PURPOSE:
Oral anticancer drug (OACD) prescriptions require extensive coordination between providers and payers, which can delay drug receipt. Specialty pharmacies facilitate communication between multiple entities. In 2018, our cancer center partnered with a freestanding organization to implement a hospital-based specialty pharmacy (HB-SP). We evaluated the time to drug receipt (TTR) before and after HB-SP implementation.
METHODS:
Data were prospectively collected on all new OACD prescriptions for adult oncology patients from January 1, 2018, to December 31, 2019. In fall 2018, a HB-SP was initiated. We collected patient sociodemographic, clinical, and prescription data. TTR was the number of days from OACD prescription to drug receipt. We used multivariable logistic regression to examine factors associated with TTR ≤ 7 days before and after HB-SP implementation.
RESULTS:
In total, 954 patients were included, representing 1,102 new OACDs. The majority of prescribed drugs were targeted OACDs (56%, n = 617), and 71% (n = 779) required prior authorization. Of all prescriptions, 84% (n = 960) were successfully received with an overall median TTR of 7 days. In unadjusted analysis, HB-SP implementation, drug class, race and ethnicity, and prior authorization requirement were significantly associated with TTR. Adjusted analyses found that patients were more likely to receive their drugs ≤ 7 days after HB-SP implementation (53% v 47%; adjusted odds ratio [aOR], 1.29; 95% CI, 1.00 to 1.68; P = .05).
CONCLUSION:
The implementation of a HB-SP in partnership with a collaborative care model contributed to a decrease in TTR for OACDs. This difference is in part attributable to improved care coordination and communication. A centralized approach may improve overall efficiency due to fewer practice disruptions.
BACKGROUND
Cancer treatment increasingly includes oral anticancer drugs (OACD), a trend expected to continue given the current number of approved OACDs and OACDs in development. Although only six oral anticancer agents received initial US Food and Drug Administration approval between 2006 and 2010, the number of approvals has increased annually, with US Food and Drug Administration approval of 11 new molecular entities in 2020 alone.1 OACDs allow patients to receive their drugs without the historical requirement of hospital visits or infusion therapy and may reduce the frequency of visits to a cancer clinic or hospital.
Procurement of OACDs, however, often requires extensive coordination across multiple entities, including patients, prescribers, and payers, due to the high costs of OACDs.2,3 These multilevel logical factors can delay drug receipt and may result in noninitiation or nonadherence to the patient's prescribed treatment regimen, ultimately risking a chance of cure or progression-free survival.4,5
Interventions or strategies focused on providers, specifically oncology pharmacists, have shown some promise in improving care coordination. Oncology pharmacists communicate directly with patients and payers and provide patient-directed education about medication. This may facilitate procurement of medication and adherence to cancer regimens, ultimately contributing to improved patient outcomes and reduction of out-of-pocket costs.6,7 To scale up the impact of individual oncology pharmacists, and to coordinate and consolidate pharmacy-specific needs, specialty pharmacies offer a potential solution. Specialty pharmacies are distinct from traditional pharmacies by offering coordination of aspects of patient care and disease management, typically with a focus on patients with chronic, complex, or rare health conditions, including cancer.8 Given the increasing use of OACDs for cancer-directed therapy, specialty pharmacies, partnered with care coordination, may build on the known benefit of oncology pharmacists by facilitating communication and coordination between multiple entities to deliver OACDs with increased efficiency.
In 2018, our cancer center partnered with Shields Health Solutions to implement a hospital-based specialty pharmacy (HB-SP). Shields Health Solutions is a freestanding organization that uses a collaborative care model, integrated care technologies, and dedicated teams to expand payer and drug access and improve care coordination through a financially sustainable model. HB-SP is a specialty pharmacy integrator, facilitating much of the care coordination that historically has been absorbed by existing staff (eg, nurses, case managers, pharmacists). Given the potential of this initiative to improve efficiency and access to OACDs, we evaluated drug receipt (TTR) within 7 days and the failure of drug receipt before and after HB-SP implementation.
METHODS
We examined all new OACD prescriptions for adult oncology patients at a large, urban NCI-designated comprehensive cancer center from January 1, 2018, to December 31, 2019. The cancer center, located in Northern Manhattan in New York City, serves a catchment area that is highly diverse, demographically and economically, with significant representation of Hispanic/Latino residents, individuals who are foreign born, and residents living below the poverty line.
