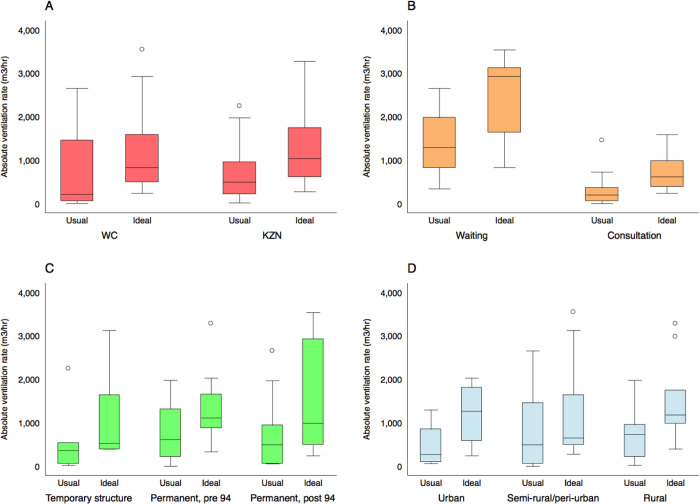
Fig 3. Box and whisker plots describing the distribution of room volumes in the clinical spaces where we conducted tracer gas release experiments.
Results are disaggregated by province (Fig 3A), room type (Fig 3B), the age of the building (Fig 3C), and clinic location (Fig 3D). Here, the boxes mark the 25th percentile, the 50th percentile (median) and the 75th percentile, with the whiskers marking the upper and lower adjacent values.
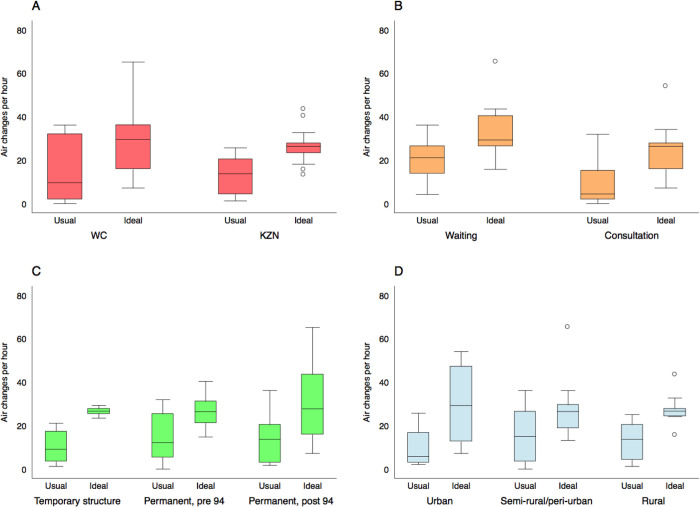
The distribution of the number of ACH under both usual and ideal conditions is presented in Fig 2B. The same data disaggregated by province, type of room, the age of the building, and whether the community is urban or rural are presented in Fig 4.
Fig 4. Box and whisker plots describing the distribution of the number of air changes per hour in the 26 clinical spaces where we conducted tracer gas release experiments.
Ventilation rates are described under usual conditions and with all doors and windows fully open. Results are disaggregated by province (Fig 4A), room type (Fig 4B), the age of the building (Fig 4C), and clinic location (Fig 4D). Here, the boxes mark the 25th percentile, the 50th percentile (median) and the 75th percentile, with the whiskers marking the upper and lower adjacent values.
Note, in one consultation room, ‘usual conditions’ were that existing windows and doors were closed leaving no effective ventilation. The tracer gas release experiments performed under these conditions saw CO2 levels rise, as air exhaled by investigators accumulated in the room. Fitting a line through these data would have yielded a negative ACH estimate. We therefore assigned this space a ventilation rate of 0 ACH and 0 m3/hr.
The number of ACH varied substantially. It was higher in waiting rooms than in consultation rooms (usual conditions: median ACH in waiting rooms = 21, range 4–36; median ACH in consultation rooms = 4, range 0–32) and, as expected, higher with existing doors and windows fully open (ideal conditions: median ACH in waiting rooms = 29, range 16–65; median ACH in consultation rooms = 26, range 7–54). Opening existing doors and windows resulted in a median 2.1-fold increase in the number of air changes per hour (range 1–25.6; note, this range does not include the consultation room with zero effective ventilation under usual conditions).
The distribution of the absolute ventilation rates is presented in Fig 2C. These data, disaggregated by province, type of room, the age of the building, and whether the community is urban or rural, are presented in Fig 5. Again, we observed substantial variation. The absolute ventilation rate was much higher in waiting rooms (usual conditions: median absolute ventilation rate = 1289 m3/hr, range 338–2651 m3/hr) than in consultation rooms (usual conditions: median absolute ventilation rate 197 m3/hr, range 0–1451 m3/hr). Temporary buildings (usual conditions: median absolute ventilation rate 360 m3/hr, range 18–2240 m3/hr) were less well ventilated than permanent structures (usual conditions: median absolute ventilation rate 493 m3/hr, range 0–2651 m3/hr).
Fig 5. Box and whisker plots describing the distribution of the absolute ventilation rate in the 26 clinical spaces where we conducted tracer gas release experiments.
