Abstract
Increases of soluble urokinase Plasminogen Activator Receptor (suPAR) were measured in both urine and plasma of a Chlorocebus aethiops (African Green Monkey; AGM) mucosal infected with SARS-CoV-2. The data indicate that elevated suPAR may be associated with renal dysfunction and pathology in the context of COVID-19.
Keywords: SARS-CoV-2, nonhuman primate, African Green Monkey, renal injury, acute kidney injury
1 ∣. CLINICAL OBSERVATIONS
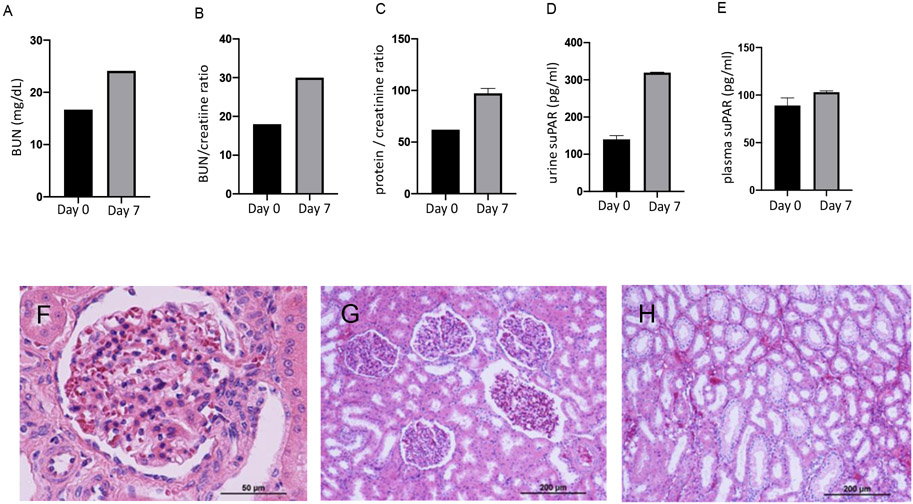
An approximately 7-yo male AGM (JK41) was mucosal inoculated with SARS-CoV-2 (WA/2020) via concomitant intratracheal/intranasal inoculation. SARS-CoV-2 was detected from mucosal swabs by TCID50 (50% Tissue Culture Infection Dose) and sub-genomic RNA using PCR. Viral loading in mucosa and lung increased and peaked at 7-days post infection and resolved at 14-days post inoculation (DPI) and persisted throughout the course of the study in pharyngeal and nasal swabs as well as bronchial brush samples. The highest levels of viral RNA were detected in the pharynx and nasal cavity at 1E+7-1E+11 and 1E+8-1E+9 sgRNA per swab, respectively. The animal (JK41) was euthanized at 28-days post exposure. Clinical findings during the first 7-days post infection included mild transient changes in saturation pressure of oxygen and appetite were also recorded. Urine was negative for albumin, glucose, or acetone. No red blood cells and no cast elements were detected in urine during this time. For protein/creatinine ratio, urine suPAR and plasma suPAR values shown in Figure 1, an average of two measurements is reported.
FIGURE 1.
Upper panels display analyses of clinical indicators of renal function and suPAR levels in plasma and urine samples at day 0 and after 7 days post infection: (A) blood urea nitrogen (BUN); (B) BUN / plasma creatinine ratio; (C) urine protein /Creatinine ratio; (D) urine suPAR (E) suPAR plasma levels. For protein/creatinine ratio, urine suPAR and plasma suPAR values, an average of two measurements is reported. Lower panels display renal histopathological findings using hematoxylin and eosin staining of kidney sections (4 μm) from an AGM after 28 days of SARS-CoV-2 intranasal/intratracheal inoculation: (F-H) show glomerular congestion (F), mild glomerular capillary tuft collapse/ shrinkage (F-G), diffuse mild tubular simplification/injury showing flattening of the proximal tubular epithelium with loss of brush border, focal peritubular capillary, small arterial and venous congestion (G-H). Microphotographs in panel F were captured using a 40x objective and G-H using a 10x objective. Scale is provided on each microphotograph.
2 ∣. KIDNEY FUNCTION ANALYSIS
Urine and blood samples were taken at days 0 and 7. Blood urea nitrogen (BUN) and creatinine were measured as indicators of renal function. To further estimate renal dysfunction, we also measured urea/plasma creatinine ratio (Figure 1A-B). Both values were elevated at 7 DPI. Furthermore, protein/creatinine ratio was elevated in urine after 7 DPI (Figure 1C). No other alterations in urine were found. A slight decrease in hemoglobin and hematocrit levels were also found after 7 DPI (Table 1).
