Abstract
P2X7 receptors are dysregulated during psychostimulant exposure. Furthermore, P2X7 receptors enhance endogenous systems (e.g., cytokines, dopamine, and glutamate) that facilitate psychostimulant addiction. Therefore, using mouse locomotor, conditioned place preference (CPP), and intracranial self-stimulation (ICSS) assays, we tested the hypothesis that methamphetamine (METH) reward and acute locomotor activation requires P2X7 receptor activity. We also investigated effects of P2X7 blockade on METH-induced changes in cytokine levels in brain reward regions. A438079 (5, 10, 50 mg/kg), a P2X7 antagonist, did not affect spontaneous locomotor activity but reduced hyperlocomotion caused by acute METH (1 mg/kg) exposure. A438079 (10 mg/kg) also prevented expression of METH CPP without causing aversive or rewarding effects. For ICSS experiments, METH (1 mg/kg) facilitated brain reward function as interpreted from reductions in baseline threshold. In the presence of A438079 (50 mg/kg), METH-induced facilitation of ICSS was reduced. Repeated METH exposure (1 mg/kg × 7 d) caused enhancement of IL-17A levels in the prefrontal cortex (PFC) that was normalized by A438070 (10 mg/kg × 7 d). The present data suggest that P2X7 receptor activity contributes to rewarding and locomotor-stimulant effects of METH through a potential mechanism involving IL-17A, which has recently been implicated in anxiety.
Keywords: purine, P2X7, psychostimulant, methamphetamine, ICSS, ATP, CPP
1. Introduction
Purinergic P2X7 receptors are ATP-sensitive ion channels expressed by neurons, microglia, oligodendrocytes and astrocytes that facilitate innate and adaptive immune responses (Miras-Portugal et al., 2021). A role for P2X7 receptors in psychostimulant addiction remains unclear despite evidence that neuroinflammation facilitates psychostimulant dependence and relapse (Gipson et al., 2021; Hutchinson and Watkins, 2014) and P2X7 activation enhances neuroinflammation by inducing pro-inflammatory cytokine and chemokine release (Oliveira-Giacomelli et al., 2021; Fernandes et al., 2016). For example, P2X7 activation is linked to increased signaling at multiple chemokine receptors, including CCR2, CCR5 and CXCR4 (Ochi-ishi et al., 2014), that contribute to the rewarding and reinforcing effects of psychostimulants (Saika et al., 2018; Nayak et al., 2020; Kim et al., 2017). P2X7 receptor activation is also coupled to an increase in mRNA and protein levels of IL-17A, which is upregulated in the prefrontal cortex (PFC) and amygdala of mice exposed to cumulative stress and facilitates anxiety- and depression-like behaviors that accompany psychostimulant abstinence (Fan et al., 2016; de Lima et al., 2020; Li et al., 2019).
In the only study that directly linked P2X7 receptors to a preclinical metric of drug addiction, P2X7 receptor blockade reduced rewarding effects of the synthetic cathinone MDPV (3,4-methylenedioxypyrovalerone) in mice (Gentile et al., 2019). In the same study, repeated MDPV exposure increased mRNA and protein levels of P2X7 receptors in the nucleus accumbens (NAC) (Gentile et al., 2019). Repeated MDPV exposure also enhanced mRNA and protein levels of mitochondrial ATP synthase, an enzyme that catalyzes synthesis of ATP, an endogenous ligand for P2X7 (Gentile et al., 2019). Evidence that ATP increases extracellular dopamine in the striatum of rats and a purinergic (P2X/Y) receptor agonist increases striatal dopamine levels and locomotor activity links enhanced purinergic signaling to dopamine systems that underlie psychostimulant reward (Krügel et al., 1999, 2001; Kittner et al., 2000, 2001; Zhang et al., 1995). P2X7 receptor antagonism or genetic deletion also reduces amphetamine-induced hyperactivity, which is often used as a preclinical model of mania, and clinical data shows efficacy for a P2X7 blocker against bipolar disorder (Gubert et al., 2016; 2020). Moreover, P2X7 receptor blockade prevents amphetamine from increasing levels of the dopamine metabolite, DOPAC, in the hippocampus of mice (Gubert et al., 2020).
Because P2X7 systems influence multiple underlying mechanisms (i.e., neuroinflammation and dopamine transmission) of addiction, we hypothesized that A438079, a competitive, reversible P2X7 antagonist, would inhibit rewarding and locomotor-activating effects of methamphetamine (METH) in mice. The hypothesis was tested using three standard assays (locomotor, conditioned place preference (CPP), and intracranial self-stimulation (ICSS)). At the cellular level, we also investigated effects of P2X7 receptor blockade on METH-induced changes in cytokines (IL-1β, IL-6, IL-10, IL-17A, CCL2, CCL5 and TNF-α) in the prefrontal cortex (PFC) and nucleus accumbens (NAC).
