PURPOSE:
Prompt recognition of acute chimeric antigen receptor T (CAR T)-cell–mediated toxicities is crucial because adequate and timely management can prevent or reverse potential life-threatening complications. In the outpatient setting, patients and informal caregivers have to recognize and report signs and symptoms marking these acute toxicities. This study provides a core set of patient- and caregiver-reported signs and symptoms (outcomes, P/CROs) and definitions of red flags warranting immediate action to include in a daily checklist for support at home, with the goal to make outpatient post–CAR T-cell care safer, optimize patient and caregiver support, and thereby facilitating an early discharge/hospital visit reduction strategy.
METHODS:
We performed a systematic review of phase II/III trials of US Food and Drug Administration–approved CAR T-cell products and selected all common and severe adverse events that could be translated into a P/CRO for inclusion in a two-round modified Delphi procedure. Eleven CAR T-cell–dedicated hematologists from the Dutch CAR T-cell tumorboard representing all treating centers selected P/CROs for inclusion in the core set and defined red flags. The final core set was evaluated with patients and caregivers.
RESULTS:
From nine clinical trials, 457 adverse events were identified of which 42 could be used as P/CRO. The final core set contains 28 items, including five signs for measurement via wearables and two signs for caregiver-performed assessments.
CONCLUSION:
This study provides a core set of P/CROs that can serve as a framework for (eHealth) tools that aim to enable patients and caregivers to more effectively recognize and report signs and symptoms of acute toxicities after CAR T-cell therapy, which will enhance safe outpatient treatment monitoring.
INTRODUCTION
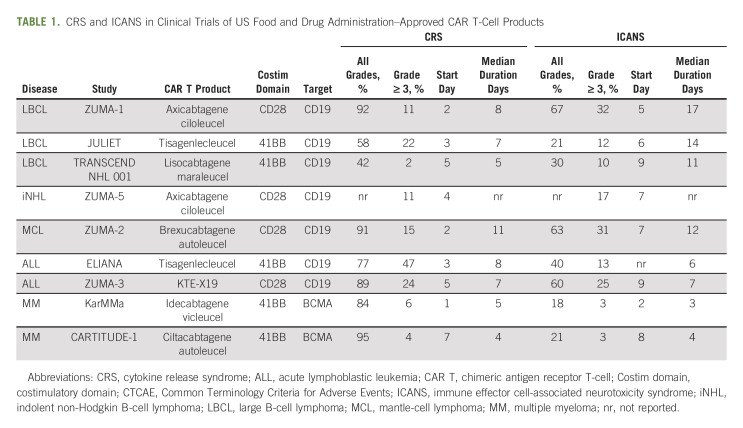
The emergence of chimeric antigen receptor T (CAR T)-cell) therapy has led to unprecedentedly high (complete) response rates across relapsed/refractory B-cell malignancies.1 The effectiveness of CAR T-cell therapy, however, comes at the cost of acute immune-mediated toxicities such as cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), cytopenias, and infections. In some patients, these toxicities can be severe, rapidly progressive, and life-threatening, especially in the acute toxicity phase.2 The occurrence and severity of these acute toxicities depend on patient, tumor, and product characteristics. More specifically, CAR T-cell products with a 41BB costimulatory domain in their design have a more favorable toxicity profile compared with products with a CD28 costimulatory domain (Table 1).3-11 Although prediction models for CAR T-cell–related toxicities are evolving, it is still not possible to determine which patients develop toxicity or not, and therefore close monitoring of all patients receiving CAR T-cell therapy is warranted.12,13 CRS and ICANS are almost always reversible if prompt recognition of signs and symptoms leads to appropriate and timely management.14,15
TABLE 1.
