Abstract
Pneumococcal disease is a major contributor to global childhood morbidity and mortality and is more common in low- and middle-income countries (LMICs) than in high-income countries. Pneumococcal carriage is a prerequisite for pneumococcal disease. Pneumococcal conjugate vaccine reduces vaccine-type carriage and disease. However, pneumococcal carriage and disease persist, and it is important to identify other potentially modifiable factors associated with pneumococcal carriage and determine if risk factors differ between low, middle, and high-income countries. This information may help inform pneumococcal disease prevention programs. This systematic literature review describes factors associated with pneumococcal carriage stratified by country income status and summarises pneumococcal carriage rates for included studies. We undertook a systematic search of English-language pneumococcal nasopharyngeal carriage studies up to 30th June 2021. Peer-reviewed studies reporting factors associated with overall pneumococcal nasopharyngeal carriage in healthy, community-based study populations were eligible for inclusion. Two researchers independently reviewed studies to determine eligibility. Results are presented as narrative summaries. This review is registered with PROSPERO, CRD42020186914. Eighty-two studies were included, and 46 (56%) were conducted in LMICs. There was heterogeneity in the factors assessed in each study. Factors positively associated with pneumococcal carriage in all income classification were young age, ethnicity, symptoms of respiratory tract infection, childcare attendance, living with young children, poverty, exposure to smoke, season, and co-colonisation with other pathogens. Breastfeeding and antibiotic use were protective against carriage in all income classifications. Median (interquartile range) pneumococcal carriage rates differed by income classification, ranging from 51% (19.3–70.2%), 38.5% (19.3–51.6%), 31.5% (19.0–51.0%), 28.5% (16.8–35.4%), (P = 0.005) in low-, lower-middle, upper-middle, and high-income classifications, respectively. Our findings suggest that where measured, factors associated with pneumococcal nasopharyngeal carriage are similar across income classifications, despite the highest pneumococcal carriage rates being in low-income classifications. Reducing viral transmission through vaccination and public health interventions to address social determinants of health would play an important role.
Introduction
Streptococcus pneumoniae is a leading cause of invasive and non-invasive diseases, including meningitis, sepsis, pneumonia, and acute otitis media [1–4]. Most cases occur in children under five and the elderly [4, 5]. The epidemiology of pneumococcal disease varies by region, with the highest incidence and mortality rates across Southeast Asia and Africa [4]. Pneumococcal colonisation precedes pneumococcal disease [6–8]. As with pneumococcal disease, pneumococcal carriage epidemiology varies by age. Carriage rates tend to be highest amongst children in the second year of life [9, 10].
Understanding the factors associated with pneumococcal carriage is essential to developing appropriate public health intervention strategies to prevent pneumococcal disease and evaluating the impact of the pneumococcal conjugate vaccine (PCV), particularly among subpopulations most at risk [11]. Previous reviews of pneumococcal carriage prevalence, serotype distribution, and impact of PCV on carriage, including data from children and adults in low- and lower-middle-income countries and sub-Saharan Africa [12, 13], reported a variety of risk factors for pneumococcal carriage, including seasonality [14, 15], rural residence, age, crowding, co-colonisation with respiratory pathogens, presence of respiratory tract infections, and comorbidities such as human immunodeficiency virus (HIV) [12, 13]. A previous systematic review reported that pneumococcal carriage rates were generally higher in low-income countries than middle-income countries; however, it is unknown if risk factors for carriage differ across low- middle-and high-income countries [12].
PCV is the most effective measure to prevent pneumococcal disease and is an intervention known to reduce carriage of vaccine serotypes [16–18]. In some settings, such as Malawi and Papua New Guinea, vaccine-serotypes have continued to circulate post-PCV introduction [19, 20]. Additionally, replacement with non-vaccine serotypes in carriage, and to a lesser extent disease, has occurred in some settings post-PCV introduction [21–23]. In addition to introducing PCV, other interventions can also reduce pneumococcal disease [24–26]. This study aims to identify risk factors for overall pneumococcal carriage to determine if there are other potentially modifiable exposures, in addition to PCV vaccination. No systematic literature review on the risk factors of pneumococcal carriage has been published previously. Therefore, we undertook a systematic literature review to describe pneumococcal nasopharyngeal carriage risk factors in community-based populations, stratified by country income classification, and summarised the reported pneumococcal carriage rates of included studies.
Methods
Protocol and registration
A systematic literature search was undertaken and reported according to the PRISMA guidelines [27]. The review protocol was registered with the International Prospective Register of Systematic Reviews, registration CRD42020186914.
Eligibility criteria
Peer-reviewed studies reporting factors associated with pneumococcal nasopharyngeal carriage, published in English before July 2021, were eligible for inclusion. As risk factors assessed in primary studies were unlikely to be similar across all pneumococcal carriage studies, we limited our review to studies of healthy, community-based populations in an attempt to select studies with as similar as possible exposures. For transparency and completeness, we also present the results for all factors assessed for association with pneumococcal carriage by each included primary study. We included community-based studies in which nasopharyngeal swabs had been taken and processed to detect pneumococci and in which statistical analyses had been conducted to determine factors associated with overall pneumococcal carriage.
We excluded animal studies, letters, case reports, editorials, comments, diagnostic articles, and intervention studies where the intervention was likely to impact the rates of pneumococcal carriage. We also excluded studies if the description of the study design and laboratory methods was inadequate (lacked inclusion/exclusion criteria, lacked an adequate description of settings, populations, or laboratory methods); if risk factors were assessed via univariable analysis only; if a P-value only was reported; if the sample size was less than 30 participants; if the total study population was non-community (e.g., hospital, prison, or childcare) or had underlying comorbidities (e.g., HIV); and if factors associated with VT or non-vaccine-type (NVT) pneumococcal carriage only were reported.
Information sources and search
We searched Medline (Ovid) and Embase (Ovid) on 30th June 2021, using thesaurus and keywords. We adapted the Medline search strategy for use in the Cochrane library. PubMed was searched using a combination of search strings for S. pneumoniae, carriage, colonisation, and nasopharynx. The search strategies are available in S1 Text.
Study selection
In the first instance, two reviewers (EFGN and JC) independently screened titles and abstracts of retrieved articles. We excluded duplicates and articles unrelated to the primary subject matter. Secondly, we assessed the eligibility of selected full texts. Reviewers resolved discordant eligibility results by discussion.
Data collection
Study data were collected and managed using REDCap electronic data capture tools hosted at the Murdoch Children’s Research Institute or entered directly into pre-prepared tables [28]. EFGN exported quality assessment and extracted study data in REDCap to Stata 16.1 for cleaning and analysis [29].
Extracted data included: first author; year of publication, research aim, study design; study year(s); country; setting (e.g., semi-urban); swab material; pneumococcal identification method; age group; sample size; citation of 2003 or 2013 World Health Organization (WHO) recommendations for detecting pneumococci in upper respiratory tracts [30, 31]; pneumococcal carriage rates; factors associated with pneumococcal carriage, with associated effect size estimates, and where reported, 95% CI and P-value; analysis method to determine associations; and variable selection method for models used to assess factors associated with pneumococcal carriage (e.g., empirical based on P-value thresholds). If the study design was not stated, we compared the described methods and classified them according to standard study design definitions [32]. For each study, we used World Bank data to determine the income status (low, lower-middle, upper-middle, and high) of the study country during the study period [33]. We categorised each study by WHO region (Africa, Americas, Eastern Mediterranean, European, South-East Asia, and Western Pacific) in which the study was conducted [34]. Reviewers contacted the authors of some eligible primary studies to obtain additional information. We used a Kruskal-Wallis test to compare overall pneumococcal nasopharyngeal carriage rates by income classification (low, lower-middle, upper-middle, and high), as this is a rank-based, non-parametric method for testing differences between two or more categorical, independent groups on a continuous (or ordinal) outcome (where study-level carriage rates were treated as continuous). We present a narrative summary of the results.
Quality of studies
The quality of each study was assessed using the relevant NIH Study Quality Assessment Tool [35]. We modified the tool by replacing the question “Were outcomes assessed using valid and reliable measures, implemented consistently across all study participants?” with an assessment of whether or not individual studies complied with the 2003 or 2013 World Health Organization standard method for detecting upper respiratory carriage of pneumococci, noting some studies were conducted or designed before the publication of these guidelines [30, 31]. One reviewer (EFGN) independently assessed quality and bias and extracted data. A second reviewer (JC) independently assessed quality and bias and extracted data from a random subset (20%) of articles for data validation. Reviewers resolved discordance through discussion.
Results
Search results
We identified 2,558 articles and excluded 1,015 as duplicates. The remaining 1,543 underwent title and abstract screening (Fig 1). Of those, 1,083 were excluded. We assessed the full text of 460 articles, retaining 82 for inclusion in this systematic review.
Fig 1. PRISMA flow diagram for study selection.
Characteristics of included studies
Most (81/82, 99%) studies were observational. Sixty-six (80%) were cross-sectional studies [14, 15, 36–98] (S1 Table). There were six (7%) cohort [99–104], four (5%) nested-cohort [105–108], two (2%) longitudinal [109, 110], and two (2%) nested-longitudinal [111, 112] study designs. There was one nested case-control [113] and one nested cross-sectional study [114]. One study reported secondary analysis using data from a randomised controlled trial [115].
All income settings and WHO regions were represented (S1 Table). The majority (36/82, 44%) were conducted in high-income countries across the Americas [66, 71–80, 84, 107], Europe [81, 85–92, 102, 108, 115], and the Western Pacific [93–95, 97, 98, 103, 104, 110]. One study was conducted across two income classifications and WHO regions: high-income Israel in the WHO European region and lower-middle-income West Bank and Gaza in the WHO Eastern Mediterranean region [96].
Study design, methods, and participant characteristics varied across studies (S1 and S2 Tables). The age groups of participants ranged from neonates to adults, however, the majority (60/82, 73%) focused on infants and toddlers [41, 47–49, 56, 60, 81, 94, 98, 99, 108, 113, 115] or children, with varying ages [14, 36, 38, 44, 45, 51, 52, 57, 59, 63–66, 70–76, 78–80, 83–87, 89, 90, 92, 93, 96, 100, 103, 104, 107, 110]. Study authors measured a variety of participant characteristics. While some, such as sex, age, and recent history of respiratory tract illness, were measured in studies from all income classifications, no single characteristic was measured in all studies. Differences in characteristics measured may reflect the cultural or income context of studies and participants’ ages. For example, childcare attendance was measured in approximately 81% (29/36) of studies conducted in high-income countries, but only 18% (3/17), 9% (1/11), and 38% (7/18) of studies conducted in low, lower-middle, and upper-middle-income countries.
