PURPOSE
To investigate the safety and efficacy of nivolumab plus cabozantinib with or without ipilimumab in patients with advanced hepatocellular carcinoma.
METHODS
In cohort 6 of the multicohort, open-label, phase I/II CheckMate 040 study, patients who were treatment-naive, sorafenib-intolerant, or had progressed on sorafenib were randomly assigned 1:1 to nivolumab 240 mg once every 2 weeks plus cabozantinib 40 mg once daily (doublet arm); or nivolumab 3 mg/kg every 2 weeks plus cabozantinib 40 mg once daily with ipilimumab 1 mg/kg once every 6 weeks (triplet arm). Primary objectives were safety and tolerability, objective response rate, and duration of response by investigator assessment per RECIST v1.1. Secondary objectives included progression-free survival (by blinded independent central review) and overall survival.
RESULTS
Seventy-one patients were randomly assigned: 36 to the doublet arm and 35 to the triplet arm. After 32.0-month median follow-up, objective response rate (95% CI) was 17% (6 to 33) and 29% (15 to 46) in the doublet and triplet arms, respectively. Median (95% CI) duration of response was 8.3 (6.9 to not estimable) months in the doublet arm and not reached (0.0 to not estimable) in the triplet arm. Median progression-free survival was 5.1 and 4.3 months, and median overall survival was 20.2 and 22.1 months for the doublet and triplet arms, respectively. Grade 3-4 treatment-related adverse events occurred in 50% and 74% of patients and treatment-related adverse events leading to discontinuation were reported for 11% and 23% in the doublet and triplet arms, respectively. There were no treatment-related deaths in either arm.
CONCLUSION
Nivolumab plus cabozantinib with or without ipilimumab showed encouraging preliminary antitumor activity and had consistent safety profiles with those established for the individual drugs in patients with advanced hepatocellular carcinoma.
INTRODUCTION
Liver cancer is the third leading cause of cancer-related deaths worldwide; up to 85% of cases are hepatocellular carcinoma (HCC).1 Most patients are diagnosed with advanced disease unsuitable for potentially curative treatments such as surgery or ablation.2
CONTEXT
Key Objective
We hypothesized that triplet therapy with nivolumab (programmed death‐1 inhibitor), cabozantinib (multikinase inhibitor), and ipilimumab (cytotoxic T-cell lymphocyte-4 inhibitor) would have an acceptable safety profile and improve clinical outcomes compared with nivolumab plus cabozantinib doublet therapy in patients with advanced hepatocellular carcinoma who were treatment-naive, sorafenib-intolerant, or had progressed on sorafenib. To our knowledge, this is the first report of dual immune checkpoint inhibitor and tyrosine kinase inhibitor therapy in this patient population.
Knowledge Generated
Both the doublet (nivolumab and cabozantinib) and triplet (nivolumab, ipilimumab, and cabozantinib) combinations demonstrated durable responses, with more frequent, deeper, and longer-lasting responses observed for the triplet therapy. Both doublet and triplet therapies had manageable safety profiles.
Relevance (A.H. Ko)
-
Although not necessarily ready for prime-time use, the combination of dual immune checkpoint inhibition plus a multikinase inhibitor warrants further clinical investigation in advanced hepatocellular carcinoma, particularly in identifying the subset of individuals who may benefit from this more aggressive therapeutic strategy.*
*Relevance section written by JCO Associate Editor Andrew H. Ko, MD.
Until recently, first-line treatment options for advanced HCC included sorafenib and lenvatinib, which inhibit cellular kinases, including vascular endothelial growth factor (VEGF) receptors (VEGFRs) and other kinases such as RET and KIT, and provide median overall survival (OS) of 12.3 and 13.6 months, respectively.3-6 Both treatments are associated with a relatively high incidence of treatment-related adverse events (TRAEs), particularly those of grade ≥ 3 (49%-57%).6 Recently, atezolizumab (programmed death ligand 1 [PD-L1] inhibitor) plus bevacizumab (VEGF inhibitor) demonstrated significant improvements versus sorafenib in median OS, median progression-free survival (PFS), and objective response rate (ORR) after 15.6-month median follow-up,7 and is now the standard first-line treatment for advanced HCC globally. Treatment options after sorafenib in the United States, Europe, and Asia include multikinase inhibitors (eg, regorafenib and cabozantinib) and the VEGFR2 inhibitor ramucirumab,8-10 which provide a median OS of < 1 year.11-13 In the United States, additional options include the immune checkpoint inhibitors (ICIs) pembrolizumab (programmed death-1 inhibitor) and nivolumab (programmed death-1 inhibitor) plus ipilimumab (cytotoxic T-cell lymphocyte-4 inhibitor), which provide ORRs between 18% and 32%.3,14,15
Nivolumab plus ipilimumab is an approved second-line treatment option for advanced HCC in the United States under the accelerated approval pathway.16,17 This was based on the results from cohort 4 of the CheckMate 040 trial, where nivolumab 1 mg/kg plus ipilimumab 3 mg/kg once every 3 weeks provided a median OS of 22.8 months and an ORR of 32%, with no new safety signals identified from the combination in this indication.14 Nivolumab and ipilimumab are known to promote antitumor immune response by complementary mechanisms,18 and further combining these agents with a multikinase inhibitor could increase antitumor activity and extend survival.19 Cabozantinib (VEGFR and TAM kinase inhibitor) is approved in the United States, European Union, and other regions as a treatment option after sorafenib for advanced HCC, on the basis of the results from the CELESTIAL trial.12,20,21 Cabozantinib also has immunomodulatory properties and can decrease tumor-induced immunosuppression and enhance tumor cell sensitivity to T-cell–mediated cell death.22,23 Nivolumab plus cabozantinib has also shown benefit in other tumor types. The combination led to improved OS, PFS, and ORR versus sunitinib in previously untreated patients with advanced renal cell carcinoma.21,24 Both doublet therapy (cabozantinib plus nivolumab) and triplet therapy (cabozantinib plus nivolumab plus ipilimumab) have shown clinical activity with manageable safety profiles in a phase I dose escalation trial in patients with advanced urothelial carcinoma and other genitourinary cancers; cabozantinib 40 mg/day was the recommended phase II dose for this combination.25 Together, these findings provide a rationale for combining cabozantinib with nivolumab and/or ipilimumab.
We report the results from cohort 6 of CheckMate 040, which investigated nivolumab plus cabozantinib with or without ipilimumab as first- or second-line treatment for patients with advanced HCC.
