Abstract
Excessive monitoring of one's performance is a characteristic of anxiety disorders that has been linked to alterations in implicit emotion regulation (ER), including elevations in neural measures of performance monitoring (i.e., error- and correct-related negativity; ERN and CRN). Elevations in ERN and CRN amplitudes have been reported consistently in anxiety disorders, suggesting that an overactive performance monitoring system is linked to ER difficulties in anxiety. Yet, the relevance of these lab-based neural measures for day-to-day emotional functioning remains poorly understood. This study examined the degree to which ERN and CRN amplitudes are associated with measures of daily ER difficulties in youth with anxiety disorders. Youth (N = 100, Mage = 11.14, SDage = 1.46) completed a computerized flanker task assessing the ERN and CRN. They then completed a 5-day ecological momentary assessment (EMA) protocol assessing their daily ER (i.e., intensity of momentary and peak negative affect, intensity of worry, reliance on maladaptive ER strategies). Results showed that more negative mean CRN amplitudes were associated with higher levels of negative emotional reactivity and more intense worries. There were no significant associations between ERN amplitude and EMA measures. Furthermore, elevations in CRN were linked to more frequent use of maladaptive ER strategies (i.e., rumination, physiological reactivity, avoidance). Together, results indicate that among youth with anxiety, individual differences in CRN, but not ERN, amplitudes are related to daily ER difficulties. Findings highlight the clinical utility of a lab-based neural measure of ER, suggesting that the CRN, rather than the ERN, reflects individual ER differences in the context of daily life among youth with pediatric anxiety disorders. As such, the CRN might serve as an important dimensional index of a treatment target that can be tracked with a validated, multi-method measure.
Keywords: Error-related negativity, Correct-related negativity, Pediatric anxiety disorders, EEG, Emotion regulation, Cognitive control, Ecological momentary assessment
Anxiety disorders are prevalent and impairing disorders that often onset in childhood and adolescence and heighten risk for later anxiety, mood, and substance use disorders (Kessler et al., 2005; Lonigan et al., 2004). Building on studies that link anxiety with alterations in emotion regulation (ER) (see Cisler and Koster, 2010, for review), researchers have sought to clarify the role that alterations in an implicit ER subprocess (Phillips et al., 2008), performance monitoring, plays in the development and maintenance of anxiety disorders (Meyer, 2017). Compared to healthy controls, youth and adults with anxiety disorders (Ladouceur et al., 2006; Meyer et al., 2013; Moser et al., 2013; Weinberg et al., 2010) exhibit heightened neural responses to errors (i.e., elevated error-related negativity, ERN), suggesting that the ERN could be a biomarker (Hanna et al., 2020; Meyer, 2017) of clinically-significant anxiety – anxiety levels that impact daily functioning. However, implications of variations in neural indices of performance monitoring on a day-to-day basis for anxious patients have not been determined, limiting our understanding of the generalizability and clinical significance of the ERN. We therefore sought to examine the degree to which ERN amplitude is associated with daily-life ER difficulties among youth diagnosed with anxiety disorders, measured by ecological momentary assessment (EMA) measures of emotional intensity, worry, and reliance on maladaptive ER strategies. We also sought to clarify whether this relationship was specific to error monitoring (i.e., the error-related negativity, or ERN), or whether neural activity following correct responses (i.e., the correct-related negativity, or CRN) was also associated with ER difficulties in daily life. Findings from this study are an important first step in understanding the implications of the ERN and/or CRN for characterizing variations in anxious youth's daily emotional functioning and for improving the treatment of anxiety disorders by targeting performance monitoring processes to enhance anxious youth's capacity for ER.
1.1. The ERN, CRN, and anxiety
The ERN and CRN are negative fronto-central deflections in the electroencephalogram (EEG) occurring within 100 ms of a response onset and are thought to be generated within a network of brain regions associated with performance monitoring that includes the anterior cingulate cortex (ACC), with the ERN following commission errors and the CRN following correct responses (Falkenstein et al., 1991; Gehring et al., 1993). Relative to the ERN, the CRN is typically a smaller and less negative deflection in the waveform (Allain et al., 2004; Burle et al., 2008; Vidal et al., 2000, 2003). Although the ERN has been consistently linked to performance monitoring, various theories exist regarding the specific functional role(s) of this neural index (Gehring et al., 2012). Some posit that the ERN reflects evaluative aspects of cognitive control (Yeung et al., 2004), while others consider it to reflect dopamine learning signals in the ACC (Holroyd and Coles, 2002), with the ERN serving as an early indicator for the need for adaptive behavior. That is, the ERN signals the need for adjustments in action, attention, and or emotional salience (Ullsperger et al., 2014).
With respect to anxiety and anxiety-related disorders (e.g., obsessive compulsive disorders, or OCD), an elevated ERN has been conceptualized as signaling heightened sensitivity to internal threat (Weinberg et al., 2015). AN elevated ERN has been associated with increased emotional arousal, or aversiveness, following error commission (Hajcak and Foti, 2008; Proudfit et al., 2013) as well as the recruitment of compensatory cognitive control processes (Moser, 2017). Each of these perspectives on why individuals with anxiety disorders exhibit an elevated ERN has implications for understanding the ER difficulties that individuals with anxiety experience in daily life. Notably, EMA studies have found that youth with anxiety report more intense negative emotional reactions to self-nominated stressors (Tan et al., 2012) and greater difficulty disengaging from repetitive negative thoughts (Newman et al., 2019), examples of emotional behaviors in daily life that might stem from increased sensitivity/reactivity to internal threat as well as deficits in cognitive control.
Although a large literature has examined the ERN in relation to anxiety (Meyer, 2017; Michael et al., 2021; Weinberg et al., 2015), fewer studies have specifically examined relations between CRN magnitude and anxiety. There is, however, some evidence to posit a similar, but less robust, association between an elevated CRN and clinical levels of anxiety (Michael et al., 2021; Moser et al., 2013). Nonetheless, much remains unknown about the role that the CRN might play in anxiety, especially in comparison to the number of studies linking the ERN to regulatory processes implicated in anxiety and anxiety-related disorders (e.g., Carthy et al., 2010; Joormann and Tanovic, 2015; Ochsner and Gross, 2005). Some theories suggest the CRN may be an artifact resulting from post-stimulus processing or partial error-processing (Coles et al., 2001). Others conceptualize the CRN as a meaningful signal, reflecting conflict resulting from a mismatch between one's intended and actual response (Luu et al., 2000), the degree to which a response was accurate but suboptimal (e.g., too slow), or uncertainty about the accuracy of one's response (Pailing and Segalowitz, 2004). An elevated CRN could, therefore, be attributed to an overactive performance monitoring system where all actions are excessively monitored due to heightened concerns about being correct (e.g., perfectionism) or an elevated negative self-focus (e.g., rumination). If this is the case, it is reasonable to expect that an elevated CRN could be associated with dimensions of anxiety such as worry and rumination.
Methodological factors, specifically the quantification of ERPs in terms of the difference in within-subject conditions (i.e., ERP activity following correct vs. erroneous responses), may have also obscured our understanding of the degree to the ERN-anxiety association is attributable to alterations in error processing. Difference-based quantification methods have been used to isolate neural activity associated with one condition relative to another in ERP studies (Luck, 2005). As such, a common conclusion of ERP studies using a subtraction-based difference score (ΔERN or ERNMamplitude – CRNMamplitude) to study anxious vs. nonanxious group differences in performance monitoring is that individuals with elevated-to-clinically levels of anxiety have an overactive error detection system. Although informative of an association between error monitoring and anxiety, subtraction-based difference scores do not assess the degree to which individual differences in performance monitoring processes following correct responses might also contribute to individual differences in anxiety. Indeed, there is some evidence to suggest that individual differences in ΔERN are driven by variability in the CRN rather than the ERN (Grammer et al., 2014; Ladouceur et al., 2018), underscoring the importance of examining both the CRN and ERN when investigating associations between individual differences in neurophysiological indices of performance monitoring and behavior. Moreover, because the ΔERN typically correlates with both the ERN and CRN in inverse directions, it is difficult to determine the relative contribution of the CRN vs. ERN (see Meyer, 2017). Consequently, there is a need to separately examine CRN and ERN amplitudes to better understand the aspects of performance monitoring that contribute to anxious symptoms.