The type of statistical model used to investigate the factors associated with pneumococcal carriage was similar across studies, most commonly (72/82 studies, 88%) logistic regression (S1 Table). The methods used for variable selection varied widely, including empirical only, a priori only, or a combination of empirical and a priori (S1 Table). Empirical variable selection (alone or in combination with a priori selection) was driven by P-values, with thresholds for inclusion ranging from <0.1 to <0.5.
Various methods were used to detect S. pneumoniae (S3 Table). Thirty-seven (45%) studies referred to either the 2003 [14, 15, 30, 36, 39, 40, 43, 49, 53–55, 60, 66, 82, 85, 86, 88, 92, 94, 95, 99, 105, 108, 111, 115] or 2013 [31, 37, 38, 44, 46–48, 50, 67–69, 106, 109, 114] WHO methods to detect pneumococci.
Quality assessment
The quality assessment results are shown in S4 Table [35]. Based on the overall assessment, approximately two-thirds of the studies were considered fair (50/82, 59%) and over a third good quality (30/82, 37%) quality. Two included studies were considered poor quality. Underlying reasons for poor quality related to a lack of sufficient detail in study methods. A cross-sectional study conducted in Hong Kong was considered poor quality as the methods were unclear, including a lack of clarity around the inclusion criteria and insufficient descriptions of pneumococcal carriage detection and statistical methods [110]. In this study, it was unclear how multivariable logistic regression models were built, there was no discussion regarding variable selection, and the only indication that a multivariable model had been used was in the abstract [110]. A cross-sectional study from Bolivia was considered poor quality, as it lacked a clear research question, the inclusion and exclusion criteria were unclear, no sample size calculation was included, and the description of statistical methods was insufficient [54]. Studies were not excluded based on poor quality to ensure transparency and completeness of reporting from all studies identified as relevant to the review [116].
Results of individual studies
S5 Table shows the factors assessed for associations with pneumococcal carriage, stratified by World Bank income classification [33]. Reported risk factors for pneumococcal carriage were demographic (e.g., young age, symptoms of respiratory tract infection, ethnicity, sex), environmental or household-related factors (e.g., living with siblings or other young children, exposure to tobacco and cooking smoke, attendance at childcare, residential location, season).
Factors assessed for association with pneumococcal carriage
Factors assessed for association with pneumococcal nasopharyngeal carriage varied by study (S5 Table). Some factors were measured and assessed in all income classifications, including age [36, 38, 40–43, 45, 46, 50, 52–62, 64–68, 70–78, 80–84, 86, 89, 90, 92–99, 101, 104, 107, 109–111, 115], antibiotic use [14, 36, 37, 40, 41, 45–47, 52, 60, 63, 66, 70, 73–79, 81, 82, 84, 92, 93, 97, 101, 104, 107, 109, 115], breastfeeding (including current, exclusive, history, and duration of) [39, 41, 49, 58, 73, 75, 83, 90, 97, 103, 104, 107, 108, 112, 115], childcare attendance [36, 41, 42, 52, 56, 57, 63, 64, 66, 70, 72–76, 78–81, 83–90, 92, 93, 96–99, 101, 103, 104, 108, 115], exposure to cigarette and cooking smoke [39–42, 46–49, 51, 53, 55, 60–62, 67, 82, 83, 86, 88, 91, 92, 97, 99, 106–108, 110, 112], ethnicity [39, 41, 47, 49, 67, 68, 78, 83, 88, 92, 100, 110], family size, and household numbers [40, 45, 52, 63, 68, 75, 96], crowding or proxies for crowding [39, 45, 55, 65, 77, 88, 100, 110], residential location [45, 47–50, 54, 57, 60, 61, 67–69, 77, 82, 88, 91, 96], respiratory tract infection symptoms [14, 15, 37, 40, 44–47, 49, 53, 56–58, 64, 67, 68, 73–75, 78, 79, 82, 84, 91, 92, 94, 95, 97, 100, 101, 104, 109, 115], participant sex [36–38, 40, 41, 45–47, 53–55, 58, 61, 62, 67, 68, 82, 83, 88–90, 92, 95, 98, 102, 107, 112, 115], and living with siblings or other young children [41, 42, 44–48, 55, 56, 63, 66, 67, 69–71, 73, 74, 78, 79, 81, 83, 84, 86–89, 92–94, 97, 98, 103, 104, 107, 108, 110, 112, 115]. Some risk factors were assessed more frequently than others, depending on income classification. For example, some form of childcare attendance was assessed for association with pneumococcal carriage in 81% (29/36) [66, 72–76, 78–81, 83–90, 92, 93, 96–98, 103, 104, 108, 115], 39% (7/18) [56, 57, 63, 64, 70, 99, 101], 9% (1/11) [52], and 18% (3/17) [36, 41, 42] of studies from high, upper-middle, lower-middle, and low-income countries, respectively. No single risk factor was assessed for association with carriage in all studies, and some risk factors, such as prematurity [41], malnutrition or body mass index [39, 44, 46, 62], and infant mode of delivery [47, 69] were assessed in one or two income classifications only.
Factors associated with pneumococcal carriage in low-income countries
In low-income countries, demographic factors commonly associated with pneumococcal carriage included young age [36, 41, 43, 45, 111, 113], and ill-health around the time of the survey, including symptoms of respiratory infections [14, 40, 45], or other unspecified illnesses [38]. Any treatment for illness and antibiotic use in the two or three weeks preceding the survey was protective against pneumococcal carriage in three studies [14, 15, 113], while five studies with broader definitions of antibiotic use reported no association with pneumococcal carriage [36, 37, 40, 41, 45].
One study reported co-colonisation with non-capsulate Haemophilus influenzae [15] and malnutrition [44] to be positively associated with carriage–no other study from low-income countries assessed these potential risk factors. Two studies in low-income countries reported an ethnicity-based association with pneumococcal carriage [39, 41]. No other studies from low-income countries assessed ethnicity in association with carriage.
Environmental and household factors positively associated with pneumococcal carriage in low-income countries included: proxies for poverty [44, 45, 113]; rainy season [15]; dry season [105, 111]; proxy for season (study time by month) [14]; living with other children or siblings [41–45, 105]; attendance at childcare [36, 42]; maternal pneumococcal carriage [105]; and frequency of social contacts [114].
Factors associated with pneumococcal carriage in lower-middle-income countries
In lower-middle-income countries, young age was associated with pneumococcal carriage [51–55, 109]. Two studies reported a positive association between male sex and pneumococcal carriage [53, 54]. Three studies reported acute respiratory illness [47, 49, 109], and one study each reported indigenous ethnicity [49] and co-colonisation with H. influenzae as independent predictors of pneumococcal carriage [46].
Environment and household-related risk factors for pneumococcal carriage in lower-middle-income countries included: residential location [47, 50, 54]; exposure to cigarette smoke [48, 51, 53]; proxies for poverty [52, 109]; season [50]; siblings [48, 51, 109]; attendance at childcare [52], and housing type [48].
Factors associated with pneumococcal carriage in upper-middle-income countries
Young age was frequently reported as positively associated with pneumococcal carriage in upper-middle-income countries [57–60, 64, 65, 99–101]. One study reported that each increasing year of age was protective against pneumococcal carriage (aOR 0.90 [95% CI 0.89–0.92]) [62]. Similarly, respiratory tract infections, variably defined as respiratory tract infection symptoms, rhinitis, infection in the month preceding survey, and previous respiratory infection, were associated with pneumococcal carriage in upper-middle-income countries [56, 57, 64, 67, 68, 100, 101].
One study reported a positive association between co-colonisation with H. influenzae and pneumococcal carriage in toddlers [99]. However, no association was found between co-colonisation with H. influenzae and pneumococcal carriage in mothers of infants and toddlers [99] or children aged under five years [59]. Similarly, one study reported co-colonisation with Staphylococcus aureus was negatively associated with pneumococcal carriage in infants and toddlers but was not associated with carriage in their mothers [99]. Other studies from upper-middle-income countries did not include co-colonisation of H. influenzae and S. aureus as potential risk factors in final models. Antibiotic use [63, 101] and breastfeeding [58] were protective against pneumococcal carriage. Three studies reported ethnicity-based differences in pneumococcal carriage [67, 68, 100]. One study reported physical contact with toddlers aged 12–23 months [68] and vaginal mode of infant delivery [69] as positively associated with pneumococcal carriage.
Environment and household-related risk factors for pneumococcal carriage in upper-middle-income countries included: attendance at childcare [56, 57, 63, 101] and school [58]; living with siblings or young children [56, 63, 64, 67, 69]; siblings attending childcare [60, 64]; poverty or proxies for poverty [62, 65, 67, 100]; season [99, 100], housing [59], and residential location [67].
Factors associated with pneumococcal carriage in high-income countries
In high-income countries, young age [66, 71–78, 80, 84, 86–88, 92–97, 103, 107, 110, 115]; ill-health, frequently involving respiratory symptoms and or otitis media [66, 73–75, 77–81, 84, 92–95, 97, 102, 104, 115]; co-colonisation with Moraxella catarrhalis, H. influenzae type b, and S. aureus [81, 95, 102, 104]; breastfeeding [73, 75, 97, 103]; ethnicity [83]; male sex [95, 102, 107]; and antibiotic use [66, 74–81, 84, 92, 101, 115] were found to be associated with pneumococcal carriage. One cohort study reported that at six months of age, bottle feeding was protective, compared with exclusive breastfeeding (adjusted hazard ratio 0.623 [95% CI 0.447–0.868] P = 0.005) [103].
Environmental and household factors associated with carriage in high-income countries included: siblings, variably defined (S5 Table) [66, 71, 73–77, 79, 81, 83, 84, 87, 88, 92–94, 97, 98, 103, 104, 107, 108, 115]; childcare attendance [66, 71–76, 78–81, 83–85, 87, 90, 92, 97, 98, 103, 104, 107, 108, 115]; proxies for poverty [75, 77, 88, 96]; residential location, defined as rural or urban, village by region of study, and community location [71, 77, 91]; exposure to cooking and cigarette smoke [86, 91, 95, 97]; and season, including autumn to winter, and summer [93, 115].
Synthesis of results
Demographic risk factors of age [36, 41, 43, 45, 51–55, 57–60, 62, 64–66, 71–78, 80, 84, 86–88, 92–97, 99–101, 103, 107, 109–111, 113, 115]; ethnicity [39, 41, 49, 67, 68, 83, 100], symptoms of respiratory tract infection [14, 15, 38, 40, 45, 47, 49, 53, 56, 57, 64, 66–68, 73–75, 77–81, 84, 92–95, 97, 100, 101, 104, 109, 113, 115], and co-colonisation with other pathogens [15, 46, 81, 95, 99, 104] were associated with carriage in all income classifications.