Ventilation rates are described under usual conditions and with all doors and windows fully open. Results are disaggregated by province (Fig 5A), room type (Fig 5B), the age of the building (Fig 5C), and clinic location (Fig 5D). Here, the boxes mark the 25th percentile, the 50th percentile (median) and the 75th percentile, with the whiskers marking the upper and lower adjacent values.
The observation that rooms in temporary structures were smaller and had a lower absolute ventilation rate should be treated with caution, as we only obtained measurements in five clinical spaces in temporary structures: 2 waiting rooms, 2 consulting rooms, and one space where vitals were measured.
Table C in S1 Text contains full results for each space in which tracer gas release experiments were performed. Fig C in S1 Text shows the association between wind speed and the absolute ventilation rate for the 26 spaces in which we undertook tracer gas release experiments.
Rebreathed fraction experiments
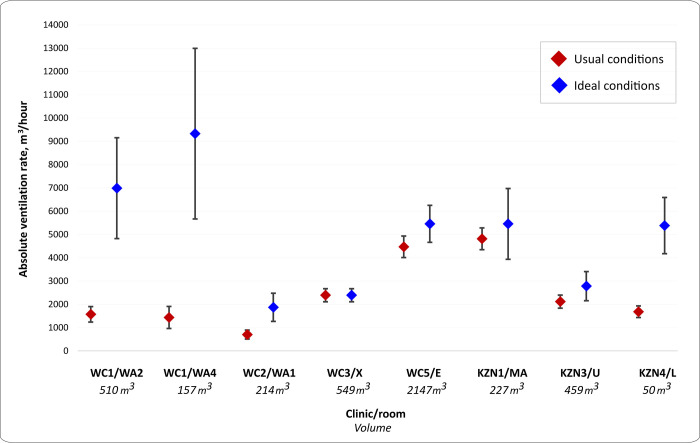
The estimated absolute ventilation rates in the eight clinic waiting rooms where the rebreathed fraction approach was used are presented in Fig 6 and Table D in S1 Text. The volume of these waiting rooms was a median 342.7 (range 50.2–2147.3) m3. Under usual conditions, there were a median 4.5 ACH (range 2.1–33.5), which improved under ideal conditions to a median 11.2 ACH [range 2.5–107.2]. Absolute ventilation rates were lower under usual conditions than under ideal conditions (median 1898.8 [range 701.5–4815.1] m3/hr vs. 5417.8 [range 1870.3–9328.4] m3/hr, respectively). Fully opening existing doors and windows improved the absolute ventilation rate by a median 2.0 fold (range 1.0–6.5).
Fig 6. Absolute ventilation rates of eight clinic waiting rooms under usual and ideal conditions, estimated by the rebreathed fraction approach.
Usual conditions = configuration of windows and doors observed when the room was in routine use. Ideal conditions = all windows and doors maximally open. Clinic WC3, room X: Usual conditions the same as ideal conditions. Vertical bars indicate upper and lower estimates of 95% confidence intervals WC: Western Cape; KZN: KwaZulu-Natal; WA2, WA4, WA1, X, E, MA, U, L are the codes for the specific waiting rooms where experiments were performed.
Note, one waiting room (KZN4, Room L) had ventilation estimated using both the tracer gas release and the rebreathed fraction approach. Where results from experiments using both methods are combined, we use the results of the tracer gas release experiments for this space.
Estimated risk of Mycobacterium tuberculosis transmission
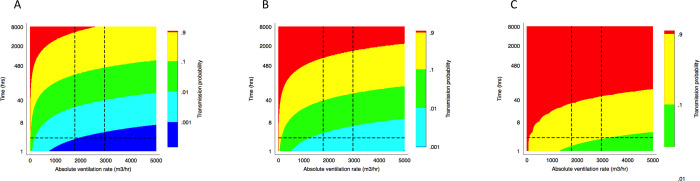
We related absolute ventilation rate and visit duration to the risk of Mtb transmission using the Wells Riley Equation [31], as described above. We produced estimates of infection risk under various assumptions about the quanta production rate (Fig 7). The horizontal lines on the figures give the median clinic visit duration of 2 hours 36 minutes [35]. The vertical lines give the median absolute ventilation rate in waiting rooms under both usual and ideal conditions. Combining data from both the tracer gas release experiments and the rebreathed fraction approach, this figure is 1769 m3/hr under usual conditions and 2950 m3/hr under ideal conditions.
Fig 7.
A set of heat maps translating ventilation rate into transmission risk, as estimated using the Wells-Riley equation at 1.25 (A), 8.2 (B) and 226 (C) quanta/hr. The horizontal lines show the median clinic visit duration. The vertical lines show the median absolute ventilation rate in clinic waiting rooms under both usual and ideal conditions.
Assuming a quanta production rate of 1.25 q/hr, that doors and windows were opened to their usual extent, and taking ventilation estimates from the consulting room with the median absolute ventilation rate, the healthcare worker in our illustrative scenario had a 2.3% risk of becoming infected with Mtb per month. The equivalent risks in the best and worst ventilated consulting rooms were 0.3% and 100% per month. Under the same assumptions, our illustrative patient had a 0.1% chance of becoming infected per visit, with the equivalent risk in the best and worst ventilated waiting rooms being 0.0% and 0.6% per visit. The risks with doors and windows fully opened were 0.8% per month (range of health worker risk across the different consulting rooms 0.3–2.0%) and 0.1% per visit (range of patient risk across the different waiting rooms 0.0–0.2%). Estimates of transmission risk were very sensitive to assumptions about the quanta production rate. For example, using 226 q/hr, and the median absolute ventilation rate, gave our illustrative patient a 17.6% risk of becoming infected per visit with doors and windows opened to their usual extent. Fully opening existing door and windows reduced this risk to 10.9%. Full results from the illustrative scenarios are presented in Tables E and F in S1 Text.
