Abstract
Background.
α1,3-Galactosyltransferase gene knockout (GTKO) pigs reduced the significance of antibody to galactose alpha 1,3-galactose (Gal) antigens but did not eliminate delayed xenograft rejection (DXR). We hypothesize that DXR of GTKO organs results from an antibody response to a limited number of non-Gal endothelial cell (EC) membrane antigens. In this study, we screened a retrovirus expression library to identify EC membrane antigens detected after cardiac xenotransplantation.
Methods.
Expression libraries were made from GT+:CD46 and GTKO porcine aortic ECs. Viral stocks were used to infect human embryonic kidney cells (HEK) that were selected by flow cytometry for IgG binding from sensitized cardiac heterotopic xenograft recipients. After three to seven rounds of selection, individual clones were assessed for non-Gal IgG binding. The porcine complementary DNA was recovered by polymerase chain reaction amplification, sequenced, and identified by homology comparisons.
Results.
A total of 199 and 317 clones were analyzed from GT+:CD46 and GTKO porcine aortic EC complementary DNA libraries, respectively. Sequence analysis identified porcine CD9, CD46, CD59, and the EC protein C receptor. We also identified porcine annexin A2 and a glycosyltransferase with homology to the human β1,4 N-acetylgalactosaminyl transferase 2 gene.
Conclusion.
The identified proteins include key EC functions and suggest that non-Gal antibody responses may compromise EC functions and thereby contribute to DXR. Recovery of the porcine β1,4 N-acetylgalactosaminyl transferase 2 suggests that an antibody response to a SDa-like carbohydrate may represent a new carbohydrate moiety involved in xenotransplantation. The identification of these porcine gene products may lead to further donor modification to enhance resistance to DXR and further reduce the level of xenograft antigenicity.
Keywords: Xenotransplantation, Cardiac, Antigen
Preformed and induced antibody directed toward the vascular endothelium is considered to be the primary immune mechanism that initiates delayed xenograft rejection (DXR). DXR is believed to result from chronic activation or injury to the vascular endothelium mediated by antibody binding, antibody-directed cell cytotoxicity, or complement-mediated damage. These processes promote the formation of a thrombogenic vasculature, which, if unchecked, leads to microvascular thrombosis, ischemic injury, and coagulative necrosis of the myocardium (1). Rejection may be further exacerbated by molecular incompatibilities in thromboregulation, which predispose the xenograft toward thrombosis (2). The preformed and induced anti-pig antibody response was believed to be primarily directed toward the α galactose (Gal) antigen that is expressed in high abundance throughout the porcine vasculature. The development of αGal-specific polymers (3) and pigs deficient in the expression of the αGal antigen (α1,3-galactosyltransferase gene knockout [GTKO]) (4) have marginalized the role for anti-Gal antibody and revealed the potential significance of new preformed and induced non-Gal antibody in DXR (5, 6).
Preformed anti-pig non-Gal antibody is present in variable levels in both humans and nonhuman primates and each exhibits a similar level of complement-dependent cytotoxicity to GTKO peripheral blood mononuclear cells (7). The induced non-Gal antibody specificity is only partially characterized. Initial studies found little evidence of anti-carbohydrate or donor-specific anti-swine leukocyte antigen reactivity but suggested that induced non-Gal antibody was targeted to pan-pig or swine leukocyte antigen conserved protein epitopes (8, 9). A systematic proteomic analysis of non-Gal antibody induced after pig-to-baboon cardiac xenotransplantation demonstrated immunoreactivity to a limited set of immunodominant GTKO porcine aortic endothelial cell (PAEC) membrane antigens (10). The identified target antigens included fibronectin and a series of stress response and inflammation-related proteins. This analysis reported the presence of additional undefined GTKO PAEC membrane antigens, which were not adequately recovered by the proteomic approach. The induced non-Gal antibody response may also react with carbohydrate or glycolipid epitopes. Consistent with this an induced primate response to an undefined acidic cardiac glycolipid has recently been reported (11, 12). In this report, we used retrovirus-encoded expression libraries, produced from GT+:CD46 and GTKO PAECs, to identify the target antigens detected by induced non-Gal antibody after pig-to-primate cardiac xenotransplantation.
