ABSTRACT
The endolysosomal system comprises a dynamic constellation of vesicles working together to sense and interpret environmental cues and facilitate homeostasis. Integrating extracellular information with the internal affairs of the cell requires endosomes and lysosomes to be proficient in decision-making: fusion or fission; recycling or degradation; fast transport or contacts with other organelles. To effectively discriminate between these options, the endolysosomal system employs complex regulatory strategies that crucially rely on reversible post-translational modifications (PTMs) with ubiquitin (Ub) and ubiquitin-like (Ubl) proteins. The cycle of conjugation, recognition and removal of different Ub- and Ubl-modified states informs cellular protein stability and behavior at spatial and temporal resolution and is thus well suited to finetune macromolecular complex assembly and function on endolysosomal membranes. Here, we discuss how ubiquitylation (also known as ubiquitination) and its biochemical relatives orchestrate endocytic traffic and designate cargo fate, influence membrane identity transitions and support formation of membrane contact sites (MCSs). Finally, we explore the opportunistic hijacking of Ub and Ubl modification cascades by intracellular bacteria that remodel host trafficking pathways to invade and prosper inside cells.
Keywords: Bacterial infection, Endosomes, Membrane contact sites, Membrane dynamics, Ubiquitin
Summary: A Review of ubiquitylation and its biochemical relatives as regulators of the endolysosomal system and its hijacking by intracellular bacteria in order for them to prosper inside cells.
Introduction
Compartmentalization of cellular processes into membrane-enclosed organelles defines the eukaryotic life form and underlies the emergence of complex (multi)cellular systems. In a living cell, compartmentalization is necessarily dynamic and thus exhibits not only spatial but also temporal constraints. This notion is exemplified by the endolysosomal system – an interactive network of diverse vesicular carriers whose identity, contents and location are continuously subject to change. To make sense of this complexity, reversible post-translational modifications (PTMs) with ubiquitin (Ub) and ubiquitin-like (Ubl) proteins come aptly into play.
Following internalization from the plasma membrane (PM), early endosomes (EEs) embark on a journey of progressive maturation (Bakker et al., 2017) characterized by luminal acidification and lipid bilayer remodeling (Maxfield and Yamashiro, 1987; Wang et al., 2019) (Fig. 1A). Maturing or sorting endosomes (SEs) are complex organelles (Klumperman and Raposo, 2014; Scott et al., 2014) that move swiftly through cellular space and sort membranes to a variety of destinations (Naslavsky and Caplan, 2018). Tubules emanating from these compartments accumulate cargoes intended for recycling back to the PM and other organelles (Cullen and Steinberg, 2018). Concomitantly, cargoes destined for lysosomal degradation or secretion become sequestered on intralumenal vesicles (ILVs) during multivesicular body (MVB) biogenesis (Gruenberg, 2020). Fusion of MVBs, also referred to as late endosomes (LEs), with proteolytic lysosomes commits ILV cargoes for degradation, whereas fusion of the same compartments with the PM expels ILVs as extracellular vesicles (EVs) (Gurung et al., 2021). A fraction of ILVs, however, manages to avoid the terminal fates of degradation and secretion by fusing back to the limiting membrane of the MVB through a process termed retrofusion (Perrin et al., 2021). MVBs and lysosomes also closely cooperate with the macroautophagy pathway to mediate clearance of cytoplasmic materials not suitable for degradation by the proteasome, which include large protein aggregates, damaged organelles and even invading pathogens (Birgisdottir and Johansen, 2020). Targeting cytosolic cargoes to the lysosome requires their sequestration into double-membrane vesicles termed autophagosomes, which subsequently fuse with proteolytic endosomes or lysosomes to achieve cargo clearance and nutrient recycling (Nakamura and Yoshimori, 2017). Together with the autophagy pathway, the endolysosomal system oversees signal transduction (Di Fiore and von Zastrow, 2014), nutrient sensing and metabolism (Efeyan et al., 2015; Lim and Zoncu, 2016), maintenance of cell polarity (Shafaq-Zadah et al., 2020), mitotic progression (Carlton et al., 2020), intercellular communication (Maas et al., 2017) and other processes. Given that cellular physiology is intimately connected to organismal wellbeing, aberrant endocytic traffic results in a broad spectrum of pathologies ranging from cancer (Mellman and Yarden, 2013) to neurodegeneration (Fleming et al., 2022; Lie and Nixon, 2019). Besides its homeostatic functions, the endolysosomal system is also the site of entry for pathogenic bacteria and viruses into the host cell (Cossart and Helenius, 2014). Consequently, endocytic compartments are ideally positioned to facilitate antigen processing and presentation (Roche and Furuta, 2015), and disturbances in endosome function are strongly linked to immune deficiencies (Unanue et al., 2016).
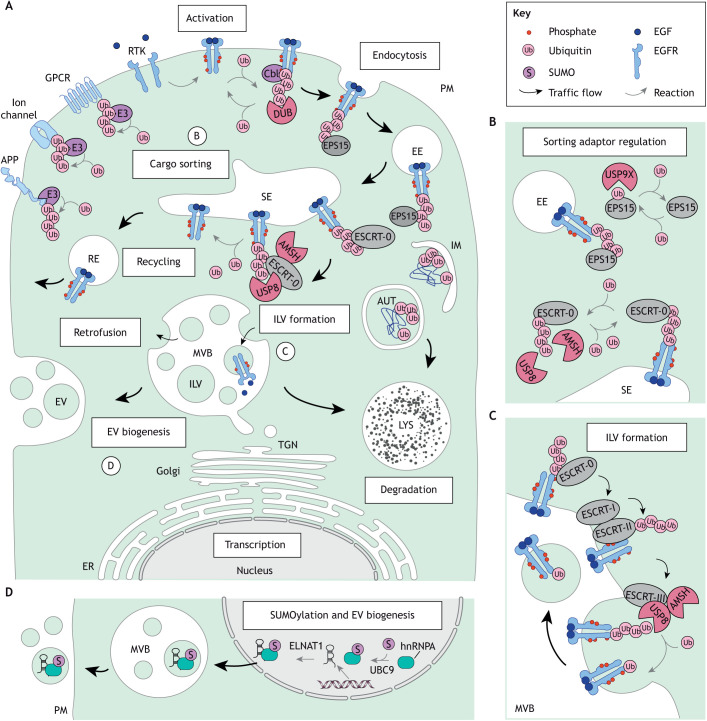
Fig. 1.
Ubiquitylation drives lysosomal turnover of the cell surface proteome. (A) Ubiquitin (Ub) modifications direct cell surface proteins for degradation, as exemplified by the epidermal growth factor receptor (EGFR) whose ligand-induced activation sets in motion its journey to lysosomes. Cargoes endocytosed in early endosomes (EEs) are partitioned between the degradative and recycling membrane pathways at the sorting endosome (SE). Ligand binding triggers EGFR phosphorylation and recruitment of the E3 ubiquitin ligase Cbl for receptor ubiquitylation. Whereas receptors lacking Ub marks traffic back to the plasma membrane (PM) via recycling endosomes (REs), ubiquitylated receptors are recognized by endosomal adaptors and incorporated into intralumenal vesicles (ILVs) of multivesicular bodies (MVBs). Subsequent fusion of MVBs with proteolytic lysosomes (LYS) commits ILV cargoes for degradation. The latter fate can, however, be averted by timely action of DUBs at various steps along the endosomal trafficking route. Besides cargo degradation, other ILV pathways exist, including return to the limiting membrane (LM) via retrofusion and release as extracellular vesicles (EVs). The autophagy pathway also feeds into the endolysosomal system via double-membrane autophagosomes (AUT) that form upon sealing of the isolation membrane (IM) around ubiquitylated cytosolic cargoes. Processes labeled B–D are shown in more detail in the other panels. (B) Ub-dependent functions of endosomal sorting adaptors are regulated by reversible ubiquitylation. Monoubiquitylation of EPS15 inhibits recognition of ubiquitylated cargoes, and deubiquitylation by USP9x restores this function. ESCRT-0 function is also positively regulated by its associated DUBs USP8 and AMSH. (C) Sequestration of ubiquitylated cargoes on ILVs is orchestrated by sequential actions of the ESCRT-0, -I, -II, and -III complexes. ESCRT-associated deubiquitylation during ILV formation ensures Ub homeostasis on endosomal membranes and maintains the cellular Ub pool. (D) SUMOylation of the RNA-binding protein hnRNPA facilitates incorporation of the long noncoding RNA ELNAT1 into ILVs for release in EVs.
Healthy traffic flow along the endolysosomal tract relies heavily on PTMs with Ub and its family members, which help designate cargo fate and direct actions of sorting and trafficking machineries in a spatially and temporally resolved manner. Ub constitutes a uniquely versatile protein modifier whose attachment to substrates is mediated by a multi-step reaction cascade (Pickart, 2001; Varshavsky, 2012) involving sequential engagement of activating (E1), conjugating (E2) (Stewart et al., 2016) and ligating (E3) enzymes (Zheng and Shabek, 2017). The two largest families of Ub ligases, the homologous to E6-AP C-terminus (HECT) and really interesting new gene (RING), differ in their mode of Ub transfer (Metzger et al., 2012; Toma-Fukai and Shimizu, 2021), but give rise to indistinguishable reaction products – Ub moieties covalently attached to (typically) lysine residues of select substrates. Because many proteins feature more than one solvent exposed lysine, the choice of modification site is often determinant of downstream outcomes. Ub itself possesses seven lysine residues (K6, K11, K27, K29, K33, K48 and K63) and an N-terminal methionine, all of which can serve as Ub acceptor sites and thus support polyubiquitin linkages of varying lengths and shapes (Akutsu et al., 2016). This spectacular diversity of Ub signals (Yau and Rape, 2016) can exert a wide range of consequences that include marking proteins for degradation (Ciechanover, 2015) or altering their interaction landscapes and activities in a matter of minutes (Swatek and Komander, 2016). The information encoded by various types of ubiquitylation can in turn be altered or removed by deubiquitylating enzymes (DUBs), hence determining how long a given Ub modification persists. Human DUBs, of which Ub-specific proteases (USPs) comprise the largest and most promiscuous family (Clague et al., 2019), are outnumbered by ligases by nearly 10-fold (Sahtoe and Sixma, 2015). Consequently, many DUBs engage in a variety of partnerships and influence diverse cellular pathways, including endocytosis and membrane trafficking (Mevissen and Komander, 2017). Ubl cascades follow the paradigm of ubiquitylation but utilize Ubl-specific activating, conjugating and ligating enzymes, and proteases (Cappadocia and Lima, 2018). Prominent among the Ubls implicated in endocytosis are small Ub-like modifier (SUMO) proteins (Su et al., 2020) and interferon-stimulated gene 15 (ISG15) (Perng and Lenschow, 2018). In this Review, we cover recent advancements in our understanding of how modifications with Ub and Ubls inform the organization, behavior and function of the endolysosomal system and delve into their exploitation by intracellular bacterial pathogens seeking to establish and maintain a fertile reproductive niche.
Ub and Ubls in endocytosis and cargo sorting
Ubiquitylation of the cell surface proteome
The first discoveries implicating ubiquitylation as a regulatory signal in endocytosis came from studies in yeast demonstrating that conjugation of a single Ub moiety to cell surface receptors is sufficient to trigger their internalization and direct them to the vacuole for degradation (Hicke and Riezman, 1996; Shih et al., 2000). Contemporaneously, connections between ubiquitylation and endocytosis were reported in mammalian cells (Levkowitz et al., 1996; Levkowitz et al., 1998). In the decades that followed, general mechanisms underlying Ub-dependent commitment of PM proteins for proteolysis across the eukaryotic kingdom began to emerge (Acconcia et al., 2009; Weinberg and Drubin, 2014). In yeast, the HECT E3 Rsp5 is the predominant Ub ligase responsible for quality control of PM proteins (Sardana et al., 2019). Rsp5 is an essential gene product that achieves substrate specificity by partnering with an array of arrestin-related trafficking adaptors, or ARTs (Barata-Antunes et al., 2021; Guiney et al., 2016), which enable controlled recognition and ubiquitylation of diverse cell surface receptors, transporters and channels (Savocco et al., 2019; Sen et al., 2020; Wawrzycka et al., 2019). In mammalian cells, ligand-induced ubiquitylation of receptor tyrosine kinases (RTKs) (Fig. 1A–C) and G protein-coupled receptors (GPCRs) has been extensively studied as a trigger for lysosomal degradation and downregulation of signaling cascades (Critchley et al., 2018; Dores and Trejo, 2019). However, in specialized contexts, ubiquitylation can exert signal-promoting functions instead (Zhang et al., 2017). Non-signaling integral PM residents, including ion channels (Estadella et al., 2020) and amyloid precursor proteins (Gireud-Goss et al., 2020), are also subject to Ub-dependent lysosomal turnover. Moreover, ubiquitylation has also been shown to guide the delivery and maturation of proteolytic enzymes in acidic compartments (Gonzalez et al., 2018), as well as regulate transport of biosynthetic cargoes between the trans-Golgi network (TGN) and endosomes (De Angelis Rigotti et al., 2017; MacDonald et al., 2014). With these insights, our appreciation of Ub as the orchestrator of endolysosomal traffic and membrane homeostasis continues to evolve.
Regulation of the mammalian cell surface proteome is enforced by a conserved group of Ub ligases. Prominent among these are the RING E3s of the Casitas B-lineage lymphoma (Cbl) (Liyasova et al., 2015) and membrane-associated RING-CH (MARCH) (Lin et al., 2019) families, as well as the HECT E3s neuronal precursor cell-expressed developmentally downregulated 4 (Nedd4) (Sicari et al., 2022) and Itchy homolog (ITCH) (Aki et al., 2015; Moser and Oliver, 2019). Ub marks conjugated to cell surface molecules usually take the form of multiple monoubiquitins or K63-linked polyubiquitin chains; however, other linkage types such as K48 and K11 have also been reported (Haglund et al., 2003; Huang et al., 2006). Ub ligase activities governing turnover of the cell surface proteome are in turn countered by DUBs and can themselves be regulated by Ub and Ubls (Nelson et al., 2016; Polo, 2012; Wang et al., 2020). Collectively, these checks and balances ensure that physiological outcomes of Ub modification cascades are finetuned in accordance with fluctuating cellular demands. In the following section, we delve into the mechanisms employed by the cell to recognize Ub and Ubl conjugates on endosomal proteins and explore their consequences for the determination of cargo fate.
Ub and SUMO as cargo fate determinants
Constitutive and ligand-induced internalization of cell surface molecules can occur via clathrin-mediated as well as clathrin-independent endocytic routes (Thottacherry et al., 2019). Much of what we know about the relationship between ubiquitylation and trafficking of endocytosed cargoes comes from studies on the epidermal growth factor receptor (EGFR) (Madshus and Stang, 2009) whose ligand-mediated activation leads to ubiquitylation of its cytoplasmic tails in a dose-dependent manner (Levkowitz et al., 1998; Sigismund et al., 2005). Whereas internalized EGFR molecules lacking Ub marks either quickly recycle back to the PM or progress gradually along the endosomal maturation route to accommodate steady state receptor turnover, robustly ubiquitylated receptors are actively committed to degradation (Holler and Dikic, 2004; Sigismund et al., 2013), as described below (Fig. 1A–C).
