Abstract
Autistic individuals exhibit significant sensorimotor difficulties. Postural stability and control are foundational motor skills for successfully performing many activities of daily living. In neurotypical development, postural stability and control develop throughout childhood and adolescence. In autistic development, previous studies have focused primarily on individual age groups (e.g., childhood, adolescence, adulthood) or only controlled for age using age-matching. Here, we examined the age trajectories of postural stability and control in autism from childhood through adolescents using standardized clinical assessments. In study 1, we tested the postural stability of autistic (n=27) and neurotypical (n=41) children, adolescents, and young adults aged 7–20 years during quiet standing on a force plate in three visual conditions: Eyes Open (EO), Eyes Closed (EC), and eyes open with the head in a translucent dome (Dome). Postural sway variability decreased as age increased for both groups, but autistic participants showed greater variability than neurotypical participants across age. In study 2, we tested autistic (n=21) and neurotypical (n=32) children and adolescents aged 7–16 years during a dynamic postural control task with 9 targets. Postural control efficiency increased as age increased for both groups, but autistic participants were less efficient compared to neurotypical participants across age. Together, these results indicate that autistic individuals have a similar age trajectory for postural stability and control compared to neurotypical individuals, but have lower postural stability and control overall.
Keywords: autism spectrum disorder, postural control, balance, sensorimotor, sensory reweighting, motor development
Lay Summary
Autistic and neurotypical children and adolescents performed a balance test and a body control test. Autistic participants had less stable balance than neurotypical participants. Autistic and neurotypical participants had less stable balance with their eyes closed and when wearing a dome on their head. Neurotypical participants had better body control than autistic participants. Autistic and neurotypical children had less stable balance and body control than adolescents.
Introduction
Motor differences are highly-prevalent in Autism Spectrum Disorder (autism/autistic), with estimates as high as 90% (Green et al., 2009; Hilton, Zhang, Whilte, Klohr, & Constantino, 2012, Miller, et al., 2021), and persistent across the lifespan (Bhat, Landa, & Galloway, 2011). In early childhood, autistic children exhibit motor delays (Davidovitch, Stein, Koren, & Friedman, 2018; Fulceri et al., 2019) and lower postural stability (Leezenbaum & Iverson, 2019). In the school years, autistic children often have lower scores on standardized assessments of gross- and fine-motor skills (Green et al., 2009; Hilton et al., 2012; Kaur, Srinivasan, & Bhat, 2018). As adults, autistic individuals show a wide range of differences in motor skills, from reaching (Campione, Piazza, Villa, & Molteni, 2016; Glazebrook, Elliott, & Lyons, 2006) to postural stability (Doumas, McKenna, & Murphy, 2016; Lim et al., 2018). Further, these differences in motor skills are related to differences in functional abilities (Licari et al., 2019; Travers et al., 2017). Despite clear motor skill differences and their effect on functional abilities across the lifespan in autism, there has been relatively little work to characterize the age trajectory (i.e., change across age, maturation, and experience) of specific domains of motor ability, such as postural stability and postural control.
Clinical relevance of postural stability and sensory reweighting
Decreased postural stability can affect the performance of simple tasks like quiet standing, which is heavily reliant on sensorimotor integration (Cuisinier et al., 2011; Shumway-Cook & Woollacott, 1985; Sparto et al., 2006; Woollacott et al., 1987). Autistic individuals exhibit lower postural stability during quiet standing (for review, see Lim, Partridge, Girdler, & Morris, 2017). Additionally, neuroimaging studies have demonstrated atypical sensory-motor neural connectivity in autism (Hanaie et al., 2013; Hau et al., 2021; Linke et al., 2020; Mostofsky et al., 2009; Oldehinkel et al., 2019; Thompson et al., 2017; Travers et al., 2015; Unruh et al,. 2019; Wang et al., 2019). Further, these differences in neural connectivity have been shown to be related to motor abilities in autistic individuals (Hanaie et al., 2013; Thompson et al., 2017; Travers et al., 2015; Unruh et al,. 2019; Wang et al.; 2019).
Prior studies of sensory reweighting in autistic children and adults have produced mixed results regarding the level of reliance on vision to maintain static postural stability, with some studies indicating an over-reliance on vision and others reporting no difference (Lim et al., 2017). Kohen-Raz et al. (1992) reported reduced anterioposterior stability in autistic participants aged 6–20 years during quiet standing under differing visual and proprioceptive conditions requiring sensory reweighting. Minshew, Sung, Jones, and Furman (2004) examined postural stability development in autistic children and adults in the anterior-posterior direction under different sensory conditions. Their findings indicated differences in postural stability between autistic and neurotypical individiuals across age, but they did not address potential differences in medial-lateral postural stability, or in the overall amount of postural sway exhibited under different sensory conditions. Together, these studies indicate that sensorimotor functioning in autistic individuals is different from neurotypical individuals and support the need for further investigation into the development of sensorimotor integration for postural stability in autistic individuals. A particular focus on medial-lateral stability is necessary, given that it is a stronger predictor of fall risk compared to anterior-posterior stability (Piirtola & Era, 2006). It is also a useful measure of change over time, given the clear developmental trajectory that can be observed across childhood and into adolescence and adulthood (Blanchet et al., 2019).
Clinical relevance of dynamic postural control
Dynamic postural control is necessary for activities of daily living including dressing and carrying objects (Haddad, Rietdyk, Claxton, & Huber, 2013), participation in physical activity (Stodden et al., 2008), and mobility (Le Mouel & Brette, 2017). A person’s limit of stability, or the distance that they are able to move their center of mass outside of their base of support without taking a compensatory step to regain balance, impacts their ability to perform functional movements. Dynamic postural control has been identified as a strong predictor of fall risk across multiple populations (Holbein-Jenny et al., 2007; Swanenburg, de Bruin, Uebelhart, & Mulder, 2010; Sun, Hseih, & Sosnoff, 2019), and difficulty engaging in well-controlled weight shifts has been implicated as a precursor of falling events (Robinovitch et al., 2013). Prior literature suggests that the neuromotor mechanisms of dynamic postural control differ from those of static postural control, and that dynamic instability may be a stronger driver of injury (e.g., Granacher & Gollhofer, 2011). Given the clinical relevance of dynamic postural control and the impact of limits of stability on functional movement, it is surprising that there is substantially less research on dynamic postural control compared to static postural stability in autism.
In the few studies on dynamic postural control in autism that have been reported, autistic individuals have exhibited less efficient dynamic postural control compared to neurotypical individuals (Bojanek, Wang, White, & Mosconi, 2020; Fournier et al., 2010; Miller, Caçola, Sherrod, Patterson, & Bugnariu, 2019; Wang et al., 2016). One metric of dynamic postural control efficiency is the directness of the path taken between a starting position and a target location during leaning. Autistic individuals use longer path lengths when leaning in the anteroposterior and mediolateral directions compared to neurotypical individuals (Wang et al., 2016). This finding is particularly suggestive of inefficiency given that in the same study, the limit of the length and width of postural stability was similar between autistic and neurotypical participants (Wang et al., 2016). This research demonstrates that dynamic postural control is different in autistic individuals and warrants further study.
While many dynamic postural control tasks require a large amount of space (e.g., gait analysis), assessments of a person’s limit of stability can be administered using the same equipment and space as quiet standing. This reduces barriers to the feasibility of administering it as part of a postural stability assessment battery that yields more comprehensive results than standard clinical assessments (e.g., Romberg’s Test). By removing barriers to assessments of postural control, we can diversify the individuals included in our research studies and improve their generalizablility.
Improving sample diversity and community-based assessments
The feasibility of conducting static and dynamic postural control assessments using computerized posturography with autistic individuals in community settings has not been established. While these assessments are common in some clinics (e.g., physical therapy, neurology), they are not often used outside of research labs or clinics, especially in autism. However, studies of other neuromotor disorders have demonstrated the efficacy of computerized posturography efficacy in detecting postural stability problems with higher sensitivity than common clinical tests like Romberg’s Test, even among minimally-impaired individuals (Melillo et al., 2017). Given the potential for “white coat effects” on performance in clinics (Cleworth, Inglis, & Carpenter, 2018; Geh et al., 2011), which could be more pronounced for autistic people (Gillis et al., 2009), there is a need to establish the feasibility of these assessments in detecting balance problems of autistic individuals in community settings. In addition, the utility of standardized assessments across a wide variety of settings and populations is an important consideration, given the historic limitation of autism research with respect to sample diversity and complexity of lab- and clinic-based assessment protocols.
Autism researchers have long relied primarily on white, middle-class, and highly educated families to participate in our research studies (West et al., 2016). Across 408 studies, West and colleagues (2016) found that of all autism research participants, between 2.1% and 6.8% identify as Black and between 0.8% and 2.5% identified as Hispanic. This has likely contributed to racial and ethnic disparities in autism diagnostic instruments (Harrison, Long, Tommet, & Jones, 2017), likelihood of diagnosis (Mandell et al., 2009), and use of services (Smith et al., 2020; Yingling, Bell, & Hock, 2019). This has led to a number of calls for improving the diversity in our samples by reducing barriers to participation in our studies (Jones & Mandell, 2020).
In the current studies, we partnered with local community organizations, used portable technology, and short, easy to understand study protocols translated in English and Spanish to include families that are often excluded from research (Sankare et al., 2015). Using portable technology and a short study protocol, we were able to meet families in locations close to where they live, work, and play. We recruited and tested participants in local schools, museums, community centers, public libraries, and at autism-focused community events. This played a substantial role in recruiting a much more diverse sample compared to prior studies, with more than 25% identifying as Hispanic and more than 10% identifying as Black. By including a more diverse sample, we hope to improve the generalizability of our findings beyond those typically included in autism research studies.
Current studies
The current studies examine age trajectories of static postural stability (Study 1) and dynamic postural control (Study 2). We used an established, portable, clinical assessment tool (i.e., Biodex Biosway) and a community-based recruitment and testing protocol to assess diverse samples of children, adolescents, and young adults. We performed two standardized postural control tasks, the Clinical Test of Sensory Integration and Balance (CTSIB) and a limits of stability (LOS) task. CTSIB and LOS can be administered quickly (<15 minutes) and with limited need for space. This makes them excellent for use in community settings. The goal of our community-based recruitment strategy was to lower potential barriers to participation in our science and improve our understanding of the autism community. By doing so, we were able to increase participation of individuals from races and ethnicities that are often underrepresented in autism research. Together, these two studies yielded excellent data for understanding age trajectories of postural stability and postural control in a more generalizable sample of autistic individuals.
Study 1
Despite well-documented differences in postural stability and sensory reweighting in autism, to our knowledge, only one study (Minshew et al., 2004) has specifically assessed their age trajectory. This is in stark contrast to the neurotypical literature, where the development of postural stability is well-characterized across childhood and adolescence (e.g., Assaiante et al., 2005; Bair et al., 2007; Verbecque, Vereek, & Hallemans, 2016). In this study, we examined the magnitude of postural sway and variability in postural sway in autistic children and adolescents. We used these measures to assess the development of postural stability and sensory reweighting among autistic and neurotypical children and adolescents. Based on previous findings (Minshew et al., 2004), we predicted that the age trajectory of sway variability would be similar in autistic and neurotypical individuals, but that autistic individuals would have consistently greater sway variability (indicating less overall postural stability) than their neurotypical peers. Additionally, we predicted that limiting the availability of visual context would have a greater effect on sway variability for autistic participants than neurotypical participants, reflecting atypical sensory reweighting.
