Abstract
Bilirubin has a number of physiological functions, both beneficial and harmful. In addition to having reactive oxygen species-scavenging activities, bilirubin has potent immunosuppressive effects associated with long-term pathophysiological sequelae. It has been recently recognized as a hormone with endocrine actions with interconnected effects on various cellular signaling pathways. Current studies show that bilirubin also decreases adiposity and prevents metabolic and cardiovascular diseases. All in all, the physiological importance of bilirubin is only now coming into light, and strategies for increasing plasma bilirubin levels to combat chronic diseases are starting to be considered. This review discusses the beneficial effects of increasing plasma bilirubin, incorporates emerging areas of bilirubin biology, and provides critical concepts to advance the field.
Keywords: Bilirubin, Hmox1, HO-1, BVRA, Blvra, cell signaling, nuclear receptors, heme oxygenase, metabolism, cardiovascular disease
Introduction
New findings in bilirubin research suggest that it has a previously unknown function; it operates as a hormone [1,2]. Published work from the past decade reveals that bilirubin at moderately elevated levels is protective against metabolic and cardiovascular diseases (reviewed in [1,3,4]). This is contrary to the past century of thinking about bilirubin being a toxic bile substance that causes liver dysfunction and the yellowing of the skin in jaundice. This new finding has driven excitement in bilirubin research and aided in focusing on new targets for obesity, diabetes, and cardiovascular research, which we discuss further below.
Recent studies show that bilirubin activates the nuclear receptor, PPARα [5–8], which drives fat burning, lowering adiposity and blood glucose. Some have utilized this emerging concept to develop bilirubin nanoparticles as potential therapeutics for metabolic and cardiovascular diseases [1,9–12]. Others have focused on activating the heme oxygenase () system that catabolizes heme to produce bilirubin (Box 1). Newer work has also been directed at the UGT1A1 enzyme that clears bilirubin from the blood by conjugation with glucuronic acid, which after is transported to the bile and intestine [13]. Suppressing UGT1A1 to increase serum/plasma bilirubin has been considered a promising therapeutic approach [14]. This occurs naturally in with the Gilbert’s syndrome polymorphism UGT1A1*28 (TA7/7 promoter gene variant), in whom reduced expression of the UGT1A1 enzyme serum/plasma bilirubin levels [1]. Those with the Gilbert’s syndrome polymorphism have been shown to have reduced risks of metabolic and cardiovascular diseases [1]. Humanized mice with Gilbertś syndrome gene promoter polymorphism (HuUGT1A1*28) [with knocked out from the mouse locus] were shown to have mildly elevated bilirubin levels and resistant to high-fat-diet-induced fatty liver disease and glucose intolerance [15]. These indicate that mild in plasma bilirubin levels promote health.
Box 1. Bilirubin production and catabolism.
The physiological concentrations of bilirubin levels depend on many biological factors such as genetics, sex, and age (see Figure 1 in the main text) [110]. The crucial enzyme that initiates the breakdown of heme is heme oxygenase (HMOX), which has two isoforms (HMOX1, OMIM *141250; and HMOX2, OMIM *141251) [146]. HMOX1 is the inducible isoform, believed to be the most inducible enzyme in the human body, activated by a variety of endogenous and exogenous stimuli associated with the disruption of redox homeostasis and dysregulation of immune system functions [121,147]. The primary function of the HMOX isozymes is to cleave the heme molecule into the linear tetrapyrrole biliverdin while simultaneously releasing iron and carbon monoxide (CO) as additional products. Biliverdin is then reduced to bilirubin by the enzyme biliverdin reductase A (BLVRA, OMIM *109750). In addition, biliverdin appears to be an integral component of the so-called bilirubin–biliverdin redox cycle that regenerates bilirubin scavenging activities (Figure I) [148].
The lipophilic unconjugated bilirubin is transported within the circulation bound to albumin [149]. Once dissociated from albumin and taken up by the liver cells [26,27], bilirubin is conjugated with glucuronic acid by bilirubin UDP-glucuronosyltransferase (UGT1A1, OMIM *191740). Hereditary underexpression of UGT1A1 could be due to variation in the so-called TATA box of the UGT1A1 gene promoter (mostly in Caucasians) or due to mutations in the structural UGT1A1 gene (mostly in Asians) resulting in the development of mild unconjugated hyperbilirubinemia (also known as Gilbert syndrome, OMIM *143500) [150]. This condition is very frequent, reaching a prevalence of approximately 9% in Caucasians (11.6 and 6.1% in males and females, respectively) [151], but differs geographically among various other populations [107].
Conjugated bilirubin is transported by the ABCC2 protein into bile to reach the intestines [13,21]. Within the intestinal lumen, bilirubin is deconjugated by β-glucuronidase of epithelial or bacterial origin and then reduced by intestinal microbiota to a series of derivatives commonly known as urobilinoids [21]. Despite the massive production of urobilinoids (a process important for the disposal of electrons produced during fermentolytic processes in these anaerobic bacteria [152]), a bilirubin reductase has never been identified to date; thus, this enzyme is a missing puzzle in the process of bilirubin metabolism. The role of the gut microbiota in the regulation of bilirubin homeostasis is also unknown. However, a recent experimental study found that a strain of Lactobacillus can increase plasma bilirubin levels and reduce body weight [153].
Hence, UGT1A1 may influence the predisposition of fat accumulation and the development of obesity [13,16]. In fact, a recent human study showed that obese men and women had significantly higher levels of the bilirubin catabolized product, urobilin [16], which originates from the gut microbiome catabolism of conjugated bilirubin by deconjugation and then forming urobilin that is absorbed via the hepatic portal vein [13]. Individuals with Gilbert’s syndrome are known to have a lower prevalence of obesity [17], suggesting that higher bilirubin levels in the blood may mediate these actions. Indeed, plasma bilirubin levels are negatively associated with adiposity in humans [16]. The expression of UGT1A1 is under the control of hepatic nuclear receptors, such as the constitutive androstane receptor (CAR), pregnane X receptor (PXR), glucocorticoid receptor (GR), aryl hydrocarbon receptor (AHR), hepatocyte nuclear receptor 1α (HNR1α) [18], and others [14], which regulate UGT1A1 transcription via the hormone response element (HRE) in its promoter [18]. These findings open possibilities for targeting the heme-bilirubin catabolism pathway as a means of increasing bilirubin levels.
Today, bilirubin has been recognized as one of the most potent endogenous antioxidants, as a powerful immunosuppressive [19], and, quite surprisingly, as a selective cell signaling molecule [20]. Bilirubin, classically viewed as an end-product of the heme catabolic pathway, was long believed to be potentially dangerous, and it is perceived that its presence in high concentrations is an ominous sign of underlying liver disease [21]. Recent advancements support a new concept that bilirubin functions as a hormone, and several of its biological functions might be mediated his mechanism [1,2,4,13]. Here, we will discuss the new findings about bilirubin, how it functions at low (hypobilirubinemia), physiological (normobilirubinemia), mildly elevated (hyperbilirubinemia), and jaundice (severe hyperbilirubinemia) levels, and how it plays an intimate role in health and disease equilibrium.
Intracellular effects of bilirubin on different pathways
Bilirubin levels in the blood have been thought to be mostly produced by reticuloendothelial cells in the spleen [1]. However, new studies in mice lacking the biliverdin reductase-A (BVRA) enzyme [22,23], which converts bilirubin from biliverdin [24], have shown that bilirubin generation likely occurs from many tissues. Vitek first called for a clinical reclassification of bilirubin levels [25], which led Creeden et al. to propose decision limits for normobilirubinemia as ranging from 10 to 25 μmol/L depending on age, gender, and race, and hypobilirubinemia at <10 μmol/L [1]. Based on recent clinical data, the decision limits for determining the risk of pathological consequences, especially in the lower concentrations, are.
Inside the cell:
Bilirubin enters cells by multiple mechanisms [26,27]: by a still not well-defined carrier molecule(s) and another by passive diffusion. The contribution of each mechanism depends on the concentration of bilirubin [27]. Important is the intracellular binding of bilirubin to fatty acid-binding protein-1 (FABP1) [28], a fatty acid-binding transporter expressed in hepatocytes, which serves as a sink for maintaining an inward gradient. While FABP1 is almost exclusively expressed in hepatocytes, and bilirubin has actions on other tissues, bilirubin may use other FABPs or similar types of proteins, such as scavenger receptors. This is supported by bilirubin binding to other FABP proteins, such as UnaG [29,30], expressed in the Unagi freshwater eel (Anguilla japonica). UnaG interacts with unconjugated bilirubin in a lock-and-key mechanism to activate its fluorescence in the skeletal muscle [29]. There are likely other FABP proteins in humans that bilirubin can utilize for cellular entry, but these are currently unknown.
Bilirubin has been well-characterized as an antioxidant for over three decades. The antioxidant activity of bilirubin increases when normal atmospheric oxygen concentrations (20%) move toward levels found in tissues (2%) [31]. Many protective effects of bilirubin have been attributed to its role as an antioxidant, which is discussed in more detail by Sedlak et al. and Thomas et al. [32,33]. More recent studies have shown that bilirubin is also involved in regulating signaling pathways indicating that bilirubin has more properties than initially thought. Thus, recently bilirubin has been regarded as a hormone that binds to receptors [1] (Figure 1).
Figure 1. Bilirubin acts as a hormone.
