PURPOSE
With the initial analysis of POLLUX at a median follow-up of 13.5 months, daratumumab in combination with lenalidomide and dexamethasone (D-Rd) significantly prolonged progression-free survival versus lenalidomide and dexamethasone (Rd) alone in patients with relapsed or refractory multiple myeloma (RRMM). We report updated efficacy and safety results at the time of final analysis for overall survival (OS).
METHODS
POLLUX was a multicenter, randomized, open-label, phase III study during which eligible patients with ≥ 1 line of prior therapy were randomly assigned 1:1 to D-Rd or Rd until disease progression or unacceptable toxicity. After positive primary analysis and protocol amendment, patients receiving Rd were offered daratumumab monotherapy after disease progression.
RESULTS
Significant OS benefit was observed with D-Rd (hazard ratio, 0.73; 95% CI, 0.58 to 0.91; P = .0044) at a median (range) follow-up of 79.7 months (0.0-86.5). The median OS was 67.6 months for D-Rd compared with 51.8 months for Rd. Prespecified analyses demonstrated an improved OS with D-Rd versus Rd in most subgroups, including patients age ≥ 65 years and patients with one, two, or three prior lines of therapy, International Staging System stage III disease, high-risk cytogenetic abnormalities, and refractoriness to their last prior line of therapy or a proteasome inhibitor. The most common (≥ 10%) grade 3/4 treatment-emergent adverse events with D-Rd versus Rd were neutropenia (57.6% v 41.6%), anemia (19.8% v 22.4%), pneumonia (17.3% v 11.0%), thrombocytopenia (15.5% v 15.7%), and diarrhea (10.2% v 3.9%).
CONCLUSION
D-Rd significantly extended OS versus Rd alone in patients with RRMM. To our knowledge, for the first time, our findings, together with the OS benefit observed with daratumumab plus bortezomib and dexamethasone in the phase III CASTOR trial, demonstrate OS improvement with daratumumab-containing regimens in RRMM (ClinicalTrials.gov identifier: NCT02076009 [POLLUX]).
INTRODUCTION
Daratumumab is a human IgGκ monoclonal antibody targeting CD38 with a direct on-tumor1-4 and immunomodulatory5-7 mechanism of action, that induces greater cytotoxicity of multiple myeloma cells ex vivo compared with analogs of other CD38 antibodies.8 Daratumumab induces higher levels of complement-dependent cytotoxicity and similar levels of antibody-dependent cellular phagocytosis and antibody-dependent cell-mediated cytotoxicity, as well as, similar levels of cell death in the presence of Fc receptor crosslinking, which occurs physiologically in vivo.8
CONTEXT
Key Objective
To report updated efficacy and safety results, including overall survival (OS), from the phase III POLLUX study of daratumumab, lenalidomide, and dexamethasone (D-Rd) in patients with relapsed or refractory multiple myeloma after a follow-up of > 6.5 years.
Knowledge Generated
At a median follow-up of 79.7 months, D-Rd significantly prolonged OS, with a 27% reduction in the risk of death versus Rd alone (median: 67.6 v 51.8 months, respectively; hazard ratio, 0.73; 95% CI, 0.58 to 0.91; P = .0044). Prespecified analyses demonstrated OS benefit for D-Rd compared with Rd across most patient subgroups.
Relevance (S. Lentzsch)
Triplets such as D-Rd are superior to doublets such as Rd alone and therefore should be the SOC in patients with relapsed refractory multiple myeloma.*
*Relevance section written by JCO Associate Editor, Suzanne Lentzsch, MD, PhD.
Several phase III studies in newly diagnosed multiple myeloma (NDMM) and relapsed or refractory multiple myeloma (RRMM) have demonstrated that daratumumab in combination with standard-of-care (SOC) regimens significantly reduced the risk of disease progression or death and achieved deep and durable responses, including significantly higher complete response or better (≥ CR) rates and minimal residual disease (MRD) negativity rates, compared with SOC alone.9-14 Results from these pivotal studies led to the approval of daratumumab in combination with SOC regimens across lines of therapy.15,16
In the initial analysis (median follow-up, 13.5 months) of the phase III POLLUX study, daratumumab plus lenalidomide and dexamethasone (D-Rd) significantly improved progression-free survival (PFS) compared with lenalidomide and dexamethasone (Rd) alone in patients with RRMM (hazard ratio [HR], 0.37; 95% CI, 0.27 to 0.52; P < .001) and achieved higher rates of deeper responses (≥ CR: 43.1% v 19.2%; MRD negativity [10–5 sensitivity]: 22.4% v 4.6%; P < .001 for both).17 In an updated analysis at a median follow-up of 25.4 months, D-Rd continued to improve PFS (HR, 0.41; 95% CI, 0.31 to 0.53; P < .0001), and responses deepened with time, with ≥ CR and MRD negativity rates (10–5 sensitivity) of 51.2% versus 21.0% (P < .0001) and 26.2% versus 6.4%, respectively (P < .000001).18 In the most recent analysis of POLLUX (median follow-up, 54.8 months), D-Rd resulted in a 56% reduction in the risk of disease progression or death compared with Rd alone (median PFS, 45.0 v 17.5 months; HR, 0.44; 95% CI, 0.35 to 0.54; P < .0001), with continuous deepening of responses and higher MRD negativity rates.19 In patients with one prior line of therapy, the median PFS was 53.3 months with D-Rd versus 19.6 months with Rd (HR, 0.42; 95% CI, 0.31 to 0.58; P < .0001). PFS benefit with D-Rd was maintained regardless of cytogenetic risk status.
Here, we report updated efficacy and safety results at the time of final overall survival (OS) analysis of POLLUX after a follow-up of > 6.5 years.
METHODS
Trial Design and Oversight
POLLUX (ClinicalTrials.gov identifier: NCT02076009) was a multicenter, randomized, open-label, active-controlled, phase III study in patients with RRMM. The study design has been previously published.17 Eligible patients had progressive disease per International Myeloma Working Group criteria20,21 during or after receipt of their last regimen and had received and had a response to ≥ 1 previous line of therapy.17 Patients refractory to lenalidomide were ineligible. The trial protocol was approved by independent ethics committees or institutional review boards at each site. Patients provided written informed consent, and the trial was conducted in accordance with the principles of the Declaration of Helsinki and current International Conference on Harmonisation Good Clinical Practice guidelines.
