Abstract
Decision-making has been shown to suffer when circadian preference is misaligned with time-of-assessment; however, little is known about how misalignment between sleep timing and the central circadian clock impacts decision-making. This study captured naturally-occurring variation in circadian alignment (i.e., alignment of sleep-wake timing with the central circadian clock) to examine if greater misalignment predicts worse decision-making. Over the course of two weeks, 32 late adolescent drinkers (18–22 years of age; 61% female; 69% White) continuously wore actigraphs and completed two overnight in-lab visits (Thursday and Sunday) in which both dim light melatonin onset (DLMO) and behavioral decision-making (risk taking, framing, and strategic reasoning tasks) were assessed. Sleep-wake timing was assessed by actigraphic midsleep from the two nights prior to each in-lab visit. Alignment was operationalized as the phase angle (interval) between average DLMO and average midsleep. Multilevel modeling was used to predict performance on decision-making tasks from circadian alignment during each in-lab visit; nonlinear associations were also examined. Shorter DLMO-midsleep phase angle predicted greater risk-taking under conditions of potential loss (B=−.11, p=.06), but less risk-taking under conditions of potential reward (B=.14, p=.03) in a curvilinear fashion. Misalignment did not predict outcomes in the framing and strategic reasoning tasks. Findings suggest that shorter alignment in timing of sleep with the central circadian clock (e.g., phase-delayed misalignment) may impact risky decision-making, further extending accumulating evidence that sleep/circadian factors are tied to risk-taking. Future studies will need to replicate findings and experimentally probe whether manipulating alignment influences decision-making.
Keywords: Circadian rhythms, Misalignment, Decision-making, Risk-taking, Phase angle
Introduction
Optimal human health and functioning requires living in tune with the circadian timing of biological systems and processes, such as sleep. Unfortunately, people frequently do not sleep in tune with their circadian timing due to social, societal, and environmental demands that can dictate when someone has the opportunity to sleep (e.g., work or school hours). Misalignment between the timing sleep-wake schedules relative to the central circadian clock has been linked to impairments in cognition and mood (Chellappa, 2020; Chellappa, Morris, & Scheer, 2018; Lewy et al., 2007), as well as risky behaviors, such as alcohol use (Hasler, Bruce, Scharf, Ngari, & Clark, 2019; Richter et al., 2021). However, the understanding of the influence of misalignment between sleep timing and the circadian system on risky behaviors is still developing and it is unclear if misalignment may alter psychological processes beyond core cognitive functions and mood. Initial evidence indicates misalignment alters neural responses related to monetary reward and impulse control (Hasler et al., 2021), suggesting that there may be downstream alterations in decision-making. Altered decision-making may be one reason why misalignment is associated with risky behaviors. As a first step in examining this possibility, this study seeks to examine if circadian misalignment (operationalized here as misalignment between sleep timing and endogenous circadian timing) is associated with altered decision-making.
To date, literature supports that decision-making is influenced by whether the time of assessment is aligned or misaligned with individuals’ circadian preference (i.e., self-reported tendency towards morningness or eveningness)—so-called synchrony effects (May, 1999; May & Hasher, 1998). For instance, when morning types engaged in decision-making in the evening (vs. morning) and when evening types engaged in decision-making in the morning (vs. evening) individuals made more risky decisions when facing potential losses, engaged in less strategic reasoning in a novel task, took more monetary risks in selecting asset bundles, and engaged in riskier trading strategies in a simulated global asset trading environment; (Castillo, Dickinson, & Petrie, 2017; Dickinson, Chaudhuri, & Greenaway-McGrevy, 2020; Dickinson & McElroy, 2012; McElroy & Dickinson, 2010). Though such synchrony effects are not always detected (Correa, Alguacil, Ciria, Jiménez, & Ruz, 2020). Alterations in decision-making due to synchrony effects are theoretically due to decrements in cognitive capacities and increases in sleepiness that occur during troughs in diurnal fluctuations (Castillo et al., 2017; Dickinson & McElroy, 2012).
This work provides important evidence that decision-making is altered and potentially impaired during times of day that are outside of one’s optimal times of performance. However, prior studies relied on self-reports of circadian preference and sleep, with no studies to our knowledge testing whether decision-making varies according to circadian alignment based on behavioral or physiological measures, (e.g., actigraphy-based sleep timing and circadian phase based on salivary melatonin). Using behavioral or physiological measures is important because self-reports of circadian preference are imperfect indicators of biological circadian timing subjected to the limitations and biases of self-reports (Kantermann, Sung, & Burgess, 2015). Moreover, experimental studies have connected sleep loss with alterations in decision-making (Massar, 2021; McKenna, Dickinson, Orff, & Drummond, 2007), which is relevant with regards to chronotype and circadian alignment. Evening types are not only more prone to circadian misalignment in daily life (Wittmann, Dinich, Merrow, & Roenneberg, 2006), which can lead to ongoing sleep restriction, but the experimental designs of the synchrony effect studies also require the evening-types to wake up earlier than normal (e.g, 8 am in (Correa et al., 2020) and thus potentially get less sleep than morning types in order to complete an assigned experimental task in the morning hours. Thus, it is necessary to account for the confounding influence of sleep duration (Adan et al., 2012) to more clearly characterize the impact of the circadian misalignment, a problem we address in the current study.
Current Study
The present project attempts to expand our understanding the role of circadian misalignment in decision-making, extending prior decision-making research that focused on the effects of sleep loss and/or synchrony effects based on self-reported circadian preference. The project employed data from 36 late adolescent participants who completed two overnight in-lab assessments of circadian timing (i.e., Dim Light Melatonin Onset; DLMO), were administered risky decision-making tasks, and wore wrist actigraphs to assess sleep over 14 days. The DLMO and actigraphy data allow, for the first time in the decision-making literature, estimation of objective circadian alignment via the DLMO-midsleep phase angle (Emens, Lewy, Kinzie, Arntz, & Rough, 2009; Hasler et al., 2019; Lewy et al., 2007; Hasler et al, 2022). The use of three distinct decision-making tasks –the lottery choice task, the unusual disease task, and the guessing game task (Nagel, 1995; Smith, Dickhaut, McCabe, & Pardo, 2002; Tversky & Kahneman, 1981)—allows for consideration of a range of decision-making processes that relate to high-level decision making and risky choice. Overall, we hypothesized that greater circadian misalignment would predict more risky decision making.
