Abstract
Introduction:
There has been an increase in the interest and availability of products asserting to contain cannabidiol (CBD).
Objective:
To describe demographic and clinical patterns in cases involving CBD exposures documented by the America’s Poison Centers (AAPCC).
Methods:
We extracted human exposure cases involving CBD from the U.S. National Poison Data System between July 2014 and June 2021. We described monthly case counts and data on demographics, exposure reason, clinical effects, medical outcomes, and co-exposures, overall and by U.S. Food and Drug Administration (FDA) approval status.
Results:
We identified 6,496 cases, of these, 85.2% involved exposures to non-FDA approved CBD. The monthly number of cases peaked at 336 in March 2021. Cases often occurred in children ages 2–12 years (36.2%). Although in this age group unintentional exposures represented most cases (94.1%), we identified therapeutic errors (3.9%), intentional use (3.0%), and adverse reactions (1.6%) in cases involving exposures to non-FDA approved CBD. Among the 5,248 (80.8%) cases involving exposure to a single product, we identified 44 major medical outcomes, all related to exposures to non-FDA approved CBD. The most frequent clinical effects included neurological, cardiac, and gastrointestinal effects. Among the 1,248 (19.2%) involving exposure to more than one product, the most frequent co-exposures included stimulants and street drugs, sedatives-hypnotics, antipsychotics, and analgesics.
Conclusions:
This case series identified an increasing trend in CBD exposure cases managed by AAPCC. It showed serious medical outcomes in temporal association with exposure to non-FDA approved CBD products. Our findings also suggest both unintentional and intentional use of non-FDA approved CBD in children. Consumers should keep these products out of reach of children and exercise caution when purchasing and using non-FDA approved CBD products.
Keywords: Cannabidiol, CBD, poison control center, National Poison Data System, cannabis, cannabis-derived products
Introduction
Cannabidiol (CBD) is a non-psychotropic cannabinoid that binds to a wide variety of physiological targets of the endocannabinoid system [1]. Although CBD may be synthetically manufactured [2], it is usually derived from CBD-rich varieties of the cannabis plant (Cannabis sativa L.) and purified to remove some other cannabinoids, including delta-9 tetrahydrocannabinol (THC), the principal psychotropic cannabinoid.
In the United States, the legality of cannabis and cannabis-derived products (CDP), including CBD, has changed over time at both the state and federal levels [3]. At the Federal level, the Agriculture Improvement Act of 2018, Pub. L. 115–334 (2018 Farm Bill) removed “hemp” (i.e. cannabis plants and derivatives that contain no more than 0.3% THC on a dry weight basis) from the Controlled Substances Act (CSA). In June 2018, the U.S. Food and Drug Administration (FDA) approved the first cannabis-derived CBD human drug product (Epidiolex®, GW Research, Ltd., Research Triangle Park, NC), currently indicated for the treatment of seizures associated with Lennox-Gastaut syndrome, Dravet syndrome, or tuberous sclerosis complex in patients one year of age or older [4,5]. Following approval of Epidiolex, the Department of Justice and Drug Enforcement Administration (DEA) placed FDA-approved drugs containing CBD derived from cannabis with no more than 0.1% THC, like Epidiolex, under Schedule V of the CSA, which were later de-scheduled in April 2020. Although “hemp” as defined by the 2018 Farm Bill is no longer controlled under the CSA, it is still subject to the FDA’s regulatory authority under the Federal Food, Drug, and Cosmetic Act (FD&C Act) and section 351 of the Public Health Service Act. FDA concluded that CBD-containing products are excluded from the dietary supplement definition and, therefore, cannot be sold as dietary supplements.
The dramatic change in CBD use in recent years coincides with marketing claims asserting that it can treat almost any health condition [6]. Non-FDA approved CBD products are frequently mislabeled, with varying CBD concentrations, and might contain very high doses of THC and other contaminants [7,8]. We aimed to describe patterns, trends, medical outcomes, and clinical effects of cases involving CBD exposures managed by the U.S. Poison Control Centers (PCCs).
Materials and methods
The America’s Poison Centers (AAPCC) maintains the National Poison Data System (NPDS), which captures data on all cases to U.S. PCCs on a near real-time basis [9]. Each exposure case is assigned a generic code (broad group of related products) and/or a product code (exact substance). NPDS included the first product code for CBD in July 2014. Since then, NPDS has made available additional codes for CBD products, including a generic code in February 2019 [10]. For this case series, we used generic and product codes for CBD, excluding marijuana, nabiximols, and delta-8 THC, to extract data from closed human exposure cases (cases where the PCC had completed all related follow up) involving CBD from July 1, 2014, through June 30, 2021. NPDS data are considered preliminary and are subject to change until the data for a given year are locked. We downloaded the dataset on August 6, 2021, after NPDS locked case data for the 2020 calendar year.
