Abstract
Recent data support incorporation of immune checkpoint inhibitors into the treatment armamentarium for esophageal, gastroesophageal junction, and gastric (esophagogastric) cancer. This practical review focuses on clinical trials that influenced US Food and Drug Administration approvals and treatment guidelines in esophagogastric cancer, including the impact of location, stage, histology, human epidermal growth factor receptor 2 status, and PD-(L)1 expression on these guidelines. The role of immunotherapy in the locally advanced and metastatic setting is constantly expanding. Over the next few years, the many ongoing trials exploring immunotherapy are anticipated to bring new treatment regimens into the frontline setting with the potential to improve survival in patients with advanced disease.
INTRODUCTION
The management of esophagogastric (EG) cancer is a dynamic area of need in clinical oncology. Collectively, EG cancers are among the most diagnosed malignancies, and most deadly. Cancers of the esophagus (E) and stomach are often grouped together in systemic therapy clinical trials, given their similarities in underlying risk factors, biology, and overlapping management strategies. Thus, for the purposes of this review, we will consider EG cancers as cancers of the esophagus, esophagogastric junction (GEJ), and stomach.
Within a review considering EG cancers as a group, it is instructive to address the anatomic and histologic differences that direct enrollment in clinical trials. Esophageal cancer is categorized into squamous cell carcinoma (SCC) and adenocarcinoma histologic subtypes. The distribution of esophageal SCC (ESCC) is more common in the upper third of the esophagus and historically connotes a poorer prognosis. By contrast, esophageal adenocarcinoma is generally found in the distal esophagus, given its association with epithelial metaplasia and Barrett's esophagus. Esophageal junction and gastric cancers are almost always adenocarcinoma subtype. These anatomic distinctions connote surgical and radiologic management differences as well, which are beyond the scope of this review.
Considerations for systemic therapy for EG cancer depend on multiple variables that require consideration by the treating oncologist, including tumor location, histology, and several biomarkers—microsatellite instability (MSI), HER2, and programmed death-1 ligand (PD-(L)1) expression.
The concordance between MSI and MMR status is so high that either has sufficient clinical utility as a biomarker, and often these are grouped together as MSI/mismatch repair deficient (dMMR),1 hereafter referred to as MSI. These MSI tumors harbor functionally impairing mutations in the DNA mismatch repair enzyme apparatus, the most relevant of which are the MLH1, MSH2, MSH6, PMS2, and EPCAM proteins, acquired either through germline mutation, or somatic mutation or inactivation. Loss of function of any part of this apparatus impairs DNA repair capability and results in spontaneous mutations throughout the genome, including in noncoding DNA regions detectable via polymerase chain reaction, termed microsatellites. The resultant genomic insult of MSI tumors is a high burden of immunogenic neoantigens and consequent inflammatory tumor microenvironment, thought to form the basis as a predictive biomarker to immunotherapies, which has been now well demonstrated in many clinical settings. Furthermore, GI tumors expressing MSI are also recognized to respond less well to chemotherapy in certain contexts, despite having an overall more favorable prognosis.2-6
In addition to MSI portending a favorable response to immunotherapy (IO), the identification of other biomarkers to predict for response to IO has remained an area of active research. Most notably, the expression of PD-(L)1, a transmembrane surface glycoprotein acting as an immunoinhibitory checkpoint inhibitors, has been associated with IO response. In GI malignancies, tumor cells themselves do not appear to express the PD-(L)1 protein themselves at a high level; rather, infiltrating immune cells in the tumor microenvironment comprise the majority of the PD-(L)1 expression. Therefore, the combined positive score (or CPS score) is used as a measurement of PD-(L)1 status, as this value is calculated from expression of tumor cells themselves added to the expression of infiltrating immune cells. The CPS score is a reproducible scoring method that can predict response to IO in GI cancers. In general, the rate of CPS positivity (defined as ≥ 1) is thought to range from 40% to 60% of all upper GI tumors; however, controversy exists as to the true background rate of these malignancies, as there appears to be significant variation between trials and their respective assays. In one meta-analysis, the prevalence of PD-(L)1 expression in gastric or GEJ tumors was as high as 57%.7 Many recent IO approvals in the gastroesophageal space have been irrespective of CPS, although varying rates of tumor response have been noted at low levels of CPS expression.8-12
The results of recent clinical trials and subsequent approvals have advanced the field to the benefit of many patients, particularly with regards to IO options; at the same time, navigating these complexities may prove challenging to a busy clinician. Therefore, our aim is to present a concise summary of the EG cancer management landscape in the text and provide key IO trials leading to US Food and Drug Administration (FDA) approvals in Figure 1, and a flowchart of management by disease, location, and biomarker in Figure 1.
FIG 1.
