Abstract
Background.
This study compares the pathologic condition of delayed xenograft rejection in Gal-positive and Gal-knockout cardiac xenografts after pig-to-baboon heterotopic cardiac xenotransplantation when the induced anti-Gal antibody response is unregulated, blocked, or absent.
Methods.
Baboon recipients of Gal-positive, CD46 pig hearts were treated with an αGal polymer (group 1; n=11) or Gal-specific immunoapheresis (group 2; n=8) to block anti-Gal antibody. Gal-knockout cardiac xenografts recipients (group 3; n=5) received no anti-Gal therapy. Perioperative and interim biopsies were examined and antibody responses were determined.
Results.
No hyperacute rejection was seen and histologic findings were similar across the groups. All groups showed vascular antibody deposition in perioperative and interim biopsies and in explant samples. A prominent antibody response was detected only in group 2. Complement activation was evident by C3d deposition but deposition of C5b and C5b-9 was limited. Earliest evidence of myocardial injury was myocyte vacuolization in the absence of microvascular thrombosis or coagulative necrosis that developed later. Histology of explanted hearts exhibited mainly microvascular thrombosis and coagulative necrosis with little evidence of interstitial hemorrhage or edema.
Conclusions.
The histology of rejection seemed independent of the anti-Gal or non-Gal immune response. Myocyte vacuolization seems to be an early feature of delayed xenograft rejection presaging more classic pathologic features.
Keywords: Cardiac, Transplant, Antibody, Xenotransplant, Rejection
Pig-to-primate cardiac xenograft rejection is dominated by antibody-mediated processes (1). Early primate xenografts were limited by hyperacute rejection (HAR) caused by preformed anti-Gal antibody (AGAB) and complement activation, and characterized by edema, thrombosis, and hemorrhage with widespread vascular deposition of antibody and terminal complement products (2, 3). Multiple approaches were developed to block HAR including complement inhibition, antibody removal, and transgenic donors expressing high levels of human complement regulatory proteins (4–7).
When HAR of wild-type (GT+) donor organs is blocked, delayed xenograft rejection (DXR) is coincident with the induction of AGAB and characterized by venous thrombosis, congestion, edema, and interstitial hemorrhage (8). This rejection is believed to result from a combination of direct antibody-mediated damage and antibody-induced endothelial cell (EC) activation (1). Additionally, molecular incompatibilities involving porcine von Willebrand factor, tissue factor pathway inhibitor, and thrombomodulin may enhance the thrombogenic potential of the xenograft and thereby contribute to DXR (9–11).
Control of the induced AGAB response was achieved by infusion of αGal polymers (12, 13) or made irrelevant by using Gal-deficient (GTKO) donors (14, 15). These transplants show no sensitization to αGal and under appropriate immunosuppression exhibit extended survival (16–19). Rejection of GTKO hearts was accompanied by vascular antibody and sometimes vascular complement deposition with variable detection of induced non-Gal antibody in serum (17, 20, 21). Because of the widespread expression of the αGal antigen and the unknown specificity of non-Gal antibody, elimination of the immunologic impact of AGAB may affect the mechanism and histology of xenograft rejection. Herein, we compare the histology of perioperative, interim, and terminal explanted tissue samples for cardiac xenografts performed under conditions at which preformed AGAB was initially blocked but the induction of AGAB proceeded largely unabated, was controlled by an αGal polymer, or did not occur due to the use of GTKO donor organs.
RESULTS
Transplant Outcomes and Antibody Response
Median implantation time was 41, 13, and 26 days for groups 1 to 3, respectively (Table 1). The frequency of rejection ranged from 40% in group 3 to 73% in group 2. Nonrejection-related graft loss was due to bacterial or viral sepsis (n=5), hemorrhage (n=2), pulmonary embolism, and renal failure (n=1 each). There was no HAR, and xenograft contractility at the time of transplant was excellent in all groups. Palpation scores indicate that maximal graft contractility (palpation score=6) was maintained for a median duration of 23, 9, and 11 days for groups 1 to 3, respectively (Table 1). All group 1 recipients treated with a branched polyethylene glycol polymeric conjugate of α-Gal trisaccharide (TPC), and group 2 recipients treated with Gal-immunoaffinity (IA) showed reduced circulating AGAB levels before transplant (Fig. 1A, B). No evidence of an induced AGAB response was evident in postexplant serum of group 1 (n=5) recipients who survived after xenograft rejection and explant (Fig. 1A). In group 2 (n=4), all recipients who survived after xenograft explant showed an induced AGAB response that ranged from a 1.2- to 7.0-fold apparent increase of anti-Gal IgM to a 1.04- to 189-fold increase in anti-Gal IgG within 10 days of organ explant (Fig. 1B). None of the GTKO recipients survived beyond organ explant, but there was no evidence of posttransplant absorption or induction of AGAB in any of the recipients (data not shown). An induction of non-Gal antibody (defined as 2-fold or greater response above pretransplant levels) was not evident in any of the group 3 recipients before organ explants (Fig. 1C). One group 1 recipient, with organ rejection on postoperative day (POD) 20, showed an induction of non-Gal IgM. Postexplant sera from three group 2 recipients showed an induced non-Gal antibody response and were the same recipients that showed an induced AGAB response.
TABLE 1.
Summary of transplant results
| Group | Na | Rejectedb | ISc | anti-Gal controld | Survival (contractility)e (d) | Rangef (d) | Biopsiesg (d) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 11 | 8 (73%) | A | TPC | 41 (23) | 15–109 | 0, 14, 28, and 56 |
| 2 | 8 | 5 (62%) | B | Gal-IA | 13 (9) | 8–42 | 0, 7, 14, 21, and 28 |
| 3 | 5 | 2 (40%) | A | GTKO | 26 (11) | 18–71 | 0, 14, 21, and 42 |
Number of transplant recipients.
The number (%) of xenograft rejections as indicated in Materials and Methods section.
Type of maintenance immunosuppression.
Methods used for controlling anti-Gal antibody.
The median graft survival in days. The number in parenthesis is the median duration of maximal xenograft contractility in days.
