Abstract
Background:
Previous studies have suggested an association between bone mineral density (BMD) and heart failure (HF) risk that may be race-dependent.
Methods:
We evaluated the relationship between BMD and incident HF in a cohort of older adults, the Health, Aging and Body Composition (Health ABC) study (n=2835), and next performed a pooled analysis involving a second older cohort, the Cardiovascular Health Study (n=1268). Hip BMD was measured by dual-energy X-ray absorptiometry in both cohorts, and spine BMD by computed tomography in a subset from Health ABC.
Results:
In Health ABC, lower BMD at the total hip was associated with higher incident HF in Black women after multivariable adjustment. Similar associations were found for BMD at the femoral neck and spine. In both cohorts, pooled analysis again revealed an association between lower total hip BMD and increased risk of HF in Black women (HR=1.41 per 0.1-g/cm2 decrement [95% CI=1.23–1.62]), and showed the same to be true for White men (HR=1.12 [1.03–1.21]). There was a decreased risk of HF in Black men (HR 0.80 [0.70–0.91]), but no relationship in White women. The associations were numerically stronger with HFpEF for Black women and White men, and with HFrEF for Black men. Findings were similar for femoral neck BMD. Sensitivity analyses delaying HF follow-up by 2 years eliminated the association in Black men.
Conclusions:
Lower BMD was associated with higher risk of HF and especially HFpEF in older Black women and White men, highlighting the need for additional investigation into underlying mechanisms.
Keywords: Bone mineral density, DXA scan, heart failure
Introduction
Both heart failure (HF) and osteoporosis impose a heavy disease burden upon older adults. In the U.S., HF affects ~6 million people,1 and its two subtypes, HF with preserved (HFpEF) and reduced ejection fraction (HFrEF), carry a similarly dismal prognosis.2 Some 12 million Americans have osteroporosis,3 predisposing to fractures and increased mortality.4
Although HF and osteoporosis are distinct disorders, they share certain features. HF has been associated with lower bone mineral density (BMD),5 as well as a ~4-fold increased risk of osteoporotic fractures.6 There is also evidence that low BMD may lead to HF. Osteoporosis has been suggested as an independent risk factor for cardiovascular disease (CVD),7 and vascular calcification and bone demineralization may involve common underlying mechanisms.8 Aging-related processes such as inflammation,9,10 autonomic dysfunction,11 and hormone disturbances12–15 could link bone loss with heart and vascular disease, disorders that exhibit variation by sex and race.3,16
In the Cardiovascular Health Study (CHS), we previously found that lower total hip BMD by dual-energy X-ray absorptiometry (DXA) was associated with higher HF risk in White men and lower risk in Black men, yet no association was seen in women of either race.17 This study was of moderate size, however, with few overall Black participants and limited ability to evaluate HF subtypes. Given well-known sex and race differences in susceptibility, we investigated the CHS findings further in the Health, Aging, and Body Composition Study (Health ABC). Our objectives were to (1) evaluate the sex- and race-specific associations of BMD with incident HF in this larger biracial sample of older persons that underwent not just DXA of the hip but, in a subset, computed tomography (CT) of the vertebral spine; and (2) pool the DXA results across Health ABC and CHS to generate more precise estimates of association, and more suitably examine relationships with HF subyptes.
Methods
Population
Details of the study design and procedures for Health ABC and CHS are provided in Supplemental Table 1. Briefly, Health ABC is a longitudinal study of risk factors for functional deterioration in older adults.18 In 1997–1998, 3075 well-functioning community residents aged 70–79 were recruited from two U.S. sites. CHS is a population-based study of cardiovascular risk factors in non-institutionalized adults ≥65 from four U.S. communities.17 In 1989–1990, 5201 individuals were enrolled, followed in 1992–1993 by 687 Black individuals. Participants underwent standardized evaluations in both cohorts.17,18,19 Selection of participants for the current analyses is described in Figure 1. Written informed consent and institutional review board approval were obtained at all sites.
Figure 1.
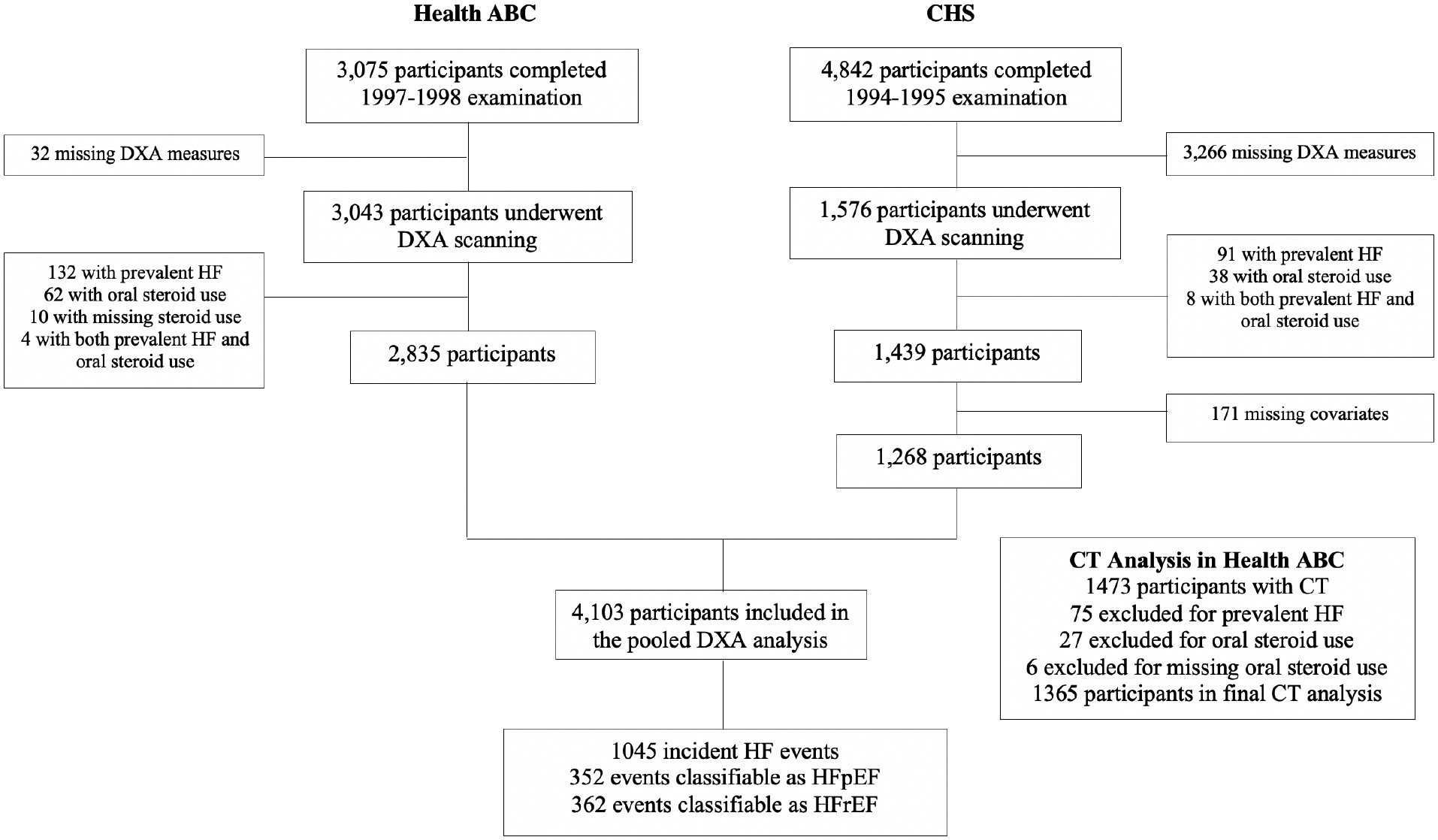
Flow diagram of participant selection in Health ABC and CHS.
