Abstract
Diabetes is a major public health concern and natural easy-going remedies are being searched. Since Cinnamomum zeylanicum Blume has a low coumarin concentration and possible insulin-enhancing properties, it is preferred over all other cinnamon species. Although similar research has been done on humans, there have been very few studies on this particular species, and none among South Asians. Moreover, no human trial that properly described their intervening agent (C. zeylanicum) and checked its efficacy at the molecular level along with clinical variables was conducted. Therefore, the current research aimed to explore the effects of C. zeylanicum on the glycemic index, lipid profile, and expression of the protein tyrosine phosphatase 1 B (PTP1B) enzyme in the peripheral blood mononuclear cells (PBMC) in type 2 diabetes.
We examined the presence of bioactive compounds in young C. zeylanicum bark (Alba grade) from native Sri Lanka using gas chromatography-mass spectrometry, high-performance thin-layer chromatography, and thin-layer chromatography before introducing it in the clinical study where trans-Cinnamaldehyde was found to be a major chemical constituent (>60%). Then, from January 2020 to March 2022, a randomized double-blinded placebo-controlled trial was carried out in the Diabetic Clinic at AIIMS Rishikesh. A total of 154 diabetic patients were enrolled and were taken either cinnamon or placebo capsules (1.5 g/day) for 120 days on an empty stomach with warm water along with their conventional treatment. Reduction in fasting blood glucose levels in the cinnamon group was found -35.50% (95% CI, -173 to 58.4), whereas in the placebo group change was 5.00% (95% CI, -165 to 224). For glycosylated hemoglobin, it differed -0.85% (95% CI, -8.2 to 1.6) in the cinnamon group compared to the placebo where it was found 0.15% (95% CI, -6.1 to 5.5). PTP1B expression in PBMC was determined from pre- and post-trial blood samples using the Western Blot, and significant inhibition was also observed (p=0.039). The study result depicts, C. zeylanicum is emerging as a beneficial plant for type 2 diabetes in Northern India and could be used as an adjunctive treatment rather than as a standalone managerial remedy.
Keywords: ceylon cinnamon, diabetes mellitus, protein tyrosine phosphatase, fasting blood glucose, cinnamomum zeylanicum, plant compound
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic complex metabolic disease with impaired insulin sensitivity and diminished insulin secretion. It is caused by multiple etiologies and the confluence of genetic, environmental, and behavioral risk factors, including inactivity, sedentary behavior, cigarette smoking, and excessive alcohol consumption [1]. The prevalence of T2DM has been progressively growing globally. Among people over the age of 18 years, it has hiked from 4.7% in 1980 to 8.5% in 2014 and was expected to reach 9.3% in 2019 [2,3]. Limited options for decreasing blood sugar, adverse effects of modern medicine, unpleasant insulin therapy, unexpected hypoglycemia, and the expense of treatment necessitate the quest for a hassle-free cure. Since the 1990s, when nuclear receptor proteins that regulate gene expression were recognized as a potential therapeutic target against diabetes, cinnamon had become the subject of research [4]. The name "cinnamon" is derived from the Latin and medieval French intermediate forms of the word "kínnamon," which means "sweet wood" [5]. Various species belonging to the Lauraceae family are cultivated as sources of cinnamon spice such as "Ceylon cinnamon" or "Cinnamomum zeylanicum," "Chinese cinnamon," or "Cinnamomum cassia," "Vietnamese cinnamon" or "Cinnamomum loureiroi," "Malabar cinnamon" or "Cinnamomum tamala," "Indonesian cinnamon" or "Cinnamomum burmannii," and "Camphor laurel" or "Cinnamomum camphora" [6]. Cinnamon extracts (CE) containing trans-Cinnamaldehyde (majorly found in cinnamon bark) and phenolic compound (majorly found in leaf) may improve insulin sensitivity, and insulin release by inhibiting the enzyme protein tyrosine phosphatase 1B [6]. CE improves type 2 diabetes by prompting GLUT 4 translocation, a major glucose transporter in skeletal muscle and adipose tissue which plays a key role in the uptake of glucose from the bloodstream, storing it as glycogen, and oxidizing it to produce energy [7]. CE affects the genes related to lipid metabolism, e.g., fatty acid synthase, sterol-responsible binding protein-1c, lipoprotein lipase, hormone-sensitive lipase, etc., in a way to control the metabolic biohazards accompanied by diabetes [8]. Pyruvate kinase (PK) and phosphoenol pyruvate carboxykinase (PEPCK) are two more important enzymes that regulate hepatic glucose metabolism by increasing glycolysis and inhibiting gluconeogenesis. In diabetes, the diminished PK activity causes a reduction of glycolysis and elevated PEPCK activity catalyzes gluconeogenesis. CE reverses the increased hepatic PEPCK mRNA expression and restoration to near-normal values of PK [9]. It also decreases plasma oxidative stress markers and delays stomach emptying [10,11].
Though animal studies had provided evidence of the positive effects of cinnamon and there is gaining popularity in the traditional use against diabetes [12], very few human clinical trials on this particular species only in the Iranian population make up the paucity of sufficient scientific evidence as well as there has been no work proving it at the biochemical level in humans [13,14]. Studies on other species of cinnamon (C. cassia or C. burmannii) had also some clashes, e.g., lack of species name, origin, quality, purity, grade, etc. Methodological differences across previous studies (small sample size, dose, duration of trial, form of intervention, e.g., grind bark to water or alcohol extract), disparities in outcomes, and lack of hands-on proof from bimolecular levels in humans have made it imperative to carry out. Using cinnamon powder as a supplement in the diet could be inexpensive and combined with other diabetes care strategies it could lower blood sugar levels more successfully in nations like India where it is widely accessible. So, a well-defined methodology was used to develop and design the current randomized clinical trial (RCT) in North India.
Materials and methods
For our research certified young C. zeylanicum Blume bark (Alba grade) was imported from the farm “True Ceylon Spice” of native Sri Lanka. After that, phytochemical analysis had been conducted using gas chromatography-mass spectrometry (GCMS), high-performance thin-layer chromatography (HPTLC), and thin-layer chromatography (TLC) in the Department of Natural products, National Institute of Pharmaceutical Education and Research (NIPER), Mohali, India. This was done to assess the purity and also to determine the active compound present in those imported barks prior to performing the trial and then crude bark powder was used for the actual randomized trial in the capsulated form.