2. Materials and methods
2.1. Animals and chemicals
Adult male C57Bl/6 mice (Jackson Laboratories; Bar Harbor, ME) were group-housed (2–5/cage) and maintained under a 12-h light/dark cycle on a regular chow diet. Studies were conducted in accordance with the Institutional Animal Care and Use Committee at Temple University. Methamphetamine hydrochloride (METH) was purchased from Sigma-Aldrich (St. Louis, MO). A438079 hydrochloride was purchased from Tocris Biosciences (St. Louis, MO, USA). Drugs were dissolved in 0.9% saline and injected intraperitoneally (ip). Mice were assigned randomly to each experimental group and used only once. Testing was done by an experimenter blinded to treatment conditions.
2.2. Locomotor experiments
Baseline locomotor activity was recorded for 60 min before A438079 (0, 5, 10, or 50 mg/kg) administration as described (Gentile et al., 2019). METH (1 mg/kg) or saline was injected 30 min later and locomotor activity was recorded for 90 min. Locomotor activity was recorded in 5-min bins.
2.3. CPP experiments
CPP experiments were conducted as described (Hicks et al., 2018). A 30-min pre-test was conducted on day 1 to determine compartment preference. The compartment in which a mouse spent less time was designated as the METH-paired side. A 4-day conditioning paradigm was then initiated. In the morning, mice were injected with METH (1 mg/kg) and confined to the METH-paired compartment for 30 min. In the afternoon, mice were injected with saline and placed in the opposite compartment for 30 min. A separate (control) group of mice were injected with saline in the morning and afternoon sessions. On day 6, a post-test was conducted whereby conditioned with METH or saline were injected with A438079 (10 mg/kg) or saline, placed into the chamber, and given access to both compartments for 30 min. A difference score was calculated as time spent in the METH-paired compartment during the post-test minus time spent in METH-paired compartment during the pre-test.
2.4. Intracranial self-stimulation (ICSS) experiments
Mice were stereotaxically implanted with a monopolar stimulating electrode (PlasticsOne; Roanoke, VA) targeting the medial forebrain bundle (from bregma: AP: −1.9, ML: +0.8, DV: −4.8 mm). Mice were trained to respond to electrical stimulation using computer-controlled operant chambers (Med Associates; St. Albans, VT) in daily sessions at the lowest current that sustained responding at a rate of one response per s ± 10%. This current was maintained through testing in which mice responded for stimulation frequencies (158–34 Hz) in descending order for 50 s. Experimental test sessions began when reward thresholds reached stable performance criteria (± 10% for 3 consecutive days). Effects of METH (0, 0.5, or 1 mg/kg) on reward thresholds were examined under a within-subjects, counterbalanced, Latin-Square design. Thereafter, A438079 (0, 5, 10, 50 mg/kg) was administered alone and, in separate sessions, as a pretreatment to METH (1 mg/kg). Thresholds were quantified from performance during 15-min test sessions. Percent baseline was computed as: (threshold after METH/threshold before A438079)*100.
2.5. Multiplex Assay of Cytokines and Chemokines by Luminex®
Mice were injected with METH (1 mg/kg) or saline for 7 d, and A438079 (50 mg/kg) or saline was administered 15 min before each METH or saline injection. Fifteen min after the last METH or saline injection, mice were euthanized by CO2 overdose. Brains were immediately extracted, and tissue from the NAC and PFC was dissected and flash-frozen after excision. For analysis, the frozen tissue samples were homogenized in 150–180 μl of phosphate-buffered saline (PBS) pH 7.2 to which an equal volume of Cell Lysis Buffer 2 was added. Samples were incubated for 30 min at room temperature with gentle shaking, then centrifuged at 13,000 × g for 20 minutes at 4° C. Supernatants were removed to a fresh 1.5 ml Eppendorf tube and immediately assayed by the Pierce BCA Protein Assay to obtain mg total protein/ml for each sample. Levels of mouse IL-1β, IL-6, IL-10, IL-17A, CCL2/MCP-1, and CCL5/RANTES were determined by the Milliplex® MAP Mouse Cytokine/Chemokine Magnetic Bead Panel Luminex® assay (MilliporeSigma, Burlington, MA). All extracts were run in duplicate. The assay was read on a BioRad BioPlex®100 Luminex® reader (BioRad Laboratories, Hercules, CA), using BioPlex® Manager 6.1 software. The cytokine/chemokine concentration values obtained, at pg/ml of extract, were divided by their respective mg total protein/ml values, to obtain pg/mg total protein values for each mediator.
2.6. Statistical analysis
Locomotor and CPP data were analyzed by two-way ANOVA followed by a Bonferroni post-hoc test. ICSS and cytokine data were analyzed by one-way ANOVA followed by Dunnett’s post-hoc analysis. Statistical significance was p < 0.05.
3. Results
3.1. A438079 reduced METH-evoked locomotor activation and CPP (Fig. 1A–B)
Fig 1. P2X7 antagonist inhibits METH-induced locomotor activation and CPP.