Discussion
We observed substantial variation between spaces in rates of natural ventilation. Under usual conditions, using the gold standard tracer gas release method, the median number of ACH was 11.7 and the median absolute ventilation rate was 447 m3/hr. There were higher ventilation rates in waiting rooms (median Q = 1289 m3/hr) than in consultation rooms (median Q = 197 m3/hr). In the eight waiting rooms where we used the rebreathed fraction approach, the median absolute ventilation rate, under usual conditions, was 1898.8 m3/hr. We showed that ventilation rates could, on average, be doubled if existing doors and windows were opened.
There are few published estimates of the absolute ventilation rate in naturally ventilated public spaces, including clinical spaces, in high burden settings. In Peruvian hospitals, Escombe and colleagues estimated an absolute ventilation rate of 2477 m3/hr in naturally ventilated spaces, and 402 m3/hr in mechanically ventilated spaces [19]. In a study of four small clinic rooms in Cape Town, South Africa, Cox and colleagues estimated a median ventilation rate of 487 m3/hr in the best ventilated space, but none of these experiments were conducted with both the window and the wind-driven roof turbines open [14].
The latest World Health Organization (WHO) guidelines on TB infection prevention and control (TB IPC), published in 2019, do not contain a recommended minimum ventilation rate for clinical spaces [10]. A 2009 WHO guideline recommended that ‘general wards and outpatient spaces’ in naturally ventilated buildings should aim for a minimum ventilation rate of 60 litres/second/person (216 m3/hr/person) [8]. For ‘airborne precaution rooms’ (small rooms for accommodating people who may be infectious) the recommended ventilation rate was 160 litres/second/person on average (576 m3/hr/person), with a minimum rate of 80 litres/second/person (288 m3/hr/person) when wind speed and direction are not favourable. In high burden settings, the transmission risk in general clinic spaces may be equivalent to that in ‘airborne precaution rooms’, given the high prevalence of undiagnosed TB [25].
Dividing the ventilation rates estimated in this study by the per person rates recommended by the WHO yields estimates of maximum occupancy that are lower than those we observed in these clinics. The median ventilation rate in waiting rooms (combining both measurement approaches) meant only eight people could be accommodated under usual conditions, or 13 under ideal conditions, whilst achieving a ventilation rate of 60 litres/second/person. The median ventilation rate in consultation rooms meant doors and windows needed to be fully opened for a health worker and one patient to be accommodated whilst achieving a ventilation rate of 60 litres/second/person. We undertook detailed analysis of patient flow in nine waiting rooms at three clinics, as part of the Umoya omuhle project. The least busy waiting area, in a rural clinic, had a median occupancy of 11 people, whereas the busiest waiting area had a median occupancy of 52 people [35]. Consultation rooms typically accommodate a single health worker plus a patient, though patients are often accompanied by a friend or family member.
We would caution against the use of binary thresholds to determine whether ventilation rates are adequate or not. As is apparent from Fig 7 and from the illustrative scenarios, Mtb infection risk depends not only on the ventilation rate but also on the duration of exposure and the infectiousness of the index case. Unacceptable levels of risk are likely with prolonged or repeated exposure, or with shorter exposure to highly infectious individuals. A nurse spending her career working only in ‘adequately’ ventilated spaces may be infected with Mtb on multiple occasions unless additional precautions, such as the use of N95 respirators, are taken. This is not to say that improvements in the natural ventilation rate may not prevent infections and consequent disease, just that they may not be sufficient to reduce risk to a level that most individuals would deem acceptable [37]. Furthermore, measurements of room ventilation taken at a single timepoint may give false reassurance, as air flow in naturally ventilated spaces will vary with changes in wind speed and direction.
We showed that natural ventilation can be improved by two-fold by fully opening available windows and doors. However, we observed that only 30% of spaces had their windows fully open and all consulting room doors were closed when consultations were taking place. Major barriers to opening existing doors and windows include concerns regards privacy and thermal comfort [38]. Detailed qualitative research exploring TB IPC related beliefs and behaviours in the same clinics will be presented separately.
Our Wells-Riley estimates of Mtb transmission risk suggest that substantial reductions in transmission risk can be achieved with both improvements in natural ventilation and reductions in duration of clinic visits (Fig 7). Using a standard assumption about infectiousness (1.25 q/hr), and using ventilation estimates from the consultation room with the median absolute ventilation rate, the estimated risk of infection in a health worker is 2.3% per month (24.8% annual risk of infection), or 0.8% per month (8.7% annual risk of infection) with existing doors and windows fully opened. This is worryingly high. To put these figures into context, standard methods estimate an annual risk of infection in the general population of 0.5–2% [39] in high-burden countries, with a slightly higher force of infection reported in community surveys in the Western Cape [40–42]. Whilst standard methods may underestimate the extent of community transmission [39], the risk to health workers is clearly much higher. Our calculations are consistent with empirical estimates of the force of infection among South African health workers, which suggest 29% are infected with Mtb per year [7]. With shorter durations of exposure and waiting rooms being better ventilated than consulting rooms, risk to patients was lower. However, patients attending at the same time as particularly infectious individuals with pulmonary TB (226 q/hr), using ventilation estimates from the waiting room with the median absolute ventilation rate, had a calculated risk of 17.4% per visit, or 10.9% if existing windows and doors were opened fully.