Method
Participants
We tested autistic (n=27, Male=22, Female=5; Mage=12.44 years, SDage=3.18 years, range=8.20–20.59 years) and neurotypical (n=41, Male=16, Female=25; Mage=10.89 years, SDage=3.50 years, range=7.10–20.90 years) children and adolescents. Participants reported their race as American Indian/Alaska Native (1.49%), Asian (4.56%), Black/African American (14.87%), or White (74.75%). Three participants declined to report their race (4.45%). A total of 25.25% of participants reported being of Hispanic ethnicity. Autistic participants had a prior diagnosis of autism or Asperger’s syndrome from an educational or healthcare professional (e.g., school psychologist, clinical psychologist, physician) according to DSM-IV or DSM-5 criteria.
Participants were recruited by passing out flyers, sending messages via listservs, and community presentations at a local science and history museum, public libraries, local schools, and public community centers in the Dallas-Fort Worth metropolitan area of the United States. Testing occurred in private spaces at each of these locations. Faculty, staff, and students from a Department of Physical Therapy and Department of Family Medicine at the University of North Texas Health Science Center performed the testing. Diagnosis, age at diagnosis, and source of diagnosis were reported verbally by parents or self-advocates during a medical history interview conducted by the research team. Because one purpose of this study was to demonstrate the feasibility of a short community-based protocol for assessment of postural control, the research team did not independently confirm each participant’s diagnosis, as this would have required a minimum of one hour. However, prior work suggests 90% concordance between reported community diagnosis and consensus diagnosis by a research team for individuals with a diagnosis of autism or Asperger’s syndrome (Hausman-Kedem, et al., 2018). We excluded participants who could not follow 2-step directions (e.g., “Stand here, look there.”), were currently taking benzodiazepines, had consumed caffeine, tobacco, or alcohol in the previous 2 hours, or weighed less than 50 lbs (22.68 kg). This study was approved by the Institutional Review Board at the University of North Texas Health Science Center. Prior to participation, all guardians gave informed consent, and all minor participants gave assent. 69% of these participants also participated in Study 2.
Clinical Test for Sensory Integration in Balance
The Clinical Test for Sensory Integration in Balance (CTSIB) is used to assess the ability to appropriately reweight sensory information to maintain standing balance under different visual conditions (Cohen, Blatchly, & Gomash, 1993; Shumway-Cook & Horak, 1986). Participants stood on the hard surface of a portable 20Hz force plate (BioSway, Biodex Corp.; Figure 1) and remained still for a single 30-second interval in each of the following conditions: eyes open (EO), eyes closed (EC), and eyes open with a translucent paper sphere placed around the participant’s head (Dome). The EO condition enabled assessment of static postural stability when all relevant sensory inputs were available and reliable, and when a visual target was displayed at approximately eye height on a screen in front of the participant. The EC condition enabled measurement of postural stability when participants did not have visual input available, requiring greater reliance on proprioceptive and vestibular input. In the Dome condition, participants had visual input, but this input lacked functional context. Participants had to maintain stability without the ability to fixate on visuospatial cues from the environment, which would typically be used by both autistic (Molloy et al., 2013) and neurotypical people (Schärli et al., 2012) to support stability.
Figure 1.
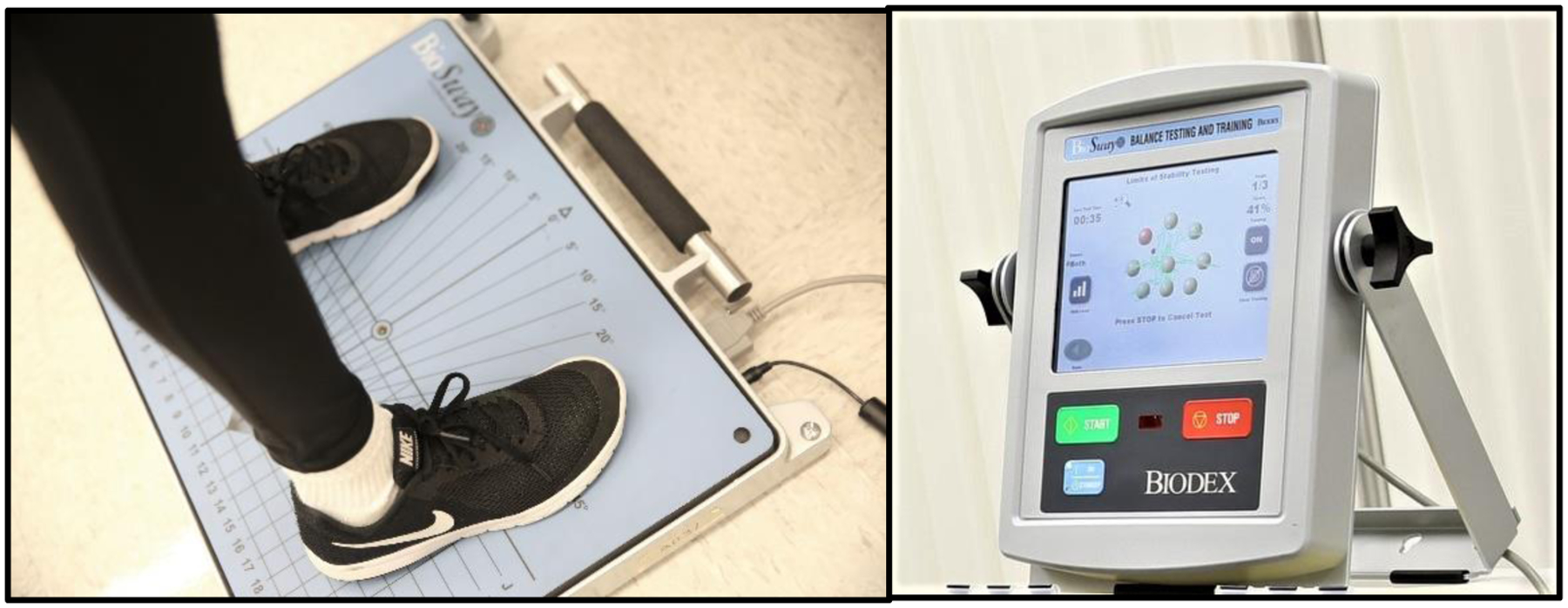
Participant standing on the portable BioSway force plate (left) and the BioSway computer displaying the Limits of Stability Task (right).
Traditionally, the CTSIB tests each of the visual conditions on a firm surface and on compliant foam (Cohen et al., 1993; Shumway-Cook & Horak, 1986). In the present study, we administered only the firm surface condition. Each participant’s foot position was standardized to the center of the force plate based on their height; any deviation from the original position was corrected prior to the start of the next condition. Individual trials in which participants did not keep their feet firmly on the surface of the force plate (e.g., took a step, raised their heels, moved their feet) were discarded and, whenever possible, readministered after correcting foot placement.
Data Analyses
The BioSway system calculates two standardized postural stability indices: Stability Index and Sway Index. We refer to these as sway magnitude and sway variability, respectively, as this more accurately reflects the nature of these indices and their particular contributions to postural stability. These indices are based on a participant’s sway angle calculated in both the X and Y directions. Sway angle is calculated by the BioSway software as the magnitude of the distance between the participant’s center of pressure and the center of the force plate, divided by 55% of the height of the individual (the estimated height of their center of mass) and scaled by a factor of 250.
Sway magnitude is the participant’s mean angular displacement from center during the trial, with higher values indicating greater displacement (Supplementary Material 1). Sway variability is the root mean square distance of the participant’s angular displacement from center (Supplementary Material 2). The higher the sway variability, the more unsteady the participant was across the duration of the trial.
Using generalized linear mixed effects models, we regressed sway magnitude and sway variability on fixed factors of group (autism, neurotypical), age (continuous), and condition (EO, EC, Dome) with a random intercept by participant (Bates, Machler, Bolker, & Walker, 2015). Sex was also tested as a factor, but we did not have any a priori hypotheses regarding sex differences and it was not statistically significant in any of our analyses. We conducted χ2-tests for fixed effects of generalized linear and linear mixed-effects models (Fox & Weisberg, 2019) and post hoc t-tests on estimated marginal means using Tukey’s method for multiple comparisons (Lenth, 2020). Estimated marginal means and standard errors are reported in response-scale, and β-weights are reported in link-scale.
Results
Sway magnitude
A generalized linear mixed-effects model with a Gamma distribution and a log link regressing sway magnitude onto group, age, and condition indicated differences by condition, but not by group, age, or their interactions (Descriptive statistics in Table 1). There was a significant main effect of condition (χ22=13.12, p=.001, Figure 2). Participants showed greater sway magnitude in the Dome (M=2.27, SE=0.19, p=.001) condition compared to the EO condition (M=1.77, SE=0.15). Sway magnitude was not significantly different between the EO and EC conditions (M=2.07, SE=0.18, p=.061) or the EC and Dome conditions (p=.361).
Table 1.
Descriptive Statistics for Postural Stability
| Condition | Autistic | Neurotypical | ||||||
|---|---|---|---|---|---|---|---|---|
| Sway Magnitude | ||||||||
| N | Mean (SD) | Median | Range | N | Mean (SD) | Median | Range | |
| Eyes open | 27 | 1.99 (1.19) | 1.63 | 0.09 – 4.70 | 41 | 2.09 (1.82) | 1.49 | 0.05 – 9.44 |
| Eyes closed | 27 | 2.52 (1.31) | 2.23 | 0.69 – 6.21 | 39 | 2.31 (1.93) | 1.85 | 0.57 – 12.13 |
| Dome | 27 | 2.88 (1.61) | 2.53 | 0.92 – 7.05 | 41 | 2.35 (1.71) | 2.09 | 0.61 – 9.78 |
| Sway Variability | ||||||||
| Eyes open | 27 | 0.98 (0.62) | 0.77 | 0.48 – 3.22 | 41 | 0.87 (0.46) | 0.71 | 0.30 – 2.34 |
| Eyes closed | 27 | 1.50 (0.57) | 1.59 | 0.64 – 2.76 | 39 | 1.19 (0.49) | 1.15 | 0.42 – 2.10 |
| Dome | 27 | 1.49 (0.79) | 1.38 | 0.59 – 4.53 | 41 | 1.26 (0.53) | 1.27 | 0.43 – 2.29 |
Figure 2.