RESULTS
Antibody Sources
Five pig-to-primate heterotopic cardiac xenotransplants were performed in the absence of standard T-cell immunosuppressants. Four transplants were performed using GT+:CD46 hearts and a αGal polymer to block anti-Gal antibody in vivo (13), and one transplant was performed using a GTKO heart. All transplants were subjected to a strong cellular and humoral immune response and rejected in 5 to 7 days. The xenografts were explanted at rejection, and the recipients were monitored for a further 2 weeks before necropsy. Serum collected at necropsy, approximately 3 weeks after transplant, was enriched in antibody that bound to GTKO PAECs. In this study, necropsy sera from the GTKO recipient and two GT+:CD46 recipients with the highest non-Gal antibody titers were used to screen retrovirus-encoded PAEC complementary DNA(cDNA) expression libraries (Fig. 1A).
FIGURE 1.

Expression library screening and analysis. (A) The non-Gal antibody response after α1,3-galactosyltransferase gene knockout (GTKO) and GT+:CD46 heterotopic cardiac xenotransplantation were determined using flow cytometry by measuring IgG binding to GTKO porcine aortic endothelial cells (PAECs). A comparison of pretransplant (white bars) and necropsy (black bars) IgG binding at 1:20-fold, 1:80-fold, and 1:320-fold dilution is shown. All recipients show an increase in anti-pig non-Gal IgG after transplant. The donor organ genotype (GTKO or GT+:CD46) is shown below each data set. (B) Flow cytometry of IgG binding to individual pRETRO-infected HEK clones. Each line represents a different pRETRO-infected clone. The lines are color coded to denote clones with similar levels of antibody binding. The filled histogram is an infected control HEK cell that does not express a non-Gal antigen. (C) Results of polymerase chain reaction (PCR) amplification of the pRETRO-encoded porcine complementary DNA (cDNA) using genomic DNA of individual pRETRO-infected HEK clones. The cDNA product was amplified using PCR primers, which flank the multiple cloning site of the pRETRO vector. These products were cloned and sequenced to identify the non-Gal antigen.
Expression Library Screening
A standard cDNA expression library was produced in the pRETRO vector using polyA-selected messenger RNA (mRNA) from primary cultured GT+:CD46 or GTKO PAECs. We performed four independent analyses of the libraries using the sensitized antibody represented in Figure 1(A). After selection, a total of 199 and 317 pRETRO-HEK-infected clones were analyzed from GT+:CD46 and GTKO libraries, respectively (Table 1). Individual pRETRO-HEK-infected clones exhibited variable level of non-Gal IgG binding (Fig. 1B). Approximately 20% to 50% of the isolated clones exhibited a viral integration based on polymerase chain reaction (PCR) amplification of the cDNA insert (Table 1 and Fig. 1C). More than 80% of the PCR-positive pRETRO-HEK infected clones showed 3-fold or greater binding of sensitized IgG compared with control pRETRO-infected HEK cells.
TABLE 1.
Summary of library screen
| Libraries |
||
|---|---|---|
| GT+:CD46 | GTKO | |
| Clones isolated | 199 | 317 |
| Detected PCR inserts | 116 (0.58) | 75 (0.24) |
| Antibody reactive | 114 (0.98) | 62 (0.83) |
Data represent the number of clones isolated from the GT+:CD46 and GTKO expression libraries, the number of clones with amplifiable inserts, and the number of antibody reactive clones. Values in parenthesis indicate the fraction of total clones with PCR inserts and the fraction of clones with PCR inserts which bound non-Gal antibody.
GTKO, α1,3-galactosyltransferase gene knockout; PCR, polymerase chain reaction; Gal, galactose.
Sequence analysis and homology comparisons to the National Center for Biotechnology Information GenBank database identified six non-Gal target genes (Table 2). Four of these non-Gal antigens are type 1 (CD46 and EC protein C receptor [PROCR]), glycophosphatidylinositol-linked (CD59) or multipass (CD9) membrane proteins, one protein (annexin A2 [ANXA2]) is closely associated with the extracellular surface, and one encodes a glycosyltransferase with strong homology to the human β1,4 N-acetylgalactosaminyl transferase 2 (B4GALNT2). A summary of the flow cytometry profile for each of the pRETRO infected HEK clone is presented in Figure 2 (a–f).
