Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
In the primary analysis of the global phase II ELIANA trial (ClinicalTrials.gov identifier: NCT02435849), tisagenlecleucel provided an overall remission rate of 81% in pediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia (R/R B-ALL), with 59% of responders remaining relapse-free at 12 months. Here, we report an update on efficacy, safety, and patient-reported quality of life in 79 pediatric and young adult patients with R/R B-ALL following a median follow-up of 38.8 months. The overall remission rate was 82%. The median event-free survival was 24 months, and the median overall survival was not reached. Event-free survival was 44% (95% CI, 31 to 57) and overall survival was 63% (95% CI, 51 to 73) at 3 years overall (most events occur within the first 2 years). The estimated 3-year relapse-free survival with and without censoring for subsequent therapy was 52% (95% CI, 37 to 66) and 48% (95% CI, 34 to 60), respectively. No new or unexpected long-term adverse events were reported. Grade 3/4 adverse events were reported in 29% of patients > 1 year after infusion; grade 3/4 infection rate did not increase > 1 year after infusion. Patients reported improvements in quality of life up to 36 months after infusion. These findings demonstrate favorable long-term safety and suggest tisagenlecleucel as a curative treatment option for heavily pretreated pediatric and young adult patients with R/R B-ALL.
INTRODUCTION
Pediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) experience increased risk of morbidity with each additional line of salvage therapy.1 Tisagenlecleucel is a CD19-directed chimeric antigen receptor (CAR) T–cell therapy2 approved for use in pediatric and young adults with R/R B-ALL and adults with R/R diffuse large B-cell lymphoma and R/R follicular lymphoma.3 In the primary analysis of the phase II ELIANA trial (ClinicalTrials.gov identifier: NCT02435849), tisagenlecleucel induced high remission rates (81%) in pediatric and young adults with R/R B-ALL.4 Furthermore, most adverse events (AEs) occurred during the first 8 weeks after infusion,4,5 and patients experienced significant quality-of-life (QOL) improvements.6
