PURPOSE
At the primary analysis of CASTOR (median follow-up, 7.4 months), daratumumab plus bortezomib and dexamethasone (D-Vd) significantly prolonged progression-free survival versus bortezomib and dexamethasone (Vd) alone in relapsed or refractory multiple myeloma (RRMM). We report updated efficacy and safety results at the final analysis for overall survival (OS).
METHODS
CASTOR was a multicenter, randomized, open-label, phase III study during which eligible patients with ≥ 1 line of prior therapy were randomly assigned to Vd (up to eight cycles) with or without daratumumab (until disease progression). After positive primary analysis and protocol amendment, patients receiving Vd were offered daratumumab monotherapy after disease progression.
RESULTS
At a median (range) follow-up of 72.6 months (0.0-79.8), significant OS benefit was observed with D-Vd (hazard ratio, 0.74; 95% CI, 0.59 to 0.92; P = .0075). Median OS was 49.6 months with D-Vd versus 38.5 months with Vd. Prespecified subgroup analyses demonstrated an OS advantage with D-Vd versus Vd for most subgroups, including patients age ≥ 65 years and patients with one or two prior lines of therapy, International Staging System stage III disease, high-risk cytogenetic abnormalities, and prior bortezomib treatment. The most common (≥ 10%) grade 3/4 treatment-emergent adverse events with D-Vd versus Vd were thrombocytopenia (46.1% v 32.9%), anemia (16.0% v 16.0%), neutropenia (13.6% v 4.6%), lymphopenia (10.3% v 2.5%), and pneumonia (10.7% v 10.1%).
CONCLUSION
D-Vd significantly prolonged OS in patients with RRMM, with the greatest OS benefit observed in patients with one prior line of therapy. To our knowledge, our results, together with the OS benefit observed with daratumumab plus lenalidomide and dexamethasone in the phase III POLLUX study, demonstrate for the first time an OS benefit with daratumumab-containing regimens in RRMM (ClinicalTrials.gov identifier: NCT02136134 [CASTOR]).
INTRODUCTION
Daratumumab is a human IgGκ monoclonal antibody targeting CD38 with a direct on-tumor1-4 and immunomodulatory5-7 mechanism of action, demonstrating greater cytotoxicity toward multiple myeloma (MM) cells ex vivo compared with analogs of other CD38 antibodies.8 Daratumumab induces higher levels of complement-dependent cytotoxicity, similar levels of antibody-dependent cell-mediated cytotoxicity and antibody-dependent cellular phagocytosis, and, in the presence of Fc receptor crosslinking, which occurs physiologically in vivo, daratumumab elicits similar levels of cell death.8 In phase III studies in newly diagnosed MM (NDMM) and relapsed or refractory MM (RRMM), the addition of daratumumab to standard-of-care regimens significantly reduced the risk of disease progression or death and achieved deep and durable responses, including significantly higher complete response or better (≥ CR) rates and minimal residual disease (MRD)-negativity rates, versus standard of care alone.9-14 On the basis of these results, daratumumab is approved in combination with standard-of-care regimens for patients with RRMM or NDMM.15,16
CONTEXT
Key Objective
To report updated efficacy and safety results, including overall survival (OS), from the phase III CASTOR study of daratumumab, bortezomib, and dexamethasone (D-Vd) in patients with relapsed or refractory multiple myeloma after approximately 6 years of follow-up.
Knowledge Generated
At a median follow-up of 72.6 months, D-Vd significantly prolonged OS, with a 26% reduction in the risk of death versus bortezomib and dexamethasone alone (median, 49.6 v 38.5 months, respectively; hazard ratio, 0.74; 95% CI, 0.59 to 0.92; P = .0075). Prespecified subgroup analyses showed OS improvement with D-Vd versus bortezomib and dexamethasone across most patient subgroups, with the greatest benefit observed in patients with one prior line of therapy.
Relevance
To our knowledge, these results, together with the OS benefit observed with daratumumab plus lenalidomide and dexamethasone in the phase III POLLUX study, demonstrate for the first time an OS benefit with daratumumab-containing regimens in relapsed or refractory multiple myeloma, with the greatest benefit observed in patients with one prior line of therapy.
At the primary analysis of the phase III CASTOR study (median follow-up, 7.4 months), daratumumab plus bortezomib and dexamethasone (D-Vd) significantly prolonged progression-free survival (PFS; hazard ratio [HR], 0.39; 95% CI, 0.28 to 0.53; P < .001) and induced higher rates of deeper responses than bortezomib and dexamethasone (Vd) alone in patients with RRMM.17 After longer follow-up (median, 19.4 months), D-Vd reduced the risk of disease progression or death by 69% (median PFS, 16.7 v 7.1 months; HR, 0.31; 95% CI, 0.24 to 0.39; P < .0001) and responses deepened, resulting in higher ≥ CR rates (28.8% v 9.8%; P < .0001) and MRD-negativity rates (10−5 sensitivity; 11.6% v 2.4%; P = .000034) versus Vd.18 In the most recent analysis of the study at a median follow-up of 50.2 months, D-Vd continued to demonstrate significant efficacy benefits versus Vd alone, inducing deep and more durable responses and improved MRD-negativity rates.19 Efficacy benefits were most pronounced in patients who received one prior line of therapy, regardless of prior treatment with lenalidomide.
We report updated efficacy and safety results at the time of the final overall survival (OS) analysis of CASTOR after approximately 6 years of follow-up.
METHODS
Trial Design and Oversight
CASTOR (ClinicalTrials.gov identifier: NCT02136134) was a multicenter, randomized, open-label, active-controlled, phase III study in patients with RRMM. The study design has been published previously.17 Eligible patients had progressive disease per International Myeloma Working Group criteria20,21 during or after completion of their last regimen, received ≥ 1 prior line of therapy, and had a partial response or better to ≥ 1 previous line of therapy.17 Patients refractory to bortezomib or another proteasome inhibitor were ineligible. The trial protocol was approved by independent ethics committees or institutional review boards at each center. Patients provided written informed consent, and the trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines.
