Abstract
Background and study aims The contamination level of ready-to-use endoscopes published in the literature varies from 0.4 % to 49.0 %. Unfortunately, the comparison and the interpretation of these results are quite impossible, given the limited number of samples and sites included and the differences observed between sampling, culturing methods, and interpretation criteria.
Methods The objective of this retrospective study was to analyze the results of 90,311 endoscope samples collected between 2004 and 2021 in 490 private or public hospitals in France.
Results Through the full test period, the mean ratio of endoscopes at the action level was 12.6 % (19.5 % including alert level). Of the endoscopy units, 23.0 % had a ratio of compliant endoscopes ≤ 70.0 %. The overall microbial quality of gastroscopes, duodenoscopes, and colonoscopes is improving year by year, whereas an opposite trend is observed for ultrasound endoscopes and bronchoscopes. In 2021, following French guidelines, 13.0 % of the endoscopes should have been quarantined and 8.1 % were at the alert level, meaning that the contamination level of 21.1 % of the endoscopes exceeded what was defined as a maximum acceptable value.
Conclusions This study demonstrates that additional efforts, including implementation of microbial surveillance strategies using a standardized sampling method and periodic observational audits, must be made to improve the overall microbiological quality of endoscopes and reduce the risk associated with their use.
Introduction
In the past 30 years, a lot of progress has been made regarding endoscope reprocessing thanks, among other things, to publication of recommendations/ guidelines 1 2 , use of automatic endoscope reprocessors compliant with ISO 15883–4 3 , use of non-fixative disinfectants 4 or changes in endoscope design. The implementation of microbiological surveillance programs, including endoscope sampling, as suggested in some guidelines 5 6 7 , may also have contributed indirectly to improving the overall quality of endoscopes.
Unfortunately, only a few studies involving a large number of endoscopes and over a long enough period of time have been published and it is difficult to evaluate the real impact of all these changes on the microbiological quality of endoscopes in real use conditions and on the risk of infection associated with these devices.
Studies published in the literature indicate that the contamination level (or non-compliance rate) of ready-to-use endoscopes varies from 0.4 % to 49.0 % 8 9 10 11 12 13 . However, the limited number of samples analyzed and the differences observed between these studies regarding the sampling method (flush vs flush-brush-flush, one channel vs all channels), nature of the sampling solution (water, 0.9 % NaCl, neutralizer), sample culturing protocols (filtration vs centrifugation) or the interpretation criteria make it difficult to compare and interpret these values 5 8 9 11 12 13 14 15 .
The objective of this retrospective study was to analyze the results of 90,311 endoscopes samples collected between 2004 and 2021 in 490 private or public hospitals in France.
Methods
The sampling method was based upon the method described in the French guidelines published in 2007 1 and 2018 16 .
As recommended, endoscopes were sampled at least 6 hours after the last reprocessing procedure. All endoscope channels were flushed with 10 to 40 mL of sampling solution NPD + thiosulfate, Biomerieux AEB611326M using sterile connectors/blockers. To increase the efficacy of the sampling method, a back-and-forth movement was performed with the sampling solution in the endoscope channels during sampling 17 . Channels were then purged with air and the sampling solution was collected in a sterile container at the distal end of the endoscopes. All samples were collected by trained laboratory technicians. Endoscope samples were stored at 5 °C ± 3 °C for not more than 24 hours and analyzed using membrane filtration method. After filtration of the entire sample volume, 0.45-µm membrane filters were incubated 5 days at 30 °C ± 2 °C on Plate Count Agar (Thermo Fisher, PO5013A). For bronchoscopes, samples were divided into two portions. The first half was filtered and incubated on Plate Count Agar and the second half on Middlebrook 7H10 agar (Becton Dickinson, 254520) for 21 days at 30 °C ± 2 °C.
After incubation, colonies were counted and bacteria isolated were identified using standard laboratory methods (Gram staining, biochemical tests). Results were expressed as a total number of colony-forming unit (CFU)/endoscope and interpreted using the criteria defined in the French guideline ( Table 1 ).
Table 1. Interpretation criteria for routine endoscope sampling as defined in French guideline 1 .
| Target level | Alert level | Action level | |
| Endoscopes introduced into sterile cavities (e. g. high-risk endoscopes: choledoscopes, hysteroscopes and cystoscopes) | Total viable count < 1 CFU/endoscope | Not applicable | Total viable count ≥ 1 CFU/endoscope or presence of indicator microorganisms * |
| Endoscopes that come in contact with mucous membranes (e. g., gastroscope, colonoscopes, bronchoscopes, duodenoscopes) | Total viable count < 5 CFU/endoscope and no indicator microorganisms | Total viable count between 5 and 25 CFU/ endoscope and no indicator microorganisms |
Total viable count > 25 CFU/endoscope or presence of indicator microorganisms |
Indicator microorganisms: Staphylococcus aureus , Enterobacteriaceae, Pseudomonas aeruginosa and other Pseudomonas, Stenotrophomonas maltophilia , Acinetobacter sp , Candida sp.
Sampling protocol for duodenoscopes
After publication in 2018 of a new guideline regarding the reprocessing of duodenoscopes in healthcare settings 16 , the sampling protocol applied to duodenoscopes was modified. Following this new protocol, two samples were collected. The first one was issued from endoscope channels using the standard method described above. The second one dedicated to the distal end of the duodenoscope included immersion of the distal end of the endoscope in the recovering solution and sampling of the inside of the elevator mechanism and the recessed area surrounding the elevator lever with a sterile brush. The two samples were then analyzed by filtration as described previously. Colonies isolated on both membranes were added and results were expressed as a total number of CFU/endoscope and interpreted using the criteria defined in the French guideline ( Table 1 ).
The ability of the sampling solution to collect small numbers of bacteria and the recovery efficiency of the sampling method were determined by exhaustive extraction according to ISO 11737–1 annex C1 18 .