Methods
Participants
Data for these analyses come from 36 healthy late adolescents (18–22 years old, Mage = 21.26 years; 22 self-reported female gender; 69% White) reporting regular alcohol use who were recruited for a study examining the relations among sleep, circadian rhythms, reward-related functioning, and alcohol use. Participants were excluded if they had a current alcohol use disorder, used substances other than alcohol, cannabis, and nicotine, had significant current medical or psychiatric conditions (e.g., cardiovascular disorder, head injury with loss of consciousness, major depression, bipolar disorder, any current sleep disorders other than insomnia), or used medication that might interfere with sleep and/or reward function. Note that individuals with insomnia were not excluded given that insomnia was expected to be prevalent among the population of greatest interest – those with regular alcohol use and/or circadian misalignment. However, no participants actually met diagnostic criteria for current Insomnia Disorder. Additionally, participants with extreme habitual sleep times (bedtimes later than 02:00 or rise times later than 10:00) or habitual sleep durations (longer than nine hours or shorter than six hours) were also excluded due to the practical challenges of studying participants with extreme sleep times and concern over having extreme outliers in a relatively small sample. This study was approved by the university Institutional Review Board and written informed consent was obtained for all participants. Note that prior work from this dataset has been previously published (Hasler et al., 2019; Hisler, Rothenberger, Clark, & Hasler, 2021), but neither of these studies examined the risk-taking decision tasks presented in this study.
Procedures
Study participation included a total of 14 consecutive days and participants kept their natural sleep schedule during this period. Each day participants completed smartphone-based ecological momentary assessments that focused on sleep, affect, and substance use. Participants continuously wore a wrist actigraph to monitor sleep-wake behavior. During this 14-day period, participants completed two overnight visits to the sleep laboratory during which assessments of DLMO and risk-taking were completed. Each participant completed an overnight visit on a Thursday and subsequent Sunday or vice versa; the order of these visits was counter-balanced to address potential habituation effects during neuroimaging during in-lab visits (because neuroimaging data is not used in this study these procedures are not discussed further). All risk-taking tasks were completing in the evening hours of the laboratory visit.
The temporal timeline of data used in this study is visually depicted in Figure 1.
Figure 1.
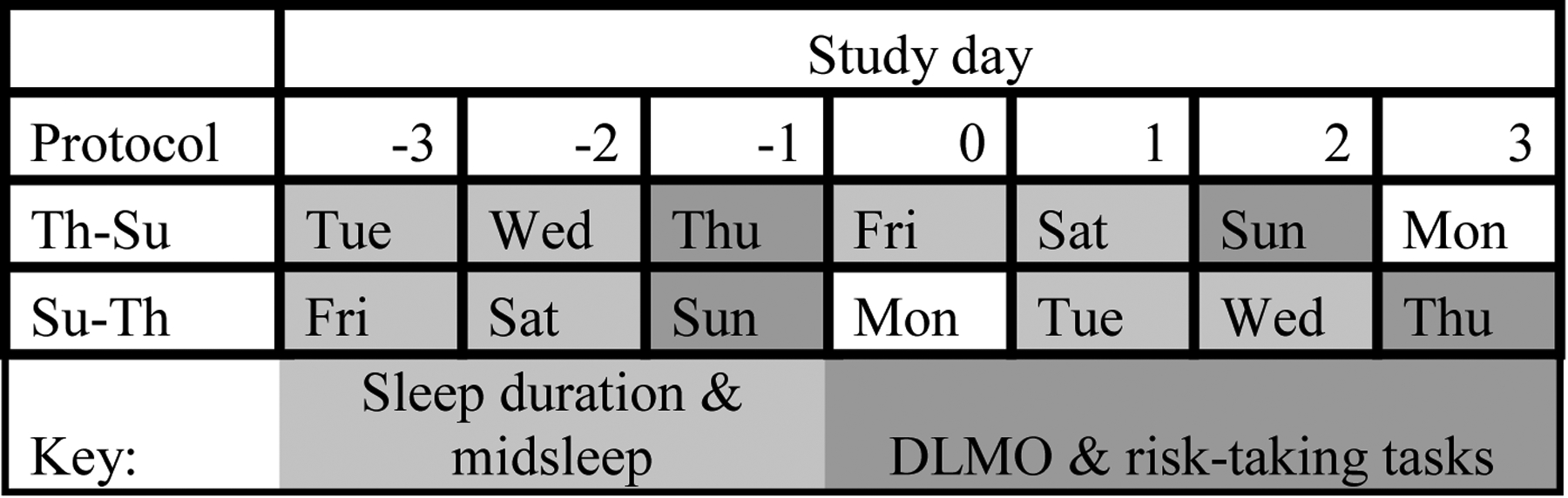
Overview of key study protocol and timepoints used in the current study.
Measures
Actigraphic sleep timing and duration.
Sleep timing (midsleep) and duration (total sleep time) were calculated from the actigraphic sleep data from the two nights prior to DLMO assessment. Participants wore an Actiwatch Spectrum Classic (Philips Respironics, Bend, OR) during the entire 14-day study period. On average, actigraphy data was available for 11.70 out of the 14 days (SD=3.26; 82.7% completion rate). Actigraphs recorded activity on medium sensitivity at 1-minute epochs. Participants received instructions to press the event recording marker on the watch to indicate when they a.) started to try to fall asleep and b.) when they woke up for the day. These event markers were used to set the start and end of the rest interval. If event markers were not available, participant self-reported bed and wake times from morning sleep diaries were used. If sleep diary data were not available, then a consensus meeting among study personnel was used to determine the rest interval based on activity and light data. After making these adjustments, midsleep (midpoint between sleep onset and sleep offset) from each of the two nights prior to the night of DLMO assessment were averaged to estimate a person’s midpoint of sleep. Total sleep time (TST) was calculated as the interval between sleep onset and sleep offset, minus wake after sleep onset, from the same two nights prior to DLMO assessment (i.e., same nights as midpoint of sleep). Sleep timing and sleep duration from the two days prior to DLMO were selected in order to best characterize sleep timing and recent sleep duration during assessment of DLMO and risk-related decision making. Because participants completed DLMO and risk-taking on Thursday and Sunday, only two days were selected so that there was not overlap between assessment of sleep timing and sleep duration from two days prior to Sunday with two days prior to Thursday. Actigraphy data was not available for two participants; therefore, these participants were excluded from analyses.
Dim Light Melatonin Onset.
Melatonin concentrations in serial saliva samples collected in dim light conditions were used to determine DLMO. During dim light conditions (<15 lux at any angle of gaze, confirmed for each visit via a light meter) in overnight laboratory visits, saliva samples were collected in Salivettes (Sarstedt, Newton, NC) every 30 minutes starting six hours before, and ending one hour after each participant’s habitual bedtime. Habitual bedtime was determined by calculating the weighted average of participants self-reported bedtime for weekdays and weekends (i.e., (weekday bedtime*5 + weekend bedtime*2)/7). Dim light conditions started one hour before the first saliva collection. During dim light procedures, participants were instructed to remain seated (other than trips to the bathroom) to control for postural effects on saliva concentrations, to refrain from eating or drinking within 10 minutes of sample collection, were instructed not to consume bananas or chocolate starting the morning of, and throughout the in-lab visits, and were instructed to limit caffeine use to the morning of the lab visit (Burgess, 2010). Otherwise, participants were asked to rinse their mouths with water 10 minutes prior to each sample collection if they had recently eaten or drank. After collection, saliva samples were frozen at −80 C and shipped overnight on dry ice for radioimmunoassaying by Solid Phase, Inc. (Portland, ME) using commercially available kits (Buhlmann, Amherst, NH). DLMO was calculated as the clock time when levels exceeded the mean of three consecutive baseline samples plus twice the standard deviation of those samples (Molina & Burgess, 2011; Voultsios, Kennaway, & Dawson, 1997). No discernable time of DLMO could be determined for four participants, and, therefore, these participants were excluded from analyses.
