Abstract
Background
Enteral nutrition (EN) interruptions due to EN intolerance impede nutrient delivery. We aimed to examine whether revising the EN intolerance definition of an algorithm would decrease EN interruptions and improve nutrient delivery in critically ill children.
Methods
We performed a cross-sectional cohort study including patients admitted to our intensive care unit (ICU) for > 24 hours and who received EN. The EN intolerance definition in our nutrition algorithm was modified to include two symptoms of EN intolerance. We compared time to 60% EN adequacy (EN delivered/ EN prescribed × 100), and EN interruptions before and after this intervention.
Results
We included 150 eligible patients, 78 and 72 patients in the pre- and post-implementation cohorts, respectively. There were no significant differences in demographics and clinical characteristics. The pre- and post-implementation cohorts achieved 60% EN adequacy 4 days (2, 5) and 3 days (2,5) after ICU admission, respectively, p=0.59. The pre-implementation cohort had a median of 1 (1,2) interruption per patient and the post-implementation cohort 2 (1, 3), p=0.08. The frequency of interruptions due to EN intolerance within the first 8 days of ICU admission was 17 in the pre-implementation and 10 in the post-implementation cohorts.
Conclusions
Modifying the EN intolerance definition of a nutrition algorithm did not change the time to 60% EN adequacy or total number of EN interruptions in critically ill children. Enteral nutrition intolerance and interruptions continue to limit nutrient delivery. Research on the best definition for EN intolerance and its effect on nutrition outcomes is needed.
Keywords: enteral nutrition, pediatrics, critical illness, intensive care unit
Introduction
Enteral nutrition (EN) is the preferred mode of nutrient delivery for critically ill children with a functional gastrointestinal tract (1). Early and optimal provision of EN in the pediatric intensive care unit (PICU) has been associated with improved clinical outcomes such as increased ventilator-free days, decreased rate of hospital-acquired infections, decreased length of stay, and decreased mortality (2, 3). However, many obstacles inhibit the delivery of early and optimal EN including EN interruptions and EN intolerance (4–7).
Enteral nutrition tolerance is often determined by bedside clinical signs or symptoms suggestive of gastrointestinal dysfunction such as emesis, diarrhea, abdominal distension, abdominal pain and gastric residual volume (GRV) (7, 8). In pediatric intensive care units (PICU), there is no clear or uniform definition for EN intolerance and this can result in prolonged and often unnecessary interruptions to EN (1, 5, 9, 10). Studies on EN intolerance in adult critically ill patients have not identified a definitive sign or symptom for EN intolerance but have found that the presence of more than one gastrointestinal symptom has a stronger association with EN intolerance than a single symptom (11).
In our institution, we have had a nutrition algorithm since 2009 which resulted in earlier attainment of EN goal, but similar frequency in total EN interruptions after implementation (12). The definition for EN intolerance in the original algorithm required the presence of only one clinical sign or symptom of EN intolerance and GRV was emphasized. Based on the best available evidence, we revised our definition for EN intolerance in the algorithm to include 2 or more of the aforementioned clinical symptoms of EN intolerance, and performed nutrition audits before and after implementation of this revised definition (11, 13, 14). In this cross-sectional cohort study, we aimed to evaluate the impact of a revised definition for EN intolerance in a nutrition algorithm on nutrition provision and EN interruptions in a PICU. We hypothesized that under the revised definition of EN intolerance, EN interruptions would decrease and EN delivery would improve resulting in faster attainment of prescribed nutrition goal.
Methods
We performed a single-center prospective, cross-sectional cohort study before and after the implementation of a revised nutrition algorithm. An initial comprehensive stepwise nutrition algorithm was implemented in our PICU in 2009 (12). In 2016, a multidisciplinary team reviewed research on nutrition for critically ill children published since the initial implementation of the algorithm and made recommendations for revising the nutrition algorithm. Primary areas of revision included the definition for EN intolerance, timing for initiation and modification of a bowel regimen, and EN recommendations for high-risk patient populations such as the stem cell transplant population and infants who had undergone general surgery. The previous nutrition algorithm defined EN intolerance as having one of the following symptoms, abdominal distension, emesis, diarrhea, abdominal discomfort and GRV, and it emphasized the use of GRV. The revised nutrition algorithm defined EN intolerance as two or more signs and/or symptoms of EN intolerance, including all of the same clinical parameters as in the initial algorithm, and de-emphasized GRV as a primary sign of intolerance. In addition, the GRV limit to consider EN intolerance was increased from a maximum GRV cutoff of 150mL to 250mL for patients greater than 50kg and remained at 3ml/kg for patients <50kg. This increase in GRV cutoff for adult-sized patients was guided by adult studies on GRV (14). The revised nutrition algorithm was implemented in 2019 and education was provided to nurses and physicians through email and in-person communications, and at the bedside, Supplemental Figure 1. An 8-week audit was performed prior to implementing the revised algorithm (June-July 2018) and after implementation (September-October 2020). This study was approved by the local Institutional Review Board, and informed consent was waived.
The study population included all patients admitted to the PICU with a length of stay greater than 24 hours and whom received EN at any time during their ICU admission, regardless of age. Patients who were already receiving care at the time of study initiation or stayed in the PICU after study period conclusion were also eligible and remained enrolled.