Recognition of ubiquitylated cargoes like EGFR is mediated by molecular machineries (Piper et al., 2014) in possession of Ub-binding domains (UBDs) and Ub-interacting motifs (UIMs) (Husnjak and Dikic, 2012). At the earliest stages of endocytosis, adaptors belonging to the EGFR pathway substrate 15 (EPS15) family interact with ubiquitylated receptors, directing their inclusion into nascent endosomes (Day et al., 2021) (Fig. 1A,B). Additionally, noncanonical modes of ubiquitylated cargo recognition at the PM are beginning to emerge (Li et al., 2017). Sorting of ubiquitylated cargoes takes place at the multifunctional SE compartment, which harbors both recycling and maturing membrane subdomains (Norris and Grant, 2020). Here, endosomal sorting complexes required for transport (ESCRTs) cluster ubiquitylated membrane proteins and execute their deposition into ILVs (Vietri et al., 2020) (Fig. 1C). The ESCRT-0 complex, consisting of the hepatocyte growth factor regulated substrate (HRS) and the signal transducing adaptor molecules 1 and 2 (STAM1 and STAM2) (Bache et al., 2003), mediates ubiquitylated cargo recognition (Raiborg et al., 2002; Roxrud et al., 2008) using its UIM (Shih et al., 2002) and VHS domains (Ren and Hurley, 2010). In line with the commonly observed signals for lysosomal degradation of transmembrane proteins, STAMs prefer K63-linked polyubiquitin chains (Lange et al., 2012). Subsequently, ESCRT-I, -II and -III act in succession to curve the limiting membrane around the cargoes selected by ESCRT-0 and perform scission of nascent ILVs into the endosomal lumen (Hurley, 2015; Tang et al., 2016). ESCRTs operating downstream of cargo selection are also capable of Ub binding (Shields et al., 2009), which ensures that sorting and ILV formation proceed as coupled processes. Importantly, the dynamic nature of endocytic progression requires ubiquitylation to be reversible (Clague et al., 2012). This activity is provided by DUBs that oppose Ub-dependent lysosomal degradation (Box 1) and modulate localization and function of endosomal proteins through mechanisms detailed in the upcoming section.
Box 1. To DUB or not to DUB? That is the question!
The reversible nature of Ub conjugation endows the processes it regulates with a dynamic character. Given the pervasiveness of Ub-mediated regulation in endosome biology and cargo traffic, it is not surprising that proteases responsible for Ub cleavage are critical for the integrity and physiology of endolysosomal systems across the eukaryotic kingdom (McCann et al., 2016). In yeast, the ubiquitin proteases Ubp2 and Ubp15 limit ubiquitylation and degradation of ARTs (Ho et al., 2017), and Ubp4 (also known as Doa4) recycles ubiquitin from endosomal cargoes prior to their inclusion into ILVs en route to the vacuole (Richter et al., 2007). In mammals, the latter function is likely performed by USP8 (Row et al., 2006), whereas the former is shared by multiple DUBs from different families. USP8 also features prominently in the context of cargo recycling, where it deubiquitylates various RTKs and thus diverts them away from lysosomal proteolysis (Berlin et al., 2010; Niendorf et al., 2007). Similarly, deubiquitylation by USP10 stimulates recycling of the cystic fibrosis transmembrane conductance regulator (CFTR) (Bomberger et al., 2009) and (together with USP7) the sodium-hydrogen exchanger 3 (NHE3; also known as SLC9A3) channel (Han and Yun, 2020), while USP9x and ubiquitin C-terminal hydrolase L1 (UCHL1) promote recycling of α5β1 integrin receptor complexes (Kharitidi et al., 2015) and MHC class I (Reinicke et al., 2019), respectively.
DUB-mediated stabilization of the cell surface proteome has direct implications for signal transduction, such as higher receptor availability and failure to degrade activated receptor complexes. Both of these effects can promote cancer, as exemplified by USP8, whose oncogenic properties are well established in Cushing's disease (Islam et al., 2021; Perez-Rivas and Reincke, 2016). However, targeting DUBs in cancer is complicated by the fact that they are involved in different cellular pathways. For instance, another cancer-associated DUB, USP15, deubiquitylates transforming growth factor-β type I receptor (TGFβRI) and thus augments Smad-mediated signaling during cancer cell metastasis (Eichhorn et al., 2012), while also attenuating insulin-like growth factor signaling (Fukushima et al., 2017). These examples underscore both the therapeutic potential of DUB interference in disease and the likely challenges associated with such pursuits.
As noted in the introductory remarks, not all intralumenal cargoes are destined for the lysosome, and some ILVs are expelled as EVs into extracellular space (Raposo and Stoorvogel, 2013). Selection of EV cargoes during MVB biogenesis relies heavily on PTMs (Atukorala and Mathivanan, 2021; Carnino et al., 2020), wherein conjugation of SUMO proteins (rather than Ub) appears to play a prominent role. For example, SUMOylation of α-synuclein aggregates enhances their excretion and pathogenic dissemination via exosomes in Parkinson's disease (Kunadt et al., 2015). Similarly, SUMOylation of RNA-binding protein hnRNPA1 facilitates inclusion of long noncoding RNAs into EVs (Chen et al., 2021) (Fig. 1D). Against the backdrop of Ub-mediated commitment of cell surface proteins for degradation, the emerging roles for SUMO in EV biogenesis illustrate that much remains to be learned about the stratification of endolysosomal cargo fates.
Regulation of endocytic machineries by Ub and Ubls
The dynamic nature of membrane remodeling and cargo sorting requires the molecular machineries that perform these tasks to repeatedly associate with and disengage from their target membranes. For endosomal adaptor proteins, this cycling is afforded by an elegant regulatory loop, wherein their Ub recognition determinants switch between cargo binding (in trans) and auto-inhibition (in cis) (Hoeller et al., 2006). Specifically, monoubiquitylation of EPS15 leads to reversible inhibition of its UIM, rendering it temporarily unable to interact with ubiquitylated cargoes (Gschweitl et al., 2016) (Fig. 1B). Given that other UIM-containing endosomal adaptors, such as HRS and STAMs, are also subject to ubiquitylation, autoinhibition is likely to constitute a general mechanism for spatiotemporal control of endosomal machinery. Interestingly, the UIM of STAM overlaps with a SUMO-interacting motif (SIM) (Guzzo and Matunis, 2013), suggesting that recognition of mixed chains containing both ubiquitin and SUMO could further finetune ESCRT-0 function. Other sorting proteins are also subject to modifications with Ub and Ubls, as exemplified by the ESCRT-I core component TSG101 (also known as VPS23). An important regulator of endocytic and exocytic traffic in plants and mammals, TSG101 is both ubiquitylated and modified by another ubiquitin-like molecule, namely ISG15, leading either to proteasomal degradation (Xia et al., 2020) or aggregation and disposal in the autolysosome (Villarroya-Beltri et al., 2016), respectively. Both outcomes transiently mimic ESCRT-I loss-of-function, resulting in impairment of MVBs biogenesis and EV secretion. Collectively, the above examples illustrate various ways in which functions of sorting complexes are regulated by the interplay between Ub and Ubl recognition and conjugation, which can be further finetuned through interferon release during inflammation.
Because addition of Ub and Ubls to cargoes and sorting proteins cannot on its own support the dynamic interplay between them, timely action of DUBs is required to complete the cycle of Ub-dependent trafficking control. Thus far, only a few DUBs have been described to directly target endosomal adaptors, thus regulating their stability and function. These include USP9x, which deubiquitylates EPS15 to relieve its autoinhibition and thereby promotes trafficking and downregulation of ubiquitylated receptors (Savio et al., 2016) (Fig. 1B). At the SE, two other DUBs USP8 and AMSH (also known as STAMBP) are recruited to the Src homology 3 (SH3) domains of STAM1 and STAM2 (Kato et al., 2000; McCullough et al., 2004) (Fig. 1B), enabling them to deubiquitylate the ESCRT-0 complex and consequently ensure the continuity of sorting function at the SE (Berlin et al., 2010; Sierra et al., 2010). USP8, also known as UBPY, is an essential protein in mammals that is required for embryonic stem cell renewal (Gu et al., 2019), growth factor receptor maintenance (Niendorf et al., 2007) and T cell development and homeostasis (Dufner et al., 2015). In T cells, USP8 foregoes binding to STAM proteins for the higher affinity SH3 domain of growth factor receptor-bound protein 2-related adaptor downstream of Shc (GADS; also known as GRAP2), demonstrating how varying the strength of modular interactions achieves functional diversification in specialized cell types. Additionally, USP8, as well as AMSH, also interact with ESCRT-III adaptors, including the accessory protein tyrosine phosphatase non-receptor type 23 (PTPN23) and charged MVB proteins (CHMPs) (Adoro et al., 2017; Ali et al., 2013; Kyuuma et al., 2007) (Fig. 1C), which enable these DUBs to participate in late endosomal sorting steps. In contrast to the linkage-promiscuous USP8 (Durcan and Fon, 2015; Row et al., 2006; Zheng et al., 2018), AMSH exhibits specificity for K63 polyubiquitin chains (Davies et al., 2011; Sato et al., 2008). This preference is shared with STAM, whose binding to AMSH promotes its DUB activity and dictates the position of Ub cleavage (Baiady et al., 2016; Hologne et al., 2016). Together, the ESCRT-associated DUBs are ideally positioned to globally survey the molecular decisions taking place at the SE and ensure accurate and efficient cargo flow.
Besides ESCRTs, other endolysosomal machineries are also subject to regulation by Ub and Ubls. One such example is the retromer complex responsible for membrane recycling from maturing endosomes (Chen et al., 2019) (Fig. 2A). Retromer comprises the core subunits VPS35, VPS26 and VPS29, which work together with membrane deforming sorting nexins (SNXs) (Hanley and Cooper, 2020) and the Wiskott–Aldrich syndrome protein and SCAR homolog (WASH) actin-polymerizing complex (Simonetti and Cullen, 2019) to identify and extract membranes carrying recycling cargoes such as the mannose-6-phosphate receptor (M6PR) to the Golgi and TGN, or the β2-adrenergic receptor to the cell surface (Seaman, 2012). Retromer complex assembly on endosomal membranes is modulated by the ovarian tumor DUB with linear linkage specificity (OTULIN) whose recruitment by SNX27 antagonizes the interaction between SNX27 and VPS26 and thus inhibits cargo loading (Stangl et al., 2019) (Fig. 2B). Furthermore, the cargo-selective retromer component VPS35 interacts with several Ub-modifying enzymes, including the RING adaptor melanoma-associated antigen gene L2 (MAGE-L2), which together with the conjugating enzyme UBE2O and the tripartite motif protein 27 (TRIM27) mediates K63-linked polyubiquitylation of WASH (Hao et al., 2013). This stimulates WASH to produce localized actin filaments at sites of tubule formation and facilitates cargo retrieval from endosomes (Fig. 2C). VPS35 is itself ubiquitylated by another Ub ligase (Fig. 2B), the RING/HECT hybrid Parkin (Williams et al., 2018). Both Parkin and VPS35 are implicated in the pathogenesis of Parkinson's disease (Panicker et al., 2021), and, although the mechanistic consequences of their interplay remain unclear (Williams et al., 2017), Parkin appears to promote retromer complex integrity, as brains of Parkin-knockout mice show marked depletion of retromer-associated WASH proteins (Williams et al., 2018). Interestingly, Parkin also cooperates with the endosomal Ub adaptor Toll-interacting protein (TOLLIP) (Katoh et al., 2004) in trafficking of stress-induced mitochondrial-derived vesicles for lysosomal clearance in a retromer-dependent manner (Ryan et al., 2020). These findings reinforce the notion that Ub-mediated interactions are broadly applicable across many membrane pathways feeding into the endolysosomal system, all of which serve important roles in the regulation of cellular homeostasis.
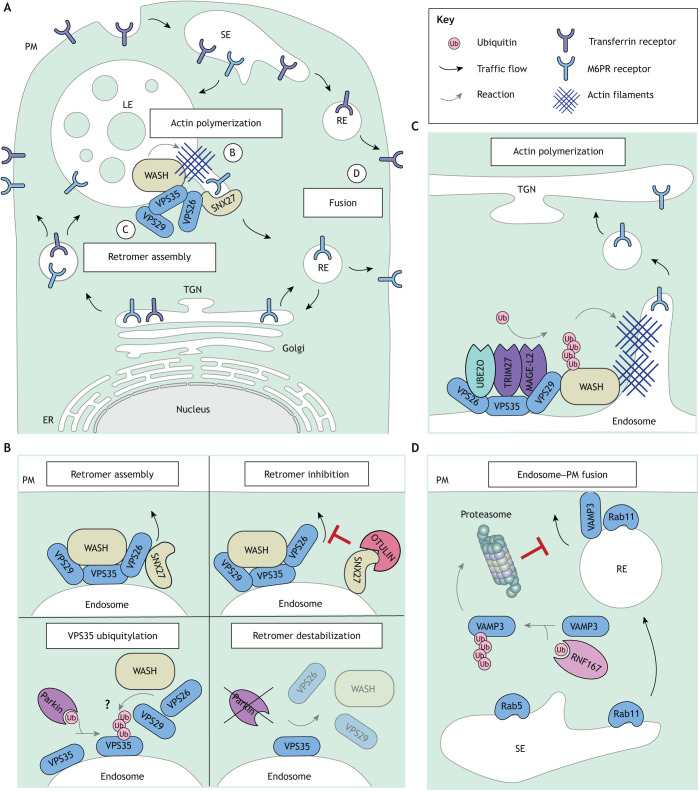
Fig. 2.
Ubiquitylation regulates retromer-mediated recycling. (A) Membrane recycling from maturing endosomes towards the plasma membrane (PM) and the trans-Golgi network (TGN) is carried out by the retromer complex comprising the cargo-selective subunit VPS35 and its partners VPS29 and VPS26. The retromer associates with the WASH complex for actin filament assembly and membrane-deforming sorting nexins (SNX) proteins. Processes labeled B–D are shown in more detail in the other panels. (B) Retromer assembly and stability are regulated by ubiquitylation. The E3 ligase Parkin ubiquitylates VPS35 and stabilizes other retromer components, however, whether ubiquitylation of VPS35 promotes retromer assembly is unclear. Association of the DUB OTULIN with SNX27 antagonizes its binding to VPS26 and thus inhibits retromer-mediated recycling to the PM. (C) Ubiquitylation of the WASH complex, mediated by the retromer-associated E3 ligase MAGE-L2 in conjunction with its substrate adaptor TRIM27 and conjugating E2 enzyme UBE2O, activates localized actin polymerization and promotes recycling from endosomes to the PM. (D) Polyubiquitylation of the SNARE VAMP3 by the E3 ligase RNF167 enhances proteasomal destruction of VAMP3 and inhibits fusion of TGN-derived recycling compartments with the PM.
Another aspect of endosome dynamics regulated by Ub is membrane fusion (Gautreau et al., 2014), which is orchestrated by soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) (Yoon and Munson, 2018). Examples of SNARE–Ub interplay include inhibition of recycling from early endosomes through ubiquitylation of vesicle-associated membrane protein 3 (VAMP3) by the RING-finger protein 167 (RNF167) (Yamazaki et al., 2013) and atypical Ub recognition by syntaxin 3 (Giovannone et al., 2020) (Fig. 2D). To accomplish fusion, SNAREs collaborate with membrane tethering complexes class C core vacuole/endosome tethering (CORVET) and homotypic fusion and vacuole protein sorting (HOPS) (Balderhaar and Ungermann, 2013), both of which contain VPS11 and VPS18 subunits with RING domains capable of E3 Ub ligase activity (Segala et al., 2019; Yogosawa et al., 2005). In vitro reconstitution of the HOPS complex suggests that these RING domains are embedded within the structure and might be unavailable for ligase function (Shvarev et al., 2022 preprint). However, several CORVET and HOPS subunits have been described to function independently (van der Beek et al., 2019), allowing for the possibility of Ub ligation by VPS11 and/or VPS18 in other cellular contexts.