Random Assignment and Study Treatment
Patients were randomly assigned (1:1) to D-Rd or Rd and stratified according to the number of lines of previous therapy (1 v 2 or 3 v > 3), International Staging System (ISS) disease stage (I v II v III), and previous lenalidomide treatment (yes v no). All patients received 28-day cycles of lenalidomide (25 mg orally once daily on days 1-21 of each cycle and 10 mg once daily if the creatinine clearance was 30-60 mL/min) and dexamethasone (40 mg once weekly) until disease progression, unacceptable toxicity, or death. For patients in the D-Rd group, daratumumab (16 mg/kg intravenously) was administered once weekly (days 1, 8, 15, and 22) in cycles 1 and 2, once every 2 weeks (days 1 and 15) during cycles 3 through 6, and once every 4 weeks thereafter. After positive primary analysis and protocol amendment, patients receiving Rd were offered daratumumab monotherapy after disease progression, after discontinuation because of toxicity, or after a washout period (defined as 2 weeks or five pharmacokinetic half-lives of the treatment, whichever is longer) if they already experienced disease progression and were receiving subsequent therapy.
End Points and Assessments
The primary end point was PFS.17 Secondary end points included overall response rate, rates of very good partial response or better and ≥ CR, MRD negativity, time to response, and OS. An exploratory end point was PFS on the subsequent line of therapy, which was defined as the time from random assignment to progression on the following line of therapy or death. Post hoc exploratory secondary analyses examined subgroups by the number of previous lines of therapy, prior treatment exposure, and cytogenetic risk assessed by next-generation sequencing, as described previously.18 Tumor response and disease progression were assessed using a validated computerized algorithm in accordance with International Myeloma Working Group response criteria.20,21 MRD was assessed using bone marrow aspirate samples and evaluated via next-generation sequencing using the clonoSEQ assay (v.2.0; Adaptive Biotechnologies, Seattle, WA). MRD was evaluated at suspected CR, at 3 and 6 months after confirmed CR, and then every 12 months (± 3 months) post-CR until the end of treatment. Patients were considered to be MRD-positive if they had an MRD-positive test result or had no MRD assessment. Cytogenetic risk was evaluated locally using local fluorescence in situ hybridization or karyotyping. High-risk patients had t(4;14), t(14;16), or del17p cytogenetic abnormalities.
Statistical Analysis
Statistical methods have been published previously.17 Time-to-event end points were compared between groups using a stratified log-rank test. HRs and 95% CIs were estimated using a stratified Cox regression model with treatment as the only explanatory variable. Stratification factors included ISS disease stage (I, II, or III), number of prior lines of therapy (1 v 2 or 3 v > 3), and prior lenalidomide treatment (no v yes). The Kaplan–Meier method was used to estimate the distributions. Overall response rates and rates of very good partial response or better and ≥ CR were compared using the stratified Cochran–Mantel–Haenszel test. MRD negativity rates were compared using Fisher's exact test.
RESULTS
Patients
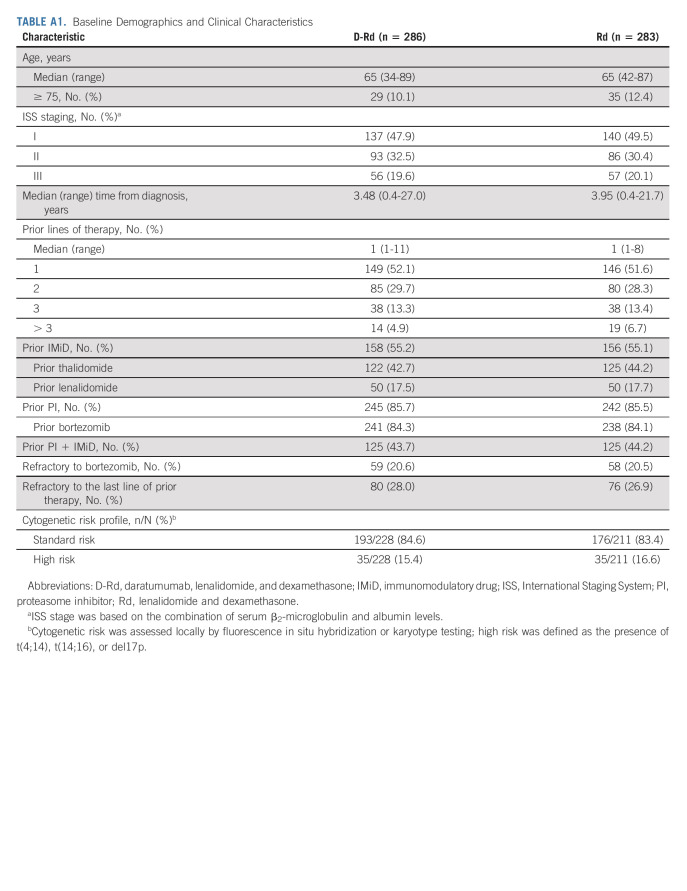
A total of 569 patients were randomly assigned to receive either D-Rd (n = 286) or Rd (n = 283; Fig 1). Patient baseline demographics and clinical characteristics were published previously and were generally well balanced between groups.17 The median (range) patient age was 65 (34-89) years (Appendix Table A1, online only). Approximately half (51.8%) of patients received one prior line of therapy, 84.2% received prior bortezomib, 17.6% received prior lenalidomide, 43.9% received both a proteasome inhibitor (PI) and an immunomodulatory drug, and 20.6% were refractory to bortezomib.
FIG 1.
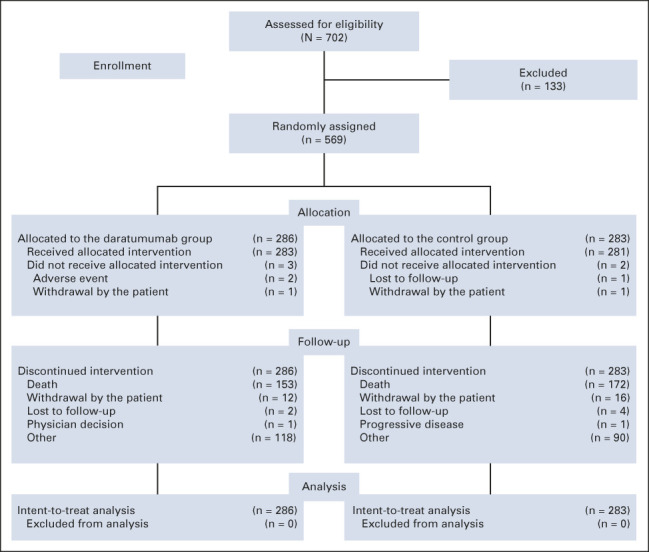
CONSORT diagram for POLLUX.
At the clinical cutoff (September 30, 2021), the median (range) study treatment duration was 34.3 months (0-85.0) for D-Rd versus 16.0 months (0.2-86.2) for Rd; the median (range) total number of treatment cycles received was 37 (1-90) and 17 (1-94), respectively. One hundred twenty-two patients in the Rd arm who received subsequent therapy received daratumumab. Sixty-six patients in the Rd arm received subsequent single-agent daratumumab after disease progression (or after a washout period if they already experienced disease progression and were receiving subsequent therapy), provided per the study protocol. Patients received a median (range) of 7.0 (1-63) cycles; the median (range) duration of daratumumab monotherapy was 5.8 months (0.0-57.3). An additional 56 patients received daratumumab as subsequent therapy not provided in the study.