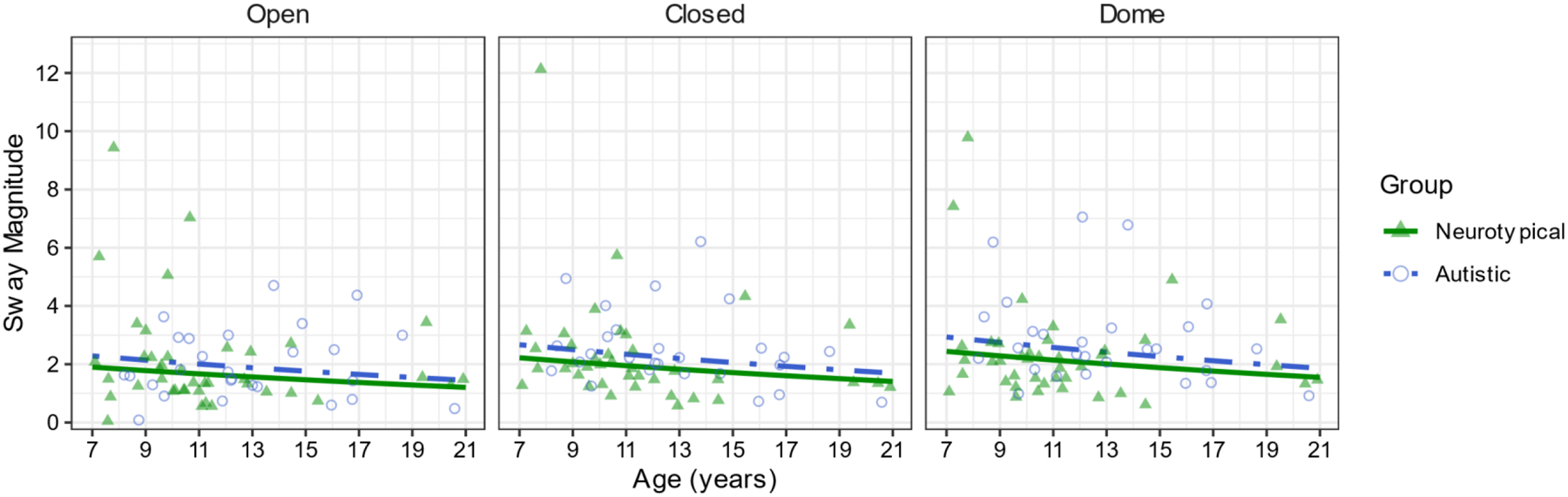
Sway magnitude differs by condition but follows a similar age trajectory in autism and neurotypical participants, with less stability in the Eyes Closed and Dome conditions compared to Eyes Open.
Sway variability
A generalized linear mixed-effects model with a Gamma distribution and a log link regressing sway variability onto group, age, and condition indicated differences within each of the factors but no interactions (Descriptive statistics in Table 1). There was a significant main effect of group (χ21=5.26, p=.022, Figure 3). Autistic participants (M=1.25, SE=0.11) showed greater sway variability compared to neurotypical participants (M=0.96, SE=0.07). There was a significant main effect of age (χ21=14.85, p.001, Figure 3), with sway variability decreasing as age increased (β=−0.06). There was a significant main effect of condition (χ22=108.87, p.001, Figure 2). Participants showed greater sway variability in the EC (M=1.24, SE=0.08, p.001) and Dome (M=1.27, SE=0.08, p.001) conditions compared to the EO condition (M=0.836, SE=0.05). Sway variability was not significantly different between the EC and Dome conditions (p=.860).
Figure 3.
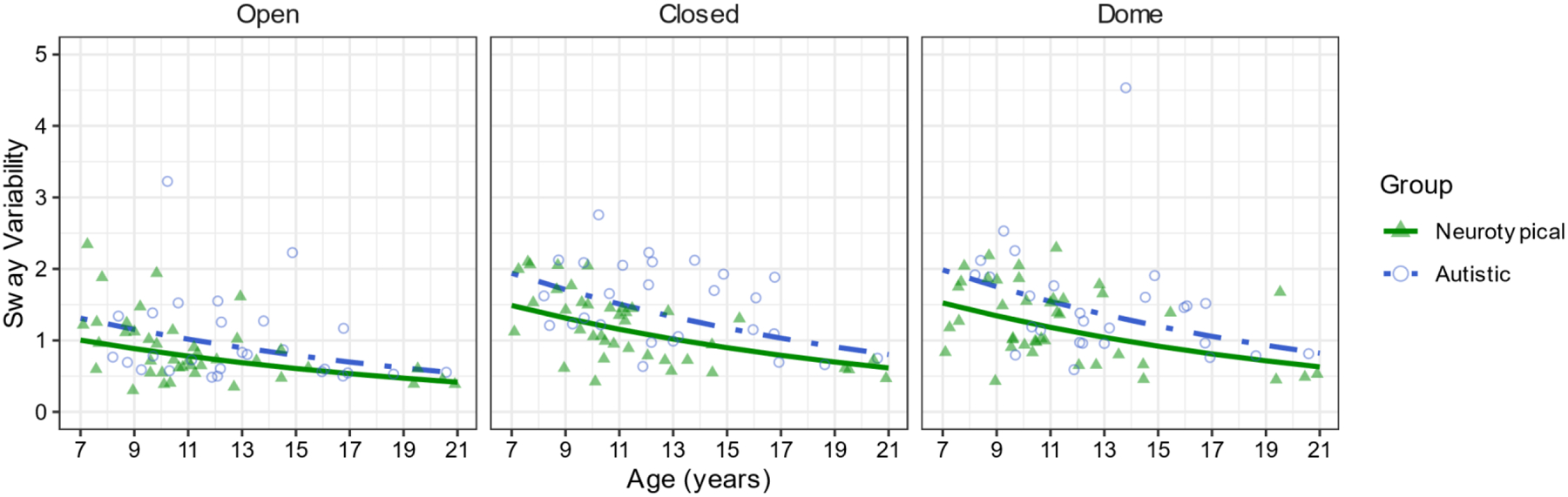
Sway variability differs by group but follows a similar age trajectory in autistic and neurotypical participants, such that sway generally decreases with age and increases with condition difficulty.
Discussion
Postural stability is critical for a wide range of activities of daily living, including those necessary for independence (Melzer et al., 2004). There is a gap in the current literature with respect to the age trajectory of postural stability in autism through childhood and adolescence, despite similar work in neurotypical children (Assaiante et al., 2005). We addressed this gap by assessing postural stability and sensory reweighting in autistic and neurotypical children and adolescents. Our findings demonstrate that autistic individuals have lower postural stability than neurotypical individuals throughout childhood and adolescence. Additionally, our findings indicate that these differences are consistent across visual conditions.
Postural stability decreases with changes or loss of visual information
Both autistic and neurotypical participants showed differing levels of postural stability across the CTSIB conditions. Participants’ sway magnitude was greater in the EC and Dome conditions compared to the EO condition. This indicates that both groups had difficulty reweighting their reliance on vision to maintain postural stability based on the lack of visual information (EC condition) or the quality of the visual information they were receiving (Dome condition).
By examining sway variability within each of the conditions (EO, EC, Dome), we characterized differences in postural stability between autistic and neurotypical participants. As expected, autistic participants demonstrated lower postural stability than neurotypical participants, suggesting that their internal feedforward models of postural control were disrupted. However, contrary to our hypothesis and previous findings (Bucci et al., 2017; Doumas et al., 2016; Goh et al., 2018), the differences in postural stability that we observed between autistic and neurotypical participants were consistent across the three visual conditions. These findings indicate that autistic participants did not rely more heavily on vision to support postural stability than neurotypical participants, suggesting that sensory reweighting ability may not have been different between groups. However, autistic individuals showed increased sway magnitude and variability across the eyes open, eyes closed, and dome conditions, suggesting that they experience typical effects of disrupted feedback information on postural stability. There is a precedent for this, as some previous findings have shown that autistic participants do not rely more on vision for postural stability compared to neurotypical participants (Morris et al., 2015; Lim et al., 2018). While autistic participants’ sway magnitude was not statistically-significantly different from that of neurotypical participants, it was numerically higher across all conditions, with the greatest mean difference in the Dome condition, where visual conflict created the greatest disruption to feedback visual information. This adds to the mixed literature on autistic people’s ability to integrate visual information into postural control. Studies with only visual manipulations, including the current study, have not detected an increased reliance on vision in autistic individuals. In contrast, studies with standing surface manipulations (Bucci et al., 2017; Doumas et al., 2016) or increased cognitive demands (Goh et al., 2018) have detected an overreliance on vision in autistic individuals. Further research is needed to determine if the reweighting differences reported in other studies only manifest under more difficult task conditions (e.g., when standing on a compliant surface, reducing the availability of reliable proprioceptive input) or are only evident in a subset of autistic individuals.
Age differences in sway variability between autistic and neurotypical individuals
Autistic participants showed more sway variability across conditions from childhood into adolescence compared to neurotypical individuals. This indicates that autistic participants not only show differences in postural stability in childhood, but that these differences continue through adolescence. This is similar to previous findings that indicate that autistic individuals have delayed postural stability development (Minshew et al., 2004) and that neurotypical individuals’ postural stability continues to develop through adolescence and into adulthood (Goulème, et al., 2018; Shams et al, 2020).
These findings indicate that autistic and neurotypical individuals follow similar age trajectories of postural stability, but autistic individuals consistently demonstrate lower postural stability as measured by sway variability. Further, these findings suggest that postural stability continues to develop throughout the second decade of life, beyond the age range of many studies of the development of postural stability. This may be a specific consequence of motor development, a reflection of the influence of cognitive development (e.g., attention), or a combination of factors. More research is needed to further elucidate the mechanisms underlying the development of postural stability in adolescence and early adulthood.
Study 2
While static postural stability is an important building-block skill for development of other motor abilities, dynamic postural control is a stronger predictor of fall risk and functional mobility (Holbein-Jenny et al., 2007; Le Mouel & Brette, 2017; Robinovitch et al., 2013). Thus, it is necessary to assess both static and dynamic stability in order to fully understand the nature of postural control in autism. Here, we examined the spatial and temporal efficiency of movements during a dynamic postural control task. We used these measures to assess the development of dynamic postural control among autistic and neurotypical children and adolescents. Based on Miller et al. (2018), we predicted that autistic individuals would have consistently less efficient postural control than their neurotypical peers.
Method
Participants
We tested autistic (n=21, Male=19, Female=2; Mage=12.73 years, SDage=2.90 years, range=8.20–16.93 years) and neurotypical (n=32, Male=13, Female=19; Mage=10.60 years, SDage=1.94 years, range=7.42–15.46 years) children and adolescents. Participants reported their race as American Indian/Alaska Native (1.92%), Asian (1.92%), Black/African American (7.69%), or White (86.54%). Some participants declined to report their race (1.92%). Additionally, 26.92% of participants reported being of Hispanic ethnicity. The same diagnostic and exclusion criteria as Study 1 were used, as were the same IRB approval, consent, and assent processes. 90% of the participants in this study also participated in Study 1.
Limits of Stability
The Limits of Stability task (LOS) is used to assess dynamic postural control by measuring how well a person can control their center of gravity outside of their base of support, without needing to move their feet or take a compensatory step to regain balance. Participants stood on the hard surface of a portable 20Hz force plate (BioSway, Biodex Corp., Figure 1). Targets and a user-controlled object were displayed at approximately eye-height on a screen in front of the participant. The participant used their center of gravity to move the user-controlled object to target locations by leaning their body without moving their feet (Figure 4). The LOS task includes a single trial for each of the nine target locations: eight at 75% of the typical limit of stability (6°: Forward, Forward Left, Forward Right, Left, Right; 4.5°: Backward Left, Backward Right; 3°: Backward), and one target at 0° (Center). Participants’ foot position was recorded prior to data collection; any deviation from the original position was corrected prior to the start of the next target. Individual trials in which participants did not keep their feet firmly on the surface of the force plate (e.g., took a step, raised their heels, moved their feet) were discarded and, whenever possible, readministered after correcting foot placement.