Circadian alignment.
Circadian alignment was operationalized as the phase angle (interval) between DLMO and midsleep, with deviations from the median DLMO-midsleep phase angle interpreted as misalignment. This operationalization has been utilized in prior research and greater misalignment (most typically shorter DLMO-midsleep phase angles, although sometimes longer DLMO-midsleep phase angle) has been linked to worse functional outcomes such as greater depressive symptomology and alcohol use, as well as altered reward-related brain function (Emens, Lewy, Kinzie, Arntz, & Rough, 2009; Hasler et al., 2019; Lewy et al., 2007; Hasler et al, 2022).
Decision-making tasks.
All decision-making tasks were completed between 18:00 and 19:00 during the overnight laboratory visits. Tasks were administered via paper and pencil as opposed to via a computer in order to ensure dim light conditions.
Lottery choice task.
The lottery choice task examines risk preference during conditions of potential gain and potential loss with real monetary incentives (Smith et al., 2002). Methodological details of the task are presented in Supplemental File. As in prior research, risk-preference scores were calculated by taking the proportion of safer-choice decisions minus riskier-choice decisions—safer choice here refers to selection of the monetary gamble with lower payoff variance from the pair of options in that trial, whereas a selection of the gamble with higher payoff variance is a riskier choice (McKenna et al., 2007; Smith et al., 2002). This risk-preference score ranges from −1 to 1, wherein more negative scores indicate greater risk-seeking, a score of zero indicates risk-neutrality, and more positive scores indicate greater risk-avoidance. Risk-preference score was calculated separately for the gain and loss conditions yielding two indices of risk-preference: 1) risk-preference under gain conditions, 2) risk-preference under loss conditions.
Unusual disease task.1
Risk preference when risk is framed in terms of potential gains or potential losses was assessed by the unusual disease task (Tversky & Kahneman, 1981). Methodological details of the task are presented in the Supplemental File. Participants completed two versions of this task across the two study visits (i.e., one on the first study visit, T1, and the second on the second study visit, T2) and the order of the tasks was counterbalanced across the two study visits. One version framed the disease response programs in terms of losses (see programs above) and the other version framed the disease response programs in terms of gains (i.e., Program A will save 200 people, Program B has a 1/3 probability that 600 people will be save and a 2/3 probability that no one will be saved). Risk preference ranges from 1 (Definitely would recommend Program A) to 7 (Definitely would recommend Program B). A response of four (the midpoint of the scale) indicates neutrality towards both programs, whereas responses greater than four indicate greater preference for the risky program (i.e., the program involving uncertainty) while responses lower than four indicate a greater preference for the risk-averse program. Thus, this task allows for measurement of risk preference when risks are framed in terms of gains versus losses, with the typical result being choices more consistent with risk avoidance in the gains domain compared to more risk seeking behavior in the loss domain.
Guessing game task.
The guessing game task assesses participants’ strategic reasoning (Nagel, 1995). Methodological details of the task are presented in the Supplemental File. Participants completed two rounds, one easy round and one difficult round, of this incentivized task during each in-lab visit. These two rounds of the task allow for examination of strategic reasoning under more versus less cognitive complexity and performance on these two rounds was examined separately (Dickinson & McElroy, 2012). Strategic reasoning is indexed by the number the participant submitted wherein lower guesses indicate higher strategic reasoning in the more difficult round and higher guesses indicate higher strategic reasoning in the cognitively easier round.
Covariates.
Age, self-identified gender (0 = male, 1 = female), number of drinking days during two days prior to overnight lab visits, and average actigraphy-measured sleep duration during two days prior to overnight lab visits were used as covariates in regression analyses to control for age, gender, alcohol use, and sleep related confounds on risk-taking. Lab visit number (T1 vs. T2), task version (version 1 vs. version 2), and the interaction between lab visit number and task version were used as covariates in all analyses to account for task practice and order effects.
Analytic Strategy
Outcomes examined in this study were 1) risk preference under gain conditions of the lottery choice task, 2) risk preferences under loss conditions of the lottery choice task, 3) risk preference during gain frame conditions of the unusual disease task, 4) risk preference during loss frame conditions of the unusual disease task, 5) strategic reasoning during the cognitively demanding round of the guessing game task, and 6) strategic reasoning during the cognitively less demanding round of the guessing game task. Analyses focused on how circadian misalignment during decision-making tasks predicted these decision-making outcomes. Because participants completed sleep, circadian phase, and decision-making assessments twice during the study, multilevel models were used to account for the non-independence of observations (i.e., repeated observations nested within each participant). Effects are reported in unstandardized effect size metric.
The effect of circadian alignment was investigated both in terms of linear and nonlinear effects. Specifically, circadian alignment was first modelled as a linear predictor of decision-making. However, prior findings suggest nonlinear effects are possible because small degrees of circadian misalignment can have smaller effects than larger degrees of circadian misalignment (Hasler et al., 2019; Lewy et al., 2007). Thus, nonlinear effects were investigated in a second set of models by first centering circadian alignment at the median (6.8 hours, which is within the 6–7 hour DLMO-midsleep phase angle suggested to be optimal for circadian functioning (Lewy, Lefler, Emens, & Bauer, 2006) and then squaring deviations from this point. Importantly, squaring these deviations will shrink the effects of small degrees of circadian misalignment while amplifying the effects of large degrees of circadian misalignment. Moreover, we sought to examine whether “phase-advanced” and “phase-delayed” types of circadian misalignment, characterized by longer and shorter DLMO-midsleep phase angles, respectively, similarly predicted decision-making. To this end, the curvilinear effects of phase-advanced and phase-delayed types of misalignment were assessed by separately modeling positive and negative deviations from 6.8 and squaring these deviations (as in (Hasler et al., 2019). In the interest of reporting full analytic choices, results are reported in a sequential fashion for each outcome. Specifically, the linear circadian misalignment effects are modelled, then the curvilinear effects separated for phase-advanced and phase-delayed types of misalignment are added to this model, and finally the robustness of results to study covariates is examine in a third model. Note that the appropriateness of these curvilinear analyses for the data were supported in diagnostic tests examining the fit of the regression models to the data as well as when utilizing data-driven approaches (i.e., spline-modeling) to more freely estimate the association between circadian alignment and risk outcomes (see Supplemental File). Finally, due to the novelty of the research question and dataset, we do not make corrections for multiple comparisons as results from this study are seen as preliminary evidence for which future research should replicate (Cao & Zhang, 2014).