TABLE 2.
Non-Gal antigens identified from a porcine cDNA expression library
| Species | Gene name | Gene symbol | NCBI ref. | Function |
|---|---|---|---|---|
| Sus scrofa | Tetraspanin 29 | CD9 | NM_214006.1 | Platelet and EC activation |
| Sus scrofa | Membrane cofactor protein | CD46 | NM_213888.1 | Complement regulation |
| Sus scrofa | Protectin | CD59 | NM_214170.1 | Complement regulation |
| Sus scrofa | EC protein C receptor | PROCR | NM_001163406 | Thrombosis |
| Sus scrofa | Annexin A2 | ANXA2 | NM_001005726.1 | Inflammation |
| Bos taurusa | β 1,4 N-acetylgalactosaminyl transferase 2 | B4GALNT2 | XM_584835.3 | Glycosylation |
The sequence with closest homology is presented.
cDNA, complementary DNA; EC, endothelial cell; Gal, galactose.
FIGURE 2.

The identification, confirmation, and expression of non-Gal porcine aortic endothelial cell (PAEC) antigens. (A) Flow cytometry of IgG binding to pRETRO-infected HEK cells for individual non-Gal antigens. (B) Flow cytometry showing IgG binding to G418-resistant pcDNA3.1/V5-His-TOPO transfected cell lines expressing individual non-Gal antigens. (C) Reverse-transcriptase polymerase chain reaction analysis of non-Gal gene expression. RNA samples: lane 1, pig heart; lane 2, GTKO PAECs; lanes 3 to 5, independent G418-resistant HEK transformants; lane 6, untransformed HEK cells; lane 7, a flow cytometry negative HEK cell line; and lanes 8 to 10, the same as lanes 3–5 but without reverse transcriptase. Non-Gal antigen expression: (a, g, and m) CD9; (b, h, and n) PROCR; (c, i, and o) CD46; (d, j, and p) CD59; (e, k, and q) ANXA2; and (f, l, and r) B4GALNT2.
Confirmation of Non-Gal Target Antigens
To insure that the identified non-Gal target antigens listed in Table 2 are the authentic targets of the induced antibody response, each cDNA was individually cloned into pcDNA3.1/V5-His-TOPO, transfected into HEK cells, and a stable G418-resistant pcDNA-HEK cell line was established. These stable cell lines were rescreened for antibody binding to validate that an induced antibody response was directed toward each of the non-Gal antigens (Fig. 2g–l). Expression of the non-Gal–encoded genes in GTKO PAECs, heart, and the pcDNA-HEK cell lines was confirmed by reverse-transcriptase (RT)-PCR (Fig. 2m–r).
Antibody Response to Individual Antigens
The antibody response to HEK cells expressing each non-Gal antigen was measured by comparing pretransplant and necropsy antibody binding for the five xenograft recipients. A representative IgG response for one baboon is shown (Fig. 3A and B) as is the average IgG response for all recipients (Fig. 3C). Induced antibody to B4GALNT2, PROCR, CD9, CD59, and CD46 was present in all or at least three of five recipients. Less consistent antibody induction was observed for ANXA2.
FIGURE 3.
