Abstract
Background
Although the SARS-CoV-2 Omicron variant is considered to induce less severe disease, there have been no consistent results on the extent of the decrease in severity.
Objectives
To compare the clinical outcomes of COVID-19–positive patients with Omicron and Delta variant infection.
Data sources
Searches were implemented up to 8 November 2022 in PubMed, Web of Science, BioRvix, and MedRvix.
Study eligibility criteria
Eligible studies were cohort studies reporting the clinical outcomes of COVID-19–positive patients with Omicron and Delta variant infection, including hospitalization, intensive care unit (ICU) admission, receiving invasive mechanical ventilation (IMV), and death.
Participants
COVID-19–positive patients with Omicron and Delta variant infection.
Assessment of risk of bias
Risk of bias was assessed employing the Newcastle-Ottawa Scale.
Methods of data synthesis
Random-effect models were employed to pool the ORs and 95% CIs to compare the risk of clinical outcome. I2 was employed to evaluate the heterogeneity between studies.
Results
A total of 33 studies with 6 037 144 COVID-19–positive patients were included in this meta-analysis. In the general population of COVID-19–positive patients, compared with Delta, Omicron variant infection resulted in a decreased risk of hospitalization (10.24% vs. 4.14%, OR = 2.91, 95% CI = 2.35–3.60), ICU admission (3.67% vs. 0.48%, OR = 3.64, 95% CI = 2.63–5.04), receiving IMV (3.93% vs. 0.34%, OR = 3.11, 95% CI = 1.76–5.50), and death (2.40% vs. 0.46%, OR = 2.97, 95% CI = 2.17–4.08). In the hospitalized patients with COVID-19, compared with Delta, Omicron variant infection resulted in a decreased risk of ICU admission (20.70% vs. 12.90%, OR = 1.63, 95% CI = 1.32–2.02), receiving IMV (10.90% vs. 5.80%, OR = 1.65, 95% CI = 1.28–2.14), and death (10.72% vs. 7.10%, OR = 1.44, 95% CI = 1.22–1.71).
Conclusions
Compared with Delta, the severity of Omicron variant infection decreased.
Keywords: Delta, Hospitalization, Meta-analysis, Omicron, SARS-CoV-2
Introduction
Like any other virus, the SARS-CoV-2 virus has the ability to evolve. Since its inception, many variants of SARS-CoV-2 have emerged in succession throughout the world, with different transmissibility and severity [1].
In December 2020, the Delta variant was first detected in India [2,3], characterized by a high viral load, a long duration of infection, and a high rate of reinfection [4]. Compared with previous variants of COVID-19, the Delta variant also has high transmissibility, associated with a mass of immune escape [5]. In November 2021, the Omicron SARS-CoV-2 variant of concern was initially detected in South Africa [6]. Subsequently, the Omicron variant replaced the Delta variant as the novel mainstream variant globally because of high transmissibility [6]. The transmissibility of the Omicron variant has been reported to vary from 3.31 to 100 times that of the Delta variant [7,8]. In addition, the resistance of Omicron to antiviral immunity has also increased [9]. However, a decrease in the severity and pathogenicity of the Omicron variant has been reported in many studies [[10], [11], [12]]. Therefore, there may be differences in clinical outcomes between patients who tested positive for the Omicron and Delta variants.
Several studies have shown that the Omicron variant caused significantly lower disease severity than the Delta variant, including hospitalization, oxygen requirements, mechanical ventilation, and death [[13], [14], [15]]. However, a review [16] came to the opposite conclusion about hospitalization and death, namely that the Omicron variant significantly affected the increase in daily hospitalization and death. Furthermore, although the current Omicron epidemic lasts less than the Delta epidemic, the absolute number of patients requiring hospitalization and death was larger in some countries [17,18].
Taking into account inconsistent reports on hospitalization, intensive care unit (ICU) admissions, receiving invasive mechanical ventilation (IMV), and death from the SARS-CoV-2 Omicron and Delta variants, this meta-analysis comprehensively summarized the available evidence and compared the clinical outcomes of the SARS-CoV-2 Omicron and Delta variants. This information is critical to systematically compare differences in clinical outcomes between Omicron and Delta variants to provide a reference for the formulation and adjustment of epidemic prevention and control policies.
Methods
This systematic review and meta-analysis was implemented in accordance with the statement of Preferred Reporting Items for Systematic Reviews and Meta-Analyses [19]. The protocol for this meta-analysis was registered at PROSPERO (registration number: CRD42022371240).
Search strategy
Four databases (PubMed, Web of Science, BioRvix, and MedRvix) were systematically searched. This search included all relevant published or preprinted articles from December 2020 to 8 November 2022. In addition, we screened the references of potential included studies for additional studies that may be relevant. Endnote software was employed for literature management and deduplication. Specific search strategies and selection criteria are provided in the Supplementary Material.
Selection criteria
The eligible articles for this review and meta-analysis met the following criteria: (a) the study was designed as a cohort study in which both Delta and Omicron patients were included; (b) participants had a definitive diagnosis of Omicron or Delta SARS-CoV-2 variant infection by PCR, gene sequencing, and other laboratory tests; and (c) studies reported the outcomes of infection, including hospitalization, ICU admission, and receiving IMV. The exclusion criteria were as follows: (a) the study design was not a cohort such as cross-sectional, case report, case-control, or randomized controlled trail; (b) participants of the study were diagnosed with infections caused by variants other than Omicron or Delta; (c) the variant was identified in the study because it was predominant in the area; and (d) the study reported only one of the Omicron and Delta variants.
Risk-of-bias assessment
The quality of identified studies was evaluated employing the Newcastle-Ottawa Scale independently by two researchers. This tool assessed the quality of the studies from three main dimensions (selection, comparability, and exposure), which are formed from eight items (the highest quality score is 9), a higher score indicating higher quality. Divergences of opinion were solved through negotiation and consensus with a third senior researcher.
Data extraction
Two researchers independently extracted and included the data in a standardized form to ensure consistency, and conflicts over the points were resolved by negotiation with a third senior researcher. The following data were extracted: author name, published year, location of study, study design, age, gender distribution, vaccination status, number of hospitalizations, ICU admission, receiving IMV, or death from Omicron and Delta infection.
