Abstract
Background
Testosterone deficiency (TD) is a prevalent condition, especially in men ≥45 years old, and testosterone therapy (TTh) can improve the quality of life in these patients.
Aim
To evaluate the safety profile of compounded subcutaneous testosterone pellets and to compare the efficacy between compounded and market brand testosterone pellets for TTh: E100 (Empower Pharmacy) and Testopel (Food and Drug Administration approved), respectively.
Methods
This was a prospective, phase 3, randomized, noninferiority clinical trial. We enrolled 75 men diagnosed with TD and randomized them 1:1 to a market brand group and a compounded pellet group. The patients were implanted with their respective testosterone pellets: Testopel (10 pellets of 75 mg) and E100 (8 pellets of 100 mg).
Outcomes
We evaluated adverse events after implantation and followed men at 2, 4, and 6 months for morning laboratory levels (prior to 10 am): serum testosterone, estradiol, hematocrit, and prostate-specific antigen.
Results
After randomization, 33 participants were enrolled in the Testopel arm and 42 in the E100 arm. Serum testosterone levels were similar between the groups at 2, 4, and 6 months, with most men (82%) dropping to <300 ng/dL by the end of the trial. Adverse events were also similar, such as elevations in prostate-specific antigen, estradiol, and hematocrit. Most dropouts were related to persistent TD symptoms and serum testosterone <300 ng/dL, with similar rates between the groups in the study.
Clinical Implications
Men treated with Testopel and E100 pellets had comparable serum testosterone levels and similar adverse event rates, providing an effective choice of long-term TTh among men with TD.
Strengths and Limitations
Strengths include the prospective, randomized, single-blinded study design and adequate follow-up. Limitations include the lack of external validity and the single-institution cohort.
Conclusion
E100 compounded testosterone pellets are a noninferior option of TTh as compared with Testopel for men presenting with TD.
Keywords: testosterone, testosterone deficiency, testosterone therapy, testosterone pellets, hypogonadism, testosterone replacement therapy
Introduction
Testosterone deficiency (TD) is characterized by a combination of low serum testosterone with symptoms such as low energy, fatigue, decreased muscle mass, reduced libido, and erectile dysfunction.1 TD prevalence can be as high as 6% in men aged 40 to 70 years.2 Testosterone therapy (TTh) in this setting has many benefits, such as increasing lean body mass, bone formation, and libido and decreasing total cholesterol, and it may even increase cognitive function.3,4
Multiple forms of TTh currently exist on the market and are available to patients, consisting of intramuscular, subdermal, transdermal, oral, and buccal formulations.5,6 These options have different dosing regimens, pharmacokinetic profiles, advantages, disadvantages, and monitoring requirements that the physician-patient team must consider. Subcutaneous testosterone pellets were first developed in the 1940s but did not gain popular adoption in the United States until Food and Drug Administration (FDA) approval for Testopel in 1972.5,7 Subcutaneous long-lasting testosterone pellets are placed in the subcutaneous hypovascular space and gradually dissolve to provide steady testosterone release.7,8
Testopel pellets (Endo International plc) are 3 × 8–mm pellets that each contain 75 mg of crystalline testosterone. Current FDA dosing guidelines recommend 150 to 450 mg every 3 to 6 months with appropriate titration required to induce pubertal changes in hypogonadal males if indicated.9 However, a large retrospective study of 380 men found that higher pellet numbers (10-12) making up 450 to >750 mg was associated with more consistent, higher, and longer maintenance of therapeutically ranged testosterone levels.8
Since FDA approval of market brand testosterone pellets, comparable pellet formulations of differing sizes and dosing regimens were studied but have not reached the market.10 In this trial, we sought to evaluate the safety profile of compounded pellets (E100) and to compare the efficacy between compounded and market brand testosterone pellets. This trial evaluated the market brand testosterone pellet Testopel (75 mg) and compared it with compounded testosterone pellets (100 mg; E100) manufactured by Empower Pharmaceuticals, an FDA-registered facility. Maintaining FDA approval and regulation ensures adherence to the FDA’s current good manufacturing practice regulations. These outline the standards for the design, monitoring, and control of manufacturing processes and facilities to ensure that products are safe and effective and to hold the approved agency subject to state and local regulations.