Taken together, there have been significant advances in our understanding of how the ERN, and to a lesser degree the CRN, marks clinical vs. non-clinical group differences in anxiety severity. These advances have significant implications for identifying clinical risk in terms of diagnostic status and the development of anxiety disorders (Meyer et al., 2018). However, variability in ERN and CRN amplitude among youth diagnosed with anxiety disorders could have important implications for understanding heterogeneity in this population. Moreover, because much of this line of research has focused on links between neural indices of performance monitoring and clinical symptoms obtained from clinical interviews or self-report rating scales, we have a limited understanding of the daily life significance of alterations in neural indices of performance monitoring (i.e., ERN and CRN) obtained from laboratory tasks as they are implemented in daily life. That is, does an over-active performance monitoring system translate to heightened emotional reactivity or maladaptive ER experienced by youth as they partake in daily activities in the rich context of their natural social environments?
Among youth with impairing levels of anxiety, an elevated ERN in the lab might, for example, reflect heightened catastrophizing of mistakes (“People are going to think I'm the worst student in the class”; “No one will talk to me after I trip on the stairs”). More negative CRN amplitudes might, on the other hand, reflect a negative self-focus about the possible correctness and/or appropriateness of one's behavior (“What if my response is actually wrong?”; “What if they were laughing at me, not at my joke?”). That is, CRN amplitude might track with an aspect within the distress dimension of anxiety – a tendency to worry. Although other clinical or self-report measures may also provide some indication of how neural indices of performance monitoring relate to clinically relevant functional domains, individuals (especially youth) may be less accurate when reporting on patterns of emotional reactivity and maladaptive ER retrospectively. Further, these rating scale measures tend to reflect broader trait tendencies to use certain types of strategies that may not reflect ER as it is naturalistically deployed in the context of day-to-day life (see McMahon and Naragon-Gainey, 2020).
1.2. Linking the ERN/CRN with emotional functioning in daily life
To our knowledge, only three studies have examined associations between the ΔERN and daily emotional functioning. Both were conducted in community samples. First, Compton et al. (2008) found that a larger ΔERN was associated with lower levels of stress reactivity, suggesting that elevated (i.e., more negative) ERNs reflect higher levels of cognitive control that also contributed to enhanced ER in adults without anxiety disorders. Second, in a follow-up study of undergraduates with elevated depressive symptoms, Compton et al. (2011) identified a trend relationship between an elevated ΔERN and reduced stress reactivity, supporting the view that an elevated ΔERN reflects higher levels of evaluative cognitive control that aid stress regulation in daily life. Third, a recent study (Overmeyer et al., 2021) reported a similar pattern of findings using a sophisticated analytic approach to identify the neurophysiological measures of performance monitoring (ERN, CRN, and ΔERN amplitudes from fronto-central electrodes quantified across the response-locked ERP waveform) that explained the most variance in self-control among a community sample of adults. Self-control, conceptualized as an aspect of self-regulation, was defined as the ability to resist a conflict-laden desire (e.g., a desire that conflicts with a long-term goal or personal standard) and was assessed using EMA. Findings from the study showed that attenuated (i.e., smaller or less negative) ΔERNs and ERN amplitudes were associated with more frequent self-control failures. Together, these findings are consistent with the view that among adults who report no psychopathology, larger ERNs reflect effective cognitive and emotion regulation (i.e., higher levels of self-control).
How, then, might researchers and clinicians understand the apparent discrepancy in the ERN-adaptive self-regulation association between individuals with normative (i.e., Compton et al., 2008, 2011; Overmeyer et al., 2021) vs. clinical levels of anxiety (see Moser et al., 2016; Weinberg et al., 2015)? That is, if more negative ERN amplitudes reflect better self-regulation processes, then why might an elevated ERN be associated with problematic and clinical levels of anxiety when evidence suggests that anxiety disorders are characterized by altered cognitive and emotional regulation (Cisler and Olatunji, 2012; Mennin et al., 2002; Eysenck et al., 2007)? One way researchers have tackled this issue is by conceptualizing the ERN as an early signal of the need for adaptive control (Ullsperger et al., 2014). The regulatory processes that occur following the early ERN warning signal, however, might not be effectively and/or efficiently executed (Moser et al., 2013) or, perhaps, even misapplied and result in maladaptive behaviors (Overmeyer et al., 2021).
Indeed, researchers have suggested that larger, more negative ERN amplitudes might not be related to adaptive behaviors among individuals with OCD (Gillan et al., 2017) because ERN amplitude could reflect misguided regulatory processes that transform a typical behavior (e.g., checking the locks) into an unhealthy compulsion (checking the locks 50×/day). Likewise, it is possible that larger ERN amplitudes among youth with clinical levels of anxiety could reflect misguided regulatory processes that transform a typical thought (“I didn't do well on that test”) into an intense and frequent worry (“I failed the course and will be held back a grade”). As such, one might expect that youth with impairing levels of anxiety who exhibit elevations in neural indices of performance monitoring (i.e., elevated, more negative ERN and CRN) would experience greater difficulties in ER in daily life relative to those with attenuated ERN and CRN amplitudes.
In the present study, we focused on early adolescence when rates of generalized and social anxiety disorders begin to increase (Beesdo et al., 2009; Kessler et al., 2005). Consistent with prior research in pediatric anxiety, we examined neural indices of performance monitoring in youth diagnosed with generalized anxiety (GAD), social anxiety (SocAD), and/or separation anxiety disorder (SAD) using an archival study examining treatment-related changes in neural and behavioral correlates of pediatric anxiety disorders (Ladouceur et al., 2018). Specifically, we examined, in youth with these anxiety disorders, the degree to which the ERN and CRN were related to daily ER difficulties using ecological momentary assessment (EMA) to measure youth's negative emotion, reliance on maladaptive ER behaviors such as avoidance, rumination, or physiological arousal, and level of worry. Considering theories suggesting that among individuals with anxiety disorders, larger (i.e., more negative) ERN amplitudes reflect greater negative reactivity to errors (Proudfit et al., 2013) and/or inefficient, or compensatory, processes (Moser et al., 2013), it is expected that elevations in ERN amplitude would be associated with ER difficulties (i.e. experience of more intense negative emotions, heightened negative emotional reactivity) in daily life among youth diagnosed with anxiety disorders. We also explored a secondary hypothesis that more negative CRN amplitudes would be associated with higher intensity worries in the daily life of youth with anxiety.
2. Methods
2.1. Participants
Participants (N = 100; 9–14 years) included youth with anxiety who provided good quality data from the Flanker Task (see Supplementary information: Fig. 1), defined as participants who showed accuracy ≥70 % and who, following initial visual inspection of EEG data had fewer than 11 electrode channels and/or the FCz or Fz electrode channels rejected for poor data quality. Youth were a subset of participants recruited as part of a randomized clinical trial study examining the neurobehavioral mechanisms of individual treatment in pediatric anxiety disorders (Silk et al., 2016). All participants met DSM-IV criteria for current Generalized Anxiety Disorder (GAD), Social Anxiety Disorder (SocAD), and/or separation anxiety disorder (SAD). Exclusion criteria included: IQ < 70 (assessed via the Block Design and Vocabulary subtests of the Wechsler Abbreviated Scale of Intelligence; WASI), use of psychoactive medications, presence of neurological impairments, current primary diagnosis of major depressive disorder and other current (e. g., PTSD) or lifetime (e.g., psychosis) Axis-I diagnoses. Table 1 outlines participant sociodemographic and clinical characteristics.
Table 1.