Outcome indicators
The outcome indicators were the comparison of the risks of hospitalization, ICU admission, receiving IMV, and death associated with SARS-CoV-2 Omicron and Delta variants. We defined hospitalization as hospital admission resulting from 2 days before the date of positive laboratory acquisition to 14 days after diagnosis, as others rating the severity of SARS-CoV-2 do [20,21]; ICU admission was defined as meeting the criteria of hospitalization and having a record of ICU admission; receiving IMV was defined as positive pressure ventilation of intratracheal or tracheostomy intubation; death was defined as demise occurring within 28 days of a positive test for COVID-19, in line with the proposal of the National Health Service [22].
Statistical analysis
Studies eligible for meta-analysis were divided into two categories: (a) the general population of COVID-19–positive patients as participants (not recruited in hospitals, with all severities, from mild to critical) and (b) the hospitalized patients with COVID-19–positive diagnosis as participants. We used pooled OR with 95% CI as the outcome measure. A random-effects model was adopted, considering the underlying heterogeneity of the included studies. The underlying heterogeneity between different studies was evaluated using the I2 statistics. Subgroup analyses were performed according to different study sites, and we classified the study sites by continent. Visual inspection of the funnel plot and the p value of Egger's test were employed to assess publication bias. Sensitivity analysis was carried out by excluding studies one by one to examine the uniformity of the consequences of meta-analyses. Furthermore, meta-regression analyses were implemented to quantify the correlation between outcome measures and other covariates, including available mean age, gender distribution, and the ratio of Omicron to Delta vaccination percentage. All statistical analyses were implemented using the Stata software package (version 17.0 for Windows).
Results
Study selection and characteristics
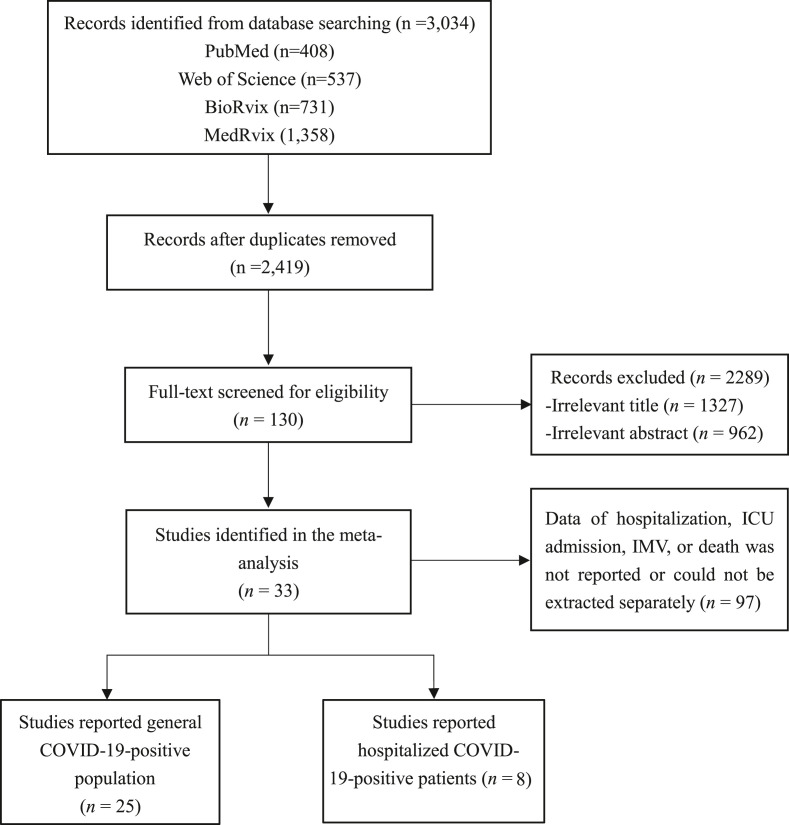
In total, 3034 unique publications were initially yielded using the search strategy; of them, 615 studies were duplicates. Further, 2289 studies were excluded because of irrelevant content via screening for titles and abstracts. The remaining 130 studies were retrieved, and the full text was browsed. An additional 96 studies were excluded because of the fact that hospitalization, ICU admission, IMV, or death data were not reported or could not be extracted separately. Ultimately, 33 studies comprising 3 367 207 patients who tested positive for Omicron and 2 672 353 for Delta from 13 countries were included in the meta-analyses, summarized in Fig. 1 and Table S1.
Fig. 1.
Flowchart of the study selection process. ICU, intensive care unit; IMV, invasive mechanical ventilation.
Of the 33 identified studies, the literature quality evaluation showed that 31 studies were considered high quality and two studies moderate quality via Newcastle-Ottawa Scale (six studies scored 8, 15 studies scored 7, ten studies scored 6, and two studies scored 5), summarized in Table S2.
The general population of COVID-19–positive patients
We identified 25 eligible studies [[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]] that included a general population of COVID-19–positive patients as participants and reported the number of hospitalizations, ICU admission, IMV, or death for Omicron and Delta infections.
Hospitalization
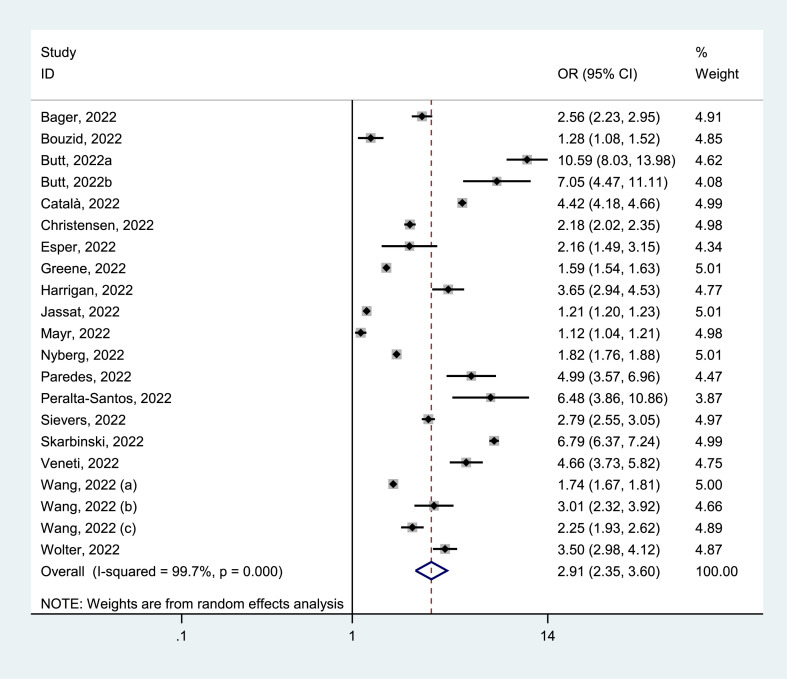
Of 25 identified studies, 21 studies [[23], [24], [25], [26], [27], [28], [29], [30],[33], [34], [35], [36], [37], [38],[40], [41], [42], [43],[45], [46], [47]] reported the hospitalization of positive patients, with 2 499 830 cases who tested positive for Delta and 3 236 005 for Omicron. Hospitalization rates because of Delta were 10.24% (95% CI = 8.41–12.07%) and Omicron were 4.14% (95% CI = 3.35–4.93%). The pooled estimates presented that the hospitalization for Delta infection was higher than that for Omicron infection (OR = 2.91, 95% CI = 2.35–3.60, p < 0.05), with a significant statistical heterogeneity (I2 = 99.7%), as shown in Fig. 2 . Our analyses of subgroup by continent, the funnel plots, and the meta-regression as well as the sensitivity analysis failed to fully detect the source of heterogeneity. Details are given in Supplementary Materials.