TABLE 1.
Blood parameters of AGM (JK41) at 0- and 7-days post inoculation (DPI).
| Day 0 | Day 7 | |
|---|---|---|
| Hb (g/dL) | 12.1 | 10.8 |
| Hct (%) | 36.1 | 32.5 |
| Na+ (mEq/L) | 147.2 | 147.3 |
| K+ (mEq/L) | 3.3 | 3.5 |
| Cl− (mEq/L) | 106.3 | 107.4 |
3 ∣. SOLUBLE UROKINASE PLASMINOGEN ACTIVATOR RECEPTOR (suPAR) IN PLASMA AND URINE INCREASED AFTER 7 DPI
The suPAR, results from the cleavage and membrane bound uPAR in inflammatory or immune mediated processes (Thuno et al., 2009). Indeed, changes in suPAR levels have been associated with the activation level of the immune system and can be used as a biomarker of immune activation in biological fluids, which is particularly important in kidney disease (Enocsson et al., 2020; Thuno et al., 2009). We found increased urine (Figure 1D) and plasma levels (Figure 1E) of suPAR after 7 DPI with SARS-CoV-2. These findings agree with previous reports in COVID-19 hospitalized patients showing increased suPAR in plasma even after increases in plasma creatinine, as indicator of renal dysfunction (Azam et al., 2020; Oulhaj et al., 2021)
4 ∣. RENAL HISTOPATHOLOGICAL FINDINGS
During clinical evolution of the case, findings were compatible with decreased renal function. These features were associated with renal histopathological abnormalities as described in Figure 1F-H.
5 ∣. DISCUSSION
The nonhuman primate and specifically the AGM species has been developed as an experimentally inducible mild to moderate animal model of COVID-19 (Munoz-Fontela et al., 2022). This species is useful in defining pathogenesis based investigations (Blair et al., 2021; Edwards et al., 2021; Rowe et al., 2022; Witt et al., 2021; Woolsey et al., 2020) and associated re-infection susceptibility studies (Woolsey et al., 2021). Accordingly, changes in renal function and related pathologic consequences in an AGM COVID-19 model may mimic the human condition and disease outcomes. Acute kidney injury (AKI) commonly occurs in patients with COVID-19 and is often associated with respiratory complications (Ardura et al., 2022). The pathogenesis of AKI in the setting of COVID-19 infection is not well understood and biomarkers for its prediction are necessary to afford better clinical decisions. Increased suPAR levels have been identified as an immunologic risk factor for AKI and the existence of an association between suPAR and decreased renal function has been suggested (Azam et al., 2020). Indeed, increased plasma suPAR in hospitalized COVID-19 patients precedes increases in serum creatinine (Azam et al., 2020; Oulhaj et al., 2021) and it may predict even other renal complications such as renal damage associated to diabetes mellitus type 2 (Guthoff et al., 2017). Nonhuman primates are the closest animal models to humans to study physiology and pathophysiology playing an essential role in the current biomedical research related to virus infections (Heald et al., 2015) (Fahlberg et al., 2020). In this report, we describe that suPAR levels increase after 7 DPI with SARS-CoV-2 and this is also associated with increases in BUN, BUN/creatinine ratio and protein/creatinine ratio in urine, all of them indicators of reduced renal function. Increases in BUN are not only associated with kidney function. Pre-renal causes of renal dysfunction may be associated to intravascular volume alterations that may recapitulate acute renal failure and increased BUN levels (Molotoris et al., 2022). In fact, an increase in BUN can be expected in patients with pneumonia including those with COVID-19 as part of the CURB65 scoring (Wang et al., 2021). The BUN, albumin, and BUN)/albumin ratio (BAR) have been found to be reliable predictors of in-hospital mortality in COVID-19 patients, however BAR is a more reliable predictor than the BUN and albumin levels (Küçükceran et al., 2021).
In the case reported, renal dysfunction was associated with slight reduction in hematocrit and hemoglobin levels and without hematuria. Renal histology after 28 DPI of SARS-CoV-2 demonstrates changes consistent with glomerular congestion, focal mild glomerular capillary tuft collapse/shrinkage, diffuse mild tubular simplification and epithelial injury, such as flattening of the proximal tubular epithelium with loss of brush border as well as focal peritubular capillary and small arterial and venous congestion in AGM. Although the pathologic correlate of the sub-nephrotic proteinuria is not demonstrated, it is possible that the endothelial and /or epithelial injury could cause altered glomerular permeability in which the glomerular and microvascular congestion may a role in this. Furthermore, despite that the time interval was only 7-days, attempting biopsy samples on the glomeruli may yield additional information and evidence of glomerular cellular injury causing any degree of proteinuria (Zee et al., 2022). These changes further support similarities of these findings with kidney histopathology reported in humans with COVID-19 who underwent a renal biopsy (Akalin et al., 2020; Chan et al., 2020; Diao et al., 2021; Su et al., 2020) and highlight the relevance of non-human primate species as models of COVID-19-associated renal injury.