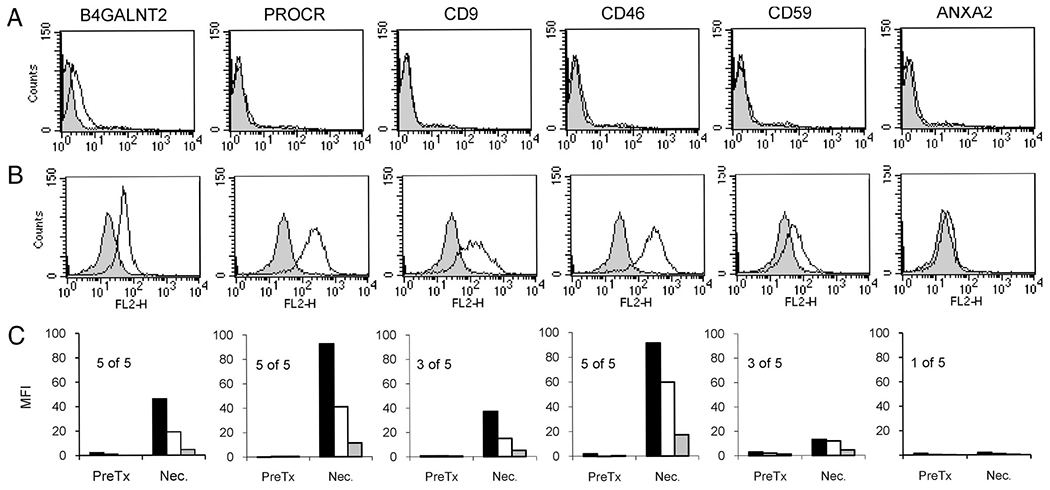
A characterization of the specific non-Gal antibody responses detected after pig-to-primate cardiac xenotransplantation. Pretransplant and sensitized necropsy serum from five cardiac xenograft recipients, not treated with T-cell immunosuppression, were individually screened for IgG reactivity to HEK cells expressing porcine non-Gal antigens. The immune response for a single transplant recipient is shown (A and B). The non-Gal antigen names are indicated above each column. (A) Pretransplant serum 1:40 dilution. (B) Necropsy serum 1:40 dilution. Filled histograms represent IgG binding to negative control HEK cells not expressing a non-Gal antigen, and lines indicate specific IgG binding to the indicated antigen. Specific antibody reactivity was calculated as indicated in the Materials and Methods. (C) The average antibody response for all five recipients to each antigen at 1:40, 1:160, and 1:640 serum dilutions (black, white, and gray filled boxes, respectively). Pretransplant serum (PreTx) and necropsy serum (Nec.). For each antigen, the number of recipients out of five showing a positive induction of antibody is indicated. A positive response was considered to be a 2-fold or greater increase in antibody binding in necropsy serum detected in two or more serum dilution.
DISCUSSION
Survival of cardiac xenografts has significantly improved during the past 10 years, yet remains limited by DXR, which seems to be initiated by preformed or induced Gal and non-Gal antibody. We hypothesized that a limited number of immunodominant non-Gal antigens were likely to be present and that immune responses to these antigens were primarily responsible for DXR of GTKO organs, at least within the current time frame of cardiac xenograft survival. Identification of these antigens is important for developing assays to monitor DXR, may be used to promote antigen-specific tolerance, may create new opportunities for genetic modification, and are essential for understanding the mechanism(s) of non-Gal–mediated DXR.
In this study, we screened GT+:CD46 and GTKO PAEC expression libraries using antibody from sensitized pig-to-primate cardiac xenograft recipients. Historically screening mammalian expression libraries by flow cytometry has successfully been used to identify both protein- and glycan-related surface antigens (14–16). The proteins identified in our study are well-defined membrane surface proteins, proteins known to associate with the extracellular surface or proteins, which alter the extracellular glycan. These proteins are involved in regulation of inflammation (ANXA2), complement (CD46 and CD59), and hemostasis (PROCR and CD9). Antibody responses to each protein would potentially be able to block important endothelial cell (EC) functions that might contribute to the pathology of DXR. CD46 and CD59 are complement regulatory proteins (CRPs) (17, 18) that act locally to establish an intrinsic barrier to complement mediated damage. Antibody directed to porcine CRPs could place the donor organ at greater risk for complement mediated damage and reinforces the significance of expressing human CD46 or other CRPs to control complement mediated injury. ANXA2 is found on the extracellular surface and functions as an EC surface receptor for plasminogen and tissue-type plasminogen activator (19). Anti-ANXA2 antibodies in patients with antiphospholipid syndrome can cause EC activation and the induction of tissue factor (20). A loss of ANXA2 function leads to reduced levels of tissue-type plasminogen activator-dependent plasmin generation and might thereby contribute to microvascular thrombosis during DXR (21). Anti-ANXA2 antibody reactivity was previously reported using a proteomic analysis (10). In our library screen, we isolated HEK expressing ANXA2 cells multiple times but see a relatively infrequent response to ANXA2 in our xenograft recipients. This may be an under estimate of the frequency of anti-ANXA2 antibody because ANXA2 surface expression on HEK cells may not accurately represent expression on ECs. CD9 is a tetraspanin protein family member. Anti-CD9 antibodies efficiently activate platelets, in some instances inducing a lethal thrombosis (22). CD9 is also expressed on ECs where antibodies to CD9 promote neutrophil adhesion (23). PROCR acts on the EC surface to enhance the formation of activated protein C by the complex of thrombin and thrombomodulin (24). Activated protein C is a potent anticoagulant. There is also a soluble form of PROCR that seems to bind to neutrophils and decrease their binding to the endothelium (25). Detection of an induced antibody response to these proteins strongly suggests that non-Gal DXR involves not only in antibody-directed injury and activation of the endothelium but also in antibody-mediated blocking of key EC functions. This suggests that substitution or transgenic expression of the corresponding human gene function may be useful for maintaining hemostasis and promoting resistance to non-Gal DXR.