Fig. 2.
Meta-analysis of the association of Delta or Omicron SASR-CoV-2 with hospitalization in the general population of COVID-19 positive.
ICU admission
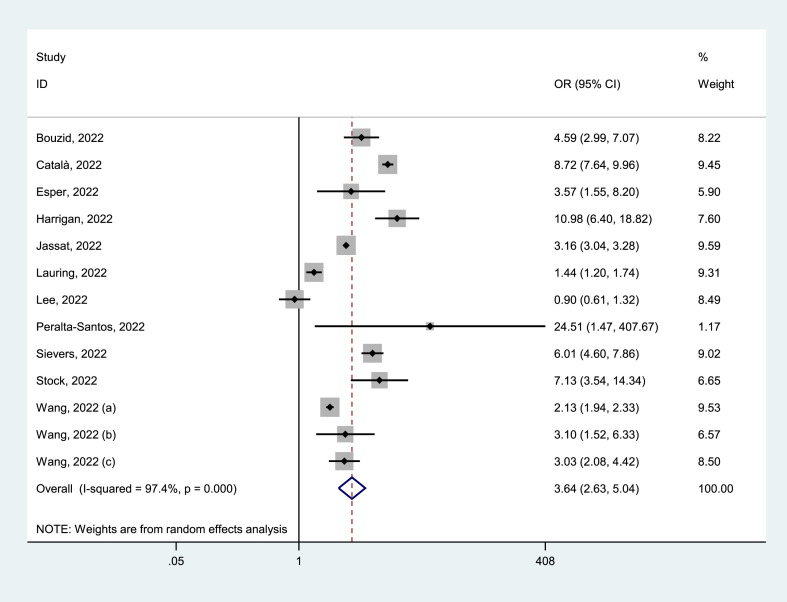
Of 25 identified studies, 13 studies [23,26,[28], [29], [30], [31], [32],36,37,39,[41], [42], [43]] reported the ICU admission of positive patients, with 1 574 082 cases who tested positive for Delta and 1 403 795 for Omicron. ICU admission rates because of Delta variant were 3.67% (95% CI = 3.17–4.17%) and Omicron variant were 0.48% (95% CI = 0.31–0.65%). The pooled estimates showed that the ICU admission for Delta infection was higher than that for Omicron infection (OR = 3.64, 95% CI = 2.63–5.04, p < 0.05), with a significant statistical heterogeneity (I2 = 97.4%), as shown in Fig. 3 . Our analyses of subgroup by continent, the funnel plots, and the meta-regression as well as the sensitivity analysis failed to fully detect the source of heterogeneity. Details are given in Supplementary Materials.
Fig. 3.
Meta-analysis of the association of Delta or Omicron SASR-CoV-2 with ICU admission in the general population of COVID-19 positive. ICU, intensive care unit.
Invasive mechanical ventilation
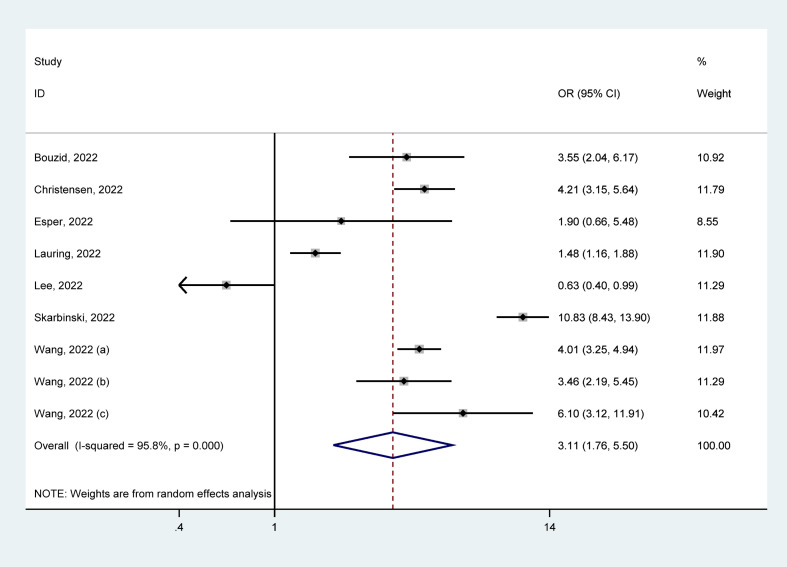
Of 25 identified studies, 9 studies [23,27,28,31,32,38,[41], [42], [43]] reported IMV in positive patients, with 238 610 cases who tested positive for Delta and 246 107 for Omicron. IMV rates because of Delta were 3.93% (95% CI = 3.14–4.71%) and Omicron were 0.34% (95% CI = 0.22–0.46%). The pooled estimates presented that patients with Delta infection had a higher risk of receiving IMV than those with Omicron infection (OR = 3.11, 95% CI = 1.76–5.50, p < 0.05), with a significant statistical heterogeneity (I2 = 95.8%), as shown in Fig. 4 . Our analyses of subgroup by continent, the funnel plots, and the meta-regression as well as the sensitivity analysis failed to fully detect the source of heterogeneity. Details are given in Supplementary Materials.
Fig. 4.
Meta-analysis of the association of Delta or Omicron SASR-CoV-2 with receiving IMV in the general population of COVID-19 positive. IMV, invasive mechanical ventilation.
