Abstract
Objectives
This study evaluates the effects of treatment with mindfulness-based stress reduction (MBSR) compared to the active control, present-centered group therapy (PCGT), on morning plasma cortisol, interleukin-6 (IL-6), and C-reactive protein (CRP) in veterans diagnosed with post-traumatic stress disorder (PTSD).
Methods
In a post hoc exploratory analysis, we pooled biomarkers and clinical outcomes of mindfulness, PTSD, and depression from two randomized controlled trials comparing MBSR (n = 104) to PCGT (n = 106) in U.S. military veterans diagnosed with PTSD. Linear mixed-effects modeling was used to evaluate associations between changes in biomarkers and clinical outcomes from baseline to 9-week primary endpoint and 16-week follow-up endpoint.
Results
Cortisol levels were inversely related to self-reported PTSD symptoms at baseline (p = 0.02). Cortisol increased from baseline to 9-week endpoint for both groups, but significantly less so in the MBSR group compared to PCGT group (mean difference 1.69 ± 0.8 SE; p = 0.035). Changes in IL-6 and CRP did not differ between groups at either baseline or week 9. From baseline to week 9, increased mindfulness was significantly associated with increased cortisol (p = 0.02) and decreased PTSD and depression severity (p < 0.01). Increased IL-6 and CRP were significantly associated with decreased PTSD severity (p < 0.05), but not depression. Pooled analysis corroborated earlier findings that MBSR is significantly better than PCGT in improving clinical outcomes. Increased mindfulness was strongly associated with improved symptoms.
Conclusions
Increased mindfulness is associated with a recalibration of cortisol levels which may be indicative of therapeutic response, especially in patients with lower baseline cortisol. Furthermore, mindfulness-based practices improve symptoms of PTSD and depression in a significant correlation with self-reported levels of mindfulness.
Clinical Trial Registration (clinicaltrials.gov)
NCT01532999 and NCT01548742.
Keywords: Post-traumatic stress disorder, Mindfulness, Meditation, Cortisol, Inflammatory markers, Mindfulness-based stress reduction, Veterans
Post-traumatic stress disorder (PTSD) is characterized by dysregulated physiologic, behavioral, and cognitive responses to triggering stimuli following a life-threatening, traumatic event. PTSD is accompanied by deleterious changes to mood, sleep, and social interactions, and is associated with increased medical morbidity, such as cardiovascular disease, metabolic disorders, muscular skeletal pain, and gastrointestinal complaints (Pacella et al., 2013). PTSD has a global lifetime prevalence of 6% in civilians (4% for men and 8% for women) and 8 to 17% in military populations (Goldstein et al., 2016; Lehavot et al., 2018); furthermore, half of these cases persist long term in the absence of treatment (Watson, 2019). United States (U.S.) military populations, such as Vietnam War veterans, living with PTSD have a twofold increase in age-related mortality related to both primary illness and health conditions (Watson, 2019).
PTSD symptoms involve a freeze, fight, or flight response, classically characterized by increased sympathetic autonomic function and acute changes in neuroendocrine function. A number of processes are implicated as predispositions to and/or downstream effects of PTSD, beginning with the sympathetic-adrenal medullary system which initiates the cascade of catecholamines and cardiovascular response to stress, the hypothalamic–pituitary–adrenal (HPA) axis which works in tandem with the catecholamine responses to stress, and the downstream immune system which can result in elevated circulating inflammatory markers (Pace & Heim, 2011). The amygdala circuits play an enormous role in regulating stress responses, particularly the HPA axis; however, chronic stress can lead to structural and functional remodeling of amygdala outputs to cortical and subcortical regions that result in heightened anxiety-related behaviors (Zhang et al., 2021). PTSD has been associated with HPA dysregulation in terms of higher levels of corticotropin-releasing hormone, age-related lower circulating cortisol levels, a flattening of diurnal cortisol patterns, and enhanced glucocorticoid sensitivity (Morris et al., 2016; Pace & Heim, 2011; Speer et al., 2019). Cortisol, secreted by the zona fasciculata in the adrenal cortex, is the end product of the HPA axis, with myriad effects intended to maintain homeostasis under conditions of threat. These functions include elevation of blood pressure, promotion of insulin resistance and higher blood glucose, and immune function suppression (Lee et al., 2015). Cortisol is secreted in a diurnal rhythm, rising before awakening, peaking shortly thereafter (a period of the rhythm called the cortisol awakening response), and declining throughout the day into the nighttime. Cortisol exerts negative feedback on its synthesis at the hypothalamus (secreting corticotropin-releasing hormone) and at the pituitary gland (which secretes adrenocorticotropic hormone). Some studies have shown cortisol to be low or unchanged at baseline with exaggerated responses to mental stress (Bremner et al., 2003; Elzinga et al., 2003) and traumatic scripts (Speer et al., 2019). The paradoxical findings of “low” or “flattened” (lower morning cortisol) in more stressed individuals has been hypothesized to be a result of chronic stress, in which prolonged exposure to high cortisol strengthens the inhibitory feedback of the HPA axis; these changes are associated withdrawal and disengagement behaviors, maladaptive to normal living and leaving the patient unable to skillfully manage life stressors (Miller et al., 2007). Inflammatory marker alterations include elevated interleukin-6 (IL-6) in survivors of life-threatening fires, motor vehicle accidents, and myocardial infarction, and a twofold increase in C-reactive protein (CRP) in patients with PTSD compared to healthy controls (Pace & Heim, 2011). Increased IL-6 responses to mental stress and traumatic scripts have been associated with PTSD (Bremner et al., 2020). In a cohort of approximately 2600 U.S. Marines deployed to warzones, higher pre-deployment CRP levels were predictive of post-deployment PTSD symptoms, suggesting inflammatory factors predisposing or mediating the development of PTSD (Eraly et al., 2014).
As such, it is plausible that interventions stabilizing these systems, as assessed by biomarker correlates, may be particularly advantageous both in attenuating further propagation of the neuropsychological processes of PTSD and in downstream neurohormonal-inflammatory effects associated with significant morbidity and mortality. For example, mindfulness-based stress reduction (MBSR) and yoga asana interventions have been shown to decrease cortisol levels; MBSR, but not non-MBSR yoga, has been shown to decrease IL-6 (Pascoe et al., 2017). One uncontrolled pilot study found a decrease in IL-6 was associated with the amount of session attendance in MBSR treatment in women with a history of interpersonal trauma before the age of 18 and current high perceived stress levels (i.e., not necessarily diagnosed with PTSD) (Gallegos et al., 2015).
MBSR combines meditative practice, body awareness, postural yoga practices (asanas), and non-judgmental attentiveness to thoughts and feelings to promote qualities of mindfulness. Mindfulness is defined by metrics, such as the Five Facet Mindfulness Questionnaire (FFMQ) that includes aspects of nonjudgment, awareness, nonreactivity, description, and observation (Carpenter et al., 2019; Cushing et al., 2018). Mindfulness resulting from MBSR or yoga practice has been shown in a meta-analysis to be associated with significant reduction in symptoms of depression, generalized anxiety disorder, and PTSD (Pascoe et al., 2017). Mindfulness may be particularly helpful not only in mitigating rumination, anxiety, and anhedonia associated with PTSD, but may promote nonjudgmental responses to stimuli that usually trigger avoidant reactions (Boyd et al., 2018). In veteran populations, mindfulness strategies have shown to be effective in alleviating PTSD symptom severity in diverse applications and settings (Boyd et al., 2018; Cushing et al., 2018; Davis et al., 2019; Polusny et al., 2015; Possemato et al., 2016). In a recent small sample of sexual minority young adults without mention of trauma or diagnosis, Manigault et al. (2017) used Internet-based survey to measure mindfulness using the Five Facet Mindfulness Inventory-Short Form and pre-paid return mailer to collect salivary samples to measure morning, mid-day, and bedtime cortisol levels. The investigators found that a greater level of mindful awareness was associated with a smaller awakening cortisol response, whereas a greater level of mindful acceptance was associated with a more robust morning cortisol response and a sharper decrease in the evening. These pilot data suggest that there may be an association between elements of mindfulness and diurnal cortisol patterns.