Given the high response rates, tisagenlecleucel has become the treatment of choice for many pediatric and young adult patients with R/R B-ALL. Herein, we report on the durability of response and potential for cure, long-term safety, and post–CAR-T cell therapy disease management on the basis of > 3 years of follow-up data from the ELIANA trial.
METHODS
Trial Design
ELIANA was a pivotal, phase II, open-label, multicenter, global study of tisagenlecleucel in pediatric and young adult patients with R/R B-ALL.2 Trial design details and outcome measures have been previously published.4,6 The median time from infusion to data cutoff reported here was 38.8 months. All patients provided written informed consent, and the study received ethics approval by local institutional review boards at each participating institution.
RESULTS
Baseline Characteristics
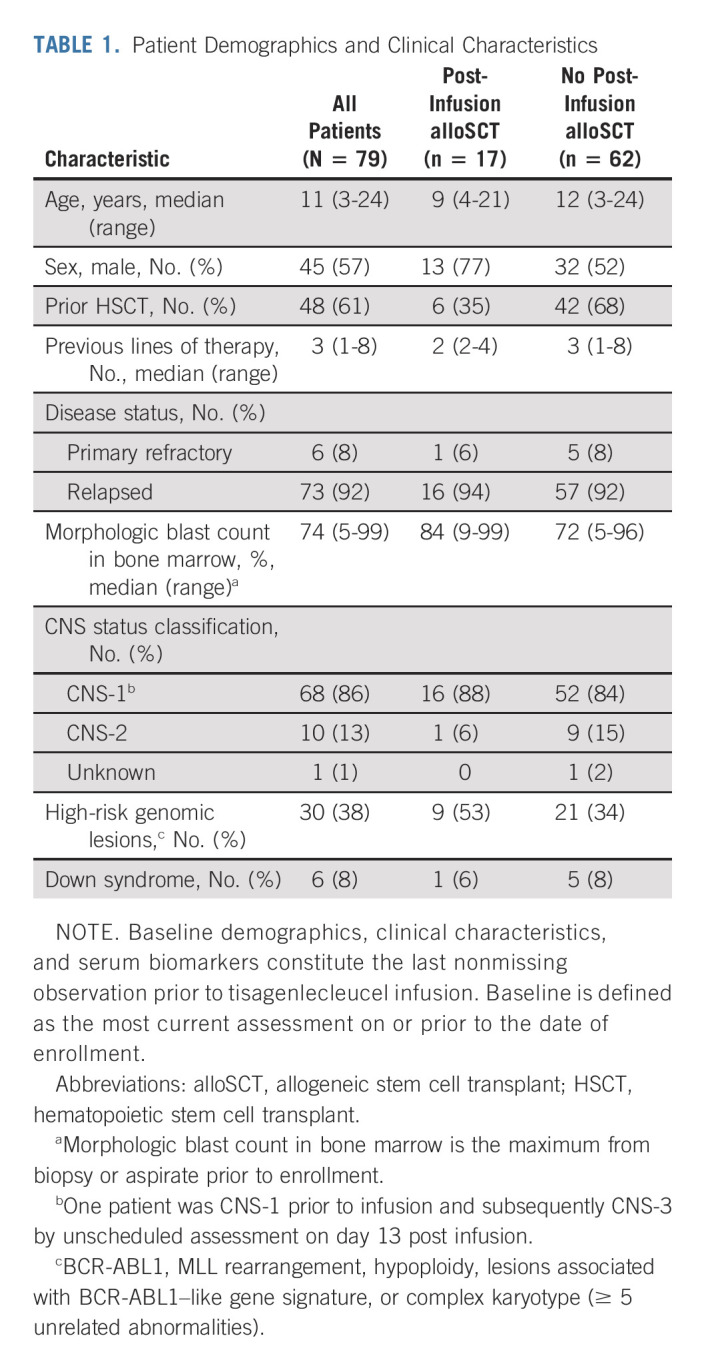
Between April 8, 2015, and July 1, 2019, 97 patients were enrolled and 79 (81%) received tisagenlecleucel, including five additional enrollments after the previously reported primary analysis.4 Patient demographics and clinical characteristics are shown in Table 1.
TABLE 1.
Patient Demographics and Clinical Characteristics
Efficacy
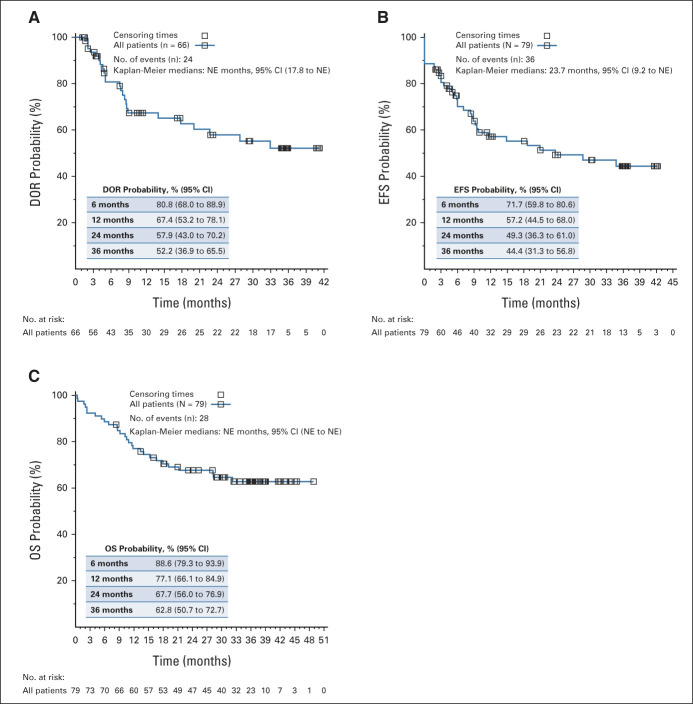
The published primary analysis (N = 75; median follow-up of 13.1 months) reported an overall remission rate (ORR) of 81% (95% CI, 71 to 89).4 In the current updated analysis (N = 79; median follow-up of 38.8 months from the date of infusion to data cutoff), the ORR was 82% within 3 months (65/79; 95% CI, 72 to 90; Data Supplement, online only). Among the 66 patients who achieved complete response/complete remission with incomplete hematologic recovery, the median duration of remission (DOR) has not been reached (Fig 1A). The estimated relapse-free survival (RFS) rate (censored for allogeneic stem-cell transplant [alloSCT] and/or further anticancer therapies) was 58% (95% CI, 43 to 70) at 24 months and 52% at 36 months (95% CI, 37 to 66); the estimated RFS rate without censoring was 52.3% (95% CI, 39 to 64) at 24 months and 47.8% (95% CI, 34.4 to 60) at 36 months. Among responders who received no subsequent therapy while in complete remission (n = 32), the estimated RFS rate was 81% (95% CI, 62 to 91) at 24 months and 76% (95% CI, 56 to 88) at 36 months.
