PURPOSE
To update a clinical practice guideline (CPG) for the empiric management of fever and neutropenia (FN) in pediatric patients with cancer and hematopoietic cell transplantation recipients.
METHODS
The International Pediatric Fever and Neutropenia Guideline Panel reconvened to conduct the second update of this CPG. We updated the previous systematic review to identify new randomized controlled trials (RCTs) evaluating any strategy for the management of FN in pediatric patients. Using the Grading of Recommendations Assessment, Development and Evaluation framework, evidence quality was classified as high, moderate, low, or very low. The panel updated recommendations related to initial management, ongoing management, and empiric antifungal therapy. Changes from the 2017 CPG were articulated, and good practice statements were considered.
RESULTS
We identified 10 new RCTs in addition to the 69 RCTs identified in previous FN CPGs to inform the 2023 FN CPG. Changes from the 2017 CPG included two conditional recommendations regarding (1) discontinuation of empiric antibacterial therapy in clinically well and afebrile patients with low-risk FN if blood cultures remain negative at 48 hours despite no evidence of marrow recovery and (2) pre-emptive antifungal therapy for invasive fungal disease in high-risk patients not receiving antimold prophylaxis. The panel created a good practice statement to initiate FN CPG-consistent empiric antibacterial therapy as soon as possible in clinically unstable febrile patients.
CONCLUSION
The updated FN CPG incorporates important modifications on the basis of recently published trials. Future work should focus on addressing knowledge gaps, improving CPG implementation, and measuring the impact of CPG-consistent care.
INTRODUCTION
Fever and neutropenia (FN) is one of the most common complications of cancer treatments. The management of pediatric FN continues to be heterogeneous across and within centers1-4; this heterogeneity can be reduced through implementation of clinical practice guidelines (CPGs). CPGs are important to direct clinical care that is evidence-based, and CPG-consistent care can improve outcomes in FN.5 Our original CPG on the management of FN in pediatric cancer and hematopoietic cell transplant (HCT) recipients was published in 20126 and updated in 2017.7 The objective was to update this CPG for the empiric management of FN in pediatric patients with cancer and HCT recipients.
METHODS
Panel Constitution
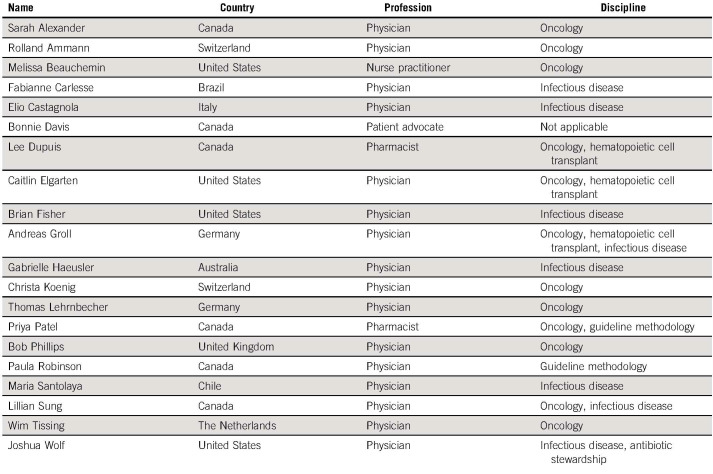
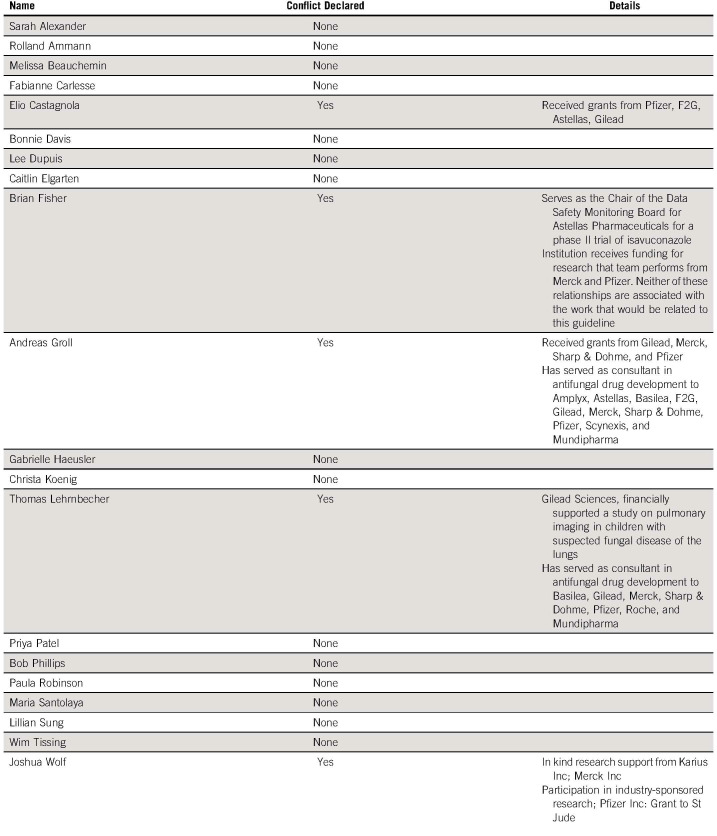
The International Pediatric Fever and Neutropenia Guideline Panel continues to include representation from pediatric oncology, pediatric infectious disease, nursing, pharmacy, a patient advocate, and two CPG methodologists representing 10 different countries (Appendix Table A1, online only). Apart from the patient advocate, panel members were selected according to content or methodological expertise. Conflicts of interest were declared by each panel member; no panel member had conflicts that precluded panel participation (Appendix Table A2, online only).
General CPG Development Approach
We followed previously validated procedures for CPG creation.8 The clinical questions addressed in the CPG update were discussed and remained unchanged from our previous CPG (Data Supplement, online only). The articulation and importance of outcomes of interest were refined by consensus and are presented in the Data Supplement. The target population consists of pediatric patients age 0-18 years receiving chemotherapy for cancer and recipients of HCT. The target users are health care professionals including physicians, nurse practitioners, physician assistants, microbiologists, antibiotic stewards, nurses, pharmacists, health care administrators, and other health care professionals who are concerned with infectious complications in pediatric patients receiving cancer treatments.