We prospectively collected data on all OACD prescriptions to patients with a diagnosis of cancer for whom a new OACD was prescribed in the electronic health record (EHR). Patients were eligible if they received oncologic care at Columbia University Medical Center (CUMC) and had an initial prescription for an OACD. Patients previously prescribed the same OACD or who received it as part of a clinical trial were excluded. This study was approved by the Columbia University Irving Medical Center Institutional Review Board (AAAR4922), and a waiver of consent was approved because the data were collected as a standard of care for all patients prescribed an OACD and due to the minimal risk of the study.
We collected patient-level data, including demographic, clinical, and insurance information, and prescription-level data, including OACD name, date prescribed, delivery date, and interactions with payers and financial assistance groups. We followed the STROBE (Strengthening Reporting of Observational Studies in Epidemiology) reporting guidelines for observational studies.9
Preimplementation OACD Procurement Procedures and Data Collection
Before the implementation in fall 2018, OACD procurement was triggered by a notification from the prescribing clinician to the outpatient clinic registered nurses or nurse practitioners who were responsible for facilitating the OACD process. The registered nurses referred all prescriptions to specialty pharmacies not within the hospital system, a process that requires multiple contacts to identify the correct specialty pharmacy on the basis of each individual's insurance coverage. The specialty pharmacy would contact the insurance company and determine the next steps for prescription fill and the cost of the medication to the patient. The procedural information was not routinely documented in individual specialty pharmacy databases.
For this research study, a paper-based case report form (CRF) was created, and the clinic nurses completed the forms to collect relevant variables and document the process until prescription delivery. The following were collected on the CRF: OACD prescription name, date of prescription, prior authorization (PA) required (yes/no), PA approval (yes/no), and date of drug delivery or failure date. The study team worked closely with the clinical staff to ensure data completion during weekly check-ins.
Postimplementation OACD Procurement Procedures and Data Collection
Following the implementation of the HB-SP, all OACD prescriptions from the outpatient oncology clinics were submitted directly to the HB-SP through the EHR. The HB-SP, in coordination with Shields Health Solutions, would facilitate procurement and delivery of the OACD, obtain further information requested from insurance companies from the clinical staff, communicate cost-related information directly to the patient, and, if necessary, facilitate the patient in obtaining financial assistance (eg, drug assistance programs, individual grants, store-based drug discounts). One comprehensive database housed all prescription information, including the variables previously collected on paper-based CRFs. During the initial implementation, both the original CRF and HB-SP database were used to ensure reliable data capture. The implementation of the HB-SP included all adult outpatient oncology clinics within our cancer center.
Outcome Variables
The primary outcome of this study was receipt of OACD within 7 days of prescription entry into the EHR (time to receipt = TTR). The secondary outcome was failure of drug receipt, defined as prescriptions that were not received within 3 months from original prescription date or by March 31, 2020. Preimplementation, date of delivery was documented by the clinic nurses and confirmed by the study team who contacted the individual specialty pharmacies to resolve any discrepancies or missing data. After implementation, date of delivery was documented in the HB-SP database. If the delivery date was not available, further investigation was conducted to determine if the prescription was received. If there was documentation that the patient was taking the OACD, but no date of delivery, the prescription was excluded from the analysis.
Data Analysis
For this prescription-level analysis, we excluded OACD prescriptions for a washout period of 4 weeks following the HB-SP initiation in each outpatient oncology clinic that participated in this study to account for the transition. To meet inclusion, primary outcome data (date of prescription receipt or confirmation of prescription failure) were required.
Descriptive statistics (proportions of categorical variables) were used to describe patient and prescription-level characteristics including drug class (chemotherapy, hormonal therapy, or targeted agent), requirement of PA, and time period of prescription (before or after implementation of HB-SP). Univariate and multivariable GEE models were used to model our primary covariate of interest, implementation status of HB-SP, against our primary outcome, TTR and secondary outcome, failure of receipt, while controlling for the fact that some patients were expected to have multiple observations. Forward selection was used to select down among other available covariates in the data set, using a threshold of P value < .1 for selection to be included in the multivariable logistic regression model. In the final logistic regression model, P values of < .05 were considered statistically significant.
Chi-square tests of independence were used to assess collinearity between demographic and clinical covariates to prevent an overfit model. All hypothesis tests were two-sided. All analyses were conducted using R. Packages used for this analysis included lme4, gee, stats, and tableone.