Collectively, these studies illustrate how selective engagement of Ub and Ubl modifying enzymes by executors of endosomal traffic controls fundamental processes in vesicle biology, namely, fusion, fission and cargo flow. Successful completion of these tasks depends in turn on appropriate designation, maintenance and exchange of vesicular membrane identity. How this aspect of endolysosomal biology is modulated by Ub and Ubls is discussed in the following section.
Ub and Ubls as influencers of membrane dynamics
Smooth transitions – ubiquitylation and SUMOylation regulate endolysosomal membrane identity
Decisive commitment of membranes and cargoes to their appropriate trafficking trajectories requires originating membranes to display different characteristics from those found at the destination site (Weeratunga et al., 2020). Intrinsic membrane identity is defined by distinct phospholipid signatures (Schink et al., 2016). In the endolysosomal system, established early and sorting compartments carry phosphatidylinositol 3-phosphate (PI3P), which is converted into phosphatidylinositol 3,5-bisphosphate (PI3,5P2) during maturation (Cullen and Carlton, 2012). Different PI species in turn drive membrane occupancy by small GTPases (Wandinger-Ness and Zerial, 2014) of the Rab (Homma et al., 2021), Arf and Arl (Sztul et al., 2019) families, whose ability to switch between active (GTP-bound) and inactive (GDP-bound) states by way of GTP hydrolysis underlies the dynamic character of membrane traffic (Pfeffer, 2017). Once activated, endosomal GTPases exert their functions through recruitment of specialized effectors that couple transport, fusion and fission machineries to their membranes of choice (Langemeyer et al., 2018). To accommodate smooth progression along the endolysosomal track, endosomes exchange their GTPases through regulated hand-over mechanisms. These include Rab5-to-Rab7 conversion on maturing endosomes (Langemeyer et al., 2020; Poteryaev et al., 2010), followed by Rab7-to-Arl8b exchange on degradative compartments (Jongsma et al., 2020; Marwaha et al., 2017) (Fig. 3A).
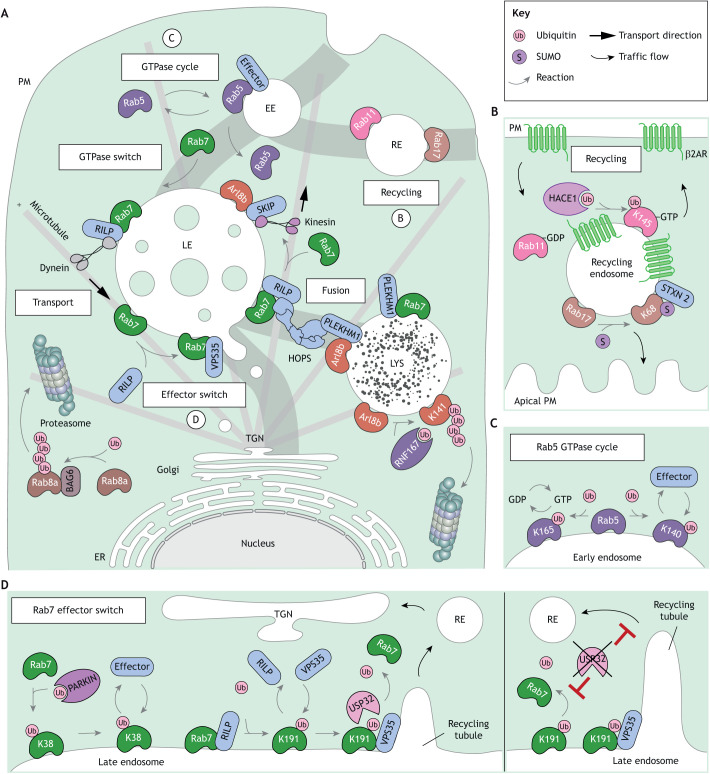
Fig. 3.
Ub and Ubls in endosomal membrane identity transitions. (A) Regulated switches between small GTPases determine endosome identity, transport and fusion through selective effector recruitment. During maturation, late endosome (LE)-associated Rab7 displaces early endosome (EE)-associated Rab5. Rab7 engages the effector RILP and the dynein motor to direct transport of late endosomes (LEs) to the perinuclear region. Here, HOPS-mediated tethering and fusion with lysosomes carrying Rab7 and/or Arl8b in complex with the effector PLEKHM1 takes place. On the other hand, displacement of Rab7 by Arl8b in complex with effector SKIP allows for kinesin motor-dependent transport to the cell periphery. Polyubiquitylation of Arl8b by E3 ligase RNF167 leads to its degradation by the proteasome. Other small GTPases including Rab8a are also turned over by the proteasome in a manner dependent on the cytosolic chaperone BAG6. Processes labeled B–D are shown in more detail in the other panels. (B) Ub and Ubl conjugation regulates Rab-mediated recycling. Endocytosed β2-adregergic receptor recruits the E3 ligase HACE1 to ubiquitylate and activate Rab11 for recycling to the plasma membrane (PM). Also, SUMOylation of Rab17 promotes SNARE syntaxin 2-mediated recycling to the apical surface. (C) Key consequences of GTPase ubiquitylation include altered GTP hydrolysis rate and effector interactions. This is illustrated by Rab5 whose monoubiquitylation on different lysine residues affects distinct aspects of its functional cycle. (D) Left, reversible ubiquitylation of Rab7 by the E3 ligase Parkin and DUB USP32 regulates effector preference. Monoubiquitylation of Rab7 disfavors interaction with RILP and promotes association with the retromer complex. Right, loss of USP32 inhibits recycling tubule resolution and blocks endosomes-to-TGN traffic. Acceptor lysine residues are indicated within some shapes.
The activity cycle of small GTPases is extensively influenced by PTMs (Shinde and Maddika, 2018), and the relevance of Ub and Ubls is rapidly emerging in this context. The first ubiquitylation on an endosomal GTPase was described for Rab11 (which has Rab11a and Rab11b isoforms), responsible for recycling from EEs, demonstrating that monoubiquitylation of Rab11 on K145 promotes recycling of β2-adrenergic receptor to the PM (Lachance et al., 2014) (Fig. 3B). Ubiquitylation of Rab11 effectors has also been implicated in cargo recycling (Sakai et al., 2019), underscoring the varied ways in which this PTM can influence the behavior and function of protein complexes on endosomes. Importantly, conjugation of Ub and Ubls to distinct lysine residues on the same GTPase can lead to different outcomes (Jung et al., 2022). For instance, monoubiquitylation of Rab5 (which has Rab5a, Rab5b and Rab5c isoforms) on K140 interferes with its binding to effectors Rabaptin5 (also known as RABEP1) and early endosome antigen 1 (EEA1), whereas the same modification on K165 affects the intrinsic GDP–GTP conversion cycle (Shin et al., 2017) (Fig. 3C). At later maturation stages, monoubiquitylation of Rab7 (which has Rab7a and Rab7b isoforms) on K38 by the E3 ligase Parkin influences its membrane association and effector interactions (Song et al., 2016). Additionally, monoubiquitylation of Rab7 on K191 (by an unknown ligase) destabilizes binding of the retrograde transport effector RILP responsible for dynein motor recruitment and promotes interaction with the retromer complex instead (Sapmaz et al., 2019) (Fig. 3D). Interestingly, both of the aforementioned Rab7 functions benefit from the deubiquitinating activity of USP32 (Sapmaz et al., 2019), suggesting that reversible ubiquitylation tunes the switching of Rab7 between its different effects in the endocytic pathway. Several endosomal GTPases are also subject to SUMOylation, including Rab7 (Mohapatra et al., 2019), Rab17 (Striz and Tuma, 2016) and Arl13b (Li et al., 2012). However, whether SUMOylation constitutes a general mode of small GTPase control remains to be established.
Besides modulating membrane association, effector binding and GTP hydrolysis, ubiquitylation can also stimulate GTPase turnover by the proteasome, as shown for Arl8b (Deshar et al., 2016) (Fig. 3A), Rab35 (Villarroel-Campos et al., 2016), Rab27a (Song et al., 2019) and Arl4C (Han et al., 2020). Furthermore, it has been speculated that some small GTPases interact with the BAG6 chaperon/holdase cytosolic quality control complex when in the GDP-bound state (Takahashi et al., 2019), and that this might constitute a general mechanism for clearance of inactive Rabs. Taken together, the above examples illustrate how Ub and Ubls offer timely controls over small GTPase abundance and function. The notion that such reversible modifications are crucial in timing dynamic membrane behaviors is further explored below where we describe the emerging roles of Ub and Ubls in formation and dissolution of specialized organellar interaction hubs termed membrane contact sites (MCSs).
Touch and go – ubiquitylation at MCSs
An abundance of physical interactions takes place between intracellular membranes in the form of MCSs (Prinz et al., 2020), which provide platforms for organellar communication and material exchange (Scorrano et al., 2019). The majority of MCSs involve the endoplasmic reticulum (ER) – an organelle whose dynamic membrane network is ideally suited to coordinate diverse cellular pathways and balance homeostasis (Nixon-Abell et al., 2016; Spang, 2018). The ER interacts extensively with endosomes, lysosomes and autophagosomes, guiding their motility, fusion and fission through various types of MCSs (Dong et al., 2016; Hoyer et al., 2018; Raiborg et al., 2015; Rocha et al., 2009; Wijdeven et al., 2016) (Fig. 4A). Thus far, only one example of Ub-dependent MCS formation between endocytic compartments and the ER has been reported (Cremer et al., 2021; Jongsma et al., 2016). Here, the RING E3 ligase RNF26, embedded within the perinuclear ER subdomain anchored by vimentin intermediate filaments (Cremer et al., 2022 preprint), cooperates with the conjugating enzyme UBE2J1 to modify the cytosolic Ub adaptor SQSTM1 (Fig. 4B). The resulting Ub-rich complex engages UBDs of endolysosomal adaptors, such as TOLLIP and EPS15, to position their cognate vesicles in the perinuclear cytoplasm. This action can subsequently be reversed by the DUB USP15, releasing vesicles for fast transport into the cell periphery. Together, these enzymes help maintain the organization of the entire endosomal repertoire (including vesicles of the TGN) in space and time, and, in so doing, facilitate efficient endolysosomal maturation and downregulation of extracellular signals (Cremer et al., 2021; Jongsma et al., 2016). Interestingly, prior to their deposition into ILVs, activated receptors, such as EGFR, undergo dephosphorylation at ER–endosome MCSs, mediated by the ER-associated phosphatase PTP1B (also known as PTPN1) (Eden et al., 2010). However, whether RNF26 and PTP1B collaborate with respect to EGFR downregulation remains unknown. Furthermore, given that the ER membrane harbors many ubiquitin ligases (Fenech et al., 2020), it is possible that additional mechanisms involving ubiquitin conjugation operate at MCSs between the ER the various members of the endolysosomal system.
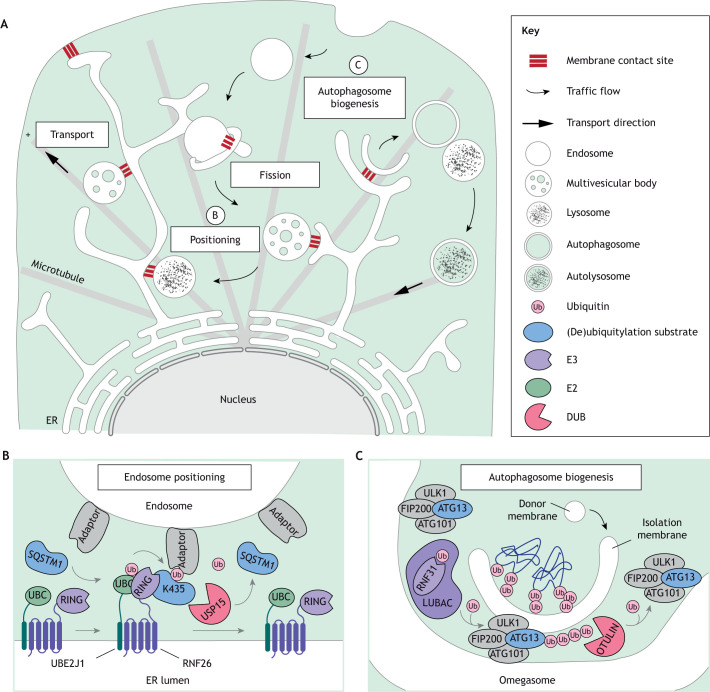
Fig. 4.
Reversible ubiquitylation at membrane contact sites. (A) ER membrane contact sites (MCSs) regulate endocytic traffic by directing endosome transport, specifying location and timing of endosome fission, and positioning endosomes (and lysosomes) in the perinuclear space. ER MCSs are also involved in autophagosome biogenesis. Processes labeled B and C are shown in more detail in the other panels. (B) Ub-dependent reversible ER-endosome MCS formation. The membrane-embedded UBE2J1–RNF26 E2–E3 pair ubiquitylates SQSTM1 on K435 to position endosomes at the perinuclear ER. Deubiquitylation by USP15 in turn dissolves this MCS, releasing endosomes for fast transport. (C) Regulation of early steps in autophagosome biogenesis by the ER. The ULK1 complex localizes at omegasome regions of the ER membrane, where it stimulates formation of the isolation membrane (IM). Timing of IM elongation is controlled by the E3 ligase RNF31 of the LUBAC complex, which mediates linear polyubiquitylation of ATG13. Once the autophagosomal double-membrane is sealed, deubiquitylation of ATG13 by OTULIN promotes autophagosome maturation.
Besides guiding endolysosomal maturation and dynamics, ER MCSs are also instrumental during early steps of autophagosome biogenesis (Kohler et al., 2020). In mammalian cells, autophagosomes are thought to originate at omega-shaped regions of the ER in response to the Unc-51-like autophagy activating kinase 1 (ULK1) (Feng et al., 2014; Nascimbeni et al., 2017). These early steps are coordinated through short-lived MCSs between the ER and the growing isolation membrane (IM), which are tethered by integral ER proteins VAPA and VAPB and IM-associated WD repeat domain phosphoinositide-interacting protein 2 (WIPI2), as well as the autophagy-related protein 2 (ATG2)–WIPI4 (WIPI4 is also known as WDR45) tethering complex (Graef, 2018; Zhao et al., 2018). To allow sufficient time for IM expansion, the linear ubiquitin assembly complex (LUBAC) modifies and stabilizes ATG13, a component of the ULK complex (Fig. 4C). Once the double-membrane of the phagophore seals around the designated (ubiquitylated) cargoes, deubiquitylation of ATG13 by OTULIN untethers the MCS, allowing the autophagosome to mature. Here again, reversible ubiquitylation provides temporal control of dynamic membrane remodeling events through short-lived interorganellar contacts. Notably, VAPA- and VAPB-mediated ER MCSs also dictate autophagosome transport and fusion with the endolysosomal system (Wijdeven et al., 2016), yet whether reversible modifications with Ub and Ubls affect these aspects is unknown. In the same vein, ER MCSs have been implicated in the maturation of phagosomes formed around intracellular bacterial invaders (Derre, 2017). Involvement of Ub and Ubls in this context (unexplored to date) is likely, given that pathogenic bacteria are expert usurpers of host Ub and Ubl conjugation cascades, as described in the section below.