Socio-demographic and clinical characteristics.
| Variable | Youth with anxiety disorders (n = 100) |
|---|---|
| Age | 11.14 (1.46) |
| Female, [n] | 54 |
| Caucasian, [n] | 88 |
| Head of household, education, [n] | |
| Some high school | 1 |
| High school graduate | 8 |
| Some college | 17 |
| College graduate | 40 |
| Graduate professional training | 34 |
| Current diagnosisa, [n] | |
| Generalized Anxiety Disorder | 71 |
| Social Anxiety Disorder | 21 |
| Separation Anxiety Disorder | 23 |
| Specific phobia | 12 |
| Major Depressive Disorder | 1 |
| ADHD (inattentive type) | 1 |
| ODD | 2 |
| Tic disorder | 4 |
Diagnostic groups are partially overlapping due to inclusion of comorbid patients. Primary/principle diagnoses were not designated, meaning that percentages for the 3 diagnostic inclusion groups will not sum to 100.
2.2. Procedures
Study procedures, including obtaining written informed consent from the primary caregiver and written assent from the participant, were approved by the University of Pittsburgh's Institutional Review Board. Interviews and rating scales were administered to the child and his/her primary caregiver before and after treatment by an independent evaluator. Following the intake assessment, participants completed an EEG assessment that assessed brain activity following responses using event-related potentials (ERPs) as well as an ecological momentary assessment (EMA) protocol to assess youth's daily emotional reactivity and regulation in their natural social environments. This study uses archival data to examine associations between ERP and EMA measures obtained prior to starting treatment. Results examining treatment-related changes in these measures have been previously reported (Ladouceur et al., 2018).
2.3. Assessment of clinical functioning
The Schedule for Affective Disorders and Schizophrenia in School-Age Children—Present and Lifetime Versions (Kaufman et al., 1997) was administered by independent evaluators to determine the presence of other Axis-I disorders. Parents and youth were interviewed separately, with independent evaluators (trained BA- and MA-level research clinicians) integrating data from both informants to arrive at final diagnoses (Silk et al., 2016). Inter-rater reliability between interviewers was calculated for 16 % of interviews, with high reliability for anxiety diagnoses (Kappa = 0.97).
2.4. Ecological momentary assessment
2.4.1. EMA protocol
We used a cellphone methodology developed for collecting EMA data on youths' day-to-day emotional functioning (Tan et al., 2012). In brief, youth received two calls each day on weekdays and four calls each day on weekends, for a total of 14 calls across 5 days. Calls occurred randomly within pre-specified 3-hour time windows and were conducted by trained research assistants. During each call, youth were asked to rate their negative emotions (herein referred to as momentary negative emotion (momentary NE)) and level of worry at the time of the call (herein referred to as momentary worry); they were also asked to nominate the most negative event that occurred within the past hour, even if they were minor events. Youth were then asked to rate emotions associated with their self-nominated “worst” event (herein referred to as peak negative emotion (peak NE) and their behavioral responses to that event (herein referred to as ER behaviors; see Silk et al., 2003). This 5-day protocol was repeated throughout treatment, but data here focus only on the pre-treatment EMA assessment.
Based on previous research, momentary and peak emotion ratings were made using four negative emotions (nervous, upset, sad, angry) (Laurent et al., 1999; Silk et al., 2003). Emotions and worries were rated on a scale from 1 (very slightly or not at all) to 5 (extremely). The ER behaviors assessed were taken from a previous EMA study (Silk et al., 2011), which was adapted from the Responses to Stress Questionnaire (Connor-Smith et al., 2000). Consistent with prior work, youth could endorse multiple ER behaviors for the same event (Silk et al., 2011). Dichotomous variables indicating the presence or absence of each ER behavior were used to create composite variables indexing the proportion of negative events in which youth used strategies that the literature has classified maladaptive (i.e., avoidance, rumination, physiological response) (Connor-Smith et al., 2000; Hampel and Petermann, 2005). Overall, the compliance rate for the EMA protocol was high, with youth completing an average of 91 % of phone surveys (M = 12.74/14 surveys, SD = 1.46, ranging from 7 to 14); EMA compliance rates did not differ for youth with and without SocAD (t = 0.27, p = .39).
2.5. Performance monitoring ERPs
2.5.1. Eriksen flanker task
An arrow version of the flanker task was administered using E-prime 2.0 software (Eprime Inc., Pittsburgh, PA). As previously described (Ladouceur et al., 2006, 2018), the task included presentation of five horizontal arrows, with 50 % congruent trials and 50 % incongruent trials. Participants were asked to respond as fast and as accurately as possible on a keyboard using their right and left index fingers according to the direction of the central arrow. Additionally, to encourage both fast and accurate responding, participants received feedback based on their performance at the end of each block. If performance was 75 % correct or lower, the message “Please try to be more accurate” was displayed; performance above 80 % correct was followed by “Please try to respond faster”; otherwise, the message “You're doing a great job” was displayed. The task comprised of 3 blocks of 200 trials each, with 30 practice trials. All stimuli were presented for 200 ms followed by an inter-trial interval that varied randomly between 500 and 1500 ms, during which a fixation cross was presented.
2.5.2. EEG data acquisition and processing
Continuous EEG activity was recorded using an the ActiveTwo 128-channel BioSemi system (BioSemi, Amsterdam, The Netherlands) sampled at 512 Hz. BioSemi ActiveTwo hardware is DC-coupled and applies digital low-pass filtering through its ADC's decimation filter which has a fifth-order sync response with a 3 dB point at one-fifth of the sample rate (i.e., approximating a low-pass filter at 100 Hz). An elastic Active Two Lycra head cap was placed on the youth's head and 128 Ag/AgCl-tipped electrodes were attached to the cap. Two electrodes were placed at supra and infra orbital sites of the right eye to monitor vertical eye movements and 2 on the outer canthi of the left and right eyes to monitor horizontal eye movements. In addition, 2 electrodes were placed on the mastoids (right and left) and 1 on the tip of the nose.
Offline, all data processing was performed using Brain Electrical Signal Analysis (BESA) software. Although EEG data were recorded using the tip of the nose as the reference (Light et al., 2010), the ActiveView system saves raw reference-free EEG data. Offline, data were re-referenced to the tip of the nose and filtered using Butterworth zero-phase filters, with a high-pass filter at 0.01 Hz with a cutoff slope of 6 dB/oct and a low-pass filter at 30 Hz with a cutoff slope of 24 dB/oct. A semi-automated pre-processing procedure was used to reject bad channels and trials with significant signal artifact. After visual inspection to identify bad channels, segments were extracted from the continuous EEG, from 200 ms prior to correct and erroneous responses to 800 ms following responses. ERP data were corrected for blinks and eye-movements using the method developed by Gratton et al. (1983). Research indicates that data that the ERN is stable after 6 trials (Olvet and Hajcak, 2009). For this study, ERN data was excluded from analyses if the EEG was contaminated by excessive artifact or the participant had fewer than 10 errors in order to better ensure more reliable signals within a sample of youth with anxiety disorders. As detailed in Ladouceur et al. (2018), a semi-automatic procedure was used to detect and reject artifacts according to the following criteria: a voltage step of >50 μV between data points, a voltage gradient of 150 μV within trials, a signal of <0.1 μV across the trial, or reaction times occurring outside of a 100–2000 ms window. Visual inspection of the data served to detect and reject any remaining artifacts. As a result, of the 130 participants with anxiety who completed the baseline ERP assessment as part of their participation in the larger RCT, 30 youth were excluded from analyses.
2.5.3. EEG data reduction and analyses
To quantify the response-locked ERPs, averages were computed separately for correct and error trials. Baseline correction was applied by subtracting from each data point the average activity in a −150 to −50 ms window prior to the response. Following artifact rejection, an average of 46 (94.6 % of total possible trials) error trials and 520 (94.51 % of total possible correct trials) correct trials were included in EEG analyses A paired-sample t-test showed that the two conditions (error and correct) did not significantly differ with respect to the percentage of trials retained, t = 0.130, p = .90. The ERN and CRN were scored as the average activity on error and correct trials, respectively, from 0 to 90 ms window after response onset at scalp site FCz, where error-related brain activity was maximal. Decisions regarding the choice of baseline and quantification of average ERN/CRN activity were informed by pervious work on the ERN in anxious clinical populations (e.g., Hajcak et al., 2005; Ridderinkhof et al., 1997; Botvinick et al., 2004) and an aim to be consistent with previously published findings reporting on the ERN from this sample (Ladouceur et al., 2018).