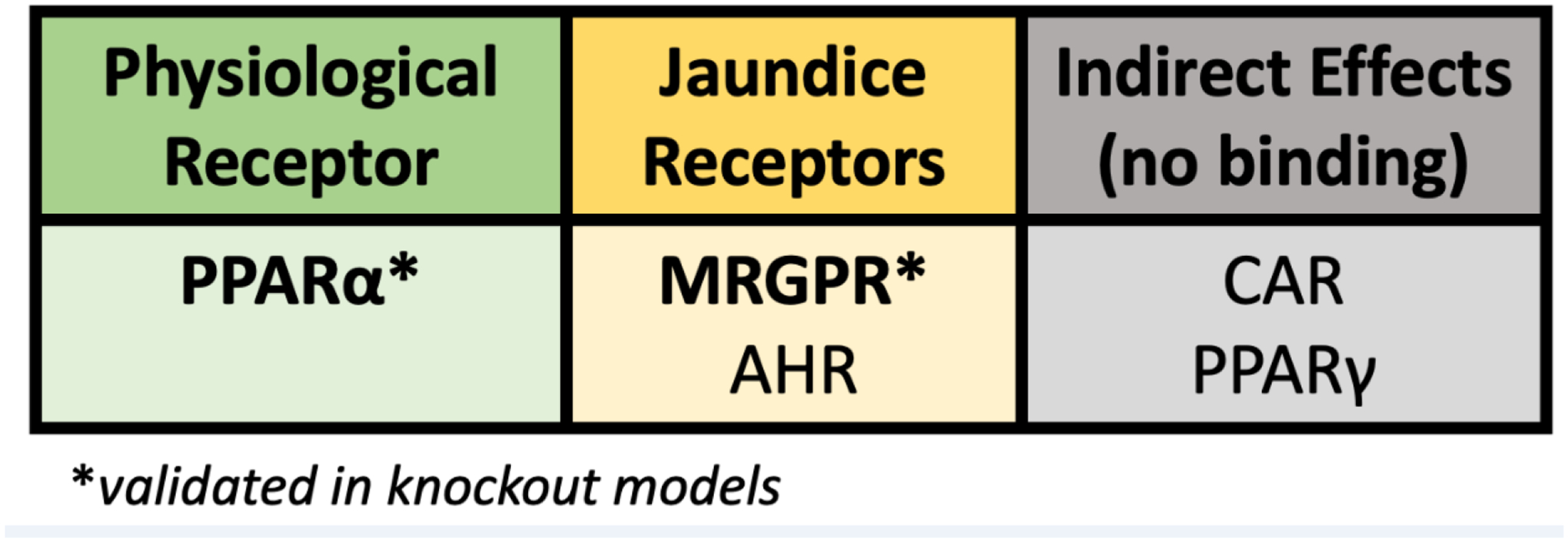
The receptors that bilirubin has been shown to activate at physiological bilirubin (10–25 μM) and pathological levels (>100 μM).
Abbreviations: Peroxisome proliferator-activated receptor alpha (PPARα), Mas-Related G-protein coupled receptor (MRGPR), aryl hydrocarbon receptor (AHR), constitutive androstane receptor (CAR), Peroxisome proliferator-activated receptor gamma (PPARγ) Asterisks: this is an indicator that studies were performed and validated in knockout animal models.
Endocrine functions of bilirubin:
By definition, a hormone is a substance that enters the bloodstream and exerts actions on cells or tissues, which occurs by direct binding to cytoplasmic or nuclear receptors. Bilirubin has been shown to have such properties at physiological levels [1,4–8] and with potential activation of other receptors at higher pathological levels [34–37]. The earliest reports that bilirubin might activate receptors are from the late 1990s and suggested that this activation might be mediated through the AHR transcription factor [35,36]. Using Hepa1c1c7 murine hepatoma cells, Sinal et al. found that high bilirubin concentrations (100 μM) enhanced Cyp1a1 expression [35], a gene thought for many years to only be controlled by AHR. The transcriptional activity of AHR requires heterodimerization with AHRT (AhR nuclear translocator) protein on DNA recognition sequences known as DREs [38], in which the Cyp1a1 promoter contains three DRE sites [39]. It also has two PPAR response elements (PPREs) that are regulated by the PPAR nuclear receptor transcription factors, which have also been shown to induce Cyp1a1 [40,41]. As the Cyp1a1 promoter contains DREs and PPREs, a relationship exists between PPARs and AHR, as PPARα has been shown to potentiate the AHR-induced Cyp1A1 expression [42]. Whether bilirubin directly acts on AHR to induce Cyp1a1 or if this might be mediated PPARα-induction of AHR is yet to be determined. The most recent investigations show that unconjugated bilirubin directly binds to the PPARα nuclear receptor [5–8] and that this interaction occurs at physiological levels with a Kd value of 5.13 μM [5].
Bilirubin acting on PPARα at physiological levels re-defines bilirubin as a metabolic hormone essential for balancing nutrient storage (Figure 1) [1]. Bilirubin binds PPARα directly and elicits a response that reduces fat accumulation in the liver (Figure 2) and adipose tissues [1,3–8,10,13,15,43–46]. Hence, bilirubin has been proposed to be potentially useful for reducing obesity and improving metabolic dysfunction [1,3,4] (see also below). The PPARα-bilirubin interaction was validated in many different studies, which included treatment of PPARα knockout (KO) animals [8], and lentiviral knockdown of PPARα in human hepatocytes that demonstrated by RNA-sequencing analysis that bilirubin-controlled gene activity is mostly PPARα-dependent (~95%) [7]. Other studies included the standard receptor-ligand binding assays that have been classically used for nuclear receptors such as competitive ligand-binding assays [6] and pull down of PPARα using bilirubin-fused sepharose beads [8]. A competitive ligand-binding study identified the amino acids in the ligand-binding domain (LBD) of PPARα that bilirubin uses for hydrogen binding, indicating a strong affinity for the receptor [6]. These studies showed that bilirubin binds to the LBD of PPARα and competes with known ligands, such as fenofibrate, for the same binding region [6]. The interaction of bilirubin-PPARα was further supported by in silico modeling of bilirubin in the PPARα LBD, which indicated that bilirubin tightly binds into the pocket [8]. Another hypothesis that bilirubin drives PPARα activity was proposed in a study that showed that bilirubin treatment drives PPARα to the Ucp1 and Cpt1 promoters in adipocytes, demonstrated by chromatin immunoprecipitation (ChIP) assays using a PPARα-specific antibody [6]. Lastly, using state-of-the-art PamGene technology for nuclear receptor coregulator interactions, it was demonstrated that bilirubin-PPARα binding causes a molecular switch from corepressor proteins to coactivators for the PPARα interactome and, thus, induces a gene response [6]. Altogether, these investigations support the idea that bilirubin directly interacts in the LBD of PPARα and causes a change in the coregulator interactome from inhibitory to stimulatory, eliciting a gene response.
Figure 2. Bilirubin responses in tissues via direct binding to receptors.
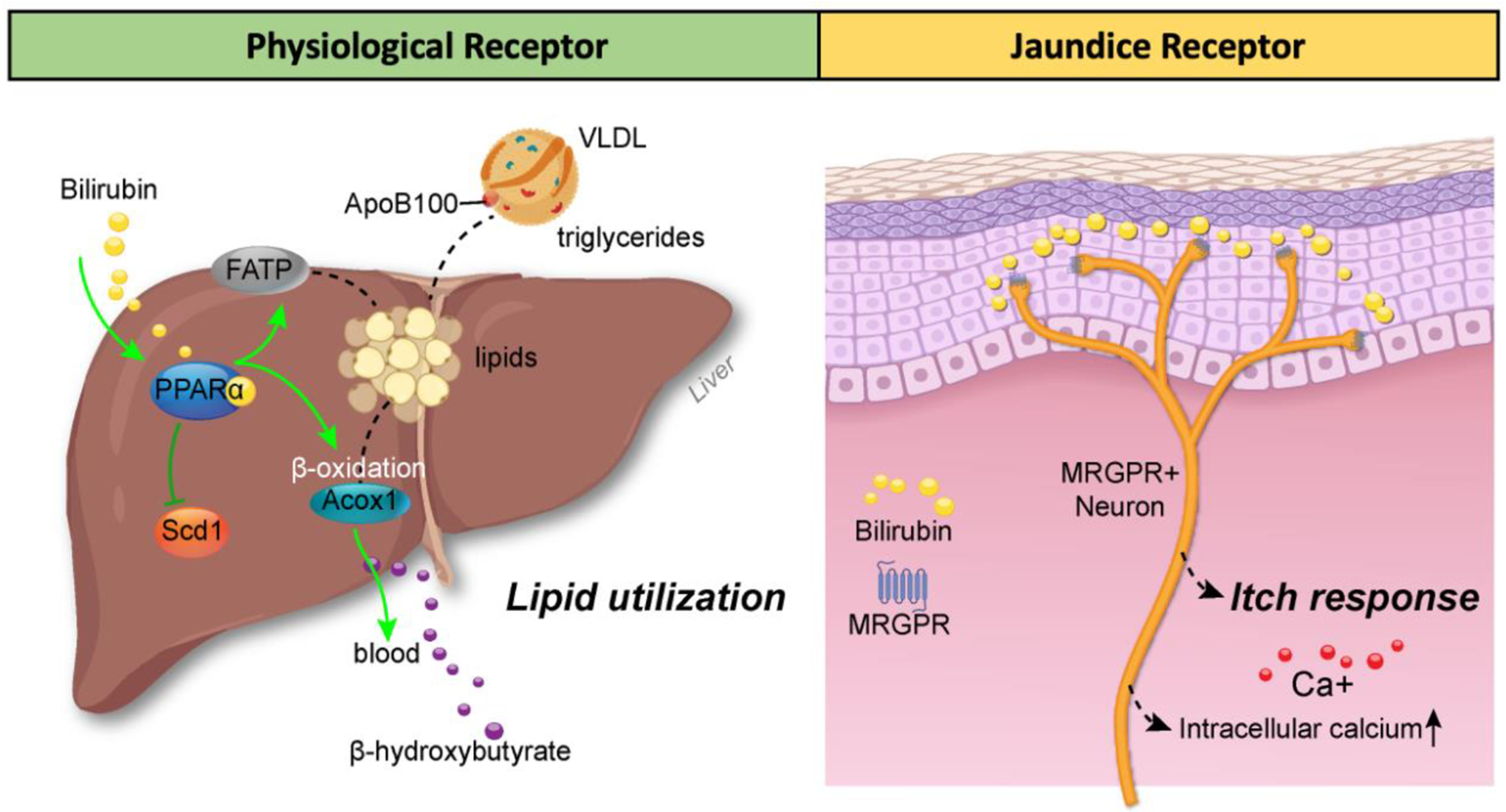
(A) Bilirubin activates the PPARα nuclear receptor transcription factor at physiological levels to regulate lipid utilization by increasing genes (ACOX1) for fat-burning β-oxidation and suppressing genes (SCD1) for de novo lipogenesis [10,15]. The PPARα-bilirubin interaction also induces lipid uptake genes such as fatty acid transporters (FATPs) and suppresses very low-density lipoprotein (VLDL) secretion from the liver and triglyceride entry to the blood to lower levels. The resultant product from PPARα-bilirubin is lipid utilization and production of the ketone β-hydroxybutyrate, which is excreted and used as an alternative energy source to glucose [146,147]. (B) The itch receptor (MRGPR) is activated by bilirubin at pathological levels (>150 μM), which increases intracellular calcium activation in MRGPR-positive neurons, activating pruritus (itching response). This figure was created by Matthew Hazzard at the University of Kentucky College of Medicine.