No actively controlled study has examined IL-6, CRP, and cortisol response to MBSR in the treatment of PTSD. The purpose of this study was to assess the effects of MBSR compared to an active control, called present-centered group therapy (PCGT), on the biomarkers IL-6, CRP, and cortisol in US military veterans diagnosed with PTSD. As this was a secondary analysis, we hypothesized that MBSR would be superior to PCGT in behavioral outcomes, and without hypothesizing directionality, that a change in biomarkers (cortisol, CRP, and IL-6) would be associated with improved PTSD clinical outcomes.
Methods
Participants
U.S. military veterans of any era who signed informed consent without requiring a surrogate or legal representative and who were 18 years of age or older; who were diagnosed with PTSD based on the Clinician-Administered PTSD Scale for DSM-IV (Blake et al., 1995); who agreed to remain on a stable regime of psychoactive medications for those on these medications at baseline; who agreed to refrain from evidence-based psychotherapies during the 8-week treatment; who were not currently suicidal, homicidal, or psychotic; and who were not diagnosed with current substance dependence (except for nicotine or caffeine), bipolar I disorder, any psychotic disorder, severe cognitive disorders, or a serious, unstable medical condition were included in the study.
Data pooled from both sites yielded a total of 210 subjects (Tuscaloosa n = 94; Minneapolis n = 116) randomized to either PCGT (n = 106) or MBSR (n = 104). As shown in Table 1, the cohort was mostly White, non-Hispanic, and male; had an average age of 55; and were represented by various military service periods of varying lengths with strong representation of Vietnam War veterans. Given the usual demographics of the U.S. veteran population, women (16%) and minorities (39%) were well-represented in this sample. Baseline demographics did not differ between groups.
Table 1.
Baseline demographics, clinical measures, and biomarker levels of participants
| Total sample N = 210 |
PCGT N = 106 |
MBSR N = 104 |
p | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Subgroup | n | % | n | % | n | % | |
| Sample size | Minneapolis | 116 | 55 | 59 | 51 | 57 | 49 | ns |
| Tuscaloosa | 94 | 45 | 47 | 50 | 47 | 50 | ns | |
| Sex | Male | 176 | 84 | 93 | 88 | 83 | 80 | ns |
| Female | 34 | 16 | 13 | 12 | 21 | 20 | ns | |
| Race | White | 129 | 61 | 66 | 51 | 63 | 49 | ns |
| Black | 67 | 32 | 35 | 52 | 32 | 48 | ns | |
| Other | 14 | 7 | 5 | 36 | 9 | 64 | ns | |
| Hispanic | No | 201 | 96 | 104 | 52 | 97 | 48 | ns |
| Yes | 8 | 4 | 2 | 25 | 6 | 75 | ns | |
| Period of service | Vietnam | 118 | 56 | 61 | 52 | 57 | 48 | ns |
| Gulf War | 35 | 17 | 21 | 60 | 14 | 40 | ns | |
| OEF-OIF | 33 | 16 | 16 | 48 | 17 | 52 | ns | |
| Other | 23 | 11 | 8 | 35 | 15 | 65 | ns | |
| Length of military (years) | < 3 | 90 | 43 | 50 | 56 | 40 | 44 | ns |
| 3 to 4 | 28 | 13 | 15 | 54 | 13 | 46 | ns | |
| > 5 | 91 | 44 | 41 | 45 | 50 | 55 | ns | |
| Mean | SD | Mean | SD | Mean | SD | |||
| Age (years) | 55 | 12 | 55 | 12 | 55 | 12 | ns | |
| CAPS-IV | 75.08 | 19.86 | 72.42 | 20.08 | 77.80 | 19.37 | 0.049 | |
| PCL | 61.68 | 12.21 | 59.27 | 12.35 | 64.14 | 11.62 | 0.004 | |
| PHQ9 | 20.68 | 8.71 | 20.04 | 8.71 | 21.34 | 8.71 | ns | |
| FFMQ | 108.56 | 17.24 | 108.51 | 16.62 | 108.61 | 17.92 | ns | |
| Cortisol (mcg/dL) | 10.90 | 5.15 | 11.78 | 5.38 | 10.07 | 4.81 | 0.037 | |
| IL-6 (pg/mL) | 2.25 | 2.30 | 1.97 | 1.81 | 2.52 | 2.67 | ns | |
| CRP (mg/L) | 2.78 | 2.20 | 2.73 | 2.20 | 2.83 | 2.22 | ns | |
Bolded values significant for p < 0.05
MBSR mindfulness-based stress reduction, PCGT present-centered group therapy, CAPS-IV Clinician-Administered PTSD Scale for DSM-IV, PCL PTSD Checklist, FFMQ Five Facet Mindfulness Questionnaire, IL-6 interleukin 6, CRP C-reactive protein, SD standard deviation, ns nonsignificant
Procedures
Interventions
The delivery of manualized MBSR (Kabat-Zinn, 1990) and PCGT (Schnurr et al., 2003) was provided by trained facilitators. Sessions were audio (Tuscaloosa)- or video (Minneapolis)- recorded for rigorous fidelity monitoring. Briefly, MBSR consisted of 8 weekly small group sessions (i.e., 2 to 8 participants/group) lasting 2.5 h/session at the Minneapolis site and 1.5 h/session at the Tuscaloosa site, and a 6- to 6.5-h silent retreat at approximately week 6. Participants received training in mindfulness (i.e., attending to the present moment feelings, thoughts, and sensations in a nonjudgmental and accepting way with an emphasis on awareness of breathing) through (a) body scan meditation, (b) sitting meditation, and (c) mindful stretching or yoga exercises practiced and intended to develop mindful awareness during movement. Participants were encouraged to practice at home with the aid of guided meditation recordings. The silent retreat included extended practice of the mindfulness body scan, sitting, walking, stretching, and eating that were learned in the weekly group sessions.
PCGT small groups met for 8 weekly 1.5-h sessions and the Tuscaloosa site provided a 1-h lunch gathering prior to week 8. The primary elements of PCGT included psychoeducation of PTSD symptoms, expectations of symptom reduction, strategies to decrease isolation, supportive sharing and problem-solving in a safe and nonjudgmental peer group, discussion to increase awareness of how PTSD affects the daily lives of each group member, and sharing perspectives about the effects of PTSD on each participant’s life. The PCGT participants kept a journal as their homework assignment.
Data Collection
Biomarkers and clinical outcomes from two randomized controlled trials (Davis et al., 2019; Polusny et al., 2015) comparing MBSR to PCGT for the treatment of PTSD in U.S. military veterans were pooled for this analysis. The pooled analysis included the Tuscaloosa Veterans Affairs (VA) Medical Center and Minneapolis VA Health Care System sites. Two sites from the Davis et al. (2019) study were excluded from the pooled analysis due to a freezer failure at one site (resulting in the loss of all baseline samples) and lack of collection of samples at the other site (resulting in only five of 57 subjects with baseline values of which only two had follow-up values). Both sites included in the analysis obtained local Institutional Review Board and Research and Development Committee approvals prior to engaging in human subject research and were monitored by an independent VA data and safety monitoring board. After reviewing and explaining the purpose, procedures, risks, benefits, and alternatives to treatment, all participants signed informed consent and privacy authorization prior to participating in research procedures. Data collection occurred between January 2012 and April 2014.
Measures
PTSD Checklist (Weathers et al., 1993)
This 17-item self-report scale was used to evaluate PTSD symptom severity. Examinees were instructed to use a 5-point (options 1–5) scale to rate how much they had been troubled by each symptom over the past month. PTSD symptom severity can be determined on a continuous scale by summing up the values across the seventeen responses. Weathers et al. (1993) reported good internal consistency, with an alpha coefficient of 0.90 for “B” symptoms, 0.89 for “C” symptoms, 0.91 for “D” symptoms, and 0.96 for all 17 symptoms.