METHODS
Study Design
CheckMate 040 is a multicohort, open-label, phase I/II study of nivolumab alone and in combination with other agents in patients with advanced HCC. In cohort 6, patients were recruited from 16 centers across five countries in Asia, Europe, and the United States. This study was conducted in accordance with Good Clinical Practice as defined by the International Council for Harmonisation. The institutional review board or independent ethics committee approved the study protocol at each site. All patients provided written informed consent to participate on the basis of the Declaration of Helsinki.
Patients
Eligibility criteria included age ≥ 18 years, histologically confirmed advanced HCC unsuitable for surgery or locoregional therapy, and Child-Pugh score of A5 or A6. Patients were either treatment-naive or had experienced progression following, or were intolerant to, sorafenib treatment. An Eastern Cooperative Oncology Group performance status score of 0 or 1, with at least one untreated tumor lesion measurable by RECIST v1.1, was required. Additional eligibility criteria are provided in the Data Supplement (online only).
Random Assignment and Blinding
Patients were enrolled via interactive voice response system (block size 4) and randomly assigned 1:1 to the doublet or triplet therapy arm. This was an open-label study.
Procedures
In the doublet arm, patients received nivolumab 240 mg once every 2 weeks plus cabozantinib 40 mg once daily; in the triplet arm, patients received intravenous infusions of nivolumab 3 mg/kg once every 2 weeks and ipilimumab 1 mg/kg once every 6 weeks plus oral cabozantinib 40 mg once daily. Treatment continued until RECIST v1.1 radiographic progression, clinical deterioration (further treatment benefit unlikely), unacceptable toxicity, or other protocol-defined criteria for discontinuation. Dose delay and reduction criteria and criteria for treatment beyond progression are provided in the Data Supplement.
Outcomes
The primary objectives were safety and tolerability, investigator-assessed ORR (RECIST v1.1),26 and duration of response (DOR). Secondary objectives included time to response, time to progression and PFS (per blinded independent central review [BICR] and/or investigator assessment), and OS. Exploratory objectives included ORR by BICR and disease control rate (DCR). See the Data Supplement for definitions of objectives.
Tumor imaging was conducted 6 weeks after random assignment and every 6 weeks until week 48, then every 12 weeks until radiographic progression or treatment discontinuation, whichever occurred later. Adverse events (AEs) and TRAEs were reported by worst grade according to National Cancer Institute Common Terminology Criteria for Adverse Events v4.0. See the Data Supplement for additional assessments.
Statistical Analyses
ORR, DCR, and corresponding 95% CIs were determined using the Clopper-Pearson method. Median (95% CI) DOR and OS were determined using the Kaplan-Meier method. Safety was analyzed by descriptive statistics, including TRAEs, discontinuation because of TRAEs, and TRAEs of potential immunologic etiology. See the Data Supplement for definitions of TRAEs and additional methods.
RESULTS
Patient Baseline Characteristics
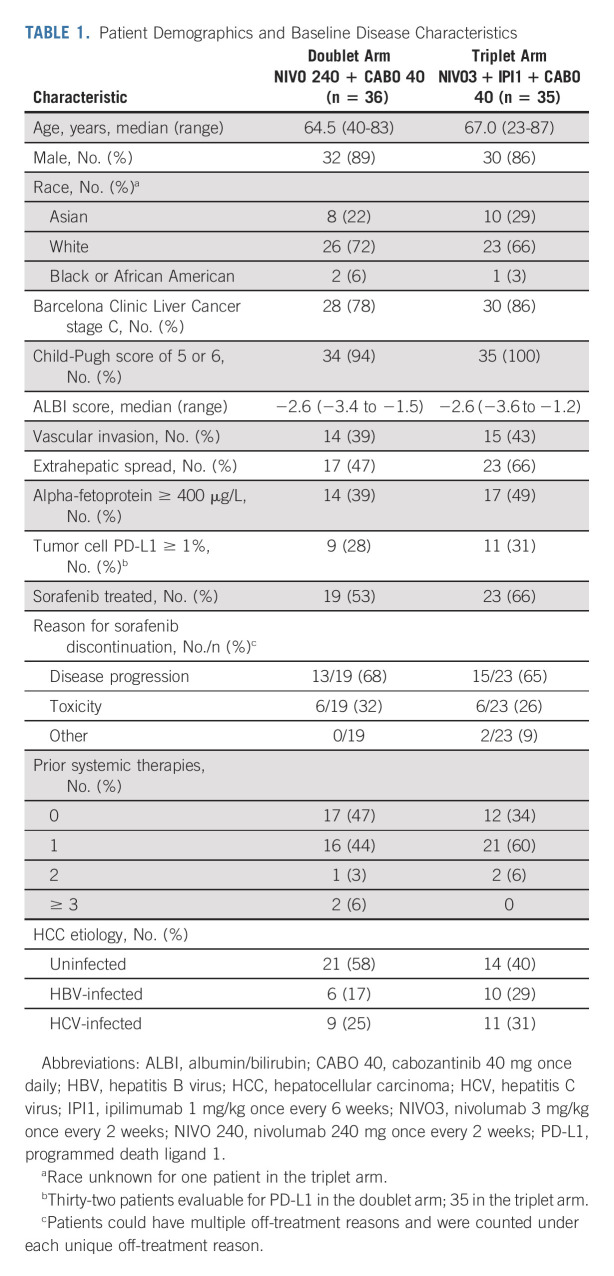
Between July 13, 2017 and January 30, 2018, 98 patients with advanced HCC were enrolled and 71 patients were randomly assigned 1:1 to the doublet (n = 36) or triplet (n = 35) arm (Fig 1). At database lock on September 30, 2020, the median (range) follow-up (from random assignment to clinical data cutoff) was 32.0 (28.5-36.2) months. Overall, 62 patients (87%) were male, and the median (range) age was 65.0 (23-87) years. Barcelona Clinic Liver Cancer (BCLC) stage C, vascular invasion, extrahepatic spread, alpha-fetoprotein ≥ 400 μg/L, and prior sorafenib treatment were less frequent in the doublet arm than in the triplet arm. Furthermore, the proportion of patients who had previously received systemic therapy was lower in the doublet arm than in the triplet arm (Table 1).
FIG 1.