One report has shown that bilirubin at very high levels (>150 μM) may stimulate responses on itch receptors, and itching is known to be associated with jaundice and cholestasis. Meixiong et al. showed that at high concentrations, bilirubin interacts with the G protein-coupled receptor (GPCR) referred to as the Mas-Related G-protein coupled receptors (MRGPRs) and activated MRGPRX4 (human) and MRGPRA1 (the mouse homolog of MRGPRX4) to elicit intracellular calcium signaling response (Figure 2) [34]. Bilirubin binding to MRGPRA1 was validated by competitive binding assays and bilirubin administration in Mrgpra1 and Blvra KO mice [34]. It was found that bilirubin had a lower kinetic binding to MRGPRA1 than PPARα, with an EC50 = 145.9 μM for MRGPRA1 excitation quantitated in MRGPRA1-overexpressing cells challenged with bilirubin to generate an intracellular calcium signaling response [34].
Other receptors that bilirubin may also activate are CAR, PPARγ, PPARδ, and possibly enzymes it might regulate, such as the angiotensin I-converting enzyme (ACE) (Figure 1). Huang et al. showed that bilirubin increases CAR target genes [47] though they concluded that bilirubin does not directly bind to CAR [47]. PPARα has been shown to increase CAR expression [48,49], and this mechanism likely affects CAR target genes. Bilirubin treatment increases PPARγ expression in diabetic mice [50], and bilirubin suppresses PPARγ expression in 3T3-L1 adipocytes [8]. Analysis of the interaction of bilirubin with PPARγ or PPARδ showed that bilirubin does not directly activate the transcriptional activity of either receptor [6] and has no direct interaction with PPARγ [5]. Danilov et al. showed that bilirubin at extremely high concentrations (about 250 μmol/L) might bind ACE to regulate the ectodomain shedding used for entering the circulating pool [37], but direct proof of bilirubin binding is lacking. More work is needed to reveal if bilirubin binds ACE and other targets at jaundice levels and how these might impact the cardiovascular system.
Data so far obtained indicates that there are at least two direct bilirubin receptor targets, one at physiological levels (PPARα) and another at higher pathological concentrations (MRGPR). Bilirubin likely activates AHR but this effect and the concentration (Kd or EC50) at which it occurs is yet to be validated in KO animals [51] or by CRISPR KO technology in cell lines. More investigations are needed to determine whether bilirubin might bind to other receptors to control normal physiological responses.
Cardiovascular and metabolic effects of bilirubin
The role of bilirubin in human health has been characterized by population studies correlating bilirubin levels with diseases [52,53], and fully supported by the recent discovery of bilirubin endocrine activities. Higher levels of bilirubin are associated with protection from the development of cardiovascular and metabolic diseases, like obesity and diabetes, as further supported by studies in individuals with Gilbert’s syndrome [17,54]. Interestingly, Gilbert’s syndrome subjects exhibit enhanced expression of PPARα, most likely from increased plasma bilirubin levels [55]. Bilirubin-induced PPARα signaling may be the reason for the significantly lower body mass index (BMI), glucose, and insulin levels observed in these individuals [55,56]. Mice that possess the human Gilbert’s syndrome polymorphism (Hu) exhibit moderate hyperbilirubinemia and are protected from high-fat diet-induced hepatic steatosis and insulin resistance [15]. These mice also have reduced inhibitory serine-73 phosphorylation of PPARα, resulting in the enhancement of PPARα signaling in the liver [15]. Treating obese and diabetic mice with bilirubin nanoparticles improved metabolic function by lowering adiposity, blood glucose levels, and hepatic steatosis [6,10]. Several mechanisms account for these effects, including increased mitochondrial activity, altered adipose tissue remodeling, and increased ketone production [6,8,10].
Gunn rats carry a loss of function mutation in their Ugt1a1 gene, resulting in moderate-to-severe hyperbilirubinemia, and adult animals are protected against hypertension and end-stage organ damage [57–61]. Moderate hyperbilirubinemia due to targeting hepatic UGT1A1 with drugs or anti-sense morpholinos prevents angiotensin-II-induced hypertension and changes in renal vascular resistance [62,63]. The antioxidant effects of hyperbilirubinemia have long been thought to be responsible for the beneficial effects on the cardiovascular system [64–66]. However, superoxide production in angiotensin II-induced hypertension was not responsible for the antihypertensive actions of moderate hyperbilirubinemia [67], indicating that other pathways may be responsible for the blood pressure-lowering actions of moderate hyperbilirubinemia in angiotensin II-dependent hypertension. Bilirubin improves vascular function by increasing the bioavailability of nitric oxide (NO) either through direct savaging of superoxide or blockade of superoxide production by inhibition of NAD(P)H oxidase [68,69]. Additionally, bilirubin may impact NO production through actions on endothelial nitric oxide synthase (eNOS) PARα signaling. PPARα agonists stimulate AMP-activated protein kinase (AMPK) to increase eNOS serine 1177 phosphorylation and mitogen-activated protein kinase (MAPK) to enhance eNOS serine 1179 phosphorylation as well as decrease eNOS threonine 497 phosphorylation ivation of ERK and Akt [70,71]. These modifications of eNOS result in enhanced NO production, which is beneficial in conditions such as hypertension and atherosclerosis.
Modulation of bilirubin levels
Slightly higher serum bilirubin concentrations are associated with apparent health benefits, particularly decreasing the risk of developing various diseases. Mildly raising serum bilirubin levels might reduce vascular and cancer risk [72]. Importantly, even small incremental elevations in serum bilirubin concentrations may provide such benefits. Based primarily on clinical studies, each micromolar increase in serum bilirubin concentration is associated with a substantial decrease in the risks of developing atherosclerosis [73,74], arterial hypertension [75], diabetes mellitus [76], colorectal cancer [77], Crohńs disease [78], systemic lupus erythematosus [79], Fabry disease [80], or schizophrenia [81]. Hence, modulation of the bilirubin levels appears to be a reasonable potential treatment strategy (Figure 3).
Figure 3. Modulation of bilirubin level.
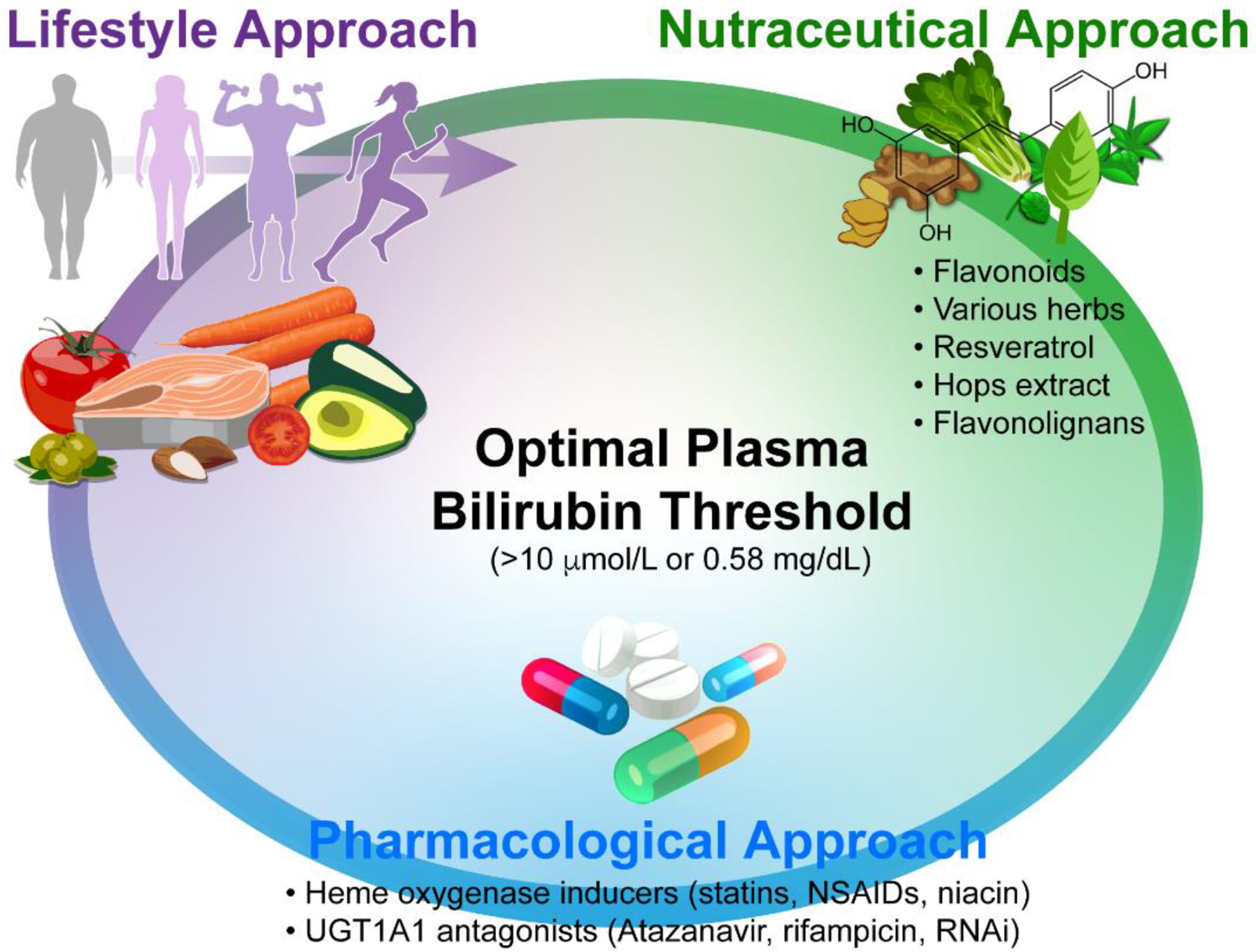
Several approaches can be used to mildly elevate the plasma bilirubin level above the optimal threshold. These include (i) Lifestyle approach (proper body composition, modest calorie restriction with a healthy diet rich in carbohydrates, fruit and vegetables, and sufficient aerobic activities); (ii) Nutraceutical approach, and finally; (iii) Pharmacological approach including nutraceuticals and drugs modulating HMOX/UGT1A1 activities as well as hepatic bilirubin transporting functions.
This figure was created by Matthew Hazzard at the University of Kentucky College of Medicine.