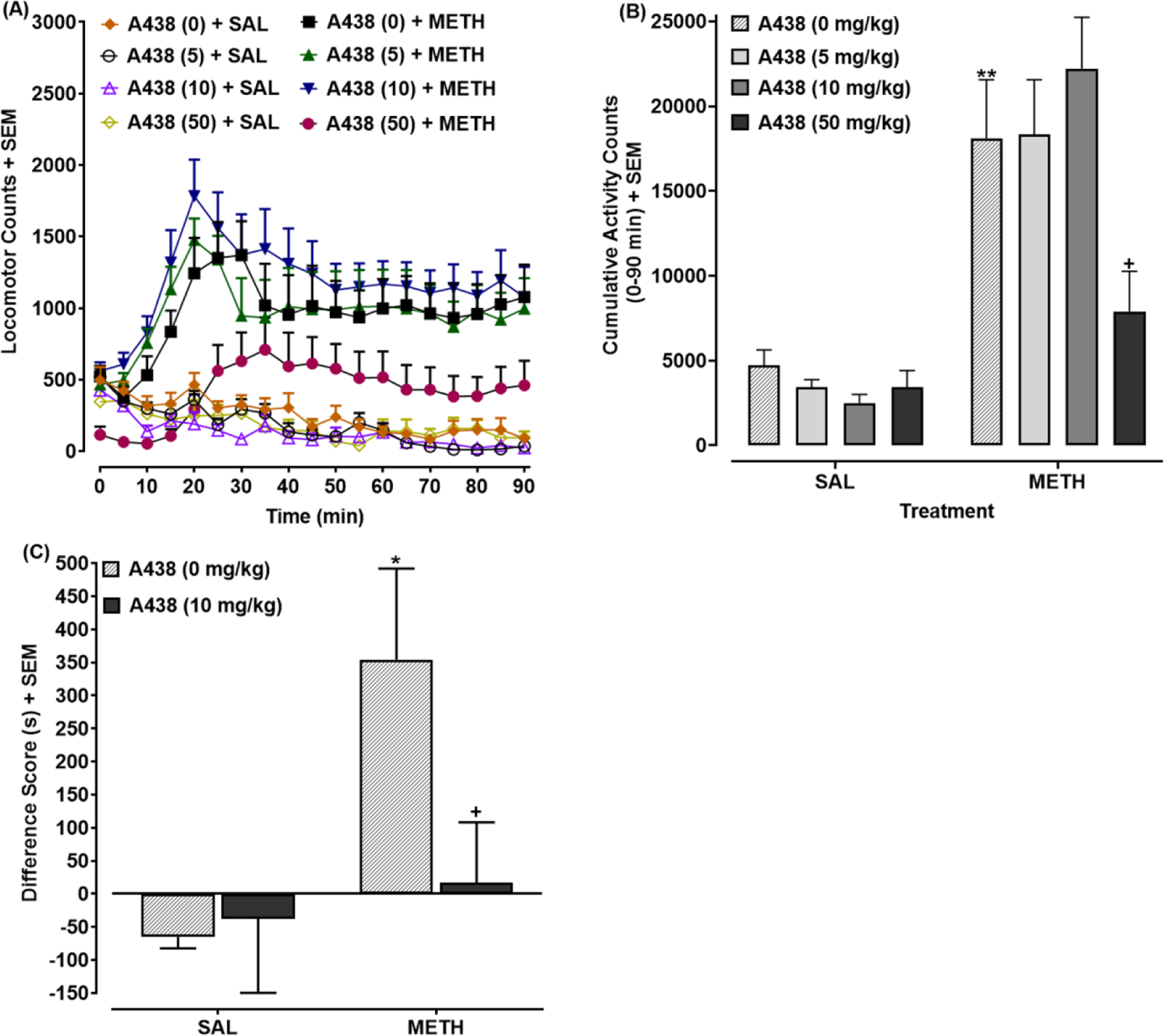
(A) Locomotor time course: Mice pretreated with A438079 (0, 5, 10, or 50 mg/kg) were injected with METH (1 mg/kg) or saline 30 min later (at time 0). Locomotor activity presented as mean locomotor counts. N=8 mice/group. (B) Cumulative locomotor activity: Time-course data from (A) were quantified and presented as cumulative locomotor counts for the 90 min-interval following METH injection. **p < 0.01 versus drug-naïve mice (A438 (0 mg) + SAL) or +p < 0.05 versus mice treated with METH by itself (A438 (0 mg) + METH). N=8 mice/group. (C) CPP: Data are presented as a difference score (difference in time spent on METH-paired side between post-test and pre-test). N=7–8 mice/group. *p < 0.05 versus drug-naïve mice (A438 (0 mg) + SAL) or +p < 0.05 versus mice conditioned with METH alone (A438 (0 mg) + METH).
For temporal locomotor data (Fig. 1A), two-way ANOVA (time × treatment) indicated effects of treatment [F(7, 56) = 13.29, p < 0.0001] and time [F(18, 1008) = 10.25, p < 0.001] and a significant treatment × time interaction [F(126, 1008) = 3.92, p < 0.0001]. For cumulative locomotor activity (Fig. 1B), two-way ANOVA (A438079 × METH) indicated effects of METH [F(1, 56) = 69.72, p < 0.0001] and A438079 [F(3, 56) = 3.66, p < 0.05] and a significant A438079 × METH interaction [F(3, 56) = 4.11, p < 0.05]. Mice injected with METH (1 mg/kg) by itself (SAL + METH) displayed greater locomotor activity counts than drug-naïve mice (SAL + SAL) (**p < 0.01) (Fig. 1B). For mice injected with A438079 (50 mg/kg) and METH (1 mg/kg), locomotor activity was lower than A438079-naïve mice injected with METH (1 mg/kg) (SAL + METH) (+p < 0.05) (Fig. 1B). Locomotor activity did not differ significantly between mice injected with A438079 (5, 10, or 50 mg/kg) or saline (p > 0.05).