CRS and ICANS in Clinical Trials of US Food and Drug Administration–Approved CAR T-Cell Products
To allow for adequate toxicity monitoring including frequent vital sign measurements, neurologic assessments, and symptom checks, most centers administer CAR T-cell therapy in-hospital and hospitalize patients during at least 7-10 days after infusion. This period is followed by an intensive outpatient follow-up period until day 28 after infusion. During this time, 24/7 presence of an informal caregiver is required to help detect potential CAR T-cell therapy–related side effects.16 There is a growing interest in reducing the need for and duration of in-hospital admission after CAR T-cell administration as its use is rapidly expanding with new CAR T-cell products and indications. This leads to increased health care resource utilization and costs, hampering affordability and accessibility.17,18 Tools such as eHealth tools that help to educate on, monitor, and report acute toxicities leading to prompt interventions are innovations that could drive broader implementation of outpatient CAR T-cell therapy.19 These tools should focus on supporting patients and informal caregivers at home in the acute toxicity phase because they are the first to recognize and report signs and symptoms that mark acute toxicities in the outpatient setting. Patient-reported symptom monitoring has already proven to be feasible and effective in oncology practice, improving early detection and management of adverse events (AEs), quality of life (QoL), and even overall survival.20,21 Adequate acute toxicity monitoring in CAR T-cell therapy includes a triplet of daily symptom checks, vital sign measurements, and neurologic assessments. Therefore a specifically designed tool should ideally combine these three components and provide specific instructions when a sign or symptom demands immediate action to enable timely management of acute toxicity (defined as red flags). A first step in creating content for this tool is to identify and select which signs and symptoms flag these acute toxicities best and can be recognized and reported by patients and their informal caregivers. The checklist needs to be concise to keep it user friendly and to increase compliance; however, it still needs to contain all items that are essential (core set).
This study provides a core set of patient- and caregiver-reported signs and symptoms (outcomes, P/CROs) and specific definitions of red flags. This provides a framework for (eHealth) tools to empower patients and caregivers during the early outpatient treatment phase to more effectively recognize and report signs and symptoms of acute CAR T-cell–mediated toxicities and facilitates a safe early discharge and/or hospital visit reduction strategy. To develop the core set, we used a structured approach of a literature search to identify all reported toxicities of US Food and Drug Administration (FDA)–approved CAR T-cell products, followed by a modified Delphi procedure among CAR T-cell therapy experts.
METHODS
Grading of CAR T-Cell–Related Toxicity Including CAR T-Cell–Mediated Syndromes Such as CRS and ICANS
CAR T-cell–related toxicities are traditionally graded according to the Common Terminology Criteria for Adverse Events (CTCAE), and in clinical trials, this grading system is universally used. However, after the first CD19-directed CAR T-cell therapy trials, it became clear that specific toxicities frequently occurred, and a new diagnosing and grading system was developed for CRS and ICANS, called the American Society for Transplantation and Cellular Therapy (ASTCT) criteria.22 The ASTCT grading is used in clinical practice to diagnose and grade CRS and ICANS. In clinical trials, both CTCAE and ASTCT grading are used. The characteristic signs and symptoms of CRS and ICANS are reported elsewhere.23,24
Developing a Core Set of P/CROs
A structured approach was followed combining a systematic literature review (step I) with consensus-based expert opinion to identify and select the core set of P/CROs (step II-IV).25
I. Systematic literature review
Adopting the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we searched MEDLINE and Embase databases for phase II-III clinical trials investigating FDA-approved CAR T-cell products published from the first report until June 14, 2021.26 We combined relevant search terms for CAR T, hematologic malignancies, and AEs (full search strategies are presented in the Data Supplement, online only). Moreover, cross-referencing was performed to identify additional relevant studies. Eligibility criteria for studies to be included were (1) adult patients ( 18 years or older), (2) phase II-III clinical trials, (3) FDA-approved CAR T-cell products, (4) reporting acute AEs using the CTCAE grading scale, and (5) studies published in English. We excluded studies investigating combination therapy. Two authors (E.R.A.P. and M.K.) independently screened titles and abstracts of all the records to identify potentially eligible articles, which were retrieved as full-text articles and subsequently independently evaluated for eligibility. Differences were discussed between the reviewers until consensus was reached.