Most studies that assessed the association between pneumococcal carriage and childcare attendance (either by the study participant or household member of the study participant) reported a positive association [36, 56, 57, 63, 64, 71–76, 78–81, 83–85, 87–90, 92, 93, 97, 98, 101, 103, 104, 108, 115]. However, some studies reported no association between childcare attendance and pneumococcal carriage [41, 42, 70, 85, 86, 88, 92, 93, 96, 99].
Poverty and proxies for poverty, including latrine distance from the household, number of rooms per household, crowding, malnutrition, and level of education, were found to increase the odds of carriage in all income classifications [44, 45, 52, 62, 65, 67, 75, 77, 88, 96, 100, 109, 113]. In three studies, lower socio-economic status, poverty, or proxies thereof, were not associated with carriage [63, 64, 78].
Breastfeeding and antibiotic use were protective against pneumococcal carriage in all income settings. Where assessed, most studies reported no association between breastfeeding and pneumococcal carriage [39, 41, 49, 83, 90, 97, 104, 108, 115]. Importantly, where an association between breastfeeding and pneumococcal carriage was found, it was protective against carriage [58, 73, 75, 107], except in a cohort study of Japanese infants as noted above, where bottle-feeding at six months was protective compared with breastfeeding [103].
Current and prior antibiotic use was protective against pneumococcal carriage in some low, upper-middle, and high-income country studies [14, 15, 46, 63, 66, 74–81, 84, 92, 101, 107, 109, 113, 115]. However other studies reported no association between prior antibiotic use and pneumococcal carriage [36, 37, 40, 41, 45, 47, 52, 60, 70, 78, 82, 93, 97, 104]. Further, one study reported that antibiotic use in the month preceding the survey was positively associated with carriage, but not at the time of the study [107].
In some studies, we identified some factors positively associated with carriage that were not associated with carriage in other studies. For example, the association between direct and indirect exposure to tobacco smoke and pneumococcal carriage varied. In some studies, no association between exposure to tobacco smoke and pneumococcal carriage was found [39, 41, 42, 46, 47, 60, 62, 67, 82, 83, 92, 99, 107, 108, 112]. However, where an association was found, the odds of carriage were increased by exposure to tobacco smoke [40, 48, 51, 53, 91, 97, 110, 112]. One study from Italy initially reported that maternal smoking was protective against pneumococcal carriage among children aged < 5 years [86]. However, a sub-analysis of children < 5 years from Milan only found the odds of carriage increased by greater than three-fold in association with exposure to maternal cigarette smoking (aOR [95% CI 1.53–8.73]).
In all income settings, living with young siblings or children was found to be positively associated with pneumococcal carriage in most studies (43/57, 75%) where it was assessed [41, 42, 44, 45, 47, 48, 56, 63, 66, 67, 69, 71, 73–75, 77, 79–81, 83, 84, 87, 88, 92–94, 97, 100, 103, 104, 108, 110, 112, 115]. However, findings varied between studies. Ten studies reported no association between living with young children/siblings and pneumococcal carriage [40, 46, 49, 55, 74, 78, 79, 86, 99, 108, 112]. In a cross-sectional study of Fijian infants aged 3–13 months which reported no association between living with two or more children and carriage, the lower bound of the 95% confidence interval approached a positive association [49]. Additionally, some findings within studies differed too. For example, a serial cross-sectional study, a nested longitudinal study, and a cohort study reported a positive association between siblings and pneumococcal carriage in some study years or age cohorts, but no association in other study years or age cohorts [74, 108, 112].
Where assessed, most studies found no association between participant sex and pneumococcal carriage [36–38, 40, 41, 45–47, 55, 58, 61, 62, 68, 82, 83, 88, 90, 92, 98, 108, 111, 112, 115]. However, sex-related associations with pneumococcal carriage were reported in low, middle, and high-income countries, with male sex being a risk factor in three high and two lower-middle-income countries [53, 54, 95, 102, 107] and female sex a risk factor in one low-income country [112] and one upper-middle-income country [67].
Pneumococcal carriage rates
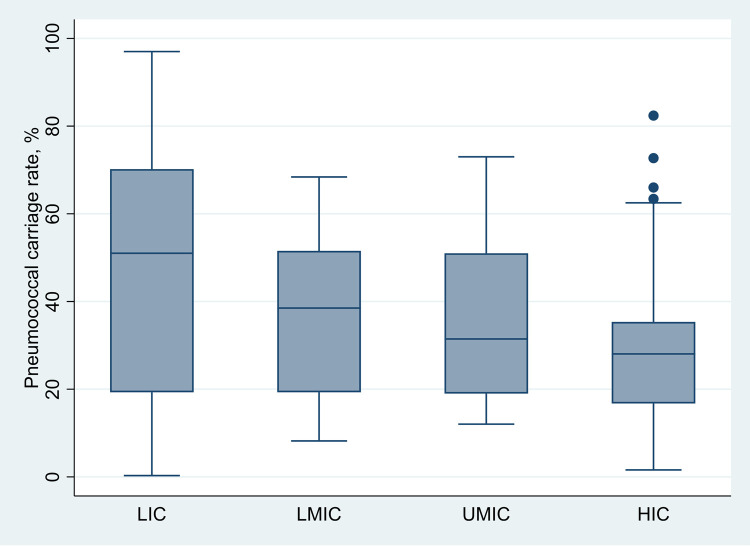
S5 Table shows pneumococcal nasopharyngeal carriage rates for individual studies. Median pneumococcal carriage rates differed by income classification, ranging from 51% (IQR 19.3–70.2), 38.5% (IQR 19.3–51.6), 31.5% (IQR 19.0–51.0), 28.5% (IQR 16.75–35.4) in low-, lower-middle, upper-middle, and high-income classifications, (χ2(3) = 17.742, P = 0.005) (Fig 2). Outliers from high-income countries include the carriage rates reported for Indigenous Australian children aged 2–4 (82.4%) and 5–8 years (72.7%) [95], children aged 6–24 months in The Netherlands (66%) [115], and Navajo and White Mountain Apache children < 6 years in the United States of America (63.4%) [107].
Fig 2. Median (interquartile range) overall pneumococcal nasopharyngeal carriage rates by income classification in all ages.
Abbreviations: LIC–lower-income classification; LMIC–lower-middle-income classification; UMIC–upper-middle-income classification; HIC–high-income classification.
Within some income classifications and countries, reported pneumococcal nasopharyngeal carriage rates were vastly different between participants of similar ages in various studies. For example, in Brazil, a pneumococcal carriage rate of 19% was reported among children aged <6 years old attending a private clinical, and 66.6% among children aged <5 years living in a slum community [57, 58]. We also noted differences in pneumococcal carriage within other countries. For example, authors reported a carriage rate of 37.2% among Gambian neonates on day 28 of life in a retrospective nested-cohort study, and 93% among neonates aged < 1 month in a cross-sectional survey of 21 villages [39, 105]. Both studies cited the 2003 WHO recommendations for detecting upper respiratory carriage of pneumococci [30]. However, in addition to differences in study design and methods, we assessed one as having good quality and the other fair (S4 Table).
Discussion
We have identified risk and protective factors for pneumococcal carriage common to all income classifications. Factors positively associated with pneumococcal nasopharyngeal carriage in all income classifications in which they were examined included: young age [36, 41, 43, 45, 51–55, 57–60, 62, 64–66, 71–78, 80, 84, 86–88, 92–97, 99–101, 103, 107, 109–111, 113, 115]; living with young children [41, 42, 44, 45, 47, 48, 56, 63, 66, 67, 69, 71, 73–75, 77, 79–81, 83, 84, 87, 88, 92–94, 97, 100, 103, 104, 108, 110, 112, 115]; symptoms of RTI [14, 15, 38, 40, 45, 47, 49, 53, 56, 57, 64, 66–68, 73–75, 77–81, 84, 92–95, 97, 100, 101, 104, 109, 113, 115]; childcare attendance [36, 56, 57, 63, 64, 71–76, 78–81, 83–85, 87–90, 92, 93, 97, 98, 101, 103, 104, 108, 115]; ethnicity [39, 41, 49, 67, 68, 83, 100]; poverty [44, 45, 52, 62, 65, 67, 75, 77, 88, 96, 100, 109, 113]; exposure to tobacco smoke [40, 48, 51, 53, 91, 97, 110, 112]; co-colonisation with H. influenzae, M. catarrhalis, and S. aureus [15, 46, 81, 95, 99, 104]. Breastfeeding [58, 73, 75, 107] and antibiotic use [14, 15, 46, 63, 66, 74–81, 84, 92, 101, 107, 109, 113, 115] were found to be protective against pneumococcal carriage in most income classifications.
PCVs are the primary intervention to control pneumococcal disease [79, 117–121]. Acknowledging PCVs are the best method of pneumococcal disease prevention, introducing PCV into national immunisation programs and ensuring high uptake is important. However, pneumococcal disease persists even with the introduction of PCV. PCV reduces vaccine serotype pneumococcal carriage in most settings. However, for unknown reasons, vaccine-serotype carriage can persist after the introduction of PCV despite high vaccination coverage. In some LMICs, such as Malawi, vaccine-serotype carriage persisted at relatively high rates compared with high-income settings after introducing PCV13, declining from 19.9% (95% CI 15.7–25.0) to 16.7% (95% CI 13.0–21.1) among vaccinated children aged 3–5 years [19]. In Fiji, the introduction of PCV10 resulted in a relative shift to higher vaccine-serotype carriage rates in older children [68]. Despite some evidence that PCV13 reduces overall carriage prevalence in some vaccinated high-income populations, generally, PCVs do not reduce overall carriage, particularly in LMICs and indigenous populations. In addition to vaccine-serotype carriage, there are other serotypes that are not included in the vaccine and rates of non-vaccine type carriage usually increases after vaccine introduction, with some level of concomitant non-vaccine type pneumococcal disease [11, 21, 22, 122, 123]. In high pneumococcal burden and transmission settings, such as LMICs, serotype replacement disease with non-vaccine serotypes could threaten pneumococcal disease control achieved with PCVs. Therefore, it remains important to consider interventions to reduce pneumococcal carriage overall.