Lifestyle modifications approach
Ideal body composition
In 2009, a clinical investigation of more than 10,000 subjects by Andersson et al. described a significant negative relationship between body composition and serum bilirubin levels [82]. In this work, they observed that bilirubin levels increased linearly with each 1% decrease in weight loss and were associated with a significantly higher bilirubin level in both men and women [82]. A negative correlation has been observed between bilirubin concentrations and BMI [17,83–85], with the lowest bilirubin concentrations observed in obese patients with visceral obesity and additional metabolic complications [84,86]. Consistent with these data, body fat percentage was the primary determinant of bilirubin concentrations in young obese subjects [87], the most likely explanation being bilirubin overconsumption due to obesity-induced oxidative stress [88], although this remains to be determined. In addition, bilirubin levels are positively associated with muscle mass, as demonstrated in patients with sarcopenia [89] and elite athletes [90]. Further supporting this is that diet-induced obese mice treated with bilirubin nanoparticles had significantly higher muscle mass and reduced adiposity [6].
Dietary factors
Fasting and calorie restriction are associated with increased serum bilirubin concentrations [91], and long-term caloric restriction is positively associated with increased lifespan [92,93]. Whether coincidental or causal, mildly elevated serum bilirubin concentrations have been associated with increased lifespan [94,95]. This has been supported by studies in people with Gilbert’s syndrome, which were reported to have only half the mortality rates compared with normobilirubinemic individuals [96]. A greater telomere length was also found in individuals with the Gilbert’s syndrome polymorphism [97].
In addition, the composition of the diet has a modulating effect on serum bilirubin concentrations. For instance, increased consumption of fruits and vegetables was positively associated with bilirubin levels in the US NHANES study [98]. Increased serum bilirubin concentrations in response to a higher carbohydrate diet were also observed in pregnant women [99], which validates previous reports [100,101]. It is noteworthy to mention that a fast-food diet was associated with significantly lower bilirubin levels [102]. Similarly, a high-fat diet that increased visceral fat content had the same bilirubin-reducing effect [103]. It is unknown whether these observations are the results of a feedback mechanism of the human body to cope with increased metabolic and oxidative stress; however, considering current knowledge, this could be a possible explanation.
Aerobic activities
In a recent study, serum bilirubin concentrations were higher in elite athletes [90], and interestingly, also the prevalence of Gilbert’s syndrome was significantly higher in athletes (9.6 vs. 22%) [90]. From these observations, one might speculate that a mild elevation of serum bilirubin predisposes one to better sports performance, as supported by the observation that regular physical activity results in an increase in serum bilirubin concentrations [104,105] (reviewed in [33,106]). This aspect was confirmed in rats where high aerobic exercise elevated plasma bilirubin concentrations [44].
bilirubin improving exercise performance (reviewed in [106]) possible and affected byoglobin levels as the greatest contributor to aerobic performance, and individuals with Gilbert’s syndrome possess increased total hemoglobin and decreased red blood cell survival [107]). Hence, it remains to be determined whether increases in circulating bilirubin predispose to or maybe increase sports performance. In addition, the antioxidant activity of bilirubin has the potential to modulate training-induced adaptation [33], similar to other antioxidant regimens [108]. However, these questions remain to be elucidated, and future studies are necessary to understand better bilirubin’s function in exercise.
Nutraceutical supplementation
Many natural compounds are often used as nutraceutical supplements that can affect bilirubin metabolism by inducing HMOX1 [109], inhibiting bilirubin uptake, suppressing UGT1A1 activity, improving the antioxidant capacity of the human body, or saving bilirubin from consumption or clearance [110]. These compounds include various plant polyphenols such as curcuminoids, flavonoids, or some flavonolignans from the silymarin complex (for a review, see [110]). Elevated serum bilirubin levels have been reported in clinical studies with patients treated with milk thistle extract (Silybum marianum (L.) Gaertn.) via the inhibition of the UGT1A1 activity by flavonolignans from the silymarin complex [111]. These data align with the results from studies in which silymarin flavonolignans dehydrosilybin A and B increased serum bilirubin concentrations [112]. On the other hand, it should be noted that some clinical trials have shown no effect of milk thistle capsules containing 140 mg silymarin on bilirubin levels when taken three times daily in healthy participants [113]. UGT1A1 has been shown to be increased in the livers of obese mice [114,115]. The differential findings in the clinical studies might be that silymarin is more effective in obese people with higher UGT1A1 and lower bilirubin levels [1]. The other explanation could be that clinically used silymarin drugs/supplements do not contain sufficient amounts of standardized silymarin. Thus, the effects of silymarin need further studies and require assessing optimal dosing and specific metabolites responsible via pharmacokinetic studies.
Inhibitory effects toward UGT1A1 were also described for epigallocatechin gallate and, to a lesser extent, for echinacea and saw palmetto [116]. It is highly likely that many other natural compounds used, especially in traditional Asian medicine, could have similar effects, although clinical data supporting these are lacking [117–119].
In contrast, the intake of certain fruits and vegetables, and coffee, is associated with a decrease in serum bilirubin concentrations due to the opposite modulation of the enzymatic and transporting mechanisms of bilirubin metabolism (for a review, see [110]).
Pharmacological applications
Many drugs used in routine clinical practice interfere with bilirubin metabolism by induction of HMOX1 that induces bilirubin production [120] or decreasing bilirubin clearance by inhibiting its uptake or biotransformation [110].
HMOX1 induction is considered one of the most inducible genes in the human body, being upregulated by numerous endogenous and exogenous factors [109,120,121]. The induction of HMOX1 has been reported for many clinically used drugs, including commonly prescribed NSAIDs, hypolipidemic, anti-aggregation drugs, H2 antihistamines, and some antihypertensive medications, as well as natural substances used as nutraceuticals [109,110]. It is important to note that the induction of HMOX1 may not significantly affect bilirubin concentrations in circulation, as implied from clinical studies on the HMOX1 gene polymorphism [122,123]. Likely, bilirubin concentrations increase intracellularly, as demonstrated in previous experimental studies [124,125]. Because of the biological importance of HMOX1 induction, several clinical trials with therapeutic drugs and nutraceuticals are currently ongoing to assess the clinical impact of this approach (www.clinicaltrials.gov, accessed January 20, 2023). The induction of HMOX1 is a promising approach to increasing bilirubin production and improving conditions affected by lower bilirubin levels (hypobilirubinemia, described in [1]).
Indeed, some drugs have clinical data showing that their use is associated with a mild increase in serum bilirubin concentrations. A typical example is atazanavir, an HIV replication inhibitor, which suppresses UGT1A1 activity and significantly increases serum bilirubin, decreases the risk of cardiovascular disease [126], and slows the progression of carotid intima-media thickness progression [127,128]. Similar UGT1A1-suppressing activities resulting in mild systemic hyperbilirubinemia have been reported for several other anticancer and antiviral pharmaceuticals [110]. Another typical example of bilirubin-inducing drugs are statins, inhibitors of HMG-CoA reductase commonly used to lower serum cholesterol levels, which interfere with bilirubin metabolism by competing for its basolateral transporting proteins on the liver cell membrane. A significant elevation of serum bilirubin has been reported in patients with hypercholesterolemia treated with rosuvastatin, simvastatin, and atorvastatin [129–131]. Similar bilirubin-increasing effects were observed in human subjects treated with niacin [132] or ursodeoxycholic acid [133].
The need for comprehensive pharmacological research with well-controlled clinical studies to test candidate drugs for their bilirubin-modulating activities is apparent.
Future applications
The potential therapeutic use of bilirubin
In the past, a direct parenteral administration of bilirubin in humans has been used frequently as a therapeutic approach. Up to 40 studies covering almost 1,500 human subjects have been reported since the 1920s in the literature using intravenous infusions of bilirubin with a relatively good safety profile (for a review of these studies, see [134]).
Recent advancements using nanotheranostics to deliver bilirubin to pathologically altered tissues and organs are currently a hot pharmacological topic in experimental medicine. Bilirubin nanoparticles bound to various carrier molecules such as polyethylene glycol, chitosan, or gold, often further modified and conjugated with other active substances such as cytostatics (for a review, see [1,135]), were constructed to treat metabolic, inflammatory, and cancer diseases [1,12,136–141]. Although important risks and hazards are associated with using these delivery particles, particularly from the long-term perspective [142], bilirubin nanoparticles have indisputable therapeutic potential, especially with the development of new self-generated and self-enhanced biodegradable materials in this rapidly evolving field [143].
Concluding remarks and future directions
As common in research, the more problems that are solved, the more questions arise. We are still not close to completely understanding the interrelated intracellular events elicited by bilirubin. The studies on experimental systems such as cell culture or organoids provide some potential answers and clues into its mechanisms. Identification of these molecular events will also allow us to understand better the interplay between bilirubin and several metabolic and immune pathways (see Outstanding questions). Defining whether modulation of bilirubin levels by dieting and exercise and possibly dietary supplementation may be useful for improving health, as suggested by population studies. Bilirubin and induction of HMOX1 to produce bilirubin may serve as new therapeutics for diseases with metabolic and inflammatory dysfunction. Newer studies to determine the impact of hypobilirubinemia [1] may offer advantages to better understanding diseases such as cardiovascular and fatty liver diseases, where low levels might be a factor.
Outstanding questions.
What are the receptor targets of bilirubin?
How do high levels of plasma/serum unconjugated bilirubin protect against cardiovascular and metabolic diseases?
How can plasma/serum levels of bilirubin be safely elevated to protect against cardiovascular and metabolic diseases?
What diseases are affected by hypobilirubinemia?
Could we reassess the current reference values of bilirubin concentrations in plasma/serum?
Figure I. Heme catabolism to bilirubin.