Essential oil extraction
Dried bark (100 g powder) was subjected to steam distillation in a Clevenger-type apparatus for 8 hours. The distillate containing water along with essential oil was transferred into a 250 mL separating funnel. Dichloromethane (DCM) was added to the separating funnel for performing partitioning. The separating funnel was then capped tightly and shaken vigorously with occasional venting. When the two layers were separated, the lower DCM layer was transferred to a clean conical flask. The extraction process was repeated with two more portions of DCM and all of the DCM fractions were collected. Then, the pooled DCM layers were washed again with distilled water followed by separating into a dry container. The traces of aqueous solvent if present in the pooled fraction were removed by passing it through anhydrous sodium sulfate (Na2SO4). The combined DCM fraction was finally evaporated using a rotary evaporator. The residue was obtained in the form of a yellow oil (0.8 mL).
TLC analysis
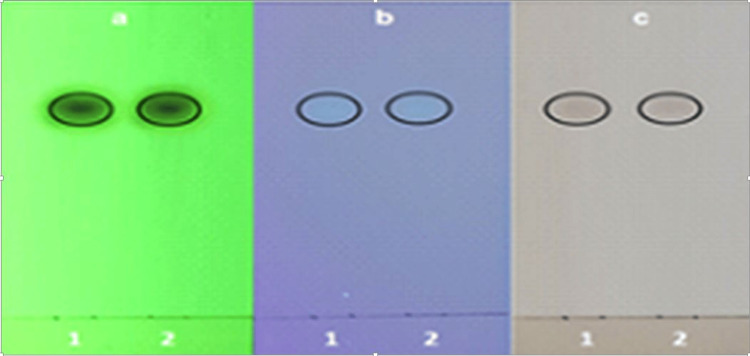
The isolated compound (cinnamaldehyde) from C. zeylanicum bark oil was dissolved in chloroform (CHCl3) and then applied on a silica gel TLC plate and developed using a mobile phase comprising of toluene:ethyl acetate (EtOAc) (8:2). The TLC plate was visualized under UV light. The standard trans-cinnamaldehyde was used as a reference standard (Figure 1).
Figure 1. TLC profile of cinnamon oil with the trans-cinnamaldehyde standard.
TLC profile at (a) 254 nm and at (b) 330 nm, (c) after derivatization (anisaldehyde sulphuric acid reagent) (1: cinnamaldehyde standard, 2: cinnamon oil).
Soxhlet extraction
A 100 g of bark material was used for the soxhlet extraction using methanol (MeOH) solvent. After completion of the soxhlet extraction (24 hours) methanol was concentrated using a rotary evaporator to give 8.66 g of the dried extract. Of the total extract, 6 g was further used for partitioning using hexane, EtOAc, and n-butanol (n-BuOH) in the order of increasing polarities. The amount of hexane extract, EtOAc extract and n-BuOH extract obtained after partitioning are 700 µL, 1.71 g, and 3.2 g, respectively.
HPTLC analysis
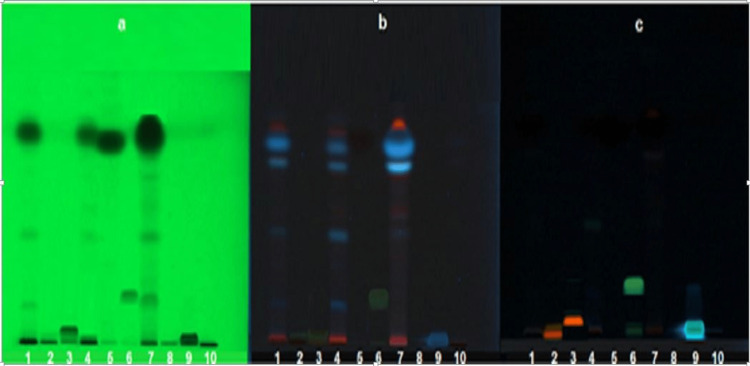
It was performed using two different mobile phases. The results with each of the mobile phases were recorded in Figures 2, 3.
Figure 2. HPTLC profile at 254 nm (a), HPTLC profile at 330 nm (b), TLC profile after derivatizing with natural product (NP) reagent (c).
1: methanol extract, 2: rutin standard, 3: quercetin standard, 4: EtOAc extract, 5: cinnamaldehyde standard, 6: kaempferol standard, 7: hexane extract, 8: catechin standard, 9: caffeic acid standard, and 10: butanol extract.
TLC Plate 1: Mobile Phase used: Toluene: EtOAc (8:2)
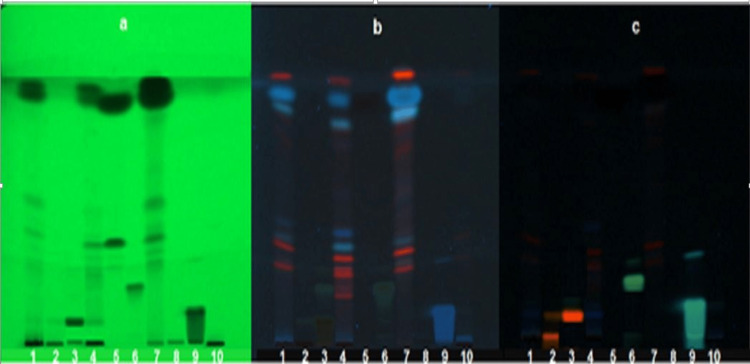
Figure 3. TLC profile at 254 nm (a), TLC profile at 330 nm (b), TLC profile after derivatizing with NP reagent (c).
1: methanol extract, 2: rutin standard, 3: quercetin standard, 4: EtOAc extract, 5: cinnamaldehyde standard, 6: kaempferol standard, 7: hexane extract, 8: catechin standard, 9: caffeic acid standard, and 10: butanol extract.
TLC Plate 2: Mobile Phase: Chloroform: Methanol: Formic acid (9.5:0.4:0.1).
HPTLC result
The TLC plates developed in two different mobile phases did not match the spots of standards which included rutin, quercetin, kaempferol, catechin, and caffeic acid. Only the presence of trans-cinnamaldehyde was confirmed with its standard using both HPTLC and TLC. The cinnamon oil percentage was calculated using the formula given as follows: % Oil = essential oil weight/sample weight × 100 × specific gravity = 0.8 mL/100g × 100 × 1.01 = 0.808. The percentage of cinnamon oil pre-reported in the bark of C. zeylanicum is 0.5-1%. The percentage of oil isolated was found to be 0.8% which is in the specified range [15].
