Abstract
Background:
Inflammatory cytokines play a role in atrial fibrillation (AF). Interleukin (IL)-1β, which is targeted in the treatment of ischemic heart disease, has not been well-studied in relation to AF.
Methods:
Postmenopausal women from the Women’s Health Initiative were included. Cox proportional hazards regression models were used to evaluate the association between log-transformed baseline cytokine levels and future AF incidence. Models were adjusted for body mass index, age, race, education, hypertension, diabetes, hyperlipidemia, current smoking, and history of coronary heart disease, congestive heart failure, or peripheral artery disease.
Results:
Of 16,729 women, 3,943 developed AF over an average of 8.5 years. Racial and ethnic groups included White (77.4%), Black/African-American (16.1%), Asian (2.7%), American Indian/Alaska Native (1.0%), and Hispanic (5.5%). Baseline IL-1β log continuous levels were not significantly associated with incident AF (HR 0.86 per 1 log (pg/mL) increase, p=0.24), similar to those of other inflammatory cytokines, IL-7, IL-8, IL-10, IGF-1, and TNF-α. There were significant associations between C-reactive protein (CRP) and IL-6 with incident AF.
Conclusions:
In this large cohort of postmenopausal women, there was no significant association between IL-1β and incident AF, although downstream effectors, CRP and IL-6, were associated with incident AF.
Keywords: Atrial fibrillation, Cytokines, Women’s Health Initiative, IL-1β, inflammation
INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia in the United States (US) with a prevalence of 5.2 million as of 2010, which is expected to rise to 12.1 million by 20301 and between 6 and 12 million in 2050.2 Despite available therapies, AF confers significant risk of morbidity and mortality among patients, with a five-fold increased risk of stroke and a 20% increased risk of death.3 Women comprise 60% of individuals with AF greater than age 75; the clinical consequences of AF for women are of particular concern, as women with AF are more likely than men to develop stroke, myocardial infarction and death.1,3,4
The role of inflammatory cytokine signaling in cardiovascular disease is well-established. Cytokines such as interleukin (IL)-1 and its downstream effectors IL-6 and C-reactive protein (CRP) are known to be part of the nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing protein 3 (NLRP3) inflammasome, are known to impact cardiovascular disease progression via systemic inflammation.5 Data demonstrating the role of inflammation in atherosclerosis, coronary artery disease (CAD), myocarditis, pericarditis, and cardiomyopathy are driving modern efforts to investigate the role of anti-inflammatory drugs to treat various cardiovascular diseases.5-7
Many of these cytokines, namely IL-1, CRP, IL-6, IL-8, IL-10, and tumor necrosis factor-alpha (TNF-α), are also thought to play a role in pathogenic AF inflammation,8-10 The “AF begets AF” phenomenon suggests that inflammatory states may predispose to AF, resulting in further localized atrial inflammation, which then leads to a cyclic process of regenerant AF.8 Interestingly, some of these pro-inflammatory cytokines have been shown to correlate with body mass index (BMI) especially in menopausal women,11 which is in line with the accepted understanding of obesity as a pro-inflammatory state.12-14 The known association between obesity,15 BMI,16 total lean body mass,17-19 and AF raises important questions about the impact of BMI on mitigating cytokine levels and AF risk.
The link between CRP levels and AF has been very well-established, with multiple recent studies linking high CRP levels to incident AF.20-23 Upstream of CRP, IL-6 has also been associated with AF as well as with AF complications like stroke, thromboembolic events, and mortality.24,25 IL-1 molecules, including the most well-studied of these, IL-1β, are even further upstream from CRP in the inflammatory signaling cascade.26 There have been some recent studies demonstrating the role of serum and adipose tissue IL-1β in AF among small cohorts of humans and animals.27-30 However, these data are limited by small sample sizes: studies showing higher levels of serum29 or microvesicle-bound30 IL-1β in persistent AF are limited to data from 34 and 90 patients, respectively. Similarly, data showing that localized IL-1β levels in epicardial adipose tissue27 and atrial macrophages28 are higher in AF states are limited to 37 and 11 subjects, respectively. Nevertheless, these findings suggest that this cytokine may play a role in atrial electrical remodeling28 and myocardial AF substrate creation.27 The role of circulating serum IL-1β as a predictive marker of future AF in a population cohort has not been studied. Importantly, IL-1β itself is a challenging biomarker to study in-vivo due to its short half-life and low plasma levels. This makes CRP and IL-6 important in determining the possible involvement of IL-1β’s systemic inflammatory effects in AF.31,32,40,41 Given the existence of anti-IL-1β therapies, 31-33 the role of the IL-1 axis in AF pathogenesis is important for the potential application of targeted therapies.
This study seeks to investigate the association between various inflammatory cytokines and incident AF in a large cohort to comment on the possible predictive value of baseline cytokine measurements for delineating AF risk. Furthermore, investigating the IL-1β-AF relationship will be specifically helpful given the rise of anti-IL-1β cardiovascular therapies. Lastly, this study will evaluate associations between BMI, inflammatory cytokine levels, and AF to further characterize inflammatory AF risk.8
MATERIALS AND METHODS
Population
The Women’s Health Initiative (WHI) is a research program with clinical trial and observational arms and a total enrollment of 161,808 postmenopausal women between 50 and 79 years of age recruited from 40 US clinical centers. The observational arm enrolled 93,676 postmenopausal women between 1993 and 1998 who were unlikely to die and planned to remain in the area of their study center for three years. At enrollment in WHI, participants completed baseline questionnaires detailing their demographics, medical history, and health habits, and underwent baseline vital sign measurement and laboratory testing. Extensive descriptions of the study design, inclusion criteria, data collection, validation, and monitoring have been thoroughly documented previously.34,35 Participants from the observational arm who had cytokine measurements were included in this study. The exclusion criteria included subjects with AF at baseline, which was assessed with a self-report questionnaire administered at WHI baseline and on baseline electrocardiogram. Additionally, subjects who were determined to be underweight (BMI less than 18.5 kg/m2) or who were missing baseline variable data were excluded.
Funding
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH/NHLBI), U.S. Department of Health and Human Services through 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005. The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A listing of WHI investigators can be found at https://www.whi.org/doc/WHIInvestigator-Long-List.pdf. This study was also funded in part by the NIH /NHLBI award 1R01HL136390-01.