During both study periods, we collected demographics, anthropometrics, baseline clinical characteristics such as admission diagnoses, comorbidities, and illness severity scores. The nutrition and clinical data were collected in 12-hour shift increments by bedside nursing staff using an audit form. A bedside nurse recorded detailed nutrition delivery variables such as whether a patient was receiving nutrition, source of the nutrition (oral, specialized EN via a tube, parenteral nutrition (PN), or EN and PN), type of feeding tube used for EN delivery (gastric vs postpyloric), and nutrition advancement status (trophic feeding vs advancing feeds vs full amount of prescribed feeds by a dietitian). For EN fed patients, signs of EN intolerance and EN interruptions, including duration and reason for interruption, were also documented. Data collected from audit forms were transferred to REDCap (Research Electronic Data Capture) for storage and management. For patients who were either already admitted to the PICU at the time of audit initiation or remained after the conclusion of the audit period, we used the electronic medical record (EMR) to extract abovementioned variables outside of the audit period.
Data Analysis and Statistical Methods
We analyzed time to EN initiation, adequacy of nutrition delivery, time (days) to achieving 60% EN adequacy, cumulative incidence of patients achieving 60% EN adequacy, and number and hours of interruptions in EN delivery and reasons for EN interruptions in both pre- and post-implementation cohorts. Achieving 60% of EN adequacy during the first week of PICU admission has been associated with improved clinical outcomes, therefore we focused our analysis on the first 8 days of PICU admission (3). The first 8 days of PICU admission were included to account for partial time in the PICU on admission day 1 and full 24 hours in the PICU on the subsequent days until transfer, discharge or day 8 of PICU admission. Daily EN adequacy was calculated by dividing the volume of EN delivered by the volume of EN prescribed by a dietitian to achieve target energy goal and multiplied by 100 to display the result as a percentage.
Patient demographic, anthropometric and clinical characteristics were described using frequency (percentage) for categorical variables and measures of central tendency with spread (median and IQR) for non-categorical variables. Nutrition variables between the pre- and post-implementation cohorts were compared using non-parametric tests including the Mann-Whitney U test for continuous variables and Chi-square test for categorical variables. A competing risks multivariable analysis controlling for age, type of admission (medical vs surgical), illness severity score (PIM3), primary reason for admission, and presence of mechanical ventilation was performed where the outcome of interest was the patient achieving 60% of EN adequacy and transfer/discharge/decease was the competing risk. A corresponding cumulative risk function was included to examine differences in the proportion of patients achieving 60% of EN adequacy over the ICU stay between the pre- and post-implementation cohorts. Patients were censored after 8 days of ICU stay.
STATA version 17.0 from StataCorp, GraphPad Prism and R 4.1.3 were used to conduct the tests and statistical significance was set at a p-value <0.05.
Results
A total of 167 and 203 patients were admitted to our ICU for a minimum of 24 hours in the pre- and post-implementation audit periods, respectively. Seventy-eight patients in the pre-implementation and 72 patients in post-implementation cohorts received EN via enteric feeding tube and were included in the study. Details of demographics, anthropometrics and clinical characteristics are described in TABLE 1. There were no differences in demographics, anthropometrics, clinical characteristics, and markers of severity of illness between the pre- and post- implementation cohorts. Median age was 5 years (1, 9.3) and 3 years (0.3, 10.8) for the pre- and post-implementation cohorts, respectively, and the female sex distribution was 46% and 40%.
TABLE 1.
Patient population demographics and clinical characteristics of EN-fed patients in pre- and post-implementation cohorts.
| Variable | Pre-implementation (n=78) | Post-implementation (n=72) | p-value8 |
|---|---|---|---|
| Age at ICU admission (years), median (IQR) | 5 (1, 9.3) | 3 (0.3, 10.8) | 0.46 |
| Female gender, n (%) | 36 (46) | 29 (40) | 0.47 |
| Weight-for-age Z-score, median, (IQR) 1 | −0.37 (−1.46, 0.49) | −0.64 (−1.7, 0.14) | 0.17 |
| Height/ length for age Z-score, median, (IQR) 2 | −1.53 (−2.87, 0.01) | −2.1 (−3.5, −0.41) | 0.33 |
| Weight-for-length Z-score, median, (IQR) 3 | 0.74 (−0.08, 1.89) | 0.71 (−0.21, 1.65) | 0.70 |
| Body Mass Index Z-score, median, (IQR)4 | 0.7 (−0.27, 1.69) | 0.51 (−0.38, 1.37) | 0.44 |
| Body Mass Index, median, (IQR) 5 | 20.6 (18.2, 27) | 21.4 (20, 30.7) | 0.55 |
| 0.15 | |||
| Principal reason for ICU admission, n (%) | 0.78 | ||
| 0.62 | |||
| Respiratory support | 0.35 | ||
| Patients on ECMO, n (%) | 2 (2.6) | 5 (6.9) | 0.20 |
| ICU LOS, median (IQR) days | 6.1 (2, 18.7) | 6.0 (2.2, 23.2) | 0.77 |
| PIM 3, median (IQR) | −4.99 (−6.2, −3.8) | −4.40 (−5.4, −3.3) | 0.15 |
| Nutrition provision, n (%) | 0.53 |
For patients younger than 20 years (73 in pre-implementation cohort and 67 in post-implementation cohort)
For patients younger than 20 years (73 in pre-implementation cohort and 66 in post-implementation cohort)
For patients younger than 2 years (25 in pre-implementation cohort and 32 in post-implementation cohort)
For patients 2–20 years of age (48 in pre-implementation cohort and 34 in post-implementation cohort)
For patients older than 20 years (5 in pre-implementation cohort and 5 in post-implementation cohort)
Under the “Other” category we have included diagnoses of admission for which there were less than 5 patients per category per cohort and these included oncologic, renal, liver, gastrointestinal diagnoses
Not mutually exclusive
As determined by either the Mann-Whitney U test for continuous variables or the Chi-Square test for trend for categorical variables
BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; ECMO, extracorporeal membrane oxygenation; EN, enteral nutrition; HFNC, high flow nasal cannula; ICU, intensive care unit; IQR, interquartile range; GI, gastrointestinal; LOS, length of stay; n, number; NIPPV, non-invasive positive pressure ventilation; PIM 3, pediatric index of mortality 3; PN, parenteral nutrition; PO, per os (by mouth); SCT, stem cell transplant.