Bacteria exploit Ub and Ubls to reprogram the endolysosomal system of the host
Many intracellular pathogens have evolved sophisticated strategies of subverting host cell processes to benefit their infection programs. Notable among these tactics is manipulation of Ub and Ubl signaling to reprogram host cell organization in service of pathogen uptake and dissemination (Ashida et al., 2014; Zhou and Zhu, 2015). From the moment of infection, bacteria, as well as viruses, exploit the host Ub system to enhance their entry. For instance, Listeria, the bacterium causing listeriosis, stimulates ubiquitylation of host cell surface receptors E-cadherin and Met, which recruit the machinery for clathrin-mediated endocytosis and promote actin cytoskeleton rearrangements necessary for bacterial internalization (Pizarro-Cerda et al., 2012). A similar strategy is exploited by the dengue virus, whose entry into T cells necessitates ubiquitylation of its receptor immunoglobulin mucin 1 (TIM-1; also known as HAVCR1) (Dejarnac et al., 2018), or the Zika virus, whose envelope protein ubiquitylation increases binding to host receptors (Giraldo et al., 2020). Once inside the host cell, some pathogens strategically remain within a membrane-bound compartment (Fig. 5A), which matures into a supportive replicative niche through active remodeling of host membranes, selective harvesting of nutrients and avoidance of degradation. Below we discuss Legionella and Salmonella as examples of bacterial species that have mastered the art of hijacking host membrane traffic and Ub and Ubl conjugation pathways to build and sustain their replicative niche.
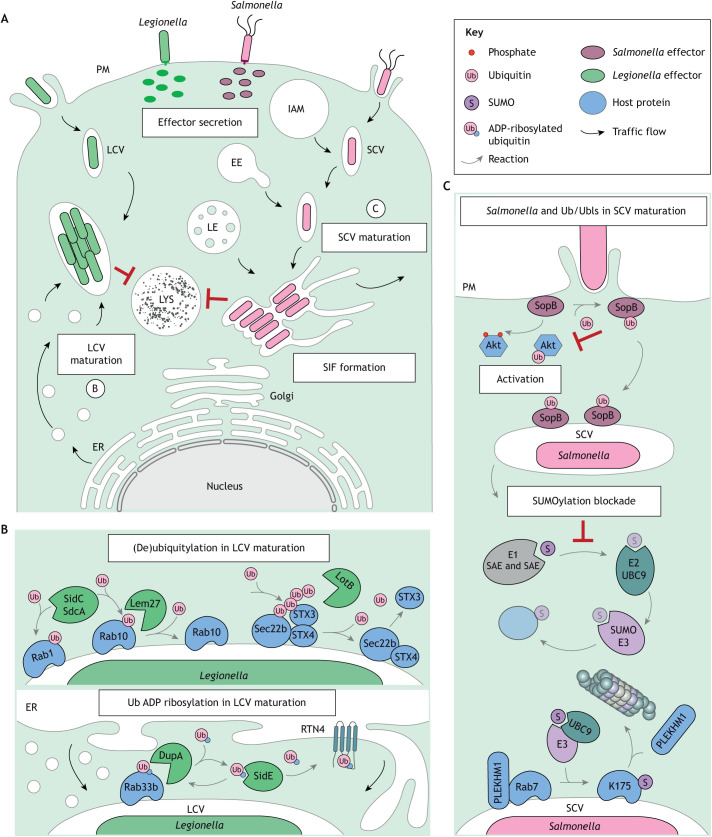
Fig. 5.
Hijacking of Ub and Ubls by intracellular bacteria to reprogram the endolysosomal system of the host. (A) Intracellular bacteria exploit reversible Ub and Ubl conjugation to reprogram the host endolysosomal system in ways benefitting their infection cycle. Both Legionella and Salmonella establish vacuolar compartments that mature into replication permissive niches while avoiding lysosomal degradation. IAM, infection associated macropinosome; EE, early endosome; LE, late endosome; LYS, lysosome. Processes labeled B and C are shown in more detail in the other panels. (B) Following bacterial entry, Legionella exploits the host ubiquitylation machinery, as well as secreting its own unique repertoire of enzymes that enable the Legionella-containing vacuole (LCV) membrane to acquire ER-like characteristics and evade the endolysosomal system. This strategy includes ubiquitylation of GTPases Rab1 and Rab10, temporal regulation of membrane expansion by reversible ubiquitylation of the SNARE Sec22b, and promotion of LCV maturation through noncanonical modifications of host factors with ADP-ribosylated Ub. (C) Ubiquitylation of the Salmonella effector SopB directs it to the Salmonella-containing vacuole (SCV), where it regulates membrane identity dynamics at the SCV. Conversely, the non-ubiquitylated form of SopB localizes predominantly to the PM and instigates activation of Akt signaling. Salmonella infection also induces a global SUMOylation blockade in the host cell through downregulation of the SUMO-conjugating enzyme UBC9. This stabilizes the key endolysosomal Rab7 that promotes maturation of the SCV. Acceptor lysine residues are indicated within some shapes.
Legionella – a master of Ub manipulation
Notably, Legionella pneumophila, the causative agent of Legionnaire's lung disease, secretes an arsenal of Ub ligases and DUBs, some of which manipulate vesicular traffic. For instance, the Legionella Ub ligases SidC and SdcA alter localization and activity of host Rab1 and Rab10 proteins (Jeng et al., 2019) (Fig. 5B). Ubiquitylation of Rab10 is further counteracted by the DUB effector Lem27, and this interplay guides the maturation of the Legionella-containing vacuole (LCV) in space and time (Liu et al., 2020). Legionella also promotes sequential addition and removal of Ub from the host SNARE Sec22b (Kitao et al., 2020). Collectively, these efforts enable the LCV to acquire ER-like characteristics and evade the endolysosomal system.
Not only does Legionella express conventional ligase and DUB effectors that cooperate with the Ub machinery of the host (Qiu and Luo, 2017), it also deploys a unique set of unconventional enzymes that utilize Ub without the need for host E1 or E2 activities (Bhogaraju and Dikic, 2016). Specifically, effectors of the SidE family conduct ADP-ribosylation of R42 on the host Ub, rendering it competent for direct attachment to serine residues of protein substrates (Qiu et al., 2016). This noncanonical Ub PTM impairs conventional ubiquitylation (Bhogaraju et al., 2016) and promotes maturation of the LCV by targeting host traffic regulators Rab33b and the ER reticulon protein Rtn4 (Kawabata et al., 2021; Kotewicz et al., 2017) (Fig. 5B). To complete this PTM cycle, Legionella also encodes phosphodiesterase effectors DupA and DupB, which reverse SidE-mediated ubiquitylation, ensuring the identity of the LCV membrane remains dynamic over time (Qiu et al., 2017). Whether other organisms can also reversibly modify proteins with ubiquitin via phosphoribosylation is unclear.
Salmonella rewires endolysosomal traffic via SUMO and Ub
Another intracellular bacterium, Salmonella Typhimurium, which causes gastroenteritis, takes a different global approach. Salmonella induces a SUMOylation blockade in the host cell by downregulating UBC9 (Verma et al., 2015). One key consequence of this blockade is the stabilization of Rab7 and enhanced binding to the fusion effector PLEKHM1, which is needed for the Salmonella-containing vacuole (SCV) to mature into a permissive replicative niche (Mohapatra et al., 2019) (Fig. 5C). Beyond Rab7, these findings raise new questions regarding the potential for broader implications of SUMOylation on the host–pathogen interaction landscape. In particular, considering that the SCV membrane undergoes several identity transitions prior to arriving at the Rab7-positive stage (Smith et al., 2007), the findings described above might represent merely the proverbial tip of the iceberg when it comes to Ub and Ubl-guided GTPase control of the SCV.
Localization and activity of bacterial effector proteins can also be regulated by Ub and Ubls. This is exemplified by the Salmonella effector SopB, which controls membrane identity of the PM, and subsequently the SCV, through phosphorylation of host phosphoinositides (Hernandez et al., 2004; Walpole et al., 2022). These sequential and locally restricted functions of SopB in promoting bacterial uptake and maturation of the SCV, respectively, are coordinated by a shift in SopB ubiquitylation (Fig. 5C). Specifically, SopB-dependent actin remodeling necessary for bacterial internalization is intensified and sustained by a SopB mutant lacking Ub acceptor lysine residues (Knodler et al., 2009; Patel et al., 2009). Conversely, ubiquitylation of SopB inhibits its early activity at the PM, prolonging the retention of this effector on the SCV instead (Knodler et al., 2009; Patel et al., 2009). In this way, Salmonella exploits the Ub and Ubl machinery to steer host and bacterial protein activities in space and time, tuning the endolysosomal landscape to optimally serve its own needs.
Ub and Ubls tune the host response
A key defense against intracellular bacterial invaders is provided by the autophagy-like pathway termed xenophagy, meaning ‘foreign-eating’ (Knodler and Celli, 2011). Cytosolic bacteria, damaged bacteria-containing vacuoles and membrane remnants resulting from vacuolar disruption can all be targeted for ubiquitylation by the LUBAC complex, marking them for autophagic clearance (Tripathi-Giesgen et al., 2021). To circumvent this fatality, the cytosolic bacterium Shigella flexneri secretes IpaH family E3 ligases that ubiquitylate and degrade LUBAC, thereby neutralizing the very machinery necessary for bacterial demise (de Jong et al., 2016; Liu et al., 2022). Legionella, on the other hand, deploys a DUB effector RavD to specifically hydrolyze linear Ub chains on the LCV (Wan et al., 2019). Using a similar strategy, Salmonella exploits a DUB effector SseL to remove Ub chains decorating cytosolic aggregates formed during infection, subduing autophagic flux and promoting bacterial replication (Mesquita et al., 2012). Salmonella also repurposes the host autophagy machinery to repair its vacuolar membrane (Kreibich et al., 2015). From the host perspective, macrophages activated by interferon γ unleash a Ub-dependent pathogen defense orchestrated by the E3 ligase RNF115 to slow maturation of the phagosome compartment and reduce Salmonella replication (Bilkei-Gorzo et al., 2022). Another interferon γ-induced E3 ligase, namely RNF213, ubiquitylates Chlamydia inclusion bodies for autolysosomal clearance, and this function is inhibited by the bacterial shielding protein GarD (Walsh et al., 2022). Collectively, these examples reveal how reversible ubiquitylation of intracellular bacteria and their phagosomal niche can be exploited by pathogens and host cells alike attempting to gain the upper hand in the conflict between them.
Conclusions and perspectives
The sheer complexity and dynamic nature of intracellular traffic necessitates subtle but decisive controls over membrane identity and cargo fate. As our appreciation for various ways in which Ub and Ubls endow the endolysosomal system with spatial and temporal constraints evolves, many exciting questions come to the forefront. For instance, whether and how the interplay between intrinsic membrane properties (i.e. the phospholipid repertoire) and secondary factors, such as small GTPases and their effectors, is regulated by Ub and Ubls is unclear. Related to this, a comprehensive view of Ub and Ubls as modulators of intracellular bacterial niches, which are strongly reliant on membrane identity control, is presently lacking. With antibiotic resistance on the rise worldwide, identification of novel druggable targets from the side of the host as well as the pathogen may offer new routes for treatment of bacterial infections (Stevenin and Neefjes, 2022).
Another glaring unknown in the field is the molecular nature of internal membrane equilibrium within endosomes. Given the importance of Ub and Ubl dynamics during ILV formation, it is reasonable to hypothesize that reversal of this process through retrofusion might also be modulated by these PTMs. Moreover, because MVB dynamics have direct implications for EV biogenesis and intercellular communication in cancer metastasis and neurodegeneration, enquiry into Ub and Ubl (de)conjugating enzymes regulating endosomes dynamics has the potential to illuminate and eventually modulate these pathological processes. Meanwhile, rapid developments in proteomic approaches (Grandi and Bantscheff, 2019; Kliza and Husnjak, 2020), genome editing (Pickar-Oliver and Gersbach, 2019) and spatiotemporally resolved imaging techniques (Guo et al., 2018) are making it possible to identify and resolve dynamic cellular behaviors with greater ease and efficiency than ever before. Obtaining such insights at the crossroads of endocytosis and the Ub system constitutes an exciting challenge of the coming decade and holds promise for the development of new therapies.
Acknowledgements
We thank Drs Piers D. Nash and Lawrence R. Dick for insightful discussions on the universe of Ub conjugation and removal.
Footnotes
Funding
Our work in this area was supported by a Spinoza grant from the Science Organization of the Netherlands (Nederlandse Organisatie voor Wetenschappelijk Onderzoek; NWO) awarded to J.N. and ICI00026 grant from the NWO Gravity Program awarded to A.S. Open Access funding provided by Leids Universitair Medisch Centrum. Deposited in PMC for immediate release.