Estimated transmission risk was much higher using ventilation estimates from less well-ventilated spaces. The substantial variation in ventilation rates observed among spaces within clinics suggest that transmission risk might be reduced by reorganising care so that patients spend more time in better ventilated spaces. Low-cost adaptations to existing structures should also be considered including, where feasible, creating covered outdoor waiting areas. A study of small, poorly-ventilated clinic rooms in Cape Town, South Africa, demonstrated improvements in natural ventilation associated with use of wind driven roof turbines or ‘Whirlybird’ fans [14]. A study in Lima, Peru, measured ventilation before and after making changes to six clinical spaces. These changes ranged in intensity from repairing windows that could not be opened at a cost of USD 25, to building a sheltered outdoor waiting area at a cost of USD 7000. The changes resulted in a median 3.0 fold increase in the ventilation rate [43].
However, as has been argued elsewhere [37], in the absence of other precautions (e.g. consistent use of N95 respirators), very high ventilation rates are needed to protect individuals with prolonged exposure, such as health workers. Where feasible, this might be achieved by moving waiting areas outdoors. The use of upper room germicidal ultraviolet (UVGI) has been advocated where there are constraints on the degree of natural ventilation that can be achieved [38]. This technology is effective at reducing Mtb transmission [44,45], and can offer protection on calm days and with doors and windows closed. However, recent experience in South Africa of low-quality installations and poor maintenance led to it falling out of favour [46]. UVGI implementation in resource limited settings is an active area of research and innovative approaches may facilitate easier installation and maintenance [47].
As part of the wider Umoya omuhle project, we have modelled the impact of TB IPC interventions implemented in clinics, including improvements in natural ventilation, on Mtb transmission both within clinics [48] and in surrounding communities [49]. These interventions are highly cost effective [50]. This work considers the infrastructural, organisational, and behavioural changes needed for such interventions to be sustainable [50]. These results support additional investment in a package of IPC interventions to limit the transmission of Mtb and other airborne pathogens. Ideally, these interventions would be introduced in manner that allowed robust estimation of their impact, as has previously been done during the national roll out of major interventions in South African healthcare system [51].
Whilst we only undertook ventilation measurements at ten clinics, these included: both new and old buildings, temporary and permanent structures, in urban and rural clinics across two provinces. We did not include clinics in higher altitude inland parts of South Africa, where the winters can be much colder. CO2 release experiments are a robust approach to measuring ventilation, though may overestimate dilutional ventilation if some of the replacement air comes from other occupied spaces (e.g., a waiting area adjoining a consultation room). However, the approach is labour intensive, technically demanding, and not possible in large open spaces or spaces that cannot be emptied of people. For this reason, we obtained ventilation estimates using this approach in a limited number of spaces and, usually, only on a single visit. As such, we will not have fully captured the variability in ventilation that might be expected with changes in weather and season. However, considering the full set of spaces that were studied, we obtained measurements over the course of a calendar year. The days on which we took our measurements were broadly representative with respect to temperature and wind speed, although the days we undertook tracer gas release experiments at clinics in KwaZulu-Natal tended to be warmer than average. Mathematically, the rebreathed fraction approach should give comparable results, though it required assumptions about occupants’ metabolic rate. Direct comparison between the two approaches, in the same space and with the same weather conditions, was not possible, because one method required the space to be occupied and other required it to be empty. It should be noted that while the confidence intervals around the estimates obtained using the rebreathed fraction approach were frequently large, this reflects changes in ventilation rates that occurred over the measurement period–for instance, due to changes in wind speed–as well as the precision of our measurements.
Simpler approaches to estimating the absolute ventilation rate are needed. Several have been proposed, such as simply measuring indoor CO2 levels [52], or estimating transmission risk using the ‘rebreathed fraction’ approach described by Rudnick and Milton [28]. However, these approaches do not partition risk into that caused by poor ventilation versus that caused by overcrowding, problems with distinct solutions. A single infectious person may transmit to a single susceptible person in a poorly ventilated space with the low room occupancy only resulting in modest rises in background CO2 levels. Furthermore, in our experience, rebreathed fraction measurements may be difficult to interpret where there is rapid flux in levels of occupancy–i.e., in small consultation rooms where patients enter and leave frequently.
Ideally, approaches to estimate absolute ventilation rates are needed that do not depend on costly equipment; that can be performed quickly by clinic staff in occupied spaces with minimal training; and with immediate results to guide risk reduction interventions, such as reducing occupancy or opening windows. A possible approach advocated in WHO documents [8,9] makes a set of simple calculations based on wind speed, which must be measured, and the area of the windows of windows and doors. No validation data are presented, and the method can only be applied in spaces with openings on opposite walls. One alternative might be a simplified tracer gas release experiment, ideally using an inert substance not usually present in room air. This might mean that small volumes of tracer gas could be released with the room in routine use. We wonder whether concentrations might then be measured using a meter adapted to feed data to a smartphone, with a phone application used to interpret the decay curve. Obtaining measurements on still days would yield an approximation of the minimum ventilation rate in these naturally ventilated spaces, which is the parameter of greatest interest when estimating transmission risk.