TABLE 2 describes nutrition characteristics in the pre- and post-implementation cohorts. Use of gastric feeding route [pre-54 (69%) vs post-43 (60%); p= 0.24], number of days from ICU admission to EN initiation [pre-1.5 (1, 4) vs post-1.5 (1, 3); p= 0.71] and percent of patients that achieved 60% of prescribed EN goal during ICU admission (pre-55.7% vs post-54.2%; p= 0.66) were similar between the pre- and post-implementation cohorts. The pre-implementation cohort achieved 60% EN adequacy a median 4 days (2, 5) of ICU admission and the post-implementation cohort 3 days (2, 5), p= 0.59, however, cumulative incidences of achieving 60% of prescribed nutrition goal on days 1 to 8 were similar in both cohorts, FIGURES 1 & 2.
TABLE 2.
Enteral nutrition characteristics in pre- and post-implementation cohorts.
| Variable | Pre-implementation N=78 | Post-implementation N=72 | p-value2 |
|---|---|---|---|
| Route of EN | |||
| Number of days from ICU admission to EN initiation, median (IQR) | 1.5 (1, 4) | 1.5 (1, 3) | 0.71 |
| Number (%) of patients achieved 60% prescribed EN goal during first 8 days of admission, n (%) | 45 (55.7) | 39 (54.2) | 0.66 |
| Number of days to achieve EN provision at 60% prescribed nutrition goal during first 8 days of admission, median (IQR) 1 | 4 (2, 5) | 3 (2, 5) | 0.59 |
Only patients who achieved 60% of prescribed nutrition goal (45 and 39 patients in pre- and post-implementation cohorts respectively) within 8 days of admission
As determined by either the Mann-Whitney U test for continuous variables or the Chi-Square test for categorical variables
EN, enteral nutrition; ICU, intensive care unit; IQR, interquartile range; n, number.
FIGURE 1. Enteral nutrition adequacy in the pre- and post-implementation cohorts during the first 8 days of ICU admission.
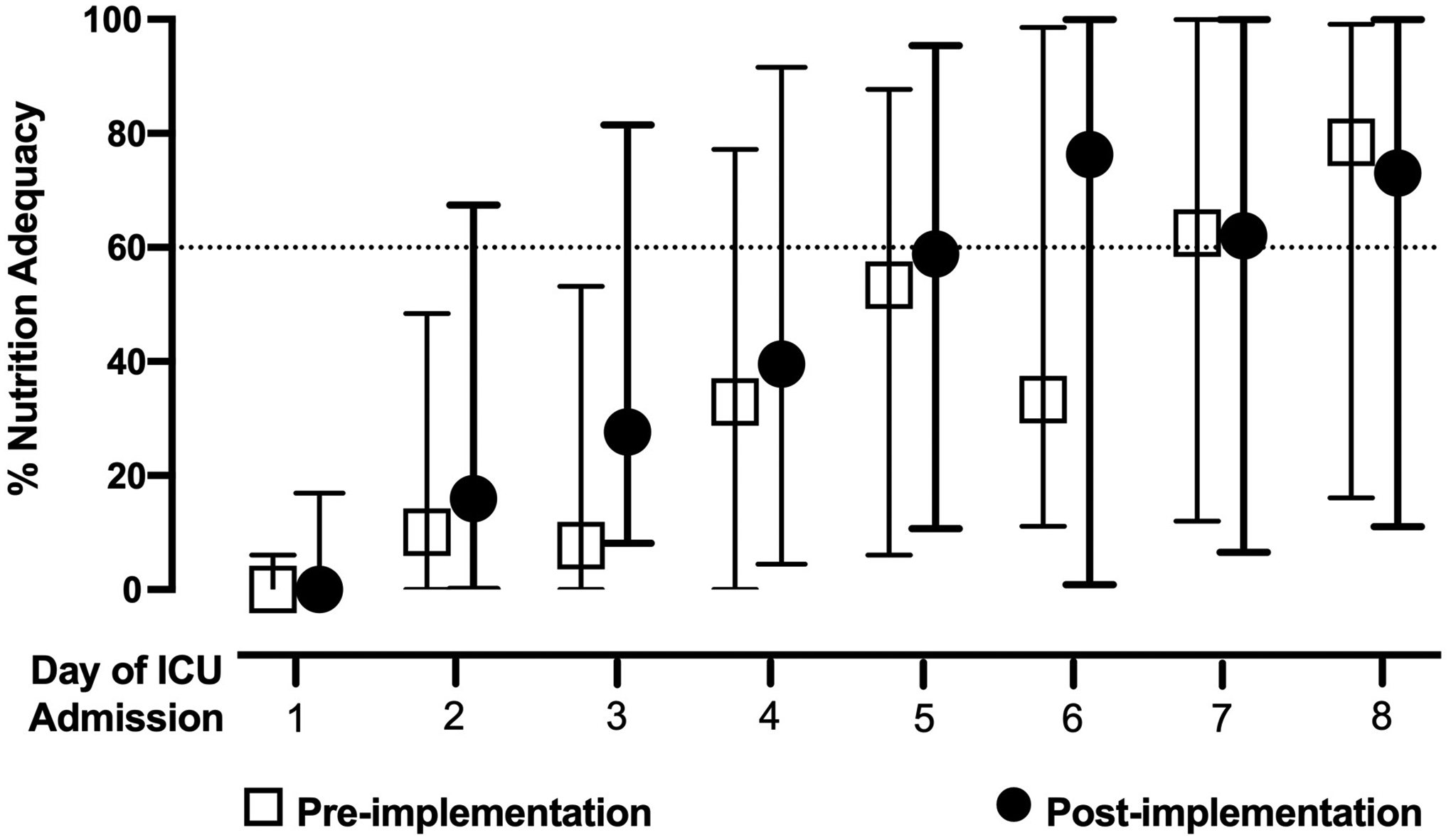
Daily EN adequacy was calculated as the delivered volume of formula divided by the prescribed volume of formula to achieve target energy for patients who received EN in the first 8 days of ICU admission. The first 8 days of ICU admission were included and accounted for partial time in the ICU on admission day 1 and full 24 hours in the ICU on the subsequent days until transfer, discharge or day 8 of ICU admission. Open squares represent the pre-implementation cohort; closed circles represent the post-implementation cohort. Open squares and closed circles represent the median percent of EN delivered and the bars the interquartile range.