Methods
Study design
This was a prospective, phase 3, randomized, noninferiority clinical trial. The trial protocol was published a priori, and it contains details about the methods.11 More information is available at ClinicalTrials.gov (NCT04523480).
Participants
Adult men presenting with a diagnosis of TD with serum total testosterone <300 ng/dL on 2 measurements and symptoms (eg, low libido, low energy, erectile dysfunction, loss of muscle mass, fatigue, gynecomastia) were screened for inclusion (Table S1). Exclusion criteria were all contraindications to receiving TTh outlined in the protocol (Table S2).11 Participants were recruited from follow-up visits of established patients, new patients with a history of TD or newly diagnosed TD, or online clinical trial recruitment advertisements. Recruitment was targeted toward patients naïve to testosterone treatment or those previously taking testosterone after stopping and completing an adequate washout period. Participants with a history of TTh (injectables or gels) were instructed to stop any current use for at least 4 weeks prior to the first treatment session and to refrain from using any other TTh during the study. Participants with a body mass index ≥40 were also excluded to better illustrate the population of men with TD.
Randomization and masking
Randomization was performed by a computer software system (REDCap) maintained by the research team.12 The participants were randomized 1:1 to the market brand pellet (75-mg; Testopel) or the compounded pellet (100-mg; E100) from FDA-approved Empower Pharmacy. A central randomization scheme was used to generate 80 randomizations, with the goal of recruiting 40 patients per group. One study coordinator was aware of the randomization scheme (Testopel vs compound group). The principal investigator and all other study coordinators were blinded.
Procedures
Following enrollment and randomization, participants were implanted with their respective testosterone pellets: Testopel (10 pellets of 75 mg) or E100 compounded (8 pellets of 100 mg). Because of COVID-19 pandemic restriction protocols, follow-ups were conducted over email and phone call at 2, 4, and 6 months; these visits included morning laboratory levels (prior to 10 am) of testosterone, estradiol, hematocrit, and prostate-specific antigen (PSA). Serum studies including sex steroid hormone evaluation and PSA were processed by Labcorp. Specifically, PSA, testosterone, and estradiol were measured via electrochemiluminescence immunoassay. Participants were also asked to report and record adverse events at each follow-up contact. At the end of the follow-up period, patients were contacted regarding continuing testosterone treatment.
Outcomes
The primary outcome was to compare total testosterone level at 2-, 4-, and 6-month follow-up bloodwork between patients who received Testopel and compounded testosterone pellets. We used current guidelines for the reference range of testosterone levels (450-600 ng/dL) to assess response to treatment.1 Patients who exhibited testosterone results <300 ng/dL at any follow-up were released from the trial and administered a different TTh. We evaluated this subgroup of men separate from those who dropped out.
Secondary outcomes included the evaluation of estradiol, hemoglobin, hematocrit, 17-hydroxyprogesterone, and PSA levels at 2, 4, and 6 months. Out-of-range results for these parameters were treated according to current guideline recommendations. Patients with estradiol levels >60 ng/dL were offered an aromatase inhibitor; patients with hematocrit levels >52% were recommended to pursue phlebotomy; and patients with significantly elevated PSA were recommended to follow up with prostate biopsy and/or magnetic resonance imaging.
Sample size and statistical analysis
A power analysis for a noninferiority trial was performed. Given the lack of comparative data in the literature comparing Testopel and compounded testosterone pellets, an estimated noninferiority limit (d) was chosen. The following metrics were chosen for this analysis: alpha, 5%; power, 80%; percentage success for Testopel, 90%; percentage success for E100, 90%; noninferiority limit, 20%. These metrics returned a need for 28 patients per arm (total 56). We aimed to recruit 33 patients per arm to account for a dropout of 10 patients. Success for each arm was defined as patients reaching a testosterone level ≥300 ng/dL at the 2-month period. Demographic data were compared between arms with Fisher’s exact test.
Normality testing was performed on all data with the Kolmogorov-Smirnov test. Nonparametric data were compared with the Mann-Whitney U test. The median and IQR were calculated for all relevant variables and baseline characteristics, as well as primary and secondary outcomes. Demographic characteristics, such as medical background and risk factors, were compared between these groups with Fisher’s exact test and Student’s t-test. All statistical analysis was performed with SAS version 9.4 software (SAS Institute).