Efficacy
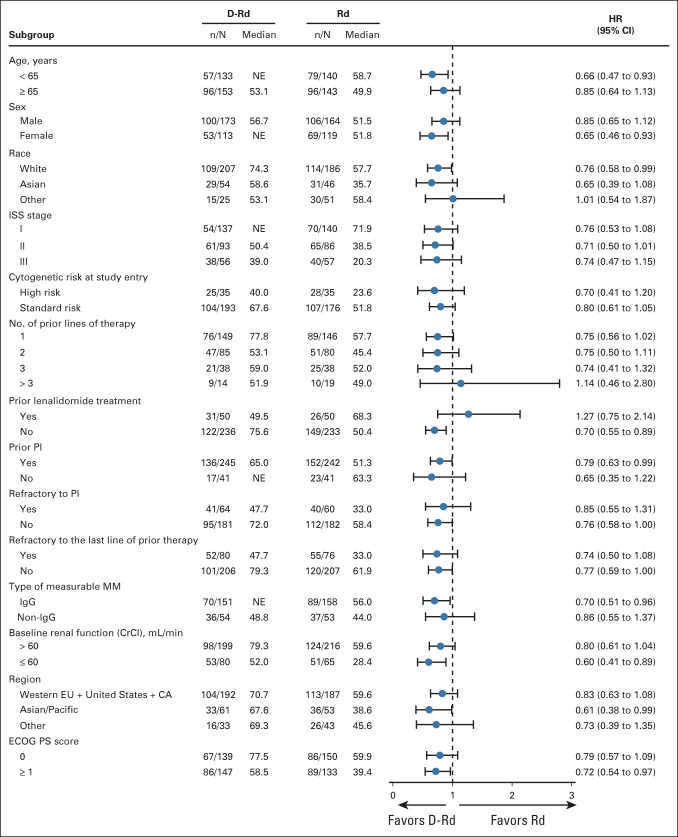
One hundred fifty-three (53.5%) of 286 patients in the D-Rd group and 175 (61.8%) of 283 patients in the Rd group had died at a median (range) follow-up of 79.7 months (0.0-86.5). The HR for death in the D-Rd group compared with the Rd group was 0.73 (95% CI, 0.58 to 0.91; P = .0044; Fig 2A), crossing the prespecified stopping boundary of P < .0331 and representing a 27% reduction in the risk of death. The median OS was 67.6 months (95% CI, 53.1 to 80.5) in the D-Rd arm versus 51.8 months (95% CI, 44.0 to 60.0) in the Rd arm. Prespecified subgroup analyses demonstrated that the OS benefit of D-Rd compared with Rd was maintained across most patient subgroups, including patients age ≥ 65 years, patients who have received one, two, or three prior lines of therapy, patients with ISS stage III disease, patients with high-risk cytogenetic abnormalities, patients with prior PI exposure, and patients who were refractory to their last line of prior therapy or to a PI (Fig 3). For patients with one prior line of therapy, the median OS was 77.8 months with D-Rd compared with 57.7 months with Rd (HR, 0.75; 95% CI, 0.56 to 1.02).
FIG 2.
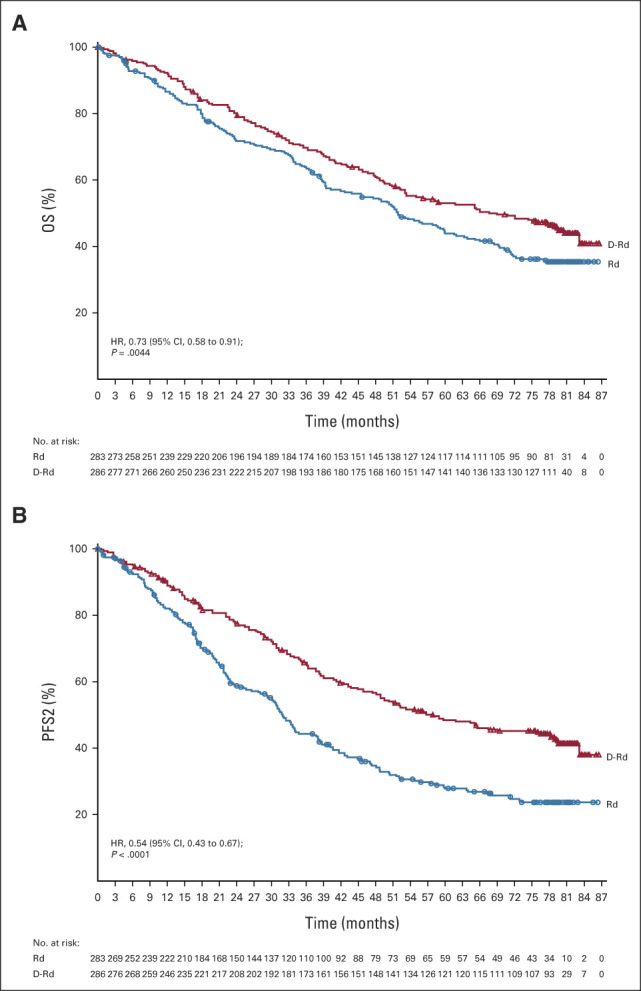
Kaplan-Meier estimates of (A) OS and (B) PFS2 in the ITT population, which included all patients who underwent random assignment. D-Rd, daratumumab, lenalidomide, and dexamethasone; HR, hazard ratio; ITT, intention-to-treat; OS, overall survival; PFS2, progression-free survival on the subsequent line of therapy; Rd, lenalidomide and dexamethasone.
FIG 3.
The results of OS in prespecified subgroups of the ITT population defined by baseline characteristics. The ISS disease stage is derived on the basis of the combination of serum β2-microglobulin and albumin levels. Higher stages indicate more severe disease. Cytogenetic risk was assessed locally by fluorescence in situ hybridization or karyotype testing; high risk was defined as the presence of t(4;14), t(14;16), or del17p. The subgroup analysis of the type of MM was performed on data from patients who had measurable disease in serum. CA, Canada; CrCl, creatinine clearance; D-Rd, daratumumab, lenalidomide, and dexamethasone; ECOG PS, Eastern Cooperative Oncology Group performance status; EU, European Union; HR, hazard ratio; IgG, immunoglobulin G; ISS, International Staging System; ITT, intention-to-treat; MM, multiple myeloma; NE, not estimable; OS, overall survival; PI, proteasome inhibitor; Rd, lenalidomide and dexamethasone.