FIGURE 2. Cumulative incidence of patients achieving 60% of prescribed EN goal during first 8 days of ICU admission.
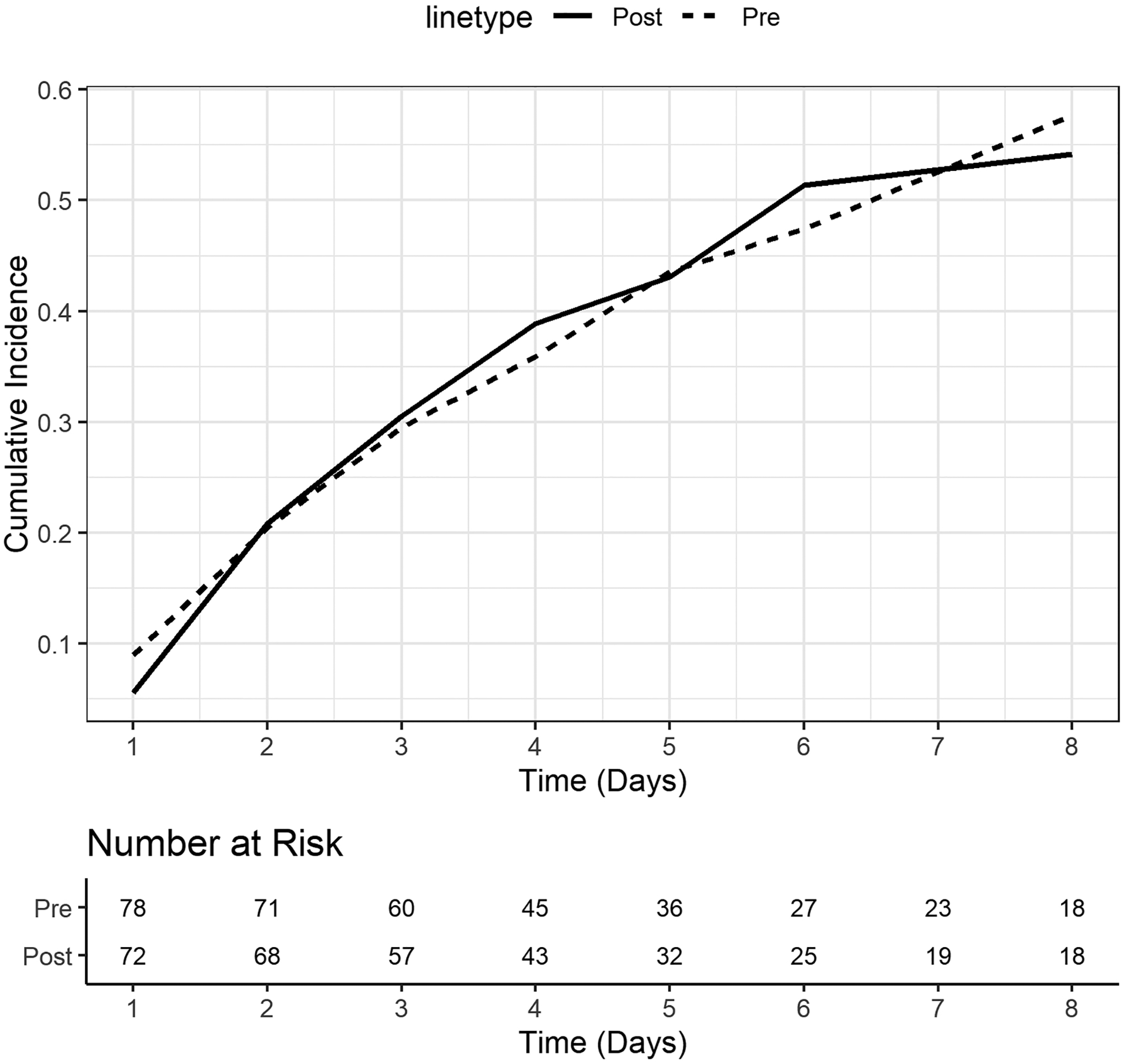
A competing risks multivariable analysis controlling for age, medical vs surgical admission, illness severity score (PIM3), primary reason for admission, and presence of mechanical ventilation was performed where the outcome of interest was the patient achieving 60% of EN adequacy and transfer/discharge/decease was the competing risk. A corresponding cumulative risk function was included to examine differences in the proportion of patients achieving 60% of EN adequacy over the ICU stay between the pre- and post-implementation cohorts. Patients were censored after 8 days of ICU stay. Dashed line represents the pre-implementation cohort (pre) and solid line the post-implementation cohort (post).