RESULTS
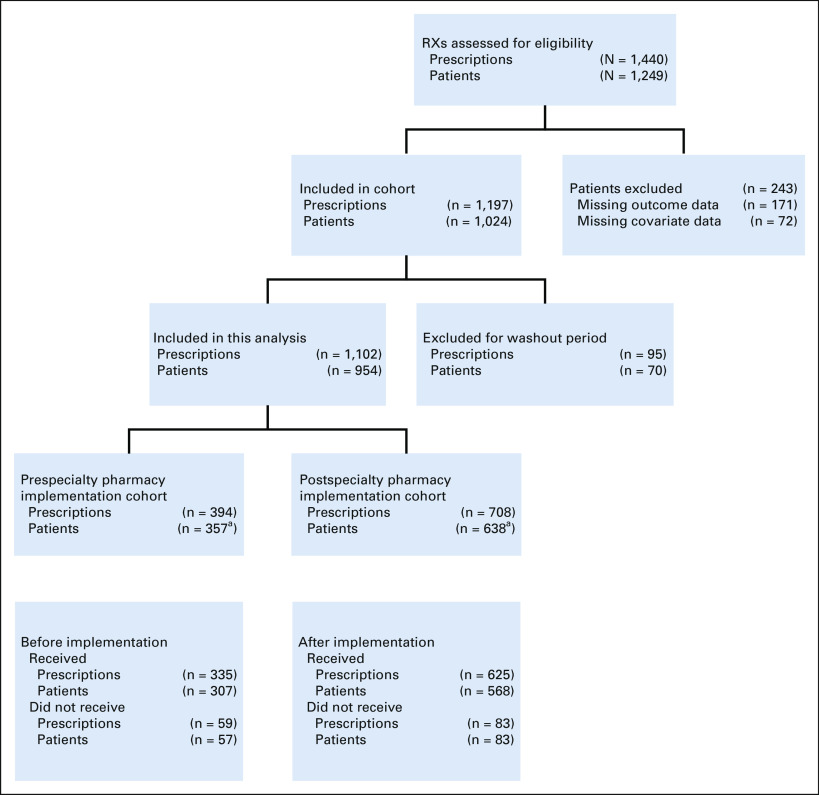
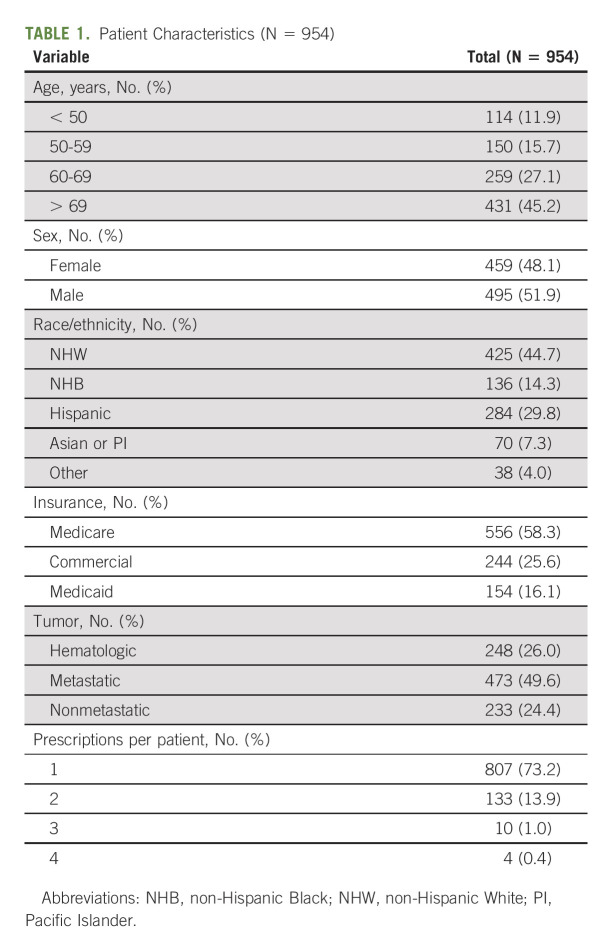
Of 1,459 OACDs that were prescribed across our institution, 954 patients, representing 1,102 OACDs, met inclusion criteria (Fig 1). Patients were equally distributed across sex (52% male), 28% were younger than 60 years. Regarding race and ethnicity, the study included 45% non-Hispanic White patients, 14% non-Hispanic Black patients, and 30% Hispanic patients of any race. Patients fell into three primary categories of medical insurance: commercial insurance only (26%), Medicaid only (16%), and Medicare with or without supplemental or secondary insurance (58%). Half of the patients (50%) had a metastatic solid tumor diagnosis, 24% had a nonmetastatic solid tumor diagnosis, and the remaining 26% had a hematologic malignancy. Most patients (73%, n = 807) were prescribed one OACD during the study period, 14% were prescribed two OACDs, and only 1% were prescribed more than two OACDs. Table 1 describes the 954 patients included in this analysis.