FIG 1.
(A) DOR, (B) EFS, and (C) OS. (A) DOR—patients who achieved a BOR of CR/CRi are included. One patient who achieved a BOR of CRi within 6 months (day 173) is included. Time is relative to detection of remission. (B) EFS—time is relative to first tisagenlecleucel infusion date. (C) OS—time is relative to first tisagenlecleucel infusion date. BOR, best overall response; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; DOR, duration of remission; EFS, event-free survival; NE, not estimable; OS, overall survival.
The median event-free survival (EFS) among all infused patients was 24 months (95% CI, 9.2 to not reached; Fig 1B) and the median EFS among responders (n = 66) has not been reached (95% CI, 18.7 to not reached; Data Supplement). The estimated EFS among all infused patients was 44% (95% CI, 31 to 57) at 36 months. A total of 24 events (all relapses) were recorded, of which six (25%) occurred > 12 months after infusion; the latest occurrence of relapse occurred 33 months after remission (Data Supplement). The median overall survival (OS) among all infused patients has not been reached, and the estimated OS rate was 63% (95% CI, 51 to 73; Fig 1C) at 36 months. Efficacy by baseline tumor burden is included in the Data Supplement.
We also examined the disease status and responses of patients who underwent alloSCT during the follow-up period. Twenty-two percent of patients (17/79) underwent alloSCT (Data Supplement); 11 were in tisagenlecleucel-mediated remission and, with a median of 18 months after alloSCT follow-up, none of these patients with available data (n = 8) relapsed. Additional details of supplemental therapy use are included in the Data Supplement.
B-Cell Recovery and Chimeric Antigen Receptor Persistence
Median time to B-cell recovery among responders was 35.3 months (95% CI, 22.9 to not estimable; Data Supplement) and the probability of persistent B-cell aplasia at 12 and 24 months after infusion was 71% (95% CI, 57.4 to 81.5) and 59% (95% CI, 43.2 to 71.2), respectively. Among patients with sustained B-cell aplasia, the median DOR was 28 months (n = 50; Fig 2). The median DOR censored for alloSCT in patients with onset of B-cell recovery < 6 months after infusion was 12.1 months (n = 10) and has not been reached in patients with onset of B-cell recovery at 6 to 12 months (n = 2) and > 12 months (n = 4).
FIG 2.
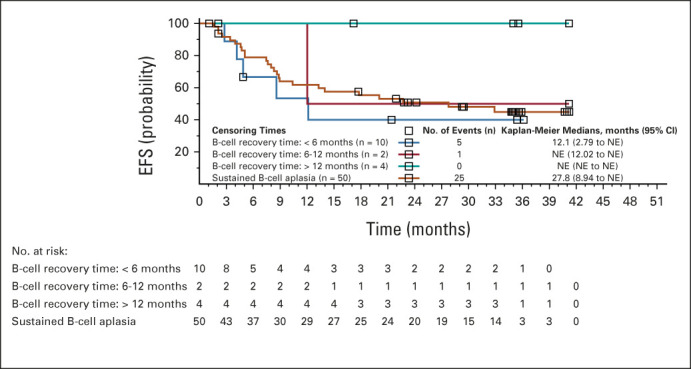
Duration of remission by B-cell recovery time point. Time is relative to first tisagenlecleucel infusion date. EFS, event-free survival; NE, not estimable.
Safety
The safety profile of tisagenlecleucel observed in this long-term follow-up analysis was consistent with published reports,4,5 and no new AEs or treatment-related mortality were observed (Data Supplement). The proportion of patients with grade 3/4 AEs declined over time (83.5% of patients < 8 weeks after infusion, 49% from 8 weeks to 1 year, and 29% > 1 year). The most frequent grade 3/4 AEs occurring > 1 year after infusion (n = 49) were infections (20.4%) and skin disorders (6.1%; all grade 3). Details of intravenous immunoglobulin use and hypogammaglobulinemia are provided in the Data Supplement.