We described CBD case counts for the entire study period and by month, age group (<2, 2–12, 13–17, 18–24, 25–44, 45–64, 65+ years), gender, exposure reason, and number of exposures (single- or multiple-substance). We calculated average annual exposure case rates per million population by first dividing the number of CBD exposure cases by the number of months in the study period and multiplying by the number of months in a year and subsequently dividing by the average population estimates from 2014–2020 prepared by the Census Bureau in collaboration with the National Center for Health Statistics. Because the global COVID-19 pandemic might have influenced CBD exposures, we examined CBD case counts and case rates before (November 1, 2018-February 29, 2020) and during the pandemic (March 1, 2020-June 30, 2021).
We also described the exposure events (scenario data) when available, and most frequent co-exposures. For single-substance exposures, we assessed counts for exposure route, and after excluding clinical effects coded as not related, unknown if related, and medical outcomes coded as unrelated effect, we described clinical effects and medical outcomes. We requested and reviewed fatality abstracts and major effects narratives (symptoms that, as a result of the exposure, were life-threatening or resulted in significant residual disability or disfigurement) at the case level from AAPCC (AAPCC coordinates all requests from the FDA).
We conducted the analyses for all CBD products and stratified by FDA approval status (FDA-approved and non-FDA approved CBD, defined thereafter as approved and non-approved CBD). For these subgroup analyses, we did not include non-categorized CBD exposures by FDA status approval.
Two analysts (SPV, SK) conducted the analyses independently using Stata/IC 13.1 (StataCorp LLC) and SAS 9.4 (SAS Institute), respectively. This study was classified as public health surveillance by the FDA and exempted from review by the institutional review board in accordance with the updated Common Rule.
Results
We identified a total of 6,496 cases meeting the inclusion criteria. They involved exposures to non-approved CBD (85.2%), approved CBD (3.8%), and non-categorized CBD exposures (11.0%). The number of cases per month ranged between zero in the early study period (first cases were recorded in November 2014) and 336 in March 2021, with an increasing trend starting in 2016 largely driven by exposures to non-approved CBD and an apparent slight downward trend starting in April 2021 (Figure 1). The monthly number of cases increased over time more markedly for children ages 2–12 years and adults ages 25–44 years (Figure 2). For the entire study period, we estimated an average annual exposure case rate of 2.8 per million population. The average annual exposure case rates during the pre-pandemic and pandemic periods were 4.9 and 8.5 per million population, respectively.
Figure 1.

Monthly counts of cannabidiol exposure cases reported to U.S. Poison Control Centers from July 1, 2014 through June 30, 2021.
Figure 2.
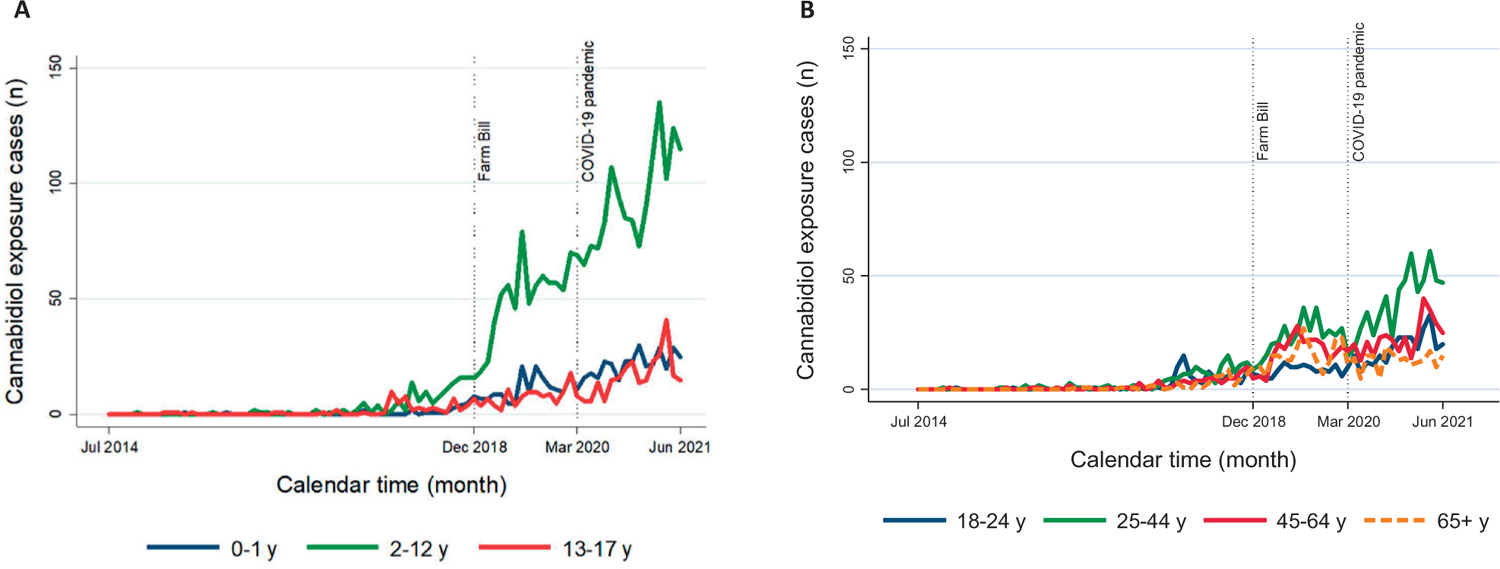
Monthly counts of cannabidiol exposure cases reported to U.S. Poison Control Centers by age group among children and adolescents (A) and adults (B) from July 1, 2014 through June 30, 2021.