BMD Determination
Areal BMD of the total hip and femoral neck (primary exposures) was assessed by DXA in Health ABC and CHS (Hologic, Waltham/Bedford, MA). In Health ABC, baseline volumetric BMD measures of the spine were determined at one site using CT (GE Medical Systems, Milwaukee, WI). Images were acquired at the L3 level to obtain trabecular BMD (secondary exposure). The DXA and CT data underwent blinded central interpretation.20,21
HF Assessment
In Health ABC and CHS, all participants attended a baseline clinic visit, followed by semi-annual phone or clinic visits for surveillance of cardiovascular events. Medical records for all potential HF events in Health ABC and CHS were centrally reviewed and adjudicated. In both studies, the diagnosis relied on documented symptoms and signs, supporting imaging evidence, and medical therapy. Based on left ventricular ejection fraction (LVEF) documentation,22 HFrEF was defined as LVEF<50% and HFpEF as LVEF≥50%. Follow-up extended to 2012 (Health ABC) or 2014 (CHS).
Covariates
All definitions were harmonized across cohorts, as reported previously.17,18,23 All variables were collected in 1997–1998 in Health ABC and 1994–1995 in CHS or, when missing, drawn from the nearest visit. Race was self-reported. Diabetes was defined by glucose levels and hypoglycemic therapy, and estimated glomerular filtration rate (eGFR) calculated from cystatin C.23 At enrollment, the presence of coronary heart disease (CHD), stroke or transient ischemic attack (TIA), peripheral artery disease (PAD), and HF, was ascertained by self-report in Health ABC and medical record review in CHS.24 In CHS, interim events preceding the 1994–1995 examination underwent standard adjudication.25 In both studies, prevalent atrial fibrillation or flutter was identified by electrocardiography or Medicare claims.23
Statistical Analysis
In sex- and race-stratified analyses, we first evaluated DXA and CT BMD measures in Health ABC, followed by a pooled individual-data analysis of DXA measures in Health ABC and CHS. Cox regression was used to evaluate BMD-HF associations. Generalized additive model plots revealed no departures from linearity. In the Health ABC-only analysis, HRs are reported per SD decrement of BMD for comparability of area and volume BMD associations, and in the pooled analysis per 0.1-g/cm2 decrement for clinical interpretability. Multivariable models were fit as previously in CHS.17 Model 1 adjusted for age and, in the pooled analysis, cohort. Model 2 (main model) additionally adjusted for body mass index (BMI), systolic blood pressure, antihypertensive medication, diabetes, smoking, alcohol, estrogen replacement, physical activity; prevalent CHD, stroke/TIA, PAD, and atrial fibrillation; forced expiratory volume in 1 second (FEV1), and eGFR. Two-way and three-way interactions of BMD with sex and race were evaluated with Wald tests.
In secondary analyses in Health ABC, we further adjusted for waist circumference or aspirin use; available mineral measures: 25-hydroxyvitamin D and phosphate; and inflammatory markers: C-reactive protein (CRP), interleukin-6 and tumor necrosis factor (TNF)-α. For the pooled cohorts, sensitivity analyses limited assessment to incident HF occurring ≥2 years after DXA or adjusted additionally for time-dependent CHD.
Analyses used SAS 9.4 (Cary, NC). P<0.05 defined statistical significance.
Results
Health ABC
Baseline characteristics for Health ABC are summarized in Table 1. Black men and women had lower education and physical activity; more hypertension, diabetes, and current smoking; lower 25-hydroxyvitamin D and TNF-α, but higher CRP and IL-6; and greater DXA and CT BMD compared to their White counterparts. Black women had higher adiposity and CHD, and less estrogen use than White women. Black men were younger, had lower waist circumference and FEV1, and exhibited higher eGFR and phosphate than White men.
Table 1.
Sex- and Race-Stratified Baseline Characteristics* of the Health ABC Cohort
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Characteristics | White (n=795) | Black (n=671) | P Value | White (n=861) | Black (n=508) | P Value |
| Age, years | 73.6 ± 2.8 | 73.4 ± 2.9 | 0.178 | 73.9 ± 2.9 | 73.5 ± 2.8 | 0.009 |
| Education, n (%) | <0.001 | <0.001 | ||||
| Less than high school | 83 (11) | 263 (39) | 122 (14) | 251 (50) | ||
| High school graduation | 339 (43) | 236 (35) | 224 (26) | 125 (25) | ||
| More than high school | 372 (47) | 168 (25) | 514 (60) | 131 (26) | ||
| Body mass index, kg/m2 | 25.9 ± 4.5 | 29.7 ± 5.8 | <0.001 | 27.0 ± 3.7 | 27.0 ± 4.3 | 0.950 |
| Waist circumference, cm | 95.6 ± 12.4 | 101.1 ± 14.8 | <0.001 | 101.9 ± 12.0 | 99.0 ± 12.5 | <0.001 |
| Smoking, n (%) | 0.001 | <0.001 | ||||
| Never smoker | 474 (60) | 371 (55) | 261 (30) | 155 (31) | ||
| Former smoker | 260 (33) | 209 (31) | 554 (65) | 245 (48) | ||
| Current smoker | 61 (8) | 90 (13) | 44 (5) | 107 (21) | ||
| Heavy alcohol use, n (%) | 43 (5) | 13 (2) | <0.001 | 40 (5) | 25 (5) | 0.804 |
| Physical activity, kcal/wk | 863 ± 1548 | 517 ± 874 | <0.001 | 1701 ± 2526 | 1022 ± 2111 | <0.001 |
| Systolic blood pressure, mm Hg | 134 ± 20 | 139 ± 22 | <0.001 | 133 ± 20 | 139 ± 22 | <0.001 |
| Antihypertensive medication, n (%) | 387 (48.7) | 449 (67) | <0.001 | 393 (45.6) | 267 (52.6) | 0.013 |
| Prevalent diabetes, n (%) | 93 (11.7) | 175 (26.1) | <0.001 | 182 (21.1) | 148 (29.1) | 0.001 |
| Estrogen replacement therapy, n (%) | 245 (30.8) | 76 (11.3) | <0.001 | NA | NA | NA |
| Aspirin use, n (%) | 305 (38.4) | 201 (30) | 0.001 | 390 (45.3) | 162 (31.9) | <0.001 |