Results
Table 1 presents means and standard deviations of key variables and raw bivariate correlations among study variables are presented in the Supplemental File. All assessments repeated over the two in-lab visits demonstrated correlations between T1 and T2 (r’s between .34 & .74, all p’s < .05), supporting the need to use multilevel statistical techniques to account for dependency between these repeated assessments. Moreover, these correlations also suggest that while there was some dependency and stability in assessments of circadian alignment and decision-making, there was also variability in circadian alignment between in-lab visits that may explain variability in decision-making.
Table 1.
Descriptive statistics key study variables (N = 31 to 36).
| M | Range | SD | |
|---|---|---|---|
| 1. Age | 21.26 | 18.66–22.88 | 1.15 |
| 2. Gender | 61% females | -- | -- |
| 3. Ethnicity | 69% White, 11% African-American, 11% Asian-American, 5% Hispanic, 8% Biracial | ||
| 4a. Average actigraphic midpoint of sleep (hh:mm) | 5:29 | 3:37–6:44 | 0:54 |
| 4b. Tuesday/Wednesday Actigraphic midpoint of sleep (hh:mm) | 4:55 | 2:49–7:44 | 1:13 |
| 4c. Friday/Saturday Actigraphic midpoint of sleep (hh:mm) | 5:28 | 3:03–8:45 | 1:00 |
| 5a. Average Dim Light Melatonin Onset (hh:mm) | 22:06 | 19:09–1:52 | 1:23 |
| 5b. Thursday Dim Light Melatonin Onset (hh:mm) | 22:02 | 18:36–1:44 | 1:29 |
| 5c. Sunday Dim Light Melatonin Onset (hh:mm) | 22:11 | 18:53–1:59 | 1:26 |
| 6a. Average circadian alignment (DLMO-midsleep phase angle) | 6.93 h | 4.88–9.78 h | 1.28 h |
| 6b. Thursday circadian alignment (DLMO-midsleep phase angle) | 6.81 h | 4.37–9.44 h | 1.45 h |
| 6c. Sunday circadian alignment (DLMO-midsleep phase angle) | 7.26 h | 4.45–10.76 h | 1.60 h |
| 7. Average drinking days from two days before in-lab visit | 0.33 | 0.00–2.00 | 0.29 |
| 8. Average actigraphic total sleep time | 6.13 h | 3.02–8.22 h | 1.16 h |
Note. Mean, range, and SD of actigraphic midpoint of sleep and Dim Light Melatonin Onset are reported in 24-hour time.
Average decision-making outcomes
In line with prospect theory and prior findings (McKenna et al., 2007; Tversky & Kahneman, 1981), participants were overall more risk seeking during loss trials (M= −0.67) and more risk averse during gain trials (M=0.50) of the lottery choice task (p<.001). A similar pattern emerged during the unusual disease task in which participants were more risk averse during the gain frame (M=3.04) and more risk seeking during the loss frame (M=3.88, p=.002). In terms of the guessing game task, participant guesses were 27.85 on average for the difficult task version and 167.74 for the easier version indicating that participants guesses were ~20–30 away from the optimal answer in both conditions.
Circadian alignment and decision-making
Risk preferences during the lottery choice task were predicted by circadian alignment (see Table 2). Specifically, there was no detected linear effect of circadian alignment nor curvilinear effect of phase-advanced circadian alignment on risk preference during loss trials (p’s>.13); however, there was a curvilinear effect of phase-delayed circadian alignment (B=−.11, p=.04) and this effect showed trend-level significance when accounting for the influence of study covariates (B=−.11, p=.06). This association is depicted in Figure 2. As illustrated, more severe phase-delayed circadian alignment (i.e., DLMO-midsleep phase angles increasingly smaller than the median of 6.8 hours) predicted greater risk-taking (i.e. more negative risk-preference score) during loss trials whereas phase-advanced circadian alignment (i.e., DLMO-midsleep phase angles longer than the median of 6.8 hours) did not predict risk preference during loss trials.
Table 2.
Multilevel regression results predicting risk preference during the lottery choice task
| Risk preference during loss trial | Risk preference during gain trial | |||||
|---|---|---|---|---|---|---|
| Model I | Model II | Model III | Model I | Model II | Model III | |
| Variables | B | B | B | B | B | B |
| Intercept | −0.62 * | −0.54 * | −.76 | 0.32 * | 0.24 * | 0.92 |
| Circadian alignment (DLMO-midsleep phase angle) | −0.04 | −0.17 † | −0.14 | 0.03 | 0.15 | 0.19 † |
| Phase-advanced circadian alignment squared | 0.04 | 0.02 | −0.03 | −0.03 | ||
| Phase-delayed circadian alignment squared | −0.11 * | −0.11 † | 0.11 † | 0.14 * | ||
| Gender | 0.10 | 0.00 | ||||
| Age | 0.02 | −0.04 | ||||
| Average drinking days from two days before in-lab visit | −.02 | 0.04 | ||||
| Average total sleep time from two days before in-lab visit | 0.00 | 0.00 | ||||
Note.
p< .10,
p<.05.
Risk-preference score ranges from −1 to 1, wherein increasing scores in the negative values indicate greater risk-seeking, a score of zero indicates risk-neutrality, and increasing scores in the positive values indicate greater risk-avoidance. Lab visit number (time 1 vs. time 2), task version (version 1 vs. version 2), and the interaction between lab visit number and task version were used as covariates in all analyses. Phase-advanced circadian alignment squared refers to the squared DLMO-midsleep alignment deviation variable for deviations > 6.8 h (and 0 otherwise) and phase-delayed circadian alignment squared refers to the squared DLMO-midsleep alignment deviation variable for deviations < 6.8 h (and 0 otherwise).
Figure 2.