Definition of Baseline Variables
Baseline variables in this study included age, race/ethnicity, income, level of education, BMI, self-reported medical history, blood pressure, and heart rate. Age was calculated from the subject’s reported birth date; race/ethnicity was self-reported; income and level of education were determined from the baseline questionnaire, as was self-reported medical history. CAD was defined as a history of myocardial infarction, coronary artery bypass surgery, or angioplasty of a coronary artery. Blood pressure and heart rate were recorded at the index clinic visit, and the diagnosis of hypertension was made based with a blood pressure greater than 140/90 at the initial visit or based on self-report of antihypertensive drug use. Finally, smoking and alcohol use status were self-reported in a baseline assessment of personal habits at study enrollment.
Ascertainment of Atrial Fibrillation
Ascertainment of incident AF in this cohort has been previously described.36 WHI data were linked with Medicare data utilizing social security numbers, birth dates, and death dates, with 97% of Medicare-eligible WHI participants successfully linked. AF incidence was defined as having at least a single ICD-9 diagnosis code of 427.31 from inpatient, outpatient, or physician diagnosis while the participant was enrolled in Medicare Fee-For-Service Parts A and B (FFS A+B). Participants enrolled in FFS A+B at WHI enrollment entered the risk set at WHI baseline, while participants that enrolled in FFS A+B after WHI enrollment were evaluated with a two-year look-back period to assess for pre-existing AF at the time of entering the risk set. Participants who were AF-free for the duration of the look-back period entered the risk set at the time of completion of the look-back period. Participants who left FFS A+B and then returned for a subsequent FFS A+B interval were not required to undergo a look-back period, as they had been established as AF-free on their initial entry into the risk set. Similarly, those participants who were younger than 65 years old at WHI study enrollment were included in this study if they had no AF at baseline and no documented AF upon enrolling in Medicare at age 65. However, these patients did undergo a period of time without surveillance given their ineligibility for Medicare before age 65. Because Medicare data is available for some participants at different time periods over WHI follow-up, a time-dependent indicator variable of Medicare coverage was added to Cox hazard models described below to adjust for possible ascertainment bias related to differential exposure to Medicare.
Cytokine Ascertainment Procedure
Cytokine levels of IL-1β, IL-6, IL-7, IL-8, IL-10, IGF-1, TNF-α, and CRP were obtained from fasting blood samples drawn at WHI enrollment, which were sent to local laboratories for centrifuging and freezing at −70°C. These samples were analyzed between September 2005 and March 2006, about 7-12 years after collection, as has been previously reported.37 Samples were then transferred to a central repository for long term storage and cytokine measurements were made. High-sensitivity CRP was measured using immunoturbidity followed by immunonephelometry, while IL-6 was measured using enzyme-linked immunosorbent assay.38,39 Of note, while cytokine levels and the above-mentioned baseline measures were assessed at WHI enrollment, Medicare FFS A+B enrollment is the time zero for this study; cytokine values utilized in this study are an extrapolation of baseline measures in some instances.
Statistical Analysis
Baseline characteristics were compared across AF status using the Student’s t-test for continuous variables and the χ2 test for categorical variables.
Multivariate-adjusted Cox proportional hazards regression analysis was performed to evaluate the relationship of incident AF with cytokine levels. This model was adjusted for covariates with known association with AF, including age, race/ethnicity (using four-level race/ethnicity modeling including White, Black, Hispanic, Other/Not Reported), level of education, smoking status, BMI, hypertension history, diabetes mellitus history, hyperlipidemia history, CAD history, congestive heart failure history, peripheral artery disease history, and smoking status. A secondary analysis without BMI as a covariate was performed to assess for confounding of the cytokine-AF association. The primary analyses were performed using continuous log-transformed cytokine values. Secondary categorical analyses with cytokine level tertiles, using the first tertile as a reference, were performed. Linear trends across incremental tertiles were estimated. To adjust for differences in measurement methods and the distribution of cytokine levels among different study sites and laboratories, all models were normalized by weighting to the WHI observational study (WHI OS) using inverse probability weighting (IPW). Associations are reported as hazard ratios (HRs) with 95% confidence intervals (CIs). A p-value of less than 0.05 was considered statistically significant. Cubic spline linear regression analyses, using three knots, were performed to explore the presence of inflection points in the continuous relationships between cytokine levels and incident AF.
The effect of BMI on the association between cytokine tertiles and incident AF was evaluated through further subgroup analyses with stratification of BMI into two groups: < 30 kg/m2 and ≥ 30 kg/m2. An interaction term was added to the multivariate-adjusted Cox proportional hazards regression analysis for BMI and each of the cytokine levels to assess for interaction. Again, all models were normalized by weighting to the WHI OS using IPW, and, in this case, events and annualized rates were also normalized. All statistical analyses were performed in Statistical Analysis Software (SAS).
RESULTS
Baseline Characteristics
This study included 16,729, postmenopausal women who had measurement of at least one of the cytokines of interest and were enrolled in FFS A+B. There were 19,032 subjects in the observational study initially identified to have any cytokine measurements. However, 1,193 were excluded for prevalent AF at enrollment or during the two-year look-back window for subjects that enrolled in FFS A+B after their WHI enrollment, 415 were excluded for being underweight (less than 18.5 kg), and 695 were excluded due to missing model covariate data. The self-reported race of participants was 77.4% White, 16.1% Black or African-American, 2.7% Asian, 0.7% American Indian or Alaska Native, 0.04% Native Hawaiian or Pacific Islander, 1.1% more than one race, and 1.9% unknown. There were 3,943 subjects (23.6% of subjects) who developed AF during an average follow up period of 8.5 (SD 5.0) years. Among the study cohort, cytokine measurements were available as follows: IL-1β from 3,011 subjects, IL-7 from 1,439 subjects, IL-8 from 1442 subjects, IL-10 from 2,452 subjects, IGF-1 from 2,281 subjects, TNF-α from 5420 subjects, CRP from 13,711 subjects, IL-6 from 9,943 subjects. The baseline demographics and medical history of the subjects are summarized in Table 1 and Supplementary Table 1. Subjects with incident AF were significantly older (68.6 years vs. 64.6 years, p < 0.001), significantly more likely to be white (83.1% vs. 70.8%, p < 0.001), had a “higher BMI (28.1 kg/m2. 27.7 kg/m2, p < 0.001), and had several comorbidities classically associated with AF (Supplementary Table 1).