TABLE 3 describes EN interruptions for patients who received EN during the first 8 days of ICU admission. The pre-implementation cohort had a median of 1 (1,2) interruption per patient, and the post-implementation cohort 2 (1,3), p= 0.08, FIGURE 3A. The total number of hours of interruptions per patient was 9 hours (4.8, 14.5) in the pre-implementation cohort and 12 hours (5.8, 18) in the post-implementation cohort, p= 0.18, FIGURE 3B. The frequency of interruptions due to EN intolerance was 17 in pre-implementation cohort and 10 in the post-implementation cohort, FIGURE 4.
TABLE 3.
Enteral Nutrition interruptions in pre- and post-implementation cohorts during the first 8 days of ICU admission.
| Variable | Pre-implementation N=63 | Post-implementation N=59 | p-value3 |
|---|---|---|---|
| Total number of patients with EN interruptions, n (%) | 33 (49.2) | 24 (40.6) | 0.20 |
| Total number of interruptions | 59 | 58 | NA |
| Median number of EN interruptions per patient, median (IQR) 1 | 1 (1, 2) | 2 (1, 3) | 0.08 |
| Total number of hours of interruptions in whole cohort | 326.25 | 323.25 | NA |
| Median number of hours of interruptions per patient, median (IQR) | 9 (4.8, 14.5) | 12 (5.8, 18) | 0.18 |
| Number of patients with particular reason for EN interruption, n 2 | 0.96 | ||
| Incidence of reasons of EN interruptions, n 2 | 0.88 | ||
| Distribution of reasons for EN intolerance,n 2 | 0.55 |
The total number of interruptions in each cohort divided by the total number of patients in the corresponding cohort who had interruptions.
Not mutually exclusive as patients could have met more than one criteria within these variables
As determined by either the Mann-Whitney U test for continuous variables or the Chi-Square test for categorical variables
EN, enteral nutrition; GRV, gastric residual volume; IQR, interquartile range; n, number; NA, not applicable
FIGURE 3. Enteral nutrition interruptions per patient during first 8 days of ICU admission.
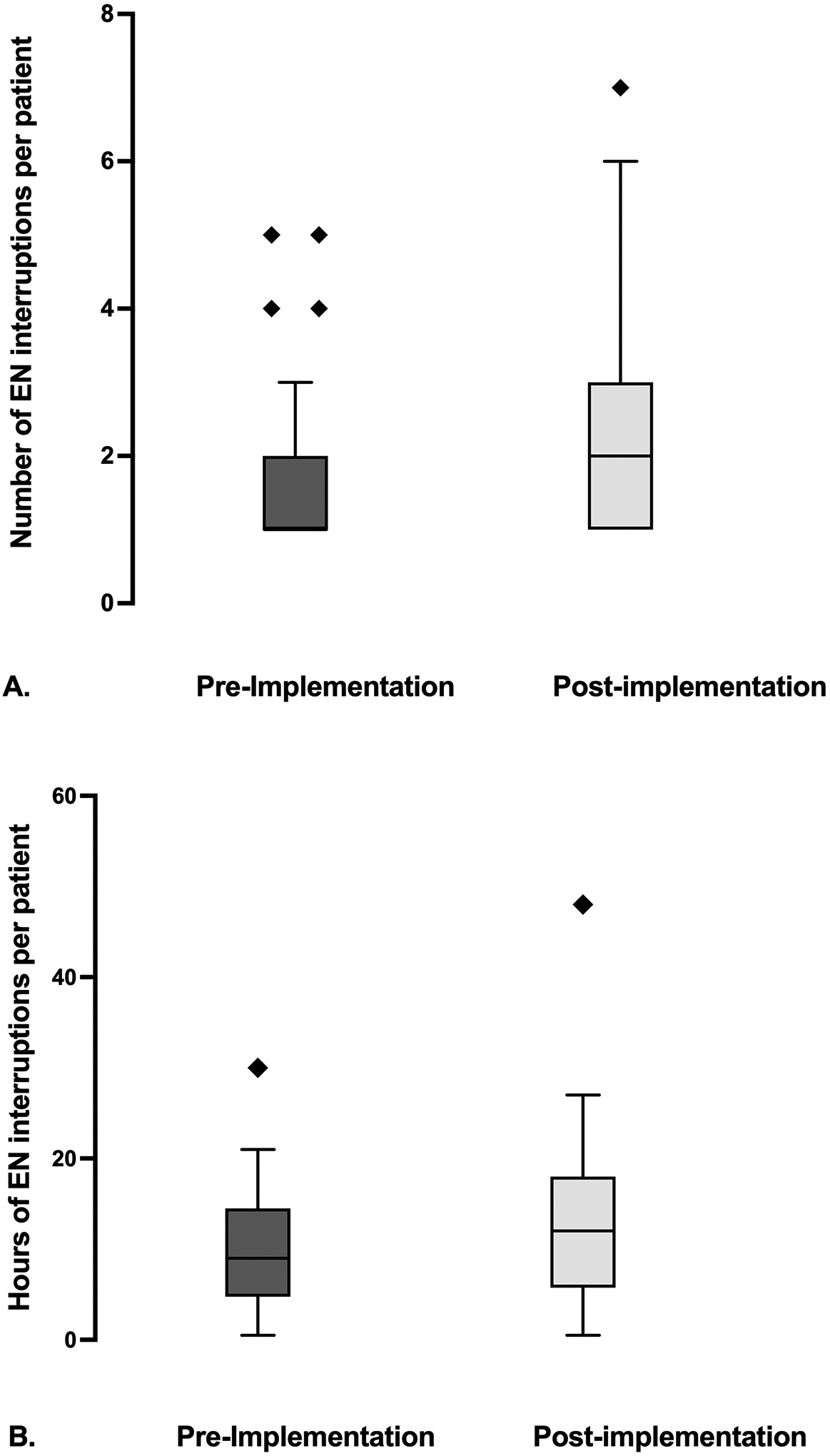
Panel A depicts the frequency of enteral nutrition (EN) interruptions per patient in the first 8 days of ICU admission. [pre-implementation- 1, (1, 2) vs post-implementation- 2 (1, 3), Mann Whitney U test p value of 0.08]. Panel B depicts number of hours of EN interruption per patient in the first 8 days of ICU admission. [pre-implementation- 9, (4.8, 14.5) vs post-implementation- 12 (5.8, 18)], Mann Whitney U test p value of 0.18]. Pre-implementation cohort (n=33) is represented in the dark gray and post-implementation cohort (n=24) in the light gray, and diamond symbols represent outliers in both panels.