References
- Acconcia, F., Sigismund, S. and Polo, S. (2009). Ubiquitin in trafficking: the network at work. Exp. Cell Res. 315, 1610-1618. 10.1016/j.yexcr.2008.10.014 [DOI] [PubMed] [Google Scholar]
- Adoro, S., Park, K. H., Bettigole, S. E., Lis, R., Shin, H. R., Seo, H., Kim, J. H., Knobeloch, K. P., Shim, J. H. and Glimcher, L. H. (2017). Post-translational control of T cell development by the ESCRT protein CHMP5. Nat. Immunol. 18, 780-790. 10.1038/ni.3764 [DOI] [PubMed] [Google Scholar]
- Aki, D., Zhang, W. and Liu, Y. C. (2015). The E3 ligase Itch in immune regulation and beyond. Immunol. Rev. 266, 6-26. 10.1111/imr.12301 [DOI] [PubMed] [Google Scholar]
- Akutsu, M., Dikic, I. and Bremm, A. (2016). Ubiquitin chain diversity at a glance. J. Cell Sci. 129, 875-880. [DOI] [PubMed] [Google Scholar]
- Ali, N., Zhang, L., Taylor, S., Mironov, A., Urbe, S. and Woodman, P. (2013). Recruitment of UBPY and ESCRT exchange drive HD-PTP-dependent sorting of EGFR to the MVB. Curr. Biol. 23, 453-461. 10.1016/j.cub.2013.02.033 [DOI] [PubMed] [Google Scholar]
- Ashida, H., Kim, M. and Sasakawa, C. (2014). Exploitation of the host ubiquitin system by human bacterial pathogens. Nat. Rev. Microbiol. 12, 399-413. 10.1038/nrmicro3259 [DOI] [PubMed] [Google Scholar]
- Atukorala, I. and Mathivanan, S. (2021). The role of post-translational modifications in targeting protein cargo to extracellular vesicles. Subcell. Biochem. 97, 45-60. 10.1007/978-3-030-67171-6_3 [DOI] [PubMed] [Google Scholar]
- Bache, K. G., Raiborg, C., Mehlum, A. and Stenmark, H. (2003). STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J. Biol. Chem. 278, 12513-12521. 10.1074/jbc.M210843200 [DOI] [PubMed] [Google Scholar]
- Baiady, N., Padala, P., Mashahreh, B., Cohen-Kfir, E., Todd, E. A., Du Pont, K. E., Berndsen, C. E. and Wiener, R. (2016). The Vps27/Hrs/STAM (VHS) domain of the signal-transducing adaptor molecule (STAM) directs associated molecule with the SH3 domain of STAM (AMSH) specificity to longer ubiquitin chains and dictates the position of cleavage. J. Biol. Chem. 291, 2033-2042. 10.1074/jbc.M115.689869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker, J., Spits, M., Neefjes, J. and Berlin, I. (2017). The EGFR odyssey - from activation to destruction in space and time. J. Cell Sci. 130, 4087-4096. [DOI] [PubMed] [Google Scholar]
- Balderhaar, H. J. and Ungermann, C. (2013). CORVET and HOPS tethering complexes - coordinators of endosome and lysosome fusion. J. Cell Sci. 126, 1307-1316. 10.1242/jcs.107805 [DOI] [PubMed] [Google Scholar]
- Barata-Antunes, C., Alves, R., Talaia, G., Casal, M., Geros, H., Mans, R. and Paiva, S. (2021). Endocytosis of nutrient transporters in fungi: The ART of connecting signaling and trafficking. Comput. Struct. Biotechnol. J. 19, 1713-1737. 10.1016/j.csbj.2021.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin, I., Schwartz, H. and Nash, P. D. (2010). Regulation of epidermal growth factor receptor ubiquitination and trafficking by the USP8.STAM complex. J. Biol. Chem. 285, 34909-34921. 10.1074/jbc.M109.016287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogaraju, S. and Dikic, I. (2016). Cell biology: Ubiquitination without E1 and E2 enzymes. Nature 533, 43-44. 10.1038/nature17888 [DOI] [PubMed] [Google Scholar]
- Bhogaraju, S., Kalayil, S., Liu, Y., Bonn, F., Colby, T., Matic, I. and Dikic, I. (2016). Phosphoribosylation of ubiquitin promotes serine ubiquitination and impairs conventional ubiquitination. Cell 167, 1636-1649.e1613. 10.1016/j.cell.2016.11.019 [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo, O., Heunis, T., Marin-Rubio, J. L., Cianfanelli, F. R., Raymond, B. B. A., Inns, J., Fabrikova, D., Peltier, J., Oakley, F., Schmid, R.et al. (2022). The E3 ubiquitin ligase RNF115 regulates phagosome maturation and host response to bacterial infection. EMBO J. 41, e108970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgisdottir, A. B. and Johansen, T. (2020). Autophagy and endocytosis - interconnections and interdependencies. J. Cell Sci. 133, jcs228114. 10.1242/jcs.228114 [DOI] [PubMed] [Google Scholar]
- Bomberger, J. M., Barnaby, R. L. and Stanton, B. A. (2009). The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. J. Biol. Chem. 284, 18778-18789. 10.1074/jbc.M109.001685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappadocia, L. and Lima, C. D. (2018). Ubiquitin-like protein conjugation: structures, chemistry, and mechanism. Chem. Rev. 118, 889-918. 10.1021/acs.chemrev.6b00737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton, J. G., Jones, H. and Eggert, U. S. (2020). Membrane and organelle dynamics during cell division. Nat. Rev. Mol. Cell Biol. 21, 151-166. 10.1038/s41580-019-0208-1 [DOI] [PubMed] [Google Scholar]
- Carnino, J. M., Ni, K. and Jin, Y. (2020). Post-translational modification regulates formation and cargo-loading of extracellular vesicles. Front. Immunol. 11, 948. 10.3389/fimmu.2020.00948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K. E., Healy, M. D. and Collins, B. M. (2019). Towards a molecular understanding of endosomal trafficking by Retromer and Retriever. Traffic 20, 465-478. 10.1111/tra.12649 [DOI] [PubMed] [Google Scholar]
- Chen, C., Zheng, H., Luo, Y., Kong, Y., An, M., Li, Y., He, W., Gao, B., Zhao, Y., Huang, H.et al. (2021). SUMOylation promotes extracellular vesicle-mediated transmission of lncRNA ELNAT1 and lymph node metastasis in bladder cancer. J. Clin. Invest. 131, e146431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover, A. (2015). The unravelling of the ubiquitin system. Nat. Rev. Mol. Cell Biol. 16, 322-324. 10.1038/nrm3982 [DOI] [PubMed] [Google Scholar]
- Clague, M. J., Liu, H. and Urbe, S. (2012). Governance of endocytic trafficking and signaling by reversible ubiquitylation. Dev. Cell 23, 457-467. 10.1016/j.devcel.2012.08.011 [DOI] [PubMed] [Google Scholar]
- Clague, M. J., Urbe, S. and Komander, D. (2019). Breaking the chains: deubiquitylating enzyme specificity begets function. Nat. Rev. Mol. Cell Biol. 20, 338-352. 10.1038/s41580-019-0099-1 [DOI] [PubMed] [Google Scholar]
- Cossart, P. and Helenius, A. (2014). Endocytosis of viruses and bacteria. Cold Spring Harb. Perspect Biol. 6, a016972. 10.1101/cshperspect.a016972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer, T., Jongsma, M. L. M., Trulsson, F., Vertegaal, A. C. O., Neefjes, J. and Berlin, I. (2021). The ER-embedded UBE2J1/RNF26 ubiquitylation complex exerts spatiotemporal control over the endolysosomal pathway. Cell Rep. 34, 108659. 10.1016/j.celrep.2020.108659 [DOI] [PubMed] [Google Scholar]
- Cremer, T., Voortman, L. M., Bos, E., van Elsland, D. M., ter Haar, L. R., Koning, R. I., Berlin, I. and Neefjes, J. (2022). Vimentin intermediate filaments organize organellar architecture in response to ER stress. bioRxiv, 10.1101/2022.03.24.485587. 10.1101/2022.03.24.485587 [DOI] [Google Scholar]
- Critchley, W. R., Pellet-Many, C., Ringham-Terry, B., Harrison, M. A., Zachary, I. C. and Ponnambalam, S. (2018). Receptor tyrosine kinase ubiquitination and de-ubiquitination in signal transduction and receptor trafficking. Cells 7, 22. 10.3390/cells7030022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen, P. J. and Carlton, J. G. (2012). Phosphoinositides in the mammalian endo-lysosomal network. Subcell. Biochem. 59, 65-110. 10.1007/978-94-007-3015-1_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen, P. J. and Steinberg, F. (2018). To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 19, 679-696. 10.1038/s41580-018-0053-7 [DOI] [PubMed] [Google Scholar]
- Davies, C. W., Paul, L. N., Kim, M. I. and Das, C. (2011). Structural and thermodynamic comparison of the catalytic domain of AMSH and AMSH-LP: nearly identical fold but different stability. J. Mol. Biol. 413, 416-429. 10.1016/j.jmb.2011.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, K. J., Kago, G., Wang, L., Richter, J. B., Hayden, C. C., Lafer, E. M. and Stachowiak, J. C. (2021). Liquid-like protein interactions catalyse assembly of endocytic vesicles. Nat. Cell Biol. 23, 366-376. 10.1038/s41556-021-00646-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis Rigotti, F., De Gassart, A., Pforr, C., Cano, F., N'Guessan, P., Combes, A., Camossetto, V., Lehner, P. J., Pierre, P. and Gatti, E. (2017). MARCH9-mediated ubiquitination regulates MHC I export from the TGN. Immunol. Cell Biol. 95, 753-764. 10.1038/icb.2017.44 [DOI] [PubMed] [Google Scholar]
- de Jong, M. F., Liu, Z., Chen, D. and Alto, N. M. (2016). Shigella flexneri suppresses NF-kappaB activation by inhibiting linear ubiquitin chain ligation. Nat. Microbiol. 1, 16084. 10.1038/nmicrobiol.2016.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejarnac, O., Hafirassou, M. L., Chazal, M., Versapuech, M., Gaillard, J., Perera-Lecoin, M., Umana-Diaz, C., Bonnet-Madin, L., Carnec, X., Tinevez, J. Y.et al. (2018). TIM-1 ubiquitination mediates dengue virus entry. Cell Rep. 23, 1779-1793. 10.1016/j.celrep.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Derre, I. (2017). Hijacking of membrane contact sites by intracellular bacterial pathogens. Adv. Exp. Med. Biol. 997, 211-223. 10.1007/978-981-10-4567-7_16 [DOI] [PubMed] [Google Scholar]
- Deshar, R., Moon, S., Yoo, W., Cho, E. B., Yoon, S. K. and Yoon, J. B. (2016). RNF167 targets Arl8B for degradation to regulate lysosome positioning and endocytic trafficking. FEBS J. 283, 4583-4599. 10.1111/febs.13947 [DOI] [PubMed] [Google Scholar]
- Di Fiore, P. P. and von Zastrow, M. (2014). Endocytosis, signaling, and beyond. Cold Spring Harb. Perspect Biol. 6, a016865. 10.1101/cshperspect.a016865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, R., Saheki, Y., Swarup, S., Lucast, L., Harper, J. W. and De Camilli, P. (2016). Endosome-ER contacts control actin nucleation and retromer function through VAP-dependent regulation of PI4P. Cell 166, 408-423. 10.1016/j.cell.2016.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dores, M. R. and Trejo, J. (2019). Endo-lysosomal sorting of G-protein-coupled receptors by ubiquitin: Diverse pathways for G-protein-coupled receptor destruction and beyond. Traffic 20, 101-109. 10.1111/tra.12619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufner, A., Kisser, A., Niendorf, S., Basters, A., Reissig, S., Schonle, A., Aichem, A., Kurz, T., Schlosser, A., Yablonski, D.et al. (2015). The ubiquitin-specific protease USP8 is critical for the development and homeostasis of T cells. Nat. Immunol. 16, 950-960. 10.1038/ni.3230 [DOI] [PubMed] [Google Scholar]
- Durcan, T. M. and Fon, E. A. (2015). USP8 and PARK2/parkin-mediated mitophagy. Autophagy 11, 428-429. 10.1080/15548627.2015.1009794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden, E. R., White, I. J., Tsapara, A. and Futter, C. E. (2010). Membrane contacts between endosomes and ER provide sites for PTP1B-epidermal growth factor receptor interaction. Nat. Cell Biol. 12, 267-272. 10.1038/ncb2026 [DOI] [PubMed] [Google Scholar]
- Efeyan, A., Comb, W. C. and Sabatini, D. M. (2015). Nutrient-sensing mechanisms and pathways. Nature 517, 302-310. 10.1038/nature14190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn, P. J., Rodon, L., Gonzalez-Junca, A., Dirac, A., Gili, M., Martinez-Saez, E., Aura, C., Barba, I., Peg, V., Prat, A.et al. (2012). USP15 stabilizes TGF-beta receptor I and promotes oncogenesis through the activation of TGF-beta signaling in glioblastoma. Nat. Med. 18, 429-435. 10.1038/nm.2619 [DOI] [PubMed] [Google Scholar]
- Estadella, I., Pedrós-Gámez, O., Colomer-Molera, M., Bosch, M., Sorkin, A. and Felipe, A. (2020). Endocytosis: a turnover mechanism controlling ion channel function. Cells 9, 1833. 10.3390/cells9081833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenech, E. J., Lari, F., Charles, P. D., Fischer, R., Laetitia-Thezenas, M., Bagola, K., Paton, A. W., Paton, J. C., Gyrd-Hansen, M., Kessler, B. M.et al. (2020). Interaction mapping of endoplasmic reticulum ubiquitin ligases identifies modulators of innate immune signalling. Elife 9, e57306. 10.7554/eLife.57306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y., He, D., Yao, Z. and Klionsky, D. J. (2014). The machinery of macroautophagy. Cell Res. 24, 24-41. 10.1038/cr.2013.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming, A., Bourdenx, M., Fujimaki, M., Karabiyik, C., Krause, G. J., Lopez, A., Martin-Segura, A., Puri, C., Scrivo, A., Skidmore, J.et al. (2022). The different autophagy degradation pathways and neurodegeneration. Neuron 110, 935-966. 10.1016/j.neuron.2022.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima, T., Yoshihara, H., Furuta, H., Hakuno, F., Iemura, S. I., Natsume, T., Nakatsu, Y., Kamata, H., Asano, T., Komada, M.et al. (2017). USP15 attenuates IGF-I signaling by antagonizing Nedd4-induced IRS-2 ubiquitination. Biochem. Biophys. Res. Commun. 484, 522-528. 10.1016/j.bbrc.2017.01.101 [DOI] [PubMed] [Google Scholar]
- Gautreau, A., Oguievetskaia, K. and Ungermann, C. (2014). Function and regulation of the endosomal fusion and fission machineries. Cold Spring Harb. Perspect Biol. 6, a016832. 10.1101/cshperspect.a016832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannone, A. J., Reales, E., Bhattaram, P., Nackeeran, S., Monahan, A. B., Syed, R. and Weimbs, T. (2020). The Habc domain of syntaxin 3 is a ubiquitin binding domain. Sci. Rep. 10, 21350. 10.1038/s41598-020-78412-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo, M. I., Xia, H., Aguilera-Aguirre, L., Hage, A., van Tol, S., Shan, C., Xie, X., Sturdevant, G. L., Robertson, S. J., McNally, K. L.et al. (2020). Envelope protein ubiquitination drives entry and pathogenesis of Zika virus. Nature 585, 414-419. 10.1038/s41586-020-2457-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gireud-Goss, M., Reyes, S., Tewari, R., Patrizz, A., Howe, M. D., Kofler, J., Waxham, M. N., McCullough, L. D. and Bean, A. J. (2020). The ubiquitin ligase UBE4B regulates amyloid precursor protein ubiquitination, endosomal trafficking, and amyloid β42 generation and secretion. Mol. Cell. Neurosci. 108, 103542. 10.1016/j.mcn.2020.103542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, A. C., Schweizer, M., Jagdmann, S., Bernreuther, C., Reinheckel, T., Saftig, P. and Damme, M. (2018). Unconventional trafficking of mammalian phospholipase D3 to lysosomes. Cell Rep 22, 1040-1053. 10.1016/j.celrep.2017.12.100 [DOI] [PubMed] [Google Scholar]