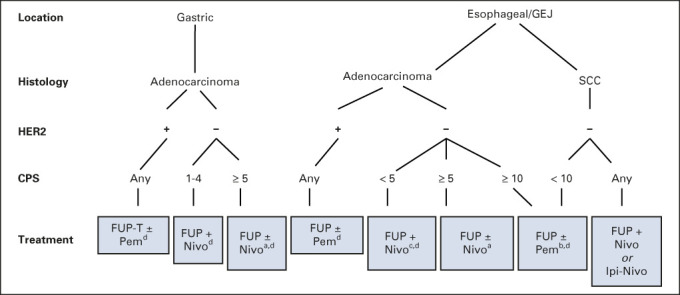
Treatment algorithm of immunotherapy-based regimens in esophagogastric cancer. Treatment algorithm for the use of immunotherapy in the metastatic setting for esophageal, gastroesophageal, and gastric cancer. IO is used with or without chemotherapy as mentioned and not all combinations are FDA-approved. aNCCN category 1 recommendation; bNCCN category 2A recommendation; cNCCN category 2B recommendation; dFDA-approved. CPS, combined positive score; FDA, US Food and Drug Administration; FUP, fluorouracil + platinum; GEJ, gastroesophageal junction; HER2, human epidermal growth factor receptor 2; IO, immunotherapy; Ipi, ipilimumab; Nivo, nivolumab; Pem, pembrolizumab; SCC, squamous cell carcinoma; T, trastuzumab.
DISCUSSION
Locally Advanced EG Cancer
For cancers of the esophagus and GEJ, two non–IO-containing treatment paradigms—neoadjuvant carboplatin-paclitaxel and radiation (CROSS trial)13 and perioperative FLOT14—are well established and comparable in overall survival (OS).15 The phase III randomized CROSS trial enrolled patients with E/GEJ cancer to surgery alone or to weekly carboplatin and paclitaxel concurrent with 23 fractions of external-beam radiation followed by surgery. OS favored the chemotherapy-containing arm over the surgery-only arm (49 v 24 months, hazard ratio [HR], 0.657, P = .003). Despite this relative improvement, the 5-year survival remained only about 50%.13 Attempts to improve outcomes using adjuvant chemotherapy alone, on the basis of a meta-analysis in the pre-CROSS era16 and a large institution's propensity-matched analysis in the post-CROSS era,17 showed modest improvement in outcomes. Currently, there is lack of randomized data to support the use of IO in the neoadjuvant setting for locally advanced gastric cancer.
EG Cancer.
Advances in the use of IO in the metastatic setting influenced incorporation into locally advanced disease in the adjuvant setting. CheckMate 577 is the landmark trial that led to adjuvant IO approval in patients with locally advanced disease.12 This global phase III trial enrolled patients with esophageal and GEJ adenocarcinoma or SCC who received neoadjuvant carboplatin and paclitaxel OR fluorouracil (FU) and cisplatin before resection. Patients were then randomly assigned 2:1 to nivolumab or placebo for up to 1 year of treatment. Median disease-free survival (DFS) was 22.4 months in the nivolumab arm and 11 months in the placebo arm (HR, 0.69, P < .001).12 Subgroup analysis suggests differential benefits according to several factors. First, whereas DFS was reported regardless of CPS, the impact of CPS score on survival appears to be substantial as the difference in DFS between the treatment arms was 19.2 months (29.4 v 10.2 months; HR, 0.62) in CPS ≥ 5, but only 5.2 months in CPS < 5 (16.3 v 11.1; HR, 0.89).12 Stratification by CPS was not provided, so it is not possible to determine the effect PD-(L)1 expression at these levels would have had on the results. Furthermore, the adenocarcinoma subgroup had a DFS of 19.4 months versus 11.1 in placebo (HR, 0.75; CI, 0.59 to 0.96) and SCC subgroup had a DFS of 29.7 months versus 11.0 months in placebo (HR, 0.61; CI, 0.42 to 0.88). Grade 3 or 4 adverse events (AEs) occurred in 34% in the nivolumab group and 32% in the placebo group. The most common AEs related to the trial regimen were fatigue, diarrhea, pruritus, and rash in the nivolumab group.12 Given the attenuated effect of IO in adenocarcinoma subtype, GEJ location, and a low/absent CPS score, it raises the question of whether aggregated DFS benefits are applicable to GEJ/adenocarcinoma and CPS < 1, as addressed in more detail elsewhere.18 However, despite some concerns of subgroups, the overall primary outcome was impressive. CheckMate 577 has changed the treatment paradigm in the adjuvant setting, leading to FDA approval of nivolumab for patients with residual pathologic disease (regardless of CPS or location or histology)19 and National Comprehensive Cancer Network (NCCN) guideline recommendation for adjuvant nivolumab in this setting.20
Ongoing Trials
Several phase III studies are ongoing to further evaluate the use of IO frontline in the locally advanced and metastatic setting to continue to improve OS and progression-free survival (PFS).