FIG 1.
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) diagram of prescription cohort. aMay have had one or more prescription, see Appendix Table A1 for patient characteristics, including the number of prescriptions. RX, treatment.
TABLE 1.
Patient Characteristics (N = 954)
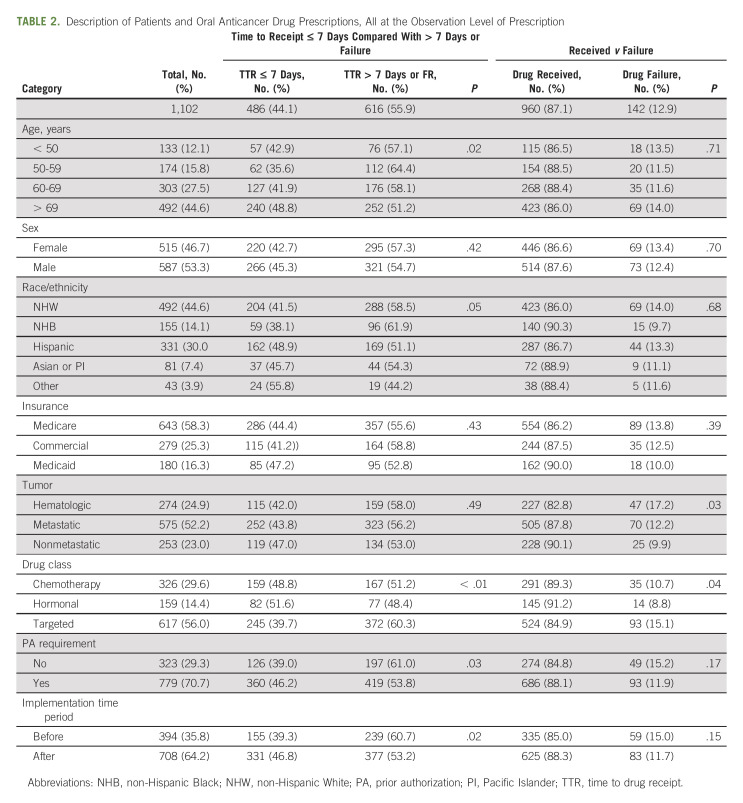
The majority of OACD prescriptions were for targeted treatment (56%, n = 617), and 71% (n = 779) required PA. Of the 1,102 OACD prescriptions, 36% (n = 394) were prescribed before the implementation of the HB-SP and 64% (n = 708) were prescribed after implementation. Table 2 describes the cohort characteristics at the prescription level, representing the 1,102 OACDs included in this analysis.
TABLE 2.
Description of Patients and Oral Anticancer Drug Prescriptions, All at the Observation Level of Prescription
Of 1,102 included OACD prescriptions, 84% (n = 960) were successfully received, and the median TTR was 7 days (range 0-85 days). Before implementation of the HB-SP, 39% (n = 155) of OACDs were received within 7 days compared with 331 (47%) after the implementation. In unadjusted analysis, patients were more likely to receive their prescription within 7 days after HB-SP implementation compared with before (odds ratio [OR], 1.33; 95% CI, 1.03 to 1.72; P = .03). Other significant factors at P < .10 in univariate analysis included race and ethnicity: OACDs prescribed to Hispanic/Latino patients were more likely to be received within 7 days compared with those prescribed to non-Hispanic White patients (OR, 1.36; 95% CI, 1.02 to 1.81; P = .04); drug class: targeted OACDs were less likely to be received within 7 days compared with chemotherapy OACDs (OR, 0.67; 95% CI, 0.52 to 0.88; P = .003); and PA requirement: OACDs requiring a PA were more likely to be received within 7 days compared with those that did not require a PA (OR, 1.35; 95% CI, 1.04 to 1.75; P = .03).