We used the Grading of Recommendations Assessment, Development and Evaluation approach to describe the level of evidence and to formulate recommendations.9 Evidence quality was classified as high, moderate, low, or very low on the basis of certainty of effects as applied to our target population.9,10 Recommendations may be strong or conditional.9 A strong recommendation for a strategy reflects high certainty that its benefits outweigh its downsides, whereas a strong recommendation against a strategy reflects high certainty that its downsides outweigh its benefits. By contrast, a conditional recommendation is made when there is uncertainty regarding benefits and downsides of a strategy or when benefits and downsides are more closely matched.
The panel also judiciously considered making good practice statements.11 Such statements can be made in situations where compelling indirect evidence from multiple comparisons strongly supports the benefit of the recommended action. The Grading of Recommendations Assessment, Development and Evaluation's suggested approach to recognizing a good practice statement is to ask whether the alternative action would be absurd or clearly not conform to ethical norms.11
Searching, Selecting, and Describing the Evidence
Although both the original 2012 CPG and 2017 CPG update used systematic reviews to inform the evidence base, the nature of those systematic reviews differed. The original CPG was based on systematic reviews and meta-analyses of nonrandomized comparisons because of the limited number of pediatric randomized controlled trials (RCTs) at that time. With the 2017 update, systematic reviews of both observational studies and RCTs were performed in acknowledgment of the increasing number of pediatric RCTs.
The approach taken in the 2023 update differed again. We hypothesized that direct, high-quality data would generally be required to substantially alter existing recommendations, and thus, we planned to restrict the systematic review to RCTs evaluating any strategy for the management of pediatric FN.
The library scientist–assisted literature search was performed in the following databases: MEDLINE including Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Embase, Cochrane Central Register of Controlled Trials, and PubMed. The Data Supplement shows the full search strategy and details specific eligibility criteria. Titles and abstracts of articles identified by the search strategy were independently screened by two reviewers (P.D.R. and H.H.), and articles potentially meeting eligibility criteria were evaluated at full text by the same two reviewers. In the event of disagreement, adjudication was performed by a third reviewer (L.S.). The Data Supplement shows the flow diagram of study identification, selection, and reasons for exclusion. We described agreement in study inclusion using the Kappa statistic.12
Study characteristics abstracted are as follows: year of publication, country of study conduct, age of participants, cancer diagnosis or HCT type, and number of randomized participants. We also collected information about the intervention and control groups and outcomes considered important (Data Supplement). We used the Cochrane Collaboration's tool for assessing risk of bias in randomized trials.13