Vaccines and other public health interventions that modify viral respiratory pathogen infection are also likely to be important in preventing pneumococcal disease. In our review, we found that having symptoms of an acute respiratory tract infection (which are mostly viral in origin) was a risk factor for pneumococcal carriage in most studies where it was measured. Co-colonisation with respiratory viruses is associated with increased pneumococcal density, which increases the risk of acute respiratory infections and in some settings severe pneumonia [124, 125]. In Israel, France, and South Korea, pneumococcal disease and community-acquired pneumonia declined during the public health measures used to control SARS-CoV-2 transmission [24–26]. Many countries reported declines in respiratory syncytial virus (RSV) during this period [126, 127]. In Israel, pneumonia admissions declined despite pneumococcal carriage and density remaining unchanged. However, the circulation of other co-colonising viruses which are known to increase the virulence of pneumococci declined substantially during the lockdown periods [24, 128]. Although not assessed in primary studies in this review, pneumococcal carriage is more frequent in young children during infection with RSV than with other viruses [129, 130]. Further, RSV stimulates substantial growth of pneumococci, and co-colonisation with RSV is associated with increased pneumococcal density and severity of acute respiratory tract infections [129, 131, 132]. Additionally, co-colonisation with influenza and parainfluenza have been found to increase the probability of pneumococcal acquisition [133]. This suggests that public health interventions that modify the transmission of viral respiratory pathogens are also very important in preventing pneumococcal disease, including vaccines against RSV and influenza, and other interventions that reduce viral pathogen transmission. Co-colonisation with H. influenzae and M. catarrhalis were also found to be risk factors for pneumococcal carriage in this review.
Identifying factors associated with pneumococcal carriage in certain settings may help inform other public health interventions that may be needed. Some risk factors are not modifiable, such as age, living with young children (however this is most likely due to increased viral transmission in this age group), and ethnicity. However, the risk of pneumococcal carriage, transmission, and disease may be reduced by public health programs and policies that target particular age groups [134, 135], to reduce transmission, such as, such as increased access to improved sanitation and hygiene [136, 137], or that are tailored to address socio-economic differences and social determinants of health which promote tranmission [138]. Reducing environmental risk factors for pneumococcal carriage and viral transmission includes improving breastfeeding, reducing malnutrition, preventing overcrowding, enhancing respiratory etiquette, and reducing smoke exposure. Public health programs that promote birth spacing (which may reduce the number of young siblings living in the same household), breastfeeding, interventions to reduce poverty, and which ensure high coverage of infant vaccination, may reduce the risk of pneumococcal disease [24–26]. Many of these modifiable factors are included in the WHO integrated Global Action Plan for the Prevention and Control of Pneumonia and Diarrhoea [139]. Having programs to address these factors would also help prevent other infectious diseases that are a common cause of child morbidity and mortality in LMICs.
There was considerable heterogeneity in the factors collected and the definitions of the factors included in studies. Consequently, the absence of reported associations between potential risk factors and pneumococcal carriage may be because factors were not measured or included in models. For example, studies in high-income countries frequently measured childcare attendance or similar. However, this was measured in three low- [36, 41, 42] and one lower-middle-income country [52]. The heterogeneity of collected data hindered the comparison of rates of risk factors across income classifications and countries. Most studies were conducted in high-income countries, yet the burden of pneumococcal disease is highest in low- and lower-middle-income countries [4]. The relatively limited risk factors reported from low- and lower-middle-income classifications reflect the limited number and range of studies investigating risk factors for pneumococcal carriage in such settings, rather than a lack of risk factors.
Pneumococcal carriage rates varied by income classification and within countries. The highest pneumococcal carriage rate was in low-income classifications. Higher carriage rates may be due to higher rates of risk factors in low-income classifications compared with high-income classifications. This systematic review has brought together diverse studies from around the globe. For example, diversity is evident in the quality, inclusion criteria, study duration, and methods of pneumococcal detection and risk factor assessment. A meta-regression to understand drivers of variation in carriage across studies would have been possible had we comparable population-level information on studies. However, few studies measured the same exposure variables or measured them with similar definitions, and population-level risk factors were not documented, preventing comparison of risk factor rates by income classifications, and limiting conclusions that could be drawn regarding differences in overall carriage rates across populations.
Nonetheless, there was some evidence of higher rates of risk factors associated with pneumococcal carriage, such as living with siblings or young children, in low-income classifications compared with high-income classifications. Further, some factors associated with pneumococcal carriage, such as exposure to tobacco smoke, are known to have higher rates in LMICs compared with HICs. In 2021, the WHO reported 1.3 billion tobacco users, 80% of whom live in LMICs [140]. A complex relationship between multiple intertwined factors may explain the higher rates of pneumococcal carriage in low-income classifications. Pneumococcal carriage rates also varied between studies within the same countries, perhaps due to differences in sampled populations, the prevalence of potential risk factors for pneumococcal carriage [57, 58]; age groups sampled [14, 44, 51, 58, 86, 87, 100] study designs and microbiological methods [30, 31, 72, 107]; and study quality [39, 105]. Indigenous ethnicity was identified as a risk factor for pneumococcal carriage [67, 68]. Further, notably high carriage rates were identified in Indigenous Australian and Navajo and White Mountain Apache children in the United States of America [95, 107]. Social determinants of health likely affect differential pneumococcal carriage (and disease) burden within countries, however comprehensive analysis of factors predisposing towards differences in pneumococcal carriage between indigenous and non-indigenous populations living in the same settings remain largely unqualified and unquantified.
Limitations
This review has some limitations. Although articles from low- and lower-middle-income countries were included in this review, most primary studies were conducted in high-income countries. Low- and lower-middle-income countries were proportionally underrepresented, limiting the potential representativeness of studies for these income settings. Further, most studies used convenience sampling. For these reasons, the studies for which pneumococcal nasopharyngeal carriage rates were available may not be representative of regional, country, or within-country populations. Therefore, we caution against using the reported rates by income classification as population or sub-population rates. The methods used to detect pneumococcal nasopharyngeal carriage differed, and most studies did not report using the WHO guidelines [31]. Consequently, differences in swab materials transport, storage, and laboratory methods may impact the measurement of pneumococcal carriage in addition to reported risk factors. Further, our review was not a systematic review of rates of pneumococcal carriage. Instead, we opportunistically used data reported in studies reporting risk factors associated with carriage in varying age groups. This may mean that the summary of reported pneumococcal carriage rates by income classification may not be representative. Additionally, variables were not collected uniformly in each study, and those selected a priori for inclusion in models to assess factors associated with pneumococcal carriage differed by study. Due to the heterogeneity of studies, assessment of publication bias by quantitative methods was not possible.
Conclusion
PCVs are the primary intervention against pneumococcal disease. Interventions to reduce viral transmission, including RSV vaccine, are also likely to play an important role in preventing pneumococcal disease. This global systematic review fills a knowledge gap regarding risk factors for pneumococcal nasopharyngeal carriage. This review identifies factors to consider for inclusion in future carriage studies, including participant age, ethnicity, symptoms of respiratory tract infection at the time of the survey, childcare attendance and living with other young children, poverty or proxies for poverty, exposure to cooking, and cigarette smoke, season, co-colonisation with other pathogens, breastfeeding, and antibiotic use. Public health programs to address these factors would also help prevent other common infectious diseases in children, especially those living in disadvantage, and help prevent morbidity and mortality. Standard definitions and measurements of variables included in future studies would be preferable to aid comparison. Similarly, future studies should comply with the WHO methods for detecting pneumococci in the upper respiratory tract [31]. Finally, this review summarises hypothesis-generating studies and may inform future studies designed to ascertain causal relationships between factors identified as associated with pneumococcal carriage.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DTA)
(DTA)
Data Availability