| Prevalent coronary heart disease, n (%) | 86 (10.8) | 112 (16.7) | 0.001 | 215 (25) | 104 (20.5) | 0.057 |
| Prevalent stroke/TIA, n (%) | 59 (7.4) | 56 (8.4) | 0.512 | 58 (6.7) | 41 (8.1) | 0.357 |
| Prevalent peripheral artery disease, n (%) | 11 (1.4) | 12 (1.8) | 0.534 | 29 (3.4) | 13 (2.6) | 0.402 |
| Prevalent atrial fibrillation, n (%) | 24 (3) | 20 (3) | 0.966 | 41 (4.8) | 15 (3) | 0.103 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 73 ± 18 | 74 ± 19 | 0.118 | 71 ± 17 | 75 ± 20 | <0.001 |
| Forced expiratory volume in 1 s, L | 1.9 ± 0.4 | 1.7 ± 0.4 | <0.001 | 2.7 ± 0.6 | 2.3 ± 0.6 | <0.001 |
| C-reactive protein, mg/mL | 2.7 ± 4.2 | 3.7 ± 4.5 | <0.001 | 2.3 ± 4.7 | 3.2 ± 4.7 | 0.002 |
| Interleukin-6, pg/mL | 2 ± 1.5 | 2.5 ± 2.1 | <0.001 | 2.3 ± 1.8 | 2.6 ± 2 | 0.018 |
| Tumor necrosis factor-α, pg/mL | 3.4 ± 1.5 | 3.2 ± 1.7 | 0.022 | 3.7 ± 1.7 | 3.3 ± 1.9 | <0.001 |
| Phosphate, mg/dL | 3.7 ± 0.4 | 3.7 ± 0.4 | 0.909 | 3.4 ± 0.5 | 3.5 ± 0.5 | <0.001 |
| 25-hydroxy vitamin D, ng/mL | 28.9 ± 11.5 | 20.7 ± 11.5 | <0.001 | 29 ± 9.1 | 21.2 ± 8.4 | <0.001 |
| Total hip BMD, g/cm2 | 0.763 ± 0.128 | 0.859 ± 0.151 | <0.001 | 0.942 ± 0.143 | 1.015 ± 0.156 | <0.001 |
| Femoral neck BMD, g/cm2 | 0.753 ± 0.133 | 0.846 ± 0.142 | <0.001 | 0.766 ± 0.127 | 0.846 ± 0.142 | <0.001 |
| Total hip, WHO categories, n (%) | <0.001 | <0.001 | ||||
| Normal, n (%) | 258 (33) | 396 (59) | 690 (80) | 459 (90) | ||
| Osteopenia, n (%) | 408 (51) | 232 (35) | 162 (19) | 47 (9) | ||
| Osteoporosis, n (%) | 129 (16) | 43 (6) | 9 (1) | 2 (0.4) | ||
| Femoral neck, WHO categories, n (%) | <0.001 | <0.001 | ||||
| Normal, n (%) | 161 (20) | 352 (53) | 487 (57) | 396 (78) | ||
| Osteopenia, n (%) | 483 (61) | 280 (42) | 346 (40) | 104 (21) | ||
| Osteoporosis, n (%) | 151 (19) | 39 (6) | 28 (3) | 8 (2) | ||
| Trabecular BMD, mg/cm3 | 99 ± 33 | 125 ± 42 | <0.001 | 120 ± 39 | 151 ± 44 | <0.001 |
Data are given as mean ± standard deviations for continuous variables and count (percent) for categorical variables. Comparisons between race groups are with t-tests or chi-square tests, as appropriate.
BMD, bone mineral density; TIA, transient ischemic attack; WHO, World Health Organization
During follow-up of 11.3±5.1 years, 583 incident HF events occurred. Sex- and race-specific relationships between DXA and CT BMD and incident HF are presented in Table 2. Lower BMD at the total hip was associated with increased HF in Black women in the minimally adjusted model. This inverse association became stronger in the main model (HR=1.80 per SD decrement [95% CI=1.40–2.31]), reflecting negative confounding by BMI, diabetes and hypertension. By contrast, among Black men, lower BMD related to decreased HF in the minimal and main (HR=0.80 [0.63–1.01]) models, though both fell short of statistical significance. Among White participants, there were no significant associations (main model) in women (HR=0.87 [0.71–1.05]) or men (HR=1.10 [0.93–1.31]). Relationships for femoral neck BMD were similar in all groups. Likewise, lower trabecular BMD of the spine was associated with higher HF risk in Black women after full adjustment (HR=1.45 per SD decrement [1.07–1.96]), but there were no significant associations in Black men (HR=0.92 [0.70–1.22]), White women (HR=0.89 [0.71–1.12]), or White men (HR=1.18 [0.94–1.47]). There was significant interaction of total hip BMD by sex among Blacks (p=0.002), but not Whites (p=0.363), as well as by race among women (p=0.026) and men (p=0.049). Additional adjustment for waist circumference, aspirin, mineral measures, or inflammatory markers did not affect the findings.
Table 2.
Sex- and Race-Stratified Associations of DXA and QCT-derived BMD Measures and Incident HF in Health ABC
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| n | White | n | Black | n | White | n | Black | |
| Cumulative HF Incidence, n (%) | 146 (18.4) | 133 (19.8) | 193 (22.4) | 111 (21.9) | ||||
| HR* (95% CI) p | HR* (95% CI) p | HR* (95% CI) p | HR* (95% CI) p | |||||
| Total Hip BMD (Area) | ||||||||
| Age-adjusted model | 795 | 0.96 (0.81–1.14) 0.622 | 671 | 1.24 (1.04–1.48) 0.019 | 861 | 1.05 (0.91–1.22) 0.488 | 508 | 0.85 (0.70–1.03) 0.095 |
| Fully adjusted model† | 739 | 0.87 (0.71–1.05) 0.143 | 608 | 1.80 (1.40–2.31) <0.001 | 799 | 1.10 (0.93–1.31) 0.251 | 469 | 0.80 (0.63–1.01) 0.061 |
| Femoral Neck BMD (Area) | ||||||||
| Age-adjusted model | 795 | 0.95 (0.81–1.12) 0.54 | 671 | 1.18 (0.98–1.41) 0.076 | 861 | 1.02 (0.88–1.18) 0.786 | 508 | 0.94 (0.78–1.14) 0.531 |
| Fully adjusted model† | 739 | 0.87 (0.73–1.05) 0.14 | 608 | 1.50 (1.18–1.91) <0.001 | 799 | 1.07 (0.91–1.27) 0.399 | 469 | 0.83 (0.66–1.04) 0.102 |
| Trabecular BMD (Volume) | ||||||||
| Age-adjusted model | 352 | 0.88 (0.70–1.10) 0.251 | 349 | 1.16 (0.90–1.51) 0.261 | 415 | 1.13 (0.91–1.40) 0.254 | 249 | 1.03 (0.80–1.32) 0.829 |
| Fully adjusted model† | 338 | 0.89 (0.71–1.12) 0.319 | 324 | 1.45 (1.07–1.96) 0.016 | 398 | 1.18 (0.94–1.47) 0.151 | 234 | 0.92 (0.70–1.22) 0.575 |
BMD, bone mineral density; CI, confidence interval; DXA, dual-energy X-ray absorptiometry; HF, heart failure; HR, hazard ratio; QCT, quantitative computed tomography;
Per standard deviation decrement of BMD
Adjusted for age, body mass index, smoking status, heavy alcohol use, physical activity, estrogen use, systolic blood pressure, antihypertensive medication use, diabetes, prevalent coronary heart disease, prevalent stroke and transient ischemic attack, prevalent peripheral artery disease, prevalent atrial fibrillation, forced expiratory ventilation in 1 second, and estimated glomerular filtrate rate.