In conclusion, we observed substantial variation in natural ventilation in clinical spaces in primary healthcare facilities in two provinces of South Africa. The worst ventilated spaces were small rooms where doors and windows had been closed. Opening all existing doors and windows resulted in meaningful improvements in ventilation. However, there remained an unacceptable risk of infection in individuals with longer exposure and in those exposed to particularly infectious index cases. A package of IPC interventions, combining improvements in natural ventilation; reorganising care so patients wait in better ventilated spaces or outdoors; and other approaches, including clinic decongestion; are likely needed to reduce Mtb transmission to acceptable levels in these settings. In future work, we plan to model adaptations to clinic structures that maximise ventilation without compromising thermal comfort.
Supporting information
Table A: Differences between the two methods used to measure ventilation in the clinics. Table B: Definitions of usual and ideal conditions. Table B: Definitions of usual and ideal conditions. Table C: Absolute ventilation rates of the clinic spaces under usual and ideal conditions using the tracer gas release technique. Table D: Ventilation rates using the rebreathed fraction approach using the continuous measurements under usual and ideal conditions. Table E: Risk of Mycobacterium tuberculosis infection for a healthcare worker spending 15 minutes per day for 25 working days with an infectious individual, calculated using the Wells-Riley equation. The probability of infection was calculated separately for each space using the measured absolute ventilation rates under usual and ideal conditions. We calculated risk using both a standard assumption about infectiousness (1.25 quanta/hr) and using a higher estimate taken from the published literature (8.2 quanta/hr). Table F: Risk of Mycobacterium tuberculosis infection for a patient spending 2.5 hours in a waiting room with an infectious individual, calculated using the Wells-Riley equation. The probability of infection was calculated separately for each space, using the measured absolute ventilation rates under usual and ideal conditions. We used both a standard assumption about infectiousness (1.25 quanta/hr) plus two higher estimates from the published literature (8.2 quanta/hr and 226 quanta/hr). Fig A: an example of a tracer gas release experiment from Room C at KZN2 clinic (experiment 6 Monitor a). Following log transformation, the right hand side of the curve is approximately linear. Fig B: Histograms showing the distribution of temperatures and wind speeds in KwaZulu-Natal and Western Cape during working hours from January 2018 –December 2020. Vertical lines shows the mean temperatures and wind speeds on the 8 days when the rebreathed approach experiments were conducted. Fig C: Association between wind speed and the absolute ventilation rates.
(PDF)
(PDF)
Acknowledgments
We are grateful to the clinical and management staff at 10 clinics where we obtained ventilation measurements. We thank Thomas Murray, Harriet Gliddon, and Sinethemba Mabuyakhulu who assisted us with ventilation measurements in KZN. We are grateful to Rod Escombe, Ed Nardell, Jon Taylor, Don Milton, and Toby van Reenen for useful discussions about various aspects of ventilation science–they take no responsibility for the content of this manuscript.
Data Availability
The data from this paper are available via https://datacompass.lshtm.ac.uk/. Analysis of the tracer gas release experiments was undertaken in Stata version 14.2 (StataCorp, College Station, Texas, USA). This Stata code is available on GitHub (https://github.com/tayates/uo_ventilation). Analysis of paired indoor-outdoor CO2 measurements was carried out using R version 3.6.0 [29].This R code is also available on GitHub (URL: https://github.com/ArminderD/ventilation.git).
Funding Statement
The Umoya Omuhle project is supported by the Economic and Social Research Council (UK) (grant reference: ES/P008011/1 to AG). The project is partly funded by the Antimicrobial Resistance Cross Council Initiative supported by the seven research councils in partnership with other funders including support from the Global Challenges Research Fund (GCRF). TAY is an NIHR Clinical Lecturer supported by the National Institute for Health Research (UK). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care. NM is additionally funded by the Wellcome Trust (218261/Z/19/Z). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet (London, England). 2006;368: 1575–1580. doi: 10.1016/S0140-6736(06)69573-1 [DOI] [PubMed] [Google Scholar]
- 2.Bock NN, Jensen PA, Miller B, Nardell E. Tuberculosis Infection Control in Resource‐Limited Settings in the Era of Expanding HIV Care and Treatment. J Infect Dis. 2007;196: S108–S113. doi: 10.1086/518661 [DOI] [PubMed] [Google Scholar]
- 3.McCreesh N, Grant AD, Yates TA, Karat AS, White RG. Tuberculosis from transmission in clinics in high HIV settings may be far higher than contact data suggest. Int J Tuberc lung Dis. 2020;24: 403–408. doi: 10.5588/ijtld.19.0410 [DOI] [PubMed] [Google Scholar]
- 4.Mzembe T, Mclean E, Khan PY, Koole O, Sichali L, Mwinuka V, et al. Risk of Mycobacterium tuberculosis transmission in an antiretroviral therapy clinic. AIDS. 2018;32: 2417–2421. doi: 10.1097/QAD.0000000000002006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bantubani N, Kabera G, Connolly C, Rustomjee R, Reddy T, Cohen T, et al. High rates of potentially infectious tuberculosis and multidrug-resistant tuberculosis (MDR-TB) among hospital inpatients in KwaZulu Natal, South Africa indicate risk of nosocomial transmission. PLoS One. 2014;9: 1–7. doi: 10.1371/journal.pone.0090868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uden L, Barber E, Ford N, Cooke GS. Risk of Tuberculosis Infection and Disease for Health Care Workers: An Updated Meta-Analysis. Open Forum Infect Dis. 2017;4: 1–7. doi: 10.1093/ofid/ofx137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarthy KM, Scott LE, Gous N, Tellie M, Venter WDF, Stevens WS, et al. High incidence of latent tuberculous infection among South African health workers: an urgent call for action. Int J Tuberc Lung Dis. 2015;19: 647–653. doi: 10.5588/ijtld.14.0759 [DOI] [PubMed] [Google Scholar]
- 8.Atkinson J, Chartier Y, Lúcia Pessoa-Silva C, Jensen P, Li Y, Seto W-H. Natural Ventilation for Infection Control in Health-Care Settings. Geneva: World Health Organization. 2009;1. [PubMed] [Google Scholar]
- 9.Roadmap to improve and ensure good indoor ventilation in the context of COVID-19. Geneva. World Health Organization;2021. Licence: CC BY-NC-SA 3.0 IGO. Available from: https://apps.who.int/iris/handle/10665/339857. 2021.