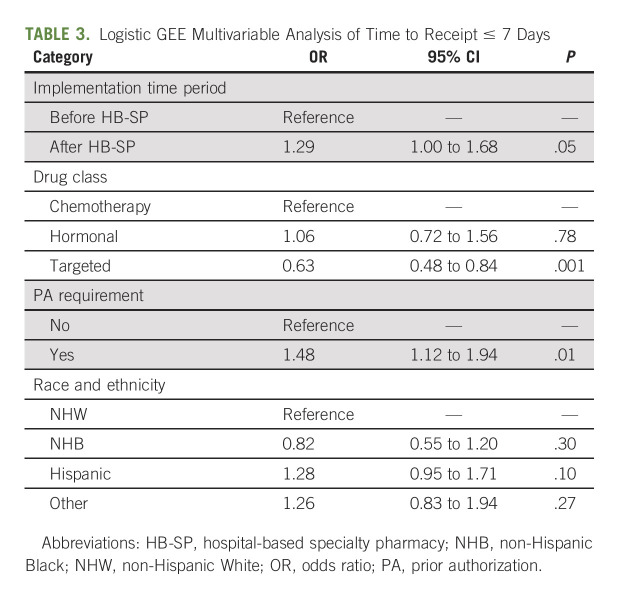
In multivariable analysis, when controlling for factors that were significant at a P value of < .1 in the univariate analysis (age, sex, race and ethnicity, PA requirement, and drug class), OACDs prescribed after the HB-SP implementation were more likely to be received within 7 days compared with those prescribed before the implementation (aOR, 1.29; 95% CI, 1.00 to 1.68; P = .05; Table 3).
TABLE 3.
Logistic GEE Multivariable Analysis of Time to Receipt ≤ 7 Days
We analyzed failure of receipt as a secondary outcome. Before implementation of the HB-SP, 59 (15%) OACDs were categorized as failure to receipt compared with 83 (12%) after implementation (OR, 0.76; 95% CI, 0.53 to 1.08; P = .13). The only covariate that was significant at P < .10 in univariate analysis was drug class (OR, 1.47; 95% CI, 0.97 to 2.23; P = .07). In the multivariable model adjusted for drug class, HB-SP implementation was not significant (aOR, 0.76; 95% CI, 0.53 to 1.08; P = .13; Appendix Table A1, online only).
DISCUSSION
In our study, the implementation of a HB-SP in partnership with a collaborative care model decreased the time to OACD receipt for patients at our institution. Following HB-SP implementation, 47% of OACDs were received sooner than the median time to OACD receipt compared with 39% before the implementation. This finding suggests that system-level interventions, specifically HB-SP implementation, may contribute to improvement in access to and initiation of OACDs.
Time to OACD receipt and initiation was facilitated in our study by the implementation of a HB-SP, highlighting the importance of system-level interventions to reduce barriers to oncology treatment. Prior studies have shown significant delays in treatment initiation, often related to process or financial barriers. Doshi et al10 examined associations between out-of-pocket (OOP) costs and delayed treatment initiation and reported an overall abandonment rate of 18% that ranged by OOP costs from 10% to 50%. Similarly, Li et al11 reported lower rates of OACD initiation in Medicare patients who were responsible for higher OOP costs compared with those who had lower OOP costs due to receipt of low-income subsidies. A recent study using more granular electronic health record data, similar to ours, examined treatment delay in patients starting a new OACD.12 With a similar median TTR of 7 days, they identified pharmacy transfers and insurance/financial issues as a primary barrier to timely receipt of newly prescribed OACDs. These studies suggest that system-level interventions are critical to improving initiation rates by reducing process and, specifically, financial barriers to drug procurement. In our study, the implementation of a HB-SP appears to contribute in part to a reduction of barriers by decreasing the time it takes to receive the medication. Failure of receipt was not significantly impacted by the implementation; however, we have previously reported that failure of receipt is often related to clinical decision making or patient choice.10
There is a critical need to move toward intervention to improve medication access, initiation, and adherence using efficient and sustainable strategies. A recent call to action from the American Society of Clinical Oncology encouraged adherence considerations early in drug development to optimize patient tolerability and ultimately, patient outcomes.1 Our study builds on this by emphasizing the importance of drug initiation, as a component of the adherence continuum, that may directly impact later negative outcomes including disease progression.