Overall, gender distribution was similar with four (0.1%) out of 3,302 females being coded as pregnant at the time of exposure (Table 1). Cases often involved CBD exposures among children ages 2–12 years (36.2%) followed by those in adults ages 25–44 years (16.9%). In children ages 2–12 years, although unintentional exposures represented most cases (94.1%), we identified therapeutic errors (3.9%), intentional use (3.0%), and adverse reactions (1.6%) in cases involving exposures to non-FDA approved CBD. A total of 1,009 cases (15.5%) included more detailed data on the reason for exposure (scenario category) where 354 (35.1%) corresponded to children ages 2–12 years. In this age group, 189 of these cases described access to somebody else’s product, child-resistant closure-related issues, or confusion about the product, with 95.8% reflecting exposures to non-approved CBD. A total of 134 cases described dosing/therapeutic errors, with 53.7% reflecting exposures to non-approved CBD. In adults ages 25–44 years, most exposures were categorized as intentional (42.0%).
Table 1.
Characteristics of cannabidiol exposure cases reported to U.S. Poison Centers by type of cannabidiol exposure from July 1, 2014 through June 30, 2021.
| Cannabidiol exposure cases, No. (%)a | |||
|---|---|---|---|
| Totalb | Non-approved cannabidiol | Approved cannabidiol | |
| Total cases | 6,496 (100) | 5,535 (100) | 244 (100) |
| Calendar year | |||
| 2014c | 3 (<0.1) | 3 (<0.1) | – |
| 2015 | 15 (0.2) | 15 (0.3) | – |
| 2016 | 34 (0.5) | 34 (0.6) | – |
| 2017 | 118 (1.8) | 118 (2.1) | – |
| 2018 | 533 (8.2) | 509 (9.2) | 24 (9.8) |
| 2019 | 1,678 (25.8) | 1,370 (24.8) | 112 (45.9) |
| 2020 | 2,381 (36.7) | 1,998 (36.1) | 70 (28.7) |
| 2021d | 1,734 (26.7) | 1,488 (26.9) | 38 (15.6) |
| COVID-19 pandemic | |||
| Pre-pandemic (July 2014-February 2020) | 2,725 (42.0) | 2,321 (41.9) | 148 (60.7) |
| Pandemic (March 2020-June 2021) | 3,771 (58.0) | 3,214 (58.1) | 96 (39.3) |
| Age group (years) | |||
| <2 | 534 (8.2) | 436 (7.9) | 18 (7.4) |
| 2–12 | 2,352 (36.2) | 1,964 (35.5) | 105 (43.0) |
| 13–17 | 442 (6.8) | 380 (6.9) | 27 (11.1) |
| 18–24 | 509 (7.8) | 443 (8.0) | 18 (7.4) |
| 25–44 | 1,099 (16.9) | 937 (16.9) | 37 (15.2) |
| 45–64 | 693 (10.7) | 605 (10.9) | 14 (5.7) |
| 65+ | 487 (7.5) | 423 (7.6) | 17 (7.0) |
| Unknown/age not categorizable | 380 (5.9) | 347 (6.3) | 8 (3.3) |
| Gender | |||
| Male | 3,150 (48.5) | 2,669 (48.2) | 114 (46.7) |
| Non-pregnant female | 3,298 (50.8) | 2,826 (51.1) | 129 (52.9) |
| Pregnant female | 4 (<0.1) | 3 (<0.1) | 1 (0.4) |
| Unknown | 44 (0.7) | 37 (0.7) | – |
| No. of substances | |||
| Multiple substance | 1,248 (19.2) | 1,056 (19.1) | 72 (29.5) |
| Single substance | 5,248 (80.8) | 4,479 (80.9) | 172 (70.5) |
| Exposure reason | |||
| Unintentional | 3,879 (59.7) | 3,244 (58.6) | 187 (76.6) |
| Intentional | 1,492 (23.0) | 1,329 (24.0) | 26 (10.7) |
| Adverse reaction | 946 (14.6) | 818 (14.8) | 28 (11.5) |
| Other | 52 (0.8) | 45 (0.8) | 2 (0.8) |
| Unknown | 127 (2.0) | 99 (1.8) | 1 (0.4) |
Percentages have been rounded up, so some percentages do not add up to exactly 100%. Percentages are column percentages (%).