Results
A total of 90,311 endoscopes were sampled between 2004 and 2021. The number of endoscopes sampled per year varied from 223 in 2004 to 18,288 in 2021 ( Table 2 ).
Table 2. Endoscope compliance rates. Number of endoscopes found at the alert and action levels and number of samples collected between 2004 and 2021.
| Endoscope compliance rates | Total no. samples | ||||||
| Action level | Alert level | Target level | |||||
| n 1 | % | n 2 | % | n 3 | % | Σn | |
| 2004 | 44 | 19,7 % | 18 | 8,1 % | 161 | 72,2 % | 223 |
| 2005 | 79 | 20,6 % | 19 | 4,9 % | 286 | 74,5 % | 384 |
| 2006 | 65 | 13,3 % | 10 | 2,0 % | 414 | 84,7 % | 489 |
| 2007 | 142 | 18,7 % | 34 | 4,5 % | 584 | 76,8 % | 760 |
| 2008 | 181 | 15,3 % | 31 | 2,6 % | 970 | 82,1 % | 1182 |
| 2009 | 379 | 22,5 % | 104 | 6,2 % | 1203 | 71,4 % | 1686 |
| 2010 | 339 | 20,0 % | 118 | 7,0 % | 1238 | 73,0 % | 1695 |
| 2011 | 245 | 14,1 % | 106 | 6,1 % | 1392 | 79,9 % | 1743 |
| 2012 | 320 | 15,4 % | 183 | 8,8 % | 1580 | 75,9 % | 2083 |
| 2013 | 385 | 13,9 % | 247 | 8,9 % | 2146 | 77,2 % | 2778 |
| 2014 | 472 | 14,1 % | 263 | 7,9 % | 2607 | 78,0 % | 3342 |
| 2015 | 635 | 13,8 % | 363 | 7,9 % | 3596 | 78,3 % | 4594 |
| 2016 | 411 | 10,8 % | 315 | 8,3 % | 3069 | 80,9 % | 3795 |
| 2017 | 863 | 8,6 % | 517 | 5,1 % | 8713 | 86,3 % | 10093 |
| 2018 | 1492 | 13,4 % | 753 | 6,7 % | 8912 | 79,9 % | 11157 |
| 2019 | 1425 | 11,5 % | 785 | 6,3 % | 10162 | 82,1 % | 12372 |
| 2020 | 1476 | 10,8 % | 849 | 6,2 % | 11322 | 83,0 % | 13647 |
| 2021 | 2384 | 13,0 % | 1480 | 8,1 % | 14424 | 78,9 % | 18288 |
| Total | 11337 | 12,6 % | 6195 | 6,9 % | 72779 | 80,6 % | 90311 |
A total of 490 private or public health facilities all located in France were included in this study. The distribution of these health facilities according to the mean compliance rate of their endoscopes ( Fig. 1 ) shows that for 51 % of the endoscopy units, at least 80 % of the endoscopes presented a contamination level considered to be acceptable (i. e. target level). However, the compliance rate reached or exceeded 90 % for only 15 % of these centers and 23 % of them had a ratio of endoscopes at the target level ≤ 70 %.
Fig. 1.
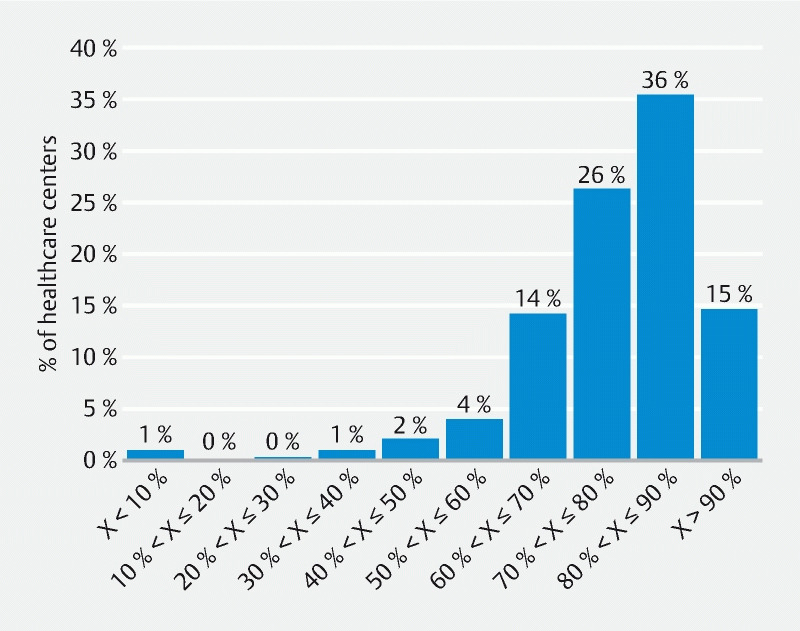
Distribution of healthcare centers included in the study between 2004 and 2021 according to their ratio of compliant endoscopes (X = percentage of endoscopes at the target level).
Through the full test period, the mean ratio of endoscopes at the action level, i.e. containing at least one indicator microorganism or more than 25 CFU/endoscope (endoscopes in contact with mucous membranes) or more than 1 CFU/endoscope (high risk endoscopes) was 12.6 %. If we include the endoscopes at the alert level, the mean ratio of endoscopes that contained a number of bacteria exceeding the range defined in the French guideline as normal would reach 19.5 % ( Table 2 ).
A more precise analysis of the results obtained between 2004 and 2021 ( Fig. 2 ) indicates that even if the evolution of the percentage of non-compliant endoscopes was irregular, the overall quality of endoscopes is improving. Thus, the mean ratio of endoscopes at the action level decreased from 19.7 % in 2004 to 13.0 % in 2021 and from 27.8 % in 2004 to 21.1 % in 2021 if we include the alert level. The ratio of non-compliant endoscopes measured in 2021 was above the global trend (represented by the regression line) and slightly higher than the data obtained in 2020 (i. e. 13.0 % at the action level in 2021 vs. 10.8 % in 2020). However, based on the data presented, it is impossible to know if the increase corresponds to a real change in the evolution or only a random variation as observed in 2006, 2009, 2010, and 2017.