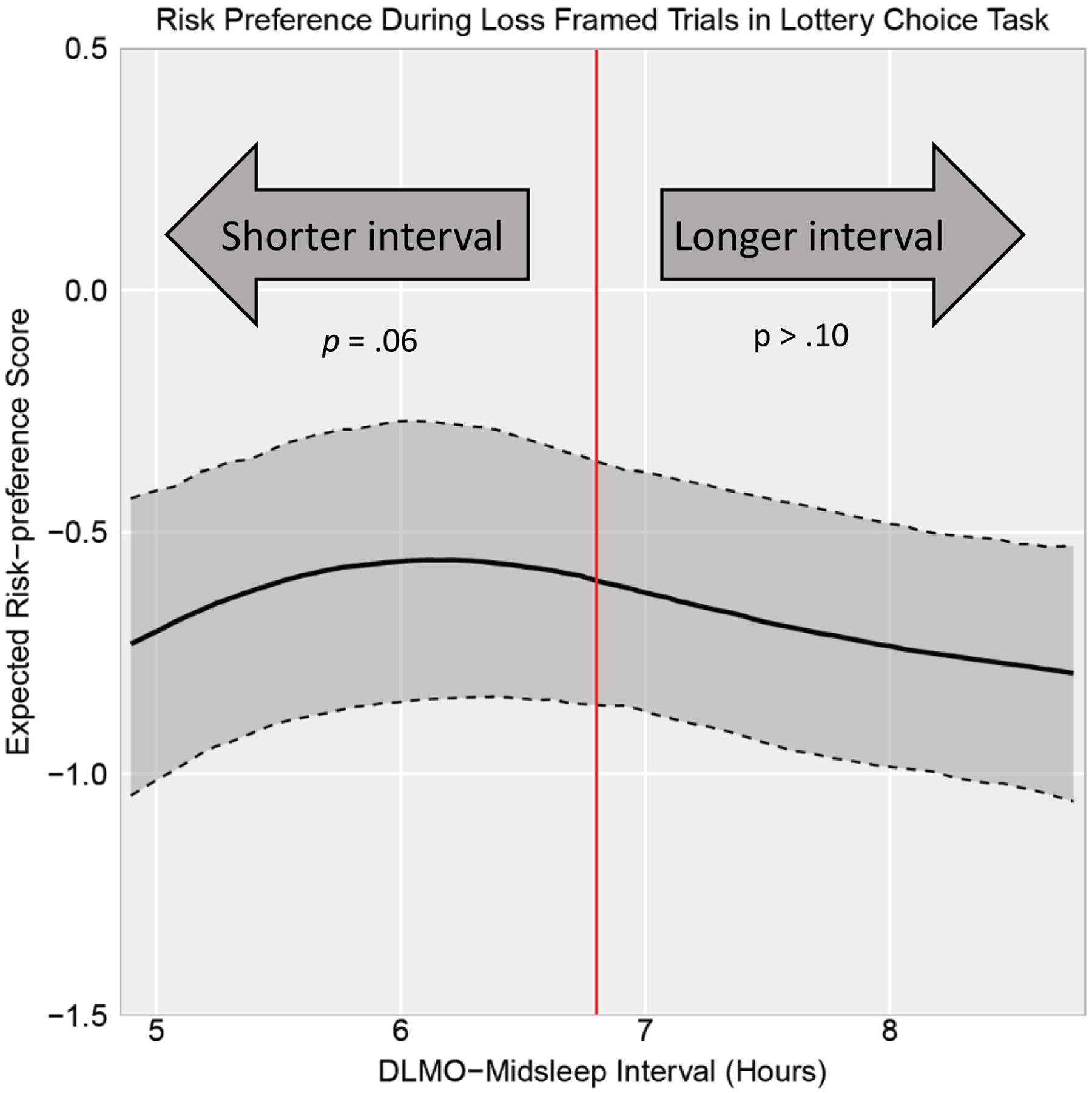
DLMO-midsleep phase angle predicting risk-preference score during loss trials in the lottery choice task. Solid black line represents estimated risk-preference score holding other covariates constant at mean values. Dark gray shading bounded by dashed lines represents the 95% pointwise confidence intervals. Red vertical line represents the median DLMO-midsleep phase angle of 6.8. DLMO-midsleep phase angles further below the median of 6.8 hours (i.e., phase-delayed circadian alignment) predicted greater risk-seeking (more negative risk-preference score) during loss framed trials. DLMO-midsleep phase angles longer than the median of 6.8 (i.e., phase-advanced circadian alignment) did not predict risk preference during loss framed trials.
There was also no detected significant linear effect of circadian alignment nor curvilinear effect of phase-advanced circadian alignment on risk preference during gain trials (p’s>.05; see Table 2); however, there was a curvilinear effect of phase-delayed circadian alignment (B =.11, p=.08) and this effect was statistically significant after accounting for study covariates (B=.14, p=.03). This association is depicted in Figure 3. As shown, more severe phase-delayed circadian alignment (i.e., DLMO-midsleep phase angles shorter than the median of 6.8 hours) predicted greater risk-avoidance (i.e. more positive risk-preference score) during gain trials whereas phase-advanced circadian alignment (i.e., DLMO-midsleep phase angles longer than the median of 6.8 hours) did not predict risk preference during these trials.
Figure 3.
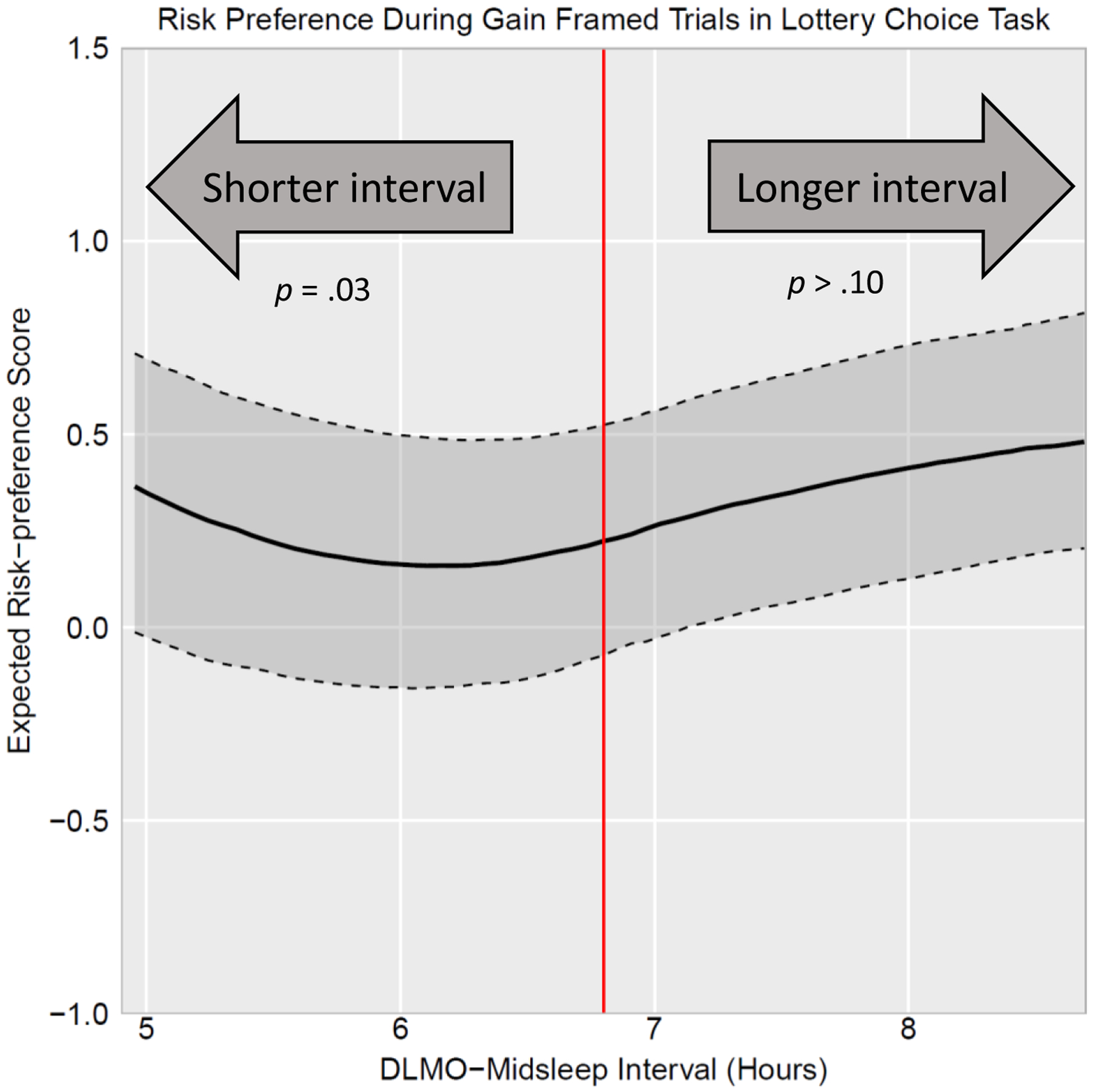
DLMO-midsleep phase angle predicting risk-preference score during gain trials in the lottery choice task. Solid black line represents estimated risk-preference score holding other covariates constant at mean values. Dark gray shading bounded by dashed lines represents the 95% pointwise confidence intervals. Red vertical line represents the median DLMO-midsleep phase angle of 6.8. DLMO-midsleep phase angles further below the median of 6.8 hours (i.e., phase-delayed circadian alignment) predicted greater risk-avoidance (more positive risk-preference score) during gain framed trials. DLMO-midsleep phase angles longer than the median of 6.8 (i.e., phase-advanced circadian alignment) did not predict risk preference during gain framed trials.