Recruitment and reasons for stopped trial
Recruitment for the clinical trial started March 2020. Most participants were recruited between June 2020 and June 2021. Recruitment was halted between March and May 2020 due to logistical complications associated with the COVID-19 pandemic.
Recruitment was stopped on October 2021 when 75 participants were reached. Date of the final follow-up bloodwork was February 2021 at 4 months and January 2022 at 6 months. Written informed consent from the participants was obtained for their participation in the study. Furthermore, Ethics Committee and Institutional Review Board approval was granted to carry out this study (No. 1933968).
Adverse events and safety
Follow-up questionnaires about treatment-emergent adverse events were sent to all patients following the procedure. The follow-up included questions about pellet extrusion, fever, pain, bruising/bleeding, medication, and emergency room visits.
Results
Baseline data
An overall 75 participants were randomly assigned to 1 of 2 groups: 33 to 75-mg Testopel and 42 to 100-mg E100. The median age was 54.5 years (range, 35.0-74.0; IQR, 15.8) for the Testopel group and 52.5 years (range, 22.0-69.0; IQR, 13.5) for the E100 group (P > .05). All 75 participants received the intended treatment. Of the 75 participants, 62, 60, and 10 performed 2-, 4-, and 6-month follow up, respectively. Figure 1 outlines participant flow, losses, and exclusions in greater detail. The baseline demographic characteristics are described in Table 1. Median baseline testosterone was similar between the groups (Testopel, 219.5 ng/dL; E100, 202.3 ng/dL; P = .25). Baseline blood levels for primary and secondary outcome parameters stratified by group are outlined in Table 2.
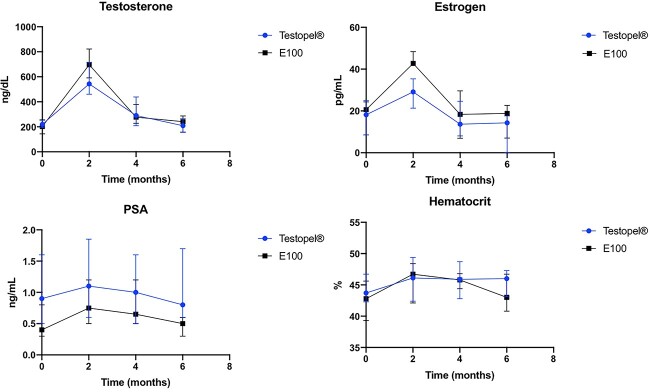
Figure 1.
Laboratory assessment at 2-month intervals comparing Testopel with compounded E100 testosterone pellet therapy. Data are displayed as median (IQR). *P < .05.
Table 1.
Demographics of the 2 experimental arms: Testopel and E100 compounded pellets.a
| Patients, No. (%) | ||||
|---|---|---|---|---|
| Demographic characteristic | Total | Testopel | E100 | P value |
| Sample | 75 (100) | 33 (44.0) | 42 (56.0) | |
| Ethnicity | >.05 | |||
| African American | 9 (12.0) | 3 (9.1) | 6 (14.3) | |
| Hispanic | 53 (70.7) | 24 (72.7) | 29 (69.0) | |
| White | 13 (17.3) | 6 (18.2) | 7 (16.7) | |
| Coronary artery disease | .08 | |||
| Yes | 3 (4.0) | 3 (9.1) | 0 (0.0) | |
| No | 72 (96.0) | 30 (90.9) | 42 (100) | |
| Hypertension | .65 | |||
| Yes | 36 (47.4) | 17 (51.5) | 19 (45.2) | |
| No | 40 (52.6) | 16 (48.5) | 23 (54.8) | |
| Hypercholesterolemia | .63 | |||
| Yes | 25 (33.3) | 12 (36.4) | 13 (31.0) | |
| No | 50 (66.7) | 21 (63.6) | 29 (69.0) | |
| Diabetes mellitus | .99 | |||
| Yes | 18 (24.0) | 8 (24.2) | 10 (23.8) | |
| No | 57 (76.0) | 25 (75.8) | 32 (76.2) | |
| Current smoker | .63 | |||
| Yes | 4 (5.3) | 1 (3.0) | 3 (7.1) | |
| No | 71 (94.7) | 32 (97.0) | 39 (92.9) | |
| History of smoking | .82 | |||
| Yes | 33 (44.0) | 14 (42.4) | 19 (45.2) | |
| No | 42 (56.0) | 19 (57.6) | 23 (54.8) | |
| Body mass index | >.05 | |||
| Underweight | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Healthy | 8 (10.7) | 2 (6.1) | 6 (14.3) | |
| Overweight | 29 (38.6) | 14 (42.4) | 15 (36.7) | |
| Obese | 38 (50.7) | 17 (51.5) | 21 (50.0) | |
| Mean ± SD | 31.19 ± 6.36 | 30.51 ± 5.11 | 31.75 ± 7.24 | .419 |
aOverall, no significant differences were reported between arms.