Figure 4.
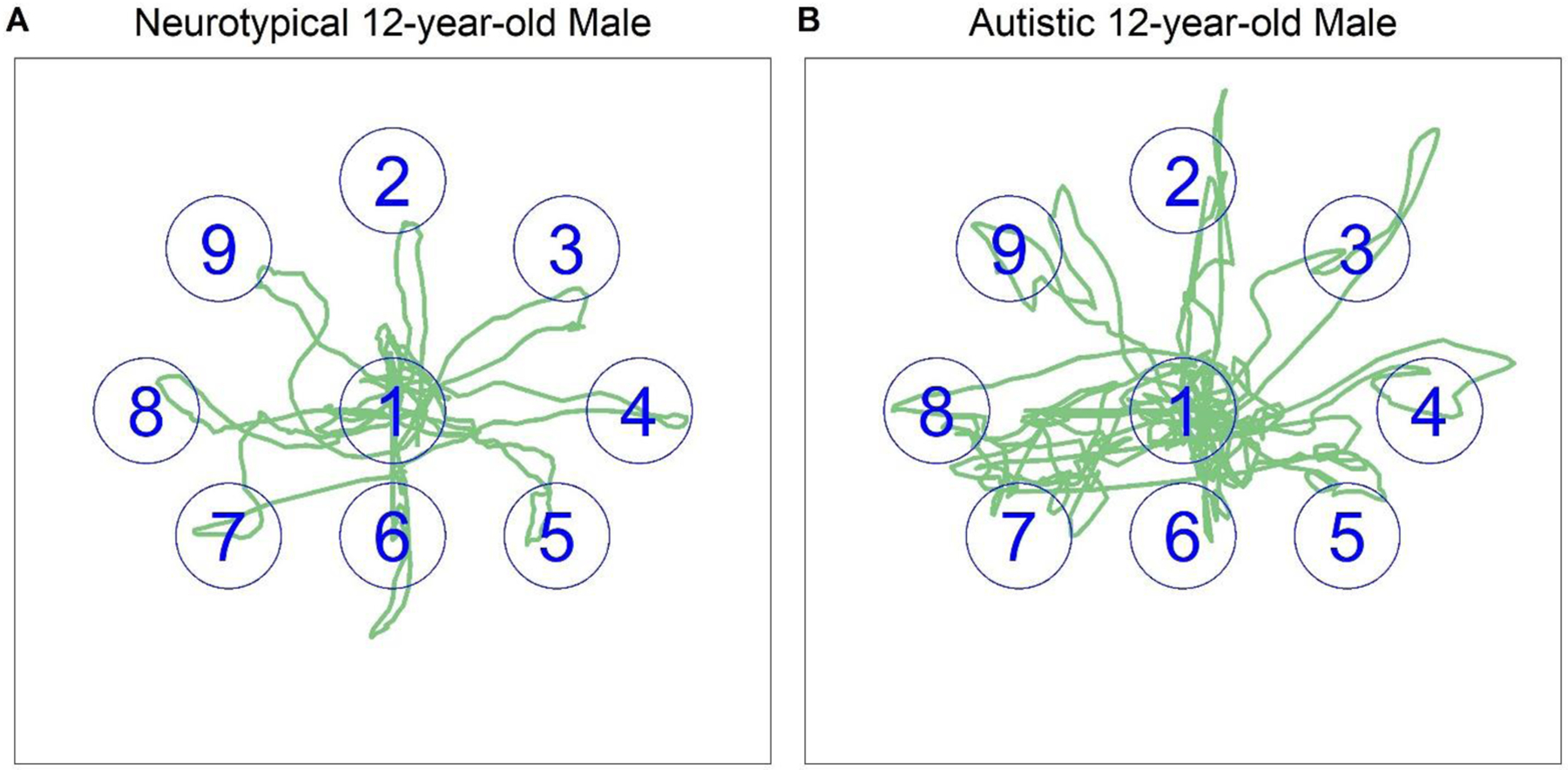
Sway plots of 12-year-old (A) neurotypical and (B) autistic male participants during the Limits of Stability Task. 1=Center, 2=Forward, 3= Forward/Right, 4=Right, 5=Backward/Right, 6=Backward, 7=Backward/Left, 8=Left, 9=Forward/Left. Note: Target position is correct, but size is approximated for plotting purposes.
Data Analysis
The BioSway system records the total task completion time (completion time) and calculates an efficiency index (movement efficiency). Movement efficiency, as defined by the BioSway system, is the straight-line distance from the center of the force plate to the target divided by the actual distance traveled by the participant to the target (Supplementary Material 3). Higher movement efficiency scores indicate more direct movement to targets. Completion time and movement efficiency were analyzed with the same approach as Study 1.
Results
Completion Time
A generalized linear model with a Gamma distribution and a log link was used to regress completion time onto group and age (Descriptive statistics in Table 2). There was a main effect of group (χ21= 13.90, p.001, Figure 5). Autistic participants (M=57.21, SE=3.99) used more time to complete the LOS task compared to neurotypical participants (M=40.59, SE=2.19). There was also a main effect of age (χ21=12.77, β=−0.06, p <.001, Figure 5), such that as participants’ age increased, the time they used to complete the LOS task decreased.
Table 2.
Descriptive Statistics for Completion Time
| Group | N | Mean (SD) | Median | Range |
|---|---|---|---|---|
| Neurotypical | 32 | 42.94 (11.24) | 40 | 28 – 77 |
| Autistic | 20 | 53.7 (21.84) | 46.5 | 30 – 95 |
Figure 5.
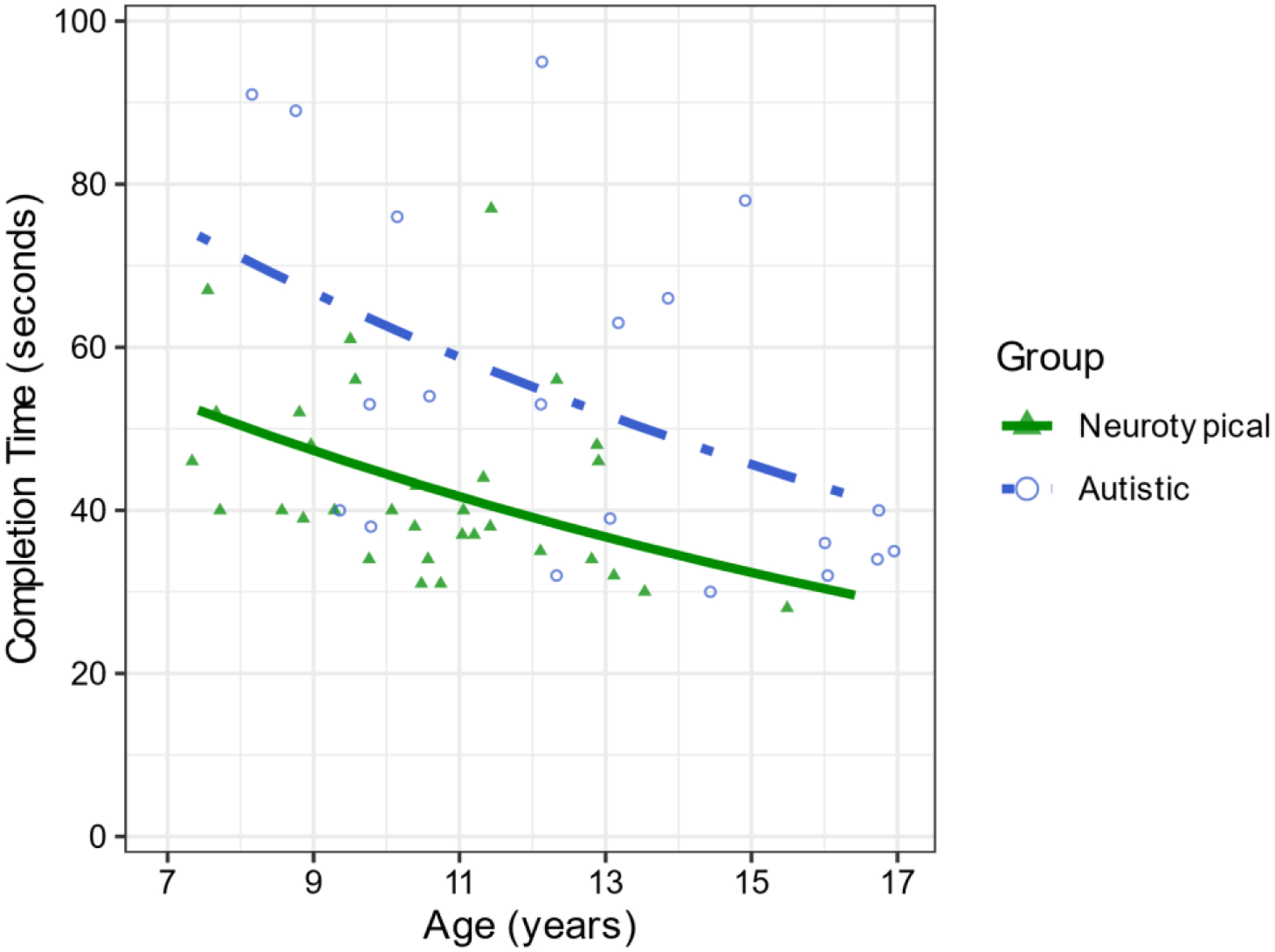
Autistic participants used more time to complete the LOS task compared to neurotypical participants, and both groups used less time as age increased.
Movement Efficiency
A linear mixed effects model was used to regress movement efficiency onto group, age, and target (center, forward, forward/right, right, backward/right, backward, backward/left, left, forward/left) with a random intercept by participant (Descriptive statistics in Table 3). There was a main effect of group (χ21=20.08, p.001, Figure 6). Neurotypical participants (M=51.88, SE=2.16) were more efficient when moving to targets than autistic participants (M=35.84, SE=2.80). There was a main effect of age (χ21=34.55, β=4.05, p.001), such that as participants’ age increased their movements were more efficient. There was a main effect of target (χ28=140.25, p.001, Figure 6). See Table 4 for target pairwise comparisons.
Table 3.
Descriptive Statistic for Movement Efficiency
| Autistic | Neurotypical | |||||||
|---|---|---|---|---|---|---|---|---|
| Target | N | Mean (SD) | Median | Range | N | Mean (SD) | Median | Range |
| Forward/Left | 20 | 47.8 (21.29) | 44.5 | 14 – 87 | 32 | 58.03 (17.68) | 58 | 26–91 |
| Forward | 20 | 39.35 (22.31) | 40.5 | 11–81 | 32 | 43.16 (14.71) | 43.5 | 10–67 |
| Forward/Right | 20 | 50.8 (18.75) | 46 | 17 – 85 | 32 | 55.69 (18.71) | 56.5 | 20–87 |
| Left | 20 | 33.8 (18.10) | 31.5 | 11–66 | 32 | 42.28 (14.88) | 42 | 19–78 |
| Center | 20 | 31.6 (19.81) | 27 | 8–66 | 32 | 40.72 (20.52) | 34.5 | 11–92 |
| Right | 20 | 47.85 (20.23) | 49 | 10–93 | 32 | 54.75 (13.77) | 53.5 | 33–90 |
| Backward/Left | 20 | 34.05 (21.68) | 27 | 5–80 | 32 | 43.16 (19.46) | 44.5 | 6–77 |
| Backward | 20 | 39.8 (19.97) | 34.5 | 13–77 | 32 | 50.78 (16.76) | 46.5 | 23–85 |
| Backward/Right | 20 | 45.55 (23.28) | 42.5 | 12–88 | 32 | 48.34 (16.68) | 49 | 16–82 |
Figure 6.