Forty-six responding patients had both Pediatric Quality-of-Life Inventory and European Quality-of-Life-5 Dimensions questionnaire visual analog scale (EQ-5D VAS) assessments at baseline and at least one postbaseline visit. The clinically meaningful improvement in health-related QOL that began as early as 3 months after infusion6 continued to improve through the subsequent 36 months (Data Supplement). In general, the proportions of patients achieving the normative mean during the postbaseline period were greater than those at baseline even when patients with missing data were assumed to not achieve the normative mean (Data Supplement).
DISCUSSION
To our knowledge, tisagenlecleucel is the only CAR-T cell therapy to be approved for pediatric and young adult patients with R/R B-ALL. The data presented here, on the basis of a median follow-up of > 3 years, demonstrate durable efficacy with tisagenlecleucel in this heavily pretreated pediatric and young adult R/R B-ALL patients, with a 36-month RFS, EFS, and OS of 52%, 44%, and 63%, respectively.
The majority of responding patients did not undergo consolidative alloSCT. Although there were no reported relapses in patients following alloSCT in tisagenlecleucel-mediated remission, larger real-world studies are needed to assess the effect of alloSCT following CAR-T cell therapy because it is not possible to directly compare the survival outcomes of those who underwent transplant with those who did not because of the small patient number and biased selection of patients for transplant.
No new safety signals were identified in this analysis, with most AEs occurring within 8 weeks after infusion. Furthermore, long-term safety-related factors, such as hypogammaglobulinemia, intravenous immunoglobulin, and infection, do not appear to diminish the clinically meaningful improvements in QOL, which are maintained up to 36 months after infusion.
The current analysis supports the recently published finding that B-cell recovery within the first 6 months after infusion predicts risk of relapse and may be an indicator for clinicians to consider subsequent therapy7; however, B-cell recovery does not always precede relapse. In addition, several studies have demonstrated that high disease burden immediately before infusion is associated with an increased risk of nonresponse and early relapse.8-10 It was not possible to explore this hypothesis because disease burden in ELIANA was only assessed before enrollment. All subjects were required to have ≥ 5% bone marrow blasts at that time but may have had a decrease before infusion. We eagerly await additional data on the effect of emerging preinfusion prognostic factors that will further inform clinical decision making, including disease burden and nonresponse to blinatumomab from the real-world setting. In conclusion, this > 3-year follow-up confirms the durable responses and manageable safety profile of tisagenlecleucel and demonstrates its curative potential in pediatric and young adult patients with R/R B-ALL.
ACKNOWLEDGMENT
The study was sponsored and designed by Novartis Pharmaceuticals Corporation and was approved by the institutional review board at each participating institution. The authors thank patients and families enrolled in this study, as well as investigators and study-site personnel. The authors also thank Andrea Chassot Agostinho, MD; David Kuzan; and Werner Earle for thoughtful discussions of the data and manuscript. Medical writing support was provided by Jacqueline R. Ward, PhD, of Healthcare Consultancy Group and was funded by Novartis Pharmaceuticals Corporation.