Random Assignment and Study Treatment
Patients were randomly assigned (1:1) to D-Vd or Vd and stratified by International Staging System disease stage (I, II, or III), number of prior lines of therapy (1 v 2 or 3 v > 3), and previous bortezomib treatment (yes v no). All patients received up to eight cycles (21 days/cycle) of bortezomib (1.3 mg/m2 subcutaneously once on days 1, 4, 8, and 11) and dexamethasone (20 mg orally or intravenously once on days 1, 2, 4, 5, 8, 9, 11, and 12). For patients in the D-Vd group, daratumumab (16 mg/kg intravenously) was administered once weekly (days 1, 8, and 15) in cycles 1-3, once every 3 weeks (on day 1) in cycles 4-8, and once every 4 weeks thereafter until disease progression or unacceptable toxicity. After positive primary analysis and protocol amendment, patients receiving Vd were offered daratumumab monotherapy after disease progression or after a washout period if they already experienced disease progression and were receiving subsequent therapy.
End Points and Assessments
PFS was the primary efficacy end point.17 Secondary end points included time to disease progression, overall response rate, MRD negativity, and OS. Exploratory secondary analyses examined subpopulations by number of lines of therapy, prior treatment exposure, and cytogenetic risk assessed by next-generation sequencing, as described previously.18 Tumor response and disease progression were assessed using a validated computerized algorithm in accordance with International Myeloma Working Group response criteria.20,21 MRD was assessed using bone marrow aspirate samples and evaluated via next-generation sequencing using the clonoSEQ assay (v.2.0; Adaptive Biotechnologies, Seattle, WA). MRD was assessed in patients with suspected CR, in patients who achieved CR (including patients with very good partial response and suspected daratumumab interference) at cycle 9 day 1 and cycle 15 day 1 (6 months after the end of the Vd backbone), and every 12 months (± 3 months) after CR until the end of treatment. Patients were considered to be MRD-positive if they had an MRD-positive test result or had no MRD assessment. Cytogenetic risk was evaluated locally using local fluorescence in situ hybridization or karyotyping. High-risk patients had t(4;14), t(14;16), or del17p cytogenetic abnormalities.
Statistical Analysis
Unless otherwise specified, efficacy analyses were based on the intention-to-treat population. Time-to-event end points were compared between groups using a stratified log-rank test. HRs and 95% CIs were estimated using a stratified Cox regression model with treatment as the sole explanatory variable. The Kaplan–Meier method was used to estimate the distributions. Binary end points, including overall response rate, were assessed using the stratified Cochran–Mantel–Haenszel test. MRD-negativity rates were compared using Fisher's exact test.
RESULTS
Patients
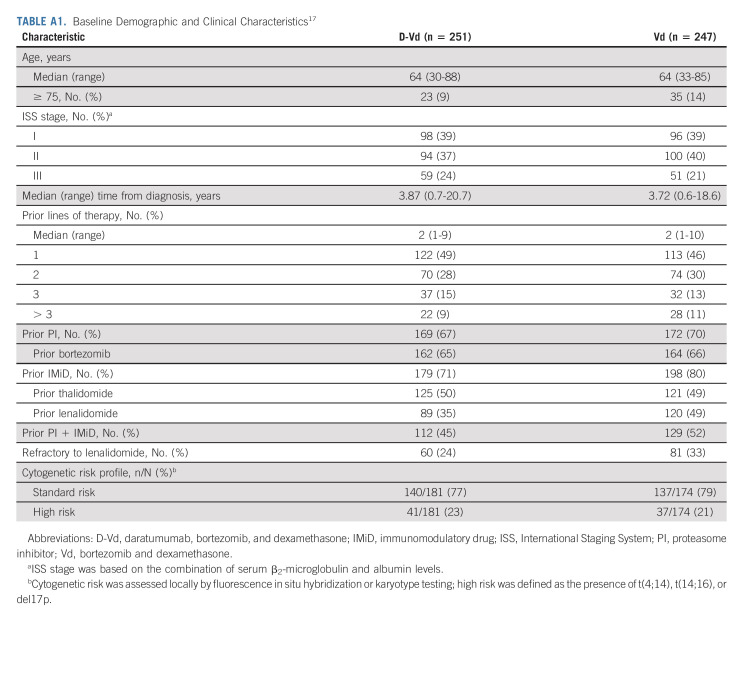
Patients were recruited between September 4, 2014, and September 24, 2015. In total, 498 patients were randomly assigned to D-Vd (n = 251) or Vd (n = 247; Fig 1). Patient demographics and baseline clinical characteristics were generally well balanced and have been published previously.17 Median (range) age was 64 (30-88) years, and median (range) number of prior lines of therapy was 2 (1-10) (Appendix Table A1, online only). Prior therapies included bortezomib (65.5%), thalidomide (49.4%), lenalidomide (42.0%), and both a proteasome inhibitor and an immunomodulatory drug (48.4%).
FIG 1.
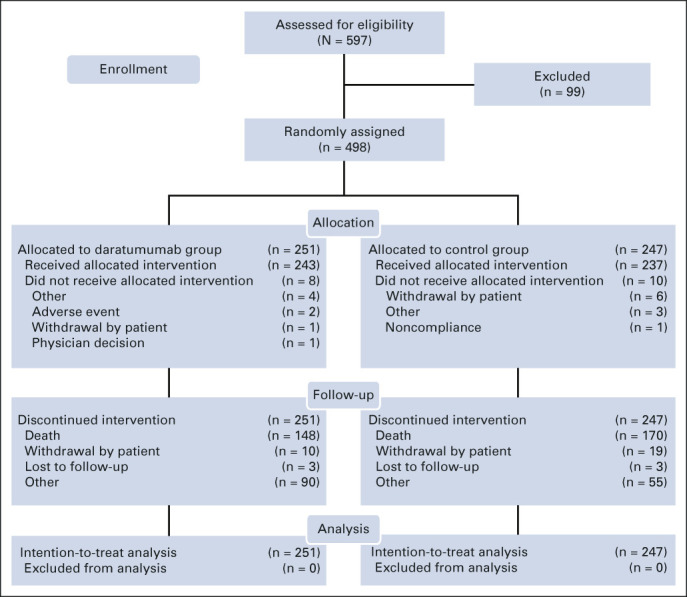
CONSORT diagram for CASTOR.
A total of 87 Vd patients subsequently received single-agent daratumumab after disease progression (or after a washout period if they already experienced disease progression and were receiving subsequent therapy), provided per study protocol. The median (range) number of daratumumab cycles received during monotherapy was 11.0 (1-63), and median (range) duration of daratumumab monotherapy was 9.2 months (0.2-57.1). An additional 38 patients received daratumumab as subsequent therapy not provided in the study.