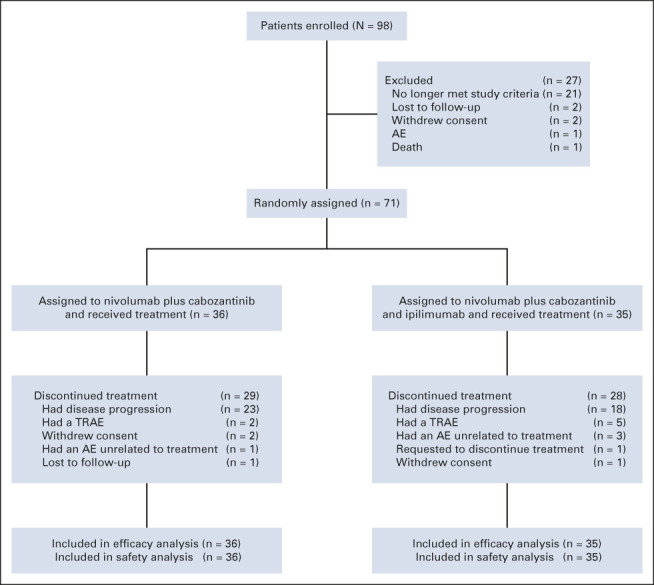
CONSORT diagram. AE, adverse event; TRAE, treatment-related adverse event.
TABLE 1.
Patient Demographics and Baseline Disease Characteristics
Patient Disposition and Study Drug Exposure
At the time of analysis, 19% of patients in the doublet arm and 20% in the triplet arm were still receiving study treatment. Disease progression was the reason for discontinuation in 64% and 51% of patients in the doublet and triplet arms, respectively.
Overall, 75% of patients in the doublet arm and 57% in the triplet arm received ≥ 90% of the intended nivolumab dose, while 42% and 46% received ≥ 90% of the intended cabozantinib dose, respectively; 71% of patients in the triplet arm received ≥ 90% of the intended ipilimumab dose. In the doublet arm, 69% of patients experienced at least one nivolumab dose delay, and 67% experienced at least one cabozantinib dose delay. In the triplet arm, 66%, 69%, and 57% of patients experienced at least one nivolumab, cabozantinib, or ipilimumab dose delay, respectively. Median (95% CI) duration of treatment overall was 7.1 months (3.9 to 13.6) in the doublet arm and 7.8 months (3.3 to 11.5) in the triplet arm. Median (range) number of doses received in the doublet arm was 13.5 (1-77) nivolumab doses and 118.0 (10-1,094) cabozantinib doses, corresponding to approximately 16 weeks of cabozantinib treatment, regardless of dose delays. Median (range) number of doses received during the treatment period in the triplet arm was 15.0 (1-79) nivolumab doses, 5.0 (1-26) ipilimumab doses, and 73.0 (14-964) cabozantinib doses, corresponding to approximately 10 weeks of cabozantinib treatment, regardless of dose delays. Median average daily cabozantinib dose received during the treatment period was 34.1 mg once daily and 30.0 mg once daily in the doublet and triplet arms, respectively. Median (range) cumulative cabozantinib dose was 4,570 mg (400-43,760) and 2,600 mg (540-38,560) in the doublet and triplet arms, respectively. Nineteen (53%) and 15 (43%) patients had cabozantinib dose reductions in the doublet and triplet arms, respectively (Data Supplement).
Thirteen patients in each arm received subsequent anticancer therapy, including radiotherapy (one patient in each arm) and systemic therapy (doublet arm: 12 patients; triplet arm: 13 patients). Some patients received more than one subsequent therapy (Data Supplement).
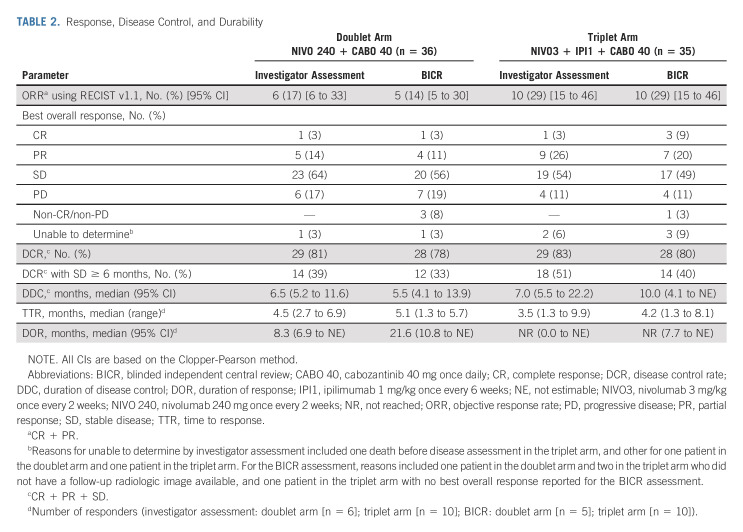
Response
In the doublet and triplet arms, investigator-assessed ORR (95% CI) was 17% (6 to 33) and 29% (15 to 46; Table 2), respectively. ORR (95% CI) by BICR was 14% (5 to 30) in the doublet arm and 29% (15 to 46) in the triplet arm. Responses per BICR were observed in both arms regardless of baseline alpha-fetoprotein status, tumor cell PD-L1 expression, or HCC etiology (Data Supplement). Investigator-assessed median (95% CI) DOR was 8.3 months (6.9 to not estimable [NE]) in the doublet arm and not reached in the triplet arm; median DOR by BICR was 21.6 months (10.8 to NE) and not reached, respectively. Of the 10 responders in the triplet arm, 90% maintained response for ≥ 6 months, 80% for ≥ 12 months, and 70% for ≥ 18 months. One patient in the doublet arm and three in the triplet arm had complete response (CR) by BICR. DCR, progressive disease (PD) rate, and median time to response were generally similar across treatment arms and assessment methods (Table 2).
TABLE 2.
Response, Disease Control, and Durability
Nineteen patients in the doublet arm and 23 in the triplet arm had received prior sorafenib. In the doublet arm, ORR (95% CI) by BICR was 6% (< 1 to 29) for sorafenib-naive patients and 21% (6 to 46) for sorafenib-pretreated patients; corresponding rates in the triplet arm were 25% (6 to 57) and 30% (13 to 53), respectively.