3. Results
3.1. Preliminary analyses
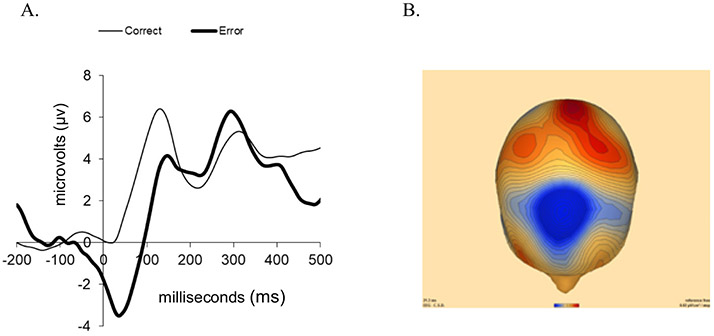
Descriptive statistics for behavioral performance on the Flanker task and main study variables (i.e., ERN/CRN mean amplitude; EMA ratings of momentary NE, peak NE, worry intensity, and reliance on maladaptive ER behaviors) can be found in Table 2. Age and sex were included as covariates based on previous research showing age and sex differences in anxiety symptoms and ERN magnitude (e.g., Grammer et al., 2014; McLean et al., 2011; Larson et al., 2011; Lewinsohn et al., 1998; Lo, 2018; Santesso et al., 2011). After controlling for age and sex, there were expected trial type and response type main effects, indicating that mean reaction times were faster for incorrect than correct responses (F = 12.12, df = 1,97, p < .01, ) and that on correct trials, mean reaction times were slower for the incongruent than congruent trial types (F = 11.14, df = 1,97, p < .01, ) and that. Post-error slowing, calculated as the average difference in reaction times from trials subsequent to an error compared to reaction times in trials following correct trials, was also marginally greater for incongruent trials than congruent ones (F = 207.09, df = 1,97 p = .03, ). Fig. 1 presents response-locked ERP data for correct and error trials at FCz for youth with anxiety (N = 100), which shows that ERP amplitude was significantly more negative for error compared to correct trials (F = 5.12, df = 1,97, p = .03, ). As shown in Table 3, we also examined bivariate associations between the two ERP indices of performance monitoring, ERN and CRN mean amplitude, and behavioral performance variables from the Flanker task; there were no significant associations between ERP and behavioral performance variables. All analyses were performed using SPSS 25.
Table 2.
Summary of behavioral performance, event-related potential (ERP), and ecological momentary assessment (EMA) measures.
| Variables | Mean (SD) |
|---|---|
| Reaction time (milliseconds) | |
| Overall | 526.16 (100.14) |
| Correct | 537.56 (99.04) |
| Error | 426.47 (115.80) |
| Congruent | 500.09 (95.13) |
| Incongruent | 552.24 (107.25) |
| Post-error slowing, overall | 114.25 (7.95) |
| Post-error slowing, congruent | 102.79 (11.86) |
| Post-error slowing, incongruent | 125.91 (8.53) |
| Percentage of errors | |
| Overall | 8.53 (4.62) |
| Congruent | 3.89 (3.50) |
| Incongruent | 13.14 (6.47) |
| ERP mean amplitude at FCz (microvolts) | |
| Correct trials | 1.35 (2.37) |
| Error trials | −2.47 (3.36) |
| ERP mean peak latency at FCz (milliseconds) | |
| Correct trials | 22.81 (19.17) |
| Error trials | 46.81 (27.31) |
| EMA data | |
| Momentary negative emotional intensity (angry, sad, nervous, and upset at time of call) | 1.32 (0.33) |
| Worry intensity | 3.17 (0.97) |
| Peak negative emotional intensity (angry, sad, nervous, and upset during a recent stressor) | 2.28 (0.58) |
| Reliance on maladaptive ER behaviors (% of total ER behaviors) | 33.99 (10.21) |
Note: ERP: event-related potentials; EMA: ecological momentary assessment, ER: emotion regulation.
Fig. 1.
A. Grand average event-related potential (ERP) waveforms are plotted at FCz following correct and error responses in anxious youth (n = 100). B. Topographic current source density (CSD) maps display the projection of the currents on the scalp surface after onset of incorrect responses at their maximal peaks between 0 and 90 ms (note: blue = more negative; red = more positive; reference free, 0.02 μV/cm2/step).
Table 3.
Bi-variate correlations between ERP mean amplitude and behavioral performance measures on the flanker task.
| ERP measure |
Flanker behavioral performance variable | ||||
|---|---|---|---|---|---|
| % Correct |
RT, correct trials |
RT, incorrect trials |
Post error slowing, congruent trials |
Post error slowing, incongruent trials |
|
| CRN | 0.02 | 0.09 | 0.09 | 0.07 | 0.06 |
| ERN | −0.16 | 0.20 | 0.21 | 0.13 | −0.01 |
Notes: ERP: event-related potential; ERN: error-related negativity; CRN: correct-related negativity; RT: reaction time.
3.2. Associations between performance monitoring ERPs and EMA measures
Because EMA data have a two-level hierarchical structure, with calls nested within individual, associations between ERP indices of performance monitoring and EMA indices of daily emotional reactivity and regulation were analyzed using multi-level linear mixed (MLM) models that included momentary negative affect, worry, and peak negative affect as time-varying dependent variables and ERN and CRN mean amplitudes independent variables as fixed effects at level-2 of the model. Continuous predictors (i.e., ERN, CRN, age) were centered at the sample mean; time was linearly entered as a fixed effect with t = 0 defined as the first EMA assessment (i.e., first EMA phone call). Age and sex were included as covariates at level-2 because of research showing that sex differences in anxiety levels (Lewinsohn et al., 1998; McLean et al., 2011) as well as sex (e.g., Larson et al., 2011) and age-related differences in ERN magnitude (e.g., Grammer et al., 2014; Lo, 2018; Santesso et al., 2011). All models included a random intercept and a within-group correlation structure of the form autoregressive order 1 (AR1) was used because calls occurred on consecutive days, resulting in correlated residuals that were expected to show decreasing dependency within increasing time lags.
Three MLM models examining associations between ERN and CRN amplitude and (1) momentary NE, (2) worry, and (3) peak NE showed that, after controlling for age and sex, there were no significant associations between individual differences in ERN amplitude and individual differences in any of the three EMA indices of daily emotional reactivity and regulation (all p's > 0.20). In contrast, greater (more negative) CRN amplitudes were significantly associated with individual differences in anxious youth's peak negative emotion (B = −0.05, t = −02.60, p = .01) and worry (B = −0.11, t = −3.22, p < .01). Specifically, for every 1 unit decrease in CRN amplitude, it expected that youth in this study experience an increase of 0.05 units in level of peak NA and an increase of 0.11 units in worry intensity. There was no significant association between CRN amplitude and momentary NE. Results from the MLM models can be found in Table 2. Associations between CRN amplitude and both peak NE (corrected p = .02) and worry intensity (corrected p = .01) remained significant following multiple comparison correction using FDR (Benjamini and Hochberg, 1995). Results from the MLM models can be found in Table 4. Overall, MLM results indicated that among youth with anxiety disorders, elevated (more negative) CRN amplitudes were associated with greater negative emotional reactivity (i.e., higher levels of negative emotion reported following a self-nominated stressor) as well as more intense worries.
Table 4.