We have also identified a porcine gene with high homology to human and mouse B4GALNT2. In humans and mice, the B4GALNT2 enzyme catalyzes the β1-4 addition of N-acetylgalactosamine (GalNAc) to terminal α2-3 sialylated Gal residues to produce the SDa antigen (also known as CAD) (26, 27). This carbohydrate structure is most abundant on the Tamm-Horsfall urinary glycoprotein. A similar carbohydrate structure, produced by a separate glycosyltransferase, is also present in the GM2 glycolipid. High levels of B4GALNT2 enzymatic activity have been reported in porcine intestinal epithelial cells, but the overall expression of B4GALNT2 RNA and distribution of SDa antigen in the pig are apparently not known (28). We show that B4GALNT2 RNA is present in porcine heart and GTKO PAECs (Fig. 2r) and have preliminary evidence of RNA expression in the kidney liver, spleen, and peripheral blood mononuclear cells (data not shown). The recovery of the porcine B4GALNT2 gene in our library screen suggests that an anti-SDa antibody or an antibody response to a SDa-like carbohydrate present on the vasculature of the xenograft may be part of the non-Gal immune response. This later interpretation seems likely as an anti-SDa antibody (KM694, Tokyo Research Laboratories, Kyowa Hakko Kirin Co. Ltd., Tokyo, Japan) binds to porcine B4GALNT2-expressing HEK cells but does not bind to GT+ or GTKO PAECs (data not shown). The lectin DBA binds to GalNAc residues and is often used for SDa staining and can distinguish SDa+ and SDa− human samples (29). This lectin also binds A1-type blood antigen. Dolichos biflorus agglutinin (DBA) has long been known to bind to porcine vascular ECs at high levels irrespective of their ABH blood group, consistent with the presence of a SDa-like antigen (30–32). We do not know the precise structure of the glycan(s) displayed by HEK cells expressing the porcine B4GALNT2 product or by GTKO PAECs, but it seems likely to include a sialylated Gal or a β-linked terminal GalNAc structure similar to the SDa carbohydrate. A carbohydrate of this type would be consistent with recent reports of induce non-Gal antibody to terminal GalNAc glycans and of baboon reactivity to acidic glycolipids (11, 12). Targeted inactivation of the porcine B4GALNT2 gene in combination with the existing α-galactosyltransferase (GGTA-1) mutation may be useful to further reduce the antigenicity of porcine xenografts.
The frequency of identifying an individual clone in this screening procedure is dependent on the abundance and size of the mRNA in PAECs, the efficiency of cloning, the level of protein expression, and the prevalence and affinity of antibody in the serum. In this study, all the clones except CD46 and PROCR were identified multiple times during the screening process. It remains possible that additional non-Gal antigens could be identified by further screening or that different antigens might be detected using sensitized sera from islet or kidney transplant recipients. These results identify for the first time a glycosyltransferase that produces a non-Gal carbohydrate antigen that may contribute to DXR. They also suggest that non-Gal–induced DXR may in part result from antibody responses that can potentially block key PAEC functions. Our results support further modification of the donor to promote resistance to non-Gal DXR or to further minimize the level of porcine antigenicity. Additional characterization of these antigen-antibody interactions and those defined by proteomic analysis will be required accurately to determine the frequency of an antibody response to each antigen and to validate their contribution to DXR.