- Graef, M. (2018). Membrane tethering by the autophagy ATG2A-WIPI4 complex. Proc. Natl. Acad. Sci. USA 115, 10540-10541. 10.1073/pnas.1814759115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi, P. and Bantscheff, M. (2019). Advanced proteomics approaches to unravel protein homeostasis. Drug Discov. Today Technol. 31, 99-108. 10.1016/j.ddtec.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Gruenberg, J. (2020). Life in the lumen: The multivesicular endosome. Traffic 21, 76-93. 10.1111/tra.12715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschweitl, M., Ulbricht, A., Barnes, C. A., Enchev, R. I., Stoffel-Studer, I., Meyer-Schaller, N., Huotari, J., Yamauchi, Y., Greber, U. F., Helenius, A.et al. (2016). A SPOPL/Cullin-3 ubiquitin ligase complex regulates endocytic trafficking by targeting EPS15 at endosomes. Elife 5, e13841. 10.7554/eLife.13841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, H., Shi, X., Liu, C., Wang, C., Sui, N., Zhao, Y., Gong, J., Wang, F., Zhang, H., Li, W.et al. (2019). USP8 maintains embryonic stem cell stemness via deubiquitination of EPG5. Nat. Commun. 10, 1465. 10.1038/s41467-019-09430-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney, E. L., Klecker, T. and Emr, S. D. (2016). Identification of the endocytic sorting signal recognized by the Art1-Rsp5 ubiquitin ligase complex. Mol. Biol. Cell 27, 4043-4054. 10.1091/mbc.E16-08-0570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y., Li, D., Zhang, S., Yang, Y., Liu, J. J., Wang, X., Liu, C., Milkie, D. E., Moore, R. P., Tulu, U. S.et al. (2018). Visualizing intracellular organelle and cytoskeletal interactions at nanoscale resolution on millisecond timescales. Cell 175, 1430-1442.e1417. 10.1016/j.cell.2018.09.057 [DOI] [PubMed] [Google Scholar]
- Gurung, S., Perocheau, D., Touramanidou, L. and Baruteau, J. (2021). The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun. Signal 19, 47. 10.1186/s12964-021-00730-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo, C. M. and Matunis, M. J. (2013). Expanding SUMO and ubiquitin-mediated signaling through hybrid SUMO-ubiquitin chains and their receptors. Cell Cycle 12, 1015-1017. 10.4161/cc.24332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund, K., Di Fiore, P. P. and Dikic, I. (2003). Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem. Sci. 28, 598-603. 10.1016/j.tibs.2003.09.005 [DOI] [PubMed] [Google Scholar]
- Han, Y. and Yun, C. C. (2020). Ubiquitin-specific peptidase 7 (USP7) and USP10 mediate deubiquitination of human NHE3 regulating its expression and activity. FASEB J. 34, 16476-16488. 10.1096/fj.202001875R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J. S., Hino, K., Li, W., Reyes, R. V., Canales, C. P., Miltner, A. M., Haddadi, Y., Sun, J., Chen, C. Y., La Torre, A.et al. (2020). CRL5-dependent regulation of the small GTPases ARL4C and ARF6 controls hippocampal morphogenesis. Proc. Natl. Acad. Sci. USA 117, 23073-23084. 10.1073/pnas.2002749117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley, S. E. and Cooper, K. F. (2020). Sorting nexins in protein homeostasis. Cells 10, 17. 10.3390/cells10010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, Y. H., Doyle, J. M., Ramanathan, S., Gomez, T. S., Jia, D., Xu, M., Chen, Z. J., Billadeau, D. D., Rosen, M. K. and Potts, P. R. (2013). Regulation of WASH-dependent actin polymerization and protein trafficking by ubiquitination. Cell 152, 1051-1064. 10.1016/j.cell.2013.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, L. D., Hueffer, K., Wenk, M. R. and Galan, J. E. (2004). Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science 304, 1805-1807. 10.1126/science.1098188 [DOI] [PubMed] [Google Scholar]
- Hicke, L. and Riezman, H. (1996). Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell 84, 277-287. 10.1016/S0092-8674(00)80982-4 [DOI] [PubMed] [Google Scholar]
- Ho, H. C., MacGurn, J. A. and Emr, S. D. (2017). Deubiquitinating enzymes Ubp2 and Ubp15 regulate endocytosis by limiting ubiquitination and degradation of ARTs. Mol. Biol. Cell 28, 1271-1283. 10.1091/mbc.e17-01-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeller, D., Crosetto, N., Blagoev, B., Raiborg, C., Tikkanen, R., Wagner, S., Kowanetz, K., Breitling, R., Mann, M., Stenmark, H.et al. (2006). Regulation of ubiquitin-binding proteins by monoubiquitination. Nat. Cell Biol. 8, 163-169. 10.1038/ncb1354 [DOI] [PubMed] [Google Scholar]
- Holler, D. and Dikic, I. (2004). Receptor endocytosis via ubiquitin-dependent and -independent pathways. Biochem. Pharmacol. 67, 1013-1017. 10.1016/j.bcp.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Hologne, M., Cantrelle, F. X., Riviere, G., Guilliere, F., Trivelli, X. and Walker, O. (2016). NMR reveals the interplay among the AMSH SH3 Binding motif, STAM2, and Lys63-linked diubiquitin. J. Mol. Biol. 428, 4544-4558. 10.1016/j.jmb.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Homma, Y., Hiragi, S. and Fukuda, M. (2021). Rab family of small GTPases: an updated view on their regulation and functions. FEBS J. 288, 36-55. 10.1111/febs.15453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer, M. J., Chitwood, P. J., Ebmeier, C. C., Striepen, J. F., Qi, R. Z., Old, W. M. and Voeltz, G. K. (2018). A novel class of ER membrane proteins regulates ER-associated endosome fission. Cell 175, 254-265.e214. 10.1016/j.cell.2018.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, F., Kirkpatrick, D., Jiang, X., Gygi, S. and Sorkin, A. (2006). Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol. Cell 21, 737-748. 10.1016/j.molcel.2006.02.018 [DOI] [PubMed] [Google Scholar]
- Hurley, J. H. (2015). ESCRTs are everywhere. EMBO J. 34, 2398-2407. 10.15252/embj.201592484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husnjak, K. and Dikic, I. (2012). Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu. Rev. Biochem. 81, 291-322. 10.1146/annurev-biochem-051810-094654 [DOI] [PubMed] [Google Scholar]
- Islam, M. T., Chen, F. and Chen, H. (2021). The oncogenic role of ubiquitin specific peptidase (USP8) and its signaling pathways targeting for cancer therapeutics. Arch. Biochem. Biophys. 701, 108811. 10.1016/j.abb.2021.108811 [DOI] [PubMed] [Google Scholar]
- Jeng, E. E., Bhadkamkar, V., Ibe, N. U., Gause, H., Jiang, L., Chan, J., Jian, R., Jimenez-Morales, D., Stevenson, E., Krogan, N. J.et al. (2019). Systematic identification of host cell regulators of legionella pneumophila pathogenesis using a genome-wide CRISPR screen. Cell Host Microbe 26, 551-563.e556. 10.1016/j.chom.2019.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma, M. L., Berlin, I., Wijdeven, R. H., Janssen, L., Janssen, G. M., Garstka, M. A., Janssen, H., Mensink, M., van Veelen, P. A., Spaapen, R. M.et al. (2016). An ER-associated pathway defines endosomal architecture for controlled cargo transport. Cell 166, 152-166. 10.1016/j.cell.2016.05.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma, M. L., Bakker, J., Cabukusta, B., Liv, N., van Elsland, D., Fermie, J., Akkermans, J. L., Kuijl, C., van der Zanden, S. Y., Janssen, L.et al. (2020). SKIP-HOPS recruits TBC1D15 for a Rab7-to-Arl8b identity switch to control late endosome transport. EMBO J. 39, e102301. 10.15252/embj.2019102301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, J., Baek, J., Tae, K., Shin, D., Han, S., Yang, W., Yu, W., Jung, S. M., Park, S. H., Choi, C. Y.et al. (2022). Structural mechanism for regulation of Rab7 by site-specific monoubiquitination. Int. J. Biol. Macromol. 194, 347-357. 10.1016/j.ijbiomac.2021.11.074 [DOI] [PubMed] [Google Scholar]
- Kato, M., Miyazawa, K. and Kitamura, N. (2000). A deubiquitinating enzyme UBPY interacts with the Src homology 3 domain of Hrs-binding protein via a novel binding motif PX(V/I)(D/N)RXXKP. J. Biol. Chem. 275, 37481-37487. 10.1074/jbc.M007251200 [DOI] [PubMed] [Google Scholar]
- Katoh, Y., Shiba, Y., Mitsuhashi, H., Yanagida, Y., Takatsu, H. and Nakayama, K. (2004). Tollip and Tom1 form a complex and recruit ubiquitin-conjugated proteins onto early endosomes. J. Biol. Chem. 279, 24435-24443. 10.1074/jbc.M400059200 [DOI] [PubMed] [Google Scholar]
- Kawabata, M., Matsuo, H., Koito, T., Murata, M., Kubori, T., Nagai, H., Tagaya, M. and Arasaki, K. (2021). Legionella hijacks the host Golgi-to-ER retrograde pathway for the association of Legionella-containing vacuole with the ER. PLoS Pathog. 17, e1009437. 10.1371/journal.ppat.1009437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitidi, D., Apaja, P. M., Manteghi, S., Suzuki, K., Malitskaya, E., Roldan, A., Gingras, M. C., Takagi, J., Lukacs, G. L. and Pause, A. (2015). Interplay of endosomal pH and ligand occupancy in integrin alpha5beta1 ubiquitination, endocytic sorting, and cell migration. Cell Rep 13, 599-609. 10.1016/j.celrep.2015.09.024 [DOI] [PubMed] [Google Scholar]
- Kitao, T., Taguchi, K., Seto, S., Arasaki, K., Ando, H., Nagai, H. and Kubori, T. (2020). Legionella Manipulates Non-canonical SNARE Pairing Using a Bacterial Deubiquitinase. Cell Rep 32, 108107. 10.1016/j.celrep.2020.108107 [DOI] [PubMed] [Google Scholar]
- Kliza, K. and Husnjak, K. (2020). Resolving the Complexity of Ubiquitin Networks. Front Mol Biosci 7, 21. 10.3389/fmolb.2020.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman, J. and Raposo, G. (2014). The complex ultrastructure of the endolysosomal system. Cold Spring Harb. Perspect Biol. 6, a016857. 10.1101/cshperspect.a016857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler, L. A. and Celli, J. (2011). Eating the strangers within: host control of intracellular bacteria via xenophagy. Cell. Microbiol. 13, 1319-1327. 10.1111/j.1462-5822.2011.01632.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler, L. A., Winfree, S., Drecktrah, D., Ireland, R. and Steele-Mortimer, O. (2009). Ubiquitination of the bacterial inositol phosphatase, SopB, regulates its biological activity at the plasma membrane. Cell. Microbiol. 11, 1652-1670. 10.1111/j.1462-5822.2009.01356.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler, V., Aufschnaiter, A. and Buttner, S. (2020). Closing the gap: membrane contact sites in the regulation of autophagy. Cells 9, 1184. 10.3390/cells9051184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotewicz, K. M., Ramabhadran, V., Sjoblom, N., Vogel, J. P., Haenssler, E., Zhang, M., Behringer, J., Scheck, R. A. and Isberg, R. R. (2017). A single legionella effector catalyzes a multistep ubiquitination pathway to rearrange tubular endoplasmic reticulum for replication. Cell Host Microbe 21, 169-181. 10.1016/j.chom.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich, S., Emmenlauer, M., Fredlund, J., Ramo, P., Munz, C., Dehio, C., Enninga, J. and Hardt, W. D. (2015). autophagy proteins promote repair of endosomal membranes damaged by the salmonella type three secretion system 1. Cell Host Microbe 18, 527-537. 10.1016/j.chom.2015.10.015 [DOI] [PubMed] [Google Scholar]
- Kunadt, M., Eckermann, K., Stuendl, A., Gong, J., Russo, B., Strauss, K., Rai, S., Kugler, S., Falomir Lockhart, L., Schwalbe, M.et al. (2015). Extracellular vesicle sorting of alpha-Synuclein is regulated by sumoylation. Acta Neuropathol. 129, 695-713. 10.1007/s00401-015-1408-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyuuma, M., Kikuchi, K., Kojima, K., Sugawara, Y., Sato, M., Mano, N., Goto, J., Takeshita, T., Yamamoto, A., Sugamura, K.et al. (2007). AMSH, an ESCRT-III associated enzyme, deubiquitinates cargo on MVB/late endosomes. Cell Struct. Funct. 31, 159-172. 10.1247/csf.06023 [DOI] [PubMed] [Google Scholar]
- Lachance, V., Degrandmaison, J., Marois, S., Robitaille, M., Genier, S., Nadeau, S., Angers, S. and Parent, J. L. (2014). Ubiquitylation and activation of a Rab GTPase is promoted by a beta(2)AR-HACE1 complex. J. Cell Sci. 127, 111-123. [DOI] [PubMed] [Google Scholar]
- Lange, A., Castaneda, C., Hoeller, D., Lancelin, J. M., Fushman, D. and Walker, O. (2012). Evidence for cooperative and domain-specific binding of the signal transducing adaptor molecule 2 (STAM2) to Lys63-linked diubiquitin. J. Biol. Chem. 287, 18687-18699. 10.1074/jbc.M111.324954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langemeyer, L., Frohlich, F. and Ungermann, C. (2018). Rab GTPase function in endosome and lysosome biogenesis. Trends Cell Biol. 28, 957-970. 10.1016/j.tcb.2018.06.007 [DOI] [PubMed] [Google Scholar]
- Langemeyer, L., Borchers, A. C., Herrmann, E., Fullbrunn, N., Han, Y., Perz, A., Auffarth, K., Kummel, D. and Ungermann, C. (2020). A conserved and regulated mechanism drives endosomal Rab transition. Elife 9, e56090. 10.7554/eLife.56090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz, G., Klapper, L. N., Tzahar, E., Freywald, A., Sela, M. and Yarden, Y. (1996). Coupling of the c-Cbl protooncogene product to ErbB-1/EGF-receptor but not to other ErbB proteins. Oncogene 12, 1117-1125. [PubMed] [Google Scholar]
- Levkowitz, G., Waterman, H., Zamir, E., Kam, Z., Oved, S., Langdon, W. Y., Beguinot, L., Geiger, B. and Yarden, Y. (1998). . c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 12, 3663-3674. 10.1101/gad.12.23.3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Létourneau, D., Holleran, B., Leduc, R., Lavigne, P. and Lavoie, C. (2017). Gα(s) protein binds ubiquitin to regulate epidermal growth factor receptor endosomal sorting. Proc. Natl. Acad. Sci. USA 114, 13477-13482. 10.1073/pnas.1708215114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Ling, K. and Hu, J. (2012). The emerging role of Arf/Arl small GTPases in cilia and ciliopathies. J. Cell. Biochem. 113, 2201-2207. 10.1002/jcb.24116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie, P. P. Y. and Nixon, R. A. (2019). Lysosome trafficking and signaling in health and neurodegenerative diseases. Neurobiol. Dis. 122, 94-105. 10.1016/j.nbd.2018.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, C. Y. and Zoncu, R. (2016). The lysosome as a command-and-control center for cellular metabolism. J. Cell Biol. 214, 653-664. 10.1083/jcb.201607005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, H., Li, S. and Shu, H. B. (2019). The membrane-associated MARCH E3 ligase family: emerging roles in immune regulation. Front Immunol 10, 1751. 10.3389/fimmu.2019.01751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S., Luo, J., Zhen, X., Qiu, J., Ouyang, S. and Luo, Z. Q. (2020). Interplay between bacterial deubiquitinase and ubiquitin E3 ligase regulates ubiquitin dynamics on Legionella phagosomes. Elife 9, e58114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Wang, Y., Wang, D., Wang, Y., Xu, X., Zhang, Y., Li, Y., Zhang, M., Gong, X., Tang, Y.et al. (2022). Mechanistic insights into the subversion of the linear ubiquitin chain assembly complex by the E3 ligase IpaH1.4 of Shigella flexneri. Proc. Natl. Acad. Sci. USA 119, e2116776119. 10.1073/pnas.2116776119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyasova, M. S., Ma, K. and Lipkowitz, S. (2015). Molecular pathways: cbl proteins in tumorigenesis and antitumor immunity-opportunities for cancer treatment. Clin. Cancer Res. 21, 1789-1794. 10.1158/1078-0432.CCR-13-2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas, S. L. N., Breakefield, X. O. and Weaver, A. M. (2017). Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 27, 172-188. 10.1016/j.tcb.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald, E., Urbe, S. and Clague, M. J. (2014). USP8 controls the trafficking and sorting of lysosomal enzymes. Traffic 15, 879-888. 10.1111/tra.12180 [DOI] [PubMed] [Google Scholar]