Table 1.
Baseline Characteristics of IL-1β Participants By Weighted1 Tertile of IL-1β
| Tertile 1 (n=1261) |
Tertile 2 (n=746) |
Tertile 3 (n=1004) |
|||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-value | |
| Demographics | |||||||
| Age, mean (SD) | 65.6 | (6.9) | 63.5 | (6.5) | 64.2 | (6.9) | <0.001 |
| Race | |||||||
| American Indian / Alaska Native | 11 | 0.9 | 6 | 0.8 | 13 | 1.3 | |
| Asian | 69 | 5.5 | 26 | 3.5 | 28 | 2.8 | |
| Native Hawaiian / Pacific Islander | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Black / African American | 192 | 15.2 | 209 | 28.0 | 260 | 25.9 | |
| White | 933 | 74.0 | 489 | 65.5 | 662 | 65.9 | |
| More than one race | 18 | 1.4 | 6 | 0.8 | 18 | 1.8 | |
| Unknown / not reported | 38 | 3.0 | 10 | 1.3 | 23 | 2.3 | |
| Ethnicity | 0.01 | ||||||
| Hispanic / Latina | 95 | 7.5 | 27 | 3.6 | 45 | 4.5 | |
| Not Hispanic / Latina | 1151 | 91.3 | 709 | 95.0 | 948 | 94.4 | |
| Unknown / not reported | 15 | 1.2 | 10 | 1.3 | 11 | 1.1 | |
| Income | 0.42 | ||||||
| <$20,000 | 255 | 20.2 | 132 | 17.7 | 171 | 17.0 | |
| $20,000-$49,999 | 526 | 41.7 | 316 | 42.4 | 431 | 42.9 | |
| $50,000-$74,999 | 213 | 16.9 | 130 | 17.4 | 179 | 17.8 | |
| ≥$75,000 | 176 | 14.0 | 127 | 17.0 | 150 | 14.9 | |
| Education | 0.06 | ||||||
| ≤High school/GED | 318 | 25.2 | 157 | 21.0 | 218 | 21.7 | |
| Some college | 486 | 38.5 | 261 | 35.0 | 380 | 37.8 | |
| College degree or higher | 457 | 36.2 | 328 | 44.0 | 406 | 40.4 | |
| Medical History | |||||||
| BMI, mean (SD) | 28.1 | (6.1) | 27.7 | (5.9) | 27.8 | (5.9) | 0.31 |
| Hypertension | |||||||
| Never treated | 656 | 52.0 | 548 | 73.5 | 650 | 64.7 | <0.001 |
| Currently untreated | 230 | 18.2 | 69 | 9.2 | 132 | 13.1 | |
| Treated | 375 | 29.7 | 129 | 17.3 | 222 | 22.1 | |
| Diabetes mellitus | 61 | 4.8 | 40 | 5.4 | 63 | 6.3 | 0.14 |
| Hyperlipidemia | 192 | 15.2 | 96 | 12.9 | 139 | 13.8 | 0.36 |
| Coronary artery disease | 43 | 3.4 | 13 | 1.7 | 14 | 1.4 | 0.002 |
| MI | 27 | 2.1 | 11 | 1.5 | 12 | 1.2 | 0.09 |
| CABG/PTCA | 27 | 2.1 | 9 | 1.2 | 10 | 1.0 | 0.03 |
| Stroke | 16 | 1.3 | 5 | 0.7 | 5 | 0.5 | 0.09 |
| Heart failure | 13 | 1.0 | 2 | 0.3 | 7 | 0.7 | 0.40 |
| Peripheral arterial disease | 37 | 2.9 | 10 | 1.3 | 13 | 1.3 | 0.008 |
| Systolic BP, mean (SD) | 128.7 | (17.5) | 124.4 | (17.2) | 125.4 | (16.4) | <0.001 |
| Diastolic BP, mean (SD) | 74.0 | (9.6) | 74.0 | (8.5) | 73.8 | (8.6) | 0.60 |
| Heart rate, mean (SD) | 69.6 | (11.1) | 69.0 | (11.5) | 70.2 | (12.3) | 0.21 |
| Habits | |||||||
| Smoking status | |||||||
| Never | 668 | 53.0 | 394 | 52.8 | 494 | 49.2 | 0.20 |
| Past | 498 | 39.5 | 304 | 40.8 | 431 | 42.9 | |
| Current | 95 | 7.5 | 48 | 6.4 | 79 | 7.9 | |
| Alcohol | |||||||
| Never | 182 | 14.4 | 98 | 13.1 | 139 | 13.8 | 0.85 |
| Past | 308 | 24.4 | 161 | 21.6 | 240 | 23.9 | |
| Current | 762 | 60.4 | 482 | 64.6 | 620 | 61.8 | |
Tertiles defined by distribution of IL-1B inverse probability weighted to the WHI observational study
p-value compares Tertile 1 vs. Tertile 3 using t-tests for continuous markers and chi-square tests for categorical markers
All values represent the total and percentage of participants unless otherwise indicated.
BMI, body mass index; GED, general educational development; MI, myocardial infarction; CABG, coronary artery bypass graft; PTCA, percutaneous transluminal coronary angioplasty.