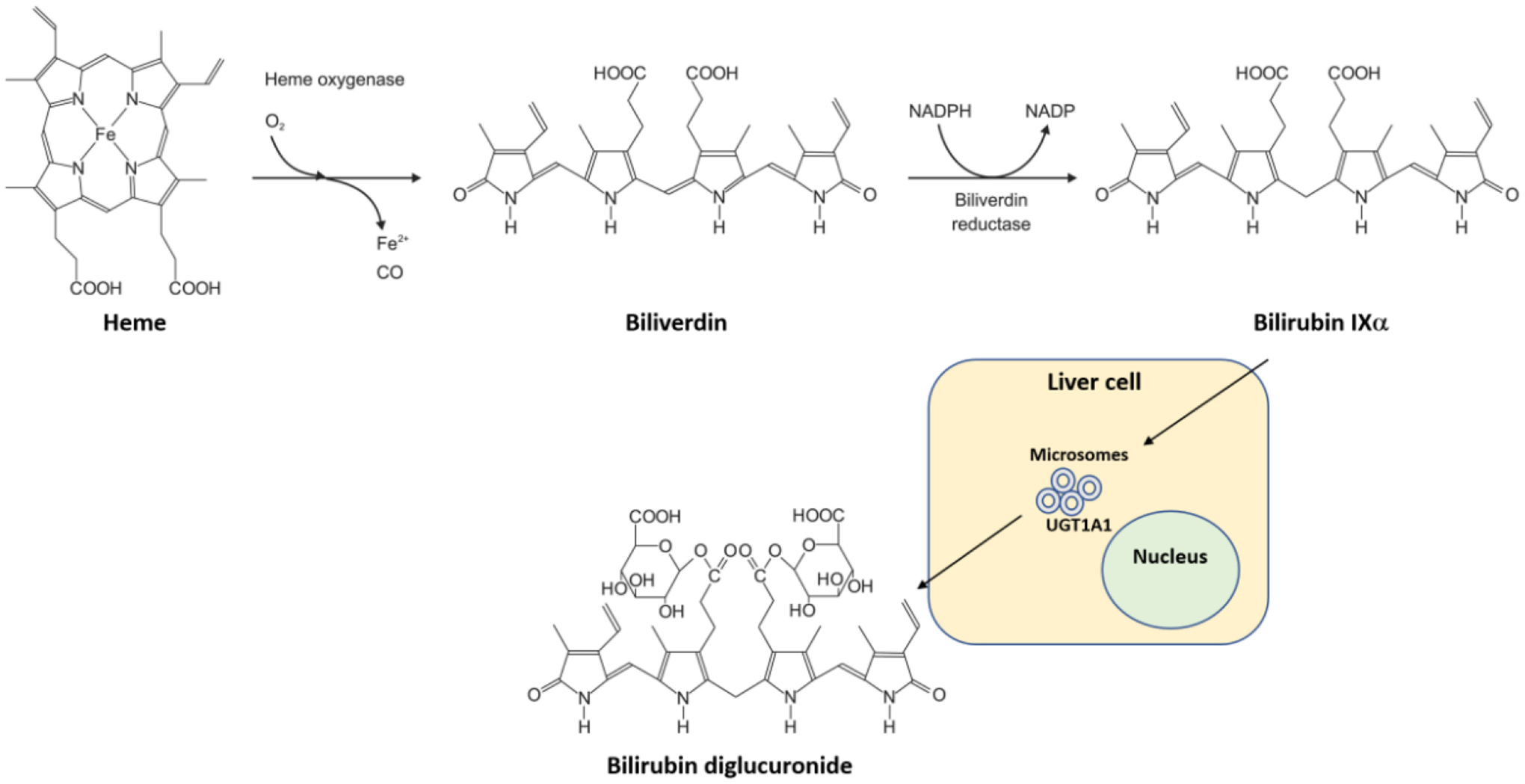
Heme is catabolized by heme oxygenase to biliverdin while releasing oxygen (O2), iron (Fe2+), and carbon monoxide (CO). Then, biliverdin reductase reduces biliverdin to bilirubin IXalpha by an NADPH to NADP mechanism. Bilirubin is cleared by hepatocytes (liver cells) via the UGT1A1 UDP-glucuronosyltransferase (UGT1A1, OMIM *191740) in microsomes by conjugation with glucuronide giving rise to bilirubin diglucuronide (also named bilirubin diglucuronoside according to IUBMB Enzyme Nomenclature), which is then excreted into the bile.
Clinician’s corner.
The serum/plasma concentration of bilirubin has traditionally been considered a marker of hepatic disease. However, emerging data show that increased levels of plasma bilirubin are protective and that low levels (0.58 mg/dl) can predispose patients to the development of cardiovascular and metabolic diseases. This raises several important questions that need to be addressed clinically. First, should bilirubin concentration be followed as a potential indicator of risk for cardiovascular and metabolic disease? Second, what decision limits should be used to define “normal” plasma bilirubin concentrations? Third, how can we effectively increase low levels of bilirubin in still-healthy subjects as well as in patients?
The results of several large-scale population studies have consistently demonstrated that plasma bilirubin concentrations correlate with protection against the development of cardiovascular and metabolic disease. These results suggest that physicians should routinely monitor the levels of bilirubin, especially in those subjects with risk factors for cardiovascular and metabolic diseases such as obesity, hypertension, type II diabetes, metabolic syndrome, metabolic dysfunction-associated liver disease (MAFLD), or those with a family history of cardiovascular and/or metabolic diseases.
The median serum/plasma bilirubin concentrations in the general population is around 10 μmol/l [1] μ [1] but the real range of “normal” bilirubin level still needs to be defined. However, the bilirubin levels can vary widely depending on the conditions in which plasma is collected. For example, prolonged fasting results in slightly increased levels of plasma bilirubin, and conditions like obesity are associated with decreased levels. Establishment of decision limits of bilirubin concentrations needs to be established so that individuals who exhibit levels below the established lower decision limit can be identified for treatments to increase plasma bilirubin levels.
There are currently no specific therapies approved for the treatment of low levels of plasma bilirubin. Potential therapies include: (i) formulations of bilirubin solubilized in a water-based formula which could be administered to patients orally or via injection; (ii) drugs that interfere with the natural conjugation of bilirubin in the liver; (iii) natural supplements that also target endogenous bilirubin metabolism, and (iv) a new concept that certain probiotics might alter the metabolism of bilirubin in the gut.
In light of the emerging data on the important role of bilirubin levels in health and disease, clinicians should recognize the need to measure bilirubin concentrations in patients at risk for cardiovascular and/or metabolic diseases. There needs to be an effort to specifically define “normal” bilirubin levels and develop a specific treatment for individuals who exhibit “low” levels of bilirubin (hypobilirubinemia).
Highlights.
Recent studies indicate that mildly elevated bilirubin levels in the blood have many health benefits.
The physiological mechanisms of bilirubin action in the human body are discussed with a focus on translational importance.
Bilirubin can be considered a hormone, in particular through its interaction with PPARα.
Increased concentrations of blood bilirubin levels above 10 μM are associated with protection from cardiovascular and metabolic diseases.
Bilirubin levels can be regulated by diet, aerobic activity, natural compounds, and some medications.
Acknowledgments
This work was supported by the National Institutes of Health R01DK121797 (T.D.H), R01DK126884 (D.E.S.), and the National Heart, Lung and Blood Institute P01 HL05197-11 (D.E.S.) and K01HL125445 (T.D.H.), and the National Institute of General Medical Sciences P20GM104357-02 (D.E.S.). This study was supported by grants MH CZ-DRO-VFN64165 (LV) from the Czech Ministry of Health, Cooperation Program, research area DIAG given by Charles University, and the project National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES LX22NPO5104) funded by European Union – Next Generation EU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
T.D.H. and D.E.S. have submitted patents on bilirubin- and obesity-related disorders. The remaining authors have no interests to declare.
References
- 1.Creeden JF et al. (2021) Bilirubin as a metabolic hormone: the physiological relevance of low levels. Am J Physiol Endocrinol Metab 320, E191–E207. 10.1152/ajpendo.00405.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vitek L and Tiribelli C (2021) Bilirubin: the yellow hormone? J Hepatol. 10.1016/j.jhep.2021.06.010 [DOI] [PubMed] [Google Scholar]
- 3.Hinds TD Jr. and Stec DE (2019) Bilirubin Safeguards Cardiorenal and Metabolic Diseases: a Protective Role in Health. Current hypertension reports 21, 87. 10.1007/s11906-019-0994-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinds TD Jr. and Stec DE (2018) Bilirubin, a Cardiometabolic Signaling Molecule. Hypertension 72, 788–795. 10.1161/HYPERTENSIONAHA.118.11130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon DM et al. (2021) Identification of Binding Regions of Bilirubin in the Ligand-Binding Pocket of the Peroxisome Proliferator-Activated Receptor-A (PPARalpha). Molecules 26. 10.3390/molecules26102975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon DM et al. (2020) Bilirubin remodels murine white adipose tissue by reshaping mitochondrial activity and the coregulator profile of peroxisome proliferator-activated receptor alpha. J Biol Chem. 10.1074/jbc.RA120.013700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon DM et al. (2019) RNA sequencing in human HepG2 hepatocytes reveals PPAR-alpha mediates transcriptome responsiveness of bilirubin. Physiol Genomics 51, 234–240. 10.1152/physiolgenomics.00028.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stec DE et al. (2016) Bilirubin Binding to PPARalpha Inhibits Lipid Accumulation. PLoS One 11, e0153427. 10.1371/journal.pone.0153427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ai W et al. (2021) Bilirubin Nanoparticles Protect Against Cardiac Ischemia/Reperfusion Injury in Mice. J Am Heart Assoc 10, e021212. 10.1161/JAHA.121.021212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinds TD Jr. et al. (2020) Bilirubin Nanoparticles Reduce Diet-Induced Hepatic Steatosis, Improve Fat Utilization, and Increase Plasma beta-Hydroxybutyrate. Front Pharmacol 11, 594574. 10.3389/fphar.2020.594574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MJ et al. (2017) PEGylated bilirubin nanoparticle as an anti-oxidative and anti-inflammatory demulcent in pancreatic islet xenotransplantation. Biomaterials 133, 242–252. 10.1016/j.biomaterials.2017.04.029 [DOI] [PubMed] [Google Scholar]
- 12.Lee Y et al. (2016) Bilirubin Nanoparticles as a Nanomedicine for Anti-inflammation Therapy. Angew Chem Int Ed Engl 55, 7460–7463. 10.1002/anie.201602525 [DOI] [PubMed] [Google Scholar]
- 13.Hamoud AR et al. (2018) Bilirubin in the Liver-Gut Signaling Axis. Trends Endocrinol Metab. 10.1016/j.tem.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundararaghavan VL et al. (2017) Glucuronidation and UGT isozymes in bladder: new targets for the treatment of uroepithelial carcinomas? Oncotarget 8, 3640–3648. 10.18632/oncotarget.12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinds TD Jr. et al. (2017) Mice with hyperbilirubinemia due to Gilbert’s Syndrome polymorphism are resistant to hepatic steatosis by decreased serine 73 phosphorylation of PPARalpha. Am J Physiol Endocrinol Metab, ajpendo 00396 02016. 10.1152/ajpendo.00396.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kipp ZA et al. (2023) Bilirubin Levels Are Negatively Correlated with Adiposity in Obese Men and Women, and Its Catabolized Product, Urobilin, Is Positively Associated with Insulin Resistance. Antioxidants 12, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seyed Khoei N et al. (2018) Mild hyperbilirubinaemia as an endogenous mitigator of overweight and obesity: Implications for improved metabolic health. Atherosclerosis 269, 306–311. 10.1016/j.atherosclerosis.2017.12.021 [DOI] [PubMed] [Google Scholar]
- 18.Sugatani J et al. (2008) Transcriptional regulation of human UGT1A1 gene expression through distal and proximal promoter motifs: implication of defects in the UGT1A1 gene promoter. N-S Arch Pharmacol 377, 597–605. 10.1007/s00210-007-0226-y [DOI] [PubMed] [Google Scholar]
- 19.Jangi S et al. (2013) The molecular basis for the immunomodulatory activities of unconjugated bilirubin. Int J Biochem Cell Biol 45, 2843–2851. 10.1016/j.biocel.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 20.Vitek L (2020) Bilirubin as a signaling molecule. Med Res Rev 40, 1335–1351. 10.1002/med.21660 [DOI] [PubMed] [Google Scholar]
- 21.Vitek L and Ostrow JD (2009) Bilirubin chemistry and metabolism; harmful and protective aspects. Current pharmaceutical design 15, 2869–2883. 10.2174/138161209789058237 [DOI] [PubMed] [Google Scholar]