Clinician Administered PTSD Scale for DSM-IV (Blake et al., 1995)
This clinician-administered scale was used to evaluate PTSD diagnosis and symptom severity and was administered by an independent assessor who remained blind to the treatment assignment. The three symptom clusters are assessed with 17 symptom questions. Four-point scales are used for both frequency (scale 0 = “none of the time” to 4 = “most or all of the time”) and intensity (scale 0 = “none” to 4 = “extreme”). Frequency and intensity scores of 1 and 2 are required for that symptom to meet the corresponding criterion for PTSD diagnosis, requiring 1 “B” criteria, 3 “C” criteria, and 2 “D” criteria overall. A severity score for each symptom is calculated by summing the frequency and intensity scores. Blake et al. (1995) reported test–retest reliabilities ranging from 0.77 to 0.96 for the three symptom clusters and from 0.90 to 0.98 for the 17-item core symptom scale.
Five Facet Mindfulness Questionnaire–Self Report (Baer et al., 2006, 2008)
This 39-item self-report instrument was used to assess 5 facets of mindfulness, i.e., observing, describing, acting with awareness, non-judging of inner experience, and nonreactivity to inner experiences. Items are rated on a 5-point Likert-type scale ranging from 1 (never or very rarely true) to 5 (very often or always true). Baer et al. (2008) reported alpha coefficients for all facets in all samples as adequate to good (range 0.72 to 0.92), with the exception of the nonreactivity to internal experience facet in the student sample, for which alpha was 0.67.
Patient Health Questionnaire–9-Item Self Report (Kroenke et al., 2001)
This 9-item self-report instrument was used to evaluate severity of depression, with a 3-point scale to establish frequency (scale 0 = not at all, 3 = nearly every day). As a severity measure, the scores from each item may be summed (with a range of 0 to 27). Kroenke et al. (2001) reported excellent internal reliability with an alpha coefficient of 0.89 in the PHQ Primary Care Study and 0.86 in the PHQ Ob-Gyn Study.
Regarding our rating scales, our dataset did not have access to the individual responses for each item, such that we are unable to report Cronbach alpha values. All instruments used have been previously validated with measures of internal reliability as stated above.
Either the Mini-International Neuropsychiatric Inventory for DSM-IV (Tuscaloosa) (Sheehan et al., 1998) or the Structured Clinical Interview for DSM-IV (Minneapolis) (First et al., 2002) was administered by a trained clinician at baseline to document PTSD and comorbid psychiatric disorders. Outcomes were assessed at baseline, at 3-week intervals during treatment (weeks 3 and 6), after treatment (week 9), and at 16-week follow-up (= ± 7 days).
Biomarkers
Blood samples were collected between 08:00 and 12:00 at baseline, week 3 (Tuscaloosa site only), 9-week end of treatment (collected at week 9 ± 7 days), and 16-week follow-up (collected at week 16 ± 7 days) for assessment of cortisol, CRP, and IL-6 levels. Time of collection was not recorded and as such is not included in analysis. Participants with known immunological conditions or taking medications that significantly alter immune function were excluded from the biosampling. Participants with CRP values > 10 mg/L, an upper limit frequently used in clinical practice, were excluded from the biomarker analysis given that such values often result from non-steady state, acute infectious, or inflammatory processes. At week 0, this excluded 4 participants in the PCGT group and 7 in the MBSR; 5 and 7 (respectively) at week 9; and 4 and 7 (respectively) at week 16. Samples for cortisol and IL-6 were collected, centrifuged, and remained frozen at each site at − 80 °C until shipment to a central laboratory at the University of Arizona for processing. Plasma samples were batch assayed for cortisol using enzyme-linked immunosorbent assay (ELISA) kits made by IBL (Hamburg, Germany), following procedures of the manufacturer. Plasma samples were batch assayed for IL-6 using ELISA kits made by R&D Systems (Minneapolis, MN) according to the manufacturer’s instructions. For the IL-6 ELISA, samples were transferred from loading plates onto assay plates with assay buffer using a TomTec Quadra Tower (TomTec, Hamden, CT, USA). An automatic plate washer (Tecan 96PW, Tecan, Männerdorf, Switzerland) was used for both the cortisol and IL-6 ELISA. Quality controls were included in each IL-6 assay, and factory controls were included in each cortisol assay. Intra- and inter-assay coefficients of variation were 7.3% and 8.3%, respectively, for the cortisol ELISA and 7.3% and 12.2%, respectively, for the IL-6 ELISA. CRP levels were measured on an Abbott Autoanalyzer using an immunoturbidimetric method at the local VA CLIA-certified laboratory.
Data Analyses
Using a blocking strategy and stratification rules, randomization was 1:1 allocation. Analyses adhered to intent-to-treatment principle for the Minneapolis site (i.e., all randomized participants included) and a modified intent-to-treat principle for the Tuscaloosa site (i.e., only randomized participants who attended at least one group therapy session included). To examine the balance across randomized treatment conditions, baseline demographics and clinical characteristics for the two conditions (MBSR vs. PCGT control) were compared using t-tests for the continuous variables, Wilcoxon tests for ordinal variables, and chi-square tests for categorical variables. Unless stated otherwise, each statistical test was conducted with a two-tailed alpha level of 0.05. For purposes of all analyses, primary outcomes were assessed at week 9 (the end of acute phase treatment) and week 16 (end of post-treatment follow-up). Statistical modeling was done using R v 4.1.1; mixed models were fit using the lme4 package, with p values from the lmerTest package, and estimated marginal means from package lsmeans (Bates et al., 2015; Kuznetsova et al., 2017; Lenth, 2016; R Core Team, 2021).
Mixed-effects linear regression analysis was used to compare MBSR and PCGT treatment conditions on the changes in PTSD, depression, mindfulness, and biomarker values over the course of the trial. Each model included up to 4 repeated assessments of the change in the outcome from baseline as the dependent variable (weeks 3, 6, 9, and 16). The models were adjusted for the baseline measure and included random effects for participant and site with fixed effects for treatment condition and time. To faithfully model temporal trends, time was treated as categorical and a treatment by time interaction was included. Differences between groups at 9 weeks were tested using predicted marginal means (also called LS means). LS means were derived from the mixed-effects regression models. The decision rule called for rejection of the null hypothesis of no treatment effect if the contrast comparing predicted marginal means between groups at 9 weeks was statistically significant (two-tailed α = 0.05).
To test whether our model-based findings are sensitive to influential or outlying observations, we conducted a parallel set of mixed effects regression models using Winsorized data, where observed “extreme” values were replaced with values that are closer to the mean. Thus, for each change outcome, we calculated the 2.5% and 97.5% percentiles of the outcome grouped by site, study arm, and week. Each observation either less than the 2.5% percentile or greater than the 97.5% percentile for its respective specific group was replaced by that bound. For example, the 2.5% percentile for change in IL-6 among all MBSR participants within one site at week 9 was − 6.87. An observed value of − 10.85 for an MBSR patient at week 16 at that site was then replaced by − 6.87 as the Winsorized value which is still “low” but will have less influence. Model results using the Winsorized outcome were compared to the main models and examined for whether any model coefficients changed either direction (sign) or statistical significance at the p < 0.05 level.
Results
Baseline Clinical Measures and Biomarkers
At baseline, unadjusted Clinician Administered PTSD Scale for DSM-IV (CAPS-IV) and PTSD Checklist (PCL) scores were significantly higher for the MBSR group compared to the PCGT group (p = 0.049 and 0.004, respectively). Patient Health Questionnaire–9-Item Self Report (PHQ9) and FFMQ showed no significant baseline differences between groups (Table 1). At baseline, unadjusted mean cortisol levels were significantly higher for the MBSR group compared to the PCGT group (p = 0.037); however, there were no differences between groups at baseline for unadjusted CRP or IL-6 (Table 1). Adding cortisol to the models described below did not meaningfully change the effect estimates or the statistical significance.