Cytokine Levels and Risk for Incident Atrial Fibrillation
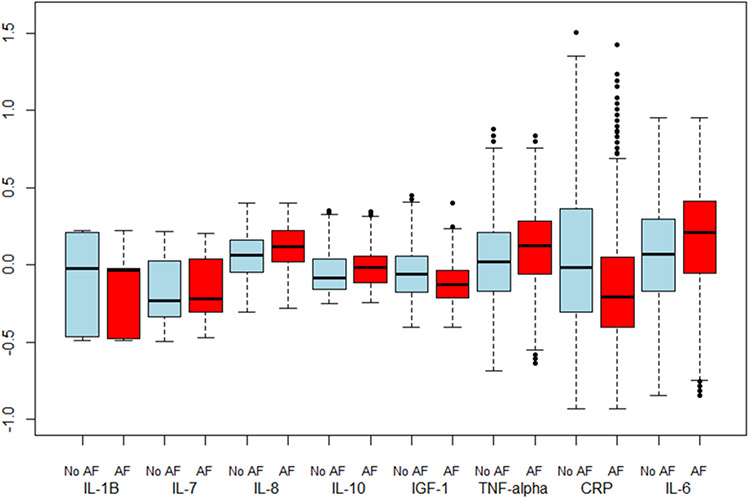
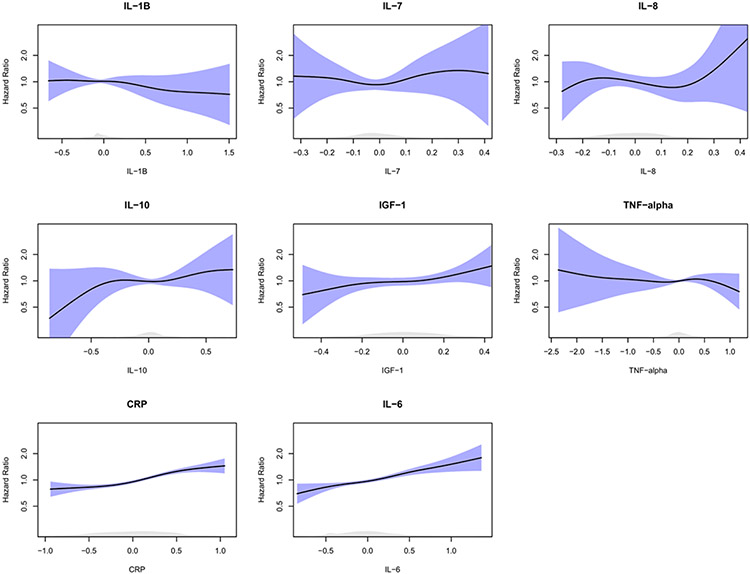
Box plots of age- and study-adjusted standardized values of the different cytokines by incident AF status are presented in Figure 1. On multivariate Cox hazard regression analysis, there was no significant association between IL-1β log continuous levels and incident AF (Table 2; HR 0.86 per 1 log (pg/mL) increase, p=0.24). AF. There were also no significant associations when analyzed by tertiles or tertile trends. In both age/race- and fully-adjusted multivariate models, levels of IL-7, IL-8, IL-10, IGF-1, and TNF-α were not associated with incident AF when analyzed in tertiles, linear trends, or as continuous log values (Table 2). In post-hoc cubic spline regression analyses of these cytokines adjusted for age and study and IPW, there was a significant association (p < 0.05) between IL-1β, IL-10, and incident AF (Figure 2).
Figure 1. Box Plots of Age and study adjusted, Standardized Biomarker Values1 by Atrial Fibrillation Status.
1CRP has an additional 49 outliers (34 no AF, 15 AF) between 1.5 and 3 standard deviations
Table 2.
Multivariate-adjusted Hazard Ratios of Incident Atrial Fibrillation
| Age / Race Adjusted | Multivariate Adjusted1 Including BMI |
Multivariate Adjusted1 Excluding BMI |
||||
|---|---|---|---|---|---|---|
| Characteristic | HR (95% CI) | P-Value | HR (95% CI) | P-Value | HR (95% CI) | P-Value |
| BMI (5 kg/m2 increase) | 1.15 (1.11, 1.19) | <0.001 | 1.11 (1.07, 1.15) | <0.001 | ||
| IL-1B, pg/mL | 0.98 | 0.98 | 0.98 | |||
| Tertile 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||
| Tertile 2 | 0.98 (0.74, 1.29) | 0.98 (0.73, 1.30) | 0.97 (0.73, 1.29) | |||
| Tertile 3 | 1.01 (0.78, 1.31) | 1.00 (0.77, 1.30) | 1.00 (0.77, 1.30) | |||
| Linear Trend | 1.01 (0.88, 1.14) | 0.94 | 1.00 (0.88, 1.14) | >0.99 | 1.00 (0.87, 1.14) | 0.99 |
| Continuous Log | 0.89 (0.70, 1.13) | 0.32 | 0.86 (0.66, 1.11) | 0.24 | 0.85 (0.66, 1.10) | 0.23 |
| IL-7, pg/mL | 0.35 | 0.22 | 0.24 | |||
| Tertile 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||
| Tertile 2 | 0.85 (0.48, 1.53) | 0.74 (0.41,1.35) | 0.76 (0.42, 1.36) | |||
| Tertile 3 | 1.27 (0.65, 2.46) | 1.22 (0.66, 2.24) | 1.21 (0.66, 2.24) | |||
| Linear Trend | 1.14 (0.79, 1.63) | 0.49 | 1.12 (0.80, 1.58) | 0.51 | 1.12 (0.79, 1.58) | 0.52 |
| Continuous Log | 1.07 (0.66, 1.71) | 0.79 | 1.04 (0.67, 1.61) | 0.87 | 1.03 (0.67, 1.61) | 0.88 |
| IL-8, pg/mL | 0.91 | 0.98 | 0.98 | |||
| Tertile 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||
| Tertile 2 | 0.89 (0.51, 1.53) | 0.95 (0.56, 1.63) | 0.95 (0.56, 1.63) | |||
| Tertile 3 | 0.92 (0.48, 1.78) | 0.98 (0.53, 1.84) | 0.98 (0.52, 1.84) | |||
| Linear Trend | 0.96 (0.68, 1.34) | 0.80 | 0.99 (0.72, 1.36) | 0.95 | 0.99 (0.72, 1.36) | 0.95 |
| Continuous Log | 0.97 (0.49, 1.92) | 0.92 | 1.06 (0.57, 1.97) | 0.87 | 1.05 (0.56, 1.97) | 0.87 |
| IL-10, pg/mL | 0.61 | 0.50 | 0.50 | |||
| Tertile 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||
| Tertile 2 | 0.89 (0.65, 1.21) | 0.85 (0.62, 1.16) | 0.85 (0.62, 1.16) | |||
| Tertile 3 | 1.02 (0.74, 1.41) | 0.98 (0.71, 1.37) | 0.98 (0.71, 1.37) | |||
| Linear Trend | 1.01 (0.85, 1.19) | 0.92 | 0.99 (0.84, 1.18) | 0.93 | 0.99 (0.84, 1.18) | 0.93 |
| Continuous Log | 1.07 (0.91, 1.27) | 0.41 | 1.05 (0.88, 1.25) | 0.56 | 1.05 (0.88, 1.25) | 0.57 |
| IGF-1, ng/mL | 0.61 | 0.61 | 0.64 | |||
| Tertile 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||
| Tertile 2 | 1.06 (0.80, 1.42) | 1.06 (0.80, 1.42) | 1.06 (0.79, 1.42) | |||
| Tertile 3 | 1.15 (0.87, 1.51) | 1.15 (0.87, 1.51) | 1.14 (0.87, 1.50) | |||
| Linear Trend | 1.07 (0.93, 1.23) | 0.32 | 1.07 (0.93, 1.23) | 0.32 | 1.07 (0.93, 1.22) | 0.35 |