Fig. 2.
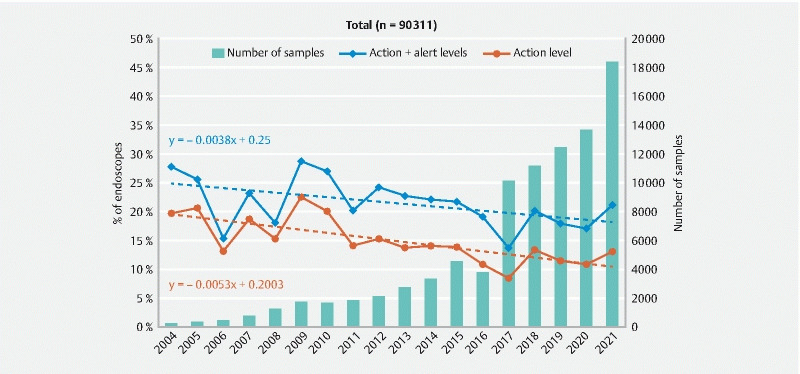
Evolution of the ratio of endoscopes at the action and alert levels and number of endoscopes sampled between 2004 and 2021.
The evolution of the quality of the endoscopes according to the nature of the devices and their regression lines which describe the behavior of the set of data through the test period are presented in Fig. 2 , Fig. 3 , Fig. 4 , Fig. 5 , Fig. 6 , Fig. 7 , Fig.8 and Fig. 9 . They were established from non-compliant rates calculated from groups of samples of at least 50 endoscopes.
Fig. 3.
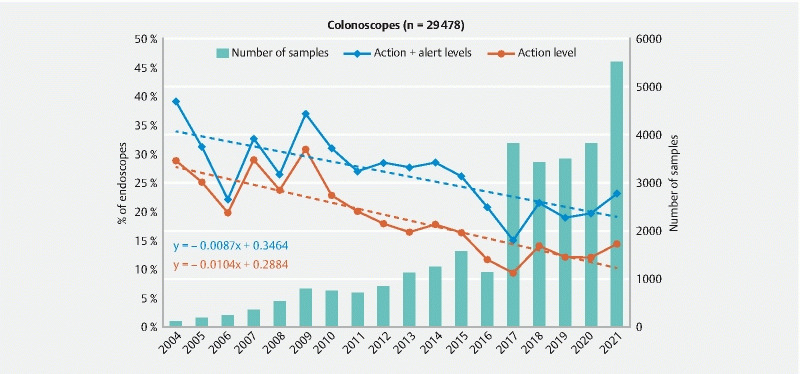
Evolution of the ratio of colonoscopes at the action and alert levels and number of endoscopes sampled between 2004 and 2021.
Fig. 4.
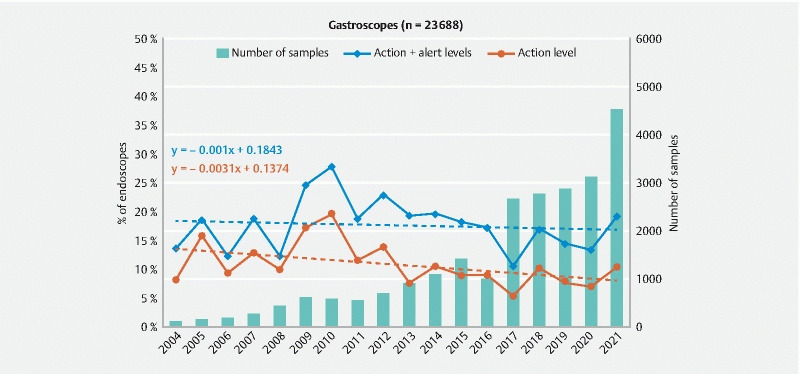
Evolution of the ratio of gastroscopes at the action and alert levels and number of endoscopes sampled between 2004 and 2021.
Fig. 5.
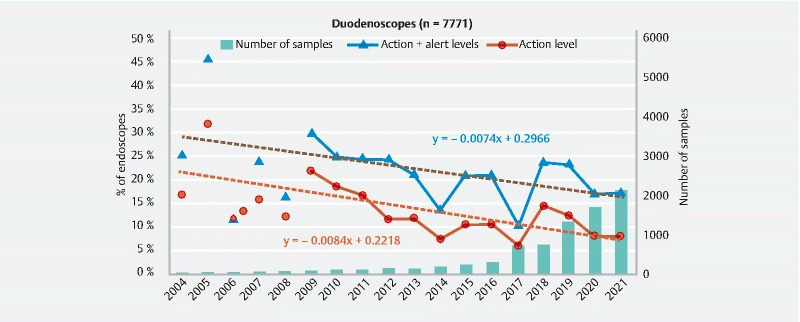
Evolution of the ratio of duodenoscopes at the action and alert levels and number of endoscopes sampled between 2004 and 2021.
Fig. 6.
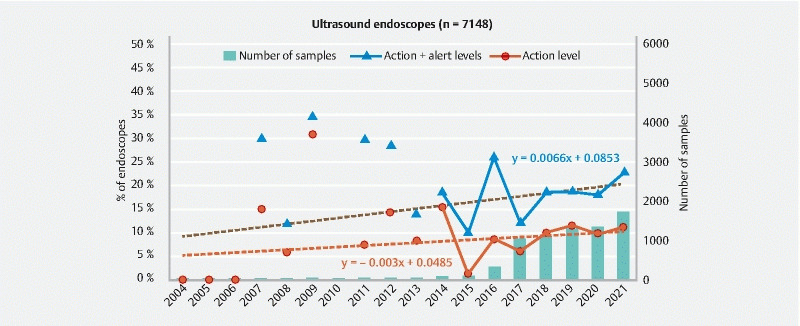
Evolution of the ratio of ultrasound endoscopes at the action and alert levels and number of endoscopes sampled between 2004 and 2021.