Death
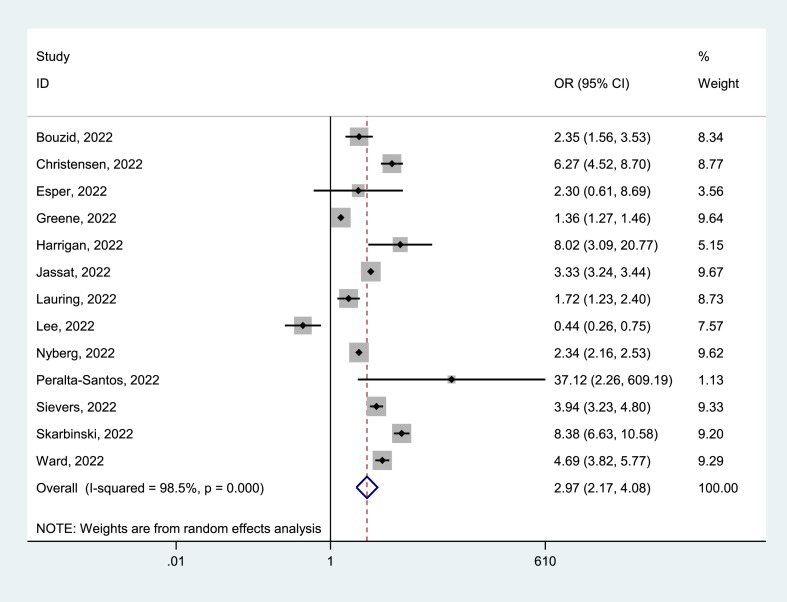
Of 25 identified studies, 13 studies [23,[27], [28], [29], [30], [31], [32],34,[36], [37], [38],44,46] reported death in positive patients, with 2 251 829 cases who tested positive for Delta and 3 119 565 for Omicron. Mortality due to Delta was 2.40% (95% CI = 1.85–2.95%) and Omicron was 0.46% (95% CI = 0.33–0.60%). The pooled estimates showed that Delta infection had a higher mortality risk than Omicron infection (OR = 2.97, 95% CI = 2.17–4.08, p < 0.05), with a significant statistical heterogeneity (I2 = 98.5%), as shown in Fig. 5 . Our analyses of subgroup by continent, the funnel plots, and the meta-regression, as well as the sensitivity analysis failed to fully detect the source of heterogeneity. Details are given in Supplementary Materials.
Fig. 5.
Meta-analysis of the association of Delta or Omicron SASR-CoV-2 with mortality risk in the general population of COVID-19 positive.
The hospitalized patients with COVID-1–positive diagnosis
We identified eight eligible studies [[48], [49], [50], [51], [52], [53], [54], [55]] that included hospitalized patients with a COVID-19–positive diagnosis as participants and reported the number of ICU admission, IMV, or death from Omicron and Delta infections.
ICU admission
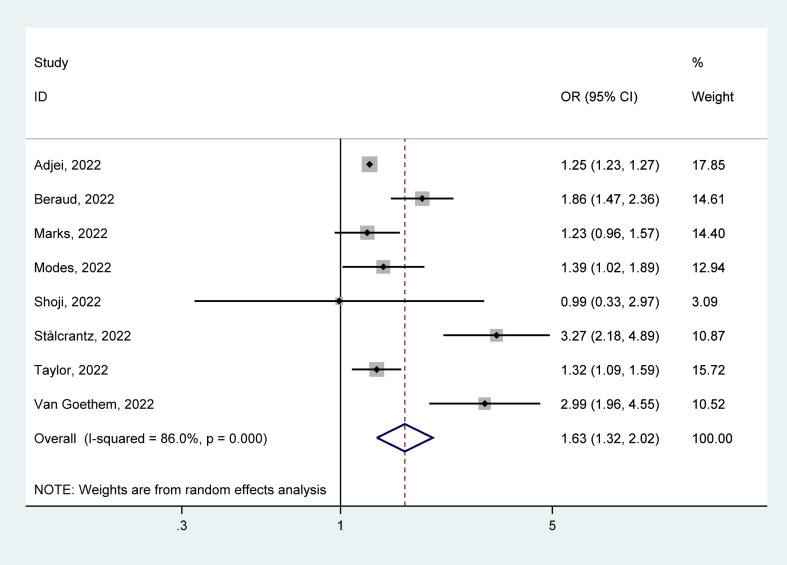
The eight studies [[48], [49], [50], [51], [52], [53], [54], [55]] reported ICU admission among hospitalized patients with COVID-19, with 171 663 cases infected with Delta and 129 646 infected with Omicron. In hospitalized patients with COVID-19, ICU admission rates because of Delta were 20.70% (95% CI = 13.30–28.20%) and Omicron were 12.90% (95% CI = 6.30–19.50%). The pooled estimates presented that the ICU admission for Delta infection was higher than that for Omicron infection (OR = 1.63, 95% CI = 1.32–2.02, p < 0.05), with a significant statistical heterogeneity (I2 = 86.0%), as shown in Fig. 6 . Our analyses of subgroup by continent, the funnel plots, and the meta-regression, as well as the sensitivity analysis failed to fully detect the source of heterogeneity. Details are given in Supplementary Materials.
Fig. 6.
Meta-analysis of the association of Delta or Omicron SASR-CoV-2 with ICU admission in the hospitalized patients with COVID-19 positive. ICU, intensive care unit.