| Continuous Log | 1.29 (0.91, 1.85) | 0.16 | 1.33 (0.93, 1.89) | 0.11 | 1.31 (0.92, 1.87) | 0.14 |
| TNF-alpha, pg/mL | 0.05 | 0.13 | 0.13 | |||
| Tertile 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||
| Tertile 2 | 1.29 (1.05, 1.58) | 1.23 (1.01, 1.51) | 1.23 (1.01, 1.51) | |||
| Tertile 3 | 1.16 (0.96, 1.41) | 1.10 (0.91, 1.34) | 1.12 (0.92, 1.37) | |||
| Linear Trend | 1.07 (0.98, 1.17) | 0.15 | 1.05 (0.95, 1.15) | 0.36 | 1.05 (0.96, 1.16) | 0.27 |
| Continuous Log | 1.02 (0.92, 1.12) | 0.77 | 0.99 (0.89, 1.10) | 0.87 | 1.00 (0.90, 1.11) | 0.99 |
| CRP, mg/L | <0.001 | <0.001 | <0.001 | |||
| Tertile 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||
| Tertile 2 | 1.10 (0.99, 1.22) | 1.01 (0.91, 1.13) | 1.05 (0.94, 1.17) | |||
| Tertile 3 | 1.47 (1.32, 1.63) | 1.25 (1.12, 1.39) | 1.33 (1.19, 1.47) | |||
| Linear Trend | 1.22 (1.15, 1.28) | <0.001 | 1.12 (1.06, 1.19) | <0.001 | 1.16 (1.10, 1.22) | <0.001 |
| Continuous Log | 1.17 (1.12, 1.22) | <0.001 | 1.09 (1.04, 1.14) | <0.001 | 1.12 (1.07, 1.17) | <0.001 |
| IL-6, pg/mL | <0.001 | 0.004 | <0.001 | |||
| Tertile 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||
| Tertile 2 | 1.08 (0.95, 1.23) | 1.02 (0.89, 1.17) | 1.05 (0.92, 1.19) | |||
| Tertile 3 | 1.36 (1.20, 1.53) | 1.21 (1.06, 1.37) | 1.27 (1.12, 1.44) | |||
| Linear Trend | 1.17 (1.10, 1.25) | <0.001 | 1.10 (1.04, 1.18) | 0.003 | 1.13 (1.06, 1.21) | <0.001 |
| Continuous Log | 1.22 (1.14, 1.31) | <0.001 | 1.16 (1.08, 1.24) | <0.001 | 1.18 (1.11, 1.27) | <0.001 |
Multivariate models are adjusted for age, race/ethnicity (Black, White, Hispanic, Other/Not Reported), education, hypertension, diabetes, hyperlipidemia, hx of CHD (MI/CABG/PTCA), hx of ChF, hx of PAD, current smoking, BMI.
BMI, body mass index; GED, general educational development; MI, myocardial infarction; CABG, coronary artery bypass graft; PTCA, percutaneous transluminal coronary angioplasty.
All models are weighted to the WHI OS using IPW weighting
Figure 2. Graphs of Age-Adjusted Splines of Standardized, Log-Transformed Biomarkers by Standard Deviation.
When analyzed as continuous log values in fully-adjusted multivariate models, levels of CRP and IL-6 were significantly associated with incident AF (HR 1.09 per 1 log (mg/L), p <0.001; HR 1.16 per 1 log (pg/mL) p < 0.001, respectively). Similarly, when analyzed categorically, compared with women in the first tertile, those in the highest tertile had a significantly elevated risk of developing incident AF (HR 1.25, p = <0.001; HR 1.21, p = 0.004, respectively). In a post-hoc cubic spline regression analysis adjusted for age and study and IPW weighting, there was a significant association between incident AF and CRP (p < 0.001) as well as IL-6 (p < 0.001). Equivalent linear models were also statistically significant for CRP (p < 0.001) and IL-6 (p < 0.001).
To assess for a confounding effect of BMI on the different cytokines, we performed additional sensitivity analyses. As we have demonstrated before, there is a significant association between BMI and incident AF after multivariate adjustment in this cohort (HR 1.11 per 5kg/m2 increase, 95% CI 1.07-1.15, p <0.001). After removing BMI from the multivariate model, there was no significant difference in the relationship between log-transformed IL-1β, IL-7, IL-8, IL-10, IGF-1 and TNF-α, and incident AF (Table 2).
Subgroup Analyses
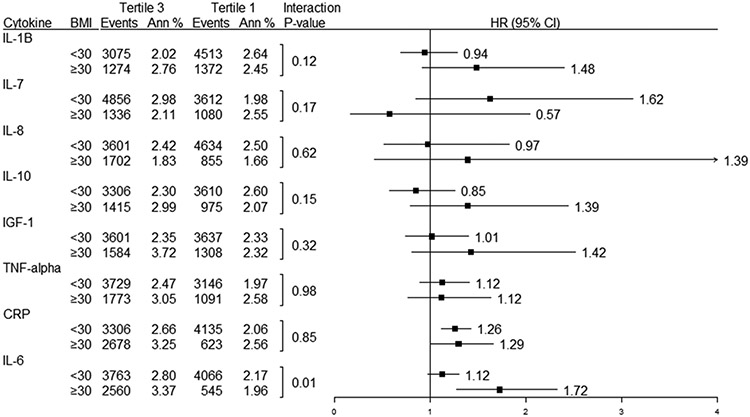
To test further for interactions between obesity and the various cytokines, we performed subgroup analyses by stratifying the participants by BMI < 30 and BMI ≥ 30 (Figure 3). There were no statistically significant interactions between BMI and the association between incident AF and the different cytokines, except for IL-6 (BMI ≥ 30: HR 1.72 for 3rd vs. 1st tertile; BMI < 30: HR 1.12 for 3rd vs. 1st tertile; p interaction = 0.01).