Fig. 7.
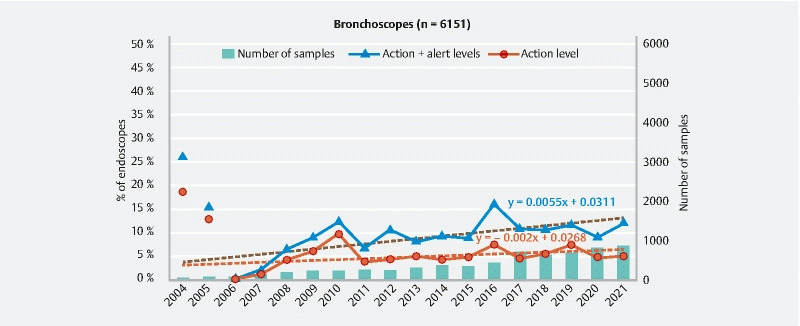
Evolution of the ratio of bronchoscopes at the action and alert levels and number of endoscopes sampled between 2004 and 2021.
Fig. 8.
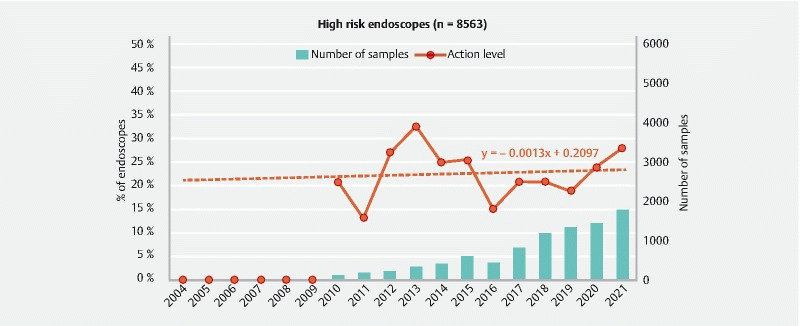
Evolution of the ratio of high-risk endoscopes at the action and alert levels and number of endoscopes sampled between 2004 and 2021.
Fig. 9.
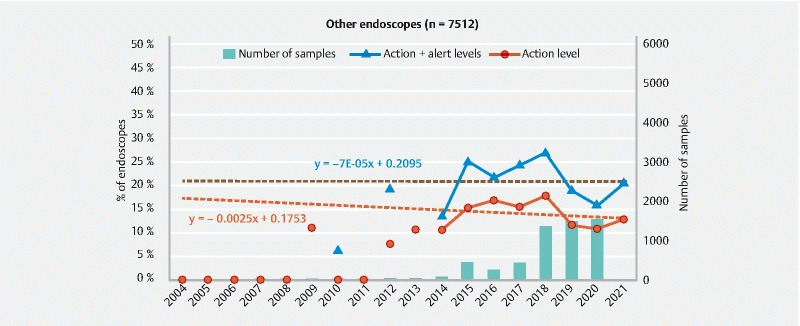
Evolution of the ratio of other endoscopes at the action and alert levels and number of endoscopes sampled between 2004 and 2021.
Analysis of these curves indicates that the ratio of colonoscopes at the action level decreased from 28.7 % in 2004 to 14.4 % in 2021( Fig. 3 ). According to the equation of the regression line, representing the mean evolution of the microbial quality of endoscopes through the test period, this variation corresponds to a reduction in the initial contamination rate of 1 % per year (0.9 % if we include the endoscopes at the alert level). In 2021, we observed an increase in the ratio of colonoscopes at the action level compared to the global trend and to 2020 (i. e. 14.4 % at the action level in 2021 vs. 12.9 % in 2020). However, it is impossible to know if this increase was due to a real change in the trend or only to data variability as observed in 2006, 2007, 2009, and 2017.
For gastroscopes ( Fig. 4 ), the decrease is much less important than for colonoscopes and according to the equation of the regression line, the mean ratio of gastroscopes at the action level was only reduced by 0.3 % per year through the test period (0.1 % if we include the alert level). These data also indicate that the mean contamination level of gastroscopes measured in 2021 was lower than for colonoscopes (10.2 % vs 14.4 % for the action level and 19.2 % vs 23.1 % if we consider endoscopes at the action and alert level).
For duodenoscopes, the curves representing the evolution of their quality through time were established from 2009, considering that the number of duodenoscope samples per year before 2009 was less than 50 ( Fig. 5 ). In 2009, the ratio of duodenoscopes at the action level was 21.9 % (29.7 % including alert level). These contamination rates are lower than the values observed the same year for colonoscopes (30.7 % and 36.7 %) but higher than for gastroscopes (17.1 % and 24.5 %). The slopes of the regression lines are comparable to those observed for colonoscopes with a reduction in the non-compliance rate of 0.84 % per year for endoscopes at the action level and 0.74 % if we include endoscopes at the alert level. However, despite the overall downward trend observed between 2009 and 2017, results obtained for duodenoscopes are also marked by an increase in the non-compliance rates in 2018 (+ 8.4 % for the action level compared to 2017). This increase was probably due to the implementation in 2018 of the enhanced sampling procedure for duodenoscopes 16 published by the French Ministry of Health as recommended by the Centers for Disease Control and Prevention few years before 7 .
Ultrasound endoscopes have appeared more recently on the market and the number of samples collected per year exceeds 50 only after 2013. The slope of the regression line representing the mean evolution of the data after 2014 indicates that the microbial quality of these endoscopes degrades over time (+ 0.7 % per year for endoscopes at the action and alert levels). This increase may also be due to the update in 2018 of the sampling protocol used for some ultrasound endoscopes (i. e. linear ultrasound endoscopes). Thus in 2021, the ratio of ultrasound endoscopes at the action level was 11.2 % (22.8 % if endoscopes at the alert levels are included). These results are comparable to those obtained for gastroscopes ( Fig. 6 ).