The range of graft survival in days.
Scheduled postoperative biopsy days.
A, tacrolimus and sirolimus; B, cyclophosphamide and cyclosporine; TPC, in vivo infusion of an αGal polymer; Gal-IA, extracorporeal αGal specific immunoapheresis; GTKO, use of Gal-deficient donor pigs.
FIGURE 1.
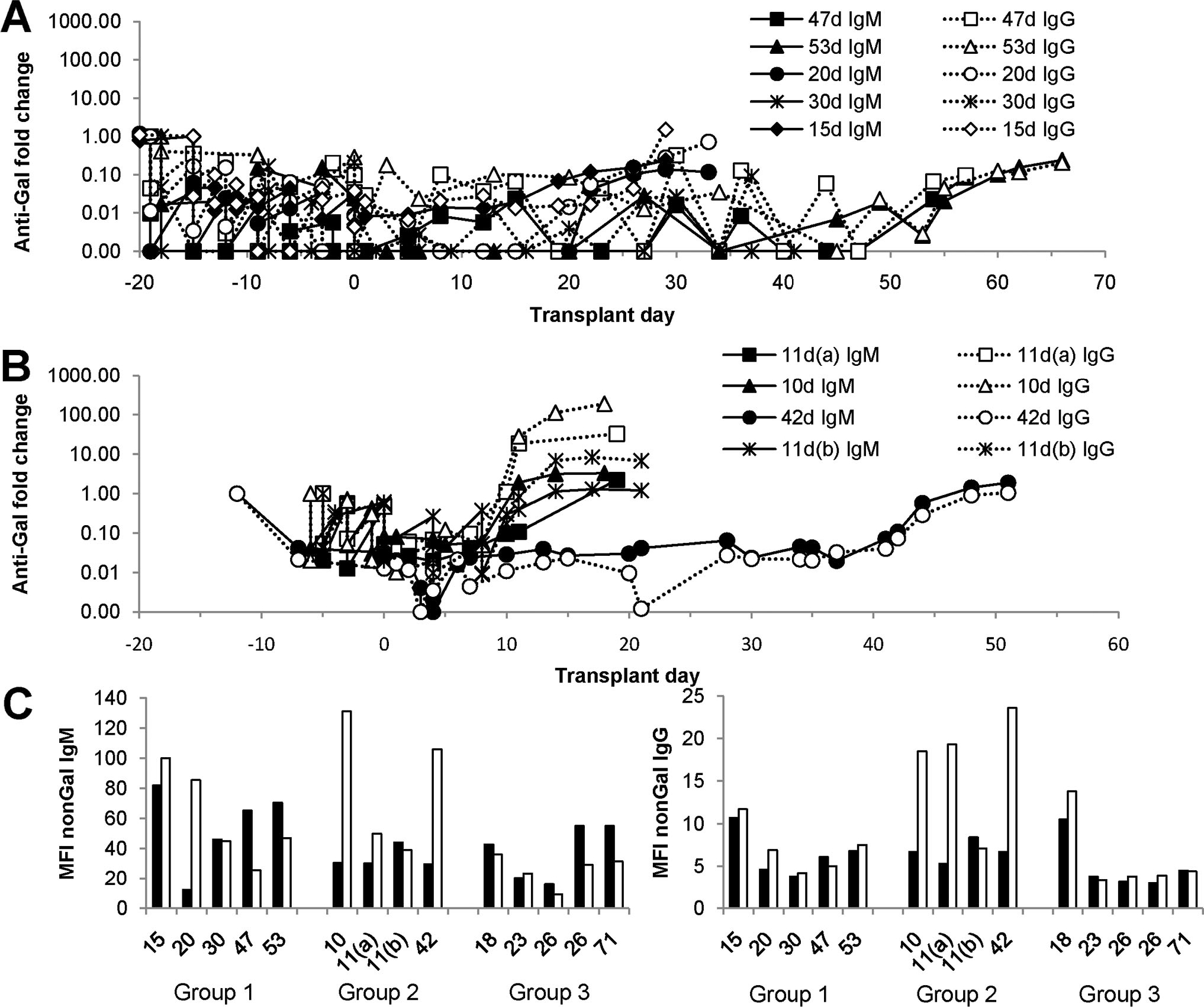
Antibody response. The graphs depict the variation in anti-Gal (A and B) and non-Gal (C) antibody after cardiac xenotransplantation. Anti-Gal IgM and IgG were measured by enzyme-linked immunosorbent assay as previously described (38) and is depicted as the fold change compared with pretransplant serum. Non-Gal antibody was determined using flow cytometry as indicated in the Materials and Methods. (A) Group 1 anti-Gal IgM and IgG. (B) Group 2 anti-Gal IgM and IgG. A and B show antibody responses in all recipients who survived xenograft explant. The day of graft rejection for each recipient is indicated in the figure. (C) Non-Gal immune responses in groups 1–3. The graph compares non-Gal IgM (left) and IgG (right) levels in pretransplant serum (filled bars) with postexplant or necropsy serum (open bars). The numbers below the bar graphs indicate the duration of the transplant for each study group (groups 1, 2, and 3) and correspond to the values in A and B for groups 1 and 2. No group 3 recipient survived explant, so the data compare non-Gal antibody levels in pretransplant and necropsy serum.
Histology of 30-Min Biopsies
There was minimal postoperative injury as 90% of evaluable 30-min biopsies (n=22) were normal with only two biopsies exhibiting a mild level of myocyte vacuolization (MV) (Fig. 2A, C). Despite a reduction in the level of AGAB before transplant, or the use of GTKO xenografts, 17 of 22 biopsies showed vascular deposition of IgM (Fig. 2B, D) and variable IgG deposition (data not shown). Biopsies obtained at 30 min showed no C5b or C5b-9 deposition (data not shown).
FIGURE 2.