Figure 3. Weighted1 Events, Annualized Rates, and Interaction between BMI and inflammatory log cytokine level.
1Events, annualized rates and models are weighted to the WHI OS using IPW weighting
DISCUSSION
This study examines the correlation between baseline cytokine levels and incident AF in a cohort of postmenopausal women. Our analyses found that CRP and IL-6 levels were associated with incident AF, although there was no significant association between baseline IL-1β log continuous levels and incident AF on multivariate-adjusted Cox regression. Importantly, CRP and IL-6 are downstream effectors of the IL-1 pathway and are affected by IL-1β activity; IL-1β itself, however, is a challenging biomarker to study in-vivo due to its short half-life and low plasma levels.31,32,40,41 Similarly, IL-7, IL-8, IL-10, IGF-1, and TNF-α continuous log levels were not associated with incident AF. Conversely, CRP and IL-6 levels were associated with incident AF.
IL-1β has recently garnered significant interest in cardiovascular literature. Novel evidence from the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) demonstrated that anti-IL-1β therapy with canakinumab may decrease rates of cardiovascular death, myocardial infarction, and stroke in patients with CAD.40 Alternate anti-IL-1β therapies such as anakinra in heart failure patients and rilonacept in pericarditis patients have also shown promising results.31-33 IL-1β is thought to play a role in AF given higher local and systemic levels in persistent AF,27-30 but has not been well-evaluated as a predictive or causative contributor to AF. The lack of a significant association between IL-1β levels and incident AF shown in this large cohort is notable but does not rule out the possibility of targeting IL-1β to prevent AF. IL-1β blockade has been shown to decrease inflammation by lowering IL-6 and CRP levels,40-44 cytokines whose levels were associated with AF incidence in our study.
Despite the well-established role of inflammation in the pathogenesis and progression of AF,8-10 data on the predictive value of certain cytokine measurements is lacking. Among inflammatory cytokines of high clinical interest, higher CRP levels have consistently been shown to be associated with incident AF,20-25 including among groups of adult women21 as in the present study. As noted, CRP is downstream of and affected by IL-1β. Our data, using multivariate Cox proportional hazards regression analysis, corroborates that CRP is associated with AF incidence. Importantly, prior Mendelian randomization analyses using CRP single nucleotide polymorphisms (SNPs) as instrument variables did not find the association between CRP and AF to be causative.20,45 However, given that CRP is only one downstream marker of inflammation, this finding does not nullify the well-established role of inflammation in incident AF or the possible value of CRP in predicting incident AF. Our findings are an important addition to the literature on CRP and AF since they were derived from a large cohort of female patients, who are underrepresented in studies on AF.
Other well-studied cytokines such as IL-6, another downstream effector of IL-1β, have been suggested to have a causal association with AF.25,46 One study found an inverse relationship between genetically-predicted IL-6 levels and AF, although they posit that this may still reflect an association between IL-6 and AF: higher genetically predicted IL-6 levels may correspond to lower levels of receptor-bound IL-6 and therefore diminished IL-6 signaling.47 While these conclusions are all suggestive of an association between IL-6 and AF, the varying results also highlight a lack of sufficient evidence on the topic, limiting the implementation of IL-6 targeted therapies for AF. Our study expands upon this data by redemonstrating an association between IL-6 and the development of future AF. Among other cytokines, TNF-α is often systemically elevated in patients with AF,48,49 while IL-8 and IL-10 have been shown to be elevated in permanent versus paroxysmal AF.10,50,51 However, studies on these cytokines have been primarily cross-sectional. Our results demonstrate that these markers may not be helpful in predicting incident AF given the lack of association between baseline serum levels and future AF development.
Cytokines, in general, have complex mechanisms of action which may lead to distinct effects depending on various factors. For example, certain cytokines such as IL-6 may have anti-inflammatory effects when released during exercise with other anti-inflammatory cytokines like IL-1ra, and soluble TNF-R but pro-inflammatory effects when released by monocyte-macrophages during infection with other common, pro-inflammatory cytokines.52 Post hoc age and study/IPW weighting adjusted splines were used to evaluate for more complex relationships between the levels of cytokines studied and AF risk: this analysis revealed potential quadratic relationships between several cytokines in addition to CRP and IL-6 that were not significantly associated in linear models. Future evaluation of concurrent cytokine levels and additive effects of cytokines could potentially elucidate intricacies in these relationships, as could additional investigation of non-linear associations between cytokine levels and AF incidence.
Given that obesity,15 a pro-inflammatory state,12-14 as well as elevated BMI16 and total lean body mass17-19 have been associated with AF,15 we also performed analyses to investigate the impact of BMI on inflammatory cytokine levels and incident AF. BMI was strongly associated with risk of AF in this study, as expected.15 However, there were no differential effects of cytokine level on AF by BMI except for among IL-6, as previously described. This apparent, overall independence from BMI suggests that the differential risk of AF by BMI is not mediated by the cytokines evaluated in this study, despite prior data showing correlation of cytokine levels and BMI in menopausal women.11 Importantly, however, given the complexity of inflammatory cytokine signaling and expression described above, there may be more complex relationships between BMI and cytokine levels that could not be assessed in this retrospective study.
Limitations
There are several limitations to this study. While using a WHI cohort provided a large dataset for analysis, the study was observational and retrospective, limited to previously collected data. In line with this, the available cytokine measurements were one-time values collected at study enrollment. This precluded an assessment of cytokine level variation over time. Additionally, because the baseline for WHI was at WHI enrollment, and time zero for this study is MCS FFS A+B enrollment, some baseline and cytokine measurements are an extrapolation of baseline. Lastly, the data was collected from exclusively postmenopausal women and therefore may not be applicable to a broader population including men and younger adults.
Conclusions
This study represents an important evaluation of the relationship between well-studied inflammatory cytokines and future AF incidence. It re-demonstrates the known association between CRP and future AF development, posits a possible role of IL-6 in predicting AF, and suggests that other cytokines, namely IL-1β, IL-7, IL-8, IL-10, IGF-1 and TNF-α, lack this predictive value. These associations persisted regardless of BMI category across all cytokines except for IL-6, wherein BMI ≥ 30 increased risk of AF. Therefore, our findings support that the IL-1 axis, namely via downstream effectors, CRP and IL-6, is likely involved in AF pathogenesis. The lack of a direct association between IL-1β levels and AF incidence may be related to challenges of IL-1β as a biomarker (short half-life, low plasma levels). Given recent emphasis on investigational anti-inflammatory therapies for cardiovascular disease, especially anti-IL-1β treatments, the lack of association demonstrated here is important for guiding pharmacologic development of AF therapies. Given the impact of anti-IL1β therapies on CRP and IL-6 as mediators of inflammation, our data highlights the possible role of these treatments in preventing AF development. Future studies using targeted IL-1β or IL-6 blockade in AF prevention may still be warranted.