Theodore W. Laetsch
Stock and Other Ownership Interests: Advanced Microbubbles
Consulting or Advisory Role: Novartis, Bayer, Cellectis, Aptitude Health, Clinical Education Alliance, Deciphera, Jumo Health, Massive Bio, Med Learning Group, Medscape, Physicans' Education Resource, Y-mAbs Therapeutics, AI Therapeutics, Jazz Pharmaceuticals, GentiBio, Menarini, Pyramid Biosciences
Research Funding: Pfizer (Inst), Novartis (Inst), Bayer (Inst), AbbVie (Inst), Amgen (Inst), Atara Biotherapeutics (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Epizyme (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Jubilant Pharmaceuticals (Inst), Novella Clinical (Inst), Servier (Inst), Foundation Medicine (Inst), Merck Sharp & Dohme (Inst), Turning Point Therapeutics (Inst)
Shannon L. Maude
Consulting or Advisory Role: Novartis, Kite
Research Funding: Novartis (Inst), Wugen, Inc (Inst)
Patents, Royalties, Other Intellectual Property: PCT/US2017/044425: Combination Therapies of Car and PD-1 Inhibitors
Travel, Accommodations, Expenses: Novartis
Susana Rives
Honoraria: Novartis, Kite/Gilead, Celgene/Bristol Myers Squibb, Shire/Servier
Consulting or Advisory Role: Servier, Amgen, Novartis, Kite/Gilead, Shire/Servier, Celgene/Bristol Myers Squibb, Cellectis, Jazz Pharmaceuticals
Speakers' Bureau: Novartis
Research Funding: Novartis (Inst)
Travel, Accommodations, Expenses: Novartis, Shire/Servier, Cellectis, Jazz Pharmaceuticals
Hidefumi Hiramatsu
Honoraria: Novar Pharma
Consulting or Advisory Role: Novartis
Henrique Bittencourt
Consulting or Advisory Role: Novartis Canada Pharmaceuticals Inc, Jazz Pharmaceuticals
Speakers' Bureau: Novartis Oncology Canada
Peter Bader
Honoraria: Medac (Inst), Riemser (Inst), Neovii (Inst)
Consulting or Advisory Role: Novartis (Inst), Amgen (Inst), Miltenyi (Inst), Servier (Inst)
Speakers' Bureau: Novartis (Inst), Amgen (Inst), Riemser (Inst), Medac (Inst), Miltenyi (Inst), Servier (Inst)
Research Funding: Medac (Inst), Neovii (Inst), Riemser (Inst)
Patents, Royalties, Other Intellectual Property: Patent on MSC licensend to Medac
André Baruchel
Leadership: DBV Technologies, lysogen, Medday Pharmaceuticals, Ascendis Pharma
Stock and Other Ownership Interests: DBV Technologies, Ascendis Pharma
Honoraria: Novartis, Kite, a Gilead company, AstraZeneca
Consulting or Advisory Role: Jazz Pharmaceuticals, Novartis, Servier, Celgene
Research Funding: Jazz Pharmaceuticals (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Sanofi, Servier
Michael Boyer
Honoraria: Novartis
Employment: Thunder Biotech
Leadership: Thunder Biotech
Barbara De Moerloose
Consulting or Advisory Role: Novartis
Uncompensated Relationships: Novartis (Inst), Gilead Sciences (Inst), Articulate Science, Jazz Pharmaceuticals
Muna Qayed
Honoraria: Novartis, Vertex, Medexus, Jazz Pharmaceuticals, Mesoblast
Consulting or Advisory Role: Novartis
Travel, Accommodations, Expenses: Novartis
Jochen Buechner
Honoraria: Novartis, Pfizer, Kite
Consulting or Advisory Role: Novartis, Janssen (Inst), Amgen (Inst)
Speakers' Bureau: Novartis
Michael A. Pulsipher
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Novartis, Mesoblast
Consulting or Advisory Role: Novartis, Medexus Pharmaceuticals, Equillium, Gentibio, Vertex, Bluebird Bio
Research Funding: Adaptive Biotechnologies, Miltenyi Biotec
Gary Douglas Myers
Honoraria: Novartis
Consulting or Advisory Role: Novartis
Speakers' Bureau: Novartis
Research Funding: Novartis (Inst)
Heather E. Stefanski
Consulting or Advisory Role: Novartis
Speakers' Bureau: Novartis
Paul L. Martin
Consulting or Advisory Role: Neovii Biotech GmbH, Genentech, Enlivex Therapeutics, and Pharmacyclics
Research Funding: Novartis (Inst), Bluebird Bio (Inst), AbGenomics (Inst)
Eneida Nemecek