Currently, the process to obtain OACD prescriptions often requires multiple steps and increases workload on clinicians and other health care staff. Among physicians surveyed for the 2020 American Medical Association PA survey, 85% reported that PAs were the most difficult pressure to their practices and 94% reported that PAs are associated with care delays.13 In oncology settings, the burden of PA requirement and financial negotiations with insurance has been reported by nurses, pharmacists, and other health care staff.14-16 The workflow scenario reflected in our preimplementation period procedures, where nurses and others are frequently disrupted to address insurance-related questions, is not uncommon.17
To alleviate these stressors, interventions to reduce workload, centralize the drug procurement process, and improve access to medications are warranted. We identified two smaller retrospective studies examining the impact of a comprehensive oral chemotherapy management clinic run by pharmacists and found early evidence of efficacy in decreasing rates of nonadherence18 and reduction in adverse effects and medication errors.7 Our study builds on these findings by examining the impact of a specialty pharmacy with the operational support of a collaborative care organization that may reduce process-related barriers related to drug access. A centralized approach to drug procurement may allow increased time for pharmacists to provide more individualized support, such as through adherence checks, review of drug-drug interaction potentials, or other educational needs. Our prospective study includes a larger cohort and strengthens the argument that medication initiation is supported by a centralized, pharmacy-led intervention.
Our study highlights the importance of complex interventions to improve medication access and initiation, a critical first step toward effective cancer treatment. Prior work focused on patient and provider factors associated with noninitiation and adherence, and our study adds to this by including a system-level lens. A recent metareview of strategies to improve and maintain medication adherence for chronic disease management found that multicomponent or multilevel interventions were more effective than standard of care.19 Pharmacists offer a critical expertise to support access to OACDs at the provider-level; however, they are limited in their ability to impact the multilevel barriers to medication initiation. Our study examines the implementation of a system-level intervention that may have had the trickle-down effect of impacting provider (pharmacist or prescribers) workflows and the overall patient experience. The improvement in TTR is likely, in part, attributable to improved care coordination and communication, suggesting that a centralized approach may improve overall efficiency due to fewer clinical practice disruptions.
We prospectively collected robust data on a cohort of patients who initiated an OACD; however, our study has some limitations. Though this is a larger sample than prior studies, our sample was small and may have been underpowered to detect the true impact of the HB-SP implementation both on TTR and on overall receipt. Owing to variability due to the limited numbers of prescription by month in the preimplementation phase, we did not have a sufficient sample size to explore the role of time and its impact on drug receipt (Appendix Fig A1, online only). Furthermore, as other research has described, the PA process is complex and fraught with challenges, and most medications that require PA are ultimately approved.16 This potential confounder is difficult to tease apart from the implementation of the HB-SP, as both are correlated but important factors to examine in this analysis. The role of PA and OACD drug receipt are a topic of interest and a focus of future studies.
Similarly, regarding the importance of valid process measures to account for the impact of the HB-SP implementation compared with the PA process, we were limited in our ability to collect additional, granular process measures. Future research should standardize how to measure the number of interactions with payers that was required for an OACD procurement, either by staff or after the implementation, through the HB-SP; the amount of time lost by staff to attend to payer interactions; and actual out-of-pocket costs by the patient, including which mechanisms were accessed (patient grants, financial assistance programs). Without these measures, we are limited in our ability to assess the multilevel potential of the HB-SP implementation. With increasingly more OACDs on the market, this study provides a framework of necessary components that future studies must include to assess implementation outcomes that ultimately impact patient clinical outcomes.20
In addition, though we collected the same data elements before and after implementation of a HB-SP, given the nature of the intervention of interest, our data source, the HB-SP database, differed from our pre-existing procedures following implementation. Our study team collaborated closely with the HB-SP during the implementation, and we co-collected data for an overlapping period to ensure consistent data collection. We also excluded a washout period to remove potentially conflicting or inconsistent data. Furthermore, our study is from a single institutional experience, potentially limiting generalizability. Our findings confirm and expand on prior studies, supporting the need to scale up this approach and test a system-level intervention across multiple institutions to refine and optimize the intervention and implementation considerations.21
In conclusion, this study examines the impact of a HB-SP implementation facilitated by a care coordination organization. We found that this intervention contributed to an improvement in time to OACD delivery. This study provides a novel system-level lens on an escalating challenge that requires intervention and scalability, particularly given the increasing number of patients on OACDs as part of their cancer treatment plan. The integration of HB-SP and care coordination in partnership with cancer treatment institutions should be a focus of future, larger, multisite, and multilevel research studies.
ACKNOWLEDGMENT
We would like to acknowledge the nurses and physicians at our institution for their contributions to this study, including workflow knowledge, data collection especially before the HB-SP implementation. We would also like to thank the pharmacists and staff from Shields Health Solutions for their insight into the procedures and clarification of workflow surrounding OACD prescription and procurement.
APPENDIX
TABLE A1.