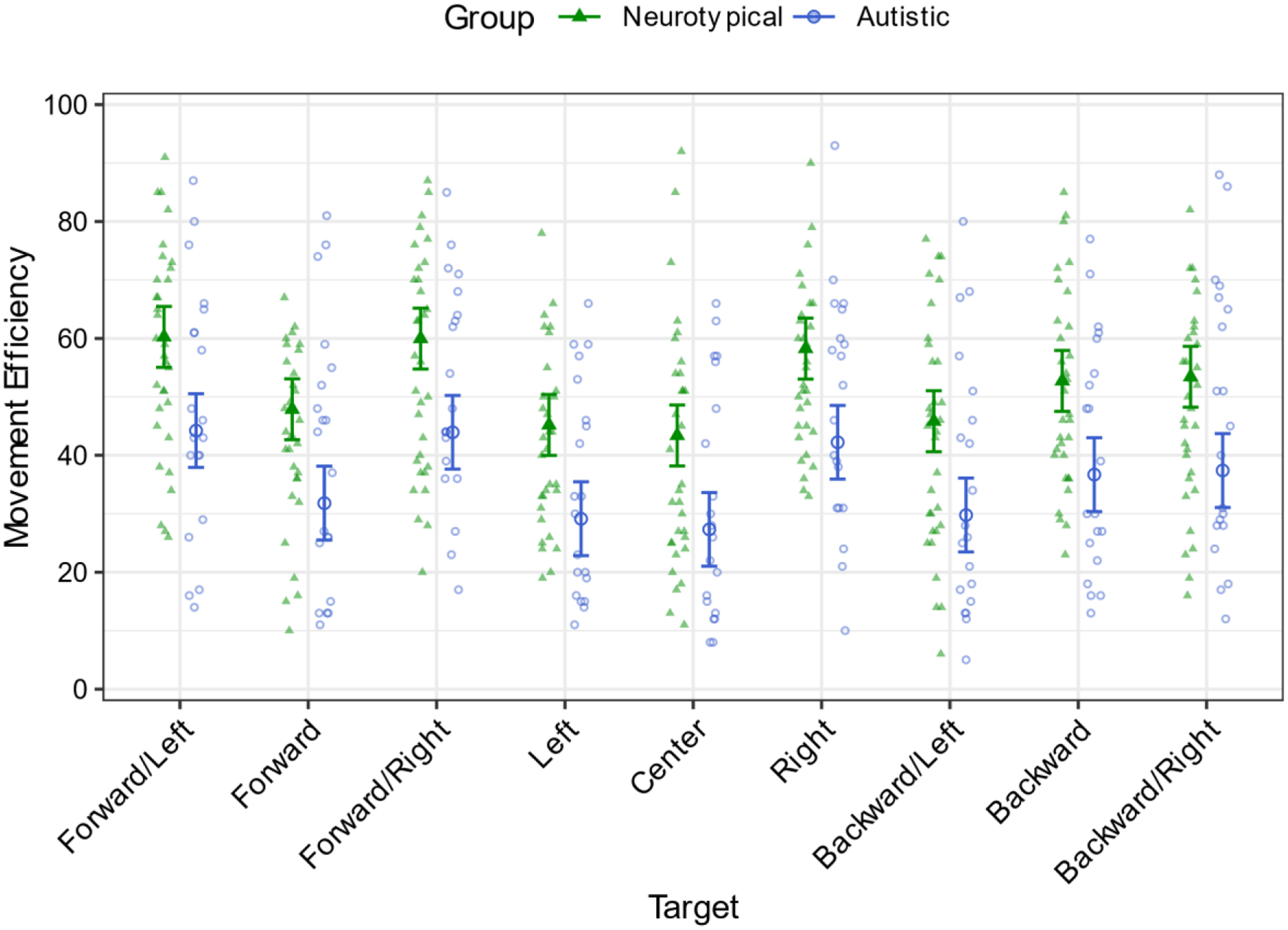
Autistic participants made less efficient movements to each of the targets compared to neurotypical participants. Error bars indicate 95% confidence limits.
Table 4.
Pairwise comparisons of Movement Efficiency between targets
| Target Contrast | Estimated Mean Difference | t ratio | p value |
|---|---|---|---|
| Forward/Left – Forward* | 12.40 | 5.50 | <.001 |
| Forward/Left - Forward/Right | 0.29 | 0.13 | 1.000 |
| Forward/Left - Left* | 15.08 | 6.69 | <.001 |
| Forward/Left - Center* | 16.88 | 7.49 | <.001 |
| Forward/Left – Right | 2.00 | 0.89 | .994 |
| Forward/Left - Backward/Left* | 14.44 | 6.41 | <.001 |
| Forward/Left - Backward* | 7.54 | 3.34 | .025 |
| Forward/Left - Backward/Right | 6.83 | 3.03 | .065 |
| Forward - Forward/Right* | −12.12 | −5.37 | <.001 |
| Forward – Left | 2.67 | 1.19 | .959 |
| Forward – Center | 4.48 | 1.99 | .553 |
| Forward - Right* | −10.40 | −4.61 | <.001 |
| Forward - Backward/Left | 2.04 | 0.90 | .993 |
| Forward - Backward | −4.87 | −2.16 | .435 |
| Forward - Backward/Right | −5.58 | −2.47 | .248 |
| Forward/Right - Left* | 14.79 | 6.56 | <.001 |
| Forward/Right - Center* | 16.60 | 7.36 | <.001 |
| Forward/Right – Right | 1.71 | 0.76 | .998 |
| Forward/Right - Backward/Left* | 14.15 | 6.28 | <.001 |
| Forward/Right - Backward* | 7.25 | 3.22 | .037 |
| Forward/Right - Backward/Right | 6.54 | 2.90 | .092 |
| Left – Center | 1.81 | 0.80 | .997 |
| Left - Right* | −13.08 | −5.80 | <.001 |
| Left - Backward/Left | −0.63 | −0.28 | 1.000 |
| Left - Backward* | −7.54 | −3.34 | .025 |
| Left - Backward/Right* | −8.25 | −3.66 | .009 |
| Center - Right* | −14.88 | −6.60 | <.001 |
| Center - Backward/Left | −2.44 | −1.08 | .976 |
| Center - Backward* | −9.35 | −4.15 | .001 |
| Center - Backward/Right* | −10.06 | −4.46 | <.001 |
| Right - Backward/Left* | 12.44 | 5.52 | <.001 |
| Right – Backward | 5.54 | 2.46 | .257 |
| Right - Backward/Right | 4.83 | 2.14 | .447 |
| Backward/Left - Backward | −6.90 | −3.06 | .059 |
| Backward/Left - Backward/Right* | −7.62 | −3.38 | .022 |
| Backward - Backward/Right | −0.71 | −0.32 | 1.000 |
Note. Standard error was 2.25 and degrees of freedom were 424.16 for all pairwise comparisons.
indicates p.05.
Discussion
Many daily living activities rely heavily on dynamic postural control (Haddad et al., 2013). Despite this importance, there is little research examining dynamic postural control in autistic individuals relative to the body of work on static postural control (Lim et al., 2017). We addressed this gap by assessing dynamic postural control in autistic and neurotypical children and adolescents. We present data demonstrating that autistic individuals use less efficient dynamic postural control compared to neurotypical individuals across late childhood and adolescence.
Decreased movement efficiency during dynamic postural tasks
Autistic individuals took ~25% more time to complete the Limits of Stability task compared to neurotypical individuals, and were notably less efficient in their paths to target positions. This was likely due to differences in overall dynamic postural control and the motor strategies autistic individuals use to compensate for these differences (Bojanek, et al 2020; Fournier et al., 2010; Miller et al., 2021). In previous work, Bojanek and colleagues demonstrated that autistic individuals constrain their postural sway by increasing the rigidity of their movements. This allows maintenance of balance, but limits movement efficiency during dynamic tasks (Bojanek et al., 2020). Difficulty with dynamic postural control in autism may be due to well-documented differences in underlying neural networks controlling movements. Specifically, the results we observed in the Limits of Stability task implicate feedback mechanisms of postural control, since participants were able to see their path represented on the screen in real-time as their user-controlled object and the trail drawn behind it. Other studies have also documented disrupted feedback integration of visual information in autism, specifically in tasks using a user-controlled cursor on a screen to represent participants’ movement (Miller et al., 2019; Miller et al., 2022; Mosconi et al., 2015). Decreased movement efficiency may indicate decreased neural connectivity between brain regions necessary for motor planning and modification of movements (Oldehinkle et al., 2020; Travers et al., 2015).
Age differences in dynamic postural control
Both autistic and neurotypical individuals used more efficient movements as age increased. Although autistic individuals used more efficient movements as age increased, their movements were continuously less efficient than that of neurotypical individuals. Thus, although autistic individuals follow expected age trajectories for motor control, they lag behind their neurotypical peers. Further research should investigate whether dynamic postural control is a malleable target for motor intervention in autism that could close the gap in motor development.
General Discussion
Research on motor difficulties in autism has received renewed attention, with existing literature suggesting the presence of differences between autistic and neurotypical development in motor abilities (Fuentes, Mostofsky, & Bastian, 2009; Mosconi et al., 2015) and their underlying neural mechanisms (Oldehinkel et al., 2020; Unruh et al., 2019; Wang et al., 2019). Although these studies demonstrate that motor difficulties in autism persist across childhood, adolescence, and adulthood (Bhat et al., 2011), there remains a dearth of literature on the long-term functional impact of motor problems or on their age trajectories. Here, we present two studies with community-derived samples demonstrating that autistic individuals (1) have lower static postural stability and (2) use less efficient dynamic postural control compared to neurotypical individuals across childhood and adolescence. Together, we find that autistic individuals show a similar, but delayed trajectory of postural control development. Specifically, autistic individuals appear to lag behind their neurotypical peers by approximately 4 years. These findings shed light on the urgent need to intervene and support autistic individuals postural control development.
Our results suggest that there are developmental differences in static and dynamic postural control, but that autistic individuals’ postural control problems persist into adulthood and do not simply resolve over time. Given these data, it is concerning that only an estimated 19.5% of autistic individuals receive physical therapy, and only 48.5% receive occupational therapy (largely in the context of academic skills like handwriting or self-care skills like dressing) (Zablotsky et al., 2015). Many of those who do receive physical therapy typically discontinue services after early motor milestones are met (e.g., crawling, standing, walking) and physical therapy service utilization drops sharply as age increases (Cidav et al., 2013), despite the fact that motor problems persist.