II Selection of relevant signs and symptoms
Three authors (A.M.S., E.R.A.P., and M.K.) extracted data on the occurrence of AEs from included studies. Because different terminologies for similar AEs were reported in the included studies, we clustered AE items if multiple different terms for similar symptomatology were used (eg, low platelets and thrombocytopenia). To create an AE shortlist, we merged all reported AEs from the different trials in one table and made a preselection of AEs with high occurrence (≥ 30% any grade), all neurological symptoms and signs that could possibly represent an underlying neurological cause (tremor but also hiccups for example) and severe AEs; grade 3 with an occurrence ≥ 5%; and all grade 4 and 5 events. There are three main categories of AEs in the CTCAE; symptomatic events, observable/measurable events, and laboratory-based events. We made a final item list containing only P/CROs to present to experts in a modified Delphi procedure using the following strategy: (1) we directly included symptomatic events and vital signs in the shortlist as they were considered to be appropriate for self-reporting/measuring; if available, we used the corresponding NCI-Patient Reported-Outcome (PRO)-CTCAE term (for example shortness of breath instead of dyspnea)27; (2) for the observable/measurable events we (E.R.A.P., M.K., A.M.S., M.J.K., and I.S.N.) determined whether and how these would most likely be reported by the patient or observed by the informal caregiver and made corresponding plain language descriptor terms for symptom items or determined appropriate functional tests (eg, the Immune Effector Cell-associated Encephalopathy (ICE) test for encephalopathy or the Alert, responsive to Voice, responsive to Pain, Unresponsive level of consiousness (AVPU) test for level of consciousness28); and (3) we excluded laboratory-based events as they were not appropriate for self-reporting/measuring.
III. Consensus procedure
We performed a two-round modified Delphi procedure. Eleven CAR T-cell therapy–dedicated hematologists, members of the national CAR T-cell tumorboard consortium who facilitate a centralized referral and eligibility assessment structure for CAR T-cell therapy for the Dutch adult population representing all Dutch CAR T treating centers for adults, participated in the consensus procedure. In the first round, the item list was presented, and participants were asked to vote for P/CROs to include or exclude in a daily checklist to monitor acute toxicity in the outpatient setting. The experts could also add any number of signs and symptoms that were, to their opinion, missing in the provided item list. After the first round, items that received more than 80% of votes for inclusion or exclusion were included in the final set or excluded, respectively. Items with 20%-80% of votes for inclusion and any new added items by the panel members were discussed in the first-panel consensus meeting, in which all panel members could state arguments to why an item should be included or excluded. Furthermore, panel members could make suggestions to cluster or rename items. A second Delphi round was organized to vote for the items discussed in the first-panel consensus meeting. The procedure in the second round was identical to the first round. Only items that received 80% of votes for inclusion in the second round were added to the final core set.
A second-panel consensus meeting was organized with the same experts to define when a sign or symptom warrants immediate action to enable timely management of acute, potentially life-threatening, toxicity (definitions of red flags).
IV. Evaluation with nurses and patients
The final core set was evaluated by five patients (patient characteristics are presented in the Data Supplement) and their informal caregivers who underwent CAR-T-cell therapy under the guidance of nurses specialized in CAR T-cell therapy. All participants provided informed consent for evaluation of the core set; the study was assessed by the Medical Research Ethics Committee of the Amsterdam University Medical Centers; W22-200#22.348app. Questions concerned clarity of the terminology used for the signs and symptoms, what they thought the sign or symptom was meaning, and whether they had suggestions to rename any. The terminology used in the definitions of red flags was also evaluated by the patients, informal caregivers, and nurses, and they were asked if any important signs or symptoms were missing on the basis of their experience with acute toxicities after CAR T-cell therapy.