Summary of results from multi-level models testing associations between CRN and ERN mean amplitudes and EMA indices of emotional functioning in youth diagnosed with anxiety disorders.
| B | SE | t | p | |
|---|---|---|---|---|
| Momentary NE | ||||
| Intercept | 1.28 (1.20; 1.36) | 0.04 | 30.50 | <0.001 |
| Time | 0.01 (−0.00; 0.02) | 0.01 | 1.15 | 0.252 |
| Age | 0.02 (1.20; 1.36) | 0.0 | 1.48 | 0.141 |
| Sex | 0.02 (−0.01; 0.11) | 0.04 | 0.48 | 0.630 |
| CRN | −0.01 (−0.02; 0.01) | 0.01 | −0.75 | 0.456 |
| ERN | 0.01 (−0.00; 0.02) | 0.01 | 0.97 | 0.334 |
| Worry | ||||
| Intercept | 3.09 (2.82; 3.35) | 0.13 | 23.00 | <0.001 |
| Time | 0.04 (0.01; 0.07) | 0.01 | 2.60 | 0.010 |
| Age | −0.09 (−0.18; 0.01) | 0.05 | −1.82 | 0.072 |
| Sex | 0.20 (−0.08; 0.48) | 0.14 | 1.41 | 0.160 |
| CRN | −0.11 (−0.18; −0.04) | 0.03 | −3.22 | 0.002 |
| ERN | 0.02 (−0.03; 0.06) | 0.02 | 0.85 | 0.399 |
| Peak NE | ||||
| Intercept | 2.29 (2.14/2.44) | 0.07 | 29.67 | <0.001 |
| Time | 0.01 (−0.01; 0.02) | 0.01 | 0.99 | 0.324 |
| Age | 0.00 (1.20; 1.36) | 0.03 | 0.10 | 0.920 |
| Sex | −0.13 (−0.29; 0.03) | 0.08 | −1.57 | 0.118 |
| CRN | −0.05 (−0.08; −0.01) | 0.01 | −2.77 | 0.006 |
| ERN | 0.02 (−0.01; 0.04) | 0.01 | 1.26 | 0.207 |
Notes: ERN: error-related negativity; CRN: correct-related negativity; EMA: ecological momentary assessment; NA: negative affect; B = unstandardized parameter estimate; SE = standard error, t = t-statistic; p = p-value. Age, CRN, and ERN were centered at the sample mean; time was centered at the start of the EMA assessment. Bolded values indicate p<.05.
A similar pattern of results was found for an ordinary least squares regression (OLS) model examining associations between ERN and CRN amplitude and the use of different ER strategies in youth diagnosed with anxiety disorders. Results showed that after controlling for age and sex, higher (more negative) CRN amplitudes (B = −0.27, B = −0.01 [−0.02; −0.00], t = −2.63, p = .01) were associated with greater reliance on maladaptive ER strategies (F = 3.20, df = 4,95, p = .02, R2 = 0.12). ERN mean amplitudes (B = 0.14, B = 0.00[−0.00; 0.01]; t = 1.36, p = .20) were not significantly associated with youth's reliance on maladaptive ER strategies.
The above MLM analyses were repeated with models that included an interaction term ERN × CRN exploring the moderating role of CRN amplitude on ERN amplitude. The interaction term was not significant in any model (see Supplemental materials, Table S1). Thus, we present results from the more parsimonious model excluding the interaction term. Finally, an additional three MLM models examined 2-way interactions between time and ERN/CRN amplitude to test the degree to which ERN and/or CRN amplitude might moderate change in worry or in momentary or peak NE. Specifically, time × ERN and time × CRN interaction variables were included as fixed effects on level 2 of the models predicting. Results from MLM models showed that neither ERN nor CRN amplitude moderated change in level of worry or negative emotionality for anxious youth (see Table 5); this pattern of results is consistent with the view that more negative CRN amplitudes are generally associated with ER difficulties that youth with anxiety disorders experience in daily life.
Table 5.
Summary of results from multi-level models testing 2-way Time × CRN and ERN mean amplitudes effects in EMA indices of emotional functioning in youth diagnosed with anxiety disorders.
| B | SE | t | p | |
|---|---|---|---|---|
| Momentary NE | ||||
| Intercept | 1.28 (1.20; 1.36) | 0.04 | 30.50 | <0.001 |
| Time | 0.01 (−0.00; 0.02) | 0.01 | 1.15 | 0.252 |
| Age | 0.02 (1.20; 1.36) | 0.0 | 1.48 | 0.141 |
| Sex | 0.02 (−0.01; 0.11) | 0.04 | 0.48 | 0.630 |
| CRN | −0.01 (−0.02; 0.01) | 0.01 | −0.75 | 0.456 |
| ERN | 0.01 (−0.00; 0.02) | 0.01 | 0.97 | 0.334 |
| Time × CRN | −0.00 (−0.00; 0.00) | 0.00 | −0.08 | 0.937 |
| Time × ERN | −0.00 (−0.00; 0.00) | 0.00 | −0.86 | 0.390 |
| Worry | ||||
| Intercept | 3.09 (2.82; 3.35) | 0.13 | 22.87 | <0.001 |
| Time | 0.04 (0.01; 0.07) | 0.02 | 2.60 | 0.010 |
| Age | −0.09 (−0.18; 0.01) | 0.05 | −1.82 | 0.072 |
| Sex | 0.21 (−0.08; 0.49) | 0.14 | 1.46 | 0.148 |
| CRN | −0.11 (−0.23; 0.01) | 0.06 | −1.87 | 0.060 |
| ERN | 0.00 (−0.08; 0.08) | 0.04 | 0.20 | 0.984 |
| Time × CRN | 0.00 (−0.01; 0.01) | 0.01 | 0.58 | 0.954 |
| Time × ERN | −0.00 (−0.01; 0.01) | 0.00 | 0.61 | 0.546 |
| Peak NE | ||||
| Intercept | 2.29 (2.14; 2.44) | 0.08 | 29.61 | <0.001 |
| Time | 0.01 (−0.01; 0.03) | 0.01 | 0.99 | 0.352 |
| Age | 0.00 (−0.05; 0.05) | 0.03 | 0.10 | 0.920 |
| Sex | −0.13 (−0.29; 0.03) | 0.08 | −1.58 | 0.116 |
| CRN | −0.05 (−0.11; 0.01) | 0.03 | −1.59 | 0.112 |
| ERN | 0.02 (−0.02; 0.07) | 0.02 | 1.13 | 0.262 |
| Time × CRN | −0.00 (−0.01; 0.01) | 0.00 | −0.46 | 0.963 |
| Time × ERN | −0.00 (−0.01; 0.00) | 0.00 | −0.47 | 0.638 |
Notes: ERN: error-related negativity; CRN: correct-related negativity; EMA: ecological momentary assessment; NA: negative affect; B = unstandardized parameter estimate; SE = standard error, t = t-statistic; p = p-value. Age, CRN, and ERN were centered at the sample mean; time was centered at the start of the EMA assessment. Bolded values indicate p<.05.
4. Discussion
Findings from this study contribute to a deeper understanding of the clinical implications of neural indices of performance monitoring for youth diagnosed with anxiety disorders (i.e., separation anxiety disorder, generalized anxiety disorder, social anxiety disorder, and/or specific phobia), providing some of the first evidence of relations between laboratory-based neural measures and behavioral measures of anxious youth's day-to-day emotional reactivity and regulation. Contrary to our hypothesis, more negative ERN amplitudes were not associated with increased levels of negative emotional reactivity and ER difficulties in daily life. Rather, we found that more negative CRN mean amplitude is linked to higher levels of peak negative emotion and a greater reliance on maladaptive ER strategies). We also found, as hypothesized, a relationship between CRN amplitude and worry intensity. Together, findings indicate that the CRN amplitude, rather than ERN amplitude, reflects individual differences in the day-to-day emotional reactivity and regulation of clinically-anxious youth, suggesting that the CRN may be particularly relevant for assessing – and understanding – variabilities in clinical presentation and impairment in pediatric anxiety disorders.
The absence of significant associations between ERN amplitude and daily emotional functioning was unexpected but may shed light on the pathophysiology and etiological processes that underlie pediatric anxiety disorders. As highlighted by previous studies of associations between neurophysiological measures of performance monitoring and behavioral measures of self-regulation in non-clinical samples (Compton et al., 2008; Overmeyer et al., 2021), the ERN may reflect evaluative aspects of error monitoring that signal need for enhanced cognitive control (Botvinick et al., 2001; Holroyd and Coles, 2002). However, the behavioral changes that involve the cognitive control subprocesses that are, in part, recruited by the ERN signal may not necessarily be adaptive (Inzlicht et al., 2015). It is possible that among clinically anxious individuals, who are thought to experience greater aversion to errors (Weinberg et al., 2015), the ERN signal may contribute to maladaptive regulatory processes, for example, over-regulation (e.g., freezing, avoidance, overcorrection), rather than adaptive behavior.