Although an explanation of the relationship between increases in suPAR and a decrease in kidney function with a single case report is a limitation of this study, we found clear evidence that several markers of renal dysfunction were affected at 7 DPI including BUN, BUN/plasma creatinine ratio, and urine protein/Creatinine ratio. However, suPAR is also increased in inflammatory diseases such as pancreatitis (Küçükceran et al., 2018) thus it has been suggested as a biomarker of systemic inflammation (Rasmussen et al., 2021)which could be considered in the design of future studies.
Taken together, these findings suggest that the AGM COVID-19 model is useful to evaluate the development of kidney injury associated with COVID-19 and its progression. Equally important, the increases in urine and plasma suPAR levels during the SARS-CoV-2 viral infection suggest a severe disease outcome as observed in clinical hospitalized patients.
ACKNOWLEDGEMENT
This work was supported, in part, by the National Institute of Allergy and Infectious Disease (NIH-NIAD) (Contract HHSN272201700033I) to CJR, and R21AI164232 to MCP, RO1DK129299, RO1DK114321, P30CA008748, DOD W81YWH-21-1-0188 to EAJ and the NIH Office of Research Infrastructure Programs, Office of the Director (OD011104). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CONFLICT OF INTEREST
The authors do not have any conflict of interest.
ETHICS APPROVAL
The Tulane University Institutional Animal Care and Use Committee approved all procedures used during this study. The Tulane National Primate Research Center (TNPRC) is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC No. 000594). The U.S. National Institutes of Health (NIH) Office of Laboratory Animal Welfare number for TNPRC is A3071-01. Tulane University Institutional Biosafety Committee approved all procedures for work in, and removal of samples from, Biosafety Level 3 laboratories.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, Ross M, Forest S, Goldstein YD, Ajaimy M, Liriano-Ward L, Pynadath C, Loarte-Campos P, Nandigam PB, Graham J, Le M, Rocca J, & Kinkhabwala M (2020, Jun 18). Covid-19 and Kidney Transplantation. N Engl J Med, 382(25), 2475–2477. 10.1056/NEJMc2011117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardura P, Lorenzo A, Manrique MM, Valencia AL, Acosta-Ochoa I, Mendiluce A, Fidalgo V, Gil JR, & Coca A (2022, May). Incidence of Aki among Hospitalized Covid-19 Patients during the First and Sixth Waves of the Pandemic. Nephrology Dialysis Transplantation, 37, I243–I244. <Go to ISI>://WOS:000813350701072 [Google Scholar]
- Azam TU, Shadid HR, Blakely P, O'Hayer P, Berlin H, Pan M, Zhao P, Zhao L, Pennathur S, Pop-Busui R, Altintas I, Tingleff J, Stauning MA, Andersen O, Adami ME, Solomonidi N, Tsilika M, Tober-Lau P, Arnaoutoglou E, Keitel V, Tacke F, Chalkias A, Loosen SH, Giamarellos-Bourboulis EJ, Eugen-Olsen J, Reiser J, Hayek SS, & International Study of Inflammation in, C. (2020, Nov). Soluble Urokinase Receptor (SuPAR) in COVID-19-Related AKI. Journal of the American Society of Nephrology, 31(11), 2725–2735. 10.1681/ASN.2020060829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RV, Vaccari M, Doyle-Meyers LA, Roy CJ, Russell-Lodrigue K, Fahlberg M, Monjure CJ, Beddingfield B, Plante KS, Plante JA, Weaver SC, Qin X, Midkiff CC, Lehmicke G, Golden N, Threeton B, Penney T, Allers C, Barnes MB, Pattison M, Datta PK, Maness NJ, Birnbaum A, Fischer T, Bohm RP, & Rappaport J (2021, Feb). Acute Respiratory Distress in Aged, SARS-CoV-2-Infected African Green Monkeys but Not Rhesus Macaques. Am J Pathol, 191(2), 274–282. 10.1016/j.ajpath.2020.