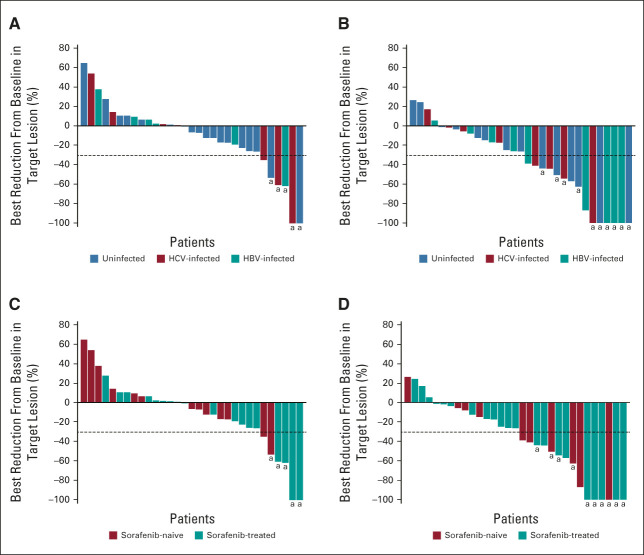
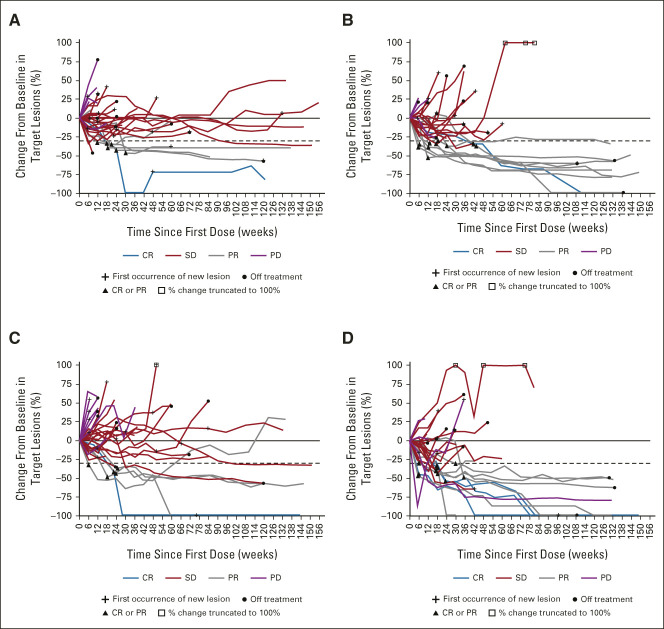
Of 31 evaluable patients in each of the doublet and triplet arms, 17 (55%) and 27 (87%), respectively, had decreases in the sum of diameters of target lesions from baseline by BICR (Fig 2). Tumor burden was reduced regardless of baseline viral etiology or prior sorafenib treatment. Among responders, target lesion reductions were deep and durable in both arms (Fig 3).
FIG 2.
Best change in target lesions (per BICR) by HCC etiology in the (A) NIVO 240 + CABO 40 treatment group and the (B) NIVO3 + IPI1 + CABO 40 treatment group, and by prior sorafenib in the (C) NIVO 240 + CABO 40 treatment group and the (D) NIVO3 + IPI1 + CABO 40 treatment group. Horizontal reference line indicates the 30% reduction consistent with a response per RECIST v1.1; aResponders. BICR, blinded independent central review; CABO 40, cabozantinib 40 mg once daily; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IPI1, ipilimumab 1 mg/kg once every 6 weeks; NIVO3, nivolumab 3 mg/kg once every 2 weeks; NIVO 240, nivolumab 240 mg once every 2 weeks.
FIG 3.
Change in target lesions over time by investigator assessment in the (A) NIVO 240 + CABO 40 treatment group and the (B) NIVO3 + IPI1 + CABO 40 treatment group, and by BICR assessment in the (C) NIVO 240 + CABO 40 treatment group and the (D) NIVO3 + IPI1 + CABO 40 treatment group. Response-evaluable patients with BOR of CR, PR, SD, or PD; target lesions assessed at baseline; and at least one on-study time point with all baseline target lesions assessed. Horizontal reference line indicates the 30% reduction consistent with a response per RECIST v1.1. BICR, blinded independent central review; BOR, best overall response; CABO 40, cabozantinib 40 mg once daily; CR, complete response; IPI1, ipilimumab 1 mg/kg once every 6 weeks; NIVO3, nivolumab 3 mg/kg once every 2 weeks; NIVO 240, nivolumab 240 mg once every 2 weeks; PD, progressive disease; PR, partial response; SD, stable disease.
PFS and OS
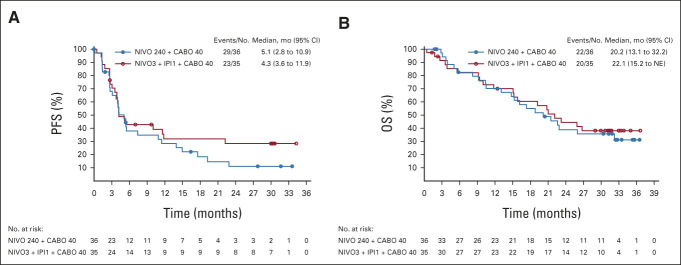
Median PFS (95% CI) by BICR was 5.1 (2.8 to 10.9) months in the doublet arm and 4.3 (3.6 to 11.9) months in the triplet arm (Fig 4A). Twelve-month PFS rates (95% CI) were 29% (15 to 44) in the doublet arm and 32% (17 to 49) in the triplet arm. Twenty-four-month PFS rates were 11% (3 to 25) and 29% (14 to 45), respectively.
FIG 4.
(A) PFS by BICR assessment and (B) OS on the basis of Kaplan-Meier estimates. BICR, blinded independent central review; CABO 40, cabozantinib 40 mg once daily; IPI1, ipilimumab 1 mg/kg once every 6 weeks; NE, not estimable; NIVO3, nivolumab 3 mg/kg once every 2 weeks; NIVO 240, nivolumab 240 mg once every 2 weeks; OS, overall survival; PFS, progression-free survival. Symbols represent censored observations.
Median OS (95% CI) was 20.2 (13.1 to 32.2) months in the doublet arm and 22.1 (15.2 to NE) months in the triplet arm (Fig 4B). Twelve-month OS rate (95% CI) was 70% for both arms (doublet arm: 52 to 83 months; triplet arm: 51 to 83 months). Corresponding 24-month rates were 39% (23 to 55) and 45% (27 to 61), respectively. Among patients with a CR or partial response, median OS was not reached for 6- or 9-month landmark analysis in either arm (Data Supplement). Among nonresponders (stable disease [SD]/PD/non-CR/non-PD), median OS (95% CI) from 6 months was 20.9 months (14.8 to 32.2) and 23.2 months (15.2 to NE) in the doublet and triplet arms, respectively; from 9 months, median OS (95% CI) was 21.5 months (15.3 to NE) and 22.1 months (15.2 to 26.7), respectively.