Baseline Biomarker Associations
Linear mixed modeling indicated that baseline cortisol levels were significantly inversely associated with baseline PCL scores; i.e., lower cortisol was associated with more severe self-reported PTSD symptoms (p = 0.02; Table 3 and Fig. 3), but not with CAPS-IV scores. There was a trend association between baseline cortisol levels and PHQ9 scores (p = 0.06; Table 3 and Fig. 3). No significant associations were found for baseline IL-6 and CRP on any baseline behavioral symptom score (Table 3).
Table 3.
LMM associations of biomarkers and symptom severity
| Baseline biomarker and behavioral symptom severity | |||||||
| Behavioral symptoms | |||||||
| CAPS-IV | PCL | PHQ9 | |||||
| Estimate | p | Estimate | p | Estimate | p | ||
| Baseline biomarker | Cortisol | − 0.12 | 0.66 | − 0.45 | 0.02 | − 0.18 | 0.06 |
| IL-6 | − 0.23 | 0.71 | − 0.44 | 0.30 | 0.16 | 0.47 | |
| CRP | − 0.12 | 0.84 | 0.08 | 0.86 | 0.14 | 0.52 | |
| Change in mindfulness to change in biomarkers at week 9 | |||||||
| Biomarkers | |||||||
| ΔCortisol | ΔIL-6 | ΔCRP | |||||
| Estimate | p | Estimate | p | Estimate | p | ||
| ΔFFMQ | 0.04 | 0.02 | − 0.01 | 0.47 | 0.00 | 0.92 | |
| Change in mindfulness or biomarkers to change in symptom severity at week 9 | |||||||
| Behavioral symptoms | |||||||
| ΔCAPS-IV | ΔPCL | ΔPHQ9 | |||||
| Estimate | p | Estimate | p | Estimate | p | ||
| ΔFFMQ | − 0.54 | 0.00 | − 0.25 | 0.00 | − 0.15 | 0.00 | |
| Biomarker | ΔCortisol | 0.05 | 0.76 | − 0.04 | 0.66 | 0.03 | 0.45 |
| ΔIL-6 | − 0.88 | 0.04 | − 0.68 | 0.00 | − 0.01 | 0.94 | |
| ΔCRP | − 1.24 | 0.01 | − 0.55 | 0.03 | − 0.21 | 0.06 | |
Bolded values significant for p < 0.05
LMM linear mixed modeling, CAPS-IV Clinician-Administered PTSD Scale for DSM-IV, PCL PTSD Checklist, FFMQ Five Facet Mindfulness Questionnaire, IL-6 interleukin 6, CRP C-reactive protein, Δ change from baseline
Fig. 3.
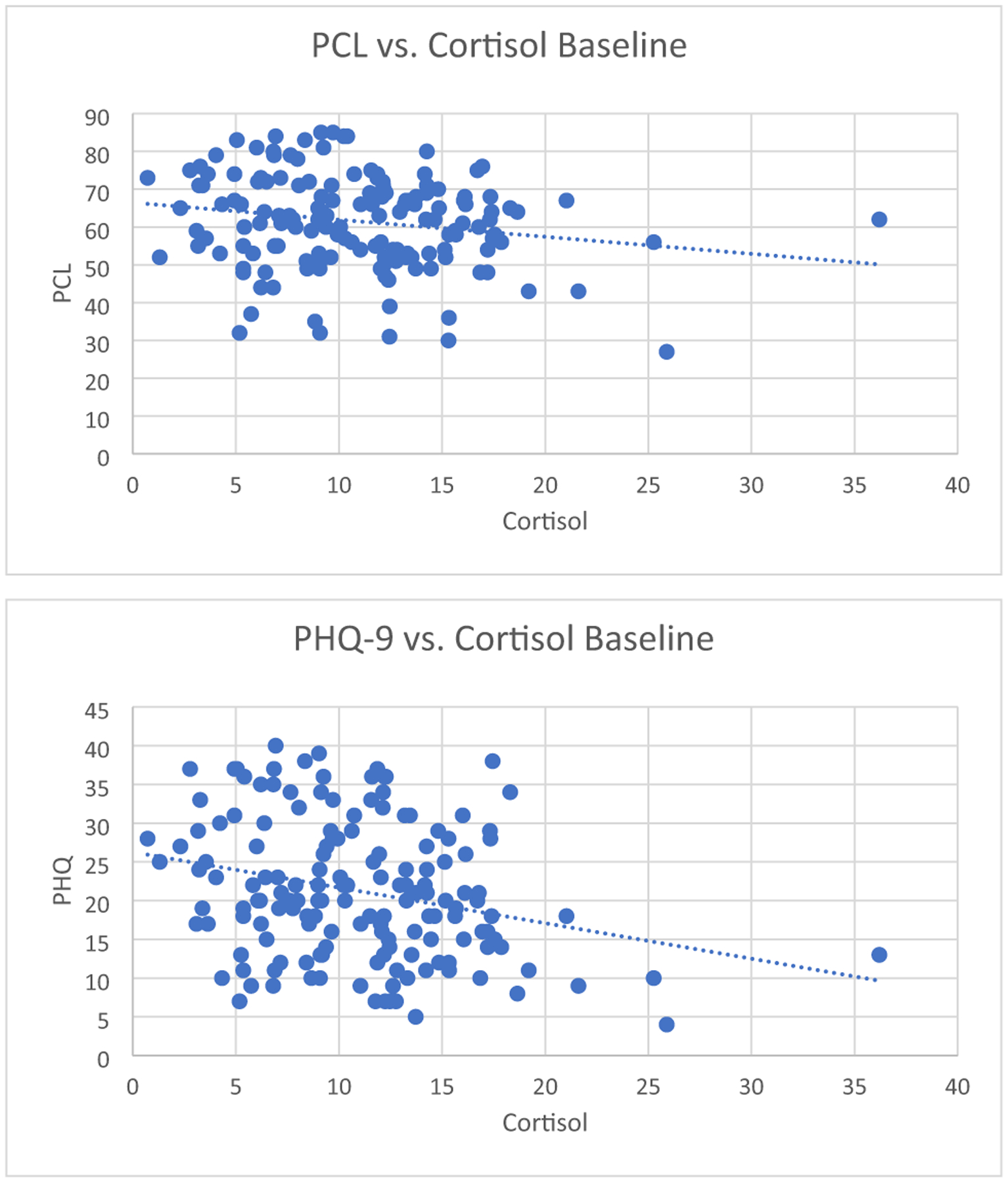
Baseline cortisol inversely associated with self-reported PTSD severity (PCL) in veterans with a diagnosis of PTSD (n = 210) as shown by linear mixed model (p = 0.018). Baseline cortisol showed a trend for an inverse association with self-reported depression severity (PHQ9) in veterans with a diagnosis of PTSD (n = 210) as shown by linear mixed model (p = 0.065)
Post-intervention Clinical Measures and Biomarker Levels
Pooled data least-squares means showed significantly greater improvement in CAPS-IV (p = 0.021), PCL (p = 0.002), and PHQ9 (p = 0.002) from baseline to the 9-week and 16-week endpoints in the MBSR compared to the PCGT group (Table 2, Fig. 1). FFMQ was significantly increased (i.e., improved mindfulness) in the MBSR group in contrast to PCGT at both 9-week and 16-week endpoints (p = 0.002; Table 2, Fig. 1). The PCGT group showed negligible change in mindfulness from baseline to week 9 and week 16. As shown in Table 4, the Cohen’s comparisons for all measures were in the range of medium effect sizes.
Table 2.