Highlights.
IL-1β levels alone are not associated with incident atrial fibrillation
CRP and IL-6, downstream of IL-1β, are associated with incident atrial fibrillation
Body mass index does not alter the effect of cytokines on atrial fibrillation risk
Declaration of Interest
Justin Parizo – Conflicts of interest: none; Funding source: none
Simon Ermakov – Conflicts of interest: none; Funding source: T32
Joseph Larson – Conflicts of interest: none; Funding source: none
Robert Wallace – Conflicts of interest: none; Funding source: none
Themistocles Assimes – Conflicts of interest: none; Funding source: none
Mark Hlatky – Conflicts of interest: none; Funding source: none
Marcia Stefanick – Conflicts of interest: none; Funding source: none
Marco Perez – Conflicts of interest: consultant Apple Inc., Biotronik, Boston Scientific, Bristol Myers
Squibb, Johnson & Johnson; Grant Funding: Apple Inc.; Funding Source: NIH/NHLBI 1R01HL136390-01
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. [DOI] [PubMed] [Google Scholar]
- 2.Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. 2021;16:217–221. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Mitchell JB, Baker CS, Kannel WB, D’Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158:229–234. [DOI] [PubMed] [Google Scholar]
- 4.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang N-Y, Tsao CW, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 5.Toldo S, Mezzaroma E, Buckley LF, Potere N, Di Nisio M, Biondi-Zoccai G, Van Tassell BW, Abbate A. Targeting the NLRP3 inflammasome in cardiovascular diseases. Pharmacol Ther. 2021;236:108053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley LF, Abbate A. Interleukin-1 blockade in cardiovascular diseases: a clinical update. Eur Heart J. 2018;39:2063–2069. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Rane M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ Res. 2021;128:1728–1746. [DOI] [PubMed] [Google Scholar]
- 8.Hu Y-F, Chen Y-J, Lin Y-J, Chen S-A. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–243. [DOI] [PubMed] [Google Scholar]
- 9.Patel P, Dokainish H, Tsai P, Lakkis N. Update on the association of inflammation and atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:1064–1070. [DOI] [PubMed] [Google Scholar]
- 10.Guo Y, Lip GYH, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–2270. [DOI] [PubMed] [Google Scholar]
- 11.Cybulska AM, Rachubińska K, Szkup M, Schneider-Matyka D, Baranowska-Bosiacka I, Chlubek D, Lubkowska A, Panczyk M, Sołek-Pastuszka J, Grochans E. Serum levels of proinflammatory cytokines and selected bioelements in perimenopausal women with regard to body mass index [Internet]. Aging. 2021;13:25025–25037. Available from: 10.18632/aging.203754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunz HE, Hart CR, Gries KJ, Parvizi M, Laurenti M, Dalla Man C, Moore N, Zhang X, Ryan Z, Polley EC, Jensen MD, Vella A, Lanza IR. Adipose tissue macrophage populations and inflammation are associated with systemic inflammation and insulin resistance in obesity. Am J Physiol Endocrinol Metab. 2021;321:E105–E121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TJ, Parise H, Levy D, D’Agostino RB Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 16.Perez MV, Wang PJ, Larson JC, Soliman EZ, Limacher M, Rodriguez B, Klein L, Manson JE, Martin LW, Prineas R, Connelly S, Hlatky M, Wassertheil-Smoller S, Stefanick ML. Risk factors for atrial fibrillation and their population burden in postmenopausal women: the Women’s Health Initiative Observational Study. Heart. 2013;99:1173–1178. [DOI] [PubMed] [Google Scholar]
- 17.Tikkanen E, Gustafsson S, Knowles JW, Perez M, Burgess S, Ingelsson E. Body composition and atrial fibrillation: a Mendelian randomization study. Eur. Heart J 2019;40:1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frost L, Benjamin EJ, Fenger-Grøn M, Pedersen A, Tjønneland A, Overvad K. Body fat, body fat distribution, lean body mass and atrial fibrillation and flutter. A Danish cohort study. Obesity . 2014;22:1546–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azarbal F, Stefanick ML, Assimes TL, Manson JE, Bea JW, Li W, Hlatky MA, Larson JC, LeBlanc ES, Albert CM, Nassir R, Martin LW, Perez MV. Lean body mass and risk of incident atrial fibrillation in post-menopausal women. Eur Heart J. 2016;37:1606–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marott SCW, Nordestgaard BG, Zacho J, Friberg J, Jensen GB, Tybjaerg-Hansen A, Benn M. Does elevated C-reactive protein increase atrial fibrillation risk? A Mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol. 2010;56:789–795. [DOI] [PubMed] [Google Scholar]
- 21.Conen D, Ridker PM, Everett BM, Tedrow UB, Rose L, Cook NR, Buring JE, Albert CM. A multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. Eur Heart J. 2010;31:1730–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, Tofler GH, Selhub J, Jacques PF, Wolf PA, Magnani JW, Ellinor PT, Wang TJ, Levy D, Vasan RS, Benjamin EJ. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. [DOI] [PubMed] [Google Scholar]
- 24.Aulin J, Siegbahn A, Hijazi Z, Ezekowitz MD, Andersson U, Connolly SJ, Huber K, Reilly PA, Wallentin L, Oldgren J. Interleukin-6 and C-reactive protein and risk for death and cardiovascular events in patients with atrial fibrillation [Internet]. American Heart Journal. 2015;170:1151–1160. Available from: 10.1016/j.ahj.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 25.Rosa M, Chignon A, Li Z, Boulanger M-C, Arsenault BJ, Bossé Y, Thériault S, Mathieu P. A Mendelian randomization study of IL6 signaling in cardiovascular diseases, immune-related disorders and longevity. NPJ Genom Med. 2019;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridker PM. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ Res. 2016;118:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q, Zhang F, Yang M, Zhong J. Increasing Level of Interleukin-1β in Epicardial Adipose Tissue Is Associated with Persistent Atrial Fibrillation. J Interferon Cytokine Res. 2020;40:64–69. [DOI] [PubMed] [Google Scholar]