Results obtained for bronchoscopes ( Fig. 7 ) demonstrate that the microbial quality of these endoscopes is better than for digestive endoscopes. In 2021, the ratio of bronchoscopes at the action level was only 5 % (12.1 % if alert level is included). Nevertheless, is important to highlight that the slope of regression line describing the general trend of the data points indicates that the quality of these endoscopes degrades over time (+ 0.6 % per year for endoscopes at the action and alert levels).
The endoscopes with the highest contamination level (27.9 % at the action level in 2021) are the high-risk endoscopes (cystoscope, ureteroscope, hysteroscope, choledoscope). Analysis of the regression line of the curve representing the data obtained between 2010 and 2021 ( Fig. 8 ) shows that the mean non-compliance rate of high-risk endoscopes was relatively stable ( + 0.1 % per year), even if important variations are observed between values measured each year. This situation is due to the interpretation criteria, which are more restrictive for the endoscopes in contact with sterile cavities (< 1 CFU/endoscope) than for the other endoscopes (> 5 CFU/endoscope or presence of indicator microorganisms).
Results presented in Fig. 10 show for each endoscope type (e. g. gastroscope, colonoscope) the distribution in 2021 of the mean non-compliance rate of each endoscope model (each point represent the non-compliance rate for the action level for a specific endoscope model). A comparison of the third-quartile values (Q3), which represent the maximum non-compliance rate observed for the lowest 75 % of data, confirm that bronchoscopes present the lowest risk. For bronchoscopes, Q3 was 6.5 %, meaning that 75 % of the 40 different models of bronchoscopes sampled had a non-compliance rate < 6.5 %. This Q3 value was 10 % for gastroscopes, 11.1 % for duodenoscopes, 13.6 % for colonoscopes and 14.9 % for ultrasound endoscopes.
Fig. 10.
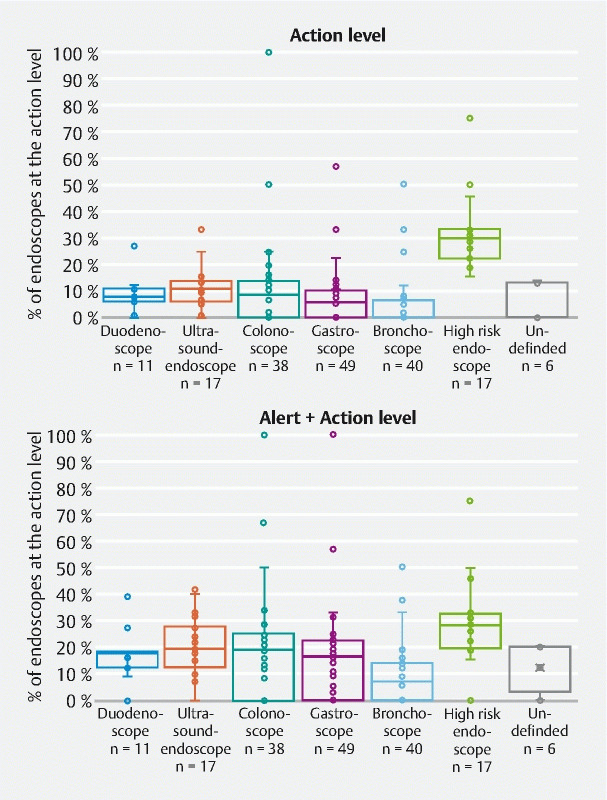
Variation in the mean ratio of non-compliant endoscopes according to the endoscope model for each endoscope type (each point represents a specific endoscope model). Quartile representation, 2021 results.
In addition, analysis of the interquartile range (IQR), which is a measure of data dispersion, shows that the widest dispersion of the data was observed for colonoscopes (IQR = 13.6 %), followed by the high-risk endoscopes (IQR = 11.1 %), gastroscopes (IQR = 10.0 %), ultrasound endoscopes (IQR = 7.9 %), bronchoscopes (IQR = 6.5 %) and duodenoscopes (IQR = 4.8 %). The same analysis, done for endoscopes at the alert and action levels ( Fig. 10 ), confirms the previous observations.
Analysis of the microorganisms recovered from endoscope channels ( Table 3 ) shows that when growth is detected, microorganisms from the environment such as fungi and Bacillus sp. were isolated in respectively 11 % and 35 % of the samples. The presence of Coagulase-negative Staphylococcus and Micrococcus sp. whose origin can be environmental or human was observed in 35 % of samples for which growth was observed. Among all microorganisms found in endoscope channels, Enterobacteriaceae were identified in 13 % of samples. Results presented in Table 3 also show that Pseudomonas aeruginosa remains one of the major endoscope contaminants with a prevalence of 13 %. Waterborne bacteria such as Pseudomonas sp . and other gram-negative rods were isolated in 6 % and 18 % of samples harboring contamination, respectively.
Table 3. Nature of the microorganisms recovered from endoscope channels. Frequency of occurrence of each microorganism in endoscope samples when a growth was observed (n = 16959).