Young preschool or school-aged children may benefit from both physical therapy and naturalistic opportunities for motor learning focused on building robust internal models of postural control, including recreational activities and sport (Ajzenman, Standeven, & Shurtleff, 2013; Kim et al., 2016; Salvador-Garcia, Valverde-Esteve, Chiva-Bartoll, & Marave-Vivas, 2022). In contrast, adolescents, young adults, and older adults may need interventions focused on explicitly teaching strategies for engaging in more effective sensory reweighting. These might include clinic-based physical therapy or fitness training to build general strength and stability, occupational therapy specifically tailored to individuals’ activities of daily living (Ozkan & Aki, 2021), and exergaming in virtual reality (Pacheco et al., 2020).
Previous work in other clinical populations demonstrates a relationship between low postural stability, limitations in activities of daily living, and reduced quality of life (e.g., cerebral palsy; Pavão, Dos Santos, de Oliveira, & Rocha, 2014; stroke, Hsieh, Sheu, Hsueh, & Wang, 2002), and low postural stability is predictive of fall risk in older adulthood (Melzer et al., 2004; Swanenburg, de Bruin, Uebelhart, & Mulder, 2010). There is little published literature on the specific relationship between postural control and activities of daily living in autism, but emerging work suggests that low postural stability is associated with poorer performance on activities of daily living, particularly for individuals with lower-than-average IQ (Fisher et al., 2018). Research is needed to determine if there is a relationship between postural stability, daily living skills, and quality of life in autism.
We used a community-based assessment approach to reduce barriers to participation (George, Duran, & Norris, 2014; Luebbert & Perez, 2015). This approach substantially increased the racial and ethnic diversity of the current samples compared to previous autism studies, with nearly twice as many Black participants and ten times as many Hispanic participants reported in other samples (West et al., 2016). This community-based approach can be used to expand the breadth of those included in autism research, improving access for participants from under-represented racial and ethnic backgrounds, lower socioeconomic backgrounds, or with limited transportation or childcare. By using short, easily-understood study protocols that limit the cognitive demands and level of language required to participate, we can also include participants with a wider range of intellectual abilities and co-occurring conditions. Thus, study samples will be more reflective of the broader autism population, including those whose resources and abilities may have prevented them from participating in traditional lab- or clinic-based research. This will further improve the generalizability of autism research and improve evidence-based clinical care.
Future research should continue to build upon the current protocol to investigate the relations between static and dynamic postural control in other areas, such as general motor performance or adaptive behavior. Recent research has indicated that general motor performance is a strong predictor of adaptive behavior in autistic individuals (Fears et al., 2022), but it is not clear the role that postural control specifically plays in this relationship. Postural control may also be independently related to general motor performance as postural control provides a stable foundation for many movements. A strength of the current protocol was its brevity, but due to this we were also limited in the types of assessments we could collect and are unable to draw conclusions regarding postural control, general motor performance, and adaptive behaviors. Prior studies suggest that 38–63% of autistic children aged 2–18 years have hypotonia (Ming, Brimacombe, & Wagner, 2007), and 9–21% have either hypertonia or a combination of hypotonia and hypertonia (Paquet et al., 2016). Dysregulated muscle tone (i.e., dystonia) is associated with atypical cerebellar function, and in turn, with both disrupted feedforward and feedback models of postural control in autism (Mosconi et al., 2015). Direct measurement of muscle tone in future studies could help to determine whether the static postural instability and dynamic postural control inefficiency differs among individuals with typical, reduced, or increased tone. Direct measurement of muscle activation through methods such as surface electromyography could also provide insight into the time course of muscle activation during visually-guided tasks, further clarifying the relative influence of feedforward and feedback control.
Static postural stability and dynamic postural control are a clinically-relevant issues that may hinder an individual’s ability to efficiently and effectively complete activities of daily living (for review, see Haddad et al., 2013). It is important to characterize the age trajectory of postural stability and control in autistic children and adolescents, both to inform interventions that target these building-block skills, and to support public policies and clinical practice guidelines that facilitate access to motor intervention across the lifespan.
Supplementary Material
Acknowledgements:
Out of respect for preferences expressed by many autistic self-advocates in our studies and in the community, we have chosen to use identity-first (rather than person-first) language throughout this manuscript. In doing so, it is not our intention to diminish or invalidate the preferences or perspectives of those who prefer person-first language. We continue to welcome feedback on ways that we can effectively partner with the autistic community to advocate for respect, acceptance, inclusion, and representation in research.
Funding:
This work was supported in part by National Science Foundation (SMA-1514495), National Institutes of Health/National Institute of Clinical and Translational Sciences (KL2-TR001103; UL1-TR001105), and National Institutes of Health/National Institute of Mental Health (K01-MH107774).
References
- Ajzenman H,F, Standeven JW, Shurtleff TL (2013). Effect of Hippotherapy on Motor Control, Adaptive Behaviors, and Participation in Children With Autism Spectrum Disorder: A Pilot Study. Am J Occup Ther, 67(6), 653–663. doi: 10.5014/ajot.2013.008383 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Autism Spectrum Disorder. In Diagnostic and Statistical Manual of Mental Disorders. (5 ed.). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Assaiante C, & Amblard B (1995). An ontogenetic model for the sensorimotor organization of balance control in humans. Human Movement Science, 14(1), 13–43. doi: 10.1016/0167-9457(94)00048-j [DOI] [Google Scholar]
- Assaiante C, Mallau S, Viel S, Jover M, & Schmitz C (2005). Development of postural control in healthy children: a functional approach. Neural Plasticity, 12(2–3), 109–118; discussion 263–172. doi: 10.1155/NP.2005.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair WN, Kiemel T, Jeka JJ, & Clark JE (2007). Development of multisensory reweighting for posture control in children. Experimental Brain Research, 183(4), 435–446. doi: 10.1007/s00221-007-1057-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barela JA, Jeka JJ, & Clark JE (2003). Postural control in children. Coupling to dynamic somatosensory information. Experimental Brain Research, 150(4), 434–442. doi: 10.1007/s00221-003-1441-5 [DOI] [PubMed] [Google Scholar]
- Bates D, Machler M, Bolker BM, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bertenthal B, & Von Hofsten C (1998). Eye, head and trunk control: the foundation for manual development. Neuroscience and Biobehavioral Reviews, 22(4), 515–520. doi: 10.1016/s0149-7634(97)00038-9 [DOI] [PubMed] [Google Scholar]
- Bhat AN, Landa RJ, & Galloway JC (2011). Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Physical Therapy, 91(7), 1116–1129. doi: 10.2522/ptj.20100294 [DOI] [PubMed] [Google Scholar]
- Blanchard Y, Carey S, Coffey J, Cohen A, Harris T, Michlik S, & Pellecchia GL (2005). The influence of concurrent cognitive tasks on postural sway in children. Pediatric Physical Therapy, 17(3), 189–193. doi: 10.1097/01.pep.0000176578.57147.5d [DOI] [PubMed] [Google Scholar]
- Blanchet M, Prince F, & Messier J (2019). Development of postural stability limits: Anteroposterior and mediolateral postural adjustment mechanisms do not follow the same maturation process. Human movement science, 63, 164–171. 10.1016/j.humov.2018.11.016 [DOI] [PubMed] [Google Scholar]
- Bojanek EK, Wang Z, White SP, & Mosconi MW (2020). Postural control processes during standing and step initiation in autism spectrum disorder. Journal of Neurodevelopmental Disorders, 12(1), 1. doi: 10.1186/s11689-019-9305-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci MP, Gouleme N, Stordeur C, Acquaviva E, Scheid I, Lefebvre A, … Delorme R (2017). Discriminant validity of spatial and temporal postural index in children with neurodevelopmental disorders. International Journal of Developmental Neuroscience, 61, 51–57. doi: 10.1016/j.ijdevneu.2017.06.010 [DOI] [PubMed] [Google Scholar]
- Campione GC, Piazza C, Villa L, & Molteni M (2016). Three-dimensional kinematic analysis of prehension movements in young children with Autism Spectrum Disorder: New insights on motor impairment. Journal of Autism and Developmental Disorders, 46(6), 1985–1999. doi: 10.1007/s10803-016-2732-6 [DOI] [PubMed] [Google Scholar]
- Chow CK, & Jacobson DH (1972). Further studies of human locomotion: Postural stability and control. Mathematical Biosciences, 15, 93–108. [Google Scholar]
- Cidav Z, Lawer L, Marcus SC, & Mandell DS (2013). Age-related variation in health service use and associated expenditures among children with autism. Journal of autism and developmental disorders, 43(4), 924–931. 10.1007/s10803-012-1637-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleworth TW, Inglis JT, & Carpenter MG (2018). Postural threat influences the conscious perception of body position during voluntary leaning. Gait & Posture, 66, 21–25. 10.1016/j.gaitpost.2018.08.003 [DOI] [PubMed] [Google Scholar]
- Cohen H, Blatchly CA, & Gombash LL (1993). A study of the clinical test of sensory interaction and balance. Physical Therapy, 73(6), 346–351. doi: 10.1093/ptj/73.6.346 [DOI] [PubMed] [Google Scholar]
- Cuisinier R, Olivier I, Vaugoyeau M, Nougier V, & Assaiante C (2011). Reweighting of sensory inputs to control quiet standing in children from 7 to 11 and in adults. Plos One, 6(5), e19697. doi: 10.1371/journal.pone.0019697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovitch M, Stein N, Koren G, & Friedman BC (2018). Deviations from typical developmental trajectories detectable at 9 months of age in low risk children later diagnosed with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 48(8), 2854–2869. doi: 10.1007/s10803-018-3549-2 [DOI] [PubMed] [Google Scholar]
- D’Mello AM, Crocetti D, Mostofsky SH, & Stoodley CJ (2015). Cerebellar gray matter and lobular volumes correlate with core autism symptoms. Neuroimage Clinical, 7, 631–639. doi: 10.1016/j.nicl.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumas M, McKenna R, & Murphy B (2016). Postural control deficits in Autism Spectrum Disorder: The role of sensory integration. Journal of Autism and Developmental Disorders, 46(3), 853–861. doi: 10.1007/s10803-015-2621-4 [DOI] [PubMed] [Google Scholar]