Pooled Analysis
Characteristics of the 4103 pooled Health ABC and CHS participants are shown in Supplemental Table 2. Corresponding associations of covariates with total hip BMD are presented in Table 3. In most race and sex groups, total hip BMD was inversely associated with age and current smoking, but positively related to adiposity and diabetes. In White women, total hip BMD exhibited positive associations with antihypertensive treatment, physical activity, estrogen use, and FEV1, as well as negative associations with atrial fibrillation and phosphate. In Black women, total hip BMD was positively associated with antihypertensive and estrogen treatment, and CRP, but negatively associated with stroke/TIA. In both White and Black men, total hip BMD was positively associated with physical activity, and FEV1. In White men, positive associations for total hip BMD were also present with heavy drinking and stroke/TIA, whereas in Black men, there was an additional positive association with aspirin use.
Table 3.
Sex- and Race-Stratified Relationships* of Baseline Covariates with Total Hip BMD Pooled for the Two Cohorts
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariates | White | Black | White | Black | ||||
| Mean±SD | p | Mean±SD | p | Mean±SD | p | Mean±SD | p | |
| Age | −0.25 | <0.001 | −0.20 | <0.001 | −0.17 | <0.001 | −0.05 | 0.184 |
| Body mass index | 0.46 | <0.001 | 0.52 | <0.001 | 0.36 | <0.001 | 0.48 | <0.001 |
| Waist circumference | 0.34 | <0.001 | 0.43 | <0.001 | 0.23 | <0.001 | 0.36 | <0.001 |
| Systolic blood pressure | 0.05 | 0.082 | 0.02 | 0.569 | 0.01 | 0.788 | 0.00 | 0.929 |
| Antihypertensive medication | <0.001 | 0.013 | 0.0629 | 0.187 | ||||
| No | 0.74 ± 0.13 | 0.84 ± 0.16 | 0.93 ± 0.15 | 1.01 ± 0.15 | ||||
| Yes | 0.76 ± 0.13 | 0.87 ± 0.15 | 0.95 ± 0.15 | 1.03 ± 0.16 | ||||
| Diabetes mellitus | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| No | 0.74 ± 0.13 | 0.84 ± 0.15 | 0.93 ± 0.15 | 1.00 ± 0.15 | ||||
| Yes | 0.80 ± 0.14 | 0.90 ± 0.15 | 0.98 ± 0.14 | 1.07 ± 0.17 | ||||
| Smoking status | <0.001 | 0.012 | 0.011 | <0.001 | ||||
| Never | 0.75 ± 0.13 | 0.86 ± 0.15 | 0.93 ± 0.15 | 1.05 ± 0.16 | ||||
| Former | 0.75 ± 0.13 | 0.86 ± 0.13 | 0.94 ± 0.15 | 1.02 ± 0.15 | ||||
| Current | 0.70 ± 0.13 | 0.82 ± 0.17 | 0.89 ± 0.17 | 0.97 ± 0.14 | ||||
| Heavy alcohol | 0.406 | 0.338 | 0.025 | 0.976 | ||||
| No | 0.75 ± 0.13 | 0.86 ± 0.15 | 0.94 ± 0.15 | 1.02 ± 0.16 | ||||
| Yes | 0.76 ± 0.13 | 0.89 ± 0.14 | 0.97 ± 0.16 | 1.02 ± 0.17 | ||||
| Physical activity | 0.07 | 0.014 | −0.00 | 0.890 | 0.09 | <0.001 | 0.10 | 0.013 |
| Estrogen replacement therapy | <0.001 | 0.008 | NA | NA | ||||
| No | 0.73 ± 0.13 | 0.85 ± 0.15 | NA | NA | ||||
| Yes | 0.78 ± 0.13 | 0.90 ± 0.13 | NA | NA | ||||
| Prevalent coronary heart disease | 0.063 | 0.650 | 0.706 | 0.655 | ||||
| No | 0.75 ± 0.13 | 0.86 ± 0.15 | 0.94 ± 0.15 | 1.02 ± 0.15 | ||||
| Yes | 0.73 ± 0.14 | 0.85 ± 0.16 | 0.94 ± 0.15 | 1.01 ± 0.17 | ||||
| Prevalent stroke/transient ischemic attack | 0.888 | 0.007 | 0.039 | 0.320 | ||||
| No | 0.75 ± 0.13 | 0.86 ± 0.15 | 0.94 ± 0.15 | 1.02 ± 0.16 | ||||
| Yes | 0.75 ± 0.14 | 0.81 ± 0.13 | 0.91 ± 0.13 | 1.04 ± 0.16 | ||||
| Prevalent peripheral arterial disease | 0.697 | 0.950 | 0.252 | 0.062 | ||||
| No | 0.75 ± 0.13 | 0.86 ± 0.15 | 0.94 ± 0.15 | 1.02 ± 0.16 | ||||
| Yes | 0.74 ± 0.17 | 0.86 ± 0.15 | 0.92 ± 0.15 | 0.95 ± 0.11 | ||||
| Prevalent atrial fibrillation | 0.009 | 0.116 | 0.246 | 0.752 | ||||
| No | 0.75 ± 0.13 | 0.86 ± 0.15 | 0.94 ± 0.15 | 1.02 ± 0.16 | ||||
| Yes | 0.70 ± 0.13 | 0.81 ± 0.15 | 0.96 ± 0.17 | 1.03 ± 0.16 | ||||
| Estimated glomerular filtration rate | −0.02 | 0.470 | 0.00 | 0.921 | 0.01 | 0.749 | 0.01 | 0.779 |
| Forced expiratory volume in 1 s | 0.22 | <0.001 | 0.04 | 0.262 | 0.17 | <0.001 | 0.13 | 0.001 |
| C-reactive protein | 0.03 | 0.324 | 0.18 | <0.001 | −0.03 | 0.344 | −0.07 | 0.100 |
| Interleukin-6 | 0.01 | 0.785 | 0.10 | 0.009 | −0.07 | 0.021 | −0.11 | 0.013 |
| Phosphate* | −0.10 | 0.008 | 0.02 | 0.630 | 0.02 | 0.666 | 0.00 | 0.938 |
| 25-hydroxyvitamin D* | −0.01 | 0.714 | −0.02 | 0.675 | −0.04 | 0.272 | 0.04 | 0.352 |
Pearson correlation coefficients for continuous variables, and means ± standard deviations for categorical variables compared using linear regression.