Table 2.
Laboratory values between treatment arms at baseline and 2, 4, and 6 months.
| Treatment arm, median (IQR) | |||
|---|---|---|---|
| Testopel | E100 | P value a | |
| Testosterone | |||
| Baseline | .2563 | ||
| ng/dL | 219.5 (194.0-252.0) | 202.3 (143.5-256.0) | |
| nmol/L | 7.6 (6.7-8.7) | 7.0 (5.0-8.9) | |
| 2 mo | .1940 | ||
| ng/dL | 543.0 (460.0-716.5) | 696.5 (591.0-822.0) | |
| nmol/L | 18.8 (16.0-24.9) | 24.2 (20.5-28.5) | |
| 4 mo | .7028 | ||
| ng/dL | 290.0 (209.0-439.0) | 277.0 (228.0-378.0) | |
| nmol/L | 10.1 (7.3-15.2) | 9.6 (7.9-13.1) | |
| 6 mo | .5947 | ||
| ng/dL | 209.0 (155.0-267.0) | 241.0 (158.0-287.0) | |
| nmol/L | 7.3 (5.4-9.3) | 8.4 (5.5-10.0) | |
| PSA, ng/mL | |||
| Baseline | 0.90 (0.50-1.60) | 0.40 (0.30-0.80) | .0037 |
| 2 mo | 1.10 (0.60-1.85) | 0.75 (0.50-1.20) | .0619 |
| 4 mo | 1.00 (0.50-1.60) | 0.65 (0.50-1.20) | .1781 |
| 6 mo | 0.80 (0.60-1.70) | 0.50 (0.30-0.60) | .0998 |
| Hematocrit, % | |||
| Baseline | 43.7 (42.3-46.7) | 42.8 (39.3-45.6) | .1697 |
| 2 mo | 46.1 (42.4-49.4) | 46.7 (42.1-48.4) | .5491 |
| 4 mo | 45.9 (42.8-48.7) | 45.8 (44.4-46.8) | .7835 |
| 6 mo | 46.0 (43.1-47.3) | 43.0 (40.8-46.7) | .2684 |
| Hemoglobin, g/dL | |||
| Baseline | 14.8 (14.0-15.6) | 14.1 (13.2-15.2) | .2052 |
| 2 mo | 15.6 (14.1-16.8) | 14.9 (14.2-16.4) | .4714 |
| 4 mo | 15.4 (14.2-16.4) | 15.2 (14.6-15.8) | .5585 |
| 6 mo | 15.7 (15.1-15.9) | 14.7 (13.3-15.6) | .2264 |
| Estradiol, pg/mL | |||
| Baseline | 18.2 (8.5-25.0) | 20.6 (8.5-24.3) | .7327 |
| 2 mo | 29.1 (21.3-35.4) | 42.7 (42.1-48.4) | .0006 |
| 4 mo | 13.7 (8.1-24.6) | 18.4 (6.9-29.6) | .5288 |
| 6 mo | 14.3 (0.1-17.6) | 18.8 (7.0-22.7) | .1726 |
| 17-OHP, ng/dL | |||
| Baseline | 45.0 (21.0-66.0) | 39.0 (27.0-63.0) | .4851 |
| 2 mo | 12.5 (0.1-17.0) | 15.0 (0.1-25.0) | .0726 |
| 4 mo | 17.0 (11.0-46.0) | 15.0 (10.0-23.0) | .1811 |
| 6 mo | 53.0 (21.0-57.0) | 38.0 (26.0-51.0) | .6377 |
Abbreviations: 17-OHP, 17-hydroxyprogesterone; PSA, prostate-specific antigen.
aBold indicates P < .05.