MATERIALS AND METHODS
Library Formation and Selection
Expression libraries were constructed in the pRetro-LIB vector (Clontech, Takara Bio, Japan) using poly (A)+ mRNA from GT+:CD46 or GTKO PAECs. The cDNA libraries contained 2.6 to 3.0×106 clones, 80% to 93% of which had cDNA insertions. The average insert size was 2.0 kb, with a size ranging from 0.8 to 4.8 kb. Library plasmid DNA was cotransfected with pVSV-G into GP2-293 packaging cells to produce a high-titer (>106 colony-forming unit) viral stock. The titer of the viral stock was estimated using an alkaline phosphatase encoding control vector according to manufacturer’s recommendations. For infection, HEK cells (1×106) were incubated with 2 mL of virus stock containing 4 μ/mL of polybrene. Infected HEK cells were collected after 48 hr, labeled with sensitized serum from a pig-to-primate cardiac xenograft recipient, and stained with Goat anti-human IgG-fluorescein isothiocyanate (FITC; Zymed Laboratories, San Francisco, CA). Approximately 5×106 cells were sorted by flow cytometry, and the brightest 10% to 30% of the cells were collected. These bright cells were culture for 48 to 72 hr before additional selection. A total of three to seven rounds of selection were performed to enrich for non-Gal antigen-expressing cells. After the final round of selection, individual cells were sorted into 96-well plates, and pRETRO-HEK infected clones were isolated. Clones were screened for antibody binding, and the cDNA insert was recovered by PCR amplification of genomic DNA. The amplified product was cloned into a TA-cloning vector and sequenced using BigDye Terminator v1.1 cycle sequencing and an ABI3730XL sequencer (Invitrogen and ABI, Life Technologies Corp., Carlsbad, CA). A basic local alignment search tool (BLAST) search of the NCBI GenBank database was used to identify the encoded gene.
Flow Cytometry
Non-Gal antibody binding to GTKO PAECs and to HEK cells expressing individual non-Gal antigens was detected by flow cytometry. Primary GTKO PAECs were isolated as described previously (10) and grown in 10% fetal bovine serum/Dulbecco’s minimum essential medium supplemented with 50 μ/mL endothelial cell growth supplement (ECGS) (BD Biosciences, San Jose, CA). Baboon serum was diluted (1:20, 1:80, and 1:320) in 1% bovine serum albumin/phosphate-buffered saline and incubated with 2×105 GTKO PAECs for 30 min at 4°C. Cells were washed with 4 mL 1% bovine serum albumin/phosphate-buffered saline and labeled with goat anti-human IgG-FITC (Invitrogen, Life Technologies Corp., Carlsbad, CA) for 30 min at 4°C, washed, and analyzed using a FACSCalibur (BD Biosciences). HEK cells expressing non-Gal antigens were cultured in 10% fetal bovine serum/Dulbecco’s minimum essential medium. Cells were incubated with baboon sera as described, and binding of IgG and IgM was detected with goat anti-human IgM-FITC (Invitrogen, Life Technologies Corp.) and goat anti-human IgG-RPE (Southern Biotech. Birmingham, AL). Specific antibody binding to non-Gal–expressing HEK cells was calculated as the difference in mean fluorescence for antibody binding to HEK cells expressing a given non-Gal antigen and antibody binding to pRETRO- infected or pcDNA3.1-transfected G418-resistant control HEK cell line, which did not express a porcine non-Gal antigen.
Expression of Specific Non-Gal Target Antigens
The cDNAs encoding non-Gal antigens identified from the pRETRO library screen were cloned into pcDNA3.1/V5-His-TOPO (Clontech) and transfected into HEK cells using Lipofectamine 2000 (Invitrogen, Life Technologies Corp.). The HEK transformants were selected for G418 resistance (500 μg/mL), and a stable cell line for each non-Gal antigen was produced. Stable cell lines were rescreened with sensitized sera as described to confirm antibody reactivity to the non-Gal target antigen.