Least-squares means with contrasts at week 9 and week 16
| PCGT | MBSR | Contrast (PCGT-MBSR) | |||||
|---|---|---|---|---|---|---|---|
| Rating scale | Mean | SE | Mean | SE | Mean | SE | p |
| Week 9 | |||||||
| ΔCAPS-IV | − 12.80 | 1.87 | − 19.10 | 1.99 | 6.29 | 2.72 | 0.021 |
| ΔPCL | − 4.63 | 1.78 | − 8.96 | 1.80 | 4.33 | 1.36 | 0.002 |
| ΔPHQ9 | − 1.08 | 1.04 | − 3.06 | 1.05 | 1.97 | 0.65 | 0.002 |
| ΔFFMQ | 1.55 | 1.31 | 7.51 | 1.36 | − 5.96 | 1.87 | 0.002 |
| Biomarker | |||||||
| ΔCortisol | 2.52 | 0.76 | 0.83 | 0.78 | 1.69 | 0.80 | 0.035 |
| ΔIL-6 | 0.20 | 0.29 | 0.61 | 0.30 | − 0.40 | 0.36 | 0.265 |
| ΔCRP | − 0.13 | 0.19 | − 0.18 | 0.20 | 0.05 | 0.27 | 0.862 |
| Week 16 | |||||||
| ΔCAPS-IV | − 14.10 | 1.93 | − 22.80 | 2.09 | 8.71 | 2.83 | 0.002 |
| ΔPCL | − 4.18 | 1.79 | − 9.69 | 1.83 | 5.51 | 1.41 | 0.000 |
| ΔPHQ9 | − 1.49 | 1.05 | − 3.20 | 1.06 | 1.71 | 0.66 | 0.010 |
| ΔFFMQ | 0.46 | 1.34 | 8.65 | 1.41 | − 8.20 | 1.92 | 0.000 |
| Biomarker | |||||||
| ΔCortisol | 0.84 | 0.78 | − 0.41 | 0.79 | 1.25 | 0.82 | 0.126 |
| ΔIL-6 | 0.28 | 0.30 | − 0.18 | 0.31 | 0.45 | 0.37 | 0.220 |
| ΔCRP | − 0.15 | 0.19 | − 0.05 | 0.22 | − 0.10 | 0.29 | 0.717 |
Bolded values significant for p < 0.05
MBSR mindfulness-based stress reduction, PCGT present-centered group therapy, CAPS-IV Clinician-Administered PTSD Scale for DSM-IV, PCL PTSD Checklist, FFMQ Five Facet Mindfulness Questionnaire, IL-6 interleukin 6, CRP C-reactive protein, SE standard error of the mean, Δ change from baseline
Fig. 1.
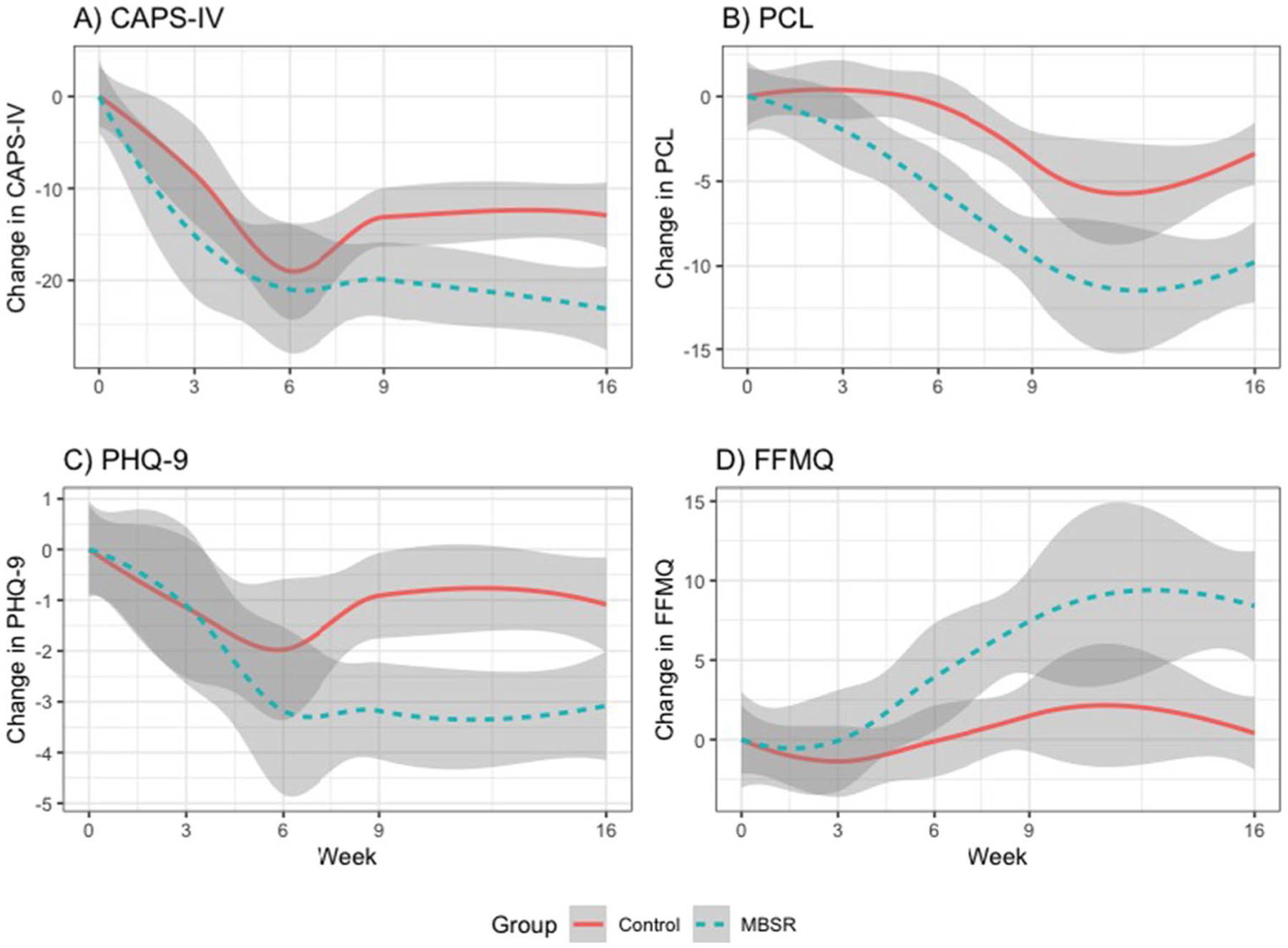
Changes in clinical measures from baseline. Compared to present-centered group therapy (PCGT), mindfulness-based stress reduction (MBSR) led to significantly greater improvement in PTSD (CAPS-IV p = 0.021; PCL p = 0.002), and depression (PHQ9 p = 0.002) at week 9 and week 16, and significantly increased mindfulness (FFMQ p = 0.002) at week 9 that was retained at the 16-week follow-up
Table 4.
Estimate of effect sizes for MBSR compared to PCGT
| Outcome | Week 9 Cohen’s d | 95% confidence interval | Week 16 Cohen’s d | 95% confidence interval |
|---|---|---|---|---|
| CAPS-IV | 0.36 | (0.07,0.65) | 0.43 | (0.11, 0.73) |
| PCL | 0.47 | (0.17, 0.76) | 0.52 | (0.21, 0.83) |
| PHQ9 | 0.44 | (0.15, 0.74) | 0.33 | (0.02, 0.63) |
| FFMQ | − 0.49 | (− 0.68, − 0.09) | − 0.49 | (− 0.79, − 0.17) |
MBSR mindfulness-based stress reduction, PCGT present-centered group therapy, CAPS-IV Clinician-Administered PTSD Scale for DSM-IV, PCL PTSD Checklist, FFMQ Five Facet Mindfulness Questionnaire
Linear mixed modeling showed that cortisol increased from baseline to 9-week endpoint for both groups, but significantly less so in the MBSR group compared to the PCGT group (p = 0.035). Cortisol levels returned to baseline levels, without between-group differences at 16-week follow-up (Table 2, Fig. 2). There were no differences in patterns between groups for IL-6 and CRP from baseline to 9-week endpoint and 16-week follow-up (Table 2, Fig. 2).
Fig. 2.