It includes exposures to approved cannabidiol (n = 244), non-approved cannabidiol (n = 5,535), and cannabidiol products not categorized as either approved or non-approved cannabidiol (n = 717). Characteristics for the 717 cases without information on their approval status were not included in a separate column.
July 1, 2014, through December 31, 2014.
January 1, 2021, through June 30, 2021; at the time of data extraction, NPDS had not locked data for 2021, so the number of cases for that period is probably incomplete.
A total of 5,248 (80.8%) cases reportedly involved single-substance exposures (i.e., CBD product). Of these, 2,039 (38.9%) described clinical effects deemed related to the exposure. The most frequent clinical effects included mild CNS depression, tachycardia, and vomiting (Table 2). Most cases (88.9%) described ingestion as the route of exposure. When exposure to more than one product was reported (19.2%), the most frequent co-exposures included stimulants and street drugs, sedatives-hypnotics, antipsychotics, analgesics, and anticonvulsants (Table 3).
Table 2.
Route of exposure, related medical outcomes, and most frequent clinical effects in cannabidiol exposure cases (single-substance exposures) reported to U.S. Poison Centers by type of cannabidiol exposure from July 1, 2014 through June 30, 2021.
| Cannabidiol exposure cases, No. (%)a | |||
|---|---|---|---|
| Totalb | Non-approved cannabidiol | Approved cannabidiol | |
| Cases with single-substance exposures, No. | 5,248 | 4,479 | 172 |
| Route of exposurec | |||
| Ingestion | 4,667 | 3,969 | 154 |
| Inhalation/nasal | 327 | 303 | 3 |
| Dermal | 268 | 230 | 6 |
| Ocular | 86 | 64 | 11 |
| Otherd | 28 | 23 | 2 |
| Unknown | 23 | 19 | – |
| Related medical outcomese | |||
| Minor effectf | 937 | 823 | 25 |
| Moderate effectg | 518 | 445 | 7 |
| Major effecth | 47 | 40 | – |
| Death/Death, indirect reporti | 1 | 1 | – |
| Not followed, minimal clinical effects possiblej | 320 | 282 | 5 |
| Unable to follow, potentially toxic exposure | 216 | 196 | 1 |
| Most frequent clinical effects (≥1.0%) | |||
| CNS depression (mild) | 561 | 493 | 10 |
| Tachycardia | 367 | 317 | 6 |
| Vomiting | 294 | 254 | 4 |
| Other – neurological | 281 | 252 | 4 |
| Dizziness/vertigo | 237 | 216 | * |
| Nausea | 233 | 206 | 2 |
| Agitation | 217 | 188 | 6 |
| Confusion | 181 | 163 | * |
| Other – miscellaneous | 169 | 153 | * |
| CNS depression (moderate) | 131 | 113 | * |
| Hypertension | 79 | 71 | * |
| Ataxia | 73 | 64 | * |
| Drowsiness/lethargy | 72 | 70 | 2 |
| Hallucinations/delusions | 68 | 67 | * |
| Tremor | 68 | 62 | * |
| Ocular – irritation/pain | 60 | 45 | 7 |
| Mydriasis | 59 | 50 | * |
| Slurred speech | * | 44 | * |
| Lacrimation | * | * | 2 |
Percentages are column percentages (%).
It includes cases with single exposures to approved cannabidiol (n = 172), non-approved cannabidiol (n = 4,479), and cannabidiol products not categorized as either approved or non-approved cannabidiol (n = 597). Characteristics for the 597 cases without information on their approval status were not included in a separate column.
Each exposure case may involve more than one route of exposure (n = 5,399).
Any other route not listed above.