Outcomes
Primary outcome
Throughout follow-up, testosterone levels for the Testopel and E100 groups were not statistically different. At 2 months, the median testosterone level was 543.0 ng/dL for the Testopel group and 696 ng/dL for the E100 group. At 4 months, the median testosterone levels were 290 and 277 ng/dL, respectively. At 6 months, they were 209 ng/dL in the Testopel group and 241 ng/dL in the E100 group. Mean testosterone levels between the groups at each follow-up were not significantly different (P = .16, .40, .72; Table 2).
Secondary outcomes
The mean PSA, hemoglobin, and hematocrit levels for Testopel and E100 at 2, 4, and 6 months were not significantly different (P > .05). The median estradiol levels for Testopel and E100 at 2 months were 29 and 43 pg/mL, respectively, which were significantly different (P = .0006). However, levels were not statistically different at the 4- and 6-month time points (Table 2). Finally, 17-hydroxyprogesterone levels were not significantly different between arms at 2, 4, and 6 months (P > .05).
Adverse events and safety
There were no cases of fever, pain, bruising/bleeding, or emergency room visits in either group. In the Testopel group, 1 participant reported pellet extrusion, as opposed to 0 participants in the E100 group.
Dropouts and losses to follow-up
Participants received the complete dosage of testosterone pellets at the beginning of the trial. Attempts were made to optimize follow-up (contacting patients to remind them of appointments). This was a source of larger dropout numbers than expected and a skew in the number of participants compared in each arm. Furthermore, the prescribed dosage of pellets was not always sufficient to keep testosterone levels within the reference range for the complete length of the study. As such, those with testosterone levels below reference by the 4-month follow-up date were not included in the comparison at 6 months, which accounted for the steep drop in participant number by the final time point.
In the Testopel and E100 groups, 18.2% and 12% dropped out due to loss to follow-up, respectively. One participant from each group was excluded due to supraphysiologic testosterone levels later confirmed to be contamination. At 4 months, 67% of the E100 group and 69% of the Testopel group had testosterone levels below the reference range and were not included at the 6-month follow-up. Contact was lost with 5% of the E100 group and 12% of the Testopel group. However, 3 participants who did not participate in follow-up in the Testopel group at the 2-month mark did follow up in the 4-month mark. By the end of the study, only 16.7% of the E100 group completed all 6 months of follow-up. In the Testopel arm, 9.1% of the original group finished the trial. Figure 2 outlines the flow of participation between time points.
Figure 2.
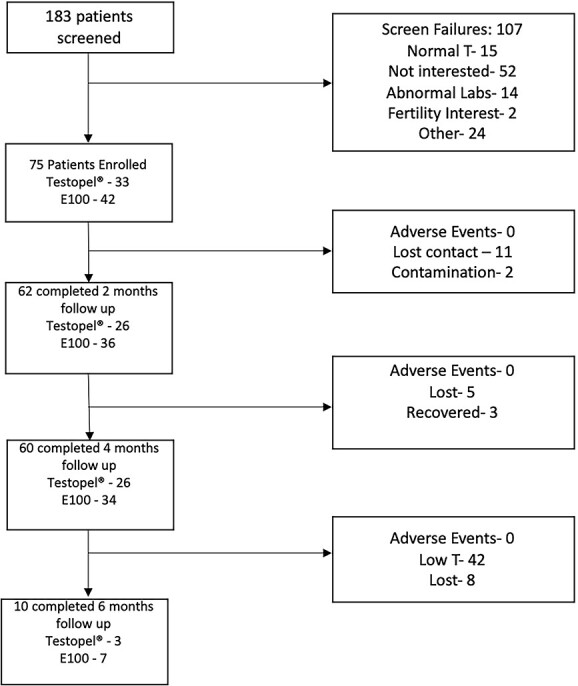
Study screening, enrollment, and participation.