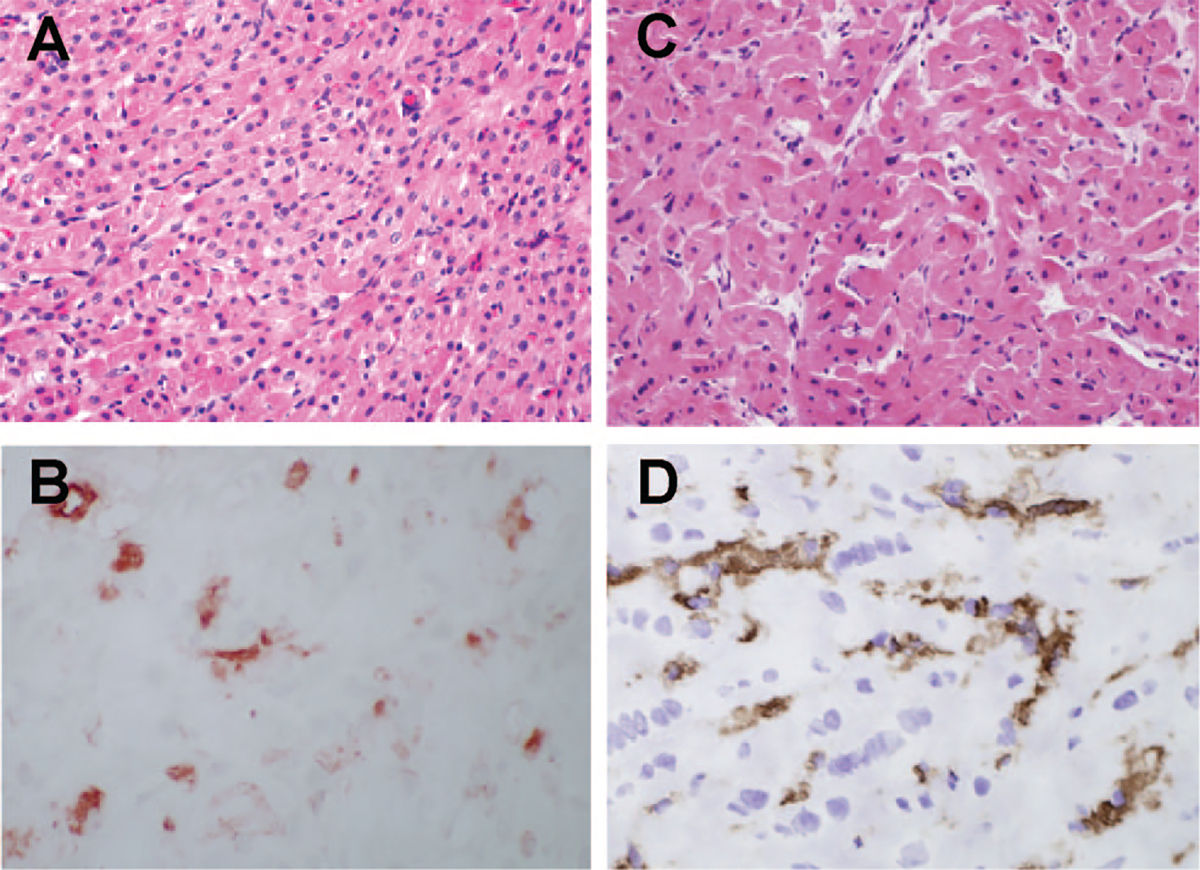
Xenograft histology in 30-min biopsies. (A, B) GT+ cardiac xenograft biopsy; (C, D) GTKO biopsy; (A, C) hematoxylin-eosin stain; (B, D) IgM staining. The IgM staining in B and D was scored diffuse 2. Parallel staining for C5d was negative (data not shown).
Histology of Interim Biopsies
We analyzed 17 interim cardiac xenograft biopsies from group 1, 15 from group 2, and 6 from group 3. Biopsies were obtained as early as POD 7 and as late as POD 47. Biopsies were staged as described by Shimizu et al. (22) into three stages; stage 1 (n=12) corresponding to biopsies obtained from hearts with vigorous contraction and minimal serum troponin; stage 2 (n=16), biopsies from hearts with reduced contractility and increased serum troponin; and stage 3 (n=10), biopsies from hearts with minimal contractility and high serum troponin. There was no significant difference in the day of biopsy for each of these stages (Fig. 3A); however, progressing from stage 1 to 3 was representative of the relative transplant time as evidenced by a significant stage-specific decrease in the average number of days to explant and a significant increase in the average proportion of transplant duration (Fig. 3A). Stage 1 biopsies showed a preponderance of MV with minimal microvascular thrombosis (MT) and no evidence of coagulative necrosis (CN), congestion, or hemorrhage (Figs. 3B and 4A). Stage 2 biopsies showed continued evidence of MV and exhibited a trend toward increased MT (Figs. 3B and 4B). High levels of MT and CN were the dominant characteristic of stage 3 biopsies (Figs. 3B and 4C). The progression of early MV to MT and eventually CN was observed in all study groups (Fig. 3C). Nearly all biopsies exhibited vascular IgM deposition (Figs. 3C and 4D, E). Biopsies stained for C3d (n=15) exhibited strong focal to diffuse vascular staining at all stages, consistent with local activation of the complement pathway, however, positive staining of C5b or C5b-9 was limited (Fig. 3D).
FIGURE 3.