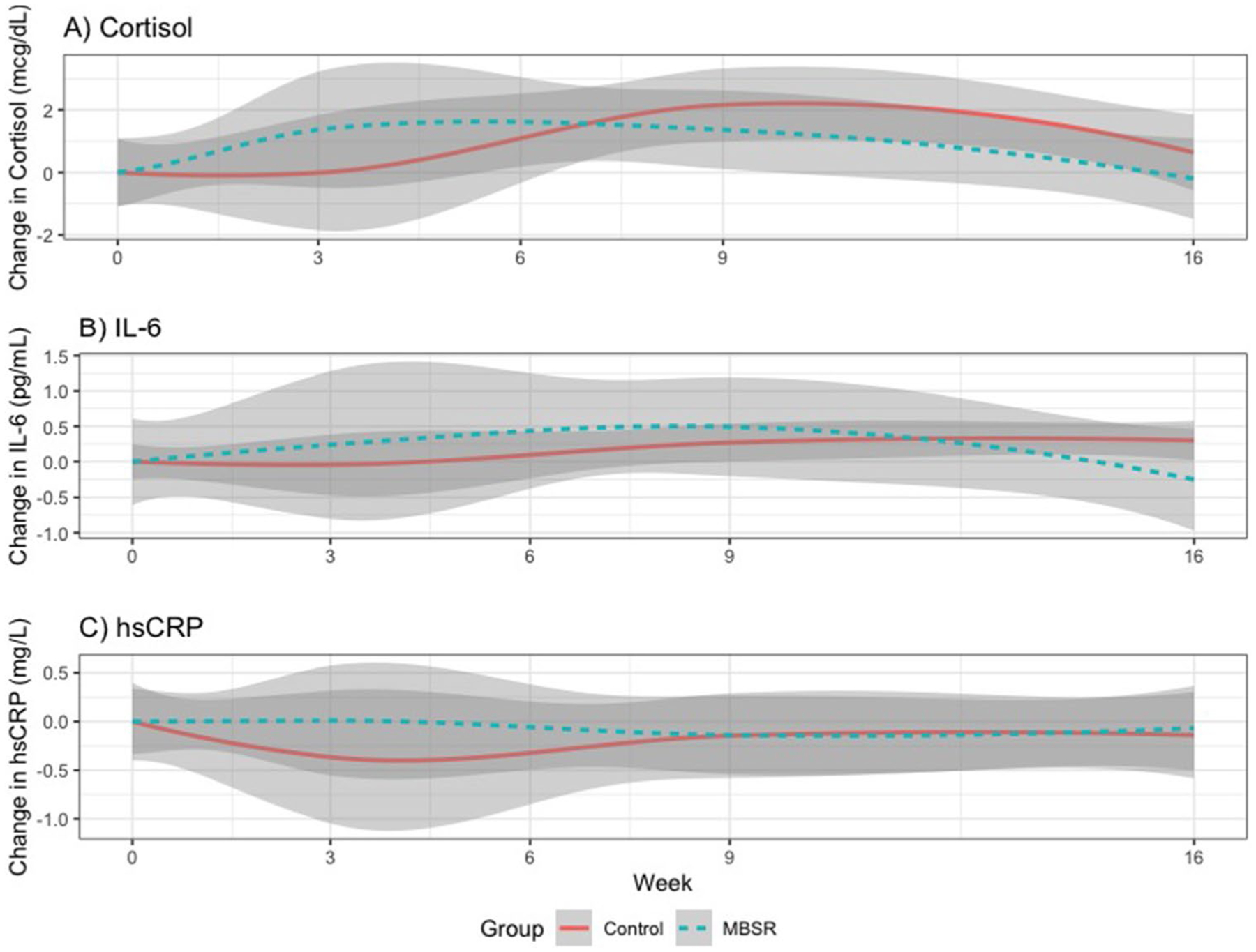
Changes over time in biomarkers. Cortisol increased from baseline to 9-week endpoint for both groups, but significantly less so in the mindfulness-based stress reduction (MBSR) group compared to (present-centered group therapy) PCGT group (p = 0.04). Interleukin-6 (IL-6) and C-reactive protein (CRP) showed no differences between groups over time at 9-week endpoint and 16-week follow-up
Biomarkers and Mindfulness Association with Symptom Improvement
As shown in Table 3, our linear mixed model accounting for study group, site, and time showed that an increase in FFMQ was significantly associated with a decrease in PCL, CAPS, and PHQ9 outcomes (p < 0.001 for all). An increase in FFMQ was significantly associated with increased cortisol (p = 0.02), but not IL-6 (p = 0.47) or CRP (p = 0.92). However, increases in both IL-6 and CRP were associated with decreases in both CAPS-IV (p = 0.04 for ΔIL-6, and p = 0.01 for ΔCRP) and PCL (p < 0.01 for ΔIL-6, and p = 0.03 for ΔCRP). Change in cortisol was not significantly associated with change in any measures of PTSD or depression.
Sensitivity Analysis
In our sensitivity analysis with Winsorized data, CRP was the only outcome where any model coefficients changed either direction (sign) or significance after Winsorization. In the CRP model, after Winsorization, the interaction term between the study group and week 3 changed direction from − 0.59 (p = 0.80) to 0.12 (p = 0.96), and the coefficient for interaction between study group and week 9 changed significance from 3.525 (p = 0.05) to 3.528 (p = 0.03).
Discussion
Our study found that lower baseline cortisol levels were associated with greater self-reported PTSD severity (Fig. 3), which is consistent with previous findings that PTSD severity is related to depressed cortisol awakening response in combat veterans (Rauch et al., 2020) and associated with hypocortisolism and diurnal rhythm abnormalities (de Kloet et al., 2007; Metzger et al., 2008; Morris et al., 2016; Pierce & Pritchard, 2016; van Liempt et al., 2013). Our modeling found that increased mindfulness was associated with an increase in morning cortisol, perhaps indicating improved cortisol flexibility, with the subjects’ HPA axis responding more vigorously to their morning stressors, a quality that is associated with greater psychologically adaptive characteristics (Mikolajczak et al., 2010). However, mindfulness practices encourage open awareness of thoughts and feelings which may inevitably include psycho-physiologically stressful PTSD triggers that can be processed during a relaxed state, which may explain the reduction in cortisol levels seen in the MBSR group at end-point (see Fig. 2).
In this study, IL-6 and CRP increase was significantly associated with decreasing CAPS-IV and PCL scores. Taken literally, this inverse relationship seems to indicate that a paradoxical increase in the inflammatory biomarkers IL-6 or CRP predicts an improvement in PTSD symptoms. There is evidence that inflammation is part of PTSD pathophysiology and that IL-6 is often elevated (Hori & Kim, 2019). It is reasonable that some increase in IL-6 would occur, given general knowledge of psychological stress (such as that in a group setting) resulting in increased acute phase reactants.
In our scenario, it is possible that less avoidance to trauma-related thoughts and sensations (MBSR) or reflections on trauma-related personal challenges (PCGT) yield increased stress-associated acute phase reactants, as well as the therapeutic process, in a relatively treatment-agnostic fashion, given a lack of significant difference between groups or an association between change in mindfulness and changes in IL-6 (Table 3). Our overall finding of a rise-fall pattern in the MBSR group is congruent with previous finding (Gallegos et al., 2015) in a non-controlled, trauma-exposed sample of elevated IL-6 immediately after a mindfulness intervention, followed by a decrease at follow-up. CRP is a downstream acute-phase reactant modulated by IL-6 and is expected to vary similarly; however, CRP is more sensitive to acute factors such as infection or even intense exercise, such that measures may be less indicative of longitudinal neuroimmunological state. Nonetheless, the overall convergence of CRP findings with IL-6 support evidence that both interventions induced a stress response associated with the therapeutic course.
Our findings corroborated prior findings regarding efficacy of mindfulness-based stress reduction in the treatment of PTSD when compared to a rigorous control intervention, and further characterize the association between mindfulness and the neuroendocrine and immune biomarkers associated with the treatment response in a veteran population, particularly that a more adaptive cortisol state is related to increased mindfulness, perhaps more so in those with lower baseline cortisol and more severe PTSD. When compared to PCGT, MBSR resulted in greater reductions in PTSD symptoms as measured by both a clinician-administered and self-report PTSD scales, as well as a greater improvement in self-reported depression (Table 4). Further, increased mindfulness (FFMQ) scores showed a clear relationship to outcome improvement as opposed to solely group assignment.