RESULTS
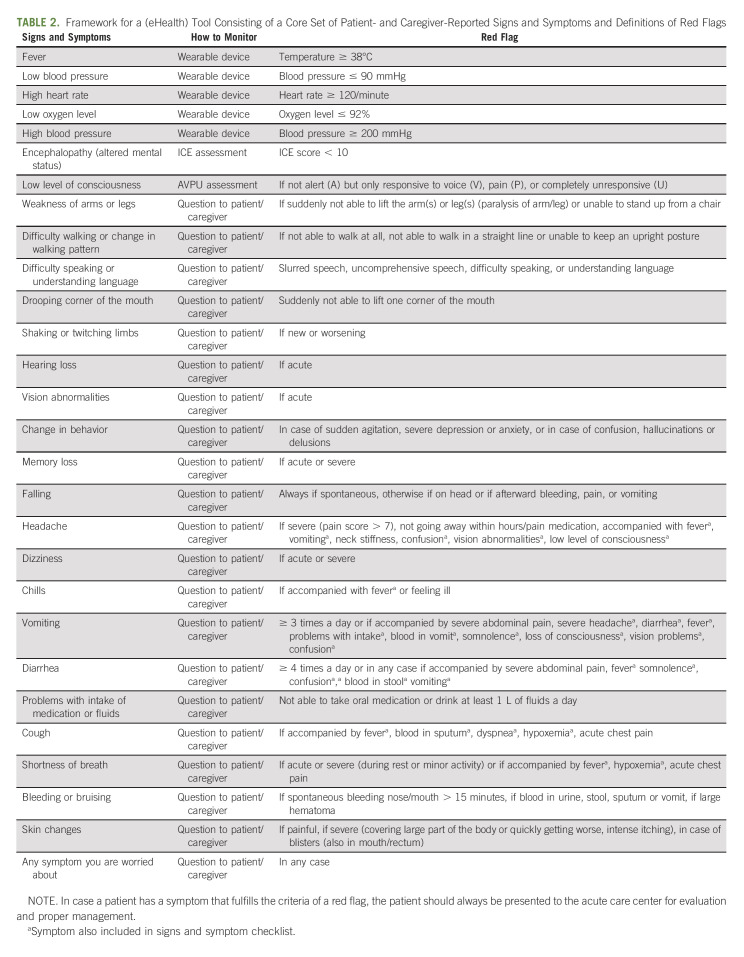
We included nine clinical trials of FDA-approved CAR T-cell products (Data Supplement). Of the 457 different AE items identified in these trials, 88 AE items were included in the AE shortlist (Data Supplement). Forty-two items were found to be appropriate to use as P/CRO for the first Delphi round and were included in the item list (Data Supplement). The results of the two-round Delphi procedure and first-panel consensus meeting are displayed in Figure 1. All expert panel members participated in the complete modified Delphi procedure (100% participation grade). In the first Delphi round, eight items were excluded and 15 items were directly included in the final core set. In the first-panel consensus meeting, the included and excluded items were presented as well as the remaining 19 items and the one suggested new item. During this first-panel consensus meeting, it was decided to cluster five neuropsychological items into one item and two GI items into one item. In total, 15 items were presented in the second Delphi round. Two items were excluded, and the remaining 13 items were included. Thus, the final core set includes 28 items: five vital signs which can be monitored via vital sign monitoring devices, two signs (encephalopathy and level of consciousness) which can be evaluated via the ICE and AVPU assessment, and 21 items which can be reported by the patient/caregiver. In the second-panel consensus meeting, suggestions when to mark a sign or symptom as a red flag were formulated. For the vital signs, hypertension/hypotension, tachycardia, hypoxemia and fever, criteria for red flags were mainly based on cutoff values used in the ASTCT criteria for CRS. Very severe hypertension was included because it can cause encephalopathy and warrants immediate treatment. General pain was excluded in the first Delphi round as the experts found this symptom nonspecific, rarely reported as severe, and if present always accompanied by more specific signs and symptoms part of CRS or an (pulmonary, GI, or skin) infection. Therefore, pain was not included in the core set but has been placed in the red flag descriptions of the GI, cardiopulmonary, and skin items. Headache, however, was included as a P/CRO as it can be the first sign of a neurological problem (meningitis/encephalitis and ICANS) and is more often severe. For the assessments performed by informal caregivers, any ICE score below 10 and any score less than alert on the AVPU scale were considered red flags, in line with the ASTCT criteria for ICANS. For most neurological signs and symptoms, the presence alone warrants immediate action, but some instructions to specify the symptoms into true red flags were given. For other signs and symptoms, severity and co-occurrence of other symptoms were provided in order for a sign or symptom to account for a true red flag (Table 2).
FIG 1.