Only available for Health ABC Cohort
Over follow-up of 11.2±5.5 years, there were 1045 HF events (352 classifiable as HFpEF, 362 as HFrEF). The associations of DXA BMD measures with incident HF are displayed in Figure 2. Lower BMD at the total hip was associated with increased HF after main-model adjustment in Black women (HR=1.41 per 0.1-g/cm2 decrement [1.23–1.62]) and White men (HR=1.12 [1.03–1.21]), along with significantly decreased HF in Black men (HR=0.80 [0.70–0.91]). No association was observed in White women (HR=0.95 [0.86–1.05]). Findings were similar for BMD at the femoral neck. Corresponding associations per 0.1-g/cm2 decrement in BMD for Health ABC are detailed in Supplemental Table 3. There was significant three-way interaction of BMD at the total hip or femoral neck with sex and race (p≤0.001).
Figure 2.
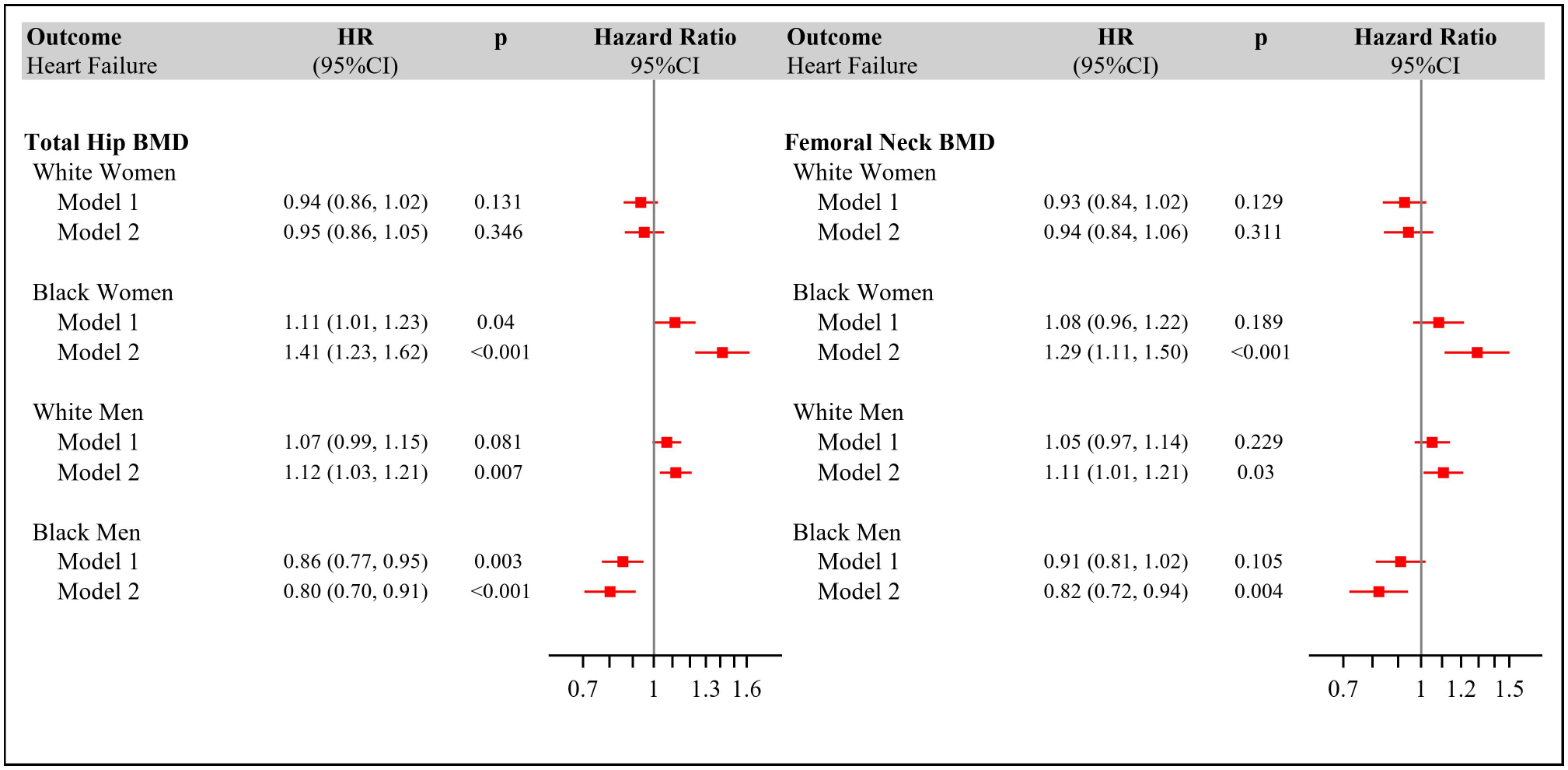
Adjusted Hazard Ratios for Incident Heart Failure by Continuous Bone Mineral Density at the Total Hip (Panel A) and Femoral Neck (Panel B) in Health ABC and CHS.
Model 1: adjusted for age and cohort.
Model 2: adjusted for age, cohort, body mass index, smoking status, heavy alcohol use, physical activity, estrogen use, systolic blood pressure, antihypertensive medication use, diabetes.
Number of incident HF events per sex/race group: White women, 351 (205 in CHS); Black women 184 (51 in CHS); White men, 364 (171 in CHS); Black men 146 (35 in CHS).
Number of individuals in White Women: Model 1=1393; Model 2=1337; Black Women: Model 1=819; Model 2=756; White Men: Model 1=1292; Model 2=1230; Black Men: Model 1=599; Model 2=560.
Regarding HF subtypes (Supplemental Table 4), total hip BMD was inversely associated with HFpEF after full adjustment in Black women (HR=1.60 per 0.1-g/cm2 decrement [1.25–2.05]) and White men (HR=1.21 [1.04–1.41]), and with HFrEF in Black women only (HR=1.35 [1.09–1.66]). Findings were similar for femoral neck BMD, although the association with HFrEF in Black women fell short of significance (HR=1.24 [0.98–1.56)]. Among Black men, BMD was not related to HFpEF, but did show a positive association with HFrEF at both the total hip (HR=0.73 per 0.1-g/cm2 decrement [0.60–0.88]) and femoral neck (HR=0.76 [0.62–0.92]). No associations were observed with either HF subtype in White women.
Delaying follow-up by 2 years abolished the BMD-HF association in Black men (total hip, HR=0.95 [0.81–1.12]) (Supplemental Table 5). Associations in Black women (HR=1.38 [1.16–1.64]) and White men (HR=1.14 [1.04–1.25]) remained similar. There was no impact in White women. Additional adjustment for time-varying CHD did not alter the results.
Discussion
Main Findings
In Health ABC’s biracial older cohort, we found that lower BMD at the total hip, femoral neck and lumbar spine was significantly associated with higher incidence of HF in Black women after extensive adjustment for potential confounders. There was also a relationship between lower BMD of the total hip and femoral neck, though not spine, and lower incidence of HF in Black men that approached significance, but no significant associations between BMD and incident HF were observed in White women or men. Pooled data from Health ABC and CHS17 revealed a consistent inverse association between BMD at the total hip and femoral neck and incident HF in Black women, as well as significant associations in Black men (positive) and White men (inverse), but none in White women. Secondary pooled analyses showed significant inverse relationships of BMD at the total hip and femoral neck with HFpEF in Black women and White men, which were significant with HFrEF only for total hip BMD in Black women. By contrast, BMD at the total hip and femoral neck revealed a significant positive association with HFrEF, but not HFpEF, in Black men.