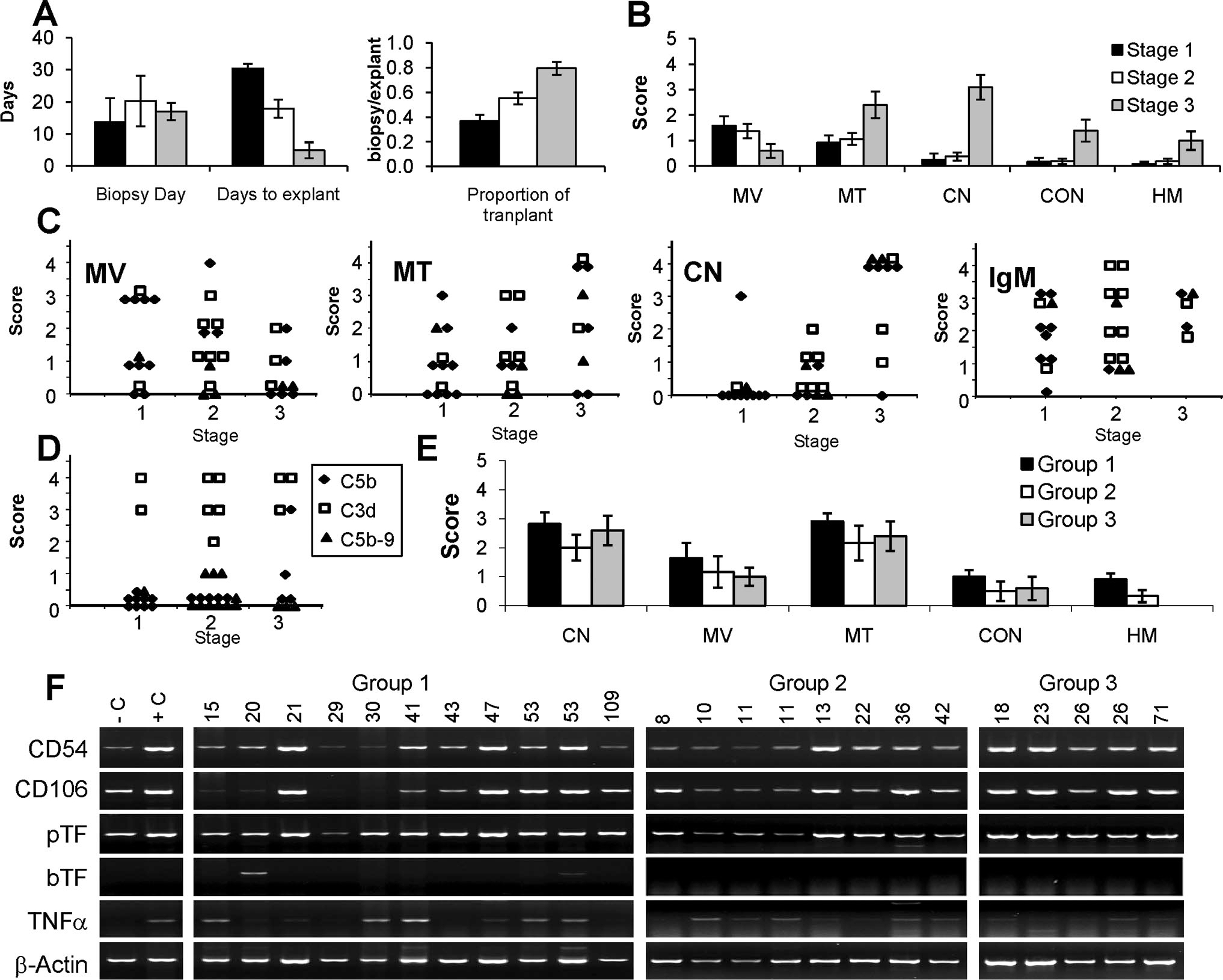
Staging and histologic features of interim biopsies and explanted left ventricle cross-sections. (A) Biopsies were staged based on levels of cardiac contraction and serum troponin (22). Stages 1 (black filled bars), 2 (white filled bars), and 3 (gray filled bars) were independent of the average biopsy day but were inversely related to the days to explants (explants day–biopsy day) and directly related to the proportion of transplant (biopsy day/explants day). (B) Average stage-dependent histologic features of interim biopsies. Each bar is the mean histologic score. (C) Stage-specific changes in interim biopsy histology for transplant group 1 (filled diamond), 2 (open square), and 3 (filled triangle). The heading for each panel (MV, MT, and CN) is the same as in (B). The IgM heading denotes vascular IgM staining. (D) Stage-specific vascular deposition of C3d, C5b, and C5b-9. (E) Average histology scores in each transplant group for explanted left ventricle cross-sections. Abbreviations are the same as in B and F. Semiquantitative reverse-transcriptase polymerase chain reaction of intragraft expression for porcine intracellular adhesion molecule CD54, vascular cellular adhesion molecule CD106, porcine tissue factor (pTF) and baboon tissue factor (bTF), and tumor necrosis factor (TNF)-α. Negative control (C) is a normal pig heart. Positive control sample (C) is an explanted pig heart after pig-to-primate heterotopic xenotransplantation performed without immunosuppression. The transplant group and survival times are indicated above each lane. In A, B, and E, the error bars are standard error of the mean. MV, myocyte vacuolization; MT, microvascular thrombosis; CN, coagulative necrosis; CON, vascular congestion; HM, hemorrhage.
FIGURE 4.