FIGURE 4. Frequency of reasons for enteral nutrition interruptions during the first 8 days of ICU admission.
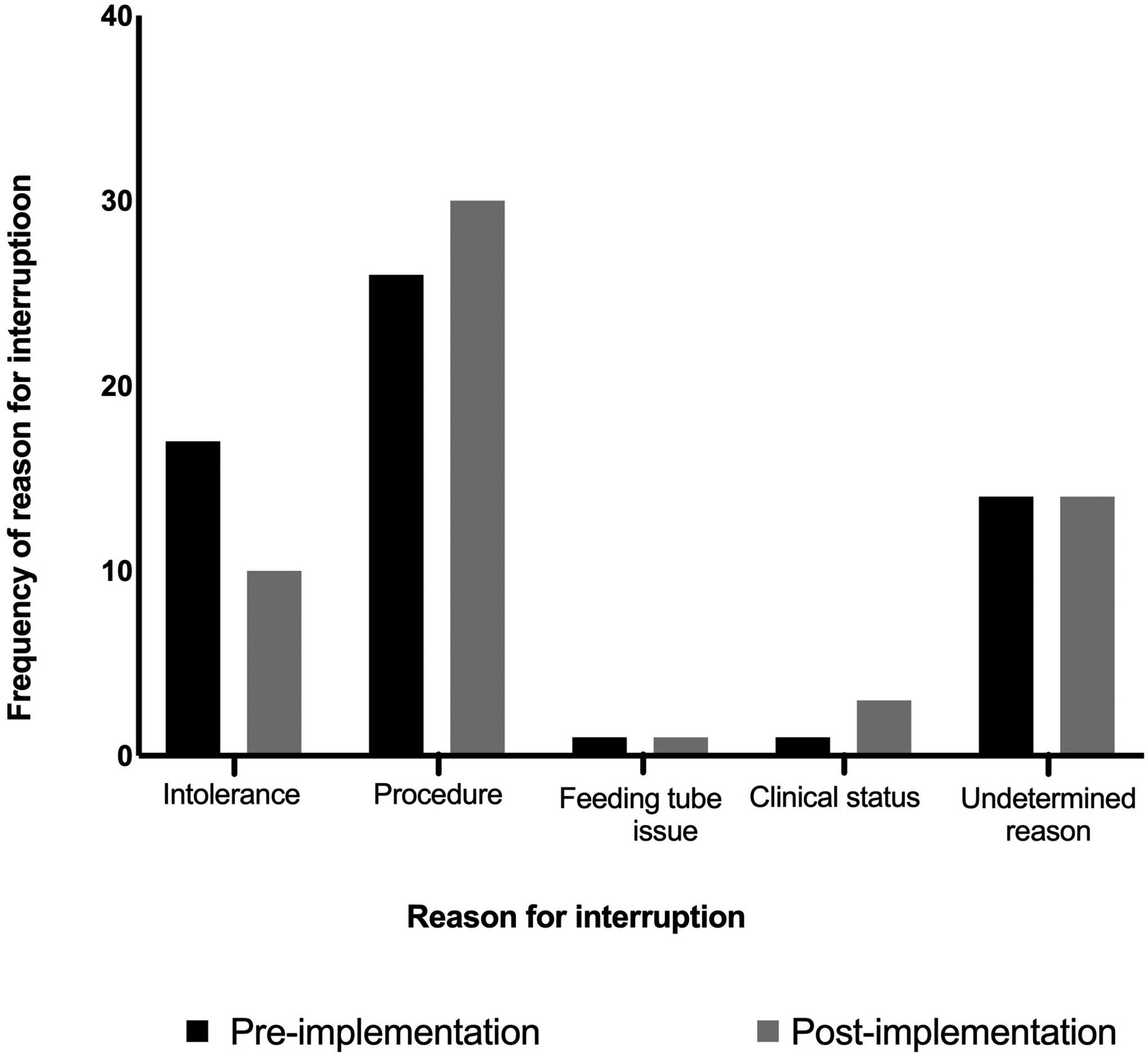
Reasons for enteral nutrition (EN) interruptions included are EN intolerance, procedure requiring NPO status, feeding tube issue, deteriorating clinical status, and undetermined reasons. Reasons are not mutually exclusive. Black bars represent the pre-implementation cohort, light gray bars the post-implementation cohort.
DISCUSSION
In this prospective, cross-sectional cohort study, we examined whether implementing a modified definition of EN intolerance in a nutrition algorithm would affect nutrient delivery and interruptions in a multidisciplinary PICU. Time to 60% EN adequacy and total EN interruptions were not statistically significant between the two cohorts. The frequency of EN interruptions due to EN intolerance was lower in the post-implementation cohort, though EN interruptions due to procedures or changes in clinical status were greater. Enteral nutrition intolerance and interruptions continue to be a challenge in pediatric critical illness, and limit optimal nutrient delivery.