| Microorganisms | % |
| Bacillus sp. | 35 |
| Coagulase-negative staphylococcus, Micrococcus sp. | 35 |
| Other Gram-negative rod ( Burkholderia sp., Stenotrophomonas , Sphinghomonas sp., Aeromonas sp., Brevundimonas sp. | 18 |
| Pseudomonas aeruginosa | 13 |
| Enterobacteriaceae ( Enterobacter sp., Escherichia coli , Klebsiella sp., Proteus sp., Serratia sp. | 13 |
| Fungi | 11 |
| Pseudomonas sp . | 6 |
| Neisseria sp . | 3 |
| Yeast ( Candida sp., Cryptococcus sp., Rhodotorula sp.) | 3 |
| Corynebacterium sp . | 2 |
| Other Gram-positif cocci ( Staphylococcus aureus , Streptococcus sp.) | 1 |
Discussion
The data collected in this study show that in 2021 in France, the mean ratio of endoscopes which should have been quarantined (i. e. at the action level) was 12.6 %. If we include the alert level, the results demonstrate that 19.5 % of endoscopes had contamination rate that was not considered to be safe for use according to French guidelines. Due to more restrictive interpretation criteria, endoscopes with the higher non-compliance rate are high-risk endoscopes (heat-sensitive endoscopes in contact with sterile cavities) with 27.9 % of them at the action level. Among endoscopes in contact with mucosa, colonoscopes had the highest non-compliance rate (14.4 % at the action level and 23.1 % if we include the alert level), followed by ultrasound gastroscopes (11.2 %-22.8 %), gastroscopes (10.2 %-19.2 %), duodenoscopes (8.0 %-17.2 %) and bronchoscopes with only 5.0 % of the endoscopes at the action level and 12.1 % at the alert or action level. Analysis of the evolution of these non-compliance rates through the full test period demonstrated that the overall microbial quality of gastroscopes, duodenoscopes, and colonoscopes is improving year by year whereas an opposite trend is observed for ultrasound endoscopes and bronchoscopes. No significant trend was observed for high-risk endoscopes and undefined endoscopes.
The results presented in this study are in line with the data published by Saviuc P et al 9 who demonstrated, using the sampling method described in the French guideline 1 , a 14 % non-compliance rate (action level) from 846 gastrointestinal endoscopes sampled during an 8-year microbiological surveillance study. This value was confirmed by Chiu KW et al 8 , who showed in 2012 that 20.8 % of colonoscopes and 10.4 % of gastroscopes were identified to be culture positive.
Data obtained for duodenoscopes are similar to those published in 2020 by Mark et al 19 , who reported, that after initial high-level disinfection, 21 of 117 duodenoscopes (18 %) were identified to be culture-positive (i. e. > 10 CFUs of low-concern organisms or any CFUs of high-concern organisms). In the same way, Rawers et al 20 showed in 2017 that 15 % of duodenoscopes harbored microorganisms with gastrointestinal or oral origin and 22 % of them had > 20 CFUs per endoscope. A more recent study based on a systematic literature review and meta-analysis identified a 15.25 % contamination rate for reprocessed patient-ready duodenoscopes, which is confirmed by our findings 21 . A few other studies demonstrating similar or even higher contamination levels have been published 11 22 .
In contrast, few others publications seem to indicate that the non-compliance rates of endoscopes are well above those observed in the present study 13 23 24 25 26 27 28 29 30 31 . Thus, in 2007, Gillespie et al 13 reported a contamination rate of only 0.4 % in a 5-year study involving 1456 endoscope samples. In their study published in 2021, Marchese et al 23 analyzed 811 samples and 12 of them were considered to be positive (i. e. 1.5 %).
However, it is important to emphasise that these studies were performed in a limited number of medical centers using a sampling method and interpretation criteria very different from those used in our study. In both studies, endoscope channels were flushed with sterile water (10 mL per channel), one part of the sample (10 mL) was centrifuged and only 0.1 mL were inoculated onto both blood and MacConkey agar incubated aerobically at 35 °C for 5 days. The detection limit for this method (which should be about 50 CFU per endoscope or more) is probably one of the factors explaining the difference observed with the data presented in our study.
The other factor that may have a critical influence on the recovery efficiency of the sampling method and which may explain the very low contamination rate observed in the Gillespie and Marchese studies is the nature of the sampling solution. In these two studies, endoscopes were sampled with sterile water or modified phosphate buffer saline, whereas data presented by Aumeran et al 32 and Richard et al 33 clearly indicated that the Tween 80-Lecithin-based solution presents a higher recovery efficiency and is more efficient than NaCl or sterile water in detecting contaminated endoscopes. Furthermore, Marchese explains that endoscopes were sampled just after reprocessing, which may also explain why they found such very low contamination rates. Indeed, when endoscopes are sampled immediately after reprocessing, it is unlikely that the 50 mL of sampling solution injected in the endoscope channels will collect more microorganisms than the few liters of reprocessing solution circulating in the endoscope channels during the previous reprocessing cycle. It is for this reason that the French guideline 1 recommends that routine microbial surveillance of endoscopes be done by sampling the instrument at least 6 hours after the last reprocessing procedure to allow microorganisms that may be present within a biofilm to grow and became detectable.
Taking into account all factors that may have an impact on the efficacy of an endoscope sampling method 6 15 , it is highly probable that some studies may have underestimated the real contamination level of endoscopes and, therefore, they should be considered with caution in any comparison or attempt to define the threshold limit for endoscope contamination level. As stated by Heuvelmans et al 34 and Kenters et al 35 , there is a real need to develop a standardized sampling protocol for endoscopes, including common interpretation criteria to allow any healthcare facility to evaluate the real risk associated with use of their endoscopes and facilitate evaluation of the impact of any new reprocessing method or process.
The comparison of the mean non-compliance rate of each endoscope gives a first indication of the risk associated with a specific endoscope type but does not permit identification of variations that may exist between endoscope models within the same type/family. Thus, analyzing the compliance rates of each endoscope model, the quartile analysis of the data, shows an important variation between endoscope models (mainly for high-risk endoscopes, colonoscopes and gastroscopes) but also that ultrasound endoscopes present a median non-compliance rate higher than colonoscopes, whereas the comparison of the mean non-compliance indicates the opposite. This observation confirms that today, given their very complex internal design, ultrasound endoscopes represent the greatest challenge in terms of reprocessing. Moreover, considering the multiple interconnections that exist between channels inside such endoscopes, the existing sampling method may not permit sampling of all channels and there is a risk that the non-compliance rate obtained for ultrasound endoscopes has been underestimated.
The results of the analysis carried out on part of the samples (n = 16,959) are in line with data previously published 8 11 26 29 and show that the contamination found in endoscopes channels is from multiple origins. The presence of microorganisms from the environment suggests that contamination may occur before and/or during sampling and underscores the importance of a well-mastered sampling technique. However, the presence of Enterobacteriaceae , Pseudomonas aeruginosa and other gram-negative rods indicates that flaws in the reprocessing procedures remain an important source of contamination.