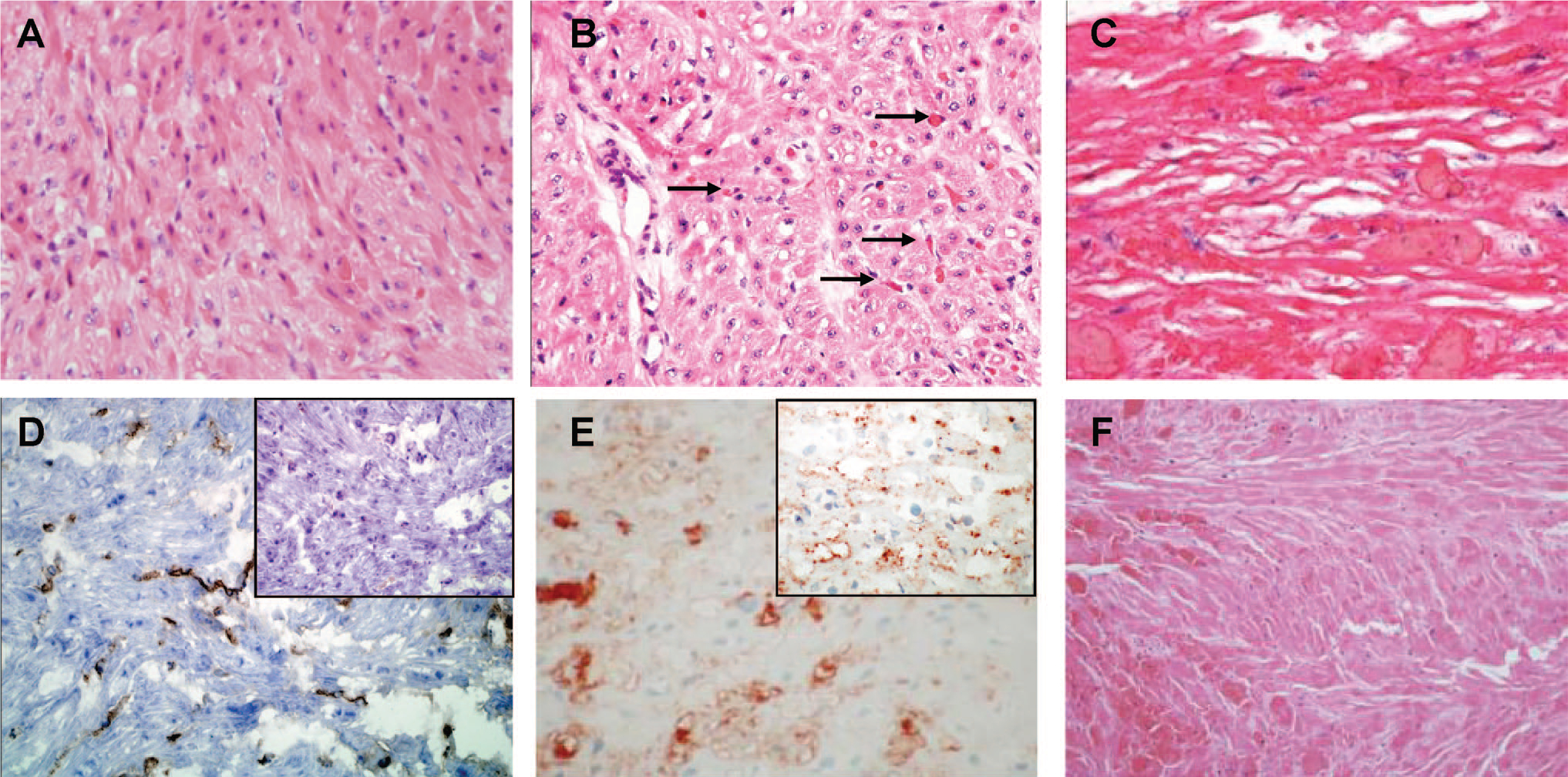
Histology of interim biopsies and explanted tissue. (A) Hematoxylin-eosin (H&E) staining of stage 1 biopsy from a group 1 recipient. High levels of myocyte vacuolization are present with minimal microvascular thrombosis or coagulative necrosis. (B) H&E stain of stage 2 biopsy from a group 1 transplant showing continued myocyte vacuolization with increased microvascular thrombosis (arrows) and low levels of coagulative necrosis. (C) H&E of stage 3 biopsy from a group 1 transplant showing widespread coagulative necrosis. (D) Vascular IgM deposition in a stage 1 biopsy from group 3. The inset shows negative staining for C5b. (E) Vascular IgM staining in a stage 2 biopsy from group 2. The inset shows positive C3d staining. (E) H&E staining of a group 1 explanted left ventricle rejected on postoperative day 53.
Histology at Explant
The histology at explant showed a predominance of CN and MT, with minimal congestion or interstitial hemorrhage (Figs. 3E and 4F). The major histologic characteristics of the left ventricle (LV) at explant (MV, MT, and CN) were not significantly different between transplant groups. Group 3 grafts exhibited somewhat less MT in the right ventricle (RV) cross-sections compared with the other groups, but group 3 also showed the lowest frequency of graft rejection (data not shown). Otherwise RV and LV histology at explant was the same. Cellular rejection was distinctly uncommon and was observed only in four interim biopsies (International Society for Heart and Lung Transplantation 2004 grade 1R [n=2] and grade 2 [n=2]) obtained on POD 8 to 15. Histologic evidence of myocardial calcification was rare; however, gross calcification of the heart at explant was common. An analysis of intragraft gene expression detected increased levels of EC activation markers CD54, CD106, and porcine tissue factor in most explanted hearts. An increased intragraft level of baboon tumor necrosis factor (TNF)-α expression was also detected.
DISCUSSION
In this report, we compared the histopathology of transplants performed under conditions that blocked the initial impact of AGAB for all recipients and either limited (group 1), did not control (group 2) or by use of GTKO donors, eliminated the induction of AGAB. The cytotoxicity of AGAB is due in part to the high abundance of the preformed antibody, its strong induction posttransplant, and the abundance of the Gal antigen on a diverse set of glycolipids and glycoproteins. In contrast, non-Gal antibody and antigens seem to be much less abundant (23) and may be predominantly EC proteins (24), although additional carbohydrate antigens may yet be defined (25). These differences suggested that the elimination of the Gal antigen (group 3) might significantly alter the histopathology or tempo of xenograft rejection. Our primary observation indicates that there is no apparent change in the histopathology of rejection as the histology of graft rejection was similar in all groups (Fig. 3B–E). Early in a transplant when all groups exhibited maximal cardiac contractility (Table 1), the dominant histologic evidence of cardiac injury was extensive vascular antibody deposition and the presence of MV. This was followed by increasing levels of MT and CN, which correlated with a loss of cardiac contractility. The terminal histology at rejection (Fig. 3E) was not affected by the presence or absence of an induced AGAB response and all groups showed strong evidence for widespread EC activation.