Statistical Analysis
Data synthesis was planned when the same strategy was evaluated by at least three studies reporting the same outcome. If a trial had three or more arms, only arms with CPG-consistent therapy7 were selected for data extraction. For binary outcomes, synthesis used the Mantel-Haenszel method and described intervention effects using the risk ratio with 95% CI. For continuous outcomes, synthesis used the inverse variance method and described intervention effects using the weighted mean difference. A random effect model was used for all analyses. The I2 value was also calculated, which describes the percentage of total variation across studies because of heterogeneity rather than by chance.13 Evaluation for publication bias was restricted to synthesis including at least 10 studies and was performed through visual inspection of funnel plots. Analysis was conducted using Review Manager 5.4.14
Formulating Recommendations
This process began with a description of newly identified RCTs published since the 2017 CPG update. Comparisons amenable to synthesis were then analyzed using all RCTs identified in the original and updated searches, with notation of whether newly added studies enabled or revised synthesis. In situations where synthesis was not possible, we also determined if findings of individual studies were likely to influence recommendation formulation. In this event, we retrieved all RCTs addressing a specific question and evaluated them together narratively.
Evidence was reviewed during two videoconference calls held in August 2022. Modified or new recommendations and good practice statements were drafted. Panel members voted; confirmation of 2017 recommendations or acceptance of new draft recommendations or statements were approved if at least 80% of panel members agreed with them. Draft versions of the recommendations and manuscript were circulated until approved by all authors.
The publication peer-review process was used as an efficient approach to external review. We plan to update this CPG in 5 years or sooner in the event of important new information.
RECOMMENDATIONS AND EXPLANATIONS
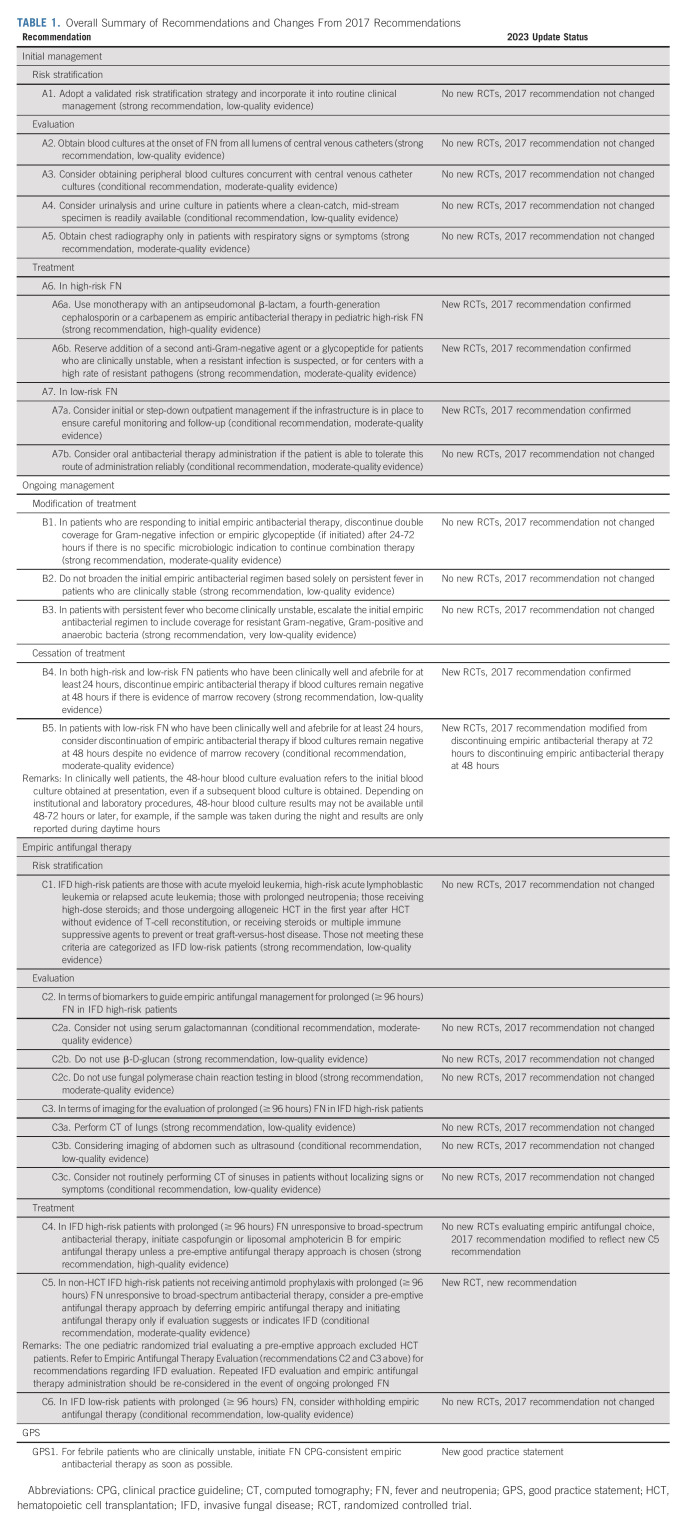
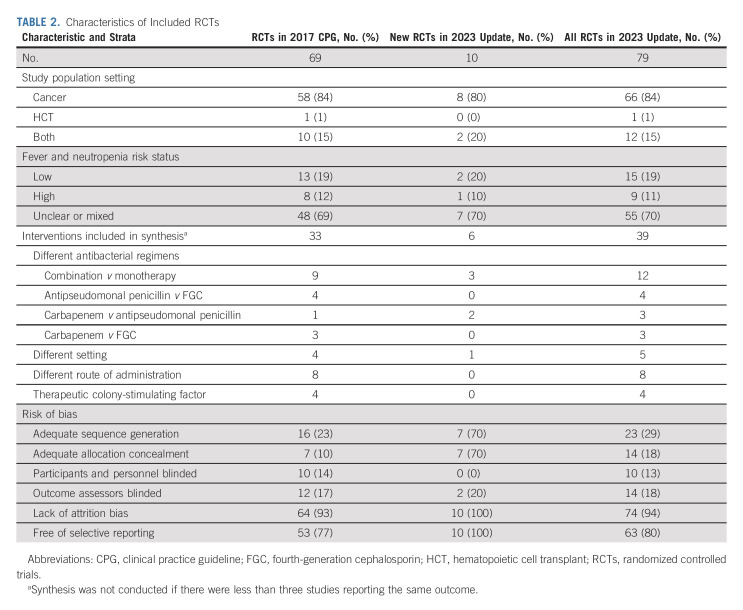
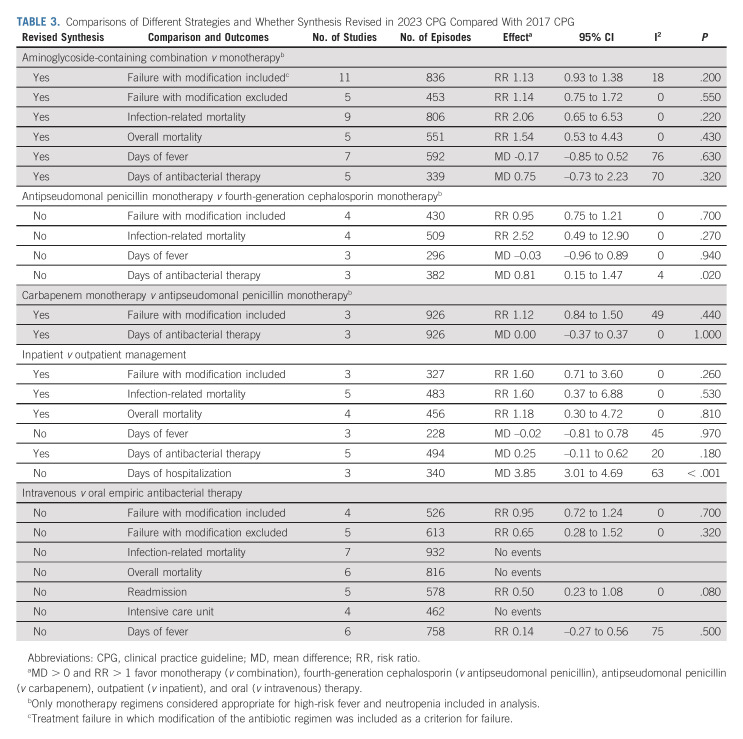
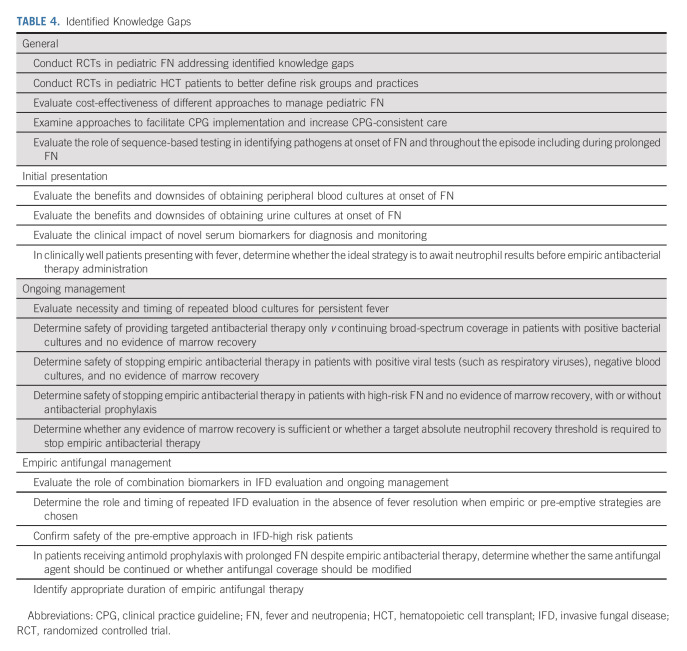
Table 1 presents the 2023 CPG update recommendations and highlights changes from the 2017 CPG. Table 2 shows the characteristics of RCTs included in the 2017 CPG (n = 69), new RCTs identified in the 2023 CPG update (n = 10), and all RCTs informing the 2023 CPG update (n = 79). Agreement in study inclusion was excellent (Kappa = 1.0). The Data Supplement provides detailed characteristics of all 79 RCTs. The Data Supplement describes the 10 new RCTs in the 2023 CPG update with a narrative summary of each study's main findings. Table 3 shows data synthesis results. No publication bias was observed (data not shown). Table 4 summarizes identified knowledge gaps.