- 10.WHO guidelines on tuberculosis infection prevention and control, 2019 update, Geneva: World Health Organization; 2019. License: CC BY-NC-SA 3.0 IGO. Available from: https://apps.who.int/iris/handle/10665/311259. [PubMed] [Google Scholar]
- 11.Li Y, Leung GM, Tang JW, Yang X, Chao CYH, Lin JZ, et al. Role of ventilation in airborne transmission of infectious agents in the built environment—a multidisciplinary systematic review. Indoor Air. 2007;17: 2–18. doi: 10.1111/j.1600-0668.2006.00445.x [DOI] [PubMed] [Google Scholar]
- 12.Yates TA, Tanser F, Abubakar I. Plan Beta for tuberculosis: It’s time to think seriously about poorly ventilated congregate settings. Int J Tuberc Lung Dis. 2016;20: 5–10. doi: 10.5588/ijtld.15.0494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor JG, Yates TA, Mthethwa M, Tanser F, Abubakar I, Altamirano H. Measuring ventilation and modelling M. Tuberculosis transmission in indoor congregate settings, rural KwaZulu-Natal. Int J Tuberc Lung Dis. 2016;20. doi: 10.5588/ijtld.16.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox H, Escombe R, McDermid C, Mtshemla Y, Spelman T, Azevedo V, et al. Wind-driven roof turbines: A novel way to improve ventilation for TB infection control in health facilities. PLoS One. 2012;7: 1–6. doi: 10.1371/journal.pone.0029589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson ET, Morrow CD, Ho T, Fürst N, Cohelia R, Tram KH, et al. Forced removals embodied as tuberculosis. Soc Sci Med. 2016;161: 13–18. doi: 10.1016/j.socscimed.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 16.Wood R, Morrow C, Ginsberg S, Piccoli E, Kalil D, Sassi A, et al. Quantification of shared air: A Social and environmental determinant of airborne disease transmission. PLoS One. 2014;9: 1–8. doi: 10.1371/journal.pone.0106622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews JR, Morrow C, Walensky RP, Wood R. Integrating social contact and environmental data in evaluating tuberculosis transmission in a South African township. J Infect Dis. 2014;210: 597–603. doi: 10.1093/infdis/jiu138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hella J, Morrow C, Mhimbira F, Ginsberg S, Chitnis N, Gagneux S, et al. Tuberculosis transmission in public locations in Tanzania: A novel approach to studying airborne disease transmission. J Infect. 2017;75: 191–197. doi: 10.1016/j.jinf.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 19.Escombe AR, Oeser CC, Gilman RH, Navincopa M, Ticona E, Pan W, et al. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4: 0309–0317. doi: 10.1371/journal.pmed.0040068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin H. Natural ventilation: A sustainable solution to infection control in healthcare settings? ASHRAE IAQ 2010 Conf.: 1–10. [Google Scholar]
- 21.Kielmann K, Karat AS, Zwama G, Colvin C, Swartz A, Voce AS, et al. Tuberculosis infection prevention and control: Why we need a whole systems approach. Infect Dis Poverty. 2020;9: 1–4. doi: 10.1186/s40249-020-00667-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umoya Omuhle group. Infection prevention and control for drug-resistant tuberculosis in South Africa in the era of decentralised care: A whole systems approach. [cited 19/08/22]. Available from https://www.lshtm.ac.uk/research/centres-projects-groups/uo. 2021. [Google Scholar]
- 23.WHO global lists of high burden countries for TB, multidrug/rifampicin-resistant TB (MDR/RR-TB) and TB/HIV, 2021–2025. Geneva: World Health Organization; 2021. Licence: CC BY-NCSA 3.0 IGO. [Cited 19/08/2022] Available from: https://cdn.who.int/media/do. [Google Scholar]
- 24.Moyo S, Ismail F, Van der Walt M, Ismail N, Mkhondo N, Dlamini S, et al. Prevalence of bacteriologically confirmed pulmonary tuberculosis in South Africa, 2017–19: a multistage, cluster-based, cross-sectional survey. Lancet Infect Dis. 2022;22: 1172–1180. doi: 10.1016/S1473-3099(22)00149-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Govender I, Karat AS, Olivier S, Baisley K, Beckwith P, Dayi N, et al. Prevalence of Mycobacterium tuberculosis in sputum and reported symptoms among clinic attendees compared to a community survey in rural South Africa. Clin Infect Dis. 2021. doi: 10.1093/cid/ciab970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallrauch C, Heller T, Lessells R, Kekana M, Bärnighausen T, Newell M. F ORUM High uptake of HIV testing for tuberculosis patients in an integrated primary health care HIV / TB programme in rural. SAMJ South African Med J. 2010;100: 146–147. [DOI] [PubMed] [Google Scholar]
- 27.Menzies R, Schwartzman K, Loo V, Pasztor J. Measuring ventilation of patient care areas in hospitals. Description of a new protocol. Am J Respir Crit Care Med. 1995;152: 1992–1999. doi: 10.1164/ajrccm.152.6.8520767 [DOI] [PubMed] [Google Scholar]