Effects of A438079 (10 mg/kg) on expression of METH (1 mg/kg) CPP are presented in Fig. 1C. Two-way ANOVA revealed a significant effect of METH conditioning [F(1, 26) = 5.48, p < 0.05] (Fig. 1C). Rats conditioned with METH (1 mg.kg) displayed greater CPP than mice conditioned with saline (**p < 0.01). CPP was reduced in mice challenged with A438079 (10 mg/kg) compared to those challenged with saline (+p < 0.05).
3.2. A438079 normalized METH-evoked changes in reward thresholds during ICSS (Fig. 2)
Fig. 2. P2X7 antagonist inhibits reduction in brain reward threshold produced by METH in ICSS assays.

Percent change in baseline reward threshold shown in (A, C,) and maximum response rate shown in (B, D). (A-B) METH (1 mg/kg) reduces brain reward threshold without affecting maximal response rate. N=5–6 mice/group. *p < 0.05 compared to saline-treated controls. (C-D) A438079 (0, 5, 10, 50 mg/kg) inhibits reduction in brain reward threshold produced by METH (1 mg/kg). N=8–9 mice/group. *p < 0.05 versus METH by itself (i.e. leftmost column).
For experiments testing METH alone (Fig. 2A–B), one-way ANOVA indicated an effect of METH dose on baseline threshold [F(2, 12) = 4.08, p < 0.05] (Fig. 2A) but no effect on maximal response rates [F(2, 12) = 3.66, p > 0.05] (Fig. 2B). The percentage of baseline threshold in mice treated with higher-dose METH (1 mg/kg) was lower than saline-injected mice (*p < 0.05; Fig. 2A). For experiments testing different doses of A438079 (0, 5, 10, or 50 mg/kg) against METH (1 mg/kg) (Fig. 2C–D), one-way ANOVA indicated a main effect on baseline threshold [F(3, 32) = 3.64, p < 0.05] (Fig. 2C) but no effect on maximal response rate (p > 0.05) (Fig. 2D). A dose of 50 mg/kg A438079 normalized METH-elicited reductions in baseline threshold (*p < 0.05), with a trend toward significance for a dose of 10 mg/kg. Prior work showed that A438709 (5 – 50 mg/kg)does not affect reward threshold or maximal response rates (Gentil et al., 2019).
3.3. A438079 normalized METH-evoked increase in IL17-A levels in PFC (Fig. 3)
Fig 3. Effects of P2X7 receptor blockade by A438079 (50 mg/kg) on METH-induced changes in cytokine and chemokine levels in the PFC (A) and NAC (B).
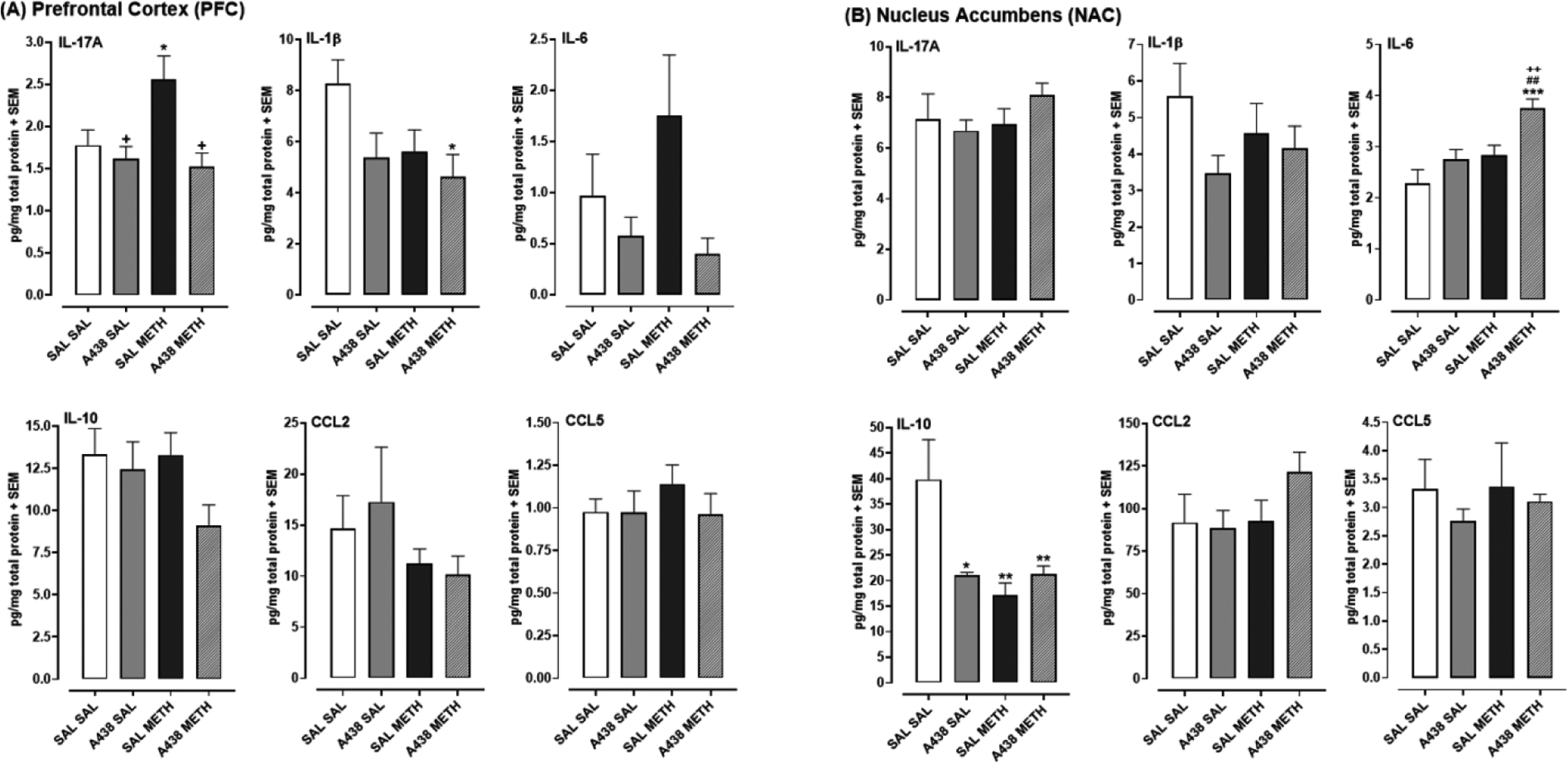
Data are presented as mean pg/mg total protein for each marker. N=7–8 mice/group. *p < 0.05 or **p < 0.01 versus drug-naïve mice (SAL SAL), +p < 0.05 or ++p < 0.01 versus METH alone (SAL METH)(A438 (0 mg) + METH), and ##p < 0.01 versus A438079 alone (A438 SAL).