Logistic GEE Multivariable Analysis of Failure to Receive
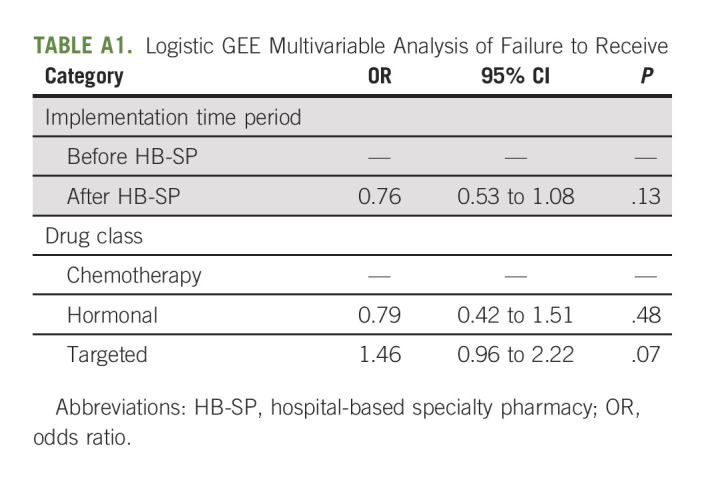
FIG A1.
TTR trend before and after shields implementation (sample size above dots). RX, treatment; TTR, time to drug receipt.
Shing Lee
Consulting or Advisory Role: PTC Therapeutics
Research Funding: Merck (Inst), Karyopharm Therapeutics (Inst), AstraZeneca (Inst), Genentech/Roche (Inst)
Melissa Accordino
Honoraria: Sermo, M3, Incrowd, Massive Bio
Other Relationship: Onclive
Elena B. Elkin
Research Funding: Pfizer
Jason D. Wright
Consulting or Advisory Role: UpToDate
Research Funding: Merck (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Dawn L. Hershman
Consulting or Advisory Role: AIM Specialty Health
No other potential conflicts of interest were reported.
SUPPORT
Supported by the National Cancer Institute at the National Institutes of Health (R21CA242044 to D.L.H., T32CA094061 to M.P.B. and M.R.L.L.); and the American Cancer Society (ACS Clinical Research Professorship to D.L.H.).
M.P.B. and M.R.L.L. contributed equally to this work.
DATA SHARING STATEMENT
Data are available from the authors upon reasonable request.
The codes utilized during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Conception and design: Melissa P. Beauchemin, Morgan R.L. Lichtenstein, Rohit Raghunathan, Sahil D. Doshi, Cynthia Law, Melissa K. Accordino, Dawn L. Hershman
Financial support: Dawn L. Hershman
Administrative support: Cynthia Law, Melissa K. Accordino, Dawn L. Hershman
Provision of study materials or patients: Cynthia Law, Melissa K. Accordino
Collection and assembly of data: Melissa P. Beauchemin, Morgan R.L. Lichtenstein, Rohit Raghunathan, Sahil D. Doshi, Cynthia Law, Melissa K. Accordino, Dawn L. Hershman
Data analysis and interpretation: Melissa P. Beauchemin, Morgan R.L. Lichtenstein, Rohit Raghunathan, Shing Lee, Cynthia Law, Melissa K. Accordino, Elena B. Elkin, Jason D. Wright, Dawn L. Hershman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of a Hospital-Based Specialty Pharmacy in Partnership with a Care Coordination Organization on Time to Delivery and Receipt of Oral Anticancer Drugs
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Shing Lee
Consulting or Advisory Role: PTC Therapeutics
Research Funding: Merck (Inst), Karyopharm Therapeutics (Inst), AstraZeneca (Inst), Genentech/Roche (Inst)
Melissa Accordino
Honoraria: Sermo, M3, Incrowd, Massive Bio
Other Relationship: Onclive
Elena B. Elkin
Research Funding: Pfizer
Jason D. Wright
Consulting or Advisory Role: UpToDate
Research Funding: Merck (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Dawn L. Hershman
Consulting or Advisory Role: AIM Specialty Health
No other potential conflicts of interest were reported.