After excluding clinical effects coded as “not related” and “unknown if related” as well as “related” medical outcomes coded as “Unrelated effect, the exposure was probably not responsible for the effect(s)”.
The patient exhibited some symptoms as a result of the exposure, but they were minimally bothersome to the patient.
The patient exhibited symptoms as a result of the exposure, which are more pronounced, more prolonged or more of a systemic nature than minor symptoms.
The patient has exhibited symptoms as a results of the exposure, which were life-threatening or resulted in significant residual disability or disfigurement.
The patient died as a result of the exposure or as a direct complication of the exposure where the complication was unlikely to have occurred had the toxic exposure not preceded the complication/a report of a fatality but not inquiry was placed to the regional poison center.
No more than minor effect possible.
Clinical effects representing <1.0%.
Table 3.
Most frequent co-exposures in cannabidiol exposure cases (multiple-substance exposures) reported to U.S. Poison Centers by type of cannabidiol exposure from July 1, 2014 through June 30, 2021.
| Cannabidiol exposure cases, No. (%)a | |||
|---|---|---|---|
| Totalb | Non-approved cannabidiol | Approved cannabidiol | |
| Cases with multiple-substance exposures, No. | 1,248 | 1,056 | 72 |
| No. of exposures per case (median [IQR]) | 2 [2, 2] | 2 [2, 2] | 2 [2, 4] |
| Total co-exposuresc | 2,244 | 1,867 | 158 |
| Most frequent co-exposures (≥1.0%)d | |||
| Stimulants and street drugs | 300 | 267 | 4 |
| Sedative/hypnotics/antipsychotics | 270 | 212 | 33 |
| Analgesics | 229 | 199 | 5 |
| Anticonvulsants | 197 | 117 | 64 |
| Dietary supplements/herbals/homeopathic | 178 | 160 | 8 |
| Antidepressants | 142 | 118 | 5 |
| Alcohols | 136 | 122 | * |
| Antihistamines | 108 | 96 | 3 |
| Cardiovascular drugs | 86 | 74 | 7 |
| Vitamins | 61 | 48 | 2 |
| Cold and cough preparations | 43 | 37 | * |
| Hormones and hormone antagonists | 42 | 32 | 2 |
| Gastrointestinal preparations | 41 | 33 | 4 |
| Tobacco/nicotine/e-cigarette products | 40 | 36 | * |
| Antimicrobials | 34 | 25 | 4 |
| Muscle relaxants | 34 | 25 | 5 |
| Plants | 32 | 30 | * |
| Electrolytes and minerals | 30 | 25 | 3 |
| Essential oils | 28 | 25 | * |
| Unknown drug | 28 | 22 | * |
| Miscellaneous drugs | 25 | * | 3 |
| Topical preparations | 22 | * | * |
| Asthma therapies | * | * | 2 |
Abbreviations: IQR, interquartile range.
Percentages are column percentages (%).
It includes cases with exposures to approved cannabidiol (n = 72), non-approved cannabidiol (n = 1,056), and cannabidiol products not categorized as either approved or non-approved cannabidiol (n = 120). Characteristics for the 120 cases without information on their approval status were not included in a separate column.
It excludes information calls (n = 5 for total cannabidiol exposures; n = 3 for non-approved cannabidiol).
Major generic code category.
Co-exposures representing <1.0%.
Non-approved cannabidiol
Most cases (85.2%) involved exposures to non-approved CBD, largely to CBD only (80.9%). Case characteristics, route of exposure, medical outcomes, and most frequent clinical effects are shown in Tables 1 and 2. Among cases reporting exposure to more than one product (19.1%), the most frequent co-exposures were stimulants and street drugs (14.3%), which often included marijuana and THC, sedative-hypnotics and antipsychotics (11.3%), mostly benzodiazepines, and analgesics (10.6%), including prescription opioids (Table 3).
Approved cannabidiol
A total of 244 (3.8%) cases involved exposure to approved CBD products, mostly in children ages 2–12 years (43.0%) followed by individuals ages 25–44 years (15.2%). A total of 172 (70.5%) cases involved single-substance exposures. Among these, the most frequently reported clinical effects included mild CNS depression, ocular irritation or pain, tachycardia, and agitation. All medical outcomes were mild or moderate (Table 2). Ingestion was the most frequent route of exposure (89.5%) followed by ocular (6.4%). Most cases (76.6%) described unintentional exposures. A total of 72 cases (29.5%) involved exposure to more than one substance, mostly anticonvulsants (Table 3).