MRD negativity rates (10–5 sensitivity threshold) were significantly higher with D-Rd compared with Rd (33.2% v 6.7%; P < .0001). MRD negativity was associated with improved OS, regardless of the treatment group (Fig 4).
FIG 4.
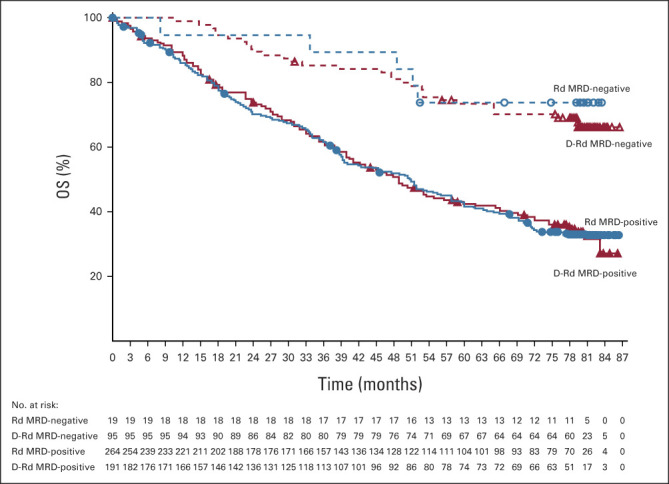
Kaplan–Meier estimates of OS by MRD status (10–5) among patients in the ITT population. D-Rd, daratumumab, lenalidomide, and dexamethasone; ITT, intention-to-treat; MRD, minimal residual disease; OS, overall survival; Rd, lenalidomide and dexamethasone.
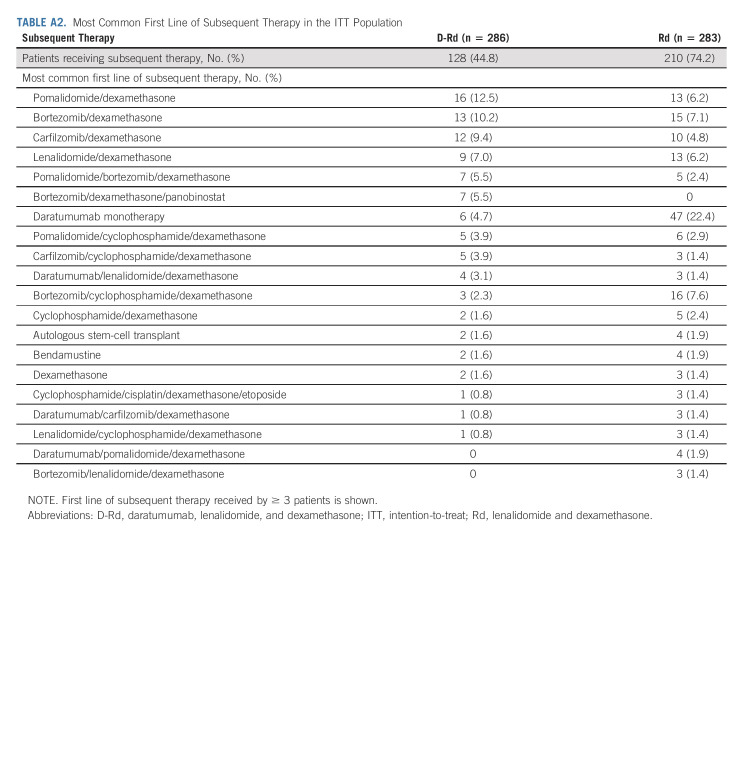
In total, 127 (44.9%) of 283 patients in the D-Rd group and 210 (74.7%) of 281 patients in the Rd group received subsequent therapy. Approximately 58% (122 of 210) of patients in the Rd arm who received subsequent therapy received daratumumab, not only primarily as monotherapy but also in combination regimens. The median (range) number of subsequent lines of therapy was 2 (1-13) in the D-Rd group and 2 (1-12) in the Rd group. Median time to subsequent treatment was significantly increased with D-Rd versus Rd (69.3 v 23.1 months; HR, 0.40; 95% CI, 0.32 to 0.50; P < .0001). For the D-Rd arm, the most common subsequent anticancer therapies were dexamethasone (39.2%), pomalidomide (23.7%), bortezomib (19.8%), cyclophosphamide (17.7%), and carfilzomib (16.6%), and for the Rd arm, they were dexamethasone (63.0%), daratumumab (43.4%), cyclophosphamide (38.1%), pomalidomide (37.0%), bortezomib (33.8%), carfilzomib (23.5%), and lenalidomide (18.5%). PFS on the subsequent line of therapy was significantly prolonged in the D-Rd arm versus the Rd arm (median, 57.9 v 32.0 months; HR, 0.54; 95% CI, 0.43 to 0.67; P < .0001; Fig 2B). First subsequent therapies for patients in both treatment groups are summarized in Appendix Table A2 (online only). The most common first subsequent therapy was pomalidomide plus dexamethasone (12.5%) or bortezomib plus dexamethasone (Vd; 10.2%) in the D-Rd group and daratumumab monotherapy (22.4%) in the Rd group. In the Rd group, 13 (6.2%) patients received daratumumab in various combination regimens as first subsequent therapy.
The median (range) time to crossover to daratumumab subsequent therapy after disease progression for patients in the Rd arm was 28.2 months (4.3-79.5). The Rd group had 66 patients who received subsequent single-agent daratumumab, provided per the study protocol after disease progression; 24 (36.4%) are still alive. The median OS is 65.6 months (95% CI, 51.3 to 73.1).