Invasive mechanical ventilation
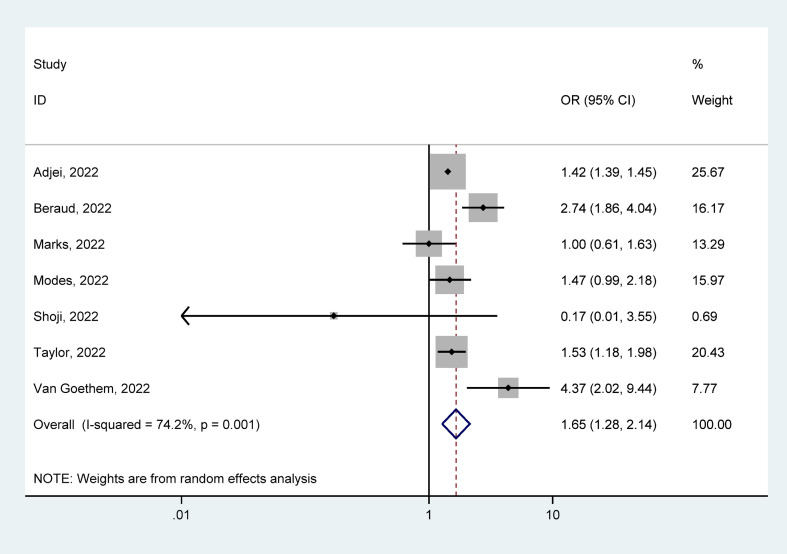
Seven studies [[48], [49], [50], [51], [52],54,55] reported receiving IMV among hospitalized patients with COVID-19, with 170 997 cases infected with Delta and 129 237 infected with Omicron. In hospitalized patients with COVID-19, ICU admission rates because of Delta were 10.90% (95% CI = 6.50–15.40%) and Omicron were 5.80% (95% CI = 0.06–10.90%). The pooled estimates presented that Delta infection had a higher risk of receiving IMV than Omicron infection (OR = 1.65, 95% CI = 1.28–2.14, p < 0.05), with significant heterogeneity of the statistics (I2 = 74.2%), as shown in Fig. 7 . Our analyses of subgroup by continent, the funnel plots, and the meta-regression as well as the sensitivity analysis failed to fully detect the source of heterogeneity. Details are given in Supplementary Materials.
Fig. 7.
Meta-analysis of the association of Delta or Omicron SARS-CoV-2 with receiving IMV in the hospitalized patients with COVID-19 positive. IMV, invasive mechanical ventilation.
Death
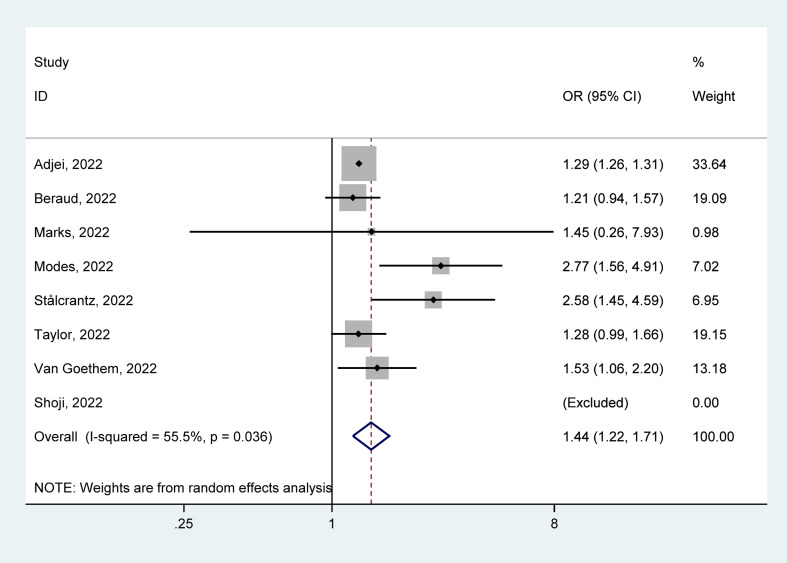
Eight studies [[48], [49], [50], [51], [52], [53], [54], [55]] reported death in hospitalized patients with COVID-19, with 171 663 cases infected with Delta and 129 646 with Omicron. In hospitalized patients with COVID-19, mortality rates because of Delta were 10.72% (95% CI = 4.28–17.17%) and Omicron were 7.10% (95% CI = 1.74–12.45%). The pooled estimates presented that Delta infection had a higher risk of death than Omicron infection (OR = 1.44, 95% CI = 1.22–1.71, p < 0.05), with significant heterogeneity of statistics (I2 = 55.5%), as shown in Fig. 8 . Our analyses of subgroup by continent, the funnel plots, and the meta-regression, as well as the sensitivity analysis failed to fully detect the source of heterogeneity. Details are given in Supplementary Materials.
Fig. 8.
Meta-analysis of the association of Delta or Omicron SASR-CoV-2 with mortality risk in the hospitalized patients with COVID-19 positive.
The incidence of clinical outcome and 95% CI of the general population and hospitalized patients who tested positive for COVID-19 are shown in Table S3.
Discussion
Our meta-analysis found that the risk of hospitalization for Omicron variant infection was low compared with Delta infection, as well as ICU admission, receiving IMV, and death. Similarly, in hospitalized patients, the risk of ICU admission, receiving IMV, and death from Omicron variant infection decreased compared with Delta variant.
In this meta-analysis, a lower inherent severity of infection was found in Omicron than in Delta, which is consistent with the vast majority of studies published elsewhere [[56], [57], [58], [59]]. COVID-19 is considered to be associated with respiratory diseases, and patients may experience symptoms ranging from asymptomatic to critical and even death [60,61]. According to Hui et al. [62], the high replication of Omicron in human bronchi may cause an increase in the amount of Omicron variant released when breathing or speaking, which, accordingly, explains the increase in its transmissibility. However, novel mutations created by evolution may have weakened the virulence of Omicron. This may explain the increased transmissibility and decreased severity of Omicron compared with Delta.
Our meta-regression analyses showed that the ratio of Omicron to Delta vaccination percentage had an insignificant effect on comparing Omicron and Delta severity, which may be associated with the increased ability of the Omicron variant to escape immunity [27,28]. This ability to escape makes it easier for Omicron to exempt the immune system from its defences, reducing the effectiveness of previous vaccinations. In addition, we found that neither the proportion of men nor the mean age had a significant effect on the comparison of the severity of the Omicron and Delta variants. This indicated that the reduction in the severity of Omicron infection was significant across age groups and that there was no significant gender difference.
Compared with the general infected population, the severity of hospitalized patients who tested positive for Omicron variant infection showed a relatively reduced decline, suggesting that the overall threat of Omicron had not declined as much as its severity. Studies have suggested that the absolute number of Omicron cases resulted in more hospitalization and other serious outcomes than what was observed in the previous variants of SARS-CoV-2, including Delta [15,29,63]. Despite the decreased severity, the Omicron variant infection has had a significant impact on COVID-19 patients and the health systems. The general threat from the Omicron variant could be grave.
The vast majority of health facilities test all individuals for SARS-CoV-2 infection upon admission, so it is unlikely that a person hospitalized with a SARS-CoV-2 infection will be undiagnosed or unreported. However, SARS-CoV-2 infection may occur in the general population but is not reported. Hence, the proportion of hospitalized patients with a COVID-19–positive could be higher than the actual proportion of hospitalization due to infection. Analogously, the sheer risk of severe clinical outcomes in patients with SARS-CoV-2 infection may be correspondingly lower than that reported in the included studies [38].