Fatalities and major effects (single-substance exposures with related medical outcomes)
There were 48 cases coded as major effect or death. None of these cases involved exposures to approved CBD products. We reviewed 45 cases as three did not involve CBD exposures. We identified one death following a non-approved CBD inhalation exposure. The AAPCC Fatality Review Committee ultimately classified this death as probably not related to the exposure due to comorbid conditions. The remaining 44 (93.6%) cases largely involved exposure to CBD via ingestion (72.7%). They were similarly distributed by gender (52.3% males) with a median age of 27 (IQR: 19–48) years. A total of 18 cases (40.9%) required admission to critical care unit, 11 (25.0%) admission to a non-critical care unit, and one case (2.3%) admission in a psychiatric facility. The most frequent clinical effects included tachycardia, major and moderate CNS depression, confusion, and vomiting. The narratives described intentional exposure in 23 of the cases (52.3%), unintentional exposure in ten cases (22.7%), and adverse reactions in eight cases (18.2%). The exposure reason for the remaining three cases was unknown. The eight cases coded as adverse reactions described various cardiac, respiratory, neurologic, and hypersensitivity events, including ventricular tachycardia/ventricular fibrillation, non-ST elevated myocardial infarction, myopericarditis, bradycardia with heart block, tachycardia, hypo/hypertension, respiratory depression, dyspnea, major CNS depression, hallucinations, and contact dermatitis with hemorrhagic edema. Seven cases reported hospitalization and treatment, with four of these cases requiring critical care treatment and intubation. These eight adult cases reported recreational and “medical” uses of CBD in various forms, including vape inhalations, oil/oral ingestions, and edibles, and occurred within hours to two days of CBD exposure. Some cases reported medical conditions that may have contributed to the events, including hypertension, heart failure, obesity, and asthma.
Discussion
This large case series displayed an increasing trend in CBD exposures managed by PCCs, most involving non-approved CBD exposures. Although rarely, it showed serious medical outcomes in temporal association with exposure to non-approved CBD products. Our findings also suggest both intentional and unintentional use of non-approved CBD in children.
The first cases involving CBD exposures were recorded in late 2014, after AAPCC made the first code for CBD available in July 2014. Since 2016, CBD cases have shown an overall increasing trend, largely driven by non-approved CBD exposures, with an apparent sharper rise that started in 2019 coinciding with the signing of the 2018 Farm Bill into law and the subsequent explosion of the CBD market [11]. The availability of a generic code for CBD in NPDS in February 2019 may have also contributed to the capture of additional CBD exposures. Cases continued to show an increasing trend through the COVID-19 pandemic period. Follow-up analyses will be required to elucidate whether the increasing trend has been reversed starting in April 2021. Similarly, in NPDS, exposures to manufactured cannabis products also increased between 2017 and 2019, while, on the contrary, PCC encounter volume data reveal an overall decreasing trend [9,12].
The increase we observed in CBD cases was notable in children ages ≤12 years, mostly due to unintentional exposures. During the pandemic period, the crude number of unintentional exposure cases notably increased in children, particularly among those ages 2–12 years (data not shown). Of note, NPDS describes unintentional exposures as those resulting from an unforeseen or unplanned event with general, therapeutic error, misuse, environmental, and unknown among the coding options available. Although only a few cases included a description of the exposure events, these cases largely reflected unintentional exposures to non-approved CBD in children. These findings are consistent with observed increases in incidence of cannabis exposures and Emergency Department visits due to unintentional exposures among children in the United States and Canada following legalization of recreational or medical cannabis and during the COVID-19 pandemic, which highlights the need for further prevention strategies [13–17]. As mentioned, the exact content of non-approved CBD products is unknown, and, importantly, long-term effects of CBD and other cannabinoids on the developing brain are still unknown [8]. Notably, we also identified cases describing dosing/therapeutic errors involving exposures to non-approved CBD, which suggests some intended use of non-approved CBD in children. Further investigations into the indications for which non-approved CBD products are being used in children might facilitate targeting appropriate preventive measures.
CBD and its primary active metabolite, 7-hydroxy CBD, are biologically active compounds, which exhibit both pharmacodynamic and pharmacokinetic properties that could lead to adverse events and drug interactions [18]. Overall, the clinical effects identified with approved CBD in our data were consistent with current U.S. labeling [5], expected effects from non-oral unintentional exposures, and effects that may in fact have been the result of underlying medical conditions. Notably, all medical outcomes were mild or moderate. Regarding non-approved CBD, the identification of cardiac and neurological effects may suggest contamination of these products. Over the past several years, the FDA has issued several warning letters to firms that market non-approved products that allegedly contain CBD. Analytical testing on non-approved CBD products found CBD concentrations differing from the contents claimed by their manufacturers [7]. Also, we identified serious medical outcomes requiring critical care treatment among individuals who reportedly used non-approved CBD. Our data showed that CBD was often used concomitantly not only with stimulants and street drugs, but also with sedative-hypnotics, antipsychotics, alcohols, opioids, dietary supplements, herbals, homeopathic products, antidepressants, and anticonvulsants. Given the unknown contents of the non-approved CBD products, drug interactions are difficult to predict. Beyond the controlled clinical trial data for Epidiolex, to date, there are still limited well-controlled safety data on CBD, thus, potential effects on underlying medical conditions and interactions with prescription medications and herbals/dietary supplements still need to be further characterized.