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Baweja M, Campbell K, Chun N, Chung M, Deshpande P, Farouk SS, Kaufman L, Kim T, Koncicki H, Lapsia V, Leisman S, Lu E, Meliambro K, Menon MC, Rein JL, Sharma S, Tokita J, Uribarri J, Vassalotti JA, Winston J, Mathews KS, Zhao S, Paranjpe I, Somani S, Richter F, Do R, Miotto R, Lala A, Kia A, Timsina P, Li L, Danieletto M, Golden E, Glowe P, Zweig M, Singh M, Freeman R, Chen R, Nestler E, Narula J, Just AC, Horowitz C, Aberg J, Loos RJF, Cho J, Fayad Z, Cordon-Cardo C, Schadt E, Levin MA, Reich DL, Fuster V, Murphy B, He JC, Charney AW, Bottinger EP, Glicksberg BS, Coca SG, & Nadkarni GN (2020, May 8). Acute Kidney Injury in Hospitalized Patients with COVID-19. medRxiv. 10.1101/2020.05.04.20090944 [DOI] [Google Scholar]
- Diao B, Wang C, Wang R, Feng Z, Zhang J, Yang H, Tan Y, Wang H, Wang C, Liu L, Liu Y, Liu Y, Wang G, Yuan Z, Hou X, Ren L, Wu Y, & Chen Y (2021, May 4). Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun, 12(1), 2506. 10.1038/s41467-021-22781-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA, Ausiello D, Salzman J, Devlin T, Langer R, Beddingfield BJ, Fears AC, Doyle-Meyers LA, Redmann RK, Killeen SZ, Maness NJ, & Roy CJ (2021, Feb 23). Exhaled aerosol increases with COVID-19 infection, age, and obesity. Proc Natl Acad Sci U S A, 118(8). 10.1073/pnas.2021830118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enocsson H, Wirestam L, Dahle C, Padyukov L, Jonsen A, Urowitz MB, Gladman DD, Romero-Diaz J, Bae SC, Fortin PR, Sanchez-Guerrero J, Clarke AE, Bernatsky S, Gordon C, Hanly JG, Wallace DJ, Isenberg DA, Rahman A, Merrill JT, Ginzler E, Alarcon GS, Chatham WW, Petri M, Khamashta M, Aranow C, Mackay M, Dooley MA, Manzi S, Ramsey-Goldman R, Nived O, Steinsson K, Zoma AA, Ruiz-Irastorza G, Lim SS, Kalunian KC, Inanc M, van Vollenhoven RF, Ramos-Casals M, Kamen DL, Jacobsen S, Peschken CA, Askanase A, Stoll T, Bruce IN, Wettero J, & Sjowall C (2020, Jan). Soluble urokinase plasminogen activator receptor (suPAR) levels predict damage accrual in patients with recent-onset systemic lupus erythematosus. Journal of Autoimmunity, 106, 102340. 10.1016/j.jaut.2019.102340 [DOI] [PubMed] [Google Scholar]
- Fahlberg MD, Blair RV, Doyle-Meyers LA, Midkiff CC, Zenere G, Russell-Lodrigue KE, Monjure CJ, Haupt EH, Penney TP, Lehmicke G, Threeton BM, Golden N, Datta PK, Roy CJ, Bohm RP, Maness NJ, Fischer T, Rappaport J, & Vaccari M (2020, Nov 27). Cellular events of acute, resolving or progressive COVID-19 in SARS-CoV-2 infected non-human primates. Nature Communications, 11(1), 6078. 10.1038/s41467-020-19967-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthoff M, Wagner R, Randrianarisoa E, Hatziagelaki E, Peter A, Haring HU, Fritsche A, & Heyne N (2017, Jan 16). Soluble urokinase receptor (suPAR) predicts microalbuminuria in patients at risk for type 2 diabetes mellitus. Sci Rep, 7, 40627. 10.1038/srep40627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald AE, Charleston JS, Iversen PL, Warren TK, Saoud JB, Al-Ibrahim M, Wells J, Warfield KL, Swenson DL, Welch LS, Sazani P, Wong M, Berry D, Kaye EM, & Bavari S (2015, Jul 23). AVI-7288 for Marburg Virus in Nonhuman Primates and Humans. N Engl J Med, 373(4), 339–348. 10.1056/NEJMoa1410345 [DOI] [PubMed] [Google Scholar]
- Küçükceran K, Ayrancı MK, Girişgin AS, Koçak S, Dündar Z,D (2021, Apr 5). The role of the BUN/albumin ratio in predicting mortality in COVID-19 patients in the emergency department. Am J Emerg Med. 48:33–37. 10.1016/j.ajem.2021.03.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küçükceran K, Ergin M, Kılınç İ, Karaibrahimoğlu A, Çolak T, Tuncar A, Dündar ZD, Koçak S, Girişgin AS, Gül M, Cander B (2018. Dec 12). The role of soluble urokinase plasminogen activator receptor (SuPAR) as an indicator of the severity of acute pancreatitis. Turk J Med Sci. 48(6)1175–1181. 10.3906/sag-1709-134 [DOI] [PubMed] [Google Scholar]