The lack of an ERN-anxiety association within our sample of youth with an anxiety disorder is also broadly consistent with other studies reporting null associations between ERN amplitude and measures of anxiety severity in clinical samples (for a review, see Meyer, 2017). However, when considering links between the ERN and day-to-day emotional functioning, it is perhaps unsurprising that a measure of error-related neural activity obtained from a situation that likely elicits a high degree of self-consciousness from everyone – performing a task while being carefully observed in a research laboratory – was unrelated to variations in daily functioning of youth with anxiety. On the other hand, because the CRN is posited to reflect higher levels of uncertainty and/or concern about one's performance (Pailing and Segalowitz, 2004; Simons, 2010), elevations in CRN amplitude could be associated with ER behaviors in daily life that involve a preoccupation with potentially negative outcomes.
A negative self-focus, particularly regarding potential threats to self, is a hallmark of anxiety disorders. Many youth with anxiety disorders are concerned with making good impressions, following the rules, and staying away from dangers that range from social embarrassment to dog bites (Kendall et al., 2010; Silverman et al., 1995) – behaviors that are supported by enhanced monitoring of one's actions that likely automatically occur even during simple tasks that have a “correct” or “incorrect” behavior. Thus, as a neural signal of enhanced performance monitoring (even) when one is correct, it is perhaps unsurprising that individual differences in the CRN, rather than the ERN, are associated with EMA measures reflecting day-to-day worry tendencies including heightened negative emotionality, greater use of rumination and avoidance as ER strategies, as well as more intense worries.
The idea that the CRN captures individual differences in the distress dimension of anxiety, such as a tendency to worry, is also consistent with results linking elevated CRN amplitudes with elevations in checking symptoms in obsessive compulsive disorder (e.g., Endrass et al., 2010; Riesel et al., 2014) and perfectionism (Meyer and Wissemann, 2020). There was also no evidence that CRN amplitude moderated change in level of negative emotional reactivity or worry in anxious youth's daily lives, suggesting that CRN is generally associated with difficulties in ER that are reflected in behavioral symptoms of anxiety and anxiety-related disorders. Additionally, research has shown that the ΔERN, an ERP measure that includes CRN amplitude, increases when there is greater uncertainty in a task (Jackson et al., 2016), providing further support for a link between the CRN and performance-related worries. Thus, clarifying the role of CRN in patterns of ER observed in youth with anxiety could yield new information about the heterogeneity in pediatric anxiety and neurodevelopmental mechanisms. For example, further study is needed to determine why variations in the CRN are linked to maladaptive ER in anxiety, including the possibility that CRN magnitude reflects (a) the degree to which youth with anxiety experience concerns and/or uncertainty about “doing a good job” interferes with ER, (b) whether CRN amplitude is specifically associated with performance-related worries vs. more generally related to all worries, and (c) if the CRN-worry association is observed only within pediatric anxious populations. Future research comparing ERN-EMA associations in youth without anxiety disorders or with other emotional disorders is also needed to clarify the functional roles of the CRN and ERN.
It is also noteworthy that findings from the current study differ from those of other studies linking ERP indices of performance monitoring and EMA indices of emotional reactivity and regulation. Unlike Compton et al. (2008, 2011) who found that greater ΔERN was associated with adaptive ER, specifically reduced stress reactivity, in community samples of young adults, we found that elevated CRN amplitude was associated with measures that likely reflect maladaptive ER processes in youth diagnosed with anxiety disorders, such as heightened negative reactivity to daily stressors, more intense worries, and a greater reliance on maladaptive ER strategies. Such discrepant findings could be attributed to differences in sample characteristics (e.g., age, severity of anxiety, severity of co-occurring depression and/or ADHD) or the paradigm used to assess neural indices of performance monitoring (Boen et al., 2021). The pattern of results from our study suggests that there may be a threshold of clinical significance (i.e., typical vs. problematic anxiety) where monitoring of one's performance hinders, rather than supports, one's ability to use performance-related feedback to regulate behavior. An additional interpretation to explore is the degree to which alterations in the ERN may contribute to inefficient, ineffective, or maladaptive regulatory behaviors (Inzlicht et al., 2015). Although we did not find that an interaction between ERN and CRN amplitude predicted variance in clinically anxious youth's day-to-day functioning, highlighting the need to for future research with larger samples to investigate multivariate, temporal associations between different aspects of cognitive control processes and day-to-day functioning in youth with and without anxiety disorders.
A strength of the current study is the use of a relatively large, well-characterized sample of youth with an anxiety disorder. Further, the study included EMA measures of daily emotional reactivity and regulation obtained from youth's natural social environments to assess clinical functioning. To our knowledge, this is the first study to identify brain-behavior relations that bridge laboratory indices of a subprocess of ER, performance monitoring, and the ER difficulties that youth with anxiety disorders experience as they go about their day-to-day activities. Replication of these findings in additional, longitudinal samples are essential to validate the role(s) of the CRN in pediatric anxiety disorders. Nonetheless, several limitations should be noted. First, because of the primary goals of the larger study, the sample consists primarily of youth with GAD, social phobia, and/or SAD. Recruiting a transdiagnostic sample of youth with other disorders (e.g., panic disorders, specific phobia, depression) could help deepen our understanding of the functional role of ERP indices of performance monitoring and how variation of these indices map on to daily life measures of emotional functioning dimensionally. Additionally, as this study used archival EEG data from a larger study examining behavioral and neurophysiological moderators of CBT response in pediatric anxiety disorders, the influence of different EEG methodological decisions such as choice of ERN baseline and of time window for ERN/CRN quantification on findings were not compared (please see Clayson et al., 2021; Klawohn et al., 2020; Sandre et al., 2020 for a discussion of ERN methodological decisions). Second, we did not have fine-grained measures of participants' levels of uncertainty and arousal during the performance monitoring task. Additionally, to ascertain the functional significance of the CRN, studies should include tasks with conditions that vary by uncertainty of response accuracy (e.g., due to ambiguity of task stimuli and/or task difficulty) and embedded ratings of participants' perceived levels of uncertainty, distress, and arousal/effort as they complete tasks assessing performance monitoring.
In sum, the associations between neural activity and daily-life behaviors observed in this study provide new insights about the relevance of variations in the magnitude of lab-based neural measures of performance monitoring for youth with anxiety disorders. By documenting associations between CRN amplitude and individual differences in worry intensity and negative emotional reactivity among youth with anxiety disorders, the study sheds light on the potential utility of ERP measures of performance monitoring, specifically, using the CRN to better understand heterogeneity within pediatric anxiety disorders. This finding is particularly interesting given results showing the lack of association between ERN/CRN amplitude and rating scales (see Meyer, 2017). Furthermore, consistent with neurocognitive perspectives on anxiety disorders, findings suggest that variability in the CRN is particularly relevant for understanding ER difficulties that are associated with emotional distress and clinical impairment among youth with anxiety disorders, including negative emotional reactivity, worry, and reliance on maladaptive ER strategies like avoidance or rumination in daily life. Finally, given the links between performance monitoring and ER, there is support for future research on the effectiveness of targeting performance monitoring to improve the ER among youth with anxiety. Future research is warranted to determine whether the CRN could be used as an assessment tool for tracking changes within the distress dimension of anxiety over the course of treatment or development.
Supplementary Material
Acknowledgments
ClinicalTrials.gov identifier is NCT00774150. We are grateful to the participants and their families. We are also grateful to Jennifer Jacubcak, Marcus Min, Jessica Wilson, Marcie Walker, Vinod Sharma, Greg Smith, Matt George, Emily Yarrison, Rachel Kolko, Erika Joyce, Danielle Gilchrist, Jillian Rodgers, Katie Burkhouse, Diana Whalen, Stephanie Davis, Simona Graur, Abby Martin, Kristin Pracht, and Grace Lee, for research assistance and data management. This research was supported by National Institute of Mental Health grant P50 MH080215 (Neal D. Ryan, P.I., Greg Siegle and Cecile Ladouceur, Project Co-Directors). Support for research participant recruitment was also provided by the Clinical and Translational Science Institute at the University of Pittsburgh (NIH/NCRR/CTSA Grant UL1 RR024153). Patricia Tan and Lauren Bylsma also received support from MH100261 and MH104325, respectively, during the preparation of this manuscript.