A trial of the ECOG-ACRIN Cancer Research Group (EA2174) is currently ongoing to evaluate IO in the neoadjuvant and adjuvant settings in patients with locoregional esophageal or GEJ cancer. In this multicenter phase II/III trial, surgical candidates with locoregional E/GEJ adenocarcinoma receive carboplatin and paclitaxel weekly × five during concurrent radiation (50.4 Gy) either with or without nivolumab 240 mg IV during weeks 1 and 3, followed by surgery. Patients with no postoperative disease receive nivolumab for 12 cycles either with or without ipilimumab for four cycles. Eligibility criteria include patients with T1-N1-3M0 or T2-3N0-2M0 disease who are candidates for surgery, no prior chemotherapy or radiation for this disease, no prior IO, and no significant autoimmune disease. Patients must be disease-free for adjuvant treatment. The primary neoadjuvant end point is pathologic complete response rate and primary adjuvant end point is DFS. Secondary end points include toxicity, DFS, and OS.21
ATTRACTION-5 is a randomized, multicenter, double-blind, placebo-controlled phase III study of nivolumab in combination with adjuvant chemotherapy in stage III gastric (G) and GEJ cancer. Patients are randomly assigned to receive adjuvant chemotherapy (S-1 or oxaliplatin and capecitabine (CAPOX) by investigator's choice) with either nivolumab or placebo. Treatment with nivolumab, placebo, and S-1 therapy will be continued for up to 1 year, and CAPOX therapy will be administered for up to 6 months. The primary end point is relapse-free survival in patients followed up to 5 years.22
KEYNOTE-585 is a phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. This study includes previously untreated G/GEJ adenocarcinoma (Siewert type 2 or 3 tumor; Siewert type 1 tumor eligibility limited to those for whom planned treatment is perioperative chemotherapy and resection), with no evidence of metastatic disease; planned surgery after preoperative chemotherapy; Eastern Cooperative Oncology Group performance status 0-1; adequate organ function; and no active autoimmune disease. Patients will be randomly assigned 1:1 to receive chemotherapy/pembrolizumab (arm 1) or chemotherapy/placebo (arm 2). Chemotherapy is with cisplatin and either capecitabine or FU. Adjuvant monotherapy is with pembrolizumab (arm 1) or placebo (arm 2). In a separate safety cohort, FU + docetaxel + oxaliplatin + leucovorin is being evaluated as a potential chemotherapy option. Primary end points are OS, event-free survival per central review, and pathologic complete response.23
KEYNOTE-975 is a phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with esophageal carcinoma. Eligibility criteria include histologically confirmed diagnosis of locally advanced unresectable (cTX N+ M0 or cT2–T4aNXM0) ESCC, GEJ cancer, esophageal adenocarcinoma, or cervical or upper thoracic esophageal carcinoma with supraclavicular lymph node metastases only (cTX N + M1). Patients are candidates for first-line treatment with definitive chemoradiotherapy. Eligible patients will be randomly assigned 1:1 to pembrolizumab or placebo in combination with definitive chemoradiotherapy. The definitive chemoradiotherapy regimen will comprise one of three options chosen at the discretion of the study site investigator: FP (cisplatin + FU) with RT at 50 Gy; FP with radiotherapy at 60 Gy; or FOLFOX with radiotherapy at 50 Gy. Treatment is continued until progression of disease, toxicity, withdrawal, or completion of 13 cycles of pembrolizumab or placebo (approximately 1 year).24
Metastatic EG Cancer
Platinum-based chemotherapy has been the standard treatment for advanced or unresectable cancers of the esophagus, GEJ, and stomach, including both SCC and adenocarcinoma, for more than 10 years.25 As platinum-doublet chemotherapy is associated with a median OS of less than 1 year,1 the introduction of agents directed against human epidermal growth factor receptor 2 (HER2) and PD-(L)1 yielded a significant advance, as, to our knowledge, these agents pushed survivals beyond 1 year in EG cancer for the first time.11,26 IO represents an exciting advance and has led to the incorporation of checkpoint inhibition therapeutics into first-line treatment as standard of care. Included herein are the trials (Fig 2) that influenced FDA approvals (Table 1) and treatment guidelines (Fig 1). We first describe early IO trials and their role in managing second- and third-line disease, then the recent seminal trials leading to approvals in the first-line setting, followed by trials in progress likely to affect the future standard of care.
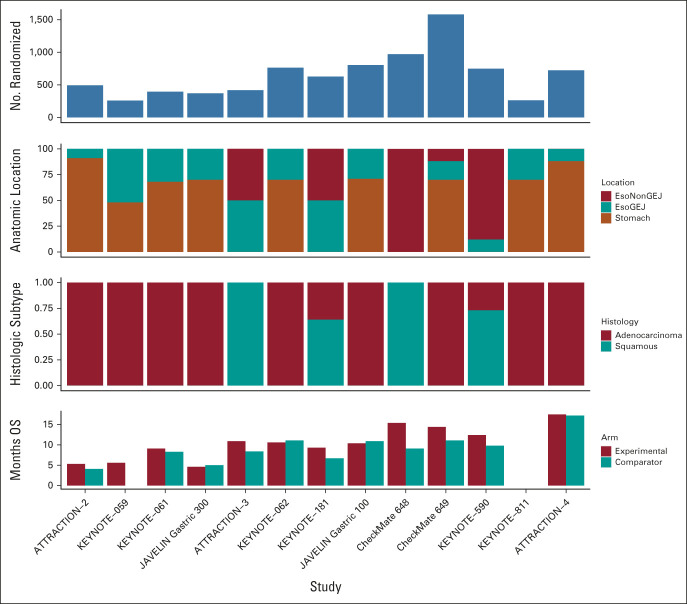
FIG 2.
Trials for metastatic esophagogastric cancer. Compilation of recent clinical trials incorporating immunotherapy in esophageal, gastroesophageal, and gastric cancer, highlighting the difference in histologic subtypes and tumor location between trials. GEJ, gastroesophageal junction.
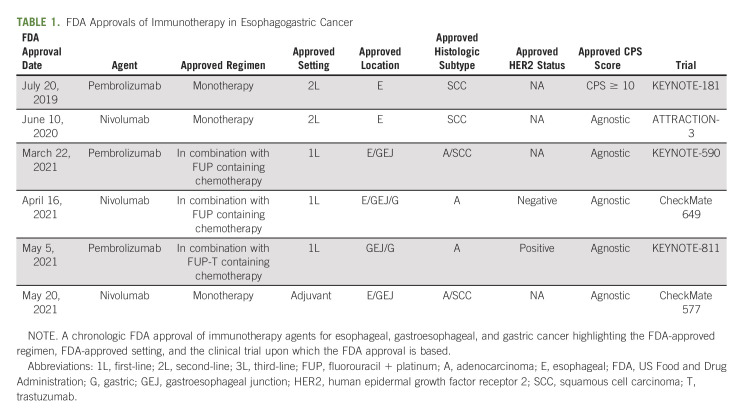
TABLE 1.