GCMS analysis
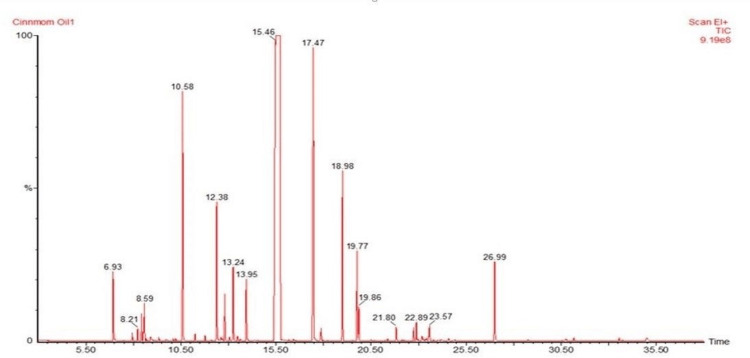
It was carried out to identify the chemical components present in the cinnamon oil obtained from the C. zeylanicum bark. Twenty-four chemical constituents were identified in the oil extracted from bark using the Clevenger apparatus viz. benzaldehyde, α-phellandrene, delta-3-carene, limonene, β-phellandrene, 3,7-dimethyl-1,6-octadien-3-ol, α-pinene, naphthalene-1,2,3,4,4a,7-hexahydro, endo-borneol, β-terpineol, d-limonene, cinnamaldehyde, eugenol, α-cubebene, copaene, α-cedrene, ylangene, caryophyllene, humulene, 3-methoxy cinnamaldehyde, caryophyllene alcohol, epiglobulol, α-santalol, and benzyl benzoate. The major components of the oil were found to be trans-cinnamaldehyde (33%) and its isomer (31%), followed by eugenol (7%), 3,7-dimethyl-1,6-octadien-3-ol (5%) caryophyllene (3%), naphthalene,1,2,3,4,4a,7-hexahydro (3%), benzyl benzoate (1.8%), d-limonene (1.5%), benzaldehyde (1.3%), and other constituents in trace amounts (Figure 4).
Figure 4. GC-MS spectrogram of cinnamon oil.
GC-MS: gas chromatography-mass spectrometry
Trans-cinnamaldehyde is present as a major chemical constituent (>60%) in the cinnamon essential oil extracted through the Clevenger apparatus, confirmed by HPTLC, TLC, and GCMS analysis by using the reference standard of cinnamaldehyde. However, HPTLC analysis revealed the absence of rutin, catechin, kaempferol, quercetin, caffeic acid, and other polyphenols in the bark extracts of C. zeylanicum.
Capsule making
After the quality checking of cinnamon bark, the authors prepared both cinnamon and placebo capsules with the help of “Green Gold Pharmaceuticals,” Haridwar, India. These pharmaceuticals are a non-profit foundation for making Ayurvedic medicines, herbal extracts, essential oils, crude herbs, etc. A manual method was used and nearly about one-week period was taken to complete the whole process. Here, the cinnamon capsule contained 500 mg C. zeylanicum grind bark powder and the placebo capsule contained 500 mg grind Bengal gram flour. Both capsules were white in color and identical in shape. Both boxes were white, identical in shape, have the same aroma spray before closing, and were labeled as “Ayurvedic medicine for diabetes.” We didn’t make any extract capsules as our aim was to use whole-product in the clinical trial to evaluate effectiveness in their natural form.
Randomized controlled trial
The RCT was conducted from January 2020 to March 2022 in the Diabetic Clinic at All India Institute of Medical Sciences (AIIMS), Rishikesh. A total of 697 T2DM patients were assessed for the eligibility criteria and finally, 154 people were recruited as per the estimated sample size. The sample size estimation was done through GPower software 3.1 with moderate effect size 0.5, α 0.05, power 0.8, and 20% attrition rate. Patients with (1) non-insulin-dependent type 2 diabetes, (2) age more than 30 years (3) glycosylated hemoglobin 6.5, (4) fasting blood glucose level 126 mg/dL, (5) cooperative and want to participate, (6) no intake of herbs or other complementary therapy in recent eight weeks, (7) no acute infection (pneumonia, urinary tract infection, otitis), and (8) no insulin therapy were included in the study. Chain smoking, heavy alcohol, pregnancy and lactation, allergy or sensitivity to cinnamon, liver disease, and critically ill patients were excluded from the trial.
The study was randomized where block randomization had been done through a computer-generated random list created by sealedenvelope.com (block size four). Participants were randomized to both groups (77 persons in each group) and made their allocation concealed. The trial was double-blinded as researchers, e.g., recruiting physicians, blood sample collectors, and outcome assessors along with participants were blind. Allocation concealment and blinding were maintained throughout the trial. After taking written informed consent from participants and completing socio-demographic proforma, the pretrial blood sample was drawn for fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), and lipid profiles. Thereafter, cinnamon 500 mg three capsules daily were taken by the experimental group in the morning on an empty stomach with warm water for 120 days or four months. The control group did the same with placebo capsules (which look identical, white in color) containing finely grounded Bengal gram flour. The outcome was re-investigated at the end of the study as a routine checkup. Conventional management (dietary modification, exercise, and oral hypoglycemic agent, e.g., metformin, glimepiride, teneligliptin, was continued for both groups during the study. The third person who was a medicine box supplier and was responsible to analyze PTP1B was open and took 10% pre- and post-trial blood samples of the experimental group in a randomized way from the sample collection center separately.
Sample collection procedure
Whole blood sample in fasting (at least eight hours) condition was drawn from a brachial vein in a sitting position with the help of a needle 22-gauge, needle holder, and tourniquet from study participants at the sample collection center of AIIMS, Rishikesh. Blood samples were collected in one ethylenediaminetetraacetic acid (EDTA) (lavender color, 2 mL), one sodium fluoride (gray color, 2 mL), and one non-anticoagulant (red, 4 mL) vials for estimation of HbA1c, FBG, and lipid profile, respectively. Thereafter study participants were undergone with their concealed allocated intervention or placebo for four months with their previous prescribed treatment. After completion of the trial, fasting blood samples of FBG, HbA1c, and lipid profiles were re-collected in the same way. The collection of blood had been performed by health care professionals only. The aseptic technique was maintained. No extravasation injury was noticed.
Fasting blood glucose analysis
Blood glucose analysis was performed with the enzymatic hexokinase method on the Fully Automated Chemistry Analyser of Beckman Coulter Inc. (Brea, CA), model no. Au480, installed at the Clinical Biochemistry Laboratory, AIIMS, Rishikesh.
Estimation of glycosylated hemoglobin
HbA1c is the greatest indicator of average glucose levels during the previous one to three months. Estimation of HbA1c was conducted on TOSOH.HLC-723 (Tessenderlo, Belgium: Tosoh Europe N.V.) ion-exchange high-performance liquid chromatography principle at the Clinical Biochemistry laboratory AIIMS Rishikesh.
Lipid profile estimation
Fasting levels of serum triacylglycerol, total cholesterol, low-density lipoprotein, and high-density lipoprotein were also estimated on AU680 fully automated clinical chemistry analyzer at the Clinical Biochemistry Laboratory, AIIMS, Rishikesh.