Consulting or Advisory Role: Novartis, Medexus, Atara Bio
Christina Peters
Honoraria: Neovii, Jazz Pharmaceuticals, Novartis
Consulting or Advisory Role: Amgen, Neovii, Novartis
Speakers' Bureau: Medac, Riemser, Amgen
Research Funding: Medac (Inst), Neovii (Inst), Riemser (Inst), Jazz Pharmaceuticals (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Neovii, Jazz Pharmaceuticals, Novartis
Seong Lin Khaw
Honoraria: Novartis
Kara L. Davis
Honoraria: Novartis
Research Funding: Jazz Pharmaceuticals
Joerg Krueger
Employment: Janssen
Consulting or Advisory Role: Novartis, Kite/Gilead, SOBI
Other Relationship: Canadian Agency for Drugs and Technologies in Health (CADTH)
Adriana Balduzzi
Speakers' Bureau: Novartis, Amgen, Medac
Travel, Accommodations, Expenses: Novartis, Medac, Neovii
Nicolas Boissel
Honoraria: Amgen, ARIAD/Incyte, Novartis, Servier, Astellas Pharma, Bristol Myers Squibb, Jazz Pharmaceuticals, Celgene, Sanofi, Pfizer
Consulting or Advisory Role: Amgen, Novartis, Servier, Pfizer
Research Funding: Amgen, Novartis, Bristol Myers Squibb, Jazz Pharmaceuticals
Expert Testimony: Amgen
Ranjan Tiwari
Employment: Novartis
Darragh O’Donovan
Employment: Novartis Ireland Ltd
Stock and Other Ownership Interests: Novartis
Stephan A. Grupp
Honoraria: TCR2 Therapeutics, Eureka Therapeutics, Cellectis
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Janssen, Cellular Biomedicine Group, Roche, Adaptimmune, Alimera Sciences, Cabaletta Bio, CRISPR Therapeutics/Vertex
Research Funding: Novartis (Inst), Kite/Gilead (Inst), Servier (Inst), Jazz Pharmaceuticals (Inst), Vertex (Inst)
Patents, Royalties, Other Intellectual Property: UPenn Toxicity management patent (Inst)
Expert Testimony: Juno Therapeutics
No other potential conflicts of interest were reported.
See accompanying editorial on page 1646
SUPPORT
Supported by Novartis Pharmaceuticals.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Theodore W. Laetsch, Shannon L. Maude, Peter Bader, Michael A. Pulsipher, Gary Douglas Meyers, Christina Peters, Gregory Yanik, Ranjan Tiwari, Stephan A. Grupp
Provision of study materials or patients: Theodore W. Laetsch, Shannon L. Maude, Susana Rives, Hidefumi Hiramatsu, Henrique Bittencourt, Peter Bader, André Baruchel, Michael Boyer, Barbara De Moerloose, Muna Qayad, Jochen Buechner, Michael A. Pulsipher, Gary Douglas Myers, Heather E. Stefanski, Paul L. Martin, Eneida Nemecek, Christina Peters, Gregory Yanik, Seong Lin Khaw, Kara L. Davis, Joerg Krueger, Adriana Balduzzi, Nicolas Boissel, Stephan A. Grupp
Collection and assembly of data: Theodore W. Laetsch, Shannon L. Maude, Susana Rives, Hidefumi Hiramatsu, Henrique Bittencourt, Peter Bader, Michael Boyer, Barbara De Moerloose, Muna Qayed, Jochen Buechner, Michael A. Pulsipher, Heather E. Stefanski, Paul L. Martin, Christina Peters, Seong Lin Khaw, Kara L. Davis, Joerg Krueger, Nicolas Boissel, Darragh O’Donovan, Stephan A. Grupp
Data analysis and interpretation: Theodore W. Laetsch, Shannon L. Maude, Susana Rives, Hidefumi Hiramatsu, Henrique Bittencourt, Peter Bader, André Baruchel, Michael Boyer, Barbara De Moerloose, Muna Qayad, Jochen Buechner, Michael A. Pulsipher, Gary Douglas Myers, Heather E. Stefanski, Paul L. Martin, Eneida Nemecek, Christina Peters, Gregory Yanik, Seong Lin Khaw, Kara L. Davis, Joerg Krueger, Adriana Balduzzi, Nicolas Boissel, Ranjan Tiwari, Darragh O’Donovan, Stephan A. Grupp
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Three-Year Update of Tisagenlecleucel in Pediatric and Young Adult Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia in the ELIANA Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Theodore W. Laetsch
Stock and Other Ownership Interests: Advanced Microbubbles