Development of the core set of P/CROs that mark acute toxicities after CAR T-cell therapy. AVPU, alert, responsive to voice, responsive to pain, unresponsive level of consciousness; CAR T, chimeric antigen receptor T; ICE, immune effector cell-associated encephalopathy; P/CROs, patient- and caregiver-reported signs and symptoms.
TABLE 2.
Framework for a (eHealth) Tool Consisting of a Core Set of Patient- and Caregiver-Reported Signs and Symptoms and Definitions of Red Flags
The evaluation with patients and informal caregivers showed no need to further translate P/CROs into more plain language descriptor terms. They also did not suggest to add any other signs, symptoms, or definitions of red flags on the basis of their own experiences. For the vital signs reported via devices, they suggested to use the terms blood pressure, heart rate, and oxygen level. The framework for the (eHealth) tool combining the core set of P/CROs and definitions of red flags is presented in Table 2.
DISCUSSION
Concerns about toxicity management create a significant barrier to CAR T-cell therapy adaptation by hemato-oncologists in the community and hampers widespread implementation of outpatient treatment.17,29,30 However, as indications expand, more CAR T-cell products with favorable toxicity profiles become available, numbers of treated patients increase and prediction of severe toxicity will be optimized; we anticipate that outpatient treatment will become the mainstay for CAR T-cell therapy.31,32 Outpatient treatment in the clinical trial setting has already proven to be feasible. However, only highly preselected patients treated with CAR T-cell products with a favorable toxicity profile were recruited to these trials.4,5,8,18 These trials facilitated safe outpatient treatment by offering extensive patient/caregiver education on recognizing CRS, ICANS, and infections, together with daily clinic visits or phone calls for the first 7 days, followed by outpatient visits at least twice per week until day 30. Such education can also be provided via (eHealth) tools with the advantage not only to educate but also to monitor and early report all relevant acute toxicities to physicians.
The current study provides consensus-based content, in the form of a core set of P/CROs and definitions of red flags that can serve as a framework for such a tool. Clinical expert knowledge on these acute toxicities was translated into signs and symptoms that can be actually reported or observed by patients and caregivers. We used a structured approach combining input from a systematic literature review and a CAR T-cell therapy expert panel and performed a modified Delphi procedure to select the essential signs and symptoms that require daily monitoring and reporting to include in this core set. In addition, consensus was reached for specific recommendations when these signs and symptoms should be considered red flags. This approach enables transparent, reproducible, and generalizable output that can be easily used or generally adapted in the hemato-oncological field.
The final core set contains 28 P/CROs of which remarkably more than half are neurological. This might be explained by the fact that the clinical spectrum of ICANS is very heterogeneous. In addition, most neurological symptoms are considered possible red flags since ICANS can start with subtle changes in mental status such as difficulty in writing or naming objects, but can rapidly progress and become life-threatening, requiring prompt management. Notably, fatigue and pain were excluded illustrating the focus of this study being on selecting specific signs and symptoms considered as possible acute CAR T-cell–related toxicity that warrant immediate action. However, when focusing on monitoring symptoms affecting QoL, fatigue and pain are among the most important items.33 A core set of patient-reported symptoms to evaluate symptom burden and the effects on QoL, that should be part of long-term follow-up, could be the topic of another Delphi study using the same item list (Data Supplement) as input in combination with items derived from interviews with patients and health care providers. For such a study, we would also recommend to include patients in the expert panel for the initial two-round Delphi procedure as they can provide essential input on what symptoms concern them most and influence QoL.
A limitation of our study is that we only included Dutch experts in the panel. Although these experts represent all Dutch CAR T-cell treating centers for adults and are all the hematologists dedicated to CAR T-cell therapy in the Netherlands with a lot of experience in clinical practice and international clinical trials, an international validation of this core set and definitions of red flags, including international evaluation with a larger group of patients and informal caregivers, might be of value. The limited number of patients involved in the evaluation of the core set is also a limitation.
Although we thoroughly looked at all reported CTCAE items in trials of FDA-approved CAR T-cell products where all AE incidences and grades were reported, we had to make a selection to have a manageable list to present to the expert panel. The criteria for selection and consensus were based on expert opinion, as guidelines are currently not available.