There are several limitations associated with this review. First, some studies included in this meta-analysis had access to publicly available data, which possibly resulted in repetitive computing in the pooled estimates. Second, for the Omicron era, many patients have been infected with previous variants and produced some antibodies, which may result in a survival bias. Third, Omicron and Delta have multiple subtypes, which may result in different clinical outcomes. We combine them for the analysis, which might have biased the results. Finally, it is difficult to extract data on the patient's underlying disease, immune system function, vaccine product, number of vaccinations, and whether they are reinfected from the included studies, making these variables difficult to include in the analysis.
In conclusion, among the general population of COVID-19–positive patients, Omicron variant infection was found to have a lower clinical severity than Delta variant infection, including lower hospitalization, ICU admission, receiving IMV, and death. In the hospitalized patients with COVID-19–positive diagnosis, the risk of ICU admission, receiving IMV, and death from Omicron variant infection decreased. Although the severity of Omicron infection has decreased, the overall threat of the Omicron variant could be severe owing to its high transmissibility. In the future, effective vaccines and treatments are needed to alleviate the harm caused by COVID-19 to public health.
Author contributions
Writing–original draft: F.H.H., Y.J.J., and D.Y.Z. Writing–review and editing: X.L.F. and W.Q.Z. Conceptualization: F.H.H. and W.T. Methodology: S.Q.H. and H.W. Visualization: M.W.G., W.D., and W.Q.S. Supervision: B.Z. and H.L.C. Funding acquisition: H.L.C. All authors declared that the work described was original research that has not been published previously and is not considered for publication elsewhere, in whole or in part. All the authors listed have approved the paper, which is enclosed.
Transparency declaration
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
This work was supported by the Humanities and Social Sciences of the Ministry of Education Planning Fund (20YJAZH007).
Data availability
All relevant data are disclosed in the paper, its associated figures, and the Supplementary Materials.
Acknowledgements
The authors thank the editor and anonymous reviewers for several insightful comments that significantly improved the paper.
Editor: J. Rodriguez-Baño
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2023.03.017.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Graham M.S., Sudre C.H., May A., Antonelli M., Murray B., Varsavsky T., et al. Changes in symptomatology, reinfection, and transmissibility associated with the SARS-CoV-2 variant B.1.1.7: an ecological study. Lancet Public Health. 2021;6:e335–e345. doi: 10.1016/s2468-2667(21)00055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Rio C., Malani P.N., Omer S.B. Confronting the Delta variant of SARS-CoV-2, summer 2021. JAMA. 2021;326:1001–1002. doi: 10.1001/jama.2021.14811. [DOI] [PubMed] [Google Scholar]
- 3.Kupferschmidt K., Wadman M. Delta variant triggers new phase in the pandemic. Science. 2021;372:1375–1376. doi: 10.1126/science.372.6549.1375. [DOI] [Google Scholar]
- 4.Wang Y., Chen R., Hu F., Lan Y., Yang Z., Zhan C., et al. Transmission, viral kinetics and clinical characteristics of the emergent SARS-CoV-2 Delta VOC in Guangzhou, China. EClinicalMedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C., Ginn H.M., Dejnirattisai W., Supasa P., Wang B., Tuekprakhon A., et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220–4236.e13. doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126–2128. doi: 10.1016/s0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki R., Yamasoba D., Kimura I., Wang L., Kishimoto M., Ito J., et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature. 2022;603:700–705. doi: 10.1038/s41586-022-04462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao S., Singh M. The newly detected B.1.1.529 (Omicron) variant of SARS-CoV-2 with multiple mutations implications for transmission, diagnostics, therapeutics, and immune evasion. DHR Proc. 2021;1:7–10. doi: 10.47488/dhrp.v1iS5.35. [DOI] [Google Scholar]
- 9.Meng B., Abdullahi A., Ferreira T.M.I., Goonawardane N., Saito A., Kimura I., et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature. 2022;603:706–714. doi: 10.1038/s41586-022-04474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bálint G., Vörös-Horváth B., Széchenyi A. Omicron: increased transmissibility and decreased pathogenicity. Signal Transduct Target Ther. 2022;7:151. doi: 10.1038/s41392-022-01009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du J., Wang J.M., Wang J., Gao Y.L., Pang X.H., Li G. Study of transmissibility of 2019-nCoV Omicron variant in Beijing. Zhonghua Liu Xing Bing Xue Za Zhi. 2022;43:1364–1369. doi: 10.3760/cma.j.cn112338-20220410-00274. [DOI] [PubMed] [Google Scholar]
- 12.Ulloa A.C., Buchan S.A., Daneman N., Brown K.A. Estimates of SARS-CoV-2 Omicron variant severity in Ontario, Canada. JAMA. 2022;327:1286–1288. doi: 10.1001/jama.2022.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyer O. COVID-19: Omicron is causing more infections but fewer hospital admissions than Delta, South African data show. BMJ. 2021;375:n3104. doi: 10.1136/bmj.n3104. [DOI] [PubMed] [Google Scholar]
- 14.Wrenn J.O., Pakala S.B., Vestal G., Shilts M.H., Brown H.M., Bowen S.M., et al. COVID-19 severity from Omicron and Delta SARS-CoV-2 variants. Influenza Other Respir Virus. 2022;16:832–836. doi: 10.1111/irv.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iuliano A.D., Brunkard J.M., Boehmer T.K., Peterson E., Adjei S., Binder A.M., et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods–United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:146–152. doi: 10.15585/mmwr.mm7104e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duong B.V., Larpruenrudee P., Fang T., Hossain S.I., Saha S.C., Gu Y., et al. Is the SARS CoV-2 Omicron variant deadlier and more transmissible than Delta variant? Int J Environ Res Public Health. 2022;19:4586. doi: 10.3390/ijerph19084586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahase E. COVID-19: hospital admission 50-70% less likely with Omicron than Delta, but transmission a major concern. BMJ. 2021;375:n3151. doi: 10.1136/bmj.n3151. [DOI] [PubMed] [Google Scholar]
- 18.Kim K., Cho K., Song J., Rahmati M., Koyanagi A., Lee S.W., et al. The case fatality rate of COVID-19 during the Delta and the Omicron epidemic phase: a meta-analysis. J Med Virol. 2023;95 doi: 10.1002/jmv.28522. [DOI] [PubMed] [Google Scholar]