Prior Studies
The EPIC-Norfolk study26 documented an inverse relationship between calcaneal BMD and incident HF in European men and women, although adjustment for eGFR was not reported. Our prior work in CHS17 evaluated BMD of the hip, detecting effect modification by both sex and race: a significant association was observed in male but not female participants, wherein lower BMD was associated with higher incidence of HF in White men, but lower incidence of HF in Black men. More recently, the HUNT study did not find an association between forearm BMD and new-onset HF in European men or women, although ascertainment of HF cases based on diagnostic coding was not validated, and events in men were relatively modest in number.27
The present investigation in Health ABC, which includes >4-fold Black individuals as the prior CHS report, newly reveals an inverse association between areal BMD of the hip, as well as volumetric BMD of the spine, and incident HF in Black women. The current analysis also confirms the positive relationship between hip BMD and new-onset HF in Black men observed in CHS. The pooled analysis of both cohorts bears out the latter association in Black men, and the previously documented inverse association in White men, which are now significant for both BMD of the total hip and femoral neck. The combined results also support the lack of a meaningful BMD-HF relationship in White women. Furthermore, the pooled analysis yields the novel finding that these associations apply more prominently or exclusively to HFpEF in Black women and White men, whereas for Black men, they pertain only to HFrEF.
Validity of Prospective Associations
A key consideration in interpreting the observed associations is the possibility of reverse causality, wherein the influence on BMD of unrecognized HF at baseline would account for the findings. As relates to the inverse association seen in Black women and White men, HF is well-documented to promote bone loss through limitation of physical activity, hormone disturbances,28 and medications such as loop diuretics.28,29 We therefore performed sensitivity analyses excluding HF events occurring in the initial 2 years of follow-up, a period in which unrecognized prevalent HF cases would likely become manifest. These analyses did not alter the inverse relationship between BMD and incident HF documented in Black women and White men (although, as discussed later, they did eliminate the positive association seen in Black men). Together with the previous result from CHS17 that exclusion of high N-terminal pro-B-type natriuretic peptide levels did not affect the inverse association for White men, these analyses reduce the likelihood that the associations documented here for this group and for Black women are attributable to reverse causality.
Potential Explanations: Women
It is well established that Black women have lower risk of osteoporosis30 and higher risk of HF compared to their White counterparts.31 The fact that an inverse relationship between BMD and HF was observed in Black, but not White, women suggests that factors involved in the pathogenesis of osteoporosis in the former group may more closely overlap or influence the development of HF.
One possible explanation for the dissimilar findings in White and Black women could be race-based differences in vitamin D and calcium metabolism. It is documented that Black Americans have lower 25-hydroxyvitamin D and higher parathyroid hormone (PTH) concentrations than White Americans.32 Such differences have been attributed to reduced synthesis of vitamin D in more melanin-rich skin, lower oral intake, and greater adipose-tissue sequestration.33 Vitamin D deficiency can promote renin-angiotensin-system activation and stimulate inflammatory pathways, and the attendant secondary hyperparathyroidism can heighten HF risk.34 Accordingly, abnormal 25-hydroxyvitamin D levels in African Americans have been proposed to explain racial disparities in CVD.35 Yet the relationships of low 25-hydroxyvitamin D and high PTH with adverse skeletal12 and cardiovascular13 outcomes seen in Whites have not been reproduced in Blacks. A previous report14 that such differences may relate to lower vitamin D binding-protein and higher associated bioavalability has been contested,36 but Blacks do exhibit higher calcium absorption and lower excretion compared with Whites.37 Thus, vitamin D metabolism and its consequences in Blacks remain incompletely understood.
We found the expected lower 25-hydroxyvitamin D levels in Black compared with White women, yet these showed no correlation with BMD, nor did adjustment for them alter the observed associations. The extent to which abnormal vitamin D metabolism contributes to osteopenia/osteoporosis in Black women, or suitable biomarkers for its detection, is not well characterized.37 But the inverse BMD-HF association identified here underscores the need to refine understanding of this relationship.
Another consideration is sex hormones. Menopause is a foremost risk factor for osteoporosis in women, and depletion of estrogen has adverse consequences not just for bone but for cardiovascular health.38 Absence of an association between BMD and HF in White women, however, argues against estrogen deficiency as a major factor tying together bone demineralization and HF risk among the post-menopausal women under study. Still, it is possible that differences in reproductive factors underlie the race-specific associations documented here. We did not account for age of menopause39 or lifetime parity,40 factors that might contribute to the differences observed.
Last, socioeconomic disparities could play a role. Lower education, physical activity, estrogen use, along with higher BMI, current smoking, CHD, and inflammatory markers, were observed in Black compared with White women. Although the associations persisted after adjustment for such factors, these reflect a more adverse health profile captured only partially in the study. Such an adverse health profile can lead to autonomic nervous system (ANS) impairment, with unfavorable effects on its regulation of bone homeostasis.11 Given that untoward health factors, along with attendant ANS dysfunction, predispose to both low BMD32 and HF,41 their accentuated levels in Black women could explain the observed link between the two conditions in this group.
Potential Explanations: Men
The inverse relationship documented between BMD and HF in White men occurred, much like the numerically stronger inverse association in Black women, in a group less prone to osteoporosis than its White female counterpart. As with Black women, this suggests that shared risk factors for skeletal and cardiovascular health, or factors affecting bone in a way that compromises cardiovascular function, may hold the answer. One possible link is aging-related oxidative stress and inflammation,9,10 which could play a greater role in BMD loss among White men or Black women. Although the BMD-HF relationship for White men was more pronounced in CHS, where it persisted after adjustment for inflammatory markers, such mechanisms remain a possibility. Similarly, ANS dysfunction, a potentially more prominent contributor to bone demineralization in White men (as in Black women) than White women, might help to account for the observed sex difference.11 Another potential link is sex-hormone deficiency, which could likewise negatively affect bone and heart homeostasis.15 Lack of ANS assessment or sex-hormone measures precludes evaluation of these premises here.
Regarding the positive BMD-HF association in Black men, combined analyses here provide further insights into its basis. As noted previously,17 osteoporosis is rare in this group, such that any untoward effects of BMD loss or its determinants would be difficult to observe. Hence, insofar as higher BMD predominantly reflected excess adiposity and its metabolic consequences in this group, the positive association likely relates to unmeasured confounding by such factors. Notably, the association was driven primarily by HFrEF, which would tend to support atherosclerosis-related myocardial injury, although adjustment for time-varying CHD did not affect the BMD-HF relationship overall. The association was abolished when follow-up was delayed by 2 years, however, suggesting that it reflected unrecognized HF at baseline, rather than a true prospective association.
Association with HFpEF
The documented association for BMD in Black women and White men was especially pronounced with HFpEF, supporting the paradigm shift away from HFpEF as strictly a disease of decreased LV compliance, and toward a systemic, multi-organ, geriatric syndrome.42 The cardinal feature of the disorder, exercise intolerance, is believed to result from inflammation and other circulating factors associated with aging, adiposity, multi-morbidity, and physical inactivity. Such factors promote loss of end-organ capillarity, mitochondrial impairment, endothelial dysfunction, and sarcopenia, representing a “common soil” for development of osteoporosis and HFpEF. Whether and how skeletal demineralization directly drives HFpEF onset will require further investigation.