REFERENCES
- 1. Levit LA, Arora S, Kluetz PG, et al. Call to action for improving oral anticancer agent adherence. J Clin Oncol. 2022;40:1036–1040. doi: 10.1200/JCO.21.02529. [DOI] [PubMed] [Google Scholar]
- 2. Shih YCT, Smieliauskas F, Geynisman DM, et al. Trends in the cost and use of targeted cancer therapies for the privately insured nonelderly: 2001 to 2011. J Clin Oncol. 2015;33:2190–2196. doi: 10.1200/JCO.2014.58.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trish E, Joyce G, Goldman DP. Specialty drug spending trends among Medicare and Medicare advantage enrollees, 2007–11. Health Aff. 2014;33:2018–2024. doi: 10.1377/hlthaff.2014.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chirgwin JH, Giobbie-Hurder A, Coates AS, et al. Treatment adherence and its impact on disease-free survival in the Breast International Group 1-98 Trial of Tamoxifen and Letrozole, alone and in sequence. J Clin Oncol. 2016;34:2452–2459. doi: 10.1200/JCO.2015.63.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holle LM, Boehnke Michaud L. Oncology pharmacists in health care delivery: Vital members of the cancer care team. JCO Oncol Pract. 2014;10:e142–e145. doi: 10.1200/JOP.2013.001257. [DOI] [PubMed] [Google Scholar]
- 7. Wong S-F, Bounthavong M, Nguyen C, et al. Implementation and preliminary outcomes of a comprehensive oral chemotherapy management clinic. Am J Health Syst Pharm. 2014;71:960–965. doi: 10.2146/ajhp130278. [DOI] [PubMed] [Google Scholar]
- 8.NASP Definitions of Specialty Pharmacy and Specialty Medications, Washington, DC. National Association of Specialty Pharmacy, 2016. https://naspnet.org/wp-content/uploads/2017/02/NASP-Defintions-final-2.16.pdf [Google Scholar]
- 9. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 10. Doshi JA, Li P, Huo H, et al. Association of patient out-of-pocket costs with prescription abandonment and delay in fills of novel oral anticancer agents. J Clin Oncol. 2018;36:476–482. doi: 10.1200/JCO.2017.74.5091. [DOI] [PubMed] [Google Scholar]
- 11. Li P, Wong Y-N, Jahnke J, et al. Association of high cost sharing and targeted therapy initiation among elderly Medicare patients with metastatic renal cell carcinoma. Cancer Med. 2018;7:75–86. doi: 10.1002/cam4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang AA, Tapia C, Bhanji Y, et al. Barriers to receipt of novel oral oncolytics: A single-institution quality improvement investigation. J Oncol Pharm Pract. 2020;26:279–285. doi: 10.1177/1078155219841424. [DOI] [PubMed] [Google Scholar]
- 13.2021 AMA Prior Authorization (PA) Physician Survey. Chicago, IL, American Medical Association; 2020. https://www.ama-assn.org/system/files/2021-04/prior-authorization-survey.pdf [Google Scholar]
- 14. Tompkinson M, Fine K, Gruber D, et al. Perceptions of health-care workers of the cost and safety of oral oncolytic agents for patients: A survey. J Adv Pract Oncol. 2020;11:354–365. doi: 10.6004/jadpro.2020.11.4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geynisman DM, Meeker CR, Doyle JL, et al. Provider and patient burdens of obtaining oral anticancer medications. Am J Manag Care. 2018;24:e128–e133. [PMC free article] [PubMed] [Google Scholar]
- 16. Dickens DS, Pollock BH. Medication prior authorization in pediatric hematology and oncology. Pediatr Blood Cancer. 2017;64:e26339. doi: 10.1002/pbc.26339. [DOI] [PubMed] [Google Scholar]
- 17. Lin NU, Bichkoff H, Hassett MJ. Increasing burden of prior authorizations in the delivery of oncology care in the United States. JCO Oncol Pract. 2018;14:525–528. doi: 10.1200/JOP.18.00428. [DOI] [PubMed] [Google Scholar]
- 18. Lam MSH, Cheung N. Impact of oncology pharmacist-managed oral anticancer therapy in patients with chronic myelogenous leukemia. J Oncol Pharm Pract. 2016;22:741–748. doi: 10.1177/1078155215608523. [DOI] [PubMed] [Google Scholar]
- 19. Wiecek E, Tonin FS, Torres-Robles A, et al. Temporal effectiveness of interventions to improve medication adherence: A network meta-analysis. PLoS One. 2019;14:e0213432. doi: 10.1371/journal.pone.0213432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Powell BJ, Fernandez ME, Williams NJ, et al. Enhancing the impact of implementation strategies in healthcare: A research agenda. Front Public Health. 2019;7:3. doi: 10.3389/fpubh.2019.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the authors upon reasonable request.
The codes utilized during the current study are available from the corresponding author on reasonable request.