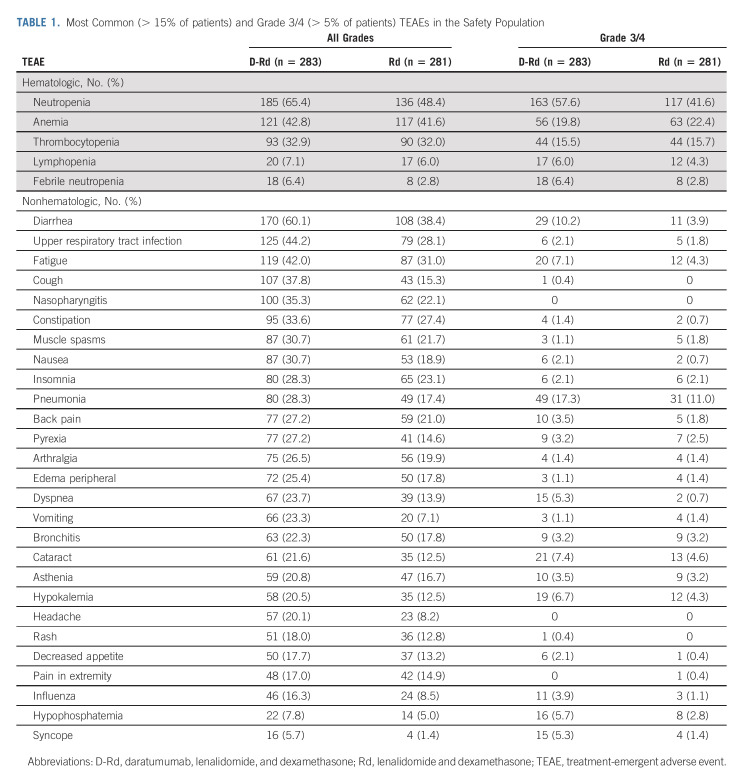
Safety
No new safety concerns were reported with extended follow-up (Table 1). The most common (≥ 10%) grade 3/4 treatment-emergent adverse events (TEAEs) in the D-Rd group versus the Rd group were neutropenia (57.6% v 41.6%), anemia (19.8% v 22.4%), pneumonia (17.3% v 11.0%), thrombocytopenia (15.5% v 15.7%), and diarrhea (10.2% v 3.9%). The incidence of grade 3/4 infections was 44.5% in the D-Rd arm and 28.1% in the Rd arm. Serious TEAEs were reported in 72.4% of patients in the D-Rd group and 52.7% of patients in the Rd group, with pneumonia being the most common (17.0% and 11.4%, respectively). The proportion of patients with TEAEs resulting in treatment discontinuation was comparable between groups (D-Rd, 19.1%; Rd, 16.0%). In the D-Rd group, 13 (4.6%) patients stopped treatment because of infections, whereas in the Rd group, 11 (3.9%) patients did so.
TABLE 1.
Most Common (> 15% of patients) and Grade 3/4 (> 5% of patients) TEAEs in the Safety Population
TEAEs that resulted in death were reported in 35 (12.4%) of 283 patients in the D-Rd arm and 24 (8.5%) of 281 patients in the Rd arm. The most common TEAEs with an outcome of death were septic shock (1.4% v 0.4%), cardiac arrest (1.1% v 0.4%), sudden death (1.1% v 0.4%), pneumonia (0.7% v 1.1%), acute kidney injury (0.4% v 1.1%), and sepsis (0% v 1.1%). There were no deaths during the study because of COVID-19 disease.
The incidence of second primary malignancies (cutaneous, invasive, and hematologic) with longer follow-up was 40 (14.1%) of 283 patients in the D-Rd arm and 30 (10.7%) of 281 patients in the Rd arm. No cancer type was predominant for second primary malignancies in either treatment arm. When adjusted for exposure to study treatment, the incidence of second primary malignancies was similar in the D-Rd (0.35 events per 100 patient-months at risk) and Rd (0.45 events per 100 patient-months at risk) groups.
DISCUSSION
After a follow-up of > 6.5 years (median follow-up, 79.7 months), adding daratumumab to the Rd regimen continued to show significant clinical benefits compared with Rd alone in patients with RRMM, including a significant OS advantage with longer follow-up. Daratumumab in combination with Rd significantly prolonged OS compared with Rd alone, with a 27% reduction in the risk of death. The OS curves separated at approximately 7 months and continued to separate with time. On the basis of prespecified subgroup analyses, an improvement in OS was also observed with D-Rd in nearly all subgroups regardless of age, ISS disease stage, cytogenetic risk, prior PI exposure, or refractoriness to PI or last line of prior therapy. When analyzed by the number of prior lines of therapy, patients with one prior line had the longest median OS of 77.8 months with D-Rd versus 57.7 months with Rd. MRD negativity rates in this final analysis were nearly five times higher with D-Rd versus Rd (33.2% v 6.7%) and are consistent with those reported in an updated analysis at a median follow-up of 54.8 months.19 Regardless of the treatment group, MRD negativity was associated with improved OS, providing further evidence of the importance of achieving MRD negativity. These long-term results from the POLLUX study complement and strengthen the OS data recently reported with daratumumab plus Vd (D-Vd) in the phase III CASTOR study in RRMM.22
It is important to note that after positive primary analysis and protocol amendment, daratumumab monotherapy was offered to patients in the Rd group after disease progression. Patients received daratumumab monotherapy because it was the approved and reimbursed regimen available to patients at the time this study was performed. We recognize that treatment options for RRMM have moved beyond daratumumab monotherapy in more recent years and allowing subsequent therapy may confound OS results. Nevertheless, an OS advantage was observed with D-Rd over Rd despite patients in the Rd arm receiving subsequent daratumumab therapy. More than half of patients (122 of 210) in the Rd arm who received subsequent therapy received salvage daratumumab, not only primarily as monotherapy but also in combination regimens; nearly half of these patients (n = 60) received daratumumab as first subsequent therapy (47 as monotherapy and 13 in various combination regimens).
With longer follow-up, no new safety concerns were observed. Although the incidence of grade 3/4 infections was higher with D-Rd versus Rd, similar proportions of patients discontinued treatment because of infections (4.6% v 3.9%, respectively). Second primary malignancies were reported more frequently in the D-Rd arm, but, when adjusted for exposure to study treatment, the incidence was similar in both arms.
Although cross-trial comparisons should be interpreted with caution because of differences in study design and population, the median OS seen with D-Rd in POLLUX (67.6 months) is the longest median OS reported to date in phase III studies of Rd-based combinations in RRMM. In the phase III TOURMALINE-MM1 trial of patients with RRMM after one to three previous lines of therapy (median follow-up, 85 months), the median OS was 53.6 months with ixazomib plus Rd versus 51.6 months with Rd alone and was not statistically different between treatment groups (HR, 0.939; 95% CI, 0.784 to 1.125; P = .495).23 In a similar population of patients with RRMM and one to three prior lines of therapy, the median OS in the phase III ASPIRE study was 48.3 months with carfilzomib plus Rd versus 40.4 months with Rd alone at a median follow-up of 67.1 months (HR, 0.79; 95% CI, 0.67 to 0.95; 1-sided P = .0045).24 In the phase III ELOQUENT-2 trial of patients with RRMM having one to three prior lines of therapy, at a minimum median follow-up of 70.6 months, the median OS was 48.3 months with elotuzumab plus Rd versus 39.6 months with Rd alone (HR, 0.82; 95.4% CI, 0.68 to 1.00; P = .0408).25 On the basis of the results of these studies, the previous benchmark for survival in RRMM of approximately 4 years has now been extended beyond 5.5 years with the POLLUX OS data.