Although time of onset, duration, severity, and frequency of AEs differ between current FDA-approved CAR T-cell products and disease types, the type of CAR T-cell toxicity is similar (eg, CRS, neurotoxicity, infections, and cytopenias), and therefore the core set can be used as a basis for different CAR T-cell constructs. As our review was performed on June, 14, 2021, and at the time of submission several new trials were published, we included an updated list of phase II-III clinical trials with FDA-approved CAR T products, and the resulting updated AE shortlist in the Data Supplement. New common, severe, and neurological AEs did not lead to any new P/CRO items and were all captured by the already included P/CROs in the core set. As new FDA-approved CAR T-cell products will become available and disease indications will extend in this rapidly evolving field, it is important to review the AEs in these new trials for new common or severe toxicities which might warrant adaptation of the core set.
A major advantage of this framework is that it can be implemented in several ways. A user-friendly way is to incorporate this checklist in a remote patient monitoring platform such as a smartphone app connected to wearable devices, enabling automated alert generation to health care providers. This can be used in conjunction with regular (telehealth) visits. Although input from patients was acquired during this study to evaluate clarity and understandability of the terms used for signs and symptoms, additional research is needed to further assess usability and feasibility when implementing such an eHealth tool in clinical practice. Another advantage of using this framework in a (eHealth) tool is automatic generation of valuable data on a set of P/CROs that might help to predict clinical outcomes. In addition, although this core set is developed for patients and informal caregivers, it could also help to educate and guide formal caregivers without extensive CAR T-cell therapy experience.
To our knowledge, this is the first evidence and consensus-based core set of P/CROs including specific definitions of red flags providing a framework for (eHealth) tools aiming to empower patients and their caregivers to more effectively recognize and report signs and symptoms of acute CAR T-cell–mediated toxicities during the early outpatient treatment phase. If this framework incorporated in an eHealth tool is proven to have adequate usability and feasibility, this tool could be used in studies investigating strategies to reduce hospital visits in the first month after CAR T-cell infusion and/or enable early discharge or even complete ambulatory treatment. As such, the core set of P/CROs including specific definitions of red flags provided in this study contributes to strategies enhancing safe outpatient monitoring and fosters wider implementation of CAR T-cell therapy. This will not only help to control costs, improving affordability and accessibility of CAR T-cell therapy, but is also believed to increase patient's satisfaction with received care and QoL.
Pieternella J. Lugtenburg
Consulting or Advisory Role: Takeda, Roche/Genentech, Genmab, Celgene, Regeneron, Incyte, AbbVie
Research Funding: Takeda (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Celgene
Tom van Meerten
Honoraria: Kite/Gilead
Consulting or Advisory Role: Janssen
Research Funding: Genentech (Inst), Celgene (Inst)
Monique C. Minnema
Consulting or Advisory Role: Janssen-Cilag (Inst)
Speakers' Bureau: Celgene/Bristol Myers Squibb (Inst), Medscape (Inst), Janssen Medical Affairs (Inst)
Joost S.P. Vermaat
Consulting or Advisory Role: Secura Bio
Marjolein W.M. van der Poel
Consulting or Advisory Role: Takeda
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Daiichi Sankyo
Martijn G.H. van Oijen
Research Funding: Roche (Inst), Nordic Group (Inst), Servier (Inst), Amgen (Inst), Merck (Inst)
Inger S. Nijhof
Consulting or Advisory Role: Janssen, Celgene/Bristol Myers Squibb
Marie José Kersten
Honoraria: Novartis, Kite, a Gilead company, Roche
Consulting or Advisory Role: Novartis, Kite, a Gilead Company, Miltenyi Biotec (Inst), Takeda (Inst)
Research Funding: Kite, a Gilead company (Inst)
Travel, Accommodations, Expenses: Novartis, Kite, a Gilead Company, Roche, Celgene
No other potential conflicts of interest were reported.
SUPPORT
This research was made possible by European Union's Horizon 2020 research and innovation program under grant agreement No. 875171, QUALITOP and by the Innovative Medicines Initiative 2 Joint Undertaking (grant agreement No. 116026), T2EVOLVE. This Joint Undertaking receives support from the European Union's Horizon 2020 Research and Innovation program and European Federation of Pharmaceutical Industries and Associations (EFPIA).