Conclusions
The results of this study demonstrate that in 2021 following French guidelines, 13.0 % of endoscopes should have been quarantined (i. e. at the action level) and if we include the alert level, the results demonstrate that 21.1 % of the endoscopes present a contamination rate not in keeping with safe use conditions. Some improvements were noted year by year, but the current microbiological quality of endoscopes remains unacceptable and the safety margin provided by the current reprocessing procedures is not high enough. Additional efforts, including periodic observational audits, must be made to improve the overall microbiological quality of our endoscopes and reduce the risk associated with their use 15 .
The presence of bacteria in the channels of an endoscope is not always synonymous with infection, but it is obvious that the higher the contamination level of an endoscope, the greater the risk of endoscopy-related transmission of infection. Therefore, microbial surveillance strategies including periodic endoscope sampling should be implemented to reduce the number of contaminated endoscopes and contribute to reduction in the infectious risk. Considering that type testing done to initially validate an endoscope washer-disinfector according to ISO 15883-4 3 are done on a limited number of endoscopes intended to represent all endoscopes that could be reprocessed in the washer, endoscope sampling is the only valid tool to verify that all endoscope models present at a specific site can be reprocessed effectively in the washer-disinfector.
However, a lot of factors may have an influence on the efficacy of an endoscope sampling method and, to be fully efficient, the method needs to be standardized and common threshold limits for it should be defined.
Footnotes
Competing interests L Pineau is an employee of Eurofins Biotech Germande which reports having been consulted and having received financial support from medical device manufacturers to performed studies on the efficacy of medical devices.
References
- 1.Beilenhoff U, Biering H, Blum R et al. ESGE-ESGENA technical specification for process validation and routine testing of endoscope reprocessing in washer-disinfectors according to EN ISO 15883, parts 1, 4, and ISO/TS 15883-5. Endoscopy. 2017;49:1262–1275. doi: 10.1055/s-0043-122073. [DOI] [PubMed] [Google Scholar]
- 2.Beilenhoff U, Biering H, Blum R et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) – Update 2018. Endoscopy. 2018;50:1205–1234. doi: 10.1055/a-0759-1629. [DOI] [PubMed] [Google Scholar]
- 3.ISO 15883-4:2018. Washer-disinfectors – Part 4: Requirements and tests for washer-disinfectors employing chemical disinfection for thermolabile endoscopes. https://www.iso.org/standard/63696.html https://www.iso.org/standard/63696.html
- 4.Pineau L, Desbuquois C, Marchetti B et al. Comparison of the fixative properties of five disinfectant solutions. J Hosp Infect. 2008;68:171–177. doi: 10.1016/j.jhin.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Instruction n° DGOS/PF2/DGS/VSS1/2016/220 du 4 juillet 2016 relative au traitement des endoscopes souples thermosensibles à canaux au sein des lieux de soins. https://www.legifrance.gouv.fr/circulaire/id/41172 https://www.legifrance.gouv.fr/circulaire/id/41172
- 6.Beilenhoff U, Neumann C, Rey J-F et al. ESGE-ESGENA Guideline for quality assurance in reprocessing: microbiological surveillance testing in endoscopy. Endoscopy. 2007;39:175–181. doi: 10.1055/s-2006-945181. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention . Interim Protocol for Healthcare Facilities Regarding Surveillance for Bacterial Contamination of Duodenoscopes after Reprocessing. http://medbox.iiab.me/modules/en-cdc/www.cdc.gov/hai/organisms/cre/cre-duodenoscope-surveillance-protocol.html http://medbox.iiab.me/modules/en-cdc/www.cdc.gov/hai/organisms/cre/cre-duodenoscope-surveillance-protocol.html
- 8.Chiu K W, Tsai M C, Wu K L et al. Surveillance cultures of samples obtained from biopsy channels and automated endoscope reprocessors after high-level disinfection of gastrointestinal endoscopes. BMC Gastroenterol. 2012;12:120. doi: 10.1186/1471-230X-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saviuc P, Picot-Guéraud R, Shum Cheong Sing J et al. Evaluation of the quality of reprocessing of gastrointestinal endoscopes. Infect Control Hosp Epidemiol. 2015;36:1017–1023. doi: 10.1017/ice.2015.123. [DOI] [PubMed] [Google Scholar]
- 10.Bader L, Blumenstock G, Birkner B et al. HYGEA (Hygiene in der Gastroenterologie - Endoskop-Aufbereitung): Studie zur Qualität der Aufbereitung von flexiblen Endoskopen in Klinik und Praxis* [HYGEA (Hygiene in gastroenterology--endoscope reprocessing): Study on quality of reprocessing flexible endoscopes in hospitals and in the practice setting] Z Gastroenterol. 2002;40:157–170. doi: 10.1055/s-2002-22326. [DOI] [PubMed] [Google Scholar]
- 11.Saliou P, Garlantézec R, Baron R et al. Microbiological investigation of endoscopes at Brest Hospital over a period from 2007 to 2009. Pathol Biol. 2011;59:88–93. doi: 10.1016/j.patbio.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto N, Sczaniecka A, Hirano M et al. A prospective, multicenter, clinical study of duodenoscope contamination after reprocessing. Infect Control Hosp Epidemiol. 2022;18:1–9. doi: 10.1017/ice.2021.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillespie E, Despina Kotsanas D, Stuart R L. Microbiological monitoring of endoscopes: 5-year review. J Gastroenterol Hepatol. 2008;23:1069–1074. doi: 10.1111/j.1440-1746.2007.05264.x. [DOI] [PubMed] [Google Scholar]