Results from subgroup analyses of POLLUX complement those recently reported from CASTOR, which showed that the greatest OS improvement with D-Vd occurred in patients with one previous line of therapy.22 Results from two phase III studies in transplant-ineligible NDMM (ALCYONE and MAIA) also demonstrate significantly prolonged OS and PFS with the addition of daratumumab to SOC regimens versus SOC alone.10,26 Given the high attrition rates observed in real-world studies in Europe27 and the United States28 and the observed OS benefits with daratumumab-containing regimens in both NDMM (ALCYONE and MAIA) and now RRMM (POLLUX and CASTOR), early use of daratumumab should be considered an SOC to achieve deep and sustained responses and prolonged disease control.
In conclusion, D-Rd significantly extended OS versus Rd alone. This updated analysis of the POLLUX study reports the longest median OS observed to date in phase III studies of Rd-based triplets in RRMM. To our knowledge, these outcomes, along with the OS results reported with D-Vd in the phase III CASTOR study, show an OS benefit with daratumumab-containing regimens in patients with RRMM for the first time.
ACKNOWLEDGMENT
We thank the patients who participated in the POLLUX study and their families and the study coinvestigators, research nurses, and coordinators at each of the clinical sites. Medical writing and editorial support were provided by Lisa Shannon, PharmD, of Lumanity Communications Inc, and were funded by Janssen Global Services LLC.
APPENDIX
TABLE A1.
Baseline Demographics and Clinical Characteristics
TABLE A2.
Most Common First Line of Subsequent Therapy in the ITT Population
Meletios A. Dimopoulos
Honoraria: Amgen, Takeda, Janssen-Cilag, Bristol Myers Squibb, BeiGene
Consulting or Advisory Role: Amgen, Janssen-Cilag, Takeda, Bristol Myers Squibb, BeiGene
Albert Oriol
Consulting or Advisory Role: Celgene, Janssen, Amgen, Sanofi, GlaxoSmithKline
Speakers' Bureau: Amgen, Celgene, Sanofi, GlaxoSmithKline
Hareth Nahi
Employment: Genmab
Stock and Other Ownership Interests: Genmab
Jesus San-Miguel
Consulting or Advisory Role: Amgen (Inst), Celgene (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Novartis (Inst), Sanofi (Inst), Janssen (Inst), Roche (Inst), AbbVie (Inst), GlaxoSmithKline (Inst), Karyopharm Therapeutics (Inst), Secura Bio (Inst), Regeneron (Inst), HaemaLogiX (Inst)
Nizar J. Bahlis
Honoraria: Celgene, Janssen, AbbVie, Amgen, Sanofi, Takeda, Karyopharm Therapeutics, GlaxoSmithKline, Genentech/Roche
Consulting or Advisory Role: Janssen, Celgene, Amgen, Sanofi, Takeda, Pfizer, Karyopharm Therapeutics
Research Funding: Janssen (Inst), Celgene (Inst)
Saad Z. Usmani
Consulting or Advisory Role: Celgene, Amgen, Janssen Oncology, Seattle Genetics, Takeda, GlaxoSmithKline, Karyopharm Therapeutics, AbbVie, Skyline Diagnostics, Merck, Oncopeptides, Genentech, Gilead Sciences, Bristol Myers Squibb/Celgene
Speakers' Bureau: Takeda, Amgen, Janssen Oncology, Sanofi, Bristol Myers Squibb/Celgene
Research Funding: Celgene, Array BioPharma, Janssen Oncology, Pharmacyclics, Sanofi, Bristol Myers Squibb, Amgen, Seattle Genetics, Merck, Skyline Diagnostics, GlaxoSmithKline
Neil Rabin
Honoraria: Janssen-Ortho, Celgene/Bristol Myers Squibb, Sanofi, GlaxoSmithKline, Takeda, Karyopharm Therapeutics
Consulting or Advisory Role: Janssen-Ortho, Celgene/Bristol Myers Squibb, Sanofi, GlaxoSmithKline, Takeda, Karyopharm Therapeutics
Speakers' Bureau: Janssen-Ortho, Celgene/Bristol Myers Squibb, Sanofi, Takeda
Robert Z. Orlowski
Stock and Other Ownership Interests: Asylia Therapeutics Inc
Consulting or Advisory Role: Bristol Myers Squibb, Janssen, Kite, a Gilead Company, Sanofi, Takeda, EcoR1 Capital LLC, Regeneron, GlaxoSmithKline, AstraZeneca, AbbVie, BioTheryX, Karyopharm Therapeutics, Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin Therapeutics, Oncopeptides
Research Funding: BioTheryX (Inst), Heidelberg Pharma (Inst), Asylia Therapeutics Inc (Inst), CARsgen Therapeutics (Inst), Sanofi/Aventis (Inst), Exelixis (Inst), Takeda (Inst), Janssen Biotech (Inst), Celgene/Bristol Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: Asylia Therapeutics Inc
Torben Plesner
Stock and Other Ownership Interests: Novo Nordisk
Consulting or Advisory Role: Janssen, Celgene, Takeda, AbbVie, Genmab, Oncopeptides, Genentech, CSL Behring
Speakers' Bureau: Janssen, Takeda
Research Funding: Janssen (Inst), Genmab (Inst), Celgene (Inst), Takeda (Inst), Oncopeptides (Inst), Genentech (Inst), AbbVie (Inst), Roche (Inst)
Sung-Soo Yoon
Honoraria: Novartis
Consulting or Advisory Role: Janssen, Takeda, Amgen, Celgene/Jazz
Research Funding: Kyowa Kirin, Roche/Genentech, Yuhan
Paul G. Richardson
Consulting or Advisory Role: Takeda, Karyopharm Therapeutics, Oncopeptides, Sanofi, Secura Bio, Bristol Myers Squibb/Celgene, GlaxoSmithKline, AstraZeneca, Novartis
Research Funding: Celgene (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), Oncopeptides (Inst), Karyopharm Therapeutics (Inst)
Hartmut Goldschmidt
Honoraria: Janssen-Cilag, Novartis, Bristol Myers Squibb, Celgene, Chugai Pharma, Sanofi, Amgen, GlaxoSmithKline
Consulting or Advisory Role: Celgene (Inst), Janssen-Cilag (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Takeda (Inst), Adaptive Biotechnologies (Inst), Sanofi (Inst)
Research Funding: Bristol Myers Squibb (Inst), Janssen (Inst), Novartis (Inst), Chugai Pharma Europe (Inst), Celgene (Inst), Amgen (Inst), Sanofi (Inst), Mundipharma (Inst), Takeda (Inst), Molecular Partners (Inst), MSD (Inst), Incyte (Inst)
Travel, Accommodations, Expenses: Janssen-Cilag, Sanofi
Other Relationship: Amgen (Inst), Celgene/Bristol Myers Squibb (Inst), Chugai Pharma Europe (Inst), Janssen (Inst), Sanofi (Inst)
Donna Reece
Honoraria: Janssen, Amgen, Bristol Myers Squibb/Celgene, Sanofi, GlaxoSmithKline, Takeda
Consulting or Advisory Role: Janssen
Research Funding: Janssen, Bristol Myers Squibb
Tahamtan Ahmadi
Employment: Genmab
Leadership: Genmab
Stock and Other Ownership Interests: Genmab
Xiang Qin
Employment: Janssen Research & Development
Xue Gai
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Janssen Research & Development
Travel, Accommodations, Expenses: Janssen Research & Development
Jodi Carey
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson
Robin Carson
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Philippe Moreau
Honoraria: Celgene, Janssen-Cilag, Amgen, GlaxoSmithKline, AbbVie, Sanofi, Oncopeptides
Consulting or Advisory Role: Celgene, Janssen, Amgen, GlaxoSmithKline, Sanofi, AbbVie, Oncopeptides
No other potential conflicts of interest were reported.