Safety
Any-grade TRAEs were reported for 32 patients (89%) in the doublet arm and 33 (94%) in the triplet arm (Table 3). The most frequently reported grade 3-4 TRAEs were diarrhea, hypertension, and AST increase in the doublet arm, and AST increase, hypertension, and lipase increase in the triplet arm. Treatment-related serious adverse events (SAEs) of any grade were reported for 11% of patients in the doublet arm and 34% in the triplet arm; grade 3-4 SAEs were reported for 8% and 34% of patients, respectively. Hyponatremia, diarrhea, immune-mediated hepatitis, ALT increase, and AST increase were each reported for 3% of patients (all grade 3-4); all other SAEs were reported in 1% of patients. Any-grade TRAEs leading to discontinuation were reported in 11% of patients in the doublet arm and 23% in the triplet arm; one (3%) and seven (20%) patients, respectively, reported grade 3-4 TRAEs leading to discontinuation. Among patients who discontinued because of TRAEs, none in the doublet arm and three (9%) in the triplet arm had CR/partial response, and three (8%) and five (14%) patients, respectively, had SD.
TABLE 3.
Treatment-Related Adverse Events
Select TRAEs of potential immunologic etiology (sTRAEs) were mostly grade 1-2 in each treatment arm (Data Supplement). The most frequent grade 3-4 sTRAEs were hepatic events (doublet arm: 11%; triplet arm: 26%), which led to one discontinuation in the doublet arm (grade 3) and four discontinuations in the triplet arm (two grade 3 and two grade 4). Most sTRAEs were treated with corticosteroids for a median of 4-6 weeks, except endocrine events, for which four patients received corticosteroids for a median of 50 weeks. Overall, five patients (20%) in the doublet arm and 10 (36%) in the triplet arm received systemic corticosteroids for sTRAEs. Corticosteroids (≥ 40 mg prednisone or equivalent) were required by two patients (8%) in the doublet arm for a median of 4.6 weeks and eight (29%) in the triplet arm for a median of 1.7 weeks.
No deaths related to study treatment occurred. The most frequently reported cause of death was disease progression (doublet arm: 53%; triplet arm: 46%). In four patients, cancer-related death was reported without radiographic (BICR or investigator assessment) or clinical progression but with liver function deterioration, indicated by worsening albumin/bilirubin grade and/or Child-Pugh score shortly before death (two patients in each arm). Another patient in the triplet arm, who died from an AE unrelated to study drug, also had liver function deterioration shortly before death. Other causes of death unrelated to study treatment included intracranial hemorrhage, stroke, lower GI bleeding, and unspecified.
DISCUSSION
ICIs and VEGF inhibitors have independently demonstrated clinical benefits in patients with advanced HCC.6,12,14,27-29 To our knowledge, this is the first report of dual ICI plus tyrosine kinase inhibitor (triplet therapy) in patients with advanced HCC. In cohort 6 of CheckMate 040, both doublet (nivolumab and cabozantinib) and triplet (nivolumab, ipilimumab, and cabozantinib) combinations demonstrated durable responses in sorafenib-naive or sorafenib-pretreated patients with advanced HCC, with more frequent, deeper, and longer-lasting responses observed for triplet therapy.
The observed ORRs for doublet and triplet arms were comparable with those of previous studies of ICI combinations in first-line (ORR, 30% by RECIST v1.1)29 and second-line settings (ORR, 15%-32%)14,15 and ICI monotherapy (ORR, 15%).27 Notably, we observed numerically longer median DOR by BICR in the triplet arm (not reached) than in the doublet arm (21.6 months) after 32-month median follow-up. With other ICIs, DOR between 13.8 and 21.7 months has been reported in first- and second-line settings.14,15,27,29 Median OS in our study (20.2-22.1 months) was consistent with that reported for first-line atezolizumab plus bevacizumab (19.2 months)29 and second-line nivolumab plus ipilimumab (22.8 months).14
Although the triplet arm had numerically higher rates of TRAEs than the doublet arm, including those leading to discontinuation, the overall safety profiles were consistent with those reported previously, with no new safety signals identified.14,30 In contrast to atezolizumab plus bevacizumab,28 no treatment-related bleeding events were reported during our study. Grade 3-4 AEs were more frequent in the triplet arm than the doublet arm, but were mainly laboratory abnormalities not expected to have major clinical impact. In both arms, TRAEs were effectively managed by nivolumab and/or ipilimumab dose delay and cabozantinib dose reduction or interruption. Similarly, most sTRAEs were effectively managed using standard treatment algorithms; 8% of patients in the doublet arm and 29% in the triplet arm required corticosteroids (≥ 40 mg prednisone or equivalent) for a median of 4.6 and 1.7 weeks, respectively. In both arms, some patients who discontinued because of TRAEs had ongoing response or SD. No treatment-related deaths occurred in either arm.
It is of interest to determine the contributions of ICIs and VEGF inhibitors when used in combination to treat advanced HCC, as immune evasion and angiogenesis are interdependent and can facilitate tumor development,31,32 and VEGF inhibition has been shown to enhance PD-L1 immunotherapy by targeting angiogenesis and regulatory T-cell proliferation.33 Triplet therapy including cabozantinib in the current CheckMate 040 cohort produced an ORR similar to that of second-line nivolumab plus ipilimumab therapy (cohort 4), a higher DCR (80% v 50%, respectively), and a lower proportion of patients with PD (11% v > 40%, respectively).14 These findings suggest that cabozantinib might work synergistically with dual ICIs in this setting. Conversely, triplet therapy in the current study increased the frequency of treatment discontinuation compared with the nivolumab plus ipilimumab arm of cohort 4.14 However, comparisons across cohorts and with other trials are not ideal because of differences in study designs and patient populations.