Interestingly, risk preference was the least extreme (i.e., expected risk-preference score closest to 0 indicating risk-neutrality) at approximately a 6-hour DLMO-midsleep phase angle in both loss and gain trials. This can be seen in Figures 2 and 3 where the expected risk preference score reaches a maximum for loss trials (Figure 2) and minimum for gain trials (Figure 3) at approximately a 6-hour DLMO-midsleep phase angle.
Circadian alignment variables did not predict risk preferences during the unusual disease task nor during the easy condition of the guessing game task (all p’s > .05; see Tables 3 & 4). The curvilinear effect of phase-delayed circadian alignment did unexpectedly predict better performance on the hard condition of the guessing game task (B=−6.09, p=.02); however, this effect was not robust to study covariates (B=−3.97, p=.11).
Table 3.
Multilevel regression results predicting risk preference during the unusual disease task
| Risk level | |||
|---|---|---|---|
| Model I | Model II | Model III | |
| Variables | B | B | B |
| Intercept | 4.84 * | 4.96 * | 1.99 |
| Circadian alignment (DLMO-midsleep phase angle) | −0.01 | 0.53 | 0.43 |
| Circadian alignment (DLMO-midsleep phase angle)*Frame version | −0.21 | −0.57 | −0.03 |
| Phase-advanced circadian alignment squared | −0.27 | −0.46 | |
| Phase-advanced circadian alignment squared*Frame version | 0.09 | 0.31 | |
| Phase-delayed circadian alignment squared | 0.18 | 0.34 | |
| Phase-delayed circadian alignment squared *Frame version | −0.21 | −0.25 | |
| Gender | −0.20 | ||
| Age | 0.10 | ||
| Average drinking days from two days before in-lab visit | −.01 | ||
| Average total sleep time from two days before in-lab visit | 0.00 | ||
Note.
p< .10,
p<.05.
Risk preference ranges from 1 (Definitely would recommend Program A) to 7 (Definitely would recommend Program B). A response of four (the midpoint of the scale) indicates neutrality towards both programs, whereas responses greater than four indicate greater preference for the risky program (i.e., the program involving uncertainty) while responses lower than four indicate a greater preference for the risk-averse program. Lab visit number (time 1 vs. time 2), task version (version 1 vs. version 2), and the interaction between lab visit number and task version were used as covariates in all analyses. Phase-advanced circadian alignment squared refers to the squared DLMO-midsleep alignment deviation variable for deviations > 6.8 h (and 0 otherwise) and phase-delayed circadian alignment squared refers to the squared DLMO-midsleep alignment deviation variable for deviations < 6.8 h (and 0 otherwise).
Table 4.
Multilevel regression results predicting strategic reasoning on guessing game task.
| Guess during hard condition | Guess during easy condition | |||||
|---|---|---|---|---|---|---|
| Model I | Model II | Model III | Model I | Model II | Model III | |
| Variables | B | B | B | B | B | B |
| Intercept | 30.77 * | 34.51 * | 38.61 | 152.28 * | 149.69 * | 208.48 * |
| Circadian alignment (DLMO-midsleep phase angle) | −0.70 | −8.19† | −4.11 | 4.51† | 8.53 | 6.55 |
| Phase-advanced circadian alignment squared | 2.25 | 1.09 | −1.01 | −3.32 | ||
| Phase-delayed circadian alignment squared | −6.09 * | −3.97 | 3.57 | 2.21 | ||
| Gender | 6.87 | −5.52 | ||||
| Age | 0.26 | −1.78 | ||||
| Average drinking days from two days before in-lab visit | 3.77 | 3.69 | ||||
| Average total sleep time from two days before in-lab visit | −0.05 | −0.04 | ||||
Note.
p< .10,
p<.05.
Strategic reasoning is indexed by the number the participant submitted wherein lower guesses indicate higher strategic reasoning in the hard condition and higher guesses indicate higher strategic reasoning in the easy condition. Lab visit number (time 1 vs. time 2), task version (version 1 vs. version 2), and the interaction between lab visit number and task version were used as covariates in all analyses. Phase-advanced circadian alignment squared refers to the squared DLMO-midsleep alignment deviation variable for deviations > 6.8 h (and 0 otherwise) and phase-delayed circadian alignment squared refers to the squared DLMO-midsleep alignment deviation variable for deviations < 6.8 h (and 0 otherwise).
Discussion
Findings from this study provide preliminary evidence that naturally-occurring patterns of phase-delayed circadian misalignment (i.e., when midpoint of sleep is soon after DLMO) predict risk-taking during conditions of potential monetary loss and potential monetary gain. Specifically, more severe phase-delayed circadian misalignment predicted greater risk-taking under conditions of monetary loss but predicted less risk-taking under conditions of monetary gain. Importantly, these effects tended to remain after separating out the confounding influence of sleep duration during the two nights prior to the task. It is important to note that the former effect (i.e., of greater risk-taking under conditions of monetary loss) was no longer under the conventional statistical p-value threshold of .05 (i.e., was .06). Given recommendations to interpret p-values in light of other contextual information (e.g., sample size, patterns across statistical tests, study precision) and to not rely exclusively on binary yes/no decisions around the threshold of .05 when reporting and interpreting results (Wasserstein & Lazar, 2016), this effect is still interpreted and discussed, though caution and replication is warranted. Overall, these findings suggest that people attempting to sleep at an earlier circadian time may have altered risk-taking, such as teenagers and young adults who typically have delayed circadian timing but have to sleep at earlier times to accommodate early school or work demands.