- Molitoris BA (2022, Jul) ow-Flow Acute Kidney Injury: The Pathophysiology of Prerenal Azotemia, Abdominal Compartment Syndrome, and Obstructive Uropathy. Clin J Am Soc Nephrol. 17(7):1039–1049. doi: 10.2215/CJN.15341121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Fontela C, Widerspick L, Albrecht RA, Beer M, Carroll MW, de Wit E, Diamond MS, Dowling WE, Funnell SGP, Garcia-Sastre A, Gerhards NM, de Jong R, Munster VJ, Neyts J, Perlman S, Reed DS, Richt JA, Riveros-Balta X, Roy CJ, Salguero FJ, Schotsaert M, Schwartz LM, Seder RA, Segales J, Vasan SS, Henao-Restrepo AM, & Barouch DH (2022, Jan). Advances and gaps in SARS-CoV-2 infection models. PLoS Pathog, 18(1), e1010161. 10.1371/journal.ppat.1010161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhaj A, Alsuwaidi AR, Suliman A, Gasmelseed H, Khan S, Alawi S, Hukan Y, George J, Alshamsi F, Sheikh F, Babiker ZOE, Prattes J, & Sourij H (2021, Jun). Admission levels of Soluble Urokinase Plasminogen Activator Receptor (suPAR) are Associated with the Development of Severe Complications in Hospitalised COVID-19 Patients: A Prospective Cohort Study. International Journal of Infectious Diseases, 107, 188–194. 10.1016/j.ijid.2021.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen LJH, Petersen JEV, Eugen-Olsen J. Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a Biomarker of Systemic Chronic Inflammation. Front Immunol. 2021. Dec 2;12:780641. doi: 10.3389/fimmu.2021.780641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe LA, Beddingfield BJ, Goff K, Killeen SZ, Chirichella NR, Melton A, Roy CJ, & Maness NJ (2022, Jan 1). Intra-Host SARS-CoV-2 Evolution in the Gut of Mucosally-Infected Chlorocebus aethiops (African Green Monkeys). Viruses, 14(1). 10.3390/v14010077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, & Zhang C (2020, Jul). Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int, 98(1), 219–227. 10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuno M, Macho B, & Eugen-Olsen J (2009). suPAR: the molecular crystal ball. Dis Markers, 27(3), 157–172. 10.3233/DMA-2009-0657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IE, Cooper G, Mousa SA (2021, Nov 9). Diagnostic Approaches for COVID-19 and Its Associated Complications. Diagnostics (Basel). 11(11):2071. doi: 10.3390/diagnostics11112071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt AN, Green RD, & Winterborn AN (2021, Oct 1). A Meta-Analysis of Rhesus Macaques (Macaca mulatta), Cynomolgus Macaques (Macaca fascicularis), African green monkeys (Chlorocebus aethiops), and Ferrets (Mustela putorius furo) as Large Animal Models for COVID-19. Comp Med, 71(5), 433–441. 10.30802/AALAS-CM-21-000032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey C, Borisevich V, Prasad AN, Agans KN, Deer DJ, Dobias NS, Heymann JC, Foster SL, Levine CB, Medina L, Melody K, Geisbert JB, Fenton KA, Geisbert TW, & Cross RW (2020, May 17). Establishment of an African green monkey model for COVID-19. bioRxiv. 10.1101/2020.05.17.100289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey C, Borisevich V, Prasad AN, Agans KN, Deer DJ, Dobias NS, Heymann JC, Foster SL, Levine CB, Medina L, Melody K, Geisbert JB, Fenton KA, Geisbert TW, & Cross RW (2021, Jan). Establishment of an African green monkey model for COVID-19 and protection against re-infection. Nat Immunol, 22(1), 86–98. 10.1038/s41590-020-00835-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee J, Liu Q, Smith AR, Hodgin JB, Rosenberg A, Gillespie BW, Holzman LB, Barisoni L, & Mariani LH (2022, May 17). Kidney Biopsy Features Most Predictive of Clinical Outcomes in the Spectrum of Minimal Change Disease and Focal Segmental Glomerulosclerosis. Journal of the American Society of Nephrology. 10.1681/Asn.2021101396 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.