Efficacy
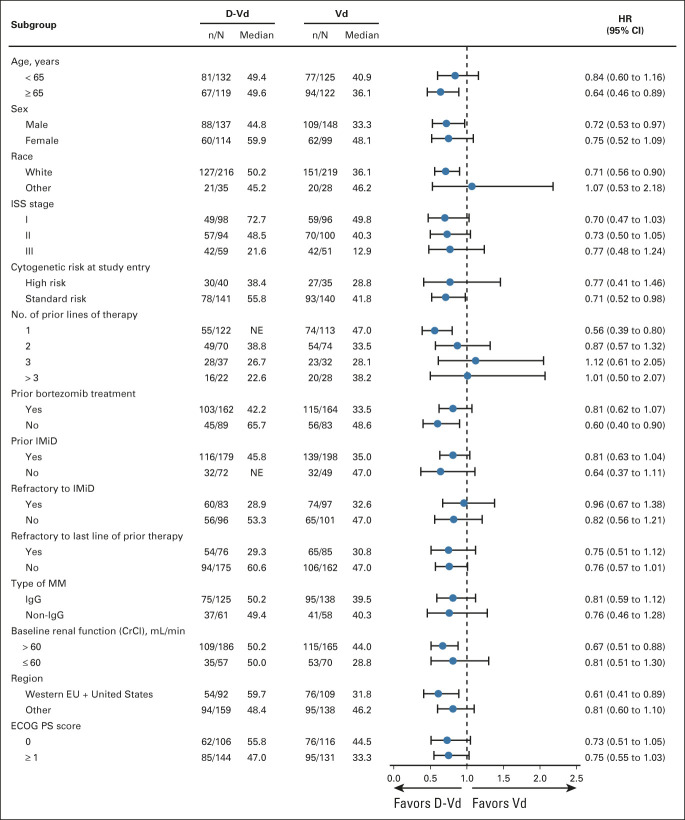
At the clinical cutoff (June 28, 2021), at a median (range) follow-up of 72.6 months (0.0-79.8), a total of 148 (59.0%) of 251 patients in the D-Vd group and 171 (69.2%) of 247 patients in the Vd group died. The HR for death for D-Vd compared with Vd was 0.74 (95% CI, 0.59 to 0.92; P = .0075; Fig 2A), crossing the prespecified stopping boundary of P = .0323 and representing a 26% reduction in the risk of death. Median OS was 49.6 months (95% CI, 42.2 to 62.3) for D-Vd versus 38.5 months (95% CI, 31.2 to 46.2) for Vd. Prespecified subgroup analyses showed OS benefit for patients in the D-Vd group versus patients in the Vd group across most patient subgroups, including patients age ≥ 65 years, patients who have received one or two prior lines of therapy, and patients with poor prognosis, such as those with advanced-stage disease (International Staging System stage III), high-risk cytogenetic abnormalities, and prior bortezomib treatment (Fig 3). It is noteworthy that OS benefit was most pronounced in patients who received one prior line of therapy (HR, 0.56; 95% CI, 0.39 to 0.80).
FIG 2.
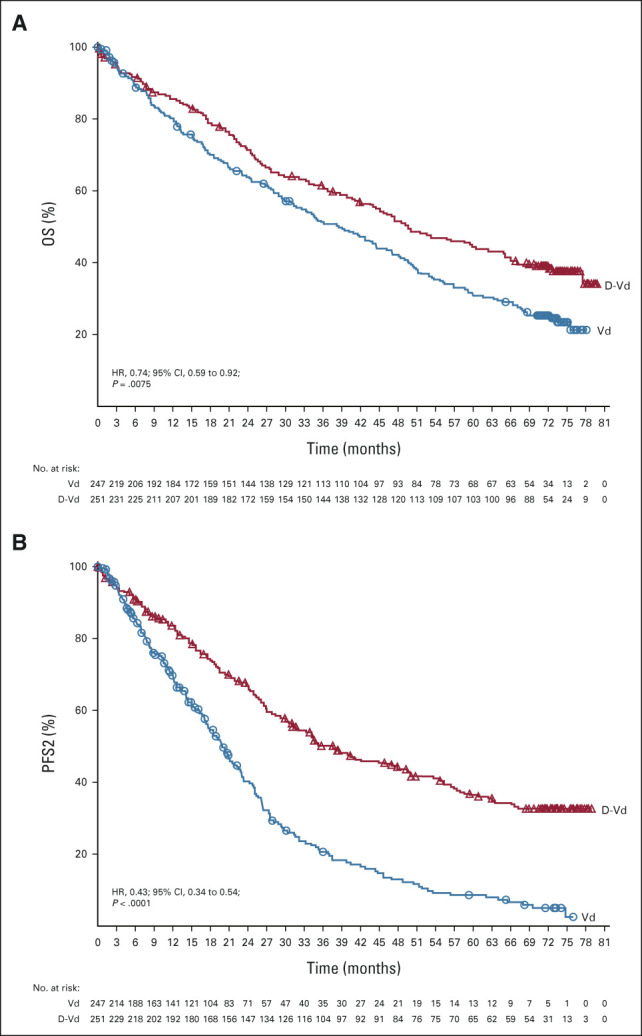
Kaplan–Meier estimates of (A) OS and (B) PFS2 in the ITT population, which included all patients who underwent random assignment. D-Vd, daratumumab, bortezomib, and dexamethasone; HR, hazard ratio; ITT, intention-to-treat; OS, overall survival; PFS2, progression-free survival on the subsequent line of therapy; Vd, bortezomib and dexamethasone.
FIG 3.
The results of OS in prespecified subgroups of the ITT population defined by baseline characteristics. The ISS disease stage is derived on the basis of the combination of serum β2-microglobulin and albumin levels. Higher stages indicate more severe disease. Cytogenetic risk was assessed locally by fluorescence in situ hybridization or karyotype testing; high risk was defined as the presence of t(4;14), t(14;16), or del17p. The subgroup analysis of the type of MM was performed on data from patients who had measurable disease in serum. CrCl, creatinine clearance; D-Vd, daratumumab, bortezomib, and dexamethasone; ECOG PS, Eastern Cooperative Oncology Group performance status; EU, European Union; HR, hazard ratio; IgG, immunoglobulin G; IMiD, immunomodulatory drug; ISS, International Staging System; ITT, intention-to-treat; MM, multiple myeloma; NE, not estimable; OS, overall survival; Vd, bortezomib and dexamethasone.
Significantly higher MRD-negativity rates (10−5 sensitivity threshold) were achieved with D-Vd versus Vd (15.1% v 1.6%; P < .0001). MRD negativity was associated with improved OS, regardless of treatment group (Fig 4).
FIG 4.