The IMbrave150 trial demonstrated the potential synergy between first-line atezolizumab plus bevacizumab versus sorafenib in patients with advanced HCC.28 Further molecular analyses of this combination versus atezolizumab alone have shown that bevacizumab might synergize with atezolizumab by targeting angiogenesis, the proliferation of regulatory T cells, and myeloid cell inflammation.33 However, recently reported results from several phase III trials of ICI plus tyrosine kinase inhibitor (TKI) combinations in the first-line setting in patients with advanced HCC are conflicting. In the COSMIC-312 trial (cabozantinib plus atezolizumab v sorafenib), PFS was significantly improved with the combination, but not interim OS.34 In the LEAP-002 trial (lenvatinib plus pembrolizumab v lenvatinib), neither PFS nor OS was significantly improved with the combination, although median OS with combination therapy was approximately 21 months.35 By contrast, in the camrelizumab plus rivoceranib trial, both PFS and OS were significantly improved with this combination (median OS approximately 22 months36). These mixed results might be partly attributed to differences between the efficacy and safety profiles of each TKI, differences in patient populations, and variations in trial design. The optimal TKI to use with ICIs in treating advanced HCC has yet to be determined.
Ideally, to determine the contribution of each component of this type of combination therapy to efficacy and safety, a multiarm trial would have to be designed in which patients are randomly assigned to receive monotherapy with an ICI, TKI, or angiogenesis inhibitor or combination therapy with one or two ICIs with different mechanisms of action, one or two ICIs plus a TKI, or one or two ICIs plus an angiogenesis inhibitor. Given the complexity of conducting such of trial, the possibility of exposing some patients to ineffective or toxic combinations, and the fact that some multiple drug combinations could not be used in patients with HCC and comorbidities, such a multiarm trial is unlikely ever to be conducted. Finally, the question of whether an antiangiogenic agent should be used in combination with ICIs as concurrent or sequential therapy can also only be addressed by future clinical trials.
Our study had some limitations. The phase II design and safety-based sample-size determination meant that patient numbers were relatively low for efficacy analyses. It was not possible to evaluate the impact of tumor burden and declining liver function on OS because few patients had high tumor burden and rapid liver function decline. Although assignment to treatment arms was randomized, no stratification was applied, and the study design did not permit formal statistical comparisons between arms, nor was an active comparator arm included. The open-label design might have influenced tumor responses by investigator assessment and assessment of AE causality. Our study population included sorafenib-naive and sorafenib-pretreated patients, limiting interpretation of the results in context with other studies.
In conclusion, nivolumab plus cabozantinib with or without ipilimumab showed clinical activity and a manageable safety profile in sorafenib-naive or sorafenib-pretreated patients with advanced HCC. Larger studies may be warranted to further characterize benefit/risk profiles of these combinations, especially triplet therapy.
ACKNOWLEDGMENT
The authors thank the patients who participated in this trial and their families; the investigators, study coordinators, study teams, and nurses who assisted; and Dako, an Agilent Technologies company, for collaborative development of the PD-L1 IHC 28-8 pharmDx assay. Bristol Myers Squibb (Princeton, NJ), Ono Pharmaceutical Company Ltd (Osaka, Japan), and Exelixis, Inc (Alameda, CA) supplied the study treatments. Medical writing support was provided by Amanda Hatton, MSc, of Parexel, funded by Bristol Myers Squibb.
PRIOR PRESENTATION
Presented in part (previous database lock) at the ASCO Gastrointestinal Cancers Symposium, San Francisco, CA, January 23-25, 2020.
SUPPORT
Supported by Bristol Myers Squibb, Ono Pharmaceutical Company, Ltd, and Exelixis, Inc.
CLINICAL TRIAL INFORMATION
Thomas Yau
Honoraria: Bristol Myers Squibb, MSD Oncology, AstraZeneca
Consulting or Advisory Role: Bristol Myers Squibb
Vittorina Zagonel
Consulting or Advisory Role: Bristol Myers Squibb, Pfizer, Mundipharma
Speakers' Bureau: Bayer, Merck, Astellas Pharma
Research Funding: Bayer (Inst)
Travel, Accommodations, Expenses: Daiichi Sankyo/Lilly
Armando Santoro
Consulting or Advisory Role: Bristol Myers Squibb, Servier, Gilead Sciences, Pfizer, Eisai, Bayer, MSD, Sanofi, ARQULE, Incyte
Speakers' Bureau: Takeda, Roche, AbbVie, Amgen, Celgene, AstraZeneca, ArQule, Lilly, Sandoz, Novartis, Bristol Myers Squibb, Servier, Gilead Sciences, Pfizer, Eisai, Bayer, MSD
Mirelis Acosta-Rivera
Consulting or Advisory Role: Merck
Speakers' Bureau: Merck, Eisai, Bristol Myers Squibb
Su Pin Choo
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene
Honoraria: Bristol Myers Squibb, AstraZeneca, Ipsen, Eisai, Roche
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Bayer, MSD Oncology, Eisai, Roche
Travel, Accommodations, Expenses: Taiho Pharmaceutical, Bristol Myers Squibb
Ana Matilla
Consulting or Advisory Role: AstraZeneca Spain, Roche, Sirtex Medical
Speakers' Bureau: Roche, Boston Scientific, Gilead Sciences
Aiwu Ruth He
Consulting or Advisory Role: Merck, Genentech/Roche, Eisai
Speakers' Bureau: Eisai, Bristol Myers Squibb, Exelixis
Research Funding: Genentech, Merck
Anthony B. El-Khoueiry
Honoraria: Bayer, Bristol Myers Squibb, Roche/Genentech, EMD Serono, EISAI, Merck, Agenus, Exelixis, Gilead Sciences, AstraZeneca/MedImmune, ABL Bio, QED Therapeutics, Servier, Tallac Therapeutics, Senti Biosciences, Qurient
Consulting or Advisory Role: Bristol Myers Squibb, Bayer, Eisai, Roche, Merck, Exelixis, Pieris Pharmaceuticals, Agenus, Gilead Sciences, AstraZeneca/MedImmune, ABL Bio, QED Therapeutics, Servier, Tallac Therapeutics, Senti Biosciences, Qurient
Research Funding: AstraZeneca, Astex Pharmaceuticals, Fulgent Genetics
Bruno Sangro
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Bayer, Adaptimmune, Sirtex Medical, Ipsen, Roche/Genentech, Eisai, Incyte, TERUMO, Boston Scientific
Speakers' Bureau: Bristol Myers Squibb, Bayer, Sirtex Medical, Ipsen, Lilly, AstraZeneca, Eisai, Incyte, Roche
Research Funding: Bristol Myers Squibb (Inst), Sirtex Medical (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Bristol Myers Squibb, Sirtex Medical, Bayer
Jordi Bruix
Consulting or Advisory Role: Bayer Schering Pharma, Sirtex Medical, Novartis, Bristol Myers Squibb, Eisai, BTG, Roche, Arqule, Basilea
Speakers' Bureau: Bayer Schering Pharma
Research Funding: Bayer Schering Pharma (Inst), Ipsen (Inst)
Travel, Accommodations, Expenses: Ipsen, AstraZeneca
Gina M. Vaccaro
Consulting or Advisory Role: Exelixis, Taiho Pharmaceutical, AstraZeneca, Incyte, Amgen, Astellas Pharma, QED Therapeutics, Pfizer, Array BioPharma, MSD, Helsinn Therapeutics, Servier, GlaxoSmithKline
Speakers' Bureau: Incyte
Research Funding: Celgene (Inst), Merck Sharp & Dohme (Inst), Astellas Pharma (Inst), EMD Serono (Inst), Incyte (Inst), Bristol Myers Squibb (Inst), Array BioPharma (Inst), AstraZeneca/MedImmune (Inst)
Travel, Accommodations, Expenses: Eisai, AstraZeneca, E.R. Squibb Sons, LLC, Foundation Medicine
Marina Tschaika
Employment: Bristol Myers Squibb/Medarex
Stock and Other Ownership Interests: Bristol Myers Squibb/Medarex
Christian Scheffold
Employment: Exelixis
Stock and Other Ownership Interests: Exelixis
Patents, Royalties, Other Intellectual Property: Patent
Jaclyn Neely
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Fabio Piscaglia
Consulting or Advisory Role: Bayer, Eisai, Tiziana Life Sciences, Roche, AstraZeneca, Ipsen, Roche, Exact Sciences
Speakers' Bureau: Bracco Diagnostics, ESAOTE, Samsung, MSD, Eisai
No other potential conflicts of interest were reported.