Western blot
Expression of protein tyrosine phosphatase 1B was compared against beta-actin expression in the Bioengineering Lab of the Indian Institute of Technology, Roorkee. Total protein lysates were prepared from the collected patient PBMCs using radioimmunoassay (RIPA) buffer (50 mM Tris-HCL, 150 mM NaCl, 1% NP40, 0.1% sodium deoxycholate, 0.1% SDS, pH 7.4) containing protease inhibitor cocktail. Protein concentrations were determined using the Bradford method (BioRad) then equal amounts of proteins were prepared in Laemmli buffer for SDS-PAGE and separated by 12% SDS-polyacrylamide gel. The separated proteins were electro-transferred into a polyvinylidene difluoride (PVDF) membrane. The membranes were blocked with 3% BSA in TBST (20 mM, Tris, pH 7.5, 150 mM NaCl, and 0.1% Tween 20) for an hour at room temperature with constant rocking. The primary antibody (PTP1B rabbit the polyclonal antibody, catalog no. 11334-1-AP, Proteintech) was diluted at 1:2000 with 3% BSA in TBST and incubated for 2 hours at room temperature. Membranes were washed three times (10 minutes each) with TBST and incubated for 1 hour in horseradish peroxidase-conjugated secondary antibodies (Goat Anti-Rabbit IgG, catalog no. SA00001-2, Proteintech) diluted at 1:5000 in 3% BSA in TBST at room temperature [16,17].
The research has followed the guidelines of the Declaration of Helsinki and Tokyo for humans and was approved by the institutional review board (#AIIMS/IEC/18/577), AIIMS Rishikesh. The trial was registered in Clinical Trial Registry, registration no. CTI/2019/04/018386.
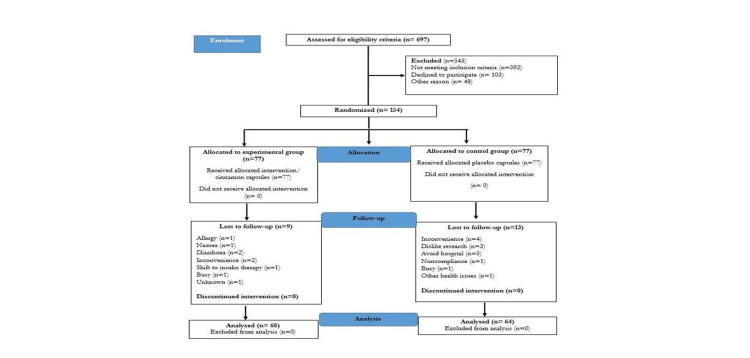
A total of 132 participants out of 154 had completed the trial. The reason for the 14% dropout was explained in Figure 5 (CONSORT flow diagram, as per 2010 guidelines). However, some of the reasons were allergies, nausea, diarrhea, inconvenience, non-compliance, avoiding the hospital, shift to insulin therapy, etc. No adverse effect was found during the trial and per protocol, analysis had been done for 68 participants in the experimental group and 64 participants in the control group.
Figure 5. CONSORT flow diagram as per 2010 guidelines.
CONSORT: Consolidated Standards of Reporting Trial
Statistical analysis
Statistical analysis had been performed based on the data. Categorical data were reported as frequency and percentages. The difference between categorical data was assessed by chi-square test. Continuous data were reported as mean±standard deviation (SD), or median (minimum to maximum) for non-normally distributed data, which was tested by use of the Kolmogorov-Smirnov and Shapiro-Wilk test. The difference between the intergroup mean±SD was tested by an independent sample t-test and the inter-group median (minimum to maximum) was tested by the Mann-Whitney U test. The differences between clinical data (non-normally distributed) were analyzed by the Mann-Whitney U test (2-tailed non-parametric) where intra-individual delta values were taken for calculating the difference. P-values less than 0.05 were regarded as statistical significance. It was a per-protocol analysis, performed by the use of IBM SPSS Statistics for Windows, version 23 (Armonk, NY: IBM Corp.) [18].
Results
Among socio-demographic details data regarding age, BMI, gender, marital status, education, occupation, religion, type of family, habitat, and duration of diabetes were obtained using a self-structured questionnaire. The data was tabulated and depicted in Table 1. Table 2 depicted the changes in fasting blood glucose, glycosylated hemoglobin, triglycerides, total cholesterol, low-density lipoprotein, and high-density lipoprotein from the initial to the end result of the trial.
Table 1. Socio-demographic characteristics of subjects from both groups.
*P-value <0.05 is considered significant.
**Independent sample t-test.
***Mann-Whitney U test.
****Chi-square test.
| S. no. | Variable | Category | f (%) | p-Value* | |
| Experimental group (n=68) | Control group (n=64) | ||||
| 1 | Age | Mean±SD | 52.09±11.88 | 53.55±10.78 | 0.463** |
| 2 | BMI | Median (min to max) | 23.90 (18.9 to 32.4) | 23.60 (17.6 to 36.9) | 0.733*** |
| 2 | Gender | Male | 47 (69.1) | 43 (67.2) | 0.812**** |
| Female | 21 (30.9) | 21 (32.8) | |||
| 3 | Marital status | Single | 4 (5.9) | 1 (1.6) | 0.164**** |
| Married | 63 (92.6) | 59 (92.2) | |||
| Widow | 1 (1.5) | 4 (6.3) | |||
| 4 | Educational status | Illiterate | 2 (2.9) | 7 (10.9) | 0.170**** |
| Primary | 6 (8.8) | 9 (14.1) | |||
| Secondary | 25 (36.8) | 17 (26.6) | |||
| Diplomat and above | 35 (51.5) | 31 (48.4) | |||
| 5 | Occupational status | Household | 15 (22.1) | 22 (34.4) | 0.537**** |
| Service | 28 (41.2) | 17 (26.6) | |||
| Daily labor | 4 (5.9) | 3 (4.7) | |||
| Retired | 10 (14.7) | 10 (15.6) | |||
| Business | 10 (14.7) | 11 (17.2) | |||
| Unemployed | 1 (1.5) | 1 (1.6) | |||
| 6 | Religion | Hindu | 65 (95.6) | 57 (89.1) | 0.157**** |
| Muslim | 3 (4.4) | 7 (10.9) | |||
| 7 | Type of family | Nuclear | 31 (45.6) | 34 (53.1) | 0.387**** |
| Joint | 37 (54.4) | 30 (46.9) | |||
| 8 | Habitat | Urban | 54 (79.4) | 47 (73.4) | 0.418**** |
| Rural | 14 (20.6) | 17 (26.6) | |||
| 9 | Duration of diabetes | <2 years | 22 (32.4) | 14 (21.9) | 0.164**** |
| 2-5 years | 15 (22.1) | 23 (35.9) | |||
| >5 years | 31 (45.6) | 27 (42.2) | |||
Table 2. Comparison of biochemical indices before and after trial.