- 19.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Bager P., Wohlfahrt J., Rasmussen M., Albertsen M., Krause T.G. Hospitalisation associated with SARS-CoV-2 Delta variant in Denmark. Lancet Infect Dis. 2021;21:1351. doi: 10.1016/s1473-3099(21)00580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheikh A., McMenamin J., Taylor B., Robertson C. Public health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/s0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NHS England COVID-19 hospital activity. https://www.england.nhs.uk/statistics/statistical-work-areas/covid-19-hospital-activity Available from:
- 23.Bouzid D., Visseaux B., Kassasseya C., Daoud A., Fémy F., Hermand C., et al. Comparison of patients infected with Delta versus Omicron COVID-19 variants presenting to Paris emergency departments: a retrospective cohort study. Ann Intern Med. 2022;175:831–837. doi: 10.7326/m22-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butt A.A., Dargham S.R., Loka S., Shaik R.M., Chemaitelly H., Tang P., et al. Coronavirus disease 2019 disease severity in children infected with the Omicron variant. Clin Infect Dis. 2022;75:e361–e367. doi: 10.1093/cid/ciac275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butt A.A., Dargham S.R., Tang P., Chemaitelly H., Hasan M.R., Coyle P.V., et al. COVID-19 disease severity in persons infected with the Omicron variant compared with the Delta variant in Qatar. J Glob Health. 2022;12 doi: 10.7189/jogh.12.05032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Català M., Coma E., Alonso S., Andrés C., Blanco I., Antón A., et al. Transmissibility, hospitalization, and intensive care admissions due to Omicron compared to Delta variants of SARS-CoV-2 in Catalonia: a cohort study and ecological analysis. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.961030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen P.A., Olsen R.J., Long S.W., Snehal R., Davis J.J., Ojeda Saavedra M., et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the Omicron variant of severe acute respiratory syndrome coronavirus 2 in Houston, Texas. Am J Pathol. 2022;192:642–652. doi: 10.1016/j.ajpath.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esper F.P., Adhikari T.M., Tu Z.J., Cheng Y.W., El-Haddad K., Farkas D.H., et al. Alpha to Omicron: disease severity and clinical outcomes of major SARS-CoV-2 variants. J Infect Dis. 2023;227:344–352. doi: 10.1093/infdis/jiac411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrigan S.P., Wilton J., Chong M., Abdia Y., Garcia H.V., Rose C., et al. Clinical severity of severe acute respiratory syndrome coronavirus 2 Omicron variant relative to Delta in British Columbia, Canada: a retrospective analysis of whole-genome sequenced cases. Clin Infect Dis. 2023;76:e18–e25. doi: 10.1093/cid/ciac705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jassat W., Abdool Karim S.S., Mudara C., Welch R., Ozougwu L., Groome M.J., et al. Clinical severity of COVID-19 in patients admitted to hospital during the Omicron wave in South Africa: a retrospective observational study. Lancet Glob Health. 2022;10:e961–e969. doi: 10.1016/s2214-109x(22)00114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauring A.S., Tenforde M.W., Chappell J.D., Gaglani M., Ginde A.A., McNeal T., et al. Clinical severity and mRNA vaccine effectiveness for Omicron, Delta, and Alpha SARS-CoV-2 variants in the United States: a prospective observational study. medRxiv. 2022 doi: 10.1101/2022.02.06.22270558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J.E., Hwang M., Kim Y.H., Chung M.J., Sim B.H., Jeong W.G., et al. SARS-CoV-2 variants infection in relationship to imaging-based pneumonia and clinical outcomes. Radiology. 2023;306 doi: 10.1148/radiol.221795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayr F.B., Talisa V.B., Castro A.D., Shaikh O.S., Omer S.B., Butt A.A. COVID-19 disease severity in US Veterans infected during Omicron and Delta variant predominant periods. Nat Commun. 2022;13:3647. doi: 10.1038/s41467-022-31402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyberg T., Ferguson N.M., Nash S.G., Webster H.H., Flaxman S., Andrews N., et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/s0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paredes M.I., Lunn S.M., Famulare M., Frisbie L.A., Painter I., Burstein R., et al. Associations between SARS-CoV-2 variants and risk of COVID-19 hospitalization among confirmed cases in Washington State: a retrospective cohort study. medRxiv. 2022 doi: 10.1101/2021.09.29.21264272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peralta-Santos A., Rodrigues E.F., Moreno J., Ricoca V., Casaca P., Fernandes E., et al. Omicron (BA.1) SARS-CoV-2 variant is associated with reduced risk of hospitalization and length of stay compared with Delta (B.1.617.2) medRxiv. 2022 doi: 10.1101/2022.01.20.22269406. 2022.01.20.22269406. [DOI] [Google Scholar]
- 37.Sievers C., Zacher B., Ullrich A., Huska M., Fuchs S., Buda S., et al. SARS-CoV-2 Omicron variants BA.1 and BA.2 both show similarly reduced disease severity of COVID-19 compared to Delta, Germany, 2021 to 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.Es.2022.27.22.2200396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skarbinski J., Wood M.S., Chervo T.C., Schapiro J.M., Elkin E.P., Valice E., et al. Risk of severe clinical outcomes among persons with SARS-CoV-2 infection with differing levels of vaccination during widespread Omicron (B.1.1.529) and Delta (B.1.617.2) variant circulation in Northern California: a retrospective cohort study. Lancet Reg Health Am. 2022;12 doi: 10.1016/j.lana.2022.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stock S.J., Moore E., Calvert C., Carruthers J., Denny C., Donaghy J., et al. Pregnancy outcomes after SARS-CoV-2 infection in periods dominated by Delta and Omicron variants in Scotland: a population-based cohort study. Lancet Respir Med. 2022;10:1129–1136. doi: 10.1016/s2213-2600(22)00360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veneti L., Bøås H., Bråthen Kristoffersen A., Stålcrantz J., Bragstad K., Hungnes O., et al. Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 Omicron BA.1 variant compared with the Delta variant, Norway, December 2021 to January 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.Es.2022.27.4.2200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L., Berger N.A., Kaelber D.C., Davis P.B., Volkow N.D., Xu R. COVID infection rates, clinical outcomes, and racial/ethnic and gender disparities before and after Omicron emerged in the US. medRxiv. 2022 doi: 10.1101/2022.02.21.22271300. [DOI] [Google Scholar]