FDA Approvals of Immunotherapy in Esophagogastric Cancer
Introduction of IO as treatment for metastatic EG cancer.
The initial trials of IO for EG cancer evaluated their use in later lines of therapy. KEYNOTE-059 was a global, phase II, single-arm trial that studied pembrolizumab monotherapy in the third-line setting for patients with advanced gastric or GEJ adenocarcinoma.27 Objective response rate (ORR) was 11.6% with complete response in 2.3% of all patients. When stratified by PD-(L)1, ORR was 15.5% versus 16.3% in patients with PD-(L)1–positive and PD-(L)1–negative tumors, respectively. Any-grade AEs were reported in 95.8% of patients, with 61.4% experiencing one or more grade 3 to grade 5 AEs. The most common any-grade AEs were fatigue, pruritus, rash, hypothyroidism, decreased appetite, anemia, nausea, diarrhea, and arthralgia.27 In 2017, these results led to the accelerated FDA approval of pembrolizumab as third-line therapy in patients with recurrent locally advanced or metastatic, gastric or GEJ adenocarcinoma whose tumors expressed PD-(L)1.28 Although, to our knowledge, this was the first FDA approval for IO in GE cancer, this FDA approval was ultimately revoked in 2021 as IO agents were introduced into earlier lines of therapy, making the benefit of pembrolizumab in the third line setting unclear.29
KEYNOTE-181 was a phase III trial that studied the benefit of pembrolizumab in the second-line setting in patients with advanced/metastatic SCC or adenocarcinoma of the esophagus.30 Patients were randomly assigned to treatment with pembrolizumab or chemotherapy. OS was prolonged with pembrolizumab versus chemotherapy for patients with CPS ≥ 10 at 9.3 months versus 6.7 (P = .0074) months, respectively. In all randomly assigned patients, OS was unchanged at 7.1 months versus 7.1 (P = .0560) months in the pembrolizumab and chemotherapy arms, respectively. Grade 3 or higher treatment-related AEs occurred in 18% of patients in the pembrolizumab group and in 40.9% of patients in the chemotherapy group. Immune-mediated AEs and infusion reactions occurred in 23.2% of patients in the pembrolizumab group and in 7.4% of patients in the chemotherapy group.30 These results led to the FDA approval of pembrolizumab in the second-line setting for patients with recurrent, locally advanced or metastatic, SCC of the esophagus whose tumors express PD-L1 CPS ≥ 1031 (Table 1).
ATTRACTION-3 was a phase III global trial among patients with esophageal and gastroesophageal SCC who had progressed beyond first-line chemotherapy.32 Patients were randomly assigned to nivolumab or investigator-choice chemotherapy. Nivolumab demonstrated a survival advantage with a median survival of 10.9 versus 8.4 months (P = .019). Eighteen percent of patients in the nivolumab group had grade 3 or 4 treatment-related AEs compared with 63% of patients in the chemotherapy group. The most frequent grade 3 or 4 treatment-related adverse events were anemia (2%) in the nivolumab group and decreased neutrophil count (28%) in the chemotherapy group.32 These findings led to FDA-approval of nivolumab for ESCC in the second-line setting.33
A more recent trial, ATTRACTION-4, was a phase II/III trial in East Asia that enrolled patients with EG adenocarcinoma.34 In the phase III portion of the trial, patients were randomly assigned to chemotherapy with oxaliplatin with either S-1 (SOX) or capecitabine (CAPOX) with nivolumab or SOX/CAPOX with placebo. The primary end point of PFS was significant, showing 10.4 versus 8.3 months (P = .0007) favoring the IO arm. Median OS was 17.4 months for the nivolumab-containing arm and 17.1 months for the chemotherapy-alone arm.34 This difference was not statistically significant; however, it has been noted that this study had a high crossover rate with IO, and the results were not stratified by PD-L1 expression, which may have confounded the results.35 The most common treatment-related grade 3-4 AEs were neutropenia (20% in the nivolumab plus chemotherapy group v 16% in the placebo plus chemotherapy group), decreased platelet count (9% v 9%), and decreased appetite (8% v 6%).34
Although the ATTRACTION trials demonstrated a role for IO in previously treated patients, patients were enrolled largely on the basis of histology rather than biomarker analysis. As it has become increasingly clear that HER2 status and PD-(L)1 CPS are associated with likelihood of response to biomarker-directed therapy, more recent trials have stratified outcomes by these factors, improving their clinical utility. One such trial, KEYNOTE-062, stratified patients on the basis of CPS score when comparing pembrolizumab versus pembrolizumab-chemotherapy versus placebo-chemotherapy in patients with untreated advanced G/GEJ cancer and CPS > 1.36 In this noninferiority study, pembrolizumab-chemotherapy was not superior to placebo-chemotherapy for OS in patients with PD-(L)1 CPS ≥ 1 or PD-(L)1 CPS ≥ 10. Moreover, in an exploratory analysis of MSI-H tumors, median OS with pembrolizumab was not reached versus 8.5 months in the chemotherapy arm for PD-(L)1 CPS ≥ 1. In the PD-(L)1 CPS ≥ 10 cohort, medians OS was prolonged in the pembrolizumab arm versus not reached in the chemotherapy arm. Crossover between treatment arms called into question the validity of this study and therefore the results of KEYNOTE-062 did not lead to FDA-approval of pembrolizumab in this patient population or a change in NCCN treatment guidelines.37 Nevertheless, subsequent superiority trials in the first line were positive and have become practice-changing.