Prospect theory predicts that people tend to be more risk-seeking under conditions of potential loss and more risk-averse under conditions of potential gain (Tversky & Kahneman, 1981); thus, the patterns observed in this study suggest that phase-delayed circadian misalignment is associated with an amplification of these general risk-taking tendencies. The amplified heuristic responses to potential gains and losses suggests that self-regulatory processes used to override these responses may be undermined by circadian misalignment. Reduced self-regulation may occur because self-regulation relies upon cognitive functions that can be impaired by circadian misalignment (Chellappa, 2020; Chellappa et al., 2018; Hofmann, Schmeichel, & Baddeley, 2012). These findings are an important extension of past literature by providing novel preliminary evidence that a physiological measure of circadian alignment is connected with altered decision-making.
One possible explanation for the association between circadian alignment and decision-making may be that conditions of misalignment impair cognitive processes that are necessary to override these more automatic responses (Dickinson & McElroy, 2012; Tversky & Kahneman, 1981). In support of this theorizing, circadian misalignment can lead to decreased attention, cognitive throughput, and information processing (Chellappa, 2020; Chellappa et al., 2018). Moreover, a single night of experimentally-imposed misalignment alters neural responses to monetary reward and impulse control in healthy adolescents (Hasler et al., 2021), raising the possibility that chronic misalignment could have even greater effects on reward- and impulsivity-related processes. Indeed, another study from this sample reported that circadian misalignment on Thursday night was associated with lower neural response to monetary reward (Hasler et al., 2022). Altogether, phase-delayed circadian misalignment may strain cognitive processes and lead to more automatic or impulsive responding, which may be why misalignment is associated with risk behaviors such as substance use (Hasler et al., 2019).
Circadian alignment was not associated with risk-related decision-making in the unusual disease nor anticipation in the guessing game tasks. These null findings may be partly surprising given prior work found that when participants completed the unusual disease task and guessing game choice problem at a non-preferred time-of-day (i.e., evening types completing the task in the morning) they were riskier in the loss frame condition of the unusual disease task and performed worse on the hard condition of the guessing game task (Dickinson & McElroy, 2012; McElroy & Dickinson, 2010). However, differences from these prior studies may be because the current study examined a different type of misalignment (i.e., biological misalignment between circadian and behavioral sleep timing) than this prior work (i.e., misalignment between time-of-task and self-reported diurnal preference). The present study focuses on a continuous measure of circadian misalignment without varying time-of-day effects; indeed, the tasks were all administered in the evening when the synchrony effect literature would suggest the late adolescent participants with their tendency towards eveningness would be functioning relatively better. It is plausible that misalignment effects in this population would be enhanced if the tasks were administered in the morning. Additionally, given that this is the first study to examine the influence of biological circadian alignment on risk-related decision-making, it is unknown what effect size to expect. It is possible that the effect of biological circadian alignment on the unusual disease task and guessing game choice problem is smaller than the current study is statistically powered to detect.
Limitations
Findings should be interpreted in light of key limitations. Most notably, circadian misalignment was not experimentally manipulated in this study but observed from naturally-occurring degrees of alignment. The lack of experimental control may allow for confounds, such as sleep duration, to bias the observed association between misalignment and decision-making. While actigraphy-assessed sleep duration was accounted for in analyses, actigraphy is not as precise as EEG assessed sleep duration and this imprecision may bias findings. Additionally, while we accounted for recent sleep duration, this provides an imperfect control for the degree of sleep restriction, which also requires consideration of inter-individual differences in sleep need. Second, findings appeared specific to risk preferences in the lottery choice task and it is unclear whether circadian misalignment may be associated with alterations in other risk- or uncertainty-related decision-making tasks not assessed in this study, such as the Balloon Analog Risk Task or Iowa Gambling Task which assess other features of risk-related behavior, such as choice under uncertainty or reward motivation and “hot” affective risky decision making. Third, the study sample consists of predominately White late adolescents who are regular alcohol users without a current substance use or psychiatric condition. While the specificity of this sample reduced the risk of substance use or psychiatric condition confounds, it is unclear if findings would generalize to other populations, such as younger adolescents or those with psychiatric conditions. Fourth, whether the effects of circadian misalignment arose due to chronic versus transient misalignment cannot be determined. Correlation between circadian misalignment at time 1 vs. time 2 (e.g., r = .65, see Table S1) suggests only moderate stability in misalignment. Longitudinal studies of circadian misalignment and risk-taking would be needed to separate the influence of chronic versus transient misalignment on risk-taking. Additionally, this is the first study to connect circadian misalignment with altered decision-making and therefore findings are novel and preliminary. In light of these limitations, future studies should seek to experimentally manipulate circadian alignment while also including multiple assessments at different circadian phases (e.g., via forced desynchrony protocols), examine if circadian misalignment influences outcomes on other decision-making tasks, examine chronic versus transient effects of circadian misalignment, and examine if the observed association replicates and extends to other populations.
Finally, the sample median in the DLMO-midsleep phase angle was selected for use as the “normative” phase angle, but there is no consensus in the literature on ideal phase angle, though prior work has fruitfully utilized a similar phase angle (Lewy et al., 2006; Emens et al., 2009; Hasler et al., 2019; Hasler et al., 2022). Risk preference during the lottery choice task in this study was least extreme (i.e., closest to risk-neutral) in both loss and gain trials at approximately a 6-hour DLMO-midsleep phase angle, which was deemed normative in prior studies of healthy adults (Lewy et al., 2006; Emens et al., 2009). Future studies with larger samples and a wider array of outcomes will be important to better characterize normative phase angles and to what extent deviations in either direction are detrimental.
Conclusion
Maintaining good health and functioning requires living in tune with the circadian clock, but people frequently do not sleep in concordance with this clock due to social, societal, and environmental demands that can influence when someone can sleep (e.g., shift work in adults, high school schedules for teenagers). Using physiological and behavioral measures of circadian and sleep timing, respectively, the current study adds to the growing literature that circadian misalignment may have consequences for psychological functioning and decision-making. In particular, phase-delayed circadian misalignment (i.e., when midpoint of sleep occurred relatively soon after dim light of melatonin onset) was associated with altered decision-making such that greater phase-delayed misalignment predicted greater risk-taking under conditions of potential loss, but less risk-taking under conditions of potential gain. These findings add to the growing understanding of the consequences of living out of sync with the circadian clock by providing evidence that the phase-delayed circadian misalignment that is common among adolescents constrained by early academic and/or work schedules may alter risk-taking preferences. Given that late adolescence is also a time period in which risk-taking behaviors tend to peak (Romer, Reyna, & Satterthwaite, 2017), these findings may eventually inform prevention and/or intervention efforts.
Supplementary Material
Funding:
This work was supported by the National Institute on Alcohol Abuse and Alcoholism [R21 AA023209]; National Institute on Drug Abuse [R01DA044143, R01DA012414].
Footnotes
Conflicts of interest: Brant Hasler reports having a consulting service agreement with American Textile Company. All other authors declare that they have no conflict of interest.