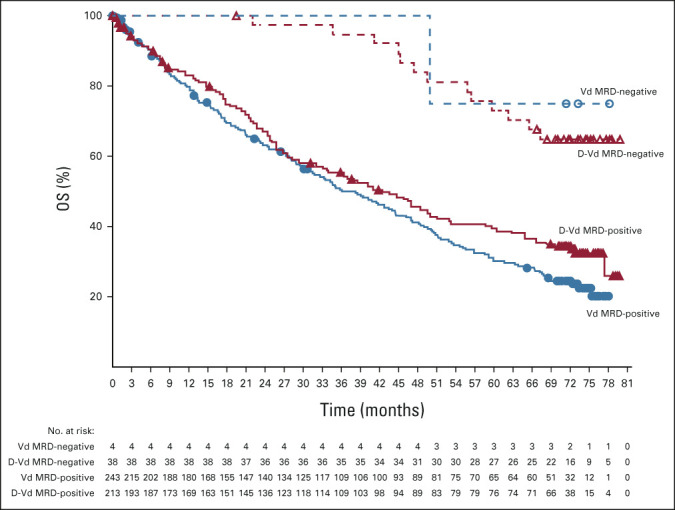
Kaplan–Meier estimates of OS by MRD status among patients in the ITT population. D-Vd, daratumumab, bortezomib, and dexamethasone; ITT, intention-to-treat; MRD, minimal residual disease; OS, overall survival; Vd, bortezomib and dexamethasone.
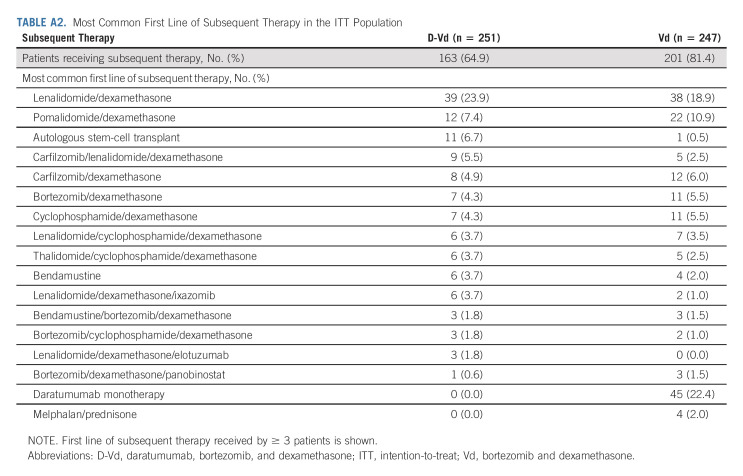
A total of 161 (66.3%) of 243 patients in the D-Vd group and 200 (84.4%) of 237 patients in the Vd group received subsequent therapy; median (range) number of subsequent lines of therapy was 2 (1-9) and 3 (1-10), respectively. Median time to subsequent therapy was significantly increased in the D-Vd arm versus the Vd arm (25.4 v 9.7 months; HR, 0.27; 95% CI, 0.21 to 0.34; P < .0001). The most common subsequent anticancer therapies included dexamethasone (59.3%), lenalidomide (39.9%), cyclophosphamide (28.0%), pomalidomide (23.0%), carfilzomib (21.0%), and bortezomib (19.8%) in the D-Vd arm and dexamethasone (66.2%), daratumumab (52.7%), lenalidomide (46.0%), cyclophosphamide (33.8%), pomalidomide (32.1%), carfilzomib (24.9%), and bortezomib (23.2%) in the Vd arm. PFS2 (defined as the time from random assignment to progression on the next line of therapy) was significantly prolonged with D-Vd versus Vd (median, 37.7 v 19.9 months; HR, 0.43; 95% CI, 0.34 to 0.54; P < .0001; Fig 2B). The most common first subsequent therapy was lenalidomide and dexamethasone (Rd) in the D-Vd arm (23.9%) and daratumumab monotherapy (22.4%) or Rd (18.9%) in the Vd arm (Appendix Table A2, online only). Median PFS for patients who started first line of subsequent therapy was 13.2 months (95% CI, 10.1 to 15.4) in the D-Vd group and 9.2 months (95% CI, 7.4 to 10.6) in the Vd group.
After disease progression, median (range) time to crossover to daratumumab subsequent therapy for patients in the Vd arm was 20.5 months (5.7-68.3). Of the 87 patients in the Vd group who received subsequent daratumumab monotherapy after disease progression, provided per study protocol, 35 (40.2%) are still alive; median OS is 63.4 months (95% CI, 51.2 to 72.4).
Safety
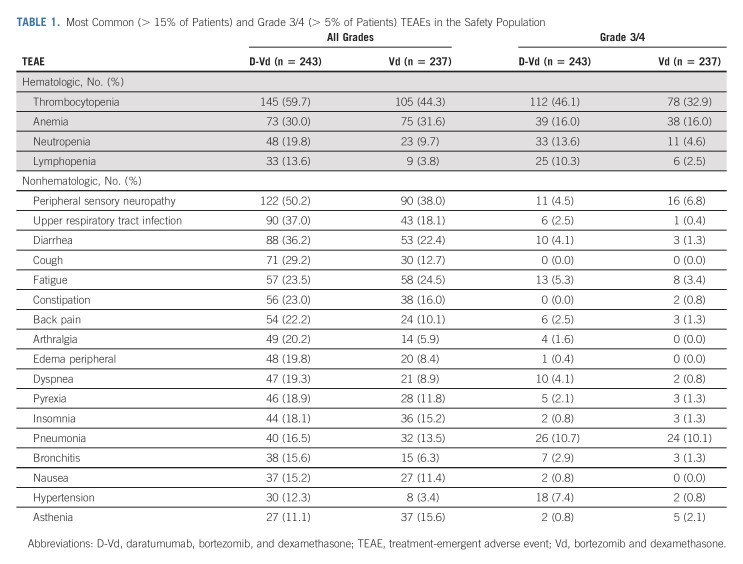
With longer follow up, no new safety concerns were reported (Table 1). The most common (≥ 10%) grade 3/4 treatment-emergent adverse events (TEAEs) with D-Vd versus Vd were thrombocytopenia (46.1% v 32.9%), anemia (16.0% v 16.0%), neutropenia (13.6% v 4.6%), lymphopenia (10.3% v 2.5%), and pneumonia (10.7% v 10.1%). Grade 3/4 infections occurred in 72 (29.6%) patients in the D-Vd arm and 45 (19.0%) patients in the Vd arm. Serious TEAEs occurred in 134 (55.1%) patients who received D-Vd and 81 (34.2%) patients who received Vd, the most common being pneumonia (10.7% and 10.1%, respectively). The percentage of patients with TEAEs leading to discontinuation remained low and similar between groups (D-Vd, 10.7%; Vd, 9.3%). Seven (2.9%) patients in the D-Vd arm and 5 (2.1%) patients in the Vd arm discontinued treatment because of infections.