Enhancing the FDA safety surveillance and signal detection capabilities is critically important to understand the safety data gaps for cannabis and CDP, including CBD. Consumers and healthcare providers can report adverse events associated with cannabis or CDP via the FDA’s MedWatch reporting system [19,20]. As of today, information from passive surveillance reports regarding CDP use is extremely limited, requiring safety surveillance from multiple data systems, including NPDS.
To our knowledge, this is the largest case series of CBD exposures in the United States. Prior assessments in NPDS included earlier study periods, were limited to exposures identified through the generic code for CBD, and/or did not exclude non-CBD products such as delta-8 THC [21, 22]. Nonetheless, our findings should be interpreted with caution as NPDS data only capture exposures reported to PCCs, lack population denominators, and rely on self-reported and electively shared information [9]. The nature of these data did not permit us to detect long-term effects, but acute events in close temporal association with the exposure. Also for any given case, there is no certainty that a suspected substance caused the event. In the early study period, despite the apparent large variety of non-approved CBD-containing products available in the market, only a few products could be identified. However, AAPCC made available additional codes for non-approved CBD products over time, so their inclusion may have helped increase granularity and capture of information that otherwise may have been overlooked. Most substance classification is based on history alone and does not involve biologic confirmation [23], and importantly, the exact content and concentration of non-approved CBD products is unknown. Also, at the time of data extraction, NPDS had not locked data for 2021, so, although reportedly AAPCC and the PCCs make every effort to close as many cases as possible before the database for the year is locked, the number of cases for that period is probably still incomplete. AAPCC is not able to verify the accuracy of every report, so despite PCCs place emphasis on accurate data collection and coding [9], some data errors might have remained. Finally, a large proportion of CBD cases had missing formulation or clinical and medical outcome follow-up data, which also limits the interpretability of the results.
Conclusions
This case series identified an overall increasing trend in CBD exposures cases managed by AAPCC, largely driven by exposures to non-approved CBD. Data suggest both unintentional and intentional use of non-approved CBD in children. Some non-approved CBD exposures led to serious outcomes, including hospitalizations. Given the limited safety data available, potential effects on underlying medical conditions, drug interactions, and unknown contents and concentrations of non-approved CBD products, consumers should safely keep these products out of reach of children and exercise caution when purchasing and using non-approved CBD products.
Acknowledgments
The authors thank their FDA colleagues, Dr. Rose Radin for valuable suggestions on NPDS analyses and Drs. Ellen Pinnow, Sukhminder K Sandhu, Cindy Kortepeter, and Cassandra Taylor for insightful review of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The manuscript was subjected to administrative review before submission, but this review did not alter its content.
Footnotes
Disclaimers
This manuscript reflects the views of the authors and should not be construed to represent FDA’s views or policies.