RT-PCR Analysis of Non-Gal Antigen Expression
Expression of non-Gal antigens was determined by RT-PCR of RNA samples from HEK cell lines, porcine hearts, and cultured PAECs. Total RNA was extracted from cells and pig heart tissue using RNeasy Mini Kit (QIAGEN, Valencia, CA) and RNA-STAT (Iso-Tex Diagnostics Inc., Friendswood, TX), respectively. For RT-PCR, 500 ng of total RNA was used in a 25 μL One-step RT-PCR reaction (USB Corp. Cleveland, OH). The reverse transcriptase reaction was performed at 42°C for 30 min, followed by 30 cycles of amplification (95°C for 30 min, 58°C for 3 min, and 72°C for 2 hr). The amplified products were electrophoresed through 1% agarose gels, and the ethidium bromide-stained DNA was imaged using a GelDoc-It imaging system (UVP Inc., Upland, CA). Primers for detections were CD9: forward, ACCATGGTAATGCCGGTCAAAGGAGGCA and reverse, ATTCTAGACCATCTCTCGGCTC; CD46: forward, ACCATGGTAATGATGGCGTTTTGCGCGCT and reverse, ATTCCACGTCCTCTCAGCAAC; CD59: forward, ACGATGGGAAGCAAAGGAGGGTT and reverse, CAGTTAGAGACAAAAGTGCCAGG; PROCR: forward, ACCATGGCGATGTTGACAACATTGCTG and reverse, AATTAACATCGCCGCCGTCCAC; ANXA2: forward, ACCATGGCAATGTCTACCGTTCATGA and reverse, CTTCAGTCATCCCCACCACACAGGTAC; and B4GALNT2: forward, ACCATGGAGATGACTTCGTACAGCCCTAG and reverse, CAGATACCTTAGGTGGCACATTGGAG.
Acknowledgments
This work was supported by NIH grant AI66310.
Footnotes
G.W.B. and C.G.A.M. are the inventors of technology related to xenotransplantation that has been licensed by the Mayo Clinic to a commercial entity. The other authors declare no conflict of interest.
REFERENCES
- 1.Platt JL, Lin SS, McGregor CG. Acute vascular rejection. Xenotransplantation 1998; 5: 169. [DOI] [PubMed] [Google Scholar]
- 2.Cowan PJ. Coagulation and the xenograft endothelium. Xenotransplantation 2007; 14: 7. [DOI] [PubMed] [Google Scholar]
- 3.Byrne GW, Schwarz A, Fesi JR, et al. Evaluation of different alpha-galactosyl glycoconjugates for use in xenotransplantation. Bioconjug Chem 2002; 13: 571. [DOI] [PubMed] [Google Scholar]
- 4.Lai L, Kolber-Simonds D, Park KW, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 2002; 295: 1089. [DOI] [PubMed] [Google Scholar]
- 5.Lam TT, Paniagua R, Shivaram G, et al. Anti-non-Gal porcine endothelial cell antibodies in acute humoral xenograft rejection of hDAF-transgenic porcine hearts in cynomolgus monkeys. Xenotransplantation 2004; 11: 531. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med 2005; 11: 1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rood PP, Hara H, Ezzelarab M, et al. Preformed antibodies to alpha1,3-galactosyltransferase gene-knockout (GT-KO) pig cells in humans, baboons, and monkeys: Implications for xenotransplantation. Transplant Proc 2005; 37: 3514. [DOI] [PubMed] [Google Scholar]
- 8.Buhler L, Xu Y, Li W, et al. An investigation of the specificity of induced anti-pig antibodies in baboons. Xenotransplantation 2003; 10: 88. [DOI] [PubMed] [Google Scholar]
- 9.Ezzelarab M, Hara H, Busch J, et al. Antibodies directed to pig non-Gal antigens in naïve and sensitized baboons. Xenotransplantation 2006; 13: 400. [DOI] [PubMed] [Google Scholar]
- 10.Byrne GW, Stalboerger PG, Davila E, et al. Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation. Xenotransplantation 2008; 15: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diswall M, Angström J, Karlsson H, et al. Structural characterization of alpha1,3-galactosyltransferase knockout pig heart and kidney glycolipids and their reactivity with human and baboon antibodies. Xenotransplantation 2010; 17: 48. [DOI] [PubMed] [Google Scholar]
- 12.Blixt O, Kumagai-Braesch M, Tibell A, et al. Anticarbohydrate antibody repertoires in patients transplanted with fetal pig islets revealed by glycan arrays. Am J Transplant 2009; 9: 83. [Google Scholar]
- 13.Davila E, Byrne GW, LaBreche PT, et al. T-cell responses during pig-to-primate xenotransplantation. Xenotransplantation 2006; 13: 31. [DOI] [PubMed] [Google Scholar]