TABLE 1.
Most Common (> 15% of Patients) and Grade 3/4 (> 5% of Patients) TEAEs in the Safety Population
TEAEs that resulted in death were reported in 17 (7.0%) patients in the D-Vd group and 14 (5.9%) patients in the Vd group. The most frequent TEAEs with an outcome of death were pneumonia (0.8% each) and general physical health deterioration (0.4% v 1.3%, respectively). Three patients died during the study due to COVID-19 disease (one in the D-Vd group and two in the Vd group). With extended follow up, the incidence of second primary malignancies (cutaneous, invasive, and hematologic) was 20 (8.2%) patients in the D-Vd arm (6 new cases since the 3-year follow-up analysis) and 5 (2.1%) patients in the Vd arm (no new cases). There was no predominant cancer type for second primary malignancies in either treatment arm. When adjusted for exposure to study treatment, the rate of second primary malignancies was similar between the D-Vd (0.35 events per 100 patient-months at risk) and Vd (0.46 events per 100 patient-months at risk) groups.
DISCUSSION
It is our understanding that these long-term results from the CASTOR study represent the first OS data with daratumumab in the RRMM population and provide further support of a survival advantage with a daratumumab-containing regimen in multiple myeloma.10,22 For the first time to our knowledge, the combination of daratumumab and a standard-of-care regimen significantly improved OS in patients with RRMM. The addition of daratumumab to Vd prolonged OS in patients with RRMM, with a 26% reduction in the risk of death versus Vd alone after a median follow-up of 72.6 months. The OS curves separated at approximately 8 months and continued to separate with time. The final MRD-negativity rate analysis results (median follow-up, 72.6 months) were consistent with those reported in the updated analysis (median follow-up, 19.4 months).18 OS was improved in patients who achieved MRD negativity compared with patients who were MRD-positive. It appears that the superiority in OS is derived from patients who achieved MRD negativity in the D-Vd group because OS was similar in the D-Vd and Vd groups in patients who were MRD-positive. This further reinforces the importance of achieving MRD negativity. A significant PFS2 benefit was maintained for the D-Vd group, with a 57% reduction in the risk of disease progression or death. These results build on the significant PFS benefit previously reported with D-Vd, with a 69% reduction in the risk of disease progression or death compared with Vd alone (median follow-up, 50.2 months).19 Median PFS was 16.7 months in the D-Vd group versus 7.1 months in the Vd group (HR, 0.31; 95% CI, 0.24 to 0.39; P < .0001).19
In prespecified subgroup analyses of OS, an OS advantage was observed with D-Vd compared with Vd for most subgroups, including patients age ≥ 65 years, patients who have received one or two prior lines of therapy, and patients with prior immunomodulatory drug or bortezomib treatment. It is noteworthy that OS benefit was most pronounced in patients who received one prior line of therapy (HR, 0.56; 95% CI, 0.39 to 0.80), supporting early use of daratumumab in treatment. These OS results also reinforce those from the previous updated PFS analysis (median follow-up, 50.2 months), which showed the most pronounced PFS benefit of D-Vd in patients who received one prior line of therapy, with a 79% reduction in the risk of disease progression or death versus Vd alone (median, 27.0 v 7.9 months; HR, 0.21; 95% CI, 0.15 to 0.31; P < .0001).19
Importantly, OS benefit was observed despite crossover in the Vd arm to daratumumab subsequent therapy. As noted earlier in the article, patients in the Vd group were offered daratumumab monotherapy after disease progression or a washout period. Approximately 63% of patients (125/200) in the Vd arm who received subsequent therapy received salvage daratumumab, primarily as monotherapy but also in combination regimens; 49 patients received first subsequent therapy with daratumumab (45 as monotherapy, four in various combination regimens). We realize that allowing this type of crossover per study protocol may confound OS results. Patients received daratumumab monotherapy as this was the approved and reimbursed regimen available to patients at the time this study was performed.
No new safety concerns were reported after extended follow-up. Grade 3/4 infections were reported more frequently with D-Vd versus Vd, but the rate of infections leading to treatment discontinuation was low and similar between groups. Second primary malignancies were reported more commonly in the D-Vd group than in the Vd group; however, the incidence was similar between groups when adjusted for exposure to study treatment.
While cross-trial comparisons should be interpreted cautiously, the CASTOR OS data reported herein (median OS, 49.6 months) are consistent with previously reported data in RRMM (ASPIRE and ELOQUENT-2),23,24 despite differences in patient demographics. In the phase III ASPIRE study of patients with RRMM and one to three prior lines of therapy, at a median follow-up of 67.1 months, median OS was 48.3 months with carfilzomib-Rd versus 40.4 months with Rd alone (HR, 0.79; 95% CI, 0.67 to 0.95; 1-sided P = .0045).23 Similarly, in the phase III ELOQUENT-2 study, at a minimum follow-up of 70.6 months, the combination of elotuzumab and Rd significantly improved OS versus Rd alone (median, 48.3 v 39.6 months, respectively; HR, 0.82; 95.4% CI, 0.68 to 1.00; P = .0408) in patients with RRMM and one to three prior lines of therapy.24 On the basis of the results of these three trials, the benchmark for survival in RRMM is approximately 4 years. It is also noteworthy that the median OS in the Vd arm of CASTOR (38.5 months) was consistent with that observed in the Vd arm of the phase III ENDEAVOR study (38.8 months).25
Similar to CASTOR, the results from the phase III POLLUX study also support utilizing a daratumumab-containing regimen after first relapse in patients with RRMM who are not refractory to lenalidomide.13,26,27 Moreover, the results from the phase III ALCYONE and MAIA trials demonstrate that adding daratumumab to standard-of-care regimens significantly improves OS and PFS compared with standard of care alone in patients with transplant-ineligible NDMM.10,22 Real-world data from a patient chart review in Europe reported high attrition rates that increased with each subsequent line of therapy, with 95% of patients receiving first-line therapy and only 61% and 38% receiving second-line and third-line therapy, respectively.28 In a retrospective analysis of 3 US databases, attrition rates were high after first (57%) and each subsequent line of therapy (43%-46%) in nontransplant elderly patients with NDMM.29 Taken together, these real-world data, in conjunction with the prolonged OS observed with daratumumab-containing regimens in both the NDMM (ALCYONE and MAIA) and now RRMM (CASTOR and POLLUX) treatment settings, support early use of daratumumab to induce deep and sustained responses and prolonged disease control to potentially delay clonal evolution and subsequent drug resistance.30 As use of daratumumab increases in the frontline treatment setting, the role of retreatment with daratumumab after progression will be of growing interest. Retrospective analyses suggest clinical efficacy with daratumumab retreatment,31,32 but the results of ongoing (LYNX; ClinicalTrials.gov identifier: NCT03871829) and future studies will further define the potential role of daratumumab retreatment after progression.