Effects of A438079 and METH on cytokine levels are presented in Figs. 3A (PFC) and 3B (NAC). For IL-17A levels in the PFC (Fig. 3A), one-way ANOVA revealed a main effect [F(3, 26) = 5.58, p < 0.05]. METH by itself (SAL + METH) increased IL-17A levels compared to drug-naïve mice (SAL + SAL) (*p < 0.05). For mice exposed to A438079 and METH, IL-17A levels were reduced compared to A438079-naïve mice exposed only to METH (SAL + METH) (+p < 0.05). For IL-1β levels in the PFC, one-way ANOVA revealed a main effect [F(3, 26) = 3.11, p < 0.05]. IL-1β levels in mice exposed to A438079 and METH (A438 + METH) were reduced compared to drug-naïve rats (SAL + SAL) (*p < 0.05).
NAC results are presented in Fig. 3B. For IL-6, one-way ANOVA revealed a main effect [F(3, 28) = 7.88, p < 0.001]. IL-6 levels in rats exposed to a combination of A438079 and METH were elevated compared to all other groups, including drug-naïve (SAL + SAL) (***p < 0.001), A438079 by itself (A438 + SAL) (##p < 0.01), and METH by itself (SAL + METH) (++p < 0.01). For IL-10, one-way ANOVA revealed a main effect [F(3, 26) = 5.48, p < 0.01]. Compared to drug-naïve mice (SAL + SAL), IL-10 levels were reduced in all treatment groups, including mice exposed to A438079 by itself (A438 + SAL) (*p < 0.05), METH by itself (**p < 0.01), and a combination of A438079 and METH (**p < 0.01).
4. Discussion
A P2X7 antagonist (A438079) inhibited three behavioral effects of METH (locomotor activation, CPP, and facilitation of ICSS) and normalized the elevation of IL-17A levels in the PFC caused by repeated METH exposure. A438079 is brain-penetrable, P2X7-selective (e.g., IC50 = 100 and 300 nm at rat and human P2X7 receptors, respectively, and IC50 ≫ 10 μM for other P2 receptors), and lacks activity at non-purinergic receptors and channels (Donnelly-Roberts and Jarvis, 2007). This suggests that A438079 efficacy against METH was due to P2X7 antagonism and active P2X7 receptors are required for the full rewarding and locomotor-activating effects of METH. Because P2X7 receptors are activated by ATP, METH exposure may have caused a rapid increase in extracellular ATP that prompted P2X7 activation (Wilhelm et al., 2010). This interpretation is supported by evidence that mRNA and protein levels of ATP synthase, an enzyme that catalyzes ATP synthesis, and P2X7 in the NAC of mice are elevated by repeated MDPV exposure (Gentile et al., 2019).