Multiple previous studies have demonstrated the benefits of protocolized nutrition delivery in both pediatric and adult ICUs (12, 15, 16). We have published our own experience implementing a stepwise nutrition algorithm which resulted in a significant improvement in EN provision such as achievement of prescribed EN intake goal at an earlier time, decrease in avoidable EN interruptions, and a decrease in PN use (12). However, studies examining whether modifications of already existing nutrition algorithms are associated with nutrition outcomes remain scarce. In this study, we examined whether modifying an existing nutrition algorithm could further improve nutrition outcomes. In particular, implementation of the original algorithm did not result in an overall decrease in EN interruptions or number of patients with EN interruptions. Therefore, we modified the definition for EN intolerance based on up to date research, and found no difference in overall frequency of EN interruptions. We identified fewer interruptions due to EN intolerance after the implementation of the revised EN intolerance definition, though this result is descriptive and limited by a small sample size. The median number of days to achieve 60% EN adequacy was one day less in the post-implementation cohort, though this was not statistically significant. These results suggest that periodic revision of nutrition algorithms is feasible and may be necessary but may not result in improved clinical outcomes. Determining when and which components of a nutrition algorithm to revise will be institution-specific and will likely have different effects across PICUs. Furthermore, we identified that procedures was a prevalent reason for EN interruptions. Enteral nutrition interruptions for procedures may not be avoidable but may be modifiable, including considering shortening NPO times for post-pyloric fed patients or considering volume-based or bolus feeding to allow time for catching-up on feeds.
Enteral nutrition intolerance is a common reason for EN interruptions (5, 7). There is no unifying definition for EN intolerance in critically ill children, yet how it’s defined can significantly affect EN interruptions and delivery. Gastric residual volume measurement is the most common clinical sign for EN intolerance included in nutrition algorithms and in practice in PICUs (7) (8, 10). Although, noted to be an unreliable marker of EN intolerance in observational studies, the lack of randomized controlled trials (RCTs) examining the effect of using GRV to determine EN tolerance and advancement has resulted in the continued use of this marker with great variability in practice (13, 17). Randomized controlled trials have been completed in critically ill adults and have found no difference in clinical outcomes such as ventilator-associated pneumonia and greater EN delivery in patients for whom GRV was not assessed compared to patients for whom GRV was utilized to guide EN advancement (14). In addition, studies examining EN intolerance in critically ill adult populations identified that the presence of multiple gastrointestinal symptoms, including GRV as one of them, had a stronger association with true EN intolerance and EN delivery rather than a single symptom (11). The initial algorithm implemented in our PICU defined EN intolerance as one clinical sign or symptom and emphasized GRV. The use of a single symptom and emphasis on GRV as markers for EN intolerance, given their limitations, could result in unnecessary and frequent EN interruptions resulting in poor EN delivery for patients whom had a low likelihood of EN intolerance. Therefore, given the evidence to date, from adult and pediatric studies, we modified the definition for EN intolerance in our nutrition algorithm to include 2 or more clinical signs of EN intolerance. We kept GRV as one of the markers of EN intolerance given the lack of pediatric RCTs to address the role of GRV in defining EN intolerance, but increased the maximum GRV cutoff from 150mL to 250mL for adult-sized patients greater than 50kg based on published practices for adult patients (14). We hypothesized this change in definition would result in less frequent EN interruptions and improved delivery. Modifying our definition did not result in an overall decrease in the number of interruptions or improvement in nutrient delivery. Research is needed to examine the best definition for EN intolerance in critically ill children that could be applied widely across PICUs, and result in improved nutritional and clinical outcomes. Novel approaches to examine GRV are also being implemented such as gastric ultrasound, and we await results from a RCT examining GRV measurement and EN delivery in the PICU (18–20).
This study is unique in that it examined the effect of modifying an existing nutrition algorithm in critically ill children. It was a prospective, cross-sectional cohort study, with cohorts similar in their characteristics and size, and similar duration and season of the year for the pre- and post-implementation study periods. The study cohorts were heterogeneous, as all patients who were admitted to our PICU for longer than 24 hours and who received EN via tube were eligible with no exceptions regardless of age, prior medical history, or reason for ICU admission, thereby representing a diverse PICU population reflective of a large, multidisciplinary PICU. However, this study is based on a single center experience only. Our patient population is likely comparable in size and in the demographic and clinical complexity of the patients in other large academic center PICUs. However, as a referral center for patients with complex esophageal and large airway pathology, and congenital diaphragmatic hernia the distribution of certain pathologies that affect nutrition delivery may differ from other PICUs. Last, nutrition algorithms can be specific to each institution’s resources, clinical experience and unique patient populations, such that our modified EN intolerance definition and approach to nutrition may not be applicable in other PICUs. Furthermore, the modified EN definition was based on available research, which is limited, and nutrition practices of our institution.
Conclusions
Modifying the EN intolerance definition of a nutrition algorithm did not change the time to 60% EN adequacy or total number of EN interruptions per patients in critically ill children. Enteral nutrition intolerance and interruptions continue to be limiting-factors in providing optimal nutrient delivery for critically ill children. Further research is needed to develop a uniform and optimal definition for EN intolerance and how implementing such a definition impacts nutrition and clinical outcomes.
Supplementary Material
Supplemental Figure 1. Revised MSICU & MICU Nutritional Algorithm that was implemented in 2019.
Acknowledgements-
We would like to thank the Medical-Surgical Intensive Care Unit Multidisciplinary Nutrition Committee and all of the unit’s nurses and dietitians who participated in the daily bedside audits.