Clinical Implications
Although low BMD is less common in Black women and White men than in White women, low bone mass/osteoporosis nonetheless is still substantial.43 Black women are a particularly underserved group as relates to osteoporosis detection and treatment.32 Our findings raise the question of whether leaving low BMD unaddressed in this group, and also in White men, could have adverse cardiovascular implications that go beyond its risks of fracture, functional disability and mortality. Clinicians caring for Black women and White men with osteoporosis should pay particular attention to cardiovascular risk factor control, as recommended in the latest guidelines generally for prevention of HF in groups at risk.44 Furthermore, the present results should motivate expanded efforts to elucidate the causes of osteoporosis in Black women, as well as White men, and determine the mechanisms linking skeletal and cardiovascular health. Such mechanistic insights could lead to new approaches to prevent one or both disorders in these and possibly other sex/race groups, which may be of particular relevance to HFpEF, a disorder for which therapeutic options remain limited.45
Limitations
Health ABC comprised Black and White older adults who were disability-free at baseline, and the current findings may not be generalizable to other groups. Nearly one-third of HF cases could not be classified as HFpEF or HFrEF, limiting power for individual subtypes. Measurement of circulating calcium and PTH levels was not applied across the Health ABC cohort, precluding their evaluation.
Conclusions
We report an association between lower BMD and higher incident HF that is specific to Black women and White men, and an association between lower BMD and decreased incident HF in Black men, the latter appearing to reflect subclinical HF at baseline. These findings should serve to raise awareness of osteoporosis and its potential cardiovascular consequences in Black women and White men. The findings also support further research into possible pathways linking these two conditions, including Vitamin D metabolism, autonomic dysregulation and endogenous sex hormones, as potentially fruitful to identifying new preventive strategies for these major aging-related disorders.
Supplementary Material
Key Points:
What is the association between bone mineral density (BMD) and incidence of heart failure (HF) and its subtypes in older adults of different race and sex characteristics?
In this pooled analysis of two prospective cohort studies of older adults, we found that lower BMD at the total hip and femoral neck was associated with increased risk of HF in Black women and White men, and decreased risk of HF in Black men, with no association seen in White women. These relationships persisted for Black women and White men, but were eliminated for Black men, when the start of follow-up was delayed by 2-years, suggesting reverse causality in the latter group. The inverse associations among Black women and White men were seen especially with HFpEF.
The associations of low BMD with HF, and particularly HFpEF, documented in Black women and White men underscore the potential cardiovascular implications of bone demineralization and its determinants in these groups. These findings suggest that improved understanding of the mechanisms linking BMD and HF could translate to novel preventive strategies for these disorders.
Why Does This Paper Matter?
This paper documents an association between low BMD and increased incidence of HF in Black women that is novel; the paper confirms the same relationship in White men, while also newly showing that this relates particularly to HFpEF in both groups. The current analyses reveal no such association for White women, but show that the previously documented relationship between low BMD and lower incidence of HF in Black men appears attributable to reverse causality. Although osteopenia and osteoporosis are less common in Black women and White men than White women, their prevalence is substantial, yet these groups often go unscreened or untreated. The present findings suggest that addressing bone loss or its determinants in these sex/race groups could have benefits not just for skeletal health, but potentially also for cardiovacular health. These results should motivate further efforts to understand underlying mechanisms, efforts that could translate to novel therapeutics.
Acknowledgements:
Sponsor’s Role:
Sponsors of this study, including the NIH, NHLBI, NINDS, and NIA, were not involved in the preparation and revision of this manuscript.
Funding:
CHS was supported by R01 AG053325 from the National Institute on Aging; and by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. Health ABC was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. The research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging. JRK was supported by K24 HL135413 from the National Heart, Lung, and Blood Institute.
Abbreviations:
- BMD
Bone mineral density
- CHD
Coronary heart disease
- CT
Computed tomography
- DXA
Dual-energy X-ray absorptiometry
- eGFR
Estimated glomerular filtration rate
- FEV1
Forced expiratory volume in 1 second
- HF
Heart failure
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
Footnotes
Disclosures: JRK reports stock ownership in Abbott, Bristol-Myers Squibb, Johnson & Johnson, Medtronic, Merck and Pfizer.
Conflicts of Interest: Jorge Kizer reports stock ownership in Abbott, Bristol-Myers Squibb, Johnson & Johnson, Medtronic, Merck and Pfizer. No other authors have identified potential conflicts of interest relevant to this article.
Brief Descriptive Title of Supplementary Materials: Details of the Cohort Studies and Additional Analyses
References
- 1.Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation. 2021;143(8). doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 2.Shah KS, Xu H, Matsouaka RA, et al. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction. J Am Coll Cardiol. 2017;70(20):2476–2486. doi: 10.1016/j.jacc.2017.08.074 [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(24):2521. doi: 10.1001/jama.2018.7498 [DOI] [PubMed] [Google Scholar]
- 4.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and Mortality of Hip Fractures in the United States. Published online 1986:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jankowska EA, Jakubaszko J, Cwynar A, et al. Bone mineral status and bone loss over time in men with chronic systolic heart failure and their clinical and hormonal determinants. Eur J Heart Fail. 2009;11(1):28–38. doi: 10.1093/eurjhf/hfn004 [DOI] [PubMed] [Google Scholar]
- 6.van Diepen S, Majumdar SR, Bakal JA, McAlister FA, Ezekowitz JA. Heart Failure Is a Risk Factor for Orthopedic Fracture: A Population-Based Analysis of 16 294 Patients. Circulation. 2008;118(19):1946–1952. doi: 10.1161/CIRCULATIONAHA.108.784009 [DOI] [PubMed] [Google Scholar]
- 7.Marcovitz PA, Tran HH, Franklin BA, et al. Usefulness of Bone Mineral Density to Predict Significant Coronary Artery Disease. Am J Cardiol. 2005;96(8):1059–1063. doi: 10.1016/j.amjcard.2005.06.034 [DOI] [PubMed] [Google Scholar]
- 8.Sprini D Correlation between osteoporosis and cardiovascular disease. Clin Cases Miner Bone Metab. Published online 2014. doi: 10.11138/ccmbm/2014.11.2.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franceschi C, Campisi J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–S9. doi: 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 10.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11(3):234–250. doi: 10.1038/nrd3669 [DOI] [PubMed] [Google Scholar]