Physiological mechanisms by which P2X7 signaling influenced METH-evoked behavioral effects are unclear. Because P2X7 receptor activation upregulates at least two correlates of METH addiction (i.e., enhanced mesolimbic dopamine transmission and neuroinflammation) (Gipson et al., 2021), possible mechanisms include P2X7 crosstalk with dopamine or cytokine systems. Dopamine release in the NAC is increased by ICSS, and ICSS is further enhanced by drugs that enhance extracellular dopamine (e.g., METH) but inhibited by drugs that block dopamine transmission (Negus and Miller, 2014). ATP enhances extracellular dopamine in the rat striatum, and a P2X/Y receptor agonist (2-methylthio ATP) increases striatal dopamine levels and locomotor activity (Krügel et al., 1999, 2001; Kittner et al., 2000, 2001; Zhang et al., 1995). Amphetamine-evoked hyperactivity, which is also mediated by increased dopamine transmission, is reduced by P2X7 receptor antagonism or genetic deletion (Gubert et al., 2016, 2020; Bhattacharya et al., 2013; Csölle et al., 2013). Moreover, in the context of a 7-day dosing paradigm like the one used in our study, a P2X7 antagonist reduces amphetamine-evoked levels of a dopamine metabolite, DOPAC, in the hippocampus of mice (Gubert et al., 2020). Because these results suggest that enhanced P2X7 signaling increases mesolimbic dopamine transmission, a P2X7 block by A438079 in our experiments may have prevented the normal METH-induced enhancement of extracellular dopamine that facilitates ICSS, leading to inhibition of METH-induced reward.
Repeated METH exposure caused an enhancement of IL-17A levels in the PFC that was completely normalized by a P2X7 receptor block. The effect was specific to the PFC, as IL-17A levels in the NAC were not affected by METH exposure. IL-17A is produced by immune cells, including CD4+ Th17 cells, CD8+ T and B cells, meningeal γδ T cells, natural killer cells, and innate lymphoid cells, and induces production of proinflammatory cytokines and chemokines (Li et al., 2019). Evidence linking IL17-A to anxiety and depression suggests that IL-17A signaling has physiological and pathophysiological functions in the CNS (de Lima et al., 2020; Li et al., 2019). For example, in mice exposed to cumulative stress, IL-17A is upregulated in the PFC and amygdala and anti-IL-17 treatment recuses anxiety- and depression-like behaviors (Kim et al., 2021). IL-17A knockout mice also spend more time in the open arm area of the elevated plus maze than the wild type mice, suggesting reduced anxiety (de Lima et al., 2020). Similar to our findings, the PFC was identified by de Lima et al. (2020) as an important anatomical site of IL-17A action, as selective knockdown of IL-17A in the PFC reduced anxiety (de Lima et al., 2020). Cellular data suggest that IL-17A receptors are primarily localized to glutamatergic neurons in the PFC, and loss of endogenous IL-17A signaling affects GABA and glutamate signaling pathways (de Lima et al., 2020). Glutamate systems in corticolimbic substrates are also dysregulated during METH exposure and contribute to dependence and relapse (Mishra et al., 2017). Thus, in our experiments, the METH-induced elevation in cortical IL-17A levels may have caused dysregulation of glutamate signaling in the PFC that contributed to the rewarding effects of METH detected in CPP and ICSS assays, although this interpretation is speculative given that behavioral and cytokine data were collected from separate groups of mice.
The ability of a P2X7 block to both reduce behavioral effects of METH and abolish the METH-induced elevation of cortical IL-17A levels suggests that crosstalk between P2X7 and IL-17A systems influences METH-evoked behaviors. Prior work shows that P2X7 blockade with A438079 abolishes the increase in mRNA and protein levels of IL-17A in splenoctyes derived from mice with experimental arthritis and reduces Th17 cell differentiation (Fan et al., 2016), although P2X4 receptors also play an important role (Hamoudi et al., 2022). In our experiments, other cytokines were also impacted by METH and A438079 exposure. For instance, cortical levels of IL-10 were reduced by METH. Because IL-10 inhibits IL-17A secretion from Th17 cells (Gu et al., 2008), the METH-induced reduction in IL-10 levels may have contributed to the elevation in cortical IL-17A levels. IL-6 mRNA levels in the NAC or PFC were not increased by METH, which is consistent with previous evidence that METH increases IL-6 gene expression in the VTA but not in the PFC or NAC (Wang et al., 2019). However, in the case in which METH was administered with A438079, we detected a robust increase in IL-6 mRNA levels in the NAC. This augmentation was surprising given that a reduction in IL-6 gene expression would be predicted in the presence of a P2X7 blockade and suggests that (1) mechanisms other than IL-6 signaling mediate the ability of P2X7 blockade to attenuate METH-evoked rewarding effects and (2) enhanced IL-6 levels in the NAC contribute to effects of METH other than reward and reinforcement, such as hyperthermia or neurotoxicity. Additionally, as noted above, effects of METH on IL-6 levels are brain-region specific, as systemic METH causes an increase in IL-6 levels in the VTA that contributes to enhanced extracellular dopamine levels in the NAC (Wang et al., 2019). Although cytokine levels in the VTA were not analyzed in our study, future studies will determine if METH causes an increase in IL-6 mRNA and protein levels that are dependent on P2X7 receptor activation.