- Madshus, I. H. and Stang, E. (2009). Internalization and intracellular sorting of the EGF receptor: a model for understanding the mechanisms of receptor trafficking. J. Cell Sci. 122, 3433-3439. 10.1242/jcs.050260 [DOI] [PubMed] [Google Scholar]
- Marwaha, R., Arya, S. B., Jagga, D., Kaur, H., Tuli, A. and Sharma, M. (2017). The Rab7 effector PLEKHM1 binds Arl8b to promote cargo traffic to lysosomes. J. Cell Biol. 216, 1051-1070. 10.1083/jcb.201607085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield, F. R. and Yamashiro, D. J. (1987). Endosome acidification and the pathways of receptor-mediated endocytosis. Adv. Exp. Med. Biol. 225, 189-198. 10.1007/978-1-4684-5442-0_16 [DOI] [PubMed] [Google Scholar]
- McCann, A. P., Scott, C. J., Van Schaeybroeck, S. and Burrows, J. F. (2016). Deubiquitylating enzymes in receptor endocytosis and trafficking. Biochem. J. 473, 4507-4525. 10.1042/BCJ20160826 [DOI] [PubMed] [Google Scholar]
- McCullough, J., Clague, M. J. and Urbe, S. (2004). AMSH is an endosome-associated ubiquitin isopeptidase. J. Cell Biol. 166, 487-492. 10.1083/jcb.200401141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman, I. and Yarden, Y. (2013). Endocytosis and cancer. Cold Spring Harb. Perspect Biol. 5, a016949. 10.1101/cshperspect.a016949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita, F. S., Thomas, M., Sachse, M., Santos, A. J., Figueira, R. and Holden, D. W. (2012). The Salmonella deubiquitinase SseL inhibits selective autophagy of cytosolic aggregates. PLoS Pathog. 8, e1002743. 10.1371/journal.ppat.1002743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger, M. B., Hristova, V. A. and Weissman, A. M. (2012). HECT and RING finger families of E3 ubiquitin ligases at a glance. J. Cell Sci. 125, 531-537. 10.1242/jcs.091777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevissen, T. E. T. and Komander, D. (2017). Mechanisms of deubiquitinase specificity and regulation. Annu. Rev. Biochem. 86, 159-192. 10.1146/annurev-biochem-061516-044916 [DOI] [PubMed] [Google Scholar]
- Mohapatra, G., Gaur, P., Mujagond, P., Singh, M., Rana, S., Pratap, S., Kaur, N., Verma, S., Krishnan, V., Singh, N.et al. (2019). A SUMOylation-dependent switch of RAB7 governs intracellular life and pathogenesis of Salmonella Typhimurium. J. Cell Sci. 132, jcs222612. [DOI] [PubMed] [Google Scholar]
- Moser, E. K. and Oliver, P. M. (2019). Regulation of autoimmune disease by the E3 ubiquitin ligase Itch. Cell. Immunol. 340, 103916. 10.1016/j.cellimm.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, S. and Yoshimori, T. (2017). New insights into autophagosome-lysosome fusion. J. Cell Sci. 130, 1209-1216. [DOI] [PubMed] [Google Scholar]
- Nascimbeni, A. C., Codogno, P. and Morel, E. (2017). Phosphatidylinositol-3-phosphate in the regulation of autophagy membrane dynamics. FEBS J. 284, 1267-1278. 10.1111/febs.13987 [DOI] [PubMed] [Google Scholar]
- Naslavsky, N. and Caplan, S. (2018). The enigmatic endosome - sorting the ins and outs of endocytic trafficking. J. Cell Sci. 131, jcs216499. 10.1242/jcs.216499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, J. K., Sorrentino, V., Avagliano Trezza, R., Heride, C., Urbe, S., Distel, B. and Zelcer, N. (2016). The deubiquitylase USP2 regulates the LDLR pathway by counteracting the E3-ubiquitin ligase IDOL. Circ. Res. 118, 410-419. 10.1161/CIRCRESAHA.115.307298 [DOI] [PubMed] [Google Scholar]
- Niendorf, S., Oksche, A., Kisser, A., Lohler, J., Prinz, M., Schorle, H., Feller, S., Lewitzky, M., Horak, I. and Knobeloch, K. P. (2007). Essential role of ubiquitin-specific protease 8 for receptor tyrosine kinase stability and endocytic trafficking in vivo. Mol. Cell. Biol. 27, 5029-5039. 10.1128/MCB.01566-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon-Abell, J., Obara, C. J., Weigel, A. V., Li, D., Legant, W. R., Xu, C. S., Pasolli, H. A., Harvey, K., Hess, H. F., Betzig, E.et al. (2016). Increased spatiotemporal resolution reveals highly dynamic dense tubular matrices in the peripheral ER. Science 354, aaf3928. 10.1126/science.aaf3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, A. and Grant, B. D. (2020). Endosomal microdomains: Formation and function. Curr. Opin. Cell Biol. 65, 86-95. 10.1016/j.ceb.2020.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker, N., Ge, P., Dawson, V. L. and Dawson, T. M. (2021). The cell biology of Parkinson's disease. J. Cell Biol. 220, e202012095. 10.1083/jcb.202012095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, J. C., Hueffer, K., Lam, T. T. and Galan, J. E. (2009). Diversification of a Salmonella virulence protein function by ubiquitin-dependent differential localization. Cell 137, 283-294. 10.1016/j.cell.2009.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Rivas, L. G. and Reincke, M. (2016). Genetics of Cushing's disease: an update. J. Endocrinol. Invest. 39, 29-35. 10.1007/s40618-015-0353-0 [DOI] [PubMed] [Google Scholar]
- Perng, Y. C. and Lenschow, D. J. (2018). ISG15 in antiviral immunity and beyond. Nat. Rev. Microbiol. 16, 423-439. 10.1038/s41579-018-0020-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin, P., Janssen, L., Janssen, H., van den Broek, B., Voortman, L. M., van Elsland, D., Berlin, I. and Neefjes, J. (2021). Retrofusion of intralumenal MVB membranes parallels viral infection and coexists with exosome release. Curr. Biol. 31, 3884-3893.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer, S. R. (2017). Rab GTPases: master regulators that establish the secretory and endocytic pathways. Mol. Biol. Cell 28, 712-715. 10.1091/mbc.e16-10-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickar-Oliver, A. and Gersbach, C. A. (2019). The next generation of CRISPR-Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 20, 490-507. 10.1038/s41580-019-0131-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart, C. M. (2001). Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503-533. 10.1146/annurev.biochem.70.1.503 [DOI] [PubMed] [Google Scholar]
- Piper, R. C., Dikic, I. and Lukacs, G. L. (2014). Ubiquitin-dependent sorting in endocytosis. Cold Spring Harb Perspect Biol. 6, a016808. 10.1101/cshperspect.a016808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Cerda, J., Kuhbacher, A. and Cossart, P. (2012). Entry of Listeria monocytogenes in mammalian epithelial cells: an updated view. Cold Spring Harb Perspect Med 2, a010009. 10.1101/cshperspect.a010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo, S. (2012). Signaling-mediated control of ubiquitin ligases in endocytosis. BMC Biol. 10, 25. 10.1186/1741-7007-10-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteryaev, D., Datta, S., Ackema, K., Zerial, M. and Spang, A. (2010). Identification of the switch in early-to-late endosome transition. Cell 141, 497-508. 10.1016/j.cell.2010.03.011 [DOI] [PubMed] [Google Scholar]
- Prinz, W. A., Toulmay, A. and Balla, T. (2020). The functional universe of membrane contact sites. Nat. Rev. Mol. Cell Biol. 21, 7-24. 10.1038/s41580-019-0180-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, J. and Luo, Z. Q. (2017). Hijacking of the Host Ubiquitin Network by Legionella pneumophila. Front. Cell Infect. Microbiol. 7, 487. 10.3389/fcimb.2017.00487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, J., Sheedlo, M. J., Yu, K., Tan, Y., Nakayasu, E. S., Das, C., Liu, X. and Luo, Z. Q. (2016). Ubiquitination independent of E1 and E2 enzymes by bacterial effectors. Nature 533, 120-124. 10.1038/nature17657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, J., Yu, K., Fei, X., Liu, Y., Nakayasu, E. S., Piehowski, P. D., Shaw, J. B., Puvar, K., Das, C., Liu, X.et al. (2017). A unique deubiquitinase that deconjugates phosphoribosyl-linked protein ubiquitination. Cell Res. 27, 865-881. 10.1038/cr.2017.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg, C., Bache, K. G., Gillooly, D. J., Madshus, I. H., Stang, E. and Stenmark, H. (2002). Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 4, 394-398. 10.1038/ncb791 [DOI] [PubMed] [Google Scholar]
- Raiborg, C., Wenzel, E. M., Pedersen, N. M., Olsvik, H., Schink, K. O., Schultz, S. W., Vietri, M., Nisi, V., Bucci, C., Brech, A.et al. (2015). Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature 520, 234-238. 10.1038/nature14359 [DOI] [PubMed] [Google Scholar]
- Raposo, G. and Stoorvogel, W. (2013). Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373-383. 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinicke, A. T., Raczkowski, F., Muhlig, M., Schmucker, P., Lischke, T., Reichelt, J., Schneider, E., Zielinski, S., Sachs, M., Jurack, E.et al. (2019). Deubiquitinating enzyme UCH-L1 promotes dendritic cell antigen cross-presentation by favoring recycling of MHC Class I molecules. J. Immunol. 203, 1730-1742. 10.4049/jimmunol.1801133 [DOI] [PubMed] [Google Scholar]
- Ren, X. and Hurley, J. H. (2010). VHS domains of ESCRT-0 cooperate in high-avidity binding to polyubiquitinated cargo. EMBO J. 29, 1045-1054. 10.1038/emboj.2010.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, C., West, M. and Odorizzi, G. (2007). Dual mechanisms specify Doa4-mediated deubiquitination at multivesicular bodies. EMBO J. 26, 2454-2464. 10.1038/sj.emboj.7601692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha, N., Kuijl, C., van der Kant, R., Janssen, L., Houben, D., Janssen, H., Zwart, W. and Neefjes, J. (2009). Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J. Cell Biol. 185, 1209-1225. 10.1083/jcb.200811005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche, P. A. and Furuta, K. (2015). The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 15, 203-216. 10.1038/nri3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row, P. E., Prior, I. A., McCullough, J., Clague, M. J. and Urbe, S. (2006). The ubiquitin isopeptidase UBPY regulates endosomal ubiquitin dynamics and is essential for receptor down-regulation. J. Biol. Chem. 281, 12618-12624. 10.1074/jbc.M512615200 [DOI] [PubMed] [Google Scholar]
- Roxrud, I., Raiborg, C., Pedersen, N. M., Stang, E. and Stenmark, H. (2008). An endosomally localized isoform of Eps15 interacts with Hrs to mediate degradation of epidermal growth factor receptor. J. Cell Biol. 180, 1205-1218. 10.1083/jcb.200708115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, T. A., Phillips, E. O., Collier, C. L., Jb Robinson, A., Routledge, D., Wood, R. E., Assar, E. A. and Tumbarello, D. A. (2020). Tollip coordinates Parkin-dependent trafficking of mitochondrial-derived vesicles. EMBO J. 39, e102539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahtoe, D. D. and Sixma, T. K. (2015). Layers of DUB regulation. Trends Biochem. Sci. 40, 456-467. 10.1016/j.tibs.2015.05.002 [DOI] [PubMed] [Google Scholar]
- Sakai, R., Fukuda, R., Unida, S., Aki, M., Ono, Y., Endo, A., Kusumi, S., Koga, D., Fukushima, T., Komada, M.et al. (2019). The integral function of the endocytic recycling compartment is regulated by RFFL-mediated ubiquitylation of Rab11 effectors. J. Cell Sci. 132, jcs228007. [DOI] [PubMed] [Google Scholar]
- Sapmaz, A., Berlin, I., Bos, E., Wijdeven, R. H., Janssen, H., Konietzny, R., Akkermans, J. J., Erson-Bensan, A. E., Koning, R. I., Kessler, B. M.et al. (2019). USP32 regulates late endosomal transport and recycling through deubiquitylation of Rab7. Nat. Commun. 10, 1454. 10.1038/s41467-019-09437-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardana, R., Zhu, L. and Emr, S. D. (2019). Rsp5 Ubiquitin ligase-mediated quality control system clears membrane proteins mistargeted to the vacuole membrane. J. Cell Biol. 218, 234-250. 10.1083/jcb.201806094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, Y., Yoshikawa, A., Yamagata, A., Mimura, H., Yamashita, M., Ookata, K., Nureki, O., Iwai, K., Komada, M. and Fukai, S. (2008). Structural basis for specific cleavage of Lys 63-linked polyubiquitin chains. Nature 455, 358-362. 10.1038/nature07254 [DOI] [PubMed] [Google Scholar]
- Savio, M. G., Wollscheid, N., Cavallaro, E., Algisi, V., Di Fiore, P. P., Sigismund, S., Maspero, E. and Polo, S. (2016). USP9X controls EGFR Fate by deubiquitinating the endocytic adaptor Eps15. Curr. Biol. 26, 173-183. 10.1016/j.cub.2015.11.050 [DOI] [PubMed] [Google Scholar]
- Savocco, J., Nootens, S., Afokpa, W., Bausart, M., Chen, X., Villers, J., Renard, H. F., Prévost, M., Wattiez, R. and Morsomme, P. (2019). Yeast α-arrestin Art2 is the key regulator of ubiquitylation-dependent endocytosis of plasma membrane vitamin B1 transporters. PLoS Biol. 17, e3000512. 10.1371/journal.pbio.3000512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schink, K. O., Tan, K. W. and Stenmark, H. (2016). Phosphoinositides in control of membrane dynamics. Annu. Rev. Cell Dev. Biol. 32, 143-171. 10.1146/annurev-cellbio-111315-125349 [DOI] [PubMed] [Google Scholar]
- Scorrano, L., De Matteis, M. A., Emr, S., Giordano, F., Hajnoczky, G., Kornmann, B., Lackner, L. L., Levine, T. P., Pellegrini, L., Reinisch, K.et al. (2019). Coming together to define membrane contact sites. Nat. Commun. 10, 1287. 10.1038/s41467-019-09253-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, C. C., Vacca, F. and Gruenberg, J. (2014). Endosome maturation, transport and functions. Semin. Cell Dev. Biol. 31, 2-10. 10.1016/j.semcdb.2014.03.034 [DOI] [PubMed] [Google Scholar]
- Seaman, M. N. (2012). The retromer complex - endosomal protein recycling and beyond. J. Cell Sci. 125, 4693-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segala, G., Bennesch, M. A., Ghahhari, N. M., Pandey, D. P., Echeverria, P. C., Karch, F., Maeda, R. K. and Picard, D. (2019). Vps11 and Vps18 of Vps-C membrane traffic complexes are E3 ubiquitin ligases and fine-tune signalling. Nat. Commun. 10, 1833. 10.1038/s41467-019-09800-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, A., Hsieh, W. C., Hanna, C. B., Hsu, C. C., Pearson, M., II, Tao, W. A. and Aguilar, R. C. (2020). The Na(+) pump Ena1 is a yeast epsin-specific cargo requiring its ubiquitylation and phosphorylation sites for internalization. J. Cell Sci. 133, jcs245415. [DOI] [PubMed] [Google Scholar]