- 28.Sun Z, Zhou D, Xie X, Wang S, Wang Z, Zhao W, Xu H, Zheng L. Cross-talk between macrophages and atrial myocytes in atrial fibrillation. Basic Res Cardiol. 2016; 111:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung Y, Cho Y, Kim N, Oh I-Y, Kang SW, Choi E-K, Hwang G-S. Lipidomic profiling reveals free fatty acid alterations in plasma from patients with atrial fibrillation. PLoS One. 2018;13:e0196709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Yan H-M, Tang M-X, Wang Z-H, Zhong M, Zhang Y, Deng J-T, Zhang W. Increased serum levels of microvesicles in nonvalvular atrial fibrillation determinated by ELISA using a specific monoclonal antibody AD-1. Clin Chim Acta. 2010;411:1700–1704. [DOI] [PubMed] [Google Scholar]
- 31.Abbate A, Wohlford GF, Del Buono MG, Chiabrando JG, Markley R, Turlington J, Kadariya D, Trankle CR, Biondi-Zoccai G, Lipinski MJ, Others. Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: results from a pooled analysis of the VCUART clinical trials. European Heart Journal-Cardiovascular Pharmacotherapy [Internet]. 2021;Available from: https://academic.oup.com/ehjcvp/advance-article-abstract/doi/10.1093/ehjcvp/pvab075/6382993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Tassell BW, Trankle CR, Canada JM, Carbone S, Buckley L, Kadariya D, Del Buono MG, Billingsley H, Wohlford G, Viscusi M, Oddi-Erdle C, Abouzaki NA, Dixon D, Biondi-Zoccai G, Arena R, Abbate A. IL-1 Blockade in Patients With Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2018;11:e005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein AL, Imazio M, Paolini JF. Phase 3 Trial of Interleukin-1 Trap Rilonacept in Recurrent Pericarditis. Reply. N. Engl. J. Med 2021;384:1475–1476. [DOI] [PubMed] [Google Scholar]
- 34.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–21. [DOI] [PubMed] [Google Scholar]
- 35.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 36.Boursiquot BC, Larson JC, Shalash OA, Vitolins MZ, Soliman EZ, Perez MV. Vitamin D with calcium supplementation and risk of atrial fibrillation in postmenopausal women. Am Heart J. 2019;209:68–78. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan RC, McGinn AP, Baird AE, Hendrix SL, Kooperberg C, Lynch J, Rosenbaum DM, Johnson KC, Strickler HD, Wassertheil-Smoller S. Inflammation and hemostasis biomarkers for predicting stroke in postmenopausal women: the Women’s Health Initiative Observational Study. J Stroke Cerebrovasc Dis. 2008;17:344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ermakov S, Azarbal F, Stefanick ML, LaMonte MJ, Li W, Tharp KM, Martin LW, Nassir R, Salmoirago-Blotcher E, Albert CM, Manson JE, Assimes TL, Hlatky MA, Larson JC, Perez MV. The associations of leptin, adiponectin and resistin with incident atrial fibrillation in women. Heart. 2016;102:1354–1362. [DOI] [PubMed] [Google Scholar]
- 39.Khan UI, Ogorodnikova AD, Xu L, Wang D, Wassertheil-Smoller S, Ho GYF, Sowers MFR, Rajpathak SN, Allison MA, Mackey RH, Vitolins MZ, Manson JE, Wildman RP. The adipokine profile of metabolically benign obese and at-risk normal weight postmenopausal women: The Women’s Health Initiative Observational Study [Internet]. Obesity. 2014;22:786–794. Available from: 10.1002/oby.20139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T, CANTOS Pilot Investigative Group. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748. [DOI] [PubMed] [Google Scholar]
- 42.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. [DOI] [PubMed] [Google Scholar]
- 43.Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation [Internet]. European Journal of Immunology. 2011;41:1203–1217. Available from: 10.1002/eji.201141550 [DOI] [PubMed] [Google Scholar]
- 45.Cao MY, Liu D, Zhang XY, Tian QY, Zhang Q, Wang YX. Association of C-reactive Protein with Cardiovascular Outcomes: A Mendelian Randomization Study in the Japanese Population. Biomed Environ Sci. 2022;35:126–132. [DOI] [PubMed] [Google Scholar]
- 46.Wu F, Feng X, Wu Y, Yu M, Ding S, Zhao H. Interleukin-6 Receptor Asp358 A>C Polymorphism Is Associated With Postoperative Atrial Fibrillation. Ann Thorac Surg. 2021;111:102–108. [DOI] [PubMed] [Google Scholar]
- 47.Yuan S, Lin A, He Q-Q, Burgess S, Larsson SC. Circulating interleukins in relation to coronary artery disease, atrial fibrillation and ischemic stroke and its subtypes: A two-sample Mendelian randomization study. Int J Cardiol. 2020;313:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng H, Xue Y-M, Zhan X-Z, Liao H-T, Guo H-M, Wu S-L. Role of tumor necrosis factor-alpha in the pathogenesis of atrial fibrillation. Chin Med J . 2011;124:1976–1982. [PubMed] [Google Scholar]
- 49.Ren M, Li X, Hao L, Zhong J. Role of tumor necrosis factor alpha in the pathogenesis of atrial fibrillation: A novel potential therapeutic target? Ann Med. 2015;47:316–324. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Solus J, Chen Q, Rho YH, Milne G, Michael Stein C, Darbar D. Role of inflammation and oxidative stress in atrial fibrillation [Internet]. Heart Rhythm. 2010;7:438–444. Available from: 10.1016/j.hrthm.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liuba I, Ahlmroth H, Jonasson L, Englund A, Jönsson A, Säfström K, Walfridsson H. Source of inflammatory markers in patients with atrial fibrillation. Europace. 2008;10:848–853. [DOI] [PubMed] [Google Scholar]
- 52.Pratesi A Skeletal muscle: an endocrine organ [Internet]. CLINICAL CASES IN MINERAL AND BONE METABOLISM. 2013;Available from: 10.11138/ccmbm/2013.10.1.011 [DOI] [PMC free article] [PubMed] [Google Scholar]