In conclusion, to our knowledge, these results, combined with the OS results reported with D-Rd in the phase III POLLUX study,27 demonstrate for the first time an OS benefit with daratumumab-containing regimens in patients with RRMM. Treatment of patients with RRMM with D-Vd results in a significant reduction in the risk of death compared with Vd alone, with the greatest OS benefit observed in patients who received one prior line of therapy. These results provide strong rationale for early use of daratumumab to maximize patient benefit.
ACKNOWLEDGMENT
The authors thank the patients who participated in the CASTOR study and their families, as well as the study coinvestigators, research nurses, and coordinators at each of the clinical sites. Medical writing and editorial support were provided by Lisa Shannon, PharmD, of Lumanity Communications Inc, and were funded by Janssen Global Services, LLC.
APPENDIX
TABLE A1.
Baseline Demographic and Clinical Characteristics17
TABLE A2.
Most Common First Line of Subsequent Therapy in the ITT Population
Pieter Sonneveld
Consulting or Advisory Role: Celgene, Janssen, Amgen, Karyopharm Therapeutics, CARsgen Therapeutics
Research Funding: Janssen (Inst), Amgen (Inst), SkylineDx (Inst), Bristol Myers Squibb/Celgene (Inst)
Asher Chanan-Khan
Leadership: Starton Therapeutics, Cellectar
Stock and Other Ownership Interests: Matthew & Asher Inc, NanoDev Therapeutics, Starton Therapeutics, Cellectar
Honoraria: BeiGene, Ascentage Pharma
Research Funding: Ascentage Pharma
Patents, Royalties, Other Intellectual Property: Patent on PSMB9 biomarker
Katja Weisel
Honoraria: Amgen, Bristol Myers Squibb/Celgene, Janssen-Cilag, GlaxoSmithKline (Inst), Adaptive Biotechnologies, Karyopharm Therapeutics, Takeda, Sanofi, AbbVie, GlaxoSmithKline, Novartis, Pfizer, Celgene, Oncopeptides, Roche
Consulting or Advisory Role: Amgen, Adaptive Biotechnologies, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Janssen-Cilag, Karyopharm Therapeutics, Sanofi, Takeda, Oncopeptides, Roche
Research Funding: Amgen (Inst), Celgene (Inst), Sanofi (Inst), Janssen-Cilag (Inst), Bristol Myers Squibb/Celgene (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: Amgen, Celgene, Bristol Myers Squibb/Celgene, Janssen-Cilag, GlaxoSmithKline, Takeda
Ajay K. Nooka
Honoraria: Amgen, Janssen Oncology, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Takeda, Oncopeptides, Karyopharm Therapeutics, Adaptive Biotechnologies, Genzyme, BeyondSpring Pharmaceuticals, Secura Bio
Consulting or Advisory Role: Amgen, Janssen Oncology, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Takeda, Oncopeptides, Karyopharm Therapeutics, Adaptive Biotechnologies, Genzyme, BeyondSpring Pharmaceuticals, Secura Bio
Research Funding: Amgen (Inst), Janssen Oncology (Inst), Takeda (Inst), Bristol Myers Squibb/Celgene (Inst), Arch Oncology (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: GlaxoSmithKline
Tamas Masszi
Consulting or Advisory Role: AbbVie, Bristol Myers Squibb/Celgene, Janssen-Cilag, Novartis, Pfizer, Takeda
Meral Beksac
Consulting or Advisory Role: Janssen Oncology (Inst), Bristol Myers Squibb/Celgene Turkey (Inst), Amgen (Inst), Takeda (Inst), Oncopeptides (Inst), Sanofi (Inst)
Speakers' Bureau: Amgen (Inst), Sanofi (Inst)
Ivan Spicka
Honoraria: Amgen, Janssen-Cilag, Takeda, Novartis
Consulting or Advisory Role: Celgene, Amgen, Janssen-Cilag, Takeda, Novartis, Sanofi
Speakers' Bureau: Celgene, Amgen, Janssen-Cilag, Takeda, Bristol Myers Squibb/Celgene, Novartis, Sanofi
Travel, Accommodations, Expenses: Celgene, Amgen, Janssen-Cilag, Bristol Myers Squibb/Celgene
Vania Hungria
Consulting or Advisory Role: AbbVie, Janssen-Cilag, Takeda, Sanofi, Bristol Myers Squibb/Celgene Brazil, Amgen, Pfizer
Speakers' Bureau: Janssen-Cilag, Takeda, Bristol Myers Squibb/Celgene Brazil, Sanofi, Amgen, GlaxoSmithKline
Markus Munder
Honoraria: Janssen Oncology, Bristol Myers Squibb/Celgene GmbH & Co KG, GlaxoSmithKline, Sanofi, Abbvie
Consulting or Advisory Role: Janssen Oncology, Bristol Myers Squibb/Celgene GmbH & Co KG, GlaxoSmithKline, Sanofi, Takeda, Amgen
Maria-Victoria Mateos
Honoraria: Janssen-Cilag, Celgene, Amgen, Takeda, GlaxoSmithKline, AbbVie/Genentech, Sanofi
Consulting or Advisory Role: Takeda, Janssen-Cilag, Celgene, Amgen, AbbVie, GlaxoSmithKline, Pfizer, Regeneron, Roche/Genentech
Tomer M. Mark
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics, Adaptive Biotechnologies, AbbVie
Consulting or Advisory Role: Adaptive Biotechnologies, Genzyme, Bristol Myers Squibb/Celgene, Amgen, Sanofi, Takeda
Research Funding: Bristol Myers Squibb/Celgene (Inst), Janssen (Inst)
Mark-David Levin
Honoraria: AbbVie, Celgene, Janssen, Takeda
Travel, Accommodations, Expenses: Takeda, Janssen, AbbVie
Tahamtan Ahmadi
Employment: Genmab
Leadership: Genmab
Stock and Other Ownership Interests: Genmab
Xiang Qin
Employment: Janssen Research & Development
Wendy Garvin Mayo
Employment: Janssen Research & Development
Xue Gai
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Janssen Research & Development
Travel, Accommodations, Expenses: Janssen Research & Development
Jodi Carey
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson
Robin Carson
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Andrew Spencer
Honoraria: Janssen-Cilag, Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Janssen-Cilag, Bristol Myers Squibb/Celgene
Speakers' Bureau: Janssen-Cilag
Research Funding: Janssen-Cilag
No other potential conflicts of interest were reported.