The original name of the unusual disease task is the Asian disease task. However, given concerns over contributing to rising xenophobia against Asian populations amidst the COVID-19 pandemic, we have elected to remove reference to “Asian” from it.
References
- Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, & Randler C (2012). Circadian typology: a comprehensive review. Chronobiology International, 29(9), 1153–1175. [DOI] [PubMed] [Google Scholar]
- Burgess HJ (2010). Partial sleep deprivation reduces phase advances to light in humans. Journal of Biological Rhythms, 25(6), 460–468. doi: 10.1177/0748730410385544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, & Zhang S (2014). Multiple comparison procedures. JAMA, 312(5), 543–544. [DOI] [PubMed] [Google Scholar]
- Castillo M, Dickinson DL, & Petrie R (2017). Sleepiness, choice consistency, and risk preferences. Theory and Decision, 82(1), 41–73. [Google Scholar]
- Chellappa S (2020). Circadian misalignment: A biological basis for mood vulnerability in shift work. European Journal of Neuroscience, 52(8), 3846–3850. [DOI] [PubMed] [Google Scholar]
- Chellappa S, Morris C, & Scheer F (2018). Daily circadian misalignment impairs human cognitive performance task-dependently. Scientific Reports, 8(1), 3041. doi: 10.1038/s41598-018-20707-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A, Alguacil S, Ciria LF, Jiménez A, & Ruz M (2020). Circadian rhythms and decision-making: a review and new evidence from electroencephalography. Chronobiology International, 37(4), 520–541. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Chaudhuri A, & Greenaway-McGrevy R (2020). Trading while sleepy? Circadian mismatch and mispricing in a global experimental asset market. Experimental Economics, 23(2), 526–553. doi: 10.1007/s10683-019-09623-0 [DOI] [Google Scholar]
- Dickinson D, & McElroy T (2012). Circadian effects on strategic reasoning. Experimental Economics, 15(3), 444–459. [Google Scholar]
- Emens J, Lewy A, Kinzie JM, Arntz D, & Rough J (2009). Circadian misalignment in major depressive disorder. Psychiatry Research, 168(3), 259–261. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Bruce S, Scharf D, Ngari W, & Clark DB (2019). Circadian misalignment and weekend alcohol use in late adolescent drinkers: preliminary evidence. Chronobiology International, 36(6), 796–810. doi: 10.1080/07420528.2019.1586720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Soehner AM, Wallace ML, Logan RW, Ngari W, Forbes EE, … Clark DB (2021). Experimentally imposed circadian misalignment alters the neural response to monetary rewards and response inhibition in healthy adolescents. Psychological Medicine, 1–9. doi: 10.1017/S0033291721000787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Graves JL, Soehner AM, Wallace ML, Clark DB. Preliminary Evidence That Circadian Alignment Predicts Neural Response to Monetary Reward in Late Adolescent Drinkers. Frontiers in neuroscience. 2022: 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisler GC, Rothenberger SD, Clark DB, & Hasler BP (2021). Is there a 24-hour rhythm in alcohol craving and does it vary by sleep/circadian timing? Chronobiology International, 38(1), 109–121. doi: 10.1080/07420528.2020.1838532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W, Schmeichel BJ, & Baddeley AD (2012). Executive functions and self-regulation. Trends in Cognitive Sciences, 16(3), 174–180. [DOI] [PubMed] [Google Scholar]
- Kantermann T, Sung H, & Burgess HJ (2015). Comparing the Morningness-Eveningness Questionnaire and Munich ChronoType Questionnaire to the Dim Light Melatonin Onset. Journal of Biological Rhythms, 30(5), 449–453. doi: 10.1177/0748730415597520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy A, Lefler B, Emens J, & Bauer V (2006). The circadian basis of winter depression. Proceedings of the National Academy of Sciences, 103(19), 7414–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy A, Rough J, Songer J, Mishra N, Yuhas K, & Emens J (2007). The phase shift hypothesis for the circadian component of winter depression. Dialogues in Clinical Neuroscience, 9(3), 291–300. doi: 10.31887/DCNS.2007.9.3/alewy [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massar SAA (2021). Sleep loss and risk-taking: new findings in a field that needs more clarity. Sleep, 44(4). doi: 10.1093/sleep/zsab013 [DOI] [PubMed] [Google Scholar]
- May CP (1999). Synchrony effects in cognition: The costs and a benefit. Psychonomic Bulletin & Review, 6(1), 142–147. [DOI] [PubMed] [Google Scholar]
- May CP, & Hasher L (1998). Synchrony effects in inhibitory control over thought and action. Journal of Experimental Psychology: Human Perception and Performance, 24(2), 363. [DOI] [PubMed] [Google Scholar]
- McElroy T, & Dickinson DL (2010). Thoughtful days and valenced nights: How much will you think about the problem? Judgment and Decision making, 5(7), 516. [Google Scholar]
- McKenna BS, Dickinson DL, Orff HJ, & Drummond SP (2007). The effects of one night of sleep deprivation on known‐risk and ambiguous‐risk decisions. Journal of Sleep Research, 16(3), 245–252. [DOI] [PubMed] [Google Scholar]
- Molina TA, & Burgess HJ (2011). Calculating the dim light melatonin onset: The impact of threshold and sampling rate. Chronobiology International, 28(8), 714–718. doi: 10.3109/07420528.2011.597531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel R (1995). Unraveling in guessing games: An experimental study. The American Economic Review, 85(5), 1313–1326. [Google Scholar]
- Richter K, Peter L, Rodenbeck A, Weess HG, Riedel-Heller SG, & Hillemacher T (2021). Shiftwork and alcohol consumption: a systematic review of the literature. European Addiction Research, 27(1), 9–15. [DOI] [PubMed] [Google Scholar]
- Romer D, Reyna VF, & Satterthwaite TD (2017). Beyond stereotypes of adolescent risk taking: Placing the adolescent brain in developmental context. Developmental Cognitive Neuroscience, 27, 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Dickhaut J, McCabe K, & Pardo JV (2002). Neuronal substrates for choice under ambiguity, risk, gains, and losses. Management science, 48(6), 711–718. [Google Scholar]
- Tversky A, & Kahneman D (1981). The framing of decisions and the psychology of choice. Science, 211(4481), 453–458. [DOI] [PubMed] [Google Scholar]
- Voultsios A, Kennaway DJ, & Dawson D (1997). Salivary melatonin as a circadian phase marker: Validation and comparison to plasma melatonin. Journal of Biological Rhythms, 12(5), 457–466. doi: 10.1177/074873049701200507 [DOI] [PubMed] [Google Scholar]
- Wassersteinm R, & Lazar NA (2016). The ASA statement on p-values: Contet, process, and purpose. The American Statistician, 70(2), 129–133. [Google Scholar]
- Wittmann M, Dinich J, Merrow M, & Roenneberg T (2006). Social jetlag: misalignment of biological and social time. Chronobiology International, 23(1–2), 497–509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