SUPPORT
Supported by Janssen Research & Development, LLC.
CLINICAL TRIAL INFORMATION
NCT02076009 (POLLUX)
DATA SHARING STATEMENT
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
AUTHOR CONTRIBUTIONS
Conception and design: Meletios A. Dimopoulos, Hareth Nahi, Jesus San-Miguel, Robert Z. Orlowski, Torben Plesner, Paul G. Richardson, Tahamtan Ahmadi, Robin Carson
Provision of study materials or patients: Jesus San-Miguel, Nizar J. Bahlis, Neil Rabin, Robert Z. Orlowski, Kenshi Suzuki, Torben Plesner, Sung-Soo Yoon, Dina Ben Yehuda, Paul G. Richardson, Philippe Moreau
Collection and assembly of data: Meletios A. Dimopoulos, Albert Oriol, Hareth Nahi, Jesus San-Miguel, Nizar J. Bahlis, Robert Z. Orlowski, Kenshi Suzuki, Torben Plesner, Sung-Soo Yoon, Dina Ben Yehuda, Paul G. Richardson, Hartmut Goldschmidt, Donna Reece, Tahamtan Ahmadi, Wendy Garvin Mayo, Jodi Carey, Robin Carson, Philippe Moreau
Data analysis and interpretation: Meletios A. Dimopoulos, Albert Oriol, Hareth Nahi, Jesus San-Miguel, Nizar J. Bahlis, Saad Z. Usmani, Neil Rabin, Robert Z. Orlowski, Kenshi Suzuki, Torben Plesner, Sung-Soo Yoon, Paul G. Richardson, Hartmut Goldschmidt, Tahamtan Ahmadi, Xiang Qin, Xue Gai, Jodi Carey, Robin Carson, Philippe Moreau
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Overall Survival With Daratumumab, Lenalidomide, and Dexamethasone in Previously Treated Multiple Myeloma (POLLUX): A Randomized, Open-Label, Phase III Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Meletios A. Dimopoulos
Honoraria: Amgen, Takeda, Janssen-Cilag, Bristol Myers Squibb, BeiGene
Consulting or Advisory Role: Amgen, Janssen-Cilag, Takeda, Bristol Myers Squibb, BeiGene
Albert Oriol
Consulting or Advisory Role: Celgene, Janssen, Amgen, Sanofi, GlaxoSmithKline
Speakers' Bureau: Amgen, Celgene, Sanofi, GlaxoSmithKline
Hareth Nahi
Employment: Genmab
Stock and Other Ownership Interests: Genmab
Jesus San-Miguel
Consulting or Advisory Role: Amgen (Inst), Celgene (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Novartis (Inst), Sanofi (Inst), Janssen (Inst), Roche (Inst), AbbVie (Inst), GlaxoSmithKline (Inst), Karyopharm Therapeutics (Inst), Secura Bio (Inst), Regeneron (Inst), HaemaLogiX (Inst)
Nizar J. Bahlis
Honoraria: Celgene, Janssen, AbbVie, Amgen, Sanofi, Takeda, Karyopharm Therapeutics, GlaxoSmithKline, Genentech/Roche
Consulting or Advisory Role: Janssen, Celgene, Amgen, Sanofi, Takeda, Pfizer, Karyopharm Therapeutics
Research Funding: Janssen (Inst), Celgene (Inst)
Saad Z. Usmani
Consulting or Advisory Role: Celgene, Amgen, Janssen Oncology, Seattle Genetics, Takeda, GlaxoSmithKline, Karyopharm Therapeutics, AbbVie, Skyline Diagnostics, Merck, Oncopeptides, Genentech, Gilead Sciences, Bristol Myers Squibb/Celgene
Speakers' Bureau: Takeda, Amgen, Janssen Oncology, Sanofi, Bristol Myers Squibb/Celgene
Research Funding: Celgene, Array BioPharma, Janssen Oncology, Pharmacyclics, Sanofi, Bristol Myers Squibb, Amgen, Seattle Genetics, Merck, Skyline Diagnostics, GlaxoSmithKline
Neil Rabin
Honoraria: Janssen-Ortho, Celgene/Bristol Myers Squibb, Sanofi, GlaxoSmithKline, Takeda, Karyopharm Therapeutics
Consulting or Advisory Role: Janssen-Ortho, Celgene/Bristol Myers Squibb, Sanofi, GlaxoSmithKline, Takeda, Karyopharm Therapeutics
Speakers' Bureau: Janssen-Ortho, Celgene/Bristol Myers Squibb, Sanofi, Takeda
Robert Z. Orlowski
Stock and Other Ownership Interests: Asylia Therapeutics Inc
Consulting or Advisory Role: Bristol Myers Squibb, Janssen, Kite, a Gilead Company, Sanofi, Takeda, EcoR1 Capital LLC, Regeneron, GlaxoSmithKline, AstraZeneca, AbbVie, BioTheryX, Karyopharm Therapeutics, Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin Therapeutics, Oncopeptides
Research Funding: BioTheryX (Inst), Heidelberg Pharma (Inst), Asylia Therapeutics Inc (Inst), CARsgen Therapeutics (Inst), Sanofi/Aventis (Inst), Exelixis (Inst), Takeda (Inst), Janssen Biotech (Inst), Celgene/Bristol Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: Asylia Therapeutics Inc
Torben Plesner
Stock and Other Ownership Interests: Novo Nordisk
Consulting or Advisory Role: Janssen, Celgene, Takeda, AbbVie, Genmab, Oncopeptides, Genentech, CSL Behring
Speakers' Bureau: Janssen, Takeda
Research Funding: Janssen (Inst), Genmab (Inst), Celgene (Inst), Takeda (Inst), Oncopeptides (Inst), Genentech (Inst), AbbVie (Inst), Roche (Inst)
Sung-Soo Yoon
Honoraria: Novartis
Consulting or Advisory Role: Janssen, Takeda, Amgen, Celgene/Jazz
Research Funding: Kyowa Kirin, Roche/Genentech, Yuhan
Paul G. Richardson
Consulting or Advisory Role: Takeda, Karyopharm Therapeutics, Oncopeptides, Sanofi, Secura Bio, Bristol Myers Squibb/Celgene, GlaxoSmithKline, AstraZeneca, Novartis
Research Funding: Celgene (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), Oncopeptides (Inst), Karyopharm Therapeutics (Inst)
Hartmut Goldschmidt
Honoraria: Janssen-Cilag, Novartis, Bristol Myers Squibb, Celgene, Chugai Pharma, Sanofi, Amgen, GlaxoSmithKline
Consulting or Advisory Role: Celgene (Inst), Janssen-Cilag (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Takeda (Inst), Adaptive Biotechnologies (Inst), Sanofi (Inst)
Research Funding: Bristol Myers Squibb (Inst), Janssen (Inst), Novartis (Inst), Chugai Pharma Europe (Inst), Celgene (Inst), Amgen (Inst), Sanofi (Inst), Mundipharma (Inst), Takeda (Inst), Molecular Partners (Inst), MSD (Inst), Incyte (Inst)
Travel, Accommodations, Expenses: Janssen-Cilag, Sanofi
Other Relationship: Amgen (Inst), Celgene/Bristol Myers Squibb (Inst), Chugai Pharma Europe (Inst), Janssen (Inst), Sanofi (Inst)
Donna Reece
Honoraria: Janssen, Amgen, Bristol Myers Squibb/Celgene, Sanofi, GlaxoSmithKline, Takeda
Consulting or Advisory Role: Janssen
Research Funding: Janssen, Bristol Myers Squibb
Tahamtan Ahmadi
Employment: Genmab
Leadership: Genmab
Stock and Other Ownership Interests: Genmab
Xiang Qin
Employment: Janssen Research & Development
Xue Gai
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Janssen Research & Development
Travel, Accommodations, Expenses: Janssen Research & Development
Jodi Carey