*P-value was calculated on intra-individual delta values and was considered as significant <0.05.
Data represented as median (minimum to maximum); difference analyzed by Mann-Whitney U test.
| S. no. | Variables | Experimental group (n=68) | Control group (n=64) | p-Value |
| 1 | FBG (mg/dL) | |||
| Initial | 162.50 (109 to 370) | 151.00 (102 to 335) | 0.000* | |
| End | 125.00 (80 to 292) | 143.50 (83 to 383) | ||
| Difference | -35.50 (-173 to 58.4) | 5.00 (-165 to 224) | ||
| 2 | HbA1C (%) | |||
| Initial | 8.95 (6.3 to 15.2) | 7.80 (5.9 to 12.8) | 0.000* | |
| End | 7.55 (5.8 to 11.6) | 7.80 (5.3 to 14.8) | ||
| Difference | -0.85 (-8.2to 1.6) | 0.15 (-6.1 to 5.5) | ||
| 3 | Triglycerides (mg/dL) | |||
| Initial | 132.00 (48 to 453) | 131.00 (48 to 574) | 0.176 | |
| End | 123.80 (34 to 737) | 143.00 (60 to 550) | ||
| Difference | -20.00 (-269 to 433) | -4.00 (-270 to 229) | ||
| 4 | Total cholesterol (mg/dL) | |||
| Initial | 196.00 (90 to 281) | 188.00 (71 to 306) | 0.74 | |
| End | 188.50 (80 to 319) | 196.00 (75 to 306) | ||
| Difference | 7.50 (-114 to 124) | 4.00 (-78 to 153) | ||
| 5 | LDL-C (mg/dL) | |||
| Initial | 109.00 (40 to 168) | 100.00 (30 to 203) | 0.047* | |
| End | 116.10 (28 to 185) | 114.00 (37 to 195) | ||
| Difference | -4.00 (-83 to 89) | 7.00 (-60 to108) | ||
| 6 | HDL-C (mg/dL) | |||
| Initial | 45.00 (25 to 84) | 46.00 (33.2 to 184.8) | 0.572 | |
| End | 46.00 (17 to 86) | 46.00 (31 to 70) | ||
| Difference | -0.50 (-48 to 23.5) | -0.50 (-134.8 to 25.8) | ||
As indicated in Table 2, there was a significant decrease in FBG, and HbA1C levels in the cinnamon group compared to the placebo (p=0.000). No such reduction was observed in the placebo group. LDL-C level was found to increase in the placebo group compared to cinnamon (p=0.047). There were no notable changes in the levels of triglycerides, total cholesterol, and HDL cholesterol (p>0.05).
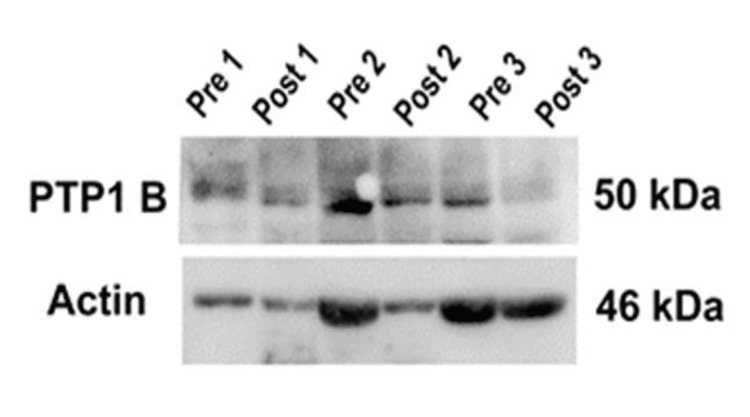
To determine the inhibition of protein tyrosine phosphatase, the western blot method was applied and beta-actin was taken as a housekeeping gene. A total of 13 participants’ blood samples from the cinnamon group who had higher glycosylated hemoglobin at the time of the pre-test and maintained good compliance throughout the trial were collected randomly. One obese non-diabetic control was also taken. Therefore, every participant had four bands of protein: pre-PTP1B, post-PTP1B, pre-beta-actin, and post-beta-actin (Figure 6).
Figure 6. Changes in protein band of protein tyrosine phosphatase 1B and beta-actin before and after the trial.
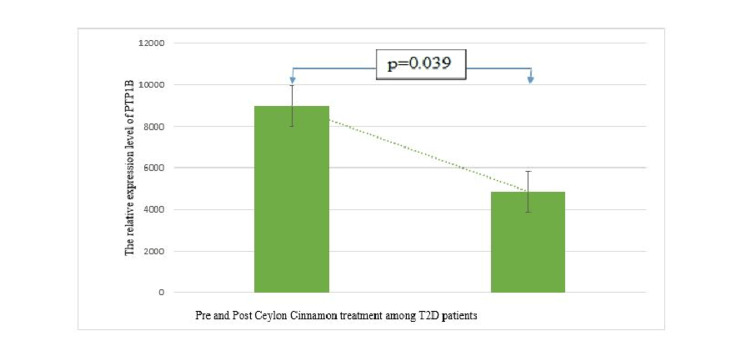
Figure 7 represents the inhibition of protein tyrosine phosphatase in peripheral blood mononuclear cells of type 2 diabetes patients after consuming C. zeylanicum for four months. Student's t-test had been used for statistical analysis between the mean pre- and post-PTP1B band’s intra-optical density. Such results depict the anti-diabetic properties of C. zeylanicum.
Figure 7. Expression of protein tyrosine phosphatase 1B in pre- and post-C. zeylanicum treatment.
Discussion
Besides the multiple etiology, complex treatment regimens, unexpected hypoglycemia, high expenditure on treatment, patients' mistaken beliefs, and the negative impacts of modern medicine have all been cited as reasons for low compliance and poor adherence to current treatment methods for diabetes. While plant resources became a top priority in the quest for new medicines, cinnamon or dalchini, the common aromatic condiment obtained from the inner bark of the tree species genus Cinnamomum was touted as a promising spice for diabetes management [19].
A 62.09-89.31% of trans-Cinnamaldehyde could be found in cinnamon bark oil as a major compound and in the present research, it was found in nearly 64% which was in the pre-specified range [20-22]. Although the use of Ceylon cinnamon against diabetes was mentioned in the Charaka Samhita and cassia was well-known in Chinese folk medicine. Khan et al. in 2003 were the first to show scientific evidence through a human trial on cassia bark powder (1 g, 3 g, and 6 g) in Pakistan, where type 2 diabetic participants' fasting blood glucose and lipid profile was significantly reduced over the course of 40 days [23]. Following that trial, Crawford in 2009 in Las Vegas, Akilen et al. in 2010 in London, and Sengsuk et al. in 2015 in Thailand conducted research with a similar methodology and approved that 1-2 g of grind cinnamon cassia for 2-3 months could decrease HbA1C in type 2 diabetes [24-26].