- 22.Stec DE et al. (2020) Biliverdin Reductase A (BVRA) Knockout in Adipocytes Induces Hypertrophy and Reduces Mitochondria in White Fat of Obese Mice. Biomolecules 10. 10.3390/biom10030387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinds TD Jr. et al. (2016) Biliverdin reductase A attenuates hepatic steatosis by inhibition of glycogen synthase kinase (GSK) 3beta phosphorylation of serine 73 of peroxisome proliferator-activated receptor (PPAR) alpha. J Biol Chem. 10.1074/jbc.M116.731703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien L et al. (2015) Biliverdin reductase isozymes in metabolism. Trends Endocrinol Metab 26, 212–220. 10.1016/j.tem.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vitek L (2019) Bilirubin as a predictor of diseases of civilization. Is it time to establish decision limits for serum bilirubin concentrations? Arch Biochem Biophys 672, 108062. 10.1016/j.abb.2019.108062 [DOI] [PubMed] [Google Scholar]
- 26.Zucker SD et al. (1999) Unconjugated bilirubin exhibits spontaneous diffusion through model lipid bilayers and native hepatocyte membranes. J Biol Chem 274, 10852–10862 [DOI] [PubMed] [Google Scholar]
- 27.Mediavilla MG et al. (1999) Uptake of [(3)H]bilirubin in freshly isolated rat hepatocytes: role of free bilirubin concentration. FEBS letters 463, 143–145. 10.1016/s0014-5793(99)01606-3 [DOI] [PubMed] [Google Scholar]
- 28.Levi AJ et al. (1969) Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest 48, 2156–2167. 10.1172/JCI106182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumagai A et al. (2013) A bilirubin-inducible fluorescent protein from eel muscle. Cell 153, 1602–1611. 10.1016/j.cell.2013.05.038 [DOI] [PubMed] [Google Scholar]
- 30.Adeosun SO et al. (2018) A Novel Fluorescence-Based Assay for the Measurement of Biliverdin Reductase Activity. React Oxyg Species (Apex) 5, 35–45. 10.20455/ros.2018.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stocker R et al. (1987) Bilirubin is an antioxidant of possible physiological importance. Science 235, 1043–1046 [DOI] [PubMed] [Google Scholar]
- 32.Sedlak TW et al. (2009) Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci U S A 106, 5171–5176. 10.1073/pnas.0813132106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas DT et al. (2022) Reactive Oxygen Species (ROS) and Antioxidants as Immunomodulators in Exercise: Implications for Heme Oxygenase and Bilirubin. Antioxidants (Basel) 11. 10.3390/antiox11020179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meixiong J et al. (2019) Identification of a bilirubin receptor that may mediate a component of cholestatic itch. eLife 8, e44116. 10.7554/eLife.44116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinal CJ and Bend JR (1997) Aryl hydrocarbon receptor-dependent induction of cyp1a1 by bilirubin in mouse hepatoma hepa 1c1c7 cells. Molecular pharmacology 52, 590–599 [DOI] [PubMed] [Google Scholar]
- 36.Phelan D et al. (1998) Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch Biochem Biophys 357, 155–163. 10.1006/abbi.1998.0814 [DOI] [PubMed] [Google Scholar]
- 37.Danilov SM et al. (2016) Lysozyme and bilirubin bind to ACE and regulate its conformation and shedding. Sci Rep 6, 34913. 10.1038/srep34913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phelan DM et al. (1998) The Ah receptor can bind ligand in the absence of receptor-associated heat-shock protein 90. Arch Biochem Biophys 353, 47–54. 10.1006/abbi.1997.0614 [DOI] [PubMed] [Google Scholar]
- 39.Denison MS et al. (1988) Inducible, receptor-dependent protein-DNA interactions at a dioxin-responsive transcriptional enhancer. Proc Natl Acad Sci U S A 85, 2528–2532. 10.1073/pnas.85.8.2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seree E et al. (2004) Evidence for a new human CYP1A1 regulation pathway involving PPAR-alpha and 2 PPRE sites. Gastroenterology 127, 1436–1445 [DOI] [PubMed] [Google Scholar]
- 41.Kim HG et al. (2008) Effect of troglitazone on CYP1A1 induction. Toxicology 246, 166–171. 10.1016/j.tox.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 42.Fallone F et al. (2005) PPARalpha activation potentiates AhR-induced CYP1A1 expression. Toxicology 216, 122–128. 10.1016/j.tox.2005.07.020 [DOI] [PubMed] [Google Scholar]
- 43.Hong S et al. (2021) Bilirubin: A Ligand of the PPARα Nuclear Receptor. In Nuclear Receptors: The Art and Science of Modulator Design and Discovery (Badr MZ, ed), pp. 463–482, Springer International Publishing [Google Scholar]
- 44.Hinds TD Jr. et al. (2020) Rats Genetically Selected for High Aerobic Exercise Capacity Have Elevated Plasma Bilirubin by Upregulation of Hepatic Biliverdin Reductase-A (BVRA) and Suppression of UGT1A1. Antioxidants (Basel) 9. 10.3390/antiox9090889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weaver L et al. (2018) Biliverdin reductase and bilirubin in hepatic disease. Am J Physiol Gastrointest Liver Physiol 314, G668–G676. 10.1152/ajpgi.00026.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundararaghavan VL et al. (2018) Bilirubin, a new therapeutic for kidney transplant? Transplant Rev (Orlando). 10.1016/j.trre.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang W et al. (2003) Induction of bilirubin clearance by the constitutive androstane receptor (CAR). Proc Natl Acad Sci U S A 100, 4156–4161. 10.1073/pnas.0630614100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wieneke N et al. (2007) PPARalpha-dependent induction of the energy homeostasis-regulating nuclear receptor NR1i3 (CAR) in rat hepatocytes: potential role in starvation adaptation. FEBS letters 581, 5617–5626. 10.1016/j.febslet.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 49.Saito K et al. (2010) Peroxisome proliferator-activated receptor alpha (PPARalpha) agonists induce constitutive androstane receptor (CAR) and cytochrome P450 2B in rat primary hepatocytes. Drug Metab Pharmacokinet 25, 108–111 [DOI] [PubMed] [Google Scholar]
- 50.Liu J et al. (2015) Bilirubin Increases Insulin Sensitivity by Regulating Cholesterol Metabolism, Adipokines and PPARgamma Levels. Sci Rep 5, 9886. 10.1038/srep09886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baker NA et al. (2015) Effects of Adipocyte Aryl Hydrocarbon Receptor Deficiency on PCB-Induced Disruption of Glucose Homeostasis in Lean and Obese Mice. Environmental health perspectives 123, 944–950. 10.1289/ehp.1408594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vitek L (2017) Bilirubin and atherosclerotic diseases. Physiol Res 66, S11–S20. 10.33549/physiolres.933581 [DOI] [PubMed] [Google Scholar]
- 53.Wagner KH et al. (2015) Looking to the horizon: the role of bilirubin in the development and prevention of age-related chronic diseases. Clin Sci (Lond) 129, 1–25. 10.1042/CS20140566 [DOI] [PubMed] [Google Scholar]
- 54.Seyed Khoei N et al. (2022) Bilirubin as an indicator of cardiometabolic health: a cross-sectional analysis in the UK Biobank. Cardiovasc Diabetol 21, 54. 10.1186/s12933-022-01484-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molzer C et al. (2016) Features of an altered AMPK metabolic pathway in Gilbert’s Syndrome, and its role in metabolic health. Sci Rep 6, 30051. 10.1038/srep30051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hinds TD Jr. et al. (2016) Does bilirubin prevent hepatic steatosis through activation of the PPARalpha nuclear receptor? Medical hypotheses 95, 54–57. 10.1016/j.mehy.2016.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nath KA et al. (2007) An analysis of the DOCA-salt model of hypertension in HO-1−/− mice and the Gunn rat. Am. J. Physiol Heart Circ. Physiol 293, H333–H342. 00870.2006 [pii]; 10.1152/ajpheart.00870.2006 [doi] [DOI] [PubMed] [Google Scholar]
- 58.Pflueger A et al. (2005) The hyperbilirubinemic Gunn rat is resistant to the pressor effects of angiotensin II. Am. J. Physiol Renal Physiol 288, F552–F558 [DOI] [PubMed] [Google Scholar]
- 59.Bakrania B et al. (2014) Hyperbilirubinemia modulates myocardial function, aortic ejection, and ischemic stress resistance in the Gunn rat. Am J Physiol Heart Circ Physiol 307, H1142–1149. 10.1152/ajpheart.00001.2014 [DOI] [PubMed] [Google Scholar]
- 60.Bakrania B et al. (2017) Chronically elevated bilirubin protects from cardiac reperfusion injury in the male Gunn rat. Acta Physiol (Oxf) 220, 461–470. 10.1111/apha.12858 [DOI] [PubMed] [Google Scholar]
- 61.Bianco A et al. (2021) Life-Long Hyperbilirubinemia Exposure and Bilirubin Priming Prevent In Vitro Metabolic Damage. Front Pharmacol 12, 646953. 10.3389/fphar.2021.646953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vera T et al. (2009) Inhibition of bilirubin metabolism induces moderate hyperbilirubinemia and attenuates ANG II-dependent hypertension in mice. Am. J. Physiol Regul. Integr. Comp Physiol 297, R738–R743. 90889.2008 [pii]; 10.1152/ajpregu.90889.2008 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vera T and Stec DE (2010) Moderate hyperbilirubinemia improves renal hemodynamics in ANG II-dependent hypertension. Am. J. Physiol Regul. Integr. Comp Physiol 299, R1044–R1049. ajpregu.00316.2010 [pii]; 10.1152/ajpregu.00316.2010 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boon AC et al. (2015) Endogenously elevated bilirubin modulates kidney function and protects from circulating oxidative stress in a rat model of adenine-induced kidney failure. Sci Rep 5, 15482. 10.1038/srep15482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallner M et al. (2013) Protection from age-related increase in lipid biomarkers and inflammation contributes to cardiovascular protection in Gilbert’s syndrome. Clin. Sci. (Lond) 125, 257–264. CS20120661 [pii]; 10.1042/CS20120661 [doi] [DOI] [PubMed] [Google Scholar]
- 66.Bulmer AC et al. (2008) Improved resistance to serum oxidation in Gilbert’s syndrome: a mechanism for cardiovascular protection. Atherosclerosis 199, 390–396. S0021–9150(07)00744–7 [pii]; 10.1016/j.atherosclerosis.2007.11.022 [doi] [DOI] [PubMed] [Google Scholar]