- Fears NE, Palmer SA, & Miller HL (2022). Motor skills predict adaptive behavior in autistic children and adolescents. Autism Research, 15(6), 1083–1089. 10.1002/aur.2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fears NE, Templin T, Sherrod GM, Bugnariu N, Patterson R, & Miller HL (2022). Autistic children use less efficient goal-directed whole body movements compared to neurotypical development. Journal of Autism & Developmental Disorders. DOI: 10.1007/s10803-022-05523-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S (2019). An R Companion to Applied Regression, Third edition. Sage, Thousand Oaks, CA. [Google Scholar]
- Fisher A, Engel C, Geist R, Lillie K, Lutman S, & Travers BG (2018). Brief report: Postural balance and daily living skills in children and adolescents with autism. Journal of Autism and Developmental Disorders, 48(9), 3210–3215. 10.1007/s10803-018-3558-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier KA, Kimberg CI, Radonovich KJ, Tillman MD, Chow JW, Lewis MH, … Hass CJ (2010). Decreased static and dynamic postural control in children with autism spectrum disorders. Gait & Posture, 32(1), 6–9. doi: 10.1016/j.gaitpost.2010.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes CT, Mostofsky SH, & Bastian AJ (2009). Children with autism show specific handwriting impairments. Neurology, 73(19), 1532–1537. doi: 10.1212/wnl.0b013e3181c0d48c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulceri F, Grossi E, Contaldo A, Narzisi A, Apicella F, Parrini I, … Muratori F (2019). Motor skills as moderators of core symptoms in Autism Spectrum Disorders: Preliminary data from an exploratory analysis With artificial neural networks. Frontiers in psychology, 9, 2683. doi: 10.3389/fpsyg.2018.02683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geh CL, Beauchamp MR, Crocker PR, & Carpenter MG (2011). Assessed and distressed: white-coat effects on clinical balance performance. Journal of psychosomatic research, 70(1), 45–51. 10.1016/j.jpsychores.2010.09.008 [DOI] [PubMed] [Google Scholar]
- George S, Duran N, & Norris K (2014). A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. American Journal of Public Health, 104(2), e16–e31. 10.2105/AJPH.2013.301706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis JM, Hammond Natof T, Lockshin SB, Romanczyk RG (2009). Fear of routine physical exams in children with Autism Spectrum Disorders: Prevalence and intervention effectiveness. Focus on Autism and Other Developmental Disabilities, 24(3):156–168. doi: 10.1177/1088357609338477 [DOI] [Google Scholar]
- Glazebrook CM, Elliott D, & Lyons J (2006). A kinematic analysis of how young adults with and without autism plan and control goal-directed movements. Motor Control, 10, 244–264. doi: 10.1123/mcj.10.3.244 [DOI] [PubMed] [Google Scholar]
- Goh KL, Morris S, Parsons R, Ring A, & Tan T (2018). Postural and cortical responses following visual occlusion in adults with and without ASD. Journal of Autism and Developmental Disorders, 48(5), 1446–1457. doi: 10.1007/s10803-017-3405-9 [DOI] [PubMed] [Google Scholar]
- Goulème N, Debue M, Spruyt K, Vanderveken C, De Siati RD, Ortega-Solis J, Petrossi J, Wiener-Vacher S, Bucci MP, Ionescu E, Thai-Van H, & Deggouj N (2018) Changes of spatial and temporal characteristics of dynamic postural control in children with typical neurodevelopment with age: Results of a multicenter pediatric study. International Journal of Pediatric Otorhinolaryngology, 113, 272–280. 10.1016/j.ijporl.2018.08.005 [DOI] [PubMed] [Google Scholar]
- Granacher U, & Gollhofer A (2011). Is there an association between variables of postural control and strength in adolescents?. The Journal of Strength & Conditioning Research, 25(6), 1718–1725. doi: 10.1519/JSC.0b013e3181dbdb08 [DOI] [PubMed] [Google Scholar]
- Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, & Baird G (2009). Impairment in movement skills of children with autistic spectrum disorders. Developmental Medicine & Child Neurology, 51(4), 311–316. doi: 10.1111/j.1469-8749.2008.03242.x [DOI] [PubMed] [Google Scholar]
- Haddad JM, Rietdyk S, Claxton LJ, & Huber JE (2013). Task-dependent postural control throughout the lifespan. Exercise and Sport Science Reviews, 41(2), 123–132. doi: 10.1097/JES.0b013e3182877cc8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaie R, Mohri I, Kagitani-Shimono K, Tachibana M, Azuma J, Matsuzaki J, … Taniike M (2013). Altered microstructural connectivity of the superior cerebellar peduncle is related to motor dysfunction in children with autistic spectrum disorders. Cerebellum, 12(5), 645–656. doi: 10.1007/s12311-013-0475-x [DOI] [PubMed] [Google Scholar]
- Hannant P, Cassidy S, Tavassoli T, & Mann F (2016). Sensorimotor difficulties are associated with the severity of Autism Spectrum Conditions. Frontiers in Integrative Neuroscience, 10, 28. doi: 10.3389/fnint.2016.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AJ, Long KA, Tommet DC, & Jones RN (2017). Examining the role of race, ethnicity, and gender on social and behavioral ratings within the Autism Diagnostic Observation Schedule. Journal of Autism and Developmental Disorders, 47(9), 2770–2782. 10.1007/s10803-017-3176-3 [DOI] [PubMed] [Google Scholar]
- Hartigan JA, & Wong MA (1979). Algorithm AS 136: A K-Means clustering algorithm. Journal of the Royal Statistical Society: Series C, 28(1), 100–108. [Google Scholar]
- Hau J, S Kohli J, Shryock I, Kinnear MK, Schadler A, Müller RA, & Carper RA (2021). Supplementary and Premotor Aspects of the Corticospinal Tract Show Links with Restricted and Repetitive Behaviors in Middle-Aged Adults with Autism Spectrum Disorder. Cerebral Cortex. 10.1093/cercor/bhab062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman-Kedem M, Kosofsky BE, Ross G et al. (2018). Accuracy of Reported Community Diagnosis of Autism Spectrum Disorder. Journal of Psychopathology and Behavioral Assessment, 40, 367–375. 10.1007/s10862-018-9642-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton CL, Zhang Y, Whilte MR, Klohr CL, & Constantino J (2012). Motor impairment in sibling pairs concordant and discordant for autism spectrum disorders. Autism, 16(4), 430–441. doi: 10.1177/1362361311423018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein-Jenny MA, McDermott K, Shaw C, & Demchak J (2007). Validity of functional stability limits as a measure of balance in adults aged 23–73 years. Ergonomics, 50(5), 631–646. 10.1080/00140130601154814 [DOI] [PubMed] [Google Scholar]
- Hsieh CL, Sheu CF, Hsueh IP, & Wang CH (2002). Trunk control as an early predictor of comprehensive activities of daily living function in stroke patients. Stroke, 33(11), 2626–2630. doi: 10.1161/01.str.0000033930.05931.93 [DOI] [PubMed] [Google Scholar]
- Johnston LM, Burns YR, Brauer SG, & Richardson CA (2002). Differences in postural control and movement performance during goal directed reaching in children with developmental coordination disorder. Human movement science, 21(5–6), 583–601. doi: 10.1016/s0167-9457(02)00153-7 [DOI] [PubMed] [Google Scholar]
- Kaur M, Srinivasan SM, & Bhat AN (2018). Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without Autism Spectrum Disorder (ASD). Research in developmental disabilities, 72, 79–95. doi: 10.1016/j.ridd.2017.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Todd T, Fujii T, Lim JC, Vrongistinos K, Jung T (2016). Effects of Taekwondo intervention on balance in children with autism spectrum disorder. J Exerc Rehabil. 31;12(4), 314–319. doi: 10.12965/jer.1632634.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen-Raz R, Volkmar FR, & Cohen DJ (1992). Postural control in children with autism. Journal of autism and developmental disorders, 22(3), 419–432. 10.1007/BF01048244 [DOI] [PubMed] [Google Scholar]
- Leezenbaum NB, & Iverson JM (2019). Trajectories of posture development in infants with and without familial risk for Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 49(8), 3257–3277. doi: 10.1007/s10803-019-04048-3 [DOI] [PubMed] [Google Scholar]
- Le Mouel C, & Brette R (2017). Mobility as the purpose of postural control. Frontiers in computational neuroscience, 11, 67. 10.3389/fncom.2017.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R (2020). emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.4.5 Retrieved from https://CRAN.R-project.org/package=emmeans
- Licari MK, Alvares GA, Varcin K, Evans KL, Cleary D, Reid SL, … Whitehouse AJO (2019). Prevalence of motor difficulties in Autism Spectrum Disorder: Analysis of a population-based cohort. Autism Research. doi: 10.1002/aur.2230 [DOI] [PubMed] [Google Scholar]
- Lim YH, Lee HC, Falkmer T, Allison GT, Tan T, Lee WL, & Morris SL (2018). Effect of visual information on postural control in adults with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-018-3634-6 [DOI] [PubMed] [Google Scholar]
- Lim YH, Partridge K, Girdler S, & Morris SL (2017). Standing postural control in individuals with Autism Spectrum Disorder: Systematic review and meta-analysis. Journal of Autism and Developmental Disorders, 47(7), 2238–2253. doi: 10.1007/s10803-017-3144-y [DOI] [PubMed] [Google Scholar]
- Linke AC, Kinnear MK, Kohli JS, Fong CH, Lincoln AJ, Carper RA, & Müller RA (2020). Impaired motor skills and atypical functional connectivity of the sensorimotor system in 40-to 65-year-old adults with autism spectrum disorders. Neurobiology of Aging, 85, 104–112. 10.1016/j.neurobiolaging.2019.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebbert R, & Perez A (2016). Barriers to clinical research participation among African Americans. Journal of Transcultural Nursing, 27(5), 456–463. https://doi.org/10.1177%2F1043659615575578 [DOI] [PubMed] [Google Scholar]
- Maki BE, Holliday PJ, & Topper AK (1994). A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. Journal of Gerontology: Medical Sciences, 49(2), M72–84. doi: 10.1093/geronj/49.2.m72 [DOI] [PubMed] [Google Scholar]
- Mallau S, Vaugoyeau M, & Assaiante C (2010). Postural strategies and sensory integration: no turning point between childhood and adolescence. Plos One, 5(9). doi: 10.1371/journal.pone.0013078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DS, Wiggins LD, Carpenter LA, Daniels J, DiGuiseppi C, Durkin MS, … & Kirby RS (2009). Racial/ethnic disparities in the identification of children with autism spectrum disorders. American journal of public health, 99(3), 493–498. 10.2105/AJPH.2007.131243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo F, Di Sapio A, Martire S, Malentacchi M, Matta M, & Bertolotto A (2017). Computerized posturography is more sensitive than clinical Romberg Test in detecting postural control impairment in minimally impaired Multiple Sclerosis patients. Multiple sclerosis and related disorders, 14, 51–55. 10.1016/j.msard.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Melzer I, Benjuya N, & Kaplanski J (2004). Postural stability in the elderly: a comparison between fallers and non-fallers. Age and Ageing, 33(6), 602–607. doi: 10.1093/ageing/afh218 [DOI] [PubMed] [Google Scholar]