All data and related metadata underlying the findings reported are included in the submitted article and its supplementary information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–16. Epub 04/12. doi: 10.1016/S0140-6736(13)60222-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Swartz S, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(11):1133–61. Epub 08/23. doi: 10.1016/S1473-3099(17)30396-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zunt JR, Kassebaum NJ, Blake N, Glennie L, Wright C, Nichols E, et al. Global, regional, and national burden of meningitis, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neuro. 2018;17(12):1061–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6(7):e744–e57. doi: 10.1016/S2214-109X(18)30247-X . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drijkoningen JJC, Rohde GGU. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20 Suppl 5:45–51. Epub 01/24. doi: 10.1111/1469-0691.12461 . [DOI] [PubMed] [Google Scholar]
- 6.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–54. Epub 2004/03/05. doi: 10.1016/S1473-3099(04)00938-7 . [DOI] [PubMed] [Google Scholar]
- 7.Simell B, Auranen K, Käyhty H, Goldblatt D, Dagan R, O’Brien KL. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines 2012;11(7):841–55. doi: 10.1586/erv.12.53 [DOI] [PubMed] [Google Scholar]
- 8.Almeida ST, Pedro T, Paulo AC, de Lencastre H, Sá-Leão R. Re-evaluation of Streptococcus pneumoniae carriage in Portuguese elderly by qPCR increases carriage estimates and unveils an expanded pool of serotypes. Sci Rep. 2020;10(1):8373. doi: 10.1038/s41598-020-65399-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray BM, Converse GM 3rd, Dillon HC Jr. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142(6):923–33. Epub 1980/12/01. doi: 10.1093/infdis/142.6.923 . [DOI] [PubMed] [Google Scholar]
- 10.Ra Wilson, Cohen JM, Reglinski M, Jose RJ, Chan WY, Marshall H, et al. Naturally acquired human immunity to pneumococcus is dependent on antibody to protein antigens. PLoS Pathog. 2017;13(1):e1006137. Epub 2017/01/31. doi: 10.1371/journal.ppat.1006137 ; PubMed Central PMCID: PMC5279798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan J, Nguyen CD, Dunne EM, Kim Mulholland E, Mungun T, Pomat WS, et al. Using pneumococcal carriage studies to monitor vaccine impact in low- and middle-income countries. Vaccine. 2019;37(43):6299–309. Epub 20190906. doi: 10.1016/j.vaccine.2019.08.073 . [DOI] [PubMed] [Google Scholar]
- 12.Adegbola RA, DeAntonio R, Hill PC, Roca A, Usuf E, Hoet B, et al. Carriage of Streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries: a systematic review and meta-analysis. PLoS One. 2014;9(8):e103293. Epub 2014/08/02. doi: 10.1371/journal.pone.0103293 ; PubMed Central PMCID: PMC4118866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usuf E, Bottomley C, Adegbola RA, Hall A. Pneumococcal carriage in sub-Saharan Africa—a systematic review. PloS one. 2014;9(1):e85001. Epub 2014/01/28. doi: 10.1371/journal.pone.0085001 ; PubMed Central PMCID: PMC3896352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdullahi O, Karani A, Tigoi CC, Mugo D, Kungu S, Wanjiru E, et al. The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS One. 2012;7(2):e30787. doi: 10.1371/journal.pone.0030787 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdullahi O, Nyiro J, Lewa P, Slack M, Scott JA. The descriptive epidemiology of Streptococcus pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi district, Kenya. Ped Infect Dis J. 2008;27(1):59–64. doi: 10.1097/INF.0b013e31814da70c . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammitt LL, Etyang AO, Morpeth SC, Ojal J, Mutuku A, Mturi N, et al. Effect of ten-valent pneumococcal conjugate vaccine on invasive pneumococcal disease and nasopharyngeal carriage in Kenya: a longitudinal surveillance study. Lancet. 2019;393(10186):2146–54. Epub 20190415. doi: 10.1016/S0140-6736(18)33005-8 ; PubMed Central PMCID: PMC6548991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roca A, Hill PC, Townend J, Egere U, Antonio M, Bojang A, et al. Effects of community-wide vaccination with PCV-7 on pneumococcal nasopharyngeal carriage in the Gambia: a cluster-randomized trial. PLoS Med. 2011;8(10):e1001107. Epub 20111018. doi: 10.1371/journal.pmed.1001107 ; PubMed Central PMCID: PMC3196470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunne EM, Satzke C, Ratu FT, Neal EFG, Boelsen LK, Matanitobua S, et al. Effect of ten-valent pneumococcal conjugate vaccine introduction on pneumococcal carriage in Fiji: results from four annual cross-sectional carriage surveys. Lancet Glob Health. 2018;6(12):e1375–e85. doi: 10.1016/S2214-109X(18)30383-8 ; PubMed Central PMCID: PMC6231327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swarthout TD, Fronterre C, Lourenço J, Obolski U, Gori A, Bar-Zeev N, et al. High residual carriage of vaccine-serotype Streptococcus pneumoniae after introduction of pneumococcal conjugate vaccine in Malawi. Nat Commun. 2020;11(1):2222. Epub 2020/05/08. doi: 10.1038/s41467-020-15786-9 ; PubMed Central PMCID: PMC7203201 Biologicals and from Takeda Pharmaceuticals outside the submitted work. No other authors declare competing interests. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Britton KJ, Pickering JL, Pomat WS, de Gier C, Nation ML, Pell CL, et al. Lack of effectiveness of 13-valent pneumococcal conjugate vaccination against pneumococcal carriage density in Papua New Guinean infants. Vaccine. 2021;39(38):5401–9. doi: 10.1016/j.vaccine.2021.07.085 [DOI] [PubMed] [Google Scholar]
- 21.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378(9807):1962–73. Epub 2011/04/16. doi: 10.1016/S0140-6736(10)62225-8 ; PubMed Central PMCID: PMC3256741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feikin DR, Kagucia EW, Loo JD, Link-Gelles R, Puhan MA, Cherian T, et al. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med. 2013;10(9):e1001517. Epub 20130924. doi: 10.1371/journal.pmed.1001517 ; PubMed Central PMCID: PMC3782411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose MA, Laurenz M, Sprenger R, Imöhl M, van der Linden M. Nasopharyngeal carriage in children after the introduction of generalized infant pneumococcal conjugate vaccine immunization in Germany. Frontiers in Medicine. 2021;8. doi: 10.3389/fmed.2021.719481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danino D, Ben-Shimol S, van der Beek BA, Givon-Lavi N, Avni YS, Greenberg D, et al. Decline in pneumococcal disease in young children during the coronavirus disease 2019 (COVID-19) pandemic in Israel associated with suppression of seasonal respiratory riruses, despite persistent pneumococcal carriage: a prospective cohort study. Clinical Infectious Diseases. 2021:ciab1014. doi: 10.1093/cid/ciab1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rybak A, Yang DD, Schrimpf C, Guedj R, Levy C, Cohen R, et al. Fall of community-acquired pneumonia in children following COVID-19 non-pharmaceutical interventions: a time series analysis. Pathogens. 2021;10(11). Epub 20211024. doi: 10.3390/pathogens10111375 ; PubMed Central PMCID: PMC8617667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DH, Nguyen TM, Kim JH. Infectious respiratory diseases decreased during the COVID-19 pandemic in South Korea. Int J Environ Res Public Health. 2021;18(11). Epub 20210603. doi: 10.3390/ijerph18116008 ; PubMed Central PMCID: PMC8199908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097–e. Epub 07/21. doi: 10.1371/journal.pmed.1000097 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. Epub 09/30. doi: 10.1016/j.jbi.2008.08.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.StataCorp. StataCorp. 2017. Stata Statistical Software: Release 15. College Station, Texas: StataCorp LP; 2017. [Google Scholar]
- 30.O’Brien KL, Nohynek H. Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Ped Infect Dis J. 2003;22(2):e1–11. Epub 2003/02/15. doi: 10.1097/01.inf.0000049347.42983.77 . [DOI] [PubMed] [Google Scholar]
- 31.Satzke C, Turner P, Virolainen-Julkunen A, Adrian PV, Antonio M, Hare KM, et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine. 2013;32(1):165–79. doi: 10.1016/j.vaccine.2013.08.062 . [DOI] [PubMed] [Google Scholar]
- 32.Porta M. A dictionary of epidemiology: Oxford university press; 2014. [Google Scholar]
- 33.World Bank Country and Lending Groups [Internet]. World Bank. 2020 [cited 2020 Jan 26].
- 34.World Health Organization. WHO/Who we are/Regional offices 2020 [cited 2020 April 3]. Available from: https://www.who.int/about/who-we-are/regional-offices.
- 35.NIH. Study Quality Assessment Tools 2014 [updated 2014 April; cited 2017 January 2]. Assessment Tools for Epidemiological studies]. Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools.
- 36.Nguyen HAT, Fujii H, Vu HTT, Parry CM, Dang AD, Ariyoshi K, et al. An alarmingly high nasal carriage rate of Streptococcus pneumoniae serotype 19F non-susceptible to multiple beta-lactam antimicrobials among Vietnamese children. BMC Infect Dis. 2019;19(1):241. Epub 2019/03/15. doi: 10.1186/s12879-019-3861-2 ; PubMed Central PMCID: PMC6416861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nackers F, Cohuet S, le Polain de Waroux O, Langendorf C, Nyehangane D, Ndazima D, et al. Carriage prevalence and serotype distribution of Streptococcus pneumoniae prior to 10-valent pneumococcal vaccine introduction: A population-based cross-sectional study in South Western Uganda, 2014. Vaccine. 2017;35(39):5271–7. Epub 2017/08/09. doi: 10.1016/j.vaccine.2017.07.081 ; PubMed Central PMCID: PMC6616034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindstrand A, Kalyango J, Alfven T, Darenberg J, Kadobera D, Bwanga F, et al. Pneumococcal carriage in children under five years in Uganda-will present pneumococcal conjugate vaccines be appropriate? PLoS One. 2016;11(11):e0166018. doi: 10.1371/journal.pone.0166018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill PC, Akisanya A, Sankareh K, Cheung YB, Saaka M, Lahai G, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian villagers. Clin Infect Dis 2006;43(6):673–9. doi: 10.1086/506941 . [DOI] [PubMed] [Google Scholar]
- 40.Usuf E, Badji H, Bojang A, Jarju S, Ikumapayi UN, Antonio M, et al. Pneumococcal carriage in rural Gambia prior to the introduction of pneumococcal conjugate vaccine: a population-based survey. Trop Med Int Health. 2015;20(7):871–9. doi: 10.1111/tmi.12505 . [DOI] [PubMed] [Google Scholar]