Our second major observation was the consistent detection of early MV. MV is a descriptive term used to denote the presence of cytoplasmic clearing. It can be due to an increase in water content or loss of myofilaments and can arise in response to ischemic and nonischemic myocyte injury. MV has been reported previously in the context of xenotransplantation, but generally as a secondary consequence of ischemic injury resulting from MT (26–28). What is different in this study is that we observed high levels of MV consistently preceding MT (Fig. 3B, C). One source of MV is transplant-associated ischemia-reperfusion (I/R) injury. In allotransplantation, perioperative ischemic myocyte injury (PIMI) reported in 89% of allograft patients within 2 weeks of transplant (29, 30) is predominantly manifest as CN followed by a polymorphonuclear infiltrate. The degree of CN is proportional to the duration of cold ischemia. Importantly, MT is not observed in PIMI. Our interim biopsies were obtained between POD 7 and 47 with a median of 14 days. PIMI related CN resultant to I/R injury would likely be evident in this time period yet over half of our interim biopsies were free of CN, and when CN was detected, it was associated with MT and thereby more consistent with the effects of DXR and not PIMI. Although we cannot exclude that some early MV may result from mild I/R, we propose that the consistent development of MV before MT and CN may reflect a nonischemic myocyte response, possibly secondary to protracted antibody-induced EC activation. Consistent with this proposal, we note that MV may result from adrenergic hyperactivity, inflammatory cytokines, disruptions in calcium, or ATP metabolism and oxidative injury (31). Inflammatory cytokines and oxidative injury are known to be produced as a result of antibody-mediated activation of the endothelium and consistent with our detection of increased intragraft expression of porcine CD54, CD106, tissue factor, and recipient TNF-α. Disruption in myocyte metabolism may also occur if the efficiency of lipid transport across the endothelium is altered by vascular antibody deposition (32). Altered mitochondrial metabolism and stress can lead to MV and eventual apoptosis. We also note that apoptosis, which correlates with the severity of cardiac DXR (33), is a more protracted process in myocytes than in other cell types and that increased caspase-3 expression can directly affect a loss of myofibril (34) leading to MV without nuclear fragmentation. Each of these mechanisms could contribute to early nonischemic MV.
The duration of xenograft survival may be affected by the quality of induced antibody (Gal vs. non-Gal) but is also dependent on many factors including the intensity and type of immunosuppression, anticoagulation, recipient background, and donor genetics. The transplant groups in this study showed the expected anti-Gal responses with induction of AGAB in group 2 recipients treated only with antibody removal and minimal induction of AGAB in group 1 treated with TPC (Fig. 1A, B). An induced non-Gal antibody response was also mainly confined to the group 2 recipients. The failure to find an induced non-Gal antibody response in groups 1 and 3 is not unusual (16–19, 21, 35), especially before xenograft explant when the graft may absorb circulating antibody. In this study, the duration of graft survival for group 1 recipients was reduced compared with our optimal studies (18). It is likely that this resulted from a combination of a reduced level of immunosuppression and the invasive nature of scheduled serial biopsies that enhanced the risk of postoperative complications that were common in this study. Similarly, repeated biopsies and a different immunosuppressive regimen, in addition to variation in donor genetics, may have limited graft survival in GTKO recipients (group 3). The reported survival of GTKO organs is variable between different groups with the extent and type of immunosuppression likely being the major determinant of survival (17, 36). Survival in group 2 was comparable or in excess to that reported by other groups (28). The increased antibody response (both anti-Gal and non-Gal) evident in group 2 recipients suggests that immune suppression in group 2 was less effective than the regimen in groups 1 and 3. Group 2 also showed the shortest duration of graft survival (Table 1). Because of variation in immunosuppression regimens, this study cannot determine whether survival of group 2 was limited due to the combined effects of concomitant anti-Gal and anti-nonGal antibody or whether apparent absence of an anti-Gal immune response in groups 1 and 3 enhanced their survival. Given the common histopathology we observe and the abundance of vascular antibody observed in all groups, it seems likely that with the current technologies, the overall effectiveness of immunosuppression and not the specificity of the induced antibody is the major determinant of graft survival.
DXR is believed to result from a combination of antibody-induced EC injury or activation coupled to the prothrombotic potential of the xenograft. Because we detect prolonged periods with maximal cardiac contractility, vascular antibody deposition, complement activation, and likely EC activation without development of MT, and because we consistently observe early MV, we propose that chronic vascular antibody deposition may affect myocyte function. This effect would be particularly important in orthotopic transplantation where cardiac function is needed to maintain the recipient. Further investigation into the potential secondary effects of vascular EC activation on cardiac myocytes and its possible effects on cardiac function and contribution to DXR are warranted.
MATERIALS AND METHODS
Heterotopic Heart Transplantation
All transplants used the pig-to-baboon heterotopic abdominal cardiac xenograft model. Three transplant groups with interim biopsies were studied (Table 1). Group 1 (n=11) received GT+;CD46 hearts (37) and were treated daily with a branched polyethylene glycol polymer of αGal trisaccharide called TPC (50 mg/kg). Details for this group have been previously described (21). Group 2 (n=8) received GT+;CD46 hearts, and AGAB was removed by αGal-specific immunoaffinity (Gal-IA) columns (Nextran, Princeton, NJ) before and at 2- to 3-day intervals posttransplant. Immunosuppression consisted of cyclosporine, targeted to 200 to 350 ng/dL and cyclophosphamide (CyP) (10 mg/kg) beginning on POD −6, −3, and at transplant (POD 0) or at 7.5 mg/kg for days −6 to 2. Later CyP doses were titrated to circulating white blood cell counts (WBC<1; hold, WBC>1.0; CyP 2.5 mg/kg, WBC>3.0; CyP 5 mg/kg, WBC>4; CyP 7.5 mg/kg). Group 3 recipients (n=5) received GTKO hearts with (n=4) or without (n=1) a CD55 transgene. Immunosuppression was similar to group 1 and consisted of ATG and rituximab induction and maintenance immunosuppression with tacrolimus (target levels, 10–20ng/mL) and sirolimus (target levels, 5–15 ng/mL). Xenograft contractility was measured at 2- to 3-day intervals by echocardiography and manual palpation. Palpation score ranged from 0, no contractility, to 6, maximal contractility. At rejection, defined by an absence of contractility, xenografts were explanted and surviving recipients were maintained on immunosuppression for at least 20 days. Treatment with TPC or Gal-IA was discontinued during the postexplant period.
Animals received humane care according to standards established by the Institutional Animal Care and Use Committee of Mayo Clinic and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources; National Institutes of Health publication No. 86–23, revised 1996).