- 11.Elefteriou F Impact of the Autonomic Nervous System on the Skeleton. Physiol Rev. 2018;98(3):1083–1112. doi: 10.1152/physrev.00014.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22(6):1745–1753. doi: 10.1007/s00198-010-1383-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial Differences in the Association of Serum 25-Hydroxyvitamin D Concentration With Coronary Heart Disease Events. JAMA. 2013;310(2):179. doi: 10.1001/jama.2013.7228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powe CE, Evans MK, Wenger J, et al. Vitamin D–Binding Protein and Vitamin D Status of Black Americans and White Americans. N Engl J Med. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke BL, Khosla S. Physiology of Bone Loss. Radiol Clin North Am. 2010;48(3):483–495. doi: 10.1016/j.rcl.2010.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25(11):1657–1666. doi: 10.1038/s41591-019-0643-8 [DOI] [PubMed] [Google Scholar]
- 17.Fohtung RB, Brown DL, Koh WJH, et al. Bone Mineral Density and Risk of Heart Failure in Older Adults: The Cardiovascular Health Study. J Am Heart Assoc. 2017;6(3). doi: 10.1161/JAHA.116.004344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, et al. Epidemiology of Incident Heart Failure in a Contemporary Elderly Cohort: The Health, Aging, and Body Composition Study. Arch Intern Med. 2009;169(7):708. doi: 10.1001/archinternmed.2009.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalogeropoulos A, Georgiopoulou V, Psaty BM, et al. Inflammatory Markers and Incident Heart Failure Risk in Older Adults. J Am Coll Cardiol. 2010;55(19):2129–2137. doi: 10.1016/j.jacc.2009.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farhat GN, Newman AB, Sutton-Tyrrell K, et al. The association of bone mineral density measures with incident cardiovascular disease in older adults. Osteoporos Int. 2007;18(7):999–1008. doi: 10.1007/s00198-007-0338-8 [DOI] [PubMed] [Google Scholar]
- 21.Mackey DC, Eby JG, Harris F, et al. Prediction of Clinical Non-Spine Fractures in Older Black and White Men and Women With Volumetric BMD of the Spine and Areal BMD of the Hip: The Health, Aging, and Body Composition Study. J Bone Miner Res. 2007;22(12):1862–1868. doi: 10.1359/jbmr.070807 [DOI] [PubMed] [Google Scholar]
- 22.Al-Kindi SG, Buzkova P, Shitole SG, et al. Soluble CD14 and Risk of Heart Failure and Its Subtypes in Older Adults. J Card Fail. 2020;26(5):410–419. doi: 10.1016/j.cardfail.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Bartz TM, Santanasto A, et al. Body Composition and Incident Heart Failure in Older Adults: Results From 2 Prospective Cohorts. J Am Heart Assoc. 2022;11(1):e023707. doi: 10.1161/JAHA.121.023707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):270–277. doi: 10.1016/1047-2797(94)00092-8 [DOI] [PubMed] [Google Scholar]
- 25.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. Ann Epidemiol. 1995;5(4):278–285. doi: 10.1016/1047-2797(94)00093-9 [DOI] [PubMed] [Google Scholar]
- 26.Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Low Bone Mineral Density Predicts Incident Heart Failure in Men and Women. JACC Heart Fail. 2014;2(4):380–389. doi: 10.1016/j.jchf.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 27.Bhatta L, Cepelis A, Vikjord SA, et al. Bone mineral density and risk of cardiovascular disease in men and women: the HUNT study. Eur J Epidemiol. 2021;36(11):1169–1177. doi: 10.1007/s10654-021-00803-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carbone L, Buzkova P, Fink HA, et al. Hip fractures and heart failure: findings from the Cardiovascular Health Study. Eur Heart J. 2010;31(1):77–84. doi: 10.1093/eurheartj/ehp483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim LS, Fink HA, Blackwell T, Taylor BC, Ensrud KE. Loop Diuretic Use and Rates of Hip Bone Loss and Risk of Falls and Fractures in Older Women: LOOP DIURETICS AND BONE LOSS, FALLS, AND FRACTURES. J Am Geriatr Soc. 2009;57(5):855–862. doi: 10.1111/j.1532-5415.2009.02195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. The Lancet. 2002;359(9319):1761–1767. doi: 10.1016/S0140-6736(02)08657-9 [DOI] [PubMed] [Google Scholar]
- 31.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial Differences in Incident Heart Failure among Young Adults. N Engl J Med. 2009;360(12):1179–1190. doi: 10.1056/NEJMoa0807265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golden SH, Brown A, Cauley JA, et al. Health Disparities in Endocrine Disorders: Biological, Clinical, and Nonclinical Factors—An Endocrine Society Scientific Statement. J Clin Endocrinol Metab. 2012;97(9):E1579–E1639. doi: 10.1210/jc.2012-2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48–54. doi: 10.1016/j.nutres.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 34.Pilz S, Verheyen N, Grübler MR, Tomaschitz A, März W. Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol. 2016;13(7):404–417. doi: 10.1038/nrcardio.2016.73 [DOI] [PubMed] [Google Scholar]
- 35.Grant WB, Peiris AN. Possible Role of Serum 25-Hydroxyvitamin D in Black–White Health Disparities in the United States. J Am Med Dir Assoc. 2010;11(9):617–628. doi: 10.1016/j.jamda.2010.03.013 [DOI] [PubMed] [Google Scholar]
- 36.Henderson CM, Lutsey PL, Misialek JR, et al. Measurement by a Novel LC-MS/MS Methodology Reveals Similar Serum Concentrations of Vitamin D–Binding Protein in Blacks and Whites. Clin Chem. 2016;62(1):179–187. doi: 10.1373/clinchem.2015.244541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shieh A, Aloia JF. Assessing Vitamin D Status in African Americans and the Influence of Vitamin D on Skeletal Health Parameters. Endocrinol Metab Clin North Am. 2017;46(1):135–152. doi: 10.1016/j.ecl.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 38.Manolagas SC. From Estrogen-Centric to Aging and Oxidative Stress: A Revised Perspective of the Pathogenesis of Osteoporosis. Endocr Rev. 2010;31(3):266–300. doi: 10.1210/er.2009-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Appiah D, Schreiner PJ, Demerath EW, Loehr LR, Chang PP, Folsom AR. Association of Age at Menopause With Incident Heart Failure: A Prospective Cohort Study and Meta‐Analysis. J Am Heart Assoc. 2016;5(8). doi: 10.1161/JAHA.116.003769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Njoroge JN, Parikh NI. Understanding Health Disparities in Cardiovascular Diseases in Pregnancy Among Black Women: Prevalence, Preventive Care, and Peripartum Support Networks. Curr Cardiovasc Risk Rep. 2020;14(7):8. doi: 10.1007/s12170-020-00641-9 [DOI] [Google Scholar]
- 41.Nayak A, Hicks AJ, Morris AA. Understanding the Complexity of Heart Failure Risk and Treatment in Black Patients. Circ Heart Fail. 2020;13(8). doi: 10.1161/CIRCHEARTFAILURE.120.007264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandey A, Shah SJ, Butler J, et al. Exercise Intolerance in Older Adults With Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2021;78(11):1166–1187. doi: 10.1016/j.jacc.2021.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright NC, Looker AC, Saag KG, et al. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine: RECENT US PREVALENCE OF OSTEOPOROSIS AND LOW BONE MASS. J Bone Miner Res. 2014;29(11):2520–2526. doi: 10.1002/jbmr.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18). doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 45.Shah SJ, Borlaug BA, Kitzman DW, et al. Research Priorities for Heart Failure With Preserved Ejection Fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation. 2020;141(12):1001–1026. doi: 10.1161/CIRCULATIONAHA.119.041886 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