Abbreviations:
- ER
emotion regulation
- ERN
error-related negativity
- CRN
correct-related negativity
- EMA
ecological momentary assessment
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpsycho.2022.06.004.
References
- Allain S, Carbonnell L, Falkenstein M, Burle B, Vidal F, 2004. The modulation of the ne-like wave on correct responses foreshadows errors. Neurosci. Lett 372 (1), 161–166. 10.1016/j.neulet.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, Pine DS, 2009. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr. Clin 32 (3), 483–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol 57 (1), 289–300. [Google Scholar]
- Boen R, Quintana D, Ladouceur C, Tamnes CK, 2021. Age-related Differences in the Error-related Negativity and Error Positivity in Children and Adolescents are Moderated by Sample and Methodological Characteristics: A Meta-analysis. PsyArXiv. 10.31234/osf.io/ykzca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD, 2001. Conflict monitoring and cognitive control. Psychol. Rev 108 (3), 624. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver TS, Yeung N, Ullsperger M, Carter CS, Cohen JD, 2004. Conflict monitoring: computational and empirical studies. Cognit. Neurosci. Atten 91–102. [Google Scholar]
- Burle B, Roger C, Allain S, Vidal F, Hasbroucq T, 2008. Error negativity does not reflect conflict: a reappraisal of conflict monitoring and anterior cingulate cortex activity. J. Cogn. Neurosci 20 (9), 1637–1655. 10.1162/jocn.2008.20110. [DOI] [PubMed] [Google Scholar]
- Carthy T, Horesh N, Apter A, Edge MD, Gross JJ, 2010. Emotional reactivity and cognitive regulation in anxious children. Behav. Res. Ther 48 (5), 384–393. 10.1016/j.brat.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW, 2010. Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review. Clin. Psychol. Rev 30 (2), 203–216. 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Olatunji BO, 2012. Emotion regulation and anxiety disorders. Curr. Psychiatry Rep 14 (3), 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson PE, Baldwin SA, Rocha HA, Larson MJ, 2021. The data-processing multiverse of event-related potentials (ERPs): a roadmap for the optimization and standardization of ERP processing and reduction pipelines. Neuroimage 245, 118712. [DOI] [PubMed] [Google Scholar]
- Coles MGH, Scheffers MK, Holroyd CB, 2001. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing? Biol. Psychol 56 (3), 173–189. 10.1016/S0301-0511(01)00076-X. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Robinson MD, Ode S, Quandt LC, Fineman SL, Carp J, 2008. Error-monitoring ability predicts daily stress regulation. Psychol. Sci 19 (7), 702–708. 10.1111/j.1467-9280.2008.02145.x. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Arnstein D, Freedman G, Dainer-Best J, Liss A, Robinson MD, 2011. Neural and behavioral measures of error-related cognitive control predict daily coping with stress. Emotion (Washington, D.C.) 11 (2), 379–390. 10.1037/a0021776. [DOI] [PubMed] [Google Scholar]
- Connor-Smith J, Compas B, Wadsworth M, Thomsen A, Saltzman H, 2000. Responses to stress in adolescence: measurement of coping and involuntary stress responses. J. Consult. Clin. Psychol 68 (6), 976–992. [PubMed] [Google Scholar]
- Endrass T, Schuermann B, Kaufmann C, Spielberg R, Kniesche R, Kathmann N, 2010. Performance monitoring and error significance in patients with obsessive-compulsive disorder. Biol. Psychol 84 (2), 257–263. 10.1016/j.biopsycho.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG, 2007. Anxiety and cognitive performance: attentional control theory. Emotion 7 (2), 336. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L, 1991. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr. Clin. Neurophysiol 78 (6), 447–455. 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E, 1993. A neural system for error detection and compensation. Psychol. Sci 4 (6), 385–390. 10.1111/j.1467-9280.1993.tb00586.x. [DOI] [Google Scholar]
- Gehring WJ, Liu Y, Orr JM, Carp J, 2012. The error-related negativity (ERN/Ne). In: Luck SJ, Kappenman E (Eds.), Oxford Handbook of Event-related Potential Components. Oxford University Press, New York, NY, pp. 231–291. [Google Scholar]
- Gillan CM, Fineberg NA, Robbins TW, 2017. A trans-diagnostic perspective on obsessive-compulsive disorder. Psychol. Med 47 (9), 1528–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammer JK, Carrasco M, Gehring WJ, Morrison FJ, 2014. Age-related changes in error processing in young children: a school-based investigation. Dev. Cogn. Neurosci 9, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E, 1983. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol 55 (4), 468–484. 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF, 2005. On the ERN and the significance of errors. Psychophysiology 42 (2), 151–160. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Foti D, 2008. Errors are aversive: defensive motivation and the error-related negativity. Psychol. Sci 19 (2), 103–108. [DOI] [PubMed] [Google Scholar]
- Hampel P, Petermann F, 2005. Age and gender effects on coping in children and adolescents. J. Youth Adolesc 34 (2), 73–83. [Google Scholar]
- Hanna GL, Liu Y, Rough HE, Surapaneni M, Hanna BS, Arnold PD, Gehring WJ, 2020. A diagnostic biomarker for pediatric generalized anxiety disorder using the error-related negativity. Child Psychiatry Hum. Dev 51 (5), 827–838. 10.1007/s10578-020-01021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG, 2002. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev 109 (4), 679. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Bartholow BD, Hirsh JB, 2015. Emotional foundations of cognitive control. Trends Cogn. Sci 19 (3), 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson F, Nelson BD, Hajcak G, 2016. The uncertainty of errors: intolerance of uncertainty is associated with error-related brain activity. Biol. Psychol 113, 52–58. 10.1016/j.biopsycho.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Joormann J, Tanovic E, 2015. Cognitive vulnerability to depression: examining cognitive control and emotion regulation. Curr. Opin. Psychol 4, 86–92. 10.1016/j.copsyc.2014.12.006. [DOI] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, Ryan N, 1997. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 36 (7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Compton SN, Walkup JT, Birmaher B, Albano AM, Sherrill J, Ginsburg G, Rynn M, McCracken J, Gosch E, Keeton C, Bergman L, Sakolsky D, Suveg C, Iyengar S, March J, Piacentini J, 2010. Clinical characteristics of anxiety disordered youth. J. Anxiety Disord 24 (3), 360–365. 10.1016/j.janxdis.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62 (6), 593–602. 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Klawohn J, Meyer A, Weinberg A, Hajcak G, 2020. Methodological choices in event-related potential (ERP) research and their impact on internal consistency reliability and individual differences: an examination of the error-related negativity (ERN) and anxiety. J. Abnorm. Psychol 129 (1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND, 2006. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. J. Child Psychol. Psychiatry 47 (10), 1073–1082. 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Tan PZ, Sharma V, Bylsma LM, Silk JS, Siegle GJ, Forbes EE, McMakin DL, Dahl RE, Kendall PC, Mannarino A, Ryan ND, 2018. Error-related brain activity in pediatric anxiety disorders remains elevated following individual therapy: a randomized clinical trial. J. Child Psychol. Psychiatry 59 (11), 1152–1161. 10.1111/jcpp.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MJ, South M, Clayson PE, 2011. Sex differences in error-related performance monitoring. Neuroreport 22 (1), 44–48. [DOI] [PubMed] [Google Scholar]
- Laurent J, Catanzaro SJ, Joiner TE Jr., Rudolph KD, Potter KI, Lambert S, Osborne L, Gathright T, 1999. A measure of positive and negative affect for children: scale development and preliminary validation. Psychol. Assess 11 (3), 326–338. 10.1037/1040-3590.11.3.326. [DOI] [Google Scholar]
- Lewinsohn PM, Gotlib IH, Lewinsohn M, Seeley JR, Allen NB, 1998. Gender differences in anxiety disorders and anxiety symptoms in adolescents. J. Abnorm. Psychol 107 (1), 109. [DOI] [PubMed] [Google Scholar]