- Miller HL, Caçola P, Sherrod G, Patterson RM, & Bugnariu NL (2019). Children with Autism Spectrum Disorder, Developmental Coordination Disorder, and typical development differ in characteristics of dynamic postural control: a preliminary study. Gait & Posture, 67, 9–11. doi: 10.1016/j.gaitpost.2018.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller HL, Sherrod GM, Mauk JE, Fears NE, Hynan LS, & Tamplain PM (2021). Shared Features or Co-occurrence? Evaluating Symptoms of Developmental Coordination Disorder in Children and Adolescents with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 1–13. 10.1007/s10803-020-04766-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller HL, Templin TN, Fears NE, Sherrod GM, Patterson RM, & Bugnariu NL (2022). Movement smoothness during dynamic postural control to a static target differs between autistic and neurotypical children. Gait & Posture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Sung K, Jones BL, & Furman JM (2004). Underdevelopment of the postural control system in autism. Neurology, 63(11), 2056–2061. doi: 10.1212/01.wnl.0000145771.98657.62 [DOI] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, & Wagner GC (2007). Prevalence of motor impairment in autism spectrum disorders. Brain and Development, 29(9), 565–570. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Dietrich KN, & Bhattacharya A (2003). Postural stability in children with autism spectrum disorder. Journal of autism and developmental disorders, 33(6), 643–652. 10.1023/B:JADD.0000006001.00667.4c. [DOI] [PubMed] [Google Scholar]
- Monsch ED, Franz CO, & Dean JC (2012). The effects of gait strategy on metabolic rate and indicators of stability during downhill walking. Journal of biomechanics, 45(11), 1928–1933. 10.1016/j.jbiomech.2012.05.024 [DOI] [PubMed] [Google Scholar]
- Morris SL, Foster CJ, Parsons R, Falkmer M, Falkmer T, & Rosalie SM (2015). Differences in the use of vision and proprioception for postural control in autism spectrum disorder. Neuroscience, 307, 273–280. doi: 10.1016/j.neuroscience.2015.08.040 [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Mohanty S, Greene RK, Cook EH, Vaillancourt DE, & Sweeney JA (2015). Feedforward and feedback motor control abnormalities implicate cerebellar dysfunctions in Autism Spectrum Disorder. Journal of Neuroscience, 35(5), 2015–2025. doi: 10.1523/jneurosci.2731-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, & Pekar JJ (2009). Decreased connectivity and cerebellar activity in autism during motor task performance. Brain, 132(9), 2413–2425. doi: 10.1093/brain/awp088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Advisory Mental Health Council Workgroup on Changes to the Research Domain Criteria Matrix. (2018). RDoC Changes to the Matrix (CMAT) Workgroup Update: Addition of the Sensorimotor Domain. Retrieved from Bethesda, MD: https://www.nimh.nih.gov/about/advisory-boards-and-groups/namhc/reports/rdoc-changes-to-the-matrix-cmat-workgroup-update-addition-of-the-sensorimotor-domain.shtml [Google Scholar]
- Oldehinkel M, Mennes M, Marquand A, Charman T, Tillmann J, Ecker C, … group, E.-A. L. (2019). Altered connectivity between cerebellum, visual, and sensory-motor networks in Autism Spectrum Disorder: Results from the EU-AIMS Longitudinal European Autism Project. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(3), 260–270. doi: 10.1016/j.bpsc.2018.11.010 [DOI] [PubMed] [Google Scholar]
- Ozkan E, & Aki E (2021). The effect of occupation-based postural stability training on postural stability and occupational performance in visually impaired individuals: A randomised controlled trial. British Journal of Occupational Therapy, 84(11), 673–683. [Google Scholar]
- Pacheco TBF, de Medeiros CSP, de Oliveira VHB, Vieira ER, & de Cavalcanti FAC (2020). Effectiveness of exergames for improving mobility and balance in older adults: a systematic review and meta-analysis. Systematic Reviews, 9(163), 10.1186/s13643-020-01421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palluel E, Nougier V, & Olivier I (2010). Postural control and attentional demand during adolescence. Brain Research, 1358, 151–159. doi: 10.1016/j.brainres.2010.08.051 [DOI] [PubMed] [Google Scholar]
- Paquet A, Olliac B, Bouvard M, Golse B, & Vaivre-Douret L (2016). The semiology of motor disorders in autism spectrum disorders as highlighted from a standardized neuro-psychomotor assessment. Frontiers in Psychology, 7(1292), doi: 10.3389/fpsyg.2016.01292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavão SL, Dos Santos AN, de Oliveira AB, & Rocha NA (2014). Functionality level and its relation to postural control during sitting-to-stand movement in children with cerebral palsy. Research in developmental disabilities, 35(2), 506–511. doi: 10.1016/j.ridd.2013.11.028 [DOI] [PubMed] [Google Scholar]
- Piirtola M, & Era P (2006). Force platform measurements as predictors of falls among older people - a review. Gerontology, 52(1), 1–16. doi: 10.1159/000089820 [DOI] [PubMed] [Google Scholar]
- Polastri PF, & Barela JA (2013). Adaptive visual re-weighting in children’s postural control. Plos One, 8(12), e82215. doi: 10.1371/journal.pone.0082215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinovitch SN, Feldman F, Yang Y, Schonnop R, Leung PM, Sarraf T, … & Loughin M (2013). Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. The Lancet, 381(9860), 47–54. 10.1016/S0140-6736(12)61263-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador-Garcia C, Valverde-Esteve T, Chiva-Bartoll O, & Marave-Vivas M (2022). Dynamic balance improvement in children with autism spectrum disorder after an extracurricular service-learning physical education program. Developmental Neurorehabilitation, 10.1080/17518423.2022.2131922 [DOI] [PubMed] [Google Scholar]
- Sankaré IC, Bross R, Brown AF, Del Pino HE, Jones LF, Morris DAM, … & Kahn KL (2015). Strategies to build trust and recruit African American and Latino community residents for health research: a cohort study. Clinical and Translational Science, 8(5), 412–420. 10.1111/cts.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schärli AM, van de Langenberg R, Murer K, & Muller RM (2012). The influence of gaze behaviour on postural control from early childhood into adulthood. Gait & Posture, 36(1), 78–84. doi: 10.1016/j.gaitpost.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Shams A, Vameghi R, Dehkordi PS, Allafan N, & Bayati M. (2020) The development of postural control among children: Repeatability and normative data for computerized dynamic posturography system. Gait Posture, 78, 40–47. doi: 10.1016/j.gaitpost.2020.03.002. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, & Horak FB (1986). Assessing the influence of sensory interaction on balance. Physical Therapy, 66(10), 1548–1550. doi: 10.1093/ptj/66.10.1548 [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, & Woollacott M (2000). Attentional demands and postural control: the effect of sensory context. Journal of Gerontology: Medical Sciences, 55(1), M10–16. doi: 10.1093/gerona/55.1.m10 [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, & Woollacott MH (1985). The growth of stability: postural control from a development perspective. Journal of Motor Behavior, 17(2), 131–147. doi: 10.1080/00222895.1985.10735341 [DOI] [PubMed] [Google Scholar]
- Smith KA, Gehricke JG, Iadarola S, Wolfe A, & Kuhlthau KA (2020). Disparities in service use among children with autism: a systematic review. Pediatrics, 145(Supplement 1), S35–S46. 10.1542/peds.2019-1895G [DOI] [PubMed] [Google Scholar]
- Sparto PJ, Redfern MS, Jasko JG, Casselbrant ML, Mandel EM, & Furman JM (2006). The influence of dynamic visual cues for postural control in children aged 7–12 years. Experimental Brain Research, 168(4), 505–516. doi: 10.1007/s00221-005-0109-8 [DOI] [PubMed] [Google Scholar]
- Steinley D (2006). K-means clustering: a half-century synthesis. British Journal of Mathematical and Statistical Psychology, 59(Pt 1), 1–34. doi: 10.1348/000711005X48266 [DOI] [PubMed] [Google Scholar]
- Stodden DF, Goodway JD, Langendorfer SJ, Roberton MA, Rudisill ME, Garcia C, & Garcia LE (2008). A developmental perspective on the role of motor skill competence in physical activity: An emergent relationship. Quest, 60(2), 290–306. 10.1080/00336297.2008.10483582 [DOI] [Google Scholar]
- Swanenburg J, de Bruin ED, Uebelhart D, & Mulder T (2010). Falls prediction in elderly people: a 1-year prospective study. Gait & Posture, 31(3), 317–321. doi: 10.1016/j.gaitpost.2009.11.013 [DOI] [PubMed] [Google Scholar]
- Thelen E, & Spencer JP (1998). Postural control during reaching in young infants: a dynamic systems approach. Neuroscience and Biobehavioral Reviews, 22(4), 507–514. doi: 10.1016/s0149-7634(97)00037-7 [DOI] [PubMed] [Google Scholar]
- Thompson A, Murphy D, Dell’Acqua F, Ecker C, McAlonan G, Howells H, … Marco C (2017). Impaired communication between the motor and somatosensory homunculus is associated with poor manual dexterity in Autism Spectrum Disorder. Biological Psychiatry, 81(3), 211–219. doi: 10.1016/j.biopsych.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Bigler ED, Duffield TC, Prigge MDB, Froehlich AL, Lange N, … Lainhart JE (2017). Longitudinal development of manual motor ability in autism spectrum disorder from childhood to mid-adulthood relates to adaptive daily living skills. Developmental science, 20, 1–15. doi: 10.1111/desc.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Bigler ED, Tromp DPM, Adluru N, Destiche D, Samsin D, … Lainhart JE (2015). Brainstem white matter predicts individual differences in manual motor difficulties and symptom severity in Autism. Journal of Autism and Developmental Disorders, 45(9), 3030–3040. doi: 10.1007/s10803-015-2467-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uljarevic M, Hedley D, Alvares GA, Varcin KJ, & Whitehouse AJO (2017). Relationship between early motor milestones and severity of restricted and repetitive behaviors in children and adolescents with autism spectrum disorder. Autism Research, 10(6), 1163–1168. doi: 10.1002/aur.1763 [DOI] [PubMed] [Google Scholar]
- Unruh KE, Martin LE, Magnon G, Vaillancourt DE, Sweeney JA, & Mosconi MW (2019). Cortical and subcortical alterations associated with precision visuomotor behavior in individuals with autism spectrum disorder. Journal of neurophysiology, 122(4), 1330–1341. doi: 10.1152/jn.00286.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbecque E, Vereeck L, & Hallemans A (2016). Postural sway in children: A literature review. Gait & posture, 49, 402–410. 10.1016/j.gaitpost.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Wang Z, Hallac RR, Conroy KC, White SP, Kane AA, Collinsworth AL, … & Mosconi MW (2016). Postural orientation and equilibrium processes associated with increased postural sway in autism spectrum disorder (ASD). Journal of neurodevelopmental disorders, 8(1), 1–17. 10.1186/s11689-016-9178-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang Y, Sweeney JA, Gong Q, Lui S, & Mosconi MW (2019). Resting-state brain network dysfunctions associated with visuomotor impairments in Autism Spectrum Disorder. Frontiers in Integrative Neuroscience, 13. doi: 10.3389/fnint.2019.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EA, Travers JC, Kemper TD, Liberty LM, Cote DL, McCollow MM, & Stansberry Brusnahan LL (2016). Racial and ethnic diversity of participants in research supporting evidence-based practices for learners with autism spectrum disorder. The Journal of Special Education, 50(3), 151–163. https://doi.org/10.1177%2F0022466916632495 [Google Scholar]
- Woollacott M, Debu B, & Mowatt M (1987). Neuromuscular control of posture in the infant and child: is vision dominant? Journal of Motor Behavior, 19(2), 167–186. doi: 10.1080/00222895.1987.10735406 [DOI] [PubMed] [Google Scholar]
- Yingling ME, Bell BA, & Hock RM (2019). Treatment utilization trajectories among children with autism spectrum disorder: Differences by race-ethnicity and neighborhood. Journal of Autism and Developmental Disorders, 49(5), 2173–2183. 10.1007/s10803-019-03896-3 [DOI] [PubMed] [Google Scholar]
- Zablotsky B, Pringle BA, Colpe LJ, Kogan MD, Rice C, & Blumberg SJ (2015). Service and treatment use among children diagnosed with Autism Spectrum Disorders. Journal of Developmental & Behavioral Pediatrics, 36(2), 98–105. doi: 10.1097/DBP.0000000000000127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