SUPPORT
Supported by Janssen Research & Development, LLC.
CLINICAL TRIAL INFORMATION
NCT02136134 [CASTOR]
DATA SHARING STATEMENT
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
AUTHOR CONTRIBUTIONS
Conception and design: Asher Chanan-Khan, Katja Weisel, Tahamtan Ahmadi, Robin Carson
Provision of study materials or patients: Asher Chanan-Khan, Katja Weisel, Tamas Masszi, Meral Beksac, Vania Hungria, Markus Munder, Maria-Victoria Mateos
Collection and assembly of data: Katja Weisel, Tamas Masszi, Meral Beksac, Ivan Spicka, Vania Hungria, Maria-Victoria Mateos, Tomer M. Mark, Tahamtan Ahmadi, Wendy Garvin Mayo, Robin Carson, Andrew Spencer
Data analysis and interpretation: Pieter Sonneveld, Asher Chanan-Khan, Katja Weisel, Ajay K. Nooka, Meral Beksac, Ivan Spicka, Vania Hungria, Markus Munder, Maria-Victoria Mateos, Tomer M. Mark, Mark-David Levin, Tahamtan Ahmadi, Xiang Qin, Xue Gai, Jodi Carey, Robin Carson, Andrew Spencer
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Overall Survival With Daratumumab, Bortezomib, and Dexamethasone in Previously Treated Multiple Myeloma (CASTOR): A Randomized, Open-Label, Phase III Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Pieter Sonneveld
Consulting or Advisory Role: Celgene, Janssen, Amgen, Karyopharm Therapeutics, CARsgen Therapeutics
Research Funding: Janssen (Inst), Amgen (Inst), SkylineDx (Inst), Bristol Myers Squibb/Celgene (Inst)
Asher Chanan-Khan
Leadership: Starton Therapeutics, Cellectar
Stock and Other Ownership Interests: Matthew & Asher Inc, NanoDev Therapeutics, Starton Therapeutics, Cellectar
Honoraria: BeiGene, Ascentage Pharma
Research Funding: Ascentage Pharma
Patents, Royalties, Other Intellectual Property: Patent on PSMB9 biomarker
Katja Weisel
Honoraria: Amgen, Bristol Myers Squibb/Celgene, Janssen-Cilag, GlaxoSmithKline (Inst), Adaptive Biotechnologies, Karyopharm Therapeutics, Takeda, Sanofi, AbbVie, GlaxoSmithKline, Novartis, Pfizer, Celgene, Oncopeptides, Roche
Consulting or Advisory Role: Amgen, Adaptive Biotechnologies, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Janssen-Cilag, Karyopharm Therapeutics, Sanofi, Takeda, Oncopeptides, Roche
Research Funding: Amgen (Inst), Celgene (Inst), Sanofi (Inst), Janssen-Cilag (Inst), Bristol Myers Squibb/Celgene (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: Amgen, Celgene, Bristol Myers Squibb/Celgene, Janssen-Cilag, GlaxoSmithKline, Takeda
Ajay K. Nooka
Honoraria: Amgen, Janssen Oncology, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Takeda, Oncopeptides, Karyopharm Therapeutics, Adaptive Biotechnologies, Genzyme, BeyondSpring Pharmaceuticals, Secura Bio
Consulting or Advisory Role: Amgen, Janssen Oncology, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Takeda, Oncopeptides, Karyopharm Therapeutics, Adaptive Biotechnologies, Genzyme, BeyondSpring Pharmaceuticals, Secura Bio
Research Funding: Amgen (Inst), Janssen Oncology (Inst), Takeda (Inst), Bristol Myers Squibb/Celgene (Inst), Arch Oncology (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: GlaxoSmithKline
Tamas Masszi
Consulting or Advisory Role: AbbVie, Bristol Myers Squibb/Celgene, Janssen-Cilag, Novartis, Pfizer, Takeda
Meral Beksac
Consulting or Advisory Role: Janssen Oncology (Inst), Bristol Myers Squibb/Celgene Turkey (Inst), Amgen (Inst), Takeda (Inst), Oncopeptides (Inst), Sanofi (Inst)
Speakers' Bureau: Amgen (Inst), Sanofi (Inst)
Ivan Spicka
Honoraria: Amgen, Janssen-Cilag, Takeda, Novartis
Consulting or Advisory Role: Celgene, Amgen, Janssen-Cilag, Takeda, Novartis, Sanofi
Speakers' Bureau: Celgene, Amgen, Janssen-Cilag, Takeda, Bristol Myers Squibb/Celgene, Novartis, Sanofi
Travel, Accommodations, Expenses: Celgene, Amgen, Janssen-Cilag, Bristol Myers Squibb/Celgene
Vania Hungria
Consulting or Advisory Role: AbbVie, Janssen-Cilag, Takeda, Sanofi, Bristol Myers Squibb/Celgene Brazil, Amgen, Pfizer
Speakers' Bureau: Janssen-Cilag, Takeda, Bristol Myers Squibb/Celgene Brazil, Sanofi, Amgen, GlaxoSmithKline
Markus Munder
Honoraria: Janssen Oncology, Bristol Myers Squibb/Celgene GmbH & Co KG, GlaxoSmithKline, Sanofi, Abbvie
Consulting or Advisory Role: Janssen Oncology, Bristol Myers Squibb/Celgene GmbH & Co KG, GlaxoSmithKline, Sanofi, Takeda, Amgen
Maria-Victoria Mateos
Honoraria: Janssen-Cilag, Celgene, Amgen, Takeda, GlaxoSmithKline, AbbVie/Genentech, Sanofi
Consulting or Advisory Role: Takeda, Janssen-Cilag, Celgene, Amgen, AbbVie, GlaxoSmithKline, Pfizer, Regeneron, Roche/Genentech
Tomer M. Mark
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics, Adaptive Biotechnologies, AbbVie
Consulting or Advisory Role: Adaptive Biotechnologies, Genzyme, Bristol Myers Squibb/Celgene, Amgen, Sanofi, Takeda
Research Funding: Bristol Myers Squibb/Celgene (Inst), Janssen (Inst)
Mark-David Levin
Honoraria: AbbVie, Celgene, Janssen, Takeda
Travel, Accommodations, Expenses: Takeda, Janssen, AbbVie
Tahamtan Ahmadi
Employment: Genmab
Leadership: Genmab
Stock and Other Ownership Interests: Genmab
Xiang Qin
Employment: Janssen Research & Development
Wendy Garvin Mayo
Employment: Janssen Research & Development
Xue Gai
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Janssen Research & Development
Travel, Accommodations, Expenses: Janssen Research & Development
Jodi Carey
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson
Robin Carson
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Andrew Spencer
Honoraria: Janssen-Cilag, Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Janssen-Cilag, Bristol Myers Squibb/Celgene
Speakers' Bureau: Janssen-Cilag
Research Funding: Janssen-Cilag
No other potential conflicts of interest were reported.