EG adenocarcinoma.
CheckMate 649 is a seminal trial that led to FDA-approval for IO for upper-GI adenocarcinoma in the first-line setting.11 In this phase III trial, patients with untreated, HER2-negative adenocarcinoma of the stomach, GEJ, or esophagus were randomly assigned to receive nivolumab-chemotherapy, nivolumab-ipilimumab, or chemotherapy alone. Nivolumab-chemotherapy showed an improvement in OS and PFS in the CPS ≥ 5 population, with a median OS of 14.4 months.11 Among the all-randomized cohort, OS was significantly improved with the addition of nivolumab when compared with chemotherapy alone with a 12-month survival rate of 55% versus 48%, respectively (P = .0002). Of note, a third arm of this trial comparing nivolumab-ipilimumab versus chemotherapy alone did not show a statistically significant difference in OS in either patients with CPS ≥ 5 (11.2 v 11.6 months, P = .23) or in the all-randomized population (11.7 v 11.8 months, P value not tested).38 Grade 3-4 treatment-related AEs occurred in 59% of patients in the nivolumab plus chemotherapy group and 44% of patients in the chemotherapy alone group, with the most common AEs being neutropenia, anemia, diarrhea, nausea, and neuropathy.11
The CheckMate 649 trial led to FDA-approval of nivolumab (without ipilimumab) for metastatic EG adenocarcinoma regardless of CPS score39 and to NCCN category 1 evidence,40 supporting the addition of IO into the platinum-doublet backbone of EG cancers. Although the trial showed a significant OS benefit to nivolumab in CPS ≥ 5, a significant OS benefit was not observed in the CPS < 1 (HR, 0.92; CI, 0.7 to 1.23), CPS < 5 (HR, 0.94; CI, 0.78 to 1.13),11 and CPS < 10 (HR, 0.91; CI, not stated to upper limit > 1)41 subpopulations, suggesting that OS benefit is driven by the CPS ≥ 10 population. Of note, 60% of patient population were CPS ≥ 5, whereas the initial study design assumed a much lower CPS > 5 prevalence of 35% on the basis of previous studies in similar patient populations11,42; this calls into question the external validity of the all-randomized OS benefit observed in the study as it may not be representative of the CPS distribution in the general population. In addition, the ATTRACTION-4 trial discussed above did not show a significant OS advantage in an unselected population, a finding unlikely to be driven by differences in race,43 but perhaps driven by CPS distribution. Similarly, KEYNOTE-062, as discussed above, showed that pembrolizumab-chemotherapy was not superior to placebo-chemotherapy when stratified by CPS score. The conflicting results seen in these studies could be due to differences in chemotherapy control arms, tumor location, or differences in antibody assays. PD-L1 status in KEYNOTE-062 was validated using the 22C3 pharmDx assay,36 while in Checkmate 649, this was validated using the 28-8 pharmDx assay.11 Use of the 28-8 assay has been shown to result in higher PD-L1 scores when compared with other antibody assays, including 22C3.44 Given these concerns, there remains controversy and lack of consensus45 to the use of IO in lower CPS scores.
Notwithstanding the above controversy, nivolumab with chemotherapy is currently FDA-approved for use in metastatic HER2-negative EG adenocarcinoma regardless of CPS score39 (Table 1). NCCN guidelines recommend with category 1 evidence the use of this combination in PD-(L)1 CPS ≥ 5.40
The identification of HER2 as a predictive biomarker of trastuzumab response in breast cancer led to the study and adoption of trastuzumab in EG cancer, where it was combined with platinum-doublet chemotherapy in the first-line setting.26 More recently, the addition of IO onto a backbone of chemotherapy and trastuzumab was evaluated in the phase III KEYNOTE-811 trial, which enrolled patients with metastatic HER2-positive EG adenocarcinoma. This trial demonstrated a significant benefit in ORR in HER2-chemotherapy-pembrolizumab compared with HER2-chemotherapy (74.4% v 51.9%).46 Complete responses were also more frequent in the pembrolizumab arm (11.3% v 3.1%). Grade 3-5 AEs occurred in 57% of patients in the pembrolizumab group versus 57.4% in the placebo group. The most common AEs included diarrhea, nausea, and anemia.46 Finalized end point data are forthcoming; however, on the basis of the initial promising results of KEYNOTE-811, the addition of pembrolizumab to a backbone of FU/platinum with HER2-targeted therapy has been FDA-approved for HER2-positive adenocarcinoma, regardless of CPS score.47 These results also led to a change in NCCN guidelines with the addition of pembrolizumab recommended as first-line therapy.40
ESCC.