For the various behavioral and cellular endpoints, different doses of A438079 were required to achieve efficacy. CPP was the primary outlier among the endpoints, as 10 mg/kg of A438079, given after METH conditioning, completely inhibited expression of METH CPP whereas a higher dose (50 mg/kg) was required to inhibit METH-evoked reductions in brain reward threshold, hyperlocomotion and changes in cytokine levels. One explanation is that the specific behavioral endpoints are mediated by different mechanisms, each with varying degrees of sensitivity to A438079. For example, expression of METH CPP, like relapse to psychostimulant seeking in self-administration models, is facilitated by increased glutamate transmission from the PFC to the NAC (Hicks et al., 2017; Knackstedt and Kalivas, 2009). Thus, disruption of glutamate transmission that facilitates expression of METH CPP may be more sensitive to P2X7 blockade by A438079 than behavioral effects of METH (e.g., locomotor activation and facilitation of ICSS) that rely more on enhanced dopamine transmission. Furthermore, motor and conditioned rewarding effects of amphetamines are mediated by mechanisms that are not entirely similar, as PKA inhibition blocks amphetamine-evoked CPP but not locomotor activation (Aujla and Beninger, 2003).
In summary, METH-induced reward and locomotor activation was dependent on P2X7 receptor activity and P2X7 blockade abolished the elevation in cortical IL-17A levels caused by repeated METH exposure. The identification of crosstalk between P2X7 receptors and IL-17A in the context of METH-evoked behaviors advances recent evidence that IL-17A physiology extends beyond rheumatoid arthritis, psoriasis and multiple sclerosis to include CNS diseases (Kim et al., 2021; de Lima et al., 2020; Li et al., 2019).
Research Highlights.
P2X7 antagonist inhibited methamphetamine CPP.
P2X7 antagonist inhibited facilitation of ICSS by methamphetamine.
P2X7 blockade reduced methamphetamine locomotor activation.
Repeated methamphetamine increased IL-17A levels in prefrontal cortex.
P2X7 antagonist abolished methamphetamine-evoked cortical IL-17A levels.
Acknowledgements
The authors acknowledge grant support from the National Institutes of Health (R01 DA045499, P30 DA013429, and T32 DA007237).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aujla H, Beninger RJ. Intra-accumbens protein kinase C inhibitor NPC 15437 blocks amphetamine-produced conditioned place preference in rats. Behav Brain Res. 2003; 147:41–48. [DOI] [PubMed] [Google Scholar]
- Alves de Lima K, Rustenhoven J, Da Mesquita S, Wall M, Salvador AF, Smirnov I, Martelossi Cebinelli G, Mamuladze T, Baker W, Papadopoulos Z, Lopes MB, Cao WS, Xie XS, Herz J, Kipnis J. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol. 2020; 21(11):1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007; 151(5):571–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan ZD, Zhang YY, Guo YH, Huang N, Ma HH, Huang H, Yu HG. Involvement of P2X7 receptor signaling on regulating the differentiation of Th17 cells and type II collagen-induced arthritis in mice. Sci Rep. 2016; 6:35804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes NC, Sriram U, Gofman L, Cenna JM, Ramirez SH, Potula R. Methamphetamine alters microglial immune function through P2X7R signaling. J Neuroinflammation. 2016; 13(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile TA, Simmons SJ, Tallarida CS, Su S, Rom S, Watson MN, Reitz AB, Potula R, Rawls SM. Synthetic cathinone MDPV enhances reward function through purinergic P2X7 receptor-dependent pathway and increases P2X7 gene expression in nucleus accumbens. Drug Alcohol Depend. 2019; 197:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Rawls S, Scofield MD, Siemsen BM, Bondy EO, Maher EE. Interactions of neuroimmune signaling and glutamate plasticity in addiction. J Neuroinflammation. 2021; 18(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Yang J, Ouyang X, Liu W, Li H, Yang J, Bromberg J, Chen SH, Mayer L, Unkeless JC, Xiong H. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol. 2008; 38(7):1807–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubert C, Andrejew R, Leite CE, Moritz CEJ, Scholl J, Figueiro F, Kapczinski F, da Silva Magalhães PV, Battastini AMO. P2X7 Purinergic Receptor Is Involved in the Pathophysiology of Mania: a Preclinical Study. Mol Neurobiol. 2020; 57(3):1347–1360. [DOI] [PubMed] [Google Scholar]
- Gubert C, Fries GR, Pfaffenseller B, Ferrari P, Coutinho-Silva R, Morrone FB, Kapczinski F, Battastini AMO. Role of P2X7 Receptor in an Animal Model of Mania Induced by D-Amphetamine. Mol Neurobiol. 2016; 53(1):611–620. [DOI] [PubMed] [Google Scholar]
- Hamoudi C, Zhao C, Abderrazak A, Salem M, Fortin PR, Sévigny J, Aoudjit F. The Purinergic Receptor P2X4 Promotes Th17 Activation and the Development of Arthritis. J Immunol. 2022; 208(5):1115–1127. [DOI] [PubMed] [Google Scholar]