DATA SHARING STATEMENT
Bristol Myers Squibb's policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
AUTHOR CONTRIBUTIONS
Conception and design: Thomas Yau, Fabio Piscaglia, Armando Santoro, Anthony B. El-Khoueiry, Bruno Sangro, Christian Scheffold, Jaclyn Neely
Provision of study materials or patients: Thomas Yau, Vittorina Zagonel, Mirelis Acosta-Rivera, Su Pin Choo, Aiwu Ruth He, Bruno Sangro, Jordi Bruix, Gina M. Vaccaro
Collection and assembly of data: Thomas Yau, Mirelis Acosta-Rivera, Su Pin Choo, Ana Matilla, Aiwu Ruth He, Antonio Cubillo Gracian, Tarek E. Eldawy, Giovanni Luca Frassineti, Gina M. Vaccaro, Jaclyn Neely, Fabio Piscaglia
Data analysis and interpretation: Thomas Yau, Vittorina Zagonel, Su Pin Choo, Ana Matilla, Aiwu Ruth He, Antonio Cubillo Gracian, Bruno Sangro, Jordi Bruix, Gina M. Vaccaro, Marina Tschaika, Christian Scheffold, Petra Koopmans, Jaclyn Neely, Fabio Piscaglia
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nivolumab Plus Cabozantinib With or Without Ipilimumab for Advanced Hepatocellular Carcinoma: Results From Cohort 6 of the CheckMate 040 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Thomas Yau
Honoraria: Bristol Myers Squibb, MSD Oncology, AstraZeneca
Consulting or Advisory Role: Bristol Myers Squibb
Vittorina Zagonel
Consulting or Advisory Role: Bristol Myers Squibb, Pfizer, Mundipharma
Speakers' Bureau: Bayer, Merck, Astellas Pharma
Research Funding: Bayer (Inst)
Travel, Accommodations, Expenses: Daiichi Sankyo/Lilly
Armando Santoro
Consulting or Advisory Role: Bristol Myers Squibb, Servier, Gilead Sciences, Pfizer, Eisai, Bayer, MSD, Sanofi, ARQULE, Incyte
Speakers' Bureau: Takeda, Roche, AbbVie, Amgen, Celgene, AstraZeneca, ArQule, Lilly, Sandoz, Novartis, Bristol Myers Squibb, Servier, Gilead Sciences, Pfizer, Eisai, Bayer, MSD
Mirelis Acosta-Rivera
Consulting or Advisory Role: Merck
Speakers' Bureau: Merck, Eisai, Bristol Myers Squibb
Su Pin Choo
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene
Honoraria: Bristol Myers Squibb, AstraZeneca, Ipsen, Eisai, Roche
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Bayer, MSD Oncology, Eisai, Roche
Travel, Accommodations, Expenses: Taiho Pharmaceutical, Bristol Myers Squibb
Ana Matilla
Consulting or Advisory Role: AstraZeneca Spain, Roche, Sirtex Medical
Speakers' Bureau: Roche, Boston Scientific, Gilead Sciences
Aiwu Ruth He
Consulting or Advisory Role: Merck, Genentech/Roche, Eisai
Speakers' Bureau: Eisai, Bristol Myers Squibb, Exelixis
Research Funding: Genentech, Merck
Anthony B. El-Khoueiry
Honoraria: Bayer, Bristol Myers Squibb, Roche/Genentech, EMD Serono, EISAI, Merck, Agenus, Exelixis, Gilead Sciences, AstraZeneca/MedImmune, ABL Bio, QED Therapeutics, Servier, Tallac Therapeutics, Senti Biosciences, Qurient
Consulting or Advisory Role: Bristol Myers Squibb, Bayer, Eisai, Roche, Merck, Exelixis, Pieris Pharmaceuticals, Agenus, Gilead Sciences, AstraZeneca/MedImmune, ABL Bio, QED Therapeutics, Servier, Tallac Therapeutics, Senti Biosciences, Qurient
Research Funding: AstraZeneca, Astex Pharmaceuticals, Fulgent Genetics
Bruno Sangro
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Bayer, Adaptimmune, Sirtex Medical, Ipsen, Roche/Genentech, Eisai, Incyte, TERUMO, Boston Scientific
Speakers' Bureau: Bristol Myers Squibb, Bayer, Sirtex Medical, Ipsen, Lilly, AstraZeneca, Eisai, Incyte, Roche
Research Funding: Bristol Myers Squibb (Inst), Sirtex Medical (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Bristol Myers Squibb, Sirtex Medical, Bayer
Jordi Bruix
Consulting or Advisory Role: Bayer Schering Pharma, Sirtex Medical, Novartis, Bristol Myers Squibb, Eisai, BTG, Roche, Arqule, Basilea
Speakers' Bureau: Bayer Schering Pharma
Research Funding: Bayer Schering Pharma (Inst), Ipsen (Inst)
Travel, Accommodations, Expenses: Ipsen, AstraZeneca
Gina M. Vaccaro
Consulting or Advisory Role: Exelixis, Taiho Pharmaceutical, AstraZeneca, Incyte, Amgen, Astellas Pharma, QED Therapeutics, Pfizer, Array BioPharma, MSD, Helsinn Therapeutics, Servier, GlaxoSmithKline
Speakers' Bureau: Incyte
Research Funding: Celgene (Inst), Merck Sharp & Dohme (Inst), Astellas Pharma (Inst), EMD Serono (Inst), Incyte (Inst), Bristol Myers Squibb (Inst), Array BioPharma (Inst), AstraZeneca/MedImmune (Inst)
Travel, Accommodations, Expenses: Eisai, AstraZeneca, E.R. Squibb Sons, LLC, Foundation Medicine
Marina Tschaika
Employment: Bristol Myers Squibb/Medarex
Stock and Other Ownership Interests: Bristol Myers Squibb/Medarex
Christian Scheffold
Employment: Exelixis
Stock and Other Ownership Interests: Exelixis
Patents, Royalties, Other Intellectual Property: Patent
Jaclyn Neely
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Fabio Piscaglia
Consulting or Advisory Role: Bayer, Eisai, Tiziana Life Sciences, Roche, AstraZeneca, Ipsen, Roche, Exact Sciences
Speakers' Bureau: Bracco Diagnostics, ESAOTE, Samsung, MSD, Eisai
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Park JW, Chen M, Colombo M, et al. : Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE study. Liver Int 35:2155-2166, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN) : Clinical Practice Guidelines in Oncology (NCCN Guidelines): Hepatobiliary Cancers Version 3.2022. 2022. https://www.nccn.org/guidelines/category_1 [Google Scholar]
- 4.Bayer HealthCare Pharmaceuticals Inc : NEXAVAR (sorafenib) prescribing information, July 2020. http://labeling.bayerhealthcare.com/html/products/pi/Nexavar_PI.pdf [Google Scholar]