Consulting or Advisory Role: Novartis, Bayer, Cellectis, Aptitude Health, Clinical Education Alliance, Deciphera, Jumo Health, Massive Bio, Med Learning Group, Medscape, Physicans' Education Resource, Y-mAbs Therapeutics, AI Therapeutics, Jazz Pharmaceuticals, GentiBio, Menarini, Pyramid Biosciences
Research Funding: Pfizer (Inst), Novartis (Inst), Bayer (Inst), AbbVie (Inst), Amgen (Inst), Atara Biotherapeutics (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Epizyme (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Jubilant Pharmaceuticals (Inst), Novella Clinical (Inst), Servier (Inst), Foundation Medicine (Inst), Merck Sharp & Dohme (Inst), Turning Point Therapeutics (Inst)
Shannon L. Maude
Consulting or Advisory Role: Novartis, Kite
Research Funding: Novartis (Inst), Wugen, Inc (Inst)
Patents, Royalties, Other Intellectual Property: PCT/US2017/044425: Combination Therapies of Car and PD-1 Inhibitors
Travel, Accommodations, Expenses: Novartis
Susana Rives
Honoraria: Novartis, Kite/Gilead, Celgene/Bristol Myers Squibb, Shire/Servier
Consulting or Advisory Role: Servier, Amgen, Novartis, Kite/Gilead, Shire/Servier, Celgene/Bristol Myers Squibb, Cellectis, Jazz Pharmaceuticals
Speakers' Bureau: Novartis
Research Funding: Novartis (Inst)
Travel, Accommodations, Expenses: Novartis, Shire/Servier, Cellectis, Jazz Pharmaceuticals
Hidefumi Hiramatsu
Honoraria: Novar Pharma
Consulting or Advisory Role: Novartis
Henrique Bittencourt
Consulting or Advisory Role: Novartis Canada Pharmaceuticals Inc, Jazz Pharmaceuticals
Speakers' Bureau: Novartis Oncology Canada
Peter Bader
Honoraria: Medac (Inst), Riemser (Inst), Neovii (Inst)
Consulting or Advisory Role: Novartis (Inst), Amgen (Inst), Miltenyi (Inst), Servier (Inst)
Speakers' Bureau: Novartis (Inst), Amgen (Inst), Riemser (Inst), Medac (Inst), Miltenyi (Inst), Servier (Inst)
Research Funding: Medac (Inst), Neovii (Inst), Riemser (Inst)
Patents, Royalties, Other Intellectual Property: Patent on MSC licensend to Medac
André Baruchel
Leadership: DBV Technologies, lysogen, Medday Pharmaceuticals, Ascendis Pharma
Stock and Other Ownership Interests: DBV Technologies, Ascendis Pharma
Honoraria: Novartis, Kite, a Gilead company, AstraZeneca
Consulting or Advisory Role: Jazz Pharmaceuticals, Novartis, Servier, Celgene
Research Funding: Jazz Pharmaceuticals (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Sanofi, Servier
Michael Boyer
Honoraria: Novartis
Employment: Thunder Biotech
Leadership: Thunder Biotech
Barbara De Moerloose
Consulting or Advisory Role: Novartis
Uncompensated Relationships: Novartis (Inst), Gilead Sciences (Inst), Articulate Science, Jazz Pharmaceuticals
Muna Qayed
Honoraria: Novartis, Vertex, Medexus, Jazz Pharmaceuticals, Mesoblast
Consulting or Advisory Role: Novartis
Travel, Accommodations, Expenses: Novartis
Jochen Buechner
Honoraria: Novartis, Pfizer, Kite
Consulting or Advisory Role: Novartis, Janssen (Inst), Amgen (Inst)
Speakers' Bureau: Novartis
Michael A. Pulsipher
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Novartis, Mesoblast
Consulting or Advisory Role: Novartis, Medexus Pharmaceuticals, Equillium, Gentibio, Vertex, Bluebird Bio
Research Funding: Adaptive Biotechnologies, Miltenyi Biotec
Gary Douglas Myers
Honoraria: Novartis
Consulting or Advisory Role: Novartis
Speakers' Bureau: Novartis
Research Funding: Novartis (Inst)
Heather E. Stefanski
Consulting or Advisory Role: Novartis
Speakers' Bureau: Novartis
Paul L. Martin
Consulting or Advisory Role: Neovii Biotech GmbH, Genentech, Enlivex Therapeutics, and Pharmacyclics
Research Funding: Novartis (Inst), Bluebird Bio (Inst), AbGenomics (Inst)
Eneida Nemecek
Consulting or Advisory Role: Novartis, Medexus, Atara Bio
Christina Peters
Honoraria: Neovii, Jazz Pharmaceuticals, Novartis
Consulting or Advisory Role: Amgen, Neovii, Novartis