Limitations and Future Directions
Adequate characterization of HPA axis output and cortisol state is complex and there are various means to describe the cortisol diurnal rhythm, such as the waking cortisol, cortisol awakening response, rate of decrease over the day, and resultant evening cortisol levels. A limitation of our study is characterization of a single random sampling of morning plasma cortisol. There is heterogeneity in cortisol responsiveness particularly evident in trauma survivors, as evidence shows that epigenetic changes affecting cortisol levels may be involved in and predispose to development of PTSD, further influenced by factors such as exposure to childhood trauma (Boks et al., 2016). Both decreases in cortisol responsiveness to stress and daily cortisol output (as assessed with hair cortisol concentration) are risk factors for development of PTSD (Steudte-Schmiedgen et al., 2015), and relatedly, both traumatic stress burden and PTSD enhance HPA axis negative feedback (Morris et al., 2012). Although our study does not measure cortisol responsiveness or cumulative output, our findings of mindfulness related, co-occurring improvements to PTSD symptoms and increases in morning cortisol suggest response of the HPA axis related to the changes occasioned by trauma exposure or PTSD. Given both the natural diurnal variation in cortisol and the complex changes occurring in cortisol accumulated traumatic stress, better characterization of our findings with other measures of cortisol, such as responsiveness and total output, would be helpful to future research.
Taken together, our findings suggest that MBSR provokes a stress response associated with a therapeutic effect in patients and improved HPA axis reactivity, possibly more so in those with more severe PTSD and greater hypocortisolemia at baseline. Increased glucocorticoids and decreased glucocorticoid receptor sensitivity (possibly due to increased available glucocorticoids) in peripheral mononuclear blood cells post-treatment has been reported in studies of prolonged exposure therapy (PE) and shows a relationship to improved PTSD outcomes (Yehuda et al., 2015), and PE may offer a particularly suitable, trauma-appropriate context for utilization of mindfulness-based HPA axis recovery. Mindfulness-based exposure therapy (MBET) may be similarly potentiated and reciprocally improve neuroendocrine outputs of the hypothalamus through increased strength of corticolimbic pathways and top-down resolution of limbic dysregulation (King et al., 2016). Further development of mindfulness-based interventions should focus on both general development of the mindfulness trait in patients, and active, synergistic utilization within other therapies.
Acknowledgements
This work was supported by the Department of Veterans Affairs (VA) Office of Research and Development, Clinical Science Research and Development and National Institutes of Health.
Funding
The research leading to these results received funding from the VA Office of Clinical Science Research & Development under Award No 1-I01-CX000687-01 and 1-I01-CX000683-01. Mr. Shapira received funding from a National Institute of Health (NIH) training grant (NIH T35 HL00747). Dr. Richman received research funding from VA and NIH. Dr. Pace received research funding from NIH. Dr. Lim received research funding from VA and NIH. Dr. Polusny received research funding from received research funding from VA and NIH National Center for Complementary and Integrative Health (NIH-NCCIH). Dr. Hamner received consulting fees from Otsuka, research funding from VA, National Institute of Mental Health, and Otsuka. Dr. Bremner received research funding from VA and NIH, and device support from ElectroCore LLC. Dr. Mumba received research funding from VA and NIH-NCCIH. Dr. Jacobs received research funding from VA. Dr. Pilkinton received research funding and/or materials from Department of Defense, VA, SAMSHA, Aptinyx, Tonix, and Avanir Pharmaceuticals. Dr. Davis received consulting fees from Otsuka, Lundbeck, Janssen, Boehringer Ingelheim and Signant Health, and research funding and/or materials from Department of Defense, VA, SAMSHA, Aptinyx, Alkermes, Avanir Pharmaceuticals, and Tonix.
Footnotes
Research Involving Human Participants and Informed Consent Both sites included in the analysis obtained approvals from the Tuscaloosa VA and Minneapolis VA Medical Centers’ Institutional Review Boards and Research and Development Committees prior to engaging in human subject research and were monitored by an independent VA data and safety monitoring board. After reviewing and explaining the purpose, procedures, risks, benefits, and alternatives to treatment, all participants signed informed consent and privacy authorization prior to participating in research procedures. Data collection occurred between January 2012 and April 2014.
Conflict of Interest The authors declare no competing interests.
Disclaimer Opinions herein are those of the individual authors and the contents do not represent the views of the United States Government or VA.
References
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, & Toney L (2006). Using self-report assessment methods to explore facets of mindfulness. Assessment, 13(1), 27–45. 10.1177/1073191105283504 [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Lykins E, Button D, Krietemeyer J, Sauer S, Walsh E, Duggan D, & Williams JM (2008). Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment, 15(3), 329–342. 10.1177/1073191107313003 [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a clinician-administered PTSD Scale. Journal of Traumatic Stress, 8(1), 75–90. 10.1007/bf02105408 [DOI] [PubMed] [Google Scholar]
- Boks MP, Rutten BP, Geuze E, Houtepen LC, Vermetten E, Kaminsky Z, & Vinkers CH (2016). SKA2 methylation is involved in cortisol stress reactivity and predicts the development of post-traumatic stress disorder (PTSD) after military deployment. Neuropsychopharmacology, 41(5), 1350–1356. 10.1038/npp.2015.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JE, Lanius RA, & McKinnon MC (2018). Mindfulness-based treatments for posttraumatic stress disorder: A review of the treatment literature and neurobiological evidence. Journal of Psychiatry and Neuroscience, 43(1), 7–25. 10.1503/jpn.170021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Gurel NZ, Jiao Y, Wittbrodt MT, Levantsevych OM, Huang M, Jung H, Shandhi MH, Beckwith J, Herring I, Rapaport MH, Murrah N, Driggers E, Ko Y-A, Alkhalaf ML, Soudan M, Song J, Ku BS, Shallenberger L, Hankus AN, Nye JA, Park J, Vaccarino V, Shah AJ, Inan OT, & Pearce BD (2020). Transcutaneous vagal nerve stimulation blocks stress-induced activation of Interleukin-6 and interferon-γ in posttraumatic stress disorder: A double-blind, randomized, sham-controlled trial. Brain, Behavior, & Immunity - Health, 11(9), 100138. 10.1016/j.bbih.2020.100138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, Afzal N, McGlashan T, Elzinga B, Anderson GM, Heninger G, Southwick SM, & Charney DS (2003). Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology, 28(6), 733–750. 10.1016/s0306-4530(02)00067-7 [DOI] [PubMed] [Google Scholar]
- Carpenter JK, Conroy K, Gomez AF, Curren LC, & Hofmann SG (2019). The relationship between trait mindfulness and affective symptoms: A meta-analysis of the Five Facet Mindfulness Questionnaire (FFMQ). Clinical Psychology Review, 74, 101785. 10.1016/j.cpr.2019.101785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing RE, Braun KL, Alden CISW, & Katz AR (2018). Military-tailored yoga for veterans with post-traumatic stress disorder. Military Medicine, 183(5–6), e223–e231. 10.1093/milmed/usx071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LL, Whetsell C, Hamner MB, Carmody J, Rothbaum BO, Allen RS, Bartolucci A, Southwick SM, & Bremner JD (2019). A multisite randomized controlled trial of mindfulness-based stress reduction in the treatment of posttraumatic stress disorder. Psychiatric Research and Clinical Practice, 1(2), 39–48. 10.1176/appi.prcp.20180002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Heijnen CJ, Geuze E, Lentjes EGWM, & Westenberg HGM (2007). Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology, 32(3), 215–226. 10.1016/j.psyneuen.2006.12.009 [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CG, Vermetten E, van Dyck R, & Bremner JD (2003). Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology, 28(9), 1656–1665. 10.1038/sj.npp.1300226 [DOI] [PubMed] [Google Scholar]
- Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O’Connor DT, Baker DG, Marine Resiliency Study T (2014). Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry, 71(4), 423–431. 10.1001/jamapsychiatry.2013.4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon ME, & Williams JB (2002). Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. (SCID-I/P) New York State Psychiatric Institute. [Google Scholar]
- Gallegos AM, Lytle MC, Moynihan JA, & Talbot NL (2015). Mindfulness-based stress reduction to enhance psychological functioning and improve inflammatory biomarkers in trauma-exposed women: A pilot study. Psychological Trauma: Theory, Research, Practice and Policy, 7(6), 525–532. 10.1037/tra0000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RB, Smith SM, Chou SP, Saha TD, Jung J, Zhang H, Pickering RP, Ruan WJ, Huang B, & Grant BF (2016). The epidemiology of DSM-5 posttraumatic stress disorder in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Social Psychiatry and Psychiatric Epidemiology, 51(8), 1137–1148. 10.1007/s00127-016-1208-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H, & Kim Y (2019). Inflammation and post-traumatic stress disorder. Psychiatry and Clinical Neurosciences, 73(4), 143–153. 10.1111/pcn.12820 [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J (1990). Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. Delacorte Press. [Google Scholar]
- King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, Angstadt M, Kessler D, Welsh R, & Liberzon I (2016). Altered default mode network (DMN) resting state functional connectivity following a mindfulness-based exposure therapy for posttraumatic stress disorder (PTSD) in combat veterans of Afghanistan and Iraq. Depression and Anxiety, 33(4), 289–299. 10.1002/da.22481 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Lee DY, Kim E, & Choi MH (2015). Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Reports, 48(4), 209–216. 10.5483/bmbrep.2015.48.4.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehavot K, Katon JG, Chen JA, Fortney JC, & Simpson TL (2018). Post-traumatic stress disorder by gender and veteran status. American Journal of Preventive Medicine, 54(1), e1–e9. 10.1016/j.amepre.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth RV (2016). Least-squares means: The R package lsmeans. Journal of Statistical Software, 69(1), 1–33. 10.18637/jss.v069.i01 [DOI] [Google Scholar]
- Manigault AW, Figueroa WS, Hollenbeck CR, Mendlein AE, Woody A, Sinegar SE, Hamilton KR, Scanlin MC, Johnson RC, & Zoccola PM (2017). A Test of the association between mindfulness subcomponents and diurnal cortisol patterns. Mindfulness, 9(3), 897–904. 10.1007/s12671-017-0829-4 [DOI] [Google Scholar]
- Metzger LJ, Carson MA, Lasko NB, Paulus LA, Orr SP, Pitman RK, & Yehuda R (2008). Basal and suppressed salivary cortisol in female Vietnam nurse veterans with and without PTSD. Psychiatry Research, 161(3), 330–335. 10.1016/j.psychres.2008.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczak M, Quoidbach J, Vanootighem V, Lambert F, Lahaye M, Fillee C, & de Timary P (2010). Cortisol awakening response (CAR)’s flexibility leads to larger and more consistent associations with psychological factors than CAR magnitude. Psychoneuroendocrinology, 35(5), 752–757. 10.1016/j.psyneuen.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133(1), 25–45. 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Morris MC, Compas BE, & Garber J (2012). Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clinical Psychology Review, 32(4), 301–315. 10.1016/j.cpr.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Hellman N, Abelson JL, & Rao U (2016). Cortisol, heart rate, and blood pressure as early markers of PTSD risk: A systematic review and meta-analysis. Clinical Psychology Review, 49, 79–91. 10.1016/j.cpr.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, & Heim CM (2011). A short review on the psychoneuroimmunology of posttraumatic stress disorder: From risk factors to medical comorbidities. Brain, Behavior, and Immunity, 25(1), 6–13. 10.1016/j.bbi.2010.10.003 [DOI] [PubMed] [Google Scholar]
- Pacella ML, Hruska B, & Delahanty DL (2013). The physical health consequences of PTSD and PTSD symptoms: A meta-analytic review. Journal of Anxiety Disorders, 27(1), 33–46. 10.1016/j.janxdis.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Pascoe MC, Thompson DR, & Ski CF (2017). Yoga, mindfulness-based stress reduction and stress-related physiological measures: A meta-analysis. Psychoneuroendocrinology, 86, 152–168. 10.1016/j.psyneuen.2017.08.008 [DOI] [PubMed] [Google Scholar]
- Pierce ME, & Pritchard LM (2016). Lower stress-reactive cortisol in female veterans associated with military status but not PTSD. Stress, 19(5), 486–491. 10.1080/10253890.2016.1217841 [DOI] [PubMed] [Google Scholar]
- Polusny MA, Erbes CR, Thuras P, Moran A, Lamberty GJ, Collins RC, Rodman JL, & Lim KO (2015). Mindfulness-based stress reduction for posttraumatic stress disorder among veterans: A randomized clinical trial. JAMA, 314(5), 456–465. 10.1001/jama.2015.8361 [DOI] [PubMed] [Google Scholar]
- Possemato K, Bergen-Cico D, Treatman S, Allen C, Wade M, & Pigeon W (2016). Randomized clinical trial of primary care brief mindfulness training for veterans with PTSD. Journal of Clinical Psychology, 72(3), 179–193. 10.1002/jclp.22241 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2021). R: A language and environment for statistical computing. In Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Rauch SAM, Sripada R, Burton M, Michopoulos V, Kerley K, Marx CE, Kilts JD, Naylor JC, Rothbaum BO, McLean CP, Smith A, Norrholm SD, Jovanovic T, Liberzon I, Williamson DE, Yarvis CJS, Dondanville KA, Young-McCaughan S, Keane TM, … the SSC. (2020). Neuroendocrine biomarkers of prolonged exposure treatment response in military-related PTSD. Psychoneuroendocrinology, 119, 104749. 10.1016/j.psyneuen.2020.104749 [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Friedman MJ, Foy DW, Shea MT, Hsieh FY, Lavori PW, Glynn SM, Wattenberg M, & Bernardy NC (2003). Randomized trial of trauma-focused group therapy for posttraumatic stress disorder: Results from a department of veterans affairs cooperative study. Archives of General Psychiatry, 60(5), 481–489. 10.1001/archpsyc.60.5.481 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, & Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(Suppl 20), 22–33. quiz 34–57. [PubMed] [Google Scholar]
- Speer KE, Semple S, Naumovski N, D’Cunha NM, & McKune AJ (2019). HPA axis function and diurnal cortisol in post-traumatic stress disorder: A systematic review. Neurobiol Stress, 11, 100180. 10.1016/j.ynstr.2019.100180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudte-Schmiedgen S, Stalder T, Schönfeld S, Wittchen HU, Trautmann S, Alexander N, Miller R, & Kirschbaum C (2015). Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology, 59, 123–133. 10.1016/j.psyneuen.2015.05.007 [DOI] [PubMed] [Google Scholar]
- van Liempt S, Arends J, Cluitmans PJM, Westenberg HGM, Kahn RS, & Vermetten E (2013). Sympathetic activity and hypothalamo-pituitary–adrenal axis activity during sleep in post-traumatic stress disorder: A study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology, 38(1), 155–165. 10.1016/j.psyneuen.2012.05.015 [DOI] [PubMed] [Google Scholar]
- Watson P (2019). PTSD as a public mental health priority. Current Psychiatry Reports, 21(7), 61. 10.1007/s11920-019-1032-1 [DOI] [PubMed] [Google Scholar]
- Weathers F, Litz B, Herman D, Huska JA, & Keane T (1993). The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Paper Presented at the Annual Convention of the International Society for Traumatic Stress Studies. [Google Scholar]
- Yehuda R, Bierer LM, Pratchett LC, Lehrner A, Koch EC, Van Manen JA, Flory JD, Makotkine I, & Hildebrandt T (2015). Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: Randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology, 51, 589–597. 10.1016/j.psyneuen.2014.08.004 [DOI] [PubMed] [Google Scholar]
- Zhang WH, Zhang JY, Holmes A, & Pan BX (2021). Amygdala circuit substrates for stress adaptation and adversity. Biological Psychiatry, 89(9), 847–856. 10.1016/j.biopsych.2020.12.026 [DOI] [PubMed] [Google Scholar]