- 14.Eléments d’assurance qualité en hygiène relatifs au contrôle microbiologique des endoscopes et à la traçabilité en endoscopie. Conseil supérieur d’hygiène publique de France. March 2007. https://solidarites-sante.gouv.fr/IMG/pdf/microbio_endoscopes-2.pdf https://solidarites-sante.gouv.fr/IMG/pdf/microbio_endoscopes-2.pdf
- 15.Alfa M J, Singh H. Contaminated flexible endoscopes: Review of impact of channel sampling methods on culture results and recommendations for root-cause analysis. Infect Control Hosp Epidemiol. 2022;43:623–628. doi: 10.1017/ice.2021.128. [DOI] [PubMed] [Google Scholar]
- 16.DGOS/PF2/DGS/VVS1/PP3/2018/195 du 2 août 2018 relative à l’actualisation du traitement des endoscopes souples thermosensibles à canaux de type duodénoscope au sein des structures de soins. https://www.legifrance.gouv.fr/download/pdf/circ?id=43880 https://www.legifrance.gouv.fr/download/pdf/circ?id=43880
- 17.Buss A, Been M, Borgers R et al. Endoscope disinfection and its pitfalls – requirement for retrograde surveillance cultures. Endoscopy. 2008;40:327–332. doi: 10.1055/s-2007-995477. [DOI] [PubMed] [Google Scholar]
- 18.ISO 11737-1:2018. Stérilisation des produits de santé — Méthodes microbiologiques — Partie 1: Détermination d'une population de microorganismes sur des produits. https://www.iso.org/fr/standard/66451.html https://www.iso.org/fr/standard/66451.html
- 19.Mark J A, Underberg K, Kramer R E. Results of duodenoscope culture and quarantine after manufacturer-recommended cleaning process. Gastrointest Endosc. 2020;91:1328–1333. doi: 10.1016/j.gie.2019.12.050. [DOI] [PubMed] [Google Scholar]
- 20.Rauwers A W, Voor In't Holt A F, Buijs J G et al. High prevalence rate of digestive tract bacteria in duodenoscopes: a nationwide study. Gut. 2018;67:1637–1645. doi: 10.1136/gutjnl-2017-315082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen S, Russell R V, Ockert L K et al. Rate and impact of duodenoscope contamination: A systematic review and meta-analysis. E Clin Med. 2020;25:100451. doi: 10.1016/j.eclinm.2020.100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cottarelli A, De Giusti M, Solimini A G et al. Microbiological surveillance of endoscopes and implications for current reprocessing procedures adopted by an Italian teaching hospital. Ann Ig. 2020;32:166–177. doi: 10.7416/ai.2020.2340. [DOI] [PubMed] [Google Scholar]
- 23.Marchese V, Di Carlo D, Fazio G et al. Microbiological surveillance of endoscopes in a southern Italian transplantation hospital: a retrospective study from 2016 to 2019. Int J Environ Res Public Health. 2021;18:3057. doi: 10.3390/ijerph18063057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartles R L, Leggett J E, Hove S et al. A randomized trial of single versus double high-level disinfection of duodenoscopes and linear echoendoscopes using standard automated reprocessing. Gastrointest Endosc. 2018;88:306–313. doi: 10.1016/j.gie.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Ma G K, Pegues D A, Kochman M L et al. Implementation of a systematic culturing program to monitor the efficacy of endoscope reprocessing: outcomes and costs. Gastrointest Endosc. 2018;87:104–109. doi: 10.1016/j.gie.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Chapman C G, Siddiqui U D, Manzano M et al. Risk of infection transmission in curvilinear array echoendoscopes: results of a prospective reprocessing and culture registry. Gastrointest Endosc. 2017;85:390–397. doi: 10.1016/j.gie.2016.07.049. [DOI] [PubMed] [Google Scholar]
- 27.Snyder G M, Wright S B, Smithey A et al. Randomized comparison of 3 high-level disinfection and sterilization procedures for duodenoscopes. Gastroenterology. 2017;153:1018–1025. doi: 10.1053/j.gastro.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 28.Paula H, Presterl E, Tribl B et al. Microbiologic surveillance of duodenoscope reprocessing at the Vienna university hospital from November 2004 through March 2015. Infect Control Hosp Epidemiol. 2015;36:1233–1235. doi: 10.1017/ice.2015.146. [DOI] [PubMed] [Google Scholar]
- 29.Ross A S, Baliga C, Verma P et al. A quarantine process for the resolution of duodenoscope-associated transmission of multidrug-resistant Escherichia coli. Gastrointest Endosc. 2015;82:477–483. doi: 10.1016/j.gie.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 30.Statement from Jeff Shuren, MD, Director of the Center for Devices and Radiological Health, on continued efforts to assess duodenoscope contamination risk. US Food and Drug Administration website. Published April 12, 2019 https://www. accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pss.cfmAccessed March 30, 2021
- 31.Decristoforo P, Kaltseis J, Fritz A et al. Tyrolean Endoscope Hygiene Surveillance Study Group. High-quality endoscope reprocessing decreases endoscope contamination. Clin Microbiol Infect. 2018;24:11010–1101. doi: 10.1016/j.cmi.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 32.Aumeran C, Thibert E, Chapelle F A et al. Assessment on experimental bacterial biofilms and in clinical practice of the efficacy of sampling solutions for microbiological testing of endoscopes. J Clin Microbiol. 2012;3:938–942. doi: 10.1128/JCM.06221-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richard M, Luu Duc D, Pineau L. Efficacy of recovery solutions for endoscopes sampling: a comparative study. SHEA 19th Annual Scientific Meeting, San Diego, March 21st 2009
- 34.Heuvelmans M, Wunderink H F, Van der Mei H C et al. A narrative review on current duodenoscope reprocessing techniques and novel developments. Antimicrob Resist Infect Control. 2021;10:171. doi: 10.1186/s13756-021-01037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenters N, Huijskens E G, Meier C et al. Infectious diseases linked to cross-contamination of flexible endoscopes. Endosc Int Open. 2015;3:E259–E265. doi: 10.1055/s-0034-1392099. [DOI] [PMC free article] [PubMed] [Google Scholar]