TABLE 1.
Overall Summary of Recommendations and Changes From 2017 Recommendations
TABLE 2.
Characteristics of Included RCTs
TABLE 3.
Comparisons of Different Strategies and Whether Synthesis Revised in 2023 CPG Compared With 2017 CPG
TABLE 4.
Identified Knowledge Gaps
SECTION A: INITIAL PRESENTATION OF FN
Initial Management: Risk Stratification
A1. Adopt a validated risk stratification strategy and incorporate it into routine clinical management (strong recommendation, low-quality evidence).
In the 2017 CPG, this recommendation was based on a systematic review of risk stratification schemas identifying multiple rules that had been validated in pediatric populations.7 No new RCTs were identified that evaluated risk stratification, and thus, the 2017 recommendation was unchanged.
Initial Management: Evaluation
A2. Obtain blood cultures at the onset of FN from all lumens of central venous catheters (strong recommendation, low-quality evidence).
A3. Consider obtaining peripheral blood cultures concurrent with central venous catheter cultures (conditional recommendation, moderate-quality evidence).
A4. Consider urinalysis and urine culture in patients where a clean-catch, mid-stream specimen is readily available (conditional recommendation, low-quality evidence).
A5. Obtain chest radiography only in patients with respiratory signs or symptoms (strong recommendation, moderate-quality evidence).
The previous recommendations focused on central and peripheral cultures were derived from a systematic review evaluating the contribution of peripheral cultures to bacteremia detection.7 The conditional recommendation to obtain peripheral cultures concurrent with central venous catheter cultures was made because peripheral cultures identified bacteremia missed by central venous catheter cultures. More specifically, the proportion of true bacteremia episodes detected by peripheral blood cultures alone, when central venous catheter cultures were negative, was 12% (95% CI, 8 to 17). It was a conditional rather than a strong recommendation because the impact of increased bacteremia detection is unknown and peripheral cultures are associated with pain and isolation of skin contaminants. More specifically, centers may choose to not perform a peripheral culture as it is uncertain how often it leads to different clinical actions or better outcomes.
The previous recommendation to consider urinalysis and urine culture where patients are old enough to provide a clean-catch mid-stream specimen readily was based on a systematic review identifying that urinary tract infections were often asymptomatic.7 It was a conditional recommendation because failure to identify urinary tract infections may not affect outcomes since empiric regimens may provide sufficient therapy to treat unidentified infection. It is important to not delay empiric antibacterial therapy to obtain urine samples. Finally, the strong recommendation to not perform a chest radiograph in asymptomatic patients was based on a systematic review, demonstrating that pulmonary infection is very rare without respiratory signs or symptoms.7 Omitting chest radiography in asymptomatic patients was not associated with adverse consequences related to undetected pneumonia.7
No new RCTs were identified that examined evaluation at the onset of pediatric FN, and thus, the 2017 recommendations were unchanged.
Initial Management: Treatment
-
A6. In high-risk FN
A6a. Use monotherapy with an antipseudomonal β-lactam, a fourth-generation cephalosporin or a carbapenem as empiric antibacterial therapy in pediatric high-risk FN (strong recommendation, high-quality evidence).
A6b. Reserve addition of a second anti-Gram-negative agent or a glycopeptide for patients who are clinically unstable, when a resistant infection is suspected, or for centers with a high rate of resistant pathogens (strong recommendation, moderate-quality evidence).
Local epidemiology and patient history of antimicrobial resistance should guide empiric antibacterial therapy choice. The updated systematic review comparing aminoglycoside-containing combination therapy versus monotherapy identified three new studies, resulting in 12 RCTs to inform this recommendation (Table 2). Table 3 shows that the revised synthesis failed to show a statistically significant difference in empiric antibacterial therapy failure, infection-related mortality, overall mortality, days of fever, or days of antibacterial therapy. Consequently, the 2017 recommendation to use monotherapy for empiric antibacterial therapy was confirmed.
In terms of specific monotherapy regimen choice, no additional studies contributed to the comparison between antipseudomonal penicillin monotherapy versus fourth-generation cephalosporin monotherapy. However, the updated systematic review was able to compare carbapenem monotherapy versus antipseudomonal penicillin monotherapy with the inclusion of two new RCTs (Table 2). No statistically significant differences in empiric therapy failure or days of empiric antibacterial therapy were demonstrated (Table 3). Consequently, institutions can choose an antipseudomonal β-lactam, a fourth-generation cephalosporin, or an antipseudomonal carbapenem as empiric therapy on the basis of local susceptibility patterns, costs, and medication availability. If possible, carbapenems should be reserved for clinically unstable patients.
-
A7. In low-risk FN
A7a. Consider initial or step-down outpatient management if the infrastructure is in place to ensure careful monitoring and follow-up (conditional recommendation, moderate-quality evidence).
A7b. Consider oral antibacterial therapy administration if the patient is able to tolerate this route of administration reliably (conditional recommendation, moderate-quality evidence).