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson
Robin Carson
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Philippe Moreau
Honoraria: Celgene, Janssen-Cilag, Amgen, GlaxoSmithKline, AbbVie, Sanofi, Oncopeptides
Consulting or Advisory Role: Celgene, Janssen, Amgen, GlaxoSmithKline, Sanofi, AbbVie, Oncopeptides
No other potential conflicts of interest were reported.
REFERENCES
- 1.de Weers M, Tai YT, van der Veer MS, et al. : Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 186:1840-1848, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Lammerts van Bueren J, Jakobs D, Kaldenhoven N, et al. : Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood 124:3474, 2014 [Google Scholar]
- 3.Overdijk MB, Verploegen S, Bogels M, et al. : Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 7:311-321, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overdijk MB, Jansen JH, Nederend M, et al. : The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcgamma receptor-mediated cross-linking. J Immunol 197:807-813, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Krejcik J, Casneuf T, Nijhof IS, et al. : Daratumumab depletes CD38+ immune-regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 128:384-394, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams HC, III, Stevenaert F, Krejcik J, et al. : High-parameter mass cytometry evaluation of relapsed/refractory multiple myeloma patients treated with daratumumab demonstrates immune modulation as a novel mechanism of action. Cytometry A 95:279-289, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casneuf T, Adams HC, III, van de Donk NWCJ, et al. : Deep immune profiling of patients treated with lenalidomide and dexamethasone with or without daratumumab. Leukemia 35:573-584, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinder M, Bahlis NJ, Malavasi F, et al. : Comparison of CD38 antibodies in vitro and ex vivo mechanisms of action in multiple myeloma. Haematologica 106:2004-2008, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreau P, Attal M, Hulin C, et al. : Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 394:29-38, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Mateos MV, Cavo M, Blade J, et al. : Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): A randomised, open-label, phase 3 trial. Lancet 395:132-141, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Facon T, Kumar S, Plesner T, et al. : Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med 380:2104-2115, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mateos MV, Sonneveld P, Hungria V, et al. : Daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in patients with previously treated multiple myeloma: Three-year follow-up of CASTOR. Clin Lymphoma Myeloma Leuk 20:509-518, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Bahlis NJ, Dimopoulos MA, White DJ, et al. : Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia 34:1875-1884, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimopoulos MA, Terpos E, Boccadoro M, et al. : Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): An open-label, randomised, phase 3 trial. Lancet Oncol 22:801-812, 2021 [DOI] [PubMed] [Google Scholar]
- 15.DARZALEX® (daratumumab) [package insert]. Horsham, PA, Janssen Biotech, Inc, 2022 [Google Scholar]
- 16.European Medicines Agency : DARZALEX 20 mg/mL concentrate for solution for infusion [summary of product characteristics]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004077/WC500207296.pdf
- 17.Dimopoulos MA, Oriol A, Nahi H, et al. : Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 375:1319-1331, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos M, San Miguel J, Belch A, et al. : Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of POLLUX. Haematologica 103:2088-2096, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman JL, Usmani SZ, San-Miguel J, et al. : Four-year follow-up of the phase 3 POLLUX study of daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) alone in relapsed or refractory multiple myeloma (RRMM). Poster presented at the 61st American Society of Hematology (ASH) Annual Meeting & Exposition, Orlando, FL, December 7-10, 2019
- 20.Durie BGM, Harousseau JL, Miguel JS, et al. : International uniform response criteria for multiple myeloma. Leukemia 20:1467-1473, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Rajkumar SV, Harousseau JL, Durie B, et al. : Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood 117:4691-4695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonneveld P, Chanan-Khan A, Weisel K, et al. : Overall survival with daratumumab, bortezomib, and dexamethasone in previously treated multiple myeloma (CASTOR): A randomized, open-label, phase III trial. J Clin Oncol 41:1600-1609, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson PG, Kumar SK, Masszi T, et al. : Final overall survival analysis of the TOURMALINE-MM1 phase III trial of ixazomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol 39:2430-2442, 2021 [DOI] [PubMed] [Google Scholar]
- 24.Siegel DS, Dimopoulos MA, Ludwig H, et al. : Improvement in overall survival with carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol 36:728-734, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Dimopoulos MA, Lonial S, White D, et al. : Elotuzumab, lenalidomide, and dexamethasone in RRMM: Final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J 10:91, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Facon T, Kumar SK, Plesner T, et al. : Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): Overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 22:1582-1596, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Yong K, Delforge M, Driessen C, et al. : Multiple myeloma: Patient outcomes in real-world practice. Br J Haematol 175:252-264, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca R, Usmani SZ, Mehra M, et al. : Frontline treatment patterns and attrition rates by subsequent lines of therapy in patients with newly diagnosed multiple myeloma. BMC Cancer 20:1087, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.