Speakers' Bureau: Medac, Riemser, Amgen
Research Funding: Medac (Inst), Neovii (Inst), Riemser (Inst), Jazz Pharmaceuticals (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Neovii, Jazz Pharmaceuticals, Novartis
Seong Lin Khaw
Honoraria: Novartis
Kara L. Davis
Honoraria: Novartis
Research Funding: Jazz Pharmaceuticals
Joerg Krueger
Employment: Janssen
Consulting or Advisory Role: Novartis, Kite/Gilead, SOBI
Other Relationship: Canadian Agency for Drugs and Technologies in Health (CADTH)
Adriana Balduzzi
Speakers' Bureau: Novartis, Amgen, Medac
Travel, Accommodations, Expenses: Novartis, Medac, Neovii
Nicolas Boissel
Honoraria: Amgen, ARIAD/Incyte, Novartis, Servier, Astellas Pharma, Bristol Myers Squibb, Jazz Pharmaceuticals, Celgene, Sanofi, Pfizer
Consulting or Advisory Role: Amgen, Novartis, Servier, Pfizer
Research Funding: Amgen, Novartis, Bristol Myers Squibb, Jazz Pharmaceuticals
Expert Testimony: Amgen
Ranjan Tiwari
Employment: Novartis
Darragh O’Donovan
Employment: Novartis Ireland Ltd
Stock and Other Ownership Interests: Novartis
Stephan A. Grupp
Honoraria: TCR2 Therapeutics, Eureka Therapeutics, Cellectis
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Janssen, Cellular Biomedicine Group, Roche, Adaptimmune, Alimera Sciences, Cabaletta Bio, CRISPR Therapeutics/Vertex
Research Funding: Novartis (Inst), Kite/Gilead (Inst), Servier (Inst), Jazz Pharmaceuticals (Inst), Vertex (Inst)
Patents, Royalties, Other Intellectual Property: UPenn Toxicity management patent (Inst)
Expert Testimony: Juno Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Crotta A, Zhang J, Keir C: Survival after stem-cell transplant in pediatric and young-adult patients with relapsed and refractory B-cell acute lymphoblastic leukemia. Curr Med Res Opin 34:435-440, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Milone MC, Fish JD, Carpenito C, et al. : Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 17:1453-1464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.KYMRIAH [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation, 2022 [Google Scholar]
- 4.Maude SL, Laetsch TW, Buechner J, et al. : Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378:439-448, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grupp SA, Maude SL, Rives S, et al. : Updated analysis of the efficacy and safety of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory (r/r) acute lymphoblastic leukemia. Blood 132, 2018. (suppl 1; abstr 895) [Google Scholar]
- 6.Laetsch TW, Myers GD, Baruchel A, et al. : Patient-reported quality of life after tisagenlecleucel infusion in children and young adults with relapsed or refractory B-cell acute lymphoblastic leukaemia: A global, single-arm, phase 2 trial. Lancet Oncol 20:1710-1718, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulsipher MA, Han X, Maude SL, et al. : Next-generation sequencing of minimal residual disease for predicting relapse after tisagenlecleucel in children and young adults with acute lymphoblastic leukemia. Blood Cancer Discov 3:66-81, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadauke S, Myers RM, Li Y, et al. : Risk-adapted preemptive tocilizumab to prevent severe cytokine release syndrome after CTL019 for pediatric B-cell acute lymphoblastic leukemia: A prospective clinical trial. J Clin Oncol 39:920-930, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz LM, Baggott C, Prabhu S, et al. : Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: A Pediatric Real-World Chimeric Antigen Receptor Consortium Report. J Clin Oncol 40:945-955, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah NN, Lee DW, Yates B, et al. : Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J Clin Oncol 39:1650-1659, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]