- 41.Ousmane S, Diallo BA, Ouedraogo R, Sanda AA, Soussou AM, Collard JM. Serotype distribution and antimicrobial sensitivity profile of Streptococcus pneumoniae carried in healthy toddlers before PCV13 introduction in Niamey, Niger. PLoS One. 2017;12(1):e0169547. doi: 10.1371/journal.pone.0169547 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haile AA, Gidebo DD, Ali MM. Colonization rate of Streptococcus pneumoniae, its associated factors and antimicrobial susceptibility pattern among children attending kindergarten school in Hawassa, southern Ethiopia. BMC Res Notes. 2019;12(1):344. Epub 2019/06/19. doi: 10.1186/s13104-019-4376-z ; PubMed Central PMCID: PMC6580519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wada FW, Tufa EG, Berheto TM, Solomon FB. Nasopharyngeal carriage of Streptococcus pneumoniae and antimicrobial susceptibility pattern among school children in South Ethiopia: post-vaccination era. BMC research notes. 2019;12(1):306. doi: 10.1186/s13104-019-4330-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gebre T, Tadesse M, Aragaw D, Feye D, Beyene HB, Seyoum D, et al. Nasopharyngeal carriage and antimicrobial susceptibility patterns of Streptococcus pneumoniae among children under five in Southwest Ethiopia. Children. 2017;4(4). doi: 10.3390/children4040027 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Assefa A, Gelaw B, Shiferaw Y, Tigabu Z. Nasopharyngeal carriage and antimicrobial susceptibility pattern of Streptococcus pneumoniae among pediatric outpatients at Gondar University Hospital, North West Ethiopia. PEDN. 2013;54(5):315–21. doi: 10.1016/j.pedneo.2013.03.017 . [DOI] [PubMed] [Google Scholar]
- 46.Uddén F, Filipe M, Slotved HC, Yamba-Yamba L, Fuursted K, Pintar Kuatoko P, et al. Pneumococcal carriage among children aged 4–12 years in Angola 4 years after the introduction of a pneumococcal conjugate vaccine. Vaccine. 2020;38(50):7928–37. Epub 2020/11/05. doi: 10.1016/j.vaccine.2020.10.060 . [DOI] [PubMed] [Google Scholar]
- 47.Dunne EM, Choummanivong M, Neal EFG, Stanhope K, Nguyen CD, Xeuatvongsa A, et al. Factors associated with pneumococcal carriage and density in infants and young children in Laos PDR. PLoS One. 2019;14(10):e0224392. doi: 10.1371/journal.pone.0224392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Mollendorf C, Dunne EM, La Vincente S, Ulziibayar M, Suuri B, Luvsantseren D, et al. Pneumococcal carriage in children in Ulaanbaatar, Mongolia before and one year after the introduction of the 13-valent pneumococcal conjugate vaccine. Vaccine. 2019;37(30):4068–75. doi: 10.1016/j.vaccine.2019.05.078 [DOI] [PubMed] [Google Scholar]
- 49.Russell FM, Carapetis JR, Ketaiwai S, Kunabuli V, Taoi M, Biribo S, et al. Pneumococcal nasopharyngeal carriage and patterns of penicillin resistance in young children in Fiji. Ann Trop Paediatr 2006;26(3):187–97. doi: 10.1179/146532806X120273 . [DOI] [PubMed] [Google Scholar]
- 50.Hu J, Sun X, Huang Z, Wagner AL, Carlson B, Yang J, et al. Streptococcus pneumoniae and Haemophilus influenzae type b carriage in Chinese children aged 12–18 months in Shanghai, China: a cross-sectional study. BMC Infect Dis. 2016;16:149. doi: 10.1186/s12879-016-1485-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farida H, Severin JA, Gasem MH, Keuter M, Wahyono H, van den Broek P, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in pneumonia-prone age groups in Semarang, Java Island, Indonesia. PLoS One. 2014;9(1):e87431. doi: 10.1371/journal.pone.0087431 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Regev-Yochay G, Raz M, Dagan R, Porat N, Shainberg B, Pinco E, et al. Nasopharyngeal carriage of Streptococcus pneumoniae by adults and children in community and family settings. Clin Infect Dis 2004;38(5):632–9. doi: 10.1086/381547 . [DOI] [PubMed] [Google Scholar]
- 53.Cardozo DM, Nascimento-Carvalho CM, Andrade AL, Silvany-Neto AM, Daltro CH, Brandao MA, et al. Prevalence and risk factors for nasopharyngeal carriage of Streptococcus pneumoniae among adolescents. Journal of Medical Microbiology. 2008;57(Pt 2):185–9. doi: 10.1099/jmm.0.47470-0 . [DOI] [PubMed] [Google Scholar]
- 54.Inverarity D, Diggle M, Ure R, Johnson P, Altstadt P, Mitchell T, et al. Molecular epidemiology and genetic diversity of pneumococcal carriage among children in Beni State, Bolivia. Trans R Soc Trop Med Hyg. 2011;105(8):445–51. doi: 10.1016/j.trstmh.2011.04.013 . [DOI] [PubMed] [Google Scholar]
- 55.Adetifa IM, Antonio M, Okoromah CA, Ebruke C, Inem V, Nsekpong D, et al. Pre-vaccination nasopharyngeal pneumococcal carriage in a Nigerian population: epidemiology and population biology. PLoS One. 2012;7(1):e30548. doi: 10.1371/journal.pone.0030548 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toledo ME, Casanova MF, Linares-Perez N, Garcia-Rivera D, Torano Peraza G, Barcos Pina I, et al. Prevalence of pneumococcal nasopharyngeal carriage among children 2–18 months of age: baseline study pre-introduction of pneumococcal vaccination in Cuba. Ped Infect Dis J. 2017;36(1):e22–e8. doi: 10.1097/INF.0000000000001341 . [DOI] [PubMed] [Google Scholar]
- 57.Neves FPG, Cardoso NT, Snyder RE, Marlow MA, Cardoso CAA, Teixeira LM, et al. Pneumococcal carriage among children after four years of routine 10-valent pneumococcal conjugate vaccine use in Brazil: The emergence of multidrug resistant serotype 6C. Vaccine. 2017;35(21):2794–800. doi: 10.1016/j.vaccine.2017.04.019 . [DOI] [PubMed] [Google Scholar]
- 58.Reis JN, Palma T, Ribeiro GS, Pinheiro RM, Ribeiro CT, Cordeiro SM, et al. Transmission of Streptococcus pneumoniae in an urban slum community. Journal of Infection. 2008;57(3):204–13. doi: 10.1016/j.jinf.2008.06.017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skosana Z, Von Gottberg A, Olorunju S, Mohale T, Du Plessis M, Adams T, et al. Non-vaccine serotype pneumococcal carriage in healthy infants in South Africa following introduction of the 13-valent pneumococcal conjugate vaccine. S Afr Med J. 2021;111(2):143–8. Epub 2021/05/05. doi: 10.7196/SAMJ.2021.v111i2.14626 . [DOI] [PubMed] [Google Scholar]
- 60.Ozdemir B, Beyazova U, Camurdan AD, Sultan N, Ozkan S, Sahin F. Nasopharyngeal carriage of Streptococcus pneumoniae in healthy Turkish infants. Journal of Infection. 2008;56(5):332–9. doi: 10.1016/j.jinf.2008.02.010 . [DOI] [PubMed] [Google Scholar]
- 61.Karami M, Hosseini SM, Hashemi SH, Ghiasvand S, Zarei O, Safari N, et al. Prevalence of nasopharyngeal carriage of Streptococcus pneumoniae in children 7 to 14 years in 2016: A survey before pneumococcal conjugate vaccine introduction in Iran. Human vaccines & immunotherapeutics. 2019;15(9):2178–82. doi: 10.1080/21645515.2018.1539601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verhagen LM, Hermsen M, Rivera-Olivero IA, Sisco MC, de Jonge MI, Hermans PW, et al. Nasopharyngeal carriage of respiratory pathogens in Warao Amerindians: significant relationship with stunting. Trop Med Int Health. 2017;22(4):407–14. doi: 10.1111/tmi.12835 . [DOI] [PubMed] [Google Scholar]
- 63.Rivera-Olivero IA, del Nogal B, Sisco MC, Bogaert D, Hermans PW, de Waard JH. Carriage and invasive isolates of Streptococcus pneumoniae in Caracas, Venezuela: the relative invasiveness of serotypes and vaccine coverage. Eur J Clin Microbiol Infect Dis. 2011;30(12):1489–95. doi: 10.1007/s10096-011-1247-5 . [DOI] [PubMed] [Google Scholar]
- 64.Ozdemir H, Ciftci E, Durmaz R, Guriz H, Aysev AD, Karbuz A, et al. Risk factors for nasopharyngeal carriage of Streptococcus pneumoniae in healthy Turkish children after the addition of heptavalent pneumococcal conjugate vaccine (PCV7) to the national vaccine schedule. Turk J Pediatr. 2013;55(6):575–83. Epub 2014/03/01. . [PubMed] [Google Scholar]
- 65.Uzuner A, Ilki A, Akman M, Gundogdu E, Erbolukbas R, Kokacya O, et al. Nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae in healthy children. Turk J Pediatr 2007;49(4):370–8. Epub 2008/02/06. . [PubMed] [Google Scholar]
- 66.Ricketson LJ, Wood ML, Vanderkooi OG, MacDonald JC, Martin IE, Demczuk WH, et al. Trends in asymptomatic nasopharyngeal colonization with Streptococcus pneumoniae after introduction of the 13-valent pneumococcal conjugate vaccine in Calgary, Canada. Ped Infect Dis J. 2014;33(7):724–30. doi: 10.1097/INF.0000000000000267 [DOI] [PubMed] [Google Scholar]
- 67.Neal EFG, Nguyen CD, Ratu FT, Dunne EM, Kama M, Ortika BD, et al. Factors associated with pneumococcal carriage and density in children and adults in Fiji, using four cross-sectional surveys. PLoS One. 2020;15(4):e0231041. doi: 10.1371/journal.pone.0231041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neal EFG, Flasche S, Nguyen CD, Ratu FT, Dunne EM, Koyamaibole L, et al. Associations between ethnicity, social contact, and pneumococcal carriage three years post-PCV10 in Fiji. Vaccine. 2020;38(2):202–11. doi: 10.1016/j.vaccine.2019.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neal EFG, Nguyen C, Ratu FT, Matanitobua S, Dunne EM, Reyburn R, et al. A comparison of pneumococcal nasopharyngeal carriage in very young Fijian infants born by vaginal or Cesarean delivery. JAMA Netw Open. 2019;2(10):e1913650. Epub 2019/10/19. doi: 10.1001/jamanetworkopen.2019.13650 ; PubMed Central PMCID: PMC6813584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arvas A, Cokugras H, Gur E, Gonullu N, Taner Z, Bahar Tokman H. Pneumococcal nasopharyngeal carriage in young healthy children after pneumococcal conjugate vaccine in Turkey. Balkan Med J. 2017. doi: 10.4274/balkanmedj.2016.1256 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samore MH, Magill MK, Alder SC, Severina E, Morrison-De Boer L, Lyon JL, et al. High rates of multiple antibiotic resistance in Streptococcus pneumoniae from healthy children living in isolated rural communities: association with cephalosporin use and intrafamilial transmission. Pediatrics. 2001;108(4):856–65. doi: 10.1542/peds.108.4.856 . [DOI] [PubMed] [Google Scholar]
- 72.Cheng Immergluck L, Kanungo S, Schwartz A, McIntyre A, Schreckenberger PC, Diaz PS. Prevalence of Streptococcus pneumoniae and Staphylococcus aureus nasopharyngeal colonization in healthy children in the United States. Epidemiol Infect. 2004;132(2):159–66. doi: 10.1017/s0950268803001791 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Finkelstein JA, Huang SS, Daniel J, Rifas-Shiman SL, Kleinman K, Goldmann D, et al. Antibiotic-resistant Streptococcus pneumoniae in the heptavalent pneumococcal conjugate vaccine era: predictors of carriage in a multicommunity sample. Pediatrics. 2003;112(4):862–9. doi: 10.1542/peds.112.4.862 . [DOI] [PubMed] [Google Scholar]