A.M.S. and E.R.A.P. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Anne M. Spanjaart, Elise R.A. Pennings, Milan Kos, Martijn G.H. van Oijen, Inger S. Nijhof, Marie José Kersten
Administrative support: Anne M. Spanjaart, Elise R.A. Pennings, Milan Kos
Provision of study materials or patients: Pieternella J. Lugtenburg, Tom van Meerten, Monique C. Minnema, Marie José Kersten
Collection and assembly of data: Anne M. Spanjaart, Elise R.A. Pennings, Milan Kos, Pim G.N.J. Mutsaers, Tom van Meerten, Jaap A. van Doesum, Monique C. Minnema, Margot Jak, Martijn G.H. van Oijen, Maria T. Kuipers, Inger S. Nijhof, Marie José Kersten
Data analysis and interpretation: Anne M. Spanjaart, Elise R.A. Pennings, Milan Kos, Pieternella J. Lugtenburg, Tom van Meerten, Monique C. Minnema, Suzanne van Dorp, Joost S.P. Vermaat, Marjolein W.M. van der Poel, Martijn G.H. van Oijen, Inger S. Nijhof, Marie José Kersten
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Development of a Core Set of Patient- and Caregiver-Reported Signs and Symptoms to Facilitate Early Recognition of Acute Chimeric Antigen Receptor T-Cell Therapy Toxicities
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Pieternella J. Lugtenburg
Consulting or Advisory Role: Takeda, Roche/Genentech, Genmab, Celgene, Regeneron, Incyte, AbbVie
Research Funding: Takeda (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Celgene
Tom van Meerten
Honoraria: Kite/Gilead
Consulting or Advisory Role: Janssen
Research Funding: Genentech (Inst), Celgene (Inst)
Monique C. Minnema
Consulting or Advisory Role: Janssen-Cilag (Inst)
Speakers' Bureau: Celgene/Bristol Myers Squibb (Inst), Medscape (Inst), Janssen Medical Affairs (Inst)
Joost S.P. Vermaat
Consulting or Advisory Role: Secura Bio
Marjolein W.M. van der Poel
Consulting or Advisory Role: Takeda
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Daiichi Sankyo
Martijn G.H. van Oijen
Research Funding: Roche (Inst), Nordic Group (Inst), Servier (Inst), Amgen (Inst), Merck (Inst)
Inger S. Nijhof
Consulting or Advisory Role: Janssen, Celgene/Bristol Myers Squibb
Marie José Kersten
Honoraria: Novartis, Kite, a Gilead company, Roche
Consulting or Advisory Role: Novartis, Kite, a Gilead Company, Miltenyi Biotec (Inst), Takeda (Inst)
Research Funding: Kite, a Gilead company (Inst)
Travel, Accommodations, Expenses: Novartis, Kite, a Gilead Company, Roche, Celgene
No other potential conflicts of interest were reported.