Detection of Gal and Non-Gal Antibody
Serum AGAB was detected using a solid-phase enzyme-linked immunosorbent assay as described previously (38). In brief, enzyme-linked immunosorbent assay plates were coated with a 30μg/mL bovine serum albumin-Gal conjugate (V-Labs, Covington, LA) and reacted with a dilution series of recipient serum. Antibody binding was detected using horseradish perioxidase conjugated anti-human IgM and IgG (Bethyl Labs, Montgomery, TX). Specific anti-Gal reactivity was determined as the difference between total antibody binding to bovine serum albumin-Gal and antibody binding in the presence of 10 mM free α-Gal trisaccharide competitor. Anti-Gal immune responses were expressed as the fold change in reactivity compared with pretransplant levels. Non-Gal antibody was detected from diluted serum (1:10–1:80 dilutions) using flow cytometry to detect serum IgM and IgG binding to GTKO porcine aortic ECs. Antibody binding was detected using goat anti-human IgG-fluorescein isothiocyanate (Zymed Laboratories, San Francisco, CA) or goat anti-human IgM-fluorescein isothiocyanate (Invitrogen, Life Technologies Corp., Carlsbad, CA). A twofold or greater increase in posttransplant antibody binding, compared with pretransplant levels, was considered as an evidence of an induced immune response. All flow cytometry was performed using an FACScalibur cytometer and Cellquest software (BD Pharmingen, San Diego, CA).
Histologic Analysis
Apical LV biopsies were taken 30 min after reperfusion. Interim LV biopsies were obtained at roughly weekly intervals (Table 1). At explant, full-thickness mid-ventricular sections of RV and LV of the explanted heart were collected (n=24). Standard hematoxylin-eosin stained sections were made for all samples. Histologic features (CN, MV, MT, congestion, and interstitial hemorrhage) were blindly graded based on the amount of involved myocardium (grade 0: 0%, 1: 1–25%, 2: 26–50%, 3: 51–75%, and 4: >75%).
Immunohistochemistry (IHC) samples were frozen and sections were stained to detect vascular IgM (A0425, Dako, Carpinteria, CA), C5b (RDI Concord, MA; clone HCC5b.1), C5b-9 (Dako; clone aE11), and C3d (ABR, Golden, CO; clone 053A-1149.3.1.4) using the Envision + dual link horseradish peroxidase polymer (K4061, Dako) and Nova Red alkaline phosphatase for detection of IgM (SK-4800, Vector Lab, Burlingame, CA) or 3.3′–diaminobenzidine (C5b). IHC slides were counterstained with modified Schmidt’s hematoxylin. Staining was assessed for intensity (0, none; 1, minimal; 2, easily recognized, characterized by smooth linear decoration of EC; and 3, intense, granular staining of EC) and the extent of reactivity (focal: <25% of vessels; or diffuse: ≥25% of vessels). For comparisons between biopsies and explant tissues, IHC stains were represented as scores ranging from 0 to 4. Negative IHC stains were scored as 0; diffuse and focal 1 stains, 1; focal 2 stain, 2; diffuse 2 stain, 3; and diffuse and focal 3 stains, 4.
Intragraft Gene Expression
Total RNA was produced from xenograft LV at explant. Semiquantitative analysis of intragraft gene expression was performed by reverse-transcriptase polymerase chain reaction using 0.5 μg RNA and primers for porcine CD54 (forward: GGCTTGGAGGTGCTGAAATCTC, reverse: TGGCTGGCAGGACATAAGTTTG), CD106 (forward: ATACTTTGGATGGTGTTTGCCG, reverse: AACTGGGTCCTTGGGTGAGATG), and tissue factor (forward: GTTTGCGGTCATCGTCTTCG, reverse: AGTCCACCAACTCCCATCTTGC) and for baboon TNF-α (forward: TTAAGCAACAAGACCACCACT, reverse: TCAAGGAAGTCTGGAAACATCT). Gene-specific amplification was performed in a MyCycler Thermal Cycler (Bio-Rad, Hercules, CA) using one-step reverse-transcriptase polymerase chain reaction (USB-Affymetrix, Santa Clara, CA) with reverse transcription performed at 42°C for 30 min. Amplification products were run in a 1.5% agarose gel.
Statistical Analysis
Group comparisons for histopathology, antibody, and complement scores (0–4) were performed using Student’s t test.
ACKNOWLEDGMENTS
The authors acknowledge the contributions of Scott Suddendorf, Paul Henke, Michael Timmons, Merilyn Walters, and Junice Thompson for technical help and Karen Schumacher for writing assistance.
This research was supported by NIH Grant AI66310.
Footnotes
Drs. Byrne and McGregor are inventors of technology related to xenotransplantation that has been licensed by the Mayo Clinic to a commercial entity.
H.D.T. participated in the performance of the research, contributed analytic tools, and participated in data analysis and writing of the article; G.W.B. and C.G.A.M.G. participated in research design, performance of the research, contributed analytic tools, and participated in data analysis and the writing of the article.