- 74.Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124(1):e1–11. doi: 10.1542/peds.2008-3099 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang SS, Finkelstein JA, Rifas-Shiman SL, Kleinman K, Platt R. Community-level predictors of pneumococcal carriage and resistance in young children. Am J Epidemiol. 2004;159(7):645–54. doi: 10.1093/aje/kwh088 . [DOI] [PubMed] [Google Scholar]
- 76.Moore MR, Hyde TB, Hennessy TW, Parks DJ, Reasonover AL, Harker-Jones M, et al. Impact of a conjugate vaccine on community-wide carriage of nonsusceptible Streptococcus pneumoniae in Alaska. Journal of Infectious Diseases. 2004;190(11):2031–8. doi: 10.1086/425422 . [DOI] [PubMed] [Google Scholar]
- 77.Reisman J, Rudolph K, Bruden D, Hurlburt D, Bruce MG, Hennessy T. Risk factors for pneumococcal colonization of the nasopharynx in Alaska native adults and children. J Pediatric Infect Dis Soc. 2014;3(2):104–11. doi: 10.1093/jpids/pit069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hsu KK, Rifas-Shiman SL, Shea KM, Kleinman KP, Lee GM, Lakoma M, et al. Do community-level predictors of pneumococcal carriage continue to play a role in the conjugate vaccine era? Epidemiol Infect. 2014;142(2):379–87. doi: 10.1017/S0950268813000794 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee GM, Kleinman K, Pelton SI, Hanage W, Huang SS, Lakoma M, et al. Impact of 13-Valent Pneumococcal Conjugate Vaccination on Streptococcus pneumoniae Carriage in Young Children in Massachusetts. J Pediatric Infect Dis Soc. 2014;3(1):23–32. doi: 10.1093/jpids/pit057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park SY, Moore MR, Bruden DL, Hyde TB, Reasonover AL, Harker-Jones M, et al. Impact of conjugate vaccine on transmission of antimicrobial-resistant Streptococcus pneumoniae among Alaskan children. Ped Infect Dis J. 2008;27(4):335–40. doi: 10.1097/INF.0b013e318161434d . [DOI] [PubMed] [Google Scholar]
- 81.Cohen R, Levy C, Bonnet E, Thollot F, Boucherat M, Fritzell B, et al. Risk factors for serotype 19A carriage after introduction of 7-valent pneumococcal vaccination. BMC Infect Dis. 2011;11:95. doi: 10.1186/1471-2334-11-95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Memish ZA, Assiri A, Almasri M, Alhakeem RF, Turkestani A, Al Rabeeah AA, et al. Impact of the Hajj on pneumococcal transmission. Clin Microbiol Infect. 2015;21(1):77.e11–8. doi: 10.1016/j.cmi.2014.07.005 . [DOI] [PubMed] [Google Scholar]
- 83.Koliou MG, Andreou K, Lamnisos D, Lavranos G, Iakovides P, Economou C, et al. Risk factors for carriage of Streptococcus pneumoniae in children. BMC Pediatr. 2018;18(1):144. Epub 2018/04/28. doi: 10.1186/s12887-018-1119-6 ; PubMed Central PMCID: PMC5921789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wroe PC, Lee GM, Finkelstein JA, Pelton SI, Hanage WP, Lipsitch M, et al. Pneumococcal carriage and antibiotic resistance in young children before 13-valent conjugate vaccine. Ped Infect Dis J. 2012;31(3):249–54. doi: 10.1097/INF.0b013e31824214ac . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Camilli R, Vescio MF, Giufre M, Daprai L, Garlaschi ML, Cerquetti M, et al. Carriage of Haemophilus influenzae is associated with pneumococcal vaccination in Italian children. Vaccine. 2015;33(36):4559–64. doi: 10.1016/j.vaccine.2015.07.009 . [DOI] [PubMed] [Google Scholar]
- 86.Camilli R, Daprai L, Cavrini F, Lombardo D, D’Ambrosio F, Del Grosso M, et al. Pneumococcal carriage in young children one year after introduction of the 13-valent conjugate vaccine in Italy. PLoS One. 2013;8(10):e76309. doi: 10.1371/journal.pone.0076309 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ansaldi F, de Florentiis D, Canepa P, Zancolli M, Martini M, Orsi A, et al. Carriage of Streptococcus pneumoniae 7 years after implementation of vaccination program in a population with very high and long-lasting coverage, Italy. Vaccine. 2012;30(13):2288–94. doi: 10.1016/j.vaccine.2012.01.067 . [DOI] [PubMed] [Google Scholar]
- 88.Navne JE, Borresen ML, Slotved HC, Andersson M, Melbye M, Ladefoged K, et al. Nasopharyngeal bacterial carriage in young children in Greenland: a population at high risk of respiratory infections. Epidemiol Infect. 2016;144(15):3226–36. doi: 10.1017/S0950268816001461 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bogaert D, van Belkum A, Sluijter M, Luijendijk A, de Groot R, Rumke HC, et al. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet. 2004;363(9424):1871–2. doi: 10.1016/S0140-6736(04)16357-5 . [DOI] [PubMed] [Google Scholar]
- 90.Alfayate Miguélez S, Yague Guirao G, Menasalvas Ruíz AI, Sanchez-Solís M, Domenech Lucas M, González Camacho F, et al. Impact of Pneumococcal Vaccination in the Nasopharyngeal Carriage of Streptococcus pneumoniae in Healthy Children of the Murcia Region in Spain. Vaccines (Basel). 2020;9(1). Epub 2021/01/01. doi: 10.3390/vaccines9010014 ; PubMed Central PMCID: PMC7823743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Almeida ST, Nunes S, Santos Paulo AC, Valadares I, Martins S, Breia F, et al. Low prevalence of pneumococcal carriage and high serotype and genotype diversity among adults over 60 years of age living in Portugal. PLoS One. 2014;9(3):e90974. doi: 10.1371/journal.pone.0090974 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zuccotti G, Mameli C, Daprai L, Garlaschi ML, Dilillo D, Bedogni G, et al. Serotype distribution and antimicrobial susceptibilities of nasopharyngeal isolates of Streptococcus pneumoniae from healthy children in the 13-valent pneumococcal conjugate vaccine era. Vaccine. 2014;32(5):527–34. doi: 10.1016/j.vaccine.2013.12.003 . [DOI] [PubMed] [Google Scholar]
- 93.Ueno M, Ishii Y, Tateda K, Anahara Y, Ebata A, Iida M, et al. Prevalence and risk factors of nasopharyngeal carriage of Streptococcus pneumoniae in healthy children in Japan. Jpn J Infect Dis. 2013;66(1):22–5. doi: 10.7883/yoken.66.22 . [DOI] [PubMed] [Google Scholar]
- 94.Chan KC, Subramanian R, Chong P, Nelson EA, Lam HS, Li AM, et al. Pneumococcal carriage in young children after introduction of PCV13 in Hong Kong. Vaccine. 2016;34(33):3867–74. doi: 10.1016/j.vaccine.2016.05.047 . [DOI] [PubMed] [Google Scholar]
- 95.Mackenzie GA, Leach AJ, Carapetis JR, Fisher J, Morris PS. Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infect Dis. 2010;10:304. doi: 10.1186/1471-2334-10-304 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Daana M, Rahav G, Hamdan A, Thalji A, Jaar F, Abdeen Z, et al. Measuring the effects of pneumococcal conjugate vaccine (PCV7) on Streptococcus pneumoniae carriage and antibiotic resistance: the Palestinian-Israeli Collaborative Research (PICR). Vaccine. 2015;33(8):1021–6. doi: 10.1016/j.vaccine.2015.01.003 . [DOI] [PubMed] [Google Scholar]
- 97.Hsieh YC, Chiu CH, Chang KY, Huang YC, Chen CJ, Kuo CY, et al. The impact of the heptavalent pneumococcal conjugate vaccine on risk factors for Streptococcus pneumoniae carriage in children. Ped Infect Dis J. 2012;31(9):e163–8. doi: 10.1097/INF.0b013e31825cb9f9 . [DOI] [PubMed] [Google Scholar]
- 98.Chang B, Akeda H, Nakamura Y, Hamabata H, Ameku K, Toma T, et al. Impact of thirteen-valent pneumococcal conjugate vaccine on nasopharyngeal carriage in healthy children under 24 months in Okinawa, Japan. Journal of infection and chemotherapy: official journal of the Japan Society of Chemotherapy. 2020;26(5):465–70. doi: 10.1016/j.jiac.2019.12.009 [DOI] [PubMed] [Google Scholar]
- 99.Shiri T, Nunes MC, Adrian PV, Van Niekerk N, Klugman KP, Madhi SA. Interrelationship of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus colonization within and between pneumococcal-vaccine naive mother-child dyads. BMC Infect Dis. 2013;13:483. doi: 10.1186/1471-2334-13-483 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Menezes AP, Azevedo J, Leite MC, Campos LC, Cunha M, Carvalho Mda G, et al. Nasopharyngeal carriage of Streptococcus pneumoniae among children in an urban setting in Brazil prior to PCV10 introduction. Vaccine. 2016;34(6):791–7. doi: 10.1016/j.vaccine.2015.12.042 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Korona-Glowniak I, Malm A. Characteristics of Streptococcus pneumoniae strains colonizing upper respiratory tract of healthy preschool children in Poland. ScientificWorldJournal. 2012. doi: 10.1100/2012/732901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoang VT, Dao TL, Ly TDA, Belhouchat K, Chaht KL, Gaudart J, et al. The dynamics and interactions of respiratory pathogen carriage among French pilgrims during the 2018 Hajj. Emerg Microbes Infect. 2019;8(1):1701–10. Epub 2019/11/22. doi: 10.1080/22221751.2019.1693247 ; PubMed Central PMCID: PMC6882464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Otsuka T, Chang B, Shirai T, Iwaya A, Wada A, Yamanaka N, et al. Individual risk factors associated with nasopharyngeal colonization with Streptococcus pneumoniae and Haemophilus influenzae: a Japanese birth cohort study. Ped Infect Dis J. 2013;32(7):709–14. doi: 10.1097/INF.0b013e31828701ea . [DOI] [PubMed] [Google Scholar]
- 104.Kuo CY, Hwang KP, Hsieh YC, Cheng CH, Huang FL, Shen YH, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Taiwan before and after the introduction of a conjugate vaccine. Vaccine. 2011;29(32):5171–7. doi: 10.1016/j.vaccine.2011.05.034 . [DOI] [PubMed] [Google Scholar]
- 105.Usuf E, Bojang A, Camara B, Jagne I, Oluwalana C, Bottomley C, et al. Maternal pneumococcal nasopharyngeal carriage and risk factors for neonatal carriage after the introduction of pneumococcal conjugate vaccines in The Gambia. Clin Microbiol Infect. 2018;24(4):389–95. Epub 2017/07/27. doi: 10.1016/j.cmi.2017.07.018 . [DOI] [PubMed] [Google Scholar]
- 106.Vanker A, Nduru PM, Barnett W, Dube FS, Sly PD, Gie RP, et al. Indoor air pollution and tobacco smoke exposure: impact on nasopharyngeal bacterial carriage in mothers and infants in an African birth cohort study. ERJ Open Res. 2019;5(1). Epub 2019/02/12. doi: ; PubMed Central PMCID: PMC6360211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Millar EV, O’Brien KL, Zell ER, Bronsdon MA, Reid R, Santosham M. Nasopharyngeal carriage of Streptococcus pneumoniae in Navajo and White Mountain Apache children before the introduction of pneumococcal conjugate vaccine. Ped Infect Dis J. 2009;28(8):711–6. Epub 2009/07/14. doi: 10.1097/INF.0b013e3181a06303 . [DOI] [PubMed] [Google Scholar]