But comparable results were not found by Vanschoonbeek et al. in 2006 in the Netherlands, Suppapitiporn et al. in 2006 in Thailand, Blevins et al. in 2007 in Oklahoma City, US, and Hasanzade et al. in 2013 in Iran [27-30]. The study of Vanschoonbeek et al. was primarily confined small sample size, a short duration, and solely female participants (post-menopausal women) who might be experiencing hormonal changes. In the study of Blevins et al., both groups were different by age, multi-ethnicity in a small study population was seen and the initial value of outcome variables was near to normal range. The study by Hasanzade et al. in 2013 majorly performed on a low socio-economic group of participants (housekeepers) which makes a methodological flaw in the research [30]. Besides those factors, the lack of a proper description of the intervening agent is a major discrepancy across all the studies. It's sometimes only described as to where it was acquired, e.g., from a local vendor. Cinnamon's origin, geographical wellness, and limit were unknown, and no validation or authentication was offered. It should be explained in detail, including the type of cinnamon, its origin, grade, whether it was new or old, and the optimal time to utilize it. Khan et al. in 2010 in Pakistan and Sharma et al. in 2012 in India didn't even specify the species' name though these studies conveyed a significant decrease in anthropometric, glycemic, and lipid index of patients with type 2 diabetes after consuming cinnamon bark [31,32].
When talking about extract, Ziegenfuss et al. in 2006 and Anderson et al. in 2016 employed cassia extract 500 mg/day for three and two months, respectively, and found reduced plasma glucose in type 2 diabetes [33,34]. The use of cassia extract for diabetes was positively impacted by intergroup differences in glycosylated hemoglobin along with fasting blood glucose levels by Lu et al. 2012 [35]. However, in those studies, there was no precise explanation of extract synthesis. Depending on the species and even the formulation, the number of active molecules (cinnamaldehyde otherwise polyphenols) may vary. Furthermore, phytochemicals may be harmed by differences in manufacturing techniques, because herbal drugs aren't subjected to similar quality control standards as other pharmacy items. Because cinnamon contains an evaporating agent, the essential oil might evaporate over time if it was old or mishandled, and there would be no active molecule to work with at that moment. The species of cinnamon was predetermined in the current investigation, along with the plant's age, location, and quality of the sticks. According to the plant list at http://www.theplantlist.org, C. zeylanicum Blume is a synonym of Cinnamomum verum J. Presl which is currently accessible in a variety of forms, including quills (usually 42-inch cinnamon sticks), quilling (broken cinnamon tubes), bark powder, bark oil, and leaf oil. The diameter of the cinnamon quills is divided into the following four classes in Sri Lankan grading: Alba (<0.24 inches), Continental (<0.63 inches), Mexican (<0.75 inches), and Hamburg (<1.3 inches). C. zeylanicum bark from native Sri Lanka was collected as young, fresh bark (Alba grade) for investigation. It was also measured whether the product was pure and legitimate and whether trans-cinnamaldehyde and eugenol, two active compounds, were present in the ground bark, and capsules were prepared just after grinding for best durability and to provide an equal quantity to the study subjects.
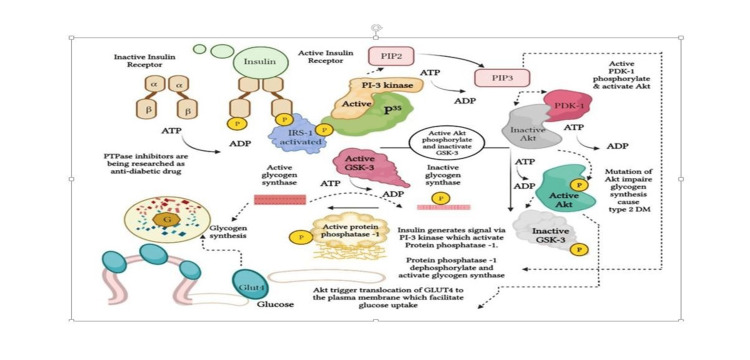
Vafa et al. carried out the first study on C. zeylanicum in Tehran, Iran, in 2012 where 44 participants with type 2 diabetes were enrolled (22 persons in each group; cinnamon and placebo), and 3g of grind C. zeylanicum was administered in the treatment group for eight weeks. Despite the study's small sample size and brief duration, the cinnamon group showed significantly lower FBG, HbA1c, and triglyceride levels than others [13]. Similar results regarding C. zeylanicum had also been found in the study by Zare et al. in 2019 in Iran [14]. The study was conducted on a relatively large sample size (138 participants) where cinnamon supplementation for three months significantly decreased levels of fasting and post-prandial blood glucose, HbA1c, triglycerides, total cholesterol, LDL cholesterol, and a significant increase in HDL cholesterol. The current research had been performed on 132 participants, and 1.5 g grind C. zeylanicum bark per day for four months resulted in a significant reduction in fasting blood sugar, and glycosylated hemoglobin among type 2 diabetic patients. Though present research didn’t observe any change in the levels of triglyceride and other lipids which might be related to a low dose of cinnamon or the near-normal value of the initial level. Along with significant inhibition of PTPase expression in peripheral blood mononuclear cells after four months of oral consumption of Ceylon cinnamon bark evidences it as an anti-diabetic herb from a biomolecular level (Figure 8).
Figure 8. Schematic representation of active cinnamon compound in insulin signaling transduction.
The image is created by the author (Anindita Mandal) of this study.
When insulin binds to α unit of the insulin receptor, phosphorylation of tyrosine protein residue of β unit takes place. Kinase enzyme helps in the addition of phosphate from ATP to tyrosine amino acid is termed tyrosine kinase. The opposite enzyme protein tyrosine phosphatase removes a phosphate group from the target molecule and causes de-phosphorylation that inactivates insulin receptors followed by insulin resistance [36-38]. There is evidence that certain diabetic individuals have a variant of PTPase that is abnormally active and for this purpose, PTPase inhibitors, e.g., cinnamon are being explored (Figure 8) [39]. The result was similar to the study by Saifudin et al. in 2013 and Imparl-Radosevich et al. in 1998. Water and methanol extracts of Cinnamomum burmannii exhibited ≥70% inhibition at 25 μg/mL (IC50, 2.47 μg/mL) which was comparable with that of the positive control, RK-682 (IC50, 2.05 μg/mL). The PTP1B inhibitory activity of the constituents of C. Burmannii was then evaluated and trans-cinnamaldehyde (5; IC50, 57.6 μM) was found as an active constituent [40,41].