- Shafaq-Zadah, M., Dransart, E. and Johannes, L. (2020). Clathrin-independent endocytosis, retrograde trafficking, and cell polarity. Curr. Opin. Cell Biol. 65, 112-121. 10.1016/j.ceb.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields, S. B., Oestreich, A. J., Winistorfer, S., Nguyen, D., Payne, J. A., Katzmann, D. J. and Piper, R. (2009). ESCRT ubiquitin-binding domains function cooperatively during MVB cargo sorting. J. Cell Biol. 185, 213-224. 10.1083/jcb.200811130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih, S. C., Sloper-Mould, K. E. and Hicke, L. (2000). Monoubiquitin carries a novel internalization signal that is appended to activated receptors. EMBO J. 19, 187-198. 10.1093/emboj/19.2.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih, S. C., Katzmann, D. J., Schnell, J. D., Sutanto, M., Emr, S. D. and Hicke, L. (2002). Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat. Cell Biol. 4, 389-393. 10.1038/ncb790 [DOI] [PubMed] [Google Scholar]
- Shin, D., Na, W., Lee, J. H., Kim, G., Baek, J., Park, S. H., Choi, C. Y. and Lee, S. (2017). Site-specific monoubiquitination downregulates Rab5 by disrupting effector binding and guanine nucleotide conversion. Elife 6, e29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde, S. R. and Maddika, S. (2018). Post translational modifications of Rab GTPases. Small GTPases 9, 49-56. 10.1080/21541248.2017.1299270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvarev, D., Schoppe, J., König, C., Perz, A., Füllbrunn, N., Kiontke, N., Langemeyer, L., Januliene, D., Schnelle, K., Kümmel, D.et al. (2022). Structure of the lysosomal membrane fusion machinery. Elife 44, e80901. 10.7554/eLife.80901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicari, D., Weber, J., Maspero, E. and Polo, S. (2022). The NEDD4 ubiquitin E3 ligase: a snapshot view of its functional activity and regulation. Biochem. Soc. Trans. 50, 473-485. 10.1042/BST20210731 [DOI] [PubMed] [Google Scholar]
- Sierra, M. I., Wright, M. H. and Nash, P. D. (2010). AMSH interacts with ESCRT-0 to regulate the stability and trafficking of CXCR4. J. Biol. Chem. 285, 13990-14004. 10.1074/jbc.M109.061309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund, S., Algisi, V., Nappo, G., Conte, A., Pascolutti, R., Cuomo, A., Bonaldi, T., Argenzio, E., Verhoef, L. G., Maspero, E.et al. (2013). Threshold-controlled ubiquitination of the EGFR directs receptor fate. EMBO J. 32, 2140-2157. 10.1038/emboj.2013.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund, S., Woelk, T., Puri, C., Maspero, E., Tacchetti, C., Transidico, P., Di Fiore, P. P. and Polo, S. (2005). Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA 102, 2760-2765. 10.1073/pnas.0409817102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti, B. and Cullen, P. J. (2019). Actin-dependent endosomal receptor recycling. Curr. Opin. Cell Biol. 56, 22-33. 10.1016/j.ceb.2018.08.006 [DOI] [PubMed] [Google Scholar]
- Smith, A. C., Heo, W. D., Braun, V., Jiang, X., Macrae, C., Casanova, J. E., Scidmore, M. A., Grinstein, S., Meyer, T. and Brumell, J. H. (2007). A network of Rab GTPases controls phagosome maturation and is modulated by Salmonella enterica serovar Typhimurium. J. Cell Biol. 176, 263-268. 10.1083/jcb.200611056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, P., Trajkovic, K., Tsunemi, T. and Krainc, D. (2016). Parkin modulates endosomal organization and function of the endo-lysosomal pathway. J. Neurosci. 36, 2425-2437. 10.1523/JNEUROSCI.2569-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, L., Tang, S., Han, X., Jiang, Z., Dong, L., Liu, C., Liang, X., Dong, J., Qiu, C., Wang, Y.et al. (2019). KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat. Commun. 10, 1639. 10.1038/s41467-019-09720-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang, A. (2018). The endoplasmic reticulum-the caring mother of the cell. Curr. Opin. Cell Biol. 53, 92-96. 10.1016/j.ceb.2018.06.004 [DOI] [PubMed] [Google Scholar]
- Stangl, A., Elliott, P. R., Pinto-Fernandez, A., Bonham, S., Harrison, L., Schaub, A., Kutzner, K., Keusekotten, K., Pfluger, P. T., El Oualid, F.et al. (2019). Regulation of the endosomal SNX27-retromer by OTULIN. Nat. Commun. 10, 4320. 10.1038/s41467-019-12309-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenin, V. and Neefjes, J. (2022). Control of host PTMs by intracellular bacteria: an opportunity toward novel anti-infective agents. Cell Chem. Biol. 29, 741-756. 10.1016/j.chembiol.2022.04.004 [DOI] [PubMed] [Google Scholar]
- Stewart, M. D., Ritterhoff, T., Klevit, R. E. and Brzovic, P. S. (2016). E2 enzymes: more than just middle men. Cell Res. 26, 423-440. 10.1038/cr.2016.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striz, A. C. and Tuma, P. L. (2016). The GTP-bound and Sumoylated Form of the rab17 small molecular weight GTPase selectively binds Syntaxin 2 in polarized hepatic WIF-B cells. J. Biol. Chem. 291, 9721-9732. 10.1074/jbc.M116.723353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, S., Zhang, Y. and Liu, P. (2020). Roles of ubiquitination and SUMOylation in DNA damage response. Curr. Issues Mol. Biol. 35, 59-84. 10.21775/cimb.035.059 [DOI] [PubMed] [Google Scholar]
- Swatek, K. N. and Komander, D. (2016). Ubiquitin modifications. Cell Res. 26, 399-422. 10.1038/cr.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul, E., Chen, P. W., Casanova, J. E., Cherfils, J., Dacks, J. B., Lambright, D. G., Lee, F. S., Randazzo, P. A., Santy, L. C., Schurmann, A.et al. (2019). ARF GTPases and their GEFs and GAPs: concepts and challenges. Mol. Biol. Cell 30, 1249-1271. 10.1091/mbc.E18-12-0820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, T., Minami, S., Tsuchiya, Y., Tajima, K., Sakai, N., Suga, K., Hisanaga, S. I., Ohbayashi, N., Fukuda, M. and Kawahara, H. (2019). Cytoplasmic control of Rab family small GTPases through BAG6. EMBO Rep. 20, e46794. 10.15252/embr.201846794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, S., Buchkovich, N. J., Henne, W. M., Banjade, S., Kim, Y. J. and Emr, S. D. (2016). ESCRT-III activation by parallel action of ESCRT-I/II and ESCRT-0/Bro1 during MVB biogenesis. Elife 5, e15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thottacherry, J. J., Sathe, M., Prabhakara, C. and Mayor, S. (2019). Spoiled for choice: diverse endocytic pathways function at the cell surface. Annu. Rev. Cell Dev. Biol. 35, 55-84. 10.1146/annurev-cellbio-100617-062710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma-Fukai, S. and Shimizu, T. (2021). Structural diversity of ubiquitin E3 ligase. Molecules 26, 6682. 10.3390/molecules26216682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi-Giesgen, I., Behrends, C. and Alpi, A. F. (2021). The ubiquitin ligation machinery in the defense against bacterial pathogens. EMBO Rep. 22, e52864. 10.15252/embr.202152864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue, E. R., Turk, V. and Neefjes, J. (2016). Variations in MHC Class II antigen processing and presentation in health and disease. Annu. Rev. Immunol. 34, 265-297. 10.1146/annurev-immunol-041015-055420 [DOI] [PubMed] [Google Scholar]
- van der Beek, J., Jonker, C., van der Welle, R., Liv, N. and Klumperman, J. (2019). CORVET, CHEVI and HOPS - multisubunit tethers of the endo-lysosomal system in health and disease. J. Cell Sci. 132, jcs189134. 10.1242/jcs.189134 [DOI] [PubMed] [Google Scholar]
- Varshavsky, A. (2012). The ubiquitin system, an immense realm. Annu. Rev. Biochem. 81, 167-176. 10.1146/annurev-biochem-051910-094049 [DOI] [PubMed] [Google Scholar]
- Verma, S., Mohapatra, G., Ahmad, S. M., Rana, S., Jain, S., Khalsa, J. K. and Srikanth, C. V. (2015). Salmonella engages host microRNAs to modulate SUMOylation: a new arsenal for intracellular survival. Mol. Cell. Biol. 35, 2932-2946. 10.1128/MCB.00397-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vietri, M., Radulovic, M. and Stenmark, H. (2020). The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 21, 25-42. 10.1038/s41580-019-0177-4 [DOI] [PubMed] [Google Scholar]
- Villarroel-Campos, D., Henríquez, D. R., Bodaleo, F. J., Oguchi, M. E., Bronfman, F. C., Fukuda, M. and Gonzalez-Billault, C. (2016). Rab35 functions in axon elongation are regulated by P53-related protein kinase in a mechanism that involves Rab35 protein degradation and the microtubule-associated protein 1B. J. Neurosci. 36, 7298-7313. 10.1523/JNEUROSCI.4064-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri, C., Baixauli, F., Mittelbrunn, M., Fernandez-Delgado, I., Torralba, D., Moreno-Gonzalo, O., Baldanta, S., Enrich, C., Guerra, S. and Sanchez-Madrid, F. (2016). ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat. Commun. 7, 13588. 10.1038/ncomms13588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walpole, G. F. W., Pacheco, J., Chauhan, N., Clark, J., Anderson, K. E., Abbas, Y. M., Brabant-Kirwan, D., Montano-Rendon, F., Liu, Z., Zhu, H.et al. (2022). Kinase-independent synthesis of 3-phosphorylated phosphoinositides by a phosphotransferase. Nat. Cell Biol. 24, 708-722. 10.1038/s41556-022-00895-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, S. C., Reitano, J. R., Dickinson, M. S., Kutsch, M., Hernandez, D., Barnes, A. B., Schott, B. H., Wang, L., Ko, D. C., Kim, S. Y.et al. (2022). The bacterial effector GarD shields Chlamydia trachomatis inclusions from RNF213-mediated ubiquitylation and destruction. Cell Host Microbe 30, 1671-1684.e1679. 10.1016/j.chom.2022.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, M., Sulpizio, A. G., Akturk, A., Beck, W. H. J., Lanz, M., Faca, V. M., Smolka, M. B., Vogel, J. P. and Mao, Y. (2019). Deubiquitination of phosphoribosyl-ubiquitin conjugates by phosphodiesterase-domain-containing Legionella effectors. Proc. Natl. Acad. Sci. USA 116, 23518-23526. 10.1073/pnas.1916287116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger-Ness, A. and Zerial, M. (2014). Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol 6, a022616. 10.1101/cshperspect.a022616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Lo, W. T. and Haucke, V. (2019). Phosphoinositide switches in endocytosis and in the endolysosomal system. Curr. Opin. Cell Biol. 59, 50-57. 10.1016/j.ceb.2019.03.011 [DOI] [PubMed] [Google Scholar]
- Wang, J. Q., Lin, Z. C., Li, L. L., Zhang, S. F., Li, W. H., Liu, W., Song, B. L. and Luo, J. (2020). SUMOylation of the ubiquitin ligase IDOL decreases LDL receptor levels and is reversed by SENP1. J. Biol. Chem. 296, 100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzycka, D., Sadlak, J., Maciaszczyk-Dziubinska, E. and Wysocki, R. (2019). Rsp5-dependent endocytosis and degradation of the arsenite transporter Acr3 requires its N-terminal acidic tail as an endocytic sorting signal and arrestin-related ubiquitin-ligase adaptors. Biochim. Biophys. Acta Biomembr. 1861, 916-925. 10.1016/j.bbamem.2019.02.004 [DOI] [PubMed] [Google Scholar]
- Weeratunga, S., Paul, B. and Collins, B. M. (2020). Recognising the signals for endosomal trafficking. Curr. Opin. Cell Biol. 65, 17-27. 10.1016/j.ceb.2020.02.005 [DOI] [PubMed] [Google Scholar]
- Weinberg, J. S. and Drubin, D. G. (2014). Regulation of clathrin-mediated endocytosis by dynamic ubiquitination and deubiquitination. Curr. Biol. 24, 951-959. 10.1016/j.cub.2014.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijdeven, R. H., Janssen, H., Nahidiazar, L., Janssen, L., Jalink, K., Berlin, I. and Neefjes, J. (2016). Cholesterol and ORP1L-mediated ER contact sites control autophagosome transport and fusion with the endocytic pathway. Nat. Commun. 7, 11808. 10.1038/ncomms11808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, E. T., Chen, X. and Moore, D. J. (2017). VPS35, the retromer complex and Parkinson's disease. J. Parkinsons Dis. 7, 219-233. 10.3233/JPD-161020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, E. T., Glauser, L., Tsika, E., Jiang, H., Islam, S. and Moore, D. J. (2018). Parkin mediates the ubiquitination of VPS35 and modulates retromer-dependent endosomal sorting. Hum. Mol. Genet. 27, 3189-3205. 10.1093/hmg/ddy224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, F. N., Zeng, B., Liu, H. S., Qi, H., Xie, L. J., Yu, L. J., Chen, Q. F., Li, J. F., Chen, Y. Q., Jiang, L.et al. (2020). SINAT E3 ubiquitin ligases mediate FREE1 and VPS23A degradation to modulate abscisic acid signaling. Plant Cell 32, 3290-3310. 10.1105/tpc.20.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki, Y., Schonherr, C., Varshney, G. K., Dogru, M., Hallberg, B. and Palmer, R. H. (2013). Goliath family E3 ligases regulate the recycling endosome pathway via VAMP3 ubiquitylation. EMBO J. 32, 524-537. 10.1038/emboj.2013.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau, R. and Rape, M. (2016). The increasing complexity of the ubiquitin code. Nat. Cell Biol. 18, 579-586. 10.1038/ncb3358 [DOI] [PubMed] [Google Scholar]
- Yogosawa, S., Hatakeyama, S., Nakayama, K. I., Miyoshi, H., Kohsaka, S. and Akazawa, C. (2005). Ubiquitylation and degradation of serum-inducible kinase by hVPS18, a RING-H2 type ubiquitin ligase. J. Biol. Chem. 280, 41619-41627. 10.1074/jbc.M508397200 [DOI] [PubMed] [Google Scholar]
- Yoon, T. Y. and Munson, M. (2018). SNARE complex assembly and disassembly. Curr. Biol. 28, R397-R401. 10.1016/j.cub.2018.01.005 [DOI] [PubMed] [Google Scholar]
- Zhang, C., Lai, M. B., Khandan, L., Lee, L. A., Chen, Z. and Junge, H. J. (2017). Norrin-induced Frizzled4 endocytosis and endo-lysosomal trafficking control retinal angiogenesis and barrier function. Nat. Commun. 8, 16050. 10.1038/ncomms16050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. G., Liu, N., Miao, G., Chen, Y., Zhao, H. and Zhang, H. (2018). The ER contact proteins VAPA/B interact with multiple autophagy proteins to modulate autophagosome biogenesis. Curr. Biol. 28, 1234-1245.e1234. 10.1016/j.cub.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Zheng, N. and Shabek, N. (2017). Ubiquitin Ligases: Structure, Function, and Regulation. Annu. Rev. Biochem. 86, 129-157. 10.1146/annurev-biochem-060815-014922 [DOI] [PubMed] [Google Scholar]
- Zheng, Y., Liu, Q., Wu, Y., Ma, L., Zhang, Z., Liu, T., Jin, S., She, Y., Li, Y. P. and Cui, J. (2018). Zika virus elicits inflammation to evade antiviral response by cleaving cGAS via NS1-caspase-1 axis. EMBO J. 37, e99347. 10.15252/embj.201899347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. and Zhu, Y. (2015). Diversity of bacterial manipulation of the host ubiquitin pathways. Cell. Microbiol. 17, 26-34. 10.1111/cmi.12384 [DOI] [PubMed] [Google Scholar]