- 14.Keusch JJ, Manzella SM, Nyame KA, et al. Expression cloning of a new member of the ABO blood group glycosyltransferases, iGb3 synthase, that directs the synthesis of Isoglobo-glycosphingolipids. J Biol Chem 2000; 275: 25308. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura T, Onishi M, Kinoshita S, et al. Efficient screening of retroviral cDNA expression libraries. Proc Natl Acad Sci USA 1995; 92: 9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorman BP, Shimizu N, Ruddle FH. Genetic analysis of the human cell surface: Antigenic marker for the human X chromosome in human-mouse hybrids. Proc Natl Acad Sci USA 1978; 75: 2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liszewski MK, Post TW, Atkinson JP. Membrane cofactor protein (MCP or CD46): Newest member of the regulators of complement activation gene cluster. Annu Rev Immunol 1991; 9: 431. [DOI] [PubMed] [Google Scholar]
- 18.Meri S, Morgan BP, Davies A, et al. Human protectin (CD59), an 18,000–20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology 1990; 71: 1. [PMC free article] [PubMed] [Google Scholar]
- 19.Ling Q, Jacovina AT, Deora A, et al. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J Clin Invest 2004; 113: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cesarman-Maus G, Ríos-Luna NP, Deora AB, et al. Autoantibodies against the fibrinolytic receptor, annexin 2, in antiphospholipid syndrome. Blood 2006; 107: 4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cockrell E, Espinola RG, McCrae KR. Annexin A2: Biology and relevance to the antiphospholipid syndrome. Lupus 2008; 17: 943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawakatsu T, Suzuki M, Kido H, et al. Antithrombotic effect of an anti-glycoprotein IIB/IIIA antibody in primate lethal thrombosis. Thromb Res 1993; 70: 245. [DOI] [PubMed] [Google Scholar]
- 23.Forsyth KD. Anti-CD9 antibodies augment neutrophil adherence to endothelium. Immunology 1991; 72: 292. [PMC free article] [PubMed] [Google Scholar]
- 24.Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein C-EPCR system: Integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol 2004; 24: 1374. [DOI] [PubMed] [Google Scholar]
- 25.Esmon CT. Structure and functions of the endothelial cell protein C receptor. Crit Care Med 2004;32(5 suppl):S298. [DOI] [PubMed] [Google Scholar]
- 26.Renton PH, Howell P, Ikin EW, et al. Anti-Sd(a), a new blood group antibody. Vox Sang 1967; 13: 493. [Google Scholar]
- 27.Montiel MD, Krzewinski-Recchi MA, Delannoy P, et al. Molecular cloning, gene organization and expression of the human UDP-GalNAc:Neu5Acalpha2–3Galbeta-R beta1,4-N-acetylgalactosaminyltransferase responsible for the biosynthesis of the blood group Sda/Cad antigen: Evidence for an unusual extended cytoplasmic domain. Biochem J 2003; 373(pt 2): 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malagolini N, Dall’Olio F, Guerrini S, et al. Identification and characterization of the Sda beta 1,4, N-acetylgalactosaminyltransferase from pig large intestine. Glycoconj J 1994; 11: 89. [DOI] [PubMed] [Google Scholar]
- 29.Wu AM, Wu JH, Watkins WM, et al. Differential binding of human blood group Sd(a+) and Sd(a−) Tamm-Horsfall glycoproteins with Dolichos biflorus and Vicia villosa-B4 agglutinins. FEBS Lett 1998; 429: 323. [DOI] [PubMed] [Google Scholar]
- 30.Smith DM, Newhouse M, Naziruddin B, et al. Blood groups and transfusions in pigs. Xenotransplantation 2006; 13: 186. [DOI] [PubMed] [Google Scholar]
- 31.Johnson EK, Schelling ME, Quitadamo IJ, et al. Cultivation and characterization of coronary microvascular endothelial cells: A novel porcine model using micropigs. Microvasc Res 2002; 64: 278. [DOI] [PubMed] [Google Scholar]
- 32.Solanes N, Rigol M, Ramirez J, et al. Histological basis of the porcine femoral artery for vascular research. Anat Histol Embryol 2005; 34: 105. [DOI] [PubMed] [Google Scholar]