- 5.Eisai R&D Management Company Ltd : LENVIMA (lenvatinib) prescribing information, November 2022. https://www.lenvima.com/-/media/Project/EIsai/Lenvima/PDF/prescribing-information.pdf? [Google Scholar]
- 6.Kudo M, Finn RS, Qin S, et al. : Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 391:1163-1173, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Finn RS, Qin S, Ikeda K, et al. : IMbrave150: Updated overall survival data from a global, randomized, open-label phase III study of atezolizumab + bevacizumab vs sorafenib in patients with unresectable hepatocellular carcinoma. Presented at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium, Virtual, January 15-17, 2021
- 8.Vogel A, Martinelli E: Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol 32:801-805, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Kudo M, Kawamura Y, Hasegawa K, et al. : Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer 10:181-223, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruix J, Chan SL, Galle PR, et al. : Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol 75:960-974, 2021 [DOI] [PubMed] [Google Scholar]
- 11.Bruix J, Qin S, Merle P, et al. : Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389:56-66, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Abou-Alfa GK, Meyer T, Cheng AL, et al. : Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 379:54-63, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu AX, Kang YK, Yen CJ, et al. : Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20:282-296, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Yau T, Kang YK, Kim TY, et al. : Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol 6:e204564, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finn RS, Ryoo BY, Merle P, et al. : Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J Clin Oncol 38:193-202, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Bristol Myers Squibb : OPDIVO (nivolumab) prescribing information, July 2022. http://packageinserts.bms.com/pi/pi_opdivo.pdf [Google Scholar]
- 17.Bristol Myers Squibb : YERVOY (ipilimumab) prescribing information, May 2022. http://packageinserts.bms.com/pi/pi_yervoy.pdf [Google Scholar]
- 18.Das R, Verma R, Sznol M, et al. : Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol 194:950-959, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apolo AB, Tomita Y, Lee M-J, et al. : Effect of cabozantinib on immunosuppressive subsets in metastatic urothelial carcinoma. J Clin Oncol 32, 2014. (suppl 15; abstr 4501) [Google Scholar]
- 20.European Medicines Agency : CABOMETYX summary of product characteristics. October 2022. https://www.ema.europa.eu/documents/product-information/cabometyx-epar-product-information_en.pdf [Google Scholar]
- 21.Exelixis : CABOMETYX (cabozantinibprescribing information. July 2022. https://www.cabometyxhcp.com/prescribing-information [Google Scholar]
- 22.Kwilas AR, Ardiani A, Donahue RN, et al. : Dual effects of a targeted small-molecule inhibitor (cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J Transl Med 12:294, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tolaney SM, Ziehr DR, Guo H, et al. : Phase II and biomarker study of cabozantinib in metastatic triple-negative breast cancer patients. Oncologist 22:25-32, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choueiri TK, Powles T, Burotto M, et al. : Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384:829-841, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apolo AB, Nadal R, Girardi DM, et al. : Phase I study of cabozantinib and nivolumab alone or with ipilimumab for advanced or metastatic urothelial carcinoma and other genitourinary tumors. J Clin Oncol 38:3672-3684, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 27.El-Khoueiry AB, Sangro B, Yau T, et al. : Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389:2492-2502, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finn RS, Qin S, Ikeda M, et al. : Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382:1894-1905, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Finn RS, Qin S, Ikeda M, et al. : IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol 39, 2021. (suppl 3; abstr 267) [Google Scholar]
- 30.Ho WJ, Zhu Q, Durham J, et al. : Neoadjuvant cabozantinib and nivolumab convert locally advanced hepatocellular carcinoma into resectable disease with enhanced antitumor immunity. Nat Cancer 2:891-903, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanahan D, Weinberg RA: Hallmarks of cancer: The next generation. Cell 144:646-674, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Khan KA, Kerbel RS: Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol 15:310-324, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Zhu AX, Abbas AR, de Galarreta MR, et al. : Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med 28:1599-1611, 2022 [DOI] [PubMed] [Google Scholar]
- 34.Kelley RK, Rimassa L, Cheng AL, et al. : Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 23:995-1008, 2022 [DOI] [PubMed] [Google Scholar]
- 35.Finn RS, Kudo M, Merle P, et al. : Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol 33:S808-S869, 2022. (suppl 7; abstr LBA34) [Google Scholar]
- 36.Qin S, Chan L, Gu S, et al. : Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): A randomized, phase III trial. Ann Oncol 33:S808-S869, 2022. (suppl 7; abstr LBA35) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Bristol Myers Squibb's policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.