- 28.Rudnick SN, Milton DK. Risk of indoor airborne infection transmission estimated from carbon dioxide concentration. Indoor Air. 2003;13: 237–245. doi: 10.1034/j.1600-0668.2003.00189.x [DOI] [PubMed] [Google Scholar]
- 29.Persily A, de Jonge L. Carbon dioxide generation rates for building occupants. Indoor Air. 2017;27: 868–879. doi: 10.1111/ina.12383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deol AK, Scarponi D, Beckwith P, Yates TA, Karat AS, Yan AWC, et al. Estimating ventilation rates in rooms with varying occupancy levels: Relevance for reducing transmission risk of airborne pathogens. PLoS One. 2021;16: e0253096. Available: doi: 10.1371/journal.pone.0253096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riley EC, Murphy G, Riley RL. Airborne spread of measles in a suburban elementary school. Am J Epidemiol. 1978;107: 421–432. doi: 10.1093/oxfordjournals.aje.a112560 [DOI] [PubMed] [Google Scholar]
- 32.Sze To GN, Chao CYH. Review and comparison between the Wells-Riley and dose-response approaches to risk assessment of infectious respiratory diseases. Indoor Air. 2010;20: 2–16. doi: 10.1111/j.1600-0668.2009.00621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riley RL, Mills CC, O’Grady F, Sultan LU, Wittstadt F, Shivpuri DN. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis. 1962;85: 511–525. doi: 10.1164/arrd.1962.85.4.511 [DOI] [PubMed] [Google Scholar]
- 34.Escombe AR, Moore DAJ, Gilman RH, Pan W, Navincopa M, Ticona E, et al. The Infectiousness of Tuberculosis Patients Coinfected with HIV. PLOS Med. 2008;5 (9):e188. doi: 10.1371/journal.pmed.0050188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karat AS, McCreesh N, Baisley K, Govender I, Kallon II, Kielmann K, et al. Estimating waiting times, patient flow, and waiting room occupancy density as part of tuberculosis infection prevention and control research in South African primary health care clinics. PLOS Glob Public Heal. 2022;2: e0000684. Available: doi: 10.1371/journal.pgph.0000684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Core Team 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available: https://www.r-project.org/. [Google Scholar]
- 37.Nardell EA, Keegan J, Cheney SA, Etkind SC. Airborne infection. Theoretical limits of protection achievable by building ventilation. Am Rev Respir Dis. 1991;144: 302–306. doi: 10.1164/ajrccm/144.2.302 [DOI] [PubMed] [Google Scholar]
- 38.Nardell E, Lederer P, Mishra H, Nathavitharana R, Theron G. Cool but dangerous: How climate change is increasing the risk of airborne infections. Indoor Air. 2020;30: 195–197. doi: 10.1111/ina.12608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dowdy DW, Behr MA. Are we underestimating the annual risk of infection with Mycobacterium tuberculosis in high-burden settings? Lancet Infect Dis. 2022. doi: 10.1016/S1473-3099(22)00153-0 [DOI] [PubMed] [Google Scholar]
- 40.Middelkoop K, Bekker L-G, Myer L, Dawson R, Wood R. Rates of tuberculosis transmission to children and adolescents in a community with a high prevalence of HIV infection among adults. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2008;47: 349–355. doi: 10.1086/589750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kritzinger FE, den Boon S, Verver S, Enarson DA, Lombard CJ, Borgdorff MW, et al. No decrease in annual risk of tuberculosis infection in endemic area in Cape Town, South Africa. Trop Med Int Health. 2009;14: 136–142. doi: 10.1111/j.1365-3156.2008.02213.x [DOI] [PubMed] [Google Scholar]
- 42.Shanaube K, Sismanidis C, Ayles H, Beyers N, Schaap A, Lawrence K-A, et al. Annual risk of tuberculous infection using different methods in communities with a high prevalence of TB and HIV in Zambia and South Africa. PLoS One. 2009;4: e7749. doi: 10.1371/journal.pone.0007749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Escombe AR, Ticona E, Chávez-Pérez V, Espinoza M, Moore DAJ. Improving natural ventilation in hospital waiting and consulting rooms to reduce nosocomial tuberculosis transmission risk in a low resource setting. BMC Infect Dis. 2019;19: 1–7. doi: 10.1186/s12879-019-3717-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Escombe AR, Moore DAJ, Gilman RH, Navincopa M, Ticona E, Mitchell B, et al. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmissio. PLoS Med. 2009;6: 0312–0323. doi: 10.1371/journal.pmed.1000043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mphaphlele M, Dharmadhikari AS, Jensen PA, Rudnick SN, van Reenen TH, Pagano MA, et al. Institutional Tuberculosis Transmission. Controlled Trial of Upper Room Ultraviolet Air Disinfection: A Basis for New Dosing Guidelines. Am J Respir Crit Care Med. 2015;192: 477–484. doi: 10.1164/rccm.201501-0060OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nardell E, Vincent R, Sliney DH. Upper-Room Ultraviolet Germicidal Irradiation (UVGI) for Air Disinfection: A Symposium in Print. Photochem Photobiol. 2013;89: 764–769. doi: 10.1111/php.12098 [DOI] [PubMed] [Google Scholar]