- 67.Stec DE et al. (2013) Antihypertensive actions of moderate hyperbilirubinemia: role of superoxide inhibition. Am. J. Hypertens 26, 918–923. hpt038 [pii]; 10.1093/ajh/hpt038 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J et al. (2015) Unconjugated Bilirubin Mediates Heme Oxygenase-1-Induced Vascular Benefits in Diabetic Mice. Diabetes 64, 1564–1575. 10.2337/db14-1391 [DOI] [PubMed] [Google Scholar]
- 69.Lanone S et al. (2005) Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats. FASEB J 19, 1890–1892 [DOI] [PubMed] [Google Scholar]
- 70.Murakami H et al. (2006) Fenofibrate activates AMPK and increases eNOS phosphorylation in HUVEC. Biochemical and biophysical research communications 341, 973–978. 10.1016/j.bbrc.2006.01.052 [DOI] [PubMed] [Google Scholar]
- 71.Wang Y et al. (2006) Effects of bezafibrate on the expression of endothelial nitric oxide synthase gene and its mechanisms in cultured bovine endothelial cells. Atherosclerosis 187, 265–273. 10.1016/j.atherosclerosis.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 72.McCarty MF (2007) “Iatrogenic Gilbert syndrome”- a strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Medical hypotheses 69, 974–994 [DOI] [PubMed] [Google Scholar]
- 73.Perlstein TS et al. (2008) Serum total bilirubin level and prevalent lower-extremity peripheral arterial disease: National Health and Nutrition Examination Survey (NHANES) 1999 to 2004. Arterioscler Thromb Vasc Biol 28, 166–172. 10.1161/ATVBAHA.107.153262 [DOI] [PubMed] [Google Scholar]
- 74.Novotny L and Vitek L (2003) Inverse relationship between serum bilirubin and atherosclerosis in men: A meta-analysis of published studies. Exp Biol Med 228, 568–571. Doi 10.1177/15353702-0322805-29 [DOI] [PubMed] [Google Scholar]
- 75.McCallum L et al. (2015) Longitudinal blood pressure control, long-term mortality, and predictive utility of serum liver enzymes and bilirubin in hypertensive patients. Hypertension 66, 37–43. 10.1161/HYPERTENSIONAHA.114.04915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiraskova A, Jovanovska J, Skrha J, Vitek L (2011) Association of low bilirubin levels and promoter variations in UGT1A1 gene with diabetes mellitus type 2 (abstract). Hepatology international 5, 37 [Google Scholar]
- 77.Jiraskova A et al. (2012) Association of serum bilirubin and promoter variations in HMOX1 and UGT1A1 genes with sporadic colorectal cancer. International journal of cancer. Journal international du cancer 131, 1549–1555. 10.1002/ijc.27412 [doi] [DOI] [PubMed] [Google Scholar]
- 78.Lenicek M et al. (2014) The relationship between serum bilirubin and Crohn’s disease. Inflamm Bowel Dis 20, 481–487. 10.1097/01.MIB.0000440817.84251.98 [DOI] [PubMed] [Google Scholar]
- 79.Vitek L et al. (2010) Association of systemic lupus erythematosus with low serum bilirubin levels. Scand J Rheumatol 39, 480–484. 10.3109/03009741003742748 [DOI] [PubMed] [Google Scholar]
- 80.Jiraskova A et al. (2017) Serum bilirubin levels and promoter variations in HMOX1 and UGT1A1 genes in patients with Fabry disease. Oxid Med Cell Longev 2017, 9478946. 10.1155/2017/9478946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vitek L et al. (2010) Serum bilirubin levels and UGT1A1 promoter variations in patients with schizophrenia. Psychiatry Res 178, 449–450. 10.1016/j.psychres.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 82.Andersson C et al. (2009) Acute effect of weight loss on levels of total bilirubin in obese, cardiovascular high-risk patients: an analysis from the lead-in period of the Sibutramine Cardiovascular Outcome trial. Metabolism 58, 1109–1115. 10.1016/j.metabol.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 83.Jenko-Praznikar Z et al. (2013) Serum bilirubin levels are lower in overweight asymptomatic middle-aged adults: An early indicator of metabolic syndrome? Metabolism 62, 976–985. 10.1016/j.metabol.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 84.Takei R et al. (2019) Bilirubin reduces visceral obesity and insulin resistance by suppression of inflammatory cytokines. PLoS One 14, e0223302. 10.1371/journal.pone.0223302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.El-Eshmawy MM et al. (2022) Association between total bilirubin levels and cardiometabolic risk factors related to obesity. Endocr Metab Immune Disord Drug Targets 22, 64–70. 10.2174/1871530321999210128201259 [DOI] [PubMed] [Google Scholar]
- 86.Fu J et al. (2022) Serum Bilirubin Level Is Increased in Metabolically Healthy Obesity. Frontiers in Endocrinology 12. 10.3389/fendo.2021.792795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Belo L et al. (2014) Body fat percentage is a major determinant of total bilirubin independently of UGT1A1*28 polymorphism in young obese. PLoS One 9, e98467. 10.1371/journal.pone.0098467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pietrocola F and Bravo-San Pedro JM (2021) Targeting autophagy to counteract obesity-associated oxidative stress. Antioxidants (Basel) 10. 10.3390/antiox10010102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang C et al. (2021) Relationship between serum bilirubin concentration and sarcopenia in patients with type 2 diabetes: a cross-sectional study. J Int Med Res 49, 3000605211004226. 10.1177/03000605211004226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Woronyczova J et al. (2022) Serum bilirubin concentrations and the prevalence of Gilbert syndrome in elite athletes. Sports Med Open Access, 1–10. 10.1186/s40798-022-00463-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kotal P et al. (1996) Fasting-related hyperbilirubinemia in rats: the effect of decreased intestinal motility. Gastroenterology 111, 217–223. 10.1053/gast.1996.v111.pm8698202 [DOI] [PubMed] [Google Scholar]
- 92.Dorling JL et al. (2020) Calorie restriction for enhanced longevity: The role of novel dietary strategies in the present obesogenic environment. Ageing Res Rev 64, 101038. 10.1016/j.arr.2020.101038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roth LW and Polotsky AJ (2012) Can we live longer by eating less? A review of caloric restriction and longevity. Maturitas 71, 315–319. 10.1016/j.maturitas.2011.12.017 [DOI] [PubMed] [Google Scholar]
- 94.Chmielewski P et al. (2017) Association of serum bilirubin with longevity: Evidence from a retrospective longitudinal study and cross-sectional data. Anthropol Rev 80, 335–348. 10.1515/anre-2017-0024 [DOI] [Google Scholar]
- 95.Aoki Y (2020) Higher serum levels of indirect bilirubin and polyunsaturated fatty acids in Japanese centenarians with better performance and nutrition status. Acta Scientifci Nutritional Health 4, 01–04. 10.31080/asnh.2020.04.0630 [DOI] [Google Scholar]
- 96.Horsfall LJ et al. (2013) Gilbert’s syndrome and the risk of death: a population-based cohort study. Journal of gastroenterology and hepatology 28, 1643–1647. 10.1111/jgh.12279 [DOI] [PubMed] [Google Scholar]
- 97.Tosevska A et al. (2016) Longer telomeres in chronic, moderate, unconjugated hyperbilirubinaemia: insights from a human study on Gilbert’s Syndrome. Sci Rep 6, 22300. 10.1038/srep22300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Loprinzi PD and Mahoney SE (2015) Association between flavonoid-rich fruit and vegetable consumption and total serum bilirubin. Angiology 66, 286–290. 10.1177/0003319714537111 [DOI] [PubMed] [Google Scholar]
- 99.He W et al. (2021) Higher serum bilirubin levels in response to higher carbohydrate intake during early pregnancy and lower gestational diabetes mellitus occurrence in overweight and obese gravidae. Front Nutr 8, 701422. 10.3389/fnut.2021.701422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.(1976) Gilbert’s syndrome: diet and starvation. Nutrition reviews 34, 328–330. 10.1111/j.1753-4887.1976.tb05811.x [DOI] [PubMed] [Google Scholar]
- 101.Gollan JL et al. (1976) Effect of dietary composition on the unconjugated hyperbilirubinaemia of Gilbert’s syndrome. Gut 17, 335–340. 10.1136/gut.17.5.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Figge A et al. (2021) Gender and gut microbiota composition determine hepatic bile acid, metabolic and inflammatory response to a single fast-food meal in healthy adults. Clin Nutr 40, 2609–2619. 10.1016/j.clnu.2021.04.008 [DOI] [PubMed] [Google Scholar]
- 103.Pallister T et al. (2017) Untangling the relationship between diet and visceral fat mass through blood metabolomics and gut microbiome profiling. International journal of obesity (2005) 41, 1106–1113. 10.1038/ijo.2017.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Swift DL et al. (2012) Effect of different doses of aerobic exercise training on total bilirubin levels. Med Sci Sports Exerc 44, 569–574. 10.1249/MSS.0b013e3182357dd4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loprinzi PD and Abbott K (2014) Physical activity and total serum bilirubin levels among insulin sensitive and insulin resistant U.S. adults. Journal of diabetes and metabolic disorders 13, 47. 10.1186/2251-6581-13-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kyle D Flack LV, Christopher Fry, Stec David E. and Hinds Terry D. Jr. (2023) Cutting Edge Concepts: Does Bilirubin Enhance Exercise Performance? Frontiers in Sports and Active Living. doi: 10.3389/fspor.2022.1040687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wagner KH et al. (2018) Diagnostic criteria and contributors to Gilbert’s syndrome. Crit Rev Clin Lab Sci 55, 129–139. 10.1080/10408363.2018.1428526 [DOI] [PubMed] [Google Scholar]
- 108.Bulmer AC et al. (2018) Bilirubin acts as a multipotent guardian of cardiovascular integrity: more than just a radical idea. American Journal of Physiology-Heart and Circulatory Physiology 315, H429–H447. 10.1152/ajpheart.00417.2017 [DOI] [PubMed] [Google Scholar]