REFERENCES
- 1.de Weers M, Tai YT, van der Veer MS, et al. : Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 186:1840-1848, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Lammerts van Bueren J, Jakobs D, Kaldenhoven N, et al. : Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood 124:3474, 2014 [Google Scholar]
- 3.Overdijk MB, Verploegen S, Bögels M, et al. : Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 7:311-321, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overdijk MB, Jansen JH, Nederend M, et al. : The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor–mediated cross-linking. J Immunol 197:807-813, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Krejcik J, Casneuf T, Nijhof IS, et al. : Daratumumab depletes CD38+ immune-regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 128:384-394, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams HC, III, Stevenaert F, Krejcik J, et al. : High-parameter mass cytometry evaluation of relapsed/refractory multiple myeloma patients treated with daratumumab demonstrates immune modulation as a novel mechanism of action. Cytometry A 95:279-289, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casneuf T, Adams HC, III, van de Donk NWCJ, et al. : Deep immune profiling of patients treated with lenalidomide and dexamethasone with or without daratumumab. Leukemia 35:573-584, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinder M, Bahlis NJ, Malavasi F, et al. : Comparison of CD38 antibodies in vitro and ex vivo mechanisms of action in multiple myeloma. Haematologica 106:2004-2008, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreau P, Attal M, Hulin C, et al. : Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 394:29-38, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Mateos MV, Cavo M, Blade J, et al. : Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): A randomised, open-label, phase 3 trial. Lancet 395:132-141, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Facon T, Kumar S, Plesner T, et al. : Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med 380:2104-2115, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mateos MV, Sonneveld P, Hungria V, et al. : Daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in patients with previously treated multiple myeloma: Three-year follow-up of CASTOR. Clin Lymphoma Myeloma Leuk 20:509-518, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Bahlis NJ, Dimopoulos MA, White DJ, et al. : Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia 34:1875-1884, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimopoulos MA, Terpos E, Boccadoro M, et al. : Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): An open-label, randomised, phase 3 trial. Lancet Oncol 22:801-812, 2021 [DOI] [PubMed] [Google Scholar]
- 15.DARZALEX® (daratumumab) [package insert]. Horsham, PA, Janssen Biotech, Inc, 2022 [Google Scholar]
- 16.European Medicines Agency : DARZALEX 20 mg/mL concentrate for solution for infusion [summary of product characteristics]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004077/WC500207296.pdf [Google Scholar]
- 17.Palumbo A, Chanan-Khan A, Weisel K, et al. : Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 375:754-766, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Spencer A, Lentzsch S, Weisel K, et al. : Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of CASTOR. Haematologica 103:2079-2087, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisel KC, Sonneveld P, Mateos MV, et al. : Efficacy and safety of daratumumab, bortezomib, and dexamethasone (D-Vd) versus bortezomib and dexamethasone (Vd) in first relapse patients (pts) with multiple myeloma (MM): Four-year update of CASTOR. Poster presented at the 61st American Society of Hematology (ASH) Annual Meeting & Exposition, Orlando, FL, December 7-10, 2019
- 20.Durie BGM, Harousseau JL, Miguel JS, et al. : International uniform response criteria for multiple myeloma. Leukemia 20:1467-1473, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Rajkumar SV, Harousseau JL, Durie B, et al. : Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood 117:4691-4695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Facon T, Kumar SK, Plesner T, et al. : Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): Overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 22:1582-1596, 2021 [DOI] [PubMed] [Google Scholar]
- 23.Siegel DS, Dimopoulos MA, Ludwig H, et al. : Improvement in overall survival with carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol 36:728-734, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Dimopoulos MA, Lonial S, White D, et al. : Elotuzumab, lenalidomide, and dexamethasone in RRMM: Final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J 10:91, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orlowski RZ, Moreau P, Niesvizky R, et al. : Carfilzomib-dexamethasone versus bortezomib-dexamethasone in relapsed or refractory multiple myeloma: Updated overall survival, safety, and subgroups. Clin Lymphoma Myeloma Leuk 19:522-530.e1, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Dimopoulos MA, Oriol A, Nahi H, et al. : Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 375:1319-1331, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Dimopoulos MA, Oriol A, Nahi H, et al. : Overall survival with daratumumab, lenalidomide, and dexamethasone in previously treated multiple myeloma (POLLUX): A randomized, open‐label, phase III trial. J Clin Oncol 41:1590-1599, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yong K, Delforge M, Driessen C, et al. : Multiple myeloma: Patient outcomes in real-world practice. Br J Haematol 175:252-264, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fonseca R, Usmani SZ, Mehra M, et al. : Frontline treatment patterns and attrition rates by subsequent lines of therapy in patients with newly diagnosed multiple myeloma. BMC Cancer 20:1087, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki K, Nishiwaki K, Yano S: Treatment strategies considering micro-environment and clonal evolution in multiple myeloma. Cancers (Basel) 13:215, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nooka AK, Joseph NS, Kaufman JL, et al. : Clinical efficacy of daratumumab, pomalidomide, and dexamethasone in patients with relapsed or refractory myeloma: Utility of re-treatment with daratumumab among refractory patients. Cancer 125:2991-3000, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Hussain MJ, Robinson MM, Hamadeh I, et al. : Daratumumab, pomalidomide and dexamethasone combination therapy in daratumumab and/or pomalidomide refractory multiple myeloma. Br J Haematol 186:140-144, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.