In considering alternative treatment approaches for patients with low-risk FN, the updated systematic review identified one additional study comparing inpatient versus outpatient management (Table 2). The revised synthesis included five RCTs and demonstrated no statistically significant differences in empiric therapy failure, infection-related mortality, overall mortality, days of fever, or days of empiric antibacterial therapy (Table 3). Not surprisingly, there were 3.85 fewer days of hospitalization associated with outpatient management (P < .001). Consequently, the 2017 conditional recommendation to consider outpatient management for centers that have an established ambulatory program for carefully selected low-risk patients was confirmed. In addition to low-risk features, social characteristics appropriate for outpatient management include history of adherence to treatment and ability to contact the clinical team reliably and return to hospital in the case of clinical deterioration. The ideal frequency and approach to monitor low-risk FN outpatients remain uncertain, but a combination of in-person and remote follow-up evaluations may be feasible and acceptable.
The previous conditional recommendation to consider oral empiric antibacterial therapy for patients who can tolerate this route of administration reliably was based on the lack of statistically significant differences in treatment failure and days of fever between oral and intravenous therapy and the absence of mortality among those randomly assigned to oral therapy.7 It was a conditional recommendation because of the increased risk of readmission among outpatients treated with oral compared with intravenous therapy. The updated systematic review did not identify new RCTs comparing intravenous versus oral empiric antibacterial therapy, and thus, the 2017 recommendation was unchanged. When oral outpatient therapy is planned, it is important to ensure that patients can receive oral antibacterial therapy from pharmacies or health plans expeditiously, and if there is the possibility of treatment delay or concerns with oral therapy adherence, intravenous therapy should be administered.
SECTION B: ONGOING MANAGEMENT OF FN EXCLUDING EMPIRIC ANTIFUNGAL THERAPY
Ongoing Management: Modification of Treatment
B1. In patients who are responding to initial empiric antibacterial therapy, discontinue double coverage for Gram-negative infection or empiric glycopeptide (if initiated) after 24-72 hours if there is no specific microbiologic indication to continue combination therapy (strong recommendation, moderate-quality evidence).
B2. Do not broaden the initial empiric antibacterial regimen based solely on persistent fever in patients who are clinically stable (strong recommendation, low-quality evidence).
B3. In patients with persistent fever who become clinically unstable, escalate the initial empiric antibacterial regimen to include coverage for resistant Gram-negative, Gram-positive and anaerobic bacteria (strong recommendation, very-low-quality evidence).
In the 2017 CPG, the rationale for early discontinuation of combination therapy was based on data supporting the initial administration of monotherapy for empiric antibacterial therapy. Although indirect, these data also influenced the recommendation to not modify the initial empiric antibacterial regimen based solely on persistent fever in patients who are clinically stable. Escalation of coverage in a patient who becomes clinically unstable was recommended to maximize the probability of providing effective antimicrobial therapy in the setting of sepsis or impending sepsis. No new RCTs were identified that examined therapy modification after initiation of empiric antibacterial therapy, and thus, the 2017 recommendations were unchanged.
Ongoing Management: Cessation of Treatment
B4. In both high-risk and low-risk FN patients who have been clinically well and afebrile for at least 24 hours, discontinue empiric antibacterial therapy if blood cultures remain negative at 48 hours if there is evidence of marrow recovery (strong recommendation, low-quality evidence).
B5. In patients with low-risk FN who have been clinically well and afebrile for at least 24 hours, consider discontinuation of empiric antibacterial therapy if blood cultures remain negative at 48 hours despite no evidence of marrow recovery (conditional recommendation, moderate-quality evidence)
In the 2017 CPG, the strong recommendation to discontinue empiric antibacterial therapy with evidence of bone marrow recovery was based on a systematic review that described a very low risk of recurrent fever with this approach.7 The updated systematic review identified two new studies evaluating discontinuation of empiric antibacterial therapy (Data Supplement). One study randomly assigned patients with low-risk FN without marrow recovery who had been afebrile for 24 hours to discontinue antibacterial therapy versus continuing oral amoxicillin-clavulanic acid or levofloxacin.15 Therapy cessation was noninferior to continuing oral antibacterial therapy in terms of treatment success. The second included patients with both high-risk and low-risk FN who had documented respiratory virus infection without evidence of bacterial infection and randomly assigned them to continue or discontinue antibacterial therapy after 48 hours if they had a favorable evolution. There was no significant difference in uneventful FN resolution.16 Two patients in the group continuing antibacterial therapy experienced Klebsiella pneumoniae bacteremia, one of whom developed sepsis. These two studies, when considered together with the two previously identified RCTs,17,18 suggest that empiric antibacterial therapy can be discontinued in clinically well patients with low-risk FN who have been afebrile for at least 24 hours if the initial blood culture remains negative at 48 hours, despite no evidence of marrow recovery (Data Supplement). The change from the 2017 recommendation was to consider discontinuation at 48 hours rather than 72 hours for patients with low-risk FN without evidence of marrow recovery. Defining evidence of marrow recovery remains elusive.
Although one study evaluated early antibacterial therapy discontinuation in patients with high-risk FN with a documented respiratory viral infection,16 the panel decided against making a recommendation regarding early discontinuation in high-risk patients. This decision stemmed from a concern that such a recommendation may inadvertently promote ordering respiratory virus testing in asymptomatic patients and recognition that patients can remain positive for respiratory viruses after resolution of the acute infection. However, safety of early empiric antibacterial therapy discontinuation in patients with high-risk FN was raised as a knowledge gap (Table 4).
SECTION C: EMPIRIC ANTIFUNGAL THERAPY
Empiric Antifungal Therapy: Risk Stratification
C1. Invasive fungal disease (IFD) high-risk patients are those with acute myeloid leukemia, high-risk acute lymphoblastic leukemia or relapsed acute leukemia; those with prolonged neutropenia; those receiving high-dose steroids; and those undergoing allogeneic HCT in the first year after HCT without evidence of T-cell reconstitution, or receiving steroids or multiple immune suppressive agents to prevent or treat graft-versus-host disease. Those not meeting these criteria are categorized as IFD low-risk patients (strong recommendation, low-quality evidence).
The previous recommendation was based on a systematic review of risk factors for IFD.19 No new RCTs were identified that evaluated risk stratification, and thus, the 2017 recommendation was unchanged.
Empiric Antifungal Therapy: Evaluation
-
C2. In terms of biomarkers to guide empiric antifungal management for prolonged (≥ 96 hours) FN in IFD high-risk patients:
C2a. Consider not using serum galactomannan (conditional recommendation, moderate-quality evidence).