KEYNOTE-590 studied a predominantly ESCC population and showed a benefit for IO in the first-line setting.48 In this phase III trial, patients with untreated, locally advanced or metastatic cancer of the esophagus or Siewert type 1 GEJ were randomly assigned to receive pembrolizumab-chemotherapy or placebo-chemotherapy. Among all randomly assigned patients, an OS benefit was observed for the pembrolizumab-chemotherapy arm (12.4 months v 9.8 months, P < .0001). Subgroup analysis showed a consistent improvement in OS in patients treated with pembrolizumab-chemotherapy versus placebo-chemotherapy in multiple subgroups: SCC and PD-(L)1 CPS ≥ 10 (31% v 15%), SCC (29% v 17%), PD-(L)1 CPS ≥ 10 (31% v 15%), and in all randomly assigned patients (28% v 16%). In a subgroup analysis of patients with adenocarcinoma, OS was longer in the pembrolizumab-chemotherapy arm than in the placebo-chemotherapy arm (11.6 months v 9.9 months), consistent with observations in the all-randomized group.48 Updates presented at ASCO GI 2022, with a follow-up of 34.8 months, showed a continued benefit: median OS was longer in the pembrolizumab-chemotherapy arm versus the placebo-chemotherapy arm in patients with ESCC CPS ≥ 10 (HR, 0.59), ESCC (HR, 0.730), CPS ≥ 10 (HR, 0.64), and all patients (HR, 0.73).49 Grade 3 or higher AEs occurred in 86% of patients in the pembrolizumab plus chemotherapy group versus 83% in the placebo plus chemotherapy group. The most common AEs included anemia and neutropenia.49 On the basis of the results of KEYNOTE-590, the addition of pembrolizumab to a FU/platinum backbone was FDA-approved in the first-line setting for metastatic or locally advanced esophageal or GEJ (tumors with epicenter 1-5 cm above the GEJ), regardless of CPS50 (Table 1). However, KEYNOTE-590 showed no advantage (medial OS 10.5 v 10.6 months) for the addition of pembrolizumab to chemotherapy in cases of CPS 1-9. Per NCCN guidelines, the additional of pembrolizumab to standard chemotherapy is a category 2A recommendation for CPS ≥ 10 and a category 2B recommendation for CPS < 10.20
Ongoing Trials
In the metastatic setting, KEYNOTE-859 is a current phase III trial studying the efficacy of pembrolizumab-chemotherapy versus placebo-chemotherapy in patients with HER2-negative advanced gastric or GEJ adenocarcinoma with a primary end point of OS.51 Initial results are pending and may show a role for pembrolizumab in HER2-negative disease. The LEAP-014 phase III trial52 is studying the addition of lenvatinib, a tyrosine kinase inhibitor, to pembrolizumab plus chemotherapy in patients with ESCC with primary end point measures of OS and PFS.
In conclusion, IO is being increasingly incorporated into treatment of EG cancer, with its role largely determined by location and CPS score in particular. In gastric and esophageal/GEJ cancer, pembrolizumab has been approved for use in HER2-positive adenocarcinoma regardless of CPS score. In HER2-negative esophageal/GEJ adenocarcinoma, pembrolizumab is approved for CPS ≥ 10, while in esophageal/GEJ SCC, it is approved for use with CPS < 10. Nivolumab is now approved for use in HER2-negative gastric adenocarcinoma with CPS ≥ 1 and in HER2-negative esophageal/GEJ adenocarcinoma with CPS < 5 (Fig 2).
Our increasing understanding of tumor factors—location, histology type, HER2 status, and CPS score—help determine optimal therapy for any individual patient. Controversy remains about the use of IO in subgroups of patients, such as those with adenocarcinoma and a lower CPS score. Physicians making these treatment decisions must balance the important considerations of treatment toxicity with a modest chance of clinical benefit. The role of IO in the locally advanced and metastatic setting is constantly expanding. Over the next few years, the many ongoing trials exploring IO are anticipated to bring new treatment regimens into the frontline setting with the potential to improve survival in patients with advanced disease.
ACKNOWLEDGMENT
The authors are grateful to Drs Sunil Reddy, Gilbert Chu, Gregory Heestand, Frank Stockdale, and Ali Raza Khaki, who precipitated this work through their mentorship and encouragement. The authors are also appreciative of Drs Craig Devoe and Lakshmi Rajdev in providing valuable feedback for this manuscript.
Tony Phillip
Consulting or Advisory Role: Foundation Medicine, Daiichi Sankyo/Astra Zeneca, Deciphera
Daniel A. King
Stock and Other Ownership Interests: Illumina
Honoraria: Skysis, Ipsen, Omni Health
No other potential conflicts of interest were reported.
S.P. and F.F. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Sunita Patruni, Fatima Fayyaz, Daniel A. King
Administrative support: Tony Phillip
Collection and assembly of data: Sunita Patruni, Fatima Fayyaz, Daniel A. King
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Immunotherapy in the Management of Esophagogastric Cancer: A Practical Review
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Tony Phillip
Consulting or Advisory Role: Foundation Medicine, Daiichi Sankyo/Astra Zeneca, Deciphera
Daniel A. King
Stock and Other Ownership Interests: Illumina
Honoraria: Skysis, Ipsen, Omni Health
No other potential conflicts of interest were reported.