- Hicks C, Gregg RA, Nayak SU, Cannella LA, Schena GJ, Tallarida CS, Reitz AB, Smith GR, Rawls SM. Glutamate carboxypeptidase II (GCPII) inhibitor 2-PMPA reduces rewarding effects of the synthetic cathinone MDPV in rats: a role for N-acetylaspartylglutamate (NAAG). Psychopharmacology 2017; 234:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Watkins LR. Why is neuroimmunopharmacology crucial for the future of addiction research? Neuropharmacology 2014; 76 Pt B(0 0):218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Connelly KL, Unterwald EM, Rawls SM. Chemokines and cocaine: CXCR4 receptor antagonist AMD3100 attenuates cocaine place preference and locomotor stimulation in rats. Brain Behav Immun. 2017; 62:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Suh YH, Chang KA. Interleukin-17 induced by cumulative mild stress promoted depression-like behaviors in young adult mice. Mol Brain. 2021; 14(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittner H, Krügel U, El-Ashmawy IM, Illes P. Suppression of feeding-evoked dopamine release in the rat nucleus accumbens by the blockade of P(2) purinoceptors. Eur J Pharmacol. 2000; 406(3):R13–4. [DOI] [PubMed] [Google Scholar]
- Kittner H, Krügel U, Illes P. The purinergic P2 receptor antagonist pyridoxalphosphate-6-azophenyl-2’4’-disulphonic acid prevents both the acute locomotor effects of amphetamine and the behavioural sensitization caused by repeated amphetamine injections in rats. Neuroscience. 2001; 102(2):241–243. [DOI] [PubMed] [Google Scholar]
- Kittner H, Krügel U, Poelchen W, Sieler D, Reinhardt R, von Kügelgen I, Illes P. P2 receptor-mediated activation of noradrenergic and dopaminergic neurons in the rat brain. Prog Brain Res. 1999; 120:223–35. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009; 9:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krügel U, Kittner H, Illes P. Adenosine 5’-triphosphate-induced dopamine release in the rat nucleus accumbens in vivo. Neurosci Lett. 1999; 265(1):49–52 [DOI] [PubMed] [Google Scholar]
- Krügel U, Kittner H, Illes P. Mechanisms of adenosine 5’-triphosphate-induced dopamine release in the rat nucleus accumbens in vivo. Synapse. 2001; 39(3):222–32. [DOI] [PubMed] [Google Scholar]
- Li YC, Chou YC, Chen HC, Lu CC, Chang DM. Interleukin-6 and interleukin-17 are related to depression in patients with rheumatoid arthritis. Int J Rheum Dis. 2019; 22(6):980–985. [DOI] [PubMed] [Google Scholar]
- Miras-Portugal MT, Ortega F, Gómez-Villafuertes R, Gualix J, Pérez-Sen R, Delicado EG. P2X7 receptors in the central nervous system. Biochem Pharmacol. 2021; 187:114472. [DOI] [PubMed] [Google Scholar]
- Mishra D, Pena-Bravo JI, Leong KC, Lavin A, Reichel CM. Methamphetamine self-administration modulates glutamate neurophysiology. Brain Struct Funct. 2017; 222(5):2031–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak SU, Cicalese S, Tallarida C, Oliver CF, Rawls SM. Chemokine CCR5 and cocaine interactions in the brain: Cocaine enhances mesolimbic CCR5 mRNA levels and produces place preference and locomotor activation that are reduced by a CCR5 antagonist. Brain Behav Immun. 2020; 83:288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev. 2014; 66(3):869–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi-ishi R, Nagata K, Inoue T, Tozaki-Saitoh H, Tsuda M, Inoue K. Involvement of the chemokine CCL3 and the purinoceptor P2X7 in the spinal cord in paclitaxel-induced mechanical allodynia. Mol Pain. 2014; 15:10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Giacomelli Á, Petiz LL, Andrejew R, Turrini N, Silva JB, Sack U, Ulrich H. Role of P2X7 Receptors in Immune Responses During Neurodegeneration. Front Cell Neurosci. 2021; 15:662935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson MJ, Turner RC, Naser ZJ, McCurdy CR, Huber JD, Matsumoto RR. SN79, a sigma receptor ligand, blocks methamphetamine-induced microglial activation and cytokine upregulation. Exp Neurol. 2013; 247:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika F, Kiguchi N, Wakida N, Kobayashi D, Fukazawa Y, Matsuzaki S, Kishioka S. Upregulation of CCL7 and CCL2 in reward system mediated through dopamine D1 receptor signaling underlies methamphetamine-induced place preference in mice. Neurosci Lett. 2018; 665:33–37. [DOI] [PubMed] [Google Scholar]
- Wang X, Northcutt AL, Cochran TA, Zhang X, Fabisiak TJ, Haas ME, Amat J, Li H, Rice KC, Maier SF, Bachtell RK, Hutchinson MR, Watkins LR. Methamphetamine Activates Toll-Like Receptor 4 to Induce Central Immune Signaling within the Ventral Tegmental Area and Contributes to Extracellular Dopamine Increase in the Nucleus Accumbens Shell. ACS Chem Neurosci. 2019; 10(8):3622–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm K, Ganesan J, Müller T, Dürr C, Grimm M, Beilhack A, Krempl CD, Sorichter S, Gerlach UV, Jüttner E, Zerweck A, Gärtner F, Pellegatti P, Di Virgilio F, Ferrari D, Kambham N, Fisch P, Finke J, Idzko M, Zeiser R. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. 2010; 16(12):1434–1438. [DOI] [PubMed] [Google Scholar]