C2b. Do not use β-D-glucan (strong recommendation, low-quality evidence).
C2c. Do not use fungal polymerase chain reaction testing in blood (strong recommendation, moderate-quality evidence).
-
C3. In terms of imaging for the evaluation of prolonged (≥ 96 hours) FN in IFD high-risk patients:
C3a. Perform computed tomography (CT) of lungs (strong recommendation, low-quality evidence).
C3b. Consider imaging of abdomen such as ultrasound (conditional recommendation, low-quality evidence).
C3c. Consider not routinely performing CT of sinuses in patients without localizing signs or symptoms (conditional recommendation, low-quality evidence).
The 2017 recommendations focused on biomarkers for evaluation of prolonged FN were derived from a systematic review of diagnostic tests for IFD.20 The conditional recommendation against the routine use of serum galactomannan was based on its poor positive predictive value and the limited utility of a high negative predictive value for diagnosing IFD. It is important to stress that these recommendations are applicable to patients at onset of prolonged FN before they have undergone radiologic evaluation. In patients with pulmonary infiltrates characteristic of IFD such as dense, well-circumscribed lesions, halo sign, air crescent sign, and cavity or wedge-shaped segmental or lobar consolidation,21 the high pretest probability of invasive aspergillosis may enhance the clinical utility of serum galactomannan testing. The strong recommendations against routine β-D-glucan and fungal polymerase chain testing in blood were based on these tests' very poor positive predictive values (49% and 17%, respectively) and negative predictive values that were not high enough to support clinical utility (96% and 95%, respectively).
The 2017 recommendations focused on imaging for the evaluation of prolonged FN were based on a systematic review of imaging studies.7 The strong recommendation to perform CT of lungs was made because lungs were the most frequent site of IFD and characteristic radiologic signs were often observed. The systematic review also identified that abdominal IFD may not be associated with signs or symptoms, leading to the conditional recommendation to consider imaging of the abdomen. Ultrasound is likely the preferred approach since it does not require sedation and is not associated with radiation exposure. The conditional recommendation against routine sinus CT was based on the common occurrence of abnormal sinus imaging and that abnormalities often did not distinguish between fungal and nonfungal sinusitis.
No new RCTs were identified that examined evaluations such as biomarkers or imaging at the onset of prolonged FN, and thus, the 2017 recommendations were unchanged.
Empiric Antifungal Therapy: Treatment
C4. In IFD high-risk patients with prolonged (≥ 96 hours) FN unresponsive to broad-spectrum antibacterial therapy, initiate caspofungin or liposomal amphotericin B for empiric antifungal therapy unless a pre-emptive antifungal therapy approach is chosen (strong recommendation, high-quality evidence).
C5. In non-HCT IFD high-risk patients not receiving anti-mold prophylaxis with prolonged (≥ 96 hours) FN unresponsive to broad-spectrum antibacterial therapy, consider a pre-emptive antifungal therapy approach by deferring empiric antifungal therapy and initiating antifungal therapy only if evaluation suggests or indicates IFD (conditional recommendation, moderate-quality evidence).
C6. In IFD low-risk patients with prolonged (≥ 96 hours) FN, consider withholding empiric antifungal therapy (conditional recommendation, low-quality evidence).
The previous strong recommendation to initiate caspofungin or liposomal amphotericin B for empiric antifungal therapy was based on three RCTs showing that caspofungin and liposomal amphotericin B had similar efficacy22,23 and that liposomal amphotericin B was less nephrotoxic than conventional amphotericin B.24 Although caspofungin was the agent tested in the RCTs, other echinocandins such as micafungin are also likely appropriate. The conditional recommendation to withhold empiric antifungal therapy in IFD low-risk patients was based on a study that randomly assigned patients with persistent fever to empiric antifungal therapy versus no empiric antifungal therapy. No benefit relative to fever resolution or IFD was detected with empiric antifungal therapy administration.22
One new RCT randomly assigned patients with high-risk and prolonged FN to pre-emptive antifungal therapy versus empiric antifungal therapy (Data Supplement).25 For those randomly assigned to pre-emptive therapy, evaluation included repeat blood cultures, serum galactomannan, chest and sinus CT, and abdominal ultrasound. Antifungal therapy was administered only in those with evidence of IFD. Pre-emptive therapy reduced the median duration of antifungal therapy (6 days v 11 days; P < .001) with no significant difference in the prevalence of IFD (9 of 76 v 9 of 73; P = .92). This RCT was designed before the publication of the 2017 CPG, and its IFD evaluation strategy differed from IFD evaluation recommendations C2 and C3. Given that those recommendations were derived from a large and systematically evaluated evidence base, IFD evaluation should follow recommendations C2 and C3, even when a pre-emptive strategy is chosen.
The panel believed that a pre-emptive approach was reasonable although it acknowledged that the trial might have limited generalizability as it excluded patients undergoing HCT and patients receiving voriconazole or posaconazole prophylaxis. Many IFD high-risk patients currently receive antimold prophylaxis.26 If patients receiving antimold prophylaxis develop prolonged FN unresponsive to broad-spectrum antibacterial therapy, it is uncertain whether antifungal coverage should be modified or whether continuing the same antimold agent should be considered pre-emptive or empiric therapy. If a pre-emptive approach is used in the absence of antimold prophylaxis, antimold therapy should be started in the presence of suggestive clinical or radiologic findings (such as pulmonary nodules) without waiting for microbiologic confirmation.
GOOD PRACTICE STATEMENT
Good practice statement 1. For febrile patients who are clinically unstable, initiate FN CPG-consistent empiric antibacterial therapy as soon as possible.
With this new good practice statement, the panel recognized the urgent need for early recognition of sepsis and expeditious administration of empiric antibacterial therapy to improve survival for patients with invasive infection. Antibacterial therapy should be administered as soon as possible, while other measures to stabilize the patient are taken and should ideally occur much sooner than 1 hour after presentation. Administration of CPG-consistent empiric antibacterial therapy is important to ensure appropriate microbial coverage against potential pathogens.