Though there's the fact that this type of human trial is difficult, as it would be among primary care patients. Trials of this nature could not be tightly controlled. Doctor shopping, medication changes, dose adjustments by patients exclusively, treatment discontinuation, participants' failure to comply with dietary restrictions, workouts, interactions with several pharmacotherapies, and lack of follow-up all had a significant impact on the trial's outcome. In the present study, researchers tried to control the extraneous variables by discussing with participants for both groups. The researchers didn’t explore about contents of the capsules, neither cinnamon nor placebo, and never prioritize anyone in front of the participants.
Major limitations of the present study are as follows: this is a single-centered study in single ethnic group and only one enzyme PTPase was studied through a single technique. Because topographical changes affect body mechanics, the study might be reproduced as a multi-centric trial including a broad population in a different geographic area. Instead of using the western blot, other biochemical tests could be utilized to acquire more specific information at the molecular level. Genes involved in the insulin pathway, rather than the insulin molecule, could also be assessed.
Conclusions
C. zeylanicum has the potential to improve the glycemic index in persons with poorly managed diabetes. Its anti-diabetic properties have made it an herbal adjuvant treatment modality. This was further substantiated by the fact that ingestion of Ceylon cinnamon inhibited protein tyrosine phosphatase in peripheral blood mononuclear cells of type 2 diabetic individuals. Triglycerides, total cholesterol, and HDL-C levels were all unaltered. The clinical relevance and durability of these effects in multi-centric trials have yet to be determined. It's still worth looking into genes linked to glycemic pathways after consumption of C. zeylanicum as future prospects of research.
Acknowledgments
Special thanks to Dr. Somanath Kundu, Postdoctoral fellow, Department of Neuroscience and Physiology, State University of New York, and Dr. Alok Goyal, Botanist, Department of Natural Products at NIPER, Mohali for their sincere help in this study.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Funding Statement
This research was supported by an institute based (All India Institute of Medical Sciences, Rishikesh) intramural research grant (#IM/RC138/2019/01).
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Institutional Review Board of AIIMS Rishikesh issued approval #AIIMS/IEC/18/577. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of All India Institute of Medical Sciences, Rishikesh
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Type 2 diabetes mellitus: a review of current trends. Olokoba AB, Obateru OA, Olokoba LB. Oman Med J. 2012;27:269–273. doi: 10.5001/omj.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO global report on diabetes: a summary. Roglic G. https://www.ijncd.org/article.asp?issn=2468-8827;year=2016;volume=1;issue=1;spage=3;epage=8;aulast=Roglic#:~:text=Although%20the%20genetic%20component%20is,factor%20is%20increased%20body%20fat. Int J Non-Commun Dis. 2016;1:3–8. [Google Scholar]
- 3.Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Saeedi P, Petersohn I, Salpea P, et al. Diabetes Res Clin Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 4.Improved insulin resistance and lipid metabolism by cinnamon extract through activation of peroxisome proliferator-activated receptors. Sheng X, Zhang Y, Gong Z, Huang C, Zang YQ. PPAR Res. 2008;2008 doi: 10.1155/2008/581348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Medagama AB. Nutr J. 2015;14 doi: 10.1186/s12937-015-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Is cinnamon efficacious for glycaemic control in type-2 diabetes mellitus. Sharma S, Mandal A, Kant R, Jachak S, Jagzape M. https://pubmed.ncbi.nlm.nih.gov/33341863/ J Pak Med Assoc. 2020;70:2065–2069. [PubMed] [Google Scholar]
- 7.Hydro-alcoholic cinnamon extract, enhances glucose transporter isotope- 4 translocation from intracellular compartments into the cytoplasmic membrane of C2C12 myotubes. Absalan A, Mohiti-Ardakani J, Hadinedoushan H, Khalili MA. Indian J Clin Biochem. 2012;27:351–356. doi: 10.1007/s12291-012-0214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cinnamon extract regulates gene expression of lipids and carbohydrate metabolism in streptozotocin-induced diabetic Wistar rats. Soliman MM, Ahmed MM, El-Shazly SA. Am J Biochem Biotechnol. 2013;9:172–182. [Google Scholar]
- 9.Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Anand P, Murali KY, Tandon V, Murthy PS, Chandra R. Chem Biol Interact. 2010;186:72–81. doi: 10.1016/j.cbi.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. Roussel AM, Hininger I, Benaraba R, Ziegenfuss TN, Anderson RA. J Am Coll Nutr. 2009;28:16–21. doi: 10.1080/07315724.2009.10719756. [DOI] [PubMed] [Google Scholar]
- 11.Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Hlebowicz J, Hlebowicz A, Lindstedt S, et al. Am J Clin Nutr. 2009;89:815–821. doi: 10.3945/ajcn.2008.26807. [DOI] [PubMed] [Google Scholar]
- 12.Ethnopharmacological Uses and Public Knowledge Regarding Cinnamomum zeylanicum in Khobar, Saudi Arabia. Almubayedh H, Ahmad R, Naqvi AA, Ahmad N. J Pharm Bioallied Sci. 2018;10:159–165. doi: 10.4103/jpbs.JPBS_71_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Effects of cinnamon consumption on glycaemic status, lipid profile and body composition in type 2 diabetic patients. Vafa M, Mohammadi F, Shidfar F, Sormaghi MS, Heidari I, Golestan B, Amiri F. https://pubmed.ncbi.nlm.nih.gov/22973482/ Int J Prev Med. 2012;3:531–536. [PMC free article] [PubMed] [Google Scholar]
- 14.Efficacy of cinnamon in patients with type II diabetes mellitus: a randomized controlled clinical trial. Zare R, Nadjarzadeh A, Zarshenas MM, Shams M, Heydari M. Clin Nutr. 2019;38:549–556. doi: 10.1016/j.clnu.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Simultaneous quantitative determination of cinnamaldehyde and methyl eugenol from stem bark of Cinnamomum zeylanicum Blume using RP-HPLC. Gursale A, Dighe V, Parekh G. J Chromatogr Sci. 2010;48:59–62. doi: 10.1093/chromsci/48.1.59. [DOI] [PubMed] [Google Scholar]