- 42.Wang L., Berger N.A., Kaelber D.C., Davis P.B., Volkow N.D., Xu R. COVID infection severity in children under 5 years old before and after Omicron emergence in the US. medRxiv. 2022 doi: 10.1101/2022.01.12.22269179. [DOI] [Google Scholar]
- 43.Wang L., Berger N.A., Kaelber D.C., Davis P.B., Volkow N.D., Xu R. Comparison of outcomes from COVID infection in pediatric and adult patients before and after the emergence of Omicron. medRxiv. 2022 doi: 10.1101/2021.12.30.21268495. [DOI] [Google Scholar]
- 44.Ward I.L., Bermingham C., Ayoubkhani D., Gethings O.J., Pouwels K.B., Yates T., et al. Risk of COVID-19 related deaths for SARS-CoV-2 Omicron (B.1.1.529) compared with Delta (B.1.617.2) medRxiv. 2022 doi: 10.1101/2022.02.24.22271466. 2022.02.24.22271466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolter N., Jassat W., Walaza S., Welch R., Moultrie H., Groome M.J., et al. Clinical severity of SARS-CoV-2 Omicron BA.4 and BA.5 lineages compared to BA.1 and Delta in South Africa. Nat Commun. 2022;13:5860. doi: 10.1038/s41467-022-33614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greene S.K., Levin-Rector A., Kyaw N.T.T., Luoma E., Amin H., McGibbon E., et al. Comparative hospitalization risk for SARS-CoV-2 Omicron and Delta variant infections, by variant predominance periods and patient-level sequencing results, New York City, August 2021-January 2022. Influenza Other Respir Virus. 2023;17 doi: 10.1111/irv.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bager P., Wohlfahrt J., Bhatt S., Stegger M., Legarth R., Møller C.H., et al. Risk of hospitalisation associated with infection with SARS-CoV-2 Omicron variant versus Delta variant in Denmark: an observational cohort study. Lancet Infect Dis. 2022;22:967–976. doi: 10.1016/s1473-3099(22)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adjei S., Hong K., Molinari N.M., Bull-Otterson L., Ajani U.A., Gundlapalli A.V., et al. Mortality risk among patients hospitalized primarily for COVID-19 during the Omicron and Delta variant pandemic periods–United States, April 2020-June 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1182–1189. doi: 10.15585/mmwr.mm7137a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beraud G., Bouetard L., Civljak R., Michon J., Tulek N., Lejeune S., et al. Impact of vaccination on the presence and severity of symptoms of hospitalised patients with an infection by the Omicron variant (B.1.1.529) of the SARS-CoV-2 (subvariant BA.1) medRxiv. 2022;2022 doi: 10.1101/2022.10.23.22281414. 10.23.22281414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marks K.J., Whitaker M., Agathis N.T., Anglin O., Milucky J., Patel K., et al. Hospitalization of infants and children aged 0-4 years with laboratory-confirmed COVID-19 –COVID-NET, 14 States, March 2020-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:429–436. doi: 10.15585/mmwr.mm7111e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Modes M.E., Directo M.P., Melgar M., Johnson L.R., Yang H., Chaudhary P., et al. Clinical characteristics and outcomes among adults hospitalized with laboratory-confirmed SARS-CoV-2 infection during periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant predominance–One Hospital, California, July 15-23 September 2021, and 21 December 2021-27 January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:217–223. doi: 10.15585/mmwr.mm7106e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shoji K., Akiyama T., Tsuzuki S., Matsunaga N., Asai Y., Suzuki S., et al. Clinical characteristics of COVID-19 in hospitalized children during the Omicron variant predominant period. J Infect Chemother. 2022;28:1531–1535. doi: 10.1016/j.jiac.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stålcrantz J., Kristoffersen A.B., Bøås H., Veneti L., Seppälä E., Aasand N., et al. Milder disease trajectory among COVID-19 patients hospitalised with the SARS-CoV-2 Omicron variant compared with the Delta variant in Norway. Scand J Public Health. 2022;50:676–682. doi: 10.1177/14034948221108548. [DOI] [PubMed] [Google Scholar]
- 54.Taylor C.A., Whitaker M., Anglin O., Milucky J., Patel K., Pham H., et al. COVID-19-associated hospitalizations among adults during SARS-CoV-2 Delta and Omicron variant predominance, by race/ethnicity and vaccination status–COVID-NET, 14 States, July 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:466–473. doi: 10.15585/mmwr.mm7112e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Goethem N., Chung P.Y.J., Meurisse M., Vandromme M., De Mot L., Brondeel R., et al. Clinical severity of SARS-CoV-2 Omicron variant compared with Delta among hospitalized COVID-19 patients in Belgium during Autumn and Winter season 2021-2022. Viruses. 2022;14:1297. doi: 10.3390/v14061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halfmann P.J., Iida S., Iwatsuki-Horimoto K., Maemura T., Kiso M., Scheaffer S.M., et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature. 2022;603:687–692. doi: 10.1038/s41586-022-04441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shuai H., Chan J.F., Hu B., Chai Y., Yuen T.T., Yin F., et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature. 2022;603:693–699. doi: 10.1038/s41586-022-04442-5. [DOI] [PubMed] [Google Scholar]
- 58.Abdullah F., Myers J., Basu D., Tintinger G., Ueckermann V., Mathebula M., et al. Decreased severity of disease during the first global Omicron variant COVID-19 outbreak in a large hospital in Tshwane, South Africa. Int J Infect Dis. 2022;116:38–42. doi: 10.1016/j.ijid.2021.12.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lauring A.S., Tenforde M.W., Chappell J.D., Gaglani M., Ginde A.A., McNeal T., et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from Omicron, Delta, and Alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376 doi: 10.1136/bmj-2021-069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 61.Cao B., Wang Y., Wen D., Liu W., Wang J.L., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hui K.P.Y., Ho J.C.W., Cheung M.C., Ng K.C., Ching R.H.H., Lai K.L., et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603:715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 63.Kawano Y., Patel N.J., Wang X., Cook C.E., Vanni K.M., Kowalski E.N., et al. Temporal trends in COVID-19 outcomes among patients with systemic autoimmune rheumatic diseases: from the first wave through the initial Omicron wave. Ann Rheum Dis. 2022;81:1742–1749. doi: 10.1136/ard-2022-222954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are disclosed in the paper, its associated figures, and the Supplementary Materials.