Discussion
TD imposes a significant detrimental effect on men’s quality of life. The estimated prevalence of TD is 6% in the American male population. Since the life expectancy of a US male is 74.5 years, many men will require long-term TTh.13 Of the various forms of testosterone replacement, testosterone pellets represent an effective, long-term treatment strategy with sustained increases in serum testosterone for up to 6 months.14 The novel findings of this single-center, open-label, randomized trial suggest that market brand and E100 compounded testosterone pellets are similar in their ability to increase serum total testosterone to within reference levels without a significant difference in side effect profile at <10% of the price. In our study, the market brand Testopel and E100 compounded pellets produced by an FDA-registered facility yielded normalization of testosterone levels with similar rates of side effects, such as polycythemia and changes in estradiol and PSA. Testopel has been shown to normalize testosterone levels and improve symptoms for at least 3 months and up to 6 months in men with TD. Specifically, Kaminetsky et al conducted a phase IV, single-center, open-label study to assess the safety and efficacy of subcutaneous insertion of testosterone pellets during a single implantation procedure. The authors determined that mean testosterone significantly increased at weeks 1, 4, and 12 as compared with preimplantation levels and then returned to baseline by week 24. The normalization of serum testosterone coincided with objective improvement in TD symptoms, as demonstrated via objective measures such as patient questionnaires.15 These findings were corroborated in a large multicenter retrospective review assessing the clinical utility of testosterone pellets for TD. In the study, Testopel elicited sustained levels of testosterone for at least 4 months and up to 6 months in men with TD, regardless of pellet number (number of Testopel pellets employed varied, with patients receiving 6 or 7, 8 or 9, or ≥10 pellets). In our trial, a significant percentage of men from both arms exhibited low levels of serum testosterone at the 4-month follow-up. While the objective of our study was not to assess dosing, it may be worth taking a closer look at optimal dosing regimens to achieve eugonadal testosterone levels for the longest period. Another aspect of the trial that should be explored is the statistically significant increase in levels of estradiol in the E100 compounded testosterone pellets as compared with Testopel. We explored different aspects of the data to help illuminate the reason for this inconsistency. Higher testosterone levels and body mass index could explain increases in estradiol; however, both parameters were not statistically different in each group. Impurities within the compounded pellets can be another explanation for the difference, but more intensive studies must be done to clarify.
This study is not without limitations. Results from a randomized controlled trial may not always emulate situations encountered in real practice and thus may not be widely applicable, especially considering our strict exclusion criteria. The lack of external validity and the single blinded design are also potential sources of inaccuracy and bias. One limitation specific to our trial includes the minor dosage difference between the pellets. We suffered a logistical dilemma between equalizing the dosages given to each group and studying their effects at or near the most common dosage. Although the difference in dose is relatively small (50 mg), it does introduce a potential confounding bias. Additionally, this study focused on the safety and noninferiority of E100 as compared with Testopel, and sexual dysfunction represents an important entity in the constellation of symptoms that patients with TD may experience. As such, an important consideration is a comparison of the effect of E100 vs Testopel on sexual symptoms after treatment. Finally, in this study, laboratory end points were assessed at 2, 4, and 6 months. Prior work on crystalline pellets has shown that peak testosterone was observed at the 1-month time frame.16 While our work did not capture this end point, we were able to capture therapeutic testosterone levels at 2 months for each arm. Future work could consider laboratory assessment at 1 month to ascertain the peak of each therapy. Finally, the relatively low power in each arm that qualified for analysis at the 6-month mark represents a limitation in long-term follow-up. Future work should examine patient- and dosing-based factors that optimize testosterone levels up to the 6-month period. This may include more frequent laboratory assessment as well as more frequent dosing (every 3 months) to better titrate treatment response and maintenance of stable testosterone levels. Nonetheless, despite these limitations, these data highlight the noninferiority and safety profile of E100 compounded testosterone pellets as compared with the more expensive Testopel in the treatment of men with TD. This is especially the case in patients who may not be able to afford the market brand or whose insurance does not cover the treatment, as the E100 compounded pellets are almost 1/10th the price.17,18 With these findings, urologists can better serve as advocates for their patients by offering an effective modality for treating the potentially debilitating effects of TD.
Conclusion
The findings of this study highlight the noninferiority of E100 compounded testosterone pellets in the treatment of male TD. Thus, E100 compounded pellets represent an alternative to Testopel. With these data, urologists can more reasonably and comfortably offer men with TD E100 compounded testosterone pellets.
Funding
This trial was funded through an investigator-initiated grant provided by Empower Pharmacy (award GR014969).
Conflicts of interest: None declared.
Ethics statement
The study protocol was approved by the institutional review board of the University of Miami Miller School of Medicine (No. 20192539). Informed consent was confirmed by the board.