- 47.Linnes JC, Rudnick SN, Hunt GM, McDevitt JJ, Nardell EA. Eggcrate UV: a whole ceiling upper-room ultraviolet germicidal irradiation system for air disinfection in occupied rooms. Indoor Air. 2014;24: 116–124. doi: 10.1111/ina.12063 [DOI] [PubMed] [Google Scholar]
- 48.McCreesh N, Karat AS, Baisley K, Diaconu K, Bozzani F, Govender I, et al. Modelling the effect of infection prevention and control measures on rate of Mycobacterium tuberculosis transmission to clinic attendees in primary health clinics in South Africa. BMJ Glob Heal. 2021;6: e007124. doi: 10.1136/bmjgh-2021-007124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCreesh N, Karat AS, Govender I, Baisley K, Diaconu K, Yates TA, et al. Estimating the contribution of transmission in primary healthcare clinics to community-wide TB disease incidence, and the impact of infection prevention and control interventions, in KwaZulu-Natal, South Africa. BMJ Glob Heal. 2022;7. doi: 10.1136/bmjgh-2021-007136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bozzani FM, DIaconu K, Gomez GB, Karat AS, Kielmann K, Grant AD, et al. Using system dynamics modelling to estimate the costs of relaxing health system constraints: A case study of tuberculosis prevention and control interventions in South Africa. Health Policy Plan. 2022;37: 369–375. doi: 10.1093/heapol/czab155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Churchyard GJ, Stevens WS, Mametja LD, McCarthy KM, Chihota V, Nicol MP, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: A cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Heal. 2015;3: e450–e457. doi: 10.1016/S2214-109X(15)00100-X [DOI] [PubMed] [Google Scholar]
- 52.Richardson ET, Morrow CD, Kalil DB, Bekker LG, Wood R. Shared air: A renewed focus on ventilation for the prevention of tuberculosis transmission. PLoS One. 2014;9: 1–7. doi: 10.1371/journal.pone.0096334 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A: Differences between the two methods used to measure ventilation in the clinics. Table B: Definitions of usual and ideal conditions. Table B: Definitions of usual and ideal conditions. Table C: Absolute ventilation rates of the clinic spaces under usual and ideal conditions using the tracer gas release technique. Table D: Ventilation rates using the rebreathed fraction approach using the continuous measurements under usual and ideal conditions. Table E: Risk of Mycobacterium tuberculosis infection for a healthcare worker spending 15 minutes per day for 25 working days with an infectious individual, calculated using the Wells-Riley equation. The probability of infection was calculated separately for each space using the measured absolute ventilation rates under usual and ideal conditions. We calculated risk using both a standard assumption about infectiousness (1.25 quanta/hr) and using a higher estimate taken from the published literature (8.2 quanta/hr). Table F: Risk of Mycobacterium tuberculosis infection for a patient spending 2.5 hours in a waiting room with an infectious individual, calculated using the Wells-Riley equation. The probability of infection was calculated separately for each space, using the measured absolute ventilation rates under usual and ideal conditions. We used both a standard assumption about infectiousness (1.25 quanta/hr) plus two higher estimates from the published literature (8.2 quanta/hr and 226 quanta/hr). Fig A: an example of a tracer gas release experiment from Room C at KZN2 clinic (experiment 6 Monitor a). Following log transformation, the right hand side of the curve is approximately linear. Fig B: Histograms showing the distribution of temperatures and wind speeds in KwaZulu-Natal and Western Cape during working hours from January 2018 –December 2020. Vertical lines shows the mean temperatures and wind speeds on the 8 days when the rebreathed approach experiments were conducted. Fig C: Association between wind speed and the absolute ventilation rates.
(PDF)
(PDF)
Data Availability Statement
The data from this paper are available via https://datacompass.lshtm.ac.uk/. Analysis of the tracer gas release experiments was undertaken in Stata version 14.2 (StataCorp, College Station, Texas, USA). This Stata code is available on GitHub (https://github.com/tayates/uo_ventilation). Analysis of paired indoor-outdoor CO2 measurements was carried out using R version 3.6.0 [29].This R code is also available on GitHub (URL: https://github.com/ArminderD/ventilation.git).