REFERENCES
- 1.Platt JL, Lin SS, McGregor CGA. Acute vascular rejection. Xenotransplantation 1998; 5: 169. [DOI] [PubMed] [Google Scholar]
- 2.Platt JL, Fischel RJ, Matas AJ, et al. Immunopathology of hyperacute xenograft rejection in a swine-to-primatemodel. Transplantation 1991; 52: 214. [DOI] [PubMed] [Google Scholar]
- 3.Schuurman H-J, Cheng J, Lam T. Pathology of xenograft rejection: A commentary. Xenotransplantation 2003; 10: 293. [DOI] [PubMed] [Google Scholar]
- 4.Weisman HF, Bartow T, Leppo MK, et al. Soluble human complement receptor type 1: In vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science 1990; 249: 146. [DOI] [PubMed] [Google Scholar]
- 5.Miyagawa S, Hirose H, Shirakura R, et al. The mechanism of discordant xenograft rejection. Transplantation 1988; 46: 825. [DOI] [PubMed] [Google Scholar]
- 6.Kozlowski T, Ierino FL, Lambrigts D, et al. Depletion of anti-Galα1–3Gal antibody in baboons by specific α-Gal immunoaffinity columns. Xenotransplantation 1998; 5: 122. [DOI] [PubMed] [Google Scholar]
- 7.Byrne GW, McCurry KR, Martin MJ, et al. Transgenic pigs expressing human CD59 and decay-accelerating factor produce an intrinsic barrier to complement-mediated damage. Transplantation 1997; 63: 149. [DOI] [PubMed] [Google Scholar]
- 8.Rose AG, Cooper DKC. Venular thrombosis is the key event in the pathogenesis of antibody-mediated cardiac rejection. Xenotransplantation 2000; 7: 31. [DOI] [PubMed] [Google Scholar]
- 9.Kopp CW, Siegel JB, Hancock WW, et al. Effect of porcine endothelial tissue factor pathway inhibitor on human coagulation factors. Transplantation 1997; 63: 749. [DOI] [PubMed] [Google Scholar]
- 10.Siegel JB, Grey ST, Lesnikoski BA, et al. Xenogeneic endothelial cells activate human prothrombin. Transplantation 1997; 64: 888. [DOI] [PubMed] [Google Scholar]
- 11.Mazzucato M, DeMarco L, Pradella P, et al. Porcine von Willebrand factor binding to human platelet GPIb induces transmembrane calcium influx. Thromb Haemost 1996; 75: 655. [PubMed] [Google Scholar]
- 12.Byrne GW, Schwarz A, Fesi JR, et al. Evaluation of different alpha-Galactosyl glycoconjugates for use in xenotransplantation. Bioconjug Chem 2002; 13: 571. [DOI] [PubMed] [Google Scholar]
- 13.Katopodis AG, Warner RG, Duthaler RO, et al. Removal of anti-Galα1,3Gal xenoantibodies with an injectable polymer. J Clin Invest 2002; 110: 1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai L, Kolber-Simonds D, Park K-W, et al. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 2002; 295: 1089. [DOI] [PubMed] [Google Scholar]
- 15.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 2003; 299: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwaki K, Knosalla C, Dor FJ, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant 2004; 4: 363. [DOI] [PubMed] [Google Scholar]
- 17.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: Initial experience. Nat Med 2005; 11: 29. [DOI] [PubMed] [Google Scholar]
- 18.McGregor CG, Davies WR, Oi K, et al. Cardiac xenotransplantation: Recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg 2005; 130: 844. [DOI] [PubMed] [Google Scholar]
- 19.McGregor CG, Teotia SS, Byrne GW, et al. Cardiac xenotransplantation: Progress toward the clinic. Transplantation 2004; 78: 1569. [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med 2005; 11: 1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byrne GW, Schirmer JM, Fass DN, et al. Warfarin or low-molecular-weight heparin therapy does not prolong pig-to-primate cardiac xenograft function. Am J Transplant 2005; 5: 1011. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol 2008; 172: 1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezzelarab M, Hara H, Busch J, et al. Antibodies directed to pig non-Gal antigens in naive and sensitized baboons. Xenotransplantation 2006; 13: 400. [DOI] [PubMed] [Google Scholar]
- 24.Byrne GW, Stalboerger PG, Davila E, et al. Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation. Xenotransplantation 2008; 15: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng YL, Moran K, Dor FJ, et al. Elicited antibodies in baboons exposed to tissues from alpha1,3-galactosyltransferase gene-knockout pigs. Transplantation 2006; 81: 1058. [DOI] [PubMed] [Google Scholar]
- 26.Schmoeckel M, Nollert G, Shahmohammadi M, et al. Prevention of hyperacute rejection by human decay accelerating factor in xenogeneic perfused working hearts. Transplantation 1996; 62: 729. [DOI] [PubMed] [Google Scholar]
- 27.Houser SL, Kuwaki K, Knosalla C, et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation 2004; 11: 416. [DOI] [PubMed] [Google Scholar]
- 28.Lin SS, Weidner BC, Byrne GW, et al. The role of antibodies in acute vascular rejection of pig-to-baboon cardiac transplants. J Clin Invest 1998; 101: 1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Billingham ME.The postsurgical heart. The pathology of cardiac transplantation. Am J Cardiovasc Pathol 1988; 1: 319. [PubMed] [Google Scholar]
- 30.Fyfe B, Loh E, Winters GL, et al. Heart transplantation-associated perioperative ischemic myocardial injury. Morphological features and clinical significance. Circulation 1996; 93: 1133. [DOI] [PubMed] [Google Scholar]
- 31.Turillazzi E, Baroldi G, Silver MD, et al. A systematic study of a myocardial lesion: Colliquative myocytolysis. Int J Cardiol 2005; 104: 152. [DOI] [PubMed] [Google Scholar]
- 32.Koonen DP, Glatz JF, Bonen A, et al. Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle. Biochim Biophys Acta 2005; 1736: 163. [DOI] [PubMed] [Google Scholar]
- 33.Weaver JG, McGregor CG, Tazelaar HD, et al. Rejection severity directly correlates with myocyte apoptosis in pig-to-baboon cardiac xenotransplantation. J Heart Lung Transplant 2005; 24: 841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Communal C, Sumandea M, de Tombe P, et al. Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci USA 2002;99:6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrne GW, Davies WR, Oi K, et al. Increased immunosuppression, not anticoagulation, extends cardiac xenograft survival. Transplantation 2006; 82: 1787. [DOI] [PubMed] [Google Scholar]
- 36.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation 2009; 87: 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diamond LE, Quinn CM, Martin MJ, et al. A human CD46 transgenic pig model system for the study of discordant xenotransplantation. Transplantation 2001; 71: 132. [DOI] [PubMed] [Google Scholar]
- 38.Diamond LE, Byrne GW, Schwarz A, et al. Analysis of the control of the anti-Gal immune response in a non-human primate by galactose alpha-1–3 galactose trisaccharide-polyethylene glycol conjugate. Transplantation 2002; 73: 1780. [DOI] [PubMed] [Google Scholar]