Supplementary Material
Contributor Information
Eliyahu Kresch, Department of Urology, University of Florida Jacksonville, Jacksonville, FL 32209, United States.
Thiago Fernandes Negris Lima, Department of Urology, Hospital Veredas, Maceió 57050, Brazil.
Manuel Molina, Desai Sethi Urological Institute, Miller School of Medicine, University of Miami, Miami, FL 33136, United States.
Nicholas A Deebel, Department of Urology, Wake Forest School of Medicine, Winston-Salem, NC 27157, United States.
Rohit Reddy, Desai Sethi Urological Institute, Miller School of Medicine, University of Miami, Miami, FL 33136, United States.
Mehul Patel, Desai Sethi Urological Institute, Miller School of Medicine, University of Miami, Miami, FL 33136, United States.
Justin Loloi, Department of Urology, Montefiore Medical Center, Bronx, NY 10467, United States.
Chase Carto, Desai Sethi Urological Institute, Miller School of Medicine, University of Miami, Miami, FL 33136, United States.
Sirpi Nackeeran, Desai Sethi Urological Institute, Miller School of Medicine, University of Miami, Miami, FL 33136, United States.
Daniel C Gonzalez, Desai Sethi Urological Institute, Miller School of Medicine, University of Miami, Miami, FL 33136, United States.
Jesse Ory, Department of Urology, Dalhousie University, Halifax B3H 4R2, Canada.
Ranjith Ramasamy, Desai Sethi Urological Institute, Miller School of Medicine, University of Miami, Miami, FL 33136, United States.
References
- 1. Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200(2):423–432. [DOI] [PubMed] [Google Scholar]
- 2. Araujo AB, O’Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2004;89(12):5920–5926. [DOI] [PubMed] [Google Scholar]
- 3. Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol. 2005;63(3):280–293. [DOI] [PubMed] [Google Scholar]
- 4. Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol. 2005;63(4):381–394. [DOI] [PubMed] [Google Scholar]
- 5. Shoskes JJ, Wilson MK, Spinner ML. Pharmacology of testosterone replacement therapy preparations. Transl Androl Urol. 2016;5(6):834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kresch E, Patel M, Lima TFN, et al. An update on the available and emerging pharmacotherapy for adults with testosterone deficiency available in the USA. Expert Opin Pharmacother. 2021;22(13):1761–1771. [DOI] [PubMed] [Google Scholar]
- 7. McCullough A. A review of testosterone pellets in the treatment of hypogonadism. Curr Sex Health Rep. 2014;6(4):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCullough AR, Khera M, Goldstein I, et al. A multi-institutional observational study of testosterone levels after testosterone pellet (Testopel) insertion. J Sex Med. 2012;9(2):594–601. [DOI] [PubMed] [Google Scholar]
- 9. Testopel. Package insert. Endo Pharmaceuticals Inc; 2015. [Google Scholar]
- 10. Jackson LM, Parker RM, Mattison DR, eds. The Clinical Utility of Compounded Bioidentical Hormone Therapy: A Review of Safety, Effectiveness, and Use. National Academies Press; 2020. [PubMed]
- 11.Testopel vs generic testosterone pellets. ClinicalTrials.gov identifier: NCT04523480. First posted August 21, 2020. Last update posted January 5, 2023. https://ClinicalTrials.gov/show/NCT04523480.
- 12. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arias E, Xu J, Tejada-Vera B, et al. US state life tables, 2019. Natl Vital Stat Rep. 2021;70(18):1–18. [PubMed] [Google Scholar]
- 14. Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6(11):3177–3192. [DOI] [PubMed] [Google Scholar]
- 15. Kaminetsky JC, Moclair B, Hemani M, et al. A phase IV prospective evaluation of the safety and efficacy of extended release testosterone pellets for the treatment of male hypogonadism. J Sex Med. 2011;8(4):1186–1196. [DOI] [PubMed] [Google Scholar]
- 16. Handelsman DJ, Conway AJ, Boylan LM. Pharmacokinetics and pharmacodynamics of testosterone pellets in man. J Clin Endocrinol Metab. 1990;71(1):216–222. [DOI] [PubMed] [Google Scholar]
- 17. Drugs.com . Testopel pellets prices, coupons and patient assistance programs. 2022. http://www.drugs.com.
- 18. Office-use catalog. Empower Pharmacy; 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.