The panel also deliberated making good practice statements focused on time to antibacterial therapy. For clinically well, patients with low-risk FN, some panel members preferred to err on the side of antibacterial therapy administration pending neutrophil count results. Others emphasized that administration of antibacterial therapy is associated with resistance, costs, and toxicity and thus preferred to defer antibacterial therapy administration until neutrophil count results were available. Regardless, institutions should be encouraged to expedite neutrophil count results to avoid unnecessary antibacterial therapy administration where feasible. More research is required in this area.
DISCUSSION
In this 2023 FN CPG update, changes from the 2017 CPG included two conditional recommendations regarding earlier discontinuation of empiric antibacterial therapy in clinically well and afebrile patients with low-risk FN if blood cultures remain negative at 48 hours despite no evidence of marrow recovery and pre-emptive antifungal therapy for IFD high-risk patients not receiving antimold prophylaxis. The panel also created a good practice statement to initiate FN CPG-consistent empiric antibacterial therapy as soon as possible in clinically unstable febrile patients.
This CPG is founded on direct pediatric cancer27 or HCT data as we believed that pediatric patients could be different from adult patients in terms of their presentation, medication availability and tolerability, and risk-benefit profile when comparing different strategies. More high-quality RCTs are required to better inform pediatric FN clinical care. CPG implementation and uptake continue to be challenging. Implementation may be improved through creation and adaptation of institution-specific care pathways28-31 on the basis of CPGs.32,33
In conclusion, this updated CPG includes important modifications on the basis of recently published trials. Future work should focus on addressing knowledge gaps, improving FN CPG implementation, and measuring the outcomes of CPG-consistent care.
APPENDIX
TABLE A1.
Guideline Panel Membership
TABLE A2.
Guideline Panel Conflict of Interest Disclosures
Thomas Lehrnbecher
Consulting or Advisory Role: Gilead Sciences, Merck Sharp & Dohme, Roche, Mundipharma, Pfizer
Speakers' Bureau: Gilead Sciences, Merck Sharp & Dohme, Sanofi, EUSA Pharma, Pfizer
Research Funding: Gilead Sciences
Travel, Accommodations, Expenses: Gilead Sciences, Merck Sharp & Dohme
Brian Fisher
Consulting or Advisory Role: Astellas Pharma
Research Funding: Pfizer (Inst), Merck (Inst), AlloVir (Inst)
Fabianne Carlesse
Honoraria: Pfizer, MSD
Consulting or Advisory Role: Pfizer, Gilead, AstraZeneca
Speakers' Bureau: Pfizer, AstraZeneca
Elio Castagnola
Honoraria: F2G, Gilead Sciences
Andreas H. Groll
Consulting or Advisory Role: Pfizer, Gilead Sciences, F2G, Mundipharma, SCYNEXIS Inc
Speakers' Bureau: Gilead Sciences, Merck Sharp & Dohme, Pfizer, F2G
Research Funding: Gilead Sciences (Inst)
Gabrielle M. Haeusler
Research Funding: Gilead Sciences
Joshua Wolf
Research Funding: Merck (Inst)
Other Relationship: Karius (Inst)
L. Lee Dupuis
Research Funding: HERON (Inst)
Lillian Sung
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
No other potential conflicts of interest were reported.
SUPPORT
Supported by the Pediatric Oncology Group of Ontario. The CPG was editorially independent of the funder. L.S. was supported by the Canada Research Chair in Pediatric Oncology Supportive Care.
AUTHOR CONTRIBUTIONS
Conception and design: Paula D. Robinson, Brian Fisher, Lillian Sung
Collection and assembly of data: Paula D. Robinson, Priya Patel, Helen Hu, Lillian Sung
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Guideline for the Management of Fever and Neutropenia in Pediatric Patients with Cancer and Hematopoietic Cell Transplantation Recipients: 2023 Update
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Thomas Lehrnbecher
Consulting or Advisory Role: Gilead Sciences, Merck Sharp & Dohme, Roche, Mundipharma, Pfizer
Speakers' Bureau: Gilead Sciences, Merck Sharp & Dohme, Sanofi, EUSA Pharma, Pfizer
Research Funding: Gilead Sciences
Travel, Accommodations, Expenses: Gilead Sciences, Merck Sharp & Dohme
Brian Fisher
Consulting or Advisory Role: Astellas Pharma
Research Funding: Pfizer (Inst), Merck (Inst), AlloVir (Inst)
Fabianne Carlesse
Honoraria: Pfizer, MSD
Consulting or Advisory Role: Pfizer, Gilead, AstraZeneca
Speakers' Bureau: Pfizer, AstraZeneca
Elio Castagnola
Honoraria: F2G, Gilead Sciences
Andreas H. Groll
Consulting or Advisory Role: Pfizer, Gilead Sciences, F2G, Mundipharma, SCYNEXIS Inc
Speakers' Bureau: Gilead Sciences, Merck Sharp & Dohme, Pfizer, F2G
Research Funding: Gilead Sciences (Inst)
Gabrielle M. Haeusler
Research Funding: Gilead Sciences
Joshua Wolf
Research Funding: Merck (Inst)
Other Relationship: Karius (Inst)
L. Lee Dupuis
Research Funding: HERON (Inst)
Lillian Sung
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
No other potential conflicts of interest were reported.