The America’s Poison Centers (AAPCC) maintains the National Poison Data System (NPDS), which houses de-identified case records of self-reported information collected from callers during exposure management and poison information calls managed by the country’s poison control centers (PCCs). NPDS data do not reflect the entire universe of exposures to a particular substance as additional exposures may go unreported to PCCs. Accordingly, NPDS data should not be construed to represent the complete incidence of U.S. exposures to any substance(s). Exposures do not necessarily represent a poisoning or overdose and AAPCC is not able to completely verify the accuracy of every report. Findings based on NPDS data do not necessarily reflect the opinions of AAPCC.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].National Center for Biotechnology Information. PubChem Compound Summary for CID 644019, Cannabidiol 2022. [cited 2022 March 13]. https://pubchem.ncbi.nlm.nih.gov/compound/Cannabidiol
- [2].Hanus LO, Tchilibon S, Ponde DE, et al. Enantiomeric cannabidiol derivatives: synthesis and binding to cannabinoid receptors. Org Biomol Chem. 2005;3(6):1116–1123. [DOI] [PubMed] [Google Scholar]
- [3].Mead A. Legal and regulatory issues governing cannabis and Cannabis-Derived products in the United States. Front Plant Sci. 2019;10:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].US Food and Drug Administration. Approval letter. Epidiolex®; GW Research, Ltd. 2022. [cited 2022 March 13]. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/210365Orig1s000Ltr.pdf
- [5].US Food and Drug Administration. Package insert. Epidiolex®; GW Research, Ltd. 2022. [cited 2022 March 13]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/210365s015lbl.pdf
- [6].Leas EC, Hendrickson EM, Nobles AL, et al. Self-reported cannabidiol (CBD) use for conditions with proven therapies. JAMA Netw Open. 2020;3(10):e2020977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].US Food and Drug Administration. Warning Letters and Test Results for Cannabidiol-Related Products. 2022. [cited 2022 March 13]. https://www.fda.gov/news-events/public-health-focus/warning-letters-and-test-results-cannabidiol-related-products
- [8].Kachru R, Perry-Lunardo C, Thompson LA. CBD use in Children-Miracle, myth, or mystery? JAMA Pediatr. 2021;175(6):652. [DOI] [PubMed] [Google Scholar]
- [9].Gummin DD, Mowry JB, Beuhler MC, et al. 2020 Annual report of the American association of poison control centers’ national poison data system (NPDS): 38th annual report. Clin Toxicol (Phila). 2021;59(12):1282–1501. [DOI] [PubMed] [Google Scholar]
- [10].Gummin DD, Mowry JB, Beuhler MC, et al. 2019 Annual report of the American association of poison control centers’ national poison data system (NPDS): 37th annual report. Clin Toxicol (Phila). 2020;58(12):1360–1541. [DOI] [PubMed] [Google Scholar]
- [11].Rubin R. Cannabidiol products are everywhere, but should people be using them? JAMA. 2019;322(22):2156–2158. [DOI] [PubMed] [Google Scholar]
- [12].Dilley JA, Graves JM, Brooks-Russell A, et al. Trends and characteristics of manufactured cannabis product and cannabis plant product exposures reported to US poison control centers, 2017–2019. JAMA Netw Open. 2021;4(5):e2110925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Laudone TW, Leonard JB, Hines EQ, et al. Changes in unintentional cannabis exposures in children 6 months to 5 years reported to United States poison centers during the first nine months of the coronavirus-19 pandemic. Clin Toxicol (Phila). 2022;60(9):1029–1031. [DOI] [PubMed] [Google Scholar]
- [14].Leonard JB, Laudone T, Hines EQ, et al. Critical care interventions in children aged 6 months to 12 years admitted to the pediatric intensive care unit after unintentional cannabis exposures. Clin Toxicol (Phila). 2022;60(8):960–965. [DOI] [PubMed] [Google Scholar]
- [15].Myran DT, Cantor N, Finkelstein Y, et al. Unintentional pediatric cannabis exposures after legalization of recreational cannabis in Canada. JAMA Netw Open. 2022;5(1):e2142521. Jan 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Radhakrishnan L, Carey K, Hartnett KP, et al. Pediatric emergency department visits before and during the COVID-19 Pandemic - United States, january 2019-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(8):313–318. [DOI] [PubMed] [Google Scholar]
- [17].Whitehill JM, Harrington C, Lang CJ, et al. Incidence of pediatric cannabis exposure among children and teenagers aged 0 to 19 years before and after medical marijuana legalization in Massachusetts. JAMA Netw Open. 2019;2(8):e199456. Aug 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brown JD, Winterstein AG. Potential adverse drug events and drug-drug interactions with medical and consumer cannabidiol (CBD) use. J Clin Med. 2019;8(7):989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].US Food and Drug Administration. MedWatch: The FDA Safety Information and Adverse Event Reporting Program 2022. [cited 2022 March 13]. Available from: https://www.fda.gov/safety/med-watch-fda-safety-information-and-adverse-event-reporting-program
- [20].US Food and Drug Administration. FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD). Questions and Answers 2022. [cited 2022 March 13]. https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program
- [21].Karami S, Perez-Vilar S, Leishear K. Cannabidiol exposure calls to U.S Poison Control Centers. Pharmacoepidemiol Drug Saf. 2020; 29(Suppl 3):499. [Google Scholar]
- [22].Berger BA, Stolz U, Colvin J, et al. Epidemiology of cannabidiol related cases reported in the national poison data system – 2019–2020. Am J Emerg Med. 2021;48:218–223. [DOI] [PubMed] [Google Scholar]
- [23].Karami S, Major JM, Calderon S, et al. Trends in dextromethorphan cough and cold products: 2000–2015 national poison data system intentional abuse exposure calls. Clin Toxicol (Phila). 2018; 56(7):656–663. [DOI] [PubMed] [Google Scholar]