EEM was funded by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease K23DK128634 Award
Footnotes
Authors have no conflicts of interest to disclose
REFERENCES
- [1].Mehta NM, Skillman HE, Irving SY, Coss-Bu JA, Vermilyea S, Farrington EA, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically Ill Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. 2017;41(5):706–742. [DOI] [PubMed] [Google Scholar]
- [2].Mehta NM, Bechard LJ, Cahill N, Wang M, Day A, Duggan CP, et al. Nutritional practices and their relationship to clinical outcomes in critically ill children--an international multicenter cohort study*. Crit Care Med. 2012;40(7):2204–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bechard LJ, Staffa SJ, Zurakowski D, and Mehta NM. Time to achieve delivery of nutrition targets is associated with clinical outcomes in critically ill children. Am J Clin Nutr, 2021;114(5):1859–1867. [DOI] [PubMed] [Google Scholar]
- [4].Brown AM, Carpenter D, Keller G, Morgan S, and Irving SY, Enteral Nutrition in the PICU: Current Status and Ongoing Challenges. J Pediatr Intensive Care. 2015;4(2):111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mehta NM, McAleer D, Hamilton S, Naples E, Leavitt K, Mitchell P, et al. , Challenges to optimal enteral nutrition in a multidisciplinary pediatric intensive care unit. JPEN J Parenter Enteral Nutr. 2010; 34(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tume L, Carter B, and Latten L, A UK and Irish survey of enteral nutrition practices in paediatric intensive care units. Br J Nutr. 2013;109(7):1304–1322. [DOI] [PubMed] [Google Scholar]
- [7].Tume LN, Eveleens RD, Verbruggen S, Harrison G, Latour JM, Valla FV, et al. , Barriers to Delivery of Enteral Nutrition in Pediatric Intensive Care: A World Survey. Pediatr Crit Care Med. 2020; 21(9): e661–e671. [DOI] [PubMed] [Google Scholar]
- [8].Eveleens RD, Joosten KFM, de Koning BAE, Hulst JM, and Verbruggen S, Definitions, predictors and outcomes of feeding intolerance in critically ill children: A systematic review. Clin Nutr. 2020;39(3):685–693. [DOI] [PubMed] [Google Scholar]
- [9].Martinez EE, Bechard LJ, and Mehta NM, Nutrition algorithms and bedside nutrient delivery practices in pediatric intensive care units: an international multicenter cohort study. Nutr Clin Pract. 2014;29(3): 360–367. [DOI] [PubMed] [Google Scholar]
- [10].Typpo KV, Irving SY, Prince JM, Pathan N, Brown AM, and Pediatric Organ Dysfunction Information Update Mandate, C, Gastrointestinal Dysfunction Criteria in Critically Ill Children: The PODIUM Consensus Conference. Pediatrics. 2022;149(1 Suppl 1): S53–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reintam Blaser A, Starkopf L, Deane AM, Poeze M, and Starkopf J, Comparison of different definitions of feeding intolerance: A retrospective observational study. Clin Nutr. 2015;34(5): 956–961. [DOI] [PubMed] [Google Scholar]
- [12].Hamilton S, McAleer DM, Ariagno K, Barrett M, Stenquist N, Duggan CP, et al. , A stepwise enteral nutrition algorithm for critically ill children helps achieve nutrient delivery goals*. Pediatr Crit Care Med. 2014;15(7): 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tume LN, Bickerdike A, Latten L, Davies S, Lefevre MH, Nicolas GW, et al. , Routine gastric residual volume measurement and energy target achievement in the PICU: a comparison study. Eur J Pediatr. 2017;176(12):1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Reignier J, Mercier E, Le Gouge A, Boulain T, Desachy A, Bellec F, et al. , Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA. 2013;309(3):249–256. [DOI] [PubMed] [Google Scholar]
- [15].Petrillo-Albarano T, Pettignano R, Asfaw M, and Easley K, Use of a feeding protocol to improve nutritional support through early, aggressive, enteral nutrition in the pediatric intensive care unit. Pediatr Crit Care Med. 2006;7(4):340–344. [DOI] [PubMed] [Google Scholar]
- [16].Heyland DK, Cahill NE, Dhaliwal R, Sun X, Day AG, and McClave SA, Impact of enteral feeding protocols on enteral nutrition delivery: results of a multicenter observational study. JPEN J Parenter Enteral Nutr. 2010;34(6):675–684. [DOI] [PubMed] [Google Scholar]
- [17].Martinez EE, Pereira LM, Gura K, Stenquist N, Ariagno K, Nurko S, et al. , Gastric Emptying in Critically Ill Children. JPEN J Parenter Enteral Nutr. 2017;41(7):1100–1109. [DOI] [PubMed] [Google Scholar]
- [18].Valla Frederic V, C E, Morice Claire, Tume Lyvonne N, Bouvet Lionel, Point-of-Care Gastric Ultrasound Confirms the Inaccuracy of Gastric Residual Volume Measurement by Aspiration in Critically Ill Children: GastriPed Study. Front Ped. 2022. Online Publication [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tume LN, Arch B, Woolfall K, Roper L, Deja E, Jones AP, et al. Determining Optimal Outcome Measures in a Trial Investigating No Routine Gastric Residual Volume Measurement in Critically Ill Children. JPEN J Parenter Enteral Nutr. 2021;45:79–86. [DOI] [PubMed] [Google Scholar]
- [20].National Institute for Health and Care Research. Funding and Awards: A Feasibility study of no gastric residual volume measurement in mechanically ventilation infants and children: the GASTRIC Study. Award ID:16/94/02. Date accessed-09/19/2022. {https://fundingawards.nihr.ac.uk/award/16/94/02}
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Revised MSICU & MICU Nutritional Algorithm that was implemented in 2019.