REFERENCES
- 1.Mueller EL, Jacob SA, Cochrane AR, et al. : Variation in hospital admission from the emergency department for children with cancer: A pediatric health information system study. Pediatr Blood Cancer 67:e28140, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haeusler GM, Slavin MA, Bryant PA, et al. : Management of fever and neutropenia in children with cancer: A survey of Australian and New Zealand practice. J Paediatr Child Health 54:761-769, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Phillips B, Selwood K, Lane SM, et al. : Variation in policies for the management of febrile neutropenia in United Kingdom Children's Cancer Study Group centres. Arch Dis Child 92:495-498, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boragina M, Patel H, Reiter S, et al. : Management of febrile neutropenia in pediatric oncology patients: A Canadian survey. Pediatr Blood Cancer 48:521-526, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Wright JD, Neugut AI, Ananth CV, et al. : Deviations from guideline-based therapy for febrile neutropenia in cancer patients and their effect on outcomes. JAMA Intern Med 173:559-568, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehrnbecher T, Phillips R, Alexander S, et al. : Guideline for the management of fever and neutropenia in children with cancer and/or undergoing hematopoietic stem-cell transplantation. J Clin Oncol 30:4427-4438, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Lehrnbecher T, Robinson P, Fisher B, et al. : Guideline for the management of fever and neutropenia in children with cancer and hematopoietic stem-cell transplantation recipients: 2017 update. J Clin Oncol 35:2082-2094, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Oxman AD, Fretheim A, Fretheim A, et al. : Improving the use of research evidence in guideline development: Introduction. Health Res Policy Syst 4:12, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brozek JL, Akl EA, Alonso-Coello P, et al. : Grading quality of evidence and strength of recommendations in clinical practice guidelines. Allergy 64:669-677, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Schünemann H, Brożek J, Guyatt G, et al. : GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. The GRADE Working Group, 2013. https://guidelinedevelopment.org/handbook [Google Scholar]
- 11.Guyatt GH, Alonso-Coello P, Schünemann HJ, et al. : Guideline panels should seldom make good practice statements: Guidance from the GRADE Working Group. J Clin Epidemiol 80:3-7, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Landis JR, Koch GG: The measurement of observer agreement for categorical data. Biometrics 33:159-174, 1977 [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S. (eds): Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. www.cochrane-handbook.org [Google Scholar]
- 14.The Nordic Cochrane Centre : The Cochrane Collaboration: RevMan (computer program). Version 5.4. https://training.cochrane.org/online-learning/coresoftware-cochrane-reviews/revman/revman-5-download [Google Scholar]
- 15.Kumar A, Biswas B, Chopra A, et al. : Early discontinuation versus continuation of antimicrobial therapy in low risk pediatric cancer patients with febrile neutropenia, before recovery of counts: A randomized controlled trial (DALFEN study). Indian J Pediatr 88:240-245, 2021 [DOI] [PubMed] [Google Scholar]
- 16.Santolaya ME, Alvarez AM, Acuna M, et al. : Efficacy and safety of withholding antimicrobial treatment in children with cancer, fever and neutropenia, with a demonstrated viral respiratory infection: A randomized clinical trial. Clin Microbiol Infect 23:173-178, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santolaya ME, Villarroel M, Avendano LF, et al. : Discontinuation of antimicrobial therapy for febrile, neutropenic children with cancer: A prospective study. Clin Infect Dis 25:92-97, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Klaassen RJ, Allen U, Doyle JJ: Randomized placebo-controlled trial of oral antibiotics in pediatric oncology patients at low-risk with fever and neutropenia. J Pediatr Hematol Oncol 22:405-411, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Fisher BT, Robinson PD, Lehrnbecher T, et al. : Risk factors for invasive fungal disease in pediatric cancer and hematopoietic stem cell transplantation: A systematic review. J Pediatr Infect Dis Soc 7:191-198, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Lehrnbecher T, Robinson PD, Fisher BT, et al. : Galactomannan, beta-D-glucan, and polymerase chain reaction-based assays for the diagnosis of invasive fungal disease in pediatric cancer and hematopoietic stem cell transplantation: A systematic review and meta-analysis. Clin Infect Dis 63:1340-1348, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Donnelly JP, Chen SC, Kauffman CA, et al. : Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 71:1367-1376, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caselli D, Cesaro S, Ziino O, et al. : A prospective, randomized study of empirical antifungal therapy for the treatment of chemotherapy-induced febrile neutropenia in children. Br J Haematol 158:249-255, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Maertens JA, Madero L, Reilly AF, et al. : A randomized, double-blind, multicenter study of caspofungin versus liposomal amphotericin B for empiric antifungal therapy in pediatric patients with persistent fever and neutropenia. Pediatr Infect Dis J 29:415-420, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Sandler ES, Mustafa MM, Tkaczewski I, et al. : Use of amphotericin B colloidal dispersion in children. J Pediatr Hematol Oncol 22:242-246, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Santolaya ME, Alvarez AM, Acuña M, et al. : Efficacy of pre-emptive versus empirical antifungal therapy in children with cancer and high-risk febrile neutropenia: A randomized clinical trial. J Antimicrob Chemother 73:2860-2866, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Lehrnbecher T, Fisher BT, Phillips B, et al. : Clinical practice guideline for systemic antifungal prophylaxis in pediatric patients with cancer and hematopoietic stem-cell transplantation recipients. J Clin Oncol 38:3205-3216, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung L, Phillips R, Lehrnbecher T: Time for paediatric febrile neutropenia guidelines—Children are not little adults. Eur J Cancer 47:811-813, 2011 [DOI] [PubMed] [Google Scholar]
- 28.National Centre for Infections in Cancer : Paediatric Low Risk Febrile Neutropenia Program. https://cancerandinfections.org/kids-low-risk-toolkit [Google Scholar]
- 29.Haeusler GM, Gaynor L, Teh B, et al. : Home-based care of low-risk febrile neutropenia in children-an implementation study in a tertiary paediatric hospital. Support Care Cancer 29:1609-1617, 2021 [DOI] [PubMed] [Google Scholar]
- 30.Tew M, De Abreu Lourenco R, Gordon JR, et al. : Cost-effectiveness of home-based care of febrile neutropenia in children with cancer. Pediatr Blood Cancer 69:e29469, 2022 [DOI] [PubMed] [Google Scholar]
- 31.Napper R: Service Evaluation of a New National Febrile Neutropaenia Protocol Using the AUS-Rule for Risk Stratification within the UK [Online Poster]. 2021. https://eacademy.escmid.org/ [Google Scholar]
- 32.Tomlinson D, Robinson PD, Gibson P, et al. : Creating and adapting an infection management care pathway in pediatric oncology. Support Care Cancer 30:7923-7934, 2022 [DOI] [PubMed] [Google Scholar]
- 33.Robinson PD, Tomlinson D, Beauchemin M, et al. : Identifying clinical practice guidelines for symptom control in pediatric oncology. Support Care Cancer 29:7049-7055, 2021 [DOI] [PubMed] [Google Scholar]