- 16.PTPN2 downregulation is associated with albuminuria and vitamin D receptor deficiency in type 2 diabetes mellitus. Zheng L, Zhang W, Li A, Liu Y, Yi B, Nakhoul F, Zhang H. J Diabetes Res. 2018;2018 doi: 10.1155/2018/3984797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MAP kinase phosphatase DUSP1 is overexpressed in obese humans and modulated by physical exercise. Khadir A, Tiss A, Abubaker J, et al. Am J Physiol Endocrinol Metab. 2015;308:71–83. doi: 10.1152/ajpendo.00577.2013. [DOI] [PubMed] [Google Scholar]
- 18.IBM Corp: released in 2015, IBM SPSS statistics for windows, version 23.0. http://www.ibm.com http://www.ibm.com
- 19.In vitro anticandidal activity of Cinnamomum verum. Vinitha M, Ballal M. J Med Sci. 2008;8:425–428. [Google Scholar]
- 20.Antimicrobial effect of cinnamon (Cinnamomum verum J. Presl) bark essential oil in cream-filled cakes and pastries. Vazirian M, Alehabib S, Jamalifar H, Fazeli MR, Toosi AN, Khanavi M. https://www.semanticscholar.org/paper/Antimicrobial-effect-of-cinnamon-(Cinnamomum-verum-Vazirian-Alehabib/1fe1e2cc1930e6fb74be20e3def5add6eab38b16 Res J Pharmacogn. 2015;2:11–16. [Google Scholar]
- 21.Extraction of cinnamon essential oil and identification of its chemical compounds. Kamaliroosta L, Gharachorloo M, Kamaliroosta Z, Alimohammad Zadeh KH. J Med Plants Res. 2012;6:609–614. [Google Scholar]
- 22.Cosmetic bio-product based on cinnamon essential oil “Cinnamomum verum” for the treatment of mycoses: preparation, chemical analysis and antimicrobial activity. Ainane T, Khammour F, Merghoub N, et al. MOJ Toxicol. 2019;5:5–8. [Google Scholar]
- 23.Cinnamon improves glucose and lipids of people with type 2 diabetes. Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Diabetes Care. 2003;26:3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 24.Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: a randomized, controlled trial. Crawford P. J Am Board Fam Med. 2009;22:507–512. doi: 10.3122/jabfm.2009.05.080093. [DOI] [PubMed] [Google Scholar]
- 25.Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic type 2 diabetic patients in the UK: a randomized, placebo-controlled, double-blind clinical trial. Akilen R, Tsiami A, Devendra D, Robinson N. Diabet Med. 2010;27:1159–1167. doi: 10.1111/j.1464-5491.2010.03079.x. [DOI] [PubMed] [Google Scholar]
- 26.Effect of cinnamon supplementation on glucose, lipids levels, glomerular filtration rate, and blood pressure of subjects with type 2 diabetes mellitus. Sengsuk C, Sanguanwong S, Tangvarasittichai O, Tangvarasittichai S. Diabetol Int. 2016;7:124–132. doi: 10.1007/s13340-015-0218-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. Vanschoonbeek K, Thomassen BJ, Senden JM, Wodzig WK, van Loon LJ. J Nutr. 2006;136:977–980. doi: 10.1093/jn/136.4.977. [DOI] [PubMed] [Google Scholar]
- 28.The effect of cinnamon cassia powder in type 2 diabetes mellitus. Suppapitiporn S, Kanpaksi N, Suppapitiporn S. https://pubmed.ncbi.nlm.nih.gov/17718288/ J Med Assoc Thai. 2006;89:200–205. [PubMed] [Google Scholar]
- 29.Effect of cinnamon on glucose and lipid levels in non insulin-dependent type 2 diabetes. Blevins SM, Leyva MJ, Brown J, Wright J, Scofield RH, Aston CE. Diabetes Care. 2007;30:2236–2237. doi: 10.2337/dc07-0098. [DOI] [PubMed] [Google Scholar]
- 30.The effect of cinnamon on glucose of type II diabetes patients. Hasanzade F, Toliat M, Emami SA, Emamimoghaadam Z. J Tradit Complement Med. 2013;3:171–174. doi: 10.4103/2225-4110.114900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cinnamon may reduce glucose, lipid, and, Cholesterol level in type 2 diabetic individuals. Khan R, Khan Z, Shah S. Pakistan J Nutr. 2010;9:430–433. [Google Scholar]
- 32.A randomized double blind placebo control trial of cinnamon supplementation on glycaemic control and lipid profile in type 2 diabetes mellitus. Sharma P, Sharma S, Agrawal RP, Agrawal V, Singhal S. https://www.researchgate.net/publication/288189632_A_randomised_double_blind_placebo_control_trial_of_cinnamon_supplementation_on_glycemic_control_and_lipid_profile_in_type_2_diabetes_mellitus Australian Journal of Herbal Medicine. 2012;24:4–9. [Google Scholar]
- 33.Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. Ziegenfuss TN, Hofheins JE, Mendel RW, Landis J, Anderson RA. J Int Soc Sports Nutr. 2006;3:45–53. doi: 10.1186/1550-2783-3-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cinnamon extract lowers glucose, insulin and cholesterol in people with elevated serum glucose. Anderson RA, Zhan Z, Luo R, et al. J Tradit Complement Med. 2016;6:332–336. doi: 10.1016/j.jtcme.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cinnamon extract improves fasting blood glucose and glycosylated hemoglobin level in Chinese patients with type 2 diabetes. Lu T, Sheng H, Wu J, Cheng Y, Zhu J, Chen Y. Nutr Res. 2012;32:408–412. doi: 10.1016/j.nutres.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Salway JG. John Wiley & Sons Ltd. Vol. 61. New York City, NY: John Wiley & Sons, Inc.; 2012. Medical Biochemistry at a Glance. Third Edition; p. 62. [Google Scholar]
- 37.Insulin receptor signaling in normal and insulin-resistant states. Boucher J, Kleinridders A, Kahn CR. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review) Ardito F, Giuliani M, Perrone D, Troiano G, Lo Muzio L. Int J Mol Med. 2017;40:271–280. doi: 10.3892/ijmm.2017.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Increased abundance of specific skeletal muscle protein-tyrosine phosphatases in a genetic model of insulin-resistant obesity and diabetes mellitus. Ahmad F, Goldstein BJ. Metabolism. 1995;44:1175–1184. doi: 10.1016/0026-0495(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 40.Protein tyrosine phosphatase 1B inhibitory activity of Indonesian herbal medicines and constituents of Cinnamomum burmannii and Zingiber aromaticum. Saifudin A, Kadota S, Tezuka Y. J Nat Med. 2013;67:264–270. doi: 10.1007/s11418-012-0674-7. [DOI] [PubMed] [Google Scholar]
- 41.Regulation of PTP-1 and insulin receptor kinase by fractions from cinnamon: implications for cinnamon regulation of insulin signalling. Imparl-Radosevich J, Deas S, Polansky MM, Baedke DA, Ingebritsen TS, Anderson RA, Graves DJ. Horm Res. 1998;50:177–182. doi: 10.1159/000023270. [DOI] [PubMed] [Google Scholar]