- 109.Stec DE and Hinds TD Jr. (2020) Natural Product Heme Oxygenase Inducers as Treatment for Nonalcoholic Fatty Liver Disease. Int J Mol Sci 21. 10.3390/ijms21249493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vitek L et al. (2019) Induction of mild hyperbilirubinemia: Hype or real therapeutic opportunity? Clin Pharmacol Ther 106, 568–575. 10.1002/cpt.1341 [DOI] [PubMed] [Google Scholar]
- 111.D’Andrea V et al. (2005) Inhibition of rat liver UDP-glucuronosyltransferase by silymarin and the metabolite silibinin-glucuronide. Life Sci 77, 683–692. 10.1016/j.lfs.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 112.Suk J et al. (2017) Milk thistle natural polyphenols increase systemic as well as hepatic concentrations of bilirubin and decrease hepatic lipoperoxidation in mice. Hepatology 66, 231a–231a [Google Scholar]
- 113.Vidimce J et al. (2021) Effect of Silymarin Treatment on Circulating Bilirubin and Cardiovascular Disease Risk Factors in Healthy Men: A Single-Blind, Randomized Crossover Trial. Clin Pharmacol Drug Dev 10, 1156–1165. 10.1002/cpdd.962 [DOI] [PubMed] [Google Scholar]
- 114.Xu J et al. (2012) UDP-glucuronosyltransferase expression in mouse liver is increased in obesity- and fasting-induced steatosis. Drug metabolism and disposition: the biological fate of chemicals 40, 259–266. 10.1124/dmd.111.039925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang P et al. (2020) Impact of obese levels on the hepatic expression of nuclear receptors and drug-metabolizing enzymes in adult and offspring mice. Acta Pharm Sin B 10, 171–185. 10.1016/j.apsb.2019.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mohamed MF et al. (2010) Inhibitory effects of commonly used herbal extracts on UGT1A1 enzyme activity. Xenobiotica 40, 663–669. 10.3109/00498254.2010.505669 [doi] [DOI] [PubMed] [Google Scholar]
- 117.Katoh M et al. (2009) Effects of Japanese herbal medicine, Kampo, on human UGT1A1 activity. Drug Metab Pharmacokinet 24, 226–234. 10.2133/dmpk.24.226 [DOI] [PubMed] [Google Scholar]
- 118.Alkharfy KM and Frye RF (2007) Effect of valerian, valerian/hops extracts, and valerenic acid on glucuronidation in vitro. Xenobiotica 37, 113–123. 10.1080/00498250601050420 [doi] [DOI] [PubMed] [Google Scholar]
- 119.Mohamed ME and Frye RF (2011) Effects of herbal supplements on drug glucuronidation. Review of clinical, animal, and in vitro studies. Planta medica 77, 311–321. 10.1055/s-0030-1250457 [DOI] [PubMed] [Google Scholar]
- 120.Bach FH (2005) Heme oxygenase-1: a therapeutic amplification funnel. FASEB J 19, 1216–1219. 10.1096/fj.04-3485cmt [DOI] [PubMed] [Google Scholar]
- 121.Maines MD (1997) The heme oxygenase system: a regulator of second messenger gases. Annual review of pharmacology and toxicology 37, 517–554. 10.1146/annurev.pharmtox.37.1.517 [DOI] [PubMed] [Google Scholar]
- 122.Exner M et al. (2004) The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med 37, 1097–1104. 10.1016/j.freeradbiomed.2004.07.008 [DOI] [PubMed] [Google Scholar]
- 123.Ma LL et al. (2022) Association between HO- 1 gene promoter polymorphisms and diseases (Review). Molecular medicine reports 25. 10.3892/mmr.2021.12545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Muchova L et al. (2007) Statin treatment increases formation of carbon monoxide and bilirubin in mice: a novel mechanism of in vivo antioxidant protection. Can J Physiol Pharmacol 85, 800–810. 10.1139/y07-077 [DOI] [PubMed] [Google Scholar]
- 125.Muchova L et al. (2015) Protective effect of heme oxygenase induction in ethinylestradiol-induced cholestasis. Journal of cellular and molecular medicine 19, 924–933. 10.1111/jcmm.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li M et al. (2020) Association between atazanavir-induced hyperbilirubinemia and cardiovascular disease in patients infected with HIV. J Am Heart Assoc 9, e016310. 10.1161/JAHA.120.016310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stein JH et al. (2015) A prospective, randomized clinical trial of antiretroviral therapies on carotid wall thickness. AIDS 29, 1775–1783. 10.1097/QAD.0000000000000762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chow D et al. (2016) Atazanavir use and carotid intima media thickness progression in HIV: potential influence of bilirubin. AIDS 30, 672–674. 10.1097/QAD.0000000000000970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu M and Tomlinson B (2012) Effects of statin treatments and polymorphisms in UGT1A1 and SLCO1B1 on serum bilirubin levels in Chinese patients with hypercholesterolaemia. Atherosclerosis 223, 427–432. S0021–9150(12)00352–8 [pii]; 10.1016/j.atherosclerosis.2012.06.002 [doi] [DOI] [PubMed] [Google Scholar]
- 130.de Sauvage Nolting PR et al. (2011) Serum bilirubin levels in familial hypercholesterolemia: a new risk marker for cardiovascular disease? J Lipid Res 52, 1755–1759. 10.1194/jlr.P013193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mirjanic-Azaric B et al. (2015) Atorvastatin treatment increases plasma bilirubin but not HMOX1 expression in stable angina patients. Scand J Clin Lab Invest 75, 382–389. 10.3109/00365513.2015.1031691 [DOI] [PubMed] [Google Scholar]
- 132.Rollinghoff W et al. (1981) Nicotinic acid test in the diagnosis of Gilbert’s syndrome: correlation with bilirubin clearance. Gut 22, 663–668. 10.1136/gut.22.8.663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.He YJ et al. (2008) Hepatic nuclear factor 1alpha inhibitor ursodeoxycholic acid influences pharmacokinetics of the organic anion transporting polypeptide 1B1 substrate rosuvastatin and bilirubin. Drug metabolism and disposition: the biological fate of chemicals 36, 1453–1456. 10.1124/dmd.108.020503 [DOI] [PubMed] [Google Scholar]
- 134.Dekker D et al. (2018) Parenteral bilirubin in healthy volunteers: a reintroduction in translational research. Br J Clin Pharmacol 84, 268–279. 10.1111/bcp.13458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yao Q et al. (2020) Therapeutic application and construction of bilirubin incorporated nanoparticles. J Control Release 328, 407–424. 10.1016/j.jconrel.2020.08.054 [DOI] [PubMed] [Google Scholar]
- 136.Kim DE et al. (2017) Bilirubin nanoparticles ameliorate allergic lung inflammation in a mouse model of asthma. Biomaterials 140, 37–44. 10.1016/j.biomaterials.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 137.Lee Y et al. (2016) Multistimuli-responsive bilirubin nanoparticles for anticancer therapy. Angew Chem Int Ed Engl 55, 10676–10680. 10.1002/anie.201604858 [DOI] [PubMed] [Google Scholar]
- 138.Lee Y et al. (2018) Biotinylated Bilirubin Nanoparticles as a Tumor Microenvironment-Responsive Drug Delivery System for Targeted Cancer Therapy. Adv Sci 5, 1–8. ARTN 1800017 10.1002/advs.201800017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fullagar B et al. (2017) Nano-encapsulation of bilirubin in pluronic F127-chitosan improves uptake in beta cells and increases islet viability and function after hypoxic stress. Cell Transplant 26, 1703–1715. 10.1177/0963689717735112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee DY et al. (2017) Black pigment gallstone-inspired platinum-chelated bilirubin nanoparticles for combined photoacoustic imaging and photothermal therapy of cancers. Angew Chem Int Ed Engl. 10.1002/anie.201707137 [DOI] [PubMed] [Google Scholar]
- 141.Vitek L and Tiribelli CT (2020) Bilirubin, intestinal integrity, the microbiome, and inflammation. New Eng J Med 383, 684–686. 10.1056/NEJMcibr2013250 [DOI] [PubMed] [Google Scholar]
- 142.De Jong WH and Borm PJ (2008) Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine 3, 133–149. 10.2147/ijn.s596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Su S and Kang PM (2020) Systemic review of biodegradable nanomaterials in nanomedicine. Nanomaterials-Basel 10. ARTN 656 10.3390/nano10040656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Newman JC and Verdin E (2014) Ketone bodies as signaling metabolites. Trends Endocrinol Metab 25, 42–52. 10.1016/j.tem.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Newman JC and Verdin E (2017) beta-Hydroxybutyrate: A Signaling Metabolite. Annu Rev Nutr 37, 51–76. 10.1146/annurev-nutr-071816-064916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ryter SW et al. (2006) Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86, 583–650. 10.1152/physrev.00011.2005 [DOI] [PubMed] [Google Scholar]
- 147.Morse D and Choi AMK (2002) Heme oxygenase-1 - The “emerging molecule” has arrived. Am J Resp Cell Mol 27, 8–16. DOI 10.1165/ajrcmb.27.1.4862 [DOI] [PubMed] [Google Scholar]
- 148.Sedlak TW and Snyder SH (2004) Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics 113, 1776–1782 [DOI] [PubMed] [Google Scholar]
- 149.Brodersen R (1980) Binding of bilirubin to albumin. CRC Crit Rev Clin Lab Sci 11, 305–399 [PubMed] [Google Scholar]
- 150.Bosma PJ et al. (1995) The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med 333, 1171–1175. 10.1056/NEJM199511023331802 [DOI] [PubMed] [Google Scholar]
- 151.Eremiasova L et al. (2020) Serum bilirubin in the Czech population. Relationship to the risk of myocardial infarction in males. Circ J. 10.1253/circj.CJ-20-0192 [DOI] [PubMed] [Google Scholar]
- 152.Vitek L et al. (2006) Identification of bilirubin reduction products formed by Clostridium perfringens isolated from human neonatal fecal flora. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 833, 149–157. 10.1016/j.jchromb.2006.01.032 [DOI] [PubMed] [Google Scholar]
- 153.Rodrigues RR et al. (2021) Transkingdom interactions between Lactobacilli and hepatic mitochondria attenuate western diet-induced diabetes. Nat Commun 12, 101. 10.1038/s41467-020-20313-x [DOI] [PMC free article] [PubMed] [Google Scholar]