REFERENCES
- 1.Svrcek M, Lascols O, Cohen R, et al. : MSI/MMR-deficient tumor diagnosis: Which standard for screening and for diagnosis? Diagnostic modalities for the colon and other sites: Differences between tumors. Bull Cancer 106:119-128, 2019 [DOI] [PubMed] [Google Scholar]
- 2.The Cancer Genome Atlas Research Network, Bass A Thorsson V Shmulevich I et al. : Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513:202-209, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willis JA, Reyes-Uribe L, Chang K, et al. : Immune activation in mismatch repair–deficient carcinogenesis: More than just mutational rate. Clin Cancer Res 26:11-17, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos EL, Maron SB, Krell RW, et al. : The interaction between microsatellite instability high (MSI-high) gastric cancer and chemotherapy on survival. J Clin Oncol 39, 2021. (suppl 3; abstr 244) [Google Scholar]
- 5.Lenz H-J, Van Cutsem E, Luisa Limon M, et al. : First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: The phase II CheckMate 142 study. J Clin Oncol 40:161-170, 2022 [DOI] [PubMed] [Google Scholar]
- 6.André T, Shiu K-K, Kim TW, et al. : Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N Engl J Med 383:2207-2218, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Kulangara K, Zhang N, Corigliano E, et al. : Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med 143:330-337, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Kong L, Wang X, Teng F, Yu J: PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 9:5023-5039, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Böger C, Behrens HM, Mathiak M, et al. : PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget 7:24269-24283, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surana R, Pant S: Immunotherapy in gastrointestinal malignancies. Adv Exp Med Biol 1342:259-272, 2021 [DOI] [PubMed] [Google Scholar]
- 11.Janjigian YY, Shitara K, Moehler M, et al. : First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 398:27-40, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly RJ, Ajani JA, Kuzdzal J, et al. : Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med 384:1191-1203, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Van Hagen P, Hulshof MCCM, van Lanschot J, et al. : Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366:2074-2084, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Al-Batran SE, Homann N, Pauligk C, et al. : Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 393:1948-1957, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Reynolds JV, Preston SR, O’Neill B, et al. : Neo-AEGIS (Neoadjuvant trial in Adenocarcinoma of the Esophagus and Esophago-Gastric Junction International Study): Preliminary results of phase III RCT of CROSS versus perioperative chemotherapy (modified MAGIC or FLOT protocol). (NCT01726452). J Clin Oncol 39, 2021. (suppl 15; abstr 4004) [Google Scholar]
- 16.Zhao P, Yan W, Fu H, et al. : Efficacy of postoperative adjuvant chemotherapy for esophageal squamous cell carcinoma: A meta-analysis. Thorac Cancer 9:1048-1055, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saeed N, Mellon EA, Meredith KL, et al. : Adjuvant chemotherapy and outcomes in esophageal carcinoma. J Clin Oncol 35, 2017. (suppl 4; abstr 181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Disease-Free Survival Benefit With Adjuvant Immunotherapy in Resectable Esophageal Cancer: Practice-Changing for All Patients? The ASCO Post. ASCO Post. June 2021. https://ascopost.com/issues/june-10-2021/disease-free-survival-benefit-with-adjuvant-immunotherapy-in-resectable-esophageal-cancer-practice-changing-for-all-patients/ [Google Scholar]
- 19.FDA Approves Nivolumab for Resected Esophageal or GEJ Cancer | US Food and Drug Administration, May 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-resected-esophageal-or-gej-cancer [Google Scholar]
- 20.McMillian N, Lenora Pluchino MA, Ajani JA, et al. : NCCN Guidelines Version 2.2022 Esophageal and Esophagogastric Junction Cancers Continue NCCN, 2022. https://www.nccn.org/home/ [Google Scholar]
- 21.Eads JR, Weitz M, Gibson MK, et al. : A phase II/III study of perioperative nivolumab and ipilimumab in patients (pts) with locoregional esophageal (E) and gastroesophageal junction (GEJ) adenocarcinoma: A trial of the ECOG-ACRIN Cancer Research Group (EA2174). J Clin Oncol 38, 2020. (suppl 15; abstr TPS4651) [Google Scholar]
- 22.Terashima M, Kim Y-W, Yeh T-S, et al. : ATTRACTION-05 (ONO-4538-38/BMS CA209844): A randomized, multicenter, double-blind, placebo- controlled phase 3 study of nivolumab (Nivo) in combination with adjuvant chemotherapy in pStage III gastric and esophagogastric junction (G/EGJ) cancer. Ann Oncol 28:v209-v268, 2017. (suppl 5) [Google Scholar]
- 23.Bang YJ, Van Cutsem E, Fuchs CS, et al. : KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol 15:943-952, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Shah MA, Bennouna J, Doi T, et al. : KEYNOTE-975 study design: A phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with esophageal carcinoma. Future Oncol 17:1143-1153, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham D, Starling N, Rao S, et al. : Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36-46, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Bang Y-J, Van Cutsem E, Feyereislova A, et al. : Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 376:687-697, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Fuchs CS, Doi T, Jang RW, et al. : Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 4:e180013, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FDA Grants Accelerated Approval to Pembrolizumab for Advanced Gastric Cancer | US Food and Drug Administration, September 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-advanced-gastric-cancer [Google Scholar]