REFERENCES
- 1.June CH, Sadelain M: Chimeric antigen receptor therapy. N Engl J Med 379:64-73, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schubert ML, Schmitt M, Wang L, et al. : Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol 32:34-48, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Locke FL, Ghobadi A, Jacobson CA, et al. : Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol 20:31-42, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster SJ, Bishop MR, Tam CS, et al. : Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 380:45-56, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Abramson JS, Palomba ML, Gordon LI, et al. : Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 396:839-852, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Jacobson CA, Chavez JC, Sehgal A, et al. : Outcomes in ZUMA-5 with axicabtagene ciloleucel (axi-cel) in patients (pts) with relapsed/refractory (R/R) indolent non-Hodgkin lymphoma (iNHL) who had the high-risk feature of progression within 24 months from initiation of first anti-CD20–containing chemoimmunotherapy (POD24). J Clin Oncol 39, 2021. (suppl 15; abstr 7515) [Google Scholar]
- 7.Wang M, Munoz J, Goy A, et al. : KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med 382:1331-1342, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maude SL, Laetsch TW, Buechner J, et al. : Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378:439-448, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah BD, Ghobadi A, Oluwole OO, et al. : KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: Phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 398:491-502, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munshi NC, Anderson LD, Jr, Shah N, et al. : Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 384:705-716, 2021 [DOI] [PubMed] [Google Scholar]
- 11.Berdeja JG, Madduri D, Usmani SZ, et al. : Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 398:314-324, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Greenbaum U, Strati P, Saliba RM, et al. : CRP and ferritin in addition to the EASIX score predict CAR-T-related toxicity. Blood Adv 5:2799-2806, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pennisi M, Sanchez-Escamilla M, Flynn JR, et al. : Modified EASIX predicts severe cytokine release syndrome and neurotoxicity after chimeric antigen receptor T cells. Blood Adv 5:3397-3406, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neelapu SS, Tummala S, Kebriaei P, et al. : Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities. Nat Rev Clin Oncol 115:47-62, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topp MS, van Meerten T, Houot R, et al. : Earlier corticosteroid use for adverse event management in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol 195:388-398, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden PJ, Roddie C, Bader P, et al. : Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann Oncol 33:259-275, 2022 [DOI] [PubMed] [Google Scholar]
- 17.Alexander M, Culos K, Roddy J, et al. : Chimeric antigen receptor T cell therapy: A comprehensive review of clinical efficacy, toxicity, and best practices for outpatient Administration. Transpl Cell Ther 27:558-570, 2021 [DOI] [PubMed] [Google Scholar]
- 18.Palomba ML, Jun MP, Lymp J, et al. : Postinfusion monitoring costs by site of care for patients with relapsed/refractory large B-cell lymphoma receiving third- or later-line treatment with lisocabtagene maraleucel in the TRANSCEND NHL 001 and OUTREACH trials. Leuk Lymphoma 62:2169-2176, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerjee R, Shah N, Dicker AP: Next-generation implementation of chimeric antigen receptor T-cell therapy using digital health. JCO Clin Cancer Inform 5:668-678, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Basch E, Deal AM, Kris MG, et al. : Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 34:557-565, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basch E, Deal AM, Dueck AC, et al. : Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 318:197-198, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DW, Santomasso BD, Locke FL, et al. : ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 25:625-638, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Morris EC, Neelapu SS, Giavridis T, et al. : Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol 22:85-96, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belin C, Devic P, Ayrignac X, et al. : Description of neurotoxicity in a series of patients treated with CAR T-cell therapy. Sci Rep 10:18997, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsey I, Eckert M, Hutchinson AD, et al. : Core outcome sets in cancer and their approaches to identifying and selecting patient-reported outcome measures: A systematic review. J Patient Rep Outcomes 4:77, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, et al. : The PRISMA 2020 Statement: An updated guideline for reporting systematic reviews. BMJ 29:372, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basch E, Reeve BB, Mitchell SA, et al. : Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 106:dju244, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romanelli D, Farrell MW: AVPU Score: StatPearls. Treasure Island, FL, StatPearls Publishing, 2022 [Google Scholar]
- 29.Nathan C, Jeune-Smith Y, Klinefelter P, et al. : Community oncologists’ perception and adaptability to emerging chimeric antigen T-cell (CAR-T) therapy. J Clin Pathways 3:31-35, 2017 [Google Scholar]
- 30.Gajra A, Jeune-Smith Y, Kish J, et al. : Perceptions of community hematologists/oncologists on barriers to chimeric antigen receptor T-cell therapy for the treatment of diffuse large B-cell lymphoma. Immunotherapy 12:725-732, 2020 [DOI] [PubMed] [Google Scholar]
- 31.Myers GD, Verneris MR, Goy A, et al. : Perspectives on outpatient administration of CAR-T cell therapy in aggressive B-cell lymphoma and acute lymphoblastic leukemia. J Immunother Cancer 9:e002056, 20212021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatwood KS, Doloria BR, Lucena M, et al. : Chimeric antigen receptor T-cell therapy: Challenges and framework of outpatient administration. EJHaem 3:3-5, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang XS, Srour SA, Whisenant M, et al. : Patient-reported symptom and functioning status during the first 12 months after chimeric antigen receptor T cell therapy for hematologic malignancies. Transplant Cell Ther 27:930.e1-930.e10, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]