- 108.Labout JA, Duijts L, Arends LR, Jaddoe VW, Hofman A, de Groot R, et al. Factors associated with pneumococcal carriage in healthy Dutch infants: the generation R study. Journal of Pediatrics. 2008;153(6):771–6. doi: 10.1016/j.jpeds.2008.05.061 . [DOI] [PubMed] [Google Scholar]
- 109.Murad C, Dunne EM, Sudigdoadi S, Fadlyana E, Tarigan R, Pell CL, et al. Pneumococcal carriage, density, and co-colonization dynamics: A longitudinal study in Indonesian infants. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2019;86:73–81. doi: 10.1016/j.ijid.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 110.Sung RY, Ling JM, Fung SM, Oppenheimer SJ, Crook DW, Lau JT, et al. Carriage of Haemophilus influenzae and Streptococcus pneumoniae in healthy Chinese and Vietnamese children in Hong Kong. Acta Paediatr. 1995;84(11):1262–7. doi: 10.1111/j.1651-2227.1995.tb13545.x . [DOI] [PubMed] [Google Scholar]
- 111.Bojang A, Jafali J, Egere U, Hill P, Antonio M, Jeffries D. Seasonality of pneumococcal nasopharyngeal carriage in rural Gambia determined within the context of a cluster randomized pneumococcal vaccine trial. PLoS One. 2015;10(7):13. CN-01130937. doi: 10.1371/journal.pone.0129649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Coles CL, Kanungo R, Rahmathullah L, Thulasiraj RD, Katz J, Santosham M, et al. Pneumococcal nasopharyngeal colonization in young South Indian infants. Ped Infect Dis J. 2001;20(3):289–95. doi: 10.1097/00006454-200103000-00014 . [DOI] [PubMed] [Google Scholar]
- 113.Coles CL, Sherchand JB, Khatry SK, Katz J, Leclerq SC, Mullany LC, et al. Nasopharyngeal carriage of S. pneumoniae among young children in rural Nepal. Trop Med Int Health. 2009;14(9):1025–33. Epub 2009/07/01. doi: 10.1111/j.1365-3156.2009.02331.x ; PubMed Central PMCID: PMC2770711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.le Polain de Waroux O, Flasche S, Kucharski AJ, Langendorf C, Ndazima D, Mwanga-Amumpaire J, et al. Identifying human encounters that shape the transmission of Streptococcus pneumoniae and other acute respiratory infections. Epidemics. 2018;25:72–9. Epub 2018/07/29. doi: 10.1016/j.epidem.2018.05.008 ; PubMed Central PMCID: PMC6227246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gils E, Veenhoven R, Rodenburg G, Hak E, Sanders E. Effect of 7-valent pneumococcal conjugate vaccine on nasopharyngeal carriage with Haemophilus influenzae and Moraxella catarrhalis in a randomized controlled trial. Vaccine. 2011;29(44):7595–8. doi: 10.1016/j.vaccine.2011.08.049 CN-00806054. [DOI] [PubMed] [Google Scholar]
- 116.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. Epub 2017/09/25. doi: 10.1136/bmj.j4008 ; PubMed Central PMCID: PMC5833365 at http://www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mulholland K, Satzke C. Serotype replacement after pneumococcal vaccination. Lancet. 2012;379(9824):1387; author reply 8–9. Epub 2012/04/17. doi: 10.1016/S0140-6736(12)60588-1 . [DOI] [PubMed] [Google Scholar]
- 118.van Hoek AJ, Sheppard CL, Andrews NJ, Waight PA, Slack MP, Harrison TG, et al. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine. 2014;32(34):4349–55. Epub 2014/03/25. doi: 10.1016/j.vaccine.2014.03.017 . [DOI] [PubMed] [Google Scholar]
- 119.Lindstrand A, Galanis I, Darenberg J, Morfeldt E, Naucler P, Blennow M, et al. Unaltered pneumococcal carriage prevalence due to expansion of non-vaccine types of low invasive potential 8years after vaccine introduction in Stockholm, Sweden. Vaccine. 2016;34(38):4565–71. Epub 2016/07/31. doi: 10.1016/j.vaccine.2016.07.031 . [DOI] [PubMed] [Google Scholar]
- 120.Desai AP, Sharma D, Crispell EK, Baughman W, Thomas S, Tunali A, et al. Decline in Pneumococcal Nasopharyngeal Carriage of Vaccine Serotypes After the Introduction of the 13-Valent Pneumococcal Conjugate Vaccine in Children in Atlanta, Georgia. The Pediatric infectious disease journal. 2015;34(11):1168–74. Epub 2015/08/01. doi: 10.1097/INF.0000000000000849 . [DOI] [PubMed] [Google Scholar]
- 121.van Gils EJ, Veenhoven RH, Hak E, Rodenburg GD, Bogaert D, Ijzerman EP, et al. Effect of reduced-dose schedules with 7-valent pneumococcal conjugate vaccine on nasopharyngeal pneumococcal carriage in children: a randomized controlled trial. Jama. 2009;302(2):159–67. Epub 2009/07/09. doi: 10.1001/jama.2009.975 . [DOI] [PubMed] [Google Scholar]
- 122.Løvlie A, Vestrheim DF, Aaberge IS, Steens A. Changes in pneumococcal carriage prevalence and factors associated with carriage in Norwegian children, four years after introduction of PCV13. BMC Infectious Diseases. 2020;20(1):29. doi: 10.1186/s12879-019-4754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Collins DA, Hoskins A, Snelling T, Senasinghe K, Bowman J, Stemberger NA, et al. Predictors of pneumococcal carriage and the effect of the 13-valent pneumococcal conjugate vaccination in the Western Australian Aboriginal population. Pneumonia. 2017;9(1):14. doi: 10.1186/s41479-017-0038-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Howard LM, Zhu Y, Griffin MR, Edwards KM, Williams JV, Gil AI, et al. Nasopharyngeal pneumococcal density during asymptomatic respiratory virus infection and risk for subsequent acute respiratory illness. Emerg Infect Dis. 2019;25(11):2040–7. doi: 10.3201/eid2511.190157 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carr OJJ, Vilivong K, Bounvilay L, Dunne EM, Lai JYR, Chan J, et al. Nasopharyngeal pneumococcal colonization density is associated with severe pneumonia in young children in the Lao PDR. J Infect Dis. 2021. Epub 20210511. doi: 10.1093/infdis/jiab239 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gastaldi A, Donà D, Barbieri E, Giaquinto C, Bont LJ, Baraldi E. COVID-19 Lesson for Respiratory Syncytial Virus (RSV): Hygiene Works. Children (Basel). 2021;8(12). Epub 20211206. doi: 10.3390/children8121144 ; PubMed Central PMCID: PMC8700687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Di Mattia G, Nenna R, Mancino E, Rizzo V, Pierangeli A, Villani A, et al. During the COVID-19 pandemic where has respiratory syncytial virus gone? Pediatr Pulmonol. 2021;56(10):3106–9. Epub 20210726. doi: 10.1002/ppul.25582 ; PubMed Central PMCID: PMC8441855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Smith CM, Sandrini S, Datta S, Freestone P, Shafeeq S, Radhakrishnan P, et al. Respiratory syncytial virus increases the virulence of Streptococcus pneumoniae by binding to penicillin binding protein 1a. A new paradigm in respiratory infection. Am J Respir Crit Care Med. 2014;190(2):196–207. doi: 10.1164/rccm.201311-2110OC ; PubMed Central PMCID: PMC4226051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brealey JC, Chappell KJ, Galbraith S, Fantino E, Gaydon J, Tozer S, et al. Streptococcus pneumoniae colonization of the nasopharynx is associated with increased severity during respiratory syncytial virus infection in young children. Respirology. 2018;23(2):220–7. doi: 10.1111/resp.13179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sender V, Hentrich K, Henriques-Normark B. Virus-induced changes of the respiratory tract environment promote secondary infections with Streptococcus pneumoniae. Front Cell Infect Microbiol. 2021;11:643326–. doi: 10.3389/fcimb.2021.643326 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weinberger DM, Klugman KP, Steiner CA, Simonsen L, Viboud C. Association between respiratory syncytial virus activity and pneumococcal disease in infants: a time series analysis of US hospitalization data. PLoS Med. 2015;12(1):e1001776. Epub 20150106. doi: 10.1371/journal.pmed.1001776 ; PubMed Central PMCID: PMC4285401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morpeth SC, Munywoki P, Hammitt LL, Bett A, Bottomley C, Onyango CO, et al. Impact of viral upper respiratory tract infection on the concentration of nasopharyngeal pneumococcal carriage among Kenyan children. Sci Rep. 2018;8(1):11030. Epub 20180723. doi: 10.1038/s41598-018-29119-w ; PubMed Central PMCID: PMC6056465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grijalva CG, Griffin MR, Edwards KM, Williams JV, Gil AI, Verastegui H, et al. The role of influenza and parainfluenza infections in nasopharyngeal pneumococcal acquisition among young children. Clinical Infectious Diseases. 2014;58(10):1369–76. doi: 10.1093/cid/ciu148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Berical AC, Harris D, Dela Cruz CS, Possick JD. Pneumococcal vaccination strategies. An update and perspective. Ann Am Thorac Soc. 2016;13(6):933–44. Epub 2016/04/19. doi: 10.1513/AnnalsATS.201511-778FR ; PubMed Central PMCID: PMC5461988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weinberger DM, Pitzer VE, Regev-Yochay G, Givon-Lavi N, Dagan R. Association between the decline in pneumococcal disease in unimmunized adults and vaccine-derived protection against colonization in toddlers and preschool-aged children. Am J Epidemiol. 2018;188(1):160–8. doi: 10.1093/aje/kwy219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mattos KJ, Eichelberger L, Warren J, Dotson A, Hawley M, Linden KG. Household Water, Sanitation, and Hygiene Practices Impact Pathogen Exposure in Remote, Rural, Unpiped Communities. Environmental Engineering Science. 2021;38(5):355–66. doi: 10.1089/ees.2020.0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gudnason T, Hrafnkelsson B, Laxdal B, Kristinsson KG. Risk factors for nasopharyngeal carriage of Streptococcus pneumoniae and effects of a hygiene intervention: repeated cross-sectional cohort study at day care centres. Scand J Infect Dis. 2014;46(7):493–501. Epub 20140430. doi: 10.3109/00365548.2014.901553 . [DOI] [PubMed] [Google Scholar]
- 138.Dunne EM, Carville K, Riley TV, Bowman J, Leach AJ, Cripps AW, et al. Aboriginal and non-Aboriginal children in Western Australia carry different serotypes of pneumococci with different antimicrobial susceptibility profiles. Pneumonia. 2016;8(1):15. doi: 10.1186/s41479-016-0015-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.World Health Organization. Ending preventable child deaths from pneumonia and diarrhoea by 2025: the integrated global action plan for pneumonia and diarrhoea (GAPPD). 2013. [DOI] [PubMed] [Google Scholar]
- 140.World Health Organization. Tobacco [Internet]. 2021 [updated 2021 July 26; cited 2021 July 29]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/tobacco.