- 29.Astor L: FDA’s ODAC Votes Against Continuation of Pembrolizumab Third-Line Indication in Gastric/GEJ Cancer. Targeted Oncol, 2021. https://www.targetedonc.com/view/fda-s-odac-votes-against-continuation-of-pembrolizumab-third-line-indication-in-gastric-gej-cancer [Google Scholar]
- 30.Kojima T, Shah MA, Muro K, et al. : Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol 38:4138-4148, 2020 [DOI] [PubMed] [Google Scholar]
- 31.FDA Approves Pembrolizumab for Advanced Esophageal Squamous Cell Cancer | US Food and Drug Administration, July 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-advanced-esophageal-squamous-cell-cancer [Google Scholar]
- 32.Kato K, Cho BC, Takahashi M, et al. : Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:1506-1517, 2019 [DOI] [PubMed] [Google Scholar]
- 33.FDA Approves Nivolumab for Esophageal Squamous Cell Carcinoma | US Food and Drug Administration, June 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-nivolumab-esophageal-squamous-cell-carcinoma [Google Scholar]
- 34.Kang Y-K, Chen L-T, Ryu M-H, et al. : Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 23:234-247, 2022 [DOI] [PubMed] [Google Scholar]
- 35.Catenacci D: Adding Immunotherapy to First-Line Chemotherapy for Gastric Cancer: Data and Implications from ATTRACTION-4 | PracticeUpdate, October 2020. https://www.practiceupdate.com/content/adding-immunotherapy-to-first-line-chemotherapy-for-gastric-cancer-data-and-implications-from-attraction-4/107395 [Google Scholar]
- 36.Shitara K, Van Cutsem E, Bang YJ, et al. : Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol 6:1571-1580, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merck pulls Keytruda’s FDA nod for third-line stomach cancer. https://pharmaphorum.com/news/merck-pulls-keytrudas-fda-nod-for-third-line-stomach-cancer/
- 38.Helwick C: CheckMate 649: Long-Term Data Support Nivolumab Plus Chemotherapy but Not Nivolumab Plus Ipilimumab in Gastric Cancer. The ASCO Post. ASCO Post. October 2021. https://ascopost.com/issues/october-25-2021/checkmate-649-long-term-data-support-nivolumab-plus-chemotherapy-but-not-nivolumab-plus-ipilimumab-in-gastric-cancer/ [Google Scholar]
- 39.FDA Approves Nivolumab in Combination With Chemotherapy for Metastatic Gastric Cancer and Esophageal Adenocarcinoma | US Food and Drug Administration, April 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-combination-chemotherapy-metastatic-gastric-cancer-and-esophageal [Google Scholar]
- 40.Nicole McMillian N, Lenora Pluchino MA, Ajani JA, et al. : NCCN Guidelines Version 2.2022 Gastric Cancer Continue NCCN Guidelines Panel Disclosures, 2022. https://www.nccn.org/home/member- [Google Scholar]
- 41.Shitara K, Janjigian YY, Moehler MH, et al. : Nivolumab (NIVO) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): Expanded efficacy, safety, and subgroup analyses from CheckMate 649. J Clin Oncol 40, 2022. (suppl 4; abstr 240) [Google Scholar]
- 42.Mehta R, Liepa AM, Zheng S, Chatterjee A: Real-world immunotherapy biomarker testing patterns and results for patients with advanced gastroesophageal cancers in the United States. J Clin Oncol 40, 2022. (suppl 4; abstr 255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen L, Bai Y, Lin X, et al. : First-line (1L) nivolumab (NIVO) plus chemotherapy (chemo) versus chemo in patients (pts) with advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): CheckMate 649 Chinese subgroup analysis. Cancer Res 81, 2021. (suppl 13; abstr CT184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeong J, Tay RYK, Teo CB, et al. : Reply to: Letter to editor on the article “Choice of PD-L1 immunohistochemistry assay influences clinical eligibility for gastric cancer immunotherapy.” Gastric Cancer 25:1133-1135, 2022 [DOI] [PubMed] [Google Scholar]
- 45.Stenger M: Study Suggests Lack of Benefit With the Addition of Immune Checkpoint Inhibition to Chemotherapy in Low PD-L1–Expressing Gastric or Esophageal Adenocarcinomas. The ASCO Post. ASCO Post. December 2021. https://ascopost.com/news/december-2021/study-suggests-lack-of-benefit-with-the-addition-of-immune-checkpoint-inhibition-to-chemotherapy-in-low-pd-l1-expressing-gastric-or-esophageal-adenocarcinomas/ [Google Scholar]
- 46.Janjigian YY, Kawazoe A, Yañez P, et al. : The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 600:727-730, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.FDA grants accelerated approval to pembrolizumab for HER2-positive gastric cancer | FDA. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-her2-positive-gastric-cancer
- 48.Sun JM, Shen L, Shah MA, et al. : Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 398:759-771, 2021 [DOI] [PubMed] [Google Scholar]
- 49.Metges J-P, Kato K, Sun J-M, et al. : First-line pembrolizumab plus chemotherapy versus chemotherapy in advanced esophageal cancer: Longer-term efficacy, safety, and quality-of-life results from the phase 3 KEYNOTE-590 study. J Clin Oncol 40, 2022. (suppl 4; abstr 241) [Google Scholar]
- 50.FDA Approves Pembrolizumab for Esophageal or GEJ Carcinoma | US Food and Drug Administration, March 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-esophageal-or-gej-carcinoma [Google Scholar]
- 51.Tabernero J, Bang YJ, Van Cutsem E, et al. : KEYNOTE-859: A phase III study of pembrolizumab plus chemotherapy in gastric/gastroesophageal junction adenocarcinoma. Future Oncol 17:2847-2855, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun J-M, Enzinger PC, Adenis A, et al. : LEAP-014: An open-label, randomized, phase 3 study of first-line lenvatinib plus pembrolizumab plus chemotherapy in esophageal squamous cell carcinoma. J Clin Oncol 40, 2022. (suppl 4; abstr TPS367) [Google Scholar]