- Light GA, Williams LE, Minow F, Sprock J, Rissling A, Sharp R, Braff DL, 2010. Electroencephalography (EEG) and event-related potentials (ERPs) with human participants. Curr. Protoc. Neurosci 52 (1), 6–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SL, 2018. A meta-analytic review of the event-related potentials (ERN and N2) in childhood and adolescence: providing a developmental perspective on the conflict monitoring theory. Dev. Rev 48, 82–112. [Google Scholar]
- Lonigan CJ, Vasey MW, Phillips BM, Hazen RA, 2004. Temperament, anxiety, and the processing of threat-relevant stimuli. J. Clin. Child Adolesc. Psychol 33 (1), 8–20. 10.1207/S15374424JCCP3301_2, 53. [DOI] [PubMed] [Google Scholar]
- Luck SJ, 2005. Ten Simple Rules for Designing and Interpreting ERP Experiments. Event-related Potentials: A Methods Handbook, 4. [Google Scholar]
- Luu P, Flaisch T, Tucker DM, 2000. Medial frontal cortex in action monitoring. J. Neurosci 20 (1), 464–469. 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG, 2011. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res 45 (8), 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon TP, Naragon-Gainey K, 2020. Ecological validity of trait emotion regulation strategy measures. Psychol. Assess 32 (8), 796. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Heimberg RG, Turk CL, Fresco DM, 2002. Applying an Emotion Regulation Framework to Integrative Approaches to Generalized Anxiety Disorder. [Google Scholar]
- Meyer A, 2017. A biomarker of anxiety in children and adolescents: a review focusing on the error-related negativity (ERN) and anxiety across development. Dev. Cogn. Neurosci 27, 58–68. 10.1016/j.dcn.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Wissemann K, 2020. Controlling parenting and perfectionism is associated with an increased error-related negativity (ERN) in young adults. Soc. Cogn. Affect. Neurosci 15 (1), 87–95. 10.1093/scan/nsaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey DC, Kujawa A, Kim J, Bufferd S, Carlson G, Klein DN, 2013. Increased error-related brain activity in six-year-old children with clinical anxiety. J. Abnorm. Child Psychol 41 (8), 1257–1266. 10.1007/S10802-013-9762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Nelson B, Perlman G, Klein DN, Kotov R, 2018. A neural biomarker, the error-related negativity, predicts the first onset of generalized anxiety disorder in a large sample of adolescent females. J. Child Psychol. Psychiatry 59 (11), 1162–1170. 10.1111/jcpp.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael JA, Wang M, Kaur M, Fitzgerald PB, Fitzgibbon BM, Hoy KE, 2021. EEG correlates of attentional control in anxiety disorders: a systematic review of error-related negativity and correct-response negativity findings. J. Affect. Disord 291, 140–153. 10.1016/j.jad.2021.04.049. [DOI] [PubMed] [Google Scholar]
- Moser JS, 2017. The nature of the relationship between anxiety and the error-related negativity across development. Curr. Behav. Neurosci. Rep 4 (4), 309–321. 10.1007/s40473-017-0132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser J, Moran T, Schroder H, Donnellan B, Yeung N, 2013. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Front. Hum. Neurosci 7 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Kneip C, Schroder HS, Larson MJ, 2016. Sex moderates the association between symptoms of anxiety, but not obsessive compulsive disorder, and error-monitoring brain activity: a meta-analytic review. Psychophysiology 53 (1), 21–29. 10.1111/psyp.12509. [DOI] [PubMed] [Google Scholar]
- Newman MG, Jacobson NC, Zainal NH, Shin KE, Szkodny LE, Sliwinski MJ, 2019. The effects of worry in daily life: an ecological momentary assessment study supporting the tenets of the contrast avoidance model. Clin. Psychol. Sci 7 (4), 794–810. 10.1177/2167702619827019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ, 2005. The cognitive control of emotion. Trends Cogn. Sci 9 (5), 242–249. 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G, 2009. The stability of error-related brain activity with increasing trials. Psychophysiology 46 (5), 957–961. 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Overmeyer R, Berghäuser J, Dieterich R, Wolff M, Goschke T, Endrass T, 2021. The error-related negativity predicts self-control failures in daily life. Front. Hum. Neurosci 14, 616. 10.3389/fnhum.2020.614979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ, 2004. The effects of uncertainty in error monitoring on associated ERPs. Brain Cogn. 56 (2), 215–233. 10.1016/j.bandc.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC, 2008. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry 13 (9), 833–857. 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH, Inzlicht M, Mennin D, 2013. Anxiety and error monitoring: the importance of motivation and emotion. Front. Hum. Neurosci 7 10.3389/fnhum.2013.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, van der Molen MW, Band GP, Bashore TR, 1997. Sources of interference from irrelevant information: a developmental study. J. Exp. Child Psychol 65 (3), 315–341. [DOI] [PubMed] [Google Scholar]
- Riesel A, Kathmann N, Endrass T, 2014. Overactive performance monitoring in obsessive–compulsive disorder is independent of symptom expression. Eur. Arch. Psychiatry Clin. Neurosci 264 (8), 707–717. 10.1007/s00406-014-0499-3. [DOI] [PubMed] [Google Scholar]
- Sandre A, Banica I, Riesel A, Flake J, Klawohn J, Weinberg A, 2020. Comparing the effects of different methodological decisions on the error-related negativity and its association with behaviour and gender. Int. J. Psychophysiol 156, 18–39. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Dzyundzyak A, Segalowitz SJ, 2011. Age, sex and individual differences in punishment sensitivity: factors influencing the feedback-related negativity. Psychophysiology 48 (11), 1481–1489. [DOI] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS, 2003. Adolescents’ emotion regulation in daily life: links to depressive symptoms and problem behavior. Child Dev. 74 (6), 1869–1880. 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Silk JS, Forbes EE, Whalen DJ, Jakubcak JL, Thompson WK, Ryan ND, Axelson DA, Birmaher B, Dahl RE, 2011. Daily emotional dynamics in depressed youth: a cell phone ecological momentary assessment study. J. Exp. Child Psychol 110 (2), 241–257. 10.1016/j.jecp.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Tan PZ, Ladouceur CD, Meller S, Siegle GJ, McMakin DL, Forbes EE, Dahl RE, Kendall PC, Mannarino A, Ryan ND, 2016. A randomized clinical trial comparing individual cognitive behavioral therapy and child-centered therapy for child anxiety disorders. J. Clin. Child Adolesc. Psychol 47 (4), 542–554. 10.1080/15374416.2016.1138408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WK, Greca AML, Wasserstein S, 1995. What do children worry about? Worries and their relation to anxiety. Child Dev. 66 (3), 671–686. 10.1111/j.1467-8624.1995.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Simons RF, 2010. The way of our errors: theme and variations. Psychophysiology 47 (1), 1–14. [DOI] [PubMed] [Google Scholar]
- Tan PZ, Forbes EE, Dahl RE, Ryan ND, Siegle GJ, Ladouceur CD, Silk JS, 2012. Emotional reactivity and regulation in anxious and nonanxious youth: a cellphone ecological momentary assessment study. J. Child Psychol. Psychiatry 53 (2), 197–206. 10.1111/j.1469-7610.2011.02469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Fischer AG, Nigbur R, Endrass T, 2014. Neural mechanisms and temporal dynamics of performance monitoring. Trends Cogn. Sci 18 (5), 259–267. [DOI] [PubMed] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J, Bonnet M, 2000. Is the ‘error negativity’ specific to errors? Biol. Psychol 51 (2), 109–128. 10.1016/S0301-0511(99)00032-0. [DOI] [PubMed] [Google Scholar]
- Vidal F, Burle B, Bonnet M, Grapperon J, Hasbroucq T, 2003. Error negativity on correct trials: a reexamination of available data. Biol. Psychol 64 (3), 265–282. 10.1016/S0301-0511(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, Hajcak G, 2010. Increased error-related brain activity in generalized anxiety disorder. Biol. Psychol 85 (3), 472–480. 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Dieterich R, Riesel A, 2015. Error-related brain activity in the age of RDoC: a review of the literature. Int. J. Psychophysiol 98 (2), 276–299. 10.1016/j.ijpsycho.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD, 2004